SUMMARY
We previously found that human NK cells lyse M. tuberculosis (M. tb)-infected monocytes and alveolar macrophages, and upregulate CD8+ T-cell responses. We also found that human NK cells produce IL-22, which inhibits intracellular growth of M. tb, and that NK cells lyse M. tb-expanded CD4+CD25+FoxP3+ T regulatory cells (Tregs). To determine the role of NK cells during the protective immune response to vaccination in vivo, we studied the NK cell and T-cell responses in a mouse model of vaccination with Bacillus Calmette-Guérin (BCG), followed by challenge with virulent M. tb H37Rv. BCG vaccination enhanced the number of IFN-γ- IL-22-producing NK cells. Depletion of NK1.1+ cells at the time of BCG vaccination increased the number of immunosuppressive Tregs (CD4+CD25hi, 95% Foxp3+) after challenge with M. tb H37Rv, and NK1.1+ cells lysed expanded but not natural Tregs in BCG-vaccinated mice. Depletion of NK1.1+ cells at the time of BCG vaccination also increased the bacillary burden and reduced T-cell responses after challenge with M. tb H37Rv. IL-22 at the time of vaccination reversed these effects and enhanced antigen-specific CD4+ cell responses in BCG-vaccinated mice after challenge with M. tb H37Rv. Our study provides the first evidence that NK1.1+ cells and IL-22 contribute to the efficacy of vaccination against microbial challenge.
Keywords: mice, bacterial, cytokine, IL-22, NK cells
INTRODUCTION
Tuberculosis causes a staggering burden of mortality, killing 1.7 million persons annually. Effective treatment in developing countries is hampered by the cost of antituberculosis drugs, inability to ensure completion of therapy, and rising drug resistance rates. Vaccination is an alternative cost-effective strategy that would contribute greatly to tuberculosis control. Development of an effective vaccine hinges on an improved understanding of immunity to Mycobacterium tuberculosis (M. tb).
NK cells are prominent components of the innate immune response, but limited information is available on the role of NK cells in mycobacterial infection. Depletion of NK cells with antibodies to NK1.1 and asialo-GM1 enhanced the growth of M. avium in mice (1), and activated NK cells that produce IFN-γ and perforin accumulate in the lungs of M. tb-infected mice (2). NK cell-derived IFN-γ also regulates the anti-mycobacterial resistance mediated by neutrophils (3). Although depletion of NK cells did not affect the bacterial burden during M. tb infection in mice, NK cells may reduce immunopathology or favor development of protective immune responses. We found that human NK cells lyse infected monocytes and alveolar macrophages through the NKp46 receptor and NKG2D (4,5), and that NK cells contribute to the capacity of CD8+ T-cells to produce IFN-γ and to lyse M. tb-infected monocytes (6). Human NK cells also produce IL-22, which inhibits intracellular growth of M. tb (7), and NK cells lyse M. tb-expanded CD4+ regulatory T cells (Tregs) (8), Human NK cells also produce IFN-γ when exposed to M. bovis bacillus Calmette-Guérin (BCG) (9), and the pleural fluid of tuberculosis patients is enriched for CD56brightCD16- NK cells, which are a prominent source of IFN-γ (9). Despite these studies of human cells in response to M. tb ex vivo, the role of NK cell during an immune response to mycobacteria in vivo remains uncertain.
We hypothesized that NK cells could contribute to BCG-induced protective immunity to M. tb through interactions with T-cells, and tested this hypothesis in a mouse model. We found that, in mice depleted of NK1.1+ cells at the time of BCG vaccination, there was increased expansion of CD4+CD25+Foxp3+ cells and a greater bacillary burden in the lungs after challenge with virulent M. tb. We also found that NK1.1+ cells lyse Tregs (CD4+CD25hi, 95% Foxp3+) in vivo during BCG vaccination, and that IL-22, produced by NK1.1+ cells, induces optimal protective immunity through enhancing antigen-specific T-cell responses after challenge with M. tb.
MATERIALS AND METHODS
Animals
All animal studies were performed on specific-pathogen-free 4-6-week-old female C57BL/6 mice and approved by The Institutional Animal Care and Use Committee of the University of Texas Health Science Center at Tyler.
Abs and other reagents
For flow cytometry, we used FITC anti-NK1.1, FITC anti-CD3, FITC anti-CD4, FITC anti-CD8, FITC anti-CCR7, PE anti-CD49b, PE anti-Foxp3, PE anti-NKp46, PE anti-CD44, PE anti-CD11b, PE-Cy5 anti-CD127, allophycocyanin anti-CD117, allophycocyanin anti-CD25, allophycocyanin anti-IL-22 (all from Biolegend). For neutralization, we used mAb to NK1.1 or isotype control antibody (Bio X cell). We used γ-irradiated M. tb H37Rv and Ag85a (both from BEI Resources), the BCG Tice strain (Organon USA Inc.) and Candida antigen (Greer Laboratories). Microbeads conjugated to Abs to CD11b, CD4 or CD8 (Miltenyi Biotec) were used for cell isolation.
Flow Cytometry
Surface and intracellular staining was performed as described (8).
BCG vaccination and aerosol infection with M. tb H37Rv
Mice were immunized subcutaneously at the base of the tail with 106 CFU of BCG in 100 μl of PBS, or with PBS alone. In some cases, mice were sacrificed 1-8 days after vaccination. For other experiments, two months after vaccination, mice were infected with M. tb H37Rv in an aerosol exposure chamber, using published methods (2). In preliminary studies, mice were exposed to different concentrations of M. tb, and CFU were enumerated in homogenized lungs 24 hrs after infection. For further studies, we selected the concentration which deposited ~50–100 bacteria in the lungs per mouse.
Depletion of NK1.1+ cells and treatment with IL-22 during BCG vaccination
Mice were given 0.3 mg of anti-NK1.1 or isotype control Ab intravenously on days 0, 1 and 2, relative to administration of BCG. Anti-NK1.1 reduced the percentage of splenic CD3- NKp46+ cells from 2.1 ± 0.3% to 0.4 ± 0.09%, (p=0.02, n=4), as measured by flow cytometry. A representative flow cytometry result is shown in supplemental Fig. 1. Some mice received recombinant IL-22 (2 ng) with each dose of anti-NK 1.1.
Culture of lung, spleen and lymph node cells
In some experiments, BCG-vaccinated mice, uninfected or infected with M. tb H37Rv, were sacrificed, and cells from the lungs, spleens or peripheral lymph nodes were cultured in 24-well plates at 2 ×106 cells/well in RPMI 1640 containing penicillin (Life Technologies) and 10% heat-inactivated FCS, with or without γ-irradiated H37Rv (10 μg/ml), Ag85a (3 μg/ml) or Candida (3 μg/ml) at 37°C and 5% CO2. After 72 h, culture supernatants were collected to determine cytokine levels. In other experiments, CD11b+ and CD4+ cells from spleens and peripheral lymph nodes were isolated by positive immunomagnetic selection (Miltenyi Biotec). CD4+ cells and CD11b+ cells were cultured at a ratio of 10:1, with or without γ-irradiated H37Rv (10 μg/ml) for 72 h. Cells were either stained to identify CD4+CD25+Foxp3+ cells, or Tregs were isolated by the Treg isolation kit (Miltenyi Biotec),using positive immunomagnetic selection for CD4+ cells, followed by positive selection for CD25hi cells. CD4+CD25– cells were also obtained. Ninety- 95% of purified CD4+CD25hi cells were FoxP3+ (Supplemental Fig. 2A), and major producers of IL-10, as determined by ELISPOT (Supplemental Fig. 2B).
Culture of monocytes, CD8+ cells, and Tregs
Mice were vaccinated with BCG. One week later, freshly isolated splenic CD8+ cells were isolated and cultured (2 × 106 cells per well) with 2 × 105 autologous monocytes per well. In some wells, 2 × 105 autologous Tregs, isolated from CD4+ cells and monocytes cultured with γ-irradiated M. tb H37Rv, were added. Cells were cultured for 72 h, CD8+ cells were isolated by positive immunomagnetic selection, and IFN-γ mRNA expression was quantified by real-time PCR.
Real-time PCR for quantification of IFN-γ mRNA
IFN-γ mRNA was quantified in CD8+ cells, using minor modifications of our published methods (7), using primers for murine IFN-γ (forward, 5’AGCTCATCCGAGTGGTCCAC 3', reverse, 5'AGCAGCGACTCCTTTTCCG 3') and β-actin (forward, 5' CCTTCAACACCCAGCCATGT 3'; reverse, 5' TGTGGACCACCAGAGGCATAC 3').
In vivo cytotoxicity assay
Mice were vaccinated with BCG. After 72 h, CD4+CD25hi cells from control and vaccinated mice were isolated from pooled spleen and lymph node cells, using the Treg isolation kit (Miltenyi Biotec). CD4+CD25hi cells from BCG-vaccinated and unvaccinated mice were labeled with 5 μM (CFSEhigh) and 0.5 μM (CFSElow) of CFSE, respectively, mixed 1:1 and inoculated intravenously (6 × 106 cells/mouse) into recipient C57BL/6 mice, 72 h after BCG vaccination. Before adoptive transfer of CD4+CD25hi cells, recipient mice were treated with anti-NK1.1 or isotype control Abs, 0, 1 and 2 days after vaccination. Eighteen h after transfer of CD4+CD25hi cells, CFSElow and CFSEhigh cells in spleens and lymph nodes draining the vaccination site were quantified by flow cytometry. The percent of in vivo lysis of BCG-expanded CD4+CD25hi cells by NK cells was calculated as 100-100 [(% target population (BCG-expanded CD4+CD25hi cells) in isotype-treated/% control CD4+CD25hi cells in isotype-treated) ÷ (% BCG-expanded CD4+CD25hi cells in NK1.1-depleted/% control CD4+CD25hi cells in NK1.1-depleted)].
Measurement of IFN-γ concentrations by ELISA
Supernatants from cultured cells were collected after 72 h and stored at -70°C until IFN-γ concentrations were measured by ELISA (Biolegend).
Frequency of lung, spleen and lymph node cells producing IFN-γ and IL-10
Lung, spleen and lymph node cells from BCG-vaccinated mice, with or without subsequent M. tb infection, were isolated, and cells (105-5 X 105, depending on the experiment) were placed on ELISPOT plates coated with 2 μg/ml of anti-IFN-γ Ab (Biolegend) in PBS. In some experiments, NK cells were isolated from spleen and lymph node cells with a negative selection kit (Miltenyi Biotech), and placed on the ELISPOT plates. Following overnight incubation at 37°C in 5% CO2, 2 μg/ml of biotinylated anti-mouse IFN-γ (Biolegend) was added. After 2 h, plates were washed with PBS and 1 μg/ml streptavidin conjugated alkaline phosphatase (Mabtech) was added. After 45 minutes, plates were washed with PBS and developed with BCIP/NBT plus substrate (R&D systems). Plates were dried and the numbers of IFN-γ cells were counted. The frequency of IL-10-producing cells was identified by using ELISPOT kits (Biolegend).
Statistical analysis
Results are shown as the mean ± SE. Comparisons between groups were performed by a paired or unpaired t test, as appropriate.
RESULTS
BCG vaccination enhances NK cell number and production of IFN-γ and IL-22
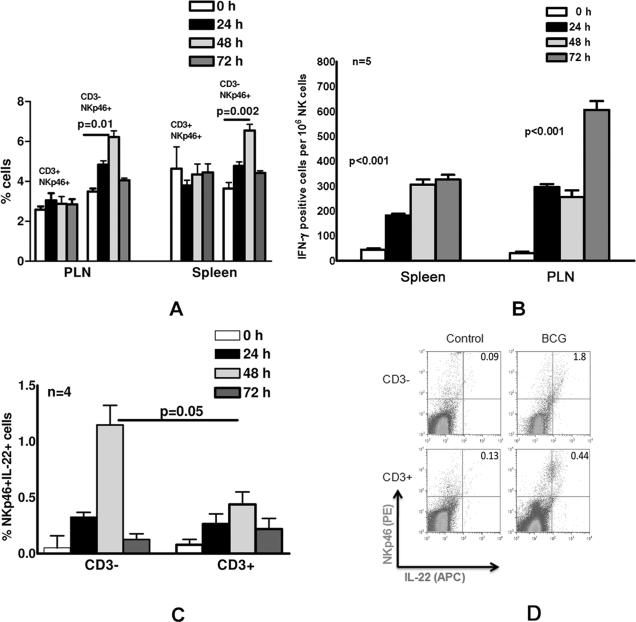
To determine whether BCG vaccination affects NK cells, mice were either vaccinated or unvaccinated with BCG. At different time points after vaccination, spleen and peripheral lymph node (PLN) cells were isolated, and the percentages of CD3+NKp46+ or CD3-NKp46+ cells were measured by flow cytometry. NK cells express NKp46 but not CD3. NKT cells are CD3+ and in mice some NKT cells express NKp46+. The percentage of CD3-NKp46+ cells in PLN increased from 3.5 ± 0.1% at baseline to 6.2 ± 0.3%, 48 h after vaccination (p=0.01, Fig. 1A), with similar increases in the spleen (p=0.002; Fig. 1A). In contrast, the number of CD3+NKp46+ cells were similar in control and BCG vaccinated mice (Fig. 1A), indicating that the number of NK cells but not NKT cells increases in PLN and spleen in response to BCG vaccination during first 72 h.
Fig. 1. BCG vaccination enhances NK cell number and production of IFN-γ and IL-22.
C57BL/6 mice (5 mice per group) were unimmunized or immunized subcutaneously with 106 CFU of BCG in 100 μl of PBS. After 24, 48 and 72 h, spleen and peripheral lymph node cells were isolated. A. The percentages of CD3+NKp46+ and CD3-NKp46+ cells were measured by flow cytometry. B. The frequency of IFN-γ-producing NK cells was determined by ELISPOT. Purified NK cells were obtained from spleen and peripheral lymph node cells by negative selection, and incubated overnight in triplicate wells on an ELISPOT plate to determine the frequency of IFN-γ-producing NK cells. C. Intracellular staining was performed to detect IL-22-producing CD3+NKp46+ and CD3-NKp46+ spleen cells. Surface staining was performed with anti-CD3 and anti-NKp46, and intracellular staining was performed with isotype control or anti-IL22. Mean values and SEs are shown. D. A representative flow cytometry result of staining for IL-22 and NKp46, after gating on CD3- and CD3+ spleen cells is shown.
We next measured the frequency of IFN-γ-producing NK cells at different time points after BCG vaccination. Purified NK cells were obtained from spleen and peripheral lymph node cells by negative selection and ELISPOT was performed, as outlined in the methods. The frequency of IFN-γ producing splenic NK cells increased 4-7-fold from 44 ± 5 per 106 cells in unvaccinated mice to 182 ± 7, 306 ± 21 and 326 ± 19 per 106 cells, 24, 48 and 72 h after vaccination, respectively (p<0.001 for all time points, compared to unvaccinated mice; Fig. 1B). Similarly, the frequency of IFN-γ-producing peripheral lymph node NK cells increased 8-20-fold, from 30 ± 6 per 106 cells in unvaccinated mice to 296 ± 12, 256 ± 27 and 606 ± 36 per 106 cells, 24, 48 and 72 h after vaccination, respectively (p<0.001 for all time points, compared to unvaccinated mice; Fig. 1B).
Next, we used intracellular staining to detect IL-22-producing CD3-NKp46+ NK cells or CD3+NKp46+ NKT cells in splenocytes after BCG vaccination. The percentages of CD3-NKp46+IL-22+ and CD3+NKp46+IL-22+ cells increased from 0.05 ± 0.1% to 1.15 ± 0.2% and 0.08 ± 0.05% to 0.44 ± 0.1% , 48 h after vaccination (p=0.05, Fig. 1C). This suggests that the majority of NKp46+ IL-22-producing cells upon BCG vaccination are NK cells and not NKT cells. A representative flow cytometry result is shown in Fig. 1D.
NK cells reduce expansion of CD4+CD25+FoxP3+ T-cells after BCG immunization
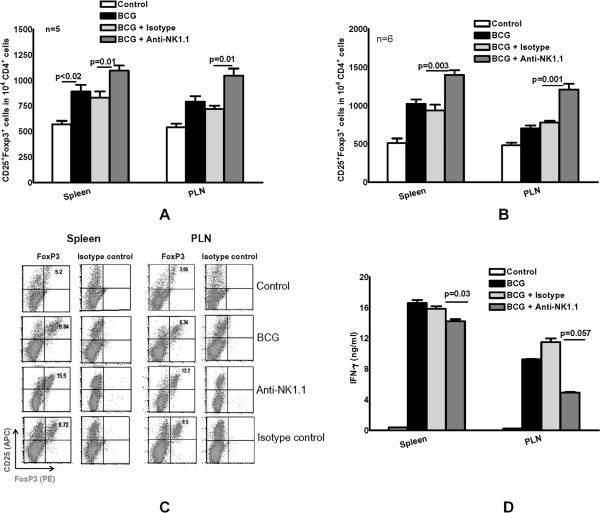
To determine the effect of NK cells on T-cell responses after BCG immunization, we immunized mice subcutaneously with 106 CFU of BCG. Some BCG-immunized mice were also treated with 3 doses of anti-NK1.1, or isotype control Ab. Anti-NK1.1 reduced the percentage of CD3-NKp46+ cells from 2.1 ± 0.3% to 0.4 ± 0.09%, (p=0.02, n=4) in spleens and a similar decrease was seen in peripheral lymph nodes, as measured by flow cytometry. A representative flow cytometry result is shown in supplemental Fig. 1. To evaluate interactions between NK1.1+ cells and Tregs, we quantified CD4+CD25+FoxP3+ cells by flow cytometry. One week after BCG vaccination, more CD4+CD25+Foxp3+ cells were present in the spleen, compared to unvaccinated mice (890 ± 66 versus 570 ± 34 cells per 104 CD4+ cells, respectively; p<0.02, Fig. 2A). Depleting NK1.1+ cells at the time of BCG vaccination further increased the number of CD4+CD25+Foxp3+ cells to 1095 ± 50 per 104 CD4+ cells, versus 838 ± 61 per 104 CD4+ cells for treatment with the isotype control Ab (Fig. 2A, p=0.01) . Similar effects were noted in the peripheral lymph nodes (Fig. 2A).
Fig. 2. NK1.1+ cells reduce expansion of CD4+CD25+FoxP3+ T-cells after BCG immunization.
C57BL/6 mice (3-6 mice per group) were unimmunized or immunized subcutaneously with 106 CFU of BCG in 100 μl of PBS. Some BCG-vaccinated mice were treated with anti-NK1.1, or isotype control Ab (0.3 mg per mouse 0, 24 and 48 h after vaccination). A. One week after vaccination, spleen and peripheral lymph node cells were isolated and CD4+CD25+FoxP3+ T-cells were measured by flow cytometry. B. One week after vaccination, CD4+ and CD11b+ cells from spleens and peripheral lymph nodes were isolated and cultured, with or without γ-irradiated M. tb H37Rv. After 5 days, CD4+CD25+FoxP3+ T-cells were measured by flow cytometry. C. A representative flow cytometry result of in vitro expanded CD4+CD25+FoxP3+ T-cells is shown. CD25+ and Foxp3+ cells were shown on gated CD4+ cells. D. One week after vaccination, CD4+ and CD11b+ cells from spleens and peripheral lymph nodes were isolated and cultured, with or without γ-irradiated M. tb H37Rv. After 5 days, supernatants were collected and IFN-γ levels were measured by ELISA. Pooled cells from 3 to 5 mice were used and the experiment was performed twice. Mean values and SEs are shown.
One week after immunization, we also determined the capacity of CD4+ cells to expand into CD4+CD25+FoxP3+ cells in the presence of γ-irradiated M. tb H37Rv. CD4+ and CD11b+ cells from spleens and peripheral lymph nodes were isolated and cultured, with or without γ-irradiated M. tb H37Rv for 5 days, as outlined in the methods. BCG vaccination induced expansion of splenic CD4+CD25+Foxp3+ cells to 1020 ± 60 per 104 CD4+ cells, compared to 510 ± 60 per 104 CD4+ cells in control mice. Depletion of NK1.1+ cells with antibody further increased expansion of CD4+CD25+Foxp3+ cells to 1400 ± 63 per 104 CD4+ cells, compared to 930 ± 76 per 104 CD4+ cells after treatment with isotype control Ab (Fig. 2B, p=0.003). Similar findings were noted in peripheral lymph nodes (Fig. 2B) where BCG vaccination induced expansion of splenic CD4+CD25+Foxp3+ cells to 700 ± 40 per 104 CD4+ cells, compared to 480 ± 36 per 104 CD4+ cells in control mice. Depletion of NK1.1+ cells further increased expansion of CD4+CD25+Foxp3+ cells to 1210 ± 75 per 104 CD4+ cells, compared to 778 ± 23 per 104 CD4+ cells after treatment with isotype control Ab (Fig. 2B, p=0.001). A representative flow cytometry result is shown in Fig. 2C.
The data above suggest that NK1.1+ cells inhibit expansion of CD4+CD25+FoxP3+ Tregs, which can in turn upregulate T-cell effector function. Therefore, we evaluated the effect of NK1.1+ cell depletion on IFN-γ production by splenocytes and lymph node cells. BCG vaccination increased M. tb-stimulated IFN-γ production by peripheral lymph node cells, but depletion of NK1.1+ cells decreased IFN-γ concentrations by 60% from 11.5 ± 0.5 ng/ml in isotype-treated mice to 4.9 ± 0.1 ng/ml (Fig. 2D, p=0.057). NK1.1+ cell depletion had similar but more modest effects on IFN-γ production by splenocytes, which fell from 15.9 ± 0.4 ng/ml in isotype-treated mice to 14.3 ± 0.3 ng/ml (Fig. 2D, p=0.03).
NK1.1+ cells inhibit expansion of Tregs after BCG vaccination and challenge with M. tb H37Rv
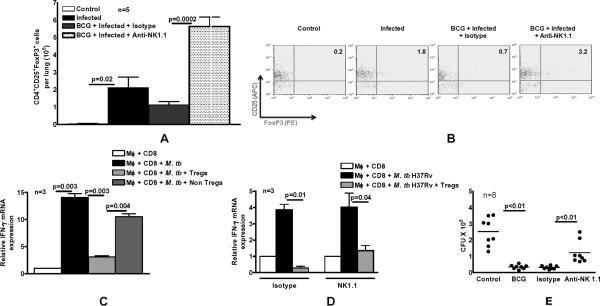
To determine if NK1.1+ cells affected Tregs during infection with M. tb H37Rv, in vivo, we evaluated the response of mice to aerosol challenge with M. tb H37Rv after vaccination with BCG. Mice were either unimmunized or immunized subcutaneously with BCG. Some immunized mice were also treated with anti-NK1.1 or isotype control Ab, as outlined in the methods. After 60 days, mice were challenged with M. tb H37Rv by aerosol. Thirty days after infection, CD4+ CD25+Foxp3+ cells in the lungs were quantified by flow cytometry. After infection with M. tb H37Rv, the number of Tregs per lung in unvaccinated mice rose 50-fold from 4.1 ± 2.3 × 103 to 2.1 ± 0.6 × 105 (p=0.02, Fig. 3A). Depletion of NK1.1+ cells at the time of BCG vaccination further increased the number of Tregs after challenge with M. tb H37Rv to 5.6 ± 0.5 × 105 cells per lung, compared to 1.1 ± 0.2 × 105 cells per lung with use of isotype control Abs (Fig. 3A, p=0.0002). A representative flow cytometry result is shown in Fig. 3B.
Fig. 3. NK1.1+ cells inhibit expansion of immunosuppressive Tregs after BCG vaccination and challenge with M. tb H37Rv.
C57BL/6 mice (3-8 mice per group) were unimmunized or immunized subcutaneously with 106 CFU of BCG in 100 μl of PBS. Some BCG-vaccinated mice were treated with anti-NK1.1, or isotype control Ab (0.3 mg per mouse 0, 24 and 48 h after vaccination). Sixty days after BCG vaccination, mice were challenged with 50–100 CFU of M. tb H37Rv by aerosol. A. Thirty days post-infection, CD4+CD25+FoxP3+ T-cells in the lungs were measured by flow cytometry. B. A representative flow cytometry result of lung cells is shown. We gated on lung CD4+ cells and then gated on CD25+ and FoxP3+ expressing cells. C. M. tb H37Rv-expanded CD4+CD25hi cells are immunosuppressive. Three C57BL/6 mice were immunized subcutaneously with 106 CFU of BCG. One week later, CD4+ and CD11b+ cells from spleens and peripheral lymph nodes were isolated and cultured with γ-irradiated M. tb H37Rv. After 72 h, CD4+CD25hi cells and CD4+CD25- cells were isolated and cultured in Transwells, within large wells containing CD8+ and CD11b+ cells obtained from mice one week after BCG vaccination and γ-irradiated M. tb H37Rv. After 72 h, the Transwells were removed, and IFN-γ mRNA was quantified in the cells in the large wells by real-time PCR. D. C57BL/6 mice (3 mice per group) were immunized subcutaneously with 106 CFU of BCG. Some BCG-vaccinated mice were treated with anti-NK1.1, or isotype control Ab (0.3 mg per mouse 0, 24 and 48 h after vaccination). Sixty days after BCG vaccination, mice were challenged with 50–100 CFU of M. tb H37Rv by aerosol. Thirty days post-infection, CD4+CD25hi Tregs from pooled lung, spleen and mediastian lymph nodes were isolated and cultured in Transwells, within large wells containing CD8+ and CD11b+ cells from BCG-vaccinated mice and γ-irradiated M. tb H37Rv. After 72 h, the Transwells were removed, and IFN-γ mRNA was quantified in the cells in the large wells by real-time PCR. Mean values and SEs are shown. E. C57BL/6 mice (8 mice per group) were unimmunized or immunized subcutaneously with 106 CFU of BCG. Some BCG-vaccinated mice were treated with anti-NK1.1, or isotype control Ab (0.3 mg per mouse 0, 24 and 48 h after vaccination). Sixty days after BCG vaccination, mice were challenged with 50–100 CFU of M. tb H37Rv by aerosol. Thirty days post-infection, lungs were homogenized and plated on 7H11 agar with THC, and CFU per lung were counted after 3 weeks.
M. tb H37Rv-expanded CD4+CD25hi cells are immunosuppressive
To determine whether the CD4+CD25+FoxP3+ cells that expand in response to M. tb H37Rv are functional Tregs, we evaluated their capacity to inhibit IFN-γ production by CD8+ cells in response to M. tb H37Rv. From mice that had been vaccinated with BCG one week previously, we isolated CD4+ and CD11b+ cells, and cultured them with γ-irradiated M. tb H37Rv. After 72 h, CD4+CD25hi cells and CD4+CD25- cells were isolated and cultured in Transwells, within large wells containing CD8+ and CD11b+ cells from BCG-vaccinated mice and γ-irradiated M. tb H37Rv. After 72 h, the Transwells were removed, and IFN-γ mRNA was quantified in the cells in the large wells by real-time PCR. Values were normalized, so that the value of IFN-γ mRNA expression by CD8+ and CD11b+ cells in the absence of M. tb H37Rv was set to 1.0. M. tb H37Rv induced a 14-fold increase in IFN-γ mRNA expression by CD8+ cells (Fig. 3C). CD4+CD25hi cells inhibited IFN-γ mRNA expression by CD8+ T-cells to a much greater extent than CD4+CD25- cells (3 ± 0.2 vs 10.5 ± 0.5, p=0.004, Fig. 3C). Therefore, the CD4+CD25hi BCG-expanded cells inhibit T-cell responses and function as Tregs.
In the above studies we used CD4+CD25hi cells that were generated in vitro. To confirm that CD4+CD25hi (90-95% Foxp3+) generated during M. tb H37Rv challenge after BCG vaccination were also immunosuppressive, we cultured in Transwells, CD4+CD25hi cells from M. tb H37Rv-infected mice that had been previously BCG-immunized and treated with either isotype control or anti-NK1.1 Abs. CD4+CD25hi cells from both groups of mice inhibited IFN-γ mRNA expression by CD8+ cells, which fell from 3.8 ± 0.3 to 0.3 ± 0.1 (Fig. 3D, p=0.01) and from 4 ± 0.2 to 1.3 ± 0.3 (Fig. 3D, p=0.04).
We determined the effects of NK1.1+ cell depletion on the bacterial burdens in the lungs. BCG vaccination reduced CFU per lung in M. tb H37Rv-infected mice from 2.5 ± 0.3 × 106 to 0.3 ± 0.04 × 106 (p=0.0002, Fig. 3E). Depletion of NK1.1+ cells at the time of vaccination reduced the protective effect of BCG vaccination, with CFU increasing from 0.3 ± 0.03 × 106 to 1.2 ± 0.2 × 106 CFU per lung (p=0.007, Fig. 3E). These findings suggest that NK1.1+ cells during BCG vaccination inhibit expansion of immunosuppressive Tregs and limit bacillary replication.
NK1.1+ cells lyse expanded Tregs during the response to BCG vaccination in vivo
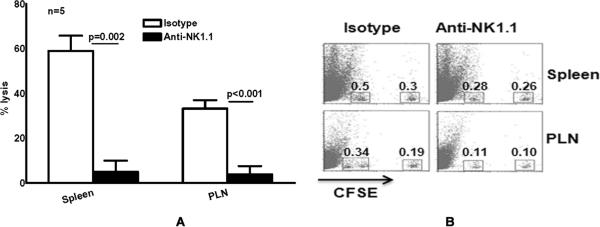
Previously, we found that activated human NK cells lyse M. tb H37Rv-expanded Tregs but not natural Tregs in vitro (6), and our above data demonstrate that depletion of NK1.1+ cells at the time of BCG vaccination increases the number of immunosuppressive Tregs and reduces the protective efficacy of BCG vaccination against challenge with M. tb H37Rv. To determine if this is due to NK1.1+ cell lysis of Tregs in vivo, we performed an in vivo cytotoxicity assay, by adoptively transferring CFSEhigh-labeled BCG-expanded CD4+CD25hi cells and CFSElow-labeled control CD4+CD25hi cells to mice, 72 h after the recipients had received BCG vaccine and treatment either with anti-NK1.1 or isotype control Ab. Eighteen h after adoptive transfer of CD4+CD25hi cells, CFSEhigh and CFSElow cells were quantified by flow cytometry, and the % lysis of expanded CD4+CD25hi cells was calculated. In isotype control Ab-treated mice, 59 ± 7 % and 33 ± 6 % of expanded CD4+CD25hi cells were lysed in the spleen and peripheral lymph nodes, respectively. NK1.1+ cell depletion reduced these values to 5 ± 5 % and 3.8 ± 3.8 %, respectively (p=0.002 and p<0.001, respectively, Fig. 4A), suggesting that NK1.1+ cells lyse substantial numbers of expanded CD4+CD25hi cells in vivo. In contrast, NK1.1+ cells did not lyse natural CD4+CD25hi cells in both spleen and peripheral lymph nodes. A representative flow cytometry result is shown in Fig. 4B.
Fig. 4. NK cells lyse expanded Tregs during the response to BCG vaccination in vivo.
A. C57BL/6 mice (5 mice per group) were unimmunized or immunized subcutaneously with 106 CFU of BCG in 100 μl of PBS. After 72 h, CD4+CD25hi cells were isolated from pooled spleen and lymph node cells, using the Treg isolation kit. CD4+CD25hi cells from BCG-vaccinated mice and control unvaccinated mice were designated as expanded and natural Tregs, respectively. Expanded and natural Tregs were labeled with 5 μM (CFSEhigh) and 0.5 μM (CFSElow) of CFSE, respectively, mixed 1:1 and inoculated intravenously (6 × 106 cells/mouse) into recipient C57BL/6 mice, 72 h after BCG vaccination. Recipient mice were treated with anti-NK1.1 or isotype control Abs, 0, 1 and 2 days after vaccination. Eighteen h after transfer of Tregs, CFSElow and CFSEhigh populations were detected by flow cytometry of single cell suspensions from spleens and peripheral lymph nodes draining the vaccination site. The percent of in vivo lysis of BCG-expanded Tregs was calculated, using the formula given in the methods. Mean values and SEs of five independent experiments are shown. B. A representative flow cytometry result of cells from a BCG-vaccinated mouse is shown. CFSEhi cells are CD4+CD25hi BCG-expanded Tregs and CFSElow cells are CD4+CD25hi natural Tregs.
NK1.1+ cells and IL-22 enhance the protective efficacy of BCG vaccination
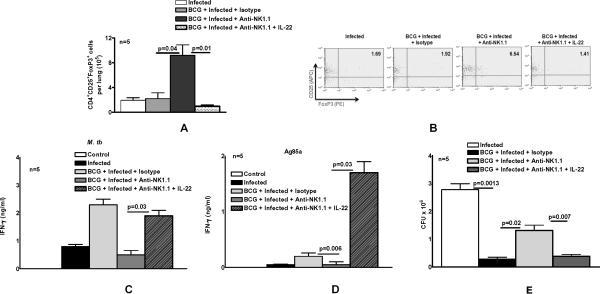
The results above show that BCG vaccination elicits IL-22 production by NK1.1+ cells (Fig. 1C) and that depletion of NK1.1+ cells at the time of BCG vaccination enhances expansion of immunosuppressive Tregs and increases bacillary replication in the lungs of mice challenged with M. tb H37Rv (Fig. 3E). To determine if IL-22 could substitute for the effects of NK1.1+ cells on CD4+ cell responses, we treated BCG-vaccinated mice with anti-NK1.1, isotype control Ab, or anti-NK1.1 plus recombinant IL-22. After 60 days, mice were challenged with M. tb H37Rv by aerosol. Sixty days after infection, we measured the number of Tregs in lungs. Similar to the findings in Fig. 3A, depletion of NK1.1+ cells at the time of BCG vaccination and challenging with M. tb H37Rv markedly increased the number of Tregs in lungs, compared to isotype control Ab-treated mice (9.2 ± 1.7 × 105 versus 2.2 ± 1.0 × 105 cells per lung, p=0.04, Fig. 5A). In contrast, IL-22 abrogated the effects of anti-NK1.1, with the number of Tregs falling from to 9.2 ± 1.7 × 105 to 1.0 ± 0.2 × 105 cells per lung (p=0.01, Fig. 5A). A representative flow cytometry result is shown in Fig. 5B.
Fig. 5. NK1.1+ cells and IL-22 enhance the protective efficacy of BCG vaccination.
C57BL/6 mice were unimmunized or immunized subcutaneously with 106 CFU of BCG. Some BCG-vaccinated mice were treated with anti-NK1.1, or isotype control Ab (0.3 mg per mouse 0, 24 and 48 h after vaccination). Some anti-NK1.1-treated mice received recombinant IL-22 (2 ng) at the same time points. Sixty days after BCG vaccination, mice were challenged with 50–100 CFU of M. tb H37Rv by aerosol. A. Thirty days post-infection, CD4+CD25+FoxP3+ T-cells in lungs were measured by flow cytometry. B. A representative flow cytometry plot of CD4+CD25+FoxP3+ T-cells is shown. Gating was performed as in Fig.3B. C and D. Thirty days post-infection, lung cells were isolated and stimulated with γ-irradiated M. tb (C) or Ag85a (D). After 72 h, IFN-γ levels were measured by ELISA. Values shown are those obtained after subtracting IFN-γ levels from wells containing medium alone. E. Sixty days post-infection, lungs were homogenized and plated on 7H11 agar with THC, and CFU per lung were counted after 3 weeks.
We next evaluated the effect of NK1.1+ cell depletion and IL-22 on mycobacterial Ag-induced IFN-γ production by lung cells. Thirty days after M. tb H37Rv infection of BCG-vaccinated mice, lung cells were isolated and stimulated with γ-irradiated M. tb H37Rv, Ag85a and Candida Ags. Depletion of NK1.1+ cells at the time of BCG vaccination inhibited IFN-γ production by lung cells in response to γ-irradiated M. tb H37Rv by 80%, and this was reversed by recombinant IL-22 (Fig. 5C). Similar findings were noted in the response to Ag85, except that IL-22 increased IFN-γ production to a greater extent (Fig. 5D). No IFN-γ was detected when cells were stimulated with Candida (not shown).
NK1.1+ cell depletion at the time of vaccination reduced the protective efficacy of BCG against challenge with M. tb H37Rv, with CFU two months after infection increasing 5-fold (p=0.02; Fig. 5E). IL-22 abrogated the effect of NK1.1+ cell depletion on CFU (p=0.007; Fig. 5E).
IL-22 expands memory CD4+ cells after BCG vaccination and M. tb H37Rv infection
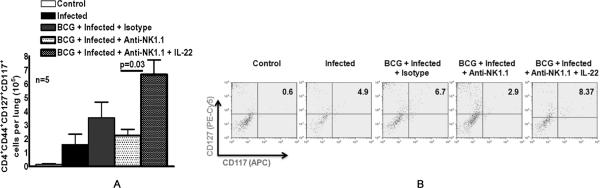
To determine whether NK1.1+ cells and IL-22 regulate antimycobacterial memory CD4+ cell responses, we immunized mice with BCG and treated some of them with anti-NK1.1, isotype control Ab, or anti-NK1.1 plus recombinant IL-22. After 60 days, mice were challenged with M. tb H37Rv by aerosol. Thirty days after infection, CD4+CD44+CD117+CD127+ cells in the lungs were measured by flow cytometry. CD117 is expressed by human CD4+ cells (10), CD117+ cells have previously been identified as major producers of IFN-γ (11) and we found that most CD4+ IFN-γ-producing cells in BCG-vaccinated mice were CD117+ (R Dhiman and R Vankayalapati, unpublished data). CD44 and CD127 were used to identify cells with a memory and activation phenotype, respectively. BCG vaccination doubled the number of CD4+CD44+CD117+CD127+ cells per lung in M. tb H37Rv-infected mice, from 1.6 ± 0.7 × 105 to 3.6 ± 1.1 × 105 (Fig. 6A). Depletion of NK1.1+ cells at the time of BCG vaccination reduced the number of CD4+CD44+CD117+CD127+ cells per lung to 2.3 ± 0.4 × 105, and IL-22 increased this number to 6.7 ± 1.1 × 105 cells per lung (p=0.03, Fig. 6A). A representative flow cytometry result is shown in Fig. 6B.
Fig. 6. IL-22 expands memory CD4+ cells after BCG vaccination and M. tb H37Rv infection.
A. C57BL/6 mice (5 mice per group) were unimmunized or immunized subcutaneously with 106 CFU of BCG. Some BCG-vaccinated mice were treated with anti-NK1.1 or isotype control Ab (0.3 mg per mouse 0, 24 and 48 h after vaccination). Some anti-NK1.1-treated mice received recombinant IL-22 (2 ng) at the same time points. Sixty days after BCG vaccination, mice were infected with 50–100 CFU of M. tb H37Rv by aerosol, and 30 days later, the number of CD4+CD44+CD127+CD117+ cells in the lungs was measured by flow cytometry. Means ±SEs are shown. B. A representative flow cytometry plot of CD4+CD44+CD127+CD117+ lung cells is shown. We gated on CD4+CD44+ cells, and then gated on CD127+ and CD117+ cells.
DISCUSSION
Murine cells that express NK1.1 include CD3- NK cells and CD3+ NKT cells, both of which contribute to immunity against cancer, viruses and bacteria, including M. tb. However, limited information is available on the role of NK1.1+ cells in vaccine-induced protective immune responses. We addressed this question in a mouse model of vaccination with BCG, followed by challenge with virulent M. tb H37Rv. Depletion of NK1.1+ cells at the time of BCG vaccination increased the number of immunosuppressive Tregs, increased the bacillary burden and reduced antigen-specific T-cell responses after challenge with M. tb H37Rv. We also found that IL-22 can reverse the effect of NK1.1+ cell depletion, reducing the number of Tregs, increasing T-cell IFN-γ production and reducing the bacillary burden after challenge with M. tb, and increasing the number of memory T-cells. Our study provides the first evidence that NK1.1+ cells and IL-22 contribute to the efficacy of vaccination against microbial challenge.
BCG vaccination resulted in infiltration of IFN-γ- and IL-22-producing NK1.1+ cells into the spleen and peripheral lymph nodes within 24 h after vaccination, paralleling previous findings that intranasal immunization with the M. tb protein, Ag85B, induced accumulation of activated and cytokine-producing NK cells in nasal-associated lymphoid tissue and cervical lymph nodes (12). BCG vaccination induced expansion of NK cells but not NKT cells (Fig. 1A), and elicited three-fold more IL-22++ NK cells than NKT cells (Fig 1C), suggesting that NK cells may play a greater role in BCG-induced responses than NKT cells. We found that NK1.1+ cells lyse M. tb H37Rv-expanded CD4CD25hi cells in vivo, and that depletion of NK1.1+ cells at the time of BCG vaccination induced expansion of immunosuppressive CD4CD25hi cells after challenge with virulent M. tb H37Rv. These findings confirm our previous work showing that activated human NK cells lyse M. tb-expanded Tregs in vitro (8), and extend this mechanism to a physiologically relevant model of vaccination-induced protection against subsequent infection. It is possible that NKT cells may also destroy Tregs, although there are no published data demonstrating this effect.
Tregs inhibit immune responses to many intracellular pathogens, including M. tb, at least in part by delaying trafficking of effector T-cells to the site of infection (13). Preventing recruitment of Tregs by antagonism of CCR4 enhanced mycobacterial antigen-specific responses to a vaccine expressing Ag85a (14), and depletion of Tregs with anti-CD25 Ab increased T-cell responses and improved protection against challenge with Herpes simplex virus and Plasmodium (15-17). In contrast, treatment with anti-CD25 prior to BCG vaccination led to a stronger T-cell response but did not enhance protection against M. tb or M. bovis (18). In the current study, depletion of NK1.1+ cells at the time of BCG vaccination increased the number of Tregs, reduced T-cell production of IFN-γ, and increased the bacillary burden after challenge with M. tb H37Rv. These findings suggest that NK1.1+ cells lyse antigen-induced Tregs that normally expand during vaccination, and may yield memory Tregs that could further expand during secondary infection (19,20).
Our in vivo cytotoxicity results showed that NK1.1+ cells in BCG-vaccinated mice lysed M. tb H37Rv-expanded but not natural Tregs. Our in vivo cytotoxicity results strongly suggest that NK1.1+ cells in BCG-vaccinated mice lysed M. tb H37Rv-expanded but not natural Tregs. While it is theoretically possible that our results could be explained by reduced homing of expanded Tregs to the spleen and lymph nodes in the absence of NK cells, no published data suggest that depletion of any cell population has a major effect on Treg homing to secondary lymphoid organs. Because BCG vaccination increased the number of IFN-γ- and IL-22-producing NK cells, we speculate that NK cells are activated after BCG vaccination and have an increased capacity to lyse expanded but not natural Tregs, paralleling findings in humans (8). NK cells lyse their targets through interactions between NK cell activating receptors and their ligands. A prominent NK cell activating receptor is NKG2D and its ligands in human cells include UL-16 binding proteins (ULBPs) and MHC class I-related chain A and B (21,22). Activated Tregs express higher levels of ULBP-1 than natural Tregs, and NK cell lysis of activated Tregs is inhibited by anti-ULBP-1 and anti-NKG2D (8). It is intriguing to speculate that murine expanded Tregs may also have increased expression of the NKG2D ligands RAE-1, H60, and MULT-1, compared to natural Tregs, and thus be more susceptible to lysis by activated NK cells.
The best studied murine NKT cells are those bearing an invariant Vα14-Jα18 TCR that recognizes α-galactosylceramide and are restricted by CD1d. Pharmacologic activation of these cells with synthetic α-galactosylceramide improves the outcome of M. tb infection (23,24). However, M. tb and M. bovis BCG do not contain natural antigens for invariant NKT cells, and infection of dendritic cells with M. bovis BCG fails to activate invariant NKT cells (25). There are also no published data demonstrating that NKT cells lyse Tregs. Combined with our current findings that BCG vaccination elicits expansion of NK cells but not NKT cells (Fig. 1A), and production of IL-22 primarily by NK cells (Fig. 1C), we believe that NK cells are likely to play the dominant role in lysing Tregs, increasing T-cell IFN-γ production and increasing protective efficacy against M. tb challenge after BCG vaccination. However, we cannot formally exclude a role for NKT cells in these processes.
IL-22 is produced by activated T-cells (26), particularly Th17 cells (27), and is a critical mediator of early mucosal defense against bacteria that cause intestinal disease and pneumonia in mouse models (28). IL-22 is also produced by murine mucosal cells that express NK cell surface markers (29, 30), by human NK cells or NK-like cells in secondary lymphoid tissue (31,32, 33), and by invariant NKT cells (34,35). IL-22 is clearly involved in the immune response to tuberculosis, as IL-22-producing M. tb-responsive CD4+ T cells are present in persons with latent tuberculosis infection (36), IL-22 levels are elevated at the site of disease in tuberculosis patients (37), and CD4+ T-cells from non-human primates and human NK cells produce IL-22, which inhibits growth of M. tb in macrophages (38, 7). However, IL-22 is not required for protection against tuberculosis, as neutralization of IL-22 did not increase susceptibility to infection with M. tb by aerosol (39). We found that BCG vaccination expands IL-22-producing NK1.1+ cells, and that recombinant IL-22 reversed the effects of depleting NK1.1+ cells by significantly reducing the number of Tregs, enhancing antigen-specific CD4+ T-cell responses upon challenge with M. tb, and increasing the number of memory T-cells (Fig. 6). In combination with prior studies, this suggests that NK1.1+ cell-derived IL-22 contributes to vaccine-induced protective immunity but not to the primary immune response to M. tb, as is the case for IL-17 (40). However, IL-17 and IL-22 appear to mediate vaccine-induced protective immune responses through distinct mechanisms. IL-17 induces local chemokine production, which leads to optimal priming of T-cells (40), whereas IL-22 inhibits expansion of induced Tregs and enhances antigen-specific T-cell responses, resulting in a reduced bacillary burden after challenge with M. tb H37Rv.
The mechanisms through which IL-22 inhibits Treg expansion and enhances T-cell responses remain uncertain. IL-22R is expressed by M. tb-infected monocyte derived macrophages (7), but not by T-cells (41,42). Dendritic cells are potent APCs that can be monocyte-derived, and different dendritic cell subpopulations favor expansion of Tregs and antigen-reactive Th1 cells (43-45). We speculate that IL-22 may skew dendritic cell subpopulations toward those that favor expansion of Th1 cells, and/or elicit migration of these subpopulations toward the site of BCG vaccination or to the lung during aerosol challenge with M. tb H37Rv.
In summary, using a mouse model of vaccination with BCG, followed by challenge with M. tb H37Rv, we found that NK1.1+ cells and IL-22 contribute to vaccine-induced protection against microbial challenge by reducing the numbers of immunosuppressive Tregs and enhancing antigen-specific T-cell responses. Further delineation of the mechanisms through which NK1.1+ cells destroy Tregs and optimize Th1 responses during BCG vaccination will facilitate development of vaccines against M. tb and other intracellular pathogens.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (AI054629, AI073612 and A1085135 to RV and the Potts Memorial Foundation grant to RD), the Cain Foundation for Infectious Disease Research, and the Center for Pulmonary and Infectious Disease Control. Peter F. Barnes holds the Margaret E. Byers Cain Chair for Tuberculosis Research.
Footnotes
Conflict of interest: The authors have no financial or commercial conflict of interest.
Reference List
- 1.Harshan KV, Gangadharam PR. In vivo depletion of natural killer cell activity leads to enhanced multiplication of Mycobacterium avium complex in mice. Infect.Immun. 1991;59:2818–2821. doi: 10.1128/iai.59.8.2818-2821.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Junqueira-Kipnis AP, Kipnis A, Jamieson A, Juarrero MG, Diefenbach A, Raulet DH, Turner J, Orme IM. NK cells respond to pulmonary infection with Mycobacterium tuberculosis, but play a minimal role in protection. J.Immunol. 2003;171:6039–6045. doi: 10.4049/jimmunol.171.11.6039. [DOI] [PubMed] [Google Scholar]
- 3.Feng CG, Kaviratne M, Rothfuchs AG, Cheever A, Hieny S, Young HA, Wynn TA, Sher A. NK cell-derived IFN-gamma differentially regulates innate resistance and neutrophil response in T cell-deficient hosts infected with Mycobacterium tuberculosis. J.Immunol. 2006;177:7086–7093. doi: 10.4049/jimmunol.177.10.7086. [DOI] [PubMed] [Google Scholar]
- 4.Vankayalapati R, Wizel B, Weis SE, Safi H, Lakey DL, Mandelboim O, Samten B, Porgador A, Barnes PF. The NKp46 receptor contributes to NK cell lysis of mononuclear phagocytes infected with an intracellular bacterium. J.Immunol. 2002;168:3451–3457. doi: 10.4049/jimmunol.168.7.3451. [DOI] [PubMed] [Google Scholar]
- 5.Vankayalapati R, Garg A, Porgador A, Griffith DE, Klucar P, Safi H, Girard WM, Cosman D, Spies T, Barnes PF. Role of NK cell-activating receptors and their ligands in the lysis of mononuclear phagocytes infected with an intracellular bacterium. J.Immunol. 2005;175:4611–4617. doi: 10.4049/jimmunol.175.7.4611. [DOI] [PubMed] [Google Scholar]
- 6.Vankayalapati R, Klucar P, Wizel B, Weis SE, Samten B, Safi H, Shams H, Barnes PF. NK cells regulate CD8+ T cell effector function in response to an intracellular pathogen. J.Immunol. 2004;172:130–137. doi: 10.4049/jimmunol.172.1.130. [DOI] [PubMed] [Google Scholar]
- 7.Dhiman R, Indramohan M, Barnes PF, Nayak RC, Paidipally P, Rao LV, Vankayalapati R. IL-22 produced by human NK cells inhibits growth of Mycobacterium tuberculosis by enhancing phagolysosomal fusion. J.Immunol. 2009;183:6639–6645. doi: 10.4049/jimmunol.0902587. [DOI] [PubMed] [Google Scholar]
- 8.Roy S, Barnes PF, Garg A, Wu S, Cosman D, Vankayalapati R. NK cells lyse T regulatory cells that expand in response to an intracellular pathogen. J.Immunol. 2008;180:1729–1736. doi: 10.4049/jimmunol.180.3.1729. [DOI] [PubMed] [Google Scholar]
- 9.Schierloh P, Yokobori N, Aleman M, Musella RM, Beigier-Bompadre M, Saab MA, Alves L, Abbate E, de la Barrera SS, Sasiain MC. Increased susceptibility to apoptosis of CD56dimCD16+ NK cells induces the enrichment of IFN-gamma-producing CD56bright cells in tuberculous pleurisy. J.Immunol. 2005;175:6852–6860. doi: 10.4049/jimmunol.175.10.6852. [DOI] [PubMed] [Google Scholar]
- 10.Bluman EM, Schnier GS, Avalos BR, Strout MP, Sultan H, Jacobson FW, Williams DE, Carson WE, Caligiuri MA. The c-kit ligand potentiates the allogeneic mixed lymphocyte reaction. Blood. 1996;88:3887–3893. [PubMed] [Google Scholar]
- 11.Matos ME, Schnier GS, Beecher MS, Ashman LK, William DE, Caligiuri MA. Expression of a functional c-kit receptor on a subset of natural killer cells. J.Exp.Med. 1993;178:1079–1084. doi: 10.1084/jem.178.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall LJ, Clare S, Dougan G. NK cells influence both innate and adaptive immune responses after mucosal immunization with antigen and mucosal adjuvant. J.Immunol. 2010;184:4327–4337. doi: 10.4049/jimmunol.0903357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shafiani S, Tucker-Heard G, Kariyone A, Takatsu K, Urdahl KB. Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J.Exp.Med. 2010;207:1409–1420. doi: 10.1084/jem.20091885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayry J, Tchilian EZ, Davies MN, Forbes EK, Draper SJ, Kaveri SV, Hill AV, Kazatchkine MD, Beverley PC, Flower DR, Tough DF. In silico identified CCR4 antagonists target regulatory T cells and exert adjuvant activity in vaccination. Proc.Natl.Acad.Sci.U.S.A. 2008;105:10221–10226. doi: 10.1073/pnas.0803453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J.Exp.Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toka FN, Suvas S, Rouse BT. CD4+ CD25+ T cells regulate vaccine-generated primary and memory CD8+ T-cell responses against herpes simplex virus type 1. J.Virol. 2004;78:13082–13089. doi: 10.1128/JVI.78.23.13082-13089.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore AC, Gallimore A, Draper SJ, Watkins KR, Gilbert SC, Hill AV. Anti-CD25 antibody enhancement of vaccine-induced immunogenicity: increased durable cellular immunity with reduced immunodominance. J.Immunol. 2005;175:7264–7273. doi: 10.4049/jimmunol.175.11.7264. [DOI] [PubMed] [Google Scholar]
- 18.Quinn KM, Rich FJ, Goldsack LM, de Lisle GW, Buddle BM, Delahunt B, Kirman JR. Accelerating the secondary immune response by inactivating CD4(+)CD25(+) T regulatory cells prior to BCG vaccination does not enhance protection against tuberculosis. Eur.J.Immunol. 2008;38:695–705. doi: 10.1002/eji.200737888. [DOI] [PubMed] [Google Scholar]
- 19.Vukmanovic-Stejic M, Zhang Y, Cook JE, Fletcher JM, McQuaid A, Masters JE, Rustin MH, Taams LS, Beverley PC, Macallan DC, Akbar AN. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J.Clin.Invest. 2006;116:2423–2433. doi: 10.1172/JCI28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vukmanovic-Stejic M, Agius E, Booth N, Dunne PJ, Lacy KE, Reed JR, Sobande TO, Kissane S, Salmon M, Rustin MH, Akbar AN. The kinetics of CD4+Foxp3+ T cell accumulation during a human cutaneous antigen-specific memory response in vivo. J.Clin.Invest. 2008;118:3639–3650. doi: 10.1172/JCI35834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 22.Cosman D, Mullberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, Kubin M, Chalupny NJ. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–133. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 23.Chackerian A, Alt J, Perera V, Behar SM. Activation of NKT cells protects mice from tuberculosis. Infect.Immun. 2002;70:6302–6309. doi: 10.1128/IAI.70.11.6302-6309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sada-Ovalle I, Skold M, Tian T, Besra GS, Behar SM. Alpha-galactosylceramide as a therapeutic agent for pulmonary Mycobacterium tuberculosis infection. Am.J.Respir.Crit Care Med. 2010;182:841–847. doi: 10.1164/rccm.200912-1921OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venkataswamy MM, Baena A, Goldberg MF, Bricard G, Im JS, Chan J, Reddington F, Besra GS, Jacobs WR, Jr., Porcelli SA. Incorporation of NKT cell-activating glycolipids enhances immunogenicity and vaccine efficacy of Mycobacterium bovis bacillus Calmette-Guerin. J.Immunol. 2009;183:1644–1656. doi: 10.4049/jimmunol.0900858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, Stinson J, Wood WI, Goddard AD, Gurney AL. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J.Biol.Chem. 2000;275:31335–31339. doi: 10.1074/jbc.M005304200. [DOI] [PubMed] [Google Scholar]
- 27.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat.Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 29.Luci C, Reynders A, Ivanov II, Cognet C, Chiche L, Chasson L, Hardwigsen J, Anguiano E, Banchereau J, Chaussabel D, Dalod M, Littman DR, Vivier E, Tomasello E. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat.Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- 30.Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat.Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, Fibbe WE, Cornelissen JJ, Spits H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat.Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 33.Hughes T, Becknell B, McClory S, Briercheck E, Freud AG, Zhang X, Mao H, Nuovo G, Yu J, Caligiuri MA. Stage 3 immature human natural killer cells found in secondary lymphoid tissue constitutively and selectively express the TH 17 cytokine interleukin-22. Blood. 2009;113:4008–4010. doi: 10.1182/blood-2008-12-192443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watarai H, Sekine-Kondo E, Shigeura T, Motomura Y, Yasuda T, Satoh R, Yoshida H, Kubo M, Kawamoto H, Koseki H, Taniguchi M. Development and function of invariant natural killer T cells producing T(h)2- and T(h)17-cytokines. PLoS.Biol. 2012;10:e1001255. doi: 10.1371/journal.pbio.1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paget C, Ivanov S, Fontaine J, Renneson J, Blanc F, Pichavant M, Dumoutier L, Ryffel B, Renauld JC, Gosset P, Gosset P, Si-Tahar M, Faveeuw C, Trottein F. Interleukin-22 is produced by invariant natural killer T lymphocytes during influenza A virus infection: potential role in protection against lung epithelial damages. J.Biol.Chem. 2012;287:8816–8829. doi: 10.1074/jbc.M111.304758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scriba TJ, Kalsdorf B, Abrahams DA, Isaacs F, Hofmeister J, Black G, Hassan HY, Wilkinson RJ, Walzl G, Gelderbloem SJ, Mahomed H, Hussey GD, Hanekom WA. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J.Immunol. 2008;180:1962–1970. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matthews K, Wilkinson KA, Kalsdorf B, Roberts T, Diacon A, Walzl G, Wolske J, Ntsekhe M, Syed F, Russell J, Mayosi BM, Dawson R, Dheda K, Wilkinson RJ, Hanekom WA, Scriba TJ. Predominance of interleukin-22 over interleukin-17 at the site of disease in human tuberculosis. Tuberculosis.(Edinb.) 2011 doi: 10.1016/j.tube.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeng G, Chen CY, Huang D, Yao S, Wang RC, Chen ZW. Membrane-Bound IL-22 after De Novo Production in Tuberculosis and Anti-Mycobacterium tuberculosis Effector Function of IL-22+ CD4+ T Cells. J.Immunol. 2011;187:190–199. doi: 10.4049/jimmunol.1004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson MS, Feng CG, Barber DL, Yarovinsky F, Cheever AW, Sher A, Grigg M, Collins M, Fouser L, Wynn TA. Redundant and pathogenic roles for IL-22 in mycobacterial, protozoan, and helminth infections. J.Immunol. 2010;184:4378–4390. doi: 10.4049/jimmunol.0903416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat.Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 41.Aujla SJ, Kolls JK. IL-22: A critical mediator in mucosal host defense. J.Mol.Med. 2009 doi: 10.1007/s00109-009-0448-1. [DOI] [PubMed] [Google Scholar]
- 42.Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22. Semin.Immunopathol. 2010;32:17–31. doi: 10.1007/s00281-009-0188-x. [DOI] [PubMed] [Google Scholar]
- 43.Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, Inaba K, Nussenzweig MC, Steinman RM. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J.Immunol. 2008;181:6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Idoyaga J, Steinman RM. SnapShot: Dendritic Cells. Cell. 2011;146:660. doi: 10.1016/j.cell.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 45.Kushwah R, Hu J. Role of dendritic cells in the induction of regulatory T cells. Cell Biosci. 2011;1:20. doi: 10.1186/2045-3701-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.