Abstract
Background
Oxytocin, classically involved in social and reproductive activities, is increasingly recognized as an antinociceptive and anxiolytic agent, effects which may be mediated via oxytocin’s interactions with the dopamine system. Thus, genetic variation within the oxytocin gene (OXT) is likely to explain variability in dopamine-related stress responses. As such, we examined how OXT variation is associated with stress-induced dopaminergic neurotransmission in a healthy human sample.
Method
Fifty-five young healthy volunteers were scanned using [11C] raclopride positron emission tomography while they underwent a standardized physical and emotional stressor that consisted of moderate levels of experimental sustained deep muscle pain, and a baseline, control state. Four haplotype tagging single nucleotide polymorphisms located in regions near OXT were genotyped. Measures of pain, affect, anxiety, well-being and interpersonal attachment were also assessed.
Results
Female rs4813625 C allele carriers demonstrated greater stress-induced dopamine release, measured as reductions in receptor availability from baseline to the pain-stress condition relative to female GG homozygotes. No significant differences were detected among males. We also observed that female rs4813625 C allele carriers exhibited higher attachment anxiety, higher trait anxiety and lower emotional well-being scores. In addition, greater stress-induced dopamine release was associated with lower emotional well-being scores in female rs4813625 C allele carriers.
Conclusions
Our results suggest that variability within the oxytocin gene appear to explain interindividual differences in dopaminergic responses to stress, which are shown to be associated with anxiety traits, including those linked to attachment style, as well as emotional well-being in women.
Keywords: oxytocin, genetics, dopamine, positron emission tomography, humans, sex differences
Introduction
For nearly a century, the neuropeptide oxytocin has been recognized as a critical social and reproductive hormone. While early descriptions of oxytocin highlighted the hypothalamic peptide’s ability to stimulate lactation and smooth muscle contraction during labor (1, 2), recent examinations reveal its capacity to facilitate a variety of social and reproductive activity from pair bonding to maternal behavior to sexual behaviors (see 3, 4). The mechanisms underlying oxytocin’s influence on these behaviors are likely complex and point to the involvement of the dopamine neurotransmitter system.
Oxytocin and its binding sites exist in areas outside of the hypothalamus, notably in regions within the mesolimbic dopamine (DA) system such as the ventral tegmental area (VTA) and the nucleus accumbens (NAc) [primate: (5–8) rat: (9–11)]. Evidence indicates that DA, in conjunction with oxytocin, is necessary for pair bonding in prairie voles (12–15), facilitates pup retrieval and licking in rats (16) and plays significant roles in social recognition and sexual behavior. Infusion of oxytocin into the VTA increases dopaminergic activity in the NAc (17) and stimulation of oxytocinergic projections within the VTA increases extracellular DA within the NAc while concurrently inducing penile erection (18). It has been postulated that oxytocin’s inducement of dopaminergic release within the mesolimbic DA system may impact incentive salience attribution to a variety of social stimuli (e.g. infants, conspecifics, mates) to ultimately influence an organism’s drive towards such objects (e.g. 18–22).
However, oxytocin’s modulation of motivational neural circuits does not appear to be limited to the social/reproductive realm. Oxytocin has antinociceptive properties when introduced into the amygdala and the NAc, both terminal fields of dopaminergic VTA projections that are involved in the integration and regulation of salient information, including that associated with painful stimuli (23–25). Along similar lines, oxytocin has been shown to alter central dopaminergic responses associated with non-social behaviors, including addictive behaviors (e.g. self-administration, tolerance, and dependence; see 26–32) and stress (33). Thus, it has been hypothesized that oxytocin, though it may have evolved as a mechanism to promote maternal, affiliative, and sexual behaviors key to an organism’s reproductive success, also impacts neurobiological mechanisms that subserve other functions, such as reward-seeking, drug taking, stress and pain responsiveness (e.g 19).
While data emerging from animal models has illustrated interactions between the oxytocinergic and dopaminergic systems, this relationship has only begun to be explored in humans. Functional magnetic resonance imaging studies have recently demonstrated that new mothers with secure attachment display enhanced ventral striatal activation when viewing pictures of their infants and activation was positively associated with changes in plasma oxytocin levels while interacting with their babies (34). Other researchers have observed that oxytocin administration increases trust behavior in humans and modulates dorsal striatum activity during participation in a trust game (35).
Given the relative paucity of data available on the potential linkage between DA and oxytocin in humans, we examined how genetic variation at the oxytocin gene relates to inter-individual variation in dopaminergic system function in humans using a direct measure of DA activity. Common polymorphisms of the oxytocin gene (OXT) were captured in a custom-made array (36). To our knowledge, no neuroimaging or behavioral studies examining OXT currently exist; however, the same polymorphisms studied here have been associated with the diagnosis of schizophrenia, a disorder whose underlying pathophysiology is hypothesized to involve abnormal dopaminergic neurotransmission in at least subsets of patients (37, 38). Subjects were scanned using Positron Emission Tomography (PET) utilizing the DA D2/D3 radioligand [11C] raclopride, under control and pain-stress conditions known to activate DA release in both the ventral and dorsal striatum (25). Under those conditions, endogenous DA release during the challenge is observed as a reduction in the in vivo availability of DA D2/D3 receptors, which was then related to OXT polymorphisms and relevant psychophysical correlates.
Methods and Materials
Participants
Fifty-five right-handed healthy volunteers (32 women, age 27 ± 6 and 23 men, age 26 ± 3, mean ± SD) participated in a pain-stress challenge described previously (see 25 and supplemental information). Protocols were approved by the University of Michigan Investigational Review Board and the Radioactive Drug Research Committee. Written informed consent was obtained from all subjects.
Genotyping
Tagging SNPs
DNA was extracted from blood samples taken at the time of scanning. A genomic region containing sequence 5 kb upstream and 1 kb downstream of OXT, mapped to chromosome 20p13, was retrieved from NCBI Human Build 35.1. Haplotype tagging single-nucleotide polymorphisms (SNPs) were identified using a previously described design pipeline (36). Four haplotype tagging SNPs (rs4813625, rs877172, rs3761248 and rs2740210), located in noncoding regions just upstream/downstream to OXT, were genotyped using the Illumina GoldenGate platform (36). Genotype and allele frequencies are shown in Table 1. The genotype frequencies for all 4 SNPs were in Hardy-Weinberg Equilibrium. Some SNP data could not be accessed due to poor assay quality so the sample sizes vary slightly for each of the analyses (Table 1).
Table 1.
OXT SNPs
| OXT SNPs | N, Genotype Frequencies | MAF | ||
|---|---|---|---|---|
| rs4813625 | 18, GG (0.36) | 24, GC (0.48) | 8, CC (0.16) | 0.40 |
| rs877172 | 23, AA (0.42) | 24, AC (0.44) | 8, CC (0.14) | 0.36 |
| rs3761248 | 33, TT (0.60) | 19, CT (0.35) | 3, CC (0.05) | 0.23 |
| rs2740210 | 30, GG (0.57) | 16, GT (0.30) | 7, TT (0.13) | 0.28 |
MAF = minor allele frequency
Blood samples were genotyped for 186 ancestry markers (AIMs) to calculate ethnic factor scores (36). This score was included as a covariate in all analyses to account for variability in allele frequencies across ethnicities (see supplemental information).
Behavioral Questionnaires
Given oxytocin’s anxiolytic effects and previous data linking plasma levels of oxytocin to attachment anxiety (39), we performed exploratory analyses assessing the potential broader impact of genetic variation on measures of attachment using the Attachment Style Questionnaire (40) and more general measures of self-reported well-being (which appear to be partially inherited, 41) using the psychological (PWB, 42), emotional (EWB, 43), and social well-being (SWB, 44) scales. Furthermore, we examined the influence of oxytocin genotype on general measures of anxiety using the State-Trait Anxiety Inventory (STAI, 45) obtaining trait measures at the time of subjects’ enrollment and state measures immediately prior to scanning.
PET Studies
Pain-Stress Challenge
In order to examine DA activity, we employed a universal physical and emotional stressor, moderate levels of sustained pain, which has previously been shown to induce significant changes in D2/D3 receptor availability (25). This experimental pain-stress challenge is particularly appropriate to probe oxytocin gene influences as oxytocin has been previously demonstrated to not only influence dopaminergic neurotransmission but also serve in antinociceptive and anxiolytic capacities.
A steady state of muscle pain was maintained over 20 min beginning 45 minutes post-tracer administration. This took place through a computer-controlled infusion of medication-grade hypertonic saline (5%) administered into the left masseter muscle. Pain intensity was rated every 15 seconds from 0 (no pain) to 100 (most intense pain imaginable) using an electronic version of 100 mm visual analog scale (VAS) and employed by the computer controller to maintain constant pain in a manner comparable across subjects using a target of 40 VAS units, as previously described (46, 47). Upon completion of the pain challenge, integrative measures of the experience were obtained using the McGill pain questionnaire (MPQ, 48), including an overall assessment of pain intensity and unpleasantness (VAS scale, 0 to 100), and the Positive and Negative Affect Schedule (PANAS, 49). Preceding the pain-stress challenge, subjects underwent a baseline control condition during which there was no expectation of pain and no infusions took place. At the time of scanning, blood samples were obtained to determine gonadal steroid hormone levels (estrogen, progesterone, testosterone). Due to difficulties placing indwelling catheters in some subjects, only 26 females (GG, n=9, GC/CC, n=17) and 21 males (GG, n=7, GC/CC, n=14) had hormone levels analyzed.
PET Acquisition and Preprocessing
Each subject underwent a 90-minute PET scan with [11C] raclopride, a DA radiotracer with affinity for both D2 and D3 receptors (50). PET scans were acquired with a Siemens (Knoxville, TN) HR+ scanner in 3-D mode (reconstructed full-width at half maximum (FWHM) resolution (~5.5 mm in-plane and 5.0 mm axially). The total activity of [11C] raclopride administered to each subject was 15.0 ± 2.2 mCi (mean ± SD). Radiotracer synthesis and image acquisition, coregistration and reconstruction protocols were identical to those used in previous publications (see 25 and supplemental information).
For each scan, two receptor-related measures were calculated, baseline DA D2/D3 binding potential at equilibrium (BPND) and the reduction in this measure with the pain-stress challenge, which reflects the release of DA and the competition between the endogenous ligand and the radiotracer (see 51 and supplemental information). Activation of the DA D2/D3 system was assessed by calculating the difference between baseline and pain-stress conditions (i.e. baseline-stress).
Magnetic resonance (MR) scans were acquired on a 3 Tesla scanner (General Electric, Milwaukee, WI) for anatomical localization and coregistration to standardized stereotactic coordinates. Acquisition sequences were axial spoiled gradient-recalled (echo time, 3.4 ms; repetition time, 10.5 ms; inversion time, 200 ms; flip angle, 25°; number of excitations, 1; 124 contiguous images; 1.5 mm thickness). MR and PET images of each subject were coregistered to each other using a mutual information algorithm as previously described (see 52 and supplemental information). To compensate for small residual anatomic variations across subjects, a three-dimensional Gaussian filter (full-width-at-half-maximum 5 mm) was applied to each image.
Statistical Analyses
Voxel-by-voxel analyses were performed using SPM5 (Wellcome Department of Cognitive Neurology, University College, London) and Matlab software (MathWorks, Natick, MA), using a flexible factorial design. Given the previously described sex differences in dopaminergic activity (53, 54) and the potential for sex differences in the effects of oxytocin, sex was incorporated as an additional factor of interest into the statistical models. Given the low numbers of minor-allele homozygotes when the sample was split by sex (Table 2), the minor-allele homozygotes and heterozygotes were combined into one group for each SNP analysis. Three of the 4 SNPs included in the array were in strong linkage disequilibrium (Figure 1), so those tests were not strictly independent. Nonetheless, we conservatively adjusted the significance threshold to p < 0.0125 to account for 4 tests.
Table 2.
PET Imaging Analyses: Right Ventromedial Caudate
| SNP | Female N | Male N | x,y,z Coordinates, mma | Cluster Sizeb | F scorec | Corrected P Valued |
|---|---|---|---|---|---|---|
| rs4813625 | (n=28GG=10, GC=12, CC=6) | n=22 (GG=8, GC=12, CC=2) | 6, 16, 2 | 500 | 12.71 | 0.01** |
| rs877172 | n=32 (AA=13, AC=16, CC=3) | n=23 (AA=10, AC=8, CC=5) | 6, 16, 3 | 128 | 5.84 | 0.78# |
| rs3761248 | (n=32TT=19, TC=13, CC=0) | n=23 (TT=14, TC=6, CC=3) | 6, 17, 2 | 729 | 9.99 | 0.06# |
| rs2740210 | n=31 (TT=4, TG=10, GG=17) | n=22 (TT=3, TG=6, GG=13) | ----- | ----- | ----- |
Indicates the Montreal Neurological Institute coordinates for the peak voxel
Cluster size expressed in 13-mm voxels
Peak-voxel statistics obtained via ANCOVA within the right ventromedial caudate.
Peak-voxel P value after multiple comparisons correction (FWE)
Significant differences between genotype groups after multiple comparisons correction (FWE) and correction for four SNPs (Bonferroni).
Effects noted within the right ventromedial caudate for these SNPs albeit below the stringent FWE threshold. Uncorrected p-values: rs3761248 (p<0.0001) and rs877172 (p=0.0016).
Figure 1. Linkage Disequilibrium.
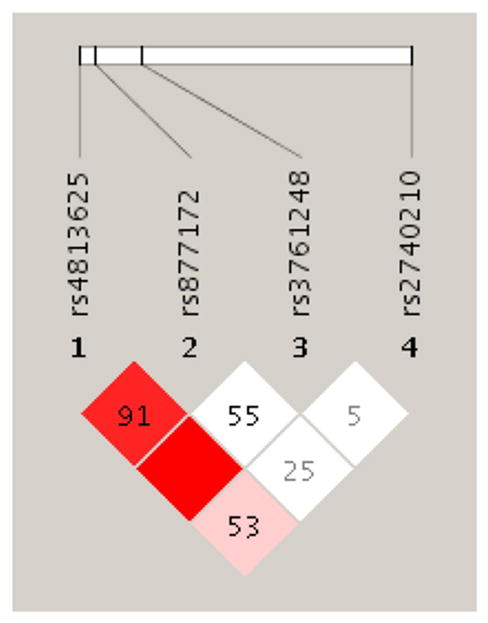
Figure. LD expressed as D’ in the study population
We performed a mixed model ANCOVA using sex and genotype as between-subject factors and stress condition (baseline-pain) as the within-subject factor with AIMs score as a covariate, with family-wise error (FWE) correction. An effects-of-interest contrast was utilized and significant regional data was extracted for post-hoc analyses. We used Bonferroni correction for post-hoc analyses to account for multiple comparisons made between groups (i.e., adjusted the threshold of significance to p=0.0125 to account for four comparisons [Male GC/CC, Male GG, Female GC/CC, Female GG]). Only regions with specific DA D2/D3 receptor binding were included in the analyses which incorporated ~42,000 voxels with BPND values > 0.2. Numerical values for each significant region were obtained by averaging the values of voxels contained in each cluster exceeding p<0.001 level (uncorrected). No global normalization was applied to the data. These data were extracted for quantification of regional changes in BPND, plotting, examination of potential outliers and further statistical analyses using SPSS for Macintosh 19.0 (SPSS Inc., Chicago, IL).
Results
Stress-Induced Activation of DA Neurotransmission
The SPM ANCOVA model for SNP rs4813625 revealed a significant peak localized in the right ventromedial caudate. Similar regional effects were noted for rs3761248 and rs877172, which are in linkage disequilibrium with rs4813625 (Table 2) however, were not significant following correction for multiple comparisons. No effects were observed for the unlinked SNP rs2740210. Given these results, stress-induced dopamine release (calculated as reductions in BPND from baseline to challenge conditions [baseline-stress], 51) was extracted from the right ventromedial caudate using the MarsBaR region of interest toolbox for SPM (55) and entered into SPSS for post-hoc analysis.
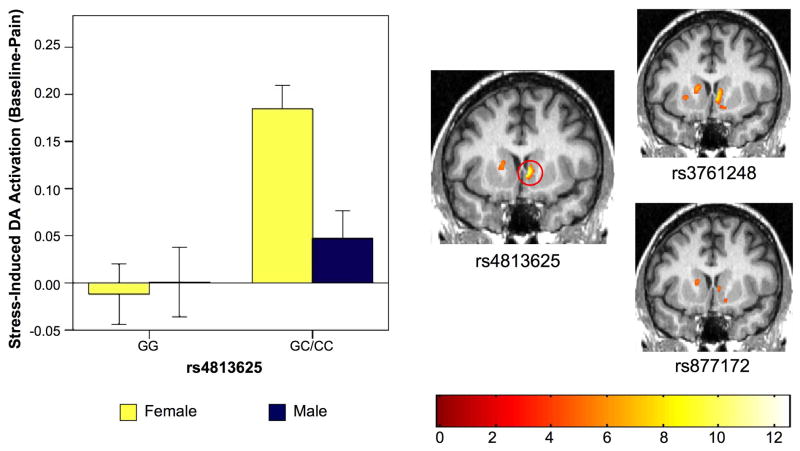
SPSS ANCOVA revealed a significant gene effect [F(1,45)=16.44, p = 0.0002], a marginally non-significant sex effect [F(1,45) = 3.70, p = 0.061] and a significant sex x genotype interaction [F(1,45) = 6.53, p = 0.014] for rs4813625. To clarify differences between groups, stress-induced activation of DA D2/D3 neurotransmission was examined pairwise using two sample t-tests. Female C allele carriers exhibited greater stress-induced DA D2/D3 activation relative to female GG homozygotes [mean change BP ± SE, Female GG: −0.019 ± 0.033, Female GC/CC: 0.1906 ± 0.025, t=−4.966, p < 0.0001, Figure 2] and relative to male C allele carriers [p=0.001, Figure 2]. No significant differences were observed between male GG homozygotes and male C allele carriers [mean difference BP ± SE, Male GG: 0.00 ± 0.036, Male GC/CC: 0.0486 ± 0.0298, t=−1.012, p = 0.324, Figure 2], or between male GG homozygotes and female GG homozygotes [t=−0.386, p = 0.705, Figure 2].
Figure 2. Impact of OXT rs4813625 on Stress-Induced DA Neurotransmission.
Left. Bar graph of extracted data from the right ventromedial caudate peak illustrating stress-induced DA activation among males and females by rs4813625 genotype, Error bars represent ± 1 standard error of the mean (Female GG, n=10; Male GG, n=8; Female CC/CG, n=18; Male CC/CG, n=14). Right. SPM ANCOVA results for OXT SNPs rs4813625, rs3761248 and rs877172. Red circle indicates location of right ventromedial caudate peak. Figure display threshold = 0.01.
Psychophysical Responses to Pain-Stress and Hormonal Influences
Given the effects of rs4813625 on DA activation, GG and C carriers were compared for differences in demographics or pain experience in both females and males. There were no significant differences in demographic, radiotracer dose or plasma levels of gonadal steroids between genotype groups for either sex (Table 3). We also did not observe any differences in STAI state anxiety scores at the time of scanning in either females or males. No significant effects of rs4813625 genotype was observed for pain-specific variables: average VAS ratings of pain intensity acquired every 15 sec for the 20 min challenge and the ratio of VAS ratings to volume of algesic substance required to maintain pain at the target level (Average VAS/Total Infusion Volume, a measure of sustained pain sensitivity). In addition, we did not observe any differences in MPQ total or MPQ sensory scores, overall pain unpleasantness, or the stress-induced change in PANAS positive or negative affect subscales for either sex (Table 4). However, female GG homozygotes recalled lower overall pain intensity immediately following the challenge though exhibited higher MPQ affective scores relative to female C carriers (Table 4). No significant differences in these measures were noted among males (Table 4).
Table 3.
Demographics & Scanning Measures - rs4813625
| Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|
| GG (n=10) | GC/CC (n=18) | tb | P Valueb | GG (n=8) | GC/CC (n=14) | tb | P Valueb | |
| Age | 26.2 (7.4) | 26.6 (5.2) | −0.17 | 0.87 | 26.5 (3.6) | 25.2 (3.1) | 0.89 | 0.39 |
| Dose (mCi) | 15.3 (1.2) | 14.8 (2.9) | 0.55 | 0.59 | 14.8 (3.1) | 15.2 (1.2) | −0.44 | 0.67 |
| Testosterone (ng/mL)# | 62.8 (21.1) | 46.7 (19.9) | 1.93 | 0.07 | 581.0 (299.1) | 621.2 (246.9) | −0.02 | 0.75 |
| Estradiol (pg/mL)# | 106.3 (60.4) | 96.3 (69.5) | 0.36 | 0.72 | ----- | ----- | ----- | |
| Progesterone (pg/mL)# | 4.8 (6.9) | 2.8 (4.3) | 0.92 | 0.37 | ----- | ----- | ----- | |
Data are expressed as mean (SD)
Calculated using 2-sample t tests for differences between genotype
Hormone assays performed in the following numbers of participants: Female GG (n=9), Female GC/CC (n=17), Males GG (n=7), Males GC/CC (n=14)
Table 4.
Pain, Attachment, Anxiety and Well-Being Measures
| Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|
| GG (n=10) | GC/CC (n=18) | tb | P Valueb | GG (n=8) | GC/CC (n=14) | tb | P Valueb | |
| Scanning Measures# | ||||||||
| Average VAS (0 to 20 minutes) | 31.03 ± 11.89 | 38.29 ± 12.80 | −1.47 | 0.15 | 33.75 ± 10.55 | 32.34 ± 9.08 | 0.33 | 0.75 |
| Total volume (mL) of hypertonic saline solution | 2.91 ± 1.26 | 2.50 ± 1.38 | 0.79 | 0.44 | 2.71 ± 1.04 | 2.68 ± 1.16 | 0.57 | 0.96 |
| Average VAS / Total Volume (0 to 20 minutes) | 2.04 ± 3.28 | 2.49 ± 2.25 | −0.43 | 0.67 | 1.57 ± 1.01 | 1.69 ± 1.37 | −0.23 | 0.82 |
| Overall Pain Intensity | 39.80 ± 11.75 | 49.17 ± 11.27 | −2.08 | 0.05* | 43.13 ± 15.80 | 40.57 ± 9.65 | 0.47 | 0.64 |
| Overall Pain Unpleasantness | 46.50 ± 22.12 | 48.61 ± 16.52 | −0.29 | 0.78 | 47.50 ± 11.95 | 42.79 ± 14.26 | 0.79 | 0.44 |
| Total MPQ | 26.20 ± 8.07 | 22.94 ± 7.75 | 1.05 | 0.30 | 26.50 ± 11.51 | 24.79 ± 13.53 | 0.30 | 0.77 |
| MPQ Sensory | 15.90 ± 5.88 | 15.39 ± 5.60 | 0.23 | 0.82 | 16.75 ± 6.36 | 16.14 ± 8.87 | 0.17 | 0.87 |
| MPQ Affect | 2.70 ± 1.83 | 1.17 ± 1.86 | 2.11 | 0.05* | 2.13 ± 4.22 | 1.57 ± 2.10 | 0.42 | 0.68 |
| Δ PANAS Negative Affect (Baseline – Pain) | 0.40 ± 1.51 | −1.06 ± 3.13 | 1.38 | 0.18 | −3.63 ± 6.89 | −1.21 ± 3.53 | −1.10 | 0.38 |
| Δ PANAS Positive Affect (Baseline – Pain) | −1.20 ± 2.86 | −0.67 ± 3.58 | −0.40 | 0.69 | −0.50 ± 3.25 | 1.14 ± 3.63 | −1.06 | 0.30 |
| STAI State | 36.60 ± 4.14 | 35.59 ± 8.91 | 0.34 | 0.74 | 31.00 ± 5.60 | 36.17 ± 14.03 | −0.70 | 0.49 |
| Attachment, Anxiety and Well-Being Measures## | ||||||||
| Attachment Avoidance | 44.33 ± 20.66 | 50.67 ± 20.10 | −0.77 | 0.45 | 41.75 ± 20.20 | 49.21 ± 22.85 | −0.77 | 0.45 |
| Attachment Anxiety | 47.22 ± 15.96 | 63.22 ± 19.62 | −2.12 | 0.05* | 54.75 ± 16.33 | 54.93 ± 18.00 | −0.02 | 0.98 |
| STAI Trait | 29.33 ± 6.52 | 35.83 ± 7.29 | −2.26 | 0.03* | 33.63 ± 8.47 | 36.21 ± 12.47 | −0.52 | 0.61 |
| Emotional Well-Being Total | 23.78 ± 2.49 | 20.11 ± 3.94 | 2.54 | 0.02* | 20.88 ± 1.25 | 18.57 ± 4.45 | 1.82 | 0.09 |
| Social Well-Being Total | 81.22 ± 4.18 | 76.50 ± 13.37 | 1.03 | 0.32 | 79.50 ± 9.90 | 78.36 ± 13.86 | 0.20 | 0.84 |
| Psychological Well-Being Total | 110.11 ± 8.22 | 103.44 ± 13.99 | 1.56 | 0.13 | 107.38 ± 15.16 | 101.50 ± 20.58 | 0.70 | 0.49 |
Data are expressed as mean (SD)
Calculated using 2-sample t tests for differences between genotype
Significant differences detected between genotype groups
1 GC/GG Female, 2 GC/CC Males and 4 GG Males did not complete the STAI State measure.
1 GG Female did not complete the additional attachment, anxiety and well-being measures.
Attachment, Anxiety and Well-Being Scales
Among females, rs4813625 C allele carriers showed higher scores in Attachment Anxiety, STAI trait anxiety, as well as lower scores on Emotional Well-Being (EWB; Table 4). No significant effect of genotype was observed for Attachment Avoidance, Social Well-Being (SWB), or Psychological Well-Being (PWB) scores in females (Table 4). No significant genotype effects were observed in the male sample (Table 4).
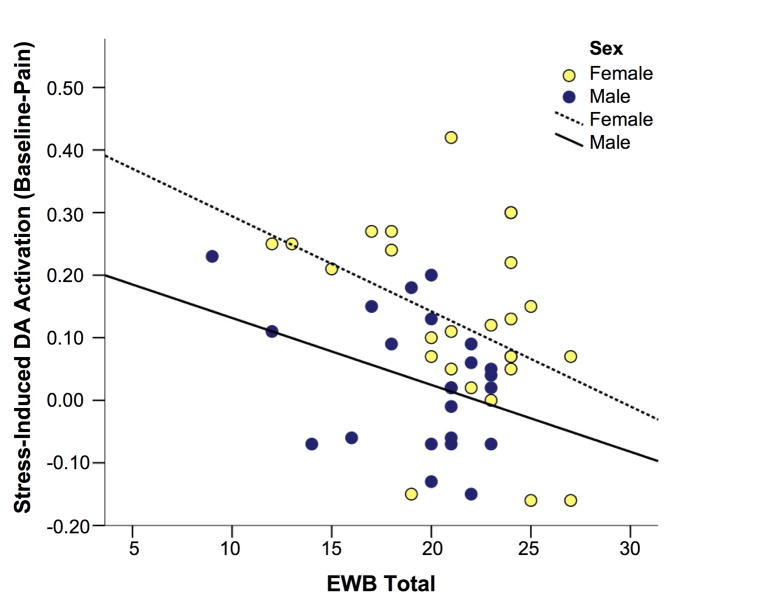
Given the effects of rs4813625 on attachment, anxiety and well-being measures, simple regression analyses were performed to determine their relationship with stress-induced DA release. We observed a significant negative relationship between EWB Total scores and stress-induced DA release within the right ventromedial caudate in our female sample (r = −0.41, p=0.03, Figure 3). A similar relationship was observed in the male sample, but did not reach statistical significance (r=−0.37, p=0.09, Figure 3). STAI trait anxiety was positively related to stress-induced DA release in both males (r=0.45, p=0.03) and females (r=0.30, p=0.13), however, this relationship only reached statistical significance in the males. Relationships between Attachment Anxiety scores and stress-induced DA release were in the positive direction in both females (r = 0.29, p = 0.15) and males (r = 0.40, p=0.07), but below statistical significance.
Figure 3. Emotional Well-Being Relationship with Stress-Induced DA Activation in the Ventromedial Caudate.
Graph. Relationship between total EWB scores and stress-induced ventromedial caudate activation in men (r=−0.370, p=0.090) and women (r=−0.406, p=0.036).
Discussion
This study examined the effects of genetic variation associated with the oxytocin gene on dopaminergic neurotransmission in humans. We observed that a SNP, rs4813625, located upstream from the coding region of the gene was associated with DA responses to a standardized physical and emotional stressor—moderate levels of sustained pain. Similar effects, but of lesser magnitude, were also observed for additional SNPs in linkage disequilibrium with rs4813625, but not with a SNP outside the haplotype block. The genetic effect was sex-specific: female but not male carriers of the C allele showed greater DA responses to the pain-stress challenge relative to GG homozygotes. Genetic variation did not appear to influence psychophysical measures specifically related to pain, or changes in affective state induced by the painful stimulus. The C allele was, however, related to low emotional well-being, high STAI trait anxiety and high attachment anxiety among females – traits which were in turn related to stress-induced striatal DA release.
Dopamine is released in response to both psychological and physiological stressors, in addition to its more traditional roles in responses to salient rewarding stimuli (25, 56–60). The magnitude of stress-induced DA release varies greatly from individual to individual (25, 59). There are likely to be many sources of this variability, including genetic influences. Strain differences in the mesocorticolimbic DA response to a forced swim test have been noted in mice whereby the mice of a hypoactive phenotype (DBA/2) show enhanced stress-induced mesoaccumbal DA release relative to their more active counterpart (C57BL/6; for review, see 61). In rats, novelty-seeking phenotypes classified as high responders (HR) or low responders (LR) on the basis of their locomotor response to novelty, which show differential DA responses in response to stress (57), can be selectively bred for this trait (62). Less work has been done to this end in humans, however polymorphisms in DA-related genes, such as DRD2 and SLC6A3 appear to increase stress-induced craving of cigarettes (63) and COMT has been shown to influence smoking induced DA release (64).
The link between oxytocin gene variation and dopaminergic responsiveness that we describe here is in line with previous research, showing a significant interplay between oxytocinergic and dopaminergic systems. Pain-induced increases in DA neurotransmission in the dorsal caudate and putamen have been positively associated with pain intensity ratings in healthy controls (25, 65). Ventral basal ganglia DA responses to pain, conversely, have been associated with increases in negative affect and fear ratings during the challenge (25). Here, variation in rs4813625 was related to DA responses to a pain-stressor within the right ventromedial caudate. This region receives afferent projections from multiple limbic structures including the hippocampus, amygdala, orbitofrontal cortex and anterior cingulate (66) and is hypothesized to act in an integrative capacity, assimilating cognitive, affective and motivational information to influence behavioral action (66–68). We did not, however, observe any significant effects of OXT genotype on measures of pain sensitivity or changes in affective state during the pain challenge. This was somewhat surprising given that exogenously applied oxytocin has well-described antinociceptive and anxiolytic properties (69–75), however, as others have pointed out (76), whether central endogenous oxytocinergic (69) mechanisms contribute significantly to sustained pain perception is not well understood. We did, however, observe differences in the subjective, integrative recall of the experience among females. Interestingly, the female GG homozygotes overall perception of the pain challenge was one of less pain intensity relative to female C carriers. However, they did use more affective terms to describe their pain experience (as measured by the affective subscale of the McGill) suggesting a greater awareness of the affective quality of the challenge, even when its sensory intensity was not enhanced.
We also noted the magnitude of stress-induced DA release was significantly and negatively correlated with EWB scores in females, not reaching statistical significance in males. Also below statistical levels of significance, positive correlations were observed with attachment anxiety ratings, in both males and females. In addition, we noted positive correlations between STAI trait anxiety and stress-induced DA release in both genders, reaching statistical significance in the male sample. This could suggest oxytocin effects are not closely associated with acute subjective psychophysical responses to our pain challenge but are more generally related to trait effects related to social or emotional function, particularly in females. This possibility is consistent with our findings for rs4813625, where female C allele carriers exhibited increased attachment anxiety, increased STAI trait anxiety and lower emotional well-being ratings than GG homozygotes. Indeed, previous studies have noted associations between changes in oxytocin plasma and attachment anxiety in humans (39). Oxytocin receptor (OXTR) polymorphisms have also been shown to be associated with autistic disorders, where a significant impairment of attachment behavior has been noted (77–80), and with adult attachment styles in patients with depression (81). These associations are not limited to patient populations; as was recently summarized by Meyer-Lindenberg and colleagues, associating OXTR polymorphisms with differences in social behavior and emotional functions as well as depression and anxiety preoccupations in control populations (82).
The direction of our findings for rs4813625 is notable in two regards. First, as described above, genetic variation within OXT was associated both with stress-induced DA activity and with measures of well-being but only among female participants. The sex-specific effects of rs4813625 on DA neurotransmission, and EWB are certainly in line with a growing literature examining oxytocin effects. Expression of the oxytocin peptide and its receptor tends to be higher in females, shows a sexual dimorphic distribution, and appears to be modulated by gonadal steroids in animal models (see 3, 83–87). Oxytocin plays sex-specific roles in reproductive behavior; for instance, oxytocin is critically involved in parturition and lactation, inducing uterine contractions during labor and milk ejection during breastfeeding in females (1, 2) and elicits erections in males (for review, see 88). Indeed, animal models suggest maternal behavior is primarily governed by oxytocin whereas paternal behavior may be more dependent on vasopressin mechanisms (89, 90). This pattern holds true for some social behaviors, such as social recognition, where vasopressin and oxytocin serve as a primary critical neuropeptides for males and females, respectively (for review, see 91). Reports of sex differences in oxytocin functioning are not limited to animal models; for instance, empathy triggered oxytocin release is higher in women than in men (92) and intranasal oxytocin attenuates amygdala responses to emotional stimuli in men (93, 94) but enhances activity in this region in women (95). Furthermore, amygdala and hypothalamic volume is related to OXTR genotype in a sex-dependent manner (96). These data and ours suggest that further studies examining sex differences in oxytocin functioning in humans are merited. Second, we found that female C-allele carriers exhibited not only the greatest stress-induced increases in DA activity but also showed lower EWB scores and higher attachment and STAI trait anxiety suggesting that this may represent a vulnerability trait by responding more prominently to salient (in this case painful stress) stimuli, or conversely, that GG homozygosity confers resiliency. From a clinical perspective, pain and stress-induced variations in DA functioning have been related to anxiety and depressive symptomatology in clinical samples (97) psychosis (98) persistent fibromyalgia pain (65), as well as the risk for the development and recurrence of drug self-administration in animal models (99).
This study represents initial research into genetic variation in the oxytocin gene and its effects on human dopaminergic mechanisms and their behavioral correlates. The data presented suggests that the oxytocinergic system influences the response of the DA system selectively in females, and as such, maybe involved in normal and pathological states that are more common among the females and where DA plays a significant role (e.g., anxiety, responses to drugs of abuse). In this context, we note that oxytocin appears to exert effects in domains beyond social and reproductive behavior, and that it influences stress-activated mechanisms in humans.
We note several limitations to this study. First, our sample size, while rather large for PET studies with neurochemical markers, necessitated the grouping of minor homozygotes and heterozygotes and thus, we were unable to assess potential additive gene effects. These findings should be followed up with a larger sample to address whether a dominant/recessive or additive model is most appropriate to describe our gene effects. Second, the proximal effects of rs4813625 on oxytocin function are presently unknown, though those studies appear warranted by the data presented. A bioinformatics analysis indicated the approximately 1KB region surrounding rs4813625 and rs3761248 has high regulatory potential. Four transcription factors (c-Jun, NR1B1, c-Fos, ctcf) have annotated binding sites near these SNPs; however, none of the binding site motifs specifically span either of the two SNPs. Though at this time there is no evidence indicating that rs4813625 is functional, it is possible that a yet undiscovered polymorphism in linkage disequilibrium with rs4813625 confers the sex-specific effects described by influencing the expression, posttranslational processing or function of oxytocin or its carrier protein neurophysin.
This study is of particular interest in regards to the potential for variation in oxytocin function or expression to influence both variation in normal behavior (attachment style, emotional stability) and resiliency and vulnerability for psychiatric disorders where DA plays a significant role. Of immediate clinical application, oxytocin has been shown to reduce tolerance, physical withdrawal and drug-self administration in animal models (29), processes that show substantial sex-dependent effects (100) and for which no sex-specific treatments are currently available. Further examination of OXT genetic variation effects on these processes appears warranted.
Supplementary Material
Acknowledgments
We thank the nuclear medicine technologists of the Positron Emission Tomography Center at the University of Michigan, Ann Arbor for their contribution to the performance of the studies. This study was supported by the National Institute of Drug Abuse grants R01 DA016423 and R01 DA022520 (Principal Investigator: Jon-Kar Zubieta), the Intramural Program of the National Institute on Alcohol Abuse and Alcoholism, and the Phil F. Jenkins Foundation.
Footnotes
Financial Disclosures
Dr. Zubieta has served as a paid consultant for Eli Lilly and Co., Johnson & Johnson, Merck, and Abbott in the 3-year period prior to submission of the manuscript. Dr. Mickey has received salary support from St. Jude Medical for research unrelated to the manuscript. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ott I, Scott JC. The galactogogue action of the thymus and corpus luteum. Proceedings of the Society for Experimental Biology and Medicine. 1910;8:49–50. [Google Scholar]
- 2.Dale HH. On some physiological actions of ergot. The Journal of Physiology. 1906;34:163–206. doi: 10.1113/jphysiol.1906.sp001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee H-J, Macbeth AH, Pagani JH, Young WS. Oxytocin: the great facilitator of life. Progress in Neurobiology. 2009;88:127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- 5.Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Research. 1991;555:220–232. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- 6.Caffé AR, Van Ryen PC, Van der Woude TP, Van Leeuwen FW. Vasopressin and oxytocin systems in the brain and upper spinal cord of Macaca fascicularis. The Journal of Comparative Neurology. 1989;287:302–325. doi: 10.1002/cne.902870304. [DOI] [PubMed] [Google Scholar]
- 7.Fliers E, Guldenaar SE, van de Wal N, Swaab DF. Extrahypothalamic vasopressin and oxytocin in the human brain; presence of vasopressin cells in the bed nucleus of the stria terminalis. Brain Research. 1986;375:363–367. doi: 10.1016/0006-8993(86)90759-6. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins JS, Ang VT, Hawthorn J, Rossor MN. Quantitative distribution of neurohypophysial hormones in human brain and spinal cord. Progress in Brain Research. 1983;60:123–128. doi: 10.1016/S0079-6123(08)64380-0. [DOI] [PubMed] [Google Scholar]
- 9.Krémarik P, Freund-Mercier MJ, Stoeckel ME. Histoautoradiographic detection of oxytocin- and vasopressin-binding sites in the telencephalon of the rat. The Journal of Comparative Neurology. 1993;333:343–359. doi: 10.1002/cne.903330304. [DOI] [PubMed] [Google Scholar]
- 10.Van Leeuwen FW, van Heerikhuize J, van der Meulen G, Wolters P. Light microscopic autoradiographic localization of [3H]oxytocin binding sites in the rat brain, pituitary and mammary gland. Brain Research. 1985;359:320–325. doi: 10.1016/0006-8993(85)91443-x. [DOI] [PubMed] [Google Scholar]
- 11.Brinton RE, Wamsley JK, Gee KW, Wan YP, Yamamura HI. [3H]oxytocin binding sites in the rat brain demonstrated by quantitative light microscopic autoradiography. European Journal of Pharmacology. 1984;102:365–367. doi: 10.1016/0014-2999(84)90270-x. [DOI] [PubMed] [Google Scholar]
- 12.Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, et al. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nature Neuroscience. 2006;9:133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- 13.Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang Z. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. The Journal of Neuroscience. 2003;23:3483–3490. doi: 10.1523/JNEUROSCI.23-08-03483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- 15.Gingrich B, Liu Y, Cascio C, Wang Z, Insel TR. Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster) Behavioral Neuroscience. 2000;114:173–183. doi: 10.1037//0735-7044.114.1.173. [DOI] [PubMed] [Google Scholar]
- 16.Keer SE, Stern JM. Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiology & Behavior. 1999;67:659–669. doi: 10.1016/s0031-9384(99)00116-x. [DOI] [PubMed] [Google Scholar]
- 17.Shahrokh DK, Zhang T-Y, Diorio J, Gratton A, Meaney MJ. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151:2276–2286. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melis MR, Melis T, Cocco C, Succu S, Sanna F, Pillolla G, et al. Oxytocin injected into the ventral tegmental area induces penile erection and increases extracellular dopamine in the nucleus accumbens and paraventricular nucleus of the hypothalamus of male rats. European Journal of Neuroscience. 2007;26:1026–1035. doi: 10.1111/j.1460-9568.2007.05721.x. [DOI] [PubMed] [Google Scholar]
- 19.Becker JB. Sexual differentiation of motivation: A novel mechanism? Hormones and behavior. 2009;55:646–654. doi: 10.1016/j.yhbeh.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Insel TR. Is social attachment an addictive disorder? Physiology & Behavior. 2003;79:351–357. doi: 10.1016/s0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- 21.Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Hormones and behavior. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behavioral Neuroscience. 1994;108:1163–1171. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- 23.Gu X-L, Yu L-C. Involvement of opioid receptors in oxytocin-induced antinociception in the nucleus accumbens of rats. The Journal of Pain. 2007;8:85–90. doi: 10.1016/j.jpain.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Han Y, Yu L-C. Involvement of oxytocin and its receptor in nociceptive modulation in the central nucleus of amygdala of rats. Neuroscience Letters. 2009;454:101–104. doi: 10.1016/j.neulet.2009.02.062. [DOI] [PubMed] [Google Scholar]
- 25.Scott D, Heitzeg M, Koeppe R, Stohler C, Zubieta J. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. The Journal of Neuroscience. 2006;26:10789–10795. doi: 10.1523/JNEUROSCI.2577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovács GL, Izbéki F, Horváth Z, Telegdy G. Effects of oxytocin and a derivative (Z-prolyl-D-leucine) on morphine tolerance/withdrawal are mediated by the limbic system. Behavioural Brain Research. 1984;14:1–8. doi: 10.1016/0166-4328(84)90014-7. [DOI] [PubMed] [Google Scholar]
- 27.Kovács GL, Horváth Z, Sarnyai Z, Faludi M, Telegdy G. Oxytocin and a C-terminal derivative (Z-prolyl-D-leucine) attenuate tolerance to and dependence on morphine and interact with dopaminergic neurotransmission in the mouse brain. Neuropharmacology. 1985;24:413–419. doi: 10.1016/0028-3908(85)90026-7. [DOI] [PubMed] [Google Scholar]
- 28.Kovàcs GL, Sarnyai Z, Barbarczi E, Szabó G, Telegdy G. The role of oxytocin-dopamine interactions in cocaine-induced locomotor hyperactivity. Neuropharmacology. 1990;29:365–368. doi: 10.1016/0028-3908(90)90095-9. [DOI] [PubMed] [Google Scholar]
- 29.Kovács GL, Sarnyai Z, Szabó G. Oxytocin and addiction: a review. Psychoneuroendocrinology. 1998;23:945–962. doi: 10.1016/s0306-4530(98)00064-x. [DOI] [PubMed] [Google Scholar]
- 30.Sarnyai Z, Szabó G, Kovács GL, Telegdy G. Oxytocin attenuates the cocaine-induced exploratory hyperactivity in mice. Neuroreport. 1990;1:200–202. doi: 10.1097/00001756-199011000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Sarnyai Z, Kovács GL. Role of oxytocin in the neuroadaptation to drugs of abuse. Psychoneuroendocrinology. 1994;19:85–117. doi: 10.1016/0306-4530(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 32.Sarnyai Z. Oxytocin as a potential mediator and modulator of drug addiction. Addiction Biology. 2011;16:199–201. doi: 10.1111/j.1369-1600.2011.00332.x. [DOI] [PubMed] [Google Scholar]
- 33.Pfister HP, Muir JL. Influence of exogenously administered oxytocin on central noradrenaline, dopamine and serotonin levels following psychological stress in nulliparous female rats (Rattus norvegicus) International Journal of Neuroscience. 1989;45:221–229. doi: 10.3109/00207458908986235. [DOI] [PubMed] [Google Scholar]
- 34.Strathearn L, Li J, Fonagy P, Montague PR. What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics. 2008;122:40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Hodgkinson CA, Yuan Q, Xu K, Shen P-H, Heinz E, Lobos EA, et al. Addictions biology: haplotype-based analysis for 130 candidate genes on a single array. Alcohol & Alcoholism. 2008;43:505–515. doi: 10.1093/alcalc/agn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proceedings of the National Academy of Sciences. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Souza RP, Ismail P, Meltzer HY, Kennedy JL. Variants in the oxytocin gene and risk for schizophrenia. Schizophrenia Research. 2010;121:279–280. doi: 10.1016/j.schres.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 39.Marazziti D, Dell’Osso B, Baroni S, Mungai F, Catena M, Rucci P, et al. A relationship between oxytocin and anxiety of romantic attachment. Clinical Practice and Epidemiology in Mental Health. 2006;2:28. doi: 10.1186/1745-0179-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brennan K, Clark C, Shaver P. Self-report measurement of adult attachment. In: Simpson J, Rholes W, editors. Attachment Theory and Close Relationships. New York, NY: The Guilford Press; 1998. pp. 46–76. [Google Scholar]
- 41.Keyes CLM, Myers JM, Kendler KS. The structure of the genetic and environmental influences on mental well-being. American Journal of Public Health. 2010;100:2379–2384. doi: 10.2105/AJPH.2010.193615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keyes CLM. The mental health continuum: from languishing to flourishing in life. Journal of Health and Social Research. 2002;43:207–222. [PubMed] [Google Scholar]
- 43.Mroczek DK, Kolarz CM. The effect of age on positive and negative affect: a developmental perspective on happiness. Journal of Personality and Social Psychology. 1998;75:1333–1349. doi: 10.1037//0022-3514.75.5.1333. [DOI] [PubMed] [Google Scholar]
- 44.Keyes C. Social Well-Being. Social Psychology Quarterly. 1998;61:121–140. [Google Scholar]
- 45.Spielberger C. State-Trait Anxiety Inventory (Form Y) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 46.Stohler C, Kowalski C. Spatial and temporal summation of sensory and affective dimensions of deep somatic pain. Pain. 1999;79:165–173. doi: 10.1016/s0304-3959(98)00171-7. [DOI] [PubMed] [Google Scholar]
- 47.Zhang X, Ashton-Miller J, Stohler C.Anonymous. A closed-loop system for maintaining constant experimental muscle pain in man. IEEE Transactions on Biomedical Engineering. 1993;40:344–352. doi: 10.1109/10.222327. [DOI] [PubMed] [Google Scholar]
- 48.Melzack R, Torgerson W. On the language of pain. Anesthesiology. 1971;34:50–59. doi: 10.1097/00000542-197101000-00017. [DOI] [PubMed] [Google Scholar]
- 49.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 50.Seeman P, Wilson A, Gmeiner P, Kapur S. Dopamine D2 and D3 receptors in human putamen, caudate nucleus, and globus pallidus. Synapse. 2006;60:205–211. doi: 10.1002/syn.20298. [DOI] [PubMed] [Google Scholar]
- 51.Innis R, Malison R, al-Tikriti M, Hoffer P, Sybirska E, Seibyl J, et al. Amphetamine-stimulated dopamine release competes in vivo for [123I]IBZM binding to the D2 receptor in nonhuman primates. Synapse. 1992;10:177–184. doi: 10.1002/syn.890100302. [DOI] [PubMed] [Google Scholar]
- 52.Meyer C, Boes J, Kim B, Bland P, Zasadny K, Kison P, et al. Demonstration of accuracy and clinical versatility of mutual information for automatic multimodality image fusion using affine and thin-plate spline warped geometric deformations. Medical Image Analysis. 1997;1:195–206. doi: 10.1016/s1361-8415(97)85010-4. [DOI] [PubMed] [Google Scholar]
- 53.Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, et al. Sex differences in striatal dopamine release in healthy adults. Biological Psychiatry. 2006;59:966–974. doi: 10.1016/j.biopsych.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 54.Riccardi P, Zald D, Li R, Park S, Ansari MS, Dawant B, et al. Sex differences in amphetamine-induced displacement of [(18)F]fallypride in striatal and extrastriatal regions: a PET study. The American Journal of Psychiatry. 2006;163:1639–1641. doi: 10.1176/ajp.2006.163.9.1639. [DOI] [PubMed] [Google Scholar]
- 55.Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox [abstract]. NeuroImage; Presented at the 8th International Conferance on Functional Mapping of the Human Brain; June 2–6, 2002; Sendai, Japan. 2002. p. 497. [Google Scholar]
- 56.Rouge-Pont F, Piazza P, Kharouby M, Le Moal M, Simon H. Higher and longer stress-induced increase in dopamine concentrations in the nucleus accumbens of animals predisposed to amphetamine self-administration. A microdialysis study. Brain Research. 1993;602:169–174. doi: 10.1016/0006-8993(93)90260-t. [DOI] [PubMed] [Google Scholar]
- 57.Rouge-Pont F, Deroche V, Le Moal M, Piazza P. Individual differences in stress-induced dopamine release in the nucleus accumbens are influenced by corticosterone. European Journal of Neuroscience. 1998;10:3903–3907. doi: 10.1046/j.1460-9568.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- 58.Thierry A, Tassin J, Blanc G, Glowinski J. Selective activation of mesocortical DA system by stress. Nature. 1976;263:242–244. doi: 10.1038/263242a0. [DOI] [PubMed] [Google Scholar]
- 59.Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. The Journal of Neuroscience. 2004;24:2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lataster J, Collip D, Ceccarini J, Haas D, Booij L, van Os J, et al. Psychosocial stress is associated with in vivo dopamine release in human ventromedial prefrontal cortex: A positron emission tomography study using [(18)F]fallypride. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 61.Cabib S, Puglisi-Allegra S, Ventura R. The contribution of comparative studies in inbred strains of mice to the understanding of the hyperactive phenotype. Behavioural Brain Research. 2002;130:103–109. doi: 10.1016/s0166-4328(01)00422-3. [DOI] [PubMed] [Google Scholar]
- 62.Stead JDH, Clinton S, Neal C, Schneider J, Jama A, Miller S, et al. Selective Breeding for Divergence in Novelty-seeking Traits: Heritability and Enrichment in Spontaneous Anxiety-related Behaviors. Behavior Genetics. 2006;36:697–712. doi: 10.1007/s10519-006-9058-7. [DOI] [PubMed] [Google Scholar]
- 63.Erblich J, Lerman C, Self DW, Diaz GA, Bovbjerg DH. Stress-induced cigarette craving: effects of the DRD2 TaqI RFLP and SLC6A3 VNTR polymorphisms. The Pharmacogenomics Journal. 2004;4:102–109. doi: 10.1038/sj.tpj.6500227. [DOI] [PubMed] [Google Scholar]
- 64.Brody AL, Mandelkern MA, Olmstead RE, Scheibal D, Hahn E, Shiraga S, et al. Gene variants of brain dopamine pathways and smoking-induced dopamine release in the ventral caudate/nucleus accumbens. Archives of General Psychiatry. 2006;63:808–816. doi: 10.1001/archpsyc.63.7.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wood PB, Schweinhardt P, Jaeger E, Dagher A, Hakyemez H, Rabiner EA, et al. Fibromyalgia patients show an abnormal dopamine response to pain. European Journal of Neuroscience. 2007;25:3576–3582. doi: 10.1111/j.1460-9568.2007.05623.x. [DOI] [PubMed] [Google Scholar]
- 66.Haber SN, Knutson B. The Reward Circuit: Linking Primate Anatomy and Human Imaging. Neuropsychopharmacology. 2009:1–23. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kalivas PW, Nakamura M. Neural systems for behavioral activation and reward. Curr Opin Neurobiol. 1999;9:223–227. doi: 10.1016/s0959-4388(99)80031-2. [DOI] [PubMed] [Google Scholar]
- 68.Mogenson G, Yang C. The contribution of basal forebrain to limbic-motor integration and the mediation of motivation to action. Adv Exp Med Biol. 1991;295:267–290. doi: 10.1007/978-1-4757-0145-6_14. [DOI] [PubMed] [Google Scholar]
- 69.Lundeberg T, Uvnäs-Moberg K, Agren G, Bruzelius G. Anti-nociceptive effects of oxytocin in rats and mice. Neuroscience Letters. 1994;170:153–157. doi: 10.1016/0304-3940(94)90262-3. [DOI] [PubMed] [Google Scholar]
- 70.Ge Y, Lundeberg T, Yu L-C. Blockade effect of mu and kappa opioid antagonists on the anti-nociception induced by intra-periaqueductal grey injection of oxytocin in rats. Brain Research. 2002;927:204–207. doi: 10.1016/s0006-8993(01)03346-7. [DOI] [PubMed] [Google Scholar]
- 71.Gao L, Yu L-C. Involvement of opioid receptors in the oxytocin-induced antinociception in the central nervous system of rats. Regulatory Peptides. 2004;120:53–58. doi: 10.1016/j.regpep.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 72.de Oliveira DCG, Zuardi AW, Graeff FG, Queiroz RHC, Crippa JAS. Anxiolytic-like effect of oxytocin in the simulated public speaking test. Journal of Psychopharmacology. 2011 doi: 10.1177/0269881111400642. [DOI] [PubMed] [Google Scholar]
- 73.Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- 74.Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, et al. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology. 2006;185:218–225. doi: 10.1007/s00213-005-0293-z. [DOI] [PubMed] [Google Scholar]
- 75.Uvnäs-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23:819–835. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- 76.Schorscher-Petcu A, Sotocinal S, Ciura S, Dupré A, Ritchie J, Sorge RE, et al. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. The Journal of Neuroscience. 2010;30:8274–8284. doi: 10.1523/JNEUROSCI.1594-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, Cook EH. Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neuroscience Letters. 2007;417:6–9. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lerer E, Levi S, Salomon S, Darvasi A, Yirmiya N, Ebstein RP. Association between the oxytocin receptor (OXTR) gene and autism: relationship to Vineland Adaptive Behavior Scales and cognition. Molecular psychiatry. 2008;13:980–988. doi: 10.1038/sj.mp.4002087. [DOI] [PubMed] [Google Scholar]
- 79.Wermter A, Kamp-Becker I, Hesse P, Schulte-Körne G, Strauch K, Remschmidt H. Evidence for the involvement of genetic variation in the oxytocin receptor gene (OXTR) in the etiology of autistic disorders on high-functioning level. American Journal of Medical Genetics Part B. 2009;153B:629–639. doi: 10.1002/ajmg.b.31032. [DOI] [PubMed] [Google Scholar]
- 80.Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, et al. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biological Psychiatry. 2005;58:74–77. doi: 10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 81.Costa B, Pini S, Gabelloni P, Abelli M, Lari L, Cardini A, et al. Oxytocin receptor polymorphisms and adult attachment style in patients with depression. Psychoneuroendocrinology. 2009;34:1506–1514. doi: 10.1016/j.psyneuen.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 82.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature Reviews Neuroscience. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 83.Carter CS. Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behavioural Brain Research. 2007;176:170–186. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 84.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiological Reviews. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 85.Insel TR, Shapiro LE. Oxytocin receptors and maternal behavior. Annals of the New York Academy of Sciences. 1992;652:122–141. doi: 10.1111/j.1749-6632.1992.tb34350.x. [DOI] [PubMed] [Google Scholar]
- 86.Nomura M, McKenna E, Korach KS, Pfaff DW, Ogawa S. Estrogen receptor-beta regulates transcript levels for oxytocin and arginine vasopressin in the hypothalamic paraventricular nucleus of male mice. Molecular Brain Research. 2002;109:84–94. doi: 10.1016/s0169-328x(02)00525-9. [DOI] [PubMed] [Google Scholar]
- 87.Witt DM, Carter CS, Lnsel TR. Oxytocin receptor binding in female prairie voles: endogenous and exogenous oestradiol stimulation. Journal of Neuroendocrinology. 1991;3:155–161. doi: 10.1111/j.1365-2826.1991.tb00258.x. [DOI] [PubMed] [Google Scholar]
- 88.Andersson KE. Pharmacology of penile erection. Pharmacological Reviews. 2001;53:417–450. [PubMed] [Google Scholar]
- 89.Bales KL, Pfeifer LA, Carter CS. Sex differences and developmental effects of manipulations of oxytocin on alloparenting and anxiety in prairie voles. Developmental psychobiology. 2004;44:123–131. doi: 10.1002/dev.10165. [DOI] [PubMed] [Google Scholar]
- 90.Wang ZX, Liu Y, Young LJ, Insel TR. Hypothalamic vasopressin gene expression increases in both males and females postpartum in a biparental rodent. Journal of neuroendocrinology. 2000;12:111–120. doi: 10.1046/j.1365-2826.2000.00435.x. [DOI] [PubMed] [Google Scholar]
- 91.Gabor CS, Phan A, Clipperton-Allen AE, Kavaliers M, Choleris E. Interplay of oxytocin, vasopressin, and sex hormones in the regulation of social recognition. Behavioral neuroscience. 2011 doi: 10.1037/a0026464. [DOI] [PubMed] [Google Scholar]
- 92.Barraza JA, Zak PJ. Empathy toward strangers triggers oxytocin release and subsequent generosity. Annals of the New York Academy of Sciences. 2009;1167:182–189. doi: 10.1111/j.1749-6632.2009.04504.x. [DOI] [PubMed] [Google Scholar]
- 93.Domes G, Heinrichs M, Gläscher J, Büchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biological psychiatry. 2007;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 94.Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. The Journal of neuroscience. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, et al. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 96.Tost H, Kolachana B, Hakimi S, Lemaitre H, Verchinski BA, Mattay VS, et al. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proceedings of the National Academy of Sciences. 2010;107:13936–13941. doi: 10.1073/pnas.1003296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Southwick SM, Vythilingam M, Charney DS. The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annual Review of Clnical Psychology. 2005;1:255–291. doi: 10.1146/annurev.clinpsy.1.102803.143948. [DOI] [PubMed] [Google Scholar]
- 98.Lodge DJ, Grace AA. Developmental pathology, dopamine, stress and schizophrenia. International Journal of Developmental Neuroscience. 2011;29:207–213. doi: 10.1016/j.ijdevneu.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ungless MA, Argilli E, Bonci A. Effects of stress and aversion on dopamine neurons: implications for addiction. Neuroscience and Biobehavioral Reviews. 2010;35:151–156. doi: 10.1016/j.neubiorev.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 100.Becker JB, Hu M. Sex differences in drug abuse. Frontiers in Neuroendocrinology. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.