Abstract
Background
Bisphosphonate-associated femur fractures have been well described but the preoperative patient factors, treatment modalities, and complications of treatment are unclear.
Questions/purposes
We asked whether a diagnosis of osteoporosis, the characteristic radiographic features of bisphosphonate-related femur fractures, and complication rates differed in patients with operatively treated femoral shaft fractures receiving bisphosphonates and in patients not receiving bisphosphonates.
Methods
We retrospectively reviewed 43 patients with bisphosphonate-associated femoral shaft fractures (including subtrochanteric) from 2002 to 2008 and 20 patients with similar fractures but not treated with bisphosphonates. Similar implants were used in both groups, but a greater number of adjuvants were used in the bisphosphonate cohort. We recorded preoperative osteoporosis and radiographic findings of the characteristic bisphosphonate femur fracture and early complications. The minimum followup was 5 months (mean, 29 months; range 5–60 months).
Results
Preoperatively a greater percentage of patients treated with bisphosphonates had confirmed osteoporosis than those not treated with bisphosphonates (24% versus 5%, respectively), a greater percentage had a proximal fracture location (48% versus 40%, respectively), and their mean cortex to shaft diameter ratio was greater (24% versus 15%, respectively). The bisphosphonate cohort had a higher rate of intraoperative fractures (21% versus 0%) and postoperative plate failures (30% versus 0%).
Conclusions
Despite low rates of other risk factors and ample use of biologic adjuvants, patients treated with bisphosphonates having femur fractures have more complications.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Bisphosphonates have become a mainstay in the treatment of osteoporosis by increasing bone mineral density and prevention of fragility fractures in patients with osteoporosis [6, 12, 13, 15, 20, 28, 32]. As a result of inhibition of bone resorption (through the inhibition of osteoclasts), bisphosphonates can cause accumulation of trabecular microdamage or perhaps contribute to the aging of collagen fibers [3, 14, 22, 23]. This may compromise the mechanical and regenerative properties of bone, resulting in fractures and delayed bone healing [3, 8, 21–23, 26]. Many studies document an increased risk of characteristic fractures of the femur in patients taking bisphosphonate medications [1, 2, 9, 14, 16–19, 24, 25, 27, 29, 30].
Although the FDA recently added a femur fracture warning to labels of bisphosphonate medications, the operative management, complications, and healing rates of this unique fracture have not been well characterized. Several studies have examined the incidence and characteristics of these fractures [9, 17–19, 25], but those that discuss operative treatment include very small numbers of patients, have no control group, and often do not address complications or healing times [3, 8, 13, 14, 20, 22, 27] (Table 1). Proper knowledge of these fractures is especially important as their biologic features, and likely compromised bone healing, may result in longer healing times and a greater number of complications than their unmedicated counterparts.
Table 1.
Literature review
| Study | Number of patients | Average age (years) | Percent female | Average followup | Implants | Adjuvants | Complications | Nonunions | Time to union |
|---|---|---|---|---|---|---|---|---|---|
| Das De et al. [7] | 12 | 63.1 | 100% | N/A | Six nails, six plates | N/A | Three plate failures | Three | N/A |
| Goh et al. [9] | 9 | 66.9 | 100% | N/A | N/A | N/A | N/A | N/A | N/A |
| Kwek et al. [17] | 17 | 66 | 100% | N/A | N/A | N/A | N/A | N/A | N/A |
| Somford et al. [31] | 3 | 73 | 100% | N/A | N/A | N/A | One contralateral fracture | N/A | N/A |
| Neviaser et al. [25] | 19 | 69.5 | 100% | N/A | N/A | N/A | N/A | N/A | N/A |
| Lenart et al. [18] | 10 | 70.4 | 100% | N/A | N/A | N/A | N/A | N/A | N/A |
| Ha et al. [11] | 11 | 68 | 100% | 27 months | Five nails, five plates | N/A | None | None | N/A |
| Capeci & Tejwani [4] | 7 | 61 | 100% | N/A | Nails in 100% | None | None | None | 4 months |
| Cermak et al. [5] | 3 | 64.3 | 100% | 8.33 months | Two nails, one plate | N/A | One delayed union | None | 7.3 months |
N/A = not available
We sought to determine whether there were: (1) differences in osteoporosis and presence of the characteristic femur fracture in patients receiving bisphosphonate therapy versus an unmedicated cohort; and (2) more complications in treatment of these fractures.
Patients and Methods
From a single-surgeon (DGL) operative database we identified all 43 patients who had sustained acutely treated fractures of the femoral shaft (from subtrochanteric to distal shaft) between 2002 and 2008 and who currently were taking a bisphosphonate, and had been doing so for at least 1 year. All patients with fractures are asked routinely by the admitting physician whether they are taking or have a history of taking a bisphosphonate, and the duration of use. We excluded 18 patients who had high-energy injuries, fractures other than those of the femoral shaft, or were nonambulatory before injury. This left a cohort of 25 patients meeting the study criteria, none of whom was included in previous studies. To create a control population we then queried the database for the following: female, acutely treated traumatic fractures of the femoral shaft (from subtrochanteric to distal shaft) treated between 2002 and 2008, no history of bisphosphonate use, no other fracture or other system injury, older than 50 years, and low-energy mechanism of injury. Twenty patients met these criteria (Table 2). All patients were female and all but one sustained a standing-height fall (she fell from several stairs). The average duration of bisphosphonate therapy was 7.6 years (range, 1–12 years; SD, 3.4 years). Fractures were consistent with the previously described bisphosphonate-related femur fracture: transverse pattern with cortical thickening and beaking typically located between the lesser trochanter and middle 1/3 of the femur [25], with a mean distance of 6 cm distal to the lesser trochanter. The minimum clinical followup was 5 months (mean, 29 months; range, 5–60 months). No patients were lost to followup. No patients were recalled specifically for this study; all data were obtained from medical records or radiographs. We had prior approval by our institutional review board.
Table 2.
Comparison of preoperative factors between bisphosphonate and control cohorts
| Parameter | Unit | BC (n = 25) | CC (n = 20) | p value | Test |
|---|---|---|---|---|---|
| Bisphosphonate | Year | 7.6 (SD = 3.4) | – | – | – |
| Age | Year | 71 (SD = 11) | 74 (SD = 14) | 0.38 | Student’s t-test |
| Low-energy mechanism | % | 96 | 84 | 0.18 | Chi-square |
| Tobacco use | % | 20 | 11 | 0.36 | Chi-square |
| Alcohol use | % | 16 | 11 | 0.6 | Chi-square |
| Diabetes | % | 12 | 5 | 0.44 | Chi-square |
| History of steroids | % | 0 | 0 | – | – |
| Osteoporosis (DEXA) | % | 24 | 5 | 0.09 | Chi-square |
| BMI | 25.5 (SD = 4.5) | 25.6 (SD = 7.0) | 0.95 | Student’s t-test | |
| Proximal 1/3 femur | % | 48 | 40 | 0.59 | Chi-square |
| Distance from LT | mm | 58 (SD = 52) | 99 (SD = 49) | 0.076 | Student’s t-test |
| Cortex /shaft | % | 24 | 15 | < 0.0001 | Student’s t-test |
BC = bisphosphonate cohort; CC = control cohort; LT = lesser trochanter; SD cited when appropriate.
The bisphosphonate cohort was similar to the control cohort for most preoperative parameters (Table 2). The bisphosphonate and control cohorts were randomly sampled using random allocation software (Random Allocation Software v. 1.0; M. Saghaei MD, Department of Anesthesia, Isfahan University of Medical Sciences, Isfahan, Iran). The bisphosphonate cohort had longer followup than the control cohort (83 versus 39 weeks). In the bisphosphonate cohort, plate and screw constructs were used in 40% (10 of 25) and an intramedullary nail in 60% (15 of 25) (Fig. 1). Teriparatide was used more frequently (p < 0.001) in the bisphosphonate cohort (64% versus 5%).
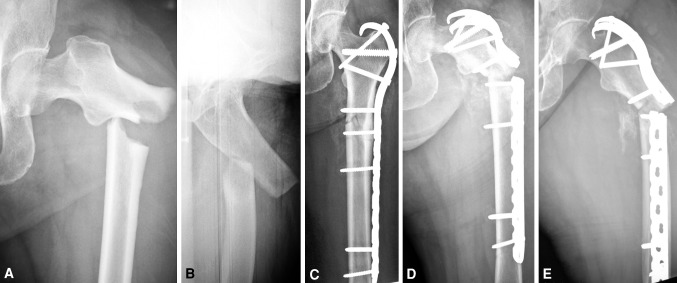
Fig. 1A–E.
(A) AP and (B) oblique radiographs show a characteristic fracture of the femur associated with bisphosphonate therapy; the lateral cortex at the fracture site is thickened, which alters the relationship between the canal and cortical surfaces. (C) An AP image is shown that was obtained after open reduction and internal fixation with a proximal femoral hook LCP Plate. (D) AP and (E) lateral radiographs obtained after hardware failure 3 months after fracture surgery show plate breakage.
All patients were assessed by the same surgeon (DGL) on presentation and underwent preoperative workup and medical optimization as needed. Patients in the bisphosphonate cohort had their bisphosphonates discontinued at the time of consultation. Cephalomedullary nailing was considered first-line treatment for all fractures. Plate fixation (large-fragment locking plate) was used for (1) revision surgery, (2) if the femoral or fracture anatomy preoperatively was deemed sufficiently abnormal to preclude placement of a nail, or (3) if required intraoperatively for adequate fracture reduction and fixation. Fractures in both groups were acutely fixed with similar implants (Table 3).
Table 3.
Comparison of operative and postoperative factors between bisphosphonate and control cohorts
| Parameter | Unit | BC (n = 25) | CC (n = 20) | p value | Test |
|---|---|---|---|---|---|
| Time to surgery | Days | 1.9 (SD = 3.9) | 2.3 (SD = 4.5) | 0.77 | Student’s t-test |
| Fixation with plate | % | 40 | 45 | 0.34 | Chi-square |
| Intraoperative grafting | % | 28 | 10 | 0.13 | Chi-square |
| Complication | % | 71 | 10 | < 0.0001 | Chi-square |
| Intraoperative fracture | % | 21 | 0 | 0.03 | Chi-square |
| Failure/nonunion | % | 13 | 0 | 0.06 | Chi-square |
| Teriparatide treatment | % | 64 | 5 | < 0.0001 | Chi-square |
| Final followup | Weeks | 83 | 39 | 0.02 | Student’s t-test |
BC = bisphosphonate cohort; CC = control cohort; SD provided when appropriate.
Followups with orthogonal radiographs obtained were performed at 2, 6, 12, 24, and 52 weeks postoperatively. All patients taking bisphosphonates before surgery received a postoperative consultation with a metabolic bone specialist with recommendation of teriparatide therapy if not contraindicated and continuation of vitamin D and calcium supplementation as needed. Major complications were defined as those requiring a change in operative plan or a secondary surgery. Healing was judged by one observer (DGL) using the following criteria: the ability to fully weight bear, lack of pain at the fracture site, and radiographic consolidation observed on orthogonal plain radiographic views. All patients included in the current study had all clinical and radiographic data available for analysis.
We determined differences in continuous variables (time receiving bisphosphonates, age, BMI, distance from the lesser trochanter, time to surgery, healing time, and length of followup) between the bisphosphonate and control cohorts using Student’s t-test. Differences in proportions of nominal data (low-energy injury mechanism, tobacco use, alcohol use, diabetes, history of steroids, osteoporosis, and BMI) were determined with the chi-square test. We did not use post hoc correction for comparison of multiple variables. A formal post hoc sample-size analysis was not performed as inclusion and exclusion criteria constrained power in this retrospective study. We examined possible univariate associations between factors of interest and healing time in the bisphosphonate cohort. The Mann-Whitney U ranked-sum test was used to compare categorical variables, and the Pearson correlation test was used for continuous variables. All statistical analyses were performed using a standard commercial package (XLStat; Addinsoft SARL, New York, NY, USA).
Results
We observed larger numbers of DEXA-diagnosed osteoporosis in the bisphosphonate cohort and the fractures tended to be more proximal (Table 2). The bisphosphonate cohort showed the characteristically thicker cortex (p < 0.001) relative to the total diameter of the diaphysis.
The bisphosphonate cohort had more complications (Table 3). The major complication rate was higher (p = 0.005) for the bisphosphonate cohort, 44% (11 of 25) versus 4% (one of 25) in the control cohort. The minor complication rate was similar (p = 0.112) in the two cohorts: 24% (6/25) for the bisphosphonate cohort versus 8% (2/25) for the control cohort. The most frequent complication was intraoperative femoral shaft comminution during nail insertion (five of 17 nailings) (Fig. 2). Three plate failures occurred (of 10 placed); none of the nails failed. The higher major complication rate in the bisphosphonate group was primarily attributable to iatrogenic fracture during nail placement (n = 5; 21% versus 0%), and postoperative plate failure (n = 3; 13% versus 0%) (Table 4).
Fig. 2A–B.
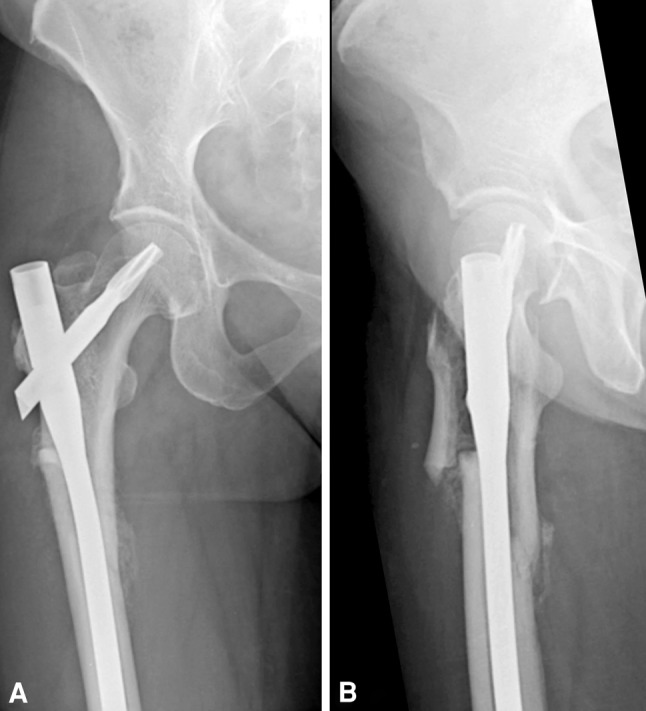
(A) AP and (B) lateral radiographs of the hip show a characteristic intraoperative iatrogenic fracture with anterolateral comminution of the proximal femur during cephalomedullary nailing. The combination of brittle bone and thickening of the cortex leading to a mismatch between the nail and proximal femur led to the iatrogenic fracture.
Table 4.
Complications in bisphosphonate and control cohorts
| Complications | BC (n = 25) | CC (n = 20) | p value |
|---|---|---|---|
| Major | 0.005 | ||
| Iatrogenic fracture | 5 | 0 | |
| Implant failure | 4 | 0 | |
| Nonunion | 1 | 0 | |
| Malunion | 1 | 0 | |
| Periprosthetic fracture | 0 | 1 | |
| Total major | 11 | 1 | |
| Minor | 0.112 | ||
| Heterotopic ossification | 1 | 0 | |
| Pain | 2 | 1 | |
| Weakness | 2 | 0 | |
| Sensory paresthesia | 1 | 0 | |
| Total minor | 6 | 1 | |
| Total major and minor | 17 | 2 |
BC = bisphosphonate cohort, CC = control cohort.
The surgeon-judged time to union in the bisphosphonate cohort was 26 weeks (range, 8–120 weeks; SD, 8 weeks) with a minimum followup of 20 weeks (range, 20–341 weeks; median, 58 weeks) and 19 weeks in the control cohort with a minimum followup of 8 weeks (median, 31 weeks; range, 8–131 weeks). The only persistent nonunion (greater than 1 year from initial treatment) at last followup occurred in the bisphosphonate cohort.
Discussion
Bisphosphonate-associated femur fractures have been reported in the literature [9, 17–19, 25], and the FDA has mandated that a warning be placed on bisphosphonate labels, but few of the specific management and complications have been well described in the literature. We sought to compare operative treatment and complications of patients with femur fractures associated with bisphosphonate therapy versus those for patients not receiving such therapy, specifically examining patient and radiographic variables and complication rates.
Our study has some limitations. First, and most importantly, we had a limited number of patients. It is difficult to obtain a large sample size with a relatively rare injury. On examination of the studies available regarding treatment of such injuries, the current study is the largest. In addition, no other study has a comparison cohort. Second, retrospective studies carry the inherent risk of observer bias, including the potential for missing data and inability to control confounding variables. Third, we did not obtain functional outcomes for the patients and therefore cannot judge whether one group had superior function to another. In addition, we were unable to rigorously judge and compare healing times.
Our current study showed these fractures are unique radiographically and result in more complications despite the use of additional medical and surgical adjuvants. The bisphosphonate cohort had a high overall complication rate (68%) in our series. When compared with the control group, this was higher, and most notably consisted of iatrogenic fracture during nail placement or postoperative plate failure. Such intraoperative complications with bisphosphonate-associated femur fractures have not been reported before (Table 4). Intramedullary nailing of these femur fractures (which otherwise is routine) could be impractical at times, especially for more proximal fractures. The thickened cortices which essentially trumpet from the metaphyseal trochanteric region to the thick diaphyseal cortex make standard reconstruction nailing difficult as the increased proximal diameter of the nail either results in iatrogenic fracture of a brittle cortex, or potentially, a varus deformity. When plates were chosen as the fixation device owing to technical impracticality of nailing, there was a high rate of implant failure likely attributable to a varus moment arm and dependence on intramembranous healing inhibited by bisphosphonates. We used similar arrays of implants in both groups, although more adjuvants were given to patients receiving bisphosphonates. In particular, more teriparatide was given postoperatively to the bisphosphonate group compared with the control group. The use of teriparatide in the treatment of bisphosphonate-associated femur fractures was suggested by Chrischilles et al. [6]. Its efficacy in such a scenario has been proposed but needs to be shown in well-designed trials [10].
Fracture healing time appeared delayed for patients receiving bisphosphonate therapy, and the only nonunion occurred in this group. In a retrospective review by Das De et al. [7], 20 patients with subtrochanteric femur fractures, of whom 12 were receiving long-term alendronate, were examined. The remaining eight patients served as a control group. There were three nonunions in the group taking alendronate versus one in the control group, but no statistical analysis was performed. Two of these three nonunions initially were plated and required revision surgery with a nail and autogenous bone grafting. The other nonunion initially was treated with a cephalomedullary nail. The authors did not mention the final outcomes for these three patients with nonunions, and there were no data presented regarding time to union. They recommended treatment with an intramedullary device, cessation of the bisphosphonate, and consideration of treatment with recombinant parathyroid hormone postoperatively [7]. Our data also suggest, when compared with similar femur fractures in patients not receiving bisphosphonate therapy, that fractures associated with bisphosphonate intake are more difficult to treat surgically and heal biologically. Because bisphosphonates used for osteoporosis result in relative inhibition of bone resorption (through the inhibition of osteoclasts), they can cause accumulation of trabecular microdamage or perhaps contribute to aging of the collagen fibers [3, 14, 22, 23]. This may compromise the mechanical and regenerative properties of bone, resulting in delayed bone healing and other complications [3, 8, 21–23, 26]. This in particular would affect intramembranous bone healing that possibly could lead to the plate failures and delayed healing times observed. In addition, the deformed, brittle bone of the proximal femur would be much less accepting of a cephalomedullary nail and might result in fracture and delayed healing.
We found the fixation and treatment of these insufficiency fractures can be challenging. Despite the low rate of other risk factors and ample use of biologic adjuvants, the altered bone quality may lead to lengthy healing times and complications requiring change in operative plan or reoperation. The increasing size of the aging population portends a growing pool of patients receiving bisphosphonates (for longer times) and therefore, increasing numbers of these types of fractures. Healthcare providers at all levels need to recognize this association, and additional study is needed to help improve treatment for this emerging problem.
Acknowledgments
We thank Omesh Paul MD, Paul Matuszewski MD, and Craig Klinger BS for assistance in formulating patient cohorts and thoughtful discussion regarding the manuscript.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at New York Presbyterian Hospital, New York, NY, USA.
References
- 1.Ahn JK, Lee J, Cha HS, Koh EM. Non-traumatic fracture of the femoral shaft in a patient taking long-term bisphosphonate therapy. Rheumatol Int. 2011;31:973–975. doi: 10.1007/s00296-010-1477-3. [DOI] [PubMed] [Google Scholar]
- 2.Aspenberg P. Bisphosphonate-induced fractures: nature strikes back? Acta Orthop. 2008;79:459–460. doi: 10.1080/17453670710015427. [DOI] [PubMed] [Google Scholar]
- 3.Cao Y, Mori S, Mashiba T, Westmore MS, Ma L, Sato M, Akiuama T, Shi L, Komatsubara S, Miyamato K, Norimatsu H. Raloxifene, estrogen, and alendronate affect the processes of fracture repair differently in ovariectomized rats. J Bone Miner Res. 2002;17:2237–2246. doi: 10.1359/jbmr.2002.17.12.2237. [DOI] [PubMed] [Google Scholar]
- 4.Capeci CM, Tejwani NC. Bilateral low-energy simultaneous or sequential femoral fractures in patients on long-term alendronate therapy. J Bone Joint Surg Am. 2009;91:2556–2561. doi: 10.2106/JBJS.H.01774. [DOI] [PubMed] [Google Scholar]
- 5.Cermak K, Shumelinsky F, Alexiou J, Gebhart MJ. Case reports: subtrochanteric femoral stress fractures after prolonged alendronate therapy. Clin Orthop Relat Res. 2010;468:1991–1996. doi: 10.1007/s11999-009-1192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chrischilles EA, Dasbach EJ, Rubenstein LM, Cook JR, Tabor HK, Black DM, the Fracture Intervention Trial Research Group The effect of alendronate on fracture-related healthcare utilization and costs: The Fracture Intervention Trial. Osteoporos Int. 2001;12:654–660. doi: 10.1007/s001980170065. [DOI] [PubMed] [Google Scholar]
- 7.Das S, Setiobudi T, Shen L, Das De S. A rational approach to management of alendronate-related subtrochanteric fractures. J Bone Joint Surg Br. 2010;92:679–686. doi: 10.1302/0301-620X.92B5.22941. [DOI] [PubMed] [Google Scholar]
- 8.Fisher JE, Rogers MJ, Halasy JM, Luckman SP, Hughes DE, Masarachia PJ, Wesolowski G, Russell RG, Rodan GA, Reszka AA. Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc Natl Acad Sci. 1999;96:133–138. doi: 10.1073/pnas.96.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goh S-K, Yang KY, Koh JS, Wong MK, Chua SY, Chua DT, Howe TS. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89:349–353. doi: 10.1302/0301-620X.89B3.18146. [DOI] [PubMed] [Google Scholar]
- 10.Gomberg SJ, Wustrack RL, Napoli N, Arnaud CD, Black DM. Teriparatide, vitamin D, and calcium healed bilateral subtrochanteric stress fractures in a postmenopausal woman with a 13-year history of continuous alendronate therapy. J Clin Endocrinol Metab. 2011;96:1627–1632. doi: 10.1210/jc.2010-2520. [DOI] [PubMed] [Google Scholar]
- 11.Ha YC, Cho MR, Park KH, Kim SY, Koo KH. Is surgery necessary for femoral insufficiency fractures after long-term bisphosphonate therapy? Clin Orthop Relat Res. 2010;468:3393–3398. doi: 10.1007/s11999-010-1583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH, 3rd, Brown J, Eriksen EF, Hoseyni MD, Axelrod DW, Miller PD. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344–1352. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 13.Hosking D, Chilvers CE, Christiansen C, Ravn P, Wasnich R, Ross P, McClung M, Balske A, Thompson D, Daley M, Yates AJ. Prevention of bone loss with alendronate in postmenopausal women under 60 years of age. Early Postmenopausal Intervention Cohort Study Group. N Engl J Med. 1998;338:485–492. doi: 10.1056/NEJM199802193380801. [DOI] [PubMed] [Google Scholar]
- 14.Isaacs JD, Shidiak L, Harris IA, Szomor ZL. Femoral insufficiency fractures associated with prolonged bisphosphonate therapy. Clin Orthop Relat Res. 2010;468:3384–3392. doi: 10.1007/s11999-010-1535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karpf DB, Shapiro DR, Seeman E, Ensrud KE, Johnston CC, Jr, Adami S, Harris ST, Santora AC, 2nd, Hirsch LJ, Oppenheimer L, Thompson D. Prevention of nonvertebral fractures by alendronate: a meta-analysis. Alendronate Osteoporosis Treatment Study Groups. JAMA. 1997;277:1159–1164. doi: 10.1001/jama.1997.03540380073035. [DOI] [PubMed] [Google Scholar]
- 16.Koh JS, Goh SK, Png MA, Kwek EB, Howe TS. Femoral cortical stress lesions in long-term bisphosphonate therapy: a herald of impending fracture? J Orthop Trauma. 2010;24:75–81. doi: 10.1097/BOT.0b013e3181b6499b. [DOI] [PubMed] [Google Scholar]
- 17.Kwek EB, Goh SK, Koh JS, Png MA, Howe TS. An emerging pattern of subtrochanteric stress fractures: a long-term complication of alendronate therapy? Injury. 2008;39:224–231. doi: 10.1016/j.injury.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 18.Lenart BA, Lorich DG, Lane JM. Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. N Engl J Med. 2008;358:1304–1306. doi: 10.1056/NEJMc0707493. [DOI] [PubMed] [Google Scholar]
- 19.Lenart BA, Neviaser AS, Lyman S, Chang CC, Edobor-Osula F, Steele B, Meulen MC, Lorich DG, Lane JM. Association of low-energy femoral fractures with prolonged bisphosphonate use: a case control study. Osteoporosis Int. 2009;20:1353–1362. doi: 10.1007/s00198-008-0805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW, Jr, Dequeker J, Favus M. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995;333:1437–1443. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- 21.Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13:581–589. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 22.Mashiba T, Hui S, Turner CH, Mori S, Johnston CC, Burr DB. Bone remodeling at the iliac crest can predict the changes in remodeling dynamics, microdamage accumulation, and mechanical properties in the lumbar vertebrae of dogs. Calcif Tissue Int. 2005;77:180–185. doi: 10.1007/s00223-005-1295-x. [DOI] [PubMed] [Google Scholar]
- 23.Mashiba T, Mori S, Burr DB, Komatsubara S, Cao Y, Manabe T, Norimatsu H. The effects of suppressed bone remodeling by bisphosphonates on microdamage accumulation and degree of mineralization in the cortical bone of dog rib. J Bone Miner Metab. 2005;23(suppl):36–42. doi: 10.1007/BF03026321. [DOI] [PubMed] [Google Scholar]
- 24.Napoli N, Novack D, Armamento-Villareal R. Bisphosphonate-associated femoral fracture: implications for management in patients with malignancies. Osteoporos Int. 2010;21:705–708. doi: 10.1007/s00198-009-1012-0. [DOI] [PubMed] [Google Scholar]
- 25.Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG. Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma. 2008;22:346–350. doi: 10.1097/BOT.0b013e318172841c. [DOI] [PubMed] [Google Scholar]
- 26.Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90:1294–1301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]
- 27.Ott SM. Atraumatic bilateral femur fracture in long-term bisphosphonate use. Comment on Orthopedics. 2009 Aug;32(8). pii: orthosupersite.com/view.asp?rID = 41933. doi:10.3928/01477447-20090624-27. Orthopedics. 2010;33:468. [DOI] [PubMed]
- 28.Riggs BL, Melton LJ., III The prevention and treatment of osteoporosis. N Engl J Med. 1992;327:620–627. doi: 10.1056/NEJM199208273270908. [DOI] [PubMed] [Google Scholar]
- 29.Sayed-Noor AS, Sjoden GO. Subtrochanteric displaced insufficiency fracture after long-term alendronate therapy: a case report. Acta Orthop. 2008;79:565–567. doi: 10.1080/17453670710015580. [DOI] [PubMed] [Google Scholar]
- 30.Sayed-Noor AS, Sjoden GO. Case reports: two femoral insufficiency fractures after long-term alendronate therapy. Clin Orthop Relat Res. 2009;467:1921–1926. doi: 10.1007/s11999-009-0725-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Somford MP, Geurts GF, Teuling JW, Thomassen BJ, Draijer WF. Long-term alendronate use not without consequences? Int J Rheumatol. 2009;2009:253432. doi: 10.1155/2009/253432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wells GA, Cranney A, Peterson J, Boucher M, Shea B, Robinson V, Coyle D, Tugwell. Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2008;CD001155. [DOI] [PubMed]