Abstract
We find that the cell surface receptor Toso is dramatically down-regulated by in vitro stimulation of human T and NK cells with IL-2 in a STAT5 dependent manner. The fact that IL-2 is known to prime NK and T cells for Fas/TNF-mediated activation induced cell death (AICD) fits nicely with the original and recent descriptions of Toso as an inhibitor of Fas/TNF-induced apoptosis. In support of this possibility, effector memory T cells express markedly lower levels of Toso than naïve T cells, indicating that activation in vivo correlates with the down-regulation of Toso. Moreover, in vitro activation of memory T cells through TCR dramatically down-regulates Toso expression compared to naïve CD4 T cells. However, over-expression of Toso in human NK cells and Jurkat T cells does not inhibit Fas-mediated apoptosis, and, in agreement with other recent reports, Toso clearly functions as an IgM receptor. Unlike CD16, Toso expression by NK cells does not convey cytotoxic potential, but its ligation does trigger intracellular signaling in NK cells. In summary, our data indicate that Toso is a functional IgM receptor that is capable of activating signaling molecules, is regulated by IL-2, and is not inherently an anti-apoptotic molecule.
INTRODUCTION
Activation induced cell death (AICD), or re-stimulation induced cell death (1), has been well documented for mature activated T cells (2) and is mediated by members of the TNF-α receptor superfamily, including Fas (3–7). IL-2 has been shown to play an important role in programming or priming Ag-stimulated T cells for AICD (8), and similar observations have been made in NK cells. NK cells that are not exposed to IL-2 exhibit less Fas-Fas ligand (FasL) mediated killing upon stimulation via the activation receptor CD16 (9). Likewise, target cells and tumor cells expressing NK receptor ligands induce apoptosis of IL-2 expanded NK cells, which is mediated by the Fas-FasL interaction (10, 11). Toso, a transmembrane protein, was first identified in activated T cells as an inhibitor of Fas- and TNF-induced apoptosis (12); hence, it is also known as Fas apoptosis inhibitory molecule (FAIM-3). The fact that Toso expression in transfected Jurkat T cells correlated with enhanced cFLIP expression led to the conclusion that the function of Toso was to potentiate cFLIP expression. A mouse ortholog of Toso was found to inhibit Fas-mediated apoptosis in murine T cells through the interaction of Toso with Fas-associated death domain protein (FADD), an adaptor molecule involved in the formation of the death inducing signaling complex (DISC) (13). This led to a model proposing that Toso binding to FADD inhibits caspase-8 activity, thereby explaining why Toso inhibits Fas- or TNFα-mediated apoptosis.
More recently, solid evidence was presented indicating that Toso is an IgM-specific Fc receptor and that the previously observed anti-apoptotic function of Toso was likely artifactual due to the routine use of an IgM anti-Fas mAb to induce Fas-mediated apoptosis (14, 15). The anti-apoptotic effects of Toso were not observed when either FasL or an IgG mAb to Fas were used to induce apoptosis (15). The evidence indicating that Toso is an IgM receptor, and hence more properly designated FcμR, is supported by its genetic location next to the genes for the polymeric immunoglobulin receptor (PIGR) and the Fcα/μR (14). Despite these reports, the function of Toso/FcμR remains a subject of active debate. In this regard, a very recent publication (16) showed that Toso is an anti-apoptotic molecule that does not bind IgM and functions by recruiting the death adaptor FADD to a Toso/RIP1 protein complex.
Our interest in Toso/FcμR began when we identified it as a gene product that is dramatically down-regulated in NK cells treated with IL-2, which led us to postulate that as an anti-apoptotic molecule its down-regulation might facilitate AICD. We have followed with interest the controversy regarding Toso/FcμR function and herein present data that indicate Toso/FcμR binds IgM and delivers an activating signal to NK cells. Moreover, we demonstrate that IL-2 down-regulates Toso/FcμR expression by both NK and T cells and that this suppression is a dynamic and reversible process. We further show that TCR activation of CD4 T cells results in down-regulation of Toso/FcμR. In accord, we found that, relative to naïve T cells, Toso/FcμR levels are low on effector and central memory T cells, which correlates with their activation status. However, in contrast to earlier studies showing that Toso/FcμR is an anti-apoptotic molecule (12, 13, 16), upon over-expressing Toso/FcμR in Jurkat T and peripheral blood NK cells, we were not able to inhibit Fas mediated apoptosis induced by FasL. We anticipate our work will extend the recognition of Toso/FcμR as an IgM receptor capable of activating signaling molecules, whose expression alone is not inherently anti-apoptotic.
MATERIALS AND METHODS
Cell culture
NK, CD4, and CD8 T cells were isolated from human peripheral blood using Stem Cell isolation kits (NK cells) or Miltenyi Biotec MACS columns (T cells), and their purity (> 95%) determined by staining with anti-CD3 mAb (eBiosciences) and anti-CD56 mAb (BD Biosciences) for NK cells, and anti-CD3 mAb and anti-CD4 mAb (eBiosciences) or anti-CD8 mAb (eBiosciences) for CD4 and CD8 T cells, respectively. Primary T cells were cultured in Iscove’s modified Dulbecco’s Medium (IMDM) containing 10% human AB serum (Valley Biomedical) and 100 IU/ml IL-2. NK cells were cultured as described for each assay.
Lentiviral vector construction
Lentiviral (LV) vectors expressing human Toso were constructed from the parent vector (pCL20c MSCV GFP), kindly provided by Dr. Arthur Nienhuis (St. Jude Children’s Research Hospital, Memphis, TN). First, an internal ribosome entry site (IRES) was inserted using Bst1107I and SacII sites. A Toso cDNA fragment flanked with ClaI and AgeI sites was PCR amplified, digested, and inserted into pCL20c MSCV IRES-GFP, thus generating pCL20c MSCV Toso-IRES-GFP. A pCL20c MSCV Toso T2A-GFP vector was generated the same way. Human Toso cDNA was also cloned into the LV vector, pCDH, which contains a puromycin resistance gene, allowing selection of positive clones. All vector constructs were confirmed by DNA sequence analysis.
Vector production and transduction
293T cells were co-transfected with viral packaging encoding constructs and either of the Toso LV expression vectors described above. Vector particles were harvested after 24 h, cleared by centrifugation, filtered, snap frozen in aliquots, and stored at −80°C. For LV transduction into NK cells, freshly isolated primary NK cells were cultured with irradiated (100 Gy) Epstein-Barr virus-transformed B cells as feeder cells in IMDM containing 10% human AB serum and 500 IU/mL of IL-2 as previously described (17). Jurkat T or NK cells were seeded with retronectin (Takara Bio Inc) and transduced (95% transduction efficiency as measured by GFP) with pCL20c MSCV Toso-IRES-GFP or pCL20c MSCV Toso-T2A-GFP, respectively, at multiplicity of infection of 10 in the presence of 8 μg/ml protamine sulfate (Sigma-Aldrich). Transduced cells were washed, cultured overnight in complete culture medium containing IL-2, and re-transduced. The transduced cells were used for assays 72 or 96 h after transduction. YTS cells were infected with pCDH-Toso in the presence of 6 μg/ml protamine sulfate and positive clones selected with 4 μg/ml puromycin.
Toso cell surface expression and IgM binding
To detect Toso expression by Jurkat T and YTS cells, cells were washed and then incubated with anti-human Toso mAb (Abnova) for 30 min on ice prior to washing and staining with PE or APC-labeled secondary Ab (eBiosciences). IgM binding was detected using APC-labeled mouse IgM (mIgM) (BD Biosciences) or by treating cells with unlabeled mIgM (Sigma Aldrich), followed by detection with PE-conjugated anti-mIgM mAb (eBioscience). For analysis of CD56bright versus CD56dim populations, fresh PMBC were stained with anti-CD3 mAb APC, anti-CD56 mAb PE, and anti-Toso mAb, and Toso expression determined as described above. To measure IgM bound to freshly isolated NK cells, PBMC were stained with anti-human IgM μ chain mAb PE (AbD Serotec), anti-human CD3 mAb, and anti-human CD56 mAb and gated on NK cells (CD3− and CD56+). To measure mIgM binding and Toso surface expression in cultured NK cells, cells were isolated from PBMC and cultured in X-vivo medium (Lonza) with 10% Ig-depleted human serum (Sunny Labs) plus 500 U/ml IL-2 for 7d, then cultured in X-vivo medium with 10% Ig-depleted serum in the absence or presence of IL-2 overnight prior to flow cytometry using the above antibodies. For blocking studies, NK cells were isolated and cultured as described above, then collected and washed with PBS prior to pretreatment with human pentameric Fcμ fragments (Fc5μ) (Athens Research). Cells were then incubated with APC-labeled mIgM, mIgM, or anti-human Toso mAb for 30 min on ice and detected as described above.
Apoptosis studies
For apoptosis induction, Jurkat T cells were maintained in cRPMI with 10% Ig-depleted serum for 1 wk prior to treatment with anti-human Fas mAb clone CH11 (IgM isotype, Upstate), recombinant FasL (Alexis Biochemicals), or anti-human Fas mAb clone 2R2 (IgG3 isotype, Kamiya Biomedicals) at the indicated concentrations for 10 h. Annexin V staining was performed using the BD Biosciences kit according to the manufacturer’s protocol. Apoptosis of the transduced NK cells, sorted into GFP+ or GFP− populations, was induced using anti-human Fas mAb clone 2R2, or FasL and crosslinking enhancer (Alexis Biochemicals) at the concentrations indicated in the figure legends for 12 h. For analysis of LV-transduced NK cells, Annexin V-APC was used according to the manufacturer’s protocol.
T cell sorting
For sorting CD4 T cells into naïve and memory cells, isolated CD4 T cells were washed with PBS containing 1% human serum and stained with anti-human CD4 mAb, CD45RO mAb, and CD62L mAb, conjugated to FITC, PE, and APC respectively (all from eBiosciences). The cells were incubated on ice for 30 min after which they were washed extensively with PBS containing 1% human serum and sorted using a FACS Aria sorter (Beckton Dickinson). Stimulation of CD4 T cells was done using plate bound anti-human CD3 mAb (1 μg/ml, BD Biosciences) for the indicated times.
Real time PCR analysis
NK cells were isolated from PBMC and cultured in IMDM with 10% human AB serum with our without IL-2 as indicated. All samples for RNA isolation were stored at 4°C in RNALater (Ambion). RNA isolation was carried out using the RNAqueous4PCR kit (Ambion) and cDNA produced using Qscript™ kit (Quanta Biosciences). Real-time PCR was done on a Roche LC480 cycler using Lightcycler® 480 SYBR green I master supermix (Roche Diagnostics). Primers for real-time PCR measurement of human Toso, actin, and 18S rRNA were purchased from Qiagen. All reactions were done in triplicate and averages were used to calculate the relative levels of each mRNA. Relative quantification of the target genes was determined using the 2nd derivative maximum using the Roche Lightcycler software and calculating the fold changes over the 18S rRNA or actin transcript levels. Melting curve analysis was performed routinely to ensure that only one product was amplified.
STAT5 and PI3K inhibitor studies
Freshly isolated human peripheral blood NK cells were pre-incubated for 30 min in IMDM plus 10% human serum with N′-((4-oxo-4H-chromen-3-yl) methylene) nicotinohydrazide (STAT5 inhibitor, EMD Biosciences) or wortmannin (PI3K inhibitor, Sigma Aldrich) at various concentrations as indicated. 500 U/ml IL-2 was added and cells were cultured for 24 h before harvesting, washing, and storing in RNALater. RNA was isolated, cDNA was synthesized, and the relative levels of Toso mRNA were quantified by normalizing to the level of 18S rRNA transcripts using real-time PCR as described above.
Measurement of cytotoxicity
NK cells were isolated and maintained in X-vivo medium with 10% Ig-depleted serum and 500 U/ml IL-2 for one wk prior to culturing in X-vivo medium plus 10% Ig-depleted medium without IL-2 overnight. P815, K562, or SK-OV3 target cells were labeled with DELFIA BATDA (TDA) cytotoxicity reagents (Perkin Elmer). Cells were washed 3 times in PBS and resuspended in X-vivo medium with 10% Ig-depleted serum. P815 cells were incubated for 30 min at room temperature with anti-human CD16 mAb (100 ng/ml, eBiosciences), anti-human NKG2D (R&D), IgG1 anti-mouse IgM mAb (1 μg/ml, BD Biosciences), anti-human Toso mAb, or control IgG (100 ng/ml, BD Biosciences). SK-OV3 cells were treated with anti-HER2 (ErbB2) mAb Trastuzumab (50 ng/ml) then spun down, resuspended in medium, and plated at 104 cells/well in triplicate in 96-well round bottom plates. NK cells were incubated with mIgM (10 μg/ml), mIgM + anti-IgM mAb, or IgM anti-Lewis Ab (10 μg/ml, Abcam) at 37°C for 30 min, then added to wells at the indicated effector-to-target (E:T) ratios. After 2 h incubation, the supernatant was collected and release of TDA was measured according to manufacturer’s instructions using a Wallac Victor2 plate reader (Perkin Elmer).
Western blots
For immunoprecipitations, YTS cells over-expressing Toso were lysed in ice-cold lysis buffer (50mM Tris-Cl pH 7.4, 150mM NaCl, 2mM EDTA, 0.5% Triton-X-100 including protease inhibitor cocktail, Sigma Aldrich), and lysates incubated overnight at 4°C with either anti-Toso mAb or control IgG Ab (Santa Cruz Biotech), followed by a 2 h incubation with protein G Dynabeads (Invitrogen) at 4°C. The beads were washed 4 times with lysis buffer and the proteins eluted with NuPAGE LDS sample buffer (Invitrogen). Precipitates were separated by 4–12% gradient Bis-Tris gels (Invitrogen) and transferred to PVDF. Blots were incubated overnight at 4°C with anti-human Toso mAb, anti-PLCγ2 polyclonal Ab (Cell Signaling), or anti-pTyr mAb (clone 4G10, Millipore), followed by incubation with HRP-conjugated secondary Ab (Santa Cruz Biotech). To detect signaling molecules, 1–2 × 106 YTS cells were cultured in cRPMI without serum overnight, then collected and resuspended in 25 μl serum-free cRMPI prior to treatment. Cultured NK cells were isolated from PBMC and cultured for one week in X-vivo medium with 10% Ig-depleted serum and 500 IU/ml IL-2 before culturing in serum-free X-Vivo medium without IL-2 overnight. Fresh NK cells were isolated from PBMC and cultured in X-vivo medium with neither serum nor IL-2 for 14 – 16 h. In either case, 5 × 106 NK cells were collected and resuspended in 15 μl X-vivo medium prior to treatment. YTS cells and NK cells were treated with PBS (control), 5 μg/ml mIgM, 5 μg/ml anti-mIgM Ab, or both for 30 min at 37°C. Ice cold lysis buffer (100mM Tris pH 7.4, 1% NP-40, 200 mM NaCl, 2mM EDTA) was added and cells lysed for 30 min prior to centrifugation to remove cellular debris. Supernatants were denatured with 4X NuPage LDS buffer with DTT, run on gels, transferred to nitrocellulose and probed with a rabbit mAb to pERK1/2 (Cell Signaling), then reprobed with a polyclonal Ab to total ERK1/2 (Cell Signaling). The blots were developed using ECL plus (Amersham Biotech) or Pierce Pico Western substrate. The integrated density of each band was measured using the gel analysis function of ImageJ (Version 1.44o), normalized to the loading control, and compared with the untreated sample (value = 1).
Statistical analysis
For all experiments, statistical analysis and graphing were done using the Graphpad Prism 5 software. Two-tailed, unpaired student’s t-test with 95% confidence interval was used to compare the statistical significance of the differences observed between treatments. In some cases, the paired t test was used for analysis as indicated in figure legends. A significance of p < 0.05 is indicated by *; p < 0.01 is indicated by **; p < 0.005 is indicated by ***.
RESULTS
Toso binds IgM
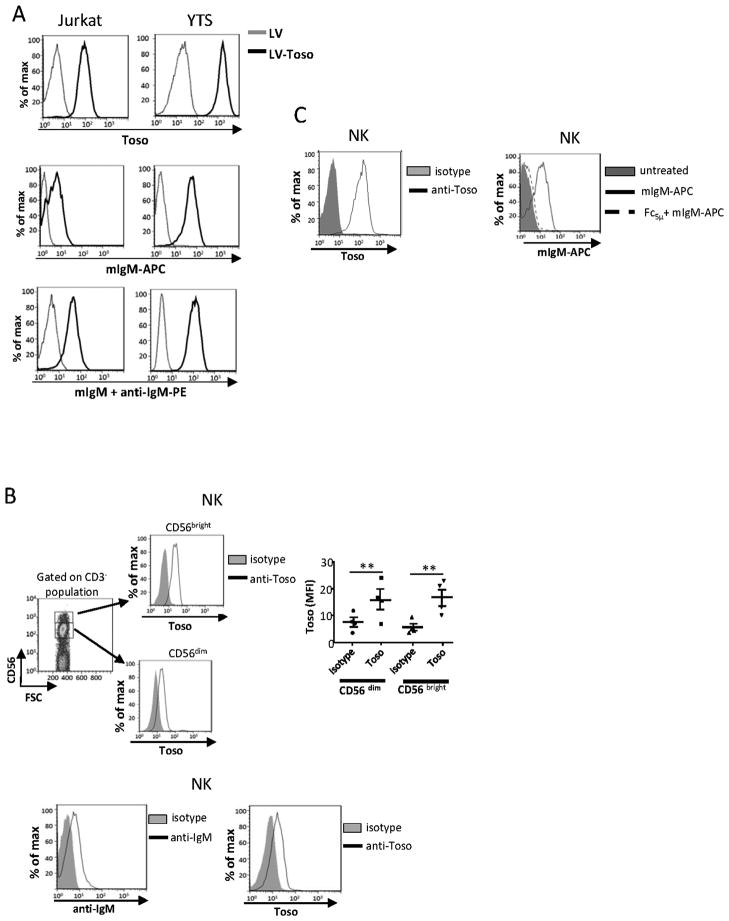
We analyzed the ability of Toso to bind IgM using Jurkat T and YTS NK cell lines over-expressing Toso, as well as NK cells isolated from PBMC. In our experiments we used mIgM because mIgM binds human Toso better than human IgM does (15). Empty LV transduced cells did not express Toso on the cell surface, as measured by flow cytometry, while the cells transduced with LV-Toso expressed the receptor (Fig. 1A). mIgM binding to these cells was detected by staining with mIgM plus anti-mIgM Ab PE or by treating cells directly with APC-labeled mIgM (Fig. 1A). [Unlike human sera, we found that any IgM present in FCS did not block the mIgM binding to cells (data not shown)]. As circulating NK cells (both CD56bright and CD56dim) express Toso (Fig. 1B, top) and are constantly exposed to serum IgM, we tested if NK cells in PBMC have IgM bound to them. PBMC were collected and gated on NK-specific markers (CD3− and CD56+). Staining with an anti-human IgM Ab indicates IgM is bound to circulating NK cells (Fig. 1B, bottom). To verify the specificity of the IgM binding, NK cells were isolated from PBMC, cultured in X-vivo medium with Ig-depleted serum plus IL-2 for 7 d, then in the same medium without IL-2 overnight. These NK cells bound mIgM-APC Ab and expressed Toso on the cell surface (Fig. 1C). NK cells exhibited essentially no binding to IgM when pretreated with Fc5μ, indicating that the binding is specific (Fig.1C). Toso expression on these same NK cells is also shown (Fig. 1C). We have found that mIgM binding to NK cells correlates with Toso expression by these cells (see Fig. 3B). These results demonstrate that Toso is expressed on NK cells and is likely the receptor responsible for IgM binding. Thus, throughout the remainder of the paper we refer to the receptor as Toso/FcμR.
Figure 1. Toso/FcμR binds IgM.
A, Jurkat T or YTS cells transduced with control lentivirus (LV) or Toso-encoding lentivirus (LV-Toso) were stained with anti-Toso mAb plus anti-IgG Ab PE or APC-conjugated mIgM (mIgM-APC) or mIgM plus anti-IgM Ab PE and analyzed by flow cytometry. B, top panel. Freshly isolated PBMC were stained with anti-Toso, anti-CD3, and anti-CD56 mAbs. Subsets of NK cells were recognized by CD56bright or CD56dim populations and analyzed for Toso expression. The right panel shows the average from 4 donors; error bars indicate SEM. Statistical analysis was done by two tailed unpaired student’s t-test. **p<0.01. Bottom panel, Freshly isolated PBMC were stained with anti-human IgM μ chain mAb (anti-IgM) or anti-Toso mAb, and gated on NK cells. These histograms are representative of 3 experiments using cells from different donors. C, NK cells that had been cultured in X-vivo medium with Ig-depleted serum were preincubated with or without Fc5μ then stained with mIgM-APC and analyzed by flow cytometry. Expression of Toso/FcμR on these NK cells is also shown. This is one representative of 3 experiments.
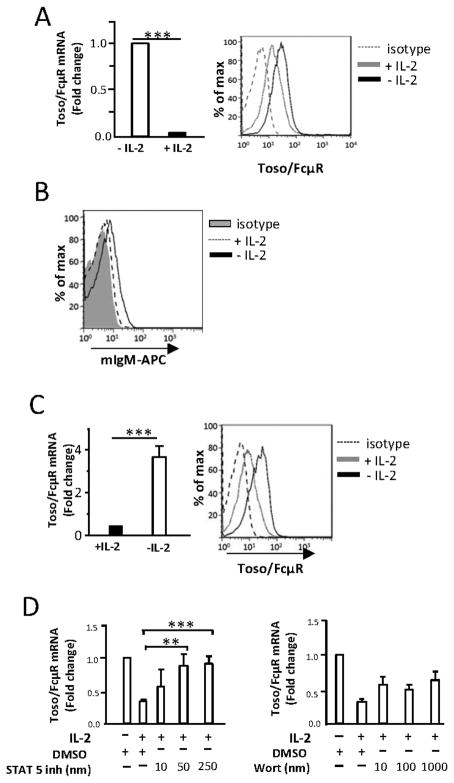
Figure 3. Toso/FcμR expression in primary human NK cells.
A, NK cells were cultured with or without IL-2 (500 U/ml) for 48 h. Toso/FcμR mRNA levels were measured by real-time PCR (left) and Toso/FcμR surface expression measured by flow cytometry using anti-Toso mAb or isotype control (mIgG2b) followed by PE-conjugated anti-mouse IgG Ab (right). The histogram is representative of results obtained from 10 donors and the bar graph shows the average. Error bars indicate SEM. B, NK cells were cultured in X-Vivo medium with Ig-depleted serum and IL-2 (500 U/ml) for 8 d (+ IL-2), or with Ig-depleted serum and IL-2 for 7 d then without IL-2 overnight (− IL-2). Cells were tested for IgM binding by adding mIgM-APC. The histogram is representative of NK cells from 12 donors. C, NK cells were cultured for 7 d with IL-2 (500 U/ml), washed, and resuspended in medium with or without IL-2 (500 U/ml) for 16 h. Expression of Toso/FcμR mRNA levels (left) and cell surface Toso/FcμR (right) are shown. The histogram is representative of results from 5 donors and the bar graph shows the average. Error bars indicate SEM. Statistical analysis was done by two tailed unpaired student’s t-test. *p<0.001. D, Freshly isolated NK cells were preincubated for 30 min with the STAT5 inhibitor or the PI3K inhibitor (wortmannin) at the indicated concentrations before adding IL-2. After 24 h, the cells were harvested and analyzed for Toso/FcμR mRNA levels. mRNA levels were normalized to 18S rRNA transcripts and presented as fold change relative to DMSO-treated cells. Data shown is relative Toso/FcμR mRNA expression from 3 donors and the error bars indicate SEM. Statistical analysis was performed using two-tailed unpaired student’s t-test. *p<0.05, **p<0.01.
Toso/FcμR expression does not inhibit apoptosis in primary human NK or Jurkat T cells
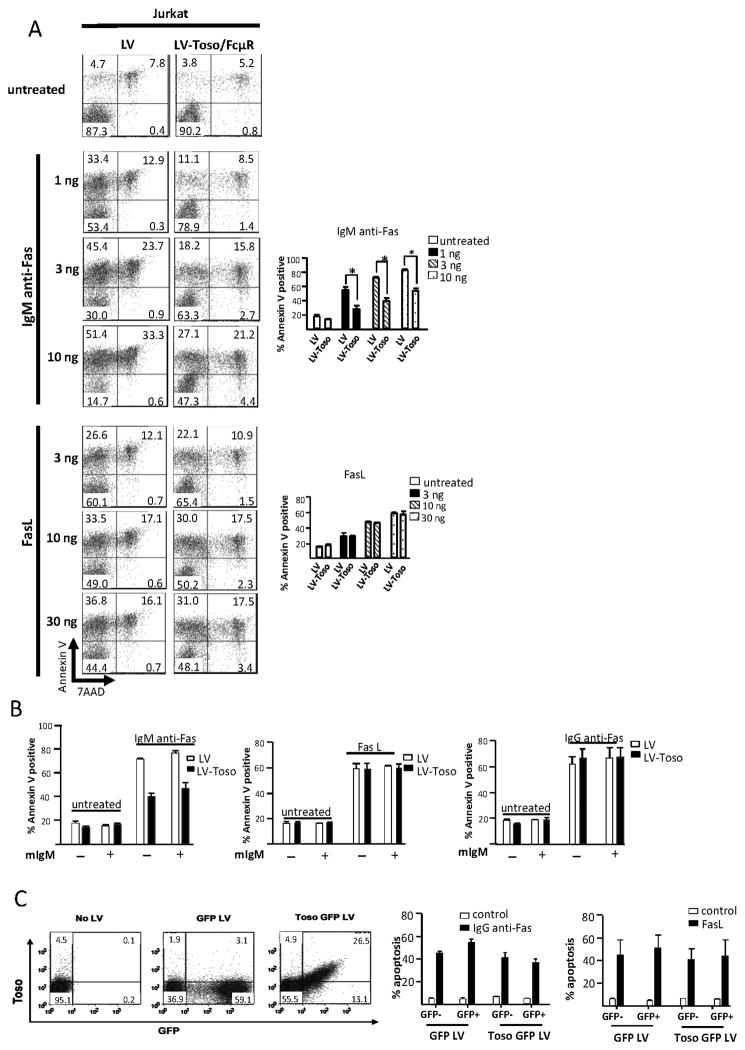
As mentioned, recent publications differ in their findings as to whether Toso/FcμR expression is inherently apoptotic (16) or not (15). To investigate the anti-apoptotic activity of Toso/FcμR, we treated control Jurkat cells or Jurkat over-expressing Toso/FcμR (see Fig. 1A) with various concentrations of FasL or anti-Fas Ab. Both control Jurkat and Toso/FcμR over-expressing cells were cultured in cRMPI with Ig-depleted serum for one week to remove any IgM. Under no circumstances did Toso/FcμR affect FasL-induced apoptosis, as evidenced by staining with 7-AAD and Annexin V, nor did it affect IgG anti-Fas Ab-mediated apoptosis (Fig 2A, B), even in the presence of mIgM. Only IgM anti-Fas Ab-treated cells exhibited reduced apoptosis, and this occurred in the presence or absence of excess mIgM (Fig. 2A, B). The effect of the IgM anti-Fas Ab is not due to the IgM isotype as mIgM (10 μg/ml) does not interfere with the anti-apoptotic effect of FasL or IgG anti-Fas Ab (Fig. 2B). This suggests that the anti-apoptotic effect of IgM anti-Fas Ab is due to the cross-linking of Toso/FcμR and Fas (see Discussion). To further verify that Toso/FcμR expression is not inherently anti-apoptotic, we transduced primary NK cells, cultured and expanded in IMDM with human serum and IL-2, with a Toso/FcμR-expressing LV that also encodes for GFP. Cells transduced with a LV expressing GFP alone were used as control. Transduction with the Toso/FcμR-GFP LV clearly produced a population of cells enriched for the surface expression of Toso/FcμR, as compared to NK cells transduced with the GFP LV control (Fig. 2C, left). Following stimulation of Fas mediated-apoptosis using IgG anti-Fas mAb or FasL, we observed that the level of apoptosis, as measured by Annexin V staining, was similar in cells transduced with GFP LV or Toso/FcμR-GFP LV (Fig. 2C, right). Thus, Toso/FcμR over-expression cannot rescue human NK cells expanded in vitro with IL-2 from Fas-mediated cell death, further indicating that, under these conditions, Toso/FcμR is not an anti-apoptotic molecule.
Figure 2. Toso/FcμR is not anti-apoptotic.
A, Jurkat T cells transduced with control LV or LV-Toso/FcμR were stimulated with IgM anti-Fas mAb or FasL at indicated concentrations for 10 h and analyzed for apoptosis by Annexin V and 7-AAD staining. One representative dot plot and bar graphs from 3 independent experiments are shown. Error bars indicate SEM. Statistics were calculated using unpaired, two-tailed student’s t-test. *p<0.05. B, Jurkat T cells transduced with control LV or LV-Toso/FcμR were untreated or stimulated with IgM anti-Fas mAb (10 ng/ml), FasL (100 ng/ml), or IgG3 anti-Fas mAb (50 ng/ml) in the absence or presence of mIgM (10 μg/ml) and the percentage of Annexin V positive stained cells determined by flow cytometry. Bar graphs indicate data from at least 4 experiments. C, Left panel, NK cells transduced with GFP-LV or Toso/FcμR-GFP-LV were stained with anti-Toso mAb. Toso/FcμR expression was analyzed by flow cytometry and shown from a representative donor. Right panel, Toso/FcμR transduced NK cells were sorted into GFP+ and GFP− cells and analyzed for Fas mediated apoptosis after culturing with IgG3 anti-Fas mAb (50 ng/ml) or recombinant FasL (100 ng/ml) and crosslinkning Ab (1μg/ml) for 12 h. Apoptotic cells were determined by staining with AnnexinV-APC using flow cytometry. Data shown is the average from 3 different donors and error bars indicate SEM.
Toso/FcμR expression by NK cells is regulated by IL-2 through a STAT5 dependent pathway
We found that NK cells isolated from human PBMC (cultured in IMDM plus human serum) with IL-2 for 48 h had a dramatic (80–90%) down-regulation of Toso/FcμR transcript levels as compared to cells cultured without IL-2 (Fig. 3A, left). This is accompanied by marked (approximately 50%) decrease in cell surface staining using an anti-Toso mAb (Fig. 3A, right). Thus, IL-2 down-regulates the expression of Toso/FcμR mRNA and cell surface expression by NK cells. We found that mIgM does not bind to NK cells cultured in X-vivo medium with Ig-depleted serum when IL-2 is present, yet when NK cells are cultured in medium without IL-2 overnight, mIgM binds (Fig. 3B). Thus, mIgM binding correlates with Toso/FcμR expression by NK cells. When culturing NK cells over a long period of time in IMDM with human serum, we observed that Toso/FcμR expression was maintained at low levels in medium containing IL-2. When NK cells cultured in this manner are washed and placed in fresh medium supplemented with 10% human serum, but lacking IL-2 for 16 h, Toso/FcμR expression increased (approximately 5 fold in mRNA and 2 fold in cell surface expression) (Fig. 3C). Upon binding of IL-2, signaling through the heterotrimeric IL-2Rα/β/γ activates multiple signaling pathways, including that of PI3 kinase (PI3K) and JAK3/STAT5 (18, 19). To determine if PI3K or JAK3/STAT5 is involved in the IL-2 mediated down-regulation of Toso/FcμR, we used specific inhibitors for PI3K or JAK3/STAT5, to block IL-2-dependent signaling from these pathways. Freshly collected NK cells were pre-incubated with wortmannin (PI3K inhibitor) or a STAT5 inhibitor, then cultured in medium with or without IL-2 for 24 h prior to mRNA isolation. STAT5 inhibition completely blocked Toso/FcμR down-regulation in a dose-dependent manner, while wortmannin could not rescue the expression of Toso/FcμR mRNA levels (Fig. 3D). Thus, the JAK/STAT pathway but not the PI3K pathway is involved in IL-2-mediated down-regulation of Toso/FcμR.
IL-2 also regulates Toso/FcμR expression in human CD4 and CD8 T cells
Since Toso/FcμR was originally discovered in T cells (12), and because AICD is a pathway that has been shown to be important in the homeostasis of peripheral T cells (20), we examined the effect of IL-2 on Toso/FcμR expression on human CD4 and CD8 T cells. CD4 and CD8 T cells were isolated from PBMC and cultured in IMDM with FCS. As with NK cells (see Fig. 3A), we observed that culturing CD4 and CD8 T cells overnight with IL-2 reduced Toso/FcμR mRNA levels significantly (~75%) (Fig. 4A). To study the regulation of Toso/FcμR expression during T cell activation, we cultured CD4 T cells in wells coated with plate-bound anti-CD3 mAb. Upon activation with anti-CD3 mAb, Toso/FcμR mRNA levels were reduced to levels similar to that regulated by IL-2 (Fig. 4B).
Figure 4. Regulation of Toso/FcμR expression on human CD4 and CD8 T cells.
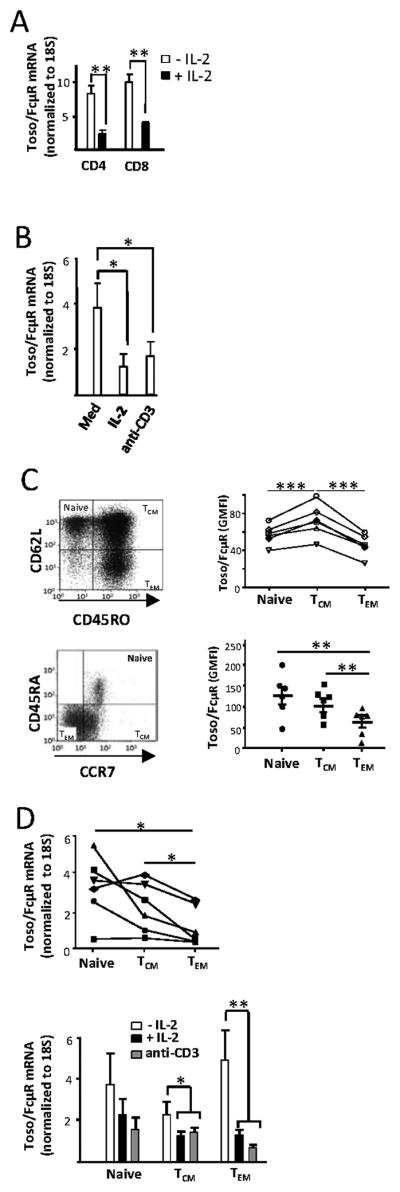
A, Human peripheral blood CD4 and CD8 T cells were isolated and cultured in the presence or absence of IL-2 (100 IU/ml) in complete IMDM overnight and Toso/FcμR mRNA expression was quantified by real-time PCR. The results shown are from 4 donors and error bars indicate SEM. Statistical analysis was done by two tailed unpaired student’s t-test. **p<0.01. B, Human CD4 T cells isolated from PBMC were cultured with plate bound anti-CD3 mAb (1 μg/ml) or IL-2 (100 IU/ml) for 24 h and analyzed for Toso/FcμR mRNA levels by real-time PCR assay. Data shown are from 5 independent experiments and the error bars indicate SEM. Statistical analysis was done by two tailed unpaired student’s t-test. *p<0.05. C, Human CD4 T cells from PBMC were stained for CD45RO and CD62L (top), or CCR7 and CD45RA (bottom), and the cell surface expression of Toso/FcμR in the naïve and memory subsets defined by these markers was determined by staining with anti-Toso/FcμR mAb followed by PE-conjugated anti-mouse secondary Ab. Data shown are geometric mean fluorescence intensity (GMFI) of anti-Toso mAb staining for 6 individual donors, each represented by a symbol on the scatter plot. D, top panel, Human CD4 T cells isolated from peripheral blood were stained for CD45RO and CD62L and the naïve and memory subsets were sorted by FACS. The expression of Toso/FcμR mRNA levels were analyzed by real-time PCR and the data are normalized to 18S rRNA expression. Data shown are mean values of triplicates from 6 individuals each represented by a symbol on the scatter plot. Statistical analysis was done by two-tailed paired t-test. D, bottom panel, Sorted cells were stimulated with plate-bound anti-CD3 mAb (1 μg/ml) or IL-2 (100 U/ml) and analyzed for Toso/FcμR expression by real-time PCR (after 24 h). Data shown is from 7 independent experiments and the error bars indicate SEM. Statistical analysis was done using two-tailed unpaired student’s t-test comparing each treatment to untreated samples (−IL2).
We hypothesized that naïve T cells, which are not activated, should have a higher level of Toso/FcμR expression than effector T cells. To test this hypothesis, we examined Toso/FcμR expression on central memory (TCM), and effector memory (TEM) CD4 T cells that were isolated from PBMC. Central and effector memory populations have been defined by the expression of CD45RO and CD62L markers (21) as well as CCR7 in combination with CD45RA (22). Regardless of the markers used to identify T cell subsets, we observed that the TEM subset had significantly lower levels of Toso/FcμR expression compared to naïve or TCM cells (Fig. 4C). These results support the hypothesis that activation and/or signaling leading to the differentiation of T cell subsets in vivo is accompanied by the down-regulation of Toso/FcμR.
We next examined if in vitro treatment of the various CD4T cell subsets with activating stimuli, IL-2 or anti-CD3 mAb, would lead to further down-regulation of Toso/FcμR expression. We isolated naïve TCM and TEM cells by sorting based on the expression levels of CD45RO and CD62L, cultured them with IL-2 or activated them with anti-CD3 mAb, and measured Toso/FcμR mRNA. In agreement with ex vivo cell surface expression levels, we found that Toso/FcμR mRNA levels were higher in freshly isolated naïve cells as compared to the effector memory cells (Fig. 4D, top). At 24 h, we again analyzed Toso/FcμR expression by real-time PCR, and while TEM cells exhibited the greatest level of down-regulation (~65%), TCM cells also showed a marked reduction (~30%) following IL-2 treatment or anti-CD3 mAb stimulation (Fig. 4D, bottom). In the case of naïve T cells, while a reduction in Toso/FcμR mRNA levels was observed, particularly in anti-CD3 treated cells compared to the untreated cells, the difference was not found to be significant (Fig.4D, bottom).
Toso/FcμR does not mediate NK cell cytotoxicity
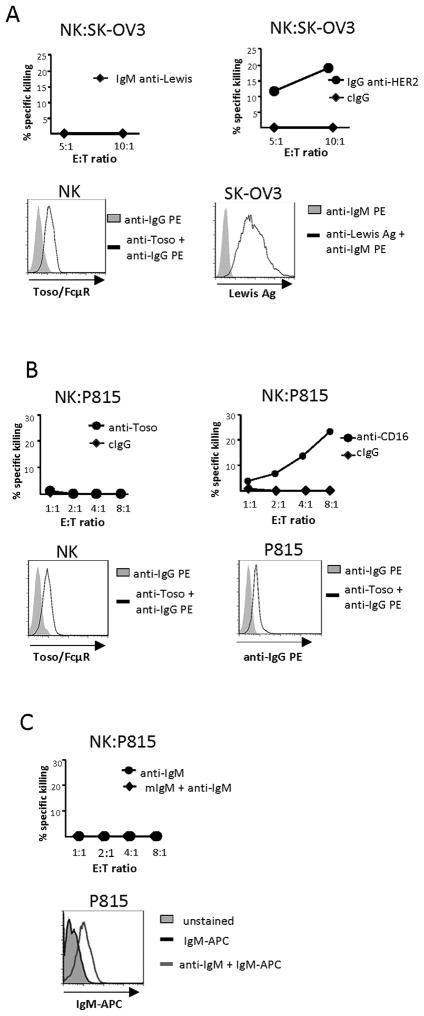
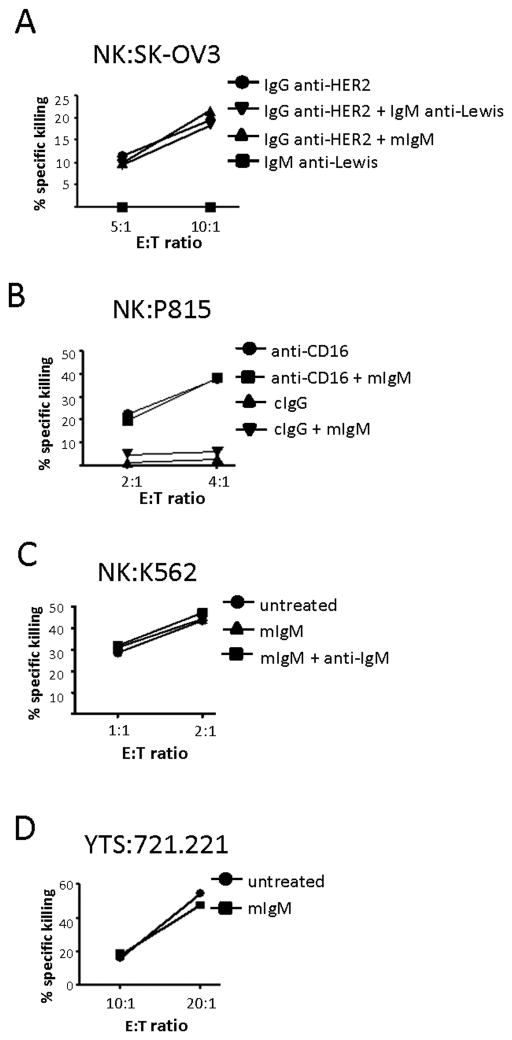
As we have observed that Toso/FcμR is not an anti-apoptotic molecule and that it specifically binds IgM, we sought to ascribe a function to this receptor in NK cells. Our first course of action was to determine if Toso/FcμR could mediate cytotoxic activity. All NK cells used in these cytotoxic assays were cultured in X-vivo medium with Ig-depleted serum and IL-2 for one week, then washed and cultured in media containing Ig-depleted serum without IL-2 overnight prior to incubation with target cells. NK cells exhibit ADCC, which is mediated by the IgG receptor CD16. We tested if Toso/FcμR could mediate a similar response, i.e. does ligation of this IgM receptor by target cell ligands trigger NK cell killing of target cells? NK cells were incubated with IgM anti-Lewis Ag Ab then added to wells containing SK-OV3 cells that express the Lewis Ag. NK cells were unable to kill targets (Fig. 5 A, top left). Flow cytometry verified the NK cells expressed Toso/FcμR and that SK-OV3 target cells could bind the IgM anti-Lewis Ab (Fig. 5A, bottom). On the other hand, ADCC utilizing CD16 recognition of IgG anti-HER2 Ab-coated SK-OV3 target cells was successful (Fig. 5A, top right). Re-directed lysis of P815 cells through their expression of Fc receptors is a common assay for determining if a given receptor expressed by NK cells can mediate cytotoxic function. To examine if Toso/FcμR could mediate re-directed lysis, NK cells were incubated with P815 target cells in the presence of control IgG Ab (cIgG) or anti-Toso mAb; no re-directed lysis of the targets occurred (Fig. 5 B, top, left). Flow cytometry verified Toso/FcμR expression on NK cells and anti-Toso mAb binding to P815 cells (Fig. 5B, bottom), illustrating that these cells expressed the correct components for NK:target cell interactions. As a positive control, the same experiment was performed with an anti-CD16 mAb, which resulted in effective killing (Fig. 5B, top right). We also examined if Toso/FcμR could mediate redirected lysis using an anti-IgM mAb instead of the anti-Toso mAb. P815 cells were coated with an anti-IgM mAb, then treated with mIgM and mixed with NK cells expressing Toso/FcμR. Again, NK cells exhibited no cytotoxicity (Fig. 5C, top), though P815 target cells could bind the anti-IgM mAb (Fig. 5C, bottom) and NK cells can bind mIgM (Fig. 1).
Figure 5. Toso/FcμR does not mediate NK cytotoxicity.
A, top panel, NK cells were incubated with IgM anti-Lewis antigen Ab (10 μg/ml), then mixed with SK-OV3 target cells (left). SK-OV3 target cells were incubated with an IgG anti-HER2 mAb (50 ng/ml), then incubated with NK cells (right). Bottom panel, Flow cytometry illustrates the expression level of Toso/FcμR on NK cells (left) and binding of the IgM anti-Lewis Ab to SK-OV3 cells (right). B, top panel, P815 cells were pretreated with anti-Toso mAb (1 μg/ml; left) or anti-CD16 mAb (100 ng/ml; right) then incubated with NK cells. Bottom panel, Flow cytometry shows expression of Toso/FcμR by NK cells (left) and binding of anti-Toso mAb to P815 cells (right). C, top panel, anti-IgM mAb (1 μg/ml) coated P815 cells were incubated with NK cells in the presence or absence of mIgM (10μg/ml). Bottom panel, Flow cytometry demonstrates P815 cells are able to bind IgM-APC via anti-IgM mAb. The graphs display representative results and are consistent with data from an at least 3 donors.
We then postulated that Toso/FcμR might act as a co-stimulatory receptor, meaning that Toso/FcμR binding to IgM would augment activation of NK cells that was mediated by other NK cell receptors. We thus tested the ability of Toso/FcμR to augment CD16-mediated ADCC. The incubation of NK cells with SK-OV3 target cells coated with anti-HER2 mAb, which binds and activates CD16, resulted in target cell killing. Neither inclusion of mIgM (to ligate Toso/FcμR) nor the IgM anti-Lewis mAb resulted in augmentation of this killing (Fig. 6A). To test if Toso/FcμR modifies NK cell cytotoxicity in a redirected lysis assay, anti-CD16 mAb-coated P815 target cells were incubated with NK cells. While NK cells were able to kill these target cells, inclusion of mIgM to ligate Toso/FcμR did not alter this killing (Fig. 6B). We also tested if Toso/FcμR could alter NK cytotoxicity mediated by the activating receptor NKG2D. P815 cells that had been coated with anti-NKG2D mAb were incubated with NK cells. While the NK cells were able to kill the P815 target cells, due to engagement of NKG2D, addition of mIgM or mIgM plus anti-IgM mAb did not augment this killing (data not shown).
Figure 6. Toso/FcμR ligation does not enhance NK cell cytotoxic function.
A, SK-OV3 target cells were coated with anti-HER2 mAb (50 ng/ml) and mixed with NK cells. The ability of NK cells to lyse SK-OV3 cells with or without mIgM (10 μg/ml) or the anti-Lewis Ag Ab (10 μg/ml; mouse IgM) was determined at the indicated E:T ratios. B. P815 target cells were coated with anti-CD16 mAb or cIgG Ab (100 ng/ml) and mixed with NK cells. The ability of NK cells to lyse P815 cells with or without mIgM (10 μg/ml) was determined at the indicated E:T ratios. The graph displays a representative result. C, NK cells were incubated with or without mIgM (10 μg/ml) in the presence or absence of anti-IgM Ab (10μg/ml). Cytotoxic activity of these cells against K562 target cells was determined at the indicated effector:target (E:T) ratios. D, Toso/FcμR-expressing YTS cells were incubated with or without mIgM (10μg/ml). Cytotoxic activity of these cells against 721.221 target cells was determined at the indicated effector:target (E:T) ratios. All graphs display representative results and are consistent with data from an additional 3 donors.
To determine if Toso/FcμR ligation could alter NK cell natural cytotoxicity against K562 cells, NK cells were incubated with K562 target cells in the absence of mIgM, the presence of mIgM, or the presence of mIgM plus anti-IgM mAb. While NK cells were successful in killing the target K562 cells, neither mIgM nor mIgM plus anti-IgM mAb had an effect (Fig. 6C). Finally, as the NK cell line YTS can lyse 721.221 cells, we tested if mIgM could augment killing of 721.221 by YTS over-expressing Toso/FcμR. However, mIgM treatment of the Toso/FcμR over-expressing YTS cells (cultured in cRPMI with Ig-depleted serum) did not affect killing of 721.221 cells (Fig. 6D). These findings indicate that Toso/FcμR ligation does not affect the cytotoxicity of NK cells. We also tested cytokine release (TNF-α, IFN-γ) and degranulation of both NK cells and Toso/FcμR over-expressing YTS cells and found that addition of mIgM did not promote cytokine release or degranulation from either cell type (data not shown).
Binding of IgM to Toso/FcμR on NK cells initiates intracellular signaling
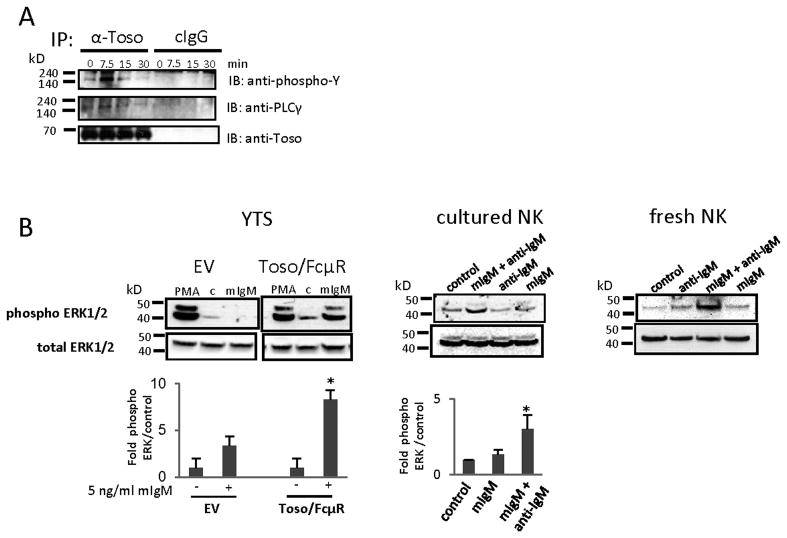
We then sought to determine if Toso/FcμR could signal at all upon ligation. YTS cells over-expressing Toso/FcμR were cultured in cRMPI with Ig-depleted serum, treated with mIgM, and lysates were immunoprecipitated using an anti-Toso mAb. Immunoprecipitates were separated on a gel and transferred to a membrane that was probed with an anti-phosphotyrosine mAb. A specific phospho-protein band of approximately 150 kD was noted and confirmed to be PLCγ by using a specific Ab to PLCγ (Fig. 7A). Moreover, the cytoplasmic tail of Toso/FcμR contains at least three consensus sites specific for binding to molecules that modulate the ERK signaling pathway as determined by Prosite scanning (Eukaryotic Linear Motif resources); therefore we tested the ability of Toso/FcμR to activate ERK. YTS cells cultured in cRPMI with FCS were washed and cultured without FCS for 16 h prior to treatment with mIgM for 30 min. Cell lysates were separated and subjected to Western blotting. mIgM treatment resulted in phosphorylation of ERK1/2 in cells overexpressing Toso/FcμR, but not in control (EV) cells (Fig. 7B, left). To investigate the signaling capacity of IgM-bound Toso/FcμR in primary cells, we isolated NK cells from PBMC and expanded them in medium containing human serum and IL-2 for two weeks. Cells were then cultured overnight without serum and IL-2 to induce Toso/FcμR expression (see Fig. 3), then treated with PBS alone (control), mIgM, anti-mIgM mAb, or mIgM plus anti-mIgM mAb. mIgM plus anti-IgM mAb-treated cells induced phosphorylation of ERK1/2 (Fig. 7B, center). Similar results were also obtained using NK cells freshly isolated from PBMC and cultured in Ig-depleted serum with no IL-2 for 16 h (Fig. 7B, right). Flow cytometry of these freshly isolated cells indicates Toso/FcμR is expressed and can bind exogenous mIgM (data not shown). Thus, the binding of IgM to Toso/FcμR generates intracellular signals indicating that Toso/FcμR has the potential to regulate cellular processes.
Figure 7. Toso/FcμR signaling.
A, YTS-Toso/FcμR cells were incubated with mIgM (10 μg/ml) for the indicated times. Cell lysates were immunoprecipitated with anti-Toso mAb (5 μg/ml) or control IgG2b Ab, followed by immunoblotting with anti-pTyr, anti-PLCγ, or anti-Toso Abs. B, YTS-Toso/FcμR, YTS-EV, NK cells cultured for one week, or freshly isolated NK cells were incubated with mIgM (5 μg/ml), anti-mIgM mAb (5 μg/ml), or mIgM plus anti-mIgM mAb for 30 min. Cell lysates were immunoblotted with anti-phosphorylated ERK1/2 (pERK1/2) or total ERK. Bands were quantified, intensities normalized to total ERK, and calibrated to values from untreated cells. Data are shown as mean + SEM from seven independent experiments. Statistical analysis was done by two-tailed unpaired student’s t-test. *p<0.05.
DISCUSSION
There is lingering controversy as to whether Toso functions to inhibit apoptosis (16) or to bind IgM (15); there is no evidence that it does both. In contrast to recently reported results (16), we provide further evidence that Toso is an IgM receptor and that its expression is not inherently anti-apoptotic; hence, we agree that it might be more appropriate to refer to it as FcμR (15). Little is known about Toso/FcμR expression by NK cells. Here we show that all circulating NK cells express Toso/FcμR and exposure of these cells to IL-2 leads to rapid down-regulation of Toso/FcμR expression that correlates with the loss of ability to bind IgM, an observation that also holds true for T cells. We analyzed the role Toso/FcμR plays in activating NK cells and found that Toso/FcμR, unlike the CD16 IgG receptor, does not mediate NK cell cytolytic activity; however, ligation of Toso/FcμR with IgM clearly leads to the phosphorylation of PLC γ and ERK.
Toso expression was originally described to be important for regulating AICD due to its inhibition of apoptosis induced by TNF receptor family members in human and mouse T cells (12, 13), a viewpoint that is supported by the recent publication of Nguyen et al. (16). These reports regarding the anti-apoptotic function of Toso/FcμR are disputed by studies showing that Toso/FcμR functions as a specific IgM receptor whose expression does not inhibit Fas ligand-induced apoptosis. Kubagawa et al. (15) showed that Toso/FcμR blocks Fas-mediated apoptosis in Jurkat T cells only when an agonistic IgM anti-Fas Ab was used, a result we confirmed (Fig. 2A, B) and extended to primary NK cells (Fig. 2C). As IgM lacking anti-Fas activity is not anti-apoptotic (Fig. 2B), the anti-apoptotic effect of IgM anti-Fas Ab must be due to the combined binding (cross-linking) of Fas and Toso/FcμR (15). As it has been shown that internalization and compartmentalization of Fas is important for apoptosis signaling (6, 11), it may be that the simultaneous binding of IgM anti-Fas Ab to Toso/FcμR prevents the internalization of the trimeric Fas receptor complex, or internalization of Fas in conjunction with Toso/FcμR alters the trafficking pattern of Fas such that anti-apoptotic signaling is suppressed. Of potential relevance is the recent observation that Toso/FcμR transports bound IgM to lysosomes for degradation (25). Alternatively, the complexing of Fas with Toso/FcμR through IgM anti-Fas Ab could alter or amplify the signals generated by binding to Toso/FcμR (Fig. 7) such that they now interfere with apoptosis. The fact that large excesses of non-specific IgM do not interfere with the anti-apoptotic effect of IgM anti-Fas Ab (Fig. 2B and (15)), indicates that the multivalent binding or Toso/FcμR and Fas conveys a large avidity advantage compared to the binding of Toso/FcμR alone. Also, we acknowledge that Toso/FcμR bound non-anti-Fas IgM, under the right circumstances, could generate anti-apoptotic signals. This could explain why Nguyen et al. (16) have observed that Toso/FcμR expression alone can interfere with death receptor signaling as their analyses were done in the presence of serum that likely contained IgM.
The expression of Toso/FcμR by NK cells provides the potential to greatly expand the arsenal of receptor specificities available to NK cells for target cell recognition, much as CD16 does for ADCC. Therefore, we examined if IgM Ab reactive with target cells could mediate ADCC, but found no evidence for this (Fig. 5A); moreover we found no evidence that IgM bound to Toso/FcμR can mediate redirected lysis (Fig. 5C) or potentiate natural cytotoxicity (Fig. 6). Furthermore, we found no evidence that Toso/FcμR ligation affects secretion of TNFα or IFNγ (data not shown). However, Toso/FcμR is known to be phosphorylated upon binding IgM (15). Investigation of the cytoplasmic tail of Toso/FcμR indicates a number of potential signaling motifs (ELM resources). These include the motif DDYINV at the very end of the cytoplasmic tail, which fits criteria for both a hemITAM (26, 27) and an ITT signaling motif (28), the prevailing characteristics for both of which are Asp or Glu residues preceding the Tyr residue in the motif. Signaling downstream of NKp65, a C-lectin type receptor containing a hemITAM, increases the cytotoxicity and IFNγ secretion of NK cells (29). ITT motifs are known to augment Grb2-mediated and PI3K signaling and receptors with this motif can act as co-stimulatory receptors that are necessary for the two-signal hypothesis of lymphocyte activation (28). Of direct relevance, when this motif was removed from ectopically expressed Toso in Jurkat T cells, protection from Fas-induced apoptosis was decreased (13).
Although we are unable to detect any effector functions, IgM binding to Toso/FcμR clearly is stimulatory for NK cells. Our results show that IgM binding to Toso/FcμR expressed endogenously on primary NK cells, and ectopically on the NK cell line YTS, results in PLCγ and ERK phosphorylation. PLCγ contains a pleckstrin homology domain, an SH3 domain, and two SH2 domains, indicating the possibility for extensive interactions with PI3 kinase and Src family kinases (see (30) for review). Thus, phosphorylation of PLCγ indicates that Toso/FcμR could influence multiple signaling events in NK cells. Our work indicates maximal stimulation of ERK after 30 min in the presence of IgM. Recently, Vire et al. reported very rapid internalization of Toso/FcμR after IgM binding in HeLa cells over-expressing the receptor and in CLL-derived B cells (25). Our signaling studies are not in contradiction to this, as there are numerous reports indicating that signaling events can continue from endocytic vesicles (for review see (31)).
We show that Toso/FcμR expression by NK cells is remarkably sensitive to down-regulation by IL-2 exposure, and this down-regulation occurs regardless of the presence of IgM (Fig. 3). Thus, if the receptor is rapidly internalized upon ligand binding (25), either the receptor is recycled or more is produced such that the presence of Toso/FcμR is maintained on the surface of NK cells, at least until the NK cells are primed with IL-2. By using appropriate inhibitors, we deduce that the IL-2 mediated regulation occurs via the STAT5 signaling pathway rather than the PI3K, both of which are utilized in IL-2 signaling. We examined if other γc cytokines might also be able to down-modulate the expression of Toso/FcμR. Indeed, IL-7 and IL-15 were able to down-regulate Toso/FcμR in NK cells (data not shown). Further, we also observed similar Toso/FcμR down-regulation by CD4 and CD8 T cells cultured with IL-2 (Fig. 4). Ex vivo analysis of T cell subsets indicated that activation in vivo correlates with Toso/FcμR down-regulation as naïve CD4+ T cells expressed higher levels of Toso/FcμR than TEM cells. If Toso/FcμR functions as an activating receptor in NK and T cells, then down-regulation of Toso/FcμR by IL-2 might be a check point in regulating the effector function of T and NK cells. This is consistent with reports that TLR-induced signaling in B cells and anti-CD3 mAb treatment of T cells both result in down-modulation of Toso/FcμR expression (15, 25), though the mechanisms of down-regulation in these cell types was not explored. On the other hand, if downstream signals from Toso/FcμR have an inhibitory effect, then IL-2 mediated down-regulation of Toso/FcμR might help T and NK cells overcome an inhibitory threshold for activation. Clearly the signal transduction of IgM-Toso/FcμR interaction needs to be studied in detail to address the function of Toso/FcμR in modulating the T and NK cell immune response.
Acknowledgments
We thank the NIH Clinical Center Department of Transfusion Medicine for blood samples from healthy human donors collected under protocol 99CC-0168. We thank Seung-Chul Choi, Giovanna Peruzzi, and Linjie Tian for their critical reading of the manuscript.
Abbreviations used in this article
- AICD
activation induced cell death
- DISC
death inducting signaling complex
- FADD
Fas-associated death domain protein
- FAIM3
Fas apoptosis inhibitory molecule
- LV
lentivirus
- mIgM
mouse IgM
- TNFR
tumor necrosis factor α receptor
Footnotes
This work was supported by the intramural program of the National Institute of Allergy and Infectious Diseases and the intramural program of the National Heart, Blood, and Lung Institute.
Y.M., S.N., F.B., K.K., J.W., and J.E.C. designed the research; Y.M., S.N., S.S., and J.W. performed the experiments; Y.M., S.N., R.C., F.B., K.K., J.W., and J.E.C. analyzed results; and F.B., J.W., K.K., and J.E.C. wrote the paper.
References
- 1.Ramaswamy M, Dumont C, Cruz AC, Muppidi JR, Gomez TS, Billadeau DD, Tybulewicz VL, Siegel RM. Cutting edge: Rac GTPases sensitize activated T cells to die via Fas. J Immunol. 2007;179:6384–6388. doi: 10.4049/jimmunol.179.10.6384. [DOI] [PubMed] [Google Scholar]
- 2.Ju ST, Panka DJ, Cui H, Ettinger R, el-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 3.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 4.Sytwu HK, Liblau RS, McDevitt HO. The roles of Fas/APO-1 (CD95) and TNF in antigen-induced programmed cell death in T cell receptor transgenic mice. Immunity. 1996;5:17–30. doi: 10.1016/s1074-7613(00)80306-4. [DOI] [PubMed] [Google Scholar]
- 5.Van Parijs L, Abbas AK. Role of Fas-mediated cell death in the regulation of immune responses. Curr Opin Immunol. 1996;8:355–361. doi: 10.1016/s0952-7915(96)80125-7. [DOI] [PubMed] [Google Scholar]
- 6.Vignaux F, Vivier E, Malissen B, Depraetere V, Nagata S, Golstein P. TCR/CD3 coupling to Fas-based cytotoxicity. J Exp Med. 1995;181:781–786. doi: 10.1084/jem.181.2.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng L, Fisher G, Miller RE, Peschon J, Lynch DH, Lenardo MJ. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 8.Lenardo MJ. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 9.Ortaldo JR, Mason AT, O’Shea JJ. Receptor-induced death in human natural killer cells: involvement of CD16. J Exp Med. 1995;181:339–344. doi: 10.1084/jem.181.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poggi A, Massaro AM, Negrini S, Contini P, Zocchi MR. Tumor-induced apoptosis of human IL-2-activated NK cells: role of natural cytotoxicity receptors. J Immunol. 2005;174:2653–2660. doi: 10.4049/jimmunol.174.5.2653. [DOI] [PubMed] [Google Scholar]
- 11.Yamauchi A, Taga K, Mostowski HS, Bloom ET. Target cell-induced apoptosis of interleukin-2-activated human natural killer cells: roles of cell surface molecules and intracellular events. Blood. 1996;87:5127–5135. [PubMed] [Google Scholar]
- 12.Hitoshi Y, Lorens J, Kitada SI, Fisher J, LaBarge M, Ring HZ, Francke U, Reed JC, Kinoshita S, Nolan GP. Toso, a cell surface, specific regulator of Fas-induced apoptosis in T cells. Immunity. 1998;8:461–471. doi: 10.1016/s1074-7613(00)80551-8. [DOI] [PubMed] [Google Scholar]
- 13.Song Y, Jacob CO. The mouse cell surface protein TOSO regulates Fas/Fas ligand-induced apoptosis through its binding to Fas-associated death domain. J Biol Chem. 2005;280:9618–9626. doi: 10.1074/jbc.M413609200. [DOI] [PubMed] [Google Scholar]
- 14.Shima H, Takatsu H, Fukuda S, Ohmae M, Hase K, Kubagawa H, Wang JY, Ohno H. Identification of TOSO/FAIM3 as an Fc receptor for IgM. Int Immunol. 2010;22:149–156. doi: 10.1093/intimm/dxp121. [DOI] [PubMed] [Google Scholar]
- 15.Kubagawa H, Oka S, Kubagawa Y, Torii I, Takayama E, Kang DW, Gartland GL, Bertoli LF, Mori H, Takatsu H, Kitamura T, Ohno H, Wang JY. Identity of the elusive IgM Fc receptor (FcmuR) in humans. J Exp Med. 2009;206:2779–2793. doi: 10.1084/jem.20091107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen XH, Lang PA, Lang KS, Adam D, Fattakhova G, Foger N, Kamal MA, Prilla P, Mathieu S, Wagner C, Mak T, Chan AC, Lee KH. Toso regulates the balance between apoptotic and nonapoptotic death receptor signaling by facilitating RIP1 ubiquitination. Blood. 2011;118:598–608. doi: 10.1182/blood-2010-10-313643. [DOI] [PubMed] [Google Scholar]
- 17.Igarashi T, Wynberg J, Srinivasan R, Becknell B, McCoy JP, Jr, Takahashi Y, Suffredini DA, Linehan WM, Caligiuri MA, Childs RW. Enhanced cytotoxicity of allogeneic NK cells with killer immunoglobulin-like receptor ligand incompatibility against melanoma and renal cell carcinoma cells. Blood. 2004;104:170–177. doi: 10.1182/blood-2003-12-4438. [DOI] [PubMed] [Google Scholar]
- 18.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 19.Kovanen PE, Leonard WJ. Cytokines and immunodeficiency diseases: critical roles of the gamma(c)-dependent cytokines interleukins 2, 4, 7, 9, 15, and 21, and their signaling pathways. Immunol Rev. 2004;202:67–83. doi: 10.1111/j.0105-2896.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- 20.Bouillet P, O’Reilly LA. CD95, BIM and T cell homeostasis. Nat Rev Immunol. 2009;9:514–519. doi: 10.1038/nri2570. [DOI] [PubMed] [Google Scholar]
- 21.Mitra DK, De Rosa SC, Luke A, Balamurugan A, Khaitan BK, Tung J, Mehra NK, Terr AI, O’Garra A, Herzenberg LA, Roederer M. Differential representations of memory T cell subsets are characteristic of polarized immunity in leprosy and atopic diseases. Int Immunol. 1999;11:1801–1810. doi: 10.1093/intimm/11.11.1801. [DOI] [PubMed] [Google Scholar]
- 22.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 23.Shibuya A, Sakamoto N, Shimizu Y, Shibuya K, Osawa M, Hiroyama T, Eyre HJ, Sutherland GR, Endo Y, Fujita T, Miyabayashi T, Sakano S, Tsuji T, Nakayama E, Phillips JH, Lanier LL, Nakauchi H. Fc alpha/mu receptor mediates endocytosis of IgM-coated microbes. Nat Immunol. 2000;1:441–446. doi: 10.1038/80886. [DOI] [PubMed] [Google Scholar]
- 24.Kaetzel CS. Polymeric Ig receptor: defender of the fort or Trojan horse? Curr Biol. 2001;11:R35–38. doi: 10.1016/s0960-9822(00)00041-5. [DOI] [PubMed] [Google Scholar]
- 25.Vire B, David A, Wiestner A. TOSO, the Fc{micro} Receptor, Is Highly Expressed on Chronic Lymphocytic Leukemia B Cells, Internalizes upon IgM Binding, Shuttles to the Lysosome, and Is Downregulated in Response to TLR Activation. J Immunol. 2011 doi: 10.4049/jimmunol.1100532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson SP, Herbert JM, Pollitt AY. GPVI and CLEC-2 in hemostasis and vascular integrity. J Thromb Haemost. 2010;8:1456–1467. doi: 10.1111/j.1538-7836.2010.03875.x. [DOI] [PubMed] [Google Scholar]
- 27.Mourao-Sa D, Robinson MJ, Zelenay S, Sancho D, Chakravarty P, Larsen R, Plantinga M, Van Rooijen N, Soares MP, Lambrecht B, Reis e Sousa C. CLEC-2 signaling via Syk in myeloid cells can regulate inflammatory responses. Eur J Immunol. 2011;41:3040–3053. doi: 10.1002/eji.201141641. [DOI] [PubMed] [Google Scholar]
- 28.Engels N, Wienands J. The signaling tool box for tyrosine-based costimulation of lymphocytes. Curr Opin Immunol. 2011;23:324–329. doi: 10.1016/j.coi.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Spreu J, Kuttruff S, Stejfova V, Dennehy KM, Schittek B, Steinle A. Interaction of C-type lectin-like receptors NKp65 and KACL facilitates dedicated immune recognition of human keratinocytes. Proc Natl Acad Sci U S A. 2010;107:5100–5105. doi: 10.1073/pnas.0913108107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carpenter G, Ji Q. Phospholipase C-gamma as a signal-transducing element. Exp Cell Res. 1999;253:15–24. doi: 10.1006/excr.1999.4671. [DOI] [PubMed] [Google Scholar]
- 31.Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]