Abstract
The adipocyte-derived hormone, leptin, plays an important role in regulating energy homeostasis and the innate immune response against bacterial infections. Leptin’s actions are mediated by signaling events initiated by phosphorylation of tyrosine residues on the long form of the leptin receptor. We recently reported that disruption of leptin receptor mediated STAT3 activation augmented host defense against pneumococcal pneumonia. In this report, we assessed leptin receptor mediated ERK activation, a pathway that was ablated in the l/l mouse through a mutation of the tyrosine 985 residue in the leptin receptor, to determine its role in host defense against bacterial pneumonia in vivo and in alveolar macrophage antibacterial functions in vitro. l/l mice exhibited increased mortality and impaired pulmonary bacterial clearance following intratracheal challenge with Klebsiella pneumoniae. The synthesis of cysteinyl-LTs was reduced and that of PGE2 enhanced in alveolar macrophages in vitro and the lungs of l/l mice following infection with K.pneumoniae in vivo. We also observed reduced phagocytosis and killing of K. pneumoniae in AMs from l/l mice that was associated with reduced reactive oxygen intermediate production in vitro. cAMP, known to suppress phagocytosis, bactericidal capacity, and reactive oxygen intermediate production, was also increased 2-fold in AMs from l/l mice. Pharmacologic blockade of PGE2 synthesis reduced cAMP levels and overcame the defective phagocytosis and killing of bacteria in AMs from l/l mice in vitro. These results demonstrate that leptin receptor mediated ERK activation plays an essential role in host defense against bacterial pneumonia and in leukocyte antibacterial effector functions.
Keywords: leptin, macrophage, pneumonia, K. pneumoniae, eicosanoids
Introduction
Pneumonia is a common consequence of malnutrition, a leading threat to human health throughout the world regardless of socioeconomic status (1). Rapid depletion of energy storage in the form of adipose tissue often occurs during periods of famine in the developing world and in hospitalized patients suffering from chronic and critical illness (2–6). Associated with the decline in fat mass is a decrease in leptin, an adipokine produced by white adipose tissue and known to regulate energy homeostasis. Under normal circumstances, leptin levels are correlated with adipose tissue mass (7). However, during acute bacterial infections and following endotoxin administration in laboratory animals, leptin levels increase disproportionately to fat mass (8–12). An important role for leptin in the regulation of immune function during periods of fasting, obesity, and in disease states mediated by inflammation is emerging.
We and others have observed that leptin plays a protective role in the host response against infectious disease (13–18). Using murine models of Klebsiella and pneumococcal pneumonia, we have found that leptin deficiency induced by genetic means or by fasting compromised pulmonary bacterial clearance and survival. This defect in pulmonary host defense was associated with abrogated alveolar macrophage (AM) and neutrophil (PMN) phagocytosis and killing of bacteria in vitro (12–14, 19). The mechanisms underlying defective leukocyte effector function in cells from leptin deficient mice were associated with a reduction in leukotriene (LT) synthesis in AMs, reduced complement receptor (CR3) expression and decreased H2O2 synthesis in PMNs (12, 14, 19). Other studies have revealed that the production of cytokines IL-6, MIP-2, and MCP-1 in leptin deficient or leptin receptor deficient mice was lower than that observed for wild type animals (13, 15). The intracellular signaling events downstream of the leptin receptor (LepR) that regulate leukocyte effector functions, in the context of bacterial pneumonia, have not been determined.
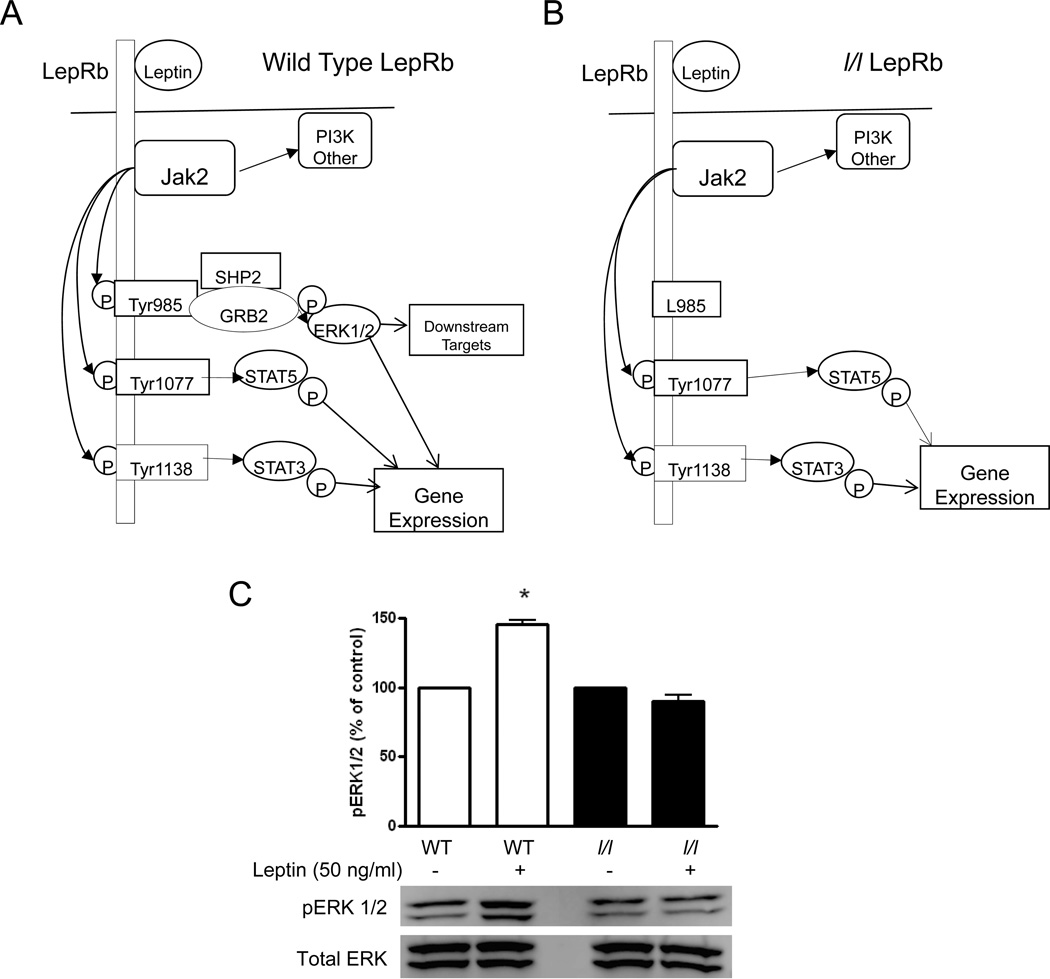
LepR signaling is mediated by the long form of the leptin receptor (LepRb) via the janus kinase and signal transducer and activator of transcription (JAK/STAT) and mitogen activated protein (MAP) kinase signaling pathways. Upon binding to its ligand (Figure 1), the LepRb activates the constitutively associated JAK2 tyrosine kinase to induce tyrosine phosphorylation-dependent signaling via several divergent pathways. JAK2 mediates phosphorylation of Tyr1138 which binds and mediates the phosphorylation-dependent activation of the latent transcription factor, STAT3. Following nuclear translocation, STAT3 activates transcription of suppressor of cytokine signaling (SOCS)-3, a protein that inhibits JAK2 and STAT3 signaling during prolonged stimulation of the LepRb (20). LepRb mediated phosphorylation of Tyr1077 activates STAT5 signaling (21). Finally, phosphorylation of Tyr985 recruits binding partners SH2-containing tyrosine phosphatase (SHP-2) and growth factor binding 2 (GRB2) which activate extracellular signal-regulated kinase 1 and 2 (ERK 1/2)(22). The generation of the l/l mouse was first described by Björnholm et al. (23) who reported that these animals lack the ability to activate the ERK 1/2 pathway via the LepRb due to a substitution mutation at Tyr985 with L985. Although a recent report by Guo et al. demonstrated that l/l mice exhibit greater susceptibility to enteric Entamoeba histolytica infection, the mechanism responsible for this defect is unknown and bacterial infections have not been studied in these mice (17).
Figure 1.
LepRb signaling events. A. In wild type (WT) mice (A), leptin binding activates the LepRb-associated janus associated kinase 2 (Jak2), a tyrosine kinase that mediates tyrosine phosphorylation of LepRb to promote several intracellular signaling events: 1) LepRb Tyr985 recruits SH2-containing tyrosine phosphatase (SHP-2) and growth factor binding 2 (GRB2) and to promote the activation of extracellular regulated kinases 1 and 2 (ERK1/2). ERK1/2 phosphorylates downstream targets and induces the transcription of genes. 2) Phosphorylated Tyr1107 binds and mediates the phosphorylation-dependent activation of STAT5 and 3) Tyr1138 recruits STAT3, which promotes the transcription many different genes. In l/l mice (B), substitution of LepRb Tyr985 with LepRb L985 prevents leptin mediated ERK 1/2 activation in AMs from l/l mice. AMs obtained from WT and l/l mice were cultured with media alone or with exogenous leptin (50 ng/ml) for 30 min and evaluated for pERK1/2 and total ERK 1/2 by immunoblot analysis. Protein levels were determined by densitometric analysis of immunoblots from 5 separate experiments (B).*, P<0.001 vs untreated WT using the students t-test.
The role of LepRb-mediated (LepRb→) signaling events in the innate immune response against bacterial infections is complex and difficult to study in vivo. Not all leptin receptor mutations result in impaired immunity. For example, we recently reported that disruption of leptin receptor mediated STAT3 signaling improved AM phagocytosis and killing of bacteria in vitro and host defense against pneumococcal pneumonia in s/s mice in vivo (18). In the current report, we assessed the contribution of intracellular signals initiated by the LepRb→ Tyr985 by comparing the responses of wild type (WT) with l/l mice in a murine model of bacterial pneumonia. We demonstrate for the first time that l/l mice exhibit increased susceptibility to gram-negative pneumonia and this pathway plays an essential role in the innate immune response against bacterial pneumonia.
Materials and Methods
Animals
Heterozygous C57Bl/6 (back-crossed for 8 generations or greater) LeprTm2mgmj/+ mice were intercrossed in the University of Michigan Unit for Laboratory Animal Medicine (ULAM, Ann Arbor, MI) to generate age- and gender-matched male and female (Lepr Tm2mgmj / Tm2mgmj) l/l and wild type (+/+;WT) (littermates) 8 to 14 weeks of age (23). Animals were genotyped by Taqman SNP allelic discrimination assays and were treated according to National Institutes of Health guidelines for the use of experimental animals with the approval of the University of Michigan Committee for the Use and Care of Animals.
Cell isolation and culture
Resident AMs were recovered from mice by bronchoalveolar lavage (BAL) as previously described, resuspended in RPMI 1640 (Life Technologies, Invitrogen, Carlsbad, CA) to a concentration of 2 × 106 cells per ml, and allowed to adhere to tissue-culture plates for 1 h (37°C, 5% CO2) (24). After replacing the media with RPMI 1640 containing 10% fetal bovine serum (Life Technologies, Invitrogen, Carlsbad, CA) and 1% penicillin-streptomycin (Pen/Strep) (Invitrogen), the cells were cultured overnight. PMNs were obtained from mice by peritoneal lavage 5 h after an intraperitoneal injection of a 1% glycogen solution in PBS as previously described (25).
Immunoblot analysis
AMs obtained from WT and l/l mice were plated at 4 × 106 cells per well and cultured overnight in RPMI 1640 containing fetal bovine serum and Pen/Strep. On the following day, the cells were prepared for lysis or cultured with media alone or leptin (50 ng/ml) for 5, 15, or 30 min for the assessment of ERK activation. The macrophages were then washed with HBSS and scraped with ice-cold lysis buffer (RIPA buffer, Sigma) and cells were disrupted with sonication (10 bursts at 20% duty/cycle). Twenty micrograms of protein, as determined by a modified Coomassie blue binding assay (Pierce Chemical, Rockford, IL), were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and transferred to nitrocellulose membranes. Membranes were probed with the rabbit polyclonal antibodies against phospho-p44/42 MAPK (ERK1/2) (Thr202/Tyr204)(1:2000), p44/42 MAPK (ERK1/2) (1:2,000), 5-lipoxygenase (5-LO) (1:250), GAPDH (1:1000) (Cell Signaling Technology, Danvers, MA), Cyclooxygenase-1(COX-1) (Bio-Rad, Hercules, CA) (1:200) and -2 (COX-2) (1:200), or microsomal prostaglandin E synthase-1 (mPGEs-1) (1:200) (Cayman Chemical, Ann Arbor, MI). Primary antibodies were detected using alkaline phosphatase-conjugated goat anti-rabbit secondary antibody (titer 1:5,000) and visualized with the ECF detection system (Amersham Pharmacia Biotech, Piscataway, NJ). The densities of the luminescent bands were quantitated in appropriately exposed nitrocellulose by using Image Reader (FujiFilm). The density value of the pERK1/2, 5-LO, COX-1, COX-2, and mPGEs-1 blots were divided by the density value of the ERK1/2 (pERK1/2) or GAPDH (5-LO, COX-1, COX-2, and mPGEs-1) blots, respectively, to normalize the relative band densities.
K. pneumoniae preparation and inoculation
K. pneumoniae strain 43816, serotype 2, was obtained from the American Type Culture Collection (Manassas, VA), and aliquots were grown in tryptic soy broth (Difco, Detroit, MI) for 18 h at 37°C. The concentration of bacteria in culture was determined spectrophotometrically (A600). K.pneumoniae was then pelleted by centrifugation (13,000 rpm for 3 min) 2×, resuspended in PBS, and serially diluted in PBS to obtain the appropriate concentration. Mice were anesthetized with ketamine and xylazine as previously described (12). A midline incision was made to expose the trachea, a 30-µl inoculum containing 5 × 103 CFU K. pneumoniae was administered via the trachea using a 26-gauge needle, and the wound was closed using surgical glue (Nexaband, Phoenix, AZ) (12).
Determination of survival and lung and spleen K. pneumoniae colony forming units (CFUs)
Following intratracheal inoculation with K. pneumoniae, mice were evaluated for survival daily for 7 days. 4 and 24 h following K. pneumoniae challenge, mice were euthanized by CO2 asphyxiation, and lungs and spleen were harvested for CFU determinations as previously described (26). Briefly, lungs and spleen were homogenized in 0.5 ml of sterile saline, serially diluted, and plated on soy-based blood agar plates (Difco). After 18 h at room temperature, CFUs were enumerated.
Blood and lung leukocyte differential and total cell count
4 and 24 h post-infection, lung leukocytes were obtained from mice by BAL following CO2 asphyxiation and differential counts were performed on cells following staining with a modified Wright-Geimsa stain (American Scientific Products, McGraw Park, IL). Blood was collected by cardiac puncture for peripheral white blood cell counts using a Hemavet cell analyzer (Drew Scientific) operated by the University of Michigan Unit for Laboratory Animal Medicine Animal Diagnostic Laboratory.
Determination of cytokines, cysteinyl-leukotrienes (cysLTs), leukotriene B4 (LTB4), prostaglandin E2 (PGE2), and leptin
In a separate group of mice, lungs obtained from euthanized mice 4 and 24 h post-infection, were homogenized and cytokine (CXCL2 [MIP-2], CCL2 [MCP-1], IL-6, IL-10, IL-12, and TNF-α) (R&D Duoset, R&D Systems, Minneapolis, MN) and cysLTs, LTB4, and PGE2 (Cayman Chemical, Ann Arbor, MI) levels were determined using commercially available EIA kits according to the manufacturer’s instructions. Blood leptin levels were determined using an EIA kit from R&D Systems according to the manufacturer’s instructions.
Fluorometric assay of AM phagocytosis
AM phagocytosis of K. pneumoniae was assessed using a previously published protocol for determining the ingestion of fluorescent, fluorescein isothiocyanate (FITC)-labeled S.pneumoniae (24). Briefly, AMs obtained by BAL were adhered and seeded in replicates of 8 to 384-well tissue culture plates with opaque sides and optically clear bottoms (Costar, Corning Inc. Life Sciences, Lowell, MA) and cultured overnight with RPMI 1640 with 1% Pen/Strep and 10% fetal calf serum (Invitrogen). On the following day, FITC-labeled K. pneumoniae were opsonized with 3% immune serum as previously described (27). AMs pretreated with RPMI media alone, with indomethacin (10 µM) (Cayman Chemical, Ann Arbor, MI) for 30 min, or cysLTs (100 nM) and LTB4 (1 nM) (alone or together) for 15 min were incubated with opsonized FITC-K. pneumoniae using an MOI of 150:1 for 60 min to allow phagocytosis to occur. Trypan blue (250 µg/ml, Molecular Probes) was added for 1 min to quench the fluorescence of extracellular bacteria and fluorescence was determined using a SPECTRAMax GEMINI EM fluorometer 485ex/535em (Molecular Devices, Sunnyvale, CA). The phagocytic index was calculated as previously described in relative fluorescence units (RFU) (18, 24). Three separate experiments were conducted with 8 replicate wells for every experimental condition and the relative fluorescence units were normalized to the control condition (untreated AMs from WT animals) in each experiment.
Bactericidal Assays
The survival of internalized K. pneumoniae within the AM was quantified using a tetrazolium dye reduction assay, as described previously (27). Briefly, 2 × 105/mL AMs, prepared as described previously, were adhered in quintuplicate in 96-well, half-area, tissue culture dishes (Corning, Inc., Lowell, MA). Following overnight culture, K pneumoniae were opsonized with 3% anti-K pneumoniae rat specific immune serum, as previously described (28). Cells were then treated with either cell culture media alone or indomethacin (10 µM) for 30 minutes and were infected with a 0.1-mL suspension of opsonized K pneumoniae (1 × 107 colony-forming units (CFU)/ml); multiplicity of infection (MOI, 50:1) for 30 minutes to allow phagocytosis to occur. The AMs were then washed 3x with PBS to remove extracellular bacteria and incubated for an additional 60 min to permit intracellular killing. The remainder of the assay was completed, as described elsewhere (27). Based on this assay, it has been determined that the intensity of the absorbance at 595 nm is directly proportional to the number of intracellular bacteria associated with the macrophages. Results were expressed as percentage of survival of ingested bacteria, where the survival of ingested bacteria = 100% × A595 control plate/A595 experimental plate.
Reactive oxygen intermediate (ROI) production
AMs were adhered to 384-well plates at a concentration of 1.25 × 105 cells/well and cultured overnight in RPMI 1640 containing 10% FCS and antibiotics. On the next day, the medium was replaced with PBS containing 10 µM H2DCF and the cells were cultured for 1 h. The medium was then replaced with warmed HBSS, and the cells were stimulated with heat-killed K. pneumoniae (K.p) opsonized with 3% specific immune serum using a multiplicity of infection of 50:1. Reactive oxygen intermediate (ROI) production was assessed every 30 min for 2 h by measuring fluorescence using a Spectramax Gemini XS fluorometer (Molecular Devices, Sunnyvale, CA) with excitation/emission setting at 493/522 nm.
Assessment of Nitric oxide (NO) production
AMs were adhered to 96-well plates at a concentration of 2 × 105 cells/well and cultured with DMEM supplemented with 1% sodium pyruvate (Invitrogen) containing 10% FCS and penicillin/streptomycin with or without 10 ng/ml lipopolysaccharide (LPS) from E. coli (Sigma-Aldrich) and 10 ng/ml IFN-γ (R&D Systems) for 24 h. NO production was determined by measuring stable nitrite (NO2−) concentrations using a modified Griess reaction with a commercially available assay kit, according to the manufacturer’s instructions (Cayman Chemical, Ann Arbor, MI).
Measurement of intracellular cyclic adenosine monophosphate (cAMP) by AMs in vitro
AMs were cultured overnight in 96-well plates in RPMI 1640 with 10% fetal calf serum and 1% Pen/Strep at concentrations of 2 × 106 cells/well. On the following day, the cell culture media was replaced with warm RPMI and AMs were incubated for 30 min in the presence or absence of indomethacin (10 µM) with the phosphodiesterase inhibitor 3-isobutyl-1-methylxantine (IBMX) (EMD Biosciences) for 30 min prior to stimulation with K.p. opsonized with 3% rat specific immune serum using a multiplicity of infection of 50:1. After 1 h, culture supernatants were aspirated and the cells were lysed by incubation for 20 min with 0.1 M HCl (22°C) for cAMP experiments. The cells were then disrupted using a cell scraper and intracellular cAMP levels were determined by enzyme-linked immunosorbent assay kit according to the manufacturer (Cayman Chemicals).
Statistical analyses
Where appropriate, mean values were compared using a paired Student t-test, a one-way or a two-way analysis of variance (ANOVA) followed by the Bonferroni correction. Survival was evaluated for differences using a log-rank test. Differences were considered significant if P ≤ 0.05 and the actual P values are mentioned in the results section. All experiments were performed on at least three separate occasions unless otherwise specified. Data are presented as mean values ± standard error of the mean unless otherwise noted.
Results
Substitution of LepRb Tyr985 with L985 in l/l mice abrogates LepRb mediated ERK1/2 activation
In order to confirm that l/l mice lack the ability to signal via LepRb Tyr985, we assessed ERK 1/2 activation using immunoblot analysis of pERK 1/2 in AMs obtained from WT and l/l mice cultured with leptin. As shown in Figure 1C, levels of total ERK 1/2 were the same for both groups of mice. However, when AMs from WT mice were cultured with exogenous leptin for 30 min, we observed an increase in pERK1/2 as determined by a 50% increase in pERK1/2 (p=0.0002). We conducted time course experiments for ERK activation (pERK) (i.e. 5, 15, and 30 mins after stimulation with leptin) and only the blots from cells stimulated for 30 min are shown since this represents the peak of this response. In contrast, we did not observe any increases in pERK 1/2 levels in AMs from l/l mice following leptin treatment for 30 min or at any other time point (p=0.12). Other signaling events initiated by this mutant receptor such as LepRb→STAT3 or STAT5 are normal as previously reported (21, 23). In addition, hypothalamic pERK activation was not observed in a previous report using l/l mice treated with much higher doses of leptin (5 µg/g of body wt) (23). Blood leptin levels were slightly lower (p=0.04) in the l/l mice (2.9 ± 0.5 ng/ml) compared with that of WT animals (4.5 ± 0.9 ng/ml) as previously reported (23). These data indicate that leptin induces phosphorylation of ERK 1/2 via the LepRb Tyr985 and that this pathway is abrogated in AMs from l/l mice.
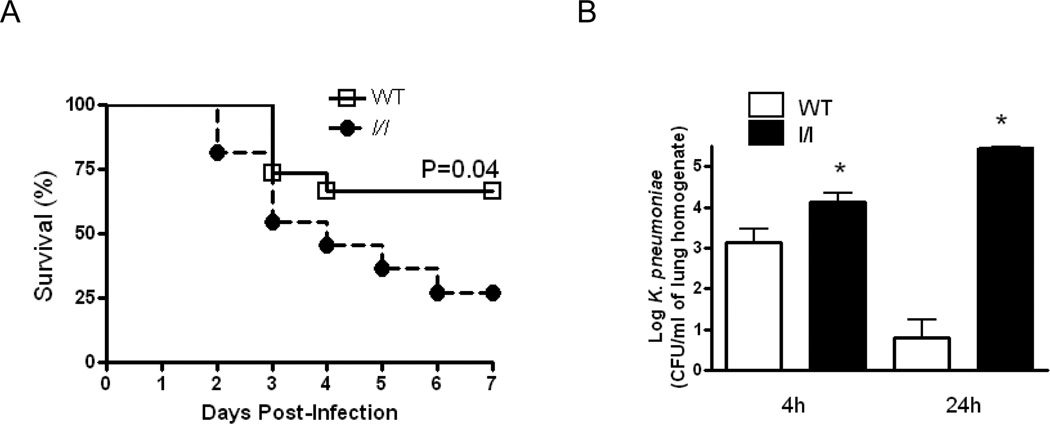
l/l mice exhibit greater mortality and reduced pulmonary bacterial clearance following K. pneumoniae challenge
We have previously demonstrated that ob/ob mice which lack functional leptin or mice rendered leptin deficient by fasting are more susceptible to both gram negative and gram positive pneumonia (12–13). In order to determine if intracellular signals arising from the LepRb Tyr985 play a role in pulmonary host defense against gram-negative pneumonia, we compared the responses of WT and l/l mice following an intratracheal challenge with K. pneumoniae. As shown in Figure 2A, l/l mice exhibited substantially lower survival (27%) as compared with WT (65%, p=0.04) following K. pneumoniae challenge 7 days post-infection. Since the differences in survival may indicate impaired pulmonary host defense in l/l mice, we assessed the bacterial burdens in the lungs and spleen of mice 4 and 24 h post-infection. We chose these time points since we observed that the first death recorded for an l/l mouse occurred 48 h after K. pneumoniae challenge. As shown in Figure 2B, bacterial burdens were approximately 1-log fold greater after 4 h (p=0.02) and 4-log fold higher at 24 h (p=0.0001) in l/l as compared with WT animals. We did not find any bacterial CFUs in spleens harvested from any of these animals 4 h (p=0.38) and 24 h (p=0.73) post-infection. These results indicate a defective pulmonary innate immune response against pulmonary bacterial infection in l/l mice.
Figure 2.
Reduced survival and impaired pulmonary bacterial clearance in l/l mice following an intratracheal challenge with K. pneumoniae. Wild type (WT) and l/l mice were challenged with 5 × 103 CFU of K. pneumoniae via the intratracheal route and monitored for survival for 7 days (A). In another group of animals, WT (open bars) and l/l (solid bars) mice were euthanized 4 and 24 h after infection and lungs were harvested and bacterial burdens in tissue homogenates were determined by counting colony forming units (CFU) as described in Materials and Methods (B). *P <0.05 compared to WT using a log-rank test. Survival curves represent an n=11–15 mice per group from 3 separate experiments. *P < 0.05 compared to WT levels at 4 and 24h after infection using a Student’s t-test. Bars represent the mean ± SEM of n=10 mice per group from three separate experiments.
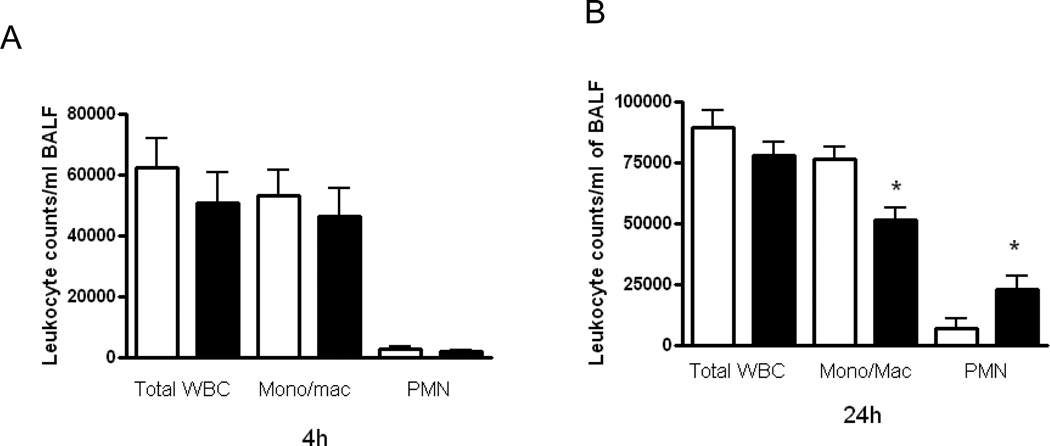
Modest differences between WT and l/l mice in leukocyte recruitment following K. pneumoniae challenge
The recruitment of leukocytes to the lungs is essential for effective host defense during bacterial pneumonia. We have previously observed increased PMN recruitment to the lungs of leptin deficient (ob/ob) mice and attenuated leukocyte recruitment in mice rendered leptin deficient by fasting in response to pneumococcal pneumonia (12–13). In order to determine if the ablation of LepRb Tyr985 signaling alters leukocyte recruitment to the lung during bacterial pneumonia, we recovered leukocytes by lavage in mice 4 and 24 h post-infection since these were the time points when we observed differences in pulmonary bacterial burdens. As shown in Figure 3, there were no differences (p=0.29) between WT and l/l mice in total or differential leukocyte counts in BALF 4 h after K. pneumoniae challenge. However, we did find lower BALF monocyte/macrophage (p=0.04) and higher neutrophil (p=0.04) counts in l/l mice at this time point. We also observed higher total peripheral blood leukocyte counts (l/l: 6.7 × 106 vs. WT: 4.1 × 106 WBC/ml) (p=0.04) and elevated PMN counts (l/l: 3.8 × 106 vs. WT: 1.9 × 106) (p=0.04) in the l/l mice 24 h post-infection. These results indicate that differences in PMN recruitment, known to play a critical role in host defense against gram-negative pneumonia (29), do not explain the limitations in Klebsiella clearance from the lungs of l/l mice.
Figure 3.
Leukocyte recruitment in wild type (WT) and l/l mice following intratracheal K.pneumoniae infection. WT (open bars) and l/l (solid bars) mice were infected via the intratracheal route with 5 × 103 CFUs of K. pneumoniae. 4 and 24 h later, cells were recovered by bronchoalveolar lavage and the total differential leukocyte, monocytes/macrophages (Mono/Mac), and neutrophil (PMN) counts were determined as described in Materials and Methods. Bars represent the mean ± SEM of n=10 mice per group. *P <0.05 compared to WT using a Student’s t-test.
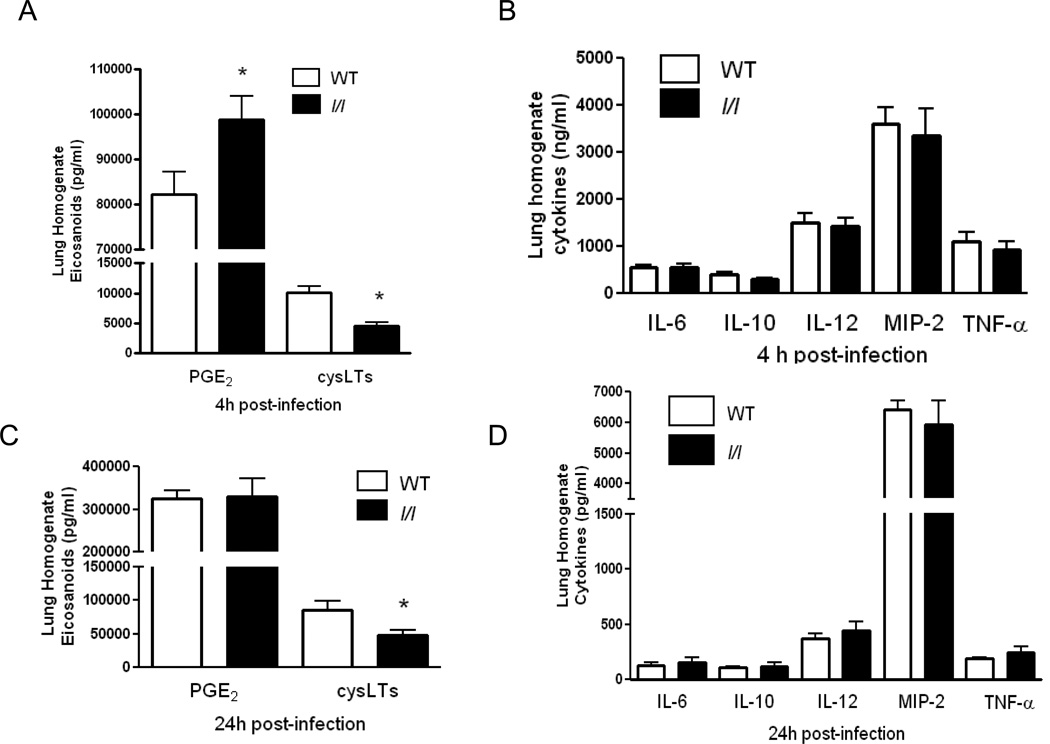
Pulmonary cytokine and eicosanoid levels post-K. pneumoniae challenge
In order to determine whether the impairment in pulmonary bacterial clearance was due to differences in proinflammatory mediators produced in the lung during infection, we assessed lung homogenate cysteinyl-LTs, PGE2, and cytokine levels after bacterial challenge. As shown in Figure 4A, there were no differences in lung homogenate cytokines known to play an important role in antibacterial host defense (IL-6, TNF-α, IL-10, IL-12, MIP-2, TNF-α) 4 h post-infection. However, we did find major differences in lipid mediators. COX derived PGE2 was elevated (p=0.02) while the 5-LO derived cysteinyl-LTs were reduced (p=0.01) in l/l mice at this time point. Reduced cysLT levels persisted 24 h post-infection in the lungs of l/l mice (p=0.02) (Figure 4C) while no differences in PGE2 or cytokines were observed (p=0.93) (Figure 4D).
Figure 4.
Elevated prostaglandin E2 and reduced cysteinyl-leukotrienes (cysLT) in l/l mice in lung homogenates following intratracheal K.pneumoniae challenge. Wild type (WT) (open bars) and l/l (solid bars) mice were infected via the intratracheal (i.t.) route with K.pneumoniae. Lung homogenates were prepared from mice 4 (A and B) and 24h (C and D) after i.t. bacterial challenge and eicosanoids, cysteinyl-leukotrienes (cysLTs) and prostaglandin E2 (PGE2), and cytokines (IL-6, IL-10, IL-12, MIP-2, and TNF-α) were determined as mentioned in Materials and Methods. *P < 0.05 compared to WT levels at 4 and 24 hr after infection using a Student’s t-test. Bars represent the mean ± SEM of n=5–10 mice per group.
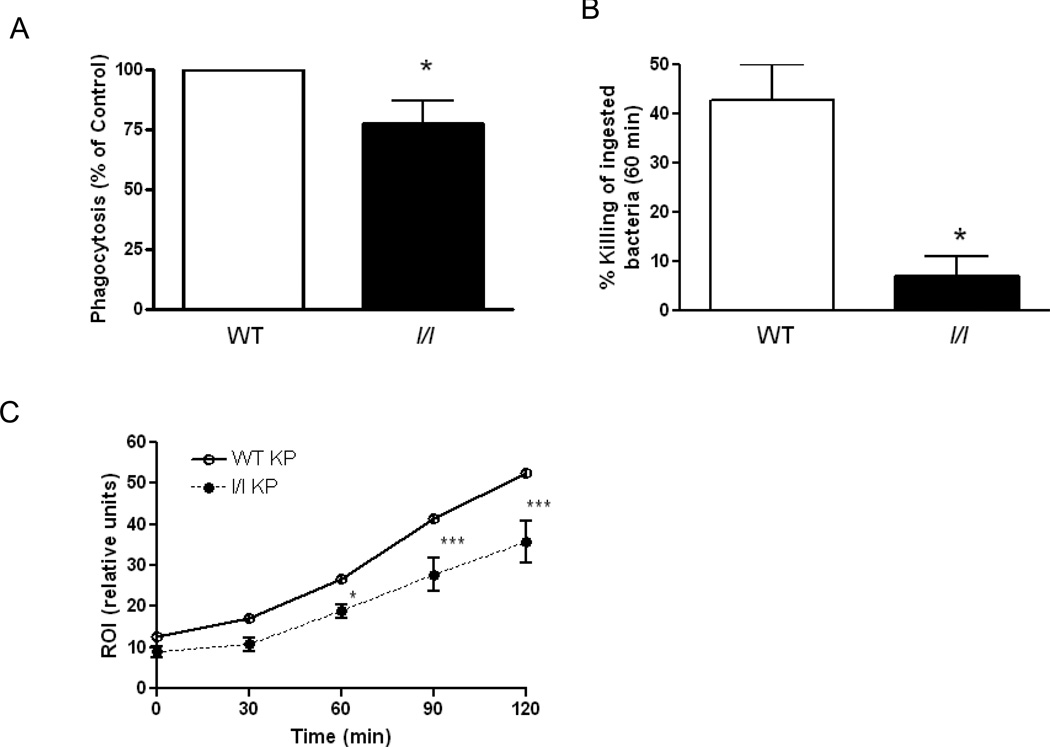
Impaired phagocytosis, killing and reactive oxygen intermediate production in AMs from l/l mice
The observed 1-log fold elevation of bacterial CFUs in l/l mice 4 h post-infection suggests a defect in the pulmonary innate immune response, and implicates the resident AM, which plays a critical role in the early stages of K. pneumoniae clearance. To test this possibility, we compared the ability of cells from WT and l/l mice to phagocytose and kill opsonized K. pneumoniae in vitro. As shown in Figure 5A, phagocytosis of K. pneumoniae opsonized with immune serum was 25% less in AMs from l/l mice than that observed in cells from WT animals (p=0.03). Next, we asked if there were differences in the ability of AMs from WT and l/l mice to kill ingested K. pneumoniae. As shown in Figure 5B, we observed that AMs from WT mice were able to kill 40% of the bacteria that had been phagocytosed during the allotted interval. In contrast, AMs from l/l mice were able to kill less than 10% of the phagocytosed bacteria during this interval, suggesting a severe defect in AM effector function (WT vs l/l, p=0.04). Previously, we had demonstrated that ROI production in neutrophils from ob/ob mice stimulated with S. pneumoniae was reduced (14). In the current study, we observed that ROI production was reduced in AMs from l/l mice, as compared with that of WT animals, by approximately 40% 120 min after stimulation with opsonized K. pneumoniae (p=0.0008). Likewise, we also observed a similar reduction in ROI production in glycogen-elicited PMNs obtained from l/l mice (p=0.01) (data not shown). We did not, however, find differences in AM nitric oxide production following stimulation overnight with LPS and IFN-γ (p=0.15) (data not shown). These results indicate that the LepRb→Tyr985 mutation in l/l mice impairs AM phagocytosis and killing of ingested bacteria and reduces the ability of AMs and PMNs to generate ROI in vitro.
Figure 5.
Impaired phagocytosis, bacterial killing, and reactive oxygen intermediate production in AMs from l/l mice. AMs obtained from WT (open bars) and l/l (solid bars) mice were assessed for their ability to phagocytose (A) and kill ingested K. pneumoniae opsonized with immune serum (B) according to the procedures described in the Materials and Methods section. *P < 0.05 compared to WT using a student t-test. Bars represent the mean ± SEM of at least 3 separate experiments. AMs from WT (open circles) and l/l (closed circles) mice were cultured with H2DCF for 1 h, and then stimulated with heat-killed K. pneumoniae opsonized with immune serum using a multiplicity of infection of 100:1 to assess reactive oxygen intermediate production (ROI) (C). ROI production was assessed using a fluorometer and expressed as relative fluorescence units. The data represent the mean of three experiments completed in quadruplicate for each time point using a two way analysis of variance. *P < 0.05 and ***P<0.001 compared WT.
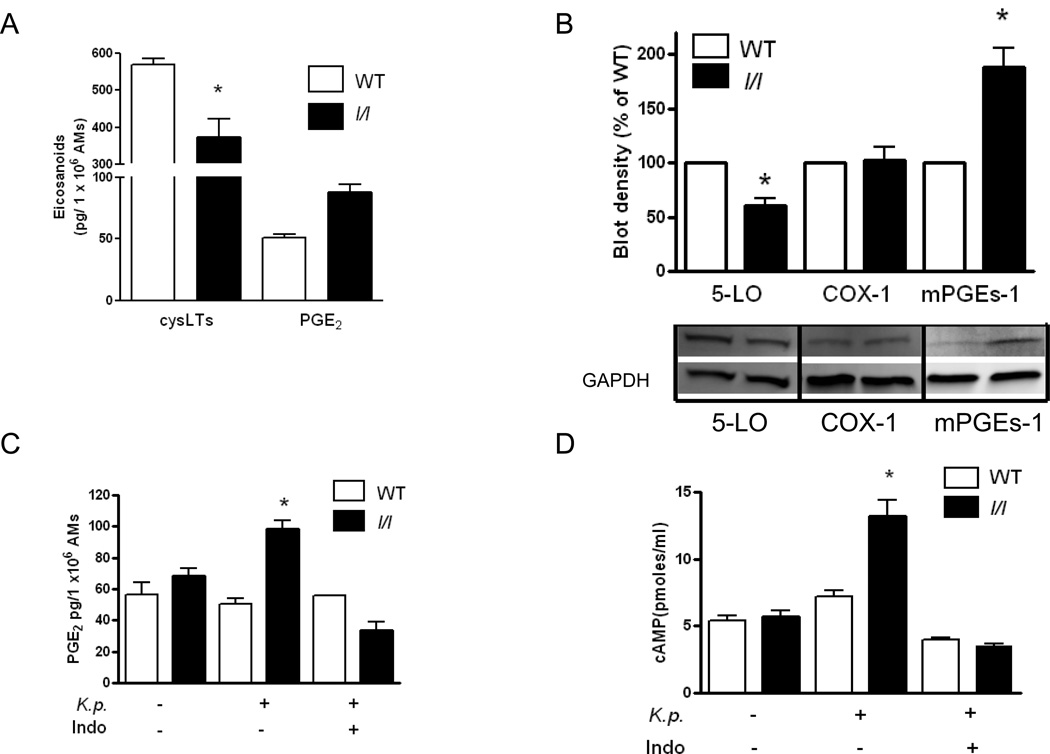
Diminished LTs and enhanced PGE2 production explained by reduced 5-LO and increased mPGEs-1 expression in AMs from l/l mice
The ability of AMs to phagocytose and kill bacteria is enhanced by LTs and reduced by PGE2 during bacterial pneumonia (14, 30–31). Since we observed differences in the levels of these eicosanoids in the lungs of mice after bacterial challenge in vivo, we asked if LT and PGE2 synthesis were altered in AMs from l/l mice in vitro. PGE2 production in AMs stimulated for 1 h with heat killed K. pneumoniae was approximately two-fold greater in cells from l/l mice (p=0.0018) (Figure 6A). In contrast, using the same stimulus, cysLT (p=0.04) and LTB4 (not shown (p=0.03)) production were decreased in AMs from l/l mice. Next, we assessed the expression of 5-LO, COX-1, COX-2, and mPGEs-1, enzymes known to play essential roles in LT and PGE2 synthesis in AMs, respectively. In comparison with cells from WT animals, 5-LO protein expression in AMs from l/l mice was reduced by approximately 40% (p=0.008). While there were no differences in COX-1 (p=0.86) or COX-2 (p=0.90) (data not shown) expression, we did find a 90% increase in the levels of mPGEs-1 in AMs from l/l mice (p=0.04). These changes in enzyme expression therefore explain the reduced LT and enhanced PGE2 production in l/l mice.
Figure 6.
Eicosanoid synthesis, eicosanoid biosynthetic enzyme expression, and cAMP levels in AMs from WT (open bars) and l/l (solid bars) mice. AMs from WT (open bars) and l/l (solid bars) mice were stimulated with heat killed K. pneumoniae (K.p.) opsonized with specific immune serum for 1 h and cysteinyl-leukotrienes (cysLT) and prostaglandin E2 (PGE2) were determined by ELISA as described in the Materials and Methods section (A). Immunoblots for 5-lipoxygenase (5-LO), cyclooxygenase-1 (COX-1), microsomal prostaglandin E synthase-1 (mPGEs-1), and glyceraldehydes-phosphate-dehydrogenase (GAPDH) (protein loading control) in AM lysates from a single experiment that is representative of a total of 3 independent experiments. Bars represent the mean arbitrary densitometric units ± SEM of immunoblots from 3 separate experiments (B). AMs cultured with and without indomethacin (Indo) (10 µM) for 30 min were stimulated with or without heat killed K. pneumoniae (K.p.) opsonized with immune serum for 1 h and the cell culture media was assayed for PGE2 (C) and AM lysates were assayed for cAMP as described in the Materials and Methods section (D). * P<0.05 vs WT using the students t-test.
Elevated PGE2 production mediates enhanced cAMP levels in AMs from l/l mice
An important mechanism by which LTs and PGE2 differentially regulate AM phagocytosis and killing of K. pneumoniae in vitro is via decreases and increases, respectively, in the levels of the second messenger cAMP, which is known to inhibit phagocytosis and bacterial killing (30–31). First, we confirmed that AMs from l/l mice produced more PGE2 than cells from WT mice (p=0.001) following stimulation with heat killed K. pneumoniae and this response could be blocked with indomethacin (Figure 6C). Next, we assessed intracellular cAMP levels and observed that AMs from l/l mice stimulated with K. pneumoniae for 1 h produced twice as much cAMP compared with AMs from WT animals (p=0.001). The increase in intracellular cAMP was completely blocked when cells from l/l mice were pretreated with indomethacin (Figure 6D). AMs from l/l mice pretreated with LTB4 did not significantly affect cAMP production following stimulation with K. pneumoniae (p=0.37) (data not shown). These results suggest that the increased production of PGE2 mediates the increased cAMP levels in AMs from l/l mice.
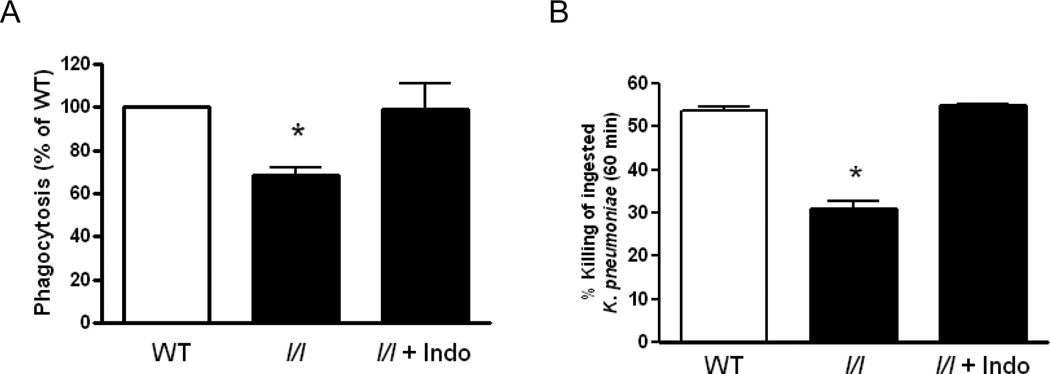
Indomethacin restores phagocytosis and bacterial killing in AMs from l/l mice
We next assessed the ability of addition or blockade of lipid mediators to restore AM effector functions in vitro. As shown in Figure 7A and B, we again observed deficient bacterial phagocytosis (p=0.002) and killing (p=0.002) in AMs from l/l mice. While we have previously reported that exogenous administration of LTB4 or cysLTs augments phagocytosis and killing in AMs from WT mice and rats (27, 32), these lipids failed to improve these endpoints in cells from l/l mice (data not shown). However, blocking PGE2 production with indomethacin restored defective antimicrobial responses in AMs from l/l mice. These results suggest that the defects in pulmonary host defense against K. pneumoniae in vivo were mainly due to the enhanced production of PGE2 in cells from l/l mice. They also imply that the LepR mutation in l/l mice impairs responsiveness to LTs.
Figure 7.
Inhibition of PGE2 synthesis with indomethacin restores bacterial phagocytosis and killing in AMs from l/l mice. AMs obtained from WT and l/l mice were cultured with and without indomethacin (Indo) (10 µM) for 30 min prior to the addition of K. pneumoniae opsonized with immune serum. Phagocytosis (A) and bacterial killing of K.pneumoniae ingested by AMs from WT and l/l mice were assessed according to procedures mentioned in the Materials and Methods section. Bars represent the mean ± SEM of at least 3 separate experiments. *P<0.01 vs WT and l/l + Indo by ANOVA using the Bonferroni test for mean separation.
Discussion
In this study, we report the novel observation that l/l mice which lack LepRb → ERK1/2 activation via Tyr985 exhibit greater susceptibility to Gram-negative pneumonia. The defect in pulmonary host defense in l/l mice was associated with impaired AM phagocytosis and killing of bacteria in vitro. In addition, we also observed increased PGE2 and reduced LTs after bacterial challenge in the lung in vivo and in AMs following culture with heat killed bacteria in vitro. Phagocytosis and killing could be restored if AMs from l/l mice were pretreated with the cyclooxygenase inhibitor, indomethacin, which normalized eicosanoid synthesis and intracellular cAMP levels. These results provide novel insights into the role of leptin receptor mediated signaling in the innate immune response against bacterial infection.
There are now a number of reports demonstrating that leptin or leptin receptor deficiency disables host defense against bacterial infections (12–15, 19, 33–35). Unlike other models of leptin or leptin receptor deficiency, the l/l mouse is neither obese nor hyperglycemic and thus provides an excellent model for assessing the importance of distinct LepR mediated signaling events in host defense against bacterial infection in the absence of endocrine abnormalities. We recently reported that LepRb–STAT3 activation is not essential for host defense, since ablating this pathway in s/s mice which possess a mutant LepRb were obese and resistant to pneumococcal pneumonia in vivo and this was associated with improved AMs antibacterial functions in vitro (18). Interestingly, these mice were protected by an enhanced ability to produce LTs and this improved AM antibacterial functions. In the current study, we observed that ablation of the LepRb→Tyr985 pathway in l/l mice, which were lean, resulted in defective host defense against K. pneumoniae in vivo and diminished AM effector functions in vitro. These differences were also most likely due to alterations in eicosanoid production.
One novel observation in this report was the enhanced synthesis of the immunosuppressive eicosanoid, PGE2, in the lungs and in AMs of l/l mice after infection. This enhancement was the result of the increased expression of mPGEs-1 as demonstrated by immunoblot analysis. The mechanism underlying this enhancement is unknown and beyond this scope of this report. PGE2 suppresses bacterial phagocytosis, ROI generation, and bacterial killing in AMs and these effects are mediated through the E-prostanoid 2 receptor (EP2), a Gαs-protein coupled receptor which activates adenylate cyclase and increases intracellular cAMP levels (30–31). We also demonstrated elevated cAMP levels in AMs from l/l mice stimulated with bacteria in vitro and this response could be blocked with the cyclooxygenase inhibitor, indomethacin. This approach also normalized impaired phagocytosis and killing of K. pneumonia in AMs from l/l mice in vitro. Other reports have also demonstrated that elevated pulmonary PGE2 synthesis suppresses host defense against bacterial pneumonia in vivo and this defect can be rescued with indomethacin or through genetic ablation of the EP2 receptor (36–37). These results suggest that the defects in host defense in l/l mice were largely due to the overproduction of PGE2 during bacterial pneumonia.
Another unexpected and novel finding in this report was the lower levels of LTs produced by l/l mice following pulmonary bacterial challenge in vivo and in AMs in vitro. This result was likely due to reduced 5-LO protein whose expression is regulated by transcription factors Sp1 and Egr1 (38). Leptin is known to enhance the expression of both these transcription factors and the lack of LepRb→Tyr985 signaling may have reduced their expression in l/l mice (22, 39–40). The LTs play a protective role in Klebsiella pneumonia since 5-LO knockout mice exhibited increased lethality and reduced pulmonary bacterial clearance (41). Furthermore, LT production was diminished in AMs from leptin-deficient (ob/ob) mice and exogenous leptin restored LT synthesis and AM phagocytosis and killing of K. pneumoniae in vitro (12, 27, 32). However, the provision of exogenous LTs did not reduce cAMP levels or restore antibacterial responses in AMs from l/l mice, suggesting a defect in LT receptor responsiveness or signaling. Further evaluation of this possibility is a focus of future investigation but beyond the scope of the current report.
Our data implicate dysregulated eicosanoid generation in AMs in the phenotype observed in l/l mice following K. pneumoniae challenge in vivo and in vitro. Based on pSTAT3 staining as a surrogate marker for the expression of the LepRb in the murine lung inflated with PBS containing leptin, we have shown that the LepRb is expressed primarily in AMs, and to a much lesser extent, in alveolar epithelial cells (18). Therefore, only those cells that express high levels of this receptor are likely to be influenced by the lack of LepRb→Tyr985 signaling. The primary sources of LTs in the lung during bacterial pneumonia are the resident AMs and PMNs known to express high levels of 5-LO. As a consequence, we observed reduced production of LTs at both time points following K. pneumoniae challenge in vivo and in AMs vitro. In contrast, the expression mPGEs-1 is not limited to AMs and would be present in alveolar epithelial cells which do not express high levels of the LepRb (18, 42). Consistent with this, we observed increased PGE2 production in AMs stimulated in vitro and 4 h post-infection in the lungs of l/l mice in vivo. Under these circumstances, the AM is the major source of PGE2. 24 h post-Klebsiella challenge, the alveolar epithelial cells are the major producers of PGE2 in vivo and there were no differences in lung PGE2 levels between WT and l/l mice. The impairment in pulmonary bacterial clearance in l/l mice in vivo was therefore most likely due to the elevated levels of PGE2 produced by AMs. PGE2, by increasing intracellular cAMP, is known to impair AM phagocytosis and killing of bacteria and to reduce ROI production, all of which are required for the elimination of K. pneumoniae (27, 30–31).
The reduced number of monocyte/macrophages recovered from the lungs of l/l mice 24h post-Klebsiella challenge was not due to impairments in either chemokine (MCP-1) production or peripheral blood monocyte counts (data not shown), which did not differ from that of WT mice. It is also unlikely that the reduction of LTs in the lungs of l/l mice was responsible for lower monocyte/macrophage counts 24 h post-K. pneumoniae challenge since no differences in lung leukocytes counts were reported in 5-LO knockout mice following K. pneumoniae challenge (41). While we did not assess cell viability, we speculate that the reduced monocyte/macrophage population in the lung of l/l mice 24 h post-K. pneumoniae challenge may reflect increased apoptosis of these cells since leptin is known to enhance the survival of human monocytes via a ERK1/2 dependent pathway (43). In support of this speculation, Guo et al. reported increased cell death and disruption of the intestinal epithelium in l/l mice following infection with E. histolytica (17). In contrast to monocytes/macrophages, the increased numbers of PMNs in BALF and the peripheral blood of l/l mice 24 h after K. pneumoniae challenge were likely due to the higher pulmonary bacterial burdens in these animals. Based on this result, it appears that the observed defect in ROI generation in PMNs (data not shown), rather than recruitment, may have contributed to the impairment in pulmonary bacterial clearance in l/l mice at this later time point. Finally, it is acknowledged that other bactericidal mechanisms may be dysfunctional in leukocytes from the l/l animals.
In summary, we report, for the first time, that LepRb→Tyr985 intracellular signaling plays a critical role in the host response against Gram-negative pneumonia in vivo and in leukocyte antibacterial functions in vitro. At present, defects in human LepRb→ERK activation have not been identified. However, a leptin receptor mutation was associated with greater susceptibility to intestinal parasitic infections in humans (44). A greater understanding of the role of leptin receptor signaling in host defense against infection will facilitate the development of targeted therapeutic interventions for the prevention and treatment of bacterial pneumonia.
Acknowledgments
The authors thank Justin Jones for his assistance in genotyping the l/l mice for these studies.
Funding: This work was supported by grants from the NIH, HL077417 to P.M., HL058897 to M. PG, DK56731 to M. G. M. Jr., T32ES007062 which supported E.O., HL10377701 to C.H.S., and a grant from the Flight Attendants Medical Research institute (FAMRI) CIA-103071 to P.M.
Non-standard abbreviations
- AM
alveolar macrophage
- BAL
bronchoalveolar lavage
- COX-1
cyclooxygenase-1
- cysLTs
cysteinyl-leukotrienes
- ERK
extracellular regulated kinase
- GRB2
growth factor binding 2
- H.K. K.p.
heat-killed K.pneumoniae
- JAK
janus kinase
- 5-LO
5-lipoxygenase
- LepRb
long isoform of the leptin receptor
- LT
leukotriene
- LTB4
leukotriene B4
- mPGEs-1
microsomal prostaglandin E synthase-1
- MOI
multiplicity of infection
- PGE2
prostaglandin E2
- STAT3
signal transducer and activator of transcription 3
- SOCS3
suppressor of cytokine signaling 3
References
- 1.Mizgerd JP. Lung infection--a public health priority. PLoS medicine. 2006;3:e76. doi: 10.1371/journal.pmed.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palacio A, Lopez M, Perez-Bravo F, Monkeberg F, Schlesinger L. Leptin levels are associated with immune response in malnourished infants. J. Clin. Clin. Endocrinol. Metab. 2002;87:3040–3046. doi: 10.1210/jcem.87.7.8636. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez L, Graniel J, Ortiz R. Effect of leptin on activation and cytokine synthesis in peripheral blood lymphocytes of malnourished infected children. Clin Exp Immunol. 2007;148:478–485. doi: 10.1111/j.1365-2249.2007.03361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Ari Z, Schafer Z, Sulkes J, Manhaim V, Tur-Kaspa R, Fainaru M. Alterations in serum leptin in chronic liver disease. Dig Dis Sci. 2002;47:183–189. doi: 10.1023/a:1013248427783. [DOI] [PubMed] [Google Scholar]
- 5.Scholze A, Rattensperger D, Zidek W, Tepel M. Low serum leptin predicts mortality in patients with chronic kidney disease stage 5. Obesity (Silver Spring) 2007;15:1617–1622. doi: 10.1038/oby.2007.191. [DOI] [PubMed] [Google Scholar]
- 6.Langouche L, Vander Perre S, Frystyk J, Flyvbjerg A, Hansen TK, Van den Berghe G. Adiponectin, retinol-binding protein 4, and leptin in protracted critical illness of pulmonary origin. Crit Care. 2009;13:R112. doi: 10.1186/cc7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maffei M, Halass J, Ravussin E, Pratlet RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern PA, Freidman JM. Leptin levels in human and rodent: measurements of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 8.Grunfeld C, Zhao C, Fuller J, Pollack A, Moser A, Friedman J, Feingold KR. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J. Clin. Clin. Invest. 1996;97:2152–2157. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarraf P, Frederich RC, Turner EM, Ma G, Jaskowiak NT, Rivet DJ, III, Flier JS, Lowell BB, Fraker DL, Alexander HR. Multiple cytokines and acute inflammation raise mouse leptin levels: Potential role in inflammatory anorexia. J. Exp. Exp. Med. 1997;185:171–176. doi: 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bornstein SR, Licinio J, Tauchnitz R, Engelmann L, Negrao A, Gold P, Chrousos GP. Plasma leptin levels are increased in survivors of acute sepsis: associated loss of diurnal rhythm in cortisol and leptin secretion. J Clin Endocrinol Metab. 1998;83:280–283. doi: 10.1210/jcem.83.1.4610. [DOI] [PubMed] [Google Scholar]
- 11.Moshyedi AK, Josephs MD, Abdalla EK, Mackay SLD, Edwards CK, Copeland EM, Moldawer LL. Increased leptin expression in mice with bacterial peritonitis is partially regulated by tumor necrosis factor alpha. Infect. Immun. 1998;66:1800–1802. doi: 10.1128/iai.66.4.1800-1802.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mancuso P, Gottschalk A, Phare SM, Peters-Golden M, Lukacs NW, Huffnagle GB. Leptin-deficient mice exhibit impaired host defense in gram-negative pneumonia. J. Immunol. 2002;168:4018–4024. doi: 10.4049/jimmunol.168.8.4018. [DOI] [PubMed] [Google Scholar]
- 13.Mancuso P, Huffnagle GB, Olszewski MA, Phipps J, Peters-Golden M. Leptin corrects host defense defects following acute starvation in murine pneumococcal pneumonia. Am J Respir Crit Care Med. 2006;173:212–218. doi: 10.1164/rccm.200506-909OC. [DOI] [PubMed] [Google Scholar]
- 14.Hsu A, Aronoff DM, Phipps J, Goel D, Mancuso P. Leptin improves pulmonary bacterial clearance and survival in ob/ob mice during pneumococcal pneumonia. Clin Exp Immunol. 2007;150:332–339. doi: 10.1111/j.1365-2249.2007.03491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikejima S, Sasaki S, Sashinami H, Mori F, Ogawa Y, Nakamura T, Abe Y, Wakabayashi K, Suda T, Nakane A. Impairment of host resistance to Listeria monocytogenes infection in liver of db/db and ob/ob mice. Diabetes. 2005;54:182–189. doi: 10.2337/diabetes.54.1.182. [DOI] [PubMed] [Google Scholar]
- 16.Wieland CW, Florquin S, Chan ED, Leemans JC, Weijer S, Verbon A, Fantuzzi G, van der Poll T. Pulmonary Mycobacterium tuberculosis infection in leptin-deficient ob/ob mice. Int. Immunol. 2005;17:1399–1408. doi: 10.1093/intimm/dxh317. [DOI] [PubMed] [Google Scholar]
- 17.Guo X, Roberts MR, Becker SM, Podd B, Zhang Y, Chua SC, Jr, Myers MG, Jr, Duggal P, Houpt ER, Petri WA., Jr Leptin signaling in intestinal epithelium mediates resistance to enteric infection by Entamoeba histolytica. Mucosal Immunol. 2011;4:294–303. doi: 10.1038/mi.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mancuso P, Peters-Golden M, Goel D, Goldberg J, Brock TG, Greenwald-Yarnell M, Myers MGJ. Disruption of leptin receptor-STAT3 signaling enhances leukotriene production and pulmonary host defense against pneumococcal pneumonia. J Immunol. 2011;186:1081–1090. doi: 10.4049/jimmunol.1001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore SI, Huffnagle GB, Chen GH, White ES, Mancuso P. Leptin modulates neutrophil phagocytosis of Klebsiella pneumoniae. Infect Immun. 2003;71:4182–4185. doi: 10.1128/IAI.71.7.4182-4185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MGJ. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 21.Gong Y, Ishida-Takahashi R, Villanueva EC, Fingar DC, Munzberg H, Myers MG., Jr The long form of the leptin receptor regulates STAT5 and ribosomal protein S6 via alternate mechanisms. J Biol Chem. 2007;282:31019–31027. doi: 10.1074/jbc.M702838200. [DOI] [PubMed] [Google Scholar]
- 22.Bjorbaek C, Buchholz RM, Davis SM, Bates SH, Pierroz DD, Gu H, Neel BG, Myers MG, Jr, Flier JS. Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem. 2001;276:4747–4755. doi: 10.1074/jbc.M007439200. [DOI] [PubMed] [Google Scholar]
- 23.Björnholm M, Munzberg H, Leshan RL, Villanueva EC, Bates SH, Louis GW, Jones JC, Ishida-Takahashi R, Bjorbaek C, Myers MG., Jr Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest. 2007;117:1354–1360. doi: 10.1172/JCI30688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phipps JC, Aronoff DM, Curtis JL, Goel D, O'Brien E, Mancuso P. Cigarette smoke exposure impairs pulmonary bacterial clearance and alveolar macrophage complement-mediated phagocytosis of streptococcus pneumoniae. Infect. Immun. 2010;78:1214–1220. doi: 10.1128/IAI.00963-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mancuso P, Nana-Sinkam P, Peters-Golden M. Leukotriene B4 augments neutrophil phagocytosis of Klebsiella pneumoniae. Infect Immun. 2001;69:2011–2016. doi: 10.1128/IAI.69.4.2011-2016.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenberger MJ, Strieter RM, Kunkel SL, Danforth JM, Goodman RE, Standiford TJ. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J. Immunol. 1995;155:722–729. [PubMed] [Google Scholar]
- 27.Serezani C, Aronoff D, Jancar S, Mancuso P, Peters-Golden M. Leukotrienes enhance the bactericidal activity of alveolar macrophages against Klebsiella pneumoniae through the activation of NADPH oxidase. Blood. 2005;106:1067–1075. doi: 10.1182/blood-2004-08-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mancuso P, Standiford TJ, Marshall T, Peters-Golden M. 5-Lipoxygenase reaction products modulate alveolar macrophage phagocytosis of Klebsiella pneumoniae. Infect Immun. 1998;66:5140–5146. doi: 10.1128/iai.66.11.5140-5146.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenberger MJ, Strieter RM, Kunkel SL, Danforth JM, Laichalk LL, McGillicuddy DC, Standiford TJ. Neutralization of MIP-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella pneumonia. J. Infect. Dis. 1996;173:159–165. doi: 10.1093/infdis/173.1.159. [DOI] [PubMed] [Google Scholar]
- 30.Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J. Immunol. 2004;173:559–565. doi: 10.4049/jimmunol.173.1.559. [DOI] [PubMed] [Google Scholar]
- 31.Serezani CH, Chung J, Ballinger MN, Moore BB, Aronoff DM, Peters-Golden M. Prostaglandin E2 suppresses bacterial killing in alveolar macrophages by inhibiting NADPH oxidase. Am J Respir Cell Mol Biol. 2007;37:562–570. doi: 10.1165/rcmb.2007-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mancuso P, Marshall T, Standiford T, Peters-Golden M. 5-Lipoxygenase reaction products modulate alveolar macrophage phagocytosis of Klebsiella pneumoniae. Infect. Immun. 1998;66:5140–5146. doi: 10.1128/iai.66.11.5140-5146.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conge GA, Gouache P, Joyeux Y, Goichot J, Fournier JM. Influence of different types of experimental obesity on resistance of the mouse to infection by Salmonella typhimurium and Klebsiella pneumoniae. Ann Nutr Metab. 1988;32:113–120. doi: 10.1159/000177423. [DOI] [PubMed] [Google Scholar]
- 34.Wehrens A, Aebischer T, Meyer TF, Walduck AK. Leptin receptor signaling is required for vaccine-induced protection against Helicobacter pylori. Helicobacter. 2008;13:94–102. doi: 10.1111/j.1523-5378.2008.00591.x. [DOI] [PubMed] [Google Scholar]
- 35.Tschop J, Nogueiras R, Haas-Lockie S, Kasten KR, Castaneda TR, Huber N, Guanciale K, Perez-Tilve D, Habegger K, Ottaway N, Woods SC, Oldfield B, Clarke I, Chua S, Jr, Farooqi IS, O'Rahilly S, Caldwell CC, Tschop MH. CNS leptin action modulates immune response and survival in sepsis. J Neurosci. 2010;30:6036–6047. doi: 10.1523/JNEUROSCI.4875-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ballinger MN, Aronoff DM, McMillan TR, Cooke KR, Olkiewicz K, Toews GB, Peters-Golden M, Moore BB. Critical role of prostaglandin E2 overproduction in impaired pulmonary host response following bone marrow transplantation. J Immunol. 2006;177:5499–5508. doi: 10.4049/jimmunol.177.8.5499. [DOI] [PubMed] [Google Scholar]
- 37.Medeiros AI, Serezani CH, Lee SP, Peters-Golden M. Efferocytosis impairs pulmonary macrophage and lung antibacterial function via PGE2/EP2 signaling. J Exp Med. 2009;206:61–68. doi: 10.1084/jem.20082058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coffey MJ, Serezani CH, Phare SM, Flamand N, Peters-Golden M. NADPH oxidase deficiency results in reduced alveolar macrophage 5-lipoxygenase expression and decreased leukotriene synthesis. J Leukoc Biol. 2007;82:1585–1591. doi: 10.1189/jlb.0107019. [DOI] [PubMed] [Google Scholar]
- 39.de Lartigue G, Lur G, Dimaline R, Varro A, Raybould H, Dockray GJ. EGR1 Is a target for cooperative interactions between cholecystokinin and leptin, and inhibition by ghrelin, in vagal afferent neurons. Endocrinology. 2010;151:3589–3599. doi: 10.1210/en.2010-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mauvoisin D, Prevost M, Ducheix S, Arnaud MP, Mounier C. Key role of the ERK1/2 MAPK pathway in the transcriptional regulation of the Stearoyl-CoA Desaturase (SCD1) gene expression in response to leptin. Mol Cell Endocrinol. 2010;319:116–128. doi: 10.1016/j.mce.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 41.Bailie MB, Standiford TJ, Laichalk LL, Coffey MJ, Strieter RM, Peters-Golden M. Leukotriene-deficient mice manifest enhanced lethality from Klebsiella pneumonia in association with decreased alveolar macrophage phagocytic and bactericidal activities. J. Immunol. 1996;157:5221–5224. [PubMed] [Google Scholar]
- 42.Lazarus M, Munday CJ, Eguchi N, Matsumoto S, Killian GJ, Kubata BK, Urade Y. Immunohistochemical Localization of Microsomal PGE Synthase-1 and Cyclooxygenases in Male Mouse Reproductive Organs. Endocrinology. 2002;143:2410–2419. doi: 10.1210/endo.143.6.8872. [DOI] [PubMed] [Google Scholar]
- 43.Najib S, Sanchez-Margalet V. Human leptin promotes survival of human circulating blood monocytes prone to apoptosis by activation of p42/44 MAPK pathway. Cell Immunol. 2002;220:143–149. doi: 10.1016/s0008-8749(03)00027-3. [DOI] [PubMed] [Google Scholar]
- 44.Duggal P, Guo X, Haque R, Peterson KM, Ricklefs S, Mondal D, Alam F, Noor Z, Verkerke HP, Marie C, Leduc CA, Chua SC, Jr, Myers MG, Jr, Leibel RL, Houpt E, Gilchrist CA, Sher A, Porcella SF, Petri WA., Jr A mutation in the leptin receptor is associated with Entamoeba histolytica infection in children. J Clin Invest. 2011;121:1191–1198. doi: 10.1172/JCI45294. [DOI] [PMC free article] [PubMed] [Google Scholar]