Abstract
Hepatitis C virus (HCV) is remarkable at disrupting human immunity to establish chronic infection. Up-regulation of inhibitory signaling pathways (such as Tim-3) and accumulation of regulatory T cells (Tregs) play pivotal roles in suppressing antiviral effector T cell (Teff) responses that are essential for viral clearance. While the Tim-3 pathway has been shown to negatively regulate Teffs, its role in regulating Foxp3+ Tregs is poorly explored. In this pilot study, we investigated whether and how the Tim-3 pathway alters Foxp3+ Treg development and function in patients with chronic HCV infection. We found that Tim-3 was up-regulated, not only on IL-2-producing CD4+CD25+Foxp3− Teffs, but also on CD4+CD25+Foxp3+ Tregs, which accumulate in the peripheral blood of chronically HCV-infected individuals when compared to healthy subjects. Tim-3 expression on Foxp3+ Tregs positively correlated with expression of the proliferation marker Ki67 on Tregs, but inversely associated with proliferation of IL-2-producing Teffs. Moreover, Foxp3+ Tregs were found to be more resistant to, and Foxp3− Teffs more sensitive to, TCR-activation-induced cell apoptosis, which was reversible by blocking Tim-3 signaling. Consistent with its role in T cell proliferation and apoptosis, blockade of Tim-3 on CD4+CD25+ T cells promoted expansion of Teffs more substantially than Tregs through improving STAT-5 signaling, thus correcting the imbalance of Foxp3+ Tregs/Foxp3− Teffs that was induced by HCV infection. Taken together, the Tim-3 pathway appears to control regulatory and effector T cell balance through altering cell proliferation and apoptosis during HCV infection.
Keywords: Tim-3, Foxp3, regulatory T cells, effector T cells, HCV, immune modulation
Introduction
Hepatitis C virus (HCV) is a global health problem characterized by persistent infection, limited therapeutic options, poor treatment responses, and no available vaccine1. Following years of intensive research into the pathogenesis of HCV, it has become evident that this virus is able to modulate host immunity, in particular T cell responses, and by doing so facilitates chronic infection2. The mechanisms by which HCV impairs antiviral T cell immunity include blunted T cell activation and proliferation by up-regulating inhibitory pathways, skewed T cell differentiation (Th1 deficiency or Th2 dominance), T cell anergy (antigen-specific hypo-responsiveness or exhaustion), T cell depletion (cell apoptosis or death), and induction of regulatory T cells (Tregs)2.
Tregs constitute a unique T-cell lineage that suppresses the function of effector T cells (Teffs) and controls the phenomenon of infectious tolerance. Foxp3 (Forkhead box P3) has been identified as a marker and transcription factor programming CD4+CD25+ Treg development and function2–3. Accumulation of Foxp3+ Tregs is a common characteristic of most chronic viral infections, and significantly suppresses antiviral CD4+ and CD8+ T cell responses4–5. The suppressive function of Foxp3+ Tregs requires TCR stimulation and additional mechanisms that include cell-cell interaction, regulatory cytokine production (TGF-β/IL-10), and IL-2 trapping6–7. In published studies, Foxp3 appears to induce a state of anergy in Tregs, being incapable of producing IL-2 or other survival cytokines; however, over-expression of CD25 (IL-2R α chain) on Tregs may absorb paracrine IL-2 that, while essential for Treg proliferation, leads to depletion or apoptosis of Teffs8–10. Thus, CD4+CD25+Foxp3+ Tregs are crucial in maintaining peripheral immune tolerance by limiting CD4+CD25+Foxp3− Teff responses, thereby minimizing T cell-dependent injury11. Despite intensive studies for the role of Tregs in T cell suppression and HCV pathogenesis, little is known about how Foxp3+ Tregs are fine-tuned in balancing T-cell-dependent immune-protection and immune-injury, a balance that may determine viral persistence versus clearance12–13.
In addition to Tregs, the recently described programmed death-1 (PD-1) and T cell immunoglobulin and mucin domain protein-3 (Tim-3) pathways represent other mechanisms to maintain the intricate balance between positive and negative signals to ensure adequate immune-protection against pathogens, and yet prevent over-activation of lymphocytes and thus immune-injury or autoimmunity14–15. Compelling evidence is emerging for the role of PD-1 and Tim-3 in peripheral immune tolerance, autoimmune response, antitumor and antiviral immune evasion, which has raised the possibility that a therapeutic strategy targeting these inhibitory pathways might be of clinical benefit, including for those with HCV infection16–17. Recently, we and others have demonstrated that PD-1 negatively regulates CD4+CD25+Foxp3+ Tregs during HCV infection18–19. The role of Tim-3 in regulation of Foxp3+ Tregs during HCV infection remains to be determined. Tim-3 is a type 1 membrane protein with a structurally conserved immunoglobulin variable (IgV) domain and mucin stalk that connects to an intracellular tail15. Initially identified as preferentially expressed on activated Th1 cells, but not Th2 cells, Tim-3 has since been found to be expressed on and have more complex functions in other immune cells, including Tregs15. Tim-3’s natural ligand, galectin-9 (Gal-9), has been shown to be up-regulated during chronic HCV infection, resulting in expansion of CD4+CD25+Foxp3+ Tregs, contraction of CD4+ Teffs, and apoptosis of HCV-specific CTLs20. Thus, this inhibitory pathway may not only regulate proliferation and differentiation of naïve T cells, but also control responses of Teffs, memory cells, and perhaps, Tregs.
Foxp3+ Treg accumulation and PD-1-or Tim-3-mediated T cell exhaustion and/or apoptosis are characteristic of chronic HCV infection1–2. While Tim-3 has been shown to play a central role in Teff dysregulation15–17, its role in Treg development and functional regulation is poorly understood. To investigate the role of Tim-3 in regulation of the balance between Foxp3+ Tregs and Foxp3− Teffs, and explore its potential role in persistent HCV infection, here we examined Tim-3 expression on CD4+CD25+Foxp3+ Tregs and CD4+CD25+Foxp3− Teffs, and its function in their regulation. In this report, we provide pilot evidence suggesting that the Tim-3 pathway alters the numbers and ratios of Foxp3+ Tregs and Foxp3− Teffs by regulating cell proliferation and apoptosis during HCV infection, and discuss the potential significance of these complex interactions in the phenomenon of viral persistence.
Material and method
Subjects
The study subjects composed of two groups of populations. The first group comprised 89 chronically HCV-infected subjects. HCV genotype and viral load were performed by Lexington VAMC and all subjects were virologically and serologically positive for HCV, prior to the IFN/RBV treatment. The second group includes age-matched 29 healthy subjects who are negative for HBV, HCV, and HIV infections. Written informed consent was obtained from all participants, and the study was approved by an institutional review board of East Tennessee State University and James H. Quillen VA Medical Center (ETSU/VA IRB).
Cell isolation and culture
Human peripheral blood mononuclear cells (PBMCs) were isolated from whole blood using Ficoll density gradient centrifugation (Atlanta biological, Lawrenceville, GA). Human CD4+ T cells were purified from PBMCs by magnetic beads with column purification according to the manufacturer’s instructions (Miltenyi Biotec Inc, Auburn CA). CD4+CD25+ T cells were purified using the CD4+CD25+ Regulatory T Cell Isolation Kit (Miltenyi Biotec). Briefly, human CD4+ T cells were negatively purified by depletion of non-CD4+ cells over a MACS column after labeling PBMCs with a cocktail of biotin-conjugated mAbs and anti-Biotin MicroBeads; then the enriched CD4+ T cell fraction was subjected to CD25 positive selection using CD25-conjugated MicroBeads and separated using a MACS column. The unlabeled CD4+CD25− T cell fraction was obtained by collecting the effluent. Isolated PBMCs, CD4+ T cells, CD4+CD25+ T cells, and CD4+CD25− T cells cultured with RPMI 1640, containing 10% fetal bovine serum (FBS, Life Technologies, Gaithersburg, MD), 100 mg/ml penicillin-streptomycin (Thermo Scientific, Logan, Utah), and 2 mM L-glutamine (Thermo Scientific, Logan, Utah) and other stimulators as described in the text.
Flow cytometry
PBMCs were stimulated by anti-CD3/CD28 (1 µg/ml of each, InvivoGen, San Diego, CA) for 24 h, in the presence of Brefeldin A (BioLegend, San Diego, CA) for the last 6 h to inhibit cytokine secretion. Cell surface staining was carried out using FITC-CD4 (Miltenyi Biotec), APC-CD25 (Miltenyi Biotec), PE-TIM-3 (R&D Systems), PE-Annexin-V (BD Biosciences), PE-CD3 and APC-CD28 (eBioscience), followed by intracellular staining for PerCP-Cy5.5-Foxp3 (eBioscience), PE-IL-2 (Miltenyi Biotec), PE-Ki67 (eBioscience) in parallel wells using the same purified PBMCs from the patients. For limited subjects depending on the available of samples, CD4+ or CD4+CD25+ T cells were purified as described above for simultaneously detecting IL-2, Ki67, Tim-3, and Foxp3 expressions in the same wells. The intracellular cytokine staining was carried out using Inside Stain kit (Miltenyi Biotec). Unstained and isotype-matched control antibodies (eBioscience) were used to determine the background level of staining and fluorescence minus one (FMO) controls were used to properly set the compensation and gate. The cells were analyzed on a FACSCalibur flow cytometry (BD, Franklin Lakes, NJ) and CELLQuest or FlowJo software.
Tim-3 blockade
Healthy and/or HCV patients’ PBMCs or purified CD4+, CD4+CD25+ T cell were incubated with LEAF™ anti-human Tim-3 antibody (10 µg/ml, BioLegend) or control IgG overnight, followed by stimulation with α-CD3/CD28 antibody (1 µg/ml each, InvivoGen) for 48 h, then subjected for flow cytometric analysis of FoxP3, Ki67, IL-2, or Annexin-V expressions. The cultures were carried out in the presence of rhIL-2 (50U/ml, R&D Systems) for 6 days in CFSE assays.
Western blot
The purified CD4+CD25+ cells were treated as described in the Tim-3 blocking assay and the expression of phosphorylated and total STAT-5 were measured by Western blot. Briefly, the CD4+CD25+ T cells were lysed in 1×RIPA lysis buffer (Boston BioproductsInc, Ashland, MA) supplied with Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Scientific, Rockfor, IL). Cell lysates were centrifuged for 30 min at 4°C and the protein concentrations were measured. Protein samples were thereafter combined with 4×Laemmli sample buffer (Boston BioProducts, Ashland, MA), denatured, and separated by SDS-PAGE. Following transfer to Amersham Hybond-P membrane (GE Healthcare, Piscataway, NJ), the membrane was blocked and probed with anti-phospho-STAT-5 or total STAT-5 antibody (Cell Signaling Technology, Inc, Danvers, MA). Then, the membrane was incubated with a horseradish peroxidase-conjugated secondary antibody (Millipore, Temecula, CA) and developed by Amersham ECL plus Western Blotting Detection Reagents (GE Healthcare Biosciences, Pittsburgh, PA) on Kodak X-OMAT-LS X-ray film (Sigma-Aldrich, St. Louis, MO). Specific bands were quantified by densitometry.
Proliferation assays
Purified CD4+CD25+ or CD4+CD25− T cells were label with CFSE (2.5 µM, Invitrogen) for 10 min at 37°C per manufacture’s instruction, washed with complete medium, and cultured (5 × 104), or co-cultured with purified Tregs or Tresp at a 1:1 cell ratio (the physiological ratio of Tregs to Teffs is about 1:16 in the peripheral blood of healthy subjects), in a 96-well plate in the presence or absence of different combinations of anti-CD3/CD28 antibodies (1 µg/ml each, InvivoGen), rhIL-2 (50U/ml, R&D Systems), LEAF™ anti-human Tim-3 antibody and control blocking antibody (10 ug/ml, BioLegend) as described in the text. After 6 d stimulation, cells were analyzed with a FACS flow cytometer (BD).
Statistical analysis
Study results are summarized for each group and results are expressed as the mean ± standard deviation (SD). Comparison between two groups is performed using multiple comparisons testing-least significant difference or Turkey’s procedure depending on the ANOVA F-test by SPSS 18 software or Prism (version 4; Graph Pad) software using nonparametric Mann-Whitney U test. Bonferonni correction is applied for those samples with multiple tests. Pair wise t-test is used to compare the significance of changes in Tim-3 blocking experiments. Correlations were analyzed by a Pearson Correlation program. Values of p < 0.05 (*), p < 0.01(**), and p < 0.001 (***) were considered significant or very significant.
Results
Accumulation of CD4+CD25+Foxp3+ Tregs in chronic HCV infection
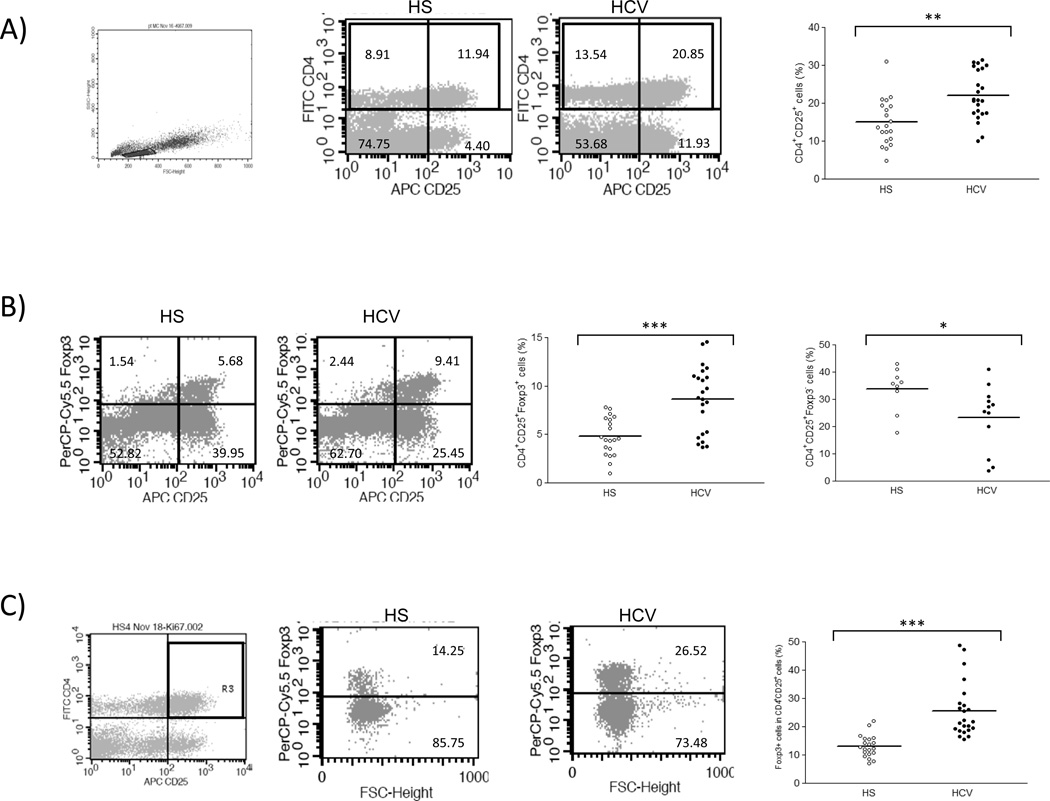
As an initial approach to characterize their functions following TCR activation, Tregs were identified in PBMCs isolated from individuals with chronic HCV infection and healthy subjects (HS). T cell receptor (TCR) stimulation by anti-CD3/CD28 activates T cells and up-regulates expressions of Tim-3, CD25, Foxp3, Ki67, and IL-2 as well as the apoptosis marker annexin V (Av) on CD4+ T cells (Fig. S1). Therefore, to simultaneously compare Tim-3 expression and cell differentiation and functions, resting PBMCs isolated from both HCV-infected patients and HS were TCR-stimulated with anti-CD3/CD28 for all experiments described in this study. As we reported previously18, CD4+CD25+ T cells accumulated in patients with chronic HCV compared to HS (Fig. 1A). Since CD25 is also a cell activation marker, Foxp3 was employed to distinguish this mixed population into CD4+CD25+Foxp3+ Tregs and CD4+CD25+Foxp3− Teffs. As shown in Fig. 1B, the absolute percentages of CD4+CD25+Foxp3+ Tregs were found to be significantly higher in chronically HCV-infected patients than in HS; conversely, the percentages of CD4+CD25+Foxp3− Teffs were significantly lower in HCV-infected patients versus HS. Likewise, the mean fluorescence intensities (MFI) of Foxp3 expression were equivalent to the cell number frequencies detected (data not shown), and the relative ratios of Foxp3+/CD4+CD25+ were also markedly elevated in the peripheral blood of HCV-infected subjects compared to that of HS (Fig. 1C).
Fig. 1. Accumulation of CD4+CD25+Foxp3+ Tregs in chronic HCV infection.
A) Flow cytometric analyses of PBMCs from healthy subjects (HS) and chronically HCV-infected patients (HCV), stained with conjugated mAbs: FITC-CD4, APC-CD25, and PerCP-Cy5.5-Foxp3. The cells were first gated on lymphocyte populations and then further gated on CD4+ T cells, with frequency of cells in each quadrant indicated in the representative dot plots. Summary percentages of CD4+CD25+ T cells detected in the gated populations of HS and HCV is shown on the right panel. Each symbol represents a single individual, and the horizontal bars represent median values. The p value (**<0.01) is denoted above the group of studied subjects. B) Representative dot plots of Foxp3 expression in CD4+CD25+ T cell populations, gated based on isotype and FMO controls, in HS and HCV are shown on the left panel. Summary data for the difference of CD4+CD25+Foxp3+ Tregs and CD4+CD25+Foxp3− Teffs in HS versus HCV are shown on the right panels. *P< 0.05, ***P< 0.001. C) The relative frequency of Foxp3+ cells in CD4+CD25+ T cell populations. The cells were gated on CD4+CD25+ T cells, and the ratio of Foxp3+ cells in CD4+CD25+ T cells from HS and HCV are shown. ***P < 0.001.
Upregulation of Tim-3 on CD4+CD25+Foxp3+ Tregs in chronic HCV infection
To determine the relationship between Tim-3 expression and Foxp3+ Treg induction, PBMCs isolated from chronically HCV-infected patients and HS were TCR-stimulated as described above, followed by flow cytometric analysis of Tim-3 expression on bulk CD4+ T cells, CD4+CD25+ mixed populations, CD4+CD25+Foxp3+ Tregs, and CD4+CD25+Foxp3− Teffs. As shown in Fig. 2A, Tim-3 expression was significantly higher in bulk CD4+ T cells and mixed CD4+CD25+ T cells of HCV patients compared with HS. Notably, Tim-3+ cell numbers were also found to be significantly higher in CD4+CD25+Foxp3+ Tregs and CD4+CD25+Foxp3− Teffs in HCV patients when compared to HS; and this held true when MFI of Tim-3 expression levels were examined (Fig. 2B). The specificity of Tim-3 expression on Tregs was confirmed by flow cytometric analysis using different source of Tim-3 McAbs (R&D system; Biolegend). Importantly, Tim-3 expression on CD4+CD25+Foxp3+ Tregs was relatively higher than those CD4+CD25+Foxp3− Teffs, in terms of positive cell number and MFI, in HCV-infected individuals; however this phenomenon was not found in HS (Fig. 2A and 2B), suggesting that HCV up-regulates Tim-3 on Foxp3+ Tregs. The relationship between Tim-3 and Foxp3 expressions in Tregs of HCV patients was determined by Pearson correlation analysis. Indeed, Tim-3 expression on Tregs was found to be closely associated and significantly correlated to Foxp3 expression in CD4+CD25+ T cells (Fig. 2C). We also analyzed Tim-3 expression on CD4− T cell (CD8) populations, and found that Tim-3 markedly increased on CD4−CD25−, CD4−CD25+, and CD4−CD25+Foxp3− T cells in HCV patients compared to that of HS (data not shown).
Fig. 2. Upregulation of Tim-3 on CD4+CD25+Foxp3+ Tregs in chronic HCV infection.
A) Flow cytometry analyses of PBMCs from HS and HCV, stained with conjugated mAbs: FITC-CD4, APC-CD25, PerCP-Cy5.5-Foxp3, and PE-Tim-3. The cells were gated on CD4+ T cells (dot plot upper column) and CD4+CD25+ subpopulations (dot plot upper right column for Foxp3+/− cell analysis) with frequency in each quadrant indicated in the representative dot plots; and summary data of Tim-3 expression on CD4+, CD4+CD25+, CD4+CD25+Foxp3+ Tregs, and CD4+CD25+Foxp3− Teffs examined in HS and HCV, calculated based on each specific cell populations, is shown below. Each symbol represents a single individual, and the horizontal bars represent median values. *P< 0.05, **P< 0.01. NS = no significance. B) Summary data (mean ± SD) of mean fluorescence intensity (MFI) of Tim-3 expression on CD4+CD25+Foxp3+ Tregs and CD4+CD25+Foxp3− Teffs from HS versus HCV patients. *P<0.05, **P<0.01. C) Correlation analysis between Foxp3 expression in CD4+CD25+ cells and Tim-3 expression on CD4+CD25+Foxp3+ Tregs in HCV-infected individuals. Pearson correlation and P values are shown above.
Tim-3 expression positively correlates with Ki67 expression and proliferation of Foxp3+ Tregs
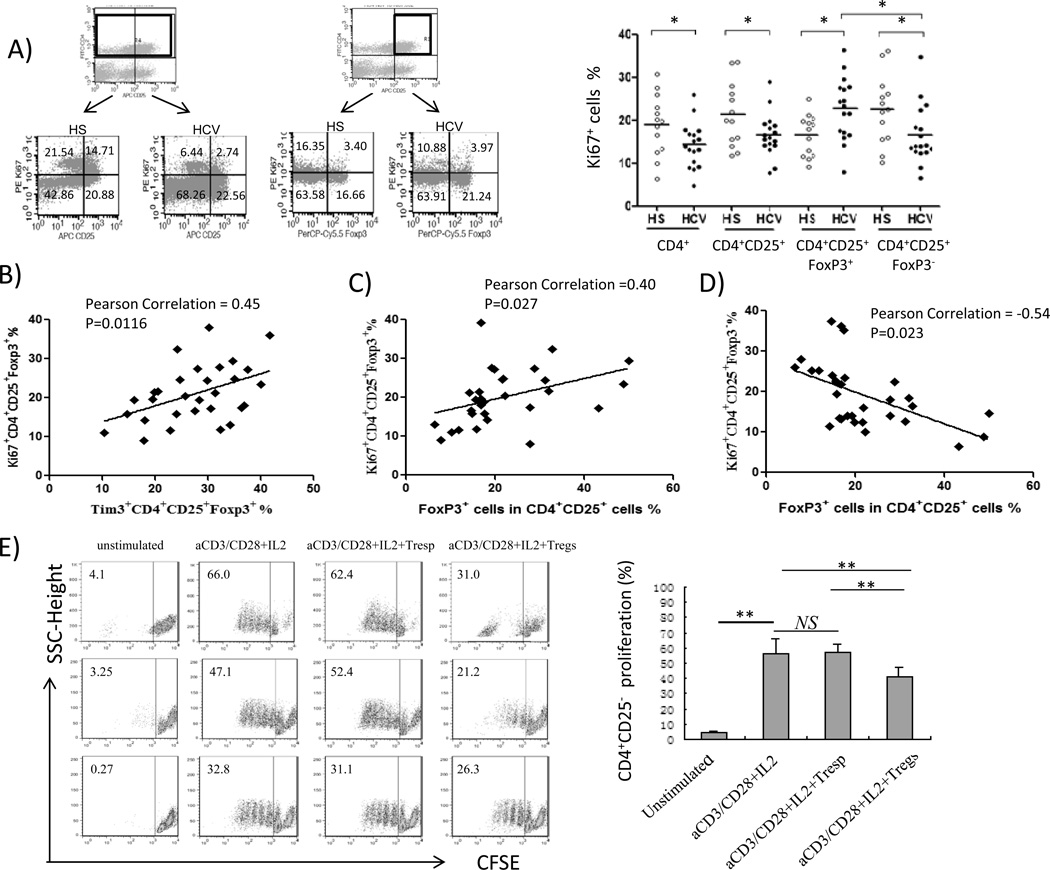
Ki67 (also known as MKI67) is a nuclear protein that is associated with ribosome RNA transcription, functioning for cell expansion and thus serving as a cell proliferation marker21. To investigate whether Foxp3+ Treg accumulation in the peripheral blood of HCV-infected patients reflects an increase of cell proliferation, we assessed Ki67 expression in Foxp3+ Tregs and Foxp3− Teffs in HCV-infected patients versus HS. As shown in Fig. 3A, representative dot plot and summary data of Ki67 expression on gated CD4+, CD4+CD25+, CD4+CD25+Foxp3+ Tregs, and CD4+CD25+Foxp3− Teffs from healthy versus HCV-infected subjects, Ki67 expression was found to be considerably lower in bulk CD4+ T cells, in mixed CD4+CD25+ T cells, and in CD4+CD25+Foxp3− Teffs in PBMCs from HCV patients compared to HS in response to ex vivo TCR stimulation. This is in consistent with our previous reports showing that Teffs exhibit an exhausted phenotype during HCV infection22–31. However, the opposite was observed in Tregs, in that Ki67 expression was significantly higher in CD4+CD25+Foxp3+ Tregs in chronically HCV-infected patients compared to HS. Remarkably, Foxp3+ Tregs exhibited higher Ki67 expression than Foxp3− Teffs in HCV-infected patients, whereas Foxp3+ Tregs exhibited lower Ki67 expression than Foxp3− Teffs in HS, suggesting greater expansion of CD4+CD25+Foxp3+ Tregs in HCV infection, but greater proliferation of CD4+CD25+Foxp3− Teffs in HS in response to TCR stimulation. Interestingly, the majority of Ki67+ cells were Tim-3−, regardless of Foxp3 expression (data not shown), suggesting that Tim-3 is indeed involved in negatively controlling both Foxp3+ and Foxp3− T cell expansion. Moreover, Tim-3 was expressed relatively higher on Foxp3+ Tregs than Foxp3− Teffs (Fig. 2), and Tim-3 expression positively correlated with Ki67 expression in Foxp3+ Tregs during HCV infection (Fig. 3B), indicating that HCV-mediated Tim-3 expression is associated with Foxp3+ Treg proliferation. Correspondingly, Foxp3 expression in CD4+CD25+ T cells was positively associated with Ki67 expression in Foxp3+ Tregs (Fig. 3C) and inversely correlated with Ki67 expression in Foxp3− Teffs (Fig. 3D), supporting the notion that expanded Tregs may inhibit Teff proliferation during HCV infection.
Fig. 3. Upregulation of Tim-3 on CD4+CD25+Foxp3+ Tregs positively correlates with the expression of Ki67 on Tregs in chronic HCV infection.
A) Flow cytometry analyses of PBMCs from HS and HCV, stained with conjugated mAbs: FITC-CD4, APC-CD25, PerCP-Cy5.5-Foxp3, and PE-Ki67. The cells were gated on CD4+ and CD4+CD25+ T cells with frequency indicated in each quadrant of representative dot plots; and summary data of Ki67 expression on CD4+, CD4+CD25+, CD4+CD25+Foxp3+ Tregs, and CD4+CD25+Foxp3− Teffs detected in HS and HCV are shown on the right. Each symbol represents a single individual, and the horizontal bars represent median values. *P<0.05. B) The correlation analysis between Ki67 and Tim-3 expressions in CD4+CD25+Foxp3+ Tregs in HS and HCV-infected individuals. Pearson correlation and P values are shown above. C) Ki67 expression by CD4+CD25+Foxp3+ Tregs positively correlated to the frequency of Foxp3 expression by CD4+CD25+ cells. D) Ki67 expression by CD4+CD25+Foxp3− Teffs inversely correlated with Foxp3 expression by CD4+CD25+ Tregs. E) Functional analysis of CD4+CD25+ T cells in suppression of CD4+CD25− T cell proliferation. CD4+CD25− T cells were isolated from HCV-infected individuals, labeled with CFSE, and then stimulated without or with anti-CD3/CD28 + IL-2 alone and in co-culture with purified CD4+CD25− T responder cells (Tresp) or CD4+CD25+ Treg-containing cells (1:1 ratio) for 6 days, followed by analysis of CFSE dilution as a means to measure T cell proliferation. Representative dot plots of cells from three HCV-infected patients under various treatments are shown on the left panel, and summary data of CFSE-labeled T cell proliferation from 8 HCV patients are shown on the right. **P<0.01. NS=no significance.
To consolidate this notion, we analyzed the inhibitory function of CD4+CD25+ T cells in our co-culture system. To this end, CFSE-labeled CD4+CD25− T responder cells (Tresps) of HCV-infected individuals were stimulated with anti-CD3/CD28 and IL-2 50 U/ml alone, or in co-culture with purified autologous CD4+CD25− Tresp- or CD4+CD25+ Treg-containing cells (1:1 ratio) for 6 days, and CFSE dilutions in Tresp cells were analyzed as a means to measure T cell proliferation. As shown in Fig. 3E, representative dot plots from 3 HCV-infected subjects, anti-CD3/CD28 and IL-2 stimulation promoted Tresp cell proliferation, while CD4+CD25+ T cells containing Foxp3+ Tregs significantly inhibited CD4+CD25−Foxp3− Tresp proliferation when compared to those co-cultured with CD4+CD25− controls. These results were reproducible in multiple independent experiments, as summary data from 8 HCV-infected individuals shown on the right.
Tim-3 expression on Foxp3+ Tregs inversely correlates with IL-2 production by Foxp3− Teffs
To further address the role of Tim-3 in regulation of T cell function, we examined the relationship between Tim-3 expression and IL-2 production by Foxp3+ Tregs and Foxp3− Teffs. To this end, PBMCs isolated from both HCV-infected and HS were TCR-stimulated as described above, followed by flow cytometric analysis of intracellular IL-2 production by bulk CD4+ T cells, CD4+CD25+ mixed populations, CD4+CD25+Foxp3+ Tregs, and CD4+CD25+Foxp3− Teffs. As shown in Fig. 4A, in HCV-infected or uninfected subjects, virtually all the IL-2-producing cells were CD4+CD25+Foxp3− Teffs; while CD4+CD25+Foxp3+ Tregs produced little if any IL-2, agreeing with the notion that Foxp3+ Tregs are in an anergic state. Consistent with the inhibited phenotype of T cells in chronically infected patients, HCV patients had significantly less IL-2-producing CD4+, CD4+CD25+, and CD4+CD25+Foxp3− Teffs than HS (Fig. 4A). In both chronically HCV-infected patients and HS, however, activated CD4+CD25+Foxp3− Teffs produced markedly higher levels of IL-2 than CD4+CD25−Foxp3− non-activated Teffs following TCR stimulation (data not shown).
Fig. 4. Upregulation of Tim-3 on CD4+CD25+Foxp3+ Tregs inversely correlates with the inhibition of IL-2-producing CD4+CD25+Foxp3− Teffs in chronic HCV infection.
A) Representative dot plots of PBMCs stained with FITC-CD4, APC-CD25, PerCP-Cy5.5-Foxp3, and PE-IL-2. The cells were gated on CD4+CD25+ T cells (upper right column) and IL-2 expression, primarily by Foxp3− Teffs, in HS versus HCV are shown. Summary data for comparison the frequency of IL-2-expressing cells in CD4+, CD4+CD25+, and CD4+CD25+Foxp3− Teffs in HS and HCV subjects is shown on the right. Each symbol represents a single individual, and the horizontal bars represent median values. *P< 0.05. B) Representative zebra plots of purified CD4+ T cells stained with FITC-CD25, APC-Tim-3, PerCP-Cy5.5-Foxp3, and PE-IL-2. The cells were gated on CD25+ T cells, and IL-2 expression, primarily by Foxp3− Teffs as shown in the upper panel, in Tim-3+ and Tim-3− cells from HS and HCV are shown below. Summary data of IL-2 expression by Tim-3+ versus Tim-3− Teffs from 8 HS and 8 HCV patients are shown on the right. **P<0.01; ***P<0.001. C) Correlation analysis between Tim-3 and IL-2 expressions by CD4+CD25+Foxp3− Teffs using purified CD4+ T cells from HS and HCV-infected individuals. D) Correlation analysis between IL-2 expression by CD4+CD25+Foxp3− Teffs and Tim-3 expression on CD4+CD25+Foxp3+ Tregs using purified CD4+ T cells from HS and HCV-infected individuals. Pearson correlation and P values are shown above.
To elucidate the relationship between Tim-3 expression on Foxp3− Teffs/Foxp3+ Tregs and IL-2 production, we purified CD4+ T cells from 8 HS and 8 HCV-infected patients and simultaneously examined IL-2, Tim-3, CD25, and Foxp3 expressions in the same cells. Again, we observed that IL-2 was primarily expressed by Foxp3− Teffs but not Foxp3+ Tregs; and decreased IL-2 production by Teffs isolated from HCV-infected subjects when compared to HS (Fig. 4B upper panel). Importantly, the majority of IL-2-producing Teffs were Tim-3−, regardless of infection status (Fig. 4B lower panel and summary data on the right), suggesting that Tim-3 negatively controls IL-2 expression in both HS and HCV infection. Moreover, IL-2-producing Teffs were found to be inversely associated with Tim-3 expression on, not only CD4+CD25+Foxp3− Teffs (Fig. 4C), but also CD4+CD25+Foxp3+ Tregs (Fig. 4D), supporting the notion that Tim-3-associated Foxp3+ Tregs may suppress the function of Foxp3− Teffs, as shown in Fig. 3D.
Foxp3+ Tregs are resistant and Foxp3− Teffs sensitive to TCR activation-mediated cell apoptosis that is reversible by blocking Tim-3 signaling
We have demonstrated that HCV infection increases Tim-3 expression (Fig. 2) that is associated with the absolute numbers of Foxp3+ Tregs (Fig. 1B), perhaps through enhanced cell proliferation by higher Ki67 expression in Foxp3+ Tregs (Fig 3B); meanwhile, HCV infection also increases the relative ratios of Foxp3 expression in CD4+CD25+ T cell populations (Fig. 1C). To explore whether this might be due to apoptosis of type-1 IL-2-producing CD4+CD25+ T cells during HCV infection, we examined whole PBMCs gated on CD4+CD25+ T cells (Fig. 5A) or purified CD4+CD25+ T cells (Fig. 5B) in response to higher doses of anti-CD3/CD28 stimulation (2~5 µg/ml, TCR over-activation-induced apoptosis), followed by flow cytometric analysis of Av expression on Foxp3− Teffs and Foxp3+ Tregs. Intriguingly, IL-2-producing Foxp3− Teffs were found to be more sensitive to TCR activation-induced cell apoptosis, in a dose-dependent manner; while non-IL-2-producing Foxp3+ Tregs were resistant to apoptosis. These data were reproducible in repeated experiments with purified CD4+CD25+ T cells in both HCV-infected and HS, but more prominently observed in HCV-infected subjects. To rule out that this lack of susceptibility to anti-CD3/CD28-mediated apoptosis in CD4+CD25+Foxp3+ Tregs was simply due to a lack of TCR, we purified CD4+ T cells from 5 HCV-infected subjects and 5 HS, stimulated as described above, and then stained for Foxp3, CD25, CD3, and CD28. As shown in Fig. S2, CD3 and CD28 are highly expressed on these cells and exhibit no expression differences in Foxp3+ Tregs versus Foxp3− Teffs.
Fig. 5. Foxp3+ Tregs are resistant, while IL-2-producing Foxp3− Teffs sensitive to, TCR activation-mediated apoptosis that is reversible by Tim-3 blockade.
A) Dose- and cell-dependent apoptosis induced by TCR stimulation. PBMCs from HCV-infected patients were stimulated with anti-CD3 and anti-CD28 at 0, 2, and 5 µg/ml of each for 48 h, stained with FITC-CD4, APC-CD25, PerCP-Cy5.5-Foxp3, and PE-Annexin-V, followed by flow cytometric analysis. The cells were gated on CD4+CD25+ T cell populations and then Foxp3+ Tregs (upper panel) and Foxp3− Teffs (lower panel), based on the isotype controls. The frequency of Annexin-V+ cells in the gated CD4+CD25+Foxp3+ Tregs or CD4+CD25+Foxp3− Teffs is indicated in the right upper corner. B) Representative dot blots of Annexin-V expression on CD4+CD25+FoxP3+ Tregs (upper panel) and CD4+CD25+FoxP3− Teffs (lower panel) by stimulation of purified CD4+CD25+ T cells from chronic HCV patients with varying doses of anti-CD3/CD28, followed by flow cytometric analysis of apoptosis as described above. The data are reproducible using purified cells from multiple HS and HCV. C) Tim-3 blockade lead to a decrease of apoptosis of purified CD4+CD25+ T cells from HCV patients. Purified CD4+CD25+ T cells from HCV-infected patients were pretreated with anti-Tim-3 or control IgG overnight, and then stimulated with anti-CD3/CD28 (2 µg/ml) for 48 h, followed by flow cytometric analysis of Annexin-V expression on CD4+CD25+FoxP3+ Tregs (upper panel) and CD4+CD25+ FoxP3− Teffs (lower panel). Summary data obtained from 8 HCV-infected patients are shown in the right panel. **P<0.01. D) Tim-3 blockade reduced apoptosis of IL-2-producing Foxp3− Teffs. Purified CD4+CD25+ T cells from HCV-infected patients were pretreated with anti-Tim-3 or control IgG overnight and then stimulated with anti-CD3/CD28 (2 µg/ml) for 48 h, followed by immunostaining and flow cytometric analysis of Annexin-V expression on IL-2− Foxp3+ Tregs (upper panel) and IL-2+ FoxP3− Teffs (lower panel). Summary data collected from 8 HCV-infected patients are shown in the right panel. **P<0.01.
Interestingly, Av expression on IL-2+ Foxp3− Teffs was observed more significantly on whole PBMCs than purified CD4+CD25+ T cells (Fig. 5A vs. Fig. 5B), suggesting that other types of cells, such as DC or NK cells and/or their secreted cytokines, might be contributing to the TCR activation-induced cell apoptosis. Most importantly, blockade of Tim-3 signaling on CD4+CD25+ cells significantly reversed the TCR-activation-mediated apoptosis of Foxp3− Teffs (Fig. 5C). We observed the same results in purified CD4+CD25+ T cells gated on IL-2+ Foxp3− Teffs and IL-2− Foxp3+ Tregs in that blocking Tim-3 signaling significantly rescued the apoptosis of IL-2-producing Foxp3− Teffs (Fig. 5D). These data were reproducible in 8 HCV-infected subjects, as summarized in Fig.5C~D right panels. In conjunction with the expression of Tim-3 on different cell populations, these results suggest that the Tim-3 pathwaynot only controls the development and function, but also the fate, of naïve CD4+ T cells, activated CD4+CD25+ T cells, Foxp3− Teffs, and Foxp3+ Tregs upon TCR activation.
Blockade of Tim-3 signaling enhances CD4+CD25+ T cell proliferation by improving STAT-5 signaling
To further investigate the role of Tim-3 pathway on T cell regulation, we examined the effect of blocking Tim-3 signaling on proliferation of CD4+CD25+ T cells. To this end, CFSE-labeled CD4+CD25+ T cells from HCV-infected patients were incubated with anti-Tim-3 blocking antibody overnight, stimulated with anti-CD3/CD28 (1 µg/ml) and IL-2 (50 µg/ml) for 6 days, followed by Foxp3 double staining and flow cytometric analysis of CFSE dilution as a means of measuring Foxp3+ Treg proliferation. As shown in Fig. 6A left panel, anti-CD3/CD28 and IL-2 stimulation promoted CD4+CD25+Foxp3+ T cell proliferation, and this effect could be significantly enhanced by blockade of Tim-3 signaling, compared to cells treated with TCR and IL-2 stimulation in the presence of IgG control. These data were reproducible in multiple experiments using purified CD4+CD25+ T cells isolated from 8 HCV-infected subjects and gated on Foxp3+ Tregs, as summarized in Fig. 6A right panel. We have previously demonstrated that dampened STAT-1 and STAT-5 proteins are involved downstream of PD-1 or Tim-3 signaling in inhibition of monocyte IL-12 production during HCV infection32–35. To elucidate the intracellular pathways that might be involved in Tim-3 signaling in the T cell lineage, purified CD4+CD25+ T cells treated as described above were also subjected to Western blot analysis for the phosphorylation of STAT proteins. In line with the increase in T cell proliferation, CD4+CD25+ T cells treated with Tim-3 blocking antibody exhibited enhanced phosphorylation of STAT-5, but not STAT-1 protein (data not shown), compared to cells treated with control IgG (Fig. 6B). These results suggest that the Tim-3 pathway negatively regulates CD4+CD25+ T cell proliferation through inhibiting STAT-5 signaling.
Fig. 6. Tim-3 blockade differentially promotes proliferation of Foxp3+ Tregs and Foxp3− Teffs through improving the phosphorylation of STAT-5 in CD4+CD25+ T cells from HCV patients.
A) Tim-3 blockade on CD4+CD25+ T cells enhances Foxp3+ Treg expansion. Left panel, single representative dot plots of CFSE-labeled CD4+CD25+ T cells from HCV-infected patients that were pretreated with a control IgG or anti-Tim-3 overnight, then stimulated without or with anti-CD3/CD28 (1 µg/ml) and IL-2 (50U/ml) for 6 d; the cells were double-stained with Foxp3 and CFSE dilutions in Foxp3+ Tregs are shown. Right panel, summary data of proliferation of CD4+CD25+Foxp3+ Tregs from 8 HCV-infected patients under various ex vivo treatments. *P<0.05, **P<0.01. B) Tim-3 blockade improves the phosphorylation of STAT-5 in CD4+CD25+ T cells from HCV-infected patients. The purified CD4+CD25+ T cells from HCV-infected individuals were pretreated with a control IgG or anti-Tim-3 antibody overnight and then stimulated with anti-CD3/CD28 (1 µg/ml) and IL-2 (50U/ml) for 6 h, followed by Western blot to detect pSTAT-5 and total STAT-5. Densitometry data of phosphorylated STAT-5, normalized by total STAT-5 as loading control, from 3 HCV-infected patients is shown below. **P<0.01. C) Tim-3 blockade on CD4+ T cells ex vivo promotes expansion of Foxp3− Teffs more substantially, thus correcting the imbalance of Foxp3+ Tregs/Foxp3− Teffs established during chronic HCV infection. Purified CD4+ T cells were treated as described in A), and summary data of the Foxp3+ Tregs/Foxp3− Teffs ratio changes under various treatments using purified CD4+ T cells from 8 HCV-infected patients is shown. **P<0.01. D) Tim-3 blockade on CD4+ T cells reduces the ratio of CD25+Foxp3+ Tregs in the CD4+ T cells from HS and HCV-infected individuals. The purified CD4+ T cells were pretreated with a control IgG or anti-Tim-3 overnight, and then stimulated with anti-CD3/CD28 (1 µg/ml) for 48 h, followed by flow cytometric analysis of Foxp3 expression in CD25+ T cells. Summary data of CD25+Foxp3+ Tregs in the purified CD4+ T cells under various treatments from 8 HS and 8 HCV-infected patients is shown in the right panel. **P<0.01.
Since it appeared that Tim-3 was up-regulated on Foxp3+ Tregs to a greater extent than Foxp3− Teffs, and the susceptibility of these two distinct type of cells to TCR-activation-mediated apoptosis differed following T cell activation (Fig. 2 and Fig. 5), we further determined whether blocking Tim-3 signaling ex vivo might expand Foxp3+ Tregs and Foxp3− Teffs differentially and, in doing so, correct the imbalance observed during chronic infection. To this end, CFSE-labeled purified CD4+ T cells from chronically HCV-infected patients were treated as described above, and then double stained for Foxp3, followed by flow cytometric analysis of CFSE dilution in Foxp3+/− cell populations. While blockade of Tim-3 signaling on CD4+ T cells enhanced the proliferation of both Foxp3+ Tregs and Foxp3− Teffs, this effect was observed more prominently in CD4+CD25+Foxp3− Teffs than CD4+CD25+Foxp3+ Tregs; i.e., Tregs were relatively less expanded than Teffs (Fig. 6C left panel). This more significant expansion of Foxp3− Teffs versus Foxp3+ Tregs following Tim-3 blockade is in concurrence with their susceptible to apoptotic rescue upon Tim-3 blockade (Fig. 5), leading to a reduction of the Foxp3+ Tregs/Foxp3− Teffs ratio. Cumulative experiments to confirm these data from 8 HCV-infected patients, as summarized in Fig. 6C right panel, demonstrated that the increased Foxp3+ Tregs/Foxp3− Teffs ratio established in vivo during HCV infection was corrected by blocking Tim-3 signaling ex vivo. We further confirmed this notion by directly examining Foxp3 frequency in CD4+CD25+ T cells, isolated from chronically HCV-infected individuals as well as HS, and treated with anti-Tim-3 versus control IgG ex vivo. As shown in Fig. 6D, representative dot plots, the up-regulation of Foxp3+ Tregs during HCV infection was significantly reversed by blockade of Tim-3 signaling. These data were reproducible with CD4+ T cells isolated from 8 HCV patients and 8 HS examined, as summarized in Fig. 6D right panel.
We have previously reported that PD-1 modulates Tregs and suppresses T cell responses during HCV infection18. Here, we also found that blocking PD-L1 ligation on CD4+CD25+ T cells promoted Foxp3− Teffs expansion more substantially than Foxp3+ Tregs, leading to reduced frequencies of Foxp3+ Tregs in bulk CD4+ T cells from HCV-infected patients following ex vivo treatment by anti-PD-L1 antibody. However, we did not observe synergistic effects on promoting CD4+CD25+ T cell proliferation by dual blockade of PD-1 and Tim-3 pathways (data not shown). We also did not find synergistic effects on reversing increased Treg frequencies established during HCV infection by dual blockade of PD-1 and Tim-3 pathways (data not shown), perhaps due to the fact that these negative pathways are linked or associated in a network of intracellular signaling28,33,34 such that blocking either PD-1 or Tim-3 alone could correct the imbalance of Foxp3+ Tregs to Foxp3− Teffs established during HCV infection.
Discussion
Both the Tim-3 pathway and Foxp3+ Tregs control the balance between an adequate protective immune response and suppression of T cell-dependent immunopathology that contribute to viral persistence. However, it remains unclear how Treg development and function are regulated to fine-tune this balance, allowing control of excessive T cell-mediated injuries without completely suppressing antiviral T cell responses. While Tim-3/Gal-9 interactions have been shown to negatively regulate Teffs, their role in regulating Foxp3+ Tregs is poorly explored. Here, we observed that chronic HCV infection was characterized by relatively higher numbers and ratios of CD4+CD25+Foxp3+ Tregs, with lower CD4+CD25+Foxp3− Teffs, upon TCR activation. Intriguingly, the i) accumulation of CD4+CD25+Foxp3+ Tregs that express the proliferation marker Ki67 but not intracellular IL-2, and ii) the contraction of CD4+CD25+Foxp3− Teffs that are the primary IL-2-producing cells, coincided with Tim-3 expression on Tregs during chronic HCV infection. Tim-3 expression on Foxp3+ Tregs positively correlated with their Ki67 expression, but was inversely associated with expansion of IL-2-producing Teffs. Moreover, the majority of Ki67+ and IL-2+ cells were Tim-3−. In addition to cell proliferation, Foxp3+ Tregs were found to be more resistant to, and Foxp3− Teffs more sensitive to, TCR activation-induced cell apoptosis. The apoptosis of IL-2-producing Foxp3− Teffs could be rescued by blocking Tim-3 signaling in purified CD4+CD25+ T cells, and this appeared to reverse the imbalance of Foxp3+ Tregs/Foxp3− Teffs established during HCV infection.
Given the relatively higher Tim-3 levelsexpressed on Foxp3+ Tregs versus Foxp3− Teffs, one would expect that blocking Tim-3 signaling could enhance proliferation of Foxp3+ Tregs to a greater extent than Foxp3− Teffs through improving STAT-5 phosphorylation. However, we observed more significant Foxp3− Teff expansion upon blocking the Tim-3 pathway, resulting in a correction of the imbalanced ratio of Foxp3+ Tregs/Foxp3− Teffs established during chronic HCV infection. It is feasible that Tim-3-mediated cell apoptosis has a more prominent effect than its inhibitory effects on cell proliferation, and it appears that the apoptotic rescue effect on Foxp3− Teffs outweighs proliferative rescue effect on Foxp3+ Tregs following Tim-3 blockade, especially given that the final result involves ratios of Foxp3+ Treg/Foxp3− Teff are much different than what is observed prior to Tim-3 blockade.
Based on these findings, we propose a model in which the Tim-3 pathway controls regulatory and effector T cell balance during HCV infection (Fig. 7). This model is plausible by providing an insight understanding of the pathogenesis of HCV persistence. The mechanisms by which HCV-mediated Tim-3 expression regulates T cell responses in vivo are likely multiple, to include induction of apoptosis of pathogenic Tim-3+ Foxp3− Teffs and induction and/or expansion of Tim-3+ Foxp3+ Tregs, leading to decreased pro-inflammatory cytokine and increased anti-inflammatory cytokine secretion during chronic HCV infection. As we have shown in this study, while more IL-2-producing CD4+CD25+Foxp3− Teffs underwent apoptosis, less non-IL-2-producing CD4+CD25+Foxp3+ Tregs were Annexin V+ (Fig. 5). One interpretation would be that Tim-3/Gal-9 interactions lead to increased sensitivity of Foxp3− Teffs and increased resistance of Foxp3+ Tregs to apoptosis, this differential susceptibility to cell apoptosis plus differential ability of Tim-3-associated Foxp3+/− cell proliferation, resulting in an altered Tregs to Teffs balance in chronic infection, which perhaps accounts in part for the counter-regulatory/anti-inflammatory effect of this inhibitory pathway.
Fig. 7. A model for Tim-3/Gal-9 interactions in regulating the HCV-mediated imbalance of Foxp3+ Tregs/Foxp3− Teffs during HCV infection.
Our data support the proposed notion that HCV infection up-regulates the expression of Tim-3 and accumulation of Foxp3+ Tregs, inhibiting Foxp3− Teff responses and resulting in blunted antiviral T cell responses with decreased pro-inflammatory cytokines and increased anti-inflammatory cytokines that contribute to viral persistence. Specifically, HCV-mediated Tim-3 inhibit proliferation and induce apoptosis of activated CD25+Foxp3− Teffs, leading to impaired protective Th1 responses as well as limited pathogenic injury. Meanwhile, as a negative feedback mechanism, Tim-3 is also up-regulated and tempers CD25+Foxp3+ Tregs, which accumulate during HCV infection and may further dampen antiviral T cell responses. Notably, Tim-3 pathway fine-tunes the development and function of Foxp3+ Tregs and blocking Tim-3 signaling may correct the imbalance of Foxp3+ Tregs/Foxp3− Teffs established during HCV infection. Therefore, the counter-regulatory effect of Tim-3 on Tregs should be taken into account when manipulating this inhibitory pathway as a novel strategy for immunotherapy against chronic viral infection, so as to balance the protective immune responses and avoid T cell-dependent injury.
In addition to cell death through apoptosis, the Tim-3 pathway could be involved in the induction and/or expansion of Foxp3+ Tregs. Recently, Tim-3 and Gal-9 mRNA levels were shown to positively correlate with Foxp3 mRNA expression in PBMCs of rheumatoid arthritis patients and were found to be significantly higher in patients with low disease activity compared to those with moderate to high disease activity. This suggests that the Tim-3/Gal-9 pathway could exert its suppressive effect on RA disease activity by modulation of Foxp3+ Tregs36. In HSV infection, Tim-3 had been found to be expressed by activated but not naïve T cells; more than 50% of T cells in HSV-induced ocular lesions in mice express Tim-3 and blocking Tim-3 signaling resulted in more severe lesions37. Importantly, Gal-9 administration could diminish the severity of ocular lesions by inhibiting Th1 cells and promoting Tregs37. Here we show that in HCV infection, Tim-3 pathway appears to control Treg/Teff development and functions. This notion is supported by a positive correlation between Tim-3 and Foxp3 (Fig. 2C) or Ki67 (Fig. 3B, 3C) expressions in Tregs, but negative association between Tim-3 or Foxp3 expressions by Tregs and Ki67 expression (Fig. 3D) or IL-2 production (Fig. 4C, 4D) by Foxp3− Teffs; and that blocking Tim-3 signaling corrected the imbalance of Foxp3+ Tregs to Foxp3− Teffs established during HCV infection (Fig. 6). Most recently in an in vitro cell co-culture system, we have also demonstrated that HCV-infected hepatocytes that express higher levels of Gal-9 and TGF-β can convert naïve CD4+ T cells into Foxp3+ Tregs (with higher levels of TGF-β/IL-10 expression) and inhibit IL-2-producing Foxp3− Teffs proliferation through the Tim-3 pathway, leading to a significant balance shift in Foxp3+ Tregs to Foxp3− Teffs (Ji X.J et al. submitted). It is possible that this balance shift of Treg and Teff responses contributes to T cell-mediated persistent viral hepatitis or viral clearance.
Although HCV is not considered to be an immunosuppressive virus in the manner of HIV, it does cause a general as well as virus-specific immunodysregulation - as evidenced for example by the fact that individuals with chronic HCV infection respond poorly to vaccinations38–40. Multiple mechanisms have been described for Treg-mediated immunosuppression. Firstly, Tregs can directly kill Teffs in a cell contact-dependent manner. Recent studies report that Tregs mediate cell death or apoptosis through a Fas/FasL interaction and a granzyme B-dependent, perforin-independent mechanism41. Indeed, Fas and FasL have been reported to be up-regulated on HCV-infected livers and Teffs to cause liver damage42–43. Secondly, Tregs can modulate the activities of Teffs by production of immunosuppressive cytokines, such as TGF-β and IL-10, which may regulate each other during T cell responses44. Enhanced TGF-β has been identified in HCV infection; its polymorphism is associated with natural clearance of HCV infection; and its level dramatically deceased in HCV patients who respond to antiviral therapy45–46.
Thirdly, Tregs over-express CD25 (IL-2R a chain), perhaps compensating for a lack of endogenous IL-2 production by absorbing high amounts of paracrine IL-2 essential for Treg proliferation, while depriving Teff of survival signaling. IL-2 triggers IL-2R downstream signaling pathways, including STAT-5 phosphorylation and translocation into the nucleus to activate gene transcription and promote cell proliferation. Our data suggest that Tim-3 negatively controls both Foxp3+ Treg and Foxp3− Teff expansion through STAT-5 signaling. From this point of view, homeostasis between Tregs and Teffs would be maintained by expression levels of Tim-3 on these cells, prohibiting exaggerated activation or suppression of Teffs. In the scenario of HCV infection, however, IL-2 mRNA transcription and translation are directly inhibited and IL-2-producing cells are prone to apoptosis; moreover, the poorly available IL-2 is also captured by IL-2-consuming-Tregs via highly expressed IL-2R to be used for expansion. Therefore, IL-2-producing Teffs may be deprived of survival cytokines and die by apoptosis9,47, as we observed in this study (Fig. 5). Our results are consistent with recent findings that support the notion of Tregs as an IL-2 “sink,” a critical mechanism for suppression of CD4+ Teffs via an IL-2-deprivation-induced apoptosis48.
Tim-3 signaling might thus hamper defense against potentially pathogenic Teffs by simultaneously harnessing two mechanisms of peripheral tolerance: 1) the direct inhibition of pro-inflammatory Teffs; and 2) the regulation of Treg development and function. Foxp3+ Tregs induced by the Tim-3/Gal-9 interactions might assist in maintaining immune homeostasis, keeping the threshold for T cell activation high enough to safeguard against autoimmunity and prevent excessive injury; meanwhile Tim-3 signaling in differentiated Tregs tempers their inhibitory function, counter-regulating Tregs to allow adequate protective immune responses to limit viral infection.
On the ligand side, Gal-9 expressed on non-hematopoietic cells in the inflamed liver, as well as hematopoetic cells in the periphery where TGF-β is present, endows Tim-3/Gal-9 interactions with the capacity to promote Treg development and regulate Treg function. While it is clear that Tim-3 is up-regulated on Tregs during HCV infection (Fig. 2), little is known about the mechanism(s) that control its expression. A recent study revealed that Tim-3 expression in the Th1 lineage is controlled by the transcription factor T-bet49, whose expression is regulated at the translational level by microRNA-146a (miR-146a)50. Whether T-bet and miRs also control Tim-3 transcription in Foxp3+ Tregs is yet to be determined and under study in our laboratory. Additionally, we have also shown that HCV-induced PD-1, SOCS-1, and Tim-3 can cross-talk with each other in inhibiting immune responses28,33,34. We thus are further determining whether a slow-degradation of Tim-3 protein is occurring as it clusters with other inhibitory molecules, resulting in its up-regulation in Tregs during HCV infection.
There are several limitations to this study and controversies in this area which must be noted. Our work focused on CD4+CD25+Foxp3+ T cells as true T regulatory cell populations with regulatory activity, as much of the literature has suggested. We believe this is supported by our data showing a suppressive role for this population in co-culture experiments (Fig. 3E), but acknowledge that additional markers to identify Treg populations (such as CD127 staining) might be more specific and can be pursued in future studies. In addition, data interpretation and understanding should take into consideration by the fact that our in vitro experiments required TCR stimulation that induce such molecules as Tim-3 and Foxp3, but which are necessary in such studies to create an experimentally level playing field for further assays.
In summary, HCV escapes host immunity by its capacity to generate quasispecies, to exert virus-specific as well as general immunosuppression, to develop Tregs, and to induce PD-1/Tim-3-mediated Teff exhaustion or apoptosis. This study represents the first efforts toward characterizing a role for HCV-induced Tim-3 expression in controlling Foxp3+ Treg development and function. Our results indicate that the Tim-3 pathway plays an important role in negative regulation of both CD4+CD25+Foxp3+ Tregs and CD4+CD25+Foxp3− Teffs, since blocking Tim-3 signaling significantly corrects the imbalance of Tregs/Teffs ratio established during HCV infection. Tim-3’s regulatory effects depend on its expression levels on T cells, on its way to interact with Gal-9 (trans- vs. cis-association), and on the milieu of other regulatory cytokines. The cellular balance shifts by altering Foxp3+ Treg to Foxp3− Teff cell number and ratio through regulation of cell proliferation and apoptosis. Since T-cell-dependent immune responses are a double-edged sword, causing harmful tissue damage while attempting to eradicate viral infection, this study is fundamental for understanding the mechanisms by which the balance of Tregs and Teffs is fine-turned through the Tim-3 pathway during HCV-host interactions. Therefore, the complex role of the Tim-3 pathway in Treg regulation must be taken into account when targeting this inhibitory pathway for immunotherapy.
Supplementary Material
Acknowledgements
We would like to thank Dr. Chuan F. Li, Department of Surgery; Dr. Donald B. Hoover, Department of Pharmacology; ETSU Quillen College of Medicine, for the use of laboratory equipments and technique support. We would also like to appreciate the supporting staffs from Hepatitis (HCV/HIV) Program at James H. Quillen VAMC. This publication is the result of work supported with resources and the use of facilities at the James H. Quillen Veterans Affairs Medical Center. The contents in this publication do not represent the views of the Department of Veterans Affairs or the United States Government.
This work was supported by an NIH NIAID grant to ZQY/JPM (R15A1084057), an NIH NIDDK grant to ZQY/JPM (R01DK093526), and an ETSU Major Research Grant to ZQY (12-002). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 2.Walker CM. Adaptive immunity to the hepatitis C virus. Adv. Virus Res. 2010;78:43–86. doi: 10.1016/B978-0-12-385032-4.00002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 4.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 5.Belkaid Y, Chen W. Regulatory ripples. Nat Immunol. 2010;11:1077–1078. doi: 10.1038/ni1210-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 7.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat. Immunol. 2005;6:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 8.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 9.Barthlott T, Moncrieffe H, Veldhoen M, Atkins CJ, Christensen J, O’Garra A, Stockinger B. CD25+CD4+ T cells compete with naïve CD4+ T cells for IL-2 and exploit it for the induction of IL-10 production. Int. Immunol. 2005;17:279–288. doi: 10.1093/intimm/dxh207. [DOI] [PubMed] [Google Scholar]
- 10.de la Rosa M, Rutz S, Dorninger H, Scheffold A. Interleukine-2 is essential for CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2480–2488. doi: 10.1002/eji.200425274. [DOI] [PubMed] [Google Scholar]
- 11.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat. Rev. Immunol. 2007;7:875–888. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 12.Shevach EM. CD4+CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 13.Haiqi H, Yong Z, Yi L. Transcriptional regulation of Foxp3 in regulatory T cells. Immunobiology. 2011;216:678–685. doi: 10.1016/j.imbio.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura H, Honjo T. PD-1: an inhibitory immunoreceptor involved in peripheral tolerance. Trends in Immunol. 2001;22:265–268. doi: 10.1016/s1471-4906(01)01888-9. [DOI] [PubMed] [Google Scholar]
- 15.Hafler DA, Kuchroo V. TIMs: central regulators of immune responses. J. Exp. Med. 2008;205:2699. doi: 10.1084/jem.20082429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, et al. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Invest. 2010;1172:4546–2557. doi: 10.1172/JCI43127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vali B, Jones RB, Sakhdari A, Sheth PM, Clayton K, et al. HCV-specific T cells in HCV/HIV co-infection show elevated frequencies of dual Tim-3/PD-1 expression that correlate with liver disease progression. Eur. J. Immunol. 2010;40:2493–2505. doi: 10.1002/eji.201040340. [DOI] [PubMed] [Google Scholar]
- 18.Ni L, Ma CJ, Zhang Y, Nandakumar S, Zhang CL, Wu XY, Borthwick T, Kumaraguru U, Moorman JP, Yao ZQ. PD-1 modulates Regulatory T cells and suppresses T cell responses in HCV-associated Lymphoma. Immunology & Cell Biology. 2011;89:535–539. doi: 10.1038/icb.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franceschini D, Paroli M, Francavilla V, Videtta M, Morrone S, Labbadia G, Cerino A, Mondelli MU, Barnaba V. PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in subjects chronically infected with HCV. J. Clin. Invest. 2009;119:551–564. doi: 10.1172/JCI36604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mengshol JA, Golden-Mason L, Arikawa T, Smith M, Niki T, et al. A crucial role for kupffer cell-derived galectin-9 in regulation of T cell immunity in hepatitis C infection. PLoS one. 5:e9504. doi: 10.1371/journal.pone.0009504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bullwinkel J, Baron-Lühr B, Lüdemann A, Wohlenberg C, Gerdes J, Scholzen T. Ki-67 protein is associated with ribosomal RNA transcription in quiescent and proliferating cells. J. Cell. Physiol. 2006;206:624–635. doi: 10.1002/jcp.20494. [DOI] [PubMed] [Google Scholar]
- 22.Kittlesen DJ, Chianese-Bullick KA, Yao ZQ, Braciale TJ, Hahn YS. Interaction between complement receptor gC1qR and hepatitis C virus core protein inhibits T lymphocyte proliferation. J. Clin. Invest. 2000;106:1239–1249. doi: 10.1172/JCI10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao ZQ, Nguyen DT, Hiotellis AI, Hahn YS. Hepatitis C virus core protein inhibits human T lymphocyte responses by a complement-dependent regulatory pathway. J. Immunol. 2001;167:5264–5272. doi: 10.4049/jimmunol.167.9.5264. [DOI] [PubMed] [Google Scholar]
- 24.Yao ZQ, Ray S, Eisen-Vandervelde A, Waggoner S, Hahn YS. Hepatitis C virus: Immunosuppression by complement regulatory pathway. Viral Immunology. 2001;14:277–295. doi: 10.1089/08828240152716547. [DOI] [PubMed] [Google Scholar]
- 25.Yao ZQ, Eisen-Vandervelde A, Ray S, Hahn YH. HCV core/gC1qR interaction arrests T cell cycle progression through stabilization of the cell cycle inhibitor p27kip1. Virology. 2003;314:271–282. doi: 10.1016/s0042-6822(03)00419-7. [DOI] [PubMed] [Google Scholar]
- 26.Yao ZQ, Eisen-Vandervelde A, Waggoner SN, Cale EM, Hahn YS. Direct binding of hepatitis C virus core to gC1qR on CD4+ and CD8+ T cells leads to impaired activation of Lck and Akt. J. Virol. 2004;78:6409–6419. doi: 10.1128/JVI.78.12.6409-6419.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao ZQ, King E, Prayther D, Yin D, Moorman J. T cell dysfunction by hepatitis C virus core protein involves PD-1/PD-L1 signaling. Viral Immunology. 2007;20:276–287. doi: 10.1089/vim.2006.0096. [DOI] [PubMed] [Google Scholar]
- 28.Frazier AD, Zhang CL, Ni L, Ma CJ, Zhang Y, Wu XY, Atia AN, Yao ZQ, Moorman JP. Program death-1 pathway affects suppressor of cytokine signaling-1 expression in T cells during hepatitis C infection. Viral Immunology. 2010;23:487–495. doi: 10.1089/vim.2010.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao ZQ, Prayther D, Trabue C, Dong ZP, Moorman J. Differential regulation of SOCS-1 signaling in B and T lymphocytes by hepatitis C virus core protein. Immunology. 2008;125:197–207. doi: 10.1111/j.1365-2567.2008.02829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao ZQ, Ni L, Zhang Y, Ma CJ, Zhang CL, Wu XY, Thayer P, Borthwick T, Chen XY, Moorman JP. Differential Regulation of T and B lymphocytes by PD-1 and SOCS-1 signaling in Hepatitis C Virus-associated non-Hodgkin’s Lymphoma. Immunol Invest. 2011;40:243–264. doi: 10.3109/08820139.2010.534218. [DOI] [PubMed] [Google Scholar]
- 31.Moorman JP, Zhang CL, Ni L, Ma CJ, Zhang Y, Wu P, Thayer T, Borthwick XY, Yao ZQ. Impaired hepatitis B vaccine responses during chronic hepatitis C infection: involvement of the PD-1 pathway in regulating CD4+ T cell responses. Vaccine. 2011;29:3169–3176. doi: 10.1016/j.vaccine.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma C, Lei N, Zhang Y, Wu X, Atia A, Thayer P, Moorman JP, Yao ZQ. PD-1 negatively regulates IL-12 expression by limiting STAT-1 phosphorylation in monocytes/macrophages during chronic hepatitis C infection. Immunology. 2011;132:421–431. doi: 10.1111/j.1365-2567.2010.03382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Ma CJ, Ni L, Wu XY, Atia AN, Thayer P, Shanker U, Li CF, Moorman JP, Yao ZQ. Crosstalk between PD-1 and SOCS-1 in HCV core-mediated IL-12 suppression. J Immunol. 2011;186:3093–3103. doi: 10.4049/jimmunol.1002006. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Ma XY, Wu CJ, Moorman JP, Yao ZQ. A Crucial Role for Tim-3 in Negative Regulation of Monocyte IL-12 Production in Chronic Hepatitis C Infection. PLoS One. 2011;6:e19664. doi: 10.1371/journal.pone.0019664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Ma CJ, Wang JM, Ji XJ, Wu XY, Moorman JP, Yao ZQ. Tim-3 regulates pro- and anti-inflammatory cytokine expression in human CD14+ Monocytes. J Leuk Biol (Cutting-edge) 2012;91:189–196. doi: 10.1189/jlb.1010591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J, Oh JM, Hwang JW, Ahn JK, Bae EK, Won J, Koh EM, Cha HS. Expression of human Tim-3 and its correlation with disease activity in rheumatoid arthritis. Scand J Rhumatol. 2011;40:334–340. doi: 10.3109/03009742.2010.547871. [DOI] [PubMed] [Google Scholar]
- 37.Sehrawat S, Suryawanshi A, Hirashima M, Rouse BT. Role of Tim-3/Galectin-9 inhibitory interaction in viral-induced immunopathology: shifting the balance toward regulators. J Immunol. 2009;182:3191–3201. doi: 10.4049/jimmunol.0803673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leroy V, Bourliere M, Durand M, Abergel A, Tran A, et al. The antibody response to hepatitis B virus vaccination is negatively influenced by the hepatitis C virus viral load in patients with chronic hepatitis C: a case-control study. Eur J Gastroenterol Hepatol. 2002;14:485–489. doi: 10.1097/00042737-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Wiedmann M, Lieberi UG, Oesen U, Porst H, Wiese M, et al. Decreased immunogenicity of recombinant hepatitis B vaccine in chronic hepatitis C. Hepatology. 2000;31:230–234. doi: 10.1002/hep.510310134. [DOI] [PubMed] [Google Scholar]
- 40.Kramer ES, Hofmann C, Smith PG, Shiffman ML, Sterling RK. Response to hepatitis A and B vaccine alone or in combination in patients with chronic hepatitis C virus and advanced fibrosis. Dig. Dis. Sci. 2009;54:2016–2025. doi: 10.1007/s10620-009-0867-4. [DOI] [PubMed] [Google Scholar]
- 41.Gondek DC, Lu LF, Quezada SA, Akaguchi SS, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783–1786. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 42.Cruise MW, Lukens JR, Nguyen AP, Lassen MG, Waggoner SN, Hahn YS. Fas ligand is responsible for CXCR3 chemokine induction in CD4+ T cell-dependent liver damage. J. Immunol. 2006;176:6235–6244. doi: 10.4049/jimmunol.176.10.6235. [DOI] [PubMed] [Google Scholar]
- 43.Cruise MW, Melief HM, Lukens J, Soguero C, Hahn YS. Increased Fas ligand expression of CD4+ T cells by HCV core induces T cell-dependent hepatic inflammation. J Leuk Biol. 2005;78:412–425. doi: 10.1189/jlb.0105005. [DOI] [PubMed] [Google Scholar]
- 44.Cottrez F, Groux H. Regulation of TGF-beta response during T cell activation is modulated by IL-10. J Immunol. 2001;167:773–778. doi: 10.4049/jimmunol.167.2.773. [DOI] [PubMed] [Google Scholar]
- 45.Tarantino G, Coppola A, Conca P, Cimino E, Di Minno G. Can serum TGF-beta I be used to evaluate the response to antiviral therapy of haemophilic patients with HCV-related chronic hepatitis? Int J Immunopathol Pharmacol. 2008;21:1007–1012. doi: 10.1177/039463200802100426. [DOI] [PubMed] [Google Scholar]
- 46.Marck B, Kajdaniuk D, Mazurek U, Janczewska-Kazek E, Kos-Kudla B, et al. TGF-beta I mRNA expression in liver biopsy specimens and TGF-beta I serum levels in patients with chronic hepatitis C before and after antiviral therapy. J Clin Pharm Ther. 2005;30:271–277. doi: 10.1111/j.1365-2710.2005.00644.x. [DOI] [PubMed] [Google Scholar]
- 47.Scheffold A, Huhn J, Hofer T. Regulation of CD4+CD25+ regulatory T cells activity: it takes (IL-) two to tango. Eur J Immunol. 2005;35:1336–1341. doi: 10.1002/eji.200425887. [DOI] [PubMed] [Google Scholar]
- 48.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 49.Anderson AC, Lord GM, Dardalhon V, Lee DH, Sabatos-Peyton CA, Glimcher LH, Kuchroo VK. T-bet, a Th1 transcription factor regulates the expression of Tim-3. Eur J Immunol. 2010;40:859–866. doi: 10.1002/eji.200939842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo M, Mao X, Ji Q, Lang M, Li S, Peng Y, Zhou W, Xiong B, Zeng Q. miR-146a in PBMCs modulates Th1 function in patients with acute coronary syndrome. Immunol Cell Biol. 2010;88:555–564. doi: 10.1038/icb.2010.16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.