Antibody catalysis has become a multifaceted field of research, involving many bridges between the biological and chemical sciences. Most recently, we see yet another example of this productive interplay in the paper by Sinha et al. in which an antibody was used to catalyze the formation of chiral β-hydroxyketones, which then were used to elaborate the complex natural product epothilone (1). Rather than using the traditional tools of chemical synthesis to probe biology, immunology was used in the service of organic chemistry.
Catalytic antibodies began with the notion that chemists should be able to use the complex machinery of the immune system, which is capable of generating tremendous chemical diversity through the processes of recombination and somatic mutation (2), to create new molecular functions, specifically highly selective catalysts. The earliest examples involved the use of transition state analogues to select antibodies with maximal binding affinity toward the rate-limiting transition state for a given reaction of interest (3, 4). Other strategies emerged shortly thereafter in which many of the basic concepts of biological catalysis (strain, proximity, general acid/base catalysis) were used in the design of molecules that could be used to guide the process of clonal expansion and somatic mutation to generate catalytic antibodies for a wide variety of reactions (5, 6). For example, antibodies were generated that catalyzed reactions ranging from acyl transfer reactions to pericyclic and redox reactions. An important demonstration was that antibodies could be used to selectively stabilize a high energy transition state relative to the lower energy favored reaction pathway, and thereby catalyze disfavored chemical reactions (7). Examples included ring forming reactions, cationic rearrangements, and redox reactions (5, 6). Antibodies also were generated for abiological reactions such as oxy-Cope and “ene” rearrangements. In a number of cases, quite efficient, highly selective catalysts were generated with rate enhancements rivaling enzymes (refs. 8–11 and K. Janda, personal communication). These experiments underscored the power of using traditional chemical tools, together with highly evolved cellular machinery, to create new function. Indeed, the same combinatorial strategies used in nature to generate antibody diversity have since been applied to other problems in biology, medicinal chemistry, and even materials science (12).
More recently, efforts have focused on detailed studies of these novel catalysts to gain new insights into the molecular mechanisms of biological catalysis and of the immune response itself. For example, kinetic, structural, and spectroscopic studies of an antibody ferrochelatase provided a textbook example of catalysis by distortion, as first proposed by Haldane over 50 years ago (13). Detailed studies of an antibody-catalyzed 3,3-sigmatropic rearrangement showed how binding energy can be used to control orbital overlap and electron distribution in the Michaelis complex to efficiently catalyze a concerted chemical rearrangement (11). Recent studies of antibody-catalyzed cationic rearrangement reactions show how the chemistry of cationic intermediates can be controlled by appropriately positioned active site groups (D. Christianson, personal communication).
Structural studies of catalytic antibodies also have resulted in important new insights into the combinatorial processes involved in the immune response itself. Structural, mutagenetic, and kinetic studies showed that the immune response to a nitrophenylphosphonate hapten involves a form of chemical instruction first proposed by Pauling over 50 years ago—binding of ligand to the germline antibody templates structural changes in the combining site that lead to increased antibody–antigen complementarity (14). Somatic mutations distant to the combining site further refine and fix the optimal active site conformation (versus folding of the remaining antibody molecule, as proposed by Pauling). This study (14) also pointed out the importance of mutations throughout the entire variable region in influencing binding affinity and specificity—a lesson that is key to the use of any combinatorial strategy in optimizing biomolecular function. Since this original study, characterization of the immunological evolution of other catalytic antibodies has provided additional immunochemical insights, such as the role of polyspecificity in the germline repertoire (15). Thus, not only can biological systems be used to create new chemical function, the detailed study of the resulting catalysts has provided important new insights into the biological process itself.
Another direction the field has taken involves efforts to recapitulate the combinatorial processes of the immune system in vitro (16). Early efforts involved antibody gene recombination by using phage lambda. Phage display then was used to carry out in vitro affinity maturation of antibodies (17, 18); this approach since has been combined with DNA shuffling methods to produce high affinity antibodies in vitro (19). Strategies now are being developed to directly select mutants with enhanced catalytic activities from large libraries of antibody mutants. These approaches include (i) the use of mechanism-based inhibitors to capture covalently active catalysts from phage-displayed libraries (20); (ii) catalytic capture or release of phage from solid support, where both antibody (or enzyme) catalyst and substrate are expressed on the phage coat (21); and (iii) in vivo selections based on complementation of auxotrophs (22–24). Such strategies are designed to provide a direct linkage between catalysis and biological amplification to evolve protein catalysts for a broad range of chemical reactions.
Among these new strategies for generating antibody catalysts is the notion of reactive immunization. In this case, the immunogen is designed to irreversibly modify any antibodies that have active site groups that can catalyze the reaction of interest (25). For example, efficient aldolase antibodies were generated that proceed by an enamine mechanism by using a β-diketone hapten that covalently modifies the ɛ amino group of an active site catalytic lysine (25). Catalytic antibodies were found that catalyze aldol reactions with efficiencies and stereoselectivities comparable to that of class I aldolases. Crystallographic studies showed the antibody active site contained a lysine residue (13). However, in contrast to the natural enzyme, these antibody aldolases have broad substrate specificity and should be of considerable practical chemical use. Indeed, these catalysts are now commercially available.
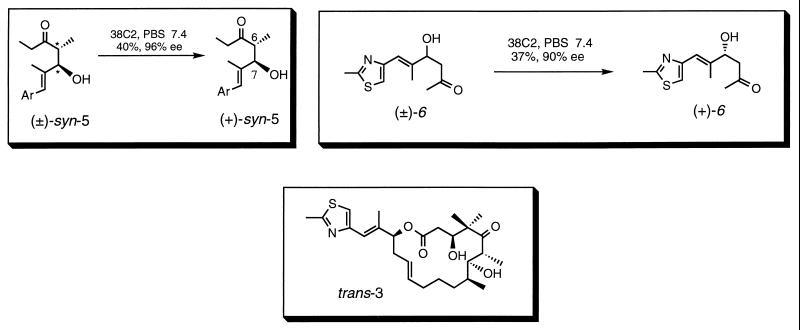
With the publication herein, we see that these aldolase antibodies can be used to carry out the synthesis of key chiral intermediates that allow the facile synthesis (by two different routes) of the natural product epothiolone, a powerful cytotoxic agent of considerable biomedical interest. In one of the synthetic routes, two different antibody-catalyzed steps afforded highly enriched enantiomers (+)-5 and (−)-6 (Scheme S1). These compounds were produced by kinetic resolutions of the corresponding achiral precursors in excellent conversions and proceeded with high enantiomeric excesses (96 and 90% ee, respectively). This is particularly impressive because the aldolase antibodies appear to be general for a broad range of aldol reactions and thus should be equally applicable to other key synthetic intermediates or products. The three chiral centers generated by these antibody-catalyzed reactions then were used to elaborate the total synthesis of epothilone A and C by previously established synthetic routes. This is not the first time that antibodies have been used effectively in total synthesis (26). Rather, this work illustrates the general use of an efficient catalyst, generated by using biological diversity, for a key chemical reaction involved in a large number of synthesis processes. Thus, the paper by Sinha et al. (1) represents yet another important chemical aspect of the field of antibody catalysis and serves to further illustrate the array of opportunities that arise by interfacing the complex machinery of nature with the synthesis and mechanistic tools of chemistry.
Scheme 1.
Footnotes
Quinkert, G., Rolf Sammet-Stiftung, May 22, 1998, Frankfurt.
A commentary on this article begins on page 14603.
References
- 1.Sinha S C, Barbas C F, III, Lerner R A. Proc Natl Acad Sci USA. 1998;95:14603–14608. doi: 10.1073/pnas.95.25.14603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tonegawa S. Nature (London) 1983;302:575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- 3.Pollack S J, Jacobs J W, Schultz P G. Science. 1986;234:1570–1573. doi: 10.1126/science.3787262. [DOI] [PubMed] [Google Scholar]
- 4.Tramontano A, Janda K D, Lerner R A. Science. 1986;234:1566–1570. doi: 10.1126/science.3787261. [DOI] [PubMed] [Google Scholar]
- 5.Schultz P G, Lerner R A. Science. 1995;269:1835–1842. doi: 10.1126/science.7569920. [DOI] [PubMed] [Google Scholar]
- 6.Lerner R A, Benkovic S J, Schultz P G. Science. 1991;252:659–667. doi: 10.1126/science.2024118. [DOI] [PubMed] [Google Scholar]
- 7.Schultz P G, Lerner R A. Acc Chem Res. 1995;26:391–395. [Google Scholar]
- 8.Wirsching P, Ashley J A, Benkovic S J, Lerner R A. Science. 1991;252:680–685. doi: 10.1126/science.2024120. [DOI] [PubMed] [Google Scholar]
- 9.Jacobsen J R, Prudent J R, Kochersperger L, Yonkovich S, Schultz P G. Science. 1992;256:365–367. doi: 10.1126/science.256.5055.365. [DOI] [PubMed] [Google Scholar]
- 10.Barbas C F, Heine A, Zhong G, Hoffmann T, Gramatikova S, Bjornestedt R, List B, Anderson J, Stura E A, Wilson I A. Science. 1997;278:2085–2092. doi: 10.1126/science.278.5346.2085. [DOI] [PubMed] [Google Scholar]
- 11.Ulrich H D, Mundorff E, Santarsiero B D, Driggers E M, Stevens R C, Schultz P G. Nature (London) 1997;389:271–275. doi: 10.1038/38470. [DOI] [PubMed] [Google Scholar]
- 12.Liu, D. R. & Schultz, P. G. Angew. Chem. Int. Ed. Eng., in press.
- 13.Romesberg F E, Santarsiero B D, Spiller B, Yin J, Barnes D, Schultz P G, Stevens R C. Biochemistry. 1998;37:14404–14409. doi: 10.1021/bi981578c. [DOI] [PubMed] [Google Scholar]
- 14.Patten P A, Gray N S, Yang P L, Marks C B, Wedemayer G J, Boniface J J, Stevens R C, Schultz P G. Science. 1996;271:1086–1091. doi: 10.1126/science.271.5252.1086. [DOI] [PubMed] [Google Scholar]
- 15.Romesberg F E, Spiller B, Schultz P G, Stevens R C. Science. 1998;279:1929–1933. doi: 10.1126/science.279.5358.1929. [DOI] [PubMed] [Google Scholar]
- 16.Huse W D, Sastry L, S, Iverson A, Kang A S, Alting-Mees M, Burton D R, Benkovic S J, Lerner R A. Science. 1989;246:1275–1281. doi: 10.1126/science.2531466. [DOI] [PubMed] [Google Scholar]
- 17.Kang A S, Barbas C F, Janda K K, Benkovic S J, Lerner R A. Proc Natl Acad Sci USA. 1991;88:4363–4366. doi: 10.1073/pnas.88.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCafferty J, Griffiths A D, Winter G, Chiswell D J. Nature (London) 1990;348:552–554. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- 19.Stemmer W P C. Nature (London) 1994;370:389–391. doi: 10.1038/370389a0. [DOI] [PubMed] [Google Scholar]
- 20.Janda K D, Lo L-C, Lo C-H, Sim M-M, Wang R, Wong C-H, Lerner R A. Science. 1997;275:945–948. doi: 10.1126/science.275.5302.945. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen H, Holder S, Sutherlin D, Schwitter U, King D, Schultz P G. Proc Natl Acad Sci USA. 1998;95:10523–10528. doi: 10.1073/pnas.95.18.10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lesley S A, Patten P A, Schultz P G. Proc Natl Acad Sci USA. 1993;90:1160–1165. doi: 10.1073/pnas.90.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang Y, Hicks J B, Hilvert D. Proc Natl Acad Sci USA. 1991;88:8784–8786. doi: 10.1073/pnas.88.19.8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smiley J A, Benkovic S J. Proc Natl Acad Sci USA. 1994;91:8319–8323. doi: 10.1073/pnas.91.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbas C F, III, Heine A, Zhong G, Hoffmann T, Gramatikova S, Bjornestedt R, List B, Anderson J, Stura E A, Wilson I A, et al. Science. 1997;278:2085–2092. doi: 10.1126/science.278.5346.2085. [DOI] [PubMed] [Google Scholar]
- 26.Sinha S C, Keinan E. J Am Chem Soc. 1995;117:3653–3654. [Google Scholar]