Abstract
Oxidative stress has been hypothesized to contribute to the development of age-related macular degeneration (AMD), the most common cause of blindness in the United States. At present, there is no treatment for early disease. Reactive oxygen species (ROS) play a physiological role in the retinal pigment epithelium (RPE), a key cell type in this disease, but with excessive ROS, oxidative damage or excessive innate immune system activation can result. The RPE has developed a robust antioxidant system driven by the transcription factor Nrf2. Impaired Nrf2 signaling can lead to oxidative damage or activate the innate immune response, both of which can lead to RPE apoptosis, a defining change in AMD. Several mouse models simulating environmental stressors or targeting specific antioxidant enzymes such as superoxide dismutase or Nrf2, have simulated some of the features of AMD. While ROS are short-lived, oxidatively damaged molecules termed oxidation specific epitopes (OSEs), can be long-lived and a source of chronic stress that activates the innate immune system through pattern recognition receptors (PRRs). The macula accumulates a number of OSEs including carboxyethylpyrrole, malondialdehyde, 4-hydroxynonenal, and advanced glycation endproducts, as well as their respective neutralizing PRRs. Excessive accumulation of OSEs results in pathologic immune activation. For example, mice immunized with the carboxyethylpyrrole develop cardinal features of AMD. Regulating ROS in the RPE by modulating antioxidant systems or neutralizing OSEs through an appropriate innate immune response are potential modalities to treat or prevent early AMD.
Keywords: Age-related macular degeneration, innate immune response, Nrf2, oxidative stress, oxidation specific epitope, retinal pigment epithelium
1. Introduction
Age-related macular degeneration (AMD) is a disease which generally afflicts people over the age of 60 years old (Gehrs et al., 2006), and is the leading cause of vision loss among the elderly (Congdon et al., 2004). At present, 1.75 million people in the United States have advanced AMD, and due to the aging population of “baby boomers”, 3 million people will be affected by 2020 (Friedman et al., 2004). Currently, 7 million people are at risk of developing advanced AMD in the United States, and 1 in 3 people over 70 years old with early AMD will develop advanced disease over the next 10 years (Gehrs et al., 2006; Mukesh et al., 2004). Needless to say, it is a significant public health problem that now costs $30 billion annually, and the cost is predicted to rise (Brown et al., 2005a). The impact of AMD to the individual is staggering. The decrease in quality of life from early AMD is similar to a person with symptomatic HIV, and with advanced AMD, to one with metastatic prostate cancer having uncontrollable pain (Brown et al., 2005b). Decreased activity (Wysong et al., 2009), depression (Augustin et al., 2007; Brody et al., 2001), and anxiety (Berman and Brodaty, 2006) contribute to this reduction in quality of life. Individuals with early AMD are equally anxious over the fear of losing vision as they are from being blind because there is no therapy for early AMD (Berman and Brodaty, 2006).
While VEGF inhibition provides treatment for the advanced neovascular form of AMD, an exact pathophysiologic understanding of the transition from chronological aging to early AMD continues to elude researchers world-wide. The title of this review was chosen because oxidative stress has long been hypothesized to contribute to AMD (Winkler et al., 1999). This review will focus on early AMD, and it discusses evidence for oxidative stress and damage in AMD, the macula’s protective mechanisms against oxidative stress and damage, and how animal models are helping to unravel the complexity of this enigmatic disease. The intention of this article is to articulate the clinical issues to scientists and to describe the scientific breakthroughs related to oxidative stress to clinicians that will improve the understanding of this disease so that clinicians and scientists will work together to develop effective preventative and treatment for early AMD.
2. Anatomical and Histological Considerations
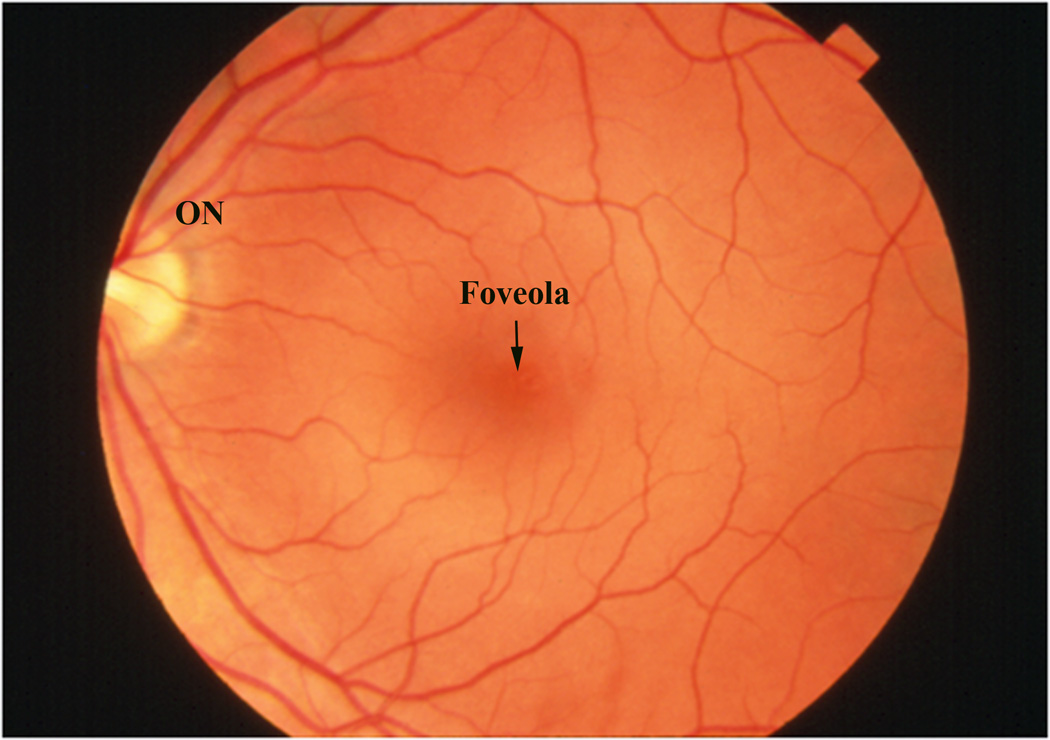
To the clinician, the macula is the central retina that is located between the major retinal vascular arcades, which nourish the inner half of the retina (Figure 1a). This area is approximately 6 mm in diameter, or the same size of a green pea. At the center of the macula is the fovea, and within the fovea is the depression known as the foveola, which enables high resolution vision. The central 250–600µm of the macula is devoid of retinal blood vessels, and derives its nutrition from the underlying choroid.
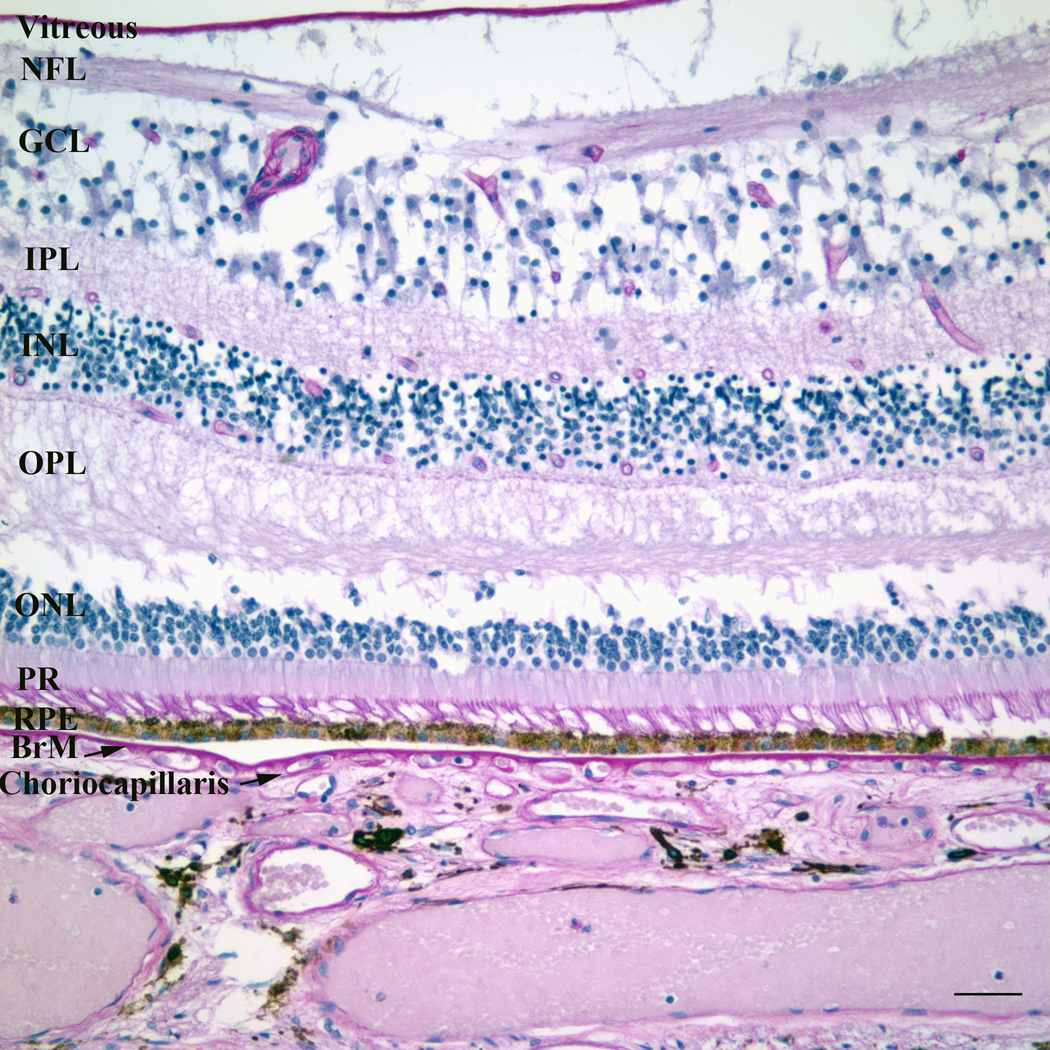
Figure 1.
a) Color fundus photograph of a normal left macula. ON, optic nerve. b) Photomicrograph of a normal macula. Note the multilayer ganglion cell layer (GCL). NFL, nerve fiber layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; PR, photoreceptor layer; RPE, retinal pigment epithelium; BrM, Bruch’s membrane. Bar=50µm.
To the scientist, the macula is defined by the region of the retina which contains more than one layer of ganglion cell nuclei (Figure 1b) (Orth et al., 1977). The neurosensory retina is a transparent membrane composed of multiple layers from the vitreous cavity, including the internal limiting membrane, nerve fiber layer, ganglion cell layer, inner plexiform layer, inner nuclear layer, outer plexiform layer, and photoreceptor layer. While the peripheral retina and much of the macula is dominated by rods in a 20 : 1 and 9 : 1 ratio, respectively, the foveola, is comprised exclusively of cones (Bron et al., 1997; Curcio et al., 1990; Jackson et al., 2002). The high density of cones accounts for the high central visual acuity.
In close proximity and communication with the photoreceptors, is the retinal pigment epithelium (RPE), a highly specialized polarized cell that maintains the health of the photoreceptors. The RPE has multiple essential functions including the daily phagocytosis of photoreceptor outer segments, absorption of light, heat exchange, vitamin A metabolism, outer blood retinal barrier, and maintainence of the choriocapillis (Ershov and Bazan, 2000). The RPE is attached to a specialized extracellular matrix called Bruch’s membrane. It is a pentalaminar structure composed of the RPE basement membrane, inner collagenous layer, middle elastin layer, outer collagenous layer, and the choriocapillaris basement membrane (Hogan, 1972). It comprises part of the outer blood retinal barrier along with the RPE. It is a semi-permeable barrier through which major metabolic exchange takes place. Adjacent to Bruch’s membrane, the choriocapillaris is a capillary plexis that contains fenestrated vascular endothelial cells. It provides the blood supply to the outer retina and RPE. The metabolic demand of the retina and RPE is high. Consistent with meeting the metabolic demands of the RPE, the choroidal circulation has the highest blood flow per unit tissue perfusion in the body, with a 7-fold greater flow in the macula than periphery (Curcio et al., 2009).
3. What is AMD?
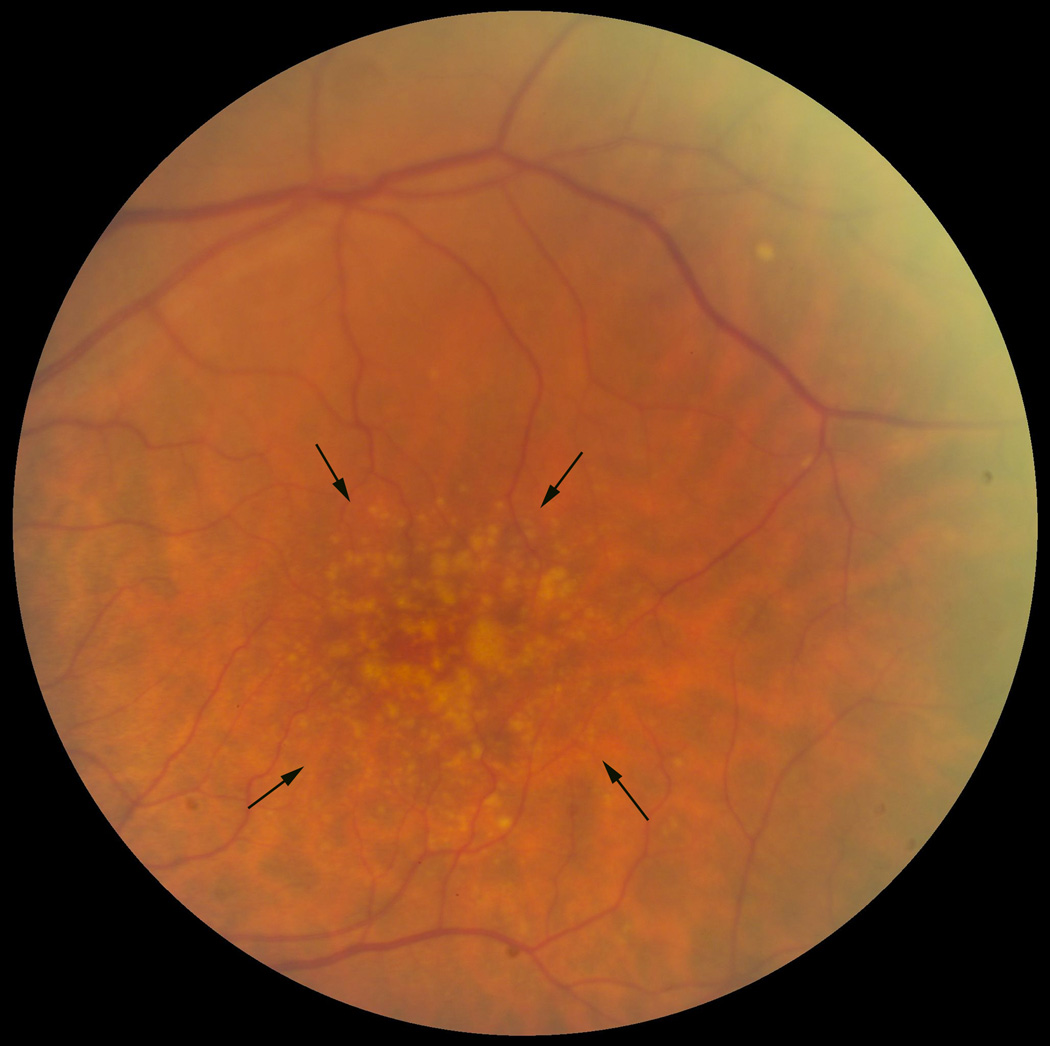
AMD is classified into two forms, a non-neovascular or “dry” form and a neovascular or “wet” form. In the wet form, vision loss can be severe when neovascular tufts develop in the subretinal space, within Bruch’s membrane, or the sub-RPE space. These vessels can hemorrhage or leak fluid, or develop fibrosis. In the dry form, which is the focus of this review, vision loss is typically gradual. To the clinician, the hallmark signs are drusen, or yellow subretinal deposits, and RPE hyper- or hypopigmentary abnormalities (Figure 2). The pigmentary abnormalities are the clinical manifestion of RPE degeneration, which ultimately culminates in apoptosis (Del Priore et al., 2002; Dunaief et al., 2002). Confluent patches of RPE apoptosis results in areas of “geographic atrophy”, a late form of dry AMD. When the geographic atrophy involves the foveola, vision loss can be severe.
Figure 2.
Fundus photograph of a left macula with many large drusen. The area of greatest involvement is outlined by the arrows.
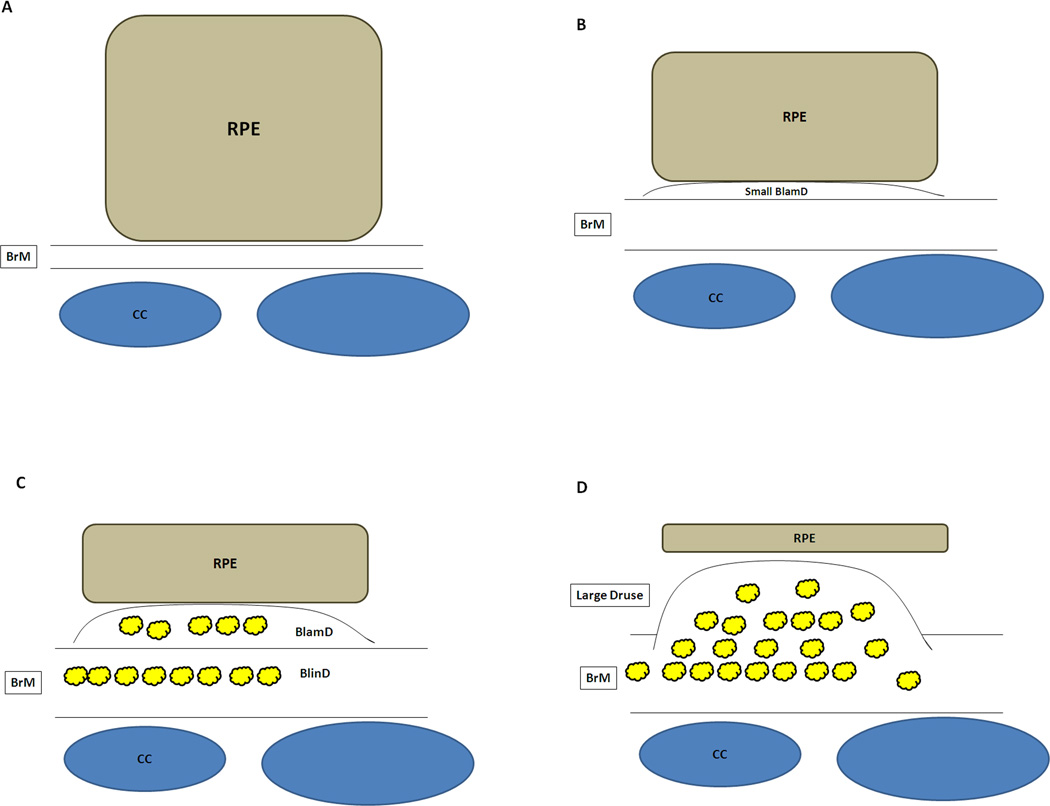
Histopathologically, early, dry AMD is characterized by degeneration of the RPE. The normal cuboidal RPE morphology becomes irregularly shaped, then flattened or atrophic, and finally dies, as delineated in Figure 3 (Green et al., 1985; Sarks, 1976; van der Schaft et al., 1992). Significant changes are also seen in Bruch’s membrane. Sarks categorized AMD severity after thorough clinical and pathological examination on eyes from patients aged 43 to 97 years (Sarks, 1976). The analysis focuses on the accumulation of heterogeneous debris within Bruch’s membrane termed basal deposits. The composition and location define whether the basal deposits are associated with aging or AMD. Basal laminar deposits (BlamD) are accumulations between the RPE cell and its basement membrane while basal linear deposits (BlinD) are heterogeneous deposits within the inner collagenous layer (Figure 3). With aging, Bruch’s membrane thickens, mainly due to accumulations in the outer collagenous layer (van der Schaft et al., 1991). BlamD are an aging change when thin and homogeneous in composition. They look identical to the RPE basement membrane on electron microscopy, and consist in part, of normal RPE basement membrane molecules such as collagen IV, laminin, and heparan sulfate proteoglycans (van der Schaft et al., 1994). In early AMD, BlamDs are thin and continuous, and are associated with RPE pigmentary disturbance. More severe basal deposits accumulate with disease progression. These changes are definitely associated with AMD and include thick BlamD, which contain heterogeneous debris including long spacing collagen and lipids, large drusen (>125microns in diameter), and basal linear deposits (BlinD) (Curcio and Millican, 1999; Spraul et al., 1996). BlinD and large drusen constitute different morphologic forms of the same lesion (Figure 4) (Abdelsalam et al., 1999; Bressler et al., 1994; Curcio and Millican, 1999; Green and Enger, 1993).
Figure 3.
Schematic diagram of degenerative changes to the RPE and Bruch’s membrane during the progression of AMD. A) Normal cuboidal morphology of the RPE. Bruch’s membrane (BrM) is normal thickness. CC, choriocapillaris. B) Flattening of the RPE with early basal laminar deposit (BlamD). C) Further flattening of the RPE with development of more advanced basal laminar deposits and development of basal linear deposits (BlinD). Heterogeneous debris (Yellow deposits) accumulates in both BlamD and BlinD. D) Atrophic RPE overlying large druse.
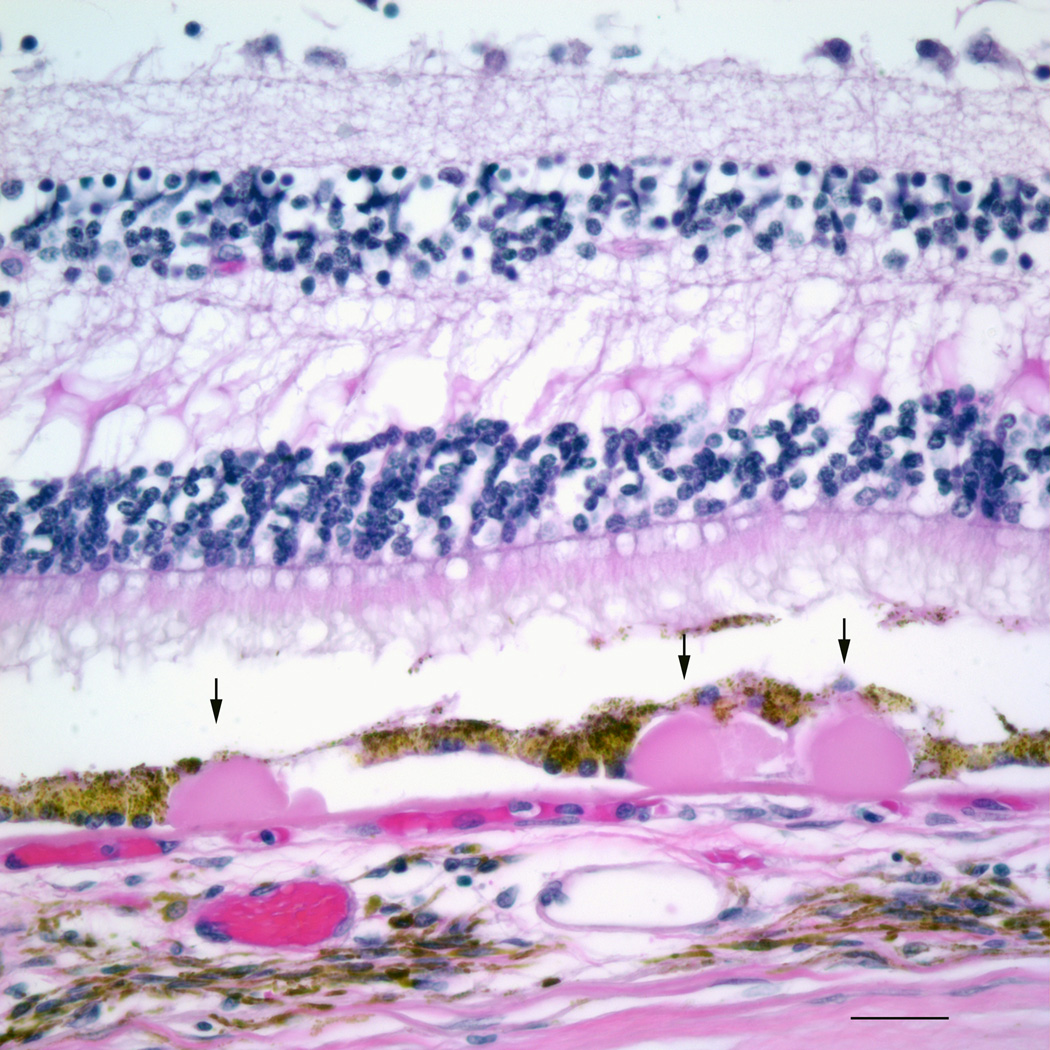
Figure 4.
Photomicrograph of drusen, highlighted by the arrows. Bar=50µm.
4. Epidemiological Evidence for AMD Risk
AMD is a complex trait disease modulated by various non-genetic or “environmental” risk factors (Heiba et al., 1994). Nonmodifiable risk factors include increasing age, gender, and family history (Heiba et al., 1994; Vingerling et al., 1995). To date, chronological age remains the strongest risk factor. A number of other risk factors have also been reported to influence AMD, including smoking, diet, higher body mass index, serum cholesterol, cataract surgery, cardiovascular disease, hypertension, nutrition, and sunlight exposure (Clemons et al., 2005; Heiba et al., 1994; Vingerling et al., 1995). Excluding age, cigarette smoking is the strongest nongenetic risk factor (Smith et al., 2001). Epidemiologic data from several large studies indicate that both dry AMD and disease progression are strongly influenced by smoking (Clemons et al., 2005; Khan et al., 2006; Klein et al., 2008; Smith et al., 2001). A “dose-response” effect has been established since “pack-years” of smoking strongly correlates with risk for AMD while smoking cessation reduces the risk for dry AMD (Khan et al., 2006). The RPE is a specific target of cigarette smoke. The Blue Mountains Eye study (Mitchell et al., 2002) showed that smoking was associated with increased RPE abnormalities and the AREDS cohort(Age-related Eye Disease Study Research Group, 2001) found that smoking was correlated with geographic atrophy, which is characterized by RPE apoptosis.
5. The Genetic Link
In the past decade, paradigm shifting discoveries have been made in identifying genetic variants that are associated with AMD risk (see review by Gorin in this issue). The most significant genes encode proteins within the alternative complement pathway. The initial discovery made by several laboratories, was the association of a single nucleotide polymorphism (SNP) rs1061170 in complement factor H (CFH) with advanced AMD (Edwards et al., 2005; Haines et al., 2005; Klein et al., 2005). Subsequent studies identified genetic variants in other complement components including C2, CFB (Gold et al., 2006; Spencer et al., 2007), C3 (Maller et al., 2007; Yates et al., 2007a), and CFI (Fagerness et al., 2009). Genome-wide linkage studies have discovered a susceptibility locus on chromosome 10q26 (Barral et al., 2006; Fisher et al., 2005). Two SNPs have been identified. One is the rs10490924 variant within gene LOC387715, which is now called ARMS2 (age-related maculopathy susceptibility 2) (Jakobsdottir et al., 2005; Rivera et al., 2005), whose function is unknown. The other is the rs11200638 SNP in the promoter of HTRA1, a serine protease that is associated with wet AMD including polypoidal choroidal vasculopathy (Dewan et al., 2006; Kondo et al., 2007; Yang et al., 2006). Two genome-wide SNP-association studies (GWAS) studies have uncovered several variants of genes involved in lipid metabolism including ABCA1, LIPC, CETP, and LPL (Chen et al., 2010; Neale et al., 2010). The genetic variants discovered to date, are estimated to account for nearly 50% of the genetic variance of AMD.
Variations in genes with function related to oxidative stress have also been associated with AMD susceptibility. These include the mitochondrial DNA polymorphism A4917G (Canter et al., 2008; SanGiovanni et al., 2009) and a polymorphism in mitochondrial superoxide dismutase 2 (SOD2) (Kimura et al., 2000). The susceptibility locus in or near the hypothetical LOC387715 gene (Jakobsdottir et al., 2005; Rivera et al., 2005) is associated with smoking, and the combination of the LOC387715 polymorphism and smoking confers a higher risk for AMD than either factor alone (Schmidt et al., 2006). Kanda et al have indicated that this locus encodes a mitochondrial protein (Kanda et al., 2007), suggesting a role for the oxidative defense response in this disease. However, the cellular location, function, and whether this protein is translated has been the subject of some controversy. Recently, Fritsche et al reported that the LOC387715 polymorphism removes the polyadenylation signal and inserts a sequence that mediates rapid mRNA turnover, which results in undetectable ARMS2 protein (Fritsche et al., 2008). The authors were able to confirm localization of the normal protein in mitochondria of photoreceptors.
6. Current Therapy for Dry AMD
Currently, there is no established prevention or therapy for early AMD. The Age-related Eye Disease Study (AREDS) was a multi-center, randomized control trial that enrolled nearly 5000 patients to evaluate the effectiveness of a combination of anti-oxidant micronutrients including β-carotene, vitamin C, vitamin E, and zinc {Age-related Eye Disease Study Research Group, 2001 #235}. Patients on this supplement over 8 years had a 25% reduction in progression to advanced AMD if they had intermediate AMD. Despite this reduction, 22% of patients on antioxidants still experienced a 15-letter decline in visual acuity. Patients with early or advanced AMD did not benefit from the treatment. In an ancillary study, Moriarty-Craige et al showed that patients on the AREDS formulation had improved plasma redox potential with increased cysteine/cystine despite no change in the glutathione/glutathione disulfide (Moriarty-Craige et al., 2005). The authors concluded that since cysteine is important for regulating apoptosis and immune function, the benefit of antioxidant supplementation on progression to advanced AMD may be partially explained by its effect on cysteine availability. These results suggest a role for antioxidants in the treatment of AMD. The AREDS antioxidant supplements are now the standard of care for treating intermediate AMD in the United States.
The use of antioxidant micronutrients is not without its critics. A meta-analysis of clinical trials from three large high quality studies of over 23,000 patients randomized to antioxidant supplementation or placebo and followed-up for AMD concluded no benefit for preventing AMD (Evans, 2008). Due to the strength of the AREDS study compared to smaller trials, the study concluded that some benefit may be derived for slowing progression, but caution was also suggested due to potential harmful effects from micronutrients. Several of the key components within the AREDS formulation have been the topic of potential unanticipated health risk. Two large randomized controlled trials have indicated that smokers who take β-carotene may be at increased risk of developing lung cancer (Beta Carotene Cancer Prevention Study Group, 1994; 1994; Omenn et al., 1996). Vitamin E has also been associated with adverse health consequences that have been uncovered in studies larger in size than the AREDS. The Heart Outcomes Prevention Evaluation (HOPE) Study, comprised of 9541 patients at least 55 years old with vascular disease or diabetes mellitus, found that vitamin E supplementation was associated with a higher risk of heart failure among people with vascular disease or diabetes (Lonn et al., 2005). Recently, the Selenium and Vitamin E Cancer Prevention Trial (SELECT) study evaluated whether selenium and vitamin E micronutrients reduced the risk of prostate cancer. While the original report found a statistically nonsignificant increase in prostate cancer with vitamin E supplementation, (Lippman et al., 2009) the group recently found with continued surveillance of 35,533 men, that the risk of prostate cancer after 7 years of follow-up was increased by 17% in men randomized to Vitamin E supplementation alone (Klein et al., 2011). No reduction or increased risk of prostate cancer was identified with the combination of vitamin E and selenium or with selenium alone. The authors suggest that caution should be used when recommending or studying high doses of micronutrients.
Neuronal cell death has been associated with elevated zinc, an effect which is magnified by oxidative stress. Recently, Sheline et al showed that intense light induced zinc accumulation in the neurosensory retina and RPE while pyruvate or nicotinamide attenuated photoreceptor cell death, presumably by restoring NAD+ levels (Sheline et al., 2010). High zinc levels have been found in proteins recovered from drusen (Lengyel et al., 2007) and in amyloid plaques of Alzheimer’s patients (Bucciarelli et al., 2002; Lee et al., 1999). Interestingly, zinc chelation has been proposed as a therapy for Alzheimer’s disease, and the DP-b99 chelator from DPharm is currently in Phase II/III clinical trial (Lee et al., 2004). This is in contrast to the zinc supplement that is part of the AREDS formulation, which was chosen for its potential antioxidant properties. Given the presence of zinc in drusen, and its role in retinal neurotoxicity, some researchers have questioned the use of zinc supplementation for AMD treatment. In contrast to pharmaceuticals, natural dietary constituents display a U-shaped-dose response curve where either deficient or supraphysiological doses may be harmful. While it is difficult to extrapolate the results of these other studies to the AREDS or the ongoing AREDSII it does raise caution and suggests that a better understanding of oxidative stress in disease is necessary. While it was considered the best formulation among experts at the time, the micronutrient composition was empiric and was not derived from pre-clinical testing. We will next critically review the pre-clinical evidence for the role of oxidative stress in AMD.
7. Reactive Oxygen Species in the Macula
The generation of reactive oxygen species (ROS) has long been considered to have harmful consequences, and has been thought to be a major factor in aging and disease. However, it is now clear that that ROS participate in a number of physiological processes that are essential to the normal functioning of cells. Redox signaling can act as an important regulator in both physiological and pathological processes. Physiological ROS appear to be generated from several sources as reviewed in (Finkel, 2011). These include NADPH-dependent oxidases, mitochondrial complex I or III cytochrome chains, and other cellular enzymes including xanthine oxidase, cyclooxygenases, lipoxygenases, and cytochrome p450 enzymes that produce ROS as part of their enzymatic function. Work on epidermal growth factor for example, established an essential role for ROS during intracellular signal transduction (Boren et al., 1998). A wide range of specific cellular responses relevant to AMD are signaled through ROS, such as feedback regulation after excess metabolism (Brunelle et al., 2005; Guzy et al., 2005), hypoxia via hypoxia inducible factor 1 (Guzy et al., 2005), regulation of autophagy through ATF4 (Scherz-Shouval et al., 2007), and regulation of the inflammatory response (Bulua et al., 2011; Nakahira et al., 2011; Zhou et al., 2011).
The macula has a high metabolic demand. This not only results in high levels of physiologic ROS for signal transduction, but also substantial ROS produced during cellular metabolism. The RPE is responsible for the routine phagocytosis of photoreceptor outer segments. In fact, the RPE phagocytizes nearly 30,000 outer segments each day (Ershov and Bazan, 2000). During outer segment phagocytosis, intracellular H2O2 resulting from NADPH oxidase in the phagosome or from β-oxidation of ROS lipids in peroxisomes is generated to the same magnitude that occurs with other phagocytic cells such as macrophages (Miceli et al., 1994; Tate et al., 1995). The RPE cell protects itself from excessive oxidative stress in part, by upregulating catalase during this process (Tate et al., 1995).
8. Unique Sources of Exogenous Oxidative Stress
The macula receives some of the highest blood flows in the body. As a result, the RPE is exposed to high ambient oxygen partial pressures of 70–90 mm Hg (Winkler et al., 1999), with the potential for exposure to abundant exogenous ROS. The macula obviously processes light for vision. As a result, photo-oxidative stress is a unique, additional source of exogenous oxidative stress. Since the work of Ham et al in 1978 (Ham et al., 1978), photo-oxidative stress has been linked with oxidative damage to the retina, RPE, and choroid, and multiple other works have substantiated this observation as reviewed in (Beatty et al., 2000)(see reviews by Sparrow, Jarett, Bhutto,, Mettu in this issue). These data correlate with the epidemiologic link of sunlight exposure and AMD risk, as delineated in the studies conducted on watermen in the Chesapeake Bay (Taylor et al., 1990; Taylor et al., 1992).
Cigarette smoke generates over 4700 chemical components, some of which are strong oxidants (Rangasamy et al., 2004; Smith and Hansch, 2000). In fact, each puff of cigarette smoke contains 1015 free radicals (Rahman and MacNee, 1996). While the exact role of oxidative damage caused by cigarette smoking in AMD is unknown, it is clear that chemical oxidants in cigarette smoke deplete tissues of ascorbic acid and protein sulfhydryl groups, causing the oxidation of DNA, lipids and proteins (Cross et al., 1993; Lykkesfeldt et al., 2000; O'Neill et al., 1994). Many of these molecular changes such as malondialdehyde, 4-hydroxynonenal, and advanced glycation endproducts (AGE), have been identified in AMD, and indicate that oxidative damage is an important factor in the mechanism of disease development.
9. Critical Evaluation of Animal Models of Oxidative Stress
Mice have become a popular animal to simulate AMD because of the mechanistic insights that result from genetically modifying a gene of interest, and because the RPE-Bruch’s membrane-choroid anatomy is very similar to humans (see review by Pennesi in this issue). When interpreting the results that have been advanced from these models, it is important to keep in mind that mice do not have maculas nor do they have a cone rich region like that of the human macula. In addition, mice have distinct differences in the regulators of complement from man that could influence the phenotype that is generated (Kim et al., 1995; Tsujimura et al., 1998; Yang et al., 2009). As a result, to date, no mouse model has completely mimicked AMD.
Importantly, some cardinal phenotypic features of AMD do manifest in mouse models of oxidative stress. Because of the epidemiological link of AMD to smoking, we exposed mice to either filtered air or cigarette smoke in a smoking chamber for 5 h/day, 5 days/week for 6 months to determine whether features of AMD could develop (Fujihara et al., 2008). Compared to mice raised in air, we found that mice exposed to smoke had increased oxidatively damaged RPE nuclei using 8-oxo-7,8-dihydro-29-deoxyguanosine (8-OHdG) immunolabeling, and profound ultrastructural injury to the RPE that included loss of RPE basal infoldings and an increase in intracellular membranous vacuoles, two signs that are seen in RPE cells overlying drusen in AMD (Anderson et al., 2002). These ultrastructural changes also correlated with increased RPE apoptosis. In a companion study, we found retinas from mice exposed to cigarette smoke contained markers for mitochondrial DNA damage, exosomes, and complement pathway components including increased C3, C5, MAC, and decreased CFH surrounding Bruch’s membrane (Wang et al., 2009). While our study found some evidence of Bruch’s membrane abnormalities, Espinosa-Heidmann et al, by exposing old mice to a high fat diet and slightly different smoking conditions fromk our study, found prominent sub-RPE deposits, thickening of Bruch's membrane, and accumulation of deposits within Bruch's membrane in mice (Espinosa-Heidmann et al., 2006). These preliminary results suggest that exposing mice to smoke uncovers some of the phenotypic changes seen in early AMD, and will provide a model for both understanding important events related to cigarette smoke mediated oxidative stress and to test new therapeutic targets.
9.1 Mouse Models of Iron Overload
Iron generates highly reactive hydroxyl radicals via the Fenton reaction to induce oxidative stress. Iron overload has been identified in a number of diseases including Alzheimer’s disease, Parkinson’s disease, and other neurodegenerative disorders (Hahn et al., 2004). Several laboratories have characterized the iron transport mechanisms in the retina and RPE(Doly et al., 1986; Hunt and Davis, 1992), and how disruption of iron regulation contributes to retinal dysfunction(Chowers et al., 2006; Yefimova et al., 2002). AMD is now included on the list of diseases with iron overload. AMD-affected maculas have increased iron in the RPE and Bruch’s membrane including within drusen (Hahn et al., 2003). Iron and the iron handling proteins ferritin and ferroportin, are found in photoreceptors within geographic atrophy. These observations in human globes have been substantiated in animal models. After photic injury, ceruloplasmin (Cp), a retinal ferroxidase, is upregulated in Muller cells and secreted into the vitreous, a response which may protect the retina from oxidative damage by decreasing the amount of ferrous iron (Chen et al., 2003). The homolog of Cp, hephaestin (Heph) is also important for retinal iron homeostasis. Mice deficient in both Cp and Heph have a striking, age-dependent increase in RPE and retinal iron (Hahn et al., 2004). Coincident with the increased iron are RPE apoptosis, photoreceptor degeneration, basal deposits, and choroidal neovascularization. These changes are consistent with iron mediated oxidative damage and regulation by Cp and Heph in the development of AMD. Chelation therapy has benefited patients with transfusional iron overload in thalassaemia and other conditions as reviewed in (Kontoghiorghes et al., 2010). Iron chelation therapy has also been tested in non-iron loaded diseases such as rheumatoid arthritis, diabetic nephropathy, and several neurodegenerative conditions without serious reported toxicity (Kontoghiorghes et al., 2010). However, these studies are small, and for the most part short-term trials, so a complete understanding of their potential remains unresolved. It does however, offer the possibility for treating iron overload in AMD.
9.2 SOD1 Deficient Mice
Superoxide dismutase (SOD) is a major free radical scavenger that exists in three isoforms. SOD1 is the major cytosolic enzyme, and is the most abundant of the three in the retina. Imamura et al studied a mouse deficient in SOD1 and found high levels of oxidative stress caused the development of drusen, Bruch’s membrane thickening, and choroidal neovascularization (Imamura et al., 2006). Interestingly, 86% of SOD1 deficient mice older than 10 months developed drusen. The number of drusen increased with age or exposure to light. The drusen were found to contain many of the marker proteins that have been identified in human drusen including vitronectin, complement regulator CD46, and complement C5. These changes support a role for oxidative stress in AMD. However, other laboratories have not reported these observations in this mouse model.
9.3 Mouse Models of Mitochondrial Dysfunction
Mitochondrial dysfunction has been implicated as a source of oxidative stress and subsequent cellular injury. Mitochondrial DNA is very susceptible to oxidative damage (Liang and Godley, 2003) and mitochondrial DNA recovered from macular RPE of AMD patients has more damage than from age-matched controls (Karunadharma et al., 2010). SOD2 is the isoform that is located in the mitochondria. Since global SOD2 deficient mice die soon after birth, Justilien et al used a ribozyme that targets SOD2 packaged in an adeno-associated virus for subretinal injection to knockdown SOD2 in the RPE (Justilien et al., 2007). Local SOD2 knockdown induced oxidative damage to proteins, vacuolization and degeneration of the RPE, thickened Bruch’s membrane, and shortened, disorganized photoreceptor outer and inner segments. As with AMD patients, SOD2 knockdown eyes had increased autofluorescence and elevated levels of lipofuscin pigments. The photoreceptor outer nuclear layer became progressively thinned from apoptotic cell death that was coincident with loss of electroretinographic signaling. Because RPE degeneration preceded photoreceptor damage and loss of retinal function, the authors concluded that RPE is the primary cell type initiating disease.
Recently, Zhao et al showed that RPE specific ablation of mitochondrial oxidative phosphorylation triggered gradual epithelial dedifferentiation using BEST1-cre recombinase mice that had been crossed with mice containing floxed Tfam, a mitochondrial transcription factor responsible for mitochondrial transcription and replication (Zhao et al., 2011). Abnormal RPE cell behavior was associated with increased glycolysis and activation of the hepatocyte growth factor/met proto-oncogene pathway. RPE dedifferentiation and hypertrophy arose through stimulation of the AKT/mammalian target of rapamycin (AKT/mTOR) pathway. The electrical response of the retina to light decreased and photoreceptors eventually degenerated. This stress response appeared to be a general reaction since administration of the oxidant NaIO3 to wild-type mice also triggered RPE dedifferentiation and mTOR activation. These events appear to have similarity to AMD. Importantly, the mTOR inhibitor rapamycin minimized the RPE dedifferentiation and preserved photoreceptor function. Rapamycin is approved for renal transplantation and treatment of renal cell carcinoma. It is currently being evaluated in a clinical trial for treating advanced non-neovascular AMD (Zhao et al., 2011). While the original intent of this study was due to its inherent anti-inflammatory properties, this recent work suggests that an additional beneficial mechanism is mTOR activation and prevention of RPE cell dedifferentiation.
9.4 Mouse Model of Glycemia
People who consume a high glycemic index diet are at enhanced risk for AMD and progression of AMD as reviewed in (Chiu et al., 2011). These observations have prompted mechanistic studies into how glycemia can influence AMD development. Uchiki et al and Weikel et al found an AMD phenotype in mice that consumed a high glycemic index diet (Uchiki et al., 2011). Mice fed a high glycemic index diet show at younger ages, ultrastructural evidence of an AMD including RPE with cytoplasmic vacuoles as well as greater extent and more frequent loss of basal infoldings, the accumulation of basal deposits within Bruch’s membrane, and sporadic damage to photoreceptors. These changes are similar to those seen in a different models of AGE formation that is described in section 12.1. A high glycemic index diet was associated with augmented formation of AGEs and protein carbonyls, a marker of oxidative stress. Of interest, one of the metabolic intermediates identified was methylglyoxal (Uchiki et al., 2011). Methylglyoxal is derived from breakdown of sugars and lipids and it reacts rapidly to form AGEs and oxidized lipid products. Its identification suggests that the high glycemic index diet results in the reaction of sugars, lipids, and oxygen to produce AGEs and oxidatively damaged molecules. Importantly, the glycemia induced compromises to the ubiquitin and lysosomal/autophagic proteolytic machinery that is causally related to the precautious onset of early AMD related phenotypes. Furthermore, the glycative stress appears to induce a vicious cycle of compromised intracellular protein editing, enhanced accumulation of AGEs and tissue dysfunction (Uchiki et al., 2011)(see review by Shang in this issue). These epidemiologic and translational laboratory data help to justify an interventional clinical trial that tests the benefit of GI diet in retinal health.
10. Nrf2 and the Antioxidant System
The models that induce an AMD phenotype through oxidative stress support a mechanistic role for oxidative stress in this disease. The cell in general, has developed multiple strategies to protect itself from the potentially damaging effects of ROS, whileallowing for physiological ROS mediated signaling. These strategies include restricted localization of oxidant producing enzymes to the intended target or the regulated entry of oxidants, such as H2O2 through aquaporin channels (Bienert et al., 2007). Due to the short half-life of free radicals, the transient production of the ROS, such as during Nox-dependent oxidant burst, is another mechanism to limit ROS to a specific locale.
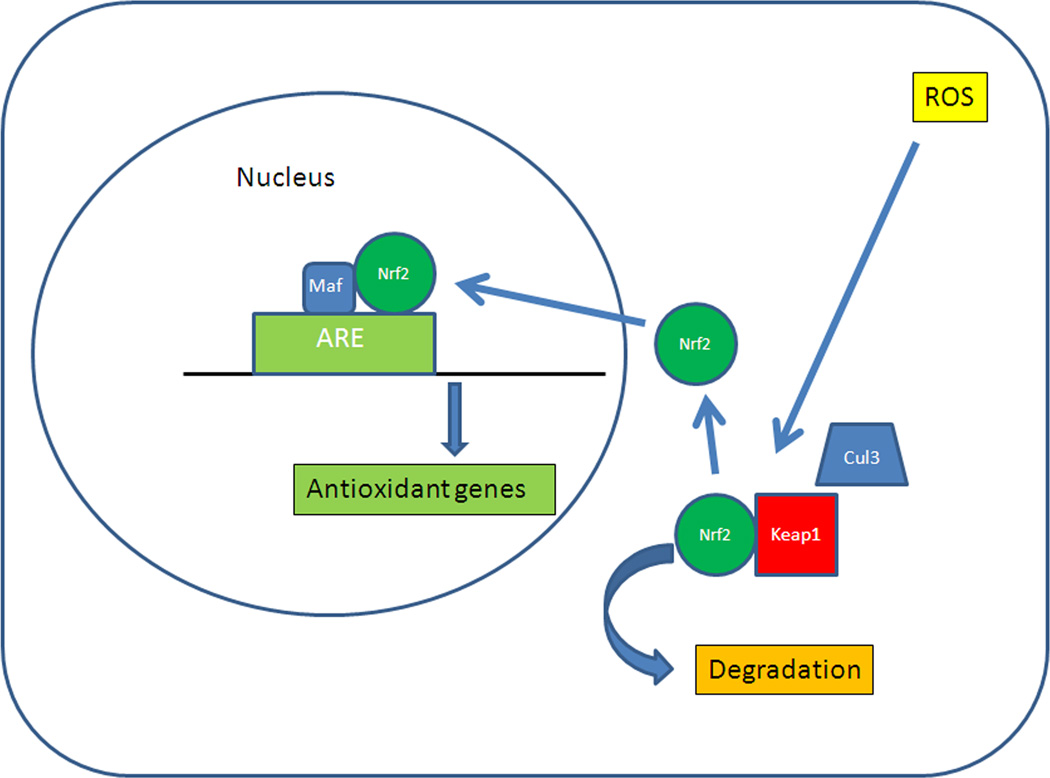
Protection against oxidative damage is mediated through a complex of antioxidant molecules. A number of antioxidant systems are regulated through various transcription factors including Nf-κB, AP1, the FoxO family, or PGC-1α. However, the most powerful transcription system is mediated through Nuclear factor erythroid-2 related factor 2 (Nrf2), a basic leucine zipper transcription factor (Biswas and Rahman, 2008). Nrf2 regulates a coordinated transcriptional program that maintains cellular redox homeostasis and protects the cell from oxidative injury (Nguyen et al., 2003; Rangasamy et al., 2004; Thimmulappa et al., 2002). Nrf2 is normally sequestered in the cytosol by interacting with Kelch-like ECH-associated protein 1 (Keap1). Keap1 also functions as a substrate adaptor protein for a Cul3-dependent E3 ubiquitin ligase complex which also helps to maintain steady-state levels of Nrf2 via proteolysis of Nrf2 by the ubiquitin-proteasome pathway (Yu and Kensler, 2005) (Figure 5) (Adams et al., 2000; Kobayashi et al., 2004; Zhang et al., 2004). Thus in the absence of stress, Keap1 constitutively suppresses Nrf2 signaling by both degrading Nrf2 and preventing Nrf2 translocation to the nucleus, which results in a low baseline expression of antioxidant genes (Thimmulappa et al., 2002). However, upon exposure to ROS, Keap1 undergoes a conformational change when its multiple cysteine residues interact with ROS, which releases Nrf2, and inhibits Keap1-mediated proteasomal degradation of Nrf2. The released Nrf2 then translocates to the nucleus, where it dimerizes with Maf proteins, and binds to the antioxidant response element (ARE) in the promoters of its target genes to initiate the transcription (Dinkova-Kostova et al., 2005; Kobayashi and Yamamoto, 2005; Wakabayashi et al., 2003). The Nrf2 signaling response regulates both an early acute phase through actions of the “direct” enzymes, such as catalase or SOD, which neutralize H2O2 and superoxide, respectively, and a chronic phase through maintenance of cellular glutathione and thioredoxin, and xenobiotic metabolism enzymes that produce reducing equivalents, such as NADPH quinine oxidoreductase (NQO-1) (Osburn et al., 2008). When ROS depletes cellular glutathione, cells can die from oxidatively mediated apoptosis (Rahman et al., 2005; Walsh et al., 1995; Will et al., 1999). Importantly, Nrf2 signaling plays an essential antioxidant role in the RPE (Gao and Talalay, 2004; Nelson et al., 2002; Nelson et al., 1999; Tanito et al., 2005).
Figure 5.
Schematic diagram of Nrf2 signaling. ARE, antioxidant response element; Cul3, Cullin3-dependent E3 ubiquitin ligase complex; Keap1, Kelch-like ECH-associated protein 1; Nrf2, Nuclear factor erythroid-2 related factor 2; Maf, musculoaponeurotic fibrosarcoma protein; ROS, reactive oxygen species.
10.1 Failure of Nrf2 Signaling in Aging and Disease
Aging can reduce Nrf2 mRNA or protein, which results in impaired Nrf2 signaling. Suzuki et al showed an age-dependent response in Nrf2 signaling to cigarette smoking (Suzuki et al., 2008). While the Nrf2 response from young patients was independent of smoking status, Nrf2 mRNA was down-regulated in macrophages of old smokers compared with old nonsmokers. Importantly, oxidized glutathione and carbonylated albumin levels in bronchoalveolar fluid were inversely correlated with Nrf2 mRNA levels. These results have been corroborated in mice, when aging suppressed the ability of Nrf2 and its target genes in alveolar macrophages, to respond to the stress of cigarette smoking (Suzuki et al., 2008).
Nrf2 also declines with disease. Nrf2 mRNA and protein, and Nrf2-dependent antioxidants and glutathione levels are reduced, and oxidative stress is increased in human emphysematous compared to normal lungs (Malhotra et al., 2008; Suzuki et al., 2008). Mice deficient in Nrf2 develop emphysema through enhanced oxidative damage from cigarette smoke (Rangasamy et al., 2004). The impaired antioxidant response can be reversed by either genetically knocking down Keap1 or increasing Nrf2 (Suzuki et al., 2008). It is clear that in AMD, the delicate balance between physiological oxidative stress and the onset of pathological oxidative stress is upset, resulting in tissue damage. Oxidatively damaged molecules are seen in all layers of the macula in AMD, as will be described below (Section 12.1). The ability to defend against oxidative stress by upregulating the antioxidant defense response is likely to be a pivotal event that mediates the initiation and progression of AMD.
10.2 A Model of AMD from Nrf2 Deficiency
Recently, Zhao et al identified many features of AMD in mice deficient of Nrf2 (Zhao et al., 2011). In these mice, exons 4 and 5 of the mouse Nrf2 gene were replaced by a LacZ reporter gene (Auerbach et al., 1996). Nrf2 deficient mice showed no distinguishing retinal features until 11 months of age when mice developed clinically and histopathologically confirmed drusen. Other hallmark features of an AMD phenotype such as autofluorescence and lipofuscin-like particles in the RPE were seen. In the RPE and Bruch’s membrane, an age-dependent increase in immunoreactivity of C3d, a marker of complement activation, serum amyloid P, vitronectin, as well as 3-nitrotyrosine, a marker of oxidatively damaged proteins, were observed. In Nrf2 deficient mice, the RPE contained ultrastructural evidence of undigested photoreceptor outer segments, autophagosomes, autolysosomes, and immunohistochemical evidence of polyubiquinated protein aggregate accumulation. Because Nrf2 signaling is an inducible system, these results suggest that while these mice were not given an oxidative stimulus, the ambient conditions may have been sufficiently pro-oxidative so that these mice were unable to sufficiently neutralize oxidative stress or degrade oxidatively damaged proteins, consistent with the hypothesis of Uchiki et al 2011 (Uchiki et al., 2011).
10.3 Nrf2 as a Therapeutic Target
The Nrf2 transcriptional and nuclear translocational response with aging can be restored in the liver by (R)-α-lipoic acid (Suh et al., 2004). Likewise, as described above, the genetic enhancement of Nrf2 improves the antioxidant response to prevent emphysema (Suzuki et al., 2008). These observations suggest that Nrf2 is a target for treating age-related oxidative stress diseases. An interesting group of candidate drugs are the triterpenoids because they are substantially more potent than other Nrf2 activators such as sulforaphane or (R)-α-lipoic acid (Yates et al., 2007b). Triterpenoids are steroid-like molecules that occur widely in hundreds of plant species, and have been widely used in Asian medicine. They have been systematically modified to enhance their cytoprotective ability. One triterpinoid, oleanolic acid, with chemical modification, can have its intrinsic anti-inflammatory activity enhanced many thousand-fold (Liby et al., 2007). The mechanism of action by the triterpenoids is mediated by their ability to form Michael adducts with cysteine residues on Keap1. Under basal conditions, Keap 1 forms a tight complex with Nrf2, but upon reaction with Michael reagents such as triterpenoids, Keap1 dissociates and allows Nrf2 translocation to the nucleus, where it activates antioxidant and cytoprotective genes. Three of the most effective derivatives synthesized from oleanolic acid are 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO), and its methyl ester and imidazolide analogs (CDDO-Me and CDDO-Im) (Liby et al., 2007). Sussan et al showed that CDDO-Im in the diet prevented emphysema in mice exposed to cigarette smoke (Sussan et al., 2008). Recently, patients with type II diabetes and chronic kidney disease were treated in a Phase II trial with CDDO-Me, bardoxolone methyl, because of the role of oxidative stress and inflammation in diabetic nephropathy (Pergola et al., 2011). This study showed improved estimated glomerular filtration rate after 24 and 52 weeks of treatment with minimal side effects. Based on the results of this study, bardoxolone methyl is being evaluated more rigorously in a phase III clinical trial.
11. Activation of the Innate Immune Response by Oxidative Stress
Oxidative stress magnifies the innate immune response, and can convert it from a protective to a pathologic response. Nrf2 signaling can protect against a dysregulated innate immune response including complement. The most striking example is septic shock. Members of our research group showed that Nrf2 is a critical host factor that determined survival from septic shock by regulating an appropriate innate immune response (Thimmulappa et al., 2006). Disrupting Nrf2 signaling markedly increased the death rate from septic shock by augmenting inflammation. We observed a similar improvement in a model of uveitis (Nagai et al., 2009). Intraperitoneally injected LPS increased ROS in the retinas of both wild-type and Nrf2 deficient mice. After LPS injection, multiple cytokines were increased more in the retinas of Nrf2 deficient mice than in those of Nrf2 competent wild-type mice. NQO-1 and glutamate-cysteine ligase, modifier subunit (GCLM), two Nrf2-responsive antioxidant enzymes, had reduced expression in Nrf2 competent mouse retinas after LPS injection, and as expected, no change in expression in Nrf2 deficient mice.
Leukocyte adhesion to the vascular endothelium is a key event that precedes leukocyte invasion into tissue (Becker et al., 2001). The number of leukocytes adherent to the retinal vascular endothelium increased after LPS treatment in both Nrf2 competent and deficient mice compared to control injections, with more adherent leukocytes in Nrf2 deficient than in Nrf2 competent mice. Pretreatment with CDDO-Im increased antioxidant gene expression in the retina, reduced inflammatory mediator expression, and reduced leukocyte adherence to retinal vasculature after LPS treatment in Nrf2 competent mice, but had no effect on Nrf2 deficient mice.
While the changes seen in the uveitis model are acute, deficient Nrf2 signaling predisposes to the development of chronic inflammation, an apparent susceptibility factor for AMD. For example, while the expression of proinflammatory cytokines is reduced at an early stage of repair, deficient Nrf2 signaling at a later stage also prolongs the inflammatory response (Braun et al., 2002). Nrf2 signaling regulates complement activation. In elderly mice, Nrf2 deficiency increases oxidative damage and complement C3 deposition in several organs including the brain (Li et al., 2004). On the other hand, Nrf2 activation prevents inflammation mediated oxidative stress. In a model of liver necrosis, Nrf2 activation by synthetic triterpenoids prevented late phase pro-inflammatory gene expression, and halted the inflammatory amplification loop (Osburn et al., 2008). Besides its direct antioxidant effects, targeting the Nrf2 pathway could minimize the accompanying pro-inflammatory response during chronic conditions like AMD.
12. Oxidation Specific Epitopes: A Consequence of Oxidative Stress
A consequence of inadequately neutralized oxidative stress is the generation of highly reactive oxidized lipids by degradative nonenzymatic reactions, such as the peroxidation of polyunsaturated fatty acids. Lipid peroxidation can lead to the formation of lipid hydroperoxides, which in the presence of transition metals such as iron, can give rise to short chain, unesterified aldehydes and a second class of aldehydes that are still esterified to the parent lipid (Esterbauer et al., 1991). Some of these lipid peroxidation products can modify proteins, lipids, and DNA on cellular and extracellular components (Chou et al., 2008; Otaki et al., 2010). These modifications in essence, result in altered self-structures that can activate the immune response. To emphasize this immunogenic property, these modifications have been designated “oxidation specific epitopes” (OSEs) (Chou et al., 2008). Importantly, while ROS are short-lived, OSEs are long-lived and can serve as a chronic inducer of oxidative stress and the innate immune response if they are not neutralized or removed. Thus, OSEs are endogenous danger signals, or a subgroup of danger-associated molecular patterns (DAMPS) or pathogen-associated molecular patterns (PAMPS). In addition, apoptotic cells have been shown to contain multiple OSEs in their membranes, making them perfect tags for the identifying biological waste by discriminating dying from viable cells. As with any immune response, there is a delicate balance between a protective and pathologic response. Inadequately neutralized oxidative stress could result in OSE formation beyond what the innate immune response can neutralize. In fact, OSEs have been identified in a wide variety of diseases, ranging from muscular dystrophy, rheumatoid arthritis, lupus, diabetes, Alzheimer’s disease to atherosclerosis where oxidative stress and inflammation are important etiologic factors (Chou et al., 2008).
We have become particularly interested in OSEs because they can activate the innate immune response through interaction with pattern recognition receptors (PRRs), which recognize highly conserved motifs on a variety of endogenous and exogenous pathogens. PRRs are germ line encoded and are the consequence of the natural selection of receptors. PRRs are focused on highly conserved motifs present not only on pathogens, but also on self-epitopes such as OSEs. They provide a vital role as a first line of defense against an invading pathogen. PRRs can be cell membrane bound or soluble. After binding to PRRs on monocytes/macrophages, dendritic cells, and natural killer cells, OSEs can be endocytosed. Through PRR-mediated signaling cascades, the OSEs elicit secretion of cytokines and chemokines that provide both targeted and generalized responses that coordinate the defense response. By engulfing OSEs, these phagocytic cells also help to remove potentially dangerous or disease causing epitopes. When bound by PRRs on macrophages, dendritic cells and other antigen-presenting cells, these antigen-presenting cells can interact with T cells, and initiate the conversion from innate and adaptive immunity. Fluid-phase PRRs can bind to OSEs and promote their neutralization or removal.
12.1 OSEs and PRRs in the Macula
A unique and perhaps signature OSE in the macula is carboxyethylpyrrole (CEP). In the retina, docosohexanoic acid (DHA) is the most abundant fatty acid in photoreceptor tips, and is also the most oxidizable fatty acid in the body because of its unsaturated structure. Due to the high oxidative stress environment, DHA is uniquely oxidatively modified to CEP (Gu et al., 2003). Matching the distribution of DHA, CEP adducts immunolocalize to photoreceptor outer segments and the RPE, and have been found in higher abundance in the outer retina of AMD patients compared to age-matched controls. In addition, CEP is found in drusen of AMD patients (Crabb et al., 2002). This distribution suggests that this OSE is formed in photoreceptor outer segments, and is phagocytozed by the RPE as the routine regeneration of outer segment processing. The RPE appears to release CEP adducts into Bruch’s membrane, presumably to clear it from the macula into the choriocapillaris for removal into the circulation. Indeed, it is likely that CEP adducts are released into the circulation and generate an immune reaction because CEP autoantibodies are found in higher concentration in the plasma of AMD patients than age-matched controls.
This profile has been simulated in a mouse model. Hollyfield et al immunized mice with CEP adducts and found elevated anti-CEP antibodies compared to controls (Hollyfield et al., 2008). In the eye, the RPE was degenerated with patches of geographic atrophy. Photoreceptors overlying atrophic RPE were edematous, while Bruch’s membrane developed prominent basal laminar deposits. These ultrastructural changes were associated with complement deposition of C3d, a marker for C3 activation, in Bruch’s membrane. In this model, the anti-CEP antibodies are both IgMs and IgGs, which suggests that both the innate (i.e. IgM) and adaptive (i.e. IgG) immune response are involved.
Our laboratory has been interested in the role that advanced glycation endproducts (AGEs), which are a heterogeneous group of OSEs produced by a series of nonenzymatic reactions between sugars, lipids and proteins. AGEs increase with aging in the macula, as well as both early and advanced AMD (Farboud et al., 1999; Handa et al., 1999). In particular, AGEs localize within basal deposits and drusen (Farboud et al., 1999; Handa et al., 1999). AGEs will bind to various pattern recognition receptors including scavenger receptors, the AGE receptor complex, and the receptor for AGEs (RAGE), which have been identified on RPE cells (Howes et al., 2004). For example, RAGE immunolabeling is increased on RPE cells overlying drusen and basal deposits compared to morphologically normal RPE and Bruch’s membrane in AMD (Yamada et al., 2006). We established a model of early AMD by giving mice low dose D-galactose, which induces AGE formation. These mice develop ultrastructural features of AMD including RPE degeneration, Bruch’s membrane thickening with basal deposit formation, and choriocapillaris endothelial injury (Ida et al., 2004). These ultrastructural changes are associated temporally with an overall transcriptome that is similar to the generalized aging response of a number of unrelated cell types (Tian et al., 2005). This includes altered expression of genes related early to inflammation, matrix expansion, and aberrant lipid processing and, later, to down-regulation of energy metabolism genes, up-regulation of crystallin genes, and altered expression of cell structure genes. Consistent with these data, systemic increases in AGEs and advanced AMD-related lesions is also observed in C57BL6 mice that consumed higher glycemic index diets (Uchiki et al., 2011)
Besides influencing the transcriptional response of the RPE, AGE accumulation in Bruch’s membrane can potentially increase the accumulation of OSEs. Recently, we showed that AGE formation in Bruch’s membrane resulted in the retention of LDLs in Bruch’s membrane (Cano et al., 2011). Using D-galactose treatment in lipoprotein lipase (LPL) deficient mice, we then found that this retention was minimized, which suggests that AGEs upregulate LPL and promote the retention of LDLs in Bruch’s membrane. The retention of LDLs would promote their oxidation, and indeed, oxidized LDLs have been identified in Bruch’s membrane including drusen and basal deposits in AMD (Yamada et al., 2008). These changes also have similarity to the response to retention hypothesis of atherosclerosis. These AGE-related changes could promote the accumulation of other OSEs, such as CEP, that might be associated with drusen formation, by reducing their transit to the choriocapillaris for removal.
MDA is an abundant OSE that accumulates in a number of oxidative stress related diseases. Chou et al recently demonstrated that 15% of all IgM natural antibodies bind MDA adducts (Chou et al., 2009). This observation illustrates the importance that the natural selection process of the innate immune system has placed on MDA. Recently, in a multi-laboratory collaboration, we discovered that CFH, traditionally considered a major regulator of the alternative complement pathway, is a novel PRR that specifically recognizes MDA (Weismann et al., 2011). Using an unbiased proteomic approach to find MDA binding proteins, pooled plasma from Rag deficient, LDLR deficient mice, which lack immunoglobulins, was incubated with beads coupled to either malondialdehyde-acetaldehyde (MAA)-modified or unmodified polylysine. More than 55% of the MDA binding proteins identified by mass spectrometry were CFH. This same phenomenon of MDA binding by CFH was seen in human plasma. As with many PRRs, the binding to an OSE species is very specific. CFH did not bind to other OSEs including oxidized phosphocholine-BSA, CEP-BSA, and 4-hydroxynonenal-BSA. CFH is composed of 20 globular short consensus repeats (SCRs) (Jozsi and Zipfel, 2008). Only the SCR7 or SCR20 fragments bound MDA. These domains have also been identified as clustering points of various disease-related mutations including the 402H variant in SCR7 that is highly associated with AMD risk. Consistent with a role for these regions of CFH that bind to lipid metabolites, plasma from patients who have the 402H variant had impaired MDA binding(Jozsi and Zipfel, 2008). This effect showed a “dose-response” effect since the plasma from homozygous patients had more impaired MDA binding than did heterozygous patients, who had impaired binding compared to patients homozygous for the protective allele. This phenomenon was irrespective of the total plasma CFH levels. CFH also bound to apoptotic debris that contained MDA epitopes which resulted in the generation of iC3b inactivation fragments. The 402H CFH variant also showed impaired generation of C3b inactivation fragments when incubated with MDA decorated apoptotic particles. This has significant implication because iC3b opsonins promote the clearance of apoptotic cells without generating inflammation (Amarilyo et al., 2010). In contrast, inadequate complement control during apoptosis can advance the apoptotic stage, which results in the release of intracellular contents that provokes tissue damaging inflammation (Fadok et al., 2001). Given the important role of apoptosis in early AMD, this finding suggests that the risk 402H CFH variant could promote the clearance of apoptotic RPE cells, but with tissue injuring inflammation. CFH blocked the uptake of MDA-modified proteins and their proinflammatory effects by macrophages in vitro. This same effect was seen in RPE cells in vitro where CFH prevented Il-8 induction. Importantly, CFH had the same inhibitory effect in vivo. When mice were given intravitreal injections of MDA, Il-8 was induced by the RPE/choroid, an effect that was neutralized by co-injection with CFH. These findings provide important mechanistic insights into innate immune responses to oxidative stress, which may be exploited in the prevention of and therapy for AMD and other chronic inflammatory diseases.
Other oxidized lipids have been identified which “tag” oxidatively damaged photoreceptors in AMD (Sun et al., 2005). The ubiquitous distribution of phospholipids in cell membranes and lipoproteins make them particularly susceptible to lipid peroxidation. Prominent among these oxidized lipids is the oxidized phospholipid, oxidized phosphatidylcholine, which appears in the retina and RPE with aging, and increases in AMD (Suzuki et al., 2007). C-reactive protein (CRP) and CD14 are PRRs that recognize oxidized phosphatidylcholine and have been identified in AMD (Elner et al., 2003; Johnson et al., 2006). CD36 is another PRR that binds to oxidized phosphatidylcholine. Picard et al found that deficiency of CD36, which is expressed by the RPE, led to Bruch’s membrane thickening and basal deposits while the CD36 agonist, EP80317, prevented Bruch’s membrane thickening in high fat diet fed apoE null mice (Picard et al., 2010). These findings suggest that CD36 is important for neutralizing oxidized phospholipid and preventing Bruch’s membrane abnormalities. Defining the role of other OSEs and their PRRs in AMD would be another worthwhile avenue of exploration.
13. Putting it Together: A Proposed Mechanism of Oxidative Stress
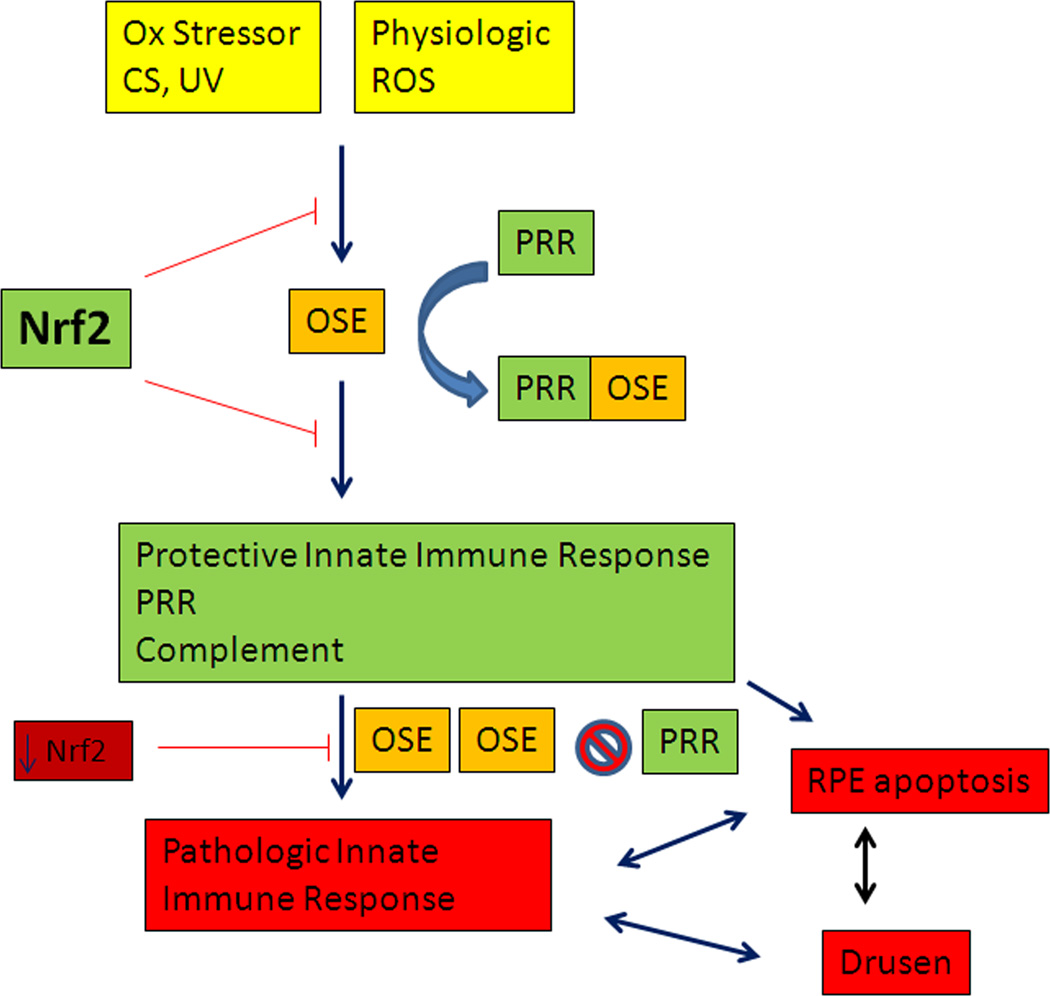
The following describes a proposed mechanism for how oxidative stress plays a role in the development of early AMD (Figure 6). The macula, and RPE in particular, has a robust antioxidant system powered by the Nrf2 signaling system, that protects the RPE from oxidative stress. In health, the generation and neutralization of ROS maintains a physiological level of oxidants and oxidant signaling molecules for essential cellular function. Excessive exogenous oxidative stress which can occur due to light exposure or cigarette smoking, stresses the antioxidant system. Chronic oxidative stress and/or aging will impair Nrf2 signaling, and render the RPE vulnerable to oxidative injury. Inadequately neutralized oxidative stress can lead to the formation of OSEs. Through the innate immune system, OSEs are neutralized by PRRs. Transmembrane PRRs may endocytose OSEs while fluid-phase PRRs may bind OSEs to either neutralize their antigenic potential or remove them from the macula. Over time, the chronic stimulation from OSEs can convert a protective into a pathologic innate immune response that results in excessive inflammation. The continued production of OSEs could overwhelm the ability of PRRs to neutralize or remove OSEs from the macula. This can result in RPE apoptosis and the accumulation of inflammatory debris within Bruch’s membrane that drives drusen formation. The aging related changes to Bruch’s membrane, such as the accumulation of AGEs, promotes the retention of OSEs and other macromolecules and/or impairs the ability of fluid-phase PRRs to remove OSEs into the circulation. This accumulation of cytotoxic moieties compromises cellular protein editing machines leading to enhanced levels of OSEs such as AGEs or oxidized lipids that will further promote drusen biogenesis. Loss of RPE function leads to impaired photoreceptor function which results in vision loss. The overlay of genetic risk, such as the 402H CFH variant, enhances the probability of exaggerated complement activation, impaired neutralization of MDA, and tissue injury.
Figure 6.
Proposed scheme of oxidative stress and its antioxidant systems including the Nrf2 signaling system and the innate immune system’s activation by oxidation specific epitopes (OSEs). CS, cigarette smoking; PRR, pattern recognition receptor; ROS, reactive oxygen species; UV, photo-oxidative stress.
14. Future Directions
The delineation of physiological and pathological ROS in the macula is in its infancy. Likewise, our understanding of how Nrf2 signaling protects the macula is also nascent. Unambiguously demonstrating an etiologic relationship between a decline in Nrf2 signaling in AMD will be an important next step. With the promising results from clinical trials of CDDO-Me, the possibility of triterpenoids to reduce oxidative stress in AMD before the innate immune system is activated is a direction that could circumvent trying to modify a specific molecule within the complement arm. The role of OSEs as chronic activators of the innate immune system through PRRs is also in its infancy. Future research needs to be directed toward understanding which OSEs and their PRRs are involved in AMD development. The unexpected discovery that CFH is a novel PRR for the ubiquitous OSE MDA opens up a number of unexplored directions that may help us to fully understand how the 402H CFH variant raises the risk of AMD. The ultimate goal would be to fully understand the events surrounding oxidative stress that cause the onset of AMD on which prevention or treatment for early disease can be designed.
Acknowledgements
This work was supported by NIH EY14005, EY019904, Edward N. & Della L. Thome Memorial Foundation Awards Program in AMD Research, Beckmann Award in Age-related Macular Degeneration, Senior Scientific Investigator Award from Research to Prevent Blindness, an unrestricted grant from Research to Prevent Blindness. Dr. Handa’s lab has also received generous gifts from the Merlau family. Dr. Handa is the Robert Bond Welch Professor. I thank Katayoon Ebrahimi, MD, for supplying the photomicrographs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelsalam A, Del Priore L, Zarbin MA. Drusen in age-related macular degeneration: pathogenesis, natural course, and laser photocoagulation-induced regression. Surv Ophthalmol. 1999;44(1):1–29. doi: 10.1016/s0039-6257(99)00072-7. [DOI] [PubMed] [Google Scholar]
- Adams J, Kelso R, Cooley L. The kelch repeat superfamily of proteins: propellers of cell function. Trends Cell Biol. 2000;10(1):17–24. doi: 10.1016/s0962-8924(99)01673-6. [DOI] [PubMed] [Google Scholar]
- Age-related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarilyo G, Verbovetski I, Atallah M, Grau A, Wiser G, Gil O, Ben-Neriah Y, Mevorach D. iC3b-opsonized apoptotic cells mediate a distinct anti-inflammatory response and transcriptional NF-kappaB-dependent blockade. Eur J Immunol. 2010;40(3):699–709. doi: 10.1002/eji.200838951. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134(3):411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- Auerbach BJ, Bisgaier CL, Wolle J, Saxena U. Oxidation of low density lipoproteins greatly enhances their association with lipoprotein lipase anchored to endothelial cell matrix. J Biol Chem. 1996;271(3):1329–1335. doi: 10.1074/jbc.271.3.1329. [DOI] [PubMed] [Google Scholar]
- Augustin A, Sahel JA, Bandello F, Dardennes R, Maurel F, Negrini C, Hieke K, Berdeaux G. Anxiety and depression prevalence rates in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007;48(4):1498–1503. doi: 10.1167/iovs.06-0761. [DOI] [PubMed] [Google Scholar]
- Barral S, Francis PJ, Schultz DW, Schain MB, Haynes C, Majewski J, Ott J, Acott T, Weleber RG, Klein ML. Expanded genome scan in extended families with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47(12):5453–5459. doi: 10.1167/iovs.06-0655. [DOI] [PubMed] [Google Scholar]
- Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45(2):115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- Becker MD, Garman K, Whitcup SM, Planck SR, Rosenbaum JT. Inhibition of leukocyte sticking and infiltration, but not rolling, by antibodies to ICAM-1 and LFA-1 in murine endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 2001;42(11):2563–2566. [PubMed] [Google Scholar]
- Berman K, Brodaty H. Psychosocial effects of age-related macular degeneration. Int Psychogeriatr. 2006;18(3):415–428. doi: 10.1017/S1041610205002905. [DOI] [PubMed] [Google Scholar]
- Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330(15):1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- Bienert GP, Moller AL, Kristiansen KA, Schulz A, Moller IM, Schjoerring JK, Jahn TP. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem. 2007;282(2):1183–1192. doi: 10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- Biswas SK, Rahman I. Environmental toxicity, redox signaling and lung inflammation: The role of glutathione. Mol Aspects Med. 2008 doi: 10.1016/j.mam.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boren J, Olin K, Lee I, Chait A, Wight TN, Innerarity TL. Identification of the principal proteoglycan-binding site in LDL. A single-point mutation in apo-B100 severely affects proteoglycan interaction without affecting LDL receptor binding. J Clin Invest. 1998;101(12):2658–2664. doi: 10.1172/JCI2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun S, Hanselmann C, Gassmann MG, auf dem Keller U, Born-Berclaz C, Chan K, Kan YW, Werner S. Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Mol Cell Biol. 2002;22(15):5492–5505. doi: 10.1128/MCB.22.15.5492-5505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler NM, Silva JC, Bressler SB, Fine SL, Green WR. Clinicopathologic correlation of drusen and retinal pigment epithelial abnormalities in age-related macular degeneration. Retina. 1994;14(2):130–142. [PubMed] [Google Scholar]
- Brody BL, Gamst AC, Williams RA, Smith AR, Lau PW, Dolnak D, Rapaport MH, Kaplan RM, Brown SI. Depression, visual acuity, comorbidity, and disability associated with age-related macular degeneration. Ophthalmology. 2001;108(10):1893–1900. doi: 10.1016/s0161-6420(01)00754-0. discussion 1900-1891. [DOI] [PubMed] [Google Scholar]
- Bron AJ, Tripathi RC, Tripathi Bl. Anatomy of the Eye and Orbit. In: Ryan S, editor. The Retina. 5th ed. London: Chapman and Hall; 1997. [Google Scholar]
- Brown GC, Brown MM, Sharma S, Stein JD, Roth Z, Campanella J, Beauchamp GR. The burden of age-related macular degeneration: a value-based medicine analysis. Trans Am Ophthalmol Soc. 2005a;103:173–184. discussion 184-176. [PMC free article] [PubMed] [Google Scholar]
- Brown MM, Brown GC, Stein JD, Roth Z, Campanella J, Beauchamp GR. Age-related macular degeneration: economic burden and value-based medicine analysis. Can J Ophthalmol. 2005b;40(3):277–287. doi: 10.1016/S0008-4182(05)80070-5. [DOI] [PubMed] [Google Scholar]
- Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1(6):409–414. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Bucciarelli LG, Wendt T, Qu W, Lu Y, Lalla E, Rong LL, Goova MT, Moser B, Kislinger T, Lee DC, Kashyap Y, Stern DM, Schmidt AM. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation. 2002;106(22):2827–2835. doi: 10.1161/01.cir.0000039325.03698.36. [DOI] [PubMed] [Google Scholar]
- Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, Kastner DL, Siegel RM. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) J Exp Med. 2011;208(3):519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano M, Fijalkowski N, Kondo N, Dike S, Handa J. Advanced Glycation Endproduct Changes to Bruch's Membrane Promotes Lipoprotein Retention by Lipoprotein Lipase. Am J Pathol. 2011;179(2):850–859. doi: 10.1016/j.ajpath.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canter JA, Olson LM, Spencer K, Schnetz-Boutaud N, Anderson B, Hauser MA, Schmidt S, Postel EA, Agarwal A, Pericak-Vance MA, Sternberg P, Jr, Haines JL. Mitochondrial DNA polymorphism A4917G is independently associated with age-related macular degeneration. PLoS ONE. 2008;3(5):e2091. doi: 10.1371/journal.pone.0002091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Dentchev T, Wong R, Hahn P, Wen R, Bennett J, Dunaief JL. Increased expression of ceruloplasmin in the retina following photic injury. Mol Vis. 2003;9:151–158. [PubMed] [Google Scholar]
- Chen W, Stambolian D, Edwards AO, Branham KE, Othman M, Jakobsdottir J, Tosakulwong N, Pericak-Vance MA, Campochiaro PA, Klein ML, Tan PL, Conley YP, Kanda A, Kopplin L, Li Y, Augustaitis KJ, Karoukis AJ, Scott WK, Agarwal A, Kovach JL, Schwartz SG, Postel EA, Brooks M, Baratz KH, Brown WL, Brucker AJ, Orlin A, Brown G, Ho A, Regillo C, Donoso L, Tian L, Kaderli B, Hadley D, Hagstrom SA, Peachey NS, Klein R, Klein BE, Gotoh N, Yamashiro K, Ferris Iii F, Fagerness JA, Reynolds R, Farrer LA, Kim IK, Miller JW, Corton M, Carracedo A, Sanchez-Salorio M, Pugh EW, Doheny KF, Brion M, Deangelis MM, Weeks DE, Zack DJ, Chew EY, Heckenlively JR, Yoshimura N, Iyengar SK, Francis PJ, Katsanis N, Seddon JM, Haines JL, Gorin MB, Abecasis GR, Swaroop A. Genetic variants near TIMP3 and high-density lipoprotein-associated loci influence susceptibility to age-related macular degeneration. Proc Natl Acad Sci U S A. 2010;107(16):7401–7406. doi: 10.1073/pnas.0912702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CJ, Liu S, Willett WC, Wolever TM, Brand-Miller JC, Barclay AW, Taylor A. Informing food choices and health outcomes by use of the dietary glycemic index. Nutr Rev. 2011;69(4):231–242. doi: 10.1111/j.1753-4887.2011.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX, Choi J, Perkmann T, Backhed F, Miller YI, Horkko S, Corr M, Witztum JL, Binder CJ. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest. 2009;119(5):1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou MY, Hartvigsen K, Hansen LF, Fogelstrand L, Shaw PX, Boullier A, Binder CJ, Witztum JL. Oxidation-specific epitopes are important targets of innate immunity. J Intern Med. 2008;263(5):479–488. doi: 10.1111/j.1365-2796.2008.01968.x. [DOI] [PubMed] [Google Scholar]
- Chowers I, Wong R, Dentchev T, Farkas RH, Iacovelli J, Gunatilaka TL, Medeiros NE, Presley JB, Campochiaro PA, Curcio CA, Dunaief JL, Zack DJ. The iron carrier transferrin is upregulated in retinas from patients with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47(5):2135–2140. doi: 10.1167/iovs.05-1135. [DOI] [PubMed] [Google Scholar]
- Clemons TE, Milton RC, Klein R, Seddon JM, Ferris FL., 3rd Risk factors for the incidence of Advanced Age-Related Macular Degeneration in the Age-Related Eye Disease Study (AREDS) AREDS report no. 19. Ophthalmology. 2005;112(4):533–539. doi: 10.1016/j.ophtha.2004.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon N, O'Colmain B, Klaver CC, Klein R, Munoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Salomon RG, Hollyfield JG. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99(23):14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross CE, O'Neill CA, Reznick AZ, Hu ML, Marcocci L, Packer L, Frei B. Cigarette smoke oxidation of human plasma constituents. Ann N Y Acad Sci. 1993;686:72–89. doi: 10.1111/j.1749-6632.1993.tb39157.x. discussion 89–90. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Johnson M, Huang JD, Rudolf M. Aging, age-related macular degeneration, and the response-to-retention of apolipoprotein B-containing lipoproteins. Prog Retin Eye Res. 2009;28(6):393–422. doi: 10.1016/j.preteyeres.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, Millican CL. Basal linear deposit and large drusen are specific for early age-related maculopathy. Arch Ophthalmol. 1999;117(3):329–339. doi: 10.1001/archopht.117.3.329. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990;292(4):497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- Del Priore LV, Kuo YH, Tezel TH. Age-related changes in human RPE cell density and apoptosis proportion in situ. Invest Ophthalmol Vis Sci. 2002;43(10):3312–3318. [PubMed] [Google Scholar]
- Dewan A, Liu M, Hartman S, Zhang S, Liu DT, Zhao C, Tam PO, Chan WM, Lam DS, Snyder M, Barnstable C, Pang CP, Hoh J. HTRA1 Promoter Polymorphism in Wet Age-Related Macular Degeneration. Science. 2006 doi: 10.1126/science.1133807. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Holtzclaw WD, Kensler TW. The role of Keap1 in cellular protective responses. Chem Res Toxicol. 2005;18(12):1779–1791. doi: 10.1021/tx050217c. [DOI] [PubMed] [Google Scholar]
- Doly M, Bonhomme B, Vennat JC. Experimental study of the retinal toxicity of hemoglobinic iron. Ophthalmic Res. 1986;18(1):21–27. doi: 10.1159/000265409. [DOI] [PubMed] [Google Scholar]
- Dunaief JL, Dentchev T, Ying GS, Milam AH. The role of apoptosis in age-related macular degeneration. Arch Ophthalmol. 2002;120(11):1435–1442. doi: 10.1001/archopht.120.11.1435. [DOI] [PubMed] [Google Scholar]
- Edwards AO, Ritter Iii R, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement Factor H Polymorphism and Age-Related Macular Degeneration. Science. 2005;308(5720):421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- Elner VM, Elner SG, Bian ZM, Kindezelskii AL, Yoshida A, Petty HR. RPE CD14 immunohistochemical, genetic, and functional expression. Exp Eye Res. 2003;76(3):321–331. doi: 10.1016/s0014-4835(02)00310-x. [DOI] [PubMed] [Google Scholar]
- Ershov AV, Bazan NG. Photoreceptor phagocytosis selectively activates PPARgamma expression in retinal pigment epithelial cells. J Neurosci Res. 2000;60(3):328–337. doi: 10.1002/(SICI)1097-4547(20000501)60:3<328::AID-JNR7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Espinosa-Heidmann DG, Suner IJ, Catanuto P, Hernandez EP, Marin-Castano ME, Cousins SW. Cigarette smoke-related oxidants and the development of sub-RPE deposits in an experimental animal model of dry AMD. Invest Ophthalmol Vis Sci. 2006;47(2):729–737. doi: 10.1167/iovs.05-0719. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11(1):81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Evans J. Antioxidant supplements to prevent or slow down the progression of AMD: a systematic review and meta-analysis. Eye (Lond) 2008;22(6):751–760. doi: 10.1038/eye.2008.100. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Guthrie L, Henson PM. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: role of proteases. J Immunol. 2001;166(11):6847–6854. doi: 10.4049/jimmunol.166.11.6847. [DOI] [PubMed] [Google Scholar]
- Fagerness JA, Maller JB, Neale BM, Reynolds RC, Daly MJ, Seddon JM. Variation near complement factor I is associated with risk of advanced AMD. Eur J Hum Genet. 2009;17(1):100–104. doi: 10.1038/ejhg.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farboud B, Aotaki-Keen A, Miyata T, Hjelmeland LM, Handa JT. Development of a polyclonal antibody with broad epitope specificity for advanced glycation endproducts and localization of these epitopes in Bruch's membrane of the aging eye. Mol Vis. 1999;5:11. [PubMed] [Google Scholar]
- Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194(1):7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SA, Abecasis GR, Yashar BM, Zareparsi S, Swaroop A, Iyengar SK, Klein BE, Klein R, Lee KE, Majewski J, Schultz DW, Klein ML, Seddon JM, Santangelo SL, Weeks DE, Conley YP, Mah TS, Schmidt S, Haines JL, Pericak-Vance MA, Gorin MB, Schulz HL, Pardi F, Lewis CM, Weber BH. Meta-analysis of genome scans of age-related macular degeneration. Hum Mol Genet. 2005;14(15):2257–2264. doi: 10.1093/hmg/ddi230. [DOI] [PubMed] [Google Scholar]
- Friedman DS, O'Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- Fritsche LG, Loenhardt T, Janssen A, Fisher SA, Rivera A, Keilhauer CN, Weber BH. Age-related macular degeneration is associated with an unstable ARMS2 (LOC387715) mRNA. Nat Genet. 2008;40(7):892–896. doi: 10.1038/ng.170. [DOI] [PubMed] [Google Scholar]
- Fujihara M, Nagai N, Sussan TE, Biswal S, Handa JT. Chronic cigarette smoke causes oxidative damage and apoptosis to retinal pigmented epithelial cells in mice. PLoS ONE. 2008;3(9):e3119. doi: 10.1371/journal.pone.0003119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Talalay P. Induction of phase 2 genes by sulforaphane protects retinal pigment epithelial cells against photooxidative damage. Proc Natl Acad Sci U S A. 2004;101(28):10446–10451. doi: 10.1073/pnas.0403886101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration--emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38(7):450–471. doi: 10.1080/07853890600946724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold B, Merriam JE, Zernant J, Hancox LS, Taiber AJ, Gehrs K, Cramer K, Neel J, Bergeron J, Barile GR, Smith RT, Hageman GS, Dean M, Allikmets R. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38(4):458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green WR, Enger C. Age-related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology. 1993;100(10):1519–1535. doi: 10.1016/s0161-6420(93)31466-1. [DOI] [PubMed] [Google Scholar]
- Green WR, McDonnell PJ, Yeo JH. Pathologic features of senile macular degeneration. Ophthalmology. 1985;92(5):615–627. [PubMed] [Google Scholar]
- Group MLP. Laser photocoagulation for juxtafoveal choroidal neovascularization. Five-year results from randomized clinical trials. Macular Photocoagulation Study Group. Arch Ophthalmol. 1994;112(4):500–509. [PubMed] [Google Scholar]
- Gu X, Meer SG, Miyagi M, Rayborn ME, Hollyfield JG, Crabb JW, Salomon RG. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J Biol Chem. 2003;278(43):42027–42035. doi: 10.1074/jbc.M305460200. [DOI] [PubMed] [Google Scholar]
- Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1(6):401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Hahn P, Milam AH, Dunaief JL. Maculas affected by age-related macular degeneration contain increased chelatable iron in the retinal pigment epithelium and Bruch's membrane. Arch Ophthalmol. 2003;121(8):1099–1105. doi: 10.1001/archopht.121.8.1099. [DOI] [PubMed] [Google Scholar]
- Hahn P, Qian Y, Dentchev T, Chen L, Beard J, Harris ZL, Dunaief JL. Disruption of ceruloplasmin and hephaestin in mice causes retinal iron overload and retinal degeneration with features of age-related macular degeneration. Proc Natl Acad Sci U S A. 2004;101(38):13850–13855. doi: 10.1073/pnas.0405146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA. Complement Factor H Variant Increases the Risk of Age-Related Macular Degeneration. Science. 2005 doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- Ham WT, Jr, Ruffolo JJ, Jr, Mueller HA, Clarke AM, Moon ME. Histologic analysis of photochemical lesions produced in rhesus retina by short-wave-length light. Invest Ophthalmol Vis Sci. 1978;17(10):1029–1035. [PubMed] [Google Scholar]
- Handa JT, Verzijl N, Matsunaga H, Aotaki-Keen A, Lutty GA, te Koppele JM, Miyata T, Hjelmeland LM. Increase in the advanced glycation end product pentosidine in Bruch's membrane with age. Invest Ophthalmol Vis Sci. 1999;40(3):775–779. [PubMed] [Google Scholar]
- Heiba IM, Elston RC, Klein BE, Klein R. Sibling correlations and segregation analysis of age-related maculopathy: the Beaver Dam Eye Study. Genet Epidemiol. 1994;11(1):51–67. doi: 10.1002/gepi.1370110106. [DOI] [PubMed] [Google Scholar]
- Hogan MJ. Role of the retinal pigment epithelium in macular disease. Trans Am Acad Ophthalmol Otolaryngol. 1972;76(1):64–80. [PubMed] [Google Scholar]
- Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008;14(2):194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes KA, Liu Y, Dunaief JL, Milam A, Frederick JM, Marks A, Baehr W. Receptor for advanced glycation end products and age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45(10):3713–3720. doi: 10.1167/iovs.04-0404. [DOI] [PubMed] [Google Scholar]
- Hunt RC, Davis AA. Release of iron by human retinal pigment epithelial cells. J Cell Physiol. 1992;152(1):102–110. doi: 10.1002/jcp.1041520114. [DOI] [PubMed] [Google Scholar]
- Ida H, Ishibashi K, Reiser K, Hjelmeland LM, Handa JT. Ultrastructural Aging of the RPE-Bruch's Membrane-Choriocapillaris Complex in the D-Galactose-Treated Mouse. Invest Ophthalmol Vis Sci. 2004;45(7):2348–2354. doi: 10.1167/iovs.03-1337. [DOI] [PubMed] [Google Scholar]
- Imamura Y, Noda S, Hashizume K, Shinoda K, Yamaguchi M, Uchiyama S, Shimizu T, Mizushima Y, Shirasawa T, Tsubota K. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: a model of age-related macular degeneration. Proc Natl Acad Sci U S A. 2006;103(30):11282–11287. doi: 10.1073/pnas.0602131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson GR, Owsley C, Curcio CA. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. Ageing Res Rev. 2002;1(3):381–396. doi: 10.1016/s1568-1637(02)00007-7. [DOI] [PubMed] [Google Scholar]
- Jakobsdottir J, Conley YP, Weeks DE, Mah TS, Ferrell RE, Gorin MB. Susceptibility genes for age-related maculopathy on chromosome 10q26. Am J Hum Genet. 2005;77(3):389–407. doi: 10.1086/444437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PT, Betts KE, Radeke MJ, Hageman GS, Anderson DH, Johnson LV. Individuals homozygous for the age-related macular degeneration risk-conferring variant of complement factor H have elevated levels of CRP in the choroid. Proc Natl Acad Sci U S A. 2006;103(46):17456–17461. doi: 10.1073/pnas.0606234103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozsi M, Zipfel PF. Factor H family proteins and human diseases. Trends Immunol. 2008;29(8):380–387. doi: 10.1016/j.it.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Justilien V, Pang JJ, Renganathan K, Zhan X, Crabb JW, Kim SR, Sparrow JR, Hauswirth WW, Lewin AS. SOD2 knockdown mouse model of early AMD. Invest Ophthalmol Vis Sci. 2007;48(10):4407–4420. doi: 10.1167/iovs.07-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda A, Chen W, Othman M, Branham KE, Brooks M, Khanna R, He S, Lyons R, Abecasis GR, Swaroop A. A variant of mitochondrial protein LOC387715/ARMS2, not HTRA1, is strongly associated with age-related macular degeneration. Proc Natl Acad Sci U S A. 2007;104(41):16227–16232. doi: 10.1073/pnas.0703933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunadharma PP, Nordgaard CL, Olsen TW, Ferrington DA. Mitochondrial DNA damage as a potential mechanism for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51(11):5470–5479. doi: 10.1167/iovs.10-5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan JC, Thurlby DA, Shahid H, Clayton DG, Yates JR, Bradley M, Moore AT, Bird AC. Smoking and age related macular degeneration: the number of pack years of cigarette smoking is a major determinant of risk for both geographic atrophy and choroidal neovascularisation. Br J Ophthalmol. 2006;90(1):75–80. doi: 10.1136/bjo.2005.073643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YU, Kinoshita T, Molina H, Hourcade D, Seya T, Wagner LM, Holers VM. Mouse complement regulatory protein Crry/p65 uses the specific mechanisms of both human decay-accelerating factor and membrane cofactor protein. J Exp Med. 1995;181(1):151–159. doi: 10.1084/jem.181.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Isashiki Y, Sonoda S, Kakiuchi-Matsumoto T, Ohba N. Genetic association of manganese superoxide dismutase with exudative age-related macular degeneration. Am J Ophthalmol. 2000;130(6):769–773. doi: 10.1016/s0002-9394(00)00552-3. [DOI] [PubMed] [Google Scholar]
- Klein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, Karp DD, Lieber MM, Walther PJ, Klotz L, Parsons JK, Chin JL, Darke AK, Lippman SM, Goodman GE, Meyskens FL, Jr, Baker LH. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306(14):1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Knudtson MD, Cruickshanks KJ, Klein BE. Further observations on the association between smoking and the long-term incidence and progression of age-related macular degeneration: the Beaver Dam Eye Study. Arch Ophthalmol. 2008;126(1):115–121. doi: 10.1001/archopht.126.1.115. [DOI] [PubMed] [Google Scholar]
- Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, Sangiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement Factor H Polymorphism in Age-Related Macular Degeneration. Science. 2005 doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24(16):7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal. 2005;7(3–4):385–394. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- Kondo N, Honda S, Ishibashi K, Tsukahara Y, Negi A. LOC387715/HTRA1 variants in polypoidal choroidal vasculopathy and age-related macular degeneration in a Japanese population. Am J Ophthalmol. 2007;144(4):608–612. doi: 10.1016/j.ajo.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Kontoghiorghes GJ, Kolnagou A, Peng CT, Shah SV, Aessopos A. Safety issues of iron chelation therapy in patients with normal range iron stores including thalassaemia, neurodegenerative, renal and infectious diseases. Expert Opin Drug Saf. 2010;9(2):201–206. doi: 10.1517/14740330903535845. [DOI] [PubMed] [Google Scholar]
- Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285(5432):1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- Lee JY, Friedman JE, Angel I, Kozak A, Koh JY. The lipophilic metal chelator DP-109 reduces amyloid pathology in brains of human beta-amyloid precursor protein transgenic mice. Neurobiol Aging. 2004;25(10):1315–1321. doi: 10.1016/j.neurobiolaging.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Lengyel I, Flinn JM, Peto T, Linkous DH, Cano K, Bird AC, Lanzirotti A, Frederickson CJ, van Kuijk FJ. High concentration of zinc in sub-retinal pigment epithelial deposits. Exp Eye Res. 2007;84(4):772–780. doi: 10.1016/j.exer.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Li J, Stein TD, Johnson JA. Genetic dissection of systemic autoimmune disease in Nrf2-deficient mice. Physiol Genomics. 2004;18(3):261–272. doi: 10.1152/physiolgenomics.00209.2003. [DOI] [PubMed] [Google Scholar]
- Liang FQ, Godley BF. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp Eye Res. 2003;76(4):397–403. doi: 10.1016/s0014-4835(03)00023-x. [DOI] [PubMed] [Google Scholar]
- Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer. 2007;7(5):357–369. doi: 10.1038/nrc2129. [DOI] [PubMed] [Google Scholar]
- Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, 3rd, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL, Jr, Baker LH, Coltman CA., Jr Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301(1):39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, Dagenais GR. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293(11):1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- Lykkesfeldt J, Christen S, Wallock LM, Chang HH, Jacob RA, Ames BN. Ascorbate is depleted by smoking and repleted by moderate supplementation: a study in male smokers and nonsmokers with matched dietary antioxidant intakes. Am J Clin Nutr. 2000;71(2):530–536. doi: 10.1093/ajcn/71.2.530. [DOI] [PubMed] [Google Scholar]
- Malhotra D, Thimmulappa R, Navas-Acien A, Sandford A, Elliott M, Singh A, Chen L, Zhuang X, Hogg J, Pare P, Tuder RM, Biswal S. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med. 2008;178(6):592–604. doi: 10.1164/rccm.200803-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Maller JB, Fagerness JA, Reynolds RC, Neale BM, Daly MJ, Seddon JM. Variation in complement factor 3 is associated with risk of age-related macular degeneration. Nat Genet. 2007;39(10):1200–1201. doi: 10.1038/ng2131. [DOI] [PubMed] [Google Scholar]
- Miceli MV, Liles MR, Newsome DA. Evaluation of oxidative processes in human pigment epithelial cells associated with retinal outer segment phagocytosis. Exp Cell Res. 1994;214(1):242–249. doi: 10.1006/excr.1994.1254. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Wang JJ, Smith W, Leeder SR. Smoking and the 5-year incidence of age-related maculopathy: the Blue Mountains Eye Study. Arch Ophthalmol. 2002;120(10):1357–1363. doi: 10.1001/archopht.120.10.1357. [DOI] [PubMed] [Google Scholar]
- Moriarty-Craige SE, Adkison J, Lynn M, Gensler G, Bressler S, Jones DP, Sternberg P., Jr Antioxidant supplements prevent oxidation of cysteine/cystine redox in patients with age-related macular degeneration. Am J Ophthalmol. 2005;140(6):1020–1026. doi: 10.1016/j.ajo.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Mukesh BN, Dimitrov PN, Leikin S, Wang JJ, Mitchell P, McCarty CA, Taylor HR. Five-year incidence of age-related maculopathy: the Visual Impairment Project. Ophthalmology. 2004;111(6):1176–1182. doi: 10.1016/j.ophtha.2003.08.042. [DOI] [PubMed] [Google Scholar]
- Nagai N, Thimmulappa RK, Cano M, Fujihara M, Izumi-Nagai K, Kong X, Sporn MB, Kensler TW, Biswal S, Handa JT. Nrf2 is a critical modulator of the innate immune response in a model of uveitis. Free Radic Biol Med. 2009 doi: 10.1016/j.freeradbiomed.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, Choi AM. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12(3):222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale BM, Fagerness J, Reynolds R, Sobrin L, Parker M, Raychaudhuri S, Tan PL, Oh EC, Merriam JE, Souied E, Bernstein PS, Li B, Frederick JM, Zhang K, Brantley MA, Jr, Lee AY, Zack DJ, Campochiaro B, Campochiaro P, Ripke S, Smith RT, Barile GR, Katsanis N, Allikmets R, Daly MJ, Seddon JM. Genome-wide association study of advanced age-related macular degeneration identifies a role of the hepatic lipase gene (LIPC) Proc Natl Acad Sci U S A. 2010;107(16):7395–7400. doi: 10.1073/pnas.0912019107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KC, Armstrong JS, Moriarty S, Cai J, Wu MW, Sternberg P, Jr, Jones DP. Protection of retinal pigment epithelial cells from oxidative damage by oltipraz, a cancer chemopreventive agent. Invest Ophthalmol Vis Sci. 2002;43(11):3550–3554. [PubMed] [Google Scholar]
- Nelson KC, Carlson JL, Newman ML, Sternberg P, Jr, Jones DP, Kavanagh TJ, Diaz D, Cai J, Wu M. Effect of dietary inducer dimethylfumarate on glutathione in cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1999;40(9):1927–1935. [PubMed] [Google Scholar]
- Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- O'Neill CA, Halliwell B, van der Vliet A, Davis PA, Packer L, Tritschler H, Strohman WJ, Rieland T, Cross CE, Reznick AZ. Aldehyde-induced protein modifications in human plasma: protection by glutathione and dihydrolipoic acid. J Lab Clin Med. 1994;124(3):359–370. [PubMed] [Google Scholar]
- Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, Barnhart S, Hammar S. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334(18):1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- Orth DH, Fine BS, Fagman W, Quirk TC. Clarification of foveomacular nomenclature and grid for quantitation of macular disorders. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1977;83(3 Pt 1):OP506–OP514. [PubMed] [Google Scholar]
- Osburn WO, Yates MS, Dolan PD, Chen S, Liby KT, Sporn MB, Taguchi K, Yamamoto M, Kensler TW. Genetic or pharmacologic amplification of nrf2 signaling inhibits acute inflammatory liver injury in mice. Toxicol Sci. 2008;104(1):218–227. doi: 10.1093/toxsci/kfn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otaki N, Chikazawa M, Nagae R, Shimozu Y, Shibata T, Ito S, Takasaki Y, Fujii J, Uchida K. Identification of a lipid peroxidation product as the source of oxidation-specific epitopes recognized by anti-DNA autoantibodies. J Biol Chem. 2010;285(44):33834–33842. doi: 10.1074/jbc.M110.165175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H, Wittes J, Warnock DG. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365(4):327–336. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- Picard E, Houssier M, Bujold K, Sapieha P, Lubell W, Dorfman A, Racine J, Hardy P, Febbraio M, Lachapelle P, Ong H, Sennlaub F, Chemtob S. CD36 plays an important role in the clearance of oxLDL and associated age-dependent sub-retinal deposits. Aging (Albany NY) 2010 doi: 10.18632/aging.100218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman I, Biswas SK, Jimenez LA, Torres M, Forman HJ. Glutathione, stress responses, and redox signaling in lung inflammation. Antioxid Redox Signal. 2005;7(1–2):42–59. doi: 10.1089/ars.2005.7.42. [DOI] [PubMed] [Google Scholar]
- Rahman I, MacNee W. Role of oxidants/antioxidants in smoking-induced lung diseases. Free Radic Biol Med. 1996;21(5):669–681. doi: 10.1016/0891-5849(96)00155-4. [DOI] [PubMed] [Google Scholar]
- Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114(9):1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera A, Fisher SA, Fritsche LG, Keilhauer CN, Lichtner P, Meitinger T, Weber BH. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum Mol Genet. 2005;14(21):3227–3236. doi: 10.1093/hmg/ddi353. [DOI] [PubMed] [Google Scholar]
- SanGiovanni JP, Arking DE, Iyengar SK, Elashoff M, Clemons TE, Reed GF, Henning AK, Sivakumaran TA, Xu X, DeWan A, Agron E, Rochtchina E, Sue CM, Wang JJ, Mitchell P, Hoh J, Francis PJ, Klein ML, Chew EY, Chakravarti A. Mitochondrial DNA variants of respiratory complex I that uniquely characterize haplogroup T2 are associated with increased risk of age-related macular degeneration. PLoS One. 2009;4(5):e5508. doi: 10.1371/journal.pone.0005508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarks SH. Ageing and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol. 1976;60(5):324–341. doi: 10.1136/bjo.60.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26(7):1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S, Hauser MA, Scott WK, Postel EA, Agarwal A, Gallins P, Wong F, Chen YS, Spencer K, Schnetz-Boutaud N, Haines JL, Pericak-Vance MA. Cigarette smoking strongly modifies the association of LOC387715 and age-related macular degeneration. Am J Hum Genet. 2006;78(5):852–864. doi: 10.1086/503822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline CT, Zhou Y, Bai S. Light-induced photoreceptor and RPE degeneration involve zinc toxicity and are attenuated by pyruvate, nicotinamide, or cyclic light. Mol Vis. 2010;16:2639–2652. [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Hansch C. The relative toxicity of compounds in mainstream cigarette smoke condensate. Food Chem Toxicol. 2000;38(7):637–646. doi: 10.1016/s0278-6915(00)00051-x. [DOI] [PubMed] [Google Scholar]
- Smith W, Assink J, Klein R, Mitchell P, Klaver CC, Klein BE, Hofman A, Jensen S, Wang JJ, de Jong PT. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology. 2001;108(4):697–704. doi: 10.1016/s0161-6420(00)00580-7. [DOI] [PubMed] [Google Scholar]
- Spencer KL, Hauser MA, Olson LM, Schmidt S, Scott WK, Gallins P, Agarwal A, Postel EA, Pericak-Vance MA, Haines JL. Protective effect of complement factor B and complement component 2 variants in age-related macular degeneration. Hum Mol Genet. 2007;16(16):1986–1992. doi: 10.1093/hmg/ddm146. [DOI] [PubMed] [Google Scholar]
- Spraul CW, Lang GE, Grossniklaus HE. Morphometric analysis of the choroid, Bruch's membrane, and retinal pigment epithelium in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37(13):2724–2735. [PubMed] [Google Scholar]
- Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A. 2004;101(10):3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Finnemann SC, Febbraio M, Shan L, Annangudi SP, Podrez EA, Hoppe G, Darrow R, Organisciak DT, Salomon RG, Silverstein RL, Hazen SL. Light-induced oxidation of photoreceptor outer segment phospholipids generates ligands for CD36-mediated phagocytosis by retinal pigment epithelium: A potential mechanism for modulating outer segment phagocytosis under oxidant stress conditions. J Biol Chem. 2005 doi: 10.1074/jbc.M509769200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussan TE, Rangasamy T, Blake DJ, Malhotra D, El-Haddad H, Bedja D, Yates MS, Kombairaju P, Yamamoto M, Liby KT, Sporn MB, Gabrielson KL, Champion HC, Tuder RM, Kensler TW, Biswal S. Targeting Nrf2 with the triterpenoid CDDO- imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0804333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Betsuyaku T, Ito Y, Nagai K, Nasuhara Y, Kaga K, Kondo S, Nishimura M. Down-regulated NF-E2-related factor 2 in pulmonary macrophages of aged smokers and patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2008;39(6):673–682. doi: 10.1165/rcmb.2007-0424OC. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kamei M, Itabe H, Yoneda K, Bando H, Kume N, Tano Y. Oxidized phospholipids in the macula increase with age and in eyes with age-related macular degeneration. Mol Vis. 2007;13:772–778. [PMC free article] [PubMed] [Google Scholar]
- Tanito M, Masutani H, Kim YC, Nishikawa M, Ohira A, Yodoi J. Sulforaphane induces thioredoxin through the antioxidant-responsive element and attenuates retinal light damage in mice. Invest Ophthalmol Vis Sci. 2005;46(3):979–987. doi: 10.1167/iovs.04-1120. [DOI] [PubMed] [Google Scholar]
- Tate DJ, Jr, Miceli MV, Newsome DA. Phagocytosis and H2O2 induce catalase and metallothionein gene expression in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1995;36(7):1271–1279. [PubMed] [Google Scholar]
- Taylor HR, Munoz B, West S, Bressler NM, Bressler SB, Rosenthal FS. Visible light and risk of age-related macular degeneration. Trans Am Ophthalmol Soc. 1990;88:163–173. discussion 173-168. [PMC free article] [PubMed] [Google Scholar]
- Taylor HR, West S, Munoz B, Rosenthal FS, Bressler SB, Bressler NM. The long-term effects of visible light on the eye. Arch Ophthalmol. 1992;110(1):99–104. doi: 10.1001/archopht.1992.01080130101035. [DOI] [PubMed] [Google Scholar]
- Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest. 2006;116(4):984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62(18):5196–5203. [PubMed] [Google Scholar]
- Tian J, Ishibashi K, Ishibashi K, Reiser K, Grebe R, Biswal S, Gehlbach P, Handa JT. Advanced glycation endproduct-induced aging of the retinal pigment epithelium and choroid: A comprehensive transcriptional response. Proc Natl Acad Sci U S A. 2005;102(33):11846–11851. doi: 10.1073/pnas.0504759102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimura A, Shida K, Kitamura M, Nomura M, Takeda J, Tanaka H, Matsumoto M, Matsumiya K, Okuyama A, Nishimune Y, Okabe M, Seya T. Molecular cloning of a murine homologue of membrane cofactor protein (CD46): preferential expression in testicular germ cells. Biochem J. 1998;330(Pt 1):163–168. doi: 10.1042/bj3300163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiki T, Weikel KA, Jiao W, Shang F, Caceres A, Pawlak D, Handa JT, Brownlee M, Nagaraj R, Taylor A. Glycation-altered proteolysis as a pathobiologic mechanism that links dietary glycemic index, aging, and age-related disease (in nondiabetics) Aging Cell. 2011 doi: 10.1111/j.1474-9726.2011.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schaft TL, de Bruijn WC, Mooy CM, Ketelaars DA, de Jong PT. Is basal laminar deposit unique for age-related macular degeneration? Arch Ophthalmol. 1991;109(3):420–425. doi: 10.1001/archopht.1991.01080030122052. [DOI] [PubMed] [Google Scholar]
- van der Schaft TL, Mooy CM, de Bruijn WC, Bosman FT, de Jong PT. Immunohistochemical light and electron microscopy of basal laminar deposit. Graefes Arch Clin Exp Ophthalmol. 1994;232(1):40–46. doi: 10.1007/BF00176436. [DOI] [PubMed] [Google Scholar]
- van der Schaft TL, Mooy CM, de Bruijn WC, Oron FG, Mulder PG, de Jong PT. Histologic features of the early stages of age-related macular degeneration. A statistical analysis. Ophthalmology. 1992;99(2):278–286. doi: 10.1016/s0161-6420(92)31982-7. [DOI] [PubMed] [Google Scholar]
- Vingerling JR, Klaver CC, Hofman A, de Jong PT. Epidemiology of age-related maculopathy. Epidemiol Rev. 1995;17(2):347–360. doi: 10.1093/oxfordjournals.epirev.a036198. [DOI] [PubMed] [Google Scholar]
- Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S, Imakado S, Kotsuji T, Otsuka F, Roop DR, Harada T, Engel JD, Yamamoto M. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet. 2003;35(3):238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- Walsh AC, Michaud SG, Malossi JA, Lawrence DA. Glutathione depletion in human T lymphocytes: analysis of activation-associated gene expression and the stress response. Toxicol Appl Pharmacol. 1995;133(2):249–261. doi: 10.1006/taap.1995.1149. [DOI] [PubMed] [Google Scholar]
- Wang AL, Lukas TJ, Yuan M, Du N, Handa JT, Neufeld AH. Changes in retinal pigment epithelium related to cigarette smoke: possible relevance to smoking as a risk factor for age-related macular degeneration. PLoS One. 2009;4(4):e5304. doi: 10.1371/journal.pone.0005304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weismann D, Hartvigsen K, Lauer N, Bennett KL, Scholl HP, Charbel Issa P, Cano M, Brandstatter H, Tsimikas S, Skerka C, Superti-Furga G, Handa JT, Zipfel PF, Witztum JL, Binder CJ. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature. 2011;478(7367):76–81. doi: 10.1038/nature10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will O, Mahler HC, Arrigo AP, Epe B. Influence of glutathione levels and heat-shock on the steady-state levels of oxidative DNA base modifications in mammalian cells. Carcinogenesis. 1999;20(2):333–337. doi: 10.1093/carcin/20.2.333. [DOI] [PubMed] [Google Scholar]
- Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Oxidative damage and age-related macular degeneration. Mol Vis. 1999;5:32. [PMC free article] [PubMed] [Google Scholar]
- Wysong A, Lee PP, Sloan FA. Longitudinal incidence of adverse outcomes of age-related macular degeneration. Arch Ophthalmol. 2009;127(3):320–327. doi: 10.1001/archophthalmol.2008.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Ishibashi K, Ishibashi K, Bhutto IA, Tian J, Lutty GA, Handa JT. The expression of advanced glycation endproduct receptors in rpe cells associated with basal deposits in human maculas. Exp Eye Res. 2006;82(5):840–848. doi: 10.1016/j.exer.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Tian J, Yang Y, Cutler RG, Wu T, Telljohann RS, Mattson MP, Handa JT. Oxidized Low Density Lipoproteins Induce a Pathologic Response by Retinal Pigmented Epithelial Cells. J Neurochem. 2008;105(4):1187–1197. doi: 10.1111/j.1471-4159.2008.05211.x. [DOI] [PubMed] [Google Scholar]
- Yang P, Tyrrell J, Han I, Jaffe GJ. Expression and modulation of RPE cell membrane complement regulatory proteins. Invest Ophthalmol Vis Sci. 2009;50(7):3473–3481. doi: 10.1167/iovs.08-3202. [DOI] [PubMed] [Google Scholar]
- Yang Z, Camp NJ, Sun H, Tong Z, Gibbs D, Cameron DJ, Chen H, Zhao Y, Pearson E, Li X, Chien J, Dewan A, Harmon J, Bernstein PS, Shridhar V, Zabriskie NA, Hoh J, Howes K, Zhang K. A Variant of the HTRA1 Gene Increases Susceptibility to Age-Related Macular Degeneration. Science. 2006 doi: 10.1126/science.1133811. [DOI] [PubMed] [Google Scholar]
- Yates JR, Sepp T, Matharu BK, Khan JC, Thurlby DA, Shahid H, Clayton DG, Hayward C, Morgan J, Wright AF, Armbrecht AM, Dhillon B, Deary IJ, Redmond E, Bird AC, Moore AT. Complement C3 Variant and the Risk of Age-Related Macular Degeneration. N Engl J Med. 2007a doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- Yates MS, Tauchi M, Katsuoka F, Flanders KC, Liby KT, Honda T, Gribble GW, Johnson DA, Johnson JA, Burton NC, Guilarte TR, Yamamoto M, Sporn MB, Kensler TW. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol Cancer Ther. 2007b;6(1):154–162. doi: 10.1158/1535-7163.MCT-06-0516. [DOI] [PubMed] [Google Scholar]
- Yefimova MG, Jeanny JC, Keller N, Sergeant C, Guillonneau X, Beaumont C, Courtois Y. Impaired retinal iron homeostasis associated with defective phagocytosis in Royal College of Surgeons rats. Invest Ophthalmol Vis Sci. 2002;43(2):537–545. [PubMed] [Google Scholar]
- Yu X, Kensler T. Nrf2 as a target for cancer chemoprevention. Mutat Res. 2005;591(1–2):93–102. doi: 10.1016/j.mrfmmm.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24(24):10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Yasumura D, Li X, Matthes M, Lloyd M, Nielsen G, Ahern K, Snyder M, Bok D, Dunaief JL, LaVail MM, Vollrath D. mTOR-mediated dedifferentiation of the retinal pigment epithelium initiates photoreceptor degeneration in mice. J Clin Invest. 2011;121(1):369–383. doi: 10.1172/JCI44303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]