Abstract
Objective
Increased plasma concentrations of the endogenous nitric oxide (NO) synthase inhibitor, asymmetric dimethylarginine (ADMA), decreased arginine bioavailability, and mitochondrial dysfunction have been reported in adult sepsis. We studied whether ADMA, arginine, and carnitine metabolism (a measure of mitochondrial dysfunction) are altered in pediatric sepsis and whether these are clinically useful biomarkers.
Design
Prospective, observational study
Setting
Pediatric intensive care unit at an academic medical center
Patients
Ninety patients ≤ 18 years-old—30 with severe sepsis or septic shock compared with thirty age-matched febrile and thirty age-matched healthy controls.
Interventions
None.
Measurements and Main Results
Plasma ADMA and whole blood arginine, citrulline, ornithine, and acylcarnitine:free carnitine (AC:FC) ratio were measured daily for septic patients and once for controls using tandem mass spectrometry. Plasma ADMA concentration (median, IQR µmol/L) on day 1 was lower in severe sepsis and septic shock (0.38, 0.30–0.56) compared with febrile (0.45, 0.40–0.59) and healthy (0.60, 0.54–0.67) controls (p<0.001), though decreased ADMA was predominantly found in neutropenic patients. Day 1 arginine was lower in septic (10, IQR 7–20 µmol/L) compared with healthy patients (32, IQR 23–40; p<0.001), and the arginine:ornithine ratio was decreased in sepsis, indicating increased arginase activity (an alternative pathway for arginine metabolism). The arginine:ADMA and AC:FC ratios did not differ between septic and control patients. ADMA was inversely correlated with organ dysfunction by PELOD score (r=−0.50, p=0.009), interleukin-6 (r=−0.55, p=0.01), and interleukin-8 (r=−0.52, p=0.03) on admission. Arginine, arginine:ADMA, and AC:FC were not associated with organ dysfunction or outcomes.
Conclusions
ADMA was decreased in pediatric sepsis and was inversely associated with inflammation and organ dysfunction. This suggests that inhibition of NO synthase by ADMA accumulation is unlikely to impact sepsis pathophysiology in septic children despite decreased arginine bioavailability. We did not find an association of ADMA with altered carnitine metabolism, nor were ADMA, arginine, and AC:FC useful as clinical biomarkers.
Keywords: Nitric oxide; nitric oxide synthase; arginine; carnitine; sepsis; intensive care units, pediatric
INTRODUCTION
The sepsis syndrome remains a major problem in pediatrics, with nearly 42,000 hospital admissions and $2 billion in health care costs in the United States annually (1). To improve outcomes and target therapies in pediatric sepsis, we must better understand biochemical changes underlying the septic response, as well as developmental differences specific to children.
The nitric oxide (NO) pathway has long been recognized in the pathogenesis of sepsis, with effects on cardiovascular, immune, and mitochondrial function, though its precise role remains poorly understood (2). NO is synthesized from L-arginine by a family of NO synthase (NOS) isoforms (3). In sepsis, an up-regulation of inducible NOS leads to an increase in systemic NO production (2). However, attempts to inhibit NOS did not improve organ function and led to increased mortality (4–6). Moreover, decreased activity of constitutive NOS isoforms may cause areas of NO deficiency and contribute to endothelial, microcirculatory, and organ dysfunction (7). Blood arginine concentration and de novo arginine synthesis are also decreased in sepsis (8–10), which may further limit NO bioavailability (11).
Endogenous NOS inhibitors, such as asymmetric dimethylarginine (ADMA), may further influence NO availability in sepsis, and their importance has been increasingly recognized in disorders of endothelial dysfunction (12). ADMA is synthesized by methylation of arginine residues on proteins, released into circulation during protein turnover, and degraded by dimethylarginine dimethylaminohydrolase (DDAH) with renal and hepatic elimination (12). ADMA competitively inhibits all NOS isoforms and competes with arginine for intracellular transport via the y+ cationic transporter (12). Thus, accumulation of ADMA restricts NO synthesis and impairs NO-mediated endothelial and vascular function (13). In adults with sepsis and organ dysfunction, elevated plasma ADMA is associated with an increased risk of mortality (13–15). Furthermore, low arginine combined with elevated ADMA may additively limit NO production, and a low arginine:ADMA ratio has been linked to vascular dysfunction in rats (16) and adults with septic shock (13). However, there are no data on the role of ADMA in pediatric sepsis. We hypothesized that plasma ADMA would be increased in children with sepsis and would be associated with severity of organ dysfunction, inflammation, and adverse outcomes.
ADMA has also been linked to mitochondrial dysfunction (17), an important mechanism of organ failure in sepsis (18). ADMA causes uncoupling of the NOS dimer, resulting in increased superoxide-mediated formation of peroxynitrite, nitration of mitochondrial proteins, and diminished ATP production (19, 20). In animal models, altered carnitine metabolism, specifically an elevated blood acylcarnitine:free carnitine (AC:FC) ratio, is reflective of intra-mitochondrial increases in the ratio of acyl-coenzyme A (acyl-CoA) to free CoA (21, 22). Alterations in these ratios are associated with decreased oxidative respiration and have been linked to impaired NO signaling (21). We therefore hypothesized that increased ADMA in pediatric sepsis would result in an elevated AC:FC ratio as a measure of mitochondrial dysfunction and would be associated with reduced NOS activity and greater organ dysfunction.
The objective of this study was to determine changes in plasma ADMA, arginine bioavailability, and carnitine metabolism in pediatric severe sepsis and septic shock, and to investigate the utility of ADMA, arginine, and AC:FC as potential biomarkers in pediatric sepsis.
MATERIALS AND METHODS
This prospective, observational study was performed in a 42-bed pediatric intensive care unit (PICU) at an academic medical center between May 2009 and December 2010. The study was approved by the local institutional review board and informed consent/assent was obtained.
Patient Population
Consecutively admitted patients to the PICU who were ≤ 18 years-old and met criteria for severe sepsis or septic shock as defined by the International Pediatric Consensus Conference (23) were eligible (septic patients). Exclusion criteria were cardiac arrest before admission, treatment with inhaled NO or sildenafil, supplementation with arginine, citrulline, or carnitine, a metabolic, urea cycle, or mitochondrial disorder, chronic renal or hepatic impairment, unrepaired cyanotic heart disease or single-ventricle anatomy, major surgery within the previous 72 hours, transfer from another facility with ongoing sepsis >24 hours, and prior study enrollment. Age-matched control patients from the same hospital were enrolled into two groups: 1) febrile (temperature ≥ 38.5°C) patients evaluated for infection without severe sepsis or shock (febrile controls) and 2) afebrile patients without evidence of an active infectious or inflammatory condition (e.g. patients undergoing endoscopy for chronic abdominal pain with normal results; healthy controls). Although various medical conditions have been reported to affect plasma ADMA levels, only disorders also listed for septic patients (e.g. chronic kidney injury) were used to exclude controls.
Data Collection
Clinical data were abstracted from the medical record onto a standardized form, and included comorbid conditions, source of infection, routine laboratory results, daily maximum inotrope score (IS) (24), mechanical ventilation, length of stay (LOS), nutritional source, and vital status. Organ dysfunction was classified using consensus criteria (23) and monitored for 28 days. Pediatric Index of Mortality (PIM)-2 score (25) and daily Pediatric Logistic Organ Dysfunction (dPELOD) scores (26) were calculated. To determine the association of ADMA with recovery from inotrope-dependent shock, septic patients were classified as having “rapid recovery” if IS was ≤ 5 or “slow recovery” if IS remained > 5 on day 3 or later.
Study Measurements
For septic patients, blood was collected within 24 hours of admission (day 1), and then daily for seven days or PICU discharge, whichever came first, for measurement of ADMA, NO pathway intermediates (arginine and citrulline), ornithine, and carnitine profile. Arginine is the amino acid substrate for NOS, citrulline is produced in a stoichiometric 1:1 relationship with NO, and ornithine is the byproduct of arginine metabolism by the enzyme arginase. Interleukin (IL)-6, IL-8, and C-reactive protein (CRP) were measured as markers of inflammation. Brain natriuretic peptide (BNP) was measured as a marker of cardiac dysfunction. If consent could not be obtained within 24 hours of admission, study labs were measured on discarded blood from the earliest clinical samples drawn on day 1. Due to limited availability of discarded blood and the requirement that blood draws be timed with clinical sampling, not all study labs were measured on every day. If renal replacement therapy was initiated, no further blood was collected since hemodialysis lowers plasma ADMA concentration (27). For control patients, a single measurement of ADMA, arginine, citrulline, ornithine, and carnitine profile was performed.
Blood specimens were processed upon collection in the main hospital laboratory. CRP and BNP were measured in plasma using a Beckman DxC600 and Siemens Centaur analyzer, respectively. Remaining samples were then stored at −70°C for batched analysis. Plasma ADMA was measured by tandem mass spectrometry (LC-MS/MS) as previously described (28), except that ADMA-d7 was incorporated as the internal standard, sample supernatant was dried to completion and reconstituted in mobile phase, and a Phenomenex Luna Silica column was used. From serum, IL-6 was measured using an enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN), and IL-8 using an electrochemiluminescent detection system (Meso-Scale Discovery, Gaithersburg, MD). Whole blood for arginine, citrulline, orthinine, AC, and FC was collected on filter paper, dried and stored at −70°C, and measured by LC-MS/MS (29). Acylcarnitine values were combined sum of short, medium, and long chain AC groups, and the AC: FC ratio was calculated as previously described (22).
Outcome Measures
The primary outcome was the difference in plasma ADMA concentration between septic patients and febrile and healthy controls. Sample size calculation based on two studies of plasma ADMA in critically ill adults (14, 15) determined that 30 septic patients, 30 febrile controls, and 30 healthy controls were needed to detect a difference in ADMA of 0.3 µmol/L with an alpha of 0.05 and 80% power. Secondary outcomes were differences in NO pathway intermediates and AC:FC between groups and the association of ADMA, arginine, and AC:FC with measures of organ dysfunction, inflammation, and clinical outcome. Three ratios were also analyzed—arginine:ADMA as a marker of arginine availability to NOS (13), citrulline:arginine as a surrogate for NOS activity (30), and arginine:ornithine as a measure of arginase activity (31).
Statistical Analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS Version 12.1, Chicago, IL) and SAS 9.2 (SAS institute Inc., Cary, NC). Since data were not normally distributed, we reported medians with interquartile range (IQR) and used Wilcoxon rank-sum or Kruskal-Wallis tests to compare continuous and chi-square or Fisher’s exact test for dichotomous variables. Longitudinal changes of repeated measurements in septic patients were analyzed using linear mixed modeling with a random intercept. Associations of ADMA, arginine, arginine:ADMA, and AC:FC with measures of organ dysfunction, inflammation, and outcome were tested using Spearman’s coefficient (rs) with Bonferroni correction for multiple comparisons. The area under the receiver operating characteristic (AUROC) curve determined the utility of ADMA to differentiate septic children from febrile controls and septic patients with rapid versus slow recovery. A post-hoc analysis of patients with neutropenia (absolute neutrophil count < 500 thous/µL) was performed after an association of ADMA with white blood cell count (WBC) was observed using two-way ANOVA. P-values ≤0.05 were considered significant.
RESULTS
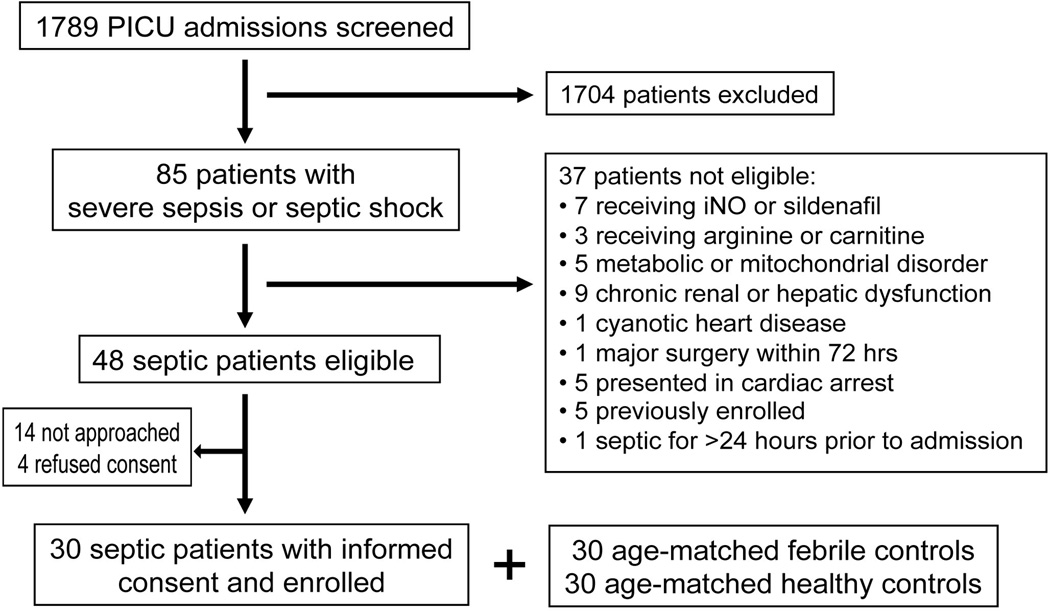
Of the 1789 patients consecutively admitted to the PICU, 85 (4.8%) met criteria for severe sepsis or septic shock. Thirty-seven of these patients were excluded, with 30 (63%) of the remaining 48 eligible septic patients consenting to enrollment (Figure 1). Thirty age-matched febrile and 30 age-matched healthy control patients were also enrolled. Patient characteristics are shown in Table 1. There was no difference in age, gender, or ethnicity between groups. Septic patients had higher PIM-2 and PELOD scores and a longer hospital LOS (all p<0.001).
Figure 1.
Patient screening and study enrollment
Table 1.
Patient characteristicsa
| Characteristic | Septic n = 30 |
Febrile Controls n = 30 |
Healthy Controls n = 30 |
|---|---|---|---|
| Age (years) | 7.2 (2.4–15.4) | 9.2 (1.8–15.7) | 8.2 (3.5–14.6) |
| Gender, % male | 50 | 40 | 70 |
| Ethnicity, n (%) | |||
| White, non-hispanic | 9 (30) | 8 (27) | 17 (57) |
| Black | 6 (20) | 6 (20) | 4 (13) |
| Hispanic | 14 (47) | 13 (43) | 5 (17) |
| Asian | 0 | 1 (3) | 3 (10) |
| Middle Eastern | 0 | 1 (3) | 0 |
| Indian | 1 (3) | 0 | 0 |
| Other/Unknown | 0 | 1 (3) | 1 (3) |
| Comorbid Conditions, n (%) | |||
| None | 4 (14) | 15 (50) | 3 (10) |
| Asthma | 1 (3) | 0 | 1 (3) |
| Cerebral palsy | 3 (10) | 0 | 0 |
| Malignancy | 16 (53) | 3 (10) | 0 |
| Bone marrow transplant | 5 (17) | 0 | 0 |
| Neutropenia | 13 (43) | 2 (6) | 0 |
| Hematologic (benign) | 1 (3) | 3 (10) | 0 |
| Gastrointestinal | 0 | 0 | 19 (64)b |
| Liver transplant | 2 (6) | 0 | 1 (3) |
| Obstructive sleep apnea | 0 | 0 | 4 (13) |
| Other | 3 (10) | 9 (30) | 2 (7) |
| Type of Infection, n (%) | |||
| Viral | 8 (27)c | 12 (40) | na |
| Bacterial | 16 (53)c,d | 11 (37)c | na |
| Fungal | 1 (3) | 2 (6) | na |
| Unknown | 7 (23)e | 5 (17) | na |
| PIM-2 | 6.8 (1.5–9.6) | 1.0 (0.5–1.6) | 0.8 (0.8–0.9) |
| PELOD, day 1 | 20 (11–21) | 0 (0–0) | 0 (0–0) |
| Hospital LOS (days) | 11 (8–18) | 1 (1–4) | 1 (1–1) |
| Hospital mortality, n (%) | 2 (7) | 0 | 0 |
na = not applicable; PIM-2, Pediatric Index of Mortality-2 score; PELOD, Pediatric Logistic Organ Dysfunction score; LOS, length of stay
Median values (interquartile range), unless indicated
All patients underwent endoscopy for chronic abdominal pain with normal findings
2 patients diagnosed with concurrent viral and bacterial infections (influenza A and staphylococcus aureus; respiratory syncytial virus and streptococcus pneumoniae)
14/16 septic patients and 6/11 febrile controls had ≥ 1 positive culture for a bacterial pathogen
Includes patients with “culture-negative” severe sepsis and septic shock
ADMA, NO pathway intermediates, and arginine:ADMA ratio
The plasma ADMA concentration (median, IQR µmol/L) on day 1 was lower in septic patients (0.38, 0.30–0.56) compared with febrile (0.45, 0.40–0.59) and healthy (0.60, 0.54–0.67) controls (p<0.001; Table 2). Arginine and citrulline on day 1 were also significantly different across the three groups (Table 2). However, arginine and citrulline (median, IQR µmol/L) were only lower in septic patients (arginine: 10, 7–20; citrulline: 8, 4–10) compared with healthy controls (arginine: 32, 23–40; citrulline: 16, 13–19; both p<0.001); there was no difference compared with febrile controls (both p>0.05).
Table 2.
Study measurements for septic and control patients
| Laboratorya | n | Septicb Median (IQR) |
n | Febrile Controls Median (IQR) |
n | Healthy Controls Median (IQR) |
pc |
|---|---|---|---|---|---|---|---|
| ADMA, day 1 | 26 | 0.38 (0.30–0.56) | 30 | 0.45 (0.40–0.59) | 30 | 0.60 (0.54–0.67) | <0.001d |
| ADMA, day 2 | 25 | 0.43 (0.35–0.54) | <0.001 | ||||
| Arginine, day 1 | 12 | 10 (7–20) | 30 | 13 (8–26) | 30 | 32 (23–40) | <0.001 |
| Arginine, day 2 | 24 | 10 (7–27) | <0.001 | ||||
| Citrulline, day 1 | 12 | 8 (4–10) | 30 | 9 (6–12) | 30 | 16 (13–19) | <0.001 |
| Citrulline, day 2 | 24 | 8 (4–17) | <0.001 | ||||
| Ornithine, day 1 | 12 | 30 (16–59) | 30 | 23 (19–72) | 30 | 45 (35–60) | 0.012 |
| Ornithine, day 2 | 24 | 43 (19–81) | 0.05 | ||||
| Arg:ADMA, day 1 | 12 | 33.2 (15.1–65.7) | 30 | 26.7 (17.8–63.4) | 30 | 54.3 (44.7–67.3) | 0.051 |
| Arg:ADMA, day 2 | 23 | 24.7 (16.9–67.7) | 0.06 | ||||
| Citr:Arg, day 1 | 12 | 0.52 (0.46–1.05) | 30 | 0.59 (0.39–0.76) | 30 | 0.52 (0.42–0.70) | 0.82 |
| Citr:Arg, day 2 | 24 | 0.58 (0.40–1.10) | 0.84 | ||||
| Arg:Orn, day 1 | 12 | 0.44 (0.24–0.56) | 30 | 0.53 (0.37–0.68) | 30 | 0.63 (0.52 (0.79) | 0.03 |
| Arg:Orn, day 2 | 24 | 0.35 (0.25–0.52) | 0.002 | ||||
| AC:FC, day 1 | 12 | 1.25 (0.44–1.98) | 30 | 0.78 (0.72–1.27) | 30 | 0.93 (0.77–1.17) | 0.67 |
| AC:FC, day 2 | 24 | 1.33 (0.60–2.03) | 0.46 | ||||
ADMA, asymmetric dimethylarginine; Arg:ADMA, arginine:asymmetric dimethylarginine ratio; Citr:Arg, citrulline:arginine ratio; Arg:Orn, arginine:ornithine ratio; AC:FC, acylcarnitine:free carnitine ratio
Units are µmol/L
Since not all measurements were available on every study day for septic patients, day 1 and 2 values are reported for comparison
Comparisons made using Kruskal-Wallis test for day 1 and day 2 values for septic patients versus control groups
ADMA (day 1) between septic and febrile patients, only, p=0.056; ADMA between septic and healthy patients, only, p<0.001; ADMA between febrile and healthy patients, only, p=0.003
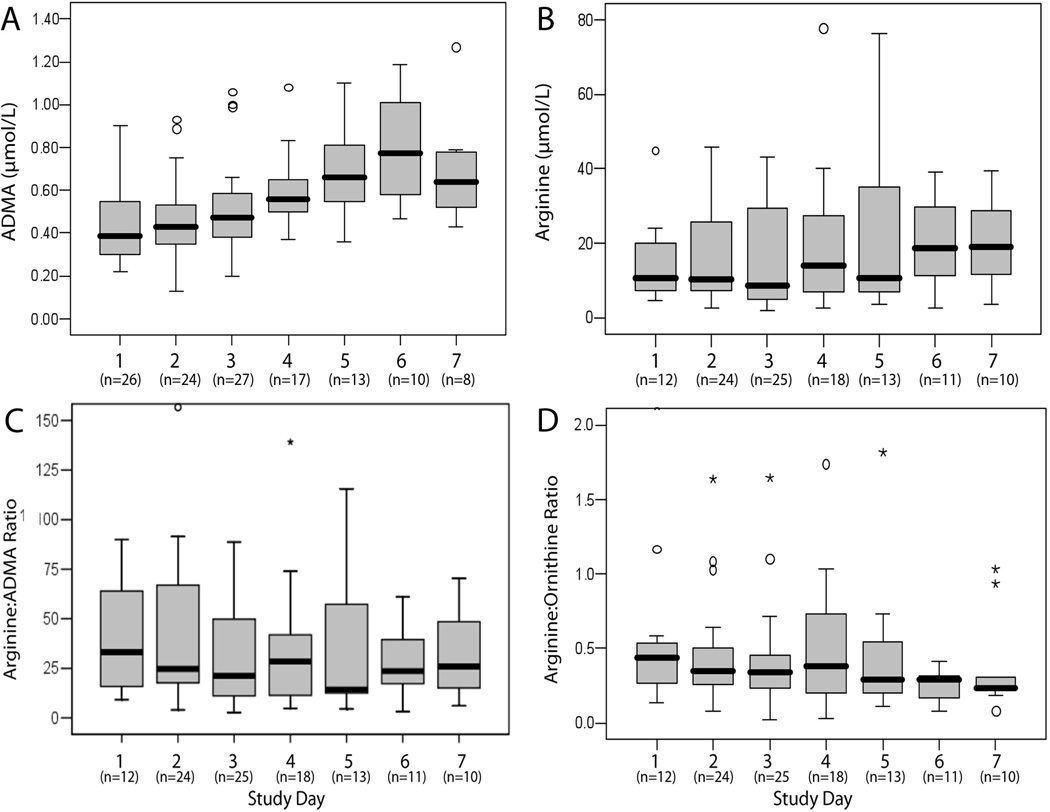
There was no difference between groups in the day 1 arginine:ADMA or citrulline:arginine ratios, though septic patients had a lower arginine:ornithine ratio than febrile and healthy control patients (p=0.03), suggesting increased arginase activity in septic patients (Table 2). Day 2 results for septic patients were also compared with control groups and yielded similar results (Table 2). Plasma ADMA increased over the study period in septic patients (p<0.0001), but there were no significant changes in arginine, arginine:ADMA, or arginine:ornithine (Figure 2) or citrulline:arginine (p=0.90) over time.
Figure 2.
Longitudinal analysis in septic patients Data are presented in box-plot analysis with central line indicating the median and grey box indicating the interquartile range. Longitudinal changes over time for septic patients were significant for asymmetric dimethylarginine (A, p<0.0001), but not for arginine (B, p=0.48), arginine:ADMA ratio (C, p=96), or arginine:ornithine ratio (D, p=0.82).
As an indirect measure of the effects of ADMA on NOS activity, we examined the relationship with ADMA levels and citrulline:arginine ratio. There was a non-significant inverse relationship between plasma ADMA and citrulline:arginine ratio for septic patients on day 1 (rs=−0.32, p=0.31). A similar trend was noted on days 3 through 6 with significance reached on day 4 (p=0.03), though this became non-significant after correction for multiple comparisons. For both febrile and healthy controls, no association was observed between ADMA and citrulline:arginine (both p>0.05).
There was a significant interaction between ADMA and WBC (p=0.01, two-way ANOVA). In the 13 septic patients who presented with neutropenia, all of whom had known malignancies, the plasma ADMA concentration (median, IQR µmol/L) on day 1 (0.30, 0.27–0.36) was lower than in the septic patients without neutropenia (0.51, 0.39–0.63; p<0.001) and remained lower through day 4. When neutropenic patients were excluded, day 1 ADMA in the septic patients did not differ from febrile controls (p=0.67) but remained lower than healthy controls (p=0.04). Although red blood cells may provide an important source for circulating ADMA (32) and could serve as a reservoir in non-neutropenic patients less likely to have concurrent anemia, ADMA was not correlated with hemoglobin in either septic (r=0.09, p=0.65) or febrile patients (r=−0.28, p=0.14; no data for healthy controls). Further comparisons between septic patients with and without neutropenia are shown in Table 3.
Table 3.
Study measurements (day 1) in septic patients with and without neutropeniaa
| Laboratorya | Septic with Neutropenia n = 13 |
Septic without Neutropenia n = 17 |
pb |
|---|---|---|---|
| ADMA (µmol/L) | 0.30 (0.27–0.36) | 0.51 (0.39–0.63) | <0.001 |
| Arginine (µmol/L) | 13 (6–20) | 8 (7–34) | 0.76 |
| Citrulline (µmol/L) | 6 (5–10) | 10 (4–13) | 0.88 |
| Ornithine (µmol/L) | 30 (20–61) | 22 (11–64) | 0.76 |
| Arg:ADMA | 41.9 (17.3–67.5) | 19.7 (14.3–53.9) | 0.53 |
| Citr:Arg | 0.54 (0.50–1.22) | 0.51 (0.40–0.86) | 0.34 |
| Arg:Orn | 0.33 (0.17–0.48) | 0.48 (0.39–0.88) | 0.20 |
| AC:FC | 0.44 (0.38–1.78) | 1.35 (0.96–2.58) | 0.40 |
| IL-6 (pg/mL) | 618 (257–5547) | 13 (0–134) | 0.001 |
| IL-8 (pg/mL) | 1115 (33–6419) | 9 (7–61) | 0.006 |
ADMA, asymmetric dimethylarginine; Arg:ADMA, arginine:asymmetric dimethylarginine ratio; Citr:Arg, citrulline:arginine ratio; Arg:Orn, arginine:ornithine ratio; AC:FC, acylcarnitine:free carnitine ratio; IL, interleukin; AC:FC, acylcarnitine:free carnitine ratio
Median values (interquartile range), unless indicated; neutropenia = absolute neutrophil count < 500 thous/µL)
Comparisons made using Fisher’s exact test
Other than higher arginine levels in septic patients receiving enteral nutrition on day 2 (enteral: 33, IQR 9–46; parenteral: 10, IQR 8–14; none: 9, IQR 7–21 µmol/L; p=0.04) and day 3 (p=0.07), there was no effect of nutrition on ADMA, arginine, or arginine:ADMA.
ADMA, arginine, and arginine:ADMA as clinical biomarkers
The AUROC curve for ADMA to differentiate between septic and febrile patients was 0.65 (95% CI 0.50–0.80). The ADMA cut-point of ≤ 0.50 µmol/L yielded a sensitivity of 69% (95% CI 56, 81%), specificity of 37% (95% CI 24, 47%), positive-predictive value of 49% (95% CI 40, 57%), and negative-predictive value of 58% (95% CI 40, 75%) to diagnose sepsis.
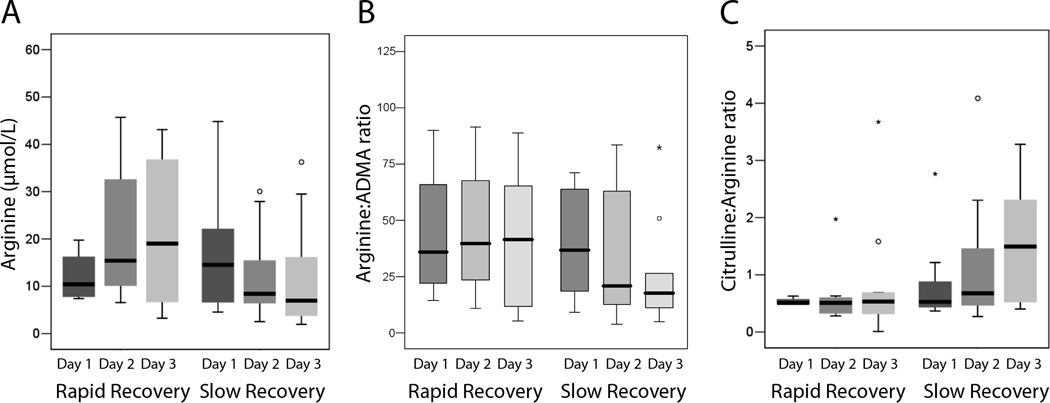
In septic patients, day 1 plasma ADMA concentration was inversely associated with day 1 PELOD score (rs=−0.50, p=0.009), IL-6 (rs=−0.55, p=0.01), and IL-8 (rs=−0.52, p=0.03) and was directly associated with WBC (rs=0.76, p=0.001). A significant association between daily ADMA and WBC was also found through study day 4 (data not shown). Day 1 plasma ADMA was not correlated with age, PIM-2 score, IS, hepatic or renal function, BNP, lactate, CRP, LOS, or inotrope- or ventilator-free days (Table 4). Neither arginine nor arginine:ADMA on day 1 were associated with measures of organ dysfunction, inflammation, or clinical outcomes (Table 4). After correction for multiple comparisons in Table 4, only the association between ADMA and WBC remained significant at the p<0.003 level. The rise in ADMA observed in septic patients over study period was not associated with changes in dPELOD, CRP, IL-6, IL-8, IS, LOS, or inotrope- or ventilator-free days (all p>0.05). There were no differences between the 11 (37%) septic patients with a rapid versus those with a slow recovery from hemodynamic support with vasoactive infusions for day 1 ADMA (median 0.33, IQR 0.29–0.45 vs 0.41, 0.35–0.59 µmol/L; p=0.24), day 2 ADMA (0.44, 0.33–0.56 vs 0.43, 0.36–0.56 µmol/L; p=0.93), or change in ADMA over time (p>0.05). Similarly, there were no differences between septic patients with rapid versus slow recovery for day 1 or day 2 arginine, arginine:ADMA, or arginine:ornithine (all p>0.05). However, there was a trend towards a decrease in both the arginine concentration and arginine:ADMA ratio in septic patients with ongoing need for vasoactive infusions on day 3 and a corresponding increase in the citrulline:arginine ratio (Figure 3). For prediction of rapid recovery, the AUROC curve for ADMA on day 1 was 0.68 (95% CI 0.45, 0.90).
Table 4.
Association of study measurements with organ dysfunction, inflammation, and clinical outcome in septic patientsa
| ADMA | Arginine | Arg:ADMA | ||||
|---|---|---|---|---|---|---|
| rs | p | rs | p | rs | p | |
| Organ Dysfunction | ||||||
| PIM-2 | −0.02 | 0.93 | 0.02 | 0.95 | 0.08 | 0.81 |
| PELOD | −0.50 | 0.009 | −0.11 | 0.74 | 0.29 | 0.37 |
| Inotrope scoreb | −0.11 | 0.60 | −0.08 | 0.81 | −0.03 | 0.92 |
| ALT | 0.25 | 0.26 | 0.30 | 0.39 | 0.09 | 0.80 |
| Total bilirubin | −0.26 | 0.24 | 0.53 | 0.12 | 0.21 | 0.56 |
| Creatinine | 0.23 | 0.26 | 0.21 | 0.52 | 0.06 | 0.86 |
| BNP | 0.07 | 0.79 | −0.09 | 0.80 | −0.42 | 0.23 |
| Lactate | 0.39 | 0.14 | 0.12 | 0.78 | −0.07 | 0.87 |
| Inflammation | ||||||
| WBC | 0.76 | <0.001c | −0.10 | 0.75 | −0.36 | 0.25 |
| CRP | −0.15 | 0.51 | 0.16 | 0.62 | 0.01 | 1.0 |
| Interleukin-6 | −0.55 | 0.01 | 0.22 | 0.50 | 0.32 | 0.31 |
| Interleukin-8 | −0.52 | 0.03 | 0.22 | 0.52 | 0.26 | 0.45 |
| Clinical Outcome | ||||||
| ICU LOS | 0.22 | 0.88 | 0.19 | 0.56 | 0.06 | 0.86 |
| Hospital LOS | 0.26 | 0.29 | 0.09 | 0.79 | 0.18 | 0.59 |
| Inotrope-free days | −0.12 | 0.57 | −0.25 | 0.44 | −0.22 | 0.48 |
| Ventilator-free days | −0.29 | 0.15 | −0.28 | 0.37 | −0.21 | 0.52 |
ADMA, asymmetric dimethylarginine; Arg:ADMA, arginine:asymmetric dimethylarginine ratio; PIM-2, Pediatric Index of Mortality-2 score, PELOD, Pediatric Logistic Organ Dysfunction score; ALT, alanine aminotransferase; BNP, brain natriuretic peptide; WBC, white blood cell count; CRP, c-reactive protein; LOS, length of stay;
Spearman’s rank correlation coefficient (rs) and coefficient of determination (r2) refer to day 1 values
Inotrope score = dopamine + dobutamine + (nor-/epinephrine × 100) + (milrinone × 10) + (vasopressin × 10) (28)
Survived correction for multiple testing at p<0.003
Figure 3.
Arginine concentrations and related ratios in septic patients with rapid versus slow recovery from hemodynamic support A progressive decline in arginine concentration (A) and the arginine:ADMA ratio (B) and an increase in the citrulline:arginine ratio (C) were observed for septic patients with an ionotrope score (IS) > 5 on day 3 (“slow recovery”) compared with septic patients with IS ≤ 5 on day 3 (“rapid recovery”); however the differences on day 3 did not reach significance for arginine (p=0.17), arginine:ADMA (p=0.28), or citrulline:arginine (p=0.07).
ADMA and carnitine metabolism
There were no differences in the AC:FC ratio, as a measure of mitochondrial dysfunction, on day 1 between septic patients and febrile and healthy controls (Table 2). Septic patients in the highest ADMA quartile did have a higher median AC:FC ratio on all study days compared to those in the lowest ADMA quartile, including day 1 (1.35, IQR 0.72–3.12 vs 0.44, IQR 0.41–2.16, p=0.57) and day 2 (1.80, IQR 0.82–2.7 vs 0.67, IQR 0.56–0.82, p=0.15); however, these differences did not reach significance. There was also no significant association of AC:FC with arginine:ADMA on any study day after correction for multiple comparisons. A trend towards an inverse association between AC:FC and citrulline:arginine was seen on day 1 (rs=−0.53, p=0.08) and on all subsequent study days, with significance reached on study days 3 (rs=−0.50, p=0.01) and 5 (rs=−0.71, p<0.001). There was no association between the daily AC:FC ratio or changes in this ratio and dPELOD score (p>0.05 on all study days).
DISCUSSION
The principal findings of this study is that plasma ADMA, a key regulatory mechanism of NO homeostasis, is decreased and the arginine:ADMA ratio is unchanged in pediatric severe sepsis and septic shock. These findings contrast with studies of septic shock in adults, in which plasma ADMA was increased and arginine:ADMA was decreased (13–15). Similar to prior investigations, we found evidence for decreased arginine bioavailability, including lower blood arginine levels and lower arginine:ornithine ratios (suggesting increased arginase activity) in septic children. Low initial plasma ADMA levels were associated with increased organ dysfunction and inflammation, although not with adverse outcomes. There was no association between changes in ADMA over time and organ dysfunction, or between ADMA and AC:FC as a measure of mitochondrial dysfunction.
Accumulation of ADMA inhibits NO-mediated vasodilatation, promotes platelet and leukocyte interactions with endothelial cells, and increases reactive oxygen species through uncoupling of the NOS dimer (17, 33). Increased ADMA has been proposed as an important mechanism of endothelial and vascular dysfunction in several disease states (28, 34–36). In critically ill adults, escalating plasma ADMA has been associated with increased mortality (14, 15). We hypothesized that a similar increase in plasma ADMA would occur in children with sepsis as a potential mechanism leading to organ dysfunction and adverse outcomes. In contrast, we found plasma ADMA to be decreased in pediatric sepsis with concurrent neutropenia and unchanged without neutropenia. In addition, despite previous studies showing ADMA to be decreased in moderate illness but increased with higher severity (13, 37), we observed a stepwise decline from healthy to febrile to septic patients indicating a “dose-response” decrease in ADMA with increasing illness severity. These findings suggest that inhibition of NO signaling through ADMA accumulation may be less likely to play a major role in the pathophysiology of sepsis in younger patients than has been postulated for adults (33).
Several important developmental differences could account for lower plasma ADMA levels in septic children compared with older patients, including decreased protein catabolism (less ADMA synthesis), reduced incidence of renal or hepatic dysfunction (enhanced ADMA clearance), and a lower rate of microangiopathic hemolysis (less red blood cell-related ADMA release). The presence of hepatic failure in particular has been suggested as a primary determinant of increased ADMA in septic adults (14, 33). Alternatively, the lower prevalence of pre-existing vascular pathology, such as hypertension and atherosclerosis, in younger patients may predispose to less ADMA accumulation (38). For example, plasma ADMA was unchanged after lipopolysaccharide (LPS) administration to “healthy young men” without preexisting cardiovascular disease (39). It is also possible that children with sepsis are better able to maintain ADMA homeostasis through a preserved feedback loop, whereby a decrease in NO leads to increased DDAH activity, faster metabolism of ADMA, and a subsequent increase in NOS activity (40). Thus, low plasma ADMA may reflect, rather than cause, decreased NO production in younger individuals.
Several physiological mechanisms may underlay the decrease in plasma ADMA observed in our septic patients. The acute inflammatory response characteristic of the early phase of sepsis may enhance DDAH activity and, thus, suppress ADMA levels. Although LPS and tumor necrosis factor-α appear to inhibit DDAH in vitro (40, 41), their effect in vivo remains unclear. While endotoxin decreased DDAH activity in a mouse model of acute lung injury (19), several clinical studies have shown plasma ADMA levels to be unchanged in the setting of acute inflammation (42), sepsis without shock (13), and moderate malaria (37), as well as reduced or unchanged following experimental administration of LPS (39, 43). In addition, our study is consistent with others demonstrating an inverse association of ADMA with inflammatory markers (e.g. IL-6) and a rise in ADMA once the infectious/inflammatory process subsides (42, 44). Other potential explanations for the low ADMA concentrations include increased cellular uptake by the y+ transporter in sepsis (45), altered activity of the methyltransferase enzymes that produce ADMA, and decreased substrate availability (i.e. diminished arginine).
Impairment of arginine homeostasis can also impact NO signaling (46), and the low blood arginine and citrulline in this study is consistent with previous reports in sepsis (8–10). Decreased arginine stems primarily from diminished de novo synthesis from citrulline, increased consumption from up-regulation of inducible NOS and arginase (8, 9, 47), and enhanced uptake via the y+ transporter (45). Although we did not find a difference in the citrulline:arginine ratio to support increased NOS activity, we did find indirect evidence of increased arginase activity (decreased arginine:ornithine ratio). Furthermore, the net effect of altered arginine bioavailability on NOS synthesis may be better determined by the arginine:ADMA ratio (13). There was a trend toward a lower ratio in septic patients compared to healthy controls, and in those with slow versus rapid recovery, but these differences were not significant.
In vitro studies have shown that ADMA-induced uncoupling of the NOS dimer leads to a decrease in NO production with a concurrent increase in reactive oxygen species and protein nitration that contribute to mitochondrial dysfunction (17, 19, 20), an important mechanism for sepsis-related organ dysfunction. This process may be accelerated in the presence of arginine deficiency (17). An increase in blood AC:FC as a surrogate for increased intra-mitochondrial acyl-CoA:CoA and mitochondrial dysfunction has been shown in pre-clinical (22) and clinical studies (29). Alterations in carnitine metabolism have also been linked to impaired NO signaling (21). This potential linkage between ADMA-induced NOS uncoupling leading to increased superoxide, altered carnitine metabolism, and deceased mitochondrial function has not previously been studied in pediatric sepsis. Although we did not find a difference in carnitine metabolism between study groups, or an association of AC:FC with ADMA, there was a suggestion that increased AC:FC may be associated with decreased NOS activity on all study days. This may reflect a connection between NOS uncoupling and mitochondrial dysfunction and merits further study.
Ideally, measurement of plasma ADMA or arginine:ADMA ratio would serve as a clinically relevant biomarker for impaired endothelial, microvascular, and mitochondrial function and identify those septic patients at highest risk for multi-organ failure and poor outcomes. While day 1 ADMA was inversely associated with organ dysfunction and the inflammatory cytokines, IL-6 and IL-8, we did not find evidence to support measurement of initial ADMA, arginine, or AC:FC to predict adverse clinical outcomes. Despite its potential for regulating NO-mediated hemodynamic changes, neither the initial nor changes in ADMA were able to predict the degree of hemodynamic support or recovery from inotrope-dependent shock. However, we did observe a slight decrease in arginine:ADMA in septic patients with “slow recovery” from vasoactive infusion therapy suggesting that following changes in ADMA relative to arginine may have clinical utility in pediatric sepsis.
The strongest association we found between ADMA and the inflammatory response was with WBC, an unexpected finding. This led to a post-hoc analysis in which we found neutropenic patients with sepsis to have lower ADMA levels than non-neutropenic patients. In conjunction with previous studies (48), these findings suggest differential regulation of the NO pathway in neutropenia. Alternatively, the higher IL-6 and IL-8 concentrations observed in neutropenic patients may have led to greater suppression of ADMA if increased inflammation does enhance DDAH activity. Although there were no differences in PIM-2 score, IS, CRP, or inotrope- or ventilator-free days, the low levels of IL-6 and IL-8 may also indicate a selectively reduced severity of illness in our non-neutropenic patients. Thus, these findings require further exploration in a larger, planned analysis.
There are several limitations to this study. Given the small sample size and lack of standardized treatment, differences in outcomes and the impact of nutrition could have been underestimated, and we cannot exclude the possibility that a subset of septic children may have increased ADMA. We also used the citrulline:arginine and arginine:ornithine ratios as surrogates for NOS and arginase activity, respectively. While a precedent exists (30, 31), these amino acids are not specific for a particular enzymatic reaction. Therefore, the absence of a strong inverse relationship between ADMA and citrulline:arginine in this study does not necessarily preclude an effect of altered ADMA on NO synthesis. Future studies incorporating measures of NO bioactivity are necessary to fully establish the effect of altered arginine and ADMA on NOS activity in pediatric sepsis. Finally, while the observed decrease in ADMA between groups was small, Cardounel et al. (49) has shown that changes in plasma ADMA of ~0.5 µmol/L have large effects on intracellular NOS inhibition, supporting the physiological importance of even slight changes in circulating ADMA.
CONCLUSIONS
We found that plasma ADMA, in contrast to adult studies, was decreased in pediatric severe sepsis and septic shock (primarily with neutropenia), and that the arginine:ADMA ratio was unchanged. This suggests that, despite diminished arginine bioavailability, ADMA accumulation and inhibition of NOS may be less likely to play a prominent role in the pathophysiology of pediatric sepsis and sepsis-related organ and mitochondrial dysfunction. While we did not find ADMA, arginine, or AC:FC ratio to be clinically useful biomarkers, this study helps to define the natural history of ADMA and arginine metabolism in pediatric sepsis and highlights a potentially important developmental difference between pediatrics and adults.
ACKNOWLEDGEMENTS
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Financial support was provided by the Medical Research Junior Board Foundation (MSW), the Department of Pathology and Laboratory Medicine (SH), the Colman Family Grant (SW), and by grant UL1RR025741 from the National Center for Research Resources, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was performed at Children’s Memorial Hospital, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
References
- 1.Watson RS, Carcillo JA, Linde-Zwirble WT, et al. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167(5):695–701. doi: 10.1164/rccm.200207-682OC. [DOI] [PubMed] [Google Scholar]
- 2.Vincent JL, Zhang H, Szabo C, et al. Effects of nitric oxide in septic shock. Am J Respir Crit Care Med. 2000;161(6):1781–1785. doi: 10.1164/ajrccm.161.6.9812004. [DOI] [PubMed] [Google Scholar]
- 3.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 4.Avontuur JA, Tutein Nolthenius RP, van Bodegom JW, et al. Prolonged inhibition of nitric oxide synthesis in severe septic shock: a clinical study. Crit Care Med. 1998;26(4):660–667. doi: 10.1097/00003246-199804000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Broccard A, Hurni JM, Eckert P, et al. Tissue oxygenation and hemodynamic response to NO synthase inhibition in septic shock. Shock. 2000;14(1):35–40. doi: 10.1097/00024382-200014010-00007. [DOI] [PubMed] [Google Scholar]
- 6.Lopez A, Lorente JA, Steingrub J, et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med. 2004;32(1):21–30. doi: 10.1097/01.CCM.0000105581.01815.C6. [DOI] [PubMed] [Google Scholar]
- 7.Trzeciak S, Cinel I, Phillip Dellinger R, et al. Resuscitating the microcirculation in sepsis: the central role of nitric oxide, emerging concepts for novel therapies, and challenges for clinical trials. Acad Emerg Med. 2008;15(5):399–413. doi: 10.1111/j.1553-2712.2008.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Argaman Z, Young VR, Noviski N, et al. Arginine and nitric oxide metabolism in critically ill septic pediatric patients. Crit Care Med. 2003;31(2):591–597. doi: 10.1097/01.CCM.0000050291.37714.74. [DOI] [PubMed] [Google Scholar]
- 9.Luiking YC, Poeze M, Ramsay G, et al. Reduced citrulline production in sepsis is related to diminished de novo arginine and nitric oxide production. Am J Clin Nutr. 2009;89(1):142–152. doi: 10.3945/ajcn.2007.25765. [DOI] [PubMed] [Google Scholar]
- 10.Davis JS, Anstey NM. Is plasma arginine concentration decreased in patients with sepsis? A systematic review and meta-analysis. Crit Care Med. 2011;39(2):380–385. doi: 10.1097/CCM.0b013e3181ffd9f7. [DOI] [PubMed] [Google Scholar]
- 11.Kalil AC, Danner RL. L-Arginine supplementation in sepsis: beneficial or harmful? Curr Opin Crit Care. 2006;12(4):303–308. doi: 10.1097/01.ccx.0000235206.92697.bf. [DOI] [PubMed] [Google Scholar]
- 12.Tran CT, Leiper JM, Vallance P. The DDAH/ADMA/NOS pathway. Atheroscler Suppl. 2003;4(4):33–40. doi: 10.1016/s1567-5688(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 13.Davis JS, Darcy CJ, Yeo TW, et al. Asymmetric dimethylarginine, endothelial nitric oxide bioavailability and mortality in sepsis. PLoS One. 2011;6(2):e17260. doi: 10.1371/journal.pone.0017260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nijveldt RJ, Teerlink T, Van Der Hoven B, et al. Asymmetrical dimethylarginine (ADMA) in critically ill patients: high plasma ADMA concentration is an independent risk factor of ICU mortality. Clin Nutr. 2003;22(1):23–30. doi: 10.1054/clnu.2002.0613. [DOI] [PubMed] [Google Scholar]
- 15.O'Dwyer MJ, Dempsey F, Crowley V, et al. Septic shock is correlated with asymmetrical dimethyl arginine levels, which may be influenced by a polymorphism in the dimethylarginine dimethylaminohydrolase II gene: a prospective observational study. Crit Care. 2006;10(5):R139. doi: 10.1186/cc5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richir MC, van Lambalgen AA, Teerlink T, et al. Low arginine/asymmetric dimethylarginine ratio deteriorates systemic hemodynamics and organ blood flow in a rat model. Crit Care Med. 2009;37(6):2010–2017. doi: 10.1097/CCM.0b013e31819ffdaf. [DOI] [PubMed] [Google Scholar]
- 17.Sydow K, Munzel T. ADMA and oxidative stress. Atheroscler Suppl. 2003;4(4):41–51. doi: 10.1016/s1567-5688(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 18.Fink MP. Bench-to-bedside review: Cytopathic hypoxia. Crit Care. 2002;6(6):491–499. doi: 10.1186/cc1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma S, Smith A, Kumar S, et al. Mechanisms of nitric oxide synthase uncoupling in endotoxin-induced acute lung injury: role of asymmetric dimethylarginine. Vascul Pharmacol. 2009;52(5–6):182–190. doi: 10.1016/j.vph.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sud N, Wells SM, Sharma S, et al. Asymmetric dimethylarginine inhibits HSP90 activity in pulmonary arterial endothelial cells: role of mitochondrial dysfunction. Am J Physiol Cell Physiol. 2008;294(6):C1407–C1418. doi: 10.1152/ajpcell.00384.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma S, Sud N, Wiseman DA, et al. Altered carnitine homeostasis is associated with decreased mitochondrial function and altered nitric oxide signaling in lambs with pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;294(1):L46–L56. doi: 10.1152/ajplung.00247.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wainwright MS, Kohli R, Whitington PF, et al. Carnitine treatment inhibits increases in cerebral carnitine esters and glutamate detected by mass spectrometry after hypoxia-ischemia in newborn rats. Stroke. 2006;37(2):524–530. doi: 10.1161/01.STR.0000198892.15269.f7. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 24.Gaies MG, Gurney JG, Yen AH, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. 2010;11(2):234–238. doi: 10.1097/PCC.0b013e3181b806fc. [DOI] [PubMed] [Google Scholar]
- 25.Slater A, Shann F, Pearson G. PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med. 2003;29(2):278–285. doi: 10.1007/s00134-002-1601-2. [DOI] [PubMed] [Google Scholar]
- 26.Leteurtre S, Martinot A, Duhamel A, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. 2003;362(9379):192–197. doi: 10.1016/S0140-6736(03)13908-6. [DOI] [PubMed] [Google Scholar]
- 27.Engelberger RP, Teta D, Henry H, et al. Hemodialysis acutely reduces the plasma levels of asymmetric dimethylarginine (ADMA) without reversing impaired nitric oxide dependent vasodilation. Clin Sci (Lond) 2009 doi: 10.1042/CS20080561. [DOI] [PubMed] [Google Scholar]
- 28.Wang S, Vicente FB, Miller A, et al. Measurement of arginine derivatives in pediatric patients with chronic kidney disease using high-performance liquid chromatography-tandem mass spectrometry. Clin Chem Lab Med. 2007;45(10):1305–1312. doi: 10.1515/CCLM.2007.277. [DOI] [PubMed] [Google Scholar]
- 29.Chace DH, Pons R, Chiriboga CA, et al. Neonatal blood carnitine concentrations: normative data by electrospray tandem mass spectometry. Pediatr Res. 2003;53(5):823–829. doi: 10.1203/01.PDR.0000059220.39578.3D. [DOI] [PubMed] [Google Scholar]
- 30.Benedetto C, Marozio L, Neri I, et al. Increased L-citrulline/L-arginine plasma ratio in severe preeclampsia. Obstet Gynecol. 2000;96(3):395–399. doi: 10.1016/s0029-7844(00)00939-x. [DOI] [PubMed] [Google Scholar]
- 31.Morris CR, Kato GJ, Poljakovic M, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. Jama. 2005;294(1):81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Billecke SS, Kitzmiller LA, Northrup JJ, et al. Contribution of whole blood to the control of plasma asymmetrical dimethylarginine. Am J Physiol Heart Circ Physiol. 2006;291(4):H1788–H1796. doi: 10.1152/ajpheart.00066.2006. [DOI] [PubMed] [Google Scholar]
- 33.Nijveldt RJ, Teerlink T, van Leeuwen PA. The asymmetrical dimethylarginine (ADMA)-multiple organ failure hypothesis. Clin Nutr. 2003;22(1):99–104. doi: 10.1054/clnu.2002.0614. [DOI] [PubMed] [Google Scholar]
- 34.Boger RH, Maas R, Schulze F, et al. Elevated levels of asymmetric dimethylarginine (ADMA) as a marker of cardiovascular disease and mortality. Clin Chem Lab Med. 2005;43(10):1124–1129. doi: 10.1515/CCLM.2005.196. [DOI] [PubMed] [Google Scholar]
- 35.Brouns R, Marescau B, Possemiers I, et al. Dimethylarginine levels in cerebrospinal fluid of hyperacute ischemic stroke patients are associated with stroke severity. Neurochem Res. 2009;34(9):1642–1649. doi: 10.1007/s11064-009-9954-3. [DOI] [PubMed] [Google Scholar]
- 36.Schulze F, Lenzen H, Hanefeld C, et al. Asymmetric dimethylarginine is an independent risk factor for coronary heart disease: results from the multicenter Coronary Artery Risk Determination investigating the Influence of ADMA Concentration (CARDIAC) study. Am Heart J. 2006;152(3):493 e491–493 e498. doi: 10.1016/j.ahj.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 37.Yeo TW, Lampah DA, Tjitra E, et al. Increased asymmetric dimethylarginine in severe falciparum malaria: association with impaired nitric oxide bioavailability and fatal outcome. PLoS Pathog. 2010;6(4):e1000868. doi: 10.1371/journal.ppat.1000868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilcken DE, Sim AS, Wang J, et al. Asymmetric dimethylarginine (ADMA) in vascular, renal and hepatic disease and the regulatory role of L-arginine on its metabolism. Mol Genet Metab. 2007;91(4):309–317. doi: 10.1016/j.ymgme.2007.04.017. discussion 308. [DOI] [PubMed] [Google Scholar]
- 39.Mittermayer F, Namiranian K, Pleiner J, et al. Acute Escherichia coli endotoxaemia decreases the plasma l-arginine/asymmetrical dimethylarginine ratio in humans. Clin Sci (Lond) 2004;106(6):577–581. doi: 10.1042/CS20030363. [DOI] [PubMed] [Google Scholar]
- 40.Palm F, Onozato ML, Luo Z, et al. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. Am J Physiol Heart Circ Physiol. 2007;293(6):H3227–H3245. doi: 10.1152/ajpheart.00998.2007. [DOI] [PubMed] [Google Scholar]
- 41.Ito A, Tsao PS, Adimoolam S, et al. Novel mechanism for endothelial dysfunction: dysregulation of dimethylarginine dimethylaminohydrolase. Circulation. 1999;99(24):3092–3095. doi: 10.1161/01.cir.99.24.3092. [DOI] [PubMed] [Google Scholar]
- 42.Zoccali C, Maas R, Cutrupi S, et al. Asymmetric dimethyl-arginine (ADMA) response to inflammation in acute infections. Nephrol Dial Transplant. 2007;22(3):801–806. doi: 10.1093/ndt/gfl719. [DOI] [PubMed] [Google Scholar]
- 43.Nijveldt RJ, Teerlink T, van Guldener C, et al. Handling of asymmetrical dimethylarginine and symmetrical dimethylarginine by the rat kidney under basal conditions and during endotoxaemia. Nephrol Dial Transplant. 2003;18(12):2542–2550. doi: 10.1093/ndt/gfg452. [DOI] [PubMed] [Google Scholar]
- 44.Iapichino G, Umbrello M, Albicini M, et al. Time course of endogenous nitric oxide inhibitors in severe sepsis in humans. Minerva Anestesiol. 2010;76(5):325–333. [PubMed] [Google Scholar]
- 45.Jia YX, Pan CS, Yang JH, et al. Altered L-arginine/nitric oxide synthase/nitric oxide pathway in the vascular adventitia of rats with sepsis. Clin Exp Pharmacol Physiol. 2006;33(12):1202–1208. doi: 10.1111/j.1440-1681.2006.04498.x. [DOI] [PubMed] [Google Scholar]
- 46.Bune AJ, Shergill JK, Cammack R, et al. L-arginine depletion by arginase reduces nitric oxide production in endotoxic shock: an electron paramagnetic resonance study. FEBS Lett. 1995;366(2–3):127–130. doi: 10.1016/0014-5793(95)00495-u. [DOI] [PubMed] [Google Scholar]
- 47.Groeneveld PH, Kwappenberg KM, Langermans JA, et al. Relation between pro- and anti-inflammatory cytokines and the production of nitric oxide (NO) in severe sepsis. Cytokine. 1997;9(2):138–142. doi: 10.1006/cyto.1996.0147. [DOI] [PubMed] [Google Scholar]
- 48.Neilly IJ, Copland M, Haj M, et al. Plasma nitrate concentrations in neutropenic and non-neutropenic patients with suspected septicaemia. Br J Haematol. 1995;89(1):199–202. doi: 10.1111/j.1365-2141.1995.tb08931.x. [DOI] [PubMed] [Google Scholar]
- 49.Cardounel AJ, Cui H, Samouilov A, et al. Evidence for the pathophysiological role of endogenous methylarginines in regulation of endothelial NO production and vascular function. J Biol Chem. 2007;282(2):879–887. doi: 10.1074/jbc.M603606200. [DOI] [PubMed] [Google Scholar]