Abstract
Increased extracellular brain glutamate has been implicated in the pathophysiology of human refractory temporal lobe epilepsy (TLE), but the cause of the excessive glutamate is unknown. Prior studies by us and others have shown that the glutamate degrading enzyme glutamine synthetase (GS) is deficient in astrocytes in the epileptogenic hippocampal formation in a subset of patients with TLE. We have postulated that the loss of GS in TLE leads to increased glutamate in astrocytes with elevated concentrations of extracellular glutamate and recurrent seizures as the ultimate end-points. Here we test the hypothesis that the deficiency in GS leads to increased glutamate in astrocytes. Rats were chronically infused with methionine sulfoximine (MSO, n=4) into the hippocampal formation to induce GS-deficiency and recurrent seizures. A separate group of rats was infused with 0.9% NaCl (saline) as a control (n=6). At least 10 days after the start of infusion, once recurrent seizures were established in the MSO-treated rats, the concentration of glutamate was assessed in CA1 of the hippocampal formation by immunogold electron microscopy. The concentration of glutamate was 47% higher in astrocytes in the MSO-treated vs. saline-treated rats (p=0.02), and the ratio of glutamate in astrocytes relative to axon terminals was increased by 74% in the MSO-treated rats (p=0.003). These data support our hypothesis that a deficiency in GS leads to increased glutamate in astrocytes. We additionally propose that the GS-deficient astrocytes in the hippocampal formation in TLE lead to elevated extracellular brain glutamate either through decreased clearance of extracellular glutamate or excessive release of glutamate into the extracellular space from these cells, or a combination of the two.
Keywords: animal models, excitotoxicity, glutamine synthetase, hippocampal sclerosis, methionine sulfoximine
Introduction
Temporal lobe epilepsy (TLE) is one of the most common types of drug-resistant epilepsies in humans, and more efficacious therapies for this disorder are therefore needed (Hauser et al., 1991). A better understanding of the molecular and cellular mechanisms that cause drug-resistant TLE is likely to facilitate the discovery of such therapies.
Several observations suggest that metabolic perturbation of glutamatergic neurotransmission may be important in the causation of TLE. Firstly, patients with drug-resistant TLE have a five-fold increase in extracellular glutamate in the epileptogenic vs. the nonepileptogenic hippocampal formation interictally (i.e. several hours away from seizures) (Cavus et al., 2005). Secondly, extracellular glutamate in the epileptogenic hippocampal formation of these patients increases six-fold above the interictal level during a seizure and remains elevated for several hours after the cessation of the seizure (During and Spencer, 1993). Finally, administration of glutamate and glutamate analogues to laboratory animals causes seizures and brain damage similar to that of human TLE (Nadler and Cuthbertson, 1980; Nadler et al., 1978; Olney, 1978; Olney et al., 1986; Olney et al., 1972). Hence, it has been postulated that increased extracellular glutamate is implicated in the pathophysiology of seizures and brain damage in TLE (Gloor, 1991; Olney et al., 1986).
We and others have previously reported that glutamine synthetase (GS) is severely deficient in a subpopulation of astrocytes in the hippocampal formation in human TLE (Eid et al., 2004; van der Hel et al., 2005). The etiology of the GS deficiency in TLE is not known, but may involve factors such as free radical damage to the enzyme (Bidmon et al., 2008; Butterfield et al., 1997), loss of neuronal input (Derouiche et al., 1993), and mechanisms involving corticosteroids (Jackson et al., 1995; Stanimirovic et al., 1999; Vardimon et al., 1999), c-Jun (Vardimon et al., 1999) and cytokines (Huang and O'Banion, 1998). Glutamate released from neurons into the extracellular space is normally taken up by astrocytes via glutamate transporters (Danbolt, 2001) and converted to glutamine by GS, which is present in the cytoplasm of astrocytes (Martinez-Hernandez et al., 1977). We have hypothesized that the deficiency in GS in TLE slows the conversion of glutamate to glutamine and leads to chronic accumulation of glutamate in astrocytes and the extracellular space (Eid et al., 2004). We have further postulated that the loss of GS in TLE is critically important in the causation of seizures and brain damage in this disorder (Eid et al., 2004). Interestingly, patients with congenital deficiency in GS due to a somatic mutation of the GS gene present with extensive brain malformations and seizures (Haberle et al., 2005; Haberle et al., 2006). Furthermore, deficiency in GS has also been reported in the brain of patients with Alzheimer’s disease (Hensley et al., 1995; Smith et al., 1991), which is associated with a higher incidence of epilepsy (Amatniek et al., 2006).
The idea that a deficiency in GS is implicated in the pathophysiology of TLE is also supported by studies of laboratory rats. Continuous (~28 days) infusion of the GS inhibitor methionine sulfoximine (MSO) unilaterally into the hippocampal formation in rats results in sustained inhibition of GS in the infused brain area with nearly normal enzyme activity in the contralateral hemisphere (Eid et al., 2008; Wang et al., 2009). Within 48 hrs of the onset of MSO infusion, the animals develop subclinical or low-grade clinical status epilepticus, which may last for several hours. The initial status epilepticus is usually followed by a latent period of approximately 7 days during which there is no clinical seizure activity (Wang et al., 2009). Recurrent, clinical seizures commence after the end of the latent period (Wang et al., 2009). The chronological development of TLE in humans frequently involves a similar sequence of events, although the latent period in humans may last for several years. Finally, about 20% of the MSO-treated animals exhibit neuropathological changes similar to hippocampal sclerosis, which is one of the hallmarks of human TLE (Eid et al., 2008; Wang et al., 2009). Thus, the MSO model has many similarities to human TLE and may prove to be a useful tool for studies of the molecular mechanisms that cause this condition.
The objective of the present study is to further characterize the MSO model by addressing the hypothesis that a deficiency in GS in the hippocampal formation results in elevated concentrations of glutamate in astrocytes. The presence of increased glutamate in astrocytes is significant because such a finding implicates astrocytes in the perturbed glutamate homeostasis in TLE. If astrocytes are critically involved in the causation of TLE, then these cells could be exploited as highly specific therapeutic targets for this disorder. Part of this work has been presented in abstract form (Perez et al., 2010).
Materials and Methods
Chemicals and animals
Chemicals were obtained from Sigma-Aldrich (St. Louis, Mo.) unless otherwise noted. Male Sprague Dawley rats were used in this study (MSO model, 200 to 250 g; Charles River Laboratories, Wilmington, Mass.). Food and water were provided ad libitum. The animal care and use procedures were approved by the Institutional Animal Care and Use Committee of Yale University. All studies were performed following current guidelines.
Rat model of GS deficiency and recurrent seizures
The procedure for creating the MSO-model has been described in detail (Eid et al., 2008; Wang et al., 2009), and therefore a brief description is provided here. Under Isoflurane anesthesia, a drug delivery cannula was introduced into the right hippocampus and connected to a subcutaneously implanted Alzet osmotic minipump (Model 2004, Durect Corp., Cupertino, Calif.). In one set of animals, the pump was filled with MSO to achieve a delivery of 0.625 µg of MSO per h for ~28 days (as per manufacturer’s specifications). In another set, the pump was filled with 0.9 % NaCl (saline). Unipolar electrodes (E363/2/SPC stainless steel electrode, Plastics One, Roanoke, Va.) with a bare diameter of 0.2 mm and an insulated diameter of 0.23 mm, were introduced into the left and right dorsal hippocampus to record EEG activity.
Video-intracranial EEG monitoring
The experimental setup for recording video-EEG was adapted from Bertram et al. (Bertram et al., 1997). The rats were housed individually in custom-made Plexiglas cages and connected to the video-EEG recording system immediately after surgery. A spring-covered, 6-channel cable connected the electrode pedestal to a commutator (Plastics One), while a second cable connected the commutator to the digital EEG recording unit (Ceegraph Vision LTM, Natus Medical Incorporated, San Carlos, Calif.). Digital cameras with infrared capacity were used to record animal behavior. The digital video signal was encoded and synchronized to the digital EEG signals. Seizures were identified by visual inspection of the EEG record. Interictal activity is essentially transient in nature and can be distinguished from seizures by its duration and signal features. Subclinical seizures can be distinguished from clinical seizures by examination of the video record. To establish the start and stop points of a seizure we determined a point that was unequivocally within the seizure, and moved backward and forward in time to the points where the EEG was characterized by normal baseline activity. The video record was examined to score the seizures on a modified Racine scale (Racine et al., 1973) as follows: Subclinical, no remarkable behavior; Stage I, immobilization, eye blinking, twitching of vibrissae and mouth movements; Stage II, head nodding, often accompanied by facial clonus; Stage III, forelimb clonus; Stage IV, rearing; Stage V, rearing, falling and generalized convulsions. Recurrent seizures were defined as ≥2 seizures of Stage III or higher at least 1 h apart.
Tissue preparation and immunogold electron microscopy
After continuous video-EEG recordings for several days, the rats were re-anesthetized with pentobarbital and perfused transcardially with 0.9% NaCl (saline) for < 10 s, followed by a solution of 2.5% glutaraldehyde and 1 % paraformaldehyde in phosphate buffer (PB; 0.1M, pH 7.4). The brains were subsequently removed and post-fixed in the same solution for at least 3 days. A Vibratome was used to cut 50 µm-thick brain sections. Every fifth section was transferred to a gelatin-coated slide and stained with cresyl violet. Subsets of the remaining sections were processed for immunogold electron microscopy.
The procedure for immunogold electron microscopy has been described in detail previously (Laake et al., 1995; Ottersen, 1987). In brief, Vibratome sections were osmicated, stained with uranyl acetate, dehydrated, and flat-embedded in Durcupan ACM (Fluka, Sigma-Aldrich). Ultrathin sections were then cut from CA1, transferred to 600 hexagonal mesh nickel grids, and processed for on-grid immunogold labeling. Following pretreatment with 9% sodium meta periodate, sections were incubated for 2 h at room temperature with a glutamate antibody (AB133, Millipore, Billerica, Mass.). The primary antibody was diluted 1:500 in tris-buffered saline with Triton-X-100 (TBST: 0.5 M Tris, 0.01 M phosphate buffer, 0.3% Triton-X-100, pH 7.4) containing 3% human serum albumin (A-1653 Sigma Chemical, St. Louis, Mo.). After several washes in TBST, sections were incubated for 2 h at room temperature with a secondary antibody (goat anti-rabbit IgG) coupled to colloidal gold particles with a diameter of 10nm (EM GAR10, BBInternational, Cardiff, U.K.). The secondary antibody was diluted 1:20 in TBST containing 10% polyethylene glycol (P-2263, Sigma-Aldrich) and 3% human serum albumin. Finally, the sections were rinsed and stained with 1% uranyl acetate for 20 min and 1% lead citrate for 1–2 min.
Electron microscopy
Using an FEI Tecnai 12 transmission electron microscope (Hillsboro, Ore.), the densities of gold particles in profiles of axon terminals with asymmetrical synapses and in profiles of astrocytes were determined. Only gold particles detected within the cytoplasm or touching the plasma membrane of the profiles were included. The cell nucleus was excluded from the analysis. At least 10 images of each profile were captured per rat. The investigator capturing the images was blinded to the identity of the experimental groups. Morphologic criteria, according to the work of Peters et al. were used to identify the different profile types (Peters et al., 1991). Astrocytes were identified by their electron-lucent cytoplasm, irregular profiles, and presence of intermediate filaments in bundles. Axon terminals were identified by their presence of synaptic vesicles. Only terminals associated with asymmetrical synaptic contacts were included in the analysis.
Quantitation and statistical analysis
ImageJ (National Institutes of Health, Bethesda, Md.) was used to quantify the gold particle density in each profile in saline treated (n=6) and MSO treated (n=4) rats. For each rat, the mean particle density in each profile was calculated by averaging the particle densities of all its corresponding images. A two-way student’s t-test was used for statistical comparisons with p < 0.05 representing a significant difference. Unless otherwise noted, the data are presented as mean ± SD.
Antibody quality assessments
The glutamate antibody was tested for specificity by incubating ultrathin sections of dialyzed brain homogenates spiked with individual aminoacids (“sandwich controls”) as previously described (Ottersen, 1987). The aminoacids tested were: GABA, glutamate, taurine, glycine, aspartate and glutamine (Fig. 3). The ultrathin sandwich control sections were processed for immunogold labeling using the same procedure as for the rat brain sections. The gold particle density over each aminoacid homogenate was determined and expressed as a percentage of the gold particle density over the brain homogenate containing glutamate. The cross-reactivity was <1% for GABA, taurine, glycine and aspartate, and <10% for glutamine. In additional controls, ultrathin rat brain sections were incubated without either the primary or the secondary antibody. No labeling was observed in either study. To minimize experimental variability, all sections were processed for immunogold labeling simultaneously.
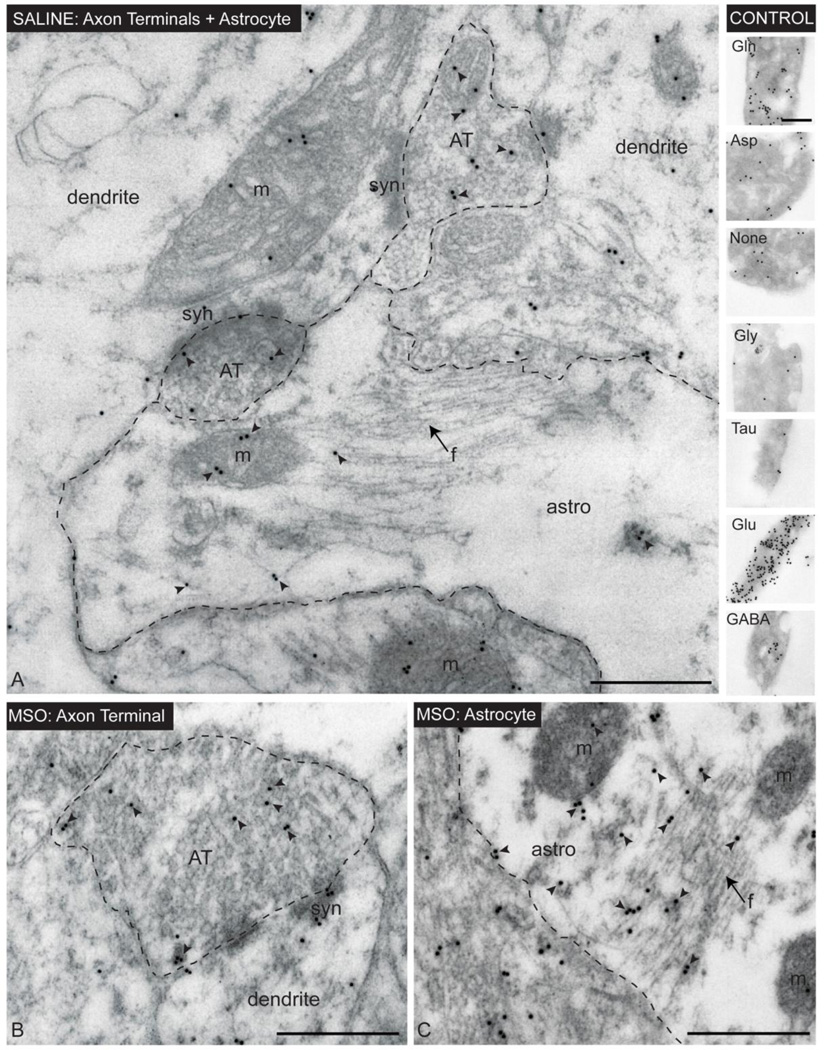
Figure 3. Immunogold electron microscopy of glutamate in CA1 of the hippocampal formation in saline- and MSO-infused rats.
Immunogold labeling for glutamate (arrowheads) is evident in astrocyte profiles (astro), axon terminals (AT) and dendrites (dendrite) in saline- (A) and MSO-infused (B, C) rats. Note the close association between astrocytes and asymmetrical (glutamatergic) synapses (syn) in A. Arrows point to bundles of intermediate filaments (f) in astrocytes. The control “sandwich” in the upper right part of the picture was processed simultaneously with the rat brain tissue for glutamate immunogold labeling. The “sandwich” represents a specificity control of the antibody used (see Materials and Methods for details). Abbreviations: astro, astrocyte; AT, axon terminal; ; m, mitochondria; syn, synaptic contact; Gln, glutamine; Asp, Aspartate; Gly, glycine; Tau, taurine, Glu, glutamate, GABA, gammaaminobutyric acid. Bars: A–C and control, 500 nm.
Results
Nissl stain
Using Nissl-stained tissue sections, we first assessed the general histopathology of the hippocampal formations from each of the two groups of rats included in this study (Fig. 1). Animals continuously infused with saline (n=6) or MSO (n=4) into the right hippocampal formation (n=6) displayed a narrow zone of neuronal loss and gliosis around the infusion cannula, consistent with Grade I pathological change [Fig. 1A, B; see (Wang et al., 2009) for an explanation of the grading system]. Five of the 6 saline-infused animals showed neither significant neuronal loss nor gliosis in the remainder of the hippocampal formation, including CA1 (Fig. 1C). One of the 6 saline-infused rats had a small area of neuronal loss and gliosis in CA2; however, all other subfields were intact. None of the MSO-infused rats exhibited significant neuronal loss or glial proliferation in the hippocampal formation away from the infusion cannula track (Fig. 1B), including CA1 (Fig. 1D).
Figure 1. Nissl-stained horizontal sections of hippocampal formations from saline- and MSO- infused rats.
Rats infused with saline (A, C) or MSO (B, D) into the right (R) hippocampal formation displayed a modest lesion around the infusion cannula track (asterisk). Outside this lesion, no significant neuronal loss was evident on visual examination. Area CA1 (C, D), which appeared morphologically intact, was used for immunogold analysis of glutamate. C and D are high power fields of the framed areas in A and B, respectively. Abbreviations: CA1, CA2, CA3: subfields of hippocampal formation; MSO: methionine sulfoximine. Bars: A–B, 500 µm; C–D, 200 µm.
Continuous video-intracranial EEG monitoring
All rats were monitored continuously by video and intracranial EEG for a period of 10 to 14 days. None of the saline-infused rats (n=6) exhibited recurrent seizures (Fig. 2A), and all of the MSO-treated rats (n=4) developed recurrent seizures (Fig. 2B). The electrographic and behavioral aspects of the seizures were similar to previously documented observations in this animal model (Eid et al., 2008; Wang et al., 2009).
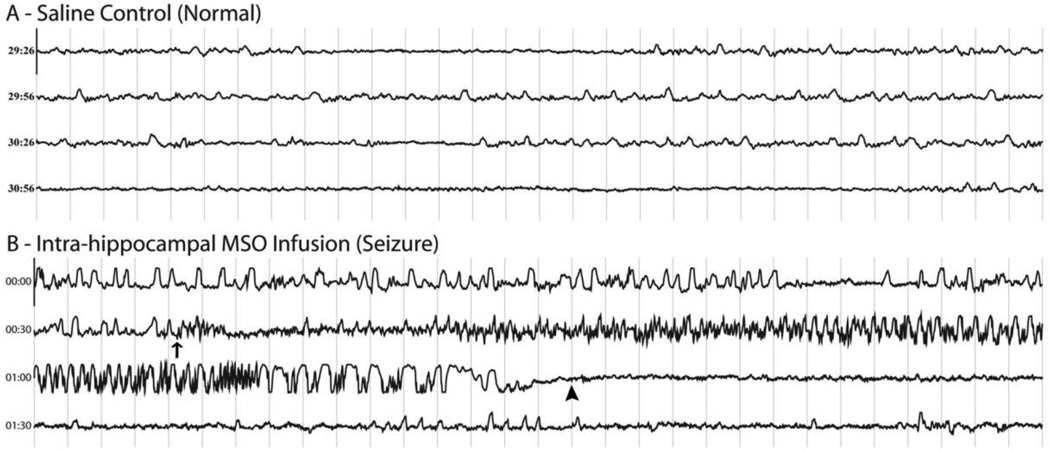
Figure 2. Intracranial EEG traces from saline- and MSO-infused rats.
Continuous 120 s EEG traces recorded from epidural screw electrodes placed over the left or right parietal cortex are displayed. Thirty seconds of EEG are shown in each horizontal trace. The light gray vertical lines denote intervals of 1 s, and the length of the Y-axis corresponds to 7 mV. Trace A depicts normal EEG activity from a saline-infused (control) rat. Trace B depicts electrographic seizure activity from an MSO-infused rat. Seizure onset in B is marked with an arrow, and seizure offset is marked with an arrowhead.
Immunogold electron microscopy
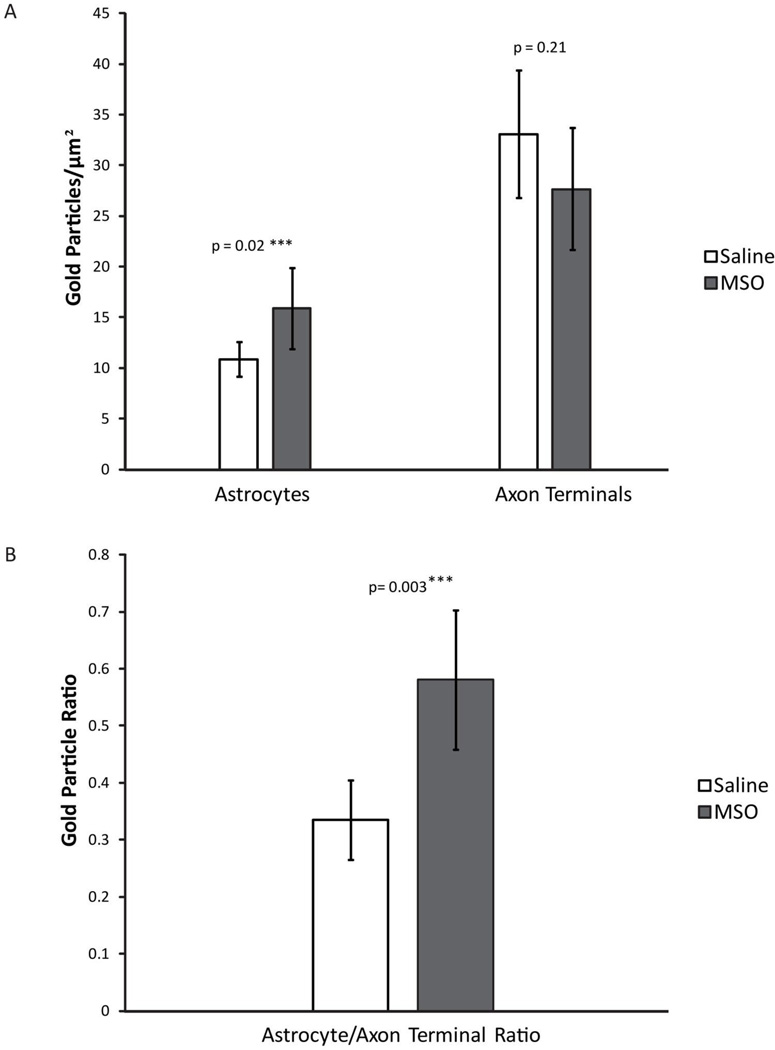
We next analyzed the tissue ultrastructure and distribution of glutamate in CA1 from saline-infused controls (n=6, Fig. 3A) and MSO-infused rats (n=4, Fig. 3B–C). Numerous profiles of morphologically intact astrocytes and axon terminals were detected in both groups (Fig. 3). Notably, many of the astrocyte profiles were closely associated with glutamatergic axon terminals (Fig 3A), as expected from studies by others (Peters et al., 1991). Although quantitation of labeling for glutamate revealed no significant difference in the glutamate density of axon terminals in the MSO-infused vs. the saline-infused hippocampus (p=0.21, Fig. 4A), there was a non-significant trend towards less glutamate label in the MSO group. However, the labeling for glutamate in astrocytes was significantly increased by 47% in animals treated with MSO vs. saline (p=0.02, Fig. 4A). To minimize the variability in labeling intensity due to fixation and tissue processing, we calculated the ratio of glutamate labeling between astrocyte profiles and axon terminals in each animal. There was a highly significant increase in the astrocyte/axon terminal glutamate ratio by 74% in the MSO-treated vs. saline-treated group (p=0.003, Fig. 4B).
Figure 4. Glutamate is redistributed from axon terminals to astrocytes in CA1 of the hippocampal formation in MSO-infused rats.
The density of immunogold labeling for glutamate in astrocytes is significantly increased in the MSO-infused vs. the saline-infused hippocampal formation (Student’s t-test p = 0.02). The ratio of glutamate in astrocytes relative to axon terminals is significantly increased in the MSO-infused vs. the saline-infused hippocampal formation (Student’s t-test: p = 0.003). Bar: mean. Error bar: SD.
Discussion
The present study demonstrates for the first time that the concentration of glutamate is increased in astrocytes relative to axon terminals in the GS-deficient hippocampal formation in laboratory rats. This finding is important because it implicates astrocytes as a possible source of the extracellular glutamate excess in patients with drug-resistant TLE, where GS has been found to be deficient (Cavus et al., 2005; During and Spencer, 1993).
It is well-established that astrocytes are key elements in the brain glutamate homeostasis. Astrocytes take up extracellular glutamate via glutamate transporter molecules located on the plasma membrane of the cells (Danbolt, 2001). Once in the astrocyte, GS converts glutamate to glutamine (Martinez-Hernandez et al., 1977), which may be shuttled to neurons and used as a precursor for the synthesis of neurotransmitter glutamate (Chaudhry et al., 2002; Svenneby, 1970). A deficiency in GS in astrocytes has been hypothesized to slow the metabolism of glutamate to glutamine and lead to accumulation of glutamate in astrocytes. High astrocytic glutamate is likely to impair the clearance of extracellular glutamate due to an increased concentration gradient across the astrocyte cytoplasm and extracellular space (Eid et al., 2004). The known stoichiometry of glutamate transport across the glial plasma membrane suggests that rapid metabolism of intracellular glutamate is a prerequisite for efficient glutamate clearance from the extracellular space (Otis and Jahr, 1998). The observation here that glutamate is increased in astrocytes in the GS-deficient hippocampal formation supports such a scenario.
A deficiency in GS is also expected to reduce the concentration of glutamate in axon terminals. This is because glutamine produced by astrocytes contributes to the synthesis of glutamate in neurons by the phosphate activated glutaminase (PAG) reaction (Jenstad et al., 2009; Svenneby, 1970). A deficiency in GS would lead to less glutamine and therefore impaired synthesis of neuronal glutamate. Studies of brain slice cultures have shown that inhibition of GS using MSO, leads to increased glutamate in astrocytes and decreased glutamate in axon terminals (Laake et al., 1995). Moreover, application of glutamine to MSO-treated brain slices increases the concentration of neuronal glutamate, suggesting that glutamine is critical for the synthesis of neurotransmitter glutamate (Laake et al., 1995). While we were unable to find a statistically significant difference in glutamate in axon terminals between the two groups, a non-significant trend towards less neuronal glutamate was evident in the MSO-infused hippocampal formation. The lack of a statistically significant difference may be explained by insufficient statistical power due to the small sample size. It is also possible that PAG is upregulated in neurons in the GS-deficient hippocampal formation, resulting in more efficient synthesis of glutamate despite low neuronal glutamine, as suggested in a recent study (Eid et al., 2007). Whether PAG is also increased in hippocampal neurons in the MSO model remains to be determined.
While the above discussion has focused on the role of GS inhibition on neuronal synthesis of glutamate, it is possible that the increased glutamate in astrocytes may be released by these cells into the extracellular space. Several lines of evidence suggest that astrocytes can release glutamate (Volterra and Meldolesi, 2005), and that such release may occur in the epileptic brain. For example, osmotic swelling of astrocytes in vitro leads to the release of astrocytic glutamate, probably via a mechanism that involves volume sensitive organic anion channels (Basarsky et al., 1999; Kimelberg et al., 1990). The possibility that swelling-induced glutamate release can occur in human TLE is indirectly supported by studies of the water channel aquaporin-4 (AQP4) in animal models and patients with TLE (Eid et al., 2005; Lee et al., 2004). The total concentration of AQP4 is increased in the epileptogenic and sclerotic hippocampal formation in human TLE, and the normal perivascular enrichment of the molecule is disrupted (Eid et al., 2005). We have postulated that the changes in AQP4 in TLE may decrease the clearance of metabolic water from the astrocyte cytoplasm to the blood and lead to astrocyte swelling and possibly glutamate release into the extracellular space (Amiry-Moghaddam and Ottersen, 2003; Anderson and Swanson, 2000; Eid et al., 2005). Other mechanisms for glutamate release by astrocytes have been proposed as well, including Ca2+ dependent mechanisms (Bezzi et al., 1998; Fellin et al., 2004; Tian et al., 2005) and release via exocytosis (Araque et al., 2000; Jourdain et al., 2007). However, most studies of astrocytic glutamate release have utilized in vitro models. Thus, it remains to be established whether glutamate release by astrocytes also occurs in the epileptogenic hippocampus in vivo and whether such release contributes to the generation of seizures in epilepsy.
It is important to note that the present study has some shortcomings because it provides a “snapshot” image of the intracellular glutamate concentrations in limited compartments of the brain (i.e. astrocytes and axon terminals in CA1). Firstly, an issue that cannot be addressed by this study is whether the intracellular glutamate concentration changes during epileptogenesis. For example, is astrocytic glutamate normal during the 7-day latency period and does it increase when the period of recurrent seizures begins? This question can be answered in more extensive studies by assessing glutamate concentrations at multiple time points starting shortly after the onset of MSO infusion. Secondly, all rats were perfusion fixed at least 12 hrs (and often much longer) after their last seizure. Thus, it is unlikely, but not impossible, that the increased astrocytic glutamate is caused by seizures alone. Studies in human TLE have shown that extracellular glutamate is chronically elevated in the epileptogenic hippocampal formation, suggesting that the increased astrocytic glutamate also is a chronic phenomenon. Nevertheless, carefully timed assessments of glutamate and seizures are required to fully resolve the relationship between seizures and astrocytic glutamate. Finally, because the increase in astrocytic glutamate may be unique to MSO-induced seizures, similar studies involving other models of TLE are warranted. Unfortunately, immunogold electron microscopy of glutamate in surgically resected brains from patients with TLE is unlikely to provide useful results. There is significant redistribution of glutamate and glutamine between neurons and glial cells after a few minutes of ischemia (Torp et al., 1991), which inevitably occurs when the blood supply is severed to the hippocampus during neurosurgical resection.
Conclusions
We have further characterized the MSO model of TLE by demonstrating increased concentrations of astrocytic glutamate in the GS-deficient epileptogenic hippocampal formation. While the biological significance of this finding remains to be established, several scenarios are possible. Firstly, increased astrocytic glutamate may impair the clearance of extracellular glutamate in the epileptogenic hippocampal formation due to a decreased concentration gradient of the neurotransmitter across the astrocyte cytoplasm and extracellular space (Eid et al., 2004). Such a scenario is important because high levels of extracellular brain glutamate may lead to epileptic seizures (Nadler and Cuthbertson, 1980; Nadler et al., 1978; Olney, 1978; Olney et al., 1986; Olney et al., 1972). Secondly, a shift in the glutamate pool from neurons to astrocytes, as demonstrated here, is also likely to slow the synthesis of neuronal GABA, possibly resulting in decreased GABAergic inhibition, increased excitability, and ultimately epileptic seizures. Finally, increased astrocytic glutamate may facilitate excessive release of glutamate by these cells, thus contributing to the extracellular glutamate excess in TLE (Bezzi et al., 1998; Fellin et al., 2004; Volterra and Meldolesi, 2005). Further studies are necessary to accurately address the questions which arise from each of these scenarios.
Highlights.
Increased extracellular brain glutamate has been implicated in the causation of epilepsy
However, the mechanism of the glutamate excess is not known
We now show that astrocytes in the epileptogenic hippocampus are enriched with glutamate
We propose that these cells may be critically involved in the glutamate excess in epilepsy
Acknowledgements
We thank Ms. Ilona Kovacs, Ms. Bjørg Riber and Ms. Karen Marie Gujord for excellent technical assistance. This work was supported by grants from the National Institutes of Health (NS058674 and NS070824 to T.E.), The Swebelius Foundation, and the Research Council of Norway (to F.L. and L.H.B.). Part of this work was also made possible by CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amatniek JC, et al. Incidence and predictors of seizures in patients with Alzheimer's disease. Epilepsia. 2006;47:867–872. doi: 10.1111/j.1528-1167.2006.00554.x. [DOI] [PubMed] [Google Scholar]
- Amiry-Moghaddam M, Ottersen OP. The molecular basis of water transport in the brain. Nat Rev Neurosci. 2003;4:991–1001. doi: 10.1038/nrn1252. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- Araque A, et al. SNARE protein-dependent glutamate release from astrocytes. J Neurosci. 2000;20:666–673. doi: 10.1523/JNEUROSCI.20-02-00666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basarsky TA, et al. Glutamate release through volume-activated channels during spreading depression. J Neurosci. 1999;19:6439–6445. doi: 10.1523/JNEUROSCI.19-15-06439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram EH, et al. Design and construction of a long-term continuous video-EEG monitoring unit for simultaneous recording of multiple small animals. Brain Res. Brain Res. Protoc. 1997;1997:85–97. doi: 10.1016/s1385-299x(97)00033-0. [DOI] [PubMed] [Google Scholar]
- Bezzi P, et al. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Bidmon HJ, et al. Glutamine synthetase becomes nitrated and its activity is reduced during repetitive seizure activity in the pentylentetrazole model of epilepsy. Epilepsia. 2008;49:1733–1748. doi: 10.1111/j.1528-1167.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, et al. Oxidatively induced structural alteration of glutamine synthetase assessed by analysis of spin label incorporation kinetics: relevance to Alzheimer's disease. J Neurochem. 1997;68:2451–2457. doi: 10.1046/j.1471-4159.1997.68062451.x. [DOI] [PubMed] [Google Scholar]
- Cavus I, et al. Extracellular metabolites in the cortex and hippocampus of epileptic patients. Ann Neurol. 2005;57:226–235. doi: 10.1002/ana.20380. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, et al. The glutamine commute: take the N line and transfer to the A. J Cell Biol. 2002;157:349–355. doi: 10.1083/jcb.200201070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog. Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Derouiche A, et al. Loss of layer-specific astrocytic glutamine synthetase immunoreactivity in slice cultures of hippocampus. Eur J Neurosci. 1993;5:122–127. doi: 10.1111/j.1460-9568.1993.tb00477.x. [DOI] [PubMed] [Google Scholar]
- During MJ, Spencer DD. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet. 1993;341:1607–1610. doi: 10.1016/0140-6736(93)90754-5. [DOI] [PubMed] [Google Scholar]
- Eid T, et al. Recurrent seizures and brain pathology after inhibition of glutamine synthetase in the hippocampus in rats. Brain. 2008;131:2061–2070. doi: 10.1093/brain/awn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid T, et al. Increased expression of phosphate-activated glutaminase in hippocampal neurons in human mesial temporal lobe epilepsy. Acta Neuropathol (Berl) 2007;113:137–152. doi: 10.1007/s00401-006-0158-5. [DOI] [PubMed] [Google Scholar]
- Eid T, et al. Loss of perivascular aquaporin 4 may underlie deficient water and K+ homeostasis in the human epileptogenic hippocampus. Proc Natl Acad Sci U S A. 2005;102:1193–1198. doi: 10.1073/pnas.0409308102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid T, et al. Loss of glutamine synthetase in the human epileptogenic hippocampus: possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet. 2004;363:28–37. doi: 10.1016/s0140-6736(03)15166-5. [DOI] [PubMed] [Google Scholar]
- Fellin T, et al. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Gloor P. Mesial temporal sclerosis: Historical background and an overview from a modern perspective. In: Luders H, editor. Epilepsy Surgery. New York: Raven Press; 1991. pp. 689–703. [Google Scholar]
- Haberle J, et al. Congenital glutamine deficiency with glutamine synthetase mutations. N Engl J Med. 2005;353:1926–1933. doi: 10.1056/NEJMoa050456. [DOI] [PubMed] [Google Scholar]
- Haberle J, et al. Inborn error of amino acid synthesis: human glutamine synthetase deficiency. J Inherit Metab Dis. 2006;29:352–358. doi: 10.1007/s10545-006-0256-5. [DOI] [PubMed] [Google Scholar]
- Hauser WA, et al. Prevalence of epilepsy in Rochester, Minnesota: 1940–1980. Epilepsia. 1991;32:429–445. doi: 10.1111/j.1528-1157.1991.tb04675.x. [DOI] [PubMed] [Google Scholar]
- Hensley K, et al. Brain regional correspondence between Alzheimer's disease histopathology and biomarkers of protein oxidation. J Neurochem. 1995;65:2146–2156. doi: 10.1046/j.1471-4159.1995.65052146.x. [DOI] [PubMed] [Google Scholar]
- Huang TL, O'Banion MK. Interleukin-1 beta and tumor necrosis factor-alpha suppress dexamethasone induction of glutamine synthetase in primary mouse astrocytes. J Neurochem. 1998;71:1436–1442. doi: 10.1046/j.1471-4159.1998.71041436.x. [DOI] [PubMed] [Google Scholar]
- Jackson MJ, et al. Effect of dibutyryl cyclic AMP and dexamethasone on glutamine synthetase gene expression in rat astrocytes in culture. Neurochem Res. 1995;20:201–207. doi: 10.1007/BF00970545. [DOI] [PubMed] [Google Scholar]
- Jenstad M, et al. System A transporter SAT2 mediates replenishment of dendritic glutamate pools controlling retrograde signaling by glutamate. Cereb Cortex. 2009;19:1092–1106. doi: 10.1093/cercor/bhn151. [DOI] [PubMed] [Google Scholar]
- Jourdain P, et al. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, et al. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J. Neurosci. 1990;10:1583–1591. doi: 10.1523/JNEUROSCI.10-05-01583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laake JH, et al. Glutamine from glial cells is essential for the maintenance of the nerve terminal pool of glutamate: immunogold evidence from hippocampal slice cultures. J Neurochem. 1995;65:871–881. doi: 10.1046/j.1471-4159.1995.65020871.x. [DOI] [PubMed] [Google Scholar]
- Lee TS, et al. Aquaporin-4 is increased in the sclerotic hippocampus in human temporal lobe epilepsy. Acta Neuropathol (Berl) 2004;108:493–502. doi: 10.1007/s00401-004-0910-7. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A, et al. Glutamine synthetase: glial localization in brain. Science. 1977;195:1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- Nadler J, Cuthbertson G. Kainic acid neurotoxicity toward the hippocampal formation: dependence on specific excitatory pathways. Brain Res. 1980;195:47–56. doi: 10.1016/0006-8993(80)90865-3. [DOI] [PubMed] [Google Scholar]
- Nadler JV, et al. Intraventricular kainic acid preferentially destroys hippocampal pyramidal cells. Nature. 1978;271:676–677. doi: 10.1038/271676a0. [DOI] [PubMed] [Google Scholar]
- Olney JW. Neurotoxicity of excitatory amino acids. In: McGeer EG, et al., editors. Kainic Acid as a Tool in Neurobiology. New York: Raven Press; 1978. pp. 37–70. [Google Scholar]
- Olney JW, et al. Excitotoxic mechanims of epileptic brain damage. In: Delgado-Escueta AV, et al., editors. Advances in neurology. New York: Raven Press; 1986. pp. 857–877. [PubMed] [Google Scholar]
- Olney JW, et al. Glutamate-induced brain damage in infant primates. J Neuropathol Exp Neurol. 1972;31:464–488. doi: 10.1097/00005072-197207000-00006. [DOI] [PubMed] [Google Scholar]
- Otis TS, Jahr CE. Anion currents and predicted glutamate flux through a neuronal glutamate transporter. J Neurosci. 1998;18:7099–7110. doi: 10.1523/JNEUROSCI.18-18-07099.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottersen OP. Postembedding light- and electron microscopic immunocytochemistry of amino acids: description of a new model system allowing identical conditions for specificity testing and tissue processing. Exp. Brain Res. 1987;69:167–174. doi: 10.1007/BF00247039. [DOI] [PubMed] [Google Scholar]
- Perez E, et al. Ultrastructural distribution of glutamate in the glutamine-synthetase-deficient epileptogenic rat hippocampus. Neuroscience Meeting Planner. 2010 254.20. [Google Scholar]
- Peters A, et al. Neurons and their supporting cells. New York: Oxford University Press; 1991. The fine structure of the nervous system. [Google Scholar]
- Racine RJ, et al. Rates of motor seizure development in rats subjected to electrical brain stimulation: strain and inter-stimulation interval effects. Electroencephalogr Clin Neurophysiol. 1973;35:553–556. doi: 10.1016/0013-4694(73)90033-3. [DOI] [PubMed] [Google Scholar]
- Smith CD, et al. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci U S A. 1991;88:10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanimirovic DB, et al. Developmental regulation of glutamate transporters and glutamine synthetase activity in astrocyte cultures differentiated in vitro. Int J Dev Neurosci. 1999;17:173–184. doi: 10.1016/s0736-5748(99)00028-3. [DOI] [PubMed] [Google Scholar]
- Svenneby G. Pig brain glutaminase: purification and identification of different enzyme forms. J Neurochem. 1970;17:1591–1599. doi: 10.1111/j.1471-4159.1970.tb03729.x. [DOI] [PubMed] [Google Scholar]
- Tian GF, et al. An astrocytic basis of epilepsy. Nat Med. 2005;11:973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torp R, et al. Cellular and subcellular redistribution of glutamate-, glutamine- and taurine-like immunoreactivities during forebrain ischemia: a semiquantitative electron microscopic study in rat hippocampus. Neuroscience. 1991;41:433–447. doi: 10.1016/0306-4522(91)90339-p. [DOI] [PubMed] [Google Scholar]
- van der Hel WS, et al. Reduced glutamine synthetase in hippocampal areas with neuron loss in temporal lobe epilepsy. Neurology. 2005;64:326–333. doi: 10.1212/01.WNL.0000149636.44660.99. [DOI] [PubMed] [Google Scholar]
- Vardimon L, et al. Glucocorticoid control of glial gene expression. J Neurobiol. 1999;40:513–527. doi: 10.1002/(sici)1097-4695(19990915)40:4<513::aid-neu8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- Wang Y, et al. The development of recurrent seizures after continuous intrahippocampal infusion of methionine sulfoximine in rats: a video-intracranial electroencephalographic study. Exp Neurol. 2009;220:293–302. doi: 10.1016/j.expneurol.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]