Abstract
Age-related macular degeneration (AMD) is the leading cause of blindness in the elderly worldwide. It affects 30–50 million individuals and clinical hallmarks of AMD are observed in at least one third of persons over the age of 75 in industrialized countries (Gehrs et al., 2006). Costs associated with AMD are in excess of $340 billion US (American-Health-Assistance-Foundation, 2012). The majority of AMD patients in the United States are not eligible for clinical treatments (Biarnes et al., 2011; Klein et al., 2011). Preventive interventions through dietary modulation are attractive strategies because many studies suggest a benefit of micro and macronutrients with respect to AMD, as well as other age-related debilities, and with few, if any, adverse effects (Chiu, 2011). Preservation of vision would enhance quality of life for millions of elderly people, and alleviate the personal and public health financial burden of AMD (Frick et al., 2007; Wood et al., 2011). Observational studies indicate that maintaining adequate levels of omega-3 fatty acids (i.e. with 2 servings/wk of fish) or a low glycemic index diet may be particularly beneficial for early AMD and that higher levels of carotenoids may be protective, most probably, against neovascular AMD. Intervention trials are needed to better understand the full effect of these nutrients and/or combinations of nutrients on retinal health. Analyses that describe effects of a nutrient on onset and/or progress of AMD are valuable because they indicate the value of a nutrient to arrest AMD at the early stages. This comprehensive summary provides essential information about the value of nutrients with regard to diminishing risk for onset or progress of AMD and can serve as a guide until data from ongoing intervention trials are available.
Keywords: AMD, antioxidants, carotenoids, nutrition, glycemic index, aging
1. INTRODUCTION AND DEFINITION OF AMD
High resolution vision is possible due to the high density of photoreceptors in the macula of the retina. Photoreceptors receive light signals and convert them to chemical and then electrical impulses that are sent to the brain. In the advanced stages of age-related macular degeneration (AMD) the central field of vision is distorted or lost due to damage or loss of photoreceptors in the macula/fovea located near the center of the retina (National Eye Institute, 2010). Photoreceptors are exposed to an extensive amount of oxidative stress in the form of light and oxygen (Zarbin, 1998) (Also see below, and articles by Handa et al., Jarrett et al., and Sparrow et al., in this issue). Each night, the outer 10% of segments of photoreceptors are shed and engulfed by the retinal pigment epithelium (RPE), which lies posterior to the photoreceptors (Sung and Chuang, 2010). Due to the requirement of one RPE cell to service thirty photoreceptors, the RPE has among the highest degradative burden in the body. In addition to the digestion of photoreceptors and their associated debris, the RPE are also involved in maintaining the nutriture of the photoreceptors. Nutrients from the choroidal blood supply must cross Bruch’s membrane, a pentalaminar structure composed of several layers of elastic and collagen, to enter the RPE and photoreceptors (Zarbin, 1998). The flow of nutrients into the retina, and debris out of the retina through the RPE is crucial, since photoreceptors do not have their own blood supply (Shakib and Zinn, 1973; Sivaprasad et al., 2005). Adequate nutritional support to the RPE also facilitates efficient turnover of photoreceptors. This is consistent with a requirement for proper nutrition for maintaining healthy vision. While we stress the function of the RPE as supporting photoreceptors, it is not clear that RPE damage is a unique initiating insult for AMD. Data also indicate that insults may occur in neural Muller cells and photoreceptors (Marc et al., 2008; Sullivan et al., 2007).
The chemical nature of nutrients should help predict which nutrients are crucial for the retina. Being a highly lipophilic tissue that is subject to environmental and age-related oxidative stress, one might anticipate that maintaining adequate levels of lipophilic antioxidants (polyunsaturated fatty acids, carotenoids, vitamin E) would bring salutary effects. To some extent this is borne out in the results discussed below. However the situation is far more complex, with hydrophilic compounds such as sugars also apparently playing significant roles in retinal homeostasis and damage.
The combination of inadequate nutrition with the inability to properly degrade and dispose of cellular debris may contribute to the formation of deposits in the RPE-Bruch’s membrane region. Basal laminar deposits are those which accumulate in the RPE cell, between the RPE basement membrane and the RPE plasma membrane. Basal linear deposits accumulate between the RPE basal lamina and the inner collagenous layer of Bruch’s membrane (Curcio and Millican, 1999). As the health of the RPE deteriorates, basal laminar deposits accumulate. These are thought to precede the formation of drusen, clinical indicators for early AMD (Al-Hussaini et al., 2009; Ding et al., 2009; Jager et al., 2008; Wang et al., 2009).
Drusen are often found between the RPE and the choroid. Mass spectrometric analysis indicates that drusen contain a variety of lipids, proteins, including ubiquitin and advanced glycation end products, as well as inflammatory mediators (Ding et al., 2009) (also see review by Shang in this issue). Early AMD is indicated by small (<63 μm) and/or a few medium-sized (<125 μm) drusen. The transition from early AMD to more advanced stages is characterized by an increase in drusen size and number as well as an increase in damage to the RPE. This damage can manifest as an increase in areas of the retina with too much or too little pigment (Davis et al., 2005a; Smiddy and Fine, 1984). If the RPE are damaged by drusen or other stressors, they may lose their ability to efficiently turn over the photoreceptors in the macula. Consequently, patients in the intermediate stages of AMD will notice a slight blur in their central field of vision.
Late AMD can manifest in two forms, geographic atrophy (also called dry AMD) and neovascular (also called wet AMD). Geographic atrophy is diagnosed when there is depigmentation in the RPE as observed by fundus photography. This depigmentation is often focal, round with sharp margins, and choroidal vessels may be visible underneath (Davis et al., 2005b). Along with this depigmentation, there is often large and abundant drusen as well as death of RPE and photoreceptors in the macula. Consequently, patients with geographic atrophy experience significant vision loss. Damaged photoreceptors and RPE cells may also accumulate and accelerate the formation of drusen, further exacerbating disease. There are currently no therapies to treat the dry form of AMD (Smiddy and Fine, 1984).
Another form of late stage AMD is manifested by formation of exudates and/or neovascularization of the retina. The latter is characterized by the development of aberrant blood vessels, originating from the choroid, that penetrate Bruch’s membrane causing damage to the RPE and overlying photoreceptors. These aberrant vessels are prone to leak: thus, the designation “wet AMD”. Such bleeding can cause the macula to bulge, causing straight lines to appear curved (AREDS, 2001). On a fundus photograph, the presence of subretinal fibrous tissue, RPE detachment, subretinal hemorrhage, or serous sensory retinal detachment would suggest that neovascular AMD is present (Davis et al., 2005b).
Approximately 90% of AMD patients in the US have early or atrophic AMD, while only 10% exhibit the more visually debilitating neovascular form. The progression from the early to the late stage of AMD can occur in only 5 years and it is also possible for dry AMD to progress to wet AMD (National Eye Institute, 2010).
The need for preventative measures against AMD is acute, in part because of the lack of effective pharmacological options, particularly for dry AMD. Additionally, while treatment regimens are available for some neovascular AMD patients, they are deployed only after the patients have already lost some vision. Presently, there are no pharmacological means to prevent AMD from reaching these later stages (See Section 2.10).
1.1 AMD STRESSORS: GENETICS, GENDER, RACE, SMOKING
Because each of these risk factors is covered in individual articles within this issue they are described only briefly here. In order to be able to critically analyze the research regarding the effect of specific nutrients on AMD, it is useful to appreciate the known or putative risk factors for AMD. Whereas some of these risk factors are modifiable, many are not. Among the latter, the most obvious risk factor is aging: 10% of those aged 66–75 yrs experience the early stages of AMD, and this rises exponentially to nearly 30% in those over the age of 75 (Friedman et al., 2004).
Genetic analyses have shown that a primary relative who has AMD can increase one’s own risk of the disease. Subsequent investigations have identified several single nucleotide polymorphisms associated with AMD risk in certain populations (see review by Gorin). The most widely known polymorphism to affect AMD risk is the Complement Factor H gene (Edwards et al., 2005; Hageman et al., 2005; Haines et al., 2005; Klein et al., 2005). Replacement of a tyrosine residue with a histidine residue at the 402 position of this gene appears to increase risk for AMD between 2.45 and 5.57 fold. The CFH gene is located on the 1q31-32 chromosome, and its protein negatively regulates the alternative pathway of complement activation.
As might be expected given roles for complement in inflammatory pathways, inflammation is thought to play an important role in AMD pathology, and dysfunction of complement proteins may lead to an uncontrollable inflammatory response that contributes to retinal damage (Haines et al., 2005). Other single nucleotide polymorphisms (SNPs) on the CFH gene such as rs12144939 and rs403846 have also been associated with small increases in risk for neovascular AMD (Hageman et al., 2011). A recent report indicates that wild type CFH is required to remove lipid oxidation products (Weismann et al., 2011).
In addition to CFH, two genes located on a different chromosome, 10q26, are also strongly associated with AMD risk. High-temperature requirement factor A1 (HTRA1) is a gene that encodes for a serine protease that is hypothesized to be involved in maintenance of Bruch’s membrane (Oka et al., 2004; Yang et al., 2006). Another gene located on the 10q26 chromosome, Pleckstrin Homology Domain-containing Protein Family A member 1/age-related maculopathy, LOC387715/ARMS2, encodes for a mitochondrial outer membrane protein that is expressed in the retina. Because mitochondria are intimately involved in energy metabolism and regulation of oxidative stress, proper mitochondrial function is required for cellular homeostasis (Crescenzo et al., 2011; Grant et al., 1997; Schild et al., 1997; Sohal et al., 1994; Takeyama et al., 1993) (Also see review by Boulton et al.). Dysfunction of the mitochondria has been demonstrated in several neurodegenerative diseases, and mitochondrial damage has been observed in AMD patients as well (Feher et al., 2006; Hartig et al., 2011; Lakatos et al., 2010; Quintanilla et al., 2012). Meta-analysis of populations from North America, Europe and Asia of the rs11200638 SNP on HTRA1, and the rs10409024 SNP on LOC387715/ARMS2 revealed that each of these gene variants is associated with nearly a 3-fold increase in risk for AMD (Kanda et al., 2007; Kondo et al., 2007; Leveziel et al., 2007; Lin et al., 2008; Tong et al., 2010; Yang et al., 2006). Recently, another analysis of a Caucasian population found that SNPs on HTRA1, especially del443ins54 were associated with increased risk for AMD (Hadley et al., 2010).
The chromosomes on which the CFH, HTRA1, and LOC387715/ARMS2 genes are located are thought to confer the most risk for AMD, but other genes involved in the complement pathway have also shown to modulate AMD risk, albeit to a smaller extent. A SNP located on the CFH receptor 5 gene, rs10922153, and rs641153 on the complement factor B gene have been shown to increase risk for neovascular AMD (Hageman et al., 2011). Conversely, several genetic variants involved in complement activation appear to be protective against AMD. Rs2274700 on CFH, rs1409153 on CFH receptor 4, rs1750311 on CFH receptor 5, rs2230199 on complement component 3, rs9332739 and rs547154 on complement component 2 and rs4151667 and rs641153 on complement factor B are associated with protective effects with regard to neovascular AMD risk in a meta-analysis of 4 cohorts, confirming the importance of the complement pathway in AMD pathology (Gold et al., 2006; Hageman et al., 2011). In addition to complement-related genes, variants in genes involved in cholesterol transport such as the TT genotype of the hepatic lipase gene, LIPC, has been associated with a 50% reduction in risk for AMD (95% CI: 0.2 0.9), compared to those with the CC variant (Hadley et al., 2010).
Additional studies have linked variants in genes for apolipoprotein E, adenosine-triphosphate-binding transporter 4, toll-like receptor 4, and vascular endothelial growth factor to AMD risk, but the associations between these gene variants and AMD risk were not as strong as that of gene variants on chromosomes 1q31-32 and 10q26 (Allikmets et al., 1997; Baird et al., 2006; Churchill et al., 2006; Zareparsi et al., 2005). Collectively, these genetic studies provide insight to help explain the early observation of increased risk for AMD among primary relatives of AMD patients. Further research is needed to understand how individual SNPs affect one another and how these genetic variants are affected by other risk factors for AMD.
Gender appears to influence risk for AMD, as described in a meta-analysis of 19 different populations. Thus, women have a slightly higher risk for developing AMD (OR = 1.13; 95% CI: 1.01, 1.28) (Rudnicka et al., 2012).
Race also appears to be a determinant of risk for AMD. While non-Hispanic Blacks (HR = 0.56; 95% CI: 0.52, 0.60) and Hispanics (HR = 0.82; 95% CI: 0.76, 0.88) have less risk for non-exudative AMD at the age of 80 than Caucasians (Cruickshanks et al., 1997; Frank et al., 2000; Hageman et al., 2011; Klein et al., 2003; Vanderbeek et al., 2011), there is a higher rate of non-exudative AMD among 60-year-old Asians (HR = 1.28; 95% CI: 1.20, 1.36) than Caucasians (Klein et al., 2006; Vanderbeek et al., 2011).
The strongest modifiable risk factor is smoking, which increases risk for AMD up to 7 fold (Klein et al., 2010). Obesity is also a significant, largely modifiable, risk factor for AMD, and it has even been shown that a 3% reduction in waist-hip ratio decreases risk for AMD by 29% (Peeters et al., 2008). Hypertension is thought to increase the risk for wet AMD due to increased strain on the vascular system, but consistent epidemiological data supporting this theory is lacking (Cruickshanks et al., 1997; Macular-Photocoagulation-Study-Group, 1997).
2. RELATIONSHIPS BETWEEN NUTRIENT INTAKE AND RISK FOR AMD
2.1 READER’S GUIDE TO READING THIS SECTION AND USE OF FIGURES
The following sections summarize (primarily) epidemiologic data that relate relationships between particular nutrients and risk for AMD. We used several criteria to organize the presentation and discussion of the data. In the text, we review first those studies that found beneficial effects of the nutrient. These are followed by those studies that found no significant effects and finally, those studies that found deleterious effects of that particular nutrient. We further divide our discussion by study design (case-control, prospective, etc.) because there are different limitations inherent in each type of study. We will first discuss case-control and cross-sectional studies. These are followed by data from prospective studies, and finally intervention studies. For simplicity, all of the endpoints for each study will be summarized together in the text, focusing on those studies which reported statistically significant effects. It is important to note differences in endpoints between studies. Some studies simply assess the presence of any type of AMD in their populations (this is denoted in the figures as simply “AMD”), while other studies differentiate between early, intermediate, or late AMD. Additionally, late AMD may be further characterized as geographic atrophy (“GA” in the figures) or neovascular (“NEO” in the figures) AMD. For clarity, those studies which used the term “exudative AMD” will be described here as “neovascular” since both terms refer to the same condition. Those studies which used the term “severe AMD” will be described here as “late AMD”. Indicators of early stages of disease include pigment abnormalities (“PIG AB” in the figures) in the retina, drusen, soft drusen, large drusen (“LG DRUSEN” in the figures). The terms AMD and age-related maculopathy (ARM) are used interchangeably. Since these terms refer to the same pathology, we will use only the term “AMD” in the text and figures, for consistency.
Data in the figures is organized by type of exposure (intake, plasma level, etc.) and outcome measure (early AMD, late AMD, etc.). Thus, for each of the nutrients, the figures are presented in the following outcome order: any type of AMD, early AMD indicators, early AMD, late AMD, and followed by geographic atrophy and neovascular AMD, if these specific types of late AMD were analyzed. Also in the figures, data regarding risk are separated from data regarding risk for progression. In order to present an unbiased, comprehensive view, results of studies which found beneficial effects of a particular nutrient on a specific outcome are reported in the figures alongside studies which found null or harmful effects of that same nutrient on the same outcome. Therefore, some figures will be referenced in the text slightly out of order. Data from studies that do not indicate odds or hazard ratios are referenced in the text, but not indicated in the figures. We begin with studies about nutrients for which the epidemiologic record is most robust.
2.2 DIETARY FATS AND FISH
Recent appreciation for the nutritional properties of the entire diet, rather than just antioxidants has led to investigations of the roles of macronutrients in AMD pathology. The relationship between risk for AMD or AMD progression and dietary fat or carbohydrate involves not only the quantity of the macronutrient, but also the quality of that macronutrient. We discuss the effects of each type of dietary fat (including omega-3 and -6 fats, mono- and polyunsaturated fats, saturated fat, total fat, trans-fats and cholesterol) as well as intake of food sources of fat such as fish on risk for AMD, and we illustrate these relationships in the figures. The figures are organized by AMD endpoint and type of study (retrospective/prospective analysis) (Figs. 1–19).
Figure 1.
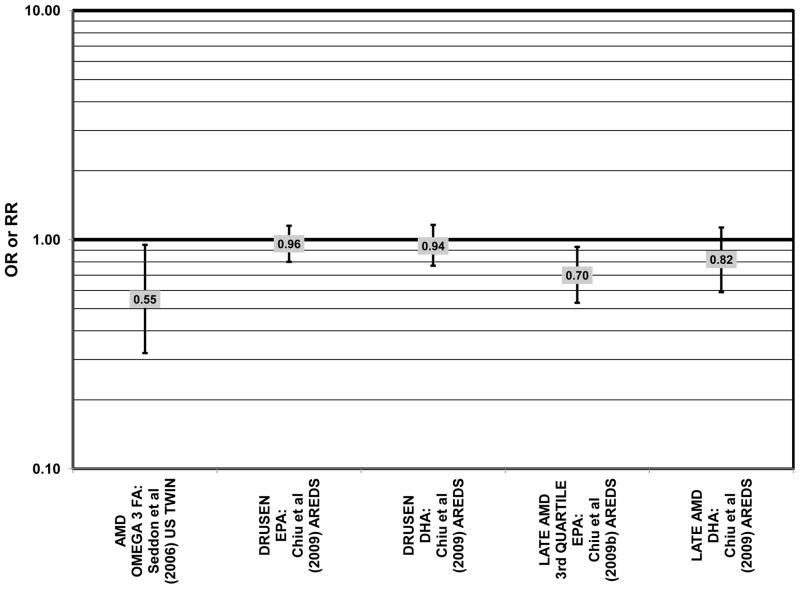
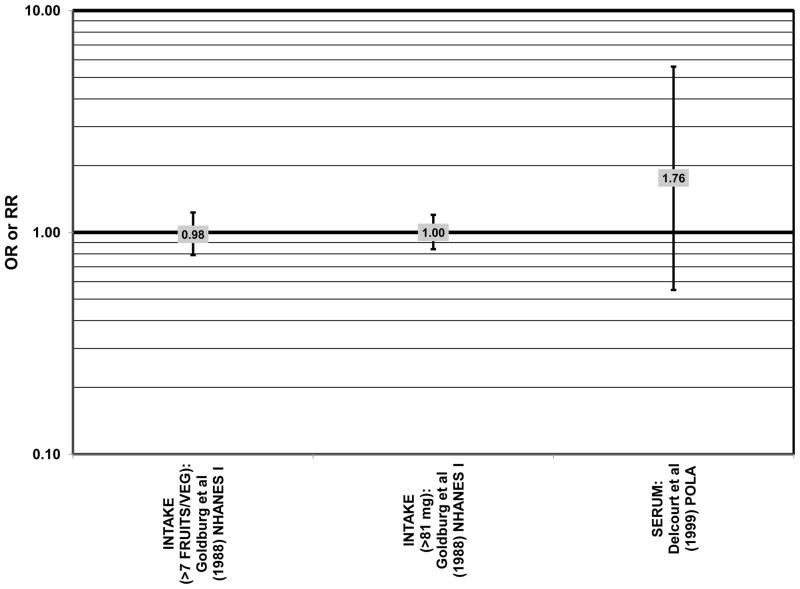
Odds or risk ratio for any stage of AMD, drusen or late AMD with high vs. low intake of omega-3 fatty acids in retrospective and cross-sectional studies.
Figure 19.
Odds or risk ratio for any stage, early, intermediate or late AMD or progression to late AMD with high vs. low intake of total fat in prospective studies.
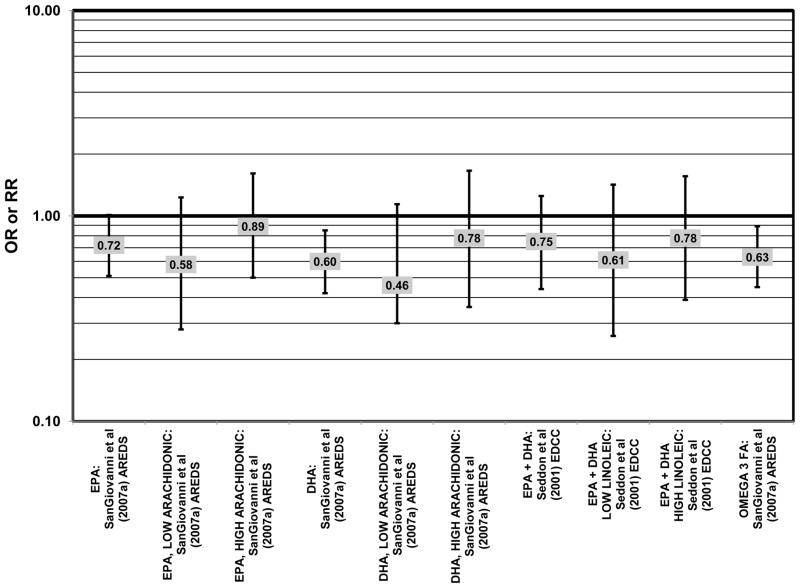
Increased intake of omega-3 fatty acids, especially long-chain omega-3 fatty acids such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) found in fish, have been associated with amelioration of a number of chronic diseases, including AMD (Bacova et al., 2010; Pilkington et al., 2011; Sapieha et al., 2011; Schweigert and Reimann, 2011). The Eye Disease Case Control Study (EDCC) consisted of 349 cases and 504 controls and found that among subjects with a low linoleic acid (omega-6 fatty acid) intake there was a trend of retinal protection in those with higher intake of omega-3 fatty acids (EPA and DHA) (p = 0.05). Without adjusting for omega-6 intake, this trend became non-significant (p = 0.29). While this trend suggests that omega-6 and omega-3 fatty acids are in a state of metabolic competition, further analysis of this cohort did not support such competition. When comparing those consuming the highest amounts of EPA and DHA with those consuming the lowest amounts (quintile 5 vs quintile 1), these omega-3 fatty acids did not confer significant protection from neovascular AMD before adjusting for linoleic acid intake (OR = 0.75; 95% CI: 0.44, 1.25) or in those with low (OR = 0.61; 95% CI: 0.26, 1.42) or high linoleic acid intake (OR = 0.78; 95% CI: 0.39, 1.56) (Seddon et al., 2001) (Fig. 2).
Figure 2.
Odds or risk ratio for neovascular AMD with high vs. low intake of omega-3 fatty acids in retrospective and cross-sectional studies.
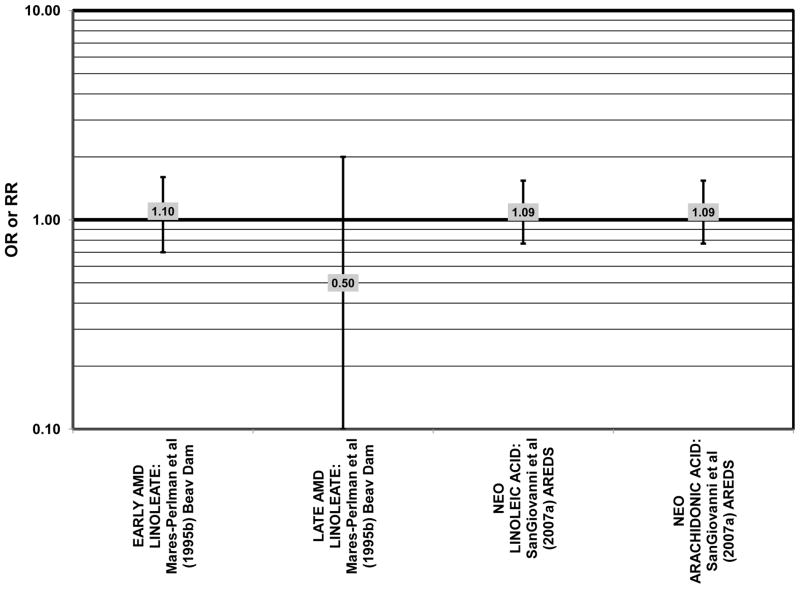
Stronger evidence for a beneficial role of omega-3 fatty acids in eye health is found in two cross-sectional studies. The US Twin Study of Age-Related Macular Degeneration (“US Twin” in the figures), a cross-sectional study comprised of 681 pairs of twins, found that compared to those consuming the least amount of omega-3 fatty acids, those consuming the highest amount had a reduced risk for any stage of AMD (OR = 0.55; 95% CI: 0.32, 0.95). This effect was driven mostly by those with a low linoleic and omega-6 fatty acid intake (p < 0.001), since the effect disappeared in those with an intake of linoleic acid above the median (Seddon et al., 2006) (Fig. 1). Analysis of the baseline data from 4,519 participants in the Age Related Eye Disease Study (AREDS) revealed that compared to those in the lowest quintile of intake, those in the highest quintile of intake for EPA (OR = 0.72; 95% CI: 0.51, 1.01; p < 0.05), DHA (OR = 0.60; 95% CI: 0.42, 0.85), and total long-chain omega-3 fatty acids (OR = 0.63; 95% CI: 0.45, 0.89) were at a reduced risk for neovascular AMD (SanGiovanni et al., 2007a) (Fig. 2). However, unlike the US Twin Study, sub-group analysis of the AREDS population revealed that the reduction of risk for neovascular AMD upon high consumption of EPA and DHA became non-significant when the cohort was separated by intake of arachidonic acid, another omega-6 fatty acid (SanGiovanni et al., 2007a) (Fig. 2).
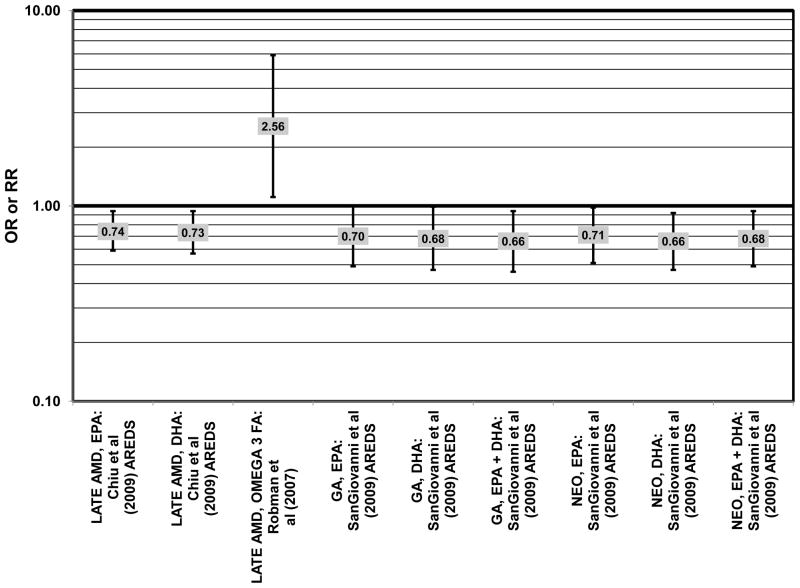
Prospective studies allow us to determine if omega-3 fatty acids can alter risk for progression of AMD. A benefit of such analysis is that it should indicate if modification of dietary practice may delay progression of non-visually impairing lesions to visually impairing lesions. Prospective analysis of 1,837 participants of AREDS found that increasing intake of DHA + EPA was associated with a decreased rate of progression to central geographic atrophy over 12 years (p = 0.026). Progression to central geographic atrophy during this period of time was lowest in subjects with intakes in the highest quintiles of intake of DHA (OR = 0.68; 95% CI: 0.47, 0.99), EPA (OR = 0.70; 95% CI: 0.49, 1.00), and DHA + EPA (OR = 0.66; 95% CI: 0.46, 0.94) (Fig. 6). Increasing intakes of DHA alone (p = 0.001) or DHA + EPA (p = 0.032) were also associated with a decrease in risk of progression to neovascular AMD (p = 0.001). Those consuming the highest amounts of DHA (OR = 0.66; 95% CI: 0.47, 0.92), EPA (OR = 0.71; 95% CI: 0.51, 0.98), and DHA + EPA (OR = 0.68; 95% CI: 0.49, 0.94) were at a reduced risk for progression to neovascular AMD compared to those consuming the lowest amounts (Sangiovanni et al., 2009) (Fig. 6).
Figure 6.
Odds or risk ratio for progression to late AMD with high vs. low intake of omega-3 fatty acids in prospective studies.
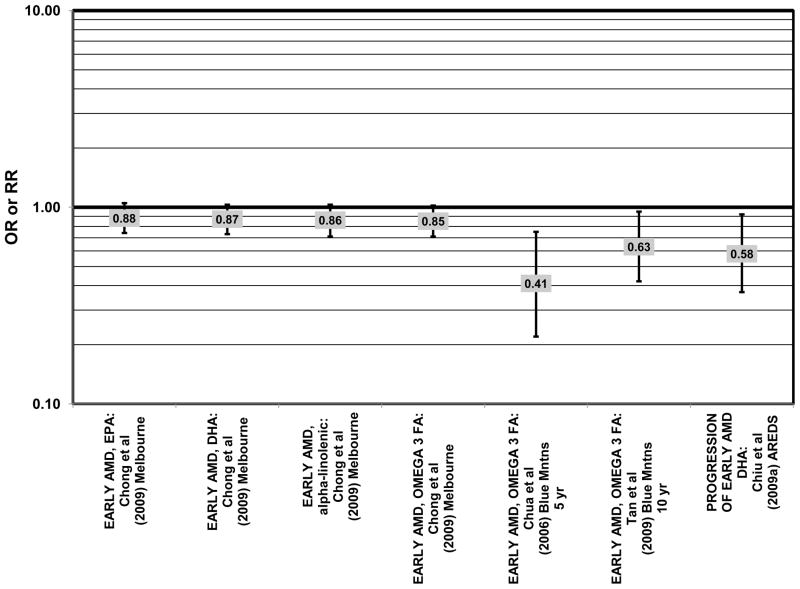
Having observed that increasing amounts of these omega-3 fatty acids were beneficial for retinal health, we undertook analyses to determine thresholds of intakes that may have a salutary effect. Analysis of 2,924 AREDS participants revealed that compared to DHA intakes < 26 mg/day, those consuming more than 64 mg/day had a reduced risk for progression to advanced AMD (HR = 0.73; 95% CI: 0.57, 0.94) (Fig. 6). Those consuming at least 42.3 mg EPA per day were at a reduced risk for progression to advanced AMD compared to those consuming less than 12.7 mg/day (HR = 0.74; 95% CI: 0.57, 0.94) (Fig. 6). Importantly, those participants who were healthy at baseline benefitted from a diet high in DHA as indicated by markedly reduced progression of early AMD (HR = 0.58; 95% CI: 0.37, 0.92) (Chiu et al., 2009a) (Fig. 4).
Figure 4.
Odds or risk ratio for early AMD or its progression with high vs. low intake of omega-3 fatty acids in prospective studies.
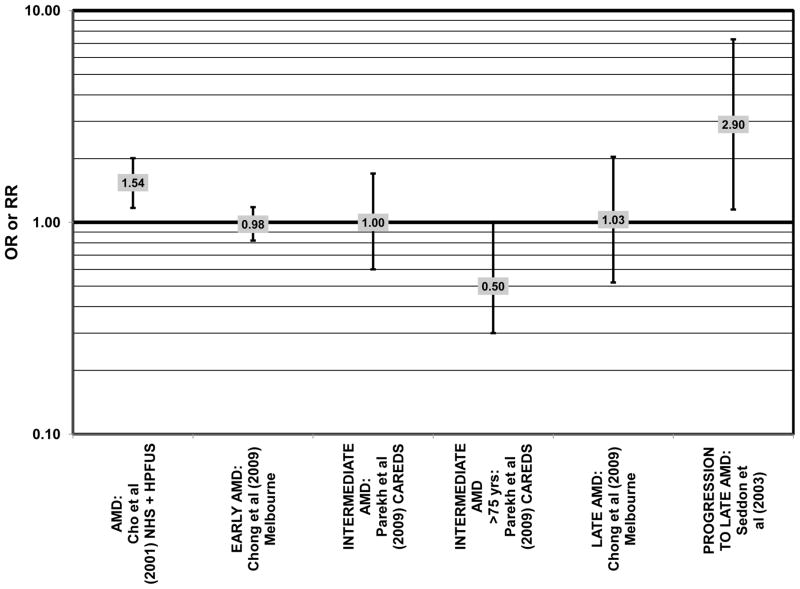
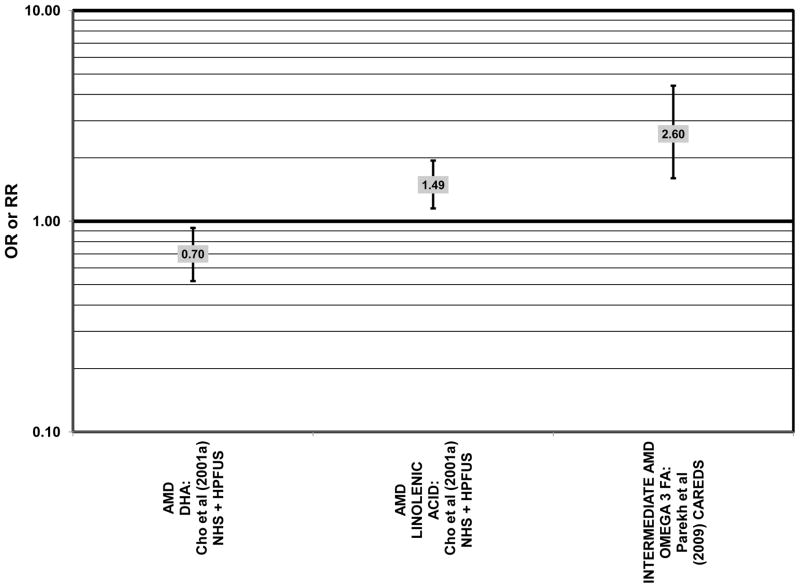
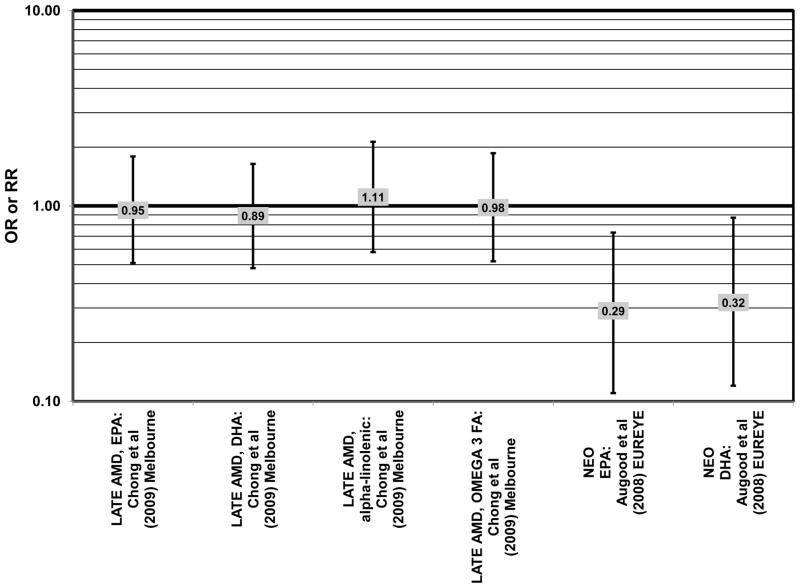
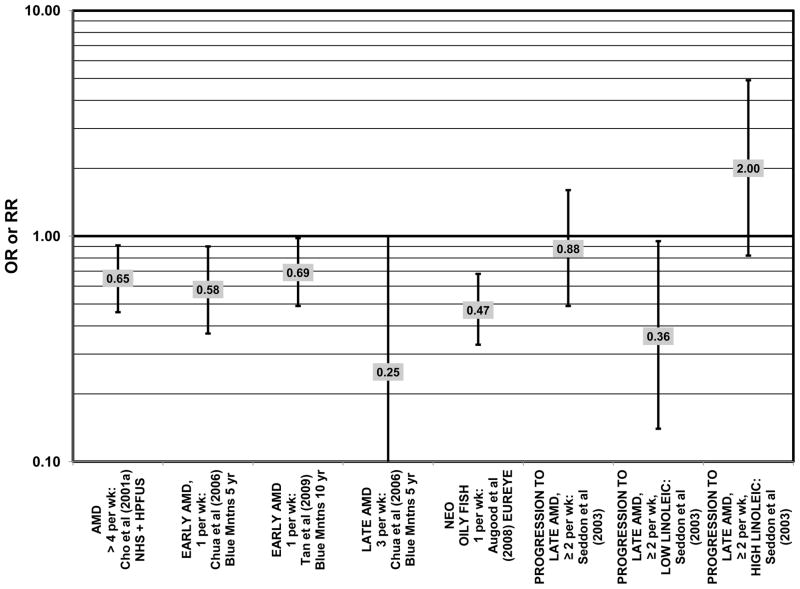
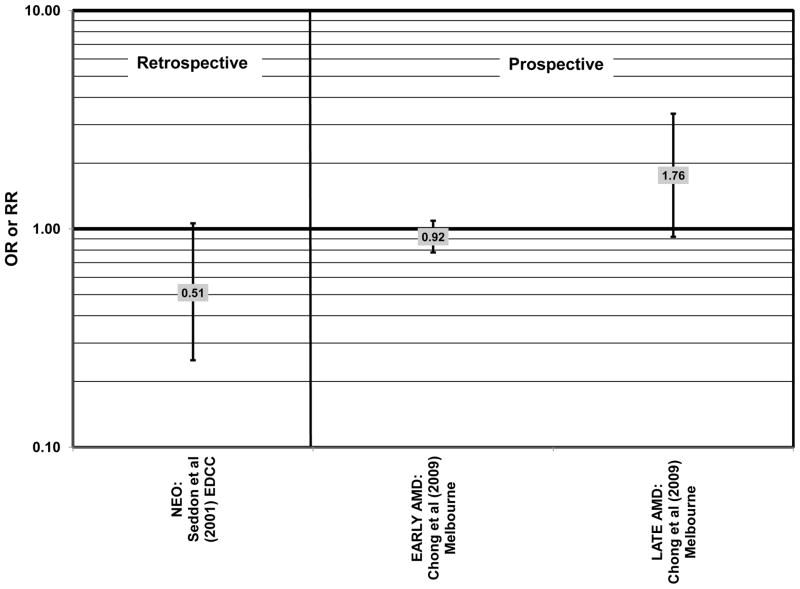
Data from several additional prospective cohorts support a beneficial role of omega-3 fatty acids in reducing risk for any grade of AMD. A prospective study of over 72,000 participants from the Nurses’ Health Study (NHS) and Health Professionals’ Follow-Up Study (HPFUS) indicated that those with the highest intakes of DHA were at a reduced risk for AMD (RR = 0.70; 95% CI: 0.52, 0.93) (Cho et al., 2001a) (Fig. 3). Participants (6,339) from the Melbourne Collaborative Cohort (“Melbourne” in the figures) indicated that those consuming the highest amounts of omega-3 fatty acids were at a slightly reduced risk for early AMD (OR = 0.85; 95% CI: 0.71, 1.02; p = 0.03 for trend) (Fig. 4). However, there was no association between particular fatty acids such as EPA, DHA and alpha-linolenic acid and early or late AMD (Chong et al., 2009). Observations of benefits of omega-3 fatty acids in general were confirmed in 2,454 participants of the Blue Mountains Eye Study (“Blue Mntns” in the figures). Compared to those with the lowest intakes of omega-3 fatty acids, those with intakes in the highest quartile had a reduced risk for incidence of early AMD (RR = 0.63; 95% CI: 0.42, 0.95) (Tan et al., 2009) (Fig. 4). These findings were corroborated in a follow-up analysis (OR = 0.41; 95% CI: 0.22, 0.75) (Chua et al., 2006) (Fig. 4). Finally, data from a European prospective cohort, EUREYE, indicated that among 2,275 participants, those with consumption levels of DHA (OR = 0.32; 95% CI: 0.12, 0.87) and EPA (OR = 0.29, 95% CI: 0.11, 0.73) in the highest quartile had a reduced risk for neovascular AMD (Augood et al., 2008) (Fig. 5).
Figure 3.
Odds or risk ratio for any stage or intermediate AMD with high vs. low intake of omega-3 fatty acids in prospective studies.
Figure 5.
Odds or risk ratio for late AMD with high vs. low intake of omega-3 fatty acids in prospective studies.
Until results from the larger AREDS2 clinical trial investigating the role of omega-3 fatty acids in AMD progression are available, findings from small intervention studies will remain of interest. In one of these trials, supplementation of 12 women with 800 mg DHA per day for 4 months significantly increased macular pigment optical density (MPOD), a surrogate marker for retina health, after 2 and 4 months of supplementation (p < 0.001). Surprisingly, women who also received 12 mg/d of lutein with the DHA didn’t experience as great an increase in MPOD at 2 months, but had similar density at 4 months (Johnson et al., 2008).
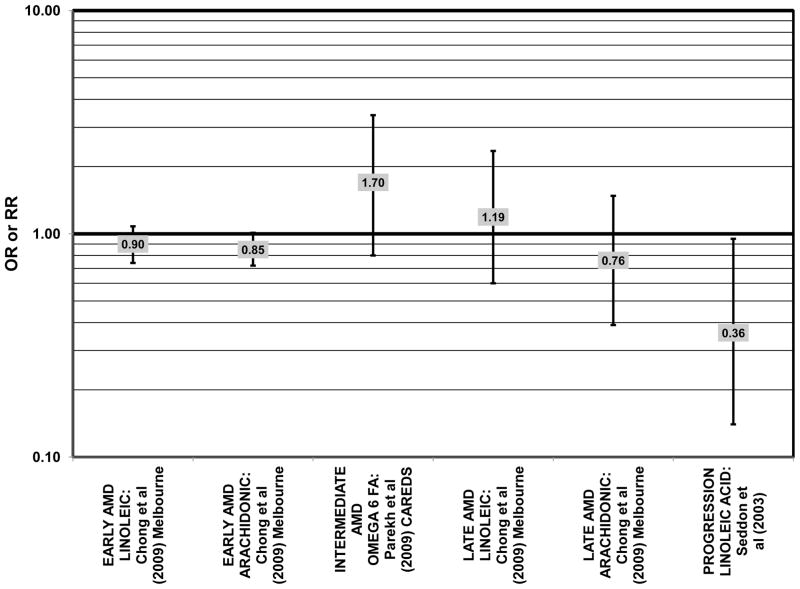
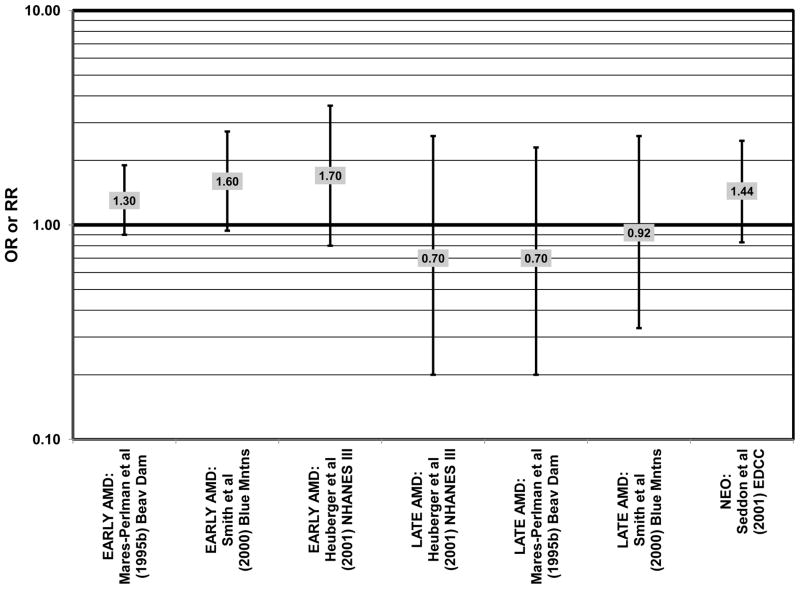
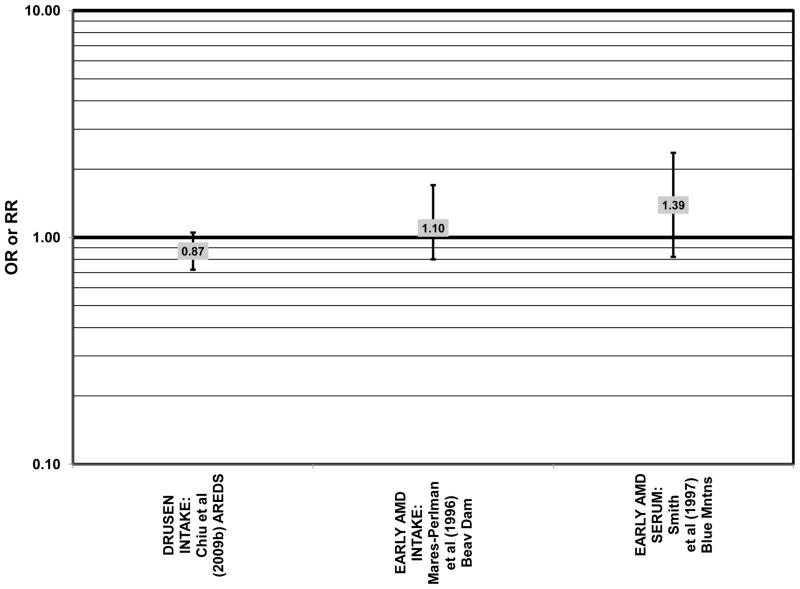
Despite these studies showing a beneficial role of omega-3 fatty acids in eye health, cross-sectional analysis of AREDS baseline data showed no effect of these nutrients. Among 4,003 participants of AREDS, there was no association between DHA intake and risk for drusen (OR = 0.94; 95% CI: 0.77, 1.16) or late AMD (OR = 0.82; 95% CI: 0.59, 1.13) (Fig. 1). There was also no association between intake of EPA and risk for drusen (OR = 0.96; 95% CI: 0.84, 1.15) (Fig. 1). Although those with intakes in the third quartile of EPA intake had 30% less risk for late AMD compared to those with the lowest intakes (95% CI: 0.53, 0.93), this association was lost at higher levels of EPA intake (Chiu et al., 2009b). In the Melbourne Collaborative Cohort, there was also no association between intake of EPA, DHA, or alpha-linolenic acid (another omega-3 fatty acid) and risk for early or late AMD (Chong et al., 2009) (Figs. 4, 5).
Several prospective studies even showed deleterious effects of omega-3 fatty acid intake on risk for AMD. The Carotenoids in Age-Related Eye Disease Study (CAREDS), an ancillary study of the Women’s Health Initiative, reported slightly harmful effects of omega-3 fatty acid consumption. Among 1,787 women, those consuming the highest amounts of omega-3 fatty acids had an increased risk for intermediate AMD compared to those women consuming the least amount of omega-3 fatty acids (OR = 2.60; 95% CI: 1.60, 4.40) (Parekh et al., 2009) (Fig. 3). Similarly, in the NHS+HPFUS, those in the highest quintile of linolenic acid intake had an increased risk for any stage of AMD (RR = 1.49; 95% CI: 1.15, 1.94; p = 0.0009 for trend) (Cho et al., 2001a) (Fig. 3). In another prospective study of early AMD patients, it was found that over 7 years, comparison of those with intakes of omega-3 fatty acids in the highest quintile with those with intakes in the lowest quintile was associated with an increased risk for AMD progression (OR = 2.56; 95% CI: 1.11, 5.91; p = 0.03 for trend) (Fig. 6). This association was based on a side-by-side comparison of photographs of retinas from baseline to study completion. More rigid definitions of AMD progression did not show such an association (Robman et al., 2007).
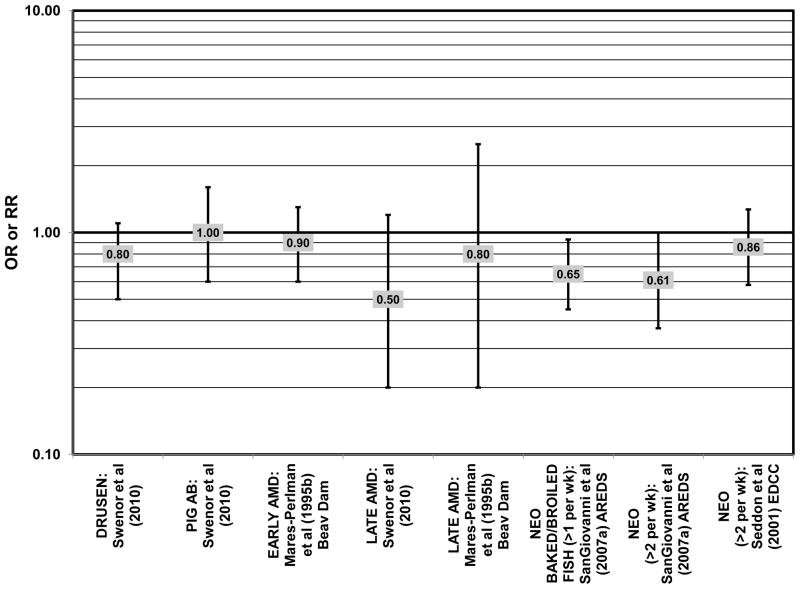
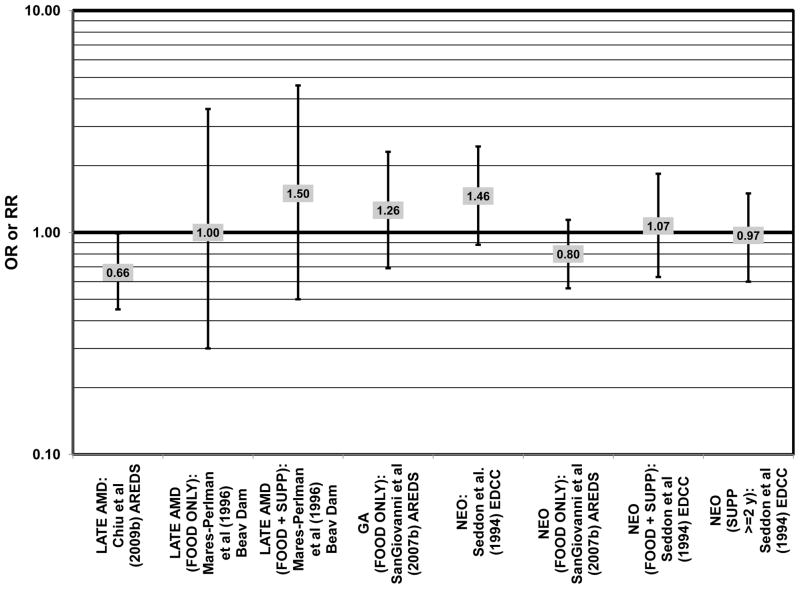
The effect of fish intake on risk for AMD has been examined because fish is one of the most common dietary sources of omega-3 fatty acids (Meyer et al., 2003). Cross-sectional analyses of intakes among AREDS participants indicated that consumption of at least 2 servings of fish per week was associated with decreased risk for neovascular AMD compared to 0 servings per week (OR = 0.61; 95% CI: 0.37, 1.00; p = 0.01 for trend), and consumption of more than 1 serving of baked or broiled fish was also associated with a decreased risk of neovascular AMD (OR = 0.65; 95% CI: 0.45, 0.93; p = 0.02 for trend) (SanGiovanni et al., 2007a) (Fig. 7).
Figure 7.
Odds or risk ratio for drusen, pigment abnormalities, early or late AMD with fish intake in retrospective and cross-sectional studies.
Two prospective studies analyzing the effect of fish intake on risk for AMD corroborated the observation of competition between omega-3 and omega-6 fatty acids in modulating eye health (that was observed in the trend analysis of the EDCC (Seddon et al., 2001). Prospective data from the Blue Mountains Eye Study showed that among 2,454 participants with low linoleic acid (omega-6) consumption, 1 serving of fish/wk was associated with a reduced risk of incident early AMD (OR = 0.69; 95% CI: 0.49, 0.98) 10 years after baseline (Tan et al., 2009) (Fig. 8). Another study reported that among those consuming at least 2 servings of fish/wk, those consuming below the median amount of linoleic acid were at a slightly reduced risk for progression to advanced AMD compared to those consuming above the median (RR = 0.36; 95% CI: 0.14, 0.95; p = 0.045 for trend) (Fig. 8). The beneficial effects of fish intake were not observed in those with higher intake or linoleic acid, nor were they observed before adjusting for linoleic acid intake (Seddon et al., 2003) (Fig. 8). Although these observations contradict those in the quintile intake analysis of the EDCC (Seddon et al., 2001), the prospective design of these studies (Seddon et al., 2003; Tan et al., 2009) increases the likelihood of a competition between omega-3 fatty acids and linoleic acid.
Figure 8.
Odds or risk ratio for any stage, early or late AMD or progression to late AMD with fish intake in prospective studies.
Additional prospective studies support the role of fish in reducing AMD risk, even without adjusting for omega-6 fatty acid intake. Analysis of the combined NHS+HPFUS indicated that those who consumed more than 4 servings of fish per week had a reduced risk for any stage of AMD relative to those consuming less than 4 servings per week (RR = 0.65; 95% CI: 0.46, 0.91) (Cho et al., 2001a) (Fig. 8). Another study reported that fish consumption once a week reduced risk for early AMD (OR = 0.58; 95% CI: 0.37, 0.90), while consumption of fish 3 times a week reduced risk for late AMD by 75% (95% CI: 0.06, 1.00) (Chua et al., 2006) (Fig. 8). Data from EUREYE also indicate that weekly consumption of oily fish was associated with a reduced risk of neovascular AMD (OR = 0.47; 95% CI: 0.33, 0.68) (Augood et al., 2008) (Fig. 8).
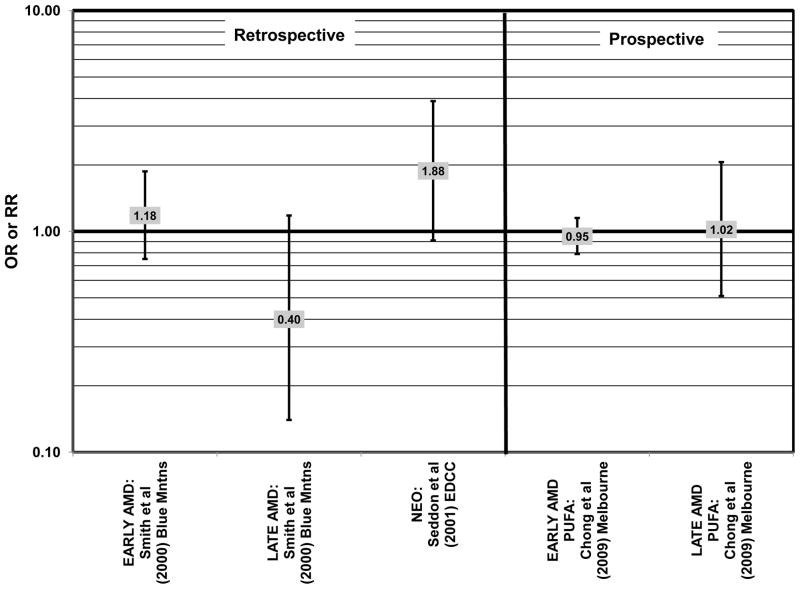
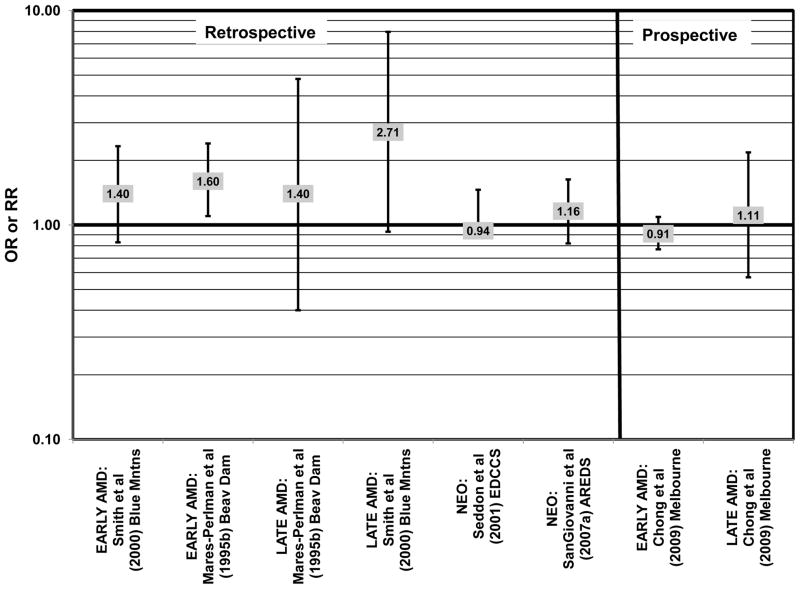
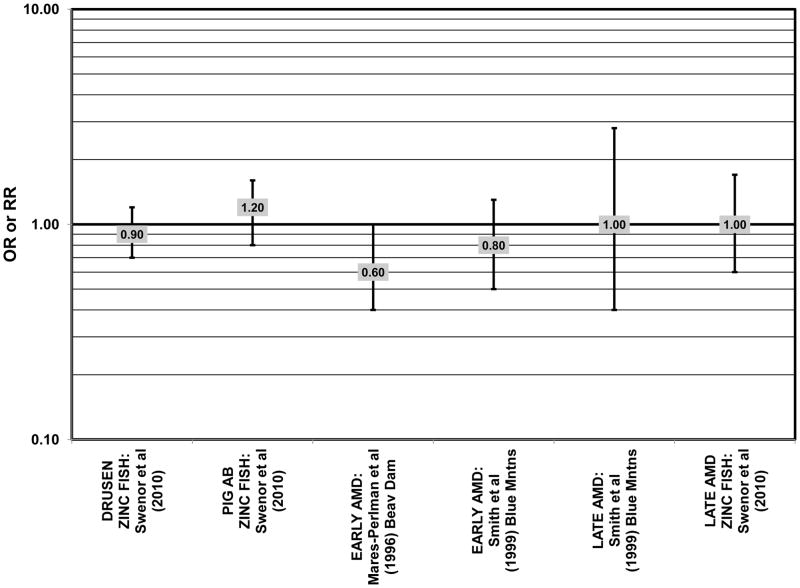
There were also a few studies that found no effect of fish intake on risk for AMD. The EDCC found no effect of fish intake on risk for neovascular AMD, nor did a retrospective study of 1,968 participants of the Beaver Dam Eye Study (“Beav Dam” in the figures) (Mares-Perlman et al., 1995b; Seddon et al., 2001) (Fig. 7). Cross-sectional data from the Blue Mountains Eye Study also did not find an association between early or late AMD and fish intake (Smith et al., 2000). Similarly, cross-sectional analysis of 2, 520 elderly men and women found that intake of at least 2 servings of fish per week did not influence risk for drusen, pigment abnormalities or late AMD (Swenor et al., 2010) (Fig. 7).
In addition to omega-3 fatty acids, analyses of intake of omega-6 fatty acids, including linoleic and arachidonic acids, and risk for AMD have been conducted. Most retrospective and prospective studies found no relationship between omega-6 fatty acid intake and AMD (Figs. 9, 10). Retrospective analysis of the Beaver Dam Eye Study did not show a relationship between linoleate consumption and risk for early or late AMD, nor did cross-sectional analysis of AREDS find an association between omega-6 fatty acid consumption and neovascular AMD risk (Mares-Perlman et al., 1995b; SanGiovanni et al., 2007a) (Fig. 9). Prospective analysis of CAREDS did not find that omega-6 fatty acid intake was related to risk for intermediate AMD, and the Melbourne Collaborative Cohort also did not find an association between consumption of linoleic or arachidonic acid and early or late AMD risk (Chong et al., 2009; Parekh et al., 2009) (Fig. 10). There was also no reported relationship between omega-6 fatty acid consumption and AMD progression in the EUREYE cohort (Robman et al., 2007). One study did indicate that omega-6 fatty acids may increase risk for disease. In the EDCC, high intakes of linoleic acid appeared to increase risk for neovascular AMD (OR = 2.00; 95% CI: 1.19, 3.37) (Seddon et al., 2001) (Fig. 9).
Figure 9.
Odds or risk ratio for early or late AMD with high vs. low intake of omega-6 fatty acids in retrospective and cross-sectional studies.
Figure 10.
Odds or risk ratio for early, intermediate or late AMD or its progression with high vs. low intake of omega-6 fatty acids in prospective studies.
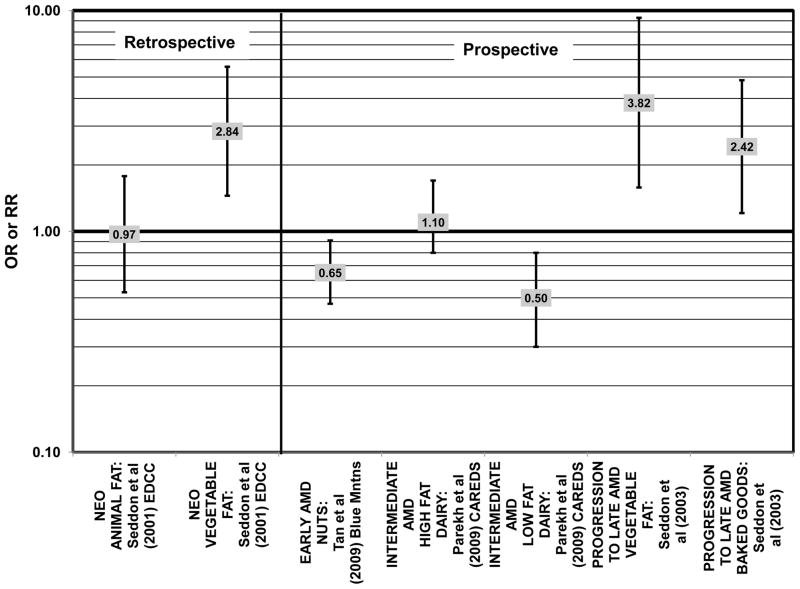
Both omega-3 and omega-6 fatty acids are polyunsaturated. Nuts are a popular source of polyunsaturated fatty acids, and in the prospective Blue Mountains Eye Study, it was found that 1–2 servings of nuts/wk was associated with a decreased risk of early AMD (OR = 0.65; 95% CI: 0.47, 0.91) among nonsmokers with low HDL and high intake of beta-carotene (Meyer et al., 2003; Tan et al., 2009) (Fig. 11). However, all other studies, such as the EDCC, Melbourne Collaborative Cohort, and cross-sectional analysis of the Blue Mountains Eye Study, which analyzed the relationship between polyunsaturated fatty acids and AMD risk did not find a significant association between these types of fatty acids and AMD risk (Chong et al., 2009; Robman et al., 2007; Seddon et al., 2001; Smith et al., 2000) (Fig. 12). Similarly, there wasn’t a significant relationship between polyunsaturated fatty acid intake and AMD risk in prospective analysis of the POLANUT cohort from southern France (a subset of the larger, POLA cohort) (Delcourt et al., 2007).
Figure 11.
Odds or risk ratio for early, intermediate or late AMD or progression to late AMD with high vs. low intake of fat-containing foods in retrospective and prospective studies.
Figure 12.
Odds or risk ratio for early or late AMD with high vs. low intake of polyunsaturated fatty acids in retrospective, cross-sectional, and prospective studies.
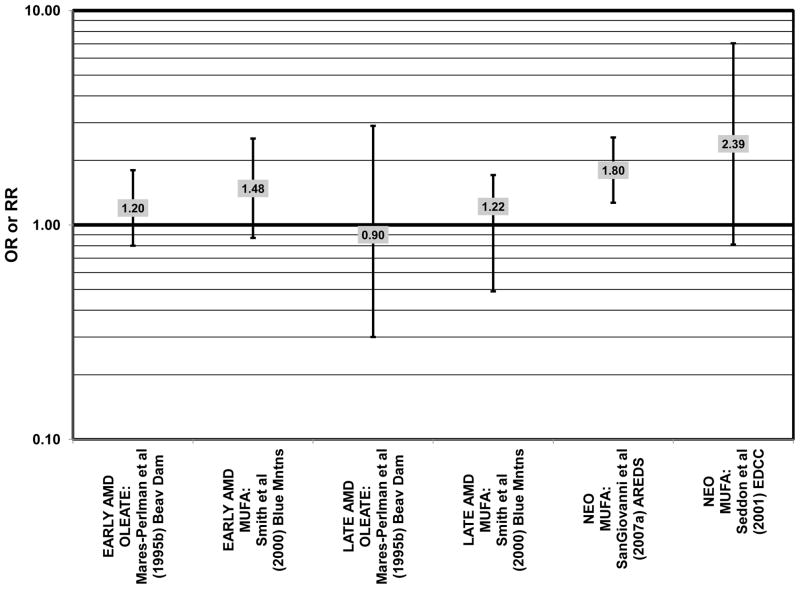
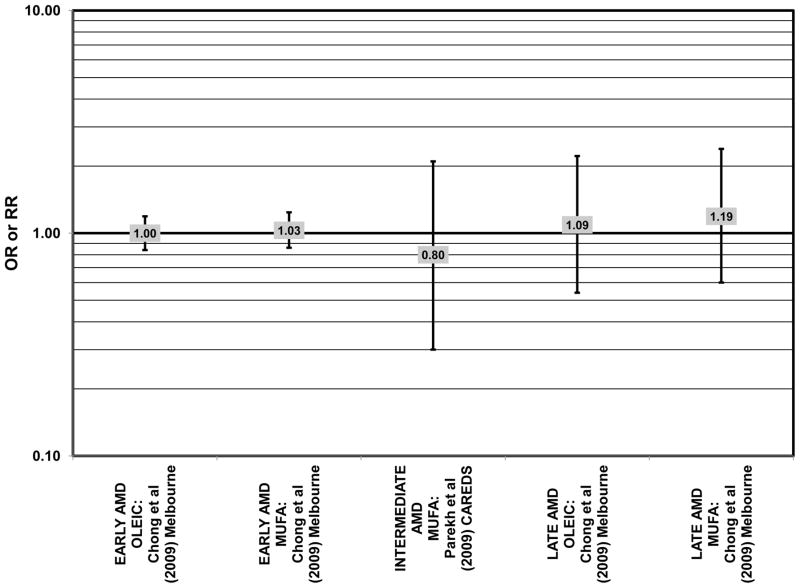
Similar to analysis of polyunsaturated fatty acids, many studies including the EDCC, Beaver Dam Eye Study, CAREDS, Melbourne Collaborative Cohort, and the POLANUT study did not find a significant association between consumption of monounsaturated fatty acids such as oleic acid and any stage of AMD risk (Chong et al., 2009; Delcourt et al., 2007; Mares-Perlman et al., 1995b; Parekh et al., 2009; Robman et al., 2007; Seddon et al., 2001) (Figs. 13, 14). However, there have been reports of increased risk for AMD with increasing consumption of monounsaturated fatty acids. In a cross-sectional analysis of 3,654 subjects from the Blue Mountains Eye Study, there was a slightly harmful trend of increasing risk for early AMD with increasing consumption of monounsaturated fatty acids (p = 0.05) (Smith et al., 2000). These potentially harmful effects of monounsaturated fatty acid consumption were also observed in a cross-sectional analysis of AREDS which indicated that compared to those consuming the lowest amounts, those consuming the highest amounts of monounsaturated fatty acids were at an increased risk for neovascular AMD (OR = 1.80; 95% CI: 1.27, 2.56) (SanGiovanni et al., 2007a) (Fig. 13). Although oleic acid is the most common source of monounsaturated fatty acids in the diet, this particular fatty acid was not significantly associated with disease risk (Mares-Perlman et al., 1995b) (Fig. 13).
Figure 13.
Odds or risk ratio for early or late AMD with high vs. low intake of monounsaturated fatty acids in retrospective and cross-sectional studies.
Figure 14.
Odds or risk ratio for early, intermediate or late AMD with high vs. low intake of monounsaturated fatty acids in prospective studies.
Elevated saturated fat intake has been related to several adverse health outcomes, as reviewed by Hooper and colleagues (Hooper et al., 2011). Consistent with this finding, no studies have reported retinal benefits through consumption of saturated fatty acids. Rather, some studies indicate that there is no effect of these fatty acids on AMD risk (Chong et al., 2009; Delcourt et al., 2007; Parekh et al., 2009; Robman et al., 2007; Seddon et al., 2001), while others indicate that these types of fats may be harmful (Mares-Perlman et al., 1995b; SanGiovanni et al., 2007a) (Fig. 15).
Figure 15.
Odds or risk ratio for early, intermediate or late AMD with high vs. low intake of saturated fat in retrospective, cross-sectional, and prospective studies.
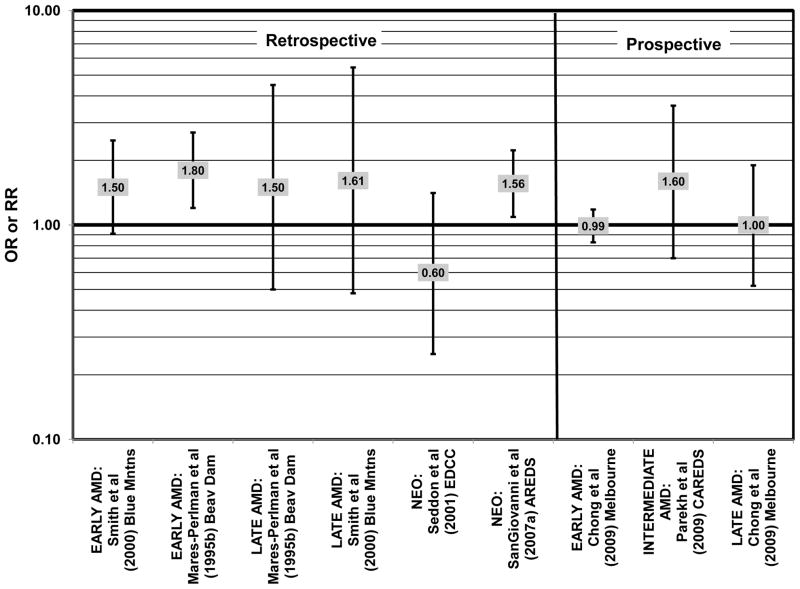
In the EDCC, saturated fat intake was not associated with neovascular AMD risk. (Seddon et al., 2001) (Fig. 15). CAREDS, Blue Mountains Eye Study and Melbourne Collaborative Cohort also did not find any association between saturated fat intake and risk for any stage, intermediate, and early or late AMD (Chong et al., 2009; Parekh et al., 2009; Smith et al., 2000) (Fig. 15). There was also no association between saturated fat intake and risk for AMD in the POLANUT study or AMD progression among 254 men and women in the Cardiovascular Health and Age Related Maculopathy Study (Delcourt et al., 2007; Robman et al., 2007). A retrospective analysis of 1,968 participants of the Beaver Dam Eye study showed that compared to intakes in the lowest quintile, those in the highest quintile of saturated fat intake were at an increased risk for early AMD (OR = 1.80; 95% CI: 1.20, 2.70; p = 0.01 for trend), although there was no risk associated with late AMD (Mares-Perlman et al., 1995b) (Fig. 15). Similarly, baseline data from AREDS showed that compared to those consuming the lowest amounts, those consuming the highest amounts of saturated fatty acids were at an increased risk for neovascular AMD (OR = 1.56; 95% CI: 1.09, 2.23). (SanGiovanni et al., 2007a) (Fig. 15).
In those studies which analyzed the role of trans-fatty acid intake in AMD risk, such as the EDCC and Melbourne Collaborative Cohort, none showed a significant relationship with early, late or neovascular AMD risk (Chong et al., 2009; Seddon et al., 2001) (Fig. 16). Trans-fat intake was also not associated with AMD progression (Robman et al., 2007).
Figure 16.
Odds or risk ratio for early or late AMD with high vs. low intake trans fatty acids in retrospective and prospective studies.
Cholesterol intake is often monitored due to relationships between poor heart health and elevated cholesterol levels (Ginsberg et al., 1994; Markus et al., 1997; Sacks et al., 1984; Terasaka et al., 2008). Cholesterol intake has also been related to risk for AMD with mixed findings. Cholesterol intake was not associated with risk for neovascular AMD in either the EDCC or a cross-sectional analysis of AREDS, nor was it associated with early AMD in the Blue Mountains cohort, or early or late AMD risk in the Melbourne Collaborative Cohort (Chong et al., 2009; SanGiovanni et al., 2007a; Seddon et al., 2001; Smith et al., 2000) (Fig. 17). However, high cholesterol intake was associated with increased risk for early AMD in a retrospective analysis of the Beaver Dam Eye Study (OR = 1.60; 95% CI: 1.10, 2.40; p = 0.03 for trend) as well as late AMD in a cross-sectional analysis of the Blue Mountains Eye Study (OR = 2.71; 95% CI: 0.93, 7.96; p = 0.04 for trend) (Mares-Perlman et al., 1995b; Smith et al., 2000) (Fig. 17).
Figure 17.
Odds or risk ratio for early or late AMD with high vs. low intake of dietary cholesterol in retrospective, cross-sectional, and prospective studies.
Risk for AMD associated with total fat intake is also not clear. There was no risk for early AMD associated with total fat intake in retrospective analysis of the Beaver Dam Eye Study, nor did the EDCC find a relationship between total fat intake and risk for neovascular AMD (Mares-Perlman et al., 1995b; Seddon et al., 2001) (Fig. 18). Cross-sectional analysis of data from the third NHANES survey also revealed that total dietary fat intake and specific types of fatty foods were not statistically related to risk for AMD (Heuberger et al., 2001). Several prospective cohorts also did not find a relationship between total fat intake and AMD risk. The Melbourne Collaborative Cohort did not find any risk for early or late AMD associated with total fat intake (Chong et al., 2009). Total fat intake was also not related to risk for AMD progression (Robman et al., 2007). CAREDS did not find any risk for intermediate AMD associated with total fat consumption. However, among those women older than 75 years of age, those with a high fat intake appeared to be protected from intermediate AMD (OR = 0.50; 95% CI: 0.30, 1.00) (Parekh et al., 2009) (Fig. 19). Contrary to the latter findings, several prospective studies found that total dietary fat might increase risk for AMD. A prospective study of 261 dry AMD patients found that over about 4.6 years, compared to those consuming the lowest levels of total fat, those consuming the highest level were at an increased risk for progression to advanced AMD (RR = 2.90; 95% CI: 1.15, 7.32; p = 0.01 for trend) (Seddon et al., 2003) (Fig. 19). Following the same trend, another prospective analysis of 832 participants in the POLANUT study suggested that increasing intake of total fat was associated with an increased risk for any stage of AMD (p = 0.007) (Delcourt et al., 2007). The NHS+HPFUS also found that compared to the lowest quintile of fat intake, those in the highest quintile had 54% increased risk for any stage of AMD (95% CI: 1.17, 2.01; p = 0.008 for trend) (Fig. 19). This association may have been driven by consumption of linolenic acid (omega-3 fatty acid), since those in the highest quintile of linolenic acid intake also had an increased risk for AMD (RR = 1.49; 95% CI: 1.15, 1.94; p = 0.0009 for trend) (Cho et al., 2001a) (Fig. 3).
Figure 18.
Odds or risk ratio for early or late AMD with high vs. low intake of total fat in retrospective and cross-sectional studies.
In addition to analyzing particular types of fatty acids, a few cohorts were used to study the effects of either animal or vegetable fats on AMD risk. The EDCC did not find an association between animal fat intake and risk for neovascular AMD (Seddon et al., 2001) (Fig. 11). However, this study did find that compared to those consuming the lowest amounts of vegetable fat, those consuming the highest amounts of vegetable fat had an increased risk for neovascular AMD (OR = 2.84; 95% CI: 1.45, 5.57; p = 0.006 for trend) (Seddon et al., 2001) (Fig. 11). Furthermore, a prospective study of 261 dry AMD patients found that over about 4.6 years, compared to those consuming the lowest levels of vegetable fat, those consuming the highest levels were at an increased risk for progression to advanced AMD (RR = 3.82; 95% CI: 1.58, 9.28; p = 0.003 for trend) (Seddon et al., 2003) (Fig. 11).
Risk for AMD was associated additional food items. In CAREDS, among those women younger than 75 years of age, those with the highest consumption of low-fat dairy products were at a reduced risk for intermediate AMD (OR = 0.50; 95% CI: 0.30, 0.80) (Parekh et al., 2009) (Fig. 11). Among 261 dry AMD patients, those consuming highest amounts of processed baked goods (which are high in fat, especially saturated and trans-fatty acids), relative to those consuming the least amount, were at an increased risk for AMD progression (RR = 2.42; 95% CI: 1.21, 4.84; p = 0.005 for trend) (Seddon et al., 2003) (Fig. 11).
In summary, a significant body of observational epidemiologic data indicates that increased consumption of long-chain omega-3 fatty acids (EPA, DHA) reduces risk for neovascular as well as early AMD. The data regarding the effect of fish consumption is not as strong as that for EPA or DHA individually, possibly suggesting that the benefits of omega-3 fatty acids might be attenuated due to interactions with other components of fish such as omega-6 fatty acids. Also, the foods consumed with the fish may confound the results of these epidemiologic studies. Some of the studies differentiated between types of fish, while others did not. The impact of monounsaturated or saturated fat consumption on AMD risk is ambiguous, but given the number of studies that have examined these nutritional variables it is likely that attempts to arrive at a definitive relationship between intakes of these food items and risk for AMD will provide clarity. Further investigation into relationships between intakes of animal and vegetable fat, specifically trans-fat, as well as cholesterol, and risk for AMD may be warranted.
2.3 DIETARY CARBOHYDRATE
In contrast to the many different types of fats, most of the research investigating the role of carbohydrates in AMD risk pertains to the glycemic index of a carbohydrate. The glycemic index is a measure of the ability of 50 g of a certain food to raise blood glucose levels, relative to the ability of 50 g of a standard food to raise blood glucose levels (Jenkins et al., 1981). As compared to a low glycemic index food, consumption of a high glycemic index food results in higher levels of glucose in the blood within two hours of consuming the food. In theory, the glycemic index is a property of the food, not the subject. Consumption of a diet with a low glycemic index has been linked to better blood glucose control in diabetics, and some data also indicates that such a diet can reduce risk for cardiovascular and kidney diseases (Chiasson et al., 2002, 2003; Chiu and Taylor, 2011; Ferland et al., 2009; Jenkins et al., 2008; Liu et al., 1999; McKeown et al., 2009; Riegersperger and Sunder-Plassmann, 2007). While still a new area of investigation, impressively, all epidemiologic data published to date indicates that consuming higher glycemic index foods is associated with a greater risk for AMD or AMD progression (Chiu, 2011; Chiu and Taylor, 2011). This was recently corroborated in carefully controlled laboratory studies (Uchiki et al., 2012; Weikel et al., 2012)
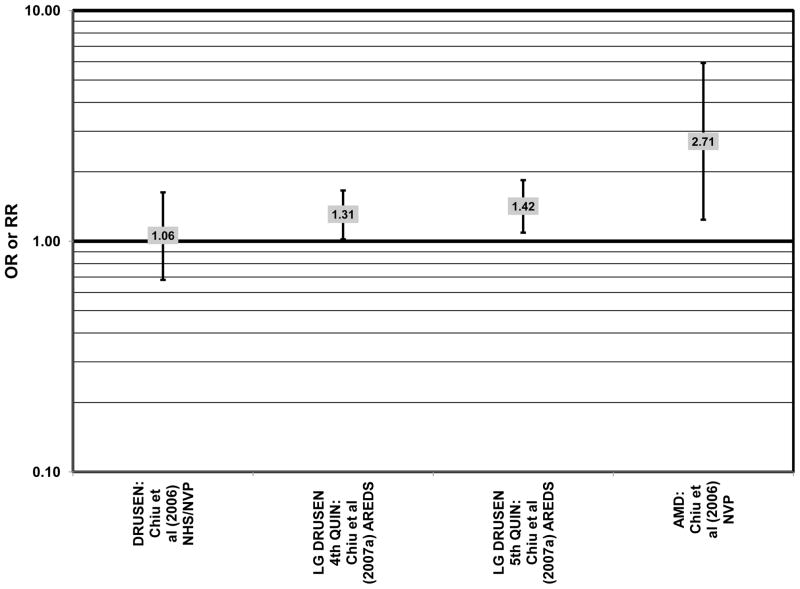
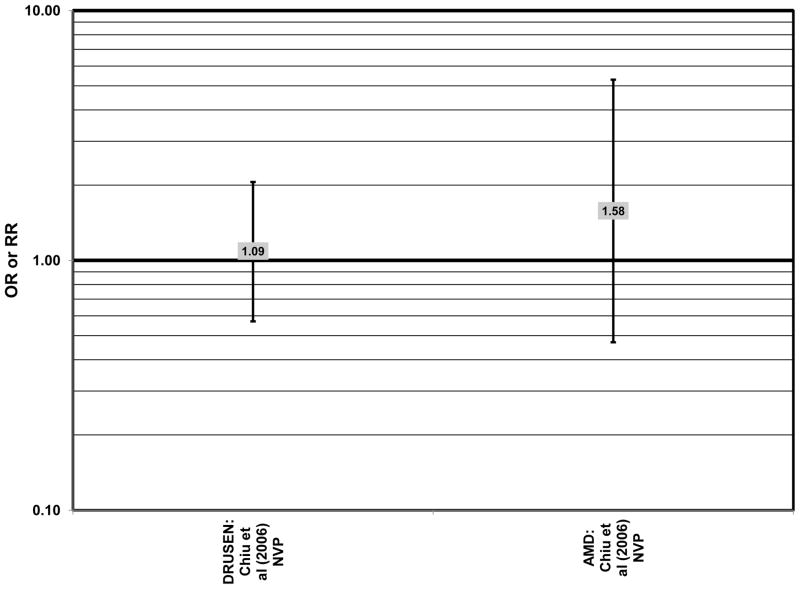
Cross-sectional analysis of baseline data from AREDS found that compared to those with dietary glycemic indexes in the first quintile, those with intakes in the fourth (OR = 1.31; 95% CI: 1.02, 1.66) or fifth quintile (OR = 1.42; 95% CI: 1.09, 1.84) were at an increased risk for the appearance of large drusen (Chiu et al., 2007a) (Fig. 20). There was also a trend for increasing glycemic index with increasing risk for large drusen (p = 0.001) (Chiu et al., 2007a). Increasing dietary glycemic index increased risk for neovascular AMD (p = 0.005), and there was a statistically significant trend of increasing dietary glycemic index with advancement of AMD stage (p < 0.001) (Chiu et al., 2007a). These observations corroborated findings from a cross-sectional analysis of the Nutrition and Vision Project (NVP), composed of 526 women in the Nurses’ Health Study. That study showed that after multivariate adjustment, women who had dietary glycemic indices in the highest tertile compared to the lowest tertile had an increased risk for AMD (OR = 2.71; 95% CI: 1.24, 5.93; p = 0.01 for trend) (Fig. 20). However, in that work, glycemic index did not affect risk for drusen (Chiu et al., 2006) (Fig. 20).
Figure 20.
Odds or risk ratio for drusen or any stage of AMD with high vs. low intake of a high glycemic index diet in cross-sectional studies.
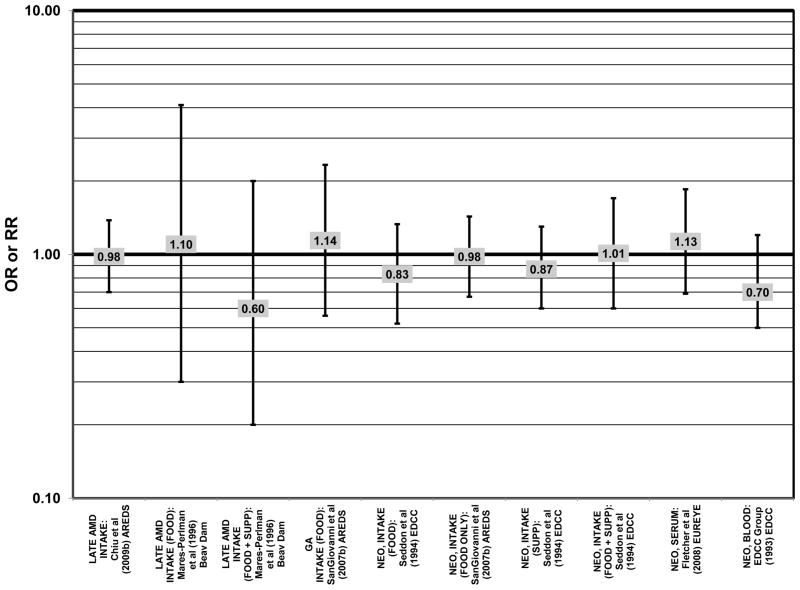
Prospective studies also indicate that higher glycemic index foods increase risk for AMD. In AREDS, risk of any progression of AMD over an 8-year period was higher in those with a higher glycemic index diet (RR = 1.10; 95% CI: 1.00, 1.20; p = 0.047) (Chiu et al., 2007b) (Fig. 21). Furthermore, those at later stages of the disease seemed to have a greater risk of progression on the higher glycemic index diet (p < 0.001) (Chiu et al., 2007b). Also, compared to those with the lowest quintile of dietary glycemic index, those in the highest quintile of dietary glycemic index had 39% higher risk of progressing to advanced AMD (95% CI: 1.08, 1.79) (Chiu et al., 2007b) (Fig. 21). Overall, the authors predict that by changing the dietary glycemic index only slightly, ~100,000 cases of AMD would be avoided in 5 years (Chiu et al., 2007a). Additional analyses (which included data regarding intake of EPA and DHA) from 2,924 AREDS participants, also revealed that compared to those consuming low glycemic index diets, consumption of high glycemic index diets increased the progression to advanced AMD by 32% (95%CI: 1.16, 1.52) (Fig. 21). Those that consumed a low glycemic index diet along with a high consumption of DHA were at an even lower risk for progression to advanced AMD (p < 0.001) (Chiu et al., 2009a).
Figure 21.
Odds or risk ratio for pigment abnormalities, drusen, early AMD or progression to late AMD with high vs. low intake of a high glycemic index diet in prospective studies.
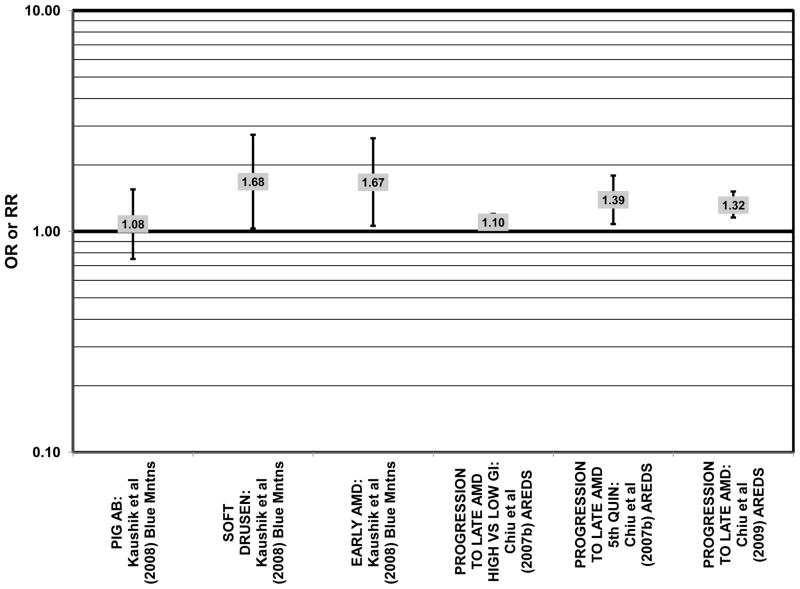
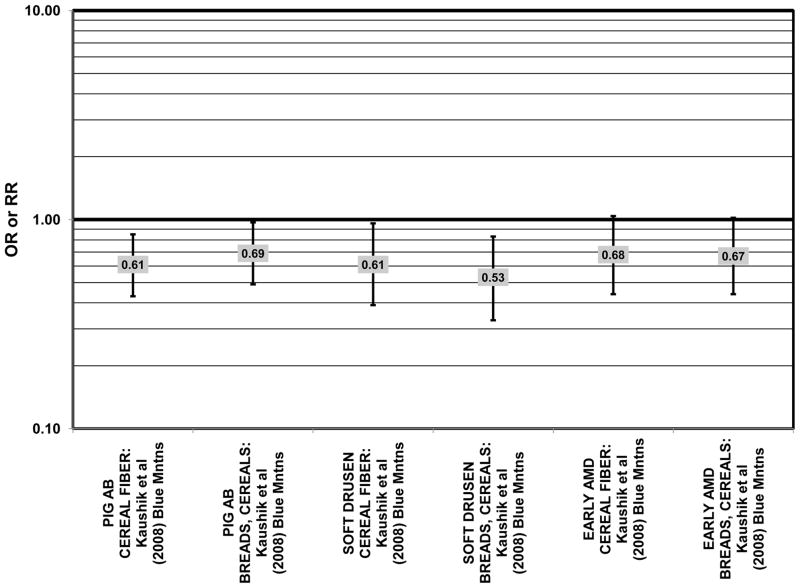
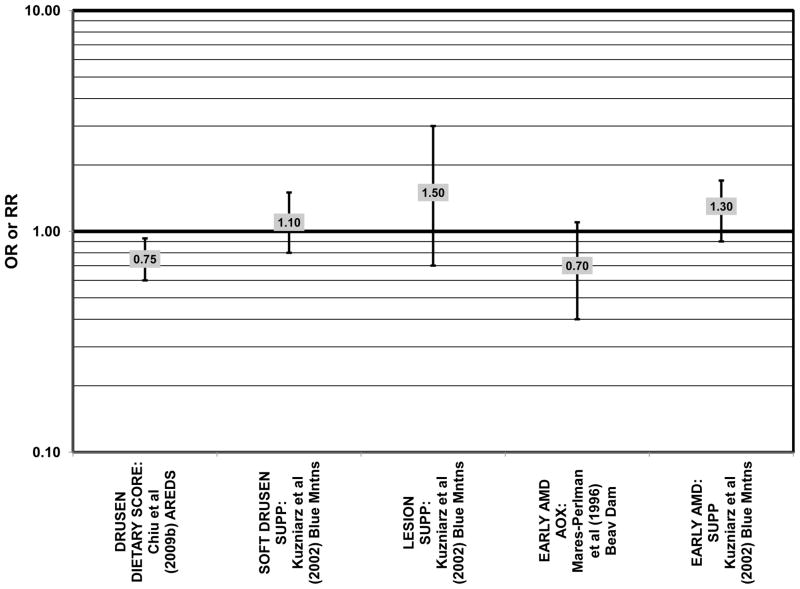
The Blue Mountains Eye Study corroborated the data from the Nurses’ Health Study and AREDS, showing that among 3,654 participants, (after adjusting for age, sex, BMI, smoking, blood pressure, history of cardiovascular disease, vegetable, fruit, and fat intake), compared to those with the lowest quartile of glycemic index, those with a dietary glycemic index in the highest quartile were at an increased risk for early AMD (RR = 1.67; 95% CI: 1.06, 2.64; p = 0.04 for trend) 10 years after baseline (Fig. 21). The authors also examined the effect of intake of cereal fiber, grains, and breads on risk for early AMD, as it was noted that most of these foods consumed by this population were of a low glycemic index. While there were no statistically significant associations with early AMD when comparing intakes in the highest to lowest quartiles (Fig. 22), there was a significant trend of decreasing risk for early AMD with increased consumption of cereal fiber (p = 0.05) and breads and grains (p = 0.03) food which are typically of a low glycemic index. Comparison of the highest to lowest quartile of glycemic index also showed an increased risk for soft drusen over 10 years (RR = 1.68; 95% CI: 1.03, 2.74; p = 0.04 for trend) (Fig. 21). Those consuming the highest amounts of cereal fiber (compared to the lowest) had a reduced risk of soft drusen (RR = 0.61; 95% CI: 0.39, 0.96; p = 0.01 for trend) and pigment abnormalities (RR = 0.61; 95% CI: 0.43, 0.85; p = 0.04 for trend) (Fig. 22). High consumption of breads and cereals also reduced risk for soft drusen (RR = 0.53; 95% CI: 0.33, 0.83; p = 0.04 for trend) and pigment abnormalities (RR = 0.69; 95% CI: 0.49, 0.97; p = 0.04 for trend) (Fig. 22). No associations were observed between early or late AMD and carbohydrate amount or intake of dietary fiber (Kaushik et al., 2008). Similarly, in the NVP, amount of carbohydrate consumed did not affect risk for AMD or drusen (Chiu et al., 2006) (Fig. 23).
Figure 22.
Odds or risk ratio for pigment abnormalities, drusen or early AMD with high vs. low intake of low glycemic index foods in a prospective study.
Figure 23.
Odds or risk ratio for drusen or any stage of AMD with high vs. low intake of total carbohydrates in cross-sectional studies.
Thus far, only three cohorts have been examined with regard to the role of carbohydrates in AMD risk. Albeit a limited number of cohorts, they were large cohorts and the evidence indicates that consumption of carbohydrates of a low glycemic index appears to lower risk for AMD and AMD progression. In comparison, total carbohydrate intake does not appear to be related to risk for or progression of AMD. Importantly, using controlled laboratory studies, the data have been corroborated and pathophysiologic mechanisms to explain the relationships between glycemic index and AMD have been proposed (Uchiki et al., 2012; Weikel et al., 2012) (See review by Shang in this issue). In short, these data indicate that glycoxidative stress results in accumulation of elevated levels of intracellular glycated proteins and compromised protein editing capacities. This leads to a viscous cycle of glycative damage, diminished proteolytic capacity, accumulation of glycated proteins, cytotoxicity and tissue dysfunction.
2.4 CAROTENOIDS AND VITAMIN A
The health benefits of carotenoids have been an area of extensive research. This interest is due, in part, to the a) observations of very high levels of carotenoids in the retina, b) indications of their salutary effects in early epidemiologic studies, and c) appreciation of their antioxidant properties, particularly in lipophilic environments. While carotenoids share similar structural properties, their biological activities and bioavailabilities are quite different (Burri et al., 2011). Lutein and zeaxanthin are the only carotenoids found at appreciable levels in the macula, as reviewed by Granado and colleagues (Granado et al., 2003). Thus, they are the major focus of this review of relations between carotenoids and risk for AMD. The order of presentation of these studies follows the guidelines noted in Section 2.0 and the risk data for each carotenoid with specified disease outcomes is distinguished in the figures (Figs. 24–55).
Figure 24.
Odds or risk ratio for any stage of AMD with high vs. low blood levels of lutein (LUT) and/or zeaxanthin (ZEA) in retrospective and cross-sectional studies.
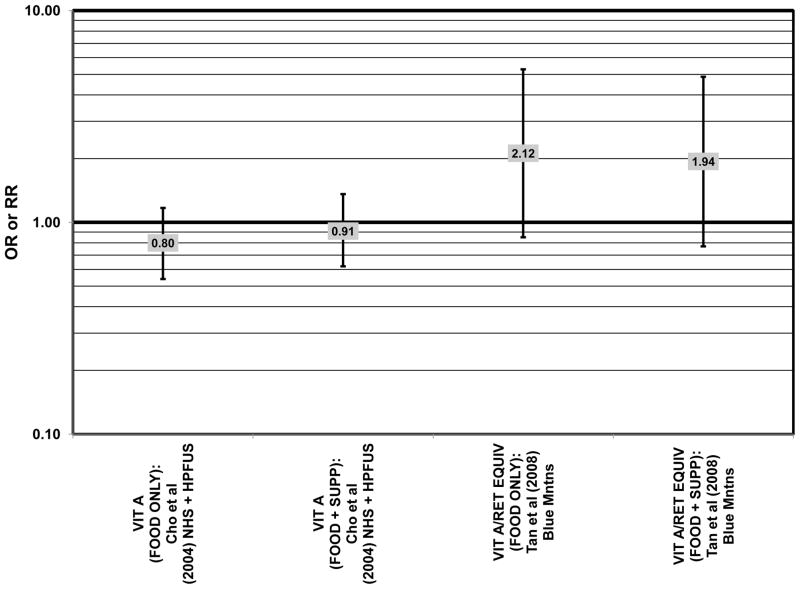
Figure 55.
Odds or risk ratio for neovascular AMD with high vs. low intake (with or without supplements) of vitamin A or retinol equivalents of vitamin A in prospective studies.
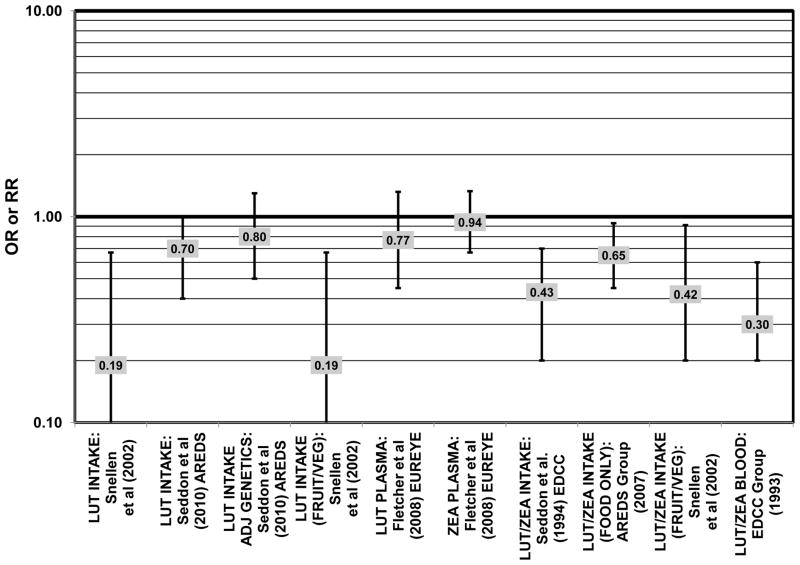
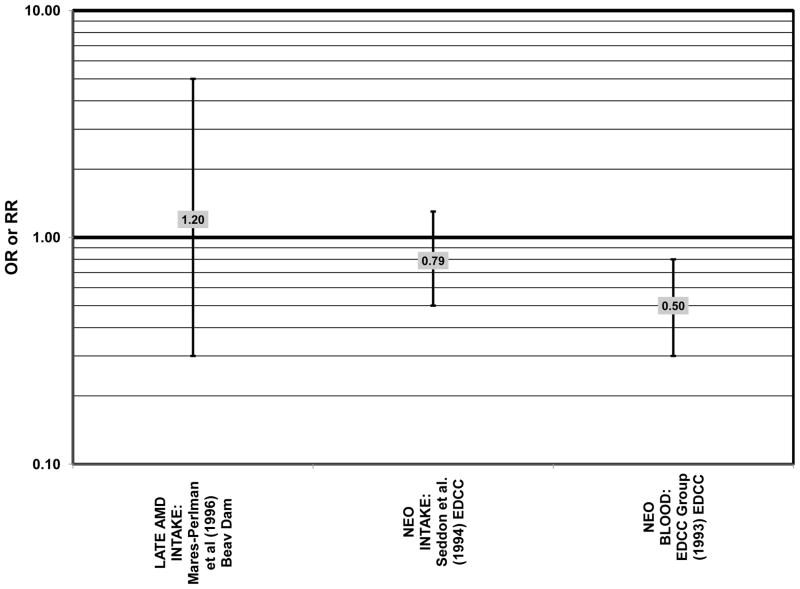
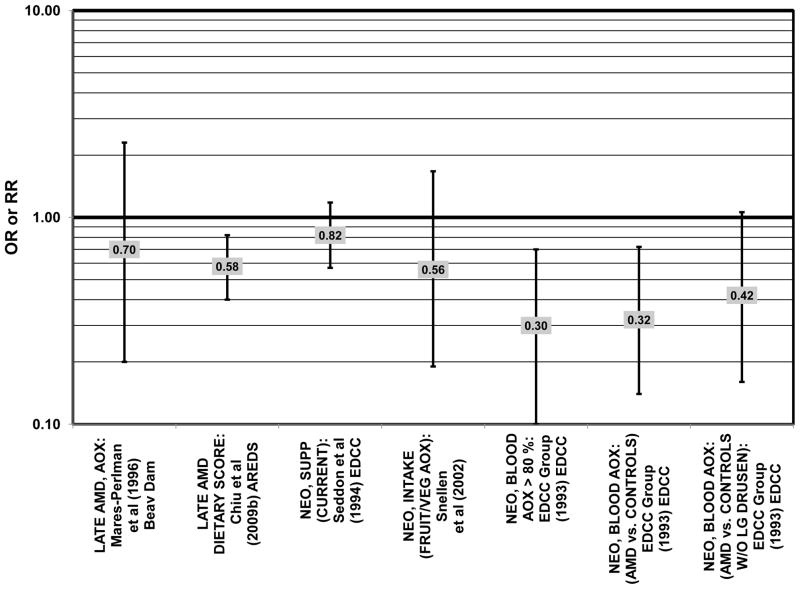
The concentration of lutein and zeaxanthin, and therefore their potential biologic function, may be modified by diet or supplement use (Connolly et al., 2010; Dietzel et al., 2011; Hammond et al., 1997; Johnson et al., 2008; Landrum et al., 1997; Schalch et al., 2007; Wenzel et al., 2007). Several case-control studies have reported that elevated lutein and zeaxanthin status is related to salutary advantage in the retina. The EDCC found that high intakes (OR = 0.43; 95% CI: 0.20, 0.70; p < 0.001) as well as blood levels (OR = 0.30; 95% CI: 0.20, 0.60; p < 0.001) of lutein/zeaxanthin were protective against neovascular AMD (Eye Disease Case-Control Study Group, 1993; Seddon et al., 1994) (Fig. 31). Another case-control study of 72 patients and 66 controls corroborated this relationship and found that the prevalence rate of neovascular AMD was reduced in patients with the highest intake of lutein compared to those with the lowest (OR = 0.19; 95% CI: 0.05, 0.67) (Snellen et al., 2002) (Fig. 31).
Figure 31.
Odds or risk ratio for neovascular AMD with high vs. low intake (with or without supplements) or blood levels of lutein (LUT) and/or zeaxanthin (ZEA) in retrospective and cross-sectional studies.
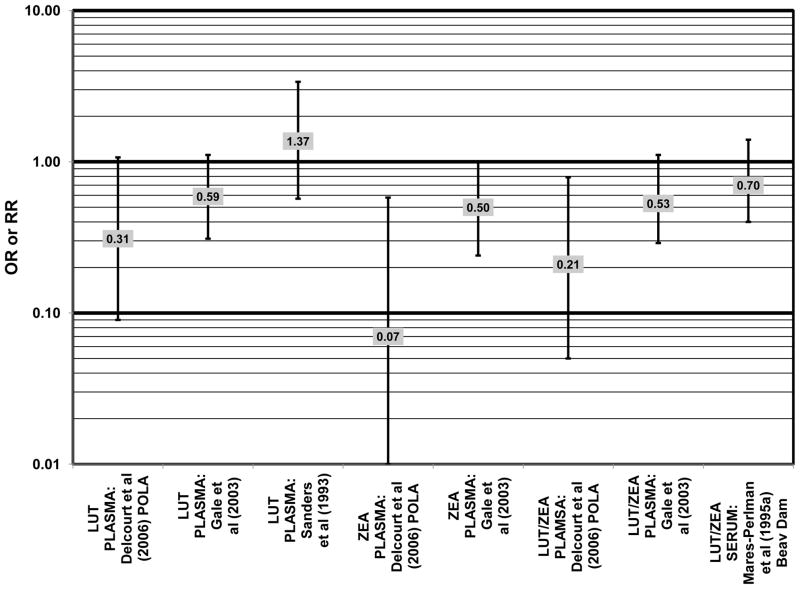
A number of cross-sectional studies support the findings from the case-control studies. A cross-sectional study of 380 elderly English men and women found that those with the highest plasma levels of zeaxanthin had less risk for any stage of AMD than those with the lowest plasma levels (OR = 0.50; 95% CI: 0.24, 1.00). The associations between AMD risk and plasma lutein or plasma lutein/zeaxanthin in this population showed the same trend, but were non-significant (Gale et al., 2003) (Fig. 24). Cross-sectional analysis of the POLA cohort (a population in Southern France, a segment of which was analyzed in the POLANUT study) revealed that those with the highest levels of zeaxanthin (OR = 0.07; 95% CI: 0.01, 0.58) or the combination of lutein and zeaxanthin (OR = 0.21; 95% CI: 0.05, 0.79) in their blood had a reduced risk for any stage of AMD, although the amount of lutein alone in the blood (OR = 0.31; 95% CI: 0.09; 1.07) was not significantly associated with disease risk (Delcourt et al., 2006) (Fig. 24).
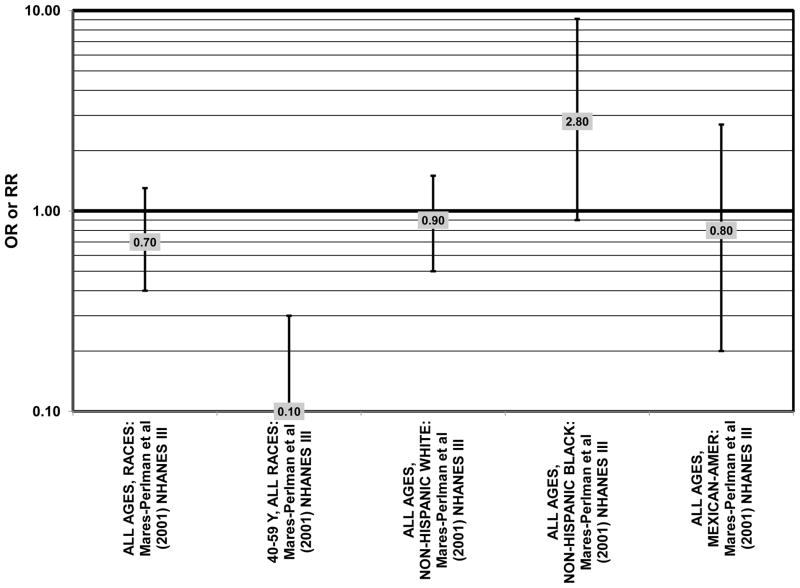
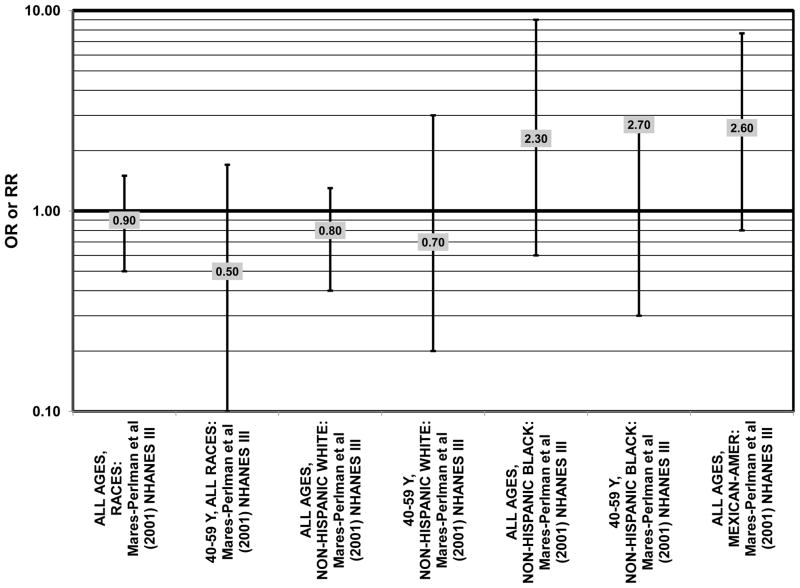
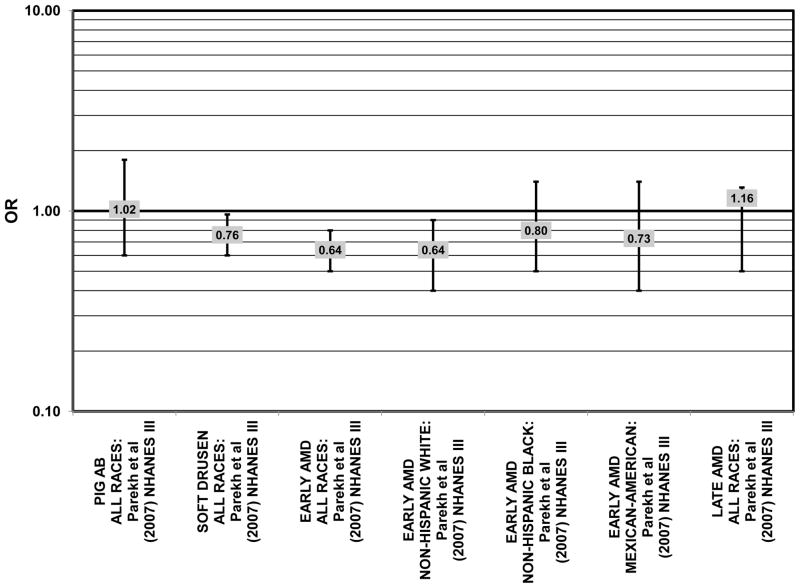
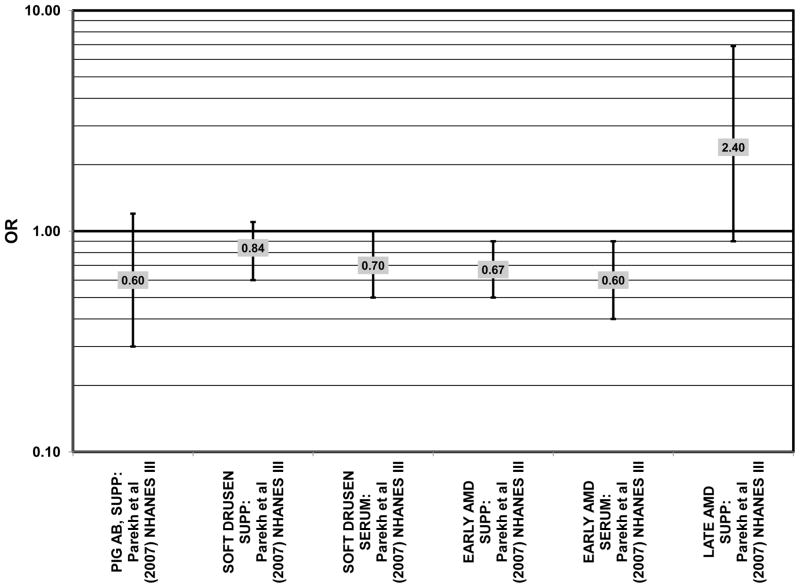
The third cohort of the National Health and Nutrition Education Evaluation Survey (NHANES III) includes data from subjects collected over six years, from 1988–1994. Cross-sectional analysis of NHANES III revealed that those aged 40–59 and consuming the highest levels of dietary lutein and zeaxanthin had less risk for pigment abnormalities compared to those consuming the lowest amounts (OR = 0.10; 95% CI: 0.10, 0.30) (Fig. 25). However, these inverse relationships were not observed in the overall study population or among specific ethnic groups for intake or blood levels of lutein and/or zeaxanthin (Figs. 25, 26). There was no relationship between lutein/ zeaxanthin blood levels and risk for soft drusen among any age group of Non-hispanic whites in the NHANES III cohort, nor was there a relationship between lutein/zeaxanthin intake and risk for soft drusen among those aged 40–79 (Figs. 27, 28). Interestingly, among 60–79 year olds, those consuming the highest level of lutein/zeaxanthin did have a reduced risk for late AMD (OR = 0.10; 95% CI: 0.00, 0.90) (Fig. 30). However, this inverse relationships was not observed in the overall NHANES III population (Mares-Perlman et al., 2001). Data from this cohort suggests that the effects of lutein and zeaxanthin on risk for disease are highly related to the age of those consuming these carotenoids.
Figure 25.
Odds or risk ratio for pigment abnormalities with high vs. low intake of lutein and zeaxanthin in a cross-sectional study.
Figure 26.
Odds or risk ratio for pigment abnormalities with high vs. low serum levels of lutein and zeaxanthin in a cross-sectional study.
Figure 27.
Odds or risk ratio for soft drusen with high vs. low intake of lutein and zeaxanthin in a cross-sectional study.
Figure 28.
Odds or risk ratio for soft drusen with high vs. low serum levels of lutein and zeaxanthin in Non-Hispanic Whites in a cross-sectional study.
Figure 30.
Odds or risk ratio for late AMD with high vs. low intake of lutein (LUT) and/or zeaxanthin (ZEA) from food in retrospective and cross-sectional studies.
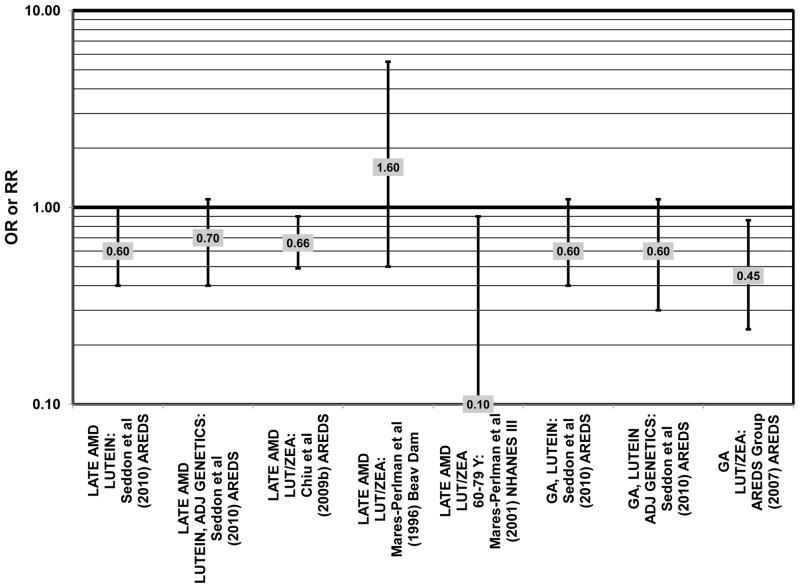
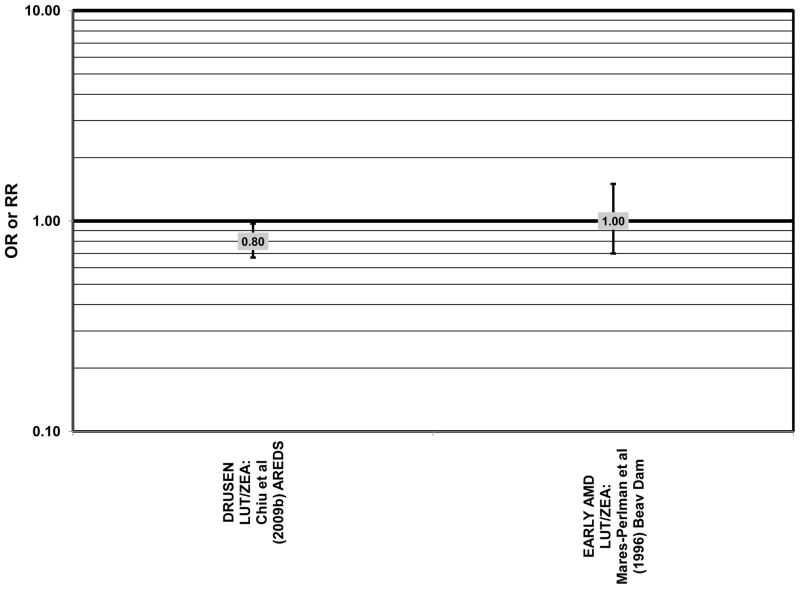
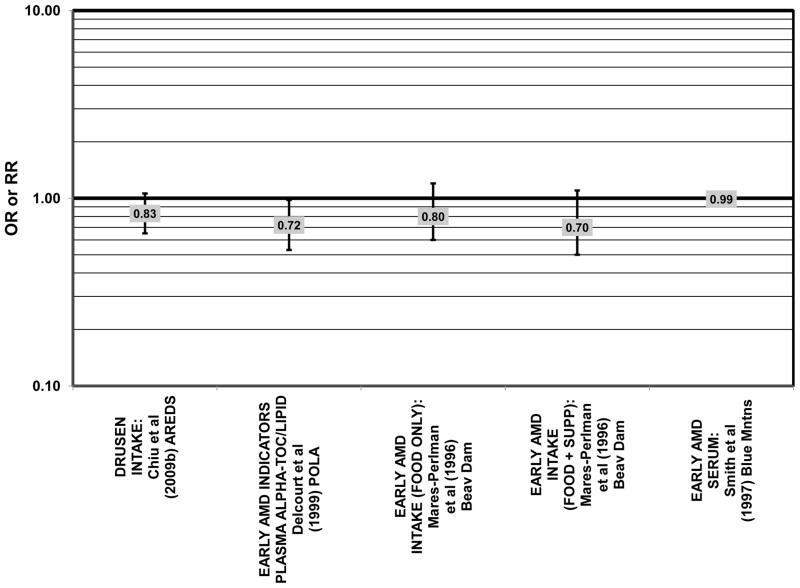
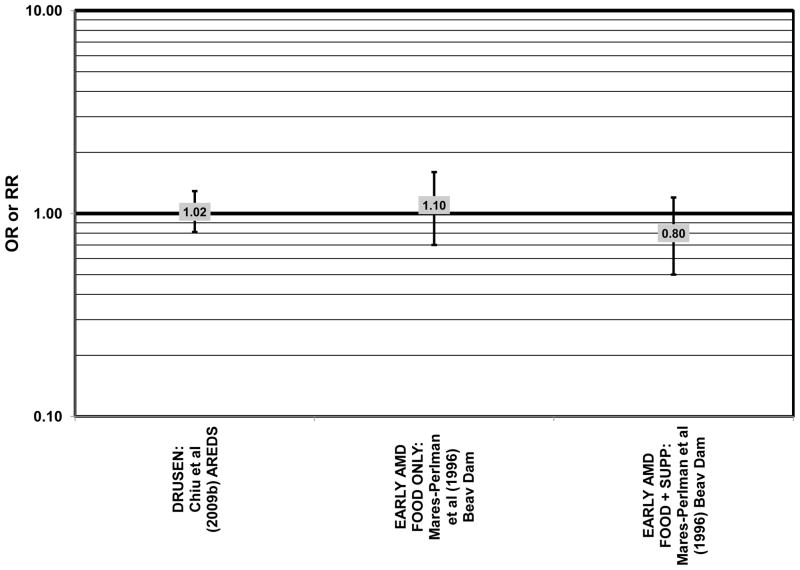
Cross sectional analysis at baseline of 4,003 participants in the AREDS study indicated that those in the third quartile of consumption of lutein/zeaxanthin had 20% reduced risk for drusen compared to those in the first quartile of intake (OR = 0.80; 95% CI: 0.67, 0.97) (Fig. 29). Those in the third quartile of lutein/zeaxanthin intake also had a reduced risk for late AMD (OR = 0.66; 0.49, 0.90) (Fig. 30). However, for both of these outcomes, the association became non-significant in those subjects with the highest consumption of lutein/zeaxanthin (Chiu et al., 2009b).
Figure 29.
Odds or risk ratio for drusen or early AMD with high vs. low intake from food of lutein and/or zeaxanthin in retrospective and cross-sectional studies.
Further support for beneficial roles of lutein and zeaxanthin in retinal health is found in data from small intervention studies. Thus, elderly men and women supplemented with 13.7 g/day lacto-wolfberry, a potent source of zeaxanthin, for 90 days not only increased serum zeaxanthin levels and antioxidant capacity in serum, but also reduced pigment changes and soft drusen accumulation relative to the placebo group (Bucheli et al., 2011). Macular pigment optical density (MPOD) is sometimes used as an indicator of macular health, a factor important in the development of AMD. A small trial of 108 German men and women supplemented daily with 12 mg lutein and 1 mg zeaxanthin for 6 months increased macular pigment optical density compared to placebo (p < 0.001), a change which was maintained even after supplementation stopped (Zeimer et al., 2009). Another placebo controlled trial of 49 elderly women showed that 12 mg/day of lutein supplementation significantly increased MPOD after 4 months (Johnson et al., 2008). It was shown in another study of 100 men and women that increases in MPOD following lutein supplementation are dose-dependent (Bone and Landrum, 2010). While encouraging, data from these trials should be regarded with caution because of their short duration and limited controls and/or clinical endpoints.
Richer and colleagues conducted a one-year randomized double-blinded placebo-controlled study to evaluate the benefits of zeaxathin, separate from lutein on visual function, as well as the effects of combining lutein and zeaxanthin in patients with atrophic AMD. Patients received either 8 mg zeaxanthin/day, 9 mg lutein/day (this was considered the “faux placebo” group), or a combination of both carotenoids. After one year, MPOD increased in all three groups, and was not different among the groups. Patients taking zeaxanthin experienced the greatest improvement in high contrast visual acuity, and clearance of central scotomas, while patients taking lutein experienced the most improvement in low contrast acuity and glare recovery. Patients supplemented with both carotenoids saw the least improvement overall, which the authors attribute to a competition between the carotenoids in the retina (Richer et al., 2011). Similarly, a majority of AMD patients given Ocuvite (supplement of 12 g lutein, 1 g zeaxanthin, and antioxidants) in the Lutein Nutritional Effects Measured by Autofluorescence (LUNA) showed elevated MPOD. However, a significant minority did not, perhaps owing to different absorption in different people.
The Carotenoids in Age-Related Maculopathy in Italians Study (CARMIS) was a randomized intervention in which patients received a combination of 10 mg lutein, 1 mg zeaxanthin, 4 mg astraxanthin and an antioxidant supplement, or did not receive any supplements. After 24 months, those receiving supplements had improved and stabilized visual acuity (p = 0.003), compared to the non-supplemented group, as well as improved contrast sensitivity at both 12 and 24 months (p = 0.001) (Piermarocchi et al., 2011).
The Lutein Antioxidant Supplement Trial (LAST) was a randomized, double-blinded, placebo-controlled trial of ninety atrophic AMD patients who either received 10 mg lutein, 10 mg lutein with an antioxidant supplement, or a maltodextrin placebo. In both groups that received lutein, there was an improvement in MPOD (p < 0.05), visual acuity (p < 0.05) and contrast sensitivity (at 6, 12, 18 degrees, p < 0.05), after 12 months of supplementation. The group receiving lutein alone also experienced improvements in the Amsler grid (p < 0.01) and in glare recovery (AREDS stage II p = 0.02); AREDS stage IV p = 0.05) (Richer et al., 2004). In a follow-up analysis of this study, it was observed that those patients with the greatest increases in MPOD with lutein supplementation were those with the lowest baseline levels, suggesting that lutein supplementation is most beneficial for high risk patients (Richer et al., 2007). The data also indicate a saturation of carotenoids in the macula (Trieschmann et al., 2007).
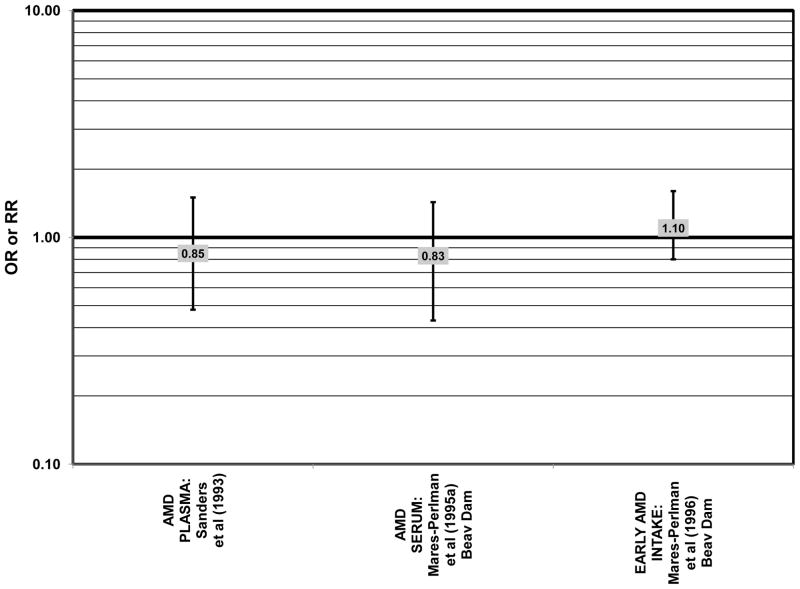
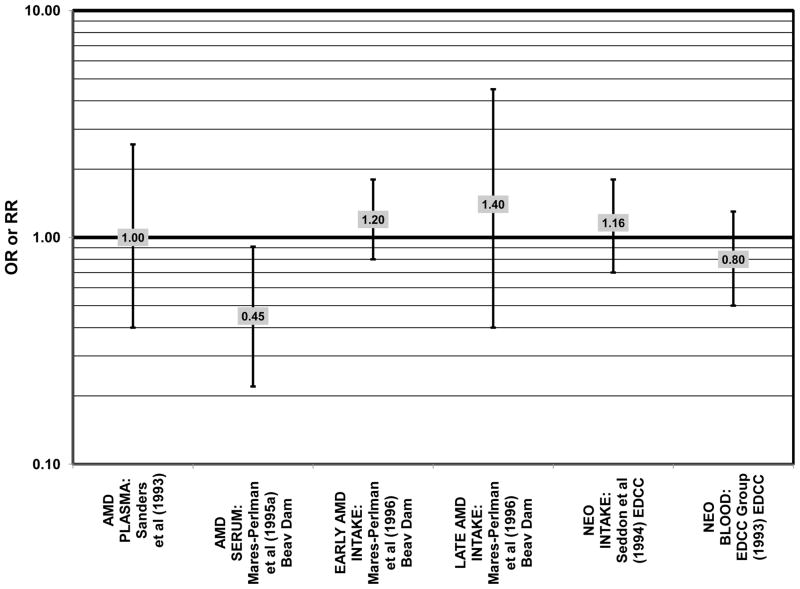
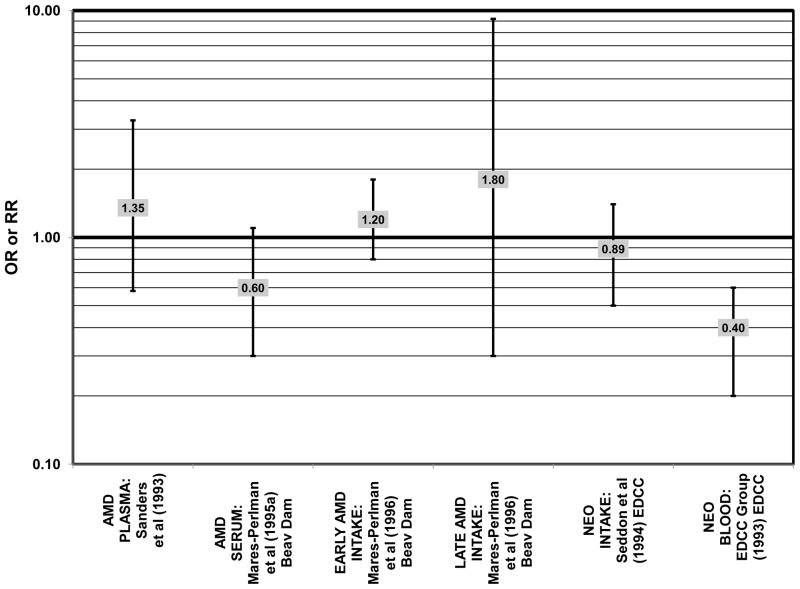
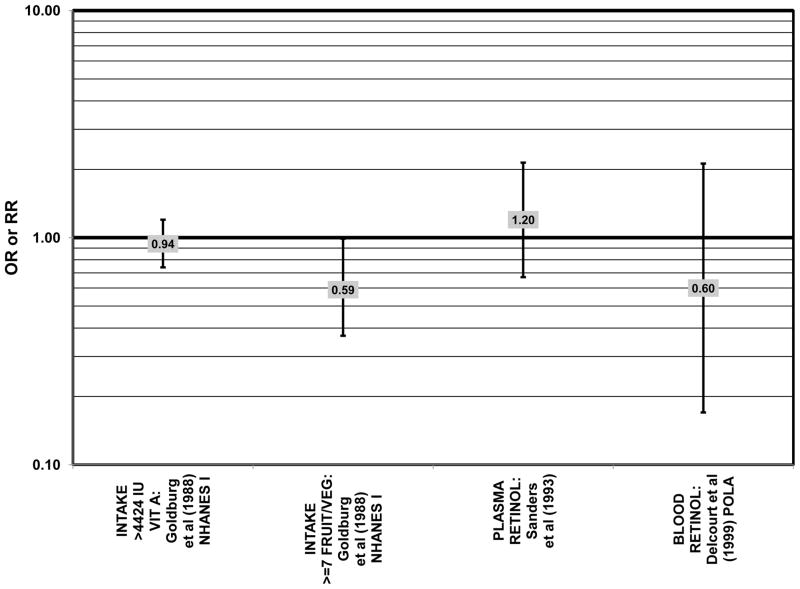
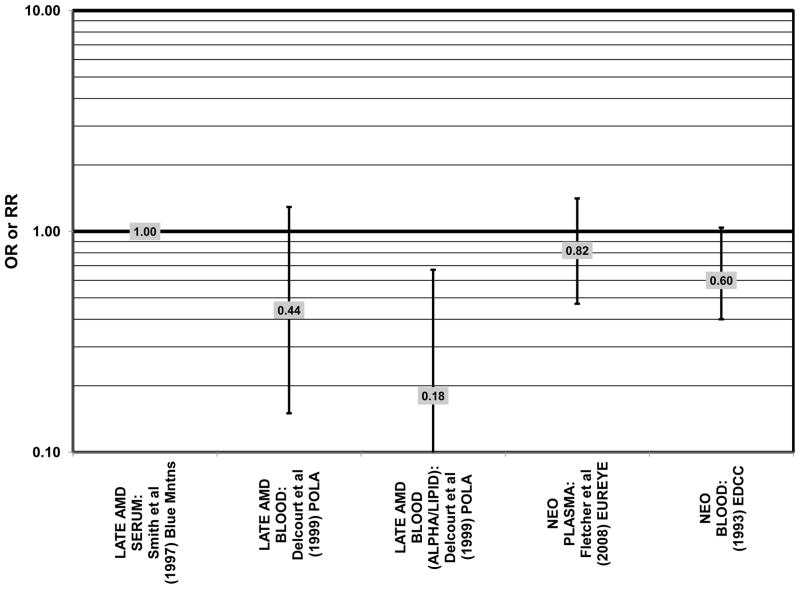
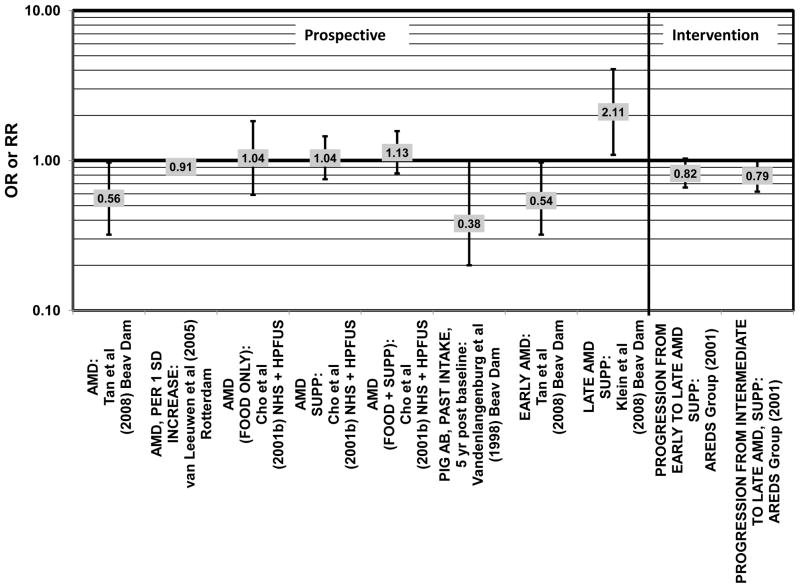
Despite these encouraging results, some studies did not find any effects of lutein and zeaxanthin on risk for AMD. A case-control study of 34 AMD patients did not find any difference in blood levels of lutein/zeaxanthin between patients and controls (Cardinault et al., 2005), nor did another case-control study consisting of 65 elderly AMD patients report any effect of plasma lutein levels on risk for AMD (OR = 1.37; 95% CI: 0.57, 3.38) (Sanders et al., 1993) (Fig. 24). One hundred sixty seven pairs of AMD patients and controls from the Beaver Dam Eye Study indicated that there was no association between serum levels of lutein/zeaxanthin (OR = 0.70; 95% CI: 0.40, 1.40) and risk for any stage of AMD (Mares-Perlman et al., 1995a) (Fig. 24). In a case-control analysis of participants in AREDS, increased lutein intake was associated with a marginally significant reduction in risk for neovascular AMD (OR = 0.70; 95% CI: 0.40, 1.00), yet this relationship became non-significant after adjusting for known genetic risk factors (OR = 0.80; 95% CI: 0.50, 1.30) (Seddon et al., 2010) (Fig. 31). Similar findings were reported in several cross-sectional studies. A cross-sectional study of 722 elderly Japanese reported that there were also no differences in serum levels of lutein/zeaxanthin between those with and without late AMD (Michikawa et al., 2009). In a cross-sectional analysis of the Beaver Dam Eye Study, intake of lutein/zeaxanthin had no effect on the risk for early or late age-related maculopathy (Mares-Perlman et al., 1996) (Figs. 29, 30). Cross-sectional analysis of 4,753 elderly men and women in the European Eye Study (“EUREYE” in the figures) also found no association between lutein or zeaxanthin plasma levels and neovascular AMD risk (Fletcher et al., 2008) (Fig. 31).
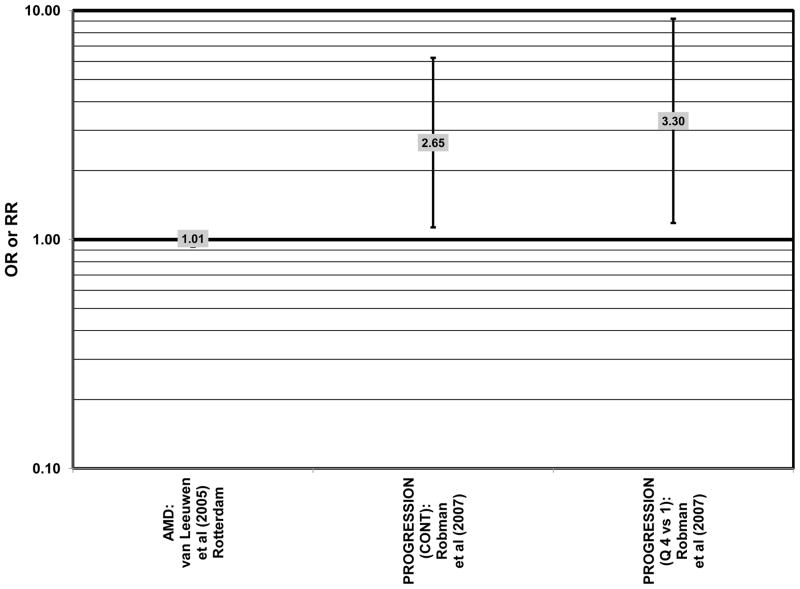
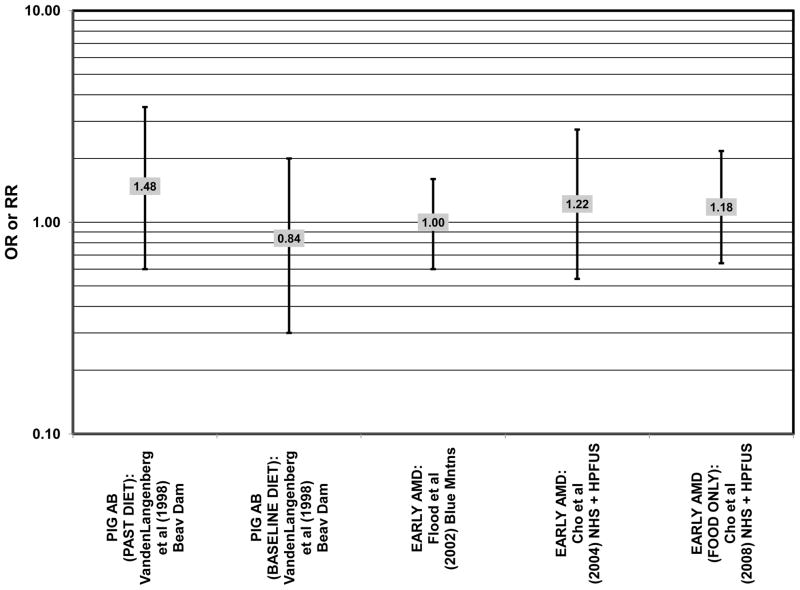
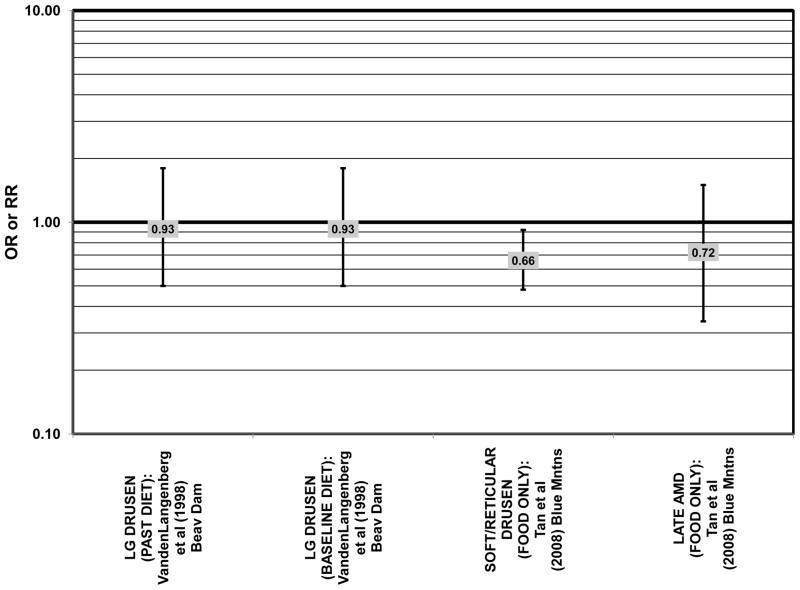
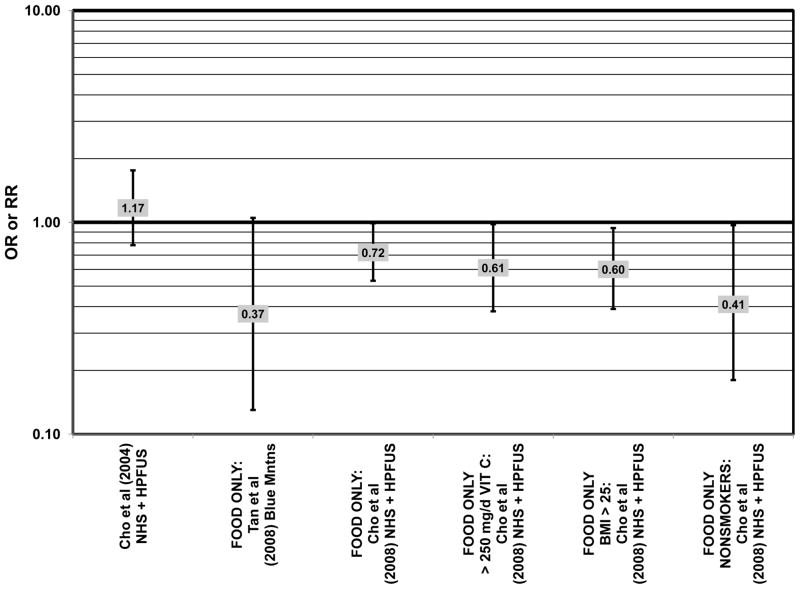
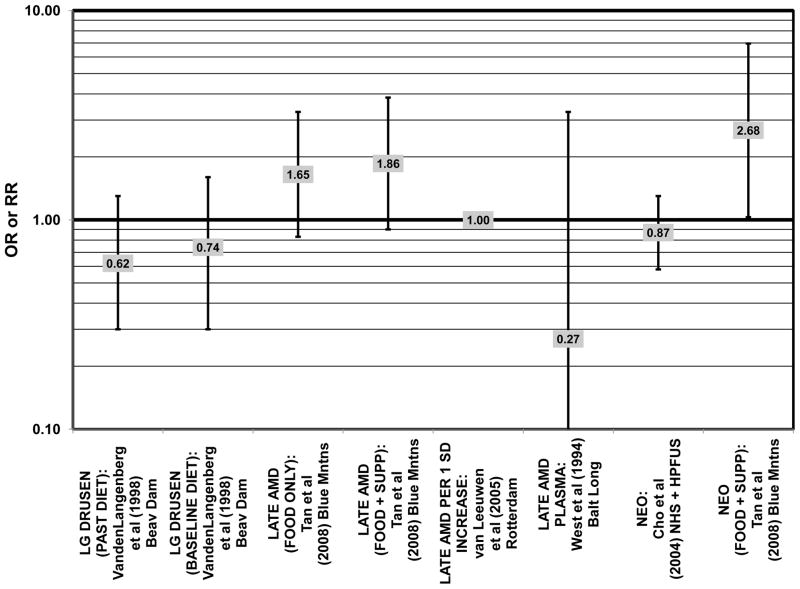
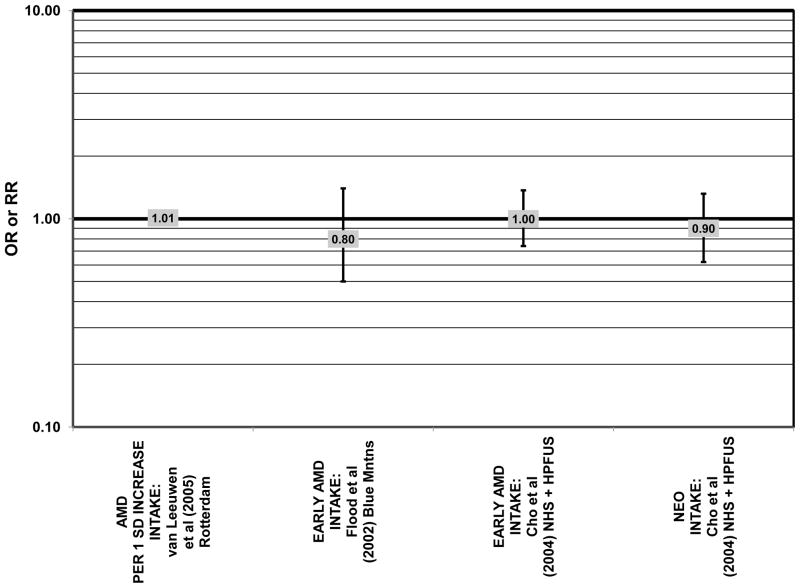
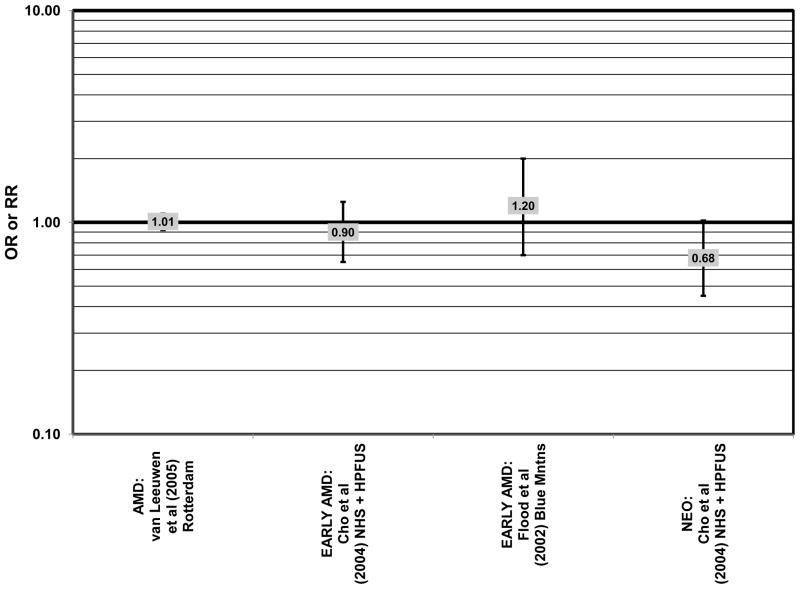
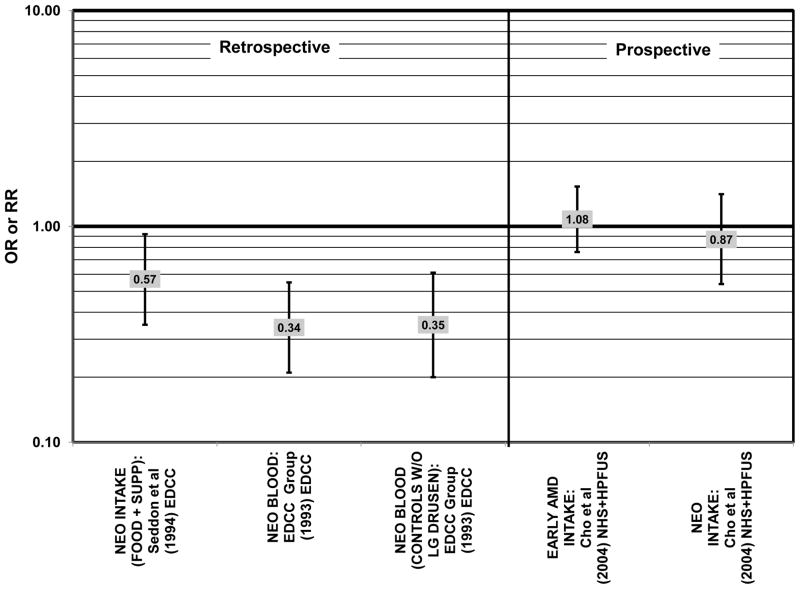
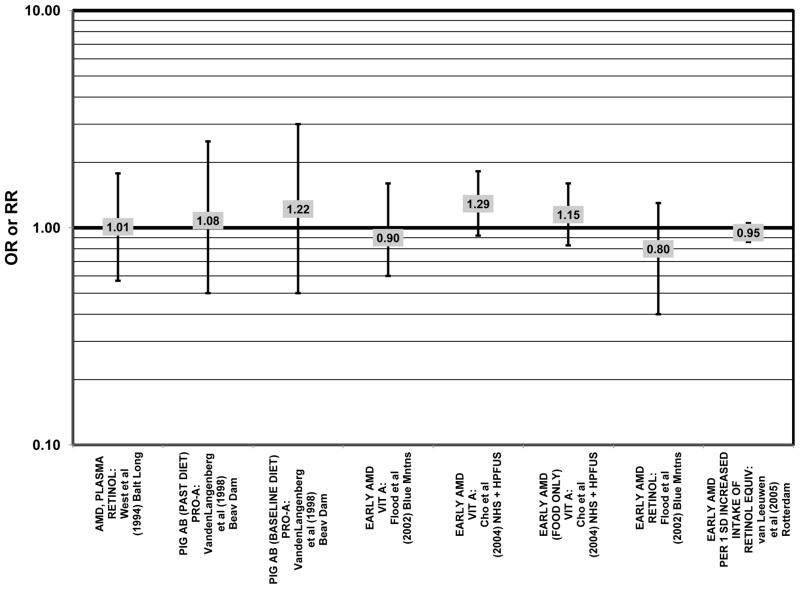
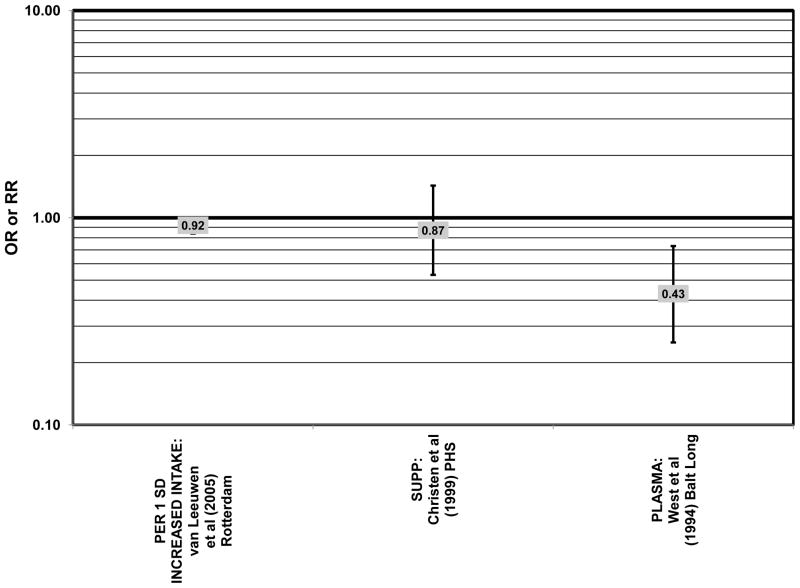
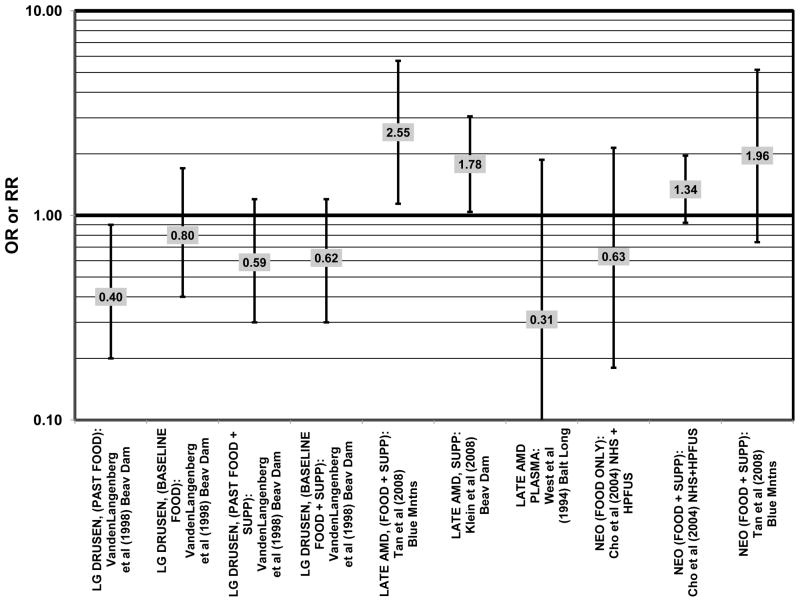
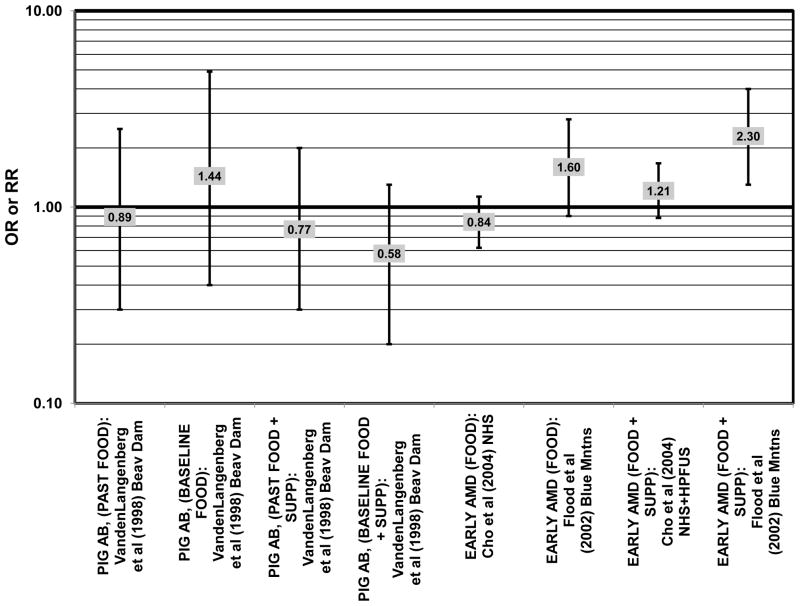
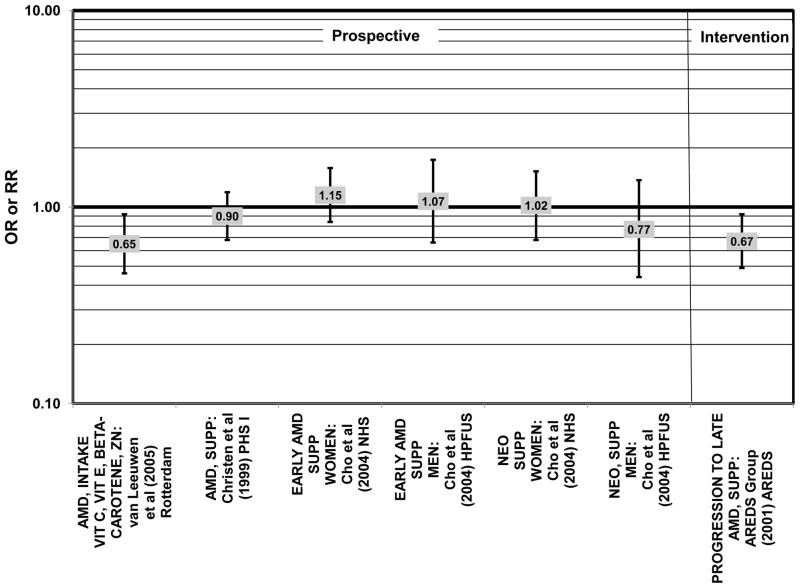
Several prospective studies also did not find an effect of lutein and zeaxanthin on AMD risk. The Rotterdam prospective cohort was composed of over 10,000 men and women over the age of 55 living in Rotterdam, Netherlands. In this cohort, there was no association between any stage of AMD and intake of lutein/zeaxanthin (van Leeuwen et al., 2005) (Fig. 32). Five years after baseline enrollment of the Blue Mountains Eye Study, data from 2,235 participants indicated that lutein/zeaxanthin intake had no effect on risk for early AMD (OR = 1.00, 95% CI: 0.60. 1.60) (Flood et al., 2002) (Fig. 33). In the same cohort, 10 years after baseline, intake of lutein and zeaxanthin in foods was without effect on risk for atrophic or neovascular AMD, although there was a reduced risk for soft/reticular drusen (OR = 0.66; 95% CI: 0.48, 0.92) (Tan et al., 2008) (Figs. 34, 35). In the Beaver Dam Eye Study, neither present nor past intake of lutein modulated the 5 year incidence of pigment abnormalities (VandenLangenberg et al., 1998) (Fig. 33). Analysis of men and women in the NHS and HPFUS revealed that those with the highest intakes of lutein and zeaxanthin did not have significantly less risk for early AMD than those with the lowest intakes after adjusting for smoking status, BMI, age, energy intake, alcohol intake, fish intake, and use of hormone replacement therapy (Cho et al., 2004) (Fig. 33). Subsequent analysis of the same cohorts also found no association between early or neovascular AMD and lutein/zeaxanthin intake (Cho et al., 2008) (Figs. 33, 35). There was slightly lower risk for neovascular AMD in this population among those who consumed high levels of lutein and zeaxanthin from food (RR = 0.72; 95% CI: 0.53, 0.99), and this effect became more robust in subgroups of this population (Fig. 35). In men and women who consumed >250 mg/day vitamin C (RR = 0.61; 95% CI: 0.38, 0.98), had a BMI of at least 25 (RR = 0.60; 95% CI: 0.39, 0.94), and were non-smokers (RR = 0.41; 95% CI: 0.18, 0.97; p = 0.07 for trend), the highest level of lutein/zeaxanthin intake was associated with a protective effect on neovascular AMD (Cho et al., 2008) (Fig. 35).
Figure 32.
Odds or risk ratio for any stage or progression through AMD with high vs. low intake of lutein and zeaxanthin in prospective studies.
Figure 33.
Odds or risk ratio for pigment abnormalities or early AMD with high vs. low intake of lutein and zeaxanthin in prospective studies.
Figure 34.
Odds or risk ratio for drusen or late AMD with high vs. low intake of lutein and zeaxanthin in prospective studies.
Figure 35.
Odds or risk ratio for neovascular AMD with high vs. low intake of lutein and zeaxanthin in prospective studies.
Finally, there are a few studies which suggested that elevated intakes of carotenoids increase risk for AMD. In retrospective analysis of the NHANES III cohort, it was observed that those who consumed the highest amounts of lutein and zeaxanthin had a slightly increased risk for the appearance of drusen (OR = 1.40; 95% CI: 1.00, 1.80) (Mares-Perlman et al., 2001) (Fig. 27). This effect appeared to be driven by the most elderly participants of this population, as the risk for drusen among those over the age of 80 who consumed the highest amounts of lutein and zeaxanthin increased (OR = 2.40; 95% CI: 1.30, 4.40) (Mares-Perlman et al., 2001) (Fig. 27). In addition, a prospective study of 254 patients with early AMD showed that after controlling for age, smoking, AMD family history, source study and follow-up duration, lutein/zeaxanthin intake (as a continuous variable) was associated with an increased risk for AMD (OR = 2.65; 95% CI: 1.13, 6.22; p = 0.02) (Fig. 32). Categorically, those consuming 880–1070 μg lutein/zeaxanthin per day had 3.3 fold risk of AMD progression compared to those consuming < 520 μg per day (95% CI: 1.18, 9.22; p = 0.02) (Robman et al., 2007) (Fig. 32).
Beta-carotene is another carotenoid that has been investigated in many studies for its potential ability to modulate AMD risk. The EDCC found that increased consumption (OR = 0.59; 95% CI: 0.40, 0.96; p = 0.03) and blood levels (OR = 0.30; 95% CI: 0.20, 0.50; p < 0.001 for trend) of beta-carotene reduced risk for neovascular AMD (Eye Disease Case-Control Study Group, 1993; Seddon et al., 1994) (Fig. 38). Further support for a beneficial role of beta-carotene was found in a cross-sectional study of 722 elderly Japanese also found that reported that those with late AMD had significantly lower serum levels of beta-carotene (p < 0.05) (Michikawa et al., 2009).
Figure 38.
Odds or risk ratio for late AMD with high vs. low intake or blood levels of beta-carotene in retrospective and cross-sectional studies.
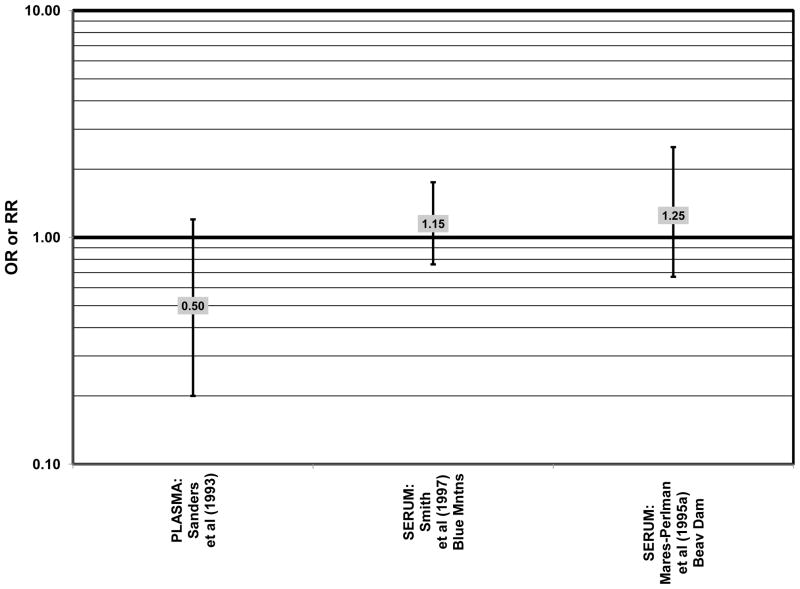
The remainder of the studies evaluating the role of beta-carotene did not find an effect of this carotenoid on risk for AMD (Figs. 36–41). A case-control study of 34 AMD patients did not find any difference in blood levels of beta-carotene between AMD patients and controls (Cardinault et al., 2005). Case-control analyses of both the Blue Mountains Eye Study and Beaver Dam Eye Study also found no association between serum beta-carotene levels and risk for any stage of AMD (Mares-Perlman et al., 1995a; Smith et al., 1997) (Fig. 36). Similarly, Sanders and colleagues did not find that plasma beta-carotene levels were associated with AMD risk in a case-control study consisting of 65 AMD patients (Sanders et al., 1993) (Fig. 36).
Figure 36.
Odds or risk ratio for any stage of AMD with high vs. low blood levels of beta-carotene in retrospective and cross-sectional studies.
Figure 41.
Odds ratio for any stage of AMD upon supplementation with beta-carotene in an intervention study.
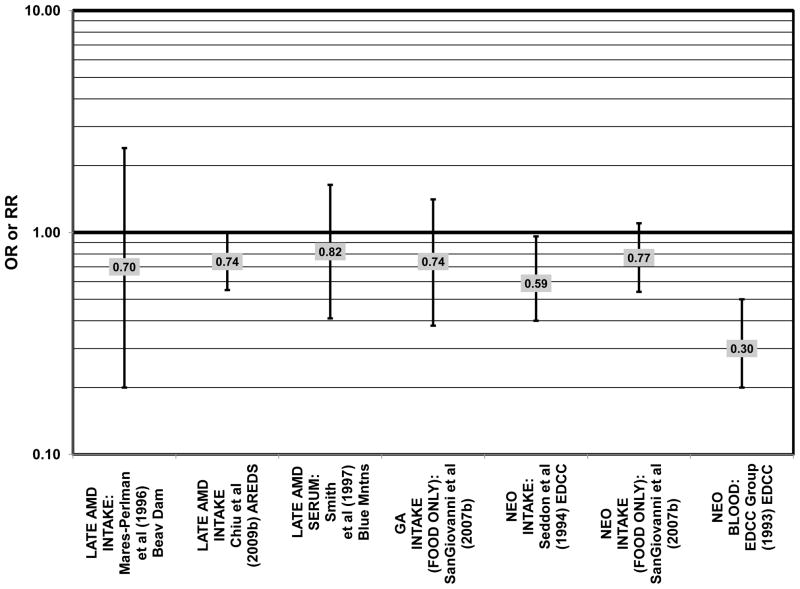
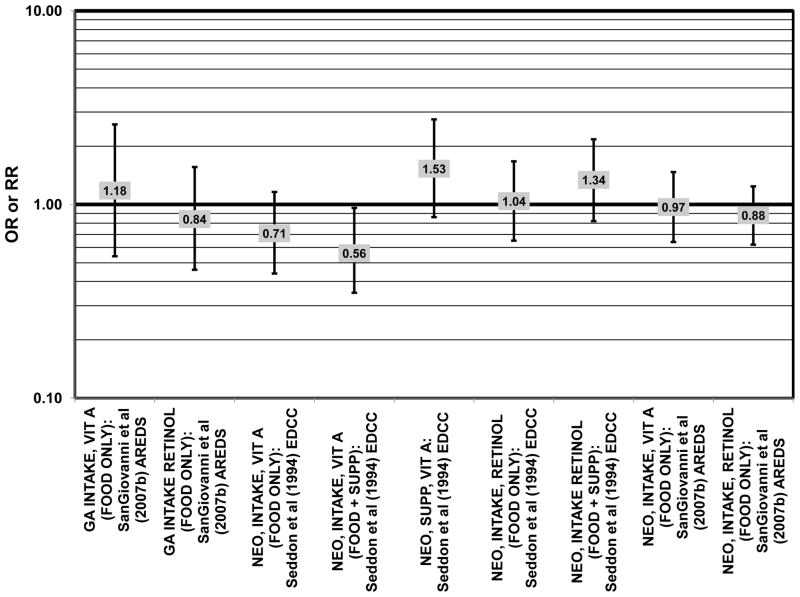
Results from these case-control studies are supported by data from cross-sectional studies. Although preliminary data from the NHS suggested that intake of beta-carotene was inversely associated with pigment abnormalities, this relationship was not confirmed in more recent data (Morris et al., 2007). A cross-sectional analysis of the Beaver Dam Eye Study showed that intake of beta-carotene had no effect on the risk for early or late age-related maculopathy (Mares-Perlman et al., 1996) (Figs. 37, 38). Baseline analysis of 4,003 participants in the AREDS study showed that beta-carotene intake was not associated with risk for drusen (OR = 0.87; 95% CI: 0.72, 1.05), yet those with beta-carotene intakes in the third quartile had a marginally reduced risk for late AMD compared to those with the lowest amount of consumption (OR = 0.74; 95% CI: 0.55, 1.00) (Chiu et al., 2009b) (Figs. 37, 38). However, intake of beta-carotene did not have any effect on risk for geographic atrophy or neovascular AMD in this same population (SanGiovanni et al., 2007b) (Fig. 38). Similarly, Smith and colleagues did not find that beta-carotene levels in serum were associated with risk for late AMD (Smith et al., 1997) (Fig. 38).
Figure 37.
. Odds or risk ratio for drusen or early AMD with high vs. low intake or blood levels of beta-carotene in retrospective and cross-sectional studies.
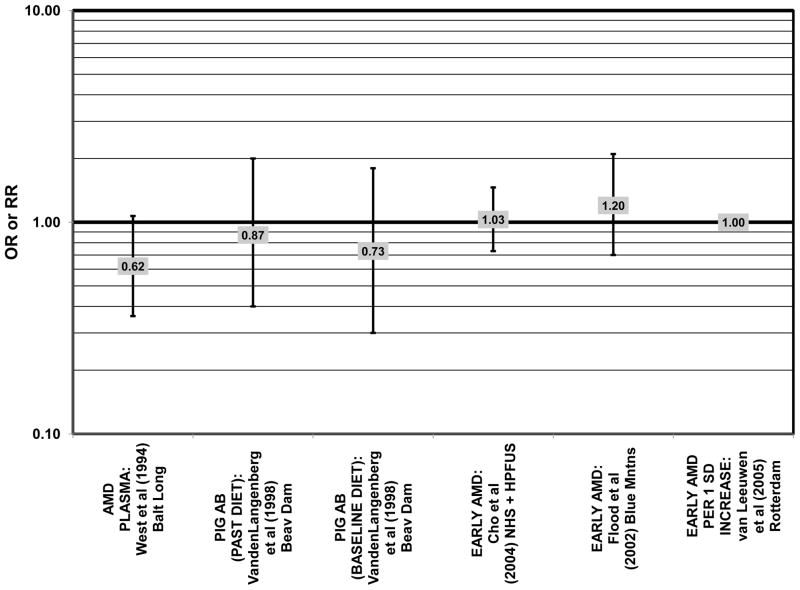
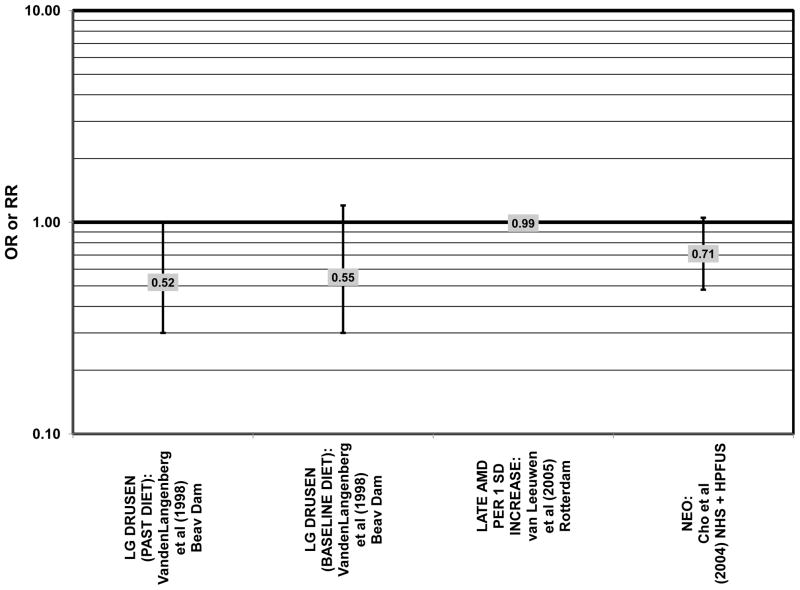
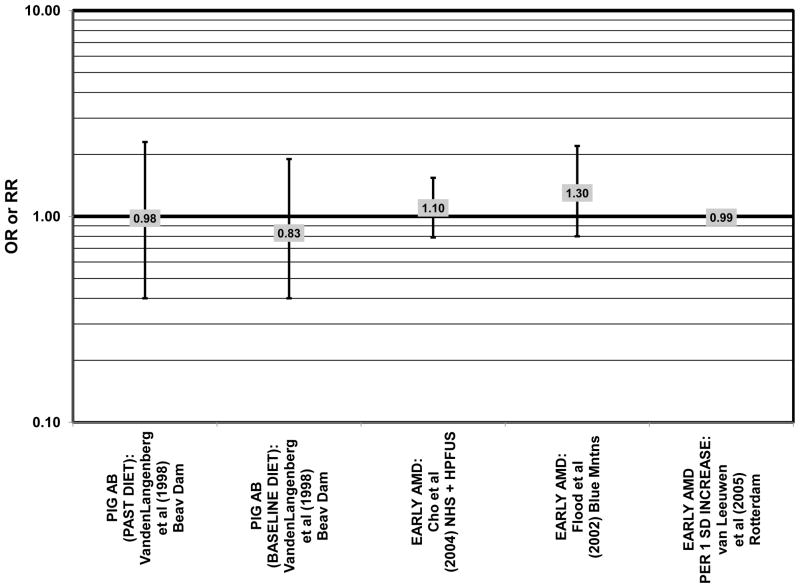
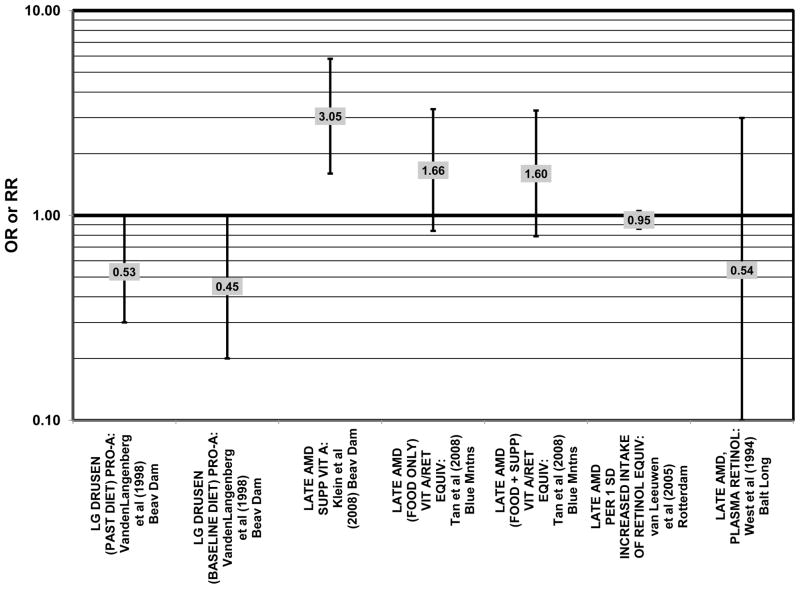
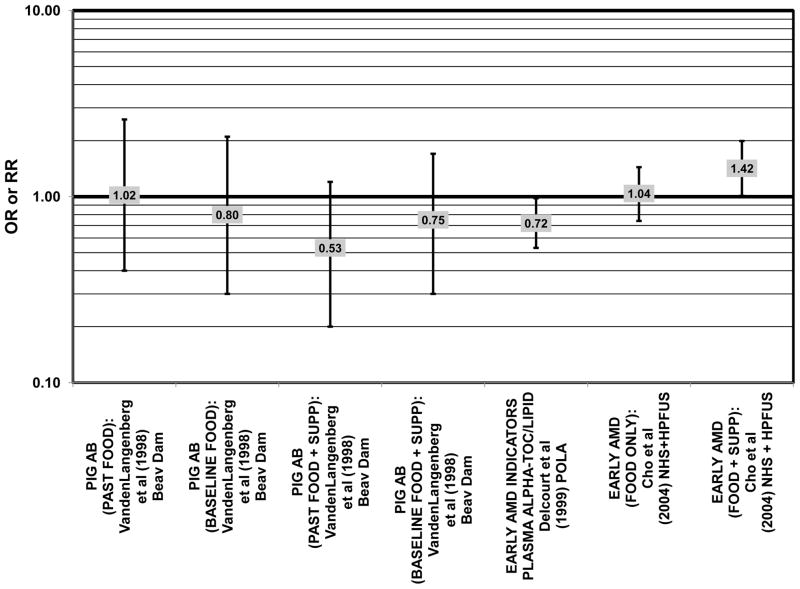
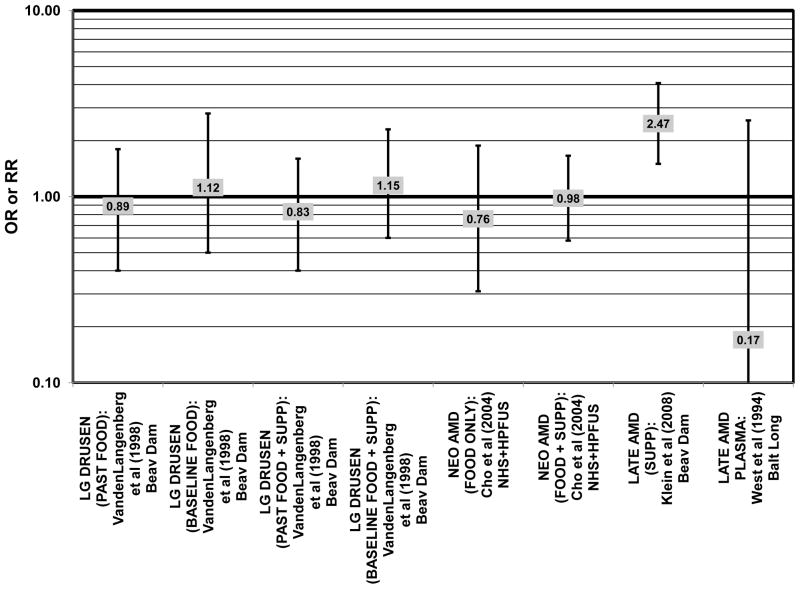
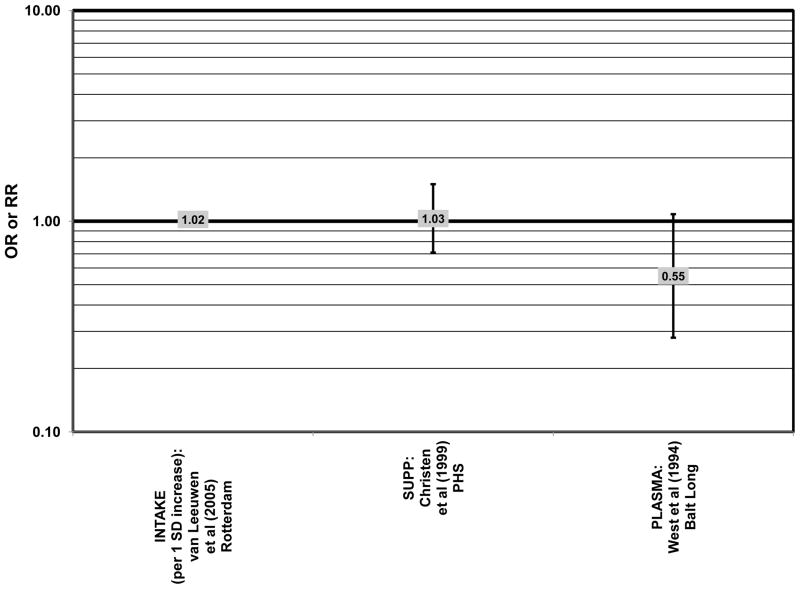
Furthermore, in prospective studies such as the Rotterdam cohort, there was no association between beta-carotene intake and risk for any stage of AMD (HR = 1.00; 95% CI: 0.94, 1.06), nor was there any association between plasma levels of beta-carotene and risk for any stage of AMD or severe AMD in the Baltimore Longitudinal Study of Aging (“Balt Long” in the figures), a prospective analysis of 916 men and women over the age of 40 (van Leeuwen et al., 2005) (West et al., 1994) (Figs. 39, 40). In the Blue Mountains Eye Study, there was no association between beta-carotene intake and risk for early AMD five years after baseline (OR = 1.20; 95% CI: 0.70, 2.10) (Flood et al., 2002) (Fig. 39). In this same cohort ten years after baseline, beta-carotene intake with (RR = 1.86; 95% CI: 0.90, 3.84) or without supplements (RR = 1.65; 95% CI: 0.83, 3.28) also had no effect on risk for any form of late AMD (Tan et al., 2008) (Fig. 40). Similarly, there was no association between beta-carotene intake and risk for early or neovascular AMD in the NHS+HPFUS (Cho et al., 2004) (Figs. 39, 40). In the Beaver Dam study, there was also no association between past or current dietary intake of beta-carotene and risk for pigment abnormalities or drusen (VandenLangenberg et al., 1998) (Figs. 39, 40).
Figure 39.
Odds or risk ratio for any stage of AMD, pigment abnormalities or early AMD with high vs. low intake or blood levels of beta-carotene in prospective studies.
Figure 40.
Odds or risk ratio for drusen or late AMD with high vs. low intake of beta-carotene in prospective studies.
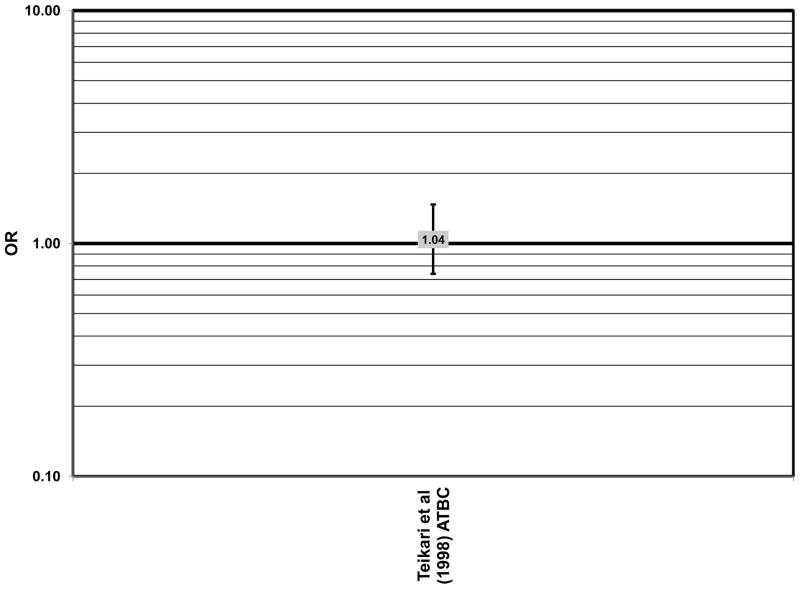
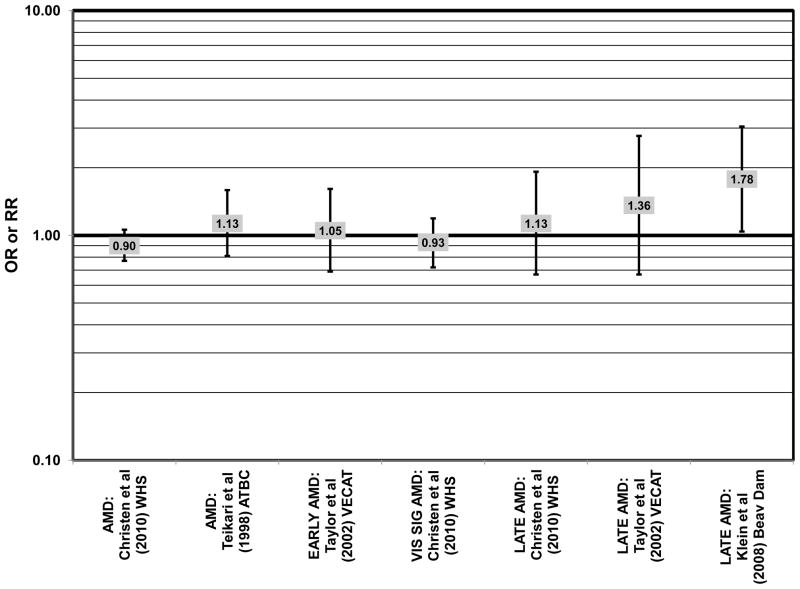
Finally, the Alpha Tocopherol Beta Carotene (ATBC) study did not show a protective effect of beta-carotene. The ATBC study found that among 29,000 Finish male smokers assigned to either daily supplements of 50 mg vitamin E, 20 mg beta-carotene, both, or placebo, 5–8 years of beta-carotene supplementation alone had no effect on risk for AMD (OR = 1.04; 95% CI: 0.74, 1.47)(Teikari et al., 1998) (Fig. 41).
In addition to beta-carotene, the EDCC also evaluated the effect of the isomer, alpha-carotene, on risk for AMD. In this study, higher blood levels of alpha-carotene (OR = 0.50; 95% CI: 0.30, 0.80; p = 0.003 for trend) were associated with decreased risk for neovascular AMD (Eye Disease Case-Control Study Group, 1993) (Fig. 43). Prospective analysis of the Beaver Dam Eye Study also found that those with an elevated past intake of dietary alpha-carotene were at a reduced risk for the appearance of large drusen (OR = 0.52; 95% CI: 0.30, 1.00) (VandenLangenberg et al., 1998) (Fig. 45).
Figure 43.
Odds or risk ratio for late AMD with high vs. low intake or blood levels of alpha-carotene in retrospective and cross-sectional studies.
Figure 45.
Odds or risk ratio for drusen on late AMD with high vs. low intake of alpha-carotene in prospective studies.
Other than these two studies, the remainder of the studies report that alpha-carotene does not significantly affect risk for AMD. Case-control analysis of patients in the Beaver Dam Eye Study did not find any association between serum levels of alpha-carotene and any stage of AMD (OR = 0.83; 95% CI: 0.43, 1.43), nor did two additional case-control studies find any difference in blood levels of alpha-carotene between AMD patients and controls (Cardinault et al., 2005; Mares-Perlman et al., 1995a; Sanders et al., 1993) (Fig. 42). In the EDCC, there was also no relationship between risk for neovascular AMD and intake of alpha-carotene (OR = 0.79; 95% CI: 0.50, 1.30, p = 0.14 for trend) (Seddon et al., 1994) (Fig. 43).
Figure 42.
Odds or risk ratio for any stage or early AMD with high vs. low intake or blood levels of alpha-carotene in retrospective and cross-sectional studies.
Two cross-sectional studies, including an elderly Japanese population as well as the Beaver Dam cohort, corroborated these results. Among 722 elderly Japanese, there was no relationship between serum levels of alpha-carotene and risk for late AMD (Michikawa et al., 2009). In the Beaver Dam cohort, there was no relationship between alpha-carotene intake and risk for early or late AMD (Mares-Perlman et al., 1996) (Figs. 42, 43).
Although preliminary data from the NHS indicated that intake of alpha-carotene and foods containing alpha-carotene was associated with decreased risk for pigment abnormalities, this relationship could not be confirmed in more current dietary data (Morris et al., 2007). Similarly, prospective analysis of the Beaver Dam Eye Study indicated that intake or alpha-carotene in the present or past diet of participants did not affect risk for pigment abnormalities or drusen (VandenLangenberg et al., 1998) (Figs. 44, 45). There was also no association between alpha-carotene intake and risk for early AMD five years after baseline analysis in the Blue Mountains Eye Study (OR = 1.30; 95% CI: 0.80, 2.20) (Flood et al., 2002) (Fig. 44). Nor was there any association between alpha-carotene intake and early (HR = 0.99; 95% CI: 0.94, 1.06) or late (OR = 0.99; 95% CI: 0.94, 1.06) AMD in the Rotterdam prospective cohort (van Leeuwen et al., 2005) (Figs. 44, 45). In the NHS+HPFUS, there was also no association between alpha-carotene intake and early or neovascular AMD (Cho et al., 2004) (Figs. 44, 45).
Figure 44.
Odds or risk ratio for pigment abnormalities or early AMD with high vs. low intake of alpha-carotene in prospective studies.
Lycopene is most commonly found in the American diet in tomato-based products, as reviewed by Maiani and colleagues(Maiani et al., 2009). Evidence for a potential role of lycopene in retinal health was found in a small case control study which found that AMD patients had less lycopene in their serum, LDL, and HDL than in healthy controls (p = 0.006) (Cardinault et al., 2005). Similarly, a nested case-control study of 167 patients from the Beaver Dam Eye Study found that higher lycopene blood levels were associated with decreased risk for any stage of AMD (OR = 0.45; 95% CI: 0.22, 0.91) (Mares-Perlman et al., 1995a) (Fig. 46).
Figure 46.
Odds or risk ratio for any stage, early or late AMD with high vs. low intake or blood levels of lycopene in retrospective and cross-sectional studies.
The remaining observational studies did not find that lycopene intake or blood levels were associated with risk for AMD. There was no association between lycopene intake (OR = 1.16; 95% CI: 0.70, 1.80; p = 0.96 for trend) or blood levels (OR = 0.80; 95% CI: 0.50, 1.30; p = 0.4 for trend) and risk for neovascular AMD in the EDCC (Eye Disease Case-Control Study Group, 1993; Seddon et al., 1994) (Fig. 46). Cross-sectional analysis of elderly Japanese found that serum levels of lycopene were not associated with risk for late AMD (Michikawa et al., 2009). Similarly, cross-sectional analysis of the Beaver Dam Eye Study showed that intake of lycopene had no effect on the risk for early or late age-related maculopathy (Mares-Perlman et al., 1996) (Fig. 46).
Prospective studies also suggest that lycopene has little role in modulating AMD risk. The Rotterdam prospective cohort found no association between any stage of AMD and intake of lycopene (HR = 1.01; 95% CI: 0.97, 1.04)(van Leeuwen et al., 2005) (Fig. 47). Recent data from the NHS found that serum levels of lycopene were not associated with risk for pigment abnormalities (Morris et al., 2007). Five years after baseline analysis of the Blue Mountains Eye Study, lycopene intake was not associated with early AMD (OR = 0.80; 95% CI: 0.50, 1.40) (Flood et al., 2002) (Fig. 47). An even larger cohort of 118,428 men and women who participated in the NHS+HPFUS found no association between early or neovascular AMD and lycopene intake (Cho et al., 2004) (Fig. 47).
Figure 47.
Odds or risk ratio for any stage, early or late AMD with high vs. low intake or blood levels of lycopene in prospective studies.
Cryptoxanthin is another carotenoid that is found in foods such as avocado, basil and mango (Maiani et al., 2009). In the EDCC, increased blood levels of cryptoxanthin were associated with decreased risk for neovascular AMD (OR = 0.40; 95% CI: 0.20, 0.60; p = 0.001 for trend) (Eye Disease Case-Control Study Group, 1993) (Fig. 48). A cross-sectional study of 722 elderly Japanese also reported that those with late AMD had significantly lower serum levels of beta-cryptoxanthin (p < 0.05) (Michikawa et al., 2009). However, another case-control study did not find any difference in blood levels of cryptoxanthin between AMD patients and healthy controls (Cardinault et al., 2005). Furthermore, case-control analysis of the Beaver Dam Eye Study revealed that beta-cryptoxanthin levels in serum were not associated with risk at any stage of AMD (OR = 0.60; 95% CI: 0.30, 1.10) (Mares-Perlman et al., 1995a) (Fig. 48). Intake of cryptoxanthin was also not associated with risk for neovascular AMD in the EDCC (OR = 0.89; 95% CI: 0.50, 1.40; p = 0.84 for trend) (Seddon et al., 1994) (Fig. 48). Cross-sectional analysis of the Beaver Dam Eye Study showed that intake of cryptoxanthin also had no effect on the risk for early or late age-related maculopathy (Mares-Perlman et al., 1996) (Fig. 48).
Figure 48.
Odds or risk ratio for any stage, early or late AMD with high vs. low intake or blood levels of cryptoxanthin in retrospective and cross-sectional studies.
Prospective analyses support the observations of little effect of cryptoxanthin intake on retinal health. The Rotterdam Study did not find any association between cryptoxanthin intake and any stage of AMD (HR = 1.01; 95% CI: 0.92, 1.10) (van Leeuwen et al., 2005) (Fig. 49). In the Blue Mountains Eye Study, five years after baseline analysis, there was no association between beta-cryptoxanthin intake and risk for early AMD (OR = 1.20; 95% CI: 0.70, 2.0), and in the NHS+HPFUS, there was no relationship between beta-cryptoxanthin intake and early (OR = 0.90; 95% CI: 0.65, 1.25) or neovascular (OR = 0.68; 95% CI: 0.45, 1.02) AMD (Cho et al., 2004; Flood et al., 2002) (Fig. 49).
Figure 49.
Odds or risk ratio for any stage, early or neovascular AMD with high vs. low intake of cryptoxanthin in prospective studies.
Levels of total carotenoids were also analyzed for their potential to confer retinal benefit. In the EDCC, increasing intake of total carotenoids was associated with decreased risk for neovascular AMD (OR = 0.57; 95% CI: 0.35, 0.92; p = 0.02 for trend) (Seddon et al., 1994) (Fig. 50). Analysis of blood levels from this same study corroborated these relationships: OR = 0.34 (95% CI: 0.21, 0.55; p < 0.001 for trend) (Fig. 50) (Eye Disease Case-Control Study Group, 1993). Similar results were obtained when AMD cases were compared to controls without large drusen. Another smaller case-control study with 48 AMD patients found that total carotenoid plasma levels were significantly lower in patients with late AMD compared to those in the early stages of the disease (p< 0.05), although there was no difference in plasma levels between AMD patients and healthy controls (Simonelli et al., 2002). This may simply imply that carotenoids play a more important role in disease progression rather than disease onset. This finding is comparable to that of another case-control study of 197 Japanese men and women that reported that eyes with early AMD and the normal eye of an AMD patient had comparable macular carotenoid levels, which were both higher than those of an eye with late AMD (p < 0.001). However, in this study macular carotenoid levels of all AMD patients were also significantly lower than those of age-matched healthy subjects (p < 0.001) as determined by Raman spectroscopy (Obana et al., 2008).
Figure 50.
Odds or risk ratio for neovascular or early AMD with high vs. low intake or blood levels of total carotenoids in retrospective and prospective studies.
Despite these reported beneficial effects of carotenoids on the retina, four case-control studies did not find any difference in blood levels of total carotenoids between AMD patients and healthy controls (Blumenkranz et al., 1986; Cardinault et al., 2005; Ishihara et al., 1997; Sanders et al., 1993). Furthermore, prospective analyses of the NHS+HPFUS found no association between early or neovascular AMD and total carotenoid intake (Cho et al., 2004) (Fig. 50).
Many carotenoids are by-products of vitamin A, which can take the form of retinol in the body. Therefore, analysis of vitamin A/retinol intake and blood levels has also been examined for its role in retinal health. NHANES I found that those who consumed increased amounts of fruits and vegetables rich in vitamin A had a decreased risk for any stage of AMD (OR = 0.59; 95% CI: 0.37, 0.99) (Goldberg et al., 1988) (Fig. 51). Also, a prospective analysis of the Beaver Dam Eye Study found that those with an elevated past intake (OR = 0.53; 95% CI: 0.30, 1.00) or current intake (OR = 0.45; 95% CI: 0.20, 1.00) of pro-vitamin A carotenoids were at a reduced risk for the appearance of large drusen (VandenLangenberg et al., 1998) (Fig. 54).
Figure 51.
Odds or risk ratio for any stage of AMD with high vs. low intake or blood levels of vitamin A or retinol in retrospective and cross-sectional studies.
Figure 54.
Odds or risk ratio for large drusen or late AMD with high vs. low intake (with or without supplements) or blood levels of pro-vitamin A carotenoids (PRO-A), vitamin A, retinol equivalents of vitamin A, or retinol in prospective studies.
All remaining analyses of vitamin A and risk for AMD did not find significant associations. A case-control study of 48 AMD patients found that AMD patients and healthy controls did not have different plasma levels of vitamin A, nor was there a difference in vitamin A levels between patients in different stages of AMD (Simonelli et al., 2002). The EDCC found that neither intake of retinol alone (OR = 1.34; 95% CI: 0.82, 2.17; p = 0.53 for trend) nor supplementation with retinol (OR = 1.53; 95% CI 0.86, 2.75; p = 0.22 for trend) modulated risk for neovascular AMD (Seddon et al., 1994) (Fig. 52). Similar findings were observed in a case-control analysis of the AREDS in which intake of vitamin A or retinol had no effect on risk for geographic atrophy or neovascular AMD (SanGiovanni et al., 2007b) (Fig. 52). Three more case-control studies also indicated that there was no difference in blood retinol levels between AMD patients and controls (Blumenkranz et al., 1986; Ishihara et al., 1997; Sanders et al., 1993).
Figure 52.
Odds or risk ratio for late AMD with high vs. low intake (with or without supplements) of vitamin A or retinol in retrospective and cross-sectional studies.
Several cross-sectional studies support these findings. There was no relationship between consumption of foods rich in vitamin A and risk for AMD in NHANES III, nor was there a relationship between intake or retinol or vitamin A and pigment abnormalities in the NHS (Goldberg et al., 1988; Morris et al., 2007). A cross-sectional study of 722 elderly Japanese also found no association between serum levels of retinol and risk for late AMD (Michikawa et al., 2009).
In prospective analysis of 2,235 participants of the Blue Mountains Eye Study, vitamin A (OR = 0.90; 95% CI: 0.60, 1.60) and retinol intake (OR = 0.80; 95% CI: 0.40, 1.30) did not affect risk for early AMD after 5 years (Flood et al., 2002) (Fig. 53). In the same cohort, 10 years after baseline, there was also no association between vitamin A intake and any form of AMD (late AMD: RR = 1.60; 95% CI: 0.79, 3.25; neovascular AMD: RR = 1.94; 95% CI: 0.77, 4.87) (Tan et al., 2008) (Figs. 54, 55). Similarly, the NHS+HPFUS found no association between early or neovascular AMD and vitamin A (Cho et al., 2004) (Figs. 53, 55). Prospective data from the Baltimore Longitudinal Study also showed that plasma levels of retinol had no effect on severe AMD risk (West et al., 1994) (Fig. 53).
Figure 53.
Odds or risk ratio for any stage of AMD, pigment abnormalities or early AMD with high vs. low intake or blood levels of pro-vitamin A carotenoids (PRO-A), vitamin A, or retinol in prospective studies.
One study did report some harmful effects of vitamin A consumption. Analysis of 4 examinations from the Beaver Dam Eye Study showed that supplementation with vitamin A was associated with an increased risk for late AMD (OR = 3.05; 95% CI: 1.60, 5.82) (Klein et al., 2008) (Fig. 54).
Taken together, these observational studies suggest that of all of the carotenoids studied, lutein and zeaxanthin may confer the most benefit to the retina. These benefits may be specific for certain types or stages of AMD, with advanced AMD showing the most consistent inverse relationships between lutein intake and decreased risk for lesions. Data concerning the effects of lutein and zeaxanthin on outcomes such as visual acuity and contrast sensitivity suggest that 10 mg of lutein/day, without zeaxanthin may confer the most retinal benefit, but intervention studies such as AREDS2 will clarify the role of these carotenoids in risk for onset and progression of specific AMD outcomes.
2.5 VITAMIN E
Similar to carotenoids, vitamin E is a lipophilic antioxidant and its role in retinal health was an early focus of research (Figs. 56–63). Some studies suggest that vitamin E (alpha-tocopherol) has a role in the diminishing risk for AMD. A case-control study of 48 AMD patients indicated that plasma vitamin E levels were significantly lower in patients with late AMD compared to patients with early AMD or healthy age-matched controls (p < 0.05). Importantly, these effects remained even after adjusting for cholesterol levels. This adjustment is of interest because being lipid soluble vitamin E associates with cholesterol on lipoprotein particles (Simonelli et al., 2002). Another case-control study of 25 elderly AMD patients found that vitamin E serum levels were significantly lower in AMD patients compared to healthy controls (p < 0.001). In this study, patients and controls had similar cholesterol levels (Belda et al., 1999). A third case-control study found that comparison of serum vitamin E levels in 35 AMD patients with 66 controls showed that vitamin E was inversely associated with risk for AMD (Ishihara et al., 1997).
Figure 56.
Odds or risk ratio for any stage of AMD with high vs. low blood levels of vitamin E in retrospective and cross-sectional studies.
Figure 63.
Odds or risk ratio for any stage, early, visually significant (“VIS SIG”) or late AMD upon supplementation with vitamin E in intervention studies.
Cross-sectional analysis of the 2,584 participants of the POLA study showed that after adjusting for plasma lipid levels, those with the highest levels of plasma vitamin E had a reduced risk for signs of early AMD such as pigment changes or soft drusen (OR = 0.72; 95% CI: 0.53, 0.98; p = 0.04 for trend) and late AMD (OR = 0.18; 95% CI: 0.05, 0.67; p = 0.004 for trend) (Delcourt et al., 1999) (Figs. 57, 59). These associations held up even in populations of different ethnicity. Thus, a cross-sectional study of 722 elderly Japanese found that patients with late AMD had marginally significant lower levels of serum alpha-tocopherol than healthy controls (p = 0.056) (Michikawa et al., 2009). Baseline analysis of 4,003 participants of the AREDS study indicated that those consuming the highest amounts of vitamin E had a marginally reduced risk for late AMD compared to those consuming the smallest amounts (OR = 0.66; 95% CI: 0.45, 0.99; p = 0.052 for trend) (Chiu et al., 2009b) (Fig. 58).
Figure 57.
Odds or risk ratio for drusen or early AMD with high vs. low intake or blood levels of vitamin E in retrospective and cross-sectional studies.
Figure 59.
Odds or risk ratio for late AMD with high vs. low blood levels of vitamin E in retrospective and cross-sectional studies.
Figure 58.
Odds or risk ratio for late AMD with high vs. low intake (with or without supplements) of vitamin E in retrospective and cross-sectional studies.
Some prospective study data corroborate the cross sectional data. Thus, the Baltimore Longitudinal Study of Aging indicated that among 976 men and women, those with the highest plasma tocopherol levels were at a reduced risk for any stage of AMD, even after adjusting for age, gender, and nuclear opacity (OR = 0.43; 95% CI: 0.25, 0.73) (West et al., 1994) (Fig. 60). In the Rotterdam prospective cohort, vitamin E intake was also associated with a slight reduction in risk for AMD (HR = 0.92; 95% CI: 0.84, 1.00) (van Leeuwen et al., 2005) (Fig. 60). Among 498 women in the NHS, increasing amounts of vitamin E intake were associated with a decreased prevalence of pigment abnormalities, an indicator of early AMD (Morris et al., 2007).
Figure 60.
Odds or risk ratio for any stage of AMD with high vs. low intake (with or without supplements) or blood levels of vitamin E in prospective studies.
There is also a significant amount of data suggesting that there is no relationship between vitamin E levels and AMD risk. A case-control study of 167 cataract patients from the Beaver Dam Eye Study revealed that there was no association between alpha-tocopherol (OR = 0.80; 95% CI: 0.40, 1.50) serum levels or gamma-tocopherol (OR = 1.30; 95% CI: 0.70, 2.40) serum levels and any stage of AMD (Mares-Perlman et al., 1995a) (Fig. 56). In a case-control study, there was no association between vitamin E serum levels and risk for AMD among 26 neovascular patients (Blumenkranz et al., 1986). The EDCC also found no association between vitamin E intake (OR = 1.07; 95% CI: 0.63, 1.84; p = 0.98 for trend) or supplementation (OR = 0.97; 95% CI: 0.6, 1.50, p = 0.28 for trend) and risk for neovascular AMD (OR = 1.46; 95% CI: 0.88, 2.44) (Seddon et al., 1994) (Fig. 58). There was also no association between blood levels of vitamin E and neovascular AMD (Eye Disease Case-Control Study Group, 1993) (Fig. 59). Case-control analysis of the AREDS population also showed that there was no association between vitamin E intake and risk for geographic atrophy or neovascular AMD (SanGiovanni et al., 2007b) (Fig. 58). A case-control studies of 65 AMD patients found no difference in blood alpha-tocopherol levels between patients and healthy controls (Sanders et al., 1993), nor did a case-control study of 165 AMD patients find that serum levels of vitamin E were associated with risk for any stage, early or late AMD (Smith et al., 1997) (Figs. 56, 57, 59).
A case-control study with 34 AMD patients analyzed the serum, LDL, and HDL of the cases and controls to determine if there were differences in antioxidant nutrients. They found no difference in serum, LDL, or HDL levels of alpha- or gamma-tocopherol between AMD patients and healthy controls (Cardinault et al., 2005). Cross-sectional analysis of the AREDS population also revealed that intake of vitamin E was not associated with risk for drusen (Chiu et al., 2009b) (Fig. 57). Retrospective analysis of the Beaver Dam cohort (n=1,968) also indicated that past intake of vitamin E with or without supplementation did not have a significant effect on early or late AMD (Mares-Perlman et al., 1996) (Figs. 57, 58). Cross-sectional analysis of 4,753 elderly men and women found no increased risk for neovascular AMD in those with the lowest plasma levels of alpha-tocopherol (OR = 0.82; 95% CI: 0.47, 1.41) (Fletcher et al., 2008) (Fig. 59).
There are also prospective studies, which cast doubt on the role of vitamin E in preventing AMD. In 1,709 participants from the Beaver Dam cohort, past intake of vitamin E was associated with a decreased risk for large drusen 5 years after baseline (OR = 0.40; 95% CI: 0.20, 0.90; p = 0.04 for trend), but there was no association with present vitamin E intake or past or present vitamin E supplementation (VandenLangenberg et al., 1998) (Figs. 62). Nor was there an association between past or present intake of vitamin E and risk for pigment abnormalities in the Beaver Dam cohort (VandenLangenberg et al., 1998) (Fig. 61). Also, in the Physicians’ Health Study (“PHS” in the figures), vitamin E supplementation was not associated with risk for any stage of AMD (RR = 0.87; 95% CI: 0.53, 1.43) (Christen et al., 1999) (Fig. 60). Similarly, a prospective analysis of 118,428 men and women who participated in the NHS+HPFUS showed that vitamin E intake had no effect on risk for early or neovascular AMD (Cho et al., 2004) (Figs. 61, 62).
Figure 62.
Odds or risk ratio for large drusen or late AMD with high vs. low intake (with or without supplements) or blood levels of vitamin E in prospective studies.
Figure 61.
Odds or risk ratio for pigment abnormalities, indicators of early AMD or early AMD with high vs. low intake (with or without supplements) or blood levels of vitamin E in prospective studies.
Driven in part by evidence suggesting that vitamin E may confer health benefits, the Women’s Health Study (WHS), a randomized double-blinded placebo controlled trial, gave 39,421 women either 600 IU vitamin E every other day, or placebo for 10 years. After adjusting for age, aspirin intake, and beta-carotene, vitamin E had no effect on risk for visually significant AMD (RR = 0.93; 95% CI: 0.72, 1.19), late AMD (RR = 1.13; 95% CI: 0.67, 1.92), or AMD with or without vision loss (RR = 0.90; 95% CI: 0.77, 1.06) (Christen et al., 2010) (Fig. 63). Another clinical trial, ATBC Study, was conducted among 29,000 Finish male smokers. Subjects received either daily supplements of 50 mg vitamin E, 20 mg beta-carotene, both, or placebo. After 5–8 years of intervention, alpha-tocopherol supplementation alone had no effect on risk for AMD (OR = 1.13; 95% CI: 0.81, 1.59) (Teikari et al., 1998) (Fig. 63). Further, the Vitamin E and age-related cataract and maculopathy (VECAT), a clinical trial of 1,193 elderly participants indicated that supplementation with 500 IU vitamin E for four years also had no effect on incidence of early AMD (RR = 1.05; 95% CI: 0.69, 1.61) or late AMD (RR = 1.36; 95% CI: 0.67, 2.77) (Taylor et al., 2002) (Fig. 63).
Interestingly, a 10-year follow up of over 2,000 subjects in the Blue Mountains Eye study showed that those consuming the highest amount of vitamin E were at a greater risk for late-stage atrophic AMD (RR = 2.55; 95% CI: 1.14, 5.70) compared to those consuming the lowest amounts of vitamin E (Fig. 62). There was no association between vitamin E intake and neovascular AMD (RR = 1.96; 95% CI: 0.74, 5.15) (Tan et al., 2008) (Fig. 62). Also, an analysis of all four examinations of the Beaver Dam Eye study indicated that there was a positive association between vitamin E supplementation and incident late AMD (OR = 1.78; 95% CI: 1.04, 3.05) (Klein et al., 2008) (Fig. 62). This was the only study reporting a positive association between vitamin E intake and AMD risk.
While there is evidence from case-control and cross-sectional studies suggesting that increased vitamin E intake may help reduce risk for AMD (such as baseline data from AREDS) (Chiu et al., 2009b), failure to see a significant effect in any of the clinical trials, as well as the possible increased risk for AMD with vitamin E supplementation suggests that vitamin E alone is not a strong preventative for AMD.
2.6 VITAMIN C
Given etiologic roles for oxidative stress in AMD and potent antioxidant properties of ascorbate (vitamin C) it might be anticipated that vitamin C status would be related to risk for AMD (Figs. 64–69). Most of the early work used case-control designs. In such a case-control study of 48 Italian AMD patients, Simonelli et al found that patients with late AMD had significantly lower plasma vitamin C levels than those patients with early AMD (p < 0.05). However, Simonelli and colleagues did not find a difference in plasma levels of vitamin C between AMD patients and healthy controls (Simonelli et al., 2002). This may be attributable to the small sample size of the study.
Figure 64.
Odds or risk ratio for any stage of AMD with high vs. low intake or blood levels of vitamin C in retrospective and cross-sectional studies.
Figure 69.
Odds or risk ratio for large drusen or late AMD with high vs. low intake (with or without supplements) or blood levels of vitamin C in prospective studies.
The remaining observational studies found little effect of vitamin C on AMD risk. A small case-control study of 26 patients with neovascular AMD did not find any relationship between serum vitamin C levels and risk for AMD (Blumenkranz et al., 1986). Similarly, in the EDCC, a case-control study consisting of 356 cataract patients and 350 healthy controls, there was no association between vitamin C blood levels and AMD risk (Eye Disease Case-Control Study Group, 1993) (Fig. 66). EDCC also found no association between vitamin C intake (OR = 0.83; 95% CI: 0.52, 1.33; p = 0.22 for trend) or vitamin C supplementation (OR = 0.87; 95% CI: 0.60, 1.30; p = 0.87 for trend) and risk for neovascular AMD (Seddon et al., 1994) (Fig. 66).
Figure 66.
Odds or risk ratio for late AMD with high vs. low intake (with or without supplements) or blood levels of vitamin C in retrospective and cross-sectional studies.
Cross-sectional studies also suggest that vitamin C does not play a large role in modulating AMD risk. Analyses of NHANES data collected from 1971–1972 indicated that after multivariate adjustment there was no association between vitamin C intake and prevalence of any stage of AMD (Goldberg et al., 1988) (Fig. 64). The POLA study also found no association between ascorbic acid plasma levels and any form of AMD (Delcourt et al., 1999) (Fig. 64). Two different cross-sectional analyses of the baseline data from AREDS of 4,519 and 4,403 participants studied the effects of a single nutrient or groups of nutrients on AMD risk. Both of these analyses indicated that after multivariate adjustment, vitamin C intake alone had no association with drusen formation, late AMD, geographic atrophy, or neovascular AMD (Chiu et al., 2009b; SanGiovanni et al., 2007b) (Figs. 65, 66). Participants (n=1,968) of the Beaver Dam Eye Study were analyzed for the role of antioxidants in the risk for early and late AMD. Past intake of vitamin C (with or without supplements) did not have a significant effect on either of these outcomes (Mares-Perlman et al., 1996) (Figs. 65, 66). Among 4,753 elderly men and women, there was no increased risk for neovascular AMD among those with the lowest serum levels of vitamin C (OR = 1.13; 95% CI: 0.69, 1.85) (Fletcher et al., 2008) (Figs. 66).
Figure 65.
Odds or risk ratio for drusen or early AMD with high vs. low vitamin C intake (with or without supplements) in retrospective and cross-sectional studies.
Prospective analyses also indicate that vitamin C intake or blood levels are not associated with risk for AMD. The PHS, a prospective cohort of 21,102 participants, did not find a significant association between vitamin C supplementation and any stage of AMD (Christen et al., 1999) (Fig. 67). The Rotterdam study also indicated that vitamin C intake did not have an association with risk for AMD (HR = 1.02; 95% CI: 0.94, 1.10) (van Leeuwen et al., 2005) (Fig. 67). Participants (n=976) of the Baltimore Longitudinal Study of Aging showed that there was no association between plasma ascorbic acid levels and risk for AMD (OR = 0.55; 95% CI: 0.28, 1.08) or late AMD (OR = 0.17; 95% CI: 0.01, 2.57) (West et al., 1994) (Figs. 67, 69). Analysis of 1,709 participants of the Beaver Dam Eye Study showed that there was no effect of past or present vitamin C intake or supplementation on 5 year incidence of pigment abnormalities or the appearance of large drusen (VandenLangenberg et al., 1998) (Figs. 68, 69). Analysis of 2,454 participants of the Blue Mountains Eye Study 10 years after baseline did not show any association between vitamin C intake and atrophic or neovascular macular degeneration. However, the 10-year analysis did not separate those subjects who supplemented with vitamin C from those who did not, so it is plausible that an effect due to vitamin C supplementation would be hidden, since an effect was seen in those who supplemented with vitamin C after 5 years (Tan et al., 2008). Similar results were observed in the NHS+HPFUS also showed that vitamin C intake had no effect on risk for early (RR = 1.21; 95% CI: 0.88, 1.67; p = 0.18) or neovascular AMD with (RR = 0.98; 95% CI: 0.58, 1.66; p = 0.52 for trend) or without supplements (RR = 0.76; 95% CI: 0.31, 1.88) (Cho et al., 2004) (Figs. 68, 69).
Figure 67.
Odds or risk ratio for any stage of AMD with high vs. low intake (with or without supplements) or blood levels of vitamin C in prospective studies.
Figure 68.
Odds or risk ratio for pigment abnormalities or early AMD with high vs. low intake (with or without supplements) of vitamin C in prospective studies.
Surprisingly, some studies even suggest that increased vitamin C intake could increase risk for disease. Data collected at 4 different examinations of the Beaver Dam Eye Study found that use of vitamin C supplements was associated with increased risk for late AMD (OR = 2.47; 95% CI: 1.40, 4.80) (Klein et al., 2008) (Fig. 69). This corroborated data from the Blue Mountains Eye Study. In that analysis, among the 2,335 participants who took vitamin C supplements, those with the highest intakes of vitamin C had an increased risk for early AMD after 5 years, compared to those with the lowest intakes of vitamin C (OR = 2.30; 95% CI: 1.30, 4.00; p = 0.002 for trend) (Flood et al., 2002) (Fig. 68).
In summary, despite the etiologic role for oxidative stress in AMD and the fact that vitamin C is a potent antioxidant, at least in aqueous systems, the majority of the many studies that have investigated relationships between vitamin C status and retinal health indicate that vitamin C status alone is not related to delayed onset or progress of AMD. Perhaps this is because the retina is largely a lipophilic environment, particularly in the photoreceptors, the major site of lesions.
2.7 ANTIOXIDANT COMBINATIONS OR MULTIVITAMINS
Nutrients often act synergistically to affect metabolism. Given these interactions, it is reasonable to hypothesize that groups of multiple nutrients would be more effective than single nutrients in modulating disease progression (Figs. 70–73). Most studies examined relationships between risk for AMD and vitamins considered to act as antioxidants.
Figure 70.
Odds or risk ratio for drusen, lesions or early AMD with high vs. low dietary score, antioxidant index (AOX) intake or use of multivitamin supplements in retrospective and cross-sectional studies.
Figure 73.
Odds or risk ratio for drusen, pigment abnormalities, early or late AMD with high vs. low intake of fish with a high zinc content or zinc in retrospective and cross-sectional studies.
Although the case-control EDCC indicated that multivitamin supplement use had no effect on risk for neovascular AMD (OR = 0.82; 95% CI 0.57, 1.18), there was an association between antioxidant index and late AMD (Seddon et al., 1994) (Fig. 71). In those participants who had the highest blood levels of at least three of either carotenoids, selenium, vitamin C, or vitamin E, there was a reduced risk for neovascular AMD (OR = 0.30; 95% CI: 0.10, 0.70; p = 0.005 for trend) compared to those who had the lowest amounts of at least 3 of the above-mentioned nutrients (Eye Disease Case-Control Study Group, 1993) (Fig. 71).
Figure 71.
Odds or risk ratio for late AMD with high vs. low antioxidant index (AOX) intake or blood levels, dietary score or use of multivitamin supplements in retrospective and cross-sectional studies.
There is only one cross-sectional study supporting a beneficial role for an antioxidant combination in AMD risk. Among 4,003 participants of the AREDS study, a compound score was created to compare overall diet to risk for AMD. The compound score included intake of vitamin E, vitamin C, zinc, lutein/zeaxanthin, DHA, EPA, and a low dietary GI. Compared to those subjects with the lowest score (lowest intake of the above-mentioned nutrients), those with the highest score were at a reduced risk for drusen (OR = 0.75; 95% CI: 0.60, 0.93; p = 0.048 for trend) and late stage AMD (OR = 0.58; 95% CI: 0.40, 0.82; p = 0.002 for trend) (Chiu et al., 2009b) (Figs. 70, 71).
In prospective analyses, the Rotterdam study indicated that after adjusting for age, sex, BMI, smoking, blood pressure, atherosclerotic score, alcohol intake, and total cholesterol, subjects that had intakes of beta-carotene, vitamin C, vitamin E, and zinc that were all above the median, had reduced risk for AMD (HR = 0.65; 95% CI: 0.46, 0.92) (van Leeuwen et al., 2005) (Fig. 72).
Figure 72.
Odds or risk ratio for any stage, early or late AMD or progression to late AMD with high vs. low intake of an antioxidant combination or use of multivitamin supplements in prospective studies and an intervention study.
Randomized, double-blinded, placebo-controlled studies are considered to be a gold standard for clinical studies. The AREDS Study (in which participants either received an antioxidant cocktail or placebo) had such a design, albeit that many of the controls were also using multivitamin supplements. Results of the AREDS indicated that supplementation with a cocktail of beta-carotene, vitamin E, vitamin C, zinc and copper reduced progression from intermediate to advanced AMD by 34% (95% CI: 0.47, 0.91) over about 6 years of follow-up (AREDS-Group, 2001; Chew et al., 2009) (Fig. 72). Another clinical trial of 27 patients with early AMD, showed that daily supplementation with 180 mg vitamin C, 30 mg vitamin E, 22.5 mg zinc, 1 mg copper, 10 mg lutein, 1 mg zeaxanthin, and 4 mg astaxanthin improved function in the central retina as measured by electroretinogram (p < 0.01)(data not shown) (Parisi et al., 2008).
In a double-blind, placebo controlled trial of dry AMD patients, it was shown that supplementation with vitamin E, zinc, magnesium, vitamin B6 and folate for 18 months maintained visual acuity, compared to placebo treatment, in which there was a decrease in visual acuity (p = 0.03). The antioxidant supplement group also reported greater vision stability in the areas of visual acuity and contrast sensitivity (p = 0.05) (Richer, 1996). Another double-blind, placebo controlled trial of dry AMD patients found that supplementation with antioxidants and omega-3 fatty acids maintained visual acuity over 6 months, while the placebo group lost visual acuity (p < 0.05) (Cangemi, 2007).
Among the evidence that challenges a role for antioxidants and AMD is a case-control study of 72 cases and 66 controls which could not find a significant association between antioxidant intake and AMD (OR = 0.56; 95% CI: 0.19, 1.67) (Snellen et al., 2002) (Fig. 71). A cross-sectional analysis of 1,968 participants of the Beaver Dam Eye Study showed that antioxidants (measured with an antioxidant index) had no association with risk for early or late AMD (Mares-Perlman et al., 1996) (Figs. 70, 71). A cross-sectional analysis of the baseline data from 2,873 participants of the Blue Mountains Eye Study found that vitamin supplements to be without effect on risk for early AMD (OR = 1.30; 95% CI: 0.90, 1.70) (Kuzniarz et al., 2002) (Fig. 70). There was also no effect on risk for soft drusen (OR = 1.10; 95% CI: 0.80, 1.50) or AMD lesions (OR = 1.50; 95% CI: 0.70, 3.00) (Kuzniarz et al., 2002) (Fig. 70). Further, prospective analysis of four examinations of the Beaver Dam Eye Study indicated that multivitamin supplementation had no effect on early or late AMD (Klein et al., 2008). Among women in the NHS as well as men in the HPFUS, there was no association between risk for early or neovascular AMD and use of a multivitamin supplement (Cho et al., 2004) (Fig. 72). Finally, the PHS reported that supplementation with a multivitamin had no effect on AMD (RR = 0.90; 95% CI: 0.68, 1.19) (Christen et al., 1999) (Fig. 72).
Taken together, epidemiologic support for the value of antioxidant combinations for diminishing risk of AMD is promising, if not robust, and deserves additional study. The AREDS intervention trial data indicates that multivitamin supplementation may be beneficial for the prevention of progression to advanced AMD. Several smaller studies corroborate results from AREDS. Since multivitamins do not appear to be harmful to the retina, use of a supplement or eating a prudent diet rich in fruits and vegetables is an advisable practice. The ongoing ARED2 trials will be valuable to determine the value of supplementation with both carotenoids and omega-3 fatty acids.
2.8 ZINC
Zinc plays an important role in a number of physiological processes including immunity, reproduction and neuronal development, as reviewed by King and colleagues (King, 2011). Concentrations of zinc are very high in the retina. Thus, many have hypothesized that zinc supplementation may aid retinal health (Figs. 73, 74).
Figure 74.
Odds or risk ratio for any stage of AMD, pigment abnormalities, early or late AMD, or progression to late AMD with high vs. low intake (with or without supplements) of zinc in prospective and intervention studies.
There have been no case-control investigations of zinc and AMD risk. A retrospective analysis of 1,968 participants of the Beaver Dam Eye study found that compared to people with the lowest amount of zinc intake from foods, those with the highest amount had a reduced risk for early AMD (OR = 0.60; 95% CI: 0.40, 1.00; p < 0.05). There was no association with late AMD (Mares-Perlman et al., 1996) (Fig. 73).
A cross-sectional study of 44 subjects indicated that while there was no difference in zinc and copper levels in the neural retina (analyzed ex vivo) between healthy subjects and those with AMD (p > 0.09), there was significantly less zinc and copper in the RPE and choroid, tissues involved in AMD development, in AMD patients compared to healthy subjects (p = 0.002) (Erie et al., 2009).
Prospective analysis of the Rotterdam study showed that among 4,170 participants, increased consumption of zinc was associated with a reduced risk for any stage of AMD (HR = 0.91; 95% CI: 0.83, 0.98) (van Leeuwen et al., 2005) (Fig. 74). Data from 1,709 participants of the Beaver Dam Eye Study indicated that there was a decrease in risk for pigment abnormalities 5 years after baseline for those with past zinc intake (food and supplements) in the highest quintile (OR = 0.38; 95% CI: 0.20, 1.00; p = 0.03 for trend) (VandenLangenberg et al., 1998) (Fig. 74). A ten year follow-up analysis of these same participants showed that those with the highest zinc intake were at a reduced risk for any type of AMD (OR = 0.56; 95% CI: 0.32, 0.97) as well as early AMD (OR = 0.54; 95% CI: 0.32, 0.97) compared to all other participants (Tan et al., 2008) (Fig. 74).
In AREDS, zinc was included in the antioxidant cocktail administered to the intervention group. Researchers evaluated AMD progression among participants in the early, intermediate and later stages (Grades 2–4) as well as among participants in the intermediate and later stages (Grades 3–4), comparing rates of progression between those who received a zinc supplement and those who did not. Among participants with grade 3 or 4 AMD, those who consumed zinc were less likely to progress to more advanced AMD than those who did not consume zinc (OR = 0.79; 95% CI: 0.62, 0.99) (Fig. 74). However, there was no significant effect of zinc supplementation on progression to late AMD when the analysis included those with grade 2 AMD (AREDS-Group, 2001) (Fig. 74).
Other than the AREDS study, which included zinc in its antioxidant supplement, there have been a few studies that analyzed the effect of zinc on AMD in clinical trials. In a study of 90 subjects, Newsome and coworkers found that 100 mg zinc twice a day for 2 years reduced vision loss (one-tailed p = 0.001) (Newsome et al., 1988). Although this dose of zinc has been thought to induce toxicity in some patients, Newsome and colleagues did not observe any adverse effects related to zinc toxicity in their study population (Newsome et al., 1988; Trempe, 1992; Yuzbasiyan-Gurkan and Brewer, 1989). Another trial with 80 dry AMD patients found that supplementation with 25 mg zinc monocysteine twice a day for 6 months improved several indicators of retinal function such as visual acuity, contrast sensitivity, and macular flash recovery time (p < 0.0001) (Newsome, 2008).
While several cross-sectional studies do indicate visual benefits for zinc, another large cross-sectional study of 2,873 participants of the Blue Mountains Eye Study found that zinc had no effect on early AMD (Kuzniarz et al., 2002). This finding was confirmed in second analysis of slightly more subjects (3,654) from the same study for early (OR = 0.80; 95% CI: 0.50, 1.30) or late AMD (OR = 1.00; 95% CI: 0.40, 2.80) (Smith et al., 1999) (Fig. 73). Similarly, 5 years after baseline, zinc intake had no effect on early AMD incidence (Flood et al., 2002). Swenor et al found that compared to those who consumed less than 0.07 servings per week of fish with a high zinc content (crab, oysters), those who consumed more than 0.07 servings per week did not have any change in risk for the appearance of drusen, pigment abnormalities, or late AMD (Swenor et al., 2010) (Fig. 73).
Contradictory data were also obtained in various studies. A prospective study of 104,208 elderly men and women also showed that there was no association between risk for AMD and ten years of zinc intake (RR = 1.13; 95% CI: 0.82, 1.57) (Cho et al., 2001b) (Fig. 74). Compared to those with the lowest amount of zinc intake from food, those with the highest food intake did not have a reduced risk of AMD (RR = 1.04; 95% CI: 0.59, 1.83) (Cho et al., 2001b) (Fig. 74). There was also no association between AMD risk and zinc supplementation (RR = 1.04; 95% CI: 0.75, 1.45) (Cho et al., 2001b) (Fig. 74).
Although two trials have shown that zinc alone can improve vision, another placebo-controlled trial of 112 AMD subjects found that 200 mg zinc per day for 2 years had no effect on vision (Stur et al., 1996). Interestingly, analysis of the Beaver Dam Eye Study indicated that use of zinc supplements was even associated with an increased risk for late AMD (OR = 2.11; 95% CI: 1.09, 4.07) (Klein et al., 2008) (Fig. 74). At present, the observational evidence supporting a role for increased zinc intake in the development of AMD is not robust. However, given the results from intervention trials such as AREDS, as described below, continued investigation is warranted (AREDS-Group, 2001; Chew et al., 2009).
2.9 VITAMIN D
The health benefits of vitamin D for various chronic diseases are being explored, but there has been only one observational study regarding the role of vitamin D in ameliorating AMD risk (Parekh et al., 2007) (Figs. 75–77). Parekh et al found that among 7,752 participants of NHANES III, non-Hispanic Whites with the highest serum levels of 1,25(OH)2 vitamin D had a reduced risk for early AMD (OR = 0.64; 95% CI: 0.40, 0.90) compared to those with the lowest serum levels (Fig. 75). However, there was no beneficial effect in non-Hispanic Blacks or Mexican Americans (Fig. 75). Combining all races, there was also a reduced risk for soft drusen in those with the highest serum levels of 1,25(OH)2 vitamin D compared to those with the lowest levels (OR = 0.76; 95% CI: 0.60, 0.96) (Fig. 75). There was no effect of serum levels of vitamin D on the appearance of pigment abnormalities or advanced AMD (Fig. 75).
Figure 75.
Odds or risk ratio for pigment abnormalities, soft drusen, early or late AMD with high vs. low serum levels of vitamin D in a retrospective study.
Figure 77.
Odds or risk ratio for pigment abnormalities, drusen, early or late AMD upon vitamin D supplementation or with high vs. low serum levels of vitamin D in those at least 40 years of age who do not consume milk daily in a retrospective study.
Milk and fish are two of the most common sources of vitamin D in the American diet, and in exploratory analyses of the NHANES III study it was found that among participants at least 40 yrs of age, those who consumed milk at least daily were at a reduced risk for early AMD (OR = 0.75; 95% CI: 0.60, 0.90) and soft drusen (OR = 0.80; 95% CI: 0.70, 0.98) compared to those who consumed it less than weekly (Fig. 76). Pigment abnormalities and advanced AMD were not affected by milk consumption (Fig. 76). Those over the age of 40 who consumed fish at least once a week were at a reduced risk for soft drusen (OR = 0.80; 95% CI: 0.70, 0.97) and advanced AMD (OR = 0.41; 95% CI: 0.20, 0.90) compared to those who consumed fish less than twice a month (Fig. 76). For those in this age group that did not consume milk daily, supplementation did attenuate early AMD risk (OR = 0.67; 95% CI: 0.50, 0.90) but not risk for soft drusen, pigment abnormalities, or advanced AMD (Parekh et al., 2007) (Fig. 77). Although risk for early AMD didn’t correspond to risks associated with early AMD indicators, results of this study do leave open the possibility that vitamin D may help decrease risk for certain forms of AMD. The effect of fish on advanced AMD may be driven more by the presence of omega-3 fatty acids in fish, rather than vitamin D, since vitamin D supplementation and milk conferred no protective effects. Further investigation in other cohorts is needed to confirm these results.
Figure 76.
Odds ratio for pigment abnormalities, soft drusen, early or late AMD with high vs. low intake of milk or fish in those at least 40 years of age in a retrospective study.
3. BRIEF REVIEW OF PHARMACOLOGIC EFFORTS TO TREAT AMD
We describe, in brief, some recent pharmacotherapeutic results, which help to highlight the need for preventative AMD interventions. The Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study group found that injection of verteporfin, a photosensitizer, followed by laser treatment can prevent leakage of newly formed retinal blood vessels that appear in cases of advanced neovascular AMD. Relative to placebo, verteporfin treatment resulted in a decreased loss of contrast sensitivity, slowed the progression of choroidal neovascularization (CNV), and slowed the loss of visual acuity over a period of 24 months. However, those receiving the photodynamic therapy experienced a significant amount of visual disturbances and pain. Furthermore, subgroup analyses revealed that this type of treatment did not benefit all cases of neovascular AMD - the greatest benefit of this therapy is limited to those with classic lesions or no occult CNV at baseline (Bressler, 2001).
Vascular endothelial growth factor (VEGF) inhibitors such as ranibizumab (Lucentis) and pegaptanib sodium (Macugen) have also been studied and are in current use to delay progress of and ameliorate CNV. Ranibizumab is a monoclonal antibody that recognizes all isoforms of VEGF. The Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age-Related Macular Degeneration (ANCHOR) trial was a multicenter, randomized, double-blind, active-treatment controlled study. In patients whose lesions contained at least 50% of the classic component (characterized by a well-defined area of new vessel growth in the macula, and often results in more severe, rapid vision loss than occult lesions), 24 months of ranibizumab treatment was more effective at delaying growth of the lesion area and even restored some visual acuity compared to patients who received photodynamic therapy (Brown et al., 2009). Rather than target all isoforms of VEGF, pegaptanib sodium (Macugen) is an RNA aptamer for a single isoform of VEGF. Two concurrent randomized, double-blinded controlled trials were conducted to evaluate the effects of Macugen in patients with subfoveal choroidal neovascularization. Compared to sham injection, in which a syringe (without a needle) was pressed against the eye wall, Macugen was shown to reduce visual acuity loss and attenuate the progression of CNV lesion and vessel leakage after 54 weeks of treatment (Gragoudas et al., 2004).
This data is encouraging, but there is also considerable concern about the use of VEGF inhibitors for AMD treatment. VEGF plays an important role in regulation of thrombogenesis, angiogenesis and vasodilation (Henry et al., 2003; Juan-Babot et al., 2003; Leung et al., 1989; Yang et al., 1996). This suggests that VEGF inhibition may increase risk for ischemic stroke, hemorrhage and hypertension, albeit thus far, clinical trials of VEGF inhibitors for the treatment of AMD haven’t found a significant increase in cardiovascular risk. However, some non-ocular hemorrhages in those patients receiving ranibizumab have been reported. Side effects such as endopthalmitis, floaters, and eye discharge have also been noted (Brown et al., 2009; Gragoudas et al., 2004; Rosenfeld et al., 2006). Since AMD patients are already at a higher risk for cardiovascular disease, these concerns cannot be easily dismissed.
In addition to the concern about the safety of VEGF inhibitors, the cost of these treatments should also be taken into consideration. Direct costs for AMD therapy in the US are estimated to be approximately 575 million dollars per year; a number which is only expected to increase if effective new therapies are not developed (Rein et al., 2006). Due to the advanced age of most AMD patients, many of these costs are covered by healthcare programs such as Medicare, spreading the financial burden of these treatments to all taxpayers.
4. CONCLUSION
AMD is the major blinding disease in developed countries. It compromises life quality and consumes significant health care resources. Because the numbers of AMD victims are growing exponentially, it is urgent to develop capacities to delay the onset or progress of AMD. Optimized nutrition appears to offer an option to achieve this goal. Consuming sufficient levels of nutrients is often associated with retained retinal integrity and intake of the levels of nutrients that are associated with benefit to the retina, particularly in the context of foods, have many salutary effects, such as diminishing risk for type 2 diabetes, diminishing risk for CVD and obesity, with no significant adverse effects.
Micronutrients
There has been extensive research to determine if micronutrients, which are thought to work as antioxidants, can delay or slow AMD progression. Data from the AREDS trial indicates that use of a high potency multivitamin supplement (vitamins C, E, beta carotene) with zinc delays progress of intermediate grades of AMD to advanced AMD. However, the current observational data does not clearly indicate a strong role for vitamins C, D or E individually in slowing AMD progression. Rather, observational studies indicate that lutein and zeaxanthin are associated with delaying progress of advanced retinal disease. The current AREDS2 trial will test this hypothesis.
Macronutrients: polyunsaturated fatty acids and carbohydrates
A robust observational study record suggests that consuming diets rich in EPA and DHA, i.e. 2 servings/wk of fish, and consuming lower glycemic index diets is protective for both early and late AMD. The former concept is being tested in the AREDS2 trial. The consistency of observational data that link consumption of lower glycemic index diets with delayed progress of early AMD as well as with prolonged retinal integrity, together with recent corroboration of these results and mechanistic findings in laboratory animals, provide compelling rationale for randomized blinded intervention trials. When completed these should confirm the salutary effects of consuming lower GI diets on retinal integrity and establish etiologic links between dietary carbohydrate and retinal function. With such data, drugs to capture the benefits of lower GI diets could be designed.
Taken together it appears that nutritional approaches, including diets that provide sufficient antioxidant nutrients, polyunsaturated fats and zinc, and which are of lower glycemic index can significantly expand the armamentarium to combat the AMD epidemic. Supplementation may be particularly important for those with limited access to nutritious diets.
Acknowledgments
We would like to thank CJ Chiu for his help compiling data, and M. Andrea Caceres for her help revising the manuscript.
FUNDING
This research was funded by USDA 1950-510000-060-01A, Johnson and Johnson Focused Giving, NIH Grant RO1 13250, NIH Grant RO1 21212. These sponsors were not involved in data collection or interpretation or in writing this manuscript.
Biographies
VITAE
Karen A Weikel conducted her thesis research and received her doctorate in Biochemical and Molecular Nutrition at Tufts University. Her work focused on characterizing the role of dietary glycemic index on retinal aging. She observed that mice fed a high glycemic index diet developed more indicators which precede early AMD than mice fed a low glycemic index diet, an effect that is associated with retinal accumulation of toxic modified proteins that impair cellular protein quality control. She is currently a post-doctoral fellow at Boston Medical Center.
Allen Taylor is the Director of the Laboratory for Nutrition and Vision Research (LNVR). Studies in the LNVR focus on the intersection of aging, proteolysis, epidemiology, and nutrition, particularly as applied to age related vision disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Hussaini H, Schneiders M, Lundh P, Jeffery G. Drusen are associated with local and distant disruptions to human retinal pigment epithelium cells. Exp Eye Res. 2009;88 (3):610–612. doi: 10.1016/j.exer.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Allikmets R, Shroyer NF, Singh N, Seddon JM, Lewis RA, Bernstein PS, Peiffer A, Zabriskie NA, Li Y, Hutchinson A, Dean M, Lupski JR, Leppert M. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science. 1997;277 (5333):1805–1807. doi: 10.1126/science.277.5333.1805. [DOI] [PubMed] [Google Scholar]
- American-Health-Assistance-Foundation. Facts on Macular Degeneration, Macular Degeneration Research. American Health Assistance Foundation; Clarksburg, MD: 2012. [Google Scholar]
- AREDS-Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch Ophthalmol. 2001;119 (10):1439–1452. doi: 10.1001/archopht.119.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AREDS. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119 (10):1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augood C, Chakravarthy U, Young I, Vioque J, de Jong PT, Bentham G, Rahu M, Seland J, Soubrane G, Tomazzoli L, Topouzis F, Vingerling JR, Fletcher AE. Oily fish consumption, dietary docosahexaenoic acid and eicosapentaenoic acid intakes, and associations with neovascular age-related macular degeneration. Am J Clin Nutr. 2008;88 (2):398–406. doi: 10.1093/ajcn/88.2.398. [DOI] [PubMed] [Google Scholar]
- Bacova B, Radosinska J, Knezl V, Kolenova L, Weismann P, Navarova J, Barancik M, Mitasikova M, Tribulova N. Omega-3 fatty acids and atorvastatin suppress ventricular fibrillation inducibility in hypertriglyceridemic rat hearts: implication of intracellular coupling protein, connexin-43. J Physiol Pharmacol. 2010;61 (6):717–723. [PubMed] [Google Scholar]
- Baird PN, Richardson AJ, Robman LD, Dimitrov PN, Tikellis G, McCarty CA, Guymer RH. Apolipoprotein (APOE) gene is associated with progression of age-related macular degeneration (AMD) Hum Mutat. 2006;27 (4):337–342. doi: 10.1002/humu.20288. [DOI] [PubMed] [Google Scholar]
- Belda JI, Roma J, Vilela C, Puertas FJ, Diaz-Llopis M, Bosch-Morell F, Romero FJ. Serum vitamin E levels negatively correlate with severity of age-related macular degeneration. Mechanisms of Ageing & Development. 1999;107 (2):159–164. doi: 10.1016/s0047-6374(98)00144-4. [DOI] [PubMed] [Google Scholar]
- Biarnes M, Mones J, Alonso J, Arias L. Update on Geographic Atrophy in Age-Related Macular Degeneration. Optom Vis Sci. 2011;88 (7):881–889. doi: 10.1097/OPX.0b013e31821988c1. [DOI] [PubMed] [Google Scholar]
- Blumenkranz MS, Russell SR, Robey MG, Kott-Blumenkranz R, Penneys N. Risk factors in age-related maculopathy complicated by choroidal neovascularization. Ophthalmology. 1986;93:552–558. doi: 10.1016/s0161-6420(86)33702-3. [DOI] [PubMed] [Google Scholar]
- Bone RA, Landrum JT. Dose-dependent response of serum lutein and macular pigment optical density to supplementation with lutein esters. Arch Biochem Biophys. 2010;504 (1):50–55. doi: 10.1016/j.abb.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler NM. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-tap report 2. Arch Ophthalmol. 2001;119 (2):198–207. [PubMed] [Google Scholar]
- Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology. 2009;116 (1):57–65. e55. doi: 10.1016/j.ophtha.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Bucheli P, Vidal K, Shen L, Gu Z, Zhang C, Miller LE, Wang J. Goji berry effects on macular characteristics and plasma antioxidant levels. Optom Vis Sci. 2011;88 (2):257–262. doi: 10.1097/OPX.0b013e318205a18f. [DOI] [PubMed] [Google Scholar]
- Burri BJ, Chang JS, Neidlinger TR. beta-Cryptoxanthin- and alpha-carotene-rich foods have greater apparent bioavailability than beta-carotene-rich foods in Western diets. Br J Nutr. 2011;105 (2):212–219. doi: 10.1017/S0007114510003260. [DOI] [PubMed] [Google Scholar]
- Cangemi FE. TOZAL Study: an open case control study of an oral antioxidant and omega-3 supplement for dry AMD. BMC Ophthalmol. 2007;7:3. doi: 10.1186/1471-2415-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinault N, Abalain JH, Sairafi B, Coudray C, Grolier P, Rambeau M, Carre JL, Mazur A, Rock E. Lycopene but not lutein nor zeaxanthin decreases in serum and lipoproteins in age-related macular degeneration patients. Clin Chim Acta. 2005;357:34–42. doi: 10.1016/j.cccn.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Chew EY, Lindblad AS, Clemons T. Summary results and recommendations from the age-related eye disease study. Arch Ophthalmol. 2009;127 (12):1678–1679. doi: 10.1001/archophthalmol.2009.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359 (9323):2072–2077. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. Jama. 2003;290 (4):486–494. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- Chiu CJ, Liu S, Willett WC, Wolever TMS, Brand-Miller JC, Barclay AC, Taylor A. Informing food choices and health outcomes by use of the dietary glycemic index. Nutrition Reviews. 2011;69 (4):231–242. doi: 10.1111/j.1753-4887.2011.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CJ, Hubbard LD, Armstrong J, Rogers G, Jacques PF, Chylack JLT, Hankinson SE, Willett WC, Taylor A. Dietary glycemic index and carbohydrate in relation to early age-related macular degeneration. Am J Clin Nutr. 2006;83:880–886. doi: 10.1093/ajcn/83.4.880. [DOI] [PubMed] [Google Scholar]
- Chiu CJ, Klein R, Milton RC, Gensler G, Taylor A. Does eating particular diets alter risk of age-related macular degeneration in users of the age-related eye disease study supplements? Br J Ophthalmol. 2009a;93 (9):1241–1246. doi: 10.1136/bjo.2008.143412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CJ, Milton RC, Gensler G, Taylor A. Association between dietary glycemic index and age-related macular degeneration in nondiabetic participants in the Age-Related Eye Disease Study. Am J Clin Nutr. 2007a;86 (1):180–188. doi: 10.1093/ajcn/86.1.180. [DOI] [PubMed] [Google Scholar]
- Chiu CJ, Milton RC, Klein R, Gensler G, Taylor A. Dietary carbohydrate and the progression of age-related macular degeneration: a prospective study from the Age-Related Eye Disease Study. Am J Clin Nutr. 2007b;86 (4):1210–1218. doi: 10.1093/ajcn/86.4.1210. [DOI] [PubMed] [Google Scholar]
- Chiu CJ, Milton RC, Klein R, Gensler G, Taylor A. Dietary compound score and risk of age-related macular degeneration in the age-related eye disease study. Ophthalmology. 2009b;116 (5):939–946. doi: 10.1016/j.ophtha.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CJ, Taylor A. Dietary hyperglycemia, glycemic index and metabolic retinal diseases. Prog Retin Eye Res. 2011;30 (1):18–53. doi: 10.1016/j.preteyeres.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho E, Hankinson SE, Rosner B, Willett WC, Colditz GA. Prospective study of lutein/zeaxanthin intake and risk of age-related macular degeneration. Am J Clin Nutr. 2008;87 (6):1837–1843. doi: 10.1093/ajcn/87.6.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho E, Hung S, Willett WC, Spiegelman D, Rimm EB, Seddon JM, Colditz GA, Hankinson SE. Prospective study of dietary fat and the risk of age-related macular degeneration. Am J Clin Nutr. 2001a;73 (2):209–218. doi: 10.1093/ajcn/73.2.209. [DOI] [PubMed] [Google Scholar]
- Cho E, Seddon JM, Rosner B, Willett WC, Hankinson SE. Prospective study of intake of fruits, vegetables, vitamins, and carotenoids and risk of age-related maculopathy. Arch Ophthalmol. 2004;122:883–892. doi: 10.1001/archopht.122.6.883. [DOI] [PubMed] [Google Scholar]
- Cho E, Stampfer MJ, Seddon JM, Hung S, Spiegelman D, Rimm EB, Willett WC, Hankinson SE. Prospective study of zinc intake and the risk of age-related macular degeneration. Ann Epidemiol. 2001b;11 (5):328–336. doi: 10.1016/s1047-2797(01)00217-4. [DOI] [PubMed] [Google Scholar]
- Chong EW, Robman LD, Simpson JA, Hodge AM, Aung KZ, Dolphin TK, English DR, Giles GG, Guymer RH. Fat consumption and its association with age-related macular degeneration. Arch Ophthalmol. 2009;127 (5):674–680. doi: 10.1001/archophthalmol.2009.60. [DOI] [PubMed] [Google Scholar]
- Christen WG, Ajani UA, Glynn RJ, Manson JE, Schaumberg DA, Chew EC, Buring JE, Hennekens CH. Prospective cohort study of antioxidant vitamin supplement use and the risk of age-related maculopathy. Am J Epidemiol. 1999;149:476–484. doi: 10.1093/oxfordjournals.aje.a009836. [DOI] [PubMed] [Google Scholar]
- Christen WG, Glynn RJ, Chew EY, Buring JE. Vitamin E and age-related macular degeneration in a randomized trial of women. Ophthalmology. 2010;117 (6):1163–1168. doi: 10.1016/j.ophtha.2009.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua B, Flood V, Rochtchina E, Wang JJ, Smith W, Mitchell P. Dietary fatty acids and the 5-year incidence of age-related maculopathy. Arch Ophthalmol. 2006;124 (7):981–986. doi: 10.1001/archopht.124.7.981. [DOI] [PubMed] [Google Scholar]
- Churchill AJ, Carter JG, Lovell HC, Ramsden C, Turner SJ, Yeung A, Escardo J, Atan D. VEGF polymorphisms are associated with neovascular age-related macular degeneration. Hum Mol Genet. 2006;15 (19):2955–2961. doi: 10.1093/hmg/ddl238. [DOI] [PubMed] [Google Scholar]
- Connolly EE, Beatty S, Thurnham DI, Loughman J, Howard AN, Stack J, Nolan JM. Augmentation of macular pigment following supplementation with all three macular carotenoids: an exploratory study. Curr Eye Res. 2010;35 (4):335–351. doi: 10.3109/02713680903521951. [DOI] [PubMed] [Google Scholar]
- Crescenzo R, Bianco F, Falcone I, Coppola P, Dulloo AG, Liverini G, Iossa S. Mitochondrial energetics in liver and skeletal muscle after energy restriction in young rats. Br J Nutr. 2011:1–11. doi: 10.1017/S0007114511005903. [DOI] [PubMed] [Google Scholar]
- Cruickshanks KJ, Hamman RF, Klein R, Nondahl DM, Shetterly SM. The prevalence of age-related maculopathy by geographic region and ethnicity. The Colorado-Wisconsin Study of Age-Related Maculopathy. Arch Ophthalmol. 1997;115 (2):242–250. doi: 10.1001/archopht.1997.01100150244015. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Millican CL. Basal linear deposit and large drusen are specific for early age-related maculopathy. Arch Ophthalmol. 1999;117 (3):329–339. doi: 10.1001/archopht.117.3.329. [DOI] [PubMed] [Google Scholar]
- Davis MD, Gangnon RE, Lee LY, Hubbard LD, Klein BE, Klein R, Ferris FL, Bressler SB, Milton RC. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol. 2005a;123 (11):1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MD, Gangnon RE, Lee LY, Hubbard LD, Klein BE, Klein R, Ferris FL, Bressler SB, Milton RC Age-Related Eye Disease Study Group. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol. 2005b;123:1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcourt C, Carriere I, Cristol JP, Lacroux A, Gerber M. Dietary fat and the risk of age-related maculopathy: the POLANUT study. Eur J Clin Nutr. 2007;61 (11):1341–1344. doi: 10.1038/sj.ejcn.1602685. [DOI] [PubMed] [Google Scholar]
- Delcourt C, Carriere I, Delage M, Barberger-Gateau P, Schalch W. Plasma lutein and zeaxanthin and other carotenoids as modifiable risk factors for age-related maculopathy and cataract: the POLA Study. Invest Ophthalmol Vis Sci. 2006;47 (6):2329–2335. doi: 10.1167/iovs.05-1235. [DOI] [PubMed] [Google Scholar]
- Delcourt C, Cristol JP, Tessier F, Leger CL, Descomps B, Papoz L. Age-related macular degeneration and antioxidant status in the POLA study. POLA Study Group. Pathologies Oculaires Liees a l’Age. Arch Ophthalmol. 1999;117 (10):1384–1390. doi: 10.1001/archopht.117.10.1384. [DOI] [PubMed] [Google Scholar]
- Dietzel M, Zeimer M, Heimes B, Claes B, Pauleikhoff D, Hense HW. Determinants of macular pigment optical density and its relation to age-related maculopathy: results from the Muenster Aging and Retina Study (MARS) Invest Ophthalmol Vis Sci. 2011;52 (6):3452–3457. doi: 10.1167/iovs.10-6713. [DOI] [PubMed] [Google Scholar]
- Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res. 2009;28 (1):1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308 (5720):421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- Erie JC, Good JA, Butz JA, Pulido JS. Reduced zinc and copper in the retinal pigment epithelium and choroid in age-related macular degeneration. Am J Ophthalmol. 2009;147 (2):276–282. e271. doi: 10.1016/j.ajo.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Eye Disease Case-Control Study Group. Antioxidant status and neovascular age-related macular degeneration. Arch Ophthalmol. 1993;111 (6358):104–109. doi: 10.1001/archopht.1993.01090010108035. [DOI] [PubMed] [Google Scholar]
- Feher J, Kovacs I, Artico M, Cavallotti C, Papale A, Balacco Gabrieli C. Mitochondrial alterations of retinal pigment epithelium in age-related macular degeneration. Neurobiol Aging. 2006;27 (7):983–993. doi: 10.1016/j.neurobiolaging.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Ferland A, Brassard P, Lemieux S, Bergeron J, Bogaty P, Bertrand F, Simard S, Poirier P. Impact of high-fat /low-carbohydrate, high-, low-glycaemic index or low-caloric meals on glucose regulation during aerobic exercise in Type 2 diabetes. Diabet Med. 2009;26 (6):589–595. doi: 10.1111/j.1464-5491.2009.02734.x. [DOI] [PubMed] [Google Scholar]
- Fletcher AE, Bentham GC, Agnew M, Young IS, Augood C, Chakravarthy U, de Jong PT, Rahu M, Seland J, Soubrane G, Tomazzoli L, Topouzis F, Vingerling JR, Vioque J. Sunlight exposure, antioxidants, and age-related macular degeneration. Arch Ophthalmol. 2008;126 (10):1396–1403. doi: 10.1001/archopht.126.10.1396. [DOI] [PubMed] [Google Scholar]
- Flood V, Smith W, Wang JJ, Manzi F, Webb K, Mitchell P. Dietary antioxidant intake and incidence of early age-related maculopathy: the Blue Mountains Eye Study. Ophthalmology. 2002;109:2272–2278. doi: 10.1016/s0161-6420(02)01263-0. [DOI] [PubMed] [Google Scholar]
- Frank RN, Puklin JE, Stock C, Canter LA. Race, iris color, and age-related macular degeneration. Trans Am Ophthalmol Soc. 2000;98:109–115. discussion 115–107. [PMC free article] [PubMed] [Google Scholar]
- Frick KD, Gower EW, Kempen JH, Wolff JL. Economic impact of visual impairment and blindness in the United States. Arch Ophthalmol. 2007;125 (4):544–550. doi: 10.1001/archopht.125.4.544. [DOI] [PubMed] [Google Scholar]
- Friedman DS, O’Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J Group., E.D.P.R. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- Gale CR, Hall NF, Phillips DIW, Martyn CN. Lutein and Zeaxanthin status and risk of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2003;44 (6):2461–2465. doi: 10.1167/iovs.02-0929. [DOI] [PubMed] [Google Scholar]
- Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration--emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38 (7):450–471. doi: 10.1080/07853890600946724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg HN, Karmally W, Siddiqui M, Holleran S, Tall AR, Rumsey SC, Deckelbaum RJ, Blaner WS, Ramakrishnan R. A dose-response study of the effects of dietary cholesterol on fasting and postprandial lipid and lipoprotein metabolism in healthy young men. Arterioscler Thromb. 1994;14 (4):576–586. doi: 10.1161/01.atv.14.4.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold B, Merriam JE, Zernant J, Hancox LS, Taiber AJ, Gehrs K, Cramer K, Neel J, Bergeron J, Barile GR, Smith RT, Hageman GS, Dean M, Allikmets R. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38 (4):458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J, Flowerdew G, Smith E, Brody JA, Tso MOM. Factors associated with age-related macular degeneration. Am JEpidemiol. 1988;128 (4633):700–710. doi: 10.1093/oxfordjournals.aje.a115023. [DOI] [PubMed] [Google Scholar]
- Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351 (27):2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- Granado F, Olmedilla B, Blanco I. Nutritional and clinical relevance of lutein in human health. Br J Nutr. 2003;90 (3):487–502. doi: 10.1079/bjn2003927. [DOI] [PubMed] [Google Scholar]
- Grant CM, MacIver FH, Dawes IW. Mitochondrial function is required for resistance to oxidative stress in the yeast Saccharomyces cerevisiae. FEBS Lett. 1997;410 (2–3):219–222. doi: 10.1016/s0014-5793(97)00592-9. [DOI] [PubMed] [Google Scholar]
- Hadley D, Orlin A, Brown G, Brucker AJ, Ho AC, Regillo CD, Donoso LA, Tian L, Kaderli B, Stambolian D. Analysis of six genetic risk factors highly associated with AMD in the region surrounding ARMS2 and HTRA1 on chromosome 10, region q26. Invest Ophthalmol Vis Sci. 2010;51 (4):2191–2196. doi: 10.1167/iovs.09-3798. [DOI] [PubMed] [Google Scholar]
- Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, Smith RJ, Silvestri G, Russell SR, Klaver CC, Barbazetto I, Chang S, Yannuzzi LA, Barile GR, Merriam JC, Smith RT, Olsh AK, Bergeron J, Zernant J, Merriam JE, Gold B, Dean M, Allikmets R. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102 (20):7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman GS, Gehrs K, Lejnine S, Bansal AT, Deangelis MM, Guymer RH, Baird PN, Allikmets R, Deciu C, Oeth P, Perlee LT. Clinical validation of a genetic model to estimate the risk of developing choroidal neovascular age-related macular degeneration. Hum Genomics. 2011;5 (5):420–440. doi: 10.1186/1479-7364-5-5-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- Hammond BR, Jr, Johnson EJ, Russell RM, Krinsky NI, Yeum K-J, Edwards RB, Snodderly DM. Dietary modification of human macular pigment density. Invest Ophthalmol Vis Sci. 1997;38:1795–1801. [PubMed] [Google Scholar]
- Hartig MB, Iuso A, Haack T, Kmiec T, Jurkiewicz E, Heim K, Roeber S, Tarabin V, Dusi S, Krajewska-Walasek M, Jozwiak S, Hempel M, Winkelmann J, Elstner M, Oexle K, Klopstock T, Mueller-Felber W, Gasser T, Trenkwalder C, Tiranti V, Kretzschmar H, Schmitz G, Strom TM, Meitinger T, Prokisch H. Absence of an orphan mitochondrial protein, c19orf12, causes a distinct clinical subtype of neurodegeneration with brain iron accumulation. Am J Hum Genet. 2011;89 (4):543–550. doi: 10.1016/j.ajhg.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, Shah PK, Willerson JT, Benza RL, Berman DS, Gibson CM, Bajamonde A, Rundle AC, Fine J, McCluskey ER. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107 (10):1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- Heuberger RA, Mares-Perlman JA, Klein R, Klein BE, Millen AE, Palta M. Relationship of dietary fat to age-related maculopathy in the Third National Health and Nutrition Examination Survey. Arch Ophthalmol. 2001;119 (12):1833–1838. doi: 10.1001/archopht.119.12.1833. [DOI] [PubMed] [Google Scholar]
- Hooper L, Summerbell CD, Thompson R, Sills D, Roberts FG, Moore H, Davey Smith G. Reduced or modified dietary fat for preventing cardiovascular disease. Cochrane Database Syst Rev. 2011;(7):CD002137. doi: 10.1002/14651858.CD002137.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara N, Yuzawa M, Tamakoshi A. Antioxidants and angiogenetic factor associated with age-related macular degeneration (exudative type) Nippon Ganka Gakkai Zasshi. 1997;101:248–251. [PubMed] [Google Scholar]
- Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358 (24):2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Kendall CW, McKeown-Eyssen G, Josse RG, Silverberg J, Booth GL, Vidgen E, Josse AR, Nguyen TH, Corrigan S, Banach MS, Ares S, Mitchell S, Emam A, Augustin LS, Parker TL, Leiter LA. Effect of a low-glycemic index or a high-cereal fiber diet on type 2 diabetes: a randomized trial. JAMA. 2008;300 (23):2742–2753. doi: 10.1001/jama.2008.808. [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34 (3):362–366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- Johnson EJ, Chung HY, Caldarella SM, Snodderly DM. The influence of supplemental lutein and docosahexaenoic acid on serum, lipoproteins, and macular pigmentation. Am J Clin Nutr. 2008;87 (5):1521–1529. doi: 10.1093/ajcn/87.5.1521. [DOI] [PubMed] [Google Scholar]
- Juan-Babot JO, Martinez-Gonzalez J, Berrozpe M, Badimon L. Neovascularization in human coronary arteries with lesions of different severity. Rev Esp Cardiol. 2003;56 (10):978–986. doi: 10.1016/s0300-8932(03)76995-4. [DOI] [PubMed] [Google Scholar]
- Kanda A, Chen W, Othman M, Branham KE, Brooks M, Khanna R, He S, Lyons R, Abecasis GR, Swaroop A. A variant of mitochondrial protein LOC387715/ARMS2, not HTRA1, is strongly associated with age-related macular degeneration. Proc Natl Acad Sci U S A. 2007;104 (41):16227–16232. doi: 10.1073/pnas.0703933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik S, Wang JJ, Flood V, Tan JS, Barclay AW, Wong TY, Brand-Miller J, Mitchell P. Dietary glycemic index and the risk of age-related macular degeneration. Am J Clin Nutr. 2008;88 (4):1104–1110. doi: 10.1093/ajcn/88.4.1104. [DOI] [PubMed] [Google Scholar]
- King JC. Zinc: an essential but elusive nutrient. Am J Clin Nutr. 2011;94 (2):679S–684S. doi: 10.3945/ajcn.110.005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein BE, Knudtson MD, Lee KE, Reinke JO, Danforth LG, Wealti AM, Moore E, Klein R. Supplements and age-related eye conditions the beaver dam eye study. Ophthalmology. 2008;115 (7):1203–1208. doi: 10.1016/j.ophtha.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Klein R, Chou CF, Klein BE, Zhang X, Meuer SM, Saaddine JB. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol. 2011;129 (1):75–80. doi: 10.1001/archophthalmol.2010.318. [DOI] [PubMed] [Google Scholar]
- Klein R, Cruickshanks KJ, Nash SD, Krantz EM, Javier Nieto F, Huang GH, Pankow JS, Klein BE. The prevalence of age-related macular degeneration and associated risk factors. Arch Ophthalmol. 2010;128 (6):750–758. doi: 10.1001/archophthalmol.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Klein BE, Knudtson MD, Wong TY, Cotch MF, Liu K, Burke G, Saad MF, Jacobs DRJ. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the multi-ethnic study of atherosclerosis. Ophthalmology. 2006;113:373–380. doi: 10.1016/j.ophtha.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Marino EK, Kuller LH, Furberg C, Burke GL, Hubbard LD. Early age-related maculopathy in the cardiovascular health study. Ophthalmology. 2003;110:25–33. doi: 10.1016/s0161-6420(02)01565-8. [DOI] [PubMed] [Google Scholar]
- Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo N, Honda S, Ishibashi K, Tsukahara Y, Negi A. LOC387715/HTRA1 variants in polypoidal choroidal vasculopathy and age-related macular degeneration in a Japanese population. Am J Ophthalmol. 2007;144 (4):608–612. doi: 10.1016/j.ajo.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Kuzniarz M, Mitchell P, Flood VM, Wang JJ. Use of vitamin and zinc supplements and age-related maculopathy: the Blue Mountains Eye Study. Ophthalmic Epidemiol. 2002;9 (4):283–295. doi: 10.1076/opep.9.4.283.1511. [DOI] [PubMed] [Google Scholar]
- Lakatos A, Derbeneva O, Younes D, Keator D, Bakken T, Lvova M, Brandon M, Guffanti G, Reglodi D, Saykin A, Weiner M, Macciardi F, Schork N, Wallace DC, Potkin SG. Association between mitochondrial DNA variations and Alzheimer’s disease in the ADNI cohort. Neurobiol Aging. 2010;31 (8):1355–1363. doi: 10.1016/j.neurobiolaging.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrum JT, Bone RA, Joa H, Kilburn MD, Moore LL, Sprague KE. A one year study of the macular pigment: The effect of 140 days of a lutein supplement. Exp Eye Res. 1997;65:57–62. doi: 10.1006/exer.1997.0309. [DOI] [PubMed] [Google Scholar]
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246 (4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Leveziel N, Souied EH, Richard F, Barbu V, Zourdani A, Morineau G, Zerbib J, Coscas G, Soubrane G, Benlian P. PLEKHA1-LOC387715-HTRA1 polymorphisms and exudative age-related macular degeneration in the French population. Mol Vis. 2007;13:2153–2159. [PubMed] [Google Scholar]
- Lin JM, Wan L, Tsai YY, Lin HJ, Tsai Y, Lee CC, Tsai CH, Tsai FJ, Tseng SH. HTRA1 polymorphism in dry and wet age-related macular degeneration. Retina. 2008;28 (2):309–313. doi: 10.1097/IAE.0b013e31814cef3a. [DOI] [PubMed] [Google Scholar]
- Liu S, Stampfer MJ, Hu FB, Giovannucci E, Rimm E, Manson JE, Hennekens CH, Willett WC. Whole-grain consumption and risk of coronary heart disease: results from the Nurses’ Health Study. Am J Clin Nutr. 1999;70 (3):412–419. doi: 10.1093/ajcn/70.3.412. [DOI] [PubMed] [Google Scholar]
- Macular-Photocoagulation-Study-Group. Risk factors for choroidal neovascularization in the second eye of patients with juxtafoveal or subfoveal choroidal neovascularization secondary to age-related macular degeneration. Arch Ophthalmol. 1997;115 (6):741–747. doi: 10.1001/archopht.1997.01100150743009. [DOI] [PubMed] [Google Scholar]
- Maiani G, Caston MJ, Catasta G, Toti E, Cambrodon IG, Bysted A, Granado-Lorencio F, Olmedilla-Alonso B, Knuthsen P, Valoti M, Bohm V, Mayer-Miebach E, Behsnilian D, Schlemmer U. Carotenoids: actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol Nutr Food Res. 2009;53(Suppl 2):S194–218. doi: 10.1002/mnfr.200800053. [DOI] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Watt CB, Vazquez-Chona F, Vaughan DK, Organisciak DT. Extreme retinal remodeling triggered by light damage: implications for age related macular degeneration. Mol Vis. 2008;14:782–806. [PMC free article] [PubMed] [Google Scholar]
- Mares-Perlman J, Klein R, Klein BEK, Greger JL, Brady WE, Palta M, Ritter LL. Association of zinc and antioxidant nutrients with age-related maculopathy. Arch Ophthalmol. 1996;114:991–997. doi: 10.1001/archopht.1996.01100140199014. [DOI] [PubMed] [Google Scholar]
- Mares-Perlman JA, Brady WE, Klein R, Klein BE, Bowen P, Stacewicz-Sapuntzakis M, Palta M. Serum antioxidants and age-related macular degeneration in a population-based case-control study. Arch Ophthalmol. 1995a;113 (12):1518–1523. doi: 10.1001/archopht.1995.01100120048007. [DOI] [PubMed] [Google Scholar]
- Mares-Perlman JA, Brady WE, Klein R, VandenLangenberg GM, Klein BE, Palta M. Dietary fat and age-related maculopathy [see comments] Archives of Ophthalmology. 1995b;113 (6):743–748. doi: 10.1001/archopht.1995.01100060069034. [DOI] [PubMed] [Google Scholar]
- Mares-Perlman JA, Fisher AI, Klein R, Palta M, Block G, Millen AE, Wright JD. Lutein and zeaxanthin in the diet and serum and their relation to age-related maculopathy in the third national health and nutrition examination survey. Am J Epidemiol. 2001;153:424–432. doi: 10.1093/aje/153.5.424. [DOI] [PubMed] [Google Scholar]
- Markus RA, Mack WJ, Azen SP, Hodis HN. Influence of lifestyle modification on atherosclerotic progression determined by ultrasonographic change in the common carotid intima-media thickness. Am J Clin Nutr. 1997;65 (4):1000–1004. doi: 10.1093/ajcn/65.4.1000. [DOI] [PubMed] [Google Scholar]
- McKeown NM, Meigs JB, Liu S, Rogers G, Yoshida M, Saltzman E, Jacques PF. Dietary carbohydrates and cardiovascular disease risk factors in the Framingham offspring cohort. J Am Coll Nutr. 2009;28 (2):150–158. doi: 10.1080/07315724.2009.10719766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer BJ, Mann NJ, Lewis JL, Milligan GC, Sinclair AJ, Howe PR. Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids. Lipids. 2003;38 (4):391–398. doi: 10.1007/s11745-003-1074-0. [DOI] [PubMed] [Google Scholar]
- Michikawa T, Ishida S, Nishiwaki Y, Kikuchi Y, Tsuboi T, Hosoda K, Ishigami A, Iwasawa S, Nakano M, Takebayashi T. Serum antioxidants and age-related macular degeneration among older Japanese. Asia Pac J Clin Nutr. 2009;18 (1):1–7. [PubMed] [Google Scholar]
- Morris MS, Jacques PF, Chylack LT, Hankinson SE, Willett WC, Hubbard LD, Taylor A. Intake of zinc and antioxidant micronutrients and early age-related maculopathy lesions. Ophthalmic Epidemiol. 2007;14 (5):288–298. doi: 10.1080/09286580601186759. [DOI] [PubMed] [Google Scholar]
- National Eye Institute, N.I.o.H. Facts About Age-Related Macular Degeneration. 2010 http://www.nei.nih.gov/health/maculardegen/armd_facts.asp.
- Newsome DA. A randomized, prospective, placebo-controlled clinical trial of a novel zinc-monocysteine compound in age-related macular degeneration. Curr Eye Res. 2008;33 (7):591–598. doi: 10.1080/02713680802178437. [DOI] [PubMed] [Google Scholar]
- Newsome DA, Swartz M, Leone NC, Elston RC, Miller E. Oral zinc in macular degeneration. Arch Ophthalmol. 1988;106:192–198. doi: 10.1001/archopht.1988.01060130202026. [DOI] [PubMed] [Google Scholar]
- Obana A, Hiramitsu T, Gohto Y, Ohira A, Mizuno S, Hirano T, Bernstein PS, Fujii H, Iseki K, Tanito M, Hotta Y. Macular carotenoid levels of normal subjects and age-related maculopathy patients in a Japanese population. Ophthalmology. 2008;115 (1):147–157. doi: 10.1016/j.ophtha.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Oka C, Tsujimoto R, Kajikawa M, Koshiba-Takeuchi K, Ina J, Yano M, Tsuchiya A, Ueta Y, Soma A, Kanda H, Matsumoto M, Kawaichi M. HtrA1 serine protease inhibits signaling mediated by Tgfbeta family proteins. Development. 2004;131 (5):1041–1053. doi: 10.1242/dev.00999. [DOI] [PubMed] [Google Scholar]
- Parekh N, Chappell RJ, Millen AE, Albert DM, Mares JA. Association between vitamin D and age-related macular degeneration in the Third National Health and Nutrition Examination Survey, 1988 through 1994. Arch Ophthalmol. 2007;125 (5):661–669. doi: 10.1001/archopht.125.5.661. [DOI] [PubMed] [Google Scholar]
- Parekh N, Voland RP, Moeller SM, Blodi BA, Ritenbaugh C, Chappell RJ, Wallace RB, Mares JA. Association between dietary fat intake and age-related macular degeneration in the Carotenoids in Age-Related Eye Disease Study (CAREDS): an ancillary study of the Women’s Health Initiative. Arch Ophthalmol. 2009;127 (11):1483–1493. doi: 10.1001/archophthalmol.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi V, Tedeschi M, Gallinaro G, Varano M, Saviano S, Piermarocchi S. Carotenoids and antioxidants in age-related maculopathy italian study: multifocal electroretinogram modifications after 1 year. Ophthalmology. 2008;115 (2):324–333. e322. doi: 10.1016/j.ophtha.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Peeters A, Magliano DJ, Stevens J, Duncan BB, Klein R, Wong TY. Changes in abdominal obesity and age-related macular degeneration: the Atherosclerosis Risk in Communities Study. Arch Ophthalmol. 2008;126 (11):1554–1560. doi: 10.1001/archopht.126.11.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piermarocchi S, Saviano S, Parisi V, Tedeschi M, Panozzo G, Scarpa G, Boschi G, Lo Giudice G. Carotenoids in Age-related Maculopathy Italian Study (CARMIS): two-year results of a randomized study. Eur J Ophthalmol. 2011:0. doi: 10.5301/ejo.5000069. [DOI] [PubMed] [Google Scholar]
- Pilkington SM, Watson RE, Nicolaou A, Rhodes LE. Omega-3 polyunsaturated fatty acids: photoprotective macronutrients. Exp Dermatol. 2011;20 (7):537–543. doi: 10.1111/j.1600-0625.2011.01294.x. [DOI] [PubMed] [Google Scholar]
- Quintanilla RA, Dolan PJ, Jin YN, Johnson GV. Truncated tau and Abeta cooperatively impair mitochondria in primary neurons. Neurobiol Aging. 2012;33 (3):619, e.625–635. doi: 10.1016/j.neurobiolaging.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein DB, Zhang P, Wirth KE, Lee PP, Hoerger TJ, McCall N, Klein R, Tielsch JM, Vijan S, Saaddine J. The economic burden of major adult visual disorders in the United States. Arch Ophthalmol. 2006;124 (12):1754–1760. doi: 10.1001/archopht.124.12.1754. [DOI] [PubMed] [Google Scholar]
- Richer S. Multicenter ophthalmic and nutritional age-related macular degeneration study--part 2: antioxidant intervention and conclusions. J Am Optom Assoc. 1996;67 (1):30–49. [PubMed] [Google Scholar]
- Richer S, Devenport J, Lang JC. LAST II: Differential temporal responses of macular pigment optical density in patients with atrophic age-related macular degeneration to dietary supplementation with xanthophylls. Optometry. 2007;78 (5):213–219. doi: 10.1016/j.optm.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Richer S, Stiles W, Statkute L, Pulido J, Frankowski J, Rudy D, Pei K, Tsipursky M, Nyland J. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial) Optometry. 2004;75 (4):216–230. doi: 10.1016/s1529-1839(04)70049-4. [DOI] [PubMed] [Google Scholar]
- Richer SP, Stiles W, Graham-Hoffman K, Levin M, Ruskin D, Wrobel J, Park DW, Thomas C. Randomized, double-blind, placebo-controlled study of zeaxanthin and visual function in patients with atrophic age-related macular degeneration: the Zeaxanthin and Visual Function Study (ZVF) FDA IND #78, 973. Optometry. 2011;82 (11):667–680. e666. doi: 10.1016/j.optm.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Riegersperger M, Sunder-Plassmann G. How to prevent progression to end stage renal disease. J Ren Care. 2007;33 (3):105–107. doi: 10.1111/j.1755-6686.2007.tb00053.x. [DOI] [PubMed] [Google Scholar]
- Robman L, Vu H, Hodge A, Tikellis G, Dimitrov P, McCarty C, Guymer R. Dietary lutein, zeaxanthin, and fats and the progression of age-related macular degeneration. Can J Ophthalmol. 2007;42 (5):720–726. doi: 10.3129/i07-116. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355 (14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- Rudnicka AR, Jarrar Z, Wormald R, Cook DG, Fletcher A, Owen CG. Age and Gender Variations in Age-related Macular Degeneration Prevalence in Populations of European Ancestry: A Meta-analysis. Ophthalmology. 2012;119 (3):571–580. doi: 10.1016/j.ophtha.2011.09.027. [DOI] [PubMed] [Google Scholar]
- Sacks FM, Salazar J, Miller L, Foster JM, Sutherland M, Samonds KW, Albers JJ, Kass EH. Ingestion of egg raises plasma low density lipoproteins in free-living subjects. Lancet. 1984;1 (8378):647–649. doi: 10.1016/s0140-6736(84)92168-8. [DOI] [PubMed] [Google Scholar]
- Sanders TAB, Haines AP, Wormald R, Wright LA, Obeid O. Essential fatty acids, plasma cholesterol, and fat-soluble vitamins in subjects with age-related maculopathy and matched control subjects. Am J Clin Nutr. 1993;57 (7114):428–433. doi: 10.1093/ajcn/57.3.428. [DOI] [PubMed] [Google Scholar]
- Sangiovanni JP, Agron E, Meleth AD, Reed GF, Sperduto RD, Clemons TE, Chew EY. {omega}-3 Long-chain polyunsaturated fatty acid intake and 12-y incidence of neovascular age-related macular degeneration and central geographic atrophy: AREDS report 30, a prospective cohort study from the Age-Related Eye Disease Study. Am J Clin Nutr. 2009;90 (6):1601–1607. doi: 10.3945/ajcn.2009.27594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SanGiovanni JP, Chew EY, Clemons TE, Davis MD, Ferris FL, 3rd, Gensler GR, Kurinij N, Lindblad AS, Milton RC, Seddon JM, Sperduto RD. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS Report No. 20. Arch Ophthalmol. 2007a;125 (5):671–679. doi: 10.1001/archopht.125.5.671. [DOI] [PubMed] [Google Scholar]
- SanGiovanni JP, Chew EY, Clemons TE, Ferris FL, 3rd, Gensler G, Lindblad AS, Milton RC, Seddon JM, Sperduto RD. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22. Arch Ophthalmol. 2007b;125 (9):1225–1232. doi: 10.1001/archopht.125.9.1225. [DOI] [PubMed] [Google Scholar]
- Sapieha P, Stahl A, Chen J, Seaward MR, Willett KL, Krah NM, Dennison RJ, Connor KM, Aderman CM, Liclican E, Carughi A, Perelman D, Kanaoka Y, Sangiovanni JP, Gronert K, Smith LE. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of omega-3 polyunsaturated fatty acids. Sci Transl Med. 2011;3(69):69ra12. doi: 10.1126/scitranslmed.3001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalch W, Cohn W, Barker FM, Kopcke W, Mellerio J, Bird AC, Robson AG, Fitzke FF, van Kuijk FJ. Xanthophyll accumulation in the human retina during supplementation with lutein or zeaxanthin - the LUXEA (LUtein Xanthophyll Eye Accumulation) study. Arch Biochem Biophys. 2007;458 (2):128–135. doi: 10.1016/j.abb.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Schild L, Reinheckel T, Wiswedel I, Augustin W. Short-term impairment of energy production in isolated rat liver mitochondria by hypoxia/reoxygenation: involvement of oxidative protein modification. Biochem J. 1997;328 (Pt 1):205–210. doi: 10.1042/bj3280205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweigert FJ, Reimann J. Micronutrients and their Relevance for the Eye - Function of Lutein, Zeaxanthin and Omega-3 Fatty Acids. Klin Monbl Augenheilkd. 2011;228 (6):537–543. doi: 10.1055/s-0029-1245527. [DOI] [PubMed] [Google Scholar]
- Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller DT, Yannuzzi LA, Willett W. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA. 1994;272 (7279):1413–1420. [PubMed] [Google Scholar]
- Seddon JM, Cote J, Rosner B. Progression of age-related macular degeneration: association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch Ophthalmol. 2003;121 (12):1728–1737. doi: 10.1001/archopht.121.12.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon JM, George S, Rosner B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: the US Twin Study of Age-Related Macular Degeneration. Arch Ophthalmol. 2006;124 (7):995–1001. doi: 10.1001/archopht.124.7.995. [DOI] [PubMed] [Google Scholar]
- Seddon JM, Reynolds R, Rosner B. Associations of smoking, body mass index, dietary lutein, and the LIPC gene variant rs10468017 with advanced age-related macular degeneration. Mol Vis. 2010;16:2412–2424. [PMC free article] [PubMed] [Google Scholar]
- Seddon JM, Rosner B, Sperduto RD, Yannuzzi L, Haller JA, Blair NP, Willett W. Dietary fat and risk for advanced age-related macular degeneration. Arch Ophthalmol. 2001;119 (8):1191–1199. doi: 10.1001/archopht.119.8.1191. [DOI] [PubMed] [Google Scholar]
- Shakib M, Zinn KM. Fine structure and function of ocular tissues. The choroid, Bruch’s membrane, and the retinal pigment epithelium. Int Ophthalmol Clin. 1973;13 (3):189–204. doi: 10.1097/00004397-197301330-00015. [DOI] [PubMed] [Google Scholar]
- Simonelli F, Zarrilli F, Mazzeo S, Verde V, Romano N, Savoia M, Testa F, Vitale DF, Rinaldi M, Sacchetti L. Serum oxidative and antioxidant parameters in a group of Italian patients with age-related maculopathy. Clin Chim Acta. 2002;320:111–115. doi: 10.1016/s0009-8981(02)00056-6. [DOI] [PubMed] [Google Scholar]
- Sivaprasad S, Bailey TA, Chong VN. Bruch’s membrane and the vascular intima: is there a common basis for age-related changes and disease? Clin Experiment Ophthalmol. 2005;33 (5):518–523. doi: 10.1111/j.1442-9071.2005.01074.x. [DOI] [PubMed] [Google Scholar]
- Smiddy WE, Fine SL. Prognosis of patients with bilateral macular drusen. Ophthalmology. 1984;91 (3):271–277. doi: 10.1016/s0161-6420(84)34309-3. [DOI] [PubMed] [Google Scholar]
- Smith W, Mitchell P, Leeder SR. Dietary fat and fish intake and age-related maculopathy. Arch Ophthalmol. 2000;118 (3):401–404. doi: 10.1001/archopht.118.3.401. [DOI] [PubMed] [Google Scholar]
- Smith W, Mitchell P, Rochester C. Serum beta carotene, alpha tocopherol, and age-related maculopathy: the Blue Mountains Eye Study. Am J Ophthalmol. 1997;124:838–840. doi: 10.1016/s0002-9394(14)71702-7. [DOI] [PubMed] [Google Scholar]
- Smith W, Mitchell P, Webb K, Leeder SR. Dietary antioxidants and age-related maculopathy: the Blue Mountains Eye Study. Ophthalmology. 1999;106 (4):761–767. doi: 10.1016/S0161-6420(99)90164-1. [DOI] [PubMed] [Google Scholar]
- Snellen EL, Verbeek AL, Van Den Hoogen GW, Cruysberg JR, Hoyng CB. Neovascular age-related macular degeneration and its relationship to antioxidant intake. Acta Ophthalmol Scand. 2002;80 (4):368–371. doi: 10.1034/j.1600-0420.2002.800404.x. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Ku HH, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech Ageing Dev. 1994;74 (1–2):121–133. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Stur M, Tittl M, Reitner A, Meisinger V. Oral zinc and the second eye in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37:1225–1235. [PubMed] [Google Scholar]
- Sullivan RK, Woldemussie E, Pow DV. Dendritic and synaptic plasticity of neurons in the human age-related macular degeneration retina. Invest Ophthalmol Vis Sci. 2007;48 (6):2782–2791. doi: 10.1167/iovs.06-1283. [DOI] [PubMed] [Google Scholar]
- Sung CH, Chuang JZ. The cell biology of vision. J Cell Biol. 2010;190 (6):953–963. doi: 10.1083/jcb.201006020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenor BK, Bressler S, Caulfield L, West SK. The impact of fish and shellfish consumption on age-related macular degeneration. Ophthalmology. 2010;117 (12):2395–2401. doi: 10.1016/j.ophtha.2010.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeyama N, Matsuo N, Tanaka T. Oxidative damage to mitochondria is mediated by the Ca(2+)-dependent inner-membrane permeability transition. Biochem J. 1993;294 (Pt 3):719–725. doi: 10.1042/bj2940719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JS, Wang JJ, Flood V, Mitchell P. Dietary fatty acids and the 10-year incidence of age-related macular degeneration: the Blue Mountains Eye Study. Arch Ophthalmol. 2009;127 (5):656–665. doi: 10.1001/archophthalmol.2009.76. [DOI] [PubMed] [Google Scholar]
- Tan JS, Wang JJ, Flood V, Rochtchina E, Smith W, Mitchell P. Dietary antioxidants and the long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Ophthalmology. 2008;115 (2):334–341. doi: 10.1016/j.ophtha.2007.03.083. [DOI] [PubMed] [Google Scholar]
- Taylor HR, Tikellis G, Robman LD, McCarty CA, McNeil JJ. Vitamin E supplementation and macular degeneration: randomised controlled trial. Bmj. 2002;325 (7354):11. doi: 10.1136/bmj.325.7354.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teikari JM, Laatikainen L, Virtamo J, Haukka J, Rautalahti M, Liesto K, Albanes D, Taylor P, Heinonen OP. Six-year supplementation with alpha-tocopherol and beta-carotene and age-related maculopathy. Acta Ophthalmol Scand. 1998;76:224–229. doi: 10.1034/j.1600-0420.1998.760220.x. [DOI] [PubMed] [Google Scholar]
- Terasaka N, Yu S, Yvan-Charvet L, Wang N, Mzhavia N, Langlois R, Pagler T, Li R, Welch CL, Goldberg IJ, Tall AR. ABCG1 and HDL protect against endothelial dysfunction in mice fed a high-cholesterol diet. J Clin Invest. 2008;118 (11):3701–3713. doi: 10.1172/JCI35470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Liao J, Zhang Y, Zhou J, Zhang H, Mao M. LOC387715/HTRA1 gene polymorphisms and susceptibility to age-related macular degeneration: A HuGE review and meta-analysis. Mol Vis. 2010;16:1958–1981. [PMC free article] [PubMed] [Google Scholar]
- Trempe CL. Zinc and macular degeneration. Arch Ophthalmol. 1992;110 (11):1517. doi: 10.1001/archopht.1992.01080230015002. [DOI] [PubMed] [Google Scholar]
- Trieschmann M, Beatty S, Nolan JM, Hense HW, Heimes B, Austermann U, Fobker M, Pauleikhoff D. Changes in macular pigment optical density and serum concentrations of its constituent carotenoids following supplemental lutein and zeaxanthin: the LUNA study. Exp Eye Res. 2007;84 (4):718–728. doi: 10.1016/j.exer.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Uchiki T, Weikel KA, Jiao W, Shang F, Caceres A, Pawlak DB, Handa JT, Brownlee M, Nagaraj R, Taylor A. Glycation-altered proteolysis as a pathobiologic mechanism that links dietary glycemic index, aging, and age-related disease (in non diabetics) Aging Cell. 2012;11 (1):1–13. doi: 10.1111/j.1474-9726.2011.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen R, Boekhoorn S, Vingerling JR, Witteman JC, Klaver CC, Hofman A, de Jong PT. Dietary intake of antioxidants and risk of age-related macular degeneration. Jama. 2005;294 (24):3101–3107. doi: 10.1001/jama.294.24.3101. [DOI] [PubMed] [Google Scholar]
- VandenLangenberg GM, Mares-Perlman JA, Klein R, Klein BE, Brady WE, Palta M. Associations between antioxidant and zinc intake and the 5-year incidence of early age-related maculopathy in the Beaver Dam Eye Study. Am J Epidemiol. 1998;148 (2):204–214. doi: 10.1093/oxfordjournals.aje.a009625. [DOI] [PubMed] [Google Scholar]
- Vanderbeek BL, Zacks DN, Talwar N, Nan B, Musch DC, Stein JD. Racial differences in age-related macular degeneration rates in the United States: a longitudinal analysis of a managed care network. Am J Ophthalmol. 2011;152 (2):273–282. e273. doi: 10.1016/j.ajo.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH. Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age-related macular degeneration. PLoS ONE. 2009;4 (1):e4160. doi: 10.1371/journal.pone.0004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weikel KA, Fitzgerald P, Shang F, Caceres MA, Bian Q, Handa JT, Stitt AW, Taylor A. Natural History of Age-Related Retinal Lesions that Precede AMD in Mice Fed High or Low Glycemic Index Diets. Invest Ophthalmol Vis Sci. 2012;53 (2):622–632. doi: 10.1167/iovs.11-8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weismann D, Hartvigsen K, Lauer N, Bennett KL, Scholl HP, Charbel Issa P, Cano M, Brandstatter H, Tsimikas S, Skerka C, Superti-Furga G, Handa JT, Zipfel PF, Witztum JL, Binder CJ. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature. 2011;478 (7367):76–81. doi: 10.1038/nature10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel AJ, Sheehan JP, Gerweck C, Stringham JM, Fuld K, Curran-Celentano J. Macular pigment optical density at four retinal loci during 120 days of lutein supplementation. Ophthalmic Physiol Opt. 2007;27 (4):329–335. doi: 10.1111/j.1475-1313.2007.00495.x. [DOI] [PubMed] [Google Scholar]
- West S, Vitale S, Hallfrisch J, Munoz B, Muller D, Bressler S, Bressler NM. Are antioxidants or supplements protective for age-related macular degeneration? Arch Ophthalmol. 1994;112:222–227. doi: 10.1001/archopht.1994.01090140098031. [DOI] [PubMed] [Google Scholar]
- Wood J, Lacherez P, Black A, Cole M, Boon M, Kerr G. Risk of falls, injurious falls, and other injuries resulting from visual impairment among older adults with Age-related Macular Degeneration. Invest Ophthalmol Vis Sci. 2011;52 (8):5088–5092. doi: 10.1167/iovs.10-6644. [DOI] [PubMed] [Google Scholar]
- Yang R, Thomas GR, Bunting S, Ko A, Ferrara N, Keyt B, Ross J, Jin H. Effects of vascular endothelial growth factor on hemodynamics and cardiac performance. J Cardiovasc Pharmacol. 1996;27 (6):838–844. doi: 10.1097/00005344-199606000-00011. [DOI] [PubMed] [Google Scholar]
- Yang Z, Camp NJ, Sun H, Tong Z, Gibbs D, Cameron DJ, Chen H, Zhao Y, Pearson E, Li X, Chien J, Dewan A, Harmon J, Bernstein PS, Shridhar V, Zabriskie NA, Hoh J, Howes K, Zhang K. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science. 2006;314 (5801):992–993. doi: 10.1126/science.1133811. [DOI] [PubMed] [Google Scholar]
- Yuzbasiyan-Gurkan V, Brewer GJ. The therapeutic use of zinc in macular degeneration. Arch Ophthalmol. 1989;107 (12):1723–1724. doi: 10.1001/archopht.1989.01070020805004. [DOI] [PubMed] [Google Scholar]
- Zarbin MA. Age-related macular degeneration: review of pathogenesis. Eur J Ophthalmol. 1998;8 (4):199–206. doi: 10.1177/112067219800800401. [DOI] [PubMed] [Google Scholar]
- Zareparsi S, Buraczynska M, Branham KE, Shah S, Eng D, Li M, Pawar H, Yashar BM, Moroi SE, Lichter PR, Petty HR, Richards JE, Abecasis GR, Elner VM, Swaroop A. Toll-like receptor 4 variant D299G is associated with susceptibility to age-related macular degeneration. Hum Mol Genet. 2005;14 (11):1449–1455. doi: 10.1093/hmg/ddi154. [DOI] [PubMed] [Google Scholar]
- Zeimer M, Hense HW, Heimes B, Austermann U, Fobker M, Pauleikhoff D. The macular pigment: short- and intermediate-term changes of macular pigment optical density following supplementation with lutein and zeaxanthin and co-antioxidants. The LUNA Study. Ophthalmologe. 2009;106 (1):29–36. doi: 10.1007/s00347-008-1773-4. [DOI] [PubMed] [Google Scholar]