Abstract
Protein kinase D acts as a major mediator of several signaling pathways related to cancer development. Aberrant PKD expression and activity have been demonstrated in multiple cancers, and novel PKD inhibitors show promising anti-cancer activities. Despite these advances, the mechanisms through which PKD contributes to the pathogenesis of cancer remain unknown. Here, we establish a novel role for PKD3, the least studied member of the PKD family, in the regulation of prostate cancer cell growth and motility through modulation of secreted tumor-promoting factors. Using both a stable inducible knockdown cell model and a transient knockdown system employing multiple siRNAs, we demonstrate that silencing of endogenous PKD3 significantly reduces prostate cancer cell proliferation, migration, and invasion. Additionally, conditioned medium from PKD3 knockdown cells exhibits less migratory potential compared to that from control cells. Further analysis indicated that depletion of PKD3 blocks secretion of multiple key tumor-promoting factors including MMP-9, IL-6, IL-8, and GROα, but does not alter mRNA transcript levels for these factors, implying impairment of the secretory pathway. More significantly, inducible depletion of PKD3 in a subcutaneous xenograft model suppresses tumor growth and decreases levels of intratumoral GROα in mice. These data validate PKD3 as a promising therapeutic target in prostate cancer and shed light on the role of secreted tumor-promoting factors in prostate cancer progression.
Keywords: Protein kinase D, prostate cancer, cytokines, secretion, inducible knockdown
INTRODUCTION
Prostate cancer is the second leading cause of cancer-related death in men (1). Recent advances in screening procedures have resulted in earlier diagnosis (2). However, despite early interventions and chemopreventive measures, therapeutic options are limited once prostate tumors have metastasized (2). A more complete understanding of the molecular mechanisms underlying prostate cancer progression is necessary to facilitate the development of novel therapies.
The protein kinase D (PKD) family of serine/threonine kinases has been implicated in the progression of a variety of cancer types including prostate cancer (3). This kinase family is comprised of 3 isoforms: PKD1/PKCμ, PKD2, and PKD3/PKCυ (4–7). PKD activation involves phosphorylation of 2 conserved serine residues in the activation loop by diacylglycerol (DAG)-responsive PKC isoenzymes, followed by autophosphorylation to confer full activity (8–10). PKD activity is maintained by both PKC-dependent and -independent mechanisms (11, 12). In addition to regulation of its catalytic activity, PKD is also subjected to spatial regulation, and can be found localized to the Golgi, plasma membrane, nucleus, or mitochondria, where it may have specialized functions (13). Aberrant PKD activity and expression have been demonstrated in a number of tumors, and PKD regulates many cancer-related cellular functions, including proliferation, survival, angiogenesis, and motility (3, 14).
As a Golgi resident protein, PKD has been shown to be essential for membrane fission and vesicle formation in the transport of cargo from the trans-Golgi network (TGN) to the plasma membrane (15–17). Although the mechanisms through which PKD regulates protein trafficking have been well studied, little is known of the identity of transported cargo, and the relevance of this regulation to tumor development remains to be determined. PKD1 has been implicated in the secretion of several tumor-promoting cytokines such as IL-6, IL-8, and GROα (18–20), and matrix metalloproteinases (MMPs) (21, 22). However, it remains to be determined 1) how PKD regulates these processes, 2) whether these regulatory mechanisms affect tumor growth, and 3) what the functions of the other PKD isoforms are in modulating these events.
In this report, we provide substantial evidence for a tumor-promoting role for PKD3 in prostate cancer progression. Our results demonstrate that PKD3 modulates proliferation and motility of prostate cancer cells, as well as growth of tumor xenografts in vivo. Furthermore, we demonstrate that PKD3 mediates the secretion of multiple factors that stimulate cancer progression both in vivo and in cells. These data validate PKD as a promising therapeutic target in the treatment of prostate cancer, and broaden our current understanding of the molecular mechanisms of PKD function in cancer progression.
MATERIALS AND METHODS
Chemicals
DMSO was purchase from Sigma. CID755673 and kb-NB142-70 were synthesized by Dr. Peter Wipf at the Department of Chemistry, University of Pittsburgh (23, 24).
Cell culture and development of stable cell lines
PC3 cells (ATCC) were maintained in Ham's F-12 medium (Cellgro) containing 10% fetal bovine serum (FBS, Gibco), 1000 units/L penicillin, and 10 mg/mL streptomycin. DU145 cells (ATCC) were maintained in RPMI 1640 (HyClone). Tetracycline (tet)-inducible PKD3 shRNA stable cell lines were generated according to the manufacturer's instructions (Invitrogen; see Supplemental Materials and Methods for details). Expression of PKD3 shRNA was induced by the addition of 1 μg/mL tet in the growth medium for 5 days, and these standard induction conditions were used for all subsequent experiments. All cell lines were authenticated by the Research Animal Diagnostic Laboratory by species-specific PCR testing within 6 months of use.
Transient knockdown of PKD3
Transient silencing of PKD3 was achieved using multiple siRNAs targeting different regions of PKD3. A total of 4 siRNA sequences were used: siPKD3-1: GCT GCT TCT CCG TGT TCA AGT CCT A (Invitrogen); siPKD3-5: CAC GAT ATG TCA GTA CTG CAA; siPKD3-6: CGG GAG AGT GTT ACC ATT GAA; and siPKD3-10: CAG ACT TGG CTT GAC CTT AGA (Qiagen). Transient transfection of PC3 cells using DharmaFECT (Dharmacon, Chicago, IL) typically resulted in 60–80% knockdown for all siRNAs excluding siPKD3-2, which was not effective at inhibiting PKD3 expression (Supplemental Figure S1).
Western blotting
Western blotting analysis was conducted as previously described (25). Primary antibodies targeted PKD3 (Cell Signaling), PKD2 (Abcam), or tubulin (Santa Cruz Biotechnology).
Quantitative real-time RT-PCR (RT-qPCR)
Total RNA was isolated from shScr, shPKD3-1 C2-1, and shPKD3-1 C2-3 cells using RNeasy Mini Kit (Qiagen, Valencia, CA). Reverse-transcription was performed using the iScript cDNA synthesis kit (BioRad, Hercules, CA), typically on 1 μg total RNA, according to the manufacturer's instructions. RT-qPCR was performed on a CFX96 Real-time Detection System with C1000 Thermal Cycler (BioRad), using SsoFast EvaGreen master mix (BioRad) in a 10 μL reaction volume. Sequences of the primer pairs and details of the RT-qPCR protocol are described in the Supplemental Materials and Methods. Relative transcript abundance was calculated by BioRad CFX96 Manager software using the ΔΔCt method with GAPDH as the reference gene.
Cell proliferation assay
Cell proliferation was determined for PC3-TR cells and stable clones expressing shScr or shPKD3-1 as previously reported (26). Growth media with or without tet was refreshed every 2 days.
Collection of conditioned medium
For the collection of conditioned medium, stable inducible clones (pretreated with tet for 5 days) or transiently-transfected prostate cancer cells (48 h post-transfection) were replated at equal high densities into serum-free medium. After 48 h, the conditioned medium was collected and stored at −80°C. Cell number was determined for each sample after condition medium collection to ensure that there was no significant discrepancy in proliferation between samples. If a significant difference in final cell number was noted between samples, the volume of conditioned medium used was normalized based on the ratio of the cell number difference between control (shScr, non-targeting siRNA, or DMSO treated cells) and experimental (PKD3 knockdown or inhibitor-treated cells) cells.
Wound healing and Matrigel invasion assays
Wound healing assay and Matrigel invasion assay were conducted as previously described (24, 27) (see Supplemental Materials and Methods for brief protocols). For wound healing assay using conditioned medium, shScr or shPKD3-1 C7 cells were treated with tet for 5 days and then replated at equal high densities into serum-free medium. After 48 h, the conditioned medium was collected, normalized based on the final cell count, and applied to confluent PC3 cells. The wound healing assay was then performed as before.
Membrane-based cytokine antibody array
Analysis of cytokine secretion into conditioned medium was performed using the Human Cytokine Array I (RayBiotech, Norcross, GA), according to the manufacturer's instructions. Collection of conditioned medium from shScr and shPKD3-1 C7 cells was performed as described above.
ELISA
Quantitative measurement of cytokines secreted into the conditioned medium was determined using ELISA, according to the manufacturer's protocol (RayBiotech, Norcross, GA). Conditioned medium was collected as described above. For experiments using the PKD inhibitors CID755673 and kb-NB142-70, cells were plated at equal high densities in serum-free medium containing the indicated concentrations of inhibitors, and conditioned medium was collected 48 h later. The volume of the condition medium used in all ELISA experiments was normalized to the final cell count as described above.
Subcutaneous xenograft mouse model
Tumor cells, resuspended 1:1 in BD Matrigel Basement Membrane Matrix (BD Biosciences) at a final cell concentration of 4 × 106 cells per 0.1 mL, were injected subcutaneously into the right flank of 8-week-old male nude mice (Charles River Laboratories, Wilmington, MA). Five days later, palpable tumors had established. Mice were randomized, and half of the mice in each group were treated with 1 mg/mL doxycycline (Dox), a tetracycline analog, via drinking water (resulting in 4 groups: shScr No Dox, shScr +Dox, shPKD3-1 C7 No Dox, shPKD3-1 C7 +Dox; n = 10–11 mice per group). Dox-containing water was changed every 2 days. Tumor volume was calculated as V (mm3) = (L × W2) / 2, where L = the largest diameter and W = the shortest diameter perpendicular to L. Twenty-five days after inoculation, mice were euthanized by CO2 inhalation, and tumors were excised. All animal studies were conducted in accordance with an IACUC-approved protocol at the University of Pittsburgh.
Statistical analysis
All statistical analyses were completed using GraphPad Prism software. Student's t-test was used to determine the significance of differences seen in Western blotting, RT-qPCR, cell proliferation, wound healing, invasion, and ELISA assays. For the animal studies, the Mann-Whitney-Wilcoxon test was used. A p-value of < 0.05 was considered statistically significant.
RESULTS
Development of multiple stable, tetracycline-inducible PKD3 knockdown prostate cancer cell lines
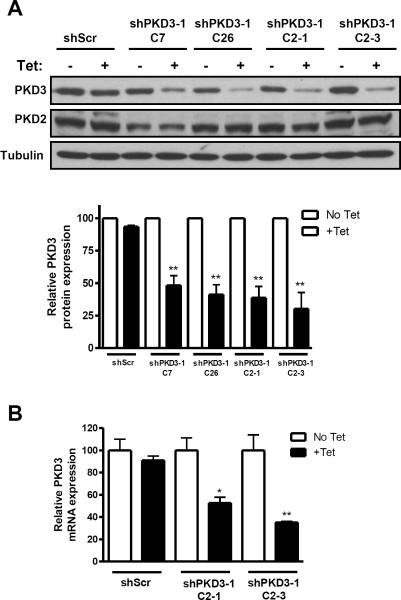
We previously demonstrated that PC3 cells express high levels of PKD3, moderate PKD2, and no detectable PKD1 (26). Additionally, PKD3 was found to be overexpressed in human prostate tumors (26). To further investigate PKD3 function in prostate cancer, we developed several PC3 cell lines stably expressing tet-inducible shRNA targeting PKD3. Four stable cell lines were isolated that showed downregulation of PKD3 upon tet treatment: shPKD3-1 C7, shPKD3-1 C26, shPKD3-1 C2-1, and shPKD3-1 C2-3. A clone expressing non-targeting, scrambled shRNA (shScr) was also isolated. Western blotting analysis revealed 50–75% knockdown of PKD3 protein from the isolated shPKD3-1 clones after tet treatment (Figure 1A). PKD3 levels of the shScr clone were unaffected, and PKD2 levels were also unchanged in all samples. Additionally, RT-qPCR revealed 50% and 65% reduction in PKD3 transcript levels for clones shPKD3-1 C2-1 and shPKD3-1 C2-3, respectively (Figure 1B).
Figure 1. Tet-induced knockdown of PKD3 in PC3 prostate cancer cells.
PC3-TR cells were transfected with the pENTR/TO/H1 vector containing shRNA targeting PKD3. After stable selection, PKD3 expression with tet treatment was analyzed by Western blotting (A). Densitometry analysis results are shown as the mean ± S.E.M. of 3 independent experiments. Additionally, PKD3 transcript levels were determined by real-time RT-qPCR (B). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Tet-induced knockdown of PKD3 reduces prostate cancer cell proliferation, migration, and invasion
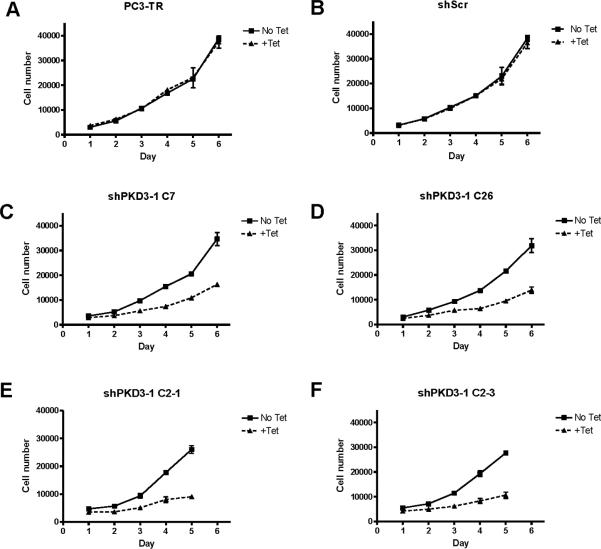
Next, we investigated whether tet-induced knockdown of PKD3 caused reduction of cell proliferation in this model system. As shown in Figures 2A–F, each of the 4 stable PKD3 knockdown clones showed a significant reduction in cell proliferation (30–38% reduction in cell numbers within 48 h, and 50% reduction in cell numbers within 3–4 days, depending on the cell line), whereas growth of both the parental PC3-TR cell line and the shScr clone were unaffected by tet treatment. This is consistent with our previous report that transient knockdown of PKD3 by siRNA causes a significant decrease in PC3 cell proliferation (26). The decreased growth rate was not accompanied by increased apoptosis in the PKD3 knockdown lines, as revealed by the absence of a sub-G0G1 apoptotic peak in cell cycle analysis (Supplemental Figures S1B–C). Furthermore, knockdown of PKD3 resulted in increased accumulation of cells in S and/or G2M and reduced numbers of cells in G0G1.
Figure 2. Tetracycline causes reduced proliferation in stable shPKD3-1 clones.
PC3-TR (A), shScr (B), shPKD3-1 C7 (C), shPKD3-1 C26 (D), shPKD3-1 C2-1 (E), or shPKD3-1 C2-3 (F) cells were plated at 5000 cell per well in 24-well plates. The total number of live cells was determined for up to 6 days, and growth media and tet were refreshed every 2 days. Experiments were repeated 3 times, and a representative graph is shown.
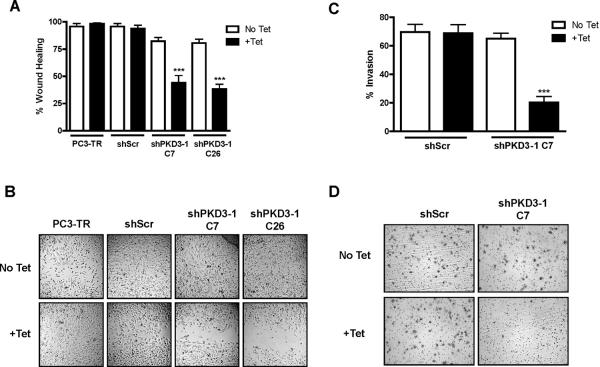
Cell motility is essential for the acquired ability of cancer cells to invade into surrounding tissues and form distant metastases, a key step in the progression of aggressive prostate cancer (28). To date, no evidence for the role of PKD3 in prostate cancer cell motility has been reported. Therefore, we investigated whether tet-induced knockdown of PKD3 could alter cell migration and invasion. First, we measured cell migration by wound healing assay in our stable clones. While the “wound” completely closed in both the parental PC3-TR cells and shScr cells, wound closure was reduced to only 45% and 35% in the PKD3 knockdown clones shPKD3-1 C7 and shPKD3-1 C26 (Figures 3A–B). This result was further confirmed using transient knockdown of PKD2 and/or PKD3 by siRNA in PC3 cells (Supplemental Figures S2B–C). Additionally, cell migration was measured using cells that had been arrested by mitomycin C treatment (Supplemental Figure S3A). In line with our previous results, knockdown of PKD3 in PC3 cells using multiple PKD3 siRNAs significantly blocked wound-induced cell migration.
Figure 3. Inducible knockdown of PKD3 leads to reduced cell migration and invasion.
A, PC3-TR, shScr, shPKD3-1 C7, or shPKD3-1 C26 cells were subjected to the wound healing assay as described in the “Materials and Methods.” Data are the mean ± S.E.M. as determined from 3 independent experiments. B, Representative images of the wounded monolayer are shown. C, shScr or shPKD3-1 C7 cells were subjected to the invasion assay as described in “Materials and Methods.” Percent invasion was calculated relative to the number of cells that migrated through control inserts in order to normalize for proliferation effects. Data are the mean ± S.E.M. of 3 independent experiments. D, Representative images are shown. ***, p < 0.001.
To measure the effects of PKD3 knockdown on cell invasion, we performed a Matrigel invasion assay using shPKD3-1 C7 and shScr cells. Invasion of tet-treated shPKD3-1 C7 cells was reduced to only 20%, compared to 65% for untreated shPKD3-1 C7 cells (Figure 3C). Invasion of shScr cells was not affected by tet treatment. This result was confirmed using transient knockdown of PKD2 and/or PKD3 (Supplemental Figure S2D). Taken together, these data demonstrate a novel role for PKD3 and PKD2 in the modulation of prostate cancer cell motility, a key capacity required for prostate cancer progression and metastasis.
Stable or transient knockdown of endogenous PKD3 by multiple siRNAs reduces secretion of several key cytokines
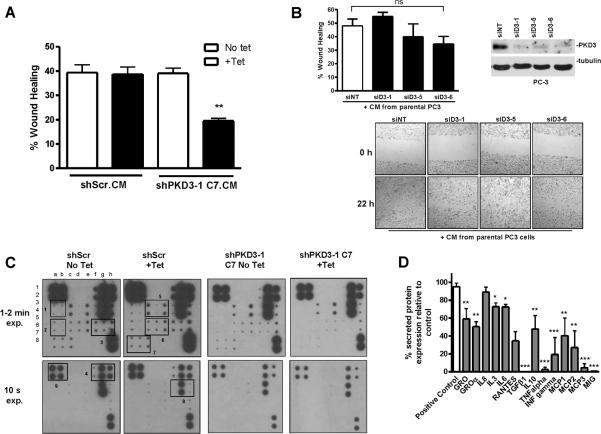
As a major traffic regulator, PKD has been shown to regulate cell motility through modulation of anterograde membrane traffic from the TGN to the plasma membrane (29), which we hypothesized may impact the secretory pathway in tumor cells. Here, we investigated whether conditioned medium from PKD3-knockdown cells could reduce the migratory potential of PC3 cells. As shown in Figure 4A, conditioned medium collected from tet-treated shPKD3-1 C7 cells significantly slowed migration of regular PC3 cells. As a control, tet did not affect the ability of shScr cells to stimulate PC3 cell migration. These results were further confirmed using condition medium obtained from mitomycin C-arrested PC3 cells with transient knockdown of PKD3 using multiple siRNAs (Supplemental Figure S3B). In further support of our hypothesis, conditioned medium collected from parental PC3 cells rescued the inhibitory effect of PKD3 knockdown on cell migration, restoring the migratory capacity of PKD3 knockdown cells to that of the control (Figure 4B). These data indicate that PKD3 promotes cell migration through the secretion of motility-stimulating factors.
Figure 4. PKD3 knockdown modulates PC3 cell migration and secretion of various cytokines.
A, Conditioned media (CM) from equal numbers of shScr or shPKD3-1 C7 cells was applied to confluent parental PC3 cells that had been “wounded”. Cell migration was measured by wound healing assay. Representative data from 1 of 3 independent experiments are shown. **, p < 0.01. B, CM from parental PC3 cells rescued PKD3 knockdown-induced inhibition of cell migration. PC3 cells were transiently transfected with siNT, siPKD3-1, siPKD3-5, or siPKD3-6. Two days later, the cells were replated at equal high densities. After overnight attachment, cells were treated with 0.5 μM mitomycin C for 2 h and “wounded”. The medium was then replenished with equal amounts of conditioned media collected from parental PC3 cells after 48 h of culture in serum-free medium in the presence of 3 nM mitomycin C. Wound healing assay was then performed as described in the “Materials and Methods.” Representative data from 3 independent experiments are shown. ns, not significant. C, PKD3 knockdown reduces cytokine secretion. Conditioned media from equal numbers of shScr or shPKD3-1 C7 cells was subjected to cytokine antibody array. Chemiluminescent detection of protein spots for each of the samples is shown. Boxes indicate spots corresponding to the following cytokines (left to right in vertical duplicate): Box 1, IL-1α, IL-2; Box 2, IL-13, IL-15; Box 3, MCP-3, MIG, RANTES; Box 4, GM-CSF, GRO, GROα; Box 5, IL-3, IL-5, Il-6; Box 6, INF-γ, MCP-1, MCP-2; Box 7, TGF-β1, TNF-α, TNF-β; Box 8, IL-7, Il-8, IL-10; Box 9, internal positive control. See Supplemental Figure S4 for an array map. D, Densitometry analysis of the cytokine array. The value for each spot was normalized to internal positive control spots on the array. The relative percent expression was determined for both shScr– vs. shScr+ and shPKD3-1 C7– vs. shPKD3-1 C7+, and the relative percent change in expression for shScr+ vs. shPKD3-1 C7+ was plotted. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
To examine whether there might be a global effect on other secretory pathways vital to cancer cell function, we performed a cytokine antibody array using conditioned medium collected from tet-induced shPKD3-1 C7 cells. The array revealed that PKD3 knockdown caused a significant reduction in most cytokines on the array (Figures 4C–D, Supplemental Figure S4). For example, IL-3 and IL-6 were reduced to ~70% of the untreated control, TGFβ and TNFα were decreased to almost undetectable levels, and the chemoattractant proteins monocyte chemotactic protein (MCP) 1, −2, and −3 were reduced to only 20–40% of the untreated control. Tet-treatment itself had no effect on cytokine secretion in shScr cells.
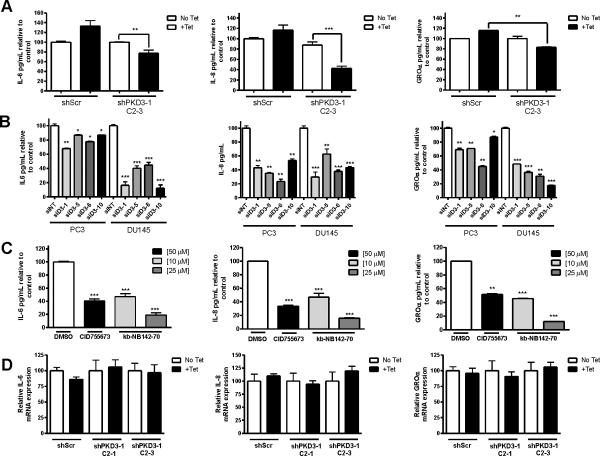
To confirm these array results, we analyzed the levels of several key cytokines secreted into the media by ELISA. Tet-induced PKD3 knockdown caused a significant reduction in the levels of IL-6, IL-8, and GROα secreted from shPKD3-1 C2-3 cells (Figure 5A). Specifically, PKD3 knockdown in shPKD3-1 C2-3 cells reduced IL-8 secretion to less than 40% of the levels detected from untreated shPKD3-1 C2-3 cells and IL-6 secretion to only 75% of the untreated control. To further confirm our results, we measured cytokine secretion in prostate cancer cells depleted of endogenous PKD3 by multiple siRNAs targeting different regions of PKD3. Transient PKD3 knockdown reduced IL-6, IL-8, and GROα secretion in both PC3 and DU145 cells (Figure 5B). To examine whether PKD kinase activity is required for this process, we investigated the effects of our previously-described novel PKD inhibitors CID755673 and kb-NB142-70 (24, 27). We found that treatment with either inhibitor caused a significant reduction in the secretion of IL-6, IL-8, and GROα (Figure 5C). In fact, treatment with 25 μM kb-NB142-70 reduced secretion of each of these cytokines to only approximately 10% of the DMSO-treated control.
Figure 5. Antagonizing PKD3 expression or activity leads to reduced secretion of IL-6, IL-8, and GROα.
A, Conditioned media from equal numbers shScr or shPKD3-1 C2-3 cells was subjected to ELISA to determine levels of secreted IL-6, IL-8, or GROα. B, PC3 and DU145 cells were transfected with siRNAs targeting different regions of PKD3 or non-targeting siRNA. Conditioned media from equal numbers of cells was then subjected to ELISA. C, PC3 cells were treated with the indicated inhibitors for 48 h. ELISA was performed on the conditioned media collected from equal numbers of cells. D, IL-6, IL-8, and GROα transcript levels relative to the GAPDH control were determined by RT-qPCR in the inducible cell lines. Each experiment was repeated with duplicates at least 2–3 times, and the data represent the mean ± S.E.M. of the independent experiments.
To determine whether the effects of PKD3 knockdown on levels of secreted cytokines were due to transcriptional regulation rather than regulation of secretion, RT-qPCR was performed on total RNA extracted from shPKD3-1 C2-1 and shPKD3-1 C2-3 cells. No changes were detected in IL-6, IL-8, or GROα transcripts in any cell line (Figure 5D), suggesting that knockdown of endogenous PKD3 or inhibition of PKD by chemical inhibitors blocks secretion, but not transcription, of key cancer-promoting factors in prostate cancer cells. Interestingly, we also found that knockdown of PKD3 using multiple siRNAs led to reduced MMP-9 activity in the conditioned medium, as measured by zymography assay, but neither MMP-9 nor MMP-14 transcript levels were affected by PKD3 knockdown (Supplemental Figure 5A–C), further suggesting an effect on secretion.
Knockdown of PKD3 halts growth of subcutaneous xenograft tumors in mice
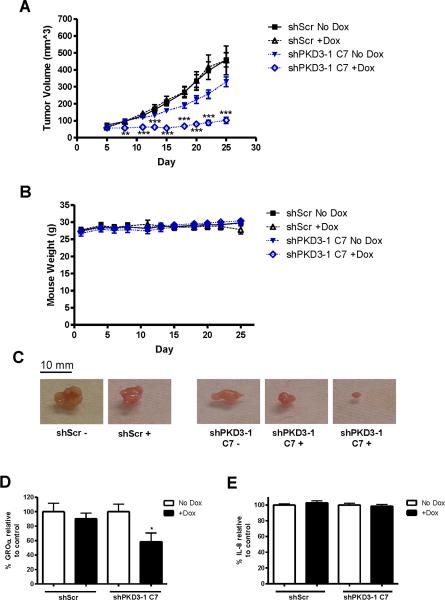
In order to validate that PKD3 is indeed a significant regulator of prostate tumor growth in vivo, we studied the ability of PKD3 knockdown to inhibit growth of PC3 tumor xenografts. We first established subcutaneous tumor xenografts in male nude mice using shScr or shPKD3-1 C7 cells from our tet-inducible PKD3 knockdown model system. Five days after inoculation of the tumor cells, doxycycline was administered to half the mice. Tumor volume was measured 3 times a week for 25 days at which time some of the tumors were nearing 20 mm in diameter, necessitating euthanasia according to IACUC guidelines. While dox itself had no effect on tumor growth in the shScr mice, a significant reduction in tumor growth was observed in dox-treated mice bearing shPKD3-1 C7 tumors (Figures 6A, C). After the 25-day experiment, tumor volume for the shPKD3-1 C7 +Dox group measured only 100 mm3 on average, compared to an average of 325 mm3 for the no Dox shPKD3-1 C7 group, a growth reduction of nearly 70%. Mice experienced no changes in weight due to tumor growth or dox treatment during the course of the study (Figure 6B). ELISA analysis of lysates prepared from the excised tumors revealed a significant reduction in GROα, but not IL-8 (Figures 6D–E). In shPKD3-1 C7 +Dox tumors, levels of GROα were reduced to approximately 55% of the no-Dox control. This significant difference indicates that PKD3 modulates levels of this cytokine in vivo, and this may contribute to the reduced tumor growth. Taken together, these results indicate that PKD3 signaling is necessary for the growth of prostate tumors in vivo.
Figure 6. Doxycycline-induced knockdown of PKD3 slows growth of subcutaneous tumor xenografts in mice.
Mice were injected subcutaneously with either shScr or shPKD3-1 C7 cells. Tumor volume (A) and mouse weight (B) were measured every 2–3 days. The experiment was performed using 5–6 mice per group and was independently repeated twice (n = 10–11 mice per group). C, Representative images of the excised tumors. D, Equal amounts of protein from tumor lysates were subjected to ELISA for detection of GROα or IL-8. For each tumor sample, duplicate measurements were determined, and tumors from 4–5 mice in each group were analyzed. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
DISCUSSION
Increasing evidence supports a major role for PKD in cancer progression. In this report, we have established an essential role for PKD3, the least-studied of the PKD family members, in the progression of prostate cancer. We have demonstrated, using both cellular and in vivo models, that inhibition of PKD activity or expression reduces proliferation, motility, and secretion of key cancer-promoting factors. These data indicate that PKD, and in particular PKD3, may be a viable target for the development of novel chemotherapeutics in prostate cancer treatment.
Using stable inducible PKD3 knockdown prostate cancer cell lines, we showed that tet-induced PKD3 knockdown led to reduced cell proliferation and motility. The effect on proliferation was in line with our previous findings obtained using a transient knockdown approach (26). Importantly, we have now provided the first evidence demonstrating that targeting PKD3 in vivo causes a significant reduction in tumor growth in a subcutaneous xenograft mouse model. The effects of PKD3 knockdown on cell proliferation and motility are in contrast to those of PKD1 reported by Balaji and colleagues, showing an almost tumor-suppressor-like function of PKD1 in prostate cancer (30, 31). As the first-discovered and most intensely studied PKD isoform, PKD1's role in cell proliferation and motility has been somewhat controversial. This is particularly evident with regard to the role of PKD1 in cell migration. Although earlier studies demonstrated that PKD1 promotes cell movement (29, 32), more recent studies have suggested that PKD1 may have an inhibitory effect on cell migration and invasion (33, 34). Notably, studies in prostate cancer cells have shown that overexpression of PKD1 promotes cell aggregation and inhibits cell migration (31), and other studies conducted in breast cancer and gastric cancer have shown that alteration of PKD1 expression or activity suppresses cell motility (22, 35). Several downstream targets of PKD1 have been implicated in this process, including slingshot phosphatase SSH1L (34, 36), RIN1 (33), and MMPs (22). Taken together, these findings highlight the complexity of PKD signaling and the importance of cellular context in shaping the biological functions of PKD in cells. The current study on PKD3, the least-studied member of the PKD family, further suggests that PKD isoforms may play specific and distinct roles in carcinogenesis and tumor progression. Accordingly, the PKD1 gene has been shown to be epigenetically silenced in primary breast and gastric cancer tissues and tumor cell lines (22, 35), while such regulation of PKD3 has not been demonstrated. Thus, despite belonging to the same kinase family and sharing high sequence homology, the PKD isoforms may have unique roles in different cancers or at different stages of cancer development.
It is noteworthy that even with incomplete knockdown (50–65%) of PKD3, potent effects on prostate cancer cell proliferation and motility were observed, implying that PKD3 may affect multiple tumor-promoting pathways. In this study, when conditioned medium from PKD3 knockdown cells was applied to normal PC3 cells, migration was significantly inhibited. Moreover, the PKD3 knockdown phenotype was rescued by applying conditioned medium collected from parental PC3 cells, indicating that PKD3 promoted the secretion of a motility-stimulating factor into the conditioned medium. Upon further investigation, we found that knockdown of PKD3 caused a global reduction in cytokine secretion in PC3 cells. These results were confirmed by ELISA for several significant targets, including IL-6, IL-8, and GROα, and were not a result of changes in the transcript levels of these proteins, suggesting that the reduced detection of cytokines was due to inhibition of secretion rather than gene expression. Thus, our data indicate that the robust effects of this partial PKD3 knockdown may be due to inhibition of the secretion of key cancer-promoting factors, thereby mediating several signaling pathways that affect proliferation and motility through reduction of autocrine and paracrine signaling. Nonetheless, our data do not exclude the possibility that PKD3 may regulate other cellular processes that impact cell growth and motility, such as cell adhesion, integrin expression, and cytoskeleton remodeling, and further studies are required to investigate these potential mechanisms.
Pro-inflammatory cytokines such as IL-6, IL-8, and GROα have well-documented, significant roles in cancer progression (37). IL-8 in particular has been demonstrated to have various functions in cancer cells that promote angiogenesis, migration, metastasis, and proliferation, and is significantly upregulated in human prostate tumors (38–40). Besides signaling in an autocrine fashion to stimulate cell growth and motility, these factors are also regulators of the tumor microenvironment (41) and are potent stimulators of angiogenesis (42). We have demonstrated that there is reduced secretion of multiple critical angiogenic cytokines with PKD3 knockdown, and this may account for the potent effects on prostate cancer cell proliferation and motility seen in our cellular studies as well as the significant reduction in tumor growth in our xenograft model, where we observed reduced levels of GROα.
A recent report showed that VEGF treatment of endothelial cells stimulated PKD1-dependent secretion of multiple cytokines, possibly mediating angiogenesis and inflammation (20). In this study, researchers found that silencing PKD1 by siRNA caused reduced VEGF-stimulated expression and secretion of IL-6, IL-8, and GROα. However, they found no effects of PKD3 knockdown on cytokine secretion, which is contrary to our findings. This discrepancy may be explained by the relatively low expression of PKD3 in endothelial cells, which is in contrast to the high expression and likely aberrant hyperactivity of PKD3 in our PC3 and DU145 cells. This may contribute to constitutive hyperactivity of the secretory pathway in these cells, leading to increased secretion of key tumor-promoting factors and enhancing autocrine growth/motility signals.
The chemokine GROα has been studied in a variety of cancers, and has been found to promote proliferation, invasion, and angiogenesis in many different contexts (43–45). In prostate cancer, GROα was shown to promote invasion and chemotaxis (44). Thus, the significant PKD3-dependent reduction in intratumoral GROα is an important observation that may explain in part the reduced tumor growth observed in shPKD3-1 C7-derived tumors. Though we do not yet know the precise mechanisms through which PKD3 mediates GROα expression in vivo, our cellular studies suggest that PKD3 knockdown impairs the secretory pathway rather than transcription of this important tumor-promoting factor. It is noteworthy that in the in vivo study, reduced GROα expression was observed in tumor tissue lysates, which suggested possible transcriptional regulation. This phenomenon may be in part explained by the very different cellular environments in culture versus in vivo, where cells must grow into tumors and become vascularized, and by the heterogeneous nature of tumor tissue. It is also possible that the duration of the in vivo experiment, with tumors experiencing PKD3 knockdown for approximately 20 days, may have triggered a feedback or autoregulatory mechanism that resulted in the reduction of GROα expression. Further studies are necessary to elucidate these mechanisms.
In conclusion, our data support a tumor-promoting function of PKD3 in prostate cancer progression. We have shown that the cumulative effects of reduced cytokine secretion can cause significant changes in cancer cell proliferation and motility. Furthermore, we have reported, for the first time, that inhibition of PKD3 expression suppresses prostate tumor growth in vivo, validating PKD as a promising target in the development of novel therapies for prostate cancer.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Peter Blumberg for supplying the PC3-TR cells, Dr. Peter Wipf for synthesizing CID755673 and kb-NB142-70, Kelly Quesnelle for assistance with the xenograft model, and Dr. Noemi Kedei and Dana Liggit for technical assistance. The authors declare no competing financial interests in the work described.
GRANT SUPPORT This study was supported in part by the National Institutes of Health (Grants R01CA129127 and R01CA142580 to Q.J. Wang) and the DOD PCRP Prostate Cancer Training Award (Grant PC102042 to C.R. LaValle).
Footnotes
Conflicts of interest: The authors declare no competing financial interests.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Shirai T. Significance of chemoprevention for prostate cancer development: experimental in vivo approaches to chemoprevention. Pathol Int. 2008;58:1–16. doi: 10.1111/j.1440-1827.2007.02182.x. [DOI] [PubMed] [Google Scholar]
- 3.LaValle CR, George KM, Sharlow ER, Lazo JS, Wipf P, Wang QJ. Protein kinase D as a potential new target for cancer therapy. Biochim Biophys Acta. 2010;1806:183–92. doi: 10.1016/j.bbcan.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayashi A, Seki N, Hattori A, Kozuma S, Saito T. PKCnu, a new member of the protein kinase C family, composes a fourth subfamily with PKCmu. Biochim Biophys Acta. 1999;1450:99–106. doi: 10.1016/s0167-4889(99)00040-3. [DOI] [PubMed] [Google Scholar]
- 5.Johannes FJ, Prestle J, Eis S, Oberhagemann P, Pfizenmaier K. PKCu is a novel, atypical member of the protein kinase C family. J Biol Chem. 1994;269:6140–8. [PubMed] [Google Scholar]
- 6.Sturany S, Van Lint J, Muller F, Wilda M, Hameister H, Hocker M, et al. Molecular cloning and characterization of the human protein kinase D2. A novel member of the protein kinase D family of serine threonine kinases. J Biol Chem. 2001;276:3310–8. doi: 10.1074/jbc.M008719200. [DOI] [PubMed] [Google Scholar]
- 7.Valverde AM, Sinnett-Smith J, Van Lint J, Rozengurt E. Molecular cloning and characterization of protein kinase D: a target for diacylglycerol and phorbol esters with a distinctive catalytic domain. Proc Natl Acad Sci U S A. 1994;91:8572–6. doi: 10.1073/pnas.91.18.8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waldron RT, Iglesias T, Rozengurt E. Phosphorylation-dependent protein kinase D activation. Electrophoresis. 1999;20:382–90. doi: 10.1002/(SICI)1522-2683(19990201)20:2<382::AID-ELPS382>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 9.Waldron RT, Rozengurt E. Protein kinase C phosphorylates protein kinase D activation loop Ser744 and Ser748 and releases autoinhibition by the pleckstrin homology domain. J Biol Chem. 2003;278:154–63. doi: 10.1074/jbc.M208075200. [DOI] [PubMed] [Google Scholar]
- 10.Zugaza JL, Sinnett-Smith J, Van Lint J, Rozengurt E. Protein kinase D (PKD) activation in intact cells through a protein kinase C-dependent signal transduction pathway. Embo J. 1996;15:6220–30. [PMC free article] [PubMed] [Google Scholar]
- 11.Jacamo R, Sinnett-Smith J, Rey O, Waldron RT, Rozengurt E. Sequential protein kinase C (PKC)-dependent and PKC-independent protein kinase D catalytic activation via Gq-coupled receptors: differential regulation of activation loop Ser(744) and Ser(748) phosphorylation. J Biol Chem. 2008;283:12877–87. doi: 10.1074/jbc.M800442200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinnett-Smith J, Jacamo R, Kui R, Wang YM, Young SH, Rey O, et al. Protein kinase D mediates mitogenic signaling by Gq-coupled receptors through protein kinase C-independent regulation of activation loop Ser744 and Ser748 phosphorylation. J Biol Chem. 2009;284:13434–45. doi: 10.1074/jbc.M806554200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang QJ. PKD at the crossroads of DAG and PKC signaling. Trends Pharmacol Sci. 2006;27:317–23. doi: 10.1016/j.tips.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Guha S, Tanasanvimon S, Sinnett-Smith J, Rozengurt E. Role of protein kinase D signaling in pancreatic cancer. Biochem Pharmacol. 2010;80:1946–54. doi: 10.1016/j.bcp.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bossard C, Bresson D, Polishchuk RS, Malhotra V. Dimeric PKD regulates membrane fission to form transport carriers at the TGN. J Cell Biol. 2007;179:1123–31. doi: 10.1083/jcb.200703166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liljedahl M, Maeda Y, Colanzi A, Ayala I, Van Lint J, Malhotra V. Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell. 2001;104:409–20. doi: 10.1016/s0092-8674(01)00228-8. [DOI] [PubMed] [Google Scholar]
- 17.Malhotra V, Campelo F. PKD Regulates Membrane Fission to Generate TGN to Cell Surface Transport Carriers. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a005280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ochi N, Tanasanvimon S, Matsuo Y, Tong Z, Sung B, Aggarwal BB, et al. Protein kinase D1 promotes anchorage-independent growth, invasion, and angiogenesis by human pancreatic cancer cells. J Cell Physiol. 2011;226:1074–81. doi: 10.1002/jcp.22421. [DOI] [PubMed] [Google Scholar]
- 19.Steiner TS, Ivison SM, Yao Y, Kifayet A. Protein kinase D1 and D2 are involved in chemokine release induced by toll-like receptors 2, 4, and 5. Cell Immunol. 2010;264:135–42. doi: 10.1016/j.cellimm.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Hao Q, Wang L, Tang H. Vascular endothelial growth factor induces protein kinase D-dependent production of proinflammatory cytokines in endothelial cells. Am J Physiol Cell Physiol. 2009;296:C821–7. doi: 10.1152/ajpcell.00504.2008. [DOI] [PubMed] [Google Scholar]
- 21.Biswas MH, Du C, Zhang C, Straubhaar J, Languino LR, Balaji KC. Protein kinase D1 inhibits cell proliferation through matrix metalloproteinase-2 and matrix metalloproteinase-9 secretion in prostate cancer. Cancer Res. 2010;70:2095–104. doi: 10.1158/0008-5472.CAN-09-4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eiseler T, Doppler H, Yan IK, Goodison S, Storz P. Protein kinase D1 regulates matrix metalloproteinase expression and inhibits breast cancer cell invasion. Breast Cancer Res. 2009;11:R13. doi: 10.1186/bcr2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bravo-Altamirano K, George KM, Frantz MC, LaValle CR, Tandon M, Leimgruber S, et al. Synthesis and Structure–Activity Relationships of Benzothienothiazepinone Inhibitors of Protein Kinase D. ACS Med Chem Lett. 2011;2:154–9. doi: 10.1021/ml100230n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaValle CR, Bravo-Altamirano K, Giridhar KV, Chen J, Sharlow E, Lazo JS, et al. Novel protein kinase D inhibitors cause potent arrest in prostate cancer cell growth and motility. BMC Chem Biol. 2010;10:5. doi: 10.1186/1472-6769-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu G, Chen J, Espinoza LA, Garfield S, Toshiyuki S, Akiko H, et al. Protein kinase D 3 is localized in vesicular structures and interacts with vesicle-associated membrane protein 2. Cell Signal. 2007;19:867–79. doi: 10.1016/j.cellsig.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Deng F, Singh SV, Wang QJ. Protein kinase D3 (PKD3) contributes to prostate cancer cell growth and survival through a PKCepsilon/PKD3 pathway downstream of Akt and ERK 1/2. Cancer Res. 2008;68:3844–53. doi: 10.1158/0008-5472.CAN-07-5156. [DOI] [PubMed] [Google Scholar]
- 27.Sharlow ER, Giridhar KV, LaValle CR, Chen J, Leimgruber S, Barrett R, et al. Potent and selective disruption of protein kinase D functionality by a benzoxoloazepinolone. J Biol Chem. 2008;283:33516–26. doi: 10.1074/jbc.M805358200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee EC, Tenniswood MP. Emergence of metastatic hormone-refractory disease in prostate cancer after anti-androgen therapy. J Cell Biochem. 2004;91:662–70. doi: 10.1002/jcb.20040. [DOI] [PubMed] [Google Scholar]
- 29.Prigozhina NL, Waterman-Storer CM. Protein kinase D-mediated anterograde membrane trafficking is required for fibroblast motility. Curr Biol. 2004;14:88–98. doi: 10.1016/j.cub.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Mak P, Jaggi M, Syed V, Chauhan SC, Hassan S, Biswas H, et al. Protein kinase D1 (PKD1) influences androgen receptor (AR) function in prostate cancer cells. Biochem Biophys Res Commun. 2008;373:618–23. doi: 10.1016/j.bbrc.2008.06.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaggi M, Rao PS, Smith DJ, Wheelock MJ, Johnson KR, Hemstreet GP, et al. E-cadherin phosphorylation by protein kinase D1/protein kinase C{mu} is associated with altered cellular aggregation and motility in prostate cancer. Cancer Res. 2005;65:483–92. [PubMed] [Google Scholar]
- 32.Bowden ET, Barth M, Thomas D, Glazer RI, Mueller SC. An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999;18:4440–9. doi: 10.1038/sj.onc.1202827. [DOI] [PubMed] [Google Scholar]
- 33.Ziegler S, Eiseler T, Scholz RP, Beck A, Link G, Hausser A. A novel protein kinase D phosphorylation site in the tumor suppressor Rab interactor 1 is critical for coordination of cell migration. Mol Biol Cell. 2011;22:570–80. doi: 10.1091/mbc.E10-05-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eiseler T, Doppler H, Yan IK, Kitatani K, Mizuno K, Storz P. Protein kinase D1 regulates cofilin-mediated F-actin reorganization and cell motility through slingshot. Nat Cell Biol. 2009;11:545–56. doi: 10.1038/ncb1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim M, Jang HR, Kim JH, Noh SM, Song KS, Cho JS, et al. Epigenetic inactivation of protein kinase D1 in gastric cancer and its role in gastric cancer cell migration and invasion. Carcinogenesis. 2008;29:629–37. doi: 10.1093/carcin/bgm291. [DOI] [PubMed] [Google Scholar]
- 36.Peterburs P, Heering J, Link G, Pfizenmaier K, Olayioye MA, Hausser A. Protein kinase D regulates cell migration by direct phosphorylation of the cofilin phosphatase slingshot 1 like. Cancer Res. 2009;69:5634–8. doi: 10.1158/0008-5472.CAN-09-0718. [DOI] [PubMed] [Google Scholar]
- 37.Mishra P, Banerjee D, Ben-Baruch A. Chemokines at the crossroads of tumor-fibroblast interactions that promote malignancy. J Leukoc Biol. 2011;89:31–9. doi: 10.1189/jlb.0310182. [DOI] [PubMed] [Google Scholar]
- 38.Murphy C, McGurk M, Pettigrew J, Santinelli A, Mazzucchelli R, Johnston PG, et al. Nonapical and cytoplasmic expression of interleukin-8, CXCR1, and CXCR2 correlates with cell proliferation and microvessel density in prostate cancer. Clin Cancer Res. 2005;11:4117–27. doi: 10.1158/1078-0432.CCR-04-1518. [DOI] [PubMed] [Google Scholar]
- 39.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–41. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 40.Araki S, Omori Y, Lyn D, Singh RK, Meinbach DM, Sandman Y, et al. Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Res. 2007;67:6854–62. doi: 10.1158/0008-5472.CAN-07-1162. [DOI] [PubMed] [Google Scholar]
- 41.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vindrieux D, Escobar P, Lazennec G. Emerging roles of chemokines in prostate cancer. Endocr Relat Cancer. 2009;16:663–73. doi: 10.1677/ERC-09-0109. [DOI] [PubMed] [Google Scholar]
- 43.Ogata H, Sekikawa A, Yamagishi H, Ichikawa K, Tomita S, Imura J, et al. GROalpha promotes invasion of colorectal cancer cells. Oncol Rep. 2010;24:1479–86. [PubMed] [Google Scholar]
- 44.Reiland J, Furcht LT, McCarthy JB. CXC-chemokines stimulate invasion and chemotaxis in prostate carcinoma cells through the CXCR2 receptor. Prostate. 1999;41:78–88. doi: 10.1002/(sici)1097-0045(19991001)41:2<78::aid-pros2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 45.Haghnegahdar H, Du J, Wang D, Strieter RM, Burdick MD, Nanney LB, et al. The tumorigenic and angiogenic effects of MGSA/GRO proteins in melanoma. J Leukoc Biol. 2000;67:53–62. doi: 10.1002/jlb.67.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.