Abstract
Background
Pelvic ring injuries with complete disruption of the posterior pelvis (AO/OTA Type C) benefit from reduction and stabilization. Open reduction in early reports had high infectious complications and many surgeons began using closed reduction and percutaneous fixation. Multiple smaller studies have reported low infection rates after a posterior approach, but these rates are not confirmed in larger series of diverse fractures.
Questions/Purposes
We therefore determined (1) the incidence of surgical site infectious complications after a posterior approach to the pelvis; and (2) whether secondary procedures other than surgical débridement are necessary as a result of the approach-related complications.
Methods
We retrospectively reviewed all 236 patients (268 surgical approaches) with C type injuries treated with a posterior approach at six institutions before 1998 and at one institution from 1998 to 2005. Posterior injuries were classified anatomically as described by Letournel and the AO/OTA system. We recorded wound complications after surgery.
Results
Surgical site infection occurred in eight of the 236 patients (3.4%) in the multicenter analysis. Treatment consisted of surgical débridement, wound closure, and antibiotics. No patients required soft tissue reconstruction as a result of the approach or infection.
Conclusion
Our data suggest with proper patient selection and the described surgical technique, there should be minimal risk for catastrophic wound complications or high infection rates as reported by others.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Complete disruption of the posterior pelvic ring (AO/OTA Type C) usually results from a high-energy mechanism. Radiographic evaluation allows diagnosis of the anatomic injury and insight into the relative stability of the injured pelvis. Multiple classification systems have been devised to describe injury patterns and the resultant instability [4, 5, 41]. Letournel [23] originally described anatomical zones of injury. The AO/OTA classification represents an evolution of classification systems into a comprehensive tool for documentation and research [10]. Type C injuries are distinguished by complete disruption of the posterior pelvic ring. The anatomic source of instability in the posterior ring may be a fracture through the iliac wing, fracture-dislocations of the sacroiliac joint, pure sacroiliac joint dislocations, or sacral fractures. These globally unstable fractures account for 20% of all pelvic ring injuries [11, 34].
Nonoperative care of unstable pelvic injuries can result in substantial residual disability as a result of chronic instability, deformity, or associated neurologic injury [9, 16, 17, 19, 29, 35, 41]. Techniques for operative stabilization were developed with the goal of improving function and avoiding subsequent deformity. In early series, the posterior approach to pelvic ring injuries was reported to have soft tissue and infectious complications of 18% to 27% [12, 22]. Letournel also described difficulties with the posterior approach when a curvilinear skin incision was used. Despite improvement in soft tissue complications with delay to surgery [3], some surgeons have been reluctant to use the posterior approach for fear of the considerable operative morbidity. External fixation can help to avoid surgical approaches to the pelvic ring, but its ability to provide adequate stability and maintain reduction of a complete posterior pelvic injury limits its application for definitive treatment in Type C injuries [6, 21, 26, 46]. Techniques for closed reduction and percutaneous iliosacral screw stabilization of the posterior pelvic ring were developed based on the desire to obtain posterior ring stability in C-type injuries and the assumption of the high morbidity associated with the posterior approach [36, 37]. Subsequent investigators have reported low rates and severity of soft tissue morbidity with percutaneous fixation [44], but recent concerns have been raised regarding the ability of percutaneous placed iliosacral screws to maintain a reduction in certain posterior ring injuries [13, 20]. Other surgeons performing the posterior approach on subsequent small series of 16 to 65 patients have not experienced high soft tissue complications with reported infection rates ranging from 0% to 10% [2, 30, 32, 33, 39, 40, 45] (Table 1). A larger, multicenter cohort of patients with a variety of injuries would help better define the rate of surgical site infections after a posterior approach to the posterior pelvic ring. This information may guide surgical decision-making as well as educate patients regarding potential complications associated with the approach.
Table 1.
Early, deep postoperative infections after posterior pelvic surgery compared with acetabulum surgery
| Author | Infection rates (number) | Number of patients | Minimum followup (months) | Injuries |
|---|---|---|---|---|
| Matta and Saucedo [29] | 3% (2) | 65 | 6 | Any Type C injury |
| Templeman et al. [40] | 5.8% (1) | 17 | 12 | Sacral fractures |
| Moed and Karges [32] | 0 % | 25 | ? | Any Type C injury |
| Borrelli et al. [2] | 0% | 22 | 12 | Sacroiliac fracture dislocations |
| Suzuki et al. [39] | 10% (2) | 20 | 12 | Sacral fractures |
| Moon and Merkle [33] | 2.4 (1) | 35 | 12 | Any Type C |
| Sagi et al. [38] | 13% (8) | 58 | 12 | Sacral fractures |
| Bellabarba et al. [1] | 16% (3) | 18 | 12 | Spinopelvic dissociations |
| Matta [28] | 4.9% (13) | 262 | 24 | Acetabulum fractures |
| Letournel et al. [24] | 2.2% (13) | 569 | 12 | Acetabulum fractures |
We therefore determined (1) the incidence of infectious complications after a posterior approach to the pelvis; and (2) whether secondary procedures other than surgical débridement were necessary as a result of the approach-related complications.
Patients and Methods
We retrospectively analyzed the medical records of all patients who had a posterior approach performed for an AO/OTA 61-C-type injury by individual surgeons at seven institutions who received training by Letournel or the senior author (JM). A total of 236 patients, including 32 individuals with bilateral Type C injuries, underwent a total of 268 posterior approaches. Thirty-five of the patients evaluated for this study were previously reported [33]. The purpose of that study was to determine the rate of soft tissue complications associated with the described posterior approach to the pelvic ring. The indications for surgery were patients diagnosed with a globally unstable pelvis (AO/OTA 61-C) in whom the injury and soft tissues were deemed appropriate for open treatment. No specific protocol for treatment was used. Patients treated with a midline posterior approach for bilateral sacral fractures were excluded from the analysis because this treatment was uncommon before 1998 and does not represent the approach as described. Mean age at the time of surgery in was 32 years (range, 2–74 years). Time to surgery averaged 7.3 days (range, 0–21 days). Fractures were classified anatomically and according to AO/OTA fracture classifications (Table 2). Eighteen percent of patients had soft tissue degloving injuries identified within the surgical field. Twenty-nine percent underwent open reduction and internal fixation of the anterior ring. The minimum followup was 3 months; we presumed all infections would have been identified within that period. Two patients did not have followup to 3 months. One patient did not return to the clinic after a prolonged hospitalization and one was lost after the initial postoperative visit 4 weeks after surgery. No patients were recalled specifically for this study; all data were obtained from medical records.
Table 2.
Classification of pelvic injuries
| AO/OTA classification | Group 1 | Group 2 | |
|---|---|---|---|
| Unilateral injuries | |||
| Ilium fractures | 61-C1.1 | 4 | 2 |
| SI dislocations | 61-C1.2 | 44 | 9 |
| SI fracture-dislocations | 61-C1.2 | 68 | 13 |
| Sacral fractures | 61-C1.3 | 49 | 15 |
| Bilateral injuries | |||
| Bilateral SI dislocations | 61-C3.1 | 6 | 1 |
| Bilateral fracture-dislocations | 61-C3.1 | 3 | 1 |
| Bilateral: different anatomic sites | 61-C3.2 | 7 | 2 |
| Bilateral sacral | 61-C3.3 | 7 | 0 |
| H type | 61-C3.3 | 5 | 0 |
| 193 | 43 | ||
SI = sacroiliac.
All patients were identified using individual surgeon databases and medical records were reviewed. We recorded patient demographic information, classification of pelvic ring injury, associated injuries, and associated complications.
The posterior approach as described by Matta [27] is performed on a radiolucent operating table. The hips are extended with pillows under the thighs to maintain the lumbar lordosis and the knees flexed to relax the sciatic nerve. The ability to obtain adequate fluoroscopic images is confirmed before preparation of the surgical field. The gluteal cleft is isolated from the operative field. The skin is antiseptically prepared. Once the field is draped, we used an adhesive occlusive drape (3M Ioban™, St Paul MN, USA) to isolate the area of the incision. Perioperative antibiotics were routinely used with specific medications and dosing determined by individual surgeons. All were approached through a common posterior incision. A longitudinal incision was placed approximately 2 cm lateral to the posterosuperior iliac spine (Fig. 1). A cutaneous flap was then raised off of gluteus fascia to the midline. If a degloving injury was identified at the time of injury, débridement of necrotic adipose tissue was performed. The abductors were released from their origin on the posterior iliac crest to the posterosuperior iliac spine (Fig. 2). The fascial origin of the gluteus maximus was released from the posterosuperior iliac spine and the dorsal fascia of the erector spinae. The glutei were then elevated from the external surface of the ilium as a flap based on the gluteal arteries and nerve. If not already disrupted by the injury, access to the sacroiliac joint and sacrum can be accomplished by elevation of the erector spinae from the dorsal sacrum (Fig. 3). Entrance into the true pelvis through the greater sciatic notch is enhanced by release of the sacrotuberous and sacrospinous ligament attachments from the lateral sacrum. Reduction of the sacroiliac joint can be confirmed by palpation anteriorly and direct visualization of the caudal portion of the joint. Reduction of sacral fractures and sacroiliac joint fracture-dislocations was by direct visualization. Before closure, further débridement of severely injured or necrotic tissues was performed. Drains deep to the gluteus flap and under the superficial flap were routinely used.
Fig. 1.
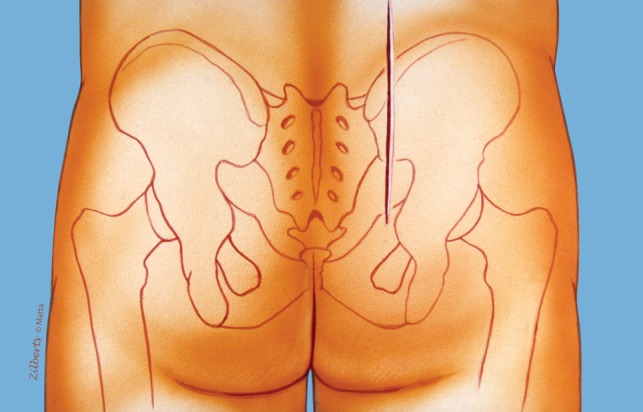
A straight incision is positioned 2 cm lateral to the posterosuperior iliac spine.
Fig. 2.
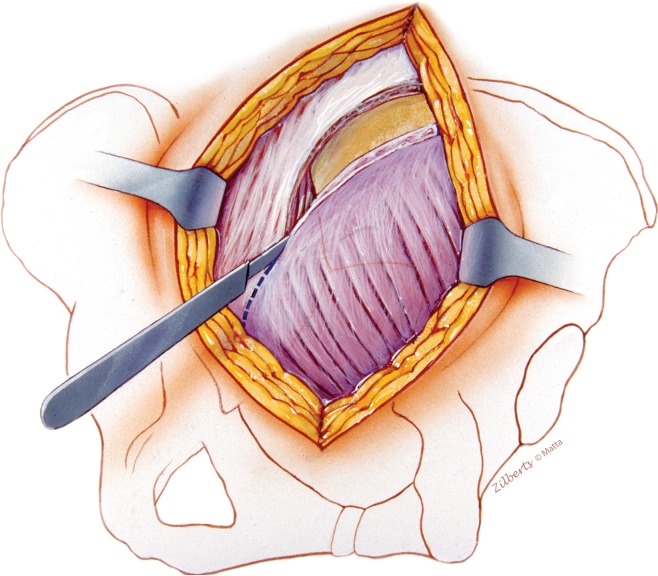
A cutaneous flap needs to be elevated to the midline to fully expose the medial origin of the gluteus maximus. Elevation of the maximus and abductors from the iliac crest initiates the deep dissection. The medial extension of the maximus muscle and its origin are carefully elevated from the erector spinae fascia and released from the midline in a proximal to distal direction.
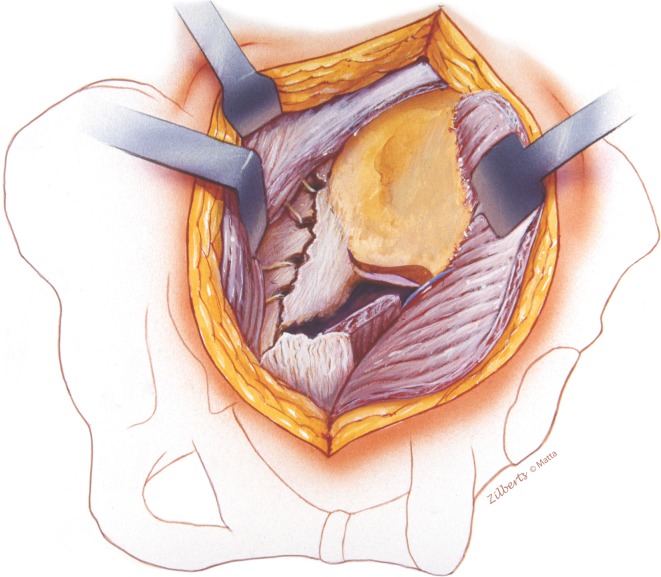
Fig. 3.
Lateral access to ilium is accomplished with release of the gluteus maximus from cristae glutae and subperiosteal elevation of the lateral musculature. Care is taken at the anterior aspect of the greater sciatic notch to avoid injury to the superior gluteal neurovascular bundle. Medial exposure is provided by elevation of erector spinae and must extend cranial to the posterior iliac spine to visualize and palpate the upper sacral segment. Injury or surgical release of ligaments (supraspinous or sacrotuberous) from the lateral sacrum will improve anterior sacral and sacroiliac joint access for palpation or clamps.
Postoperatively, patients were mobilized out of bed as associated injuries would allow. If bed-bound, a soft air mattress should be used to limit direct pressure on the wound. Isolated injuries to the hemipelvis would be mobilized with an assist device, limiting force to 30 lb foot-flat weightbearing, emphasizing a heel-toe gait pattern with supervised physical therapy. Weightbearing limitations were continued for 8 to 12 weeks postoperatively depending on injury pattern.
No specific protocol for followup was used by participating surgeons. The complication of interest was a surgical site infection deep to the skin as defined by the Centers for Disease Control and Prevention [18]. Local postoperative conditions of the surgical site (ie, warmth, erythema, swelling, pain, tenderness, and/or wound drainage or dehiscence) were recorded. The presumed diagnosis of deep surgical site infection was made based on these local changes and surgeon judgment. Deep infections after open reduction and internal fixation of the pelvic ring were classified as at least IIa complications, requiring a return to the operating room for formal débridement [7]. All infections demonstrated bacterial growth in culture from the surgical wounds. All were treated with a postoperative course of intravenous organism-specific antibiotics.
Results
Eight patients (3.4%) had a deep wound infection. Ten patients returned to the operating room and the posterior incision was surgically opened and irrigation and débridement was performed. One patient had postoperative anterior urine collection from inadequate drainage of a ruptured bladder requiring evacuation through a Pfannenstiel approach. This wound grew bacteria. The posterior wound was opened but was not actively infected and was culture-negative. An additional patient with prolonged wound drainage was taken back to the operating room for exploration of the wound and was culture-negative. Eight additional patients developed wound drainage and/or erythema involving the pelvic surgical sites postoperatively. One patient developed an infected soft tissue degloving injury from an infected anterior pin site after open reduction and internal fixation. This did not involve the posterior surgical wound. One patient had a superficial wound infection that responded to antibiotics. Six patients (3%) had prolonged (greater than 5 days) excessive wound drainage. Deep surgical site infection rates for individual surgeons at their respective institutions varied from 2.3% to 5.5%.
No additional soft tissue reconstruction was necessary after soft tissue complication or deep surgical site infection. No wound slough was documented in the series. One patient required tissue transfer for an open injury. Portions of two wounds were allowed to heal by secondary intention. Five of eight patients with deep infection underwent revision of reduction and/or internal fixation at the time of débridement, including one with isolated screw removal.
Discussion
The posterior approach to the pelvic ring continues to be associated with a high postoperative infection risk despite studies to the contrary [2, 30, 32, 33, 39, 40, 45]. We set out to define the risk of deep surgical site infection using a larger cohort of unstable pelvic injuries (Type C) operated on by a group of surgeons trained in a specific technique and to determine if infectious complications led to the need for secondary reconstructive procedures.
We recognize limitations of our study. First, we had no set indications for treating with open reduction and internal fixation through the posterior approach and no specific treatment protocol for those patients having this treatment. Although the surgeons favored the posterior approach for treating unstable posterior pelvic ring injuries, the number of patients treated with alternative approaches for stabilization of the posterior ring resulting from injury pattern, patient factors, or conditions of the posterior soft tissue envelope is not known for the entire series of patients. Furthermore, we had no protocol for type and duration of perioperative antibiotics and these data were not compiled for this study. Second, we had only short-term followup. All patients included were operated on within 21 days of injury and followed for at least 3 months postoperatively. It is possible some late infections would not have been diagnosed in patients with short-term followup, especially in the setting of internal fixation. Third, we did not determine if infections affected the subsequent course of the patient. Open reduction and internal fixation reportedly decreases the incidence of nonunion and malunion after a pelvic fracture [29] and reduces disability [14] with a higher percentage of satisfactory results [29]. Anatomic reconstruction of the pelvic ring may be important, but other factors can influence patient-reported pain, function, and ability to return to work [8, 25, 31, 42]. Therefore, we cannot comment on the impact of soft tissue complications on patient-reported results of the injury. Finally, we did not evaluate reductions and/or subsequent loss of reduction and therefore we cannot comment on whether the increased risk of infection is warranted in comparison to closed reduction and internal fixation.
Using the technique described, the posterior approach to an unstable posterior pelvic ring was associated with an infection rate of 3.4%. This rate is much less than that reported by Goldstein et al. [12] or Kellam et al. [22] in the early era of pelvic open reduction and internal fixation. These authors attributed the higher infection rates to prolonged operating times or a crushing mechanism of injury. Our rate also falls in the middle of those reported in other smaller series and is lower than that reported for the addition of spinopelvic fixation to sacral fractures (13%) or spinopelvic dissociations (16%) [1, 38]. It approximates the rate in large series of operatively treated acetabulum fractures (2.3% to 4.9%) [24, 28]. Because this series includes a wide variety of injuries and surgeons of differing experience from a spectrum of practice environments, we believe the rate reported likely represents a realistic expectation for this approach performed by individuals trained in pelvic surgery who deem the posterior soft tissue appropriate for an open procedure.
No additional soft tissue reconstructive procedures other than surgical excision, débridement, and closure were required as a result of the approach. Of those developing an infection, five of eight (62.5%) underwent some change in the pelvic fixation during treatment of the infection.
Previous studies have appropriately focused attention on the status of the soft tissues after pelvic fractures. Appropriate nursing care, an anatomic-based approach, identification and treatment of soft tissue degloving injuries [15, 43], débridement of severely contused or necrotic tissue at surgery, and changes in fixation technique may all account for the improvement in soft tissue complications. A careful overall evaluation of the patient and soft tissues will help in making appropriate choices regarding techniques for reduction and fixation of posterior ring injuries. A posterior approach as described for open reduction and internal fixation of posterior pelvic ring injuries has a 3.4% deep surgical site infection rate and no major soft tissue complications occurred in this study. The authors continue to advocate the use of the posterior approach when necessary for reduction of posterior pelvic ring injuries in patients without a severely compromised posterior soft tissue envelope.
Acknowledgments
We acknowledge the contributions of Paul Merkle MD, Steve Olson MD, Keith Mayo MD, and David Templeman for compiling and contributing data and making this study possible.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Loyola University Medical Center, Maywood, IL, USA.
References
- 1.Bellabarba C, Schildhauer TA, Vaccaro AR, Chapman JR. Complications associated with surgical stabilization of high-grade sacral fracture dislocations with spino-pelvic instability. Spine (Phila Pa 1976). 2006;31:S80–S88. doi: 10.1097/01.brs.0000217949.31762.be. [DOI] [PubMed] [Google Scholar]
- 2.Borrelli J, Jr, Koval KJ, Helfet DL. The crescent fracture: a posterior fracture dislocation of the sacroiliac joint. J Orthop Trauma. 1996;10:165–170. doi: 10.1097/00005131-199604000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Browner BD, Cole JD, Graham JM, Bondurant FJ, Nunchuck-Burns SK, Colter HB. Delayed posterior internal fixation of unstable pelvic fractures. J Trauma. 1987;27:998–1006. doi: 10.1097/00005373-198709000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Bucholz RW. The pathological anatomy of Malgaigne fracture-dislocations of the pelvis. J Bone Joint Surg Am. 1981;63:400–404. [PubMed] [Google Scholar]
- 5.Dalal SA, Burgess AR, Siegel JH, Young JW, Brumback RJ, Poka A, Dunham CM, Gens D, Bathon H. Pelvic fracture in multiple trauma: classification by mechanism is key to pattern of organ injury, resuscitative requirements, and outcome. J Trauma Inj Infect Crit Care. 1989;29:981–1000. doi: 10.1097/00005373-198907000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Dickson KF, Matta JM. Skeletal deformity after anterior external fixation of the pelvis. J Orthop Trauma. 2009;23:327–332. doi: 10.1097/BOT.0b013e3181a23f5b. [DOI] [PubMed] [Google Scholar]
- 7.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dujardin FH, Hossenbaccus M, Duparc F, Biga N, Thomine JM. Long-term functional prognosis of posterior injuries in high-energy pelvic disruption. J Orthop Trauma. 1998;12:145–150. doi: 10.1097/00005131-199803000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Dunn AW, Morris HD. Fractures and dislocations of the pelvis. J Bone Joint Surg Am. 1968;50:1639–1648. [PubMed] [Google Scholar]
- 10.Fracture and dislocation compendium. Orthopaedic Trauma Association Committee for Coding and Classification. J Orthop Trauma. 1996;10(Suppl 1):v–ix. [PubMed]
- 11.Gansslen A, Pohlemann T, Paul C, Lobenhoffer P, Tscherne H. Epidemiology of pelvic ring injuries. Injury. 1996;27(Suppl 1):S-A13–S-A20. [PubMed] [Google Scholar]
- 12.Goldstein A, Phillips T, Sclafani SJ, Scalea T, Duncan A, Goldstein J, Panetta T, Shaftan G. Early open reduction and internal fixation of the disrupted pelvic ring. J Trauma Inj Infect Crit Care. 1986;26:325–333. doi: 10.1097/00005373-198604000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Griffin DR, Starr AJ, Reinert CM, Jones AL, Whitlock S. Vertically unstable pelvic fractures fixed with percutaneous iliosacral screws: does posterior injury pattern predict fixation failure? J Orthop Trauma. 2003;17:399–405. doi: 10.1097/00005131-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Gruen GS, Leit ME, Gruen RJ, Garrison HG, Auble TE, Peitzman AB. Functional outcome of patients with unstable pelvic ring fractures stabilized with open reduction and internal fixation. J Trauma. 1995;39:838–844. doi: 10.1097/00005373-199511000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Hak DJ, Olson SA, Matta JM. Diagnosis and management of closed internal degloving injuries associated with pelvic and acetabular fractures: the Morel-Lavallee lesion. J Trauma. 1997;42:1046–1051. doi: 10.1097/00005373-199706000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Henderson RC. The long-term results of nonoperatively treated major pelvic disruptions. J Orthop Trauma. 1989;3:41–47. doi: 10.1097/00005131-198903010-00008. [DOI] [PubMed] [Google Scholar]
- 17.Holdsworth FW. Dislocation and fracture-dislocation of the pelvis. J Bone Joint Surg Br. 1948;30:461–466. [PubMed] [Google Scholar]
- 18.Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Am J Infect Control. 1992;20:271–274. doi: 10.1016/S0196-6553(05)80201-9. [DOI] [PubMed] [Google Scholar]
- 19.Huittinen VM, Slatis P. Fractures of the pelvis. Trauma mechanism, types of injury and principles of treatment. Acta Chir Scand. 1972;138:563–569. [PubMed] [Google Scholar]
- 20.Keating JF, Werier J, Blachut P, Broekhuyse H, Meek RN, O’Brien PJ. Early fixation of the vertically unstable pelvis: the role of iliosacral screw fixation of the posterior lesion. J Orthop Trauma. 1999;13:107–113. doi: 10.1097/00005131-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Kellam JF. The role of external fixation in pelvic disruptions. Clin Orthop Relat Res. 1989;241:66–82. [PubMed] [Google Scholar]
- 22.Kellam JF, McMurtry RY, Paley D, Tile M. The unstable pelvic fracture. Operative treatment. Orthop Clin North Am. 1987;18:25–41. [PubMed] [Google Scholar]
- 23.Letournel E. Pelvic fractures. Injury. 1978;10:145–148. doi: 10.1016/S0020-1383(79)80081-9. [DOI] [PubMed] [Google Scholar]
- 24.Letournel É, Judet R, Elson R. Fractures of the Acetabulum. Berlin, Germany; New York, NY, USA: Springer-Verlag; 1993. [Google Scholar]
- 25.Lindahl J, Hirvensalo E. Outcome of operatively treated type-C injuries of the pelvic ring. Acta Orthop. 2005;76:667–678. doi: 10.1080/17453670510041754. [DOI] [PubMed] [Google Scholar]
- 26.Lindahl J, Hirvensalo E, Bostman O, Santavirta S. Failure of reduction with an external fixator in the management of injuries of the pelvic ring. Long-term evaluation of 110 patients. J Bone Joint Surg Br. 1999;81:955–962. doi: 10.1302/0301-620X.81B6.8571. [DOI] [PubMed] [Google Scholar]
- 27.Matta JM. Surgical Approaches to Fractures of the Acetabulum and Pelvis. Malibu, CA, USA: Joel M. Matta, MD Inc; 1989.
- 28.Matta JM. Fractures of the acetabulum: accuracy of reduction and clinical results in patients managed operatively within three weeks after the injury. J Bone Joint Surg Am. 1996;78:1632–1645. [PubMed] [Google Scholar]
- 29.Matta JM, Saucedo T. Internal fixation of pelvic ring fractures. Clin Orthop Relat Res. 1989;242:83–97. [PubMed] [Google Scholar]
- 30.Matta JM, Tornetta P., 3rd Internal fixation of unstable pelvic ring injuries. Clin Orthop Relat Res. 1996;329:129–140. doi: 10.1097/00003086-199608000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Miranda MA, Riemer BL, Butterfield SL, Burke CJ., 3rd Pelvic ring injuries. A long term functional outcome study. Clin Orthop Relat Res. 1996;329:152–159. doi: 10.1097/00003086-199608000-00019. [DOI] [PubMed] [Google Scholar]
- 32.Moed BR, Karges DE. Techniques for reduction and fixation of pelvic ring disruptions through the posterior approach. Clin Orthop Relat Res. 1996;329:102–114. doi: 10.1097/00003086-199608000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Moon CN, Merkle PF. A level one trauma center’s experience with the posterior approach to the pelvis. Orthopedics. 2002;25:159–162. doi: 10.3928/0147-7447-20020201-20. [DOI] [PubMed] [Google Scholar]
- 34.Pohlemann T, Tscherne H, Baumgartel F, Egbers HJ, Euler E, Maurer F, Fell M, Mayr E, Quirini WW, Schlickewei W, Weinberg A. Pelvic fractures: epidemiology, therapy and long-term outcome. Overview of the multicenter study of the Pelvis Study Group [in German] Unfallchirurg. 1996;99:160–167. doi: 10.1007/s001130050049. [DOI] [PubMed] [Google Scholar]
- 35.Raf L. Double vertical fractures of the pelvis. Acta Chir Scand. 1966;131:298–305. [PubMed] [Google Scholar]
- 36.Routt ML, Jr, Kregor PJ, Simonian PT, Mayo KA. Early results of percutaneous iliosacral screws placed with the patient in the supine position. J Orthop Trauma. 1995;9:207–214. doi: 10.1097/00005131-199506000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Routt ML, Jr, Simonian PT, Mills WJ. Iliosacral screw fixation: early complications of the percutaneous technique. J Orthop Trauma. 1997;11:584–589. doi: 10.1097/00005131-199711000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Sagi HC, Militano U, Caron T, Lindvall E. A comprehensive analysis with minimum 1-year follow-up of vertically unstable transforaminal sacral fractures treated with triangular osteosynthesis. J Orthop Trauma. 2009;23:313–319. doi: 10.1097/BOT.0b013e3181a32b91. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki T, Hak DJ, Ziran BH, Adams SA, Stahel PF, Morgan SJ, Smith WR. Outcome and complications of posterior transiliac plating for vertically unstable sacral fractures. Injury. 2009;40:405–409. doi: 10.1016/j.injury.2008.06.039. [DOI] [PubMed] [Google Scholar]
- 40.Templeman D, Goulet J, Duwelius PJ, Olson S, Davidson M. Internal fixation of displaced fractures of the sacrum. Clin Orthop Relat Res. 1996;329:180–185. doi: 10.1097/00003086-199608000-00021. [DOI] [PubMed] [Google Scholar]
- 41.Tile M. Pelvic ring fractures: should they be fixed? J Bone Joint Surg Br. 1988;70:1–12. doi: 10.1302/0301-620X.70B1.3276697. [DOI] [PubMed] [Google Scholar]
- 42.Tornetta P, 3rd, Matta JM. Outcome of operatively treated unstable posterior pelvic ring disruptions. Clin Orthop Relat Res. 1996;329:186–193. doi: 10.1097/00003086-199608000-00022. [DOI] [PubMed] [Google Scholar]
- 43.Tseng S, Tornetta P., III Percutaneous management of Morel-Lavallee lesions. J Bone Joint Surg Am. 2006;88:92–96. doi: 10.2106/JBJS.E.00021. [DOI] [PubMed] [Google Scholar]
- 44.Bosch EW, Zwienen CM, Vugt AB. Fluoroscopic positioning of sacroiliac screws in 88 patients. J Trauma. 2002;53:44–48. doi: 10.1097/00005373-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Ward EF, Tomasin J. Vander Griend RA. Open reduction and internal fixation of vertical shear pelvic fractures. J Trauma. 1987;27:291–295. doi: 10.1097/00005373-198703000-00011. [DOI] [PubMed] [Google Scholar]
- 46.Wild JJ, Jr, Hanson GW, Tullos HS. Unstable fractures of the pelvis treated by external fixation. J Bone Joint Surg Am. 1982;64:1010–1020. [PubMed] [Google Scholar]