Abstract
Vascular endothelial growth factor (VEGF) is a hypoxia-induced angiogenic protein that exhibits a broad range of neurotrophic and neuroprotective effects in the central nervous system. Given that neurogenesis occurs in close proximity to blood vessels, increasing evidence has suggested that VEGF may constitute an important link between neurogenesis and angiogenesis. Although it is known that VEGF can directly stimulate the proliferation of neuronal progenitors, the underlying signaling pathways responsible in this process are not fully understood. Thus, in the present study, we set out to examine the requirement of two downstream targets of the VEGF/Flk-1 signaling network, the phosphatidylinositol 3-kinase (PI3K)/Akt and extracellular signal-regulated kinase (ERK) pathways, in producing the mitogenic effects of VEGF. Both in vivo and in vitro experiments showed that a single treatment of VEGF activated Erk1/2 and Akt signaling pathways in the adult rat hippocampus and in cultured hippocampal neuronal progenitor cells. This effect was blocked with the VEGF/Flk-1 inhibitor SU5416. Importantly, microinfusion of VEGF into the rat brain also induced pCREB expression in the dentate gyrus and increased the number of BrdU-labeled cells in the dentate subgranular zone. Double immunofluorescence labeling revealed that a large proportion of BrdU-labeled cells expressed activated forms of Flk-1, Erk1/2, and Akt. Interestingly, treatment with the SSRI fluoxetine, which is well known to stimulate neurogenesis and VEGF-signaling, also produced a similar expression pattern of Erk1/2 and Akt in proliferating cells. Finally, pharmacological experiments showed that administration of inhibitors of either MAPK/ERK (U0126) or PI3K (LY294002) blocked VEGF-stimulation of hippocampal cell proliferation in vivo and in vitro. Taken together, our findings demonstrate that the proliferative actions of VEGF require activation of both ERK and Akt signaling cascades and that these intracellular pathways are stimulated almost exclusively in actively proliferating neuronal progenitor cells of the adult hippocampus.
Keywords: VEGF, neurotrophic factors, MEK/ERK, PI3K/AKT, fluoxetine, neurogenesis, hippocampus
1. INTRODUCTION
Vascular endothelial growth factor (VEGF) is a potent endothelial cell mitogen and key regulator of angiogenesis (Leung et al., 1989; Yancopoulos et al., 2000). In addition to its well-established angiogenic effects, recent evidence has revealed an important role for VEGF in exerting trophic and protective actions on neurons. For example, VEGF stimulates neurite outgrowth and survival of superior cervical, dorsal root ganglion, and cortical neurons in culture (Jin et al., 2006; Khaibullina et al., 2004; Sondell et al., 2000), and protects both HN33 (mouse hippocampal neuron × neuroblastoma) and cortical neurons against cell death induced by hypoxic conditions (Jin et al., 2001; Jin et al., 2000; Li et al., 2005). Conversely, VEGF reduction triggers apoptosis of cultured cortical and hippocampal neurons (Matsuzaki et al., 2001; Ogunshola et al., 2002), and contributes to adult-onset motor neuron degeneration in mice (Oosthuyse et al., 2001). In light of these diverse effects, there has been increasing interest in the development of VEGF for the treatment of various neurodegenerative conditions, such as traumatic brain injury, amyotrophic lateral sclerosis, and stroke (Hermann and Zechariah, 2009; Skold and Kanje, 2008).
In the adult mammalian brain, the dentate subgranular zone (SGZ) and subventricular zone of the lateral ventricle are active sites of neurogenesis (Altman and Das, 1965; Cameron and McKay, 2001; Kempermann et al., 2004). It is well known that signals provided by the local microenvironment regulate the proliferation and differentiation of neural stem/progenitor cells (Suh et al., 2009). Of the proposed regulators, the vasculature represents an important candidate in providing the required molecular signals and metabolic demands necessary for maintaining neuronal progenitor pools throughout life. Consistent with this view, neurogenesis has been shown to occur in close proximity to growing blood vessels in the SGZ (Palmer et al., 2000), and accumulating evidence suggests that endothelial cells can influence neural stem/progenitor cell proliferation through the release of various growth factors (Li et al., 2006; Louissaint et al., 2002).
Several studies have found that VEGF can act as a direct stimulator of neurogenesis (Jin et al., 2002; Schanzer et al., 2004). VEGF exerts its biological functions through several receptors, among them VEGFR-2 (Flk-1) is believed to mediate most of the neuron-specific effects of VEGF, including neurogenesis (Ruiz de Almodovar et al., 2009), although there is recent evidence that VEGFR-1 (Flt-1) and VEGFR-3 (Flt-4) also regulate neurogenesis in the subventricular zone and dentate SGZ (Calvo et al., 2011; Wittko et al., 2009). While the exact contribution of VEGF-stimulated neurogenesis in the adult brain is unclear, a large number of studies have shown that VEGF expression is increased, particularly in the hippocampus, after various pro-neurogenic stimuli. For example, hippocampal VEGF expression is upregulated in response to antidepressant treatment, and VEGF signaling is required for the neurogenic as well as the behavioral effects of these drugs (Fournier and Duman, 2011; Greene et al., 2009; Lee et al., 2009; Warner-Schmidt and Duman, 2007). VEGF is also required for the increased cell proliferation and neurogenesis that occurs after adult mice are exposed to environmental enrichment (Cao et al., 2004) or exercise (Fabel et al., 2003), while conditions that reduce hippocampal cell proliferation, such as aging or stress, are associated with reduced levels of VEGF and Flk-1 in the hippocampus (Heine et al., 2005; Shetty et al., 2005).
Although these findings highlight the importance of VEGF/Flk-1 signaling in cell proliferation, the precise downstream intracellular signaling pathways mediating this effect on neuronal progenitor cells remain to be determined. In the present study, we set out to examine the requirement of two downstream targets of the VEGF/Flk-1 signaling network, the phosphatidylinositol 3-kinase (PI3K)/Akt and extracellular signal-regulated kinase (ERK) pathways, in producing the mitogenic effects of VEGF in the adult hippocampus. Our results reveal that VEGF increases adult hippocampal cell proliferation through activation of ERK and Akt signaling cascades, and that VEGF stimulates proliferation by directly acting on neuronal progenitor cells both in vivo and in vitro.
2. MATERIALS AND METHODS
Male Sprague-Dawley rats (Charles Rivers) weighing between 175 and 250 g at the time of arrival served as subjects. They were housed in pairs in rectangular polypropylene cages with standard laboratory bedding and kept on an artificial 12:12 h light:dark cycle with lights on at 0700 h local time. Ambient temperature in the housing facility was maintained at 20°C (±1°C). Food and water was available ad libitum throughout the duration of the experiment. Animal use and procedures were in accordance with the National Institutes of Health guidelines and approved by the Yale University Animal Care and Use Committees. All efforts were made to minimize the number of animals used in these experiments.
2.1. Drugs
Drugs used included human recombinant VEGF165 (Sigma-Aldrich), U0126 (10 mM, 4.3 μg/μl, Cell Signaling), LY294002 (10 mM, 4.6 μg/μl, Cell Signaling), and SU5416 (4 mM, 2.4 μg/μl, Sigma-Aldrich). All drugs were prepared according to the manufacturer's specification in either phosphate buffered saline (pH 7.2) or dimethyl sulfoxide (DMSO), and stored at −20°C until use. Bromodeoxyuridine (BrdU; 150 mg/kg, 20 mg/ml, Sigma-Aldrich) was dissolved in warm physiological saline (50°C) and then sterile filtered before administration.
Surgical and Microinfusion Procedure
After one week of habituation to the animal colony, rats were anesthetized with a ketamine (80 mg/kg, i.m., Fort Dodge Animal Health)-xylazine (6 mg/kg, i.m., Lloyd Laboratories) cocktail and placed into a stereotaxic apparatus. A single guide cannula (22 Ga, Plastic One) was inserted into the lateral ventricle (−0.9 mm anteroposterior, ±1.5 mm mediolateral, and −3.3 mm below dura). The cannula assembly was secured to the skull with four stainless steel screws and dental acrylic, and each animal was fitted with a dummy cannula to prevent the accumulation of debris.
Following a 7 to 9-day recovery period, compounds were delivered i.c.v. in a 2 μl volume and at a flow rate of 0.25 μl/min. The infusion cannula was left in place for an additional 3 minutes after delivery before slowly being withdrawn to facilitate diffusion of the compound and to prevent back-filling of the guide. Following the last infusion, animals were sacrificed at various time-points according to the purpose of the experiment. For inhibitor experiments, compounds (e.g., DMSO, U0126, LY294002, or SU5416) were delivered 30 minutes before VEGF or vehicle (PBS) infusion.
Western Blot Analysis
Dissected hippocampal samples were homogenized in lysis buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 2 mM EDTA, 1 mM sodium vanadate, 10 mM NaF, and 1× protease inhibitor cocktail. Protein concentration was determined by BCA assay (Pierce Biotechnology). For Western blotting, equal amounts of protein (10–30 μg) were loaded and separated on a 7.5% or 10% SDS-PAGE gel. To facilitate normalization of band intensities across different gels, the same control samples were loaded on all gels. After electrophoresis, the proteins were electrically transferred to nitrocellulose membranes. Following electro-transfer, membranes were blocked for 1 hr in 5% bovine serum albumin in TBS-T (TBS + 0.1% Tween-20) and incubated overnight at 4°C with primary antibody. The following primary antibodies were used: phospho-Akt (Ser473, 1:1000, Millipore), total Akt, phospho-ERK (Thr202/Tyr204, 1:1000, Millipore), phospho-CREB (Ser133, 1:1000, Millipore), total CREB (1:1000, Millipore), and GAPDH (1:10000, Millipore). Following incubation, membranes were washed in TBS-T and incubated for 1 h with an appropriate peroxidase-labeled secondary antibody (1:10000; Vector Laboratories). Bands were visualized with enhanced chemluminescence and exposed to Hyblot CL autoradiography film (Denville Scientific Inc.). Membranes were stripped (2% SDS, 100 mM β-mercaptoethanol, 50 mM Tris-HCl, pH 6.8) for 30 min at 50–55 °C and then received several washes with TBS-T. The stripped membranes were placed in blocking solution for 1 hr and incubated with a primary antibody direct against the total levels of the respective protein (non-phosphorylated) as a protein loading control.
The intensity of the protein bands was quantified using image analysis software (ImageJ 1.35, National Institute of Mental Health). For each blot, the background signal was determined by tracing an unlabeled area adjacent to each band and subtracting this value from the target band. Resultant values were normalized to the average signal for the total (non-phosphorylated) protein levels (also background adjusted) to simplify gel analysis and reduce inter and intra-gel variability.
Tissue Preparation and Immunohistochemistry
For in vivo analysis of cell proliferation, rats received a single injection of BrdU (150 mg/kg, i.p.) 2 hrs after VEGF or PBS microinfusion. Approximately, one hour later, the rats were deeply anesthetized with chloral hydrate (300 mg/kg, i.p.) and underwent transcardiac perfusion with 0.1 M phosphate buffer saline (PBS, pH=7.4) followed by 10% buffered formalin. The brains were extracted and postfixed in the same fixative for 48–72 hrs at 4°C before undergoing sucrose infiltration (15%, 30% sucrose) and sectioning on a freezing microtome (40 μm). All sections were collected and stored at −20°C in a cryoprotectant solution consisting of 30% sucrose, 1% polyvinylpyrrolidone, and 30% ethylene glycol in 0.1 M phosphate buffered saline (PBS; pH=7.4) until use.
For BrdU immunohistochemistry, free-floating sections were treated with 1 N HCl at 45 °C for 30–45 min to denature the DNA and expose the BrdU antigen. Sections were then incubated for 1 hr at room temperature in a blocking solution comprised of 5% normal horse serum, 1% bovine serum albumin (BSA), and 0.3% Triton X-100 dissolved in 0.1 M PBS. After blocking, the sections were treated with a primary anti-mouse BrdU monoclonal antibody (1:500, 48 hrs, 4°C, Becton Dickinson) diluted in the previously described blocking solution, followed by incubation with a secondary biotinylated antibody (horse anti-mouse, 1:500, 2 hrs, room temperature, Vector Laboratories) and then avidin-biotin peroxidase complex (1:200, 1 hr, room temperature, Vectastain ABC Elite, Vector Laboratories). Immunolabeled cells were visualized with a solution of 3,3'-diamobenzidine (DAB) according to the manufacturers specification (Vector Laboratories). Before coverslipping, mounted sections were counterstained with NovaRed (Vector Laboratories). For quantification, every 12th section throughout the hippocampus was counted using a modified unbiased stereology protocol (Kuhn et al., 1997). Briefly, BrdU-labeled cells in the dentate SGZ layer ipsilateral and contralateral to site of cannulation were counted at 400× magnification (Olympus BX60). The dentate SGZ was defined here as a two cell-body width zone along the border of the dentate granule cell layer and hilus. To avoid oversampling, cells in the outermost plane of focus were omitted. The number of BrdU-labeled cells counted was then multiplied by 12 to provide an estimate for the total number for BrdU+ cells per dentate SGZ layer.
For phospho-ERK, -Akt, -CREB, and Ki-67 immunohistochemistry, sections were treated with anti-mouse phospho-ERK (1:2000, Millipore, 24 hrs, 4°C), anti-rabbit phospho-Akt (1:1000, Millipore, 24 hrs, 4°C), anti-rabbit phospho-CREB (1:1000, Millipore, 48 hrs, 4°C), or anti-rabbit Ki-67 (1:250, Novacastra) antibodies. Sections were then treated with appropriate biotinylated secondary antibodies (all 1:500, 2 hrs, room temperature, Vector Laboratories) followed by amplification with avidin-biotin complex. Immunolabeled cells were visualized with a solution of DAB containing either nickel ammonium sulfate to yield black color precipitate or DAB-only to yield a brown precipitate (Vector Laboratories). For quantification of phospho-ERK and -Akt cells, the total number of phospho-labeled cells in the dentate SGZ was estimated using the same procedures as described above. Semi-quantitative densitometry was used to assess phospho-CREB expression in the dentate granule cell layer and SGZ, as well as in the CA1 stratum pyramidal subfield of the hippocampus. The procedure was adapted from a previously published protocol (Fournier et al., 2010). Briefly, images were captured at 8-bit resolution on a digital camera that was attached to an Olympus BX-60 microscope. Camera exposure and gain settings were held constant between animals. Using image-analysis software (ImageJ 1.35, National Institute of Mental Health), the mean relative optical density was calculated from digital images of three coronal sections. Background staining was controlled by calculating the average optical density levels from corpus callosum and subtracting these values from the region of interest. The results were then expressed as the percentage change from vehicle-treated controls. Finally, for Ki-67 labeled cells, the same protocol used to quantify BrdU+ cells was applied.
Adult hippocampal stem/progenitor cell isolation and culture
Adult rat hippocampal stem/progenitor cells used in this study were isolated from young adult Sprague-Dawley rats. The hippocampi were isolated and then enzymatically dissociated for 40 min at 37 °C in DMEM (Gibco) containing 1 U/mL Dispase II (Roche), 2.5 U/mL papain (Worthington), and 250 U/mL DNAse I (Worthington). Digested tissue was washed with DMEM/F12 (1:1) containing 10% defined FBS (Gibco), and then suspended in 35% PBS-equilibrated Percoll solution (Amersham Pharmacia Biotech). The cell suspension was fractionated by centrifugation for 10 min at 500 × g and the resultant pellet resuspended in 65% Percoll solution before being fractionated again for 10 min at 500 × g. The floating neuronal progenitors were collected and plated onto 6-well culture plates (Becton Dickinson) in DMEM/F12 (1:1) containing 10% FBS medium at a density of 1 × 105 cells cm2. The plates were coated with 10 mg/mL poly-L-ornithine (Sigma) and 5 mg/mL mouse laminin (Invitrogen). After 24 h, the medium was replaced with a growth medium consisting of DMEM/F12 (1:1) containing N2 supplement (Gibco) and 40 ng/mL human recombinant FGF-2 (Peprotech). Every other day, progenitors were fed by 50% medium exchange. At 90% confluence, the primary culture was passaged after brief trypsinization and centrifugation.
To examine proliferation, cultured adult hippocampal neural stem/progenitors were passaged onto polyornithine/laminin-coated plastic coverslips (Nalge Nunc International) placed on 24-well culture plates (Becton Dickinson) at a density of 5 × 103 cells cm2. Five days after passage, coverslips were incubated for 30 minutes with inhibitors of various pathways, including SU5416 (4 μM, Flk-1 inhibitor, Calbiochem), LY294002 (10 μM, PI3K inhibitor, Calbiochem), or U0126 (10 μM, MEK/ERK inhibitor, Upstate Biotechnology) before treatment recombinant human VEGF (Sigma-Aldrich) or PBS. After thirty minutes, BrdU (10 μM) was added and coverslips were fixed approximately 2 hrs later.
Immunofluorescence
Free-floating rat brain sections were processed as before for BrdU immunohistochemistry. Sections were incubated (for 48–72 hrs) in a cocktail containing anti-rat BrdU (1:100, Accurate) and anti-mouse pERK (1:100, Millipore), or anti-mouse BrdU (1:100, Becton Dickinson) and anti-rabbit pAkt (1:100, Millipore) or anti-goat pFlk-1 (1:100, Santa Cruz Biotechnology). After several washes in PBS, fluorescent-conjugated antibodies (Alexa Fluor 488 and 546, 1:200) were applied for 3 hrs in the dark. Between 30 and 40 BrdU+ cells per animal (3 to 4 subjects per group) were analyzed for co-localization using z-plane sectioning on a confocal microscope (Zeiss LSM 510).
For immunocytochemistry, coverslips were fixed in 4% PBS-buffered paraformaldehyde for 15 min. Coverslips were then acid hydrolyzed in 2 N HCl (X hrs) to expose the BrdU-antigen and blocked in PBS-T (0.1% Triton X-100), 10% normal goat sera, 0.5% BSA for 1 hr. Following blocking, coverslips were exposed for 2 hrs to the following primary antibody mixture: mouse anti-BrdU (1:200, Becton Dickinson) and goat anti-SOX2 (1:1000, Santa Cruz Biotechnology) diluted in 5% normal goat sera in PBS. Detection of primary antibodies was performed with a mixture of Alexa 488 or 546 secondary antibodies conjugated (1:200; Molecular Probes) for 2 hrs in the dark. Coverslips were then washed in PBS (3×, 10 min) and dried completely before being mounted with anti-fade media (VectaShield; Vector Laboratories). For quantification of the proliferation of cultured progenitor cells, 10 random areas were counted per slide using an epifluorescent microscope.
Data Analysis
All data analyses were completed using Statistical Package for the Social Sciences (SPSS v 17.00). Data were subjected to two-tailed unpaired t-tests, paired t-tests, or analysis of variance (one or two-way ANOVA with Bonferroni post hoc analyses), when appropriate. Results are presented as mean ± standard error of the mean (S.E.M.). The criterion for statistical significance was set at P < 0.05.
3. RESULTS
Activation of intracellular signaling cascades by VEGF in adult hippocampus
The PI3K-Akt and MEK-ERK signaling pathways are known to be involved in the trophic and neuroprotective effects of growth factors. This includes VEGF which is reported to activate downstream PI3K/Akt and MEK/ERK cascades via binding to its tyrosine kinase receptors (Matsumoto and Claesson-Welsh, 2001). However, the intracellular signaling cascades activated by VEGF have only been studied in cultured neuronal or endothelial preparations (Ruiz de Almodovar et al., 2009), and as such, little is known about the role of these signaling cascades in the adult brain.
To identify possible mechanisms underlying VEGF's mitogenic action in the adult hippocampus, we examined the activated/phosphorylated state of ERK1/2 and Akt 60 minutes after VEGF microinfusion. We found that VEGF microinfusion increased pERK and pAkt expression in whole hippocampal homogenates. However, the induction of these kinases was confined only to the ipsilateral (same side as microinfusion) hippocampus (paired t-tests, P<.05 Suppl Fig. 1). In contrast, there was no significant induction of either pERK or pAkt in the hippocampus on the contralateral (non-cannulated) side. This is consistent with other reports involving i.c.v. delivery of BDNF (Yan et al., 1994) and IGF-1 (Nagaraja et al., 2005). The explanation for this is unclear. However, given the unidirectional flow of cerebrospinal fluid (Fishman, 1980), the high concentration of VEGF receptors expressed within the nearby choroid plexus that would likely sequester exogenous VEGF, and that the diffusion efficacy of a molecule decreases with the square of the distance, it is likely that each or all of these factors would limit interventricular distribution of VEGF following a single acute administration. Because of these findings, all subsequent experiments will focus on the regulation of these signaling pathways and neurogenesis on the same side of cannulation (ipsilateral).
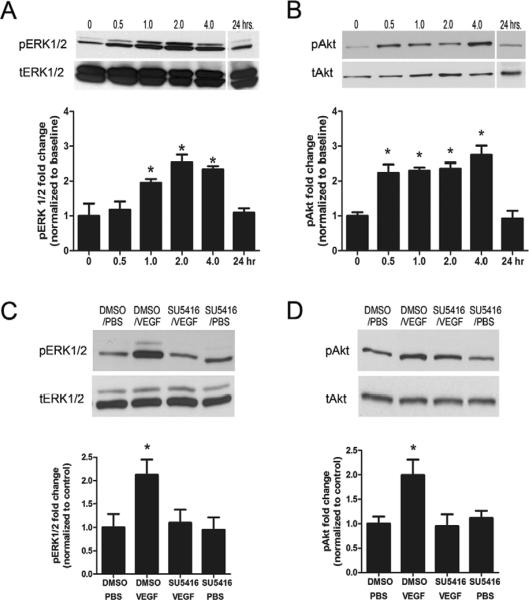
Time course studies demonstrated a significant increase in the phospho-activated form of ERK1/2 (pERK) in hippocampal homogenates 60 minutes after a single i.c.v. microinfusion of VEGF (P<.002, Fig. 1a) that persisted for at least 4 hrs after VEGF microinfusion (P<.001) and returned to baseline levels 24 hrs later (P=.481). Levels of the phospho-activated form of Akt (pAkt) were increased within 30 minutes of VEGF infusion (P<.016, Fig. 1b), persisted for at least 4 hrs (P<.010), and returned to baseline 24 hrs later (P=.643). Importantly, there was no change in the total protein levels of either total ERK1/2 or Akt at any of the measured time points.
Figure 1. Effect of VEGF on phospho-activated forms of ERK1/2 and Akt in hippocampus.
(A and B) Time-course western blot analysis of activated ERK1/2 and Akt in hippocampal homogenates at 0, 0.5 hr, 1 hr, 2 hr, 4 hr, and 24 hr after microinfusion of VEGF (50 ng/μl; n=3 per time point). Levels of phospho-ERK1/2 are increased 1 hr after VEGF microinfusion and persist for 4 hrs. In contrast, levels of phospho-Akt are increased 0.5 hr after VEGF microinfusion and persist for 4 hrs. One-way ANOVA, Bonferroni: * P<.05 vs. 0 hr. (C and D) Representative bands of phospho-ERK1/2 and phospho-Akt in the hippocampus taken 1 hr after VEGF in rats pretreated with either DMSO or the Flk-1 inhibitor SU5416. DMSO or SU5416 was i.c.v. administered 30 minutes before VEGF or PBS microinfusion and blocked the phosphorylation of ERK1/2 (C) and Akt (D). Student t-test: * P<.05 vs. DMSO/PBS controls. Error bars denote mean ± SEM
Next, to determine the spatial profile of pAkt and pERK expression in the hippocampus, rats were sacrificed 3 hrs after VEGF microinfusion. Immunostaining revealed that VEGF treatment increased pAkt labeling in cells along the SGZ (Suppl Fig. 2). There was an absence of pAkt labeling in neurons of the CA1 stratum pyramidal layer (data not shown). Likewise, pERK labeling was also increased in the dentate SGZ after VEGF (Suppl Fig. 2). However, this increase was not as robust as with pAkt, and additional pERK labeling could be detected in the CA1 stratum pyramidal layer of some VEGF-treated animals (data not shown).
VEGF activation of MEK/ERK and PI3K/Akt signaling in the adult hippocampus requires Flk-1 signaling
To assess the requirement of Flk-1 receptor signaling in the induction of phosphorylated ERK1/2 and Akt, we pre-infused the selective Flk-1 inhibitor SU5416 (2.4 μg/μl) 30 minutes before VEGF treatment. Pretreatment with SU5416 abrogated the induction of phospho-ERK1/2 (P<.05, Fig. 1c) and phospho-Akt (P<.01, Fig. 1d) in whole hippocampal homogenates examined 1 hr after VEGF treatment—a time point shown to cause robust activation of these two kinase (see Fig. 1a,b). These results highlight the importance of Flk-1 signaling in causing the rapid activation of MEK/ERK and PI3K/Akt signaling pathways after VEGF.
VEGF/Flk-1 signaling activates CREB signaling in the dentate gyrus and SGZ
Both Akt and ERK1/2 have a number of downstream effectors known to be important in proliferation, differentiation, and cell survival (Brazil and Hemmings, 2001; Hanada et al., 2004; Manning and Cantley, 2007; Roovers and Assoian, 2000; Shaul and Seger, 2007; Zhang and Liu, 2002). One common downstream target of Akt- and Erk-signaling is the cAMP-response-element binding protein (CREB). It is well known that CREB is a major regulator of progenitor cell proliferation and survival in the adult hippocampus (Merz et al., 2011; Nakagawa et al., 2002a; Nakagawa et al., 2002b). Therefore, we decided to probe the potential connection between VEGF and CREB signaling in vivo.
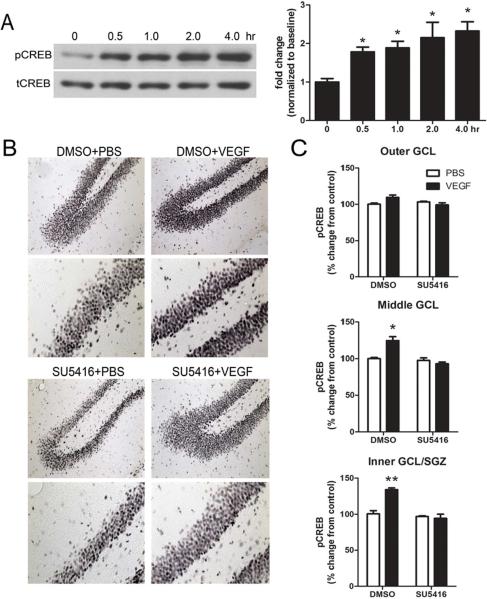
First, we examined the time course of CREB activation in the adult hippocampus following VEGF microinfusion. Hippocampal homogenates taken at 30, 60, 120, or 240 min (n=3 animals per time point) after VEGF revealed a robust 1.9 to 2.3-fold increase in the phosphorylated form of CREB (pCREB) across all time points examined (Fig. 2a). Next, to determine if CREB activation was localized to the dentate gyrus, rats were sacrificed 3 hrs after VEGF microinfusion and brain sections were stained for pCREB immunohistochemistry. Consistent with previous work (Nakagawa et al., 2002a), we observed dense pCREB staining in the inner third of the dentate granule cell layer and SGZ region (Fig. 2b). When the dentate granule cell layer was partitioned into 3 equally sized increments (e.g., outer, middle, and inner/SGZ portions of the granule cell layer), only the inner/SGZ and middle regions showed a significant increase in pCREB expression with VEGF treatment (P<.05, Fig. 2c). There was a slight increase in the expression of pCREB in the outer granule cell layer after VEGF treatment, however, this increase did not reach statistical significance (P=.09). Lastly, to assess if pretreatment with the selective Flk-1 blocker SU5416 (2.4 μg/μl) could prevent VEGF stimulation of CREB, an additional group of rats was pretreated with the inhibitor 30 min before VEGF infusion and sacrificed 3 hrs later for pCREB immunohistochemistry. Blockade of VEGF/Flk-1 signaling prevented the induction of pCREB in the inner/SGZ and middle granule cell layer with VEGF infusion (Fig. 2c). However, pretreatment with SU5416 did not significantly influence basal expression levels of pCREB in any of the dentate granule cell layer regions of vehicle-treated controls.
Figure 2. Effect of VEGF on phospho-CREB expression in the hippocampus.
(A) Time course western blot analysis of phospho-CREB expression in hippocampal homogenates at 0, 0.5 hr, 1 hr, 2 hr, and 4 hr after VEGF microinfusion. Levels of phospho-CREB in the hippocampus are increased 0.5 hr after VEGF microinfusion and persist for 4 hrs. One-way ANOVA, Bonferroni: * P<.05 vs. 0 hr. (B) Representative photomicrographs showing phospho-CREB expression in DMSO/PBS, DMSO/VEGF, SU5416/PBS, and SU5416/VEGF treated rats sacrificed 3 hrs later. DMSO or SU5416 was administered 30 minutes before treatment with PBS or VEGF. (C) Quantitative analysis of the inner dentate granule cell layer/SGZ, middle dentate granule cell layer, and outer regions of the dentate granule cell layer shows that VEGF increased phospho-CREB expression in the inner/SGZ and middle dentate granule cell layers. Pretreatment with the Flk-1 inhibitor SU5416 prevents VEGF-stimulation of CREB in these regions. Two-way ANOVA, Bonferroni: * P<.05 vs. DMSO/PBS controls. Error bars denote mean ± SEM
To determine if there were additional hippocampal region differences in pCREB induction after VEGF treatment, we also examined pCREB staining in the CA1 stratum pyramidal layer. In contrast to the dentate gyrus, there was no significant difference in pCREB labeling in the CA1 subfield 3 hrs after VEGF microinfusion (P=.15, data not shown). Taken together, our findings suggest that VEGF/Flk-1 signaling results in a rapid but selective pattern of CREB phosphorylation in the hippocampus.
VEGF-induced hippocampal cell proliferation requires MAPK/ERK and PI3K/Akt signaling
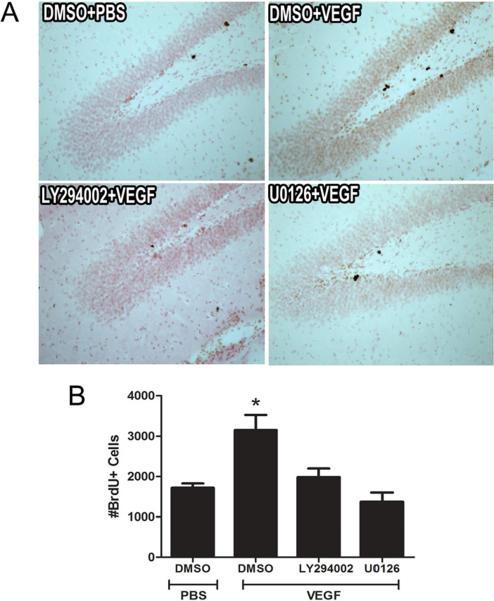
To examine whether a single microinfusion of VEGF can influence cell proliferation, dividing cells were labeled with BrdU (150 mg/kg, i.p.) 2 hrs after VEGF microinfusion. Rats were perfused 1 hr later and the number of BrdU-labeled cells was counted. This procedure permitted us to label proliferating cells within the time frame associated with robust induction of both the ERK and Akt pathways after VEGF treatment. We found that a single i.c.v. microinfusion of VEGF resulted in a 1.8-fold increase in the number of BrdU-labeled cells in the dentate SGZ compared to vehicle-treated controls (P<.002, n=5 rats per group, Fig. 3b). We then asked if cell proliferation could be detected at an earlier time point using the cell cycle marker Ki-67, which is expressed throughout all active phases of mitotic division. The results revealed a marginally significant increase in the number of actively proliferating Ki-67+ cells in the dentate SGZ (P=.064, Suppl Fig. 3). Given that Ki-67 expression persists throughout all active phases of the cell cycle (G1, S, and G2 phases), it is likely that Ki-67+ cells were dividing prior to VEGF treatment limiting our ability to detect a statistically significant difference after 1 hr of VEGF treatment. Nonetheless, our findings are consistent with the possibility of the recruitment of neuronal progenitors into the cell cycle as early as 1-hr after VEGF stimulation and overlap with the time frame associated with activation of both ERK and Akt signaling pathways (Fig. 1).
Figure 3. VEGF stimulation of hippocampal cell proliferation requires MAPK/ERK and PI3K/Akt signaling.
(A) Representative photomicrographs showing BrdU-labeled cells in the adult dentate SGZ of rats treated with DMSO, U0126 (MEK/ERK inhibitor), or LY294002 (PI3K inhibitor) 30 minutes before PBS or VEGF microinfusion. (B) Quantitative cell counting of the number of BrdU-labeled cells indicates that VEGF increased the number of BrdU-labeled cells in the dentate SGZ. However, pretreatment with either U0126 or LY294002 significantly blocked VEGF stimulation of hippocampal cell proliferation. One-way ANOVA, Bonferroni: * P <.002 vs. DMSO/PBS controls. Error bars denote mean ± SEM
Next, we set out to characterize the involvement of the MAPK/ERK or PI3K/Akt pathways in mediating the effect of VEGF on cell proliferation. In these experiments, rats were pretreated with either U0126 (MEK/ERK inhibitor) or LY294002 (PI3K inhibitor) 30 min before VEGF microinfusion and then received a single injection of BrdU 2 hrs later. As before, the number of BrdU-labeled cells in the dentate SGZ was examined 1 hr later. We found that pretreatment with either U0126 (4.3 μg/μl) or LY294002 (4.6 μg/μl) blocked the increase in BrdU-labeled cells after VEGF infusion (all Ps < .008, n=5–6 rats per group, Fig. 3a,b). Importantly, administration of either U0126 or LY294002 alone did not influence the basal level of cell proliferation (Suppl Fig. 4). Finally, we also found that pretreatment with either U0126 or LY294002 significantly blocked VEGF-induced CREB phosphorylation within the dentate granule cell layer and SGZ (Suppl Fig 5.).
pERK and pAkt expression in proliferating cells is increased after VEGF or Fluoxetine
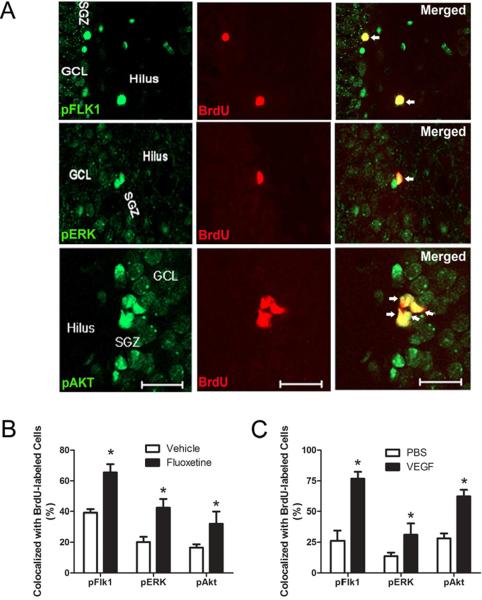
The results indicate that VEGF-signaling induces cell proliferation in the dentate SGZ through the activation of both ERK and Akt cascades. Therefore, we wanted to examine the distribution of ERK and Akt expression among proliferating cells. As shown Figure 4, immunofluorescence labeling revealed several BrdU+ cells along the dentate SGZ were co-labeled with pFlk-1, pERK, and pAkt. Quantification of BrdU-labeled cells indicated that VEGF treatment increased the proportion of BrdU+ cells co-labeled with pFlk-1, pAkt, and pERK (All Ps < .05, n=5 rats per group, Fig 4b), respectively. Although VEGF treatment increased the phosphorylated forms of both ERK and Akt in proliferating cells, there was a greater percentage of BrdU+ cells that co-labeled with pAkt than pERK (paired t-test p<.001).
Figure 4. Phospho-activated forms of Flk-1, ERK, and Akt among proliferating cells.
(A) Representative photomicrographs showing phosphorylated forms of Flk-1, ERK, and Akt of BrdU-cells located in the dentate SGZ. pFlk-1, pERK, pAkT (green) and BrdU (red). Scale bar: 50 μm (pFlk-1, pERK) and 10 μm (pAkt). (B) Quantitative analysis showing the percentage of BrdU-labeled cells co-expressing pFlk-1, pERK, and pAkt after VEGF or PBS microinfusion. Student t-test: * P < .05 vs. PBS controls. (C) Quantitative analysis showing the percentage of BrdU-labeled co-expressing pFlk-1, pERK, and pAkt after chronic fluoxetine or vehicle treatment (representative images are from fluoxetine treated rat brains). Student t-test: * P <.02 vs. Vehicle. Error bars denote mean ± SEM
To determine whether additional stimuli (e.g., antidepressants) known to induce neurogenesis and VEGF expression also increase PI3K/Akt and MEK/ERK signaling within proliferating cells of the dentate SGZ, a separate cohort of rats were given the selective serotonin reuptake inhibitor fluoxetine (5 mg/kg, i.p.) for 14 days and administered BrdU (75 mg/kg, i.p.) 2 hrs before perfusion. As expected, we found that fluoxetine treatment significantly increased the number of BrdU+ cells compared to vehicle-treated controls (fluoxetine: 1,634 ± 159 vs. Vehicle: 534 ± 67; p<.001, n=6). Quantification of BrdU+ cells further revealed that treatment with fluoxetine significantly increased the proportion of BrdU+ cells expressing pFlk-1, pERK, and pAkt markers (All Ps < .05, n=3–4 rats per group) (Figure 4c). Together, these results indicate that chronic fluoxetine treatment also activates both Erk and Akt signaling pathways in proliferating cells.
Activation of similar intracellular signal cascades by VEGF in adult hippocampal stem/progenitor culture
It is possible that the proliferative action of VEGF observed in our in vivo study could be the result of VEGF's effect on other cell types (e.g., mature neurons, astrocytes, endothelia) that in turn release growth factors (e.g., BDNF or FGF-2). These released factors would then act on receptors located on neuronal progenitor cells and activate signaling cascades that control proliferation (Emanueli et al., 2003; Louissaint et al., 2002; Ogunshola et al., 2002). To address this concern, we used cultured adult hippocampal stem/progenitor cells to examine whether VEGF can directly stimulate proliferation through activation of similar signaling pathways as was observed in vivo. Phosphorylation of Flk-1, ERK1/2, and Akt was evaluated by Western blot analysis after treatment of VEGF at different doses (0–100 ng/mL). VEGF treatment for 180 min increased the expression level of phosphorylated ERK1/2 at doses between 20 ng/mL and 100 ng/mL (Suppl Fig. 6). In contrast, Flk-1 and Akt phosphorylation was only significantly increased at the range of 50 ng/mL and 100 ng/mL of VEGF. Therefore, based on these observations, all subsequent cell culture experiments employed a dose of 50 ng/mL of VEGF.
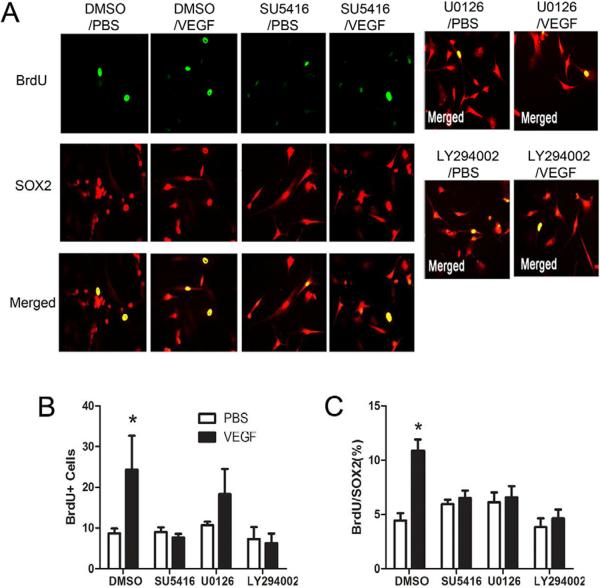
To assess the requirement of PI3K/Akt and MEK/ERK signaling pathways in mediating VEGF-induced cell proliferation, adult hippocampal stem/progenitor cells were exposed to inhibitors of PI3K and MEK/ERK 30 minutes before treatment with VEGF. Pretreatment with the PI3K inhibitor LY294002 (10 μM) significantly reduced the number of BrdU-labeled cells compared to the VEGF only-treated group (P<.006, Fig. 5a,b). There was no significant change in the number of BrdU-labeled cells after pretreatment with the ERK1/2 inhibitor U0126 (10 μM) compared to the VEGF only-treated group (P=.30). To control for differences in plating density across replicates and between groups, we used double immunofluorescence labeling with BrdU and Sox2 (a transcription factor mainly expressed by type-1 and type-2 progenitor cells (Kronenberg et al., 2003). As shown in Figure 5, pretreatment with either inhibitors significantly reduced the proportion of BrdU+/Sox+ cells after VEGF treatment (all Ps < .018, Fig. 5a,c). Finally, pretreatment with the Flk-1 inhibitor SU5416 (4 μM) blocked VEGF-induced cell proliferation in cultured hippocampal stem/progenitor cells.
Figure 5. VEGF stimulation of adult hippocampal neural stem/progenitor cell proliferation requires MAPK/ERK and PI3K/Akt signaling.
(A) Adult hippocampal neural stem/progenitor cells cultures (5 days after one passage) treated with Flk-1 (SU5416), MEK/ERK (U0126), or PI3K (LY294002) inhibitors 30 minutes before stimulation with VEGF (50 ng/mL). Cells were fixed 2 hrs later and immunostained with SOX2 (red) and BrdU (green). BrdU (10 μM) was added 30 minutes after VEGF stimulation. (B) Quantitative analysis showed that VEGF treatment significantly increased the number of BrdU-labeled cells. (C) Analysis of the percentage of BrdU-labeled cells that express Sox2 revealed that pretreatment with inhibitors of Flk-1, MEK/ERK, or Akt all blocked VEGF-stimulation of cell proliferation. One-way ANOVA, Bonferroni: * P <.05 vs. DMSO/PBS-treated cells. Error bars denote mean ± SEM
4. DISCUSSION
A growing body of evidence has demonstrated the importance of the anatomical and signaling relationship between neural stem/progenitor cells and the vasculature (Louissaint et al., 2002; Palmer et al., 2000; Shen et al., 2004). These findings provide compelling support that angiogenesis and neurogenesis may be coordinated events in the CNS. Indeed, secreted growth factors from the vasculature produce favorable conditions for promoting ongoing neurogenesis (Yang et al., 2011). VEGF is a critical factor responsible for maintaining vascular homeostasis (Thanigaimani et al., 2011) and is also a prime candidate for mediating vascular/neuronal interactions in both the developing and adult brain (Ruiz de Almodovar et al., 2009). In support of this view, past work has shown a role for VEGF in stimulating neurogenesis (Cao et al., 2004; Fabel et al., 2003; Jin et al., 2002; Maurer et al., 2003). However, downstream signaling events activated by VEGF, which trigger this process, are not well understood.
Consistent with earlier cell culture work using embryonic cortical neurons, astrocytes, or retinal progenitor cells (Hashimoto et al., 2006; Mani et al., 2005; Ogunshola et al., 2002), we found that a single microinfusion of VEGF (i.c.v.) increased the activated forms of Akt and ERK1/2 in the hippocampus, specifically in the dentate gyrus (Fig. 1 and Suppl Fig. 2). VEGF also increased cell proliferation in the dentate SGZ (Fig. 3) and trigged the activation of ERK1/2 and Akt in BrdU-labeled cells (Fig. 4). Moreover, disruption of either ERK or Akt signaling blocked VEGF-induced cell proliferation, indicating a central role for these pathways in controlling proliferation in the dentate SGZ and in culture (Fig. 3 and 5). Basal levels of proliferation were not affected by a single dose of U0126 or LY294002 treatment (Suppl Fig. 4) suggesting that ERK and Akt signaling pathways are not necessary for regulating constitutive division. Furthermore, the more rapid activation of Akt compared to ERK1/2 suggests that PI3K/Akt signaling might initiate the induction of proliferation and the activation of the ERK1/2, a process that likely helps to stabilize and maintain cell cycle progression. Indeed, there is evidence that the cell survival (PI3K/Akt) and mitogenic (Ras/Ref/MEK/ERK) cascades act as independent pathways, but that there is cross-talk between these cascades (Aksamitiene et al., 2010). Together, these results further underscore the importance of ERK and Akt pathways in mediating the actions of VEGF on hippocampal cell proliferation.
Both MEK/ERK and PI3K/Akt signaling pathways participate in the biological effects of growth factors (Manning and Cantley, 2007; Shaul and Seger, 2007). The ERK signaling cascade is reported to control the proliferation of multiple cell types, including neural stem/progenitor cells, especially in response to growth factor treatment (Li et al., 2001; Wang et al., 2009). In contrast, although Akt signaling is best known for mediating cell survival, accumulating evidence also supports its role in regulating cell cycle progression (Liang and Slingerland, 2003), stem cell self-renewal, and neuronal cell number and composition (Easton et al., 2005; Tschopp et al., 2005). Therefore, our findings that ERK and Akt activation are both required for the proliferative effects of VEGF in vivo and in vitro is consistent with the involvement of these signaling pathways in promoting neural progenitor cell proliferation by other growth factors, such as NGF, IGF-1, heparin-binding epidermal growth factor, and neurotrophin-3 (Aberg et al., 2003; Barnabe-Heider and Miller, 2003; Jin et al., 2005; Kalluri et al., 2007; Ohtsuka et al., 2009).
The involvement of either ERK or Akt pathways in mediating VEGF's effect on cell proliferation suggests that both kinases likely converge on a common set of downstream targets. One likely candidate in this regard is the nuclear transcription factor CREB. In the present study, we demonstrated that VEGF (i.c.v.) increased levels of the phosphorylated/activated form of CREB in the dentate SGZ, and that this effect could be blocked by inhibition of Flk-1, ERK, or PI3K/Akt (Fig. 2, Suppl. Fig 5). Induction of CREB was also observed in mature dentate gyrus granule cells likely reflecting the effects of VEGF on more broad neuronal functions, such as long-term potentiation (Licht et al., 2011). It is well known that the transactivation of CREB can both directly and indirectly target downstream cell proliferation factors, including the E2F family of transcription factors. The E2F family (E2F1 through 6) influences cellular proliferation and differentiation through promoting G1/S phase transition of the cell cycle (Polager and Ginsberg, 2009). E2F1 is highly expressed throughout development in proliferating undifferentiated neuroblasts (Dagnino et al., 1997) and E2F-1 deficient mice exhibit significantly lower cell proliferation and neurogenesis in the adult dentate SGZ and subventricular zone (Cooper-Kuhn et al., 2002). Interestingly, Zhu and colleagues (2003) found that VEGF stimulates the proliferation of cortical neuronal precursors in vitro through increasing nuclear expression of several E2F family transcription factors—an effect blocked by inhibitors of MEK and PI3K. With these points in mind, it will be interesting for future studies to examine the role of E2F in the proliferative effects of VEGF and/or other growth factors in vivo.
We also examined the role of ERK and Akt in the actions of antidepressant treatment. Different classes of antidepressants stimulate neurogenesis and there is evidence that the behavioral actions of these agents can be partially attributed to this neurogenic effect (David et al., 2009; Malberg et al., 2000; Santarelli et al., 2003). As such, there has been increasing interest in identifying downstream intracellular signaling networks that modulate antidepressant-induced cell proliferation and neurogenesis. Our previous work demonstrated that different antidepressants up-regulate the expression of VEGF in the hippocampus (Warner-Schmidt and Duman, 2007; Greene et al., 2009). Here we show that chronic fluoxetine administration increases pERK and pAkt activation in BrdU-labeled cells. Taken together, the findings from the present study underscore the involvement of the MAP /ERK and PI3K/Akt signaling pathways in regulating neural progenitor cell responsiveness to diverse stimuli, including the antidepressants, and further suggest that the promotion of VEGF/Flk-1 signaling could be an important component in this process.
Role of VEGF in cell proliferation
In the adult dentate gyrus, new cells are generated through a multistage process that begins with the division of radial glial stem cells to produce a population of transient amplifying progenitors that rapidly divide to produce neuronal committed intermediate progenitors that in turn eventually integrate into surrounding circuitry through an experience-dependent regulatory process (Brandt et al., 2003; Encinas et al., 2006; Jessberger et al., 2005; Kempermann et al., 2004; Kronenberg et al., 2003). Previous studies have reported colocalization of Flk-1 and the immature neuronal marker doublecortin indicating that VEGF-signaling may be important in expanding the division of late stage intermediate neuronal progenitors (Jin et al., 2002). This expansion is sensitive to various forms of hippocampal activity and physiological stimuli, such as physical exercise, seizures, and chemical antidepressants, including fluoxetine (Brandt et al., 2003; Encinas et al., 2006; Jessberger et al., 2005; Kempermann et al., 2004; Kronenberg et al., 2003). Notably, all of these stimuli increase VEGF expression and enhance VEGF-Flk-1 signaling (During and Cao, 2006; Fabel et al., 2003; Segi-Nishida et al., 2008; Warner-Schmidt and Duman, 2007). Previously, we have shown that VEGF-Flk-1 signaling plays an important role in stimulating the division of radial glial stem cells in response to electroconvulsive seizures (Segi-Nishida et al., 2008). Currently, it is not known if differential recruitment of ERK and Akt-signaling pathways by VEGF plays a role in the proliferation of different populations of neural progenitors. However, recent work has shown a preferential activation of ERK-signaling in slowly dividing radial glial cells after spontaneous seizures (Li et al., 2010), and that both ERK and Akt-signaling promote proliferation and survival of later stage neuronal progenitors (Bruel-Jungerman et al., 2009; Choi et al., 2008; Hao et al., 2004; Yan et al., 2007). It will be interesting for future studies to determine if VEGF stimulates overlapping or discrete forms of Akt and ERK-signaling in the various classes of neuronal progenitors.
An important unanswered question is whether VEGF-mediated cell proliferation in the adult brain is the result of a direct action of VEGF on neuronal progenitor cells or an indirect action via the stimulation and release of growth factors from surrounding cells (e.g., astrocytes or endothelial cells). An indirect action of VEGF on proliferation has been has shown in vivo (Louissaint et al., 2002), but studies with neurospheres from Flk-1 mutant mice suggests that direct VEGF/Flk-1 signaling is essential for both the proliferation and survival of cultured neural stem cells (Wada et al., 2006). In the current study, three lines of evidence argue for a direct effect of VEGF on the neural progenitor cells. First, we showed that VEGF treatment directly activates Flk-1 in cultured adult hippocampal stem/progenitor cells and stimulates proliferation (Suppl Fig. 6). Second, examination of hippocampal sections after VEGF microinfusion revealed colocalization of pFlk-1 labeling in BrdU+ cells (Fig. 4). However, future studies utilizing neuronal-, glial-, or endothelial-specific genetic ablation of VEGF and/or its receptors will be required to elucidate the contribution of direct vs. indirect mechanisms in this process. Our laboratory is presently examining these possibilities.
Conclusions
Although it has been previously shown that Akt and ERK play a role in cell cycle regulation and proliferation, these signaling cascades have been generally studied for their role in influencing hyper-proliferative or carcinogenic processes within a malignant cellular environment. Therefore, the results of this study underscore the importance of the ERK and Akt signaling pathways in regulating the proliferative responses of neuronal progenitors in a normal microenvironment. This information could be used to develop novel treatments that promote repair and regeneration of injured brain tissue and cognitive recovery. In this regard, there has been active interest in pursuing VEGF therapy as a possible candidate in promoting neurological recovery through its well-established effects on neurogenesis, synaptogenesis, and angiogenesis. By characterization of the downstream targets of VEGF that are important in regulating cell proliferation in the adult brain, it is possible that more effective clinical interventions could be developed to permit in vivo manipulation of endogenous neuronal progenitors as a potential therapy for brain injury. Finally, these approaches may also be effective in the treatment of neuropsychiatric disorders, including stress-related illnesses such as depression, which are characterized by atrophy and the loss of neurons and glia in brain regions that control emotion and mood.
Supplementary Material
Left: Representative bands of phospho-ERK1/2 and phospho-Akt from left or right hippocampal homogenates taken 1 hr after VEGF or PBS microinfusion into the right lateral ventricle. Right: Densitometric analysis showed that VEGF-induced changes in pERK1/2 and pAkt expression were confined only to the ipsilateral (same) side of cannulation. There was no change in the induction of ERK1/2 or Akt in the contralateral (opposite) hippocampus after VEGF. One-way ANOVA, Bonferroni: * P<.001 vs. PBS-R. Error bars denote mean ± SEM
Top: Representative photomicrographs showing pERK and pAkt expression in the dentate SGZ 3 hrs after PBS or VEGF microinfusion. Bottom: The mean number of phospho-ERK1/2 or Akt cells in the dentate SGZ per section after microinfusion with PBS or VEGF. There was an increase in the number of pERK1/2 and Akt cells in the dentate SGZ. Student t-test: * P<.05 vs. PBS control. Error bars denote mean ± SEM
Top: Representative photomicrographs show Ki-67 labeled cells in the dentate SGZ 1 hr after PBS or VEGF microinfusion. Bottom: Cell counts revealed that there was a marginally significant increase in the number of proliferating cells 1 hr following VEGF microinfusion. Student t-test: # P=.064 vs. PBS control. Error bars denote mean ± SEM
Top: Representative photomicrographs of hippocampal sections from rats pretreated with DMSO, LY294002 (PI3K inhibitor), or U0126 (MEK/ERK inhibitors) 30 minutes before microinfusion of PBS or VEGF. BrdU was administered two hours after PBS or VEGF microinfusion and all rats were sacrificed 1 hr later. Bottom: Cell counts showed that pretreatment with either MEK/ERK or PI3K inhibitors did not change the number of BrdU-labeled cells in PBS-infused rats. In contrast, pretreatment with DMSO followed by VEGF significantly increased the number of BrdU-labeled cells. One-way ANOVA, Bonferroni: * P<.05 vs. DMSO/PBS controls. Error bars denote mean ± SEM
Top: Representative photomicrographs of pCREB from hippocampal sections of rats pretreated with DMSO, LY294002 (PI3K inhibitor), or U0126 (MEK/ERK inhibitor) 30 minutes before microinfusion of PBS or VEGF. Rats were sacrificed three hours later. Bottom: Quantitative analysis of the inner dentate granule cell layer/SGZ, middle dentate granule cell layer, and outer regions of the dentate granule cell layer shows that VEGF microinfusion increased phospho-CREB express throughout all dentate granule cell layer regions. Pretreatment with the U0126 or LY294002 blocked VEGF-stimulation of CREB in these regions. Two-way ANOVA, Bonferroni: * P<.05 vs. DMSO/PBS controls. Error bars denote mean ± SEM
Top: Representative blots showing activation of pFlk-1, pAkt, and pERK1/2 following VEGF treatment at various doses (0–100 ng/ml). Total levels of ERK1/2 (tERK1/2) served as a protein loading control. Bottom: Densitometric analysis revealed that there was a significant increase in pFlk-1 and pAkt induction in cultured hippocampal stem/progenitor cells at VEGF concentrations between 50 and 100 ng/mL. In addition, the induction of pERK1/2 occurred at lower concentrations of VEGF (10–50 ng/mL). One-way ANOVA, Bonferroni: * P <.05 vs. 0 ng/ml VEGF. Error bars denote mean ± SEM
Highlights
VEGF stimulates ERK and Akt signaling in adult rat hippocampus and in cultured hippocampal neuronal progenitor cells
VEGF induces pCREB expression in the dentate gyrus
VEGF stimulates hippocampal cell proliferation at time point that corresponds with the induction of ERK, Akt, and CREB.
Inhibitors of ERK and Akt blocked the mitogenic effects of VEGF on hippocampal cell proliferation both in vivo and in vitro
ACKNOWLEDGEMENTS
N.M.F. is a James Hudson Brown-Alexander Brown Coxe postdoctoral research fellow and was supported by the Natural Sciences and Engineering Research Council of Canada and Canadian Institutes of Health Research. This work is supported by US Public Health Service grants MH45481 (R.S.D.), 2 P01 MH25642 (R.S.D.), and the Connecticut Mental Health Center (R.S.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. REFERENCES
- Aberg MA, Aberg ND, Palmer TD, Alborn AM, Carlsson-Skwirut C, Bang P, Rosengren LE, Olsson T, Gage FH, Eriksson PS. IGF-I has a direct proliferative effect in adult hippocampal progenitor cells. Mol Cell Neurosci. 2003;24:23–40. doi: 10.1016/s1044-7431(03)00082-4. [DOI] [PubMed] [Google Scholar]
- Aksamitiene E, Kholodenko BN, Kolch W, Hoek JB, Kiyatkin A. PI3K/Akt-sensitive MEK-independent compensatory circuit of ERK activation in ER-positive PI3K-mutant T47D breast cancer cells. Cell Signal. 2010;22:1369–1378. doi: 10.1016/j.cellsig.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J.Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Barnabe-Heider F, Miller FD. Endogenously produced neurotrophins regulate survival and differentiation of cortical progenitors via distinct signaling pathways. J Neurosci. 2003;23:5149–5160. doi: 10.1523/JNEUROSCI.23-12-05149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt MD, Jessberger S, Steiner B, Kronenberg G, Reuter K, Bick-Sander A, von der BW, Kempermann G. Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol.Cell Neurosci. 2003;24:603–613. doi: 10.1016/s1044-7431(03)00207-0. [DOI] [PubMed] [Google Scholar]
- Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Veyrac A, Dufour F, Horwood J, Laroche S, Davis S. Inhibition of PI3K-Akt signaling blocks exercise-mediated enhancement of adult neurogenesis and synaptic plasticity in the dentate gyrus. PLoS One 4. 2009:e7901. doi: 10.1371/journal.pone.0007901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo CF, Fontaine RH, Soueid J, Tammela T, Makinen T, Alfaro-Cervello C, Bonnaud F, Miguez A, Benhaim L, Xu Y, Barallobre MJ, Moutkine I, Lyytikka J, Tatlisumak T, Pytowski B, Zalc B, Richardson W, Kessaris N, Garcia-Verdugo JM, Alitalo K, Eichmann A, Thomas JL. Vascular endothelial growth factor receptor 3 directly regulates murine neurogenesis. Genes Dev. 2011;25:831–844. doi: 10.1101/gad.615311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J.Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Choi YS, Cho HY, Hoyt KR, Naegele JR, Obrietan K. IGF-1 receptor-mediated ERK/MAPK signaling couples status epilepticus to progenitor cell proliferation in the subgranular layer of the dentate gyrus. Glia. 2008;56:791–800. doi: 10.1002/glia.20653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper-Kuhn CM, Vroemen M, Brown J, Ye H, Thompson MA, Winkler J, Kuhn HG. Impaired adult neurogenesis in mice lacking the transcription factor E2F1. Mol Cell Neurosci. 2002;21:312–323. doi: 10.1006/mcne.2002.1176. [DOI] [PubMed] [Google Scholar]
- Dagnino L, Fry CJ, Bartley SM, Farnham P, Gallie BL, Phillips RA. Expression patterns of the E2F family of transcription factors during mouse nervous system development. Mech Dev. 1997;66:13–25. doi: 10.1016/s0925-4773(97)00083-x. [DOI] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During MJ, Cao L. VEGF, a mediator of the effect of experience on hippocampal neurogenesis. Curr Alzheimer Res. 2006;3:29–33. doi: 10.2174/156720506775697133. [DOI] [PubMed] [Google Scholar]
- Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, Lee VM, Szabolcs M, de Jong R, Oltersdorf T, Ludwig T, Efstratiadis A, Birnbaum MJ. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol Cell Biol. 2005;25:1869–1878. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanueli C, Schratzberger P, Kirchmair R, Madeddu P. Paracrine control of vascularization and neurogenesis by neurotrophins. Br J Pharmacol. 2003;140:614–619. doi: 10.1038/sj.bjp.0705458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc.Natl.Acad.Sci.U.S.A. 2006;103:8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Fishman RA. Cerebrospinal Fluid In Diseases of the Nervous System. Saunders; Philadelphia: 1980. [Google Scholar]
- Fournier NM, Andersen DR, Botterill JJ, Sterner EY, Lussier AL, Caruncho HJ, Kalynchuk LE. The effect of amygdala kindling on hippocampal neurogenesis coincides with decreased reelin and DISC1 expression in the adult dentate gyrus. Hippocampus. 2010;20:659–671. doi: 10.1002/hipo.20653. [DOI] [PubMed] [Google Scholar]
- Fournier NM, Duman RS. Role of vascular endothelial growth factor in adult hippocampal neurogenesis: Implications for the pathophysiology and treatment of depression. Behav Brain Res. 2011 doi: 10.1016/j.bbr.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene J, Banasr M, Lee B, Warner-Schmidt J, Duman RS. Vascular endothelial growth factor signaling is required for the behavioral actions of antidepressant treatment: pharmacological and cellular characterization. Neuropsychopharmacology. 2009;34:2459–2468. doi: 10.1038/npp.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT--a major therapeutic target. Biochim Biophys Acta. 2004;1697:3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Hao Y, Creson T, Zhang L, Li P, Du F, Yuan P, Gould TD, Manji HK, Chen G. Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J Neurosci. 2004;24:6590–6599. doi: 10.1523/JNEUROSCI.5747-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Zhang XM, Chen BY, Yang XJ. VEGF activates divergent intracellular signaling components to regulate retinal progenitor cell proliferation and neuronal differentiation. Development. 2006;133:2201–2210. doi: 10.1242/dev.02385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine VM, Zareno J, Maslam S, Joels M, Lucassen PJ. Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature and VEGF and Flk-1 protein expression. Eur J Neurosci. 2005;21:1304–1314. doi: 10.1111/j.1460-9568.2005.03951.x. [DOI] [PubMed] [Google Scholar]
- Hermann DM, Zechariah A. Implications of vascular endothelial growth factor for postischemic neurovascular remodeling. J Cereb Blood Flow Metab. 2009;29:1620–1643. doi: 10.1038/jcbfm.2009.100. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Romer B, Babu H, Kempermann G. Seizures induce proliferation and dispersion of doublecortin-positive hippocampal progenitor cells. Neurol. 2005;196:342–351. doi: 10.1016/j.expneurol.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Jin K, Mao XO, Batteur SP, McEachron E, Leahy A, Greenberg DA. Caspase-3 and the regulation of hypoxic neuronal death by vascular endothelial growth factor. Neuroscience. 2001;108:351–358. doi: 10.1016/s0306-4522(01)00154-3. [DOI] [PubMed] [Google Scholar]
- Jin K, Mao XO, Del Rio Guerra G, Jin L, Greenberg DA. Heparin-binding epidermal growth factor-like growth factor stimulates cell proliferation in cerebral cortical cultures through phosphatidylinositol 3'-kinase and mitogen-activated protein kinase. J Neurosci Res. 2005;81:497–505. doi: 10.1002/jnr.20510. [DOI] [PubMed] [Google Scholar]
- Jin K, Mao XO, Greenberg DA. Vascular endothelial growth factor stimulates neurite outgrowth from cerebral cortical neurons via Rho kinase signaling. J Neurobiol. 2006;66:236–242. doi: 10.1002/neu.20215. [DOI] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci U S A. 2000;97:10242–10247. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri HS, Vemuganti R, Dempsey RJ. Mechanism of insulin-like growth factor I-mediated proliferation of adult neural progenitor cells: role of Akt. Eur J Neurosci. 2007;25:1041–1048. doi: 10.1111/j.1460-9568.2007.05336.x. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Khaibullina AA, Rosenstein JM, Krum JM. Vascular endothelial growth factor promotes neurite maturation in primary CNS neuronal cultures. Brain Res Dev Brain Res. 2004;148:59–68. doi: 10.1016/j.devbrainres.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Kempermann G. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J.Comp Neurol. 2003;467:455–463. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Jang DJ, Lee N, Ko HG, Kim H, Kim YS, Kim B, Son J, Kim SH, Chung H, Lee MY, Kim WR, Sun W, Zhuo M, Abel T, Kaang BK, Son H. Induction of neuronal vascular endothelial growth factor expression by cAMP in the dentate gyrus of the hippocampus is required for antidepressant-like behaviors. J.Neurosci. 2009;29:8493–8505. doi: 10.1523/JNEUROSCI.1321-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Li BS, Ma W, Zhang L, Barker JL, Stenger DA, Pant HC. Activation of phosphatidylinositol-3 kinase (PI-3K) and extracellular regulated kinases (Erk1/2) is involved in muscarinic receptor-mediated DNA synthesis in neural progenitor cells. J Neurosci. 2001;21:1569–1579. doi: 10.1523/JNEUROSCI.21-05-01569.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Marks JD, Schumacker PT, Young RM, Brorson JR. Physiological hypoxia promotes survival of cultured cortical neurons. Eur J Neurosci. 2005;22:1319–1326. doi: 10.1111/j.1460-9568.2005.04335.x. [DOI] [PubMed] [Google Scholar]
- Li Q, Ford MC, Lavik EB, Madri JA. Modeling the neurovascular niche: VEGF- and BDNF-mediated cross-talk between neural stem cells and endothelial cells: an in vitro study. J Neurosci Res. 2006;84:1656–1668. doi: 10.1002/jnr.21087. [DOI] [PubMed] [Google Scholar]
- Li Y, Peng Z, Xiao B, Houser CR. Activation of ERK by spontaneous seizures in neural progenitors of the dentate gyrus in a mouse model of epilepsy. Exp Neurol. 2010;224:133–145. doi: 10.1016/j.expneurol.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Slingerland JM. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle. 2003;2:339–345. [PubMed] [Google Scholar]
- Licht T, Goshen I, Avital A, Kreisel T, Zubedat S, Eavri R, Segal M, Yirmiya R, Keshet E. Reversible modulations of neuronal plasticity by VEGF. Proc Natl Acad Sci U S A. 2011;108:5081–5086. doi: 10.1073/pnas.1007640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louissaint A, Jr., Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J.Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani N, Khaibullina A, Krum JM, Rosenstein JM. Astrocyte growth effects of vascular endothelial growth factor (VEGF) application to perinatal neocortical explants: receptor mediation and signal transduction pathways. Exp Neurol. 2005;192:394–406. doi: 10.1016/j.expneurol.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Claesson-Welsh L. VEGF receptor signal transduction. Sci STKE. 2001;2001:re21. doi: 10.1126/stke.2001.112.re21. [DOI] [PubMed] [Google Scholar]
- Matsuzaki H, Tamatani M, Yamaguchi A, Namikawa K, Kiyama H, Vitek MP, Mitsuda N, Tohyama M. Vascular endothelial growth factor rescues hippocampal neurons from glutamate-induced toxicity: signal transduction cascades. FASEB J. 2001;15:1218–1220. [PubMed] [Google Scholar]
- Maurer MH, Tripps WK, Feldmann RE, Jr., Kuschinsky W. Expression of vascular endothelial growth factor and its receptors in rat neural stem cells. Neurosci Lett. 2003;344:165–168. doi: 10.1016/s0304-3940(03)00407-5. [DOI] [PubMed] [Google Scholar]
- Merz K, Herold S, Lie DC. CREB in adult neurogenesis--master and partner in the development of adult-born neurons? Eur J Neurosci. 2011;33:1078–1086. doi: 10.1111/j.1460-9568.2011.07606.x. [DOI] [PubMed] [Google Scholar]
- Nagaraja TN, Patel P, Gorski M, Gorevic PD, Patlak CS, Fenstermacher JD. In normal rat, intraventricularly administered insulin-like growth factor-1 is rapidly cleared from CSF with limited distribution into brain. Cerebrospinal Fluid Res. 2005;2:5. doi: 10.1186/1743-8454-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Chen J, Fujioka T, Malberg J, Tsuji S, Duman RS. Localization of phosphorylated cAMP response element-binding protein in immature neurons of adult hippocampus. J Neurosci. 2002a;22:9868–9876. doi: 10.1523/JNEUROSCI.22-22-09868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C, Zhang YJ, Nestler EJ, Duman RS. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci. 2002b;22:3673–3682. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunshola OO, Antic A, Donoghue MJ, Fan SY, Kim H, Stewart WB, Madri JA, Ment LR. Paracrine and autocrine functions of neuronal vascular endothelial growth factor (VEGF) in the central nervous system. J Biol Chem. 2002;277:11410–11415. doi: 10.1074/jbc.M111085200. [DOI] [PubMed] [Google Scholar]
- Ohtsuka M, Fukumitsu H, Furukawa S. Neurotrophin-3 stimulates neurogenetic proliferation via the extracellular signal-regulated kinase pathway. J Neurosci Res. 2009;87:301–306. doi: 10.1002/jnr.21855. [DOI] [PubMed] [Google Scholar]
- Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D, Brusselmans K, Van Dorpe J, Hellings P, Gorselink M, Heymans S, Theilmeier G, Dewerchin M, Laudenbach V, Vermylen P, Raat H, Acker T, Vleminckx V, Van Den Bosch L, Cashman N, Fujisawa H, Drost MR, Sciot R, Bruyninckx F, Hicklin DJ, Ince C, Gressens P, Lupu F, Plate KH, Robberecht W, Herbert JM, Collen D, Carmeliet P. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001;28:131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J.Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Polager S, Ginsberg D. p53 and E2f: partners in life and death. Nat Rev Cancer. 2009;9:738–748. doi: 10.1038/nrc2718. [DOI] [PubMed] [Google Scholar]
- Roovers K, Assoian RK. Integrating the MAP kinase signal into the G1 phase cell cycle machinery. Bioessays. 2000;22:818–826. doi: 10.1002/1521-1878(200009)22:9<818::AID-BIES7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Ruiz de Almodovar C, Lambrechts D, Mazzone M, Carmeliet P. Role and therapeutic potential of VEGF in the nervous system. Physiol Rev. 2009;89:607–648. doi: 10.1152/physrev.00031.2008. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Schanzer A, Wachs FP, Wilhelm D, Acker T, Cooper-Kuhn C, Beck H, Winkler J, Aigner L, Plate KH, Kuhn HG. Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol. 2004;14:237–248. doi: 10.1111/j.1750-3639.2004.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segi-Nishida E, Warner-Schmidt JL, Duman RS. Electroconvulsive seizure and VEGF increase the proliferation of neural stem-like cells in rat hippocampus. Proc Natl Acad Sci U S A. 2008;105:11352–11357. doi: 10.1073/pnas.0710858105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta. 2007;1773:1213–1226. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51:173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- Skold MK, Kanje M. Vascular endothelial growth factor in central nervous system injuries - a vascular growth factor getting nervous? Curr Neurovasc Res. 2008;5:246–259. doi: 10.2174/156720208786413451. [DOI] [PubMed] [Google Scholar]
- Sondell M, Sundler F, Kanje M. Vascular endothelial growth factor is a neurotrophic factor which stimulates axonal outgrowth through the flk-1 receptor. Eur J Neurosci. 2000;12:4243–4254. doi: 10.1046/j.0953-816x.2000.01326.x. [DOI] [PubMed] [Google Scholar]
- Suh H, Deng W, Gage FH. Signaling in Adult Neurogenesis. Annu.Rev.Cell Dev.Biol. 2009 doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- Thanigaimani S, Kichenadasse G, Mangoni AA. The emerging role of vascular endothelial growth factor (VEGF) in vascular homeostasis: lessons from recent trials with anti-VEGF drugs. Curr Vasc Pharmacol. 2011;9:358–380. doi: 10.2174/157016111795495503. [DOI] [PubMed] [Google Scholar]
- Tschopp O, Yang ZZ, Brodbeck D, Dummler BA, Hemmings-Mieszczak M, Watanabe T, Michaelis T, Frahm J, Hemmings BA. Essential role of protein kinase B gamma (PKB gamma/Akt3) in postnatal brain development but not in glucose homeostasis. Development. 2005;132:2943–2954. doi: 10.1242/dev.01864. [DOI] [PubMed] [Google Scholar]
- Wada T, Haigh JJ, Ema M, Hitoshi S, Chaddah R, Rossant J, Nagy A, van der Kooy D. Vascular endothelial growth factor directly inhibits primitive neural stem cell survival but promotes definitive neural stem cell survival. J Neurosci. 2006;26:6803–6812. doi: 10.1523/JNEUROSCI.0526-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Gao Y, Xiao Z, Chen B, Han J, Zhang J, Wang X, Dai J. Erk1/2 promotes proliferation and inhibits neuronal differentiation of neural stem cells. Neurosci Lett. 2009;461:252–257. doi: 10.1016/j.neulet.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci U S A. 2007;104:4647–4652. doi: 10.1073/pnas.0610282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittko IM, Schanzer A, Kuzmichev A, Schneider FT, Shibuya M, Raab S, Plate KH. VEGFR-1 regulates adult olfactory bulb neurogenesis and migration of neural progenitors in the rostral migratory stream in vivo. J Neurosci. 2009;29:8704–8714. doi: 10.1523/JNEUROSCI.5527-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Matheson C, Sun J, Radeke MJ, Feinstein SC, Miller JA. Distribution of intracerebral ventricularly administered neurotrophins in rat brain and its correlation with trk receptor expression. Exp Neurol. 1994;127:23–36. doi: 10.1006/exnr.1994.1076. [DOI] [PubMed] [Google Scholar]
- Yan XB, Hou HL, Wu LM, Liu J, Zhou JN. Lithium regulates hippocampal neurogenesis by ERK pathway and facilitates recovery of spatial learning and memory in rats after transient global cerebral ischemia. Neuropharmacology. 2007;53:487–495. doi: 10.1016/j.neuropharm.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- Yang XT, Bi YY, Feng DF. From the vascular microenvironment to neurogenesis. Brain Res Bull. 2011;84:1–7. doi: 10.1016/j.brainresbull.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Jin K, Mao XO, Greenberg DA. Vascular endothelial growth factor promotes proliferation of cortical neuron precursors by regulating E2F expression. FASEB J. 2003;17:186–193. doi: 10.1096/fj.02-0515com. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Left: Representative bands of phospho-ERK1/2 and phospho-Akt from left or right hippocampal homogenates taken 1 hr after VEGF or PBS microinfusion into the right lateral ventricle. Right: Densitometric analysis showed that VEGF-induced changes in pERK1/2 and pAkt expression were confined only to the ipsilateral (same) side of cannulation. There was no change in the induction of ERK1/2 or Akt in the contralateral (opposite) hippocampus after VEGF. One-way ANOVA, Bonferroni: * P<.001 vs. PBS-R. Error bars denote mean ± SEM
Top: Representative photomicrographs showing pERK and pAkt expression in the dentate SGZ 3 hrs after PBS or VEGF microinfusion. Bottom: The mean number of phospho-ERK1/2 or Akt cells in the dentate SGZ per section after microinfusion with PBS or VEGF. There was an increase in the number of pERK1/2 and Akt cells in the dentate SGZ. Student t-test: * P<.05 vs. PBS control. Error bars denote mean ± SEM
Top: Representative photomicrographs show Ki-67 labeled cells in the dentate SGZ 1 hr after PBS or VEGF microinfusion. Bottom: Cell counts revealed that there was a marginally significant increase in the number of proliferating cells 1 hr following VEGF microinfusion. Student t-test: # P=.064 vs. PBS control. Error bars denote mean ± SEM
Top: Representative photomicrographs of hippocampal sections from rats pretreated with DMSO, LY294002 (PI3K inhibitor), or U0126 (MEK/ERK inhibitors) 30 minutes before microinfusion of PBS or VEGF. BrdU was administered two hours after PBS or VEGF microinfusion and all rats were sacrificed 1 hr later. Bottom: Cell counts showed that pretreatment with either MEK/ERK or PI3K inhibitors did not change the number of BrdU-labeled cells in PBS-infused rats. In contrast, pretreatment with DMSO followed by VEGF significantly increased the number of BrdU-labeled cells. One-way ANOVA, Bonferroni: * P<.05 vs. DMSO/PBS controls. Error bars denote mean ± SEM
Top: Representative photomicrographs of pCREB from hippocampal sections of rats pretreated with DMSO, LY294002 (PI3K inhibitor), or U0126 (MEK/ERK inhibitor) 30 minutes before microinfusion of PBS or VEGF. Rats were sacrificed three hours later. Bottom: Quantitative analysis of the inner dentate granule cell layer/SGZ, middle dentate granule cell layer, and outer regions of the dentate granule cell layer shows that VEGF microinfusion increased phospho-CREB express throughout all dentate granule cell layer regions. Pretreatment with the U0126 or LY294002 blocked VEGF-stimulation of CREB in these regions. Two-way ANOVA, Bonferroni: * P<.05 vs. DMSO/PBS controls. Error bars denote mean ± SEM
Top: Representative blots showing activation of pFlk-1, pAkt, and pERK1/2 following VEGF treatment at various doses (0–100 ng/ml). Total levels of ERK1/2 (tERK1/2) served as a protein loading control. Bottom: Densitometric analysis revealed that there was a significant increase in pFlk-1 and pAkt induction in cultured hippocampal stem/progenitor cells at VEGF concentrations between 50 and 100 ng/mL. In addition, the induction of pERK1/2 occurred at lower concentrations of VEGF (10–50 ng/mL). One-way ANOVA, Bonferroni: * P <.05 vs. 0 ng/ml VEGF. Error bars denote mean ± SEM