Abstract
Strategic exposure to donor antigens prior to transplantation can be an effective way for inducting donor-specific tolerance in allogeneic recipients. We have recently shown that pre-transplant infusion of donor splenocytes treated with the chemical cross-linker ethylcarbodiimide (ECDI-SPs) induces indefinite islet allograft survival in a full MHC-mismatched model without the need for any immunosuppression. Mechanisms of allograft protection by this strategy remain elusive. In this study, we show that the infused donor ECDI-SPs differentially target T cells with indirect versus direct allo-specificities. To target indirect allo-specific T cells, ECDI-SPs induce up-regulation of negative, but not positive, co-stimulatory molecules on recipient splenic CD11c+ DCs phagocytosing the injected ECDI-SPs. Indirect allo-specific T cells activated by such CD11c+ DCs undergo robust initial proliferation followed by rapid clonal depletion. The remaining T cells are sequestered in the spleen without homing to the graft site or the graft draining lymph node. In contrast, direct allo-specific T cells interacting with intact donor ECDI-SPs not yet phagocytosed undergo limited proliferation and are subsequently anergized. Furthermore, CD4+CD25+Foxp3+ T cells are induced in lymphoid organs and at the graft site by ECDI-SPs. We conclude that donor ECDI-SPs infusions target host allogeneic responses via a multitude of mechanisms including clonal depletion, anergy and immunoregulation, which act in a synergistic fashion to induce robust transplant tolerance. This simple form of negative vaccination has significant potential for clinical translation in human transplantation.
Keywords: ECDI (ethylene carbodiimide), Allogeneic transplantation, Tolerance, Graft rejection, Antigen presenting cells, Dendritic cells, Indirect antigen presentation pathway, Direct antigen presentation pathway, Costimulatory molecules
Introduction
Current standard practice for controlling allogeneic transplant rejection is life-long immunosuppression. These pharmacological agents have significant immunologic and metabolic toxicities. Donor-specific tolerance is an attractive concept, but has not yet been reliably and consistently achieved in humans.
One approach for inducing donor-specific tolerance is by strategic exposures of the recipient to donor antigens prior to transplantation. A critically important aspect of such exposures is to identify conditions that will ensure host tolerance rather than sensitization. Such “planned preimmunization” was experimented in the 1980s in the form of donor-specific transfusions (DST) [1–4]. Despite initial promises, unacceptably high incidences of sensitization following DST alone precluded its clinical application [2, 3]. DST in combination with co-stimulation blockade through anti-CD154 resulted in tolerance in some animal models including non-human primates. However, the pro-thrombotic effect of this monoclonal antibody has limited its clinical application [5–8].
A robust strategy for induction of antigen-specific tolerance has been described in models of autoimmune diseases such as experimental autoimmune encephalomyelitis (EAE) and autoimmune diabetes [9–12], in which peptides or proteins implicated in the specific autoimmune disease (e.g. insulin protein) are cross-linked to the cell surface of splenocytes (SPs) using a chemical cross-linker 1-ethyl-3-(3′-dimethylaminopropyl)-carbodiimide (ECDI). Infusion of such autoantigen-coupled SPs induces robust antigen-specific tolerance by preventing Th1 and Th17 mediated autoimmunity and restores self-tolerance [10–13].
Translating this strategy to transplantation tolerance, we have recently reported that infusions of ECDI-coupled donor splenocytes (ECDI-SPs) on day −7 and day +1 in recipients (with day 0 being the day of transplant) induce long-term allograft tolerance in a full MHC-mismatched mouse model of islet transplantation [14, 15]. This tolerance strategy does not require generalized T and/or B cell depletion or costimulation blockade, therefore making it highly attractive for potential clinical translation for human allogeneic transplantations. While cellular and humoral anti-donor responses are significantly suppressed by the infusion of donor ECDI-SPs, the exact mechanisms leading to such effective control of alloimmunity remain elusive.
Here we report that donor ECDI-SPs are capable of targeting both direct and indirect pathways of allo-recognition via distinct mechanisms. The majority of donor ECDI-SPs are rapidly internalized by recipient splenic antigen presenting cells (APCs), particularly the CD11c+ dendritic cells (DCs), which selectively up-regulate negative, but not positive, co-stimulatory molecules. Upon encountering with such recipient APCs, T cells with indirect allo-specificity undergo rapid expansion followed by profound clonal contraction, with the remaining T cells sequestered in the spleen without trafficking to the graft or graft draining lymph nodes (DLN). On the other hand, residual donor ECDI-SPs not internalized by host phagocytes weakly stimulate T cells with direct allo-specificity and render them resistant to subsequent stimulation (anergy). In addition, regulatory T cell population is expanded by ECDI-SPs. Thus, donor ECDI-SPs based therapy employs several distinct yet synergistic mechanisms to achieve robust and durable transplant tolerance.
Materials and Methods
Mice
8 to 20-week-old male BALB/c (H2d), congenic Thy1.1, Thy1.2, CD45.2 and CD45.1 C57BL/6 (H2b), SJL (H2s), Foxp3GPF knock-in mice, CD11c-DTR mice and IFN-γ−/− mice on a C57BL/6 background were purchased from the Jackson Laboratory. 4C mice were provided by Dr. Qizhi Tang from UCSF. All mice were housed under specific pathogen-free conditions at Northwestern University (NU). Protocols were approved by the NU IACUC.
Antibodies and FACS analysis
PE-conjugated anti-IFN-γ (XMG1.2), APC-conjugated anti-Thy1.2(104), PerCP-conjugated anti-CD4 (L3T4; GK1.5), APC-conjugated anti-CD11c (HL3), PerCP-conjugated anti-B220 (RA3-6B2), PerCP-conjugated anti-CD11b (Mac-1α chain), PE-conjugated anti-CD8α (53.6.7), PE-conjugated anti-CD86 (7-2), FITC-conjugated anti-CD40 (3/23), PE-conjugated anti-CD80 (16-10A1), PE-conjugated anti-PD-L2 (TY-25), PE-conjugated anti-PD-L1 (MIH-5), and APC-conjugated anti-CD25 (PC61) were from BD Biosciences. PE-conjugated anti-mouse Foxp3 (FJK-16a) was from eBiosciences.
Diabetes experiments
Mice were treated with streptozotocin (Sigma Aldrich) at 170 mg/kg. Confirmation of diabetes and protocol for islet transplantation are described previously[16]. Graft rejection was determined by two consecutive blood glucose readings > 250 mg/dL.
ECDI Cell coupling and tolerance induction
Tolerance was induced by i.v. injection of ECDI treated donor SPs as described [15]. For tracking of ECDI-treated splenocytes in vivo, the cells were further labeled with PKH-67 (Sigma-aldrich) at a final concentration of 2×10−6 M at 1×107 cells/ml at room temperature for 5 min, followed by washing prior to injection into untreated recipients or recipients depleted of various sub-types of phagocytes. For macrophage depletion, clodronate-loaded liposome (Encapsula NanoScience, 300 μl per mouse) was injected i.v. 18 hours prior to each dose of ECDI-SPs injection. For B cell depletion, 250 μg of anti-mCD20 depleting antibody (5D2, Genentech) was injected i.p. 72 hours prior to each dose of ECDI-SPs injection. For CD11c+ DC depletion, diphtheria toxin (DT, 10 ng/kg) was injected i.p. 18 hours before each dose of ECDI-SPs injection in CD11c-DTR mice. Depletion of respective cell populations was each verified by FACS analysis. For neutralizing IFN-γ, anti-IFN-γ (XMG1.2, BioXCell) was given at 500 μg/mouse i.p. 24 hours prior to each dose of ECDI-SPs injection.
Immunofluorescence
Frozen sections of spleens were blocked with 10% donkey serum (Sigma-Aldrich). Staining was performed with anti-CD11c mAb (Armenian Hamster IgG clone AP-MAB0814; Novus Biologicals), anti-CD45R (B220) mAb (rat IgG2a, κ clone RA3-6B2; eBiosciences) and anti-F4/80 mAb (rat IgG2a, κ clone BM8; eBiosciences). Antibody binding was visualized using secondary antibodies (Dylight 594-conjugated AffiniPure goat anti-Armenian hamster IgG for CD11c and Dylight 594-conjugated AffiniPure donkey anti-rat IgG for all other markers; Jackson ImmunoResearch). Mounting medium with dapi was used (Vector Laboratories). Images were visualized using Zeiss Axio Scope A1, acquired with Jenoptik ProgRes MFcool camera and analyzed with ProgRes Mac Capture Pro 2.7 software.
Generation and adoptive transfer of activated DCs
B6 or BALB/c bone marrow dendritic cells (BM-DCs) were generated using published protocol[17]. B6 BM-DCs were pulsed with BALB/c or SJL (third party) lysates (prepared with 3 cycles of freeze-thaw of splenocytes) for 8 hours followed by LPS (100 ng/ml) activation overnight. BALB/c BM-DCs were similarly activated with LPS overnight. CD11c+ DCs were enriched the next day using CD11c positive isolation kit (Miltenyi). 2×106 CD11c+ cells were injected i.p. into B6 recipients on indicated days.
Adoptive transfer of T cells
TCR transgenic TEa (CD45.2+) and 4C (Thy1.1+) CD4 T cells were purified from spleens of respective TCR transgenic mice using CD4+ negative isolation kit (Miltenyi). TEa and 4C CD4 T cells were labeled with 5 μM CFSE (Molecular Probes) and injected i.v. into CD45.1+Thy1.2+ B6 congenic recipients on day −8 in reference to the day of islet transplantation (day 0), and analyzed on indicated days. In some experiments, CFSE-labeled TEa (CD45.2+) CD4 T cells were injected into CD11c-DTR (CD45.2+) mice on day −8, and analyzed on indicated days using Vα2 to gate on the injected TEa cell population. For in vitro re-stimulation, harvested cells were further stimulated with PMA/ionomycin (50 ng/ml and 200 ng/ml, respectively) for 4 hours and further proliferation examined by CFSE dilution.
Statistical analysis
Graft survival was calculated by Kaplan Meier analysis. Log Rank test was used to compare survival between groups. Column statistics was performed using Student’s t-test. P values < 0.05 were considered to be statistically significant. All analyses were done with GraphPad PRISM 5 software.
Results
Donor ECDI-SPs are rapidly internalized by recipient splenic antigen presenting cells
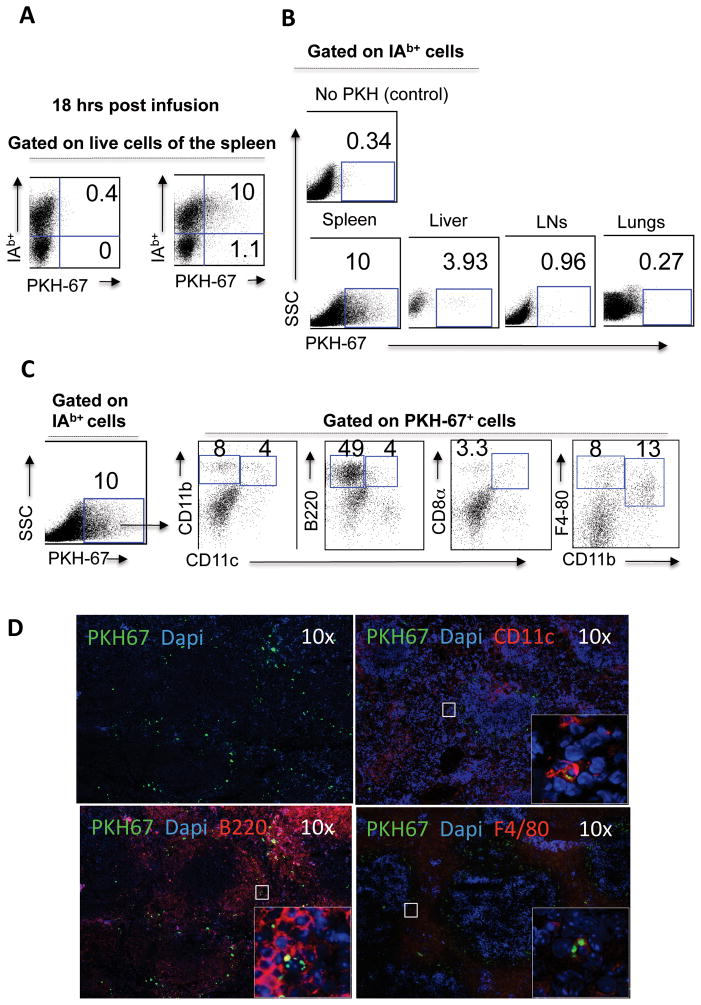
In order to track the donor (BALB/c) ECDI-SPs in vivo after injection, the cells were labeled with a membrane fluorophore (PKH-67) prior to injection. The distribution of PKH-67+ cells at 3 and 18 hrs post injection was investigated. At 3 hours post injection, over 85% of PKH-67+ cells were also positive for recipient (B6) MHC class II I-Ab, suggesting that majority of the injected donor ECDI-SPs had been internalized by recipient class II+ antigen presenting cells (APCs) (data not shown). This distribution of PKH-67+ at 18 hours post injection (Fig. 1A) was not significantly different from that at 3 hours, and therefore 18 hour data are shown for subsequent analysis. As shown in Fig. 1B, the majority of I-Ab+PKH-67+ cells were found in the spleen, and to a lesser degree in the liver, of the injected mice. Few, if any, I-Ab+PKH-67+ cells were found in peripheral lymph nodes (LNs), the lungs (Fig. 1A, right panels), the bone marrow, or the thymus (data not shown). As shown in Fig. 1C, of the I-Ab+PKH-67+ cells in the spleen, 4% were CD11b+CD11c+ DCs, 4% were B220+CD11cint DCs, and 3.3% were CD8α+CD11c+ DCs. In addition, 49% of I-Ab+PKH-67+ cells in the spleen were B220+CD11c− B cells (confirmed by cell surface expression of CD19, data not shown), and the remaining I-Ab+PKH-67+ cells were mainly F4/80+CD11b+ (13%) and F4/80+CD11b−(8%) macrophages. Anatomically, at 18 hr post injection, PKH-67+ cells were mainly distributed in the marginal zone of the spleen (Fig. 1D, left upper panel). Double fluorescent staining and confocal microscopic examination of the spleen tissue revealed PKH-67+ fragments (green, intracellular) within CD11c+, B220+ and F4/80+ cells (red, labeled with respective cell surface antibodies), confirming that PKH-67+ cells had indeed been internalized by host APCs (Fig. 1D).
Figure 1. Donor ECDI-SPs are rapidly internalized by recipient splenic APCs.
1×108 ECDI-fixed, PKH-67-labeled BALB/c splenocytes were injected into B6 mice. 18 hours later, organs were harvested, treated with collagenase, and stained with I-Ab (class II of B6), CD11c, CD11b, B220, and CD8α. IAb was used to differentiate recipient APCs picking up PKH-67+ ECDI-SPs fragments from intact PKH-67+ donor ECDI-SPs themselves. Portions of the spleen were processed for immunofluorescent staining. A, Distribution of free PKH-67+ donor ECDI-SPs (I-Ab−PKH-67+) versus internalized PKH-67+ ECDI-SPs fragments by recipient I-Ab+ APCs (I-Ab+PKH-67+) in the spleen. B, Distribution of I-Ab+PKH-67+ in the spleen, the liver, peripheral lymph nodes (LNs), and the lungs. PKH-67+ gate was set by using splenocytes from B6 mice injected with unlabeled ECDI-fixed BALB/c splenocytes (“No PKH (control)”). C, I-Ab+PKH-67+ cells were further characterized by cell surface markers of APC subtypes. D, Co-focal immunofluorescent images of B6 spleens 18 hours after injection of ECDI-fixed, PKH-67-labeled BALB/c splenocytes. Sections were stained with respective antibodies as shown. Panel 1: overall distribution of PKH-67+ cells (green) within the spleen. Panels 2–4: sub-cellular localization of PKH-67+ fragments (green, intracellular) was seen in CD11c+, B220+ and F4/80+ cells (red, cell surface). Data shown in A to C are representative of at least 3 independent experiments. Data shown in D are representative of sections from at least 3 identically treated individual spleens.
Recipients CD11c+ DCs are obligatory for allograft tolerance induced by donor ECDI-SPs infusions
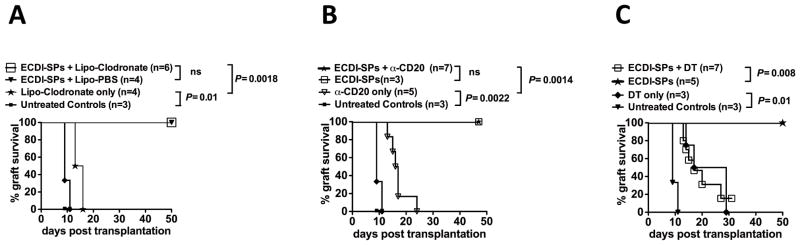
To determine which APC population internalizing the injected donor ECDI-SPs might be responsible for the induction of tolerance, individual APC population was depleted at the time of donor ECDI-SPs infusion and subsequent islet allograft survival was examined. Macrophages were depleted by injecting the mice with Lipo-Clodronate 18 hours prior to ECDI-SPs infusions. Recipient treatment with Lipo-Clodronate indeed globally depleted macrophages (data not shown) including those that had taken up ECDI-SPs (Supplementary Fig. 1A). As shown in Fig. 2A, depletion of macrophages at the time of ECDI-SPs infusions did not affect islet allograft survival. We next depleted B cells with anti-mouse CD20 monoclonal antibody. The depleting antibody was given three days prior to donor ECDI-SPs infusions and indeed depleted B cells that had internalized ECDI-SPs (Supplementary Fig. 1B). The effect of B cell depletion using this antibody was long-lasting with slow gradual return of CD19+ cells beginning at least 3–4 weeks after the treatment (data not shown). As shown in Fig. 2B, depletion of B cells at the time of ECDI-SPs injections also did affect islet allograft survival. Depletion of B cells itself in the absence of ECDI-SPs had only a modest effect on graft survival as all islet grafts were rejected between 13 to 23 days. Finally, the role of CD11c+ DCs in tolerance induction was studied using CD11c-DTR (diphtheria toxin receptor) mice. Administration of diphtheria toxin (DT) efficiently depleted CD11c+ cells in these mice including those CD11c+ cells that had taken up ECDI-SPs (Supplementary Fig. 1C). Mice were injected with DT 18 hours prior to donor ECDI-SPs infusions and islet allograft survival was examined. As shown in Fig. 2C, while depletion of CD11c+ cells itself actually had a moderate beneficial effect on islet allograft survival, it completely abolished the graft tolerance effect by donor ECDI-SPs infusions such that the majority of the mice rejected their islet allograft by day 30. These data indicate that the CD11c+ cell population plays an obligatory role in allograft tolerance induced by donor ECDI-SPs infusions.
Figure 2. Recipients CD11c+ DCs are obligatory for allograft tolerance induced by donor ECDI-SPs infusions.
Depletion of macrophage, B cells, and DCs and confirmation of depletion are described in Materials and Methods. A, Graft survival in mice depleted of macrophages by liposome clodronate (Lipo-Clodronate) at the time of donor ECDI-SPs infusions. B, Graft survival in mice depleted of B cells by anti-mCD20 at the time of donor ECDI-SPs infusions. C, Graft survival in CD11c-DTR mice depleted of CD11c+ DCs by treatment with DT at the time of donor ECDI-SPs infusions. P values for significant differences are shown.
Splenic CD11c+ DCs from recipients treated with donor ECDI-SPs selectively up-regulate negative co-stimulatory molecules
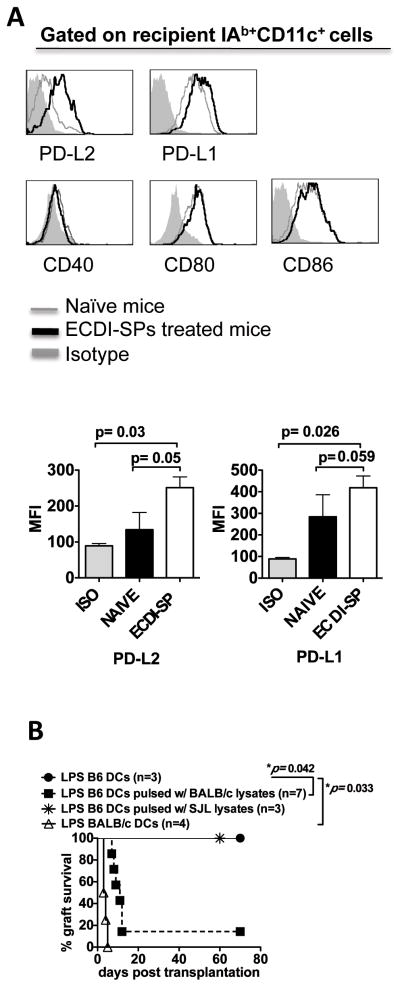
As CD11c+ DCs appeared to be the pivotal APC population mediating the tolerogenic effect of ECDI-SPs, we next examined the phenotype of splenic CD11c+ DCs from recipients treated with donor ECDI-SPs. As shown in Fig. 3A, compared with CD11c+ DCs from untreated recipients, DCs from recipients treated with ECDI-SPs up-regulated the expression of the negative co-stimulatory molecule programmed death ligand-2 (PD-L2), and to a lesser degree programmed death ligand-1 (PD-L1). The mean fluorescent intensities (MFIs) for PD-L2 and PD-L1 are shown in the bar graph below to the histograms. In contrast, expression of positive co-stimulatory molecules CD40, CD80 and CD86 was not up-regulated. We therefore hypothesized that this altered pattern of negative versus positive co-stimulatory pattern was critical in mediating the tolerogenic effect of ECDI-SPs. If so, providing activated DCs carrying the alloantigens would provide the missing positive co-stimulation signals in trans and effectively abrogate tolerance induced by donor ECDI-SPs infusions. To test this hypothesis, B6 BM-DCs were pulsed with BALB/c splenocyte lysate, activated with LPS overnight, and injected to B6 recipients (2×106 cells per mouse) on the same day of the first ECDI-fixed donor cell infusion, and islet allograft survival was examined. The uptake of BALB/c lysate by B6 BM-DCs and the up-regulation of CD80, CD86 and CD40 after LPS treatment of B6 BM-DCs were both confirmed by FACS analysis (data not shown). As shown in Fig. 3B, transferring of activated B6 BM-DCs pulsed with BALB/c lysate (activating the indirect pathway of allo-recognition) effectively abolished tolerance induction. This process was exquisitely donor-specific, and not due to non-specific production of inflammatory cytokines by the activated DCs, because transferring of activated but un-pulsed DCs or DCs pulsed with third party (SJL) splenocyte lysate was unable to affect tolerance induced by donor (BALB/c) ECDI-SPs infusions. Interestingly, transferring of LPS-activated donor (BALB/c) BM-DCs (activating the direct pathway of allo-recognition) was also able to abolish tolerance induced by donor ECDI-SPs (Fig. 3B). These findings indicate that effectively control of both the indirect and direct allo-antigen presentation pathways is one of the critical mechanisms by which ECDI-SPs infusions induce transplant tolerance and mediate allograft protection.
Figure 3. Splenic CD11c+ DCs from recipients treated with donor ECDI-SPs selectively up-regulate negative co-stimulatory molecules.
A, B6 mice were injected with 1×108 BALB/c ECDI-SPs. Untreated naive mice served as control. 18 hours later, the spleen was treated with collagenase, processed into single cell suspension, stained with respective antibodies as indicated. Top panels: gray line: untreated naïve control mice; black line: mice injected with BALB/c ECDI-SPs; shaded histogram: isotype control. Data shown are representative of 3 independent experiments. Bottom panels: bar graphs showing average MFIs of PD-L1 and PD-L2 from 3 independent experiments with P values listed to indicate statistical significance. B, LPS activated DCs abrogate tolerance induction by ECDI-SPs infusions. For indirect allo-specificities, B6 BM-DCs were pulsed with BALB/c lysate, activated overnight with LPS, and injected into B6 mice on the same days as the first dose of ECDI-SPs injection. B6 BM-DCs not pulsed with lysate or pulsed with third party (SJL) lysate were used as controls. For direct allo-specificities, BALB/c BM-DCs were activated overnight with LPS and injected into B6 mice on the same day as the first dose of ECDI-fixed donor cell injection.
Donor ECDI-SPs infusions deplete T cells with indirect allo-antigen specificity
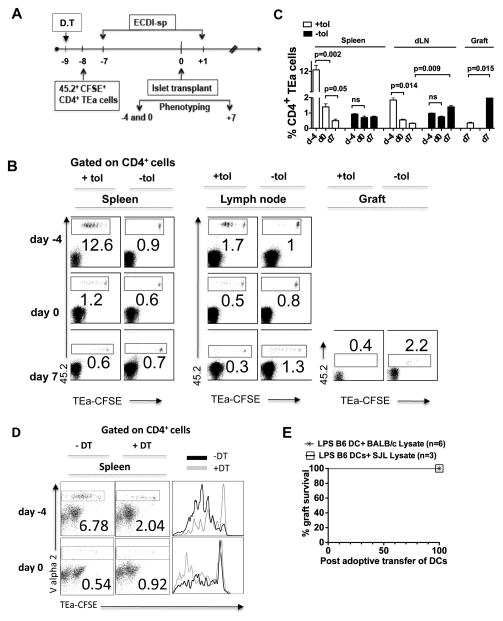
We next examined the effect of ECDI-SPs infusions on CD4 T cells with indirect allo-antigen specificity. To do so, TEa T cell receptor (TCR) transgenic mice were used. CD4+ T cells from TEa mice carry a transgenic TCR specific for the I-Eαd (BALB/c) allopeptide 52–68 cross-presented by B6 MHC class II I-Ab, therefore carrying indirect donor antigen-specificity [18]. As illustrated in Fig. 4A, 4×106 CD45.2+CD4+ TEa T cells were labeled with CFSE and adoptively transferred to CD45.1+ B6 recipients 1 day prior to the first dose of donor ECDI-SPs infusion on day −7. Mice were subsequently transplanted with islet grafts on day 0, tolerized again on day +1 according to our standard protocol, sacrificed on days −4, 0 and +7 for examination of the injected TEa cells. Comparisons were made to untreated control mice. As shown in representative dot plots in Fig. 4B, in tolerized mice (“+tol”), within 72 hrs of donor ECDI-SPs infusion (day −4), TEa cells underwent 6–7 cycles of cell divisions and expanded significantly (12.6%) in the spleen (Fig. 4B, left panels). The majority of the expanded TEa cells remained in the spleen rather than traveling to peripheral LNs (Fig. 4B, middle panels). However, by day 0 (i.e., the day of islet transplantation and 7 days post the first donor ECDI-SPs infusion), these cells underwent ~10-fold contraction (from 12.6% to 1.2%). Furthermore, following the second donor ECDI-SPs infusion on day +1, by day +7 their number in the spleen further diminished (0.6%). Moreover, only a small fraction of total TEa cells were able to be detected in the graft dLN (0.3%, middle panel) or the islet graft (0.4%, right panel) at this time point. In contrast, in untreated naïve mice (“−tol”), TEa cells remained quiescent prior to islet graft transplantation and were distributed equally in the spleen and peripheral LNs (0.9% and 1%, respectively). After islet graft transplantation at day +7, a significantly higher percentage of TEa cells homed to the graft dLN (1.3%) and the islet graft (2.2%), and proliferated rigorously there compared with those remaining in the spleen (0.7%). A summary of data from 3–5 independent experiments is presented in Fig. 4C with P values listed to indicate statistical significance. These data indicate that donor ECDI-SPs infusions lead to an initial expansion followed by profound depletion of CD4 T cells with indirect allo-antigen specificities in the spleen. More importantly, the remaining T cells are preferentially retained in the spleen rather than trafficking to the graft dLN or the islet graft to mediate anti-donor responses and graft destruction. One possible explanation for such splenic sequestration is the large burden of cognate antigens in the spleen provided by the infused donor ECDI-SPs.
Figure 4. Donor ECDI-SPs infusions deplete T cells with indirect allo-antigen specificity.
A, Schematic treatment plan. 4×106 CFSE-labeled 45.2+ TEa T cells were adoptively transferred into 45.1 congenic recipients on day −8, followed by standard tolerance induction with ECDI-SPs infusions (day −7, +1) and islet transplantation (day 0). TEa T cells were examined on day −4, 0 and day +7 as indicated. In some experiments, DT injection was performed on day −9 to deplete CD11c+ DCs from the CD11c-DTR mice. B, Plots were gated on total CD4+ T cells. Percentages of CD45.2+ TEa T cells in the spleen (left panels), the kidney (draining) LNs (middle panels), and the graft (right panels) are shown. +tol: with ECDI-SPs infusions; −tol: without ECDI-SPs infusions. Data shown are representative of 3–5 individual experiments. C, Summary of data from 3–5 individual experiments performed as in B. The averages of percentages of 45.2+ (TEa) cells among total CD4+ cells were calculated and depicted in the bar graph, and P values are listed to indicate statistical significance. D, CD11c-DTR mice were injected with DT to deplete CD11c+ cells (+DT) or not (−DT) prior to injection of CD4+ TEa cells and ECDI-SPs as schematically shown in Fig. 4A. TEa T cells were examined in the spleen on day −4 and day 0 by gating on Vα2+ cells. Left panels: representative dot plots. Right panel: histogram overlay. E, Long-term tolerized mice by ECDI-SPs infusions (>100 days graft survival post initial islet transplantation) were adoptively transferred with LPS-activated B6 BM-DCs pulsed with donor (BALB/c) or a third party (SJL) lysate. Graft survival was monitored by blood glucose measurements.
Given the critical importance of CD11c+ cells in the graft protection induced by ECDI-SPs as shown in Fig. 2C, we next examined the effect of CD11c+ cell depletion on the behavior of the injected TEa cells. As shown in Fig. 4D, when CD11c+ cells were depleted from the ECDI-SPs treated recipients, the initial (day −4) TEa cell division and expansion seen in the spleen were much diminished compared with those seen in recipients not depleted of CD11c+ cells (2.04% in depleted vs. 6.78% in un-depleted recipients). Consequently, on day 0, a much less profound contraction was seen in the depleted recipients (~2-fold, from 2.04% to 0.92%) compared with that seen in the un-depleted recipients (~13-fold, from 6.78% to 0.54%). We speculate that the remaining TEa cells in the CD11c+ cell depleted recipients are fully capable of responding to further antigenic stimuli of an islet transplantation, and trafficking to the graft and the graft draining lymph node to mediate effector function.
Consistent with the notion that T cells with indirect allo-specificities are by-and-large depleted by our strategy, in long-term tolerized mice by donor-ECDI-SPs infusions (>100 days after the initial transplant and with functioning islet grafts), adoptive transfer of LPS-activated recipient CD11c+DCs pulsed with donor lysate (therefore presenting to and activating T cells with indirect allo-specificities) could not break established graft tolerance to precipitate islet graft rejection (Fig. 4E). This is in sharp contrast to the ability of such activated DCs to prevent tolerance at the induction stage (Fig 3B) when T cells with indirect allo-specificities are still abundantly present.
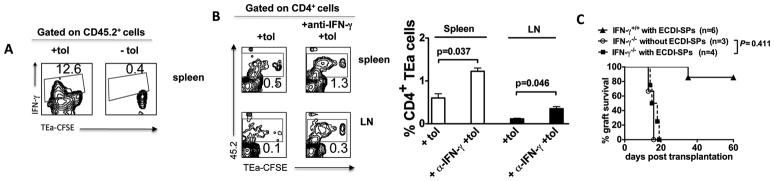
IFN-γ contributes to the depletion of allo-specific T cells and is obligatory for tolerance induced by donor ECDI-SPs infusions
Examination of cytokine expression in the spleen after donor ECDI-SPs injection revealed that IFN-γ was expressed by a significant percentage (12.6%) of the proliferating TEa cells (Fig. 5A). IFN-γ has been previously implicated in activation induced cell death (AICD) of T cells through caspase pathway activation [19]. We therefore tested whether production of this cytokine by proliferating TEa T cells contributed to the ultimate decrease in numbers of these cells. To do so, mice were treated with a neutralizing anti-IFN-γ concomitant with donor ECDI-SPs infusion, and TEa cells were examined 7 days later. As shown in Fig. 5B (representative contour plots on left, and summary bar graph on right with P values listed to indicate statistical significance), treatment with anti-IFN-γ mAb increased the number of TEa cells in the spleen and LNs by ~2–3 fold. To further ascertain the role of IFN-γ in the induction of tolerance by donor ECDI-SPs infusions, we attempted to tolerize IFN-γ recipients using the same regimen. As shown in Fig. 5C, IFN-γ recipients were resistant to tolerance by this regimen and rejected their islet grafts 15–20 days post transplantation in a manner similar to untreated IFN-γ recipients. These data suggest that IFN-γ plays an obligatory role in the tolerance induced by donor ECDI-SPs infusions, potentially through depletion of donor-specific T cells during initial activation.
Figure 5. IFN-γ contributes to the depletion of allo-specific T cells and is obligatory for tolerance induced by donor ECDI-SPs infusions.
4 × 106 CFSE-labeled CD45.2+ TEa T cells were injected into CD45.1+ congenic B6 recipients 1 day prior to treatment with donor ECDI-SPs. A, The spleen was obtained 72 hours after the injection of ECDI-SPs and analyzed by FACS. Plots shown were gated on CD45.2+ cells. IFN-γ expression was increased in proliferating TEa T cells in response to ECDI-fixed donor cell infusion. B, Neutralizing anti-IFN-γ mAb (500 μg × 1) was injected on the day of ECDI-fixed donor cell infusion. The spleen and peripheral LNs were obtained 7 days after the injection of ECDI-SPs and analyzed by FACS. Plots shown were gated on CD4+ cells. Anti-IFN-γ treatment partially rescued the depletion of TEa T cells in the spleen and peripheral LNs. The averages of percentages of 45.2+ (TEa) cells among total CD4+ cells in the spleen and peripheral LNs with or without anti-IFN-γ treatment were calculated and depicted in the bar graph, and P values are listed to indicate statistical significance. C, IFN-γ−/− recipients were not able to be tolerized by donor ECDI-SPs infusions. IFN-γ−/− recipients were transplanted with islet allografts with or without donor ECDI-SPs infusions. Graft survival was not different between IFN-γ−/− recipients with or without ECDI-SPs treatment (*P = 0.411). Data shown in A and B are representative or average of 3 individual experiments.
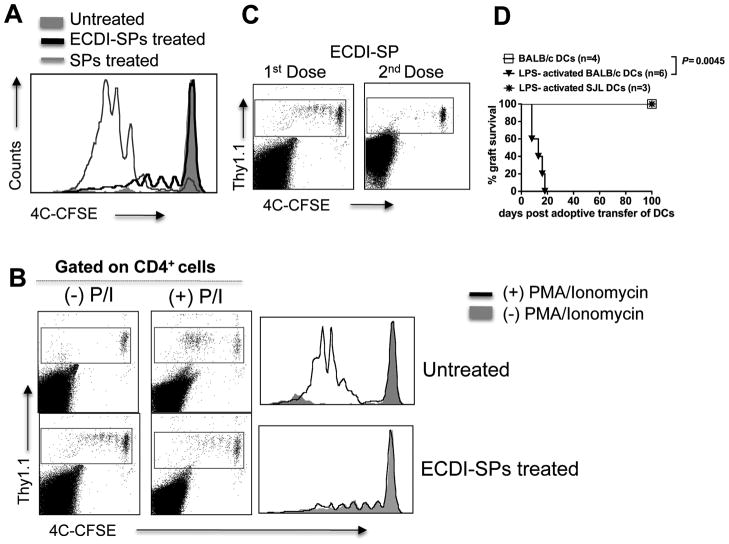
Donor ECDI-SPs infusions induce anergy in T cells with direct allo-antigen specificity
Post injection, a small percentage of the donor ECDI-SPs remained free without being internalized by recipient APCs (1.1%, Fig, 1A). These donor cells are likely able to transiently activate host T cells with direct allo-antigen specificities. To examine this, we took advantage of the 4C transgenic mice. CD4+ cells from these mice carry a transgenic TCR specific for a BALB/c allopeptide presented by BALB/c class II MHC I-Ad, and therefore display direct donor antigen-specificity [20]. Similar to what is shown schematically in Fig. 4A, 4×106 Thy1.1+CD4+ 4C T cells were labeled with CFSE and adoptively transferred to Th1.2+ B6 recipients one day prior to the first dose of donor ECDI-SPs infusion. Mice were subsequently transplanted with islet grafts on day 0 and tolerized again on day +1 according to our standard protocol. Comparisons were made to untreated control mice and mice injected with BALB/c SPs without ECDI treatment. As shown in Fig. 6A, 72 hrs after donor ECDI-SPs infusion, a small fraction of the 4C cells underwent up to three cycles of cell divisions. 4C cells in untreated mice remained completely undivided due to the lack of stimulator cells. In contrast, in mice injected with BALB/c SPs without ECDI treatment, the majority of the 4C T cells underwent five to seven cycles of cell division. Interestingly, 4C T cells isolated from mice receiving donor ECDI-SPs entered a state of unresponsiveness as they were completely resistant to further activation when stimulated ex vivo with phorbol myristate acetate (PMA) and Ionomycin (Fig. 6B, bottom panels). This was in sharp contrast to the undivided 4C T cells recovered from untreated mice, which proliferated vigorously in response to PMA and Ionomycin stimulation (Fig. 6B, top panels). Furthermore, after receiving an islet transplant and following the second donor ECDI-SPs infusion on day +1, the previous undivided 4C cells in the ECDI-SPs treated mice showed no further division in the spleen (Fig. 6C) or peripheral and draining lymph nodes (data not shown) on day +7. These data indicate that donor ECDI-SPs infusions induce a state of unresponsiveness (anergy) in T cells with direct allo-antigen specificity.
Figure 6. Donor ECDI-SPs infusions induce anergy in T cells with direct allo-antigen specificity.
4×106 CFSE-labeled Thy1.1+CD4+ 4C T cells were adoptively transferred into Thy1.2+ recipients on day −8, followed by our standard tolerance induction with ECDI-SPs infusions (day −7, +1) and islet transplantation (day 0). 4C T cells were examined on day −4 (for A, B) and day +7 (for C). A, Histogram shows 4C T cell proliferation by CFSE dilution in mice: treated with donor ECDI-SPs (thick black line), with donor SPs not treated with ECDI (thin gray line), and untreated (shaded gray). B, T cells were isolated from the spleen of untreated mice (top panels) or mice treated with donor ECDI-SPs (bottom panels), and stimulated ex vivo with PMA and Ionomycin for 4 hours. Plots were gated on total CD4+ T cells. Dot plots show CSFE dilution of 4C cells before ((−)P/I) and after ((+)P/I) PMA/Ionomycin stimulation. Comparison of before and after PMA/Ionomycin stimulation is shown by histogram overlay. C, Further proliferation of the Thy1.1+ 4C T cells after receiving the 2nd dose of donor ECDI-SPs (day +1) was examined on day +7, and compared with that after the 1st dose of donor ECDI-SPs (examined on day −4). D, Long-term tolerized mice by ECDI-SPs infusions (>100 days graft survival post initial islet transplantation) were adoptively transferred with un-activated donor (BALB/c) BM-DCs, or LPS-activated donor (BALB/c) or third party (SJL) BM-DCs. Graft survival was monitored by blood glucose measurements. Data shown in A–C are representative of at least 3 individual experiments.
Consistent with this notion, in long-term protected mice by donor-ECDI-SPs infusions (>100 days after the initial transplant and with functioning islet grafts), transfer of LPS-activated, but not un-activated, BALB/c (donor) BM-DCs (therefore presenting to and activating T cells with direct allo-specificities) broke the established tolerance and precipitated graft rejection (Fig. 6D), suggesting that such a state of unresponsiveness of T cells with direct allo-antigen specificity can be reversed when appropriate stimuli are present.
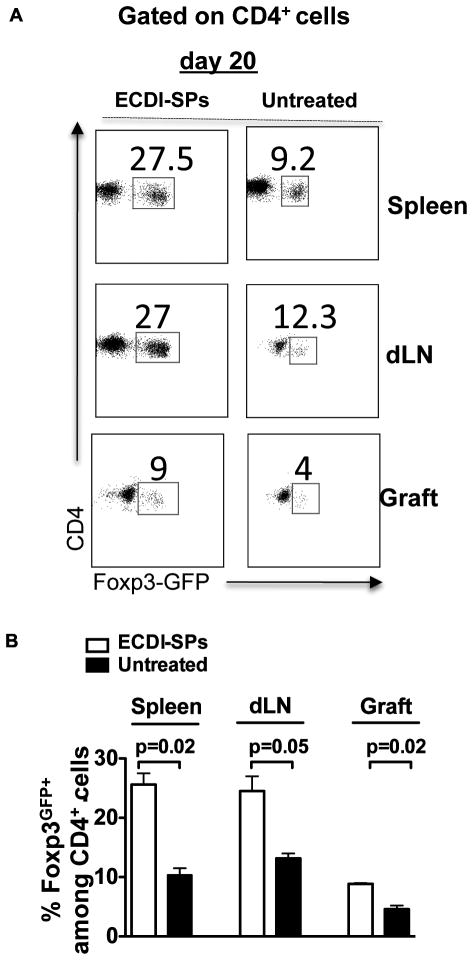
Donor ECDI-SPs infusions induce regulatory T cells in secondary lymphoid organs and the islet graft
As a third mechanism for tolerance (in addition to clonal deletion and anergy), immunoregulation was next examined. To do so, Foxp3-GFP knock-in mice were used as islet graft recipients, and CD4+Foxp3GFP+ cells in secondary lymphoid organs (SLOs) and the graft were examined on 20 days post transplant in recipients with or without donor ECDI-SPs infusions. As shown in Fig. 7A (representative dot plots) and 7B (summary bar graph with P values listed to indicate statistical significance), recipients treated with donor ECDI-SPs displayed higher percentages of CD4+Foxp3GFP+ cells (among total CD4+ cells) in the spleen, the draining lymph nodes, as well as the graft as compared with untreated recipients. Therefore, donor ECDI-SPs infusions lead to an early increase of CD4+Foxp3+ regulatory T cells in all SLOs and the graft. This finding is consistent with our previous observation that tolerance by this regimen is abrogated by treatment of recipients with a depleting anti-CD25 antibody (PC61) at the time of the first donor ECDI-SPs infusion [15, 21].
Figure 7. Donor ECDI-SPs infusions induce regulatory T cells in secondary lymphoid organs and the islet graft.
Foxp3-GFP knock-in mice were transplanted with islet allograft with or without our standard treatment with donor ECDI-SPs infusions. Presence of CD4+Foxp3GFP+ T regulatory cells were examined in the spleen, the graft draining lymph node (dLN), and the graft on day 20 post transplantation. Percentages of Foxp3GFP+ T cells among all CD4+ cells on day 20 are shown. A, Representative dot plots were gated on CD4+ T cells. B, Data from 3 individual experiments were averaged and depicted in the bar graph, and P values are listed to indicate statistical significance.
Discussion
Antigen-coupled ECDI-SPs have been used to induce tolerance to auto-antigens and allo-antigens in several disease models including multiple sclerosis (MS), autoimmune diabetes, and allogeneic islet transplantation [10–12, 15], and has recently been shown to be effective in controlling allergic airway disease and food allergy [22]. Therefore, this tolerance strategy has high potential for translating to clinical practice for various applications including transplantation. However, allo-reactive T cell repertoire is distinct from auto-reactive T cell repertoire. First, the precursor frequency is likely to be much larger [23]. Second, recipient T cells may be directly activated by allo-antigens presented on donor MHCs by donor passenger leukocytes; or indirectly activated by allo-antigens processed and cross-presented on recipient MHCs. Therefore, successful tolerance strategies for transplant will need to be able control both pathways effectively.
In the current report, we attempt to dissect the mechanisms of donor-specific graft protection provided by donor ECDI-SPs infusions. We demonstrate that donor ECDI-SPs infusions target both pathways of antigen presentation via distinct mechanisms. Recipient CD11c+ DCs internalizing donor ECDI-SPs selectively up-regulate negative co-stimulatory molecules. Indirect presentation of allo-antigens to recipient T cells by these CD11c+ DCs results in an initial expansion followed by profound contraction of T cells with indirect antigen specificities. This pattern of short-term, un-sustained T cell effector responses may also suggest the inability of these T cells to develop into long-lived memory cells [24]. On the other hand, donor ECDI-SPs anergize T cells with direct antigen specificities, likely because of delivery of signal 1 in the absence of signal 2 [25]. This notion is consistent with our observation that splenocytes after ECDI-treatment are unable to up-regulate B7-1, B7-2, and CD40 when cultured in vitro (Bryant J., unpublished observations). Therefore, unlike the tolerance induced by ECDI-coupled peptide infusion in models of autoimmunity where the main mechanism of tolerance occurs through indirect antigen presentation to auto-reactive T cells [10, 25], tolerance induction to allografts benefits from targeting the direct as well as the indirect pathways. As different organ or tissue grafts may carry different types and number of donor passenger leukocytes, ECDI-SPs may have different efficacy in controlling the direct pathway in these different models. This is an important consideration for designing ECDI-SPs based tolerance strategy in other models of transplantation such as allogeneic cardiac transplantation or xenogeneic islet transplantation. On the other hand, indirect antigen presentation is one of the main contributors responsible for chronic rejection [26] and for antibody-mediated rejection [27, 28]. Consistent with this notion is our previous report of lack of anti-donor antibody production in tolerized mice treated with donor ECDI-SPs [15]. The ability of ECDI-SPs to effectively target the indirect pathway therefore overcomes an important clinical problem that contributes to chronic and antibody-mediated rejections.
We observed a significant early expansion of Foxp3+ Tregs in all SLOs as well as the islet graft in ECDI-SPs treated recipients (Fig. 7A and B). One unresolved issue here is whether the observed increase of Foxp3+ Tregs arises from the direct or the indirect allo-reactive T cell repertoires. Neither the TEa nor the 4C T cells in our hands were observed to express Foxp3 in any of the SLOs examined (data not shown). One possibility is that the TCR affinity of TEa or 4C T cells is such that in our system they do not become Foxp3 expressing Tregs. However, this does not exclude the possibility of other allo-specific T cells with different affinities for donor antigens to become Foxp3+ Tregs. It is also possible that the initial increased Foxp3+ cells (Fig. 7) are not donor-specific, such as those seen to be induced in the presence of large numbers of apoptotic cells [29], and the donor-specificity of this tolerance strategy is determined by its ability to control donor-specific effector T cell function seen with the 4C and TEa cells. It has also been observed by others that conversion of TEa T cells to Foxp3+ cells in vivo is exquisite to the timing of the injection of these cells in relation to the injection of the toleragen [30]. Furthermore, we observed that in contrast to the critical role of the CD25+Foxp3+ T cells during tolerance induction [15], in long-term tolerized recipient (>100 days), depletion of these cells by PC-61 no longer had an effect on graft survival (Kheradmand et al, unpublished observation), suggesting that the role of these cells during tolerance maintenance is less prominent. Therefore, we postulate that the TCR repertoire of CD25+Foxp3+ T cells induced by ECDI-SPs may evolve over time, so as their role in the induction and maintenance of tolerance by this strategy.
DCs, macrophages, and B cells as APCs are the major components for initiating immune responses. A critically important outcome of such responses is the decision between antigen-specific immunity versus tolerance. Interestingly, ECDI-fixed donor cells appear to be interacting with all of these populations in vivo upon i.v. injection. However, selective depletion of specific populations suggest that only the CD11c+ DCs are obligatory for the tolerance induced by donor ECDI-SPs infusions. Tolerogenic DCs have been described in several models of transplantation [31, 32] and numerous secretory factors have been implicated in mediating downstream tolerogenic effects [33–36]. Our published data and ongoing experiments reveal that TGF-β [21], IL-10 [37] and indoleamine 2,3-dioxygenase (IDO) (Kheradmand et al, unpublished observations) each plays an obligatory role in tolerance induced by this regimen. The exact sources of these soluble factors are currently under investigation.
An interesting observation in our study is the pattern of expressions of negative versus positive co-stimulatory molecules by the CD11c+ DCs following infusions of donor ECDI-SPs. There was a complete lack of up-regulation of B7-1 and B7-2 molecules by DCs uptaking ECDI-fixed donor cells, whereas PD-L1, and more prominently PD-L2 were both up-regulated. The molecular mechanism by which DCs express this specific pattern of negative versus positive co-stimulatory molecules is currently under investigation using genome-wide gene expression profiling and proteomics approaches. Consistent with a critical role of negative co-stimulatory molecules in our tolerance regimen, we have previously shown that PD-L1−/− mice were resistant to tolerance induction by donor ECDI-SPs infusions [15]. Lack of co-stimulation by B7-1/B7-2-CD28 interactions between APCs and T cells has been shown to induce a rapid but transient T cell activation that is characterized by production of IFN-γ and IL-10 but a lack of production of IL-2, IL-6 and TNF-α [38, 39]. This distinct pattern of cytokine production is thought to subsequently contribute to apoptosis and deletion of the antigen-specific T cell population via both activation induced cell death and passive cell death [40, 41]. Our observation that TEa cells underwent initial expansion in cell numbers followed by rapid clonal contraction with donor ECDI-SPs infusions in an IFN-γ dependent fashion is consistent with this notion. The role of IFN-γ in tolerance induced by this regimen is further ascertained by the inability of IFN-γ−/− recipients to be tolerized. The source of the IFN-γ participating in tolerance induction here is not entirely clear. It is possible that the effector cells are the primary producers of IFN-γ as shown in Fig. 5A, which then acts in an autocrine fashion and promotes AICD via STAT1 and caspase dependent pathways [19]. Ultimately, elucidation of the precise mechanisms by which IFN-γ exerts tolerogenic effects in donor ECDI-SPs infusions awaits studies utilizing cell-specific IFN-γ or IFN-γ receptor knockouts.
Allo-antigen specific TCR repertoire represents up to 5–10% of total host TCR repertoire [23]. Studies using sensitive MHC class II tetramer technology reveal that T cell clones expressing TCRs specific for different foreign peptide-MHC II complex ligands in general do not have cross-reactivities [42, 43]. Therefore, tolerizing the entire allo-antigen specific T cell repertoire by individually tolerizing each allo-reactive T cell clone may be impractical. On the other hand, APCs interacting with allo-specific T cells may simultaneously express multiple allopeptide-MHC complexes of interest. Consequently, inducing tolerogenic APCs in vivo by regimens such as ours has the potential to tolerize multiple allo-reactive T cell clones whose peptide-MHC complex ligands are co-expressed on the same APCs. Therefore, regimens targeting APCs may allow more efficient induction of tolerance via mechanisms including linked suppression [44, 45] and epitope spreading [46].
In conclusion, donor ECDI-SPs infusions target host allogeneic responses via a multitude of mechanisms including clonal depletion, anergy and immunoregulation, thereby providing potent donor-specific allograft protection. Our studies highlighted multiple advantages of this strategy for transplant tolerance over other regimens: i) Generalized T cell depletion is not required, as ECDI-SPs effectively deplete and anergize donor-specific T cells; ii) Generalized costimulation blockade is not required, as ECDI-SPs ensure defective positive co-stimulatory interactions [47] and enhance PD-L1/2-mediated negative costimulation; iii) Adoptive transfer of ex-vivo expanded Tregs is not required, as ECDI-SPs directly promote enhanced Treg function in vivo [15]; iv) ECDI-SPs allow pre-transplant donor antigen presentation to a quiescent immune system promoting preemptive tolerization [48, 49], thereby allowing engraftment of transplanted tissues/organs in the presence of greatly reduced alloreactivity and immunosuppressive drug toxicity; and finally, v) As autoantigen-coupled leukocytes are known to restore tolerance in animal models of autoimmunity, both allo- and autoantigens can be coupled to carrier cells for tolerance induction to transplanted tissue/organs where both alloimmunity and recurrent autoimmunity may be detrimental to the graft. We therefore believe that this simple form of negative vaccination has significant potential for clinical translation to human transplantation.
Supplementary Material
Acknowledgments
We wish to acknowledge the Northwestern University Interdepartmental ImmunoBiology Flow Cytometry Core Facility for its support of this work.
Footnotes
This work was supported by grants from the Juvenile Diabetes Research Foundation Postdoctoral Fellowship Grant 3-2010-447 (T.K.), Regular Research Grant 1-2007-1055 (X.L., S.D.M), the National Institutes of Health Training Grant T32 DK077662 (J.L.H., N.L.) and NIH Directors New Innovator Award DP2 DK083099 (X.L.).
Contribution: T.K., X.L. designed research; T.K., S.W., J.B., J.J.T., J.L.H. and K.L.P. performed experiments; T.K., J.B., S.D.M., Z.Z. and X.L. analyzed results; T.K., J.B., N.L. and X.L. wrote the manuscript.
References
- 1.Goulmy E, Blokland E, Fassbinder W, Persijn G, van Rood JJ. Occurrence of posttransplant donor-specific cell-mediated lympholysis nonreactivity in renal allograft recipients with perioperative transfusions only. Transplantation. 1985;39(1):105–7. [PubMed] [Google Scholar]
- 2.Bucin D. Adverse effect of blood transfusion on the long-term outcome of kidney transplantation. Exp Clin Immunogenet. 1988;5(1):39–47. [PubMed] [Google Scholar]
- 3.Bucin D. Blood transfusion in renal transplantation--the induction of tolerance by incompatibility for class I antigen. Med Hypotheses. 1988;27(1):19–27. doi: 10.1016/0306-9877(88)90077-1. [DOI] [PubMed] [Google Scholar]
- 4.van Twuyver E, Mooijaart RJ, ten Berge IJ, van der Horst AR, Wilmink JM, Kast WM, Melief CJ, de Waal LP. Pretransplantation blood transfusion revisited. N Engl J Med. 1991;325(17):1210–3. doi: 10.1056/NEJM199110243251704. [DOI] [PubMed] [Google Scholar]
- 5.Stumpf C, Lehner C, Eskafi S, Raaz D, Yilmaz A, Ropers S, Schmeisser A, Ludwig J, Daniel WG, Garlichs CD. Enhanced levels of CD154 (CD40 ligand) on platelets in patients with chronic heart failure. Eur J Heart Fail. 2003;5(5):629–37. doi: 10.1016/s1388-9842(03)00110-7. [DOI] [PubMed] [Google Scholar]
- 6.Stumpf C, Lehner C, Yilmaz A, Daniel WG, Garlichs CD. Decrease of serum levels of the anti-inflammatory cytokine interleukin-10 in patients with advanced chronic heart failure. Clin Sci (Lond) 2003;105(1):45–50. doi: 10.1042/CS20020359. [DOI] [PubMed] [Google Scholar]
- 7.Knosalla C, Gollackner B, Cooper DK. Anti-CD154 monoclonal antibody and thromboembolism revisted. Transplantation. 2002;74(3):416–7. doi: 10.1097/00007890-200208150-00024. [DOI] [PubMed] [Google Scholar]
- 8.Knosalla C, Gollackner B, Dor FJ, Cooper DK. Therapeutic interventions in xenotransplantation. Curr Drug Targets Cardiovasc Haematol Disord. 2002;2(2):105–19. doi: 10.2174/1568006023337574. [DOI] [PubMed] [Google Scholar]
- 9.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165(2):302–19. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turley DM, Miller SD. Peripheral tolerance induction using ethylenecarbodiimide-fixed APCs uses both direct and indirect mechanisms of antigen presentation for prevention of experimental autoimmune encephalomyelitis. J Immunol. 2007;178(4):2212–20. doi: 10.4049/jimmunol.178.4.2212. [DOI] [PubMed] [Google Scholar]
- 11.Miller SD, Turley DM, Podojil JR. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat Rev Immunol. 2007;7(9):665–77. doi: 10.1038/nri2153. [DOI] [PubMed] [Google Scholar]
- 12.Fife BT, Guleria I, Gubbels Bupp M, Eagar TN, Tang Q, Bour-Jordan H, Yagita H, Azuma M, Sayegh MH, Bluestone JA. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006;203(12):2737–47. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eagar TN, Turley DM, Padilla J, Karandikar NJ, Tan L, Bluestone JA, Miller SD. CTLA-4 regulates expansion and differentiation of Th1 cells following induction of peripheral T cell tolerance. J Immunol. 2004;172(12):7442–50. doi: 10.4049/jimmunol.172.12.7442. [DOI] [PubMed] [Google Scholar]
- 14.Kheradmand T, Wang S, Gibly RF, Zhang X, Holland S, Tasch J, Graham JG, Kaufman DB, Miller SD, Shea LD, Luo X. Permanent protection of PLG scaffold transplanted allogeneic islet grafts in diabetic mice treated with ECDI-fixed donor splenocyte infusions. Biomaterials. 2011 doi: 10.1016/j.biomaterials.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo X, Pothoven KL, McCarthy D, DeGutes M, Martin A, Getts DR, Xia G, He J, Zhang X, Kaufman DB, Miller SD. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proc Natl Acad Sci U S A. 2008;105(38):14527–32. doi: 10.1073/pnas.0805204105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo X, Yang H, Kim IS, Saint-Hilaire F, Thomas DA, De BP, Ozkaynak E, Muthukumar T, Hancock WW, Crystal RG, Suthanthiran M. Systemic transforming growth factor-beta1 gene therapy induces Foxp3+ regulatory cells, restores self-tolerance, and facilitates regeneration of beta cell function in overtly diabetic nonobese diabetic mice. Transplantation. 2005;79(9):1091–6. doi: 10.1097/01.tp.0000161223.54452.a2. [DOI] [PubMed] [Google Scholar]
- 17.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176(6):1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grubin CE, Kovats S, deRoos P, Rudensky AY. Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II-bound self-peptides. Immunity. 1997;7(2):197–208. doi: 10.1016/s1074-7613(00)80523-3. [DOI] [PubMed] [Google Scholar]
- 19.Refaeli Y, Van Parijs L, Alexander SI, Abbas AK. Interferon gamma is required for activation-induced death of T lymphocytes. J Exp Med. 2002;196(7):999–1005. doi: 10.1084/jem.20020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brennan TV, Hoang V, Garrod KR, Liu FC, Hayden T, Kim J, Kang SM. A new T-cell receptor transgenic model of the CD4+ direct pathway: level of priming determines acute versus chronic rejection. Transplantation. 2008;85(2):247–55. doi: 10.1097/TP.0b013e31815e883e. [DOI] [PubMed] [Google Scholar]
- 21.Luo X, Zhang Q, Liu V, Xia Z, Pothoven KL, Lee C. Cutting Edge: TGF-{beta}-Induced Expression of Foxp3 in T cells Is Mediated through Inactivation of ERK. J Immunol. 2008;180(5):2757–61. doi: 10.4049/jimmunol.180.5.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smarr CB, Hsu CL, Byrne AJ, Miller SD, Bryce PJ. Antigen-fixed leukocytes tolerize Th2 responses in mouse models of allergy. J Immunol. 2011;187(10):5090–8. doi: 10.4049/jimmunol.1100608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watson D, Hu M, Zhang GY, Wang YM, Alexander SI. Tolerance induction by removal of alloreactive T cells: in-vivo and pruning strategies. Curr Opin Organ Transplant. 2009;14(4):357–63. doi: 10.1097/mot.0b013e32832ceef4. [DOI] [PubMed] [Google Scholar]
- 24.Wood SC, Lu G, Burrell BE, Bishop DK. Transplant acceptance following anti-CD4 versus anti-CD40L therapy: evidence for differential maintenance of graft-reactive T cells. Am J Transplant. 2008;8(10):2037–48. doi: 10.1111/j.1600-6143.2008.02372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jenkins MK, Ashwell JD, Schwartz RH. Allogeneic non-T spleen cells restore the responsiveness of normal T cell clones stimulated with antigen and chemically modified antigen-presenting cells. J Immunol. 1988;140(10):3324–30. [PubMed] [Google Scholar]
- 26.Gokmen MR, Lombardi G, Lechler RI. The importance of the indirect pathway of allorecognition in clinical transplantation. Curr Opin Immunol. 2008;20(5):568–74. doi: 10.1016/j.coi.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Taylor AL, Negus SL, Negus M, Bolton EM, Bradley JA, Pettigrew GJ. Pathways of helper CD4 T cell allorecognition in generating alloantibody and CD8 T cell alloimmunity. Transplantation. 2007;83(7):931–7. doi: 10.1097/01.tp.0000257960.07783.e3. [DOI] [PubMed] [Google Scholar]
- 28.Noorchashm H, Reed AJ, Rostami SY, Mozaffari R, Zekavat G, Koeberlein B, Caton AJ, Naji A. B cell-mediated antigen presentation is required for the pathogenesis of acute cardiac allograft rejection. J Immunol. 2006;177(11):7715–22. doi: 10.4049/jimmunol.177.11.7715. [DOI] [PubMed] [Google Scholar]
- 29.Perruche S, Zhang P, Liu Y, Saas P, Bluestone JA, Chen W. CD3-specific antibody-induced immune tolerance involves transforming growth factor-beta from phagocytes digesting apoptotic T cells. Nat Med. 2008;14(5):528–35. doi: 10.1038/nm1749. [DOI] [PubMed] [Google Scholar]
- 30.Burrell BE, Bromberg JS. Fates of CD4+ T cells in a tolerant environment depend on timing and place of antigen exposure. Am J Transplant. 2012;12(3):576–89. doi: 10.1111/j.1600-6143.2011.03879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomson AW. Tolerogenic dendritic cells: all present and correct? Am J Transplant. 2010;10(2):214–9. doi: 10.1111/j.1600-6143.2009.02955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill M, Cuturi MC. Negative vaccination by tolerogenic dendritic cells in organ transplantation. Curr Opin Organ Transplant. 2010 doi: 10.1097/MOT.0b013e32833f7114. [DOI] [PubMed] [Google Scholar]
- 33.Wahl SM, Chen W. Transforming growth factor-beta-induced regulatory T cells referee inflammatory and autoimmune diseases. Arthritis Res Ther. 2005;7(2):62–8. doi: 10.1186/ar1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 35.Palafox D, Llorente L, Alberu J, Torres-Machorro A, Camorlinga N, Rodriguez C, Granados J. The role of indoleamine 2,3 dioxygenase in the induction of immune tolerance in organ transplantation. Transplant Rev (Orlando) 2010;24(3):160–5. doi: 10.1016/j.trre.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Jia L, Tian P, Ding C. Immunoregulatory effects of indoleamine 2, 3-dioxygenase in transplantation. Transpl Immunol. 2009;21(1):18–22. doi: 10.1016/j.trim.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Getts DR, Turley DM, Smith CE, Harp CT, McCarthy D, Feeney EM, Getts MT, Martin AJ, Luo X, Terry RL, King NJ, Miller SD. Tolerance Induced by Apoptotic Antigen-Coupled Leukocytes Is Induced by PD-L1+ and IL-10-Producing Splenic Macrophages and Maintained by T Regulatory Cells. J Immunol. 2011;187(5):2405–17. doi: 10.4049/jimmunol.1004175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blair PJ, Riley JL, Harlan DM, Abe R, Tadaki DK, Hoffmann SC, White L, Francomano T, Perfetto SJ, Kirk AD, June CH. CD40 ligand (CD154) triggers a short-term CD4(+) T cell activation response that results in secretion of immunomodulatory cytokines and apoptosis. J Exp Med. 2000;191(4):651–60. doi: 10.1084/jem.191.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yashiro Y, Tai XG, Toyo-oka K, Park CS, Abe R, Hamaoka T, Kobayashi M, Neben S, Fujiwara H. A fundamental difference in the capacity to induce proliferation of naive T cells between CD28 and other co-stimulatory molecules. Eur J Immunol. 1998;28(3):926–35. doi: 10.1002/(SICI)1521-4141(199803)28:03<926::AID-IMMU926>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 40.Wekerle T, Kurtz J, Sayegh M, Ito H, Wells A, Bensinger S, Shaffer J, Turka L, Sykes M. Peripheral deletion after bone marrow transplantation with costimulatory blockade has features of both activation-induced cell death and passive cell death. J Immunol. 2001;166(4):2311–6. doi: 10.4049/jimmunol.166.4.2311. [DOI] [PubMed] [Google Scholar]
- 41.Lehnert AM, Yi S, Burgess JS, O’Connell PJ. Pancreatic islet xenograft tolerance after short-term costimulation blockade is associated with increased CD4+ T cell apoptosis but not immune deviation. Transplantation. 2000;69(6):1176–85. doi: 10.1097/00007890-200003270-00024. [DOI] [PubMed] [Google Scholar]
- 42.Chu HH, Moon JJ, Kruse AC, Pepper M, Jenkins MK. Negative selection and peptide chemistry determine the size of naive foreign peptide-MHC class II-specific CD4+ T cell populations. J Immunol. 2010;185(8):4705–13. doi: 10.4049/jimmunol.1002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jenkins MK, Chu HH, McLachlan JB, Moon JJ. On the composition of the preimmune repertoire of T cells specific for Peptide-major histocompatibility complex ligands. Annu Rev Immunol. 2010;28:275–94. doi: 10.1146/annurev-immunol-030409-101253. [DOI] [PubMed] [Google Scholar]
- 44.Davies JD, Leong LY, Mellor A, Cobbold SP, Waldmann H. T cell suppression in transplantation tolerance through linked recognition. J Immunol. 1996;156(10):3602–7. [PubMed] [Google Scholar]
- 45.Cobbold SP, Adams E, Nolan KF, Regateiro FS, Waldmann H. Connecting the mechanisms of T-cell regulation: dendritic cells as the missing link. Immunol Rev. 2010;236:203–18. doi: 10.1111/j.1600-065X.2010.00913.x. [DOI] [PubMed] [Google Scholar]
- 46.Yang L, DuTemple B, Gorczynski RM, Levy G, Zhang L. Evidence for epitope spreading and active suppression in skin graft tolerance after donor-specific transfusion. Transplantation. 1999;67(11):1404–10. doi: 10.1097/00007890-199906150-00003. [DOI] [PubMed] [Google Scholar]
- 47.Martin AJ, McCarthy D, Waltenbaugh C, Goings G, Luo X, Miller SD. Ethylenecarbodiimide-Treated Splenocytes Carrying Male CD4 Epitopes Confer Histocompatability Y Chromosome Antigen Transplant Protection by Inhibiting CD154 Upregulation. J Immunol. 2010 doi: 10.4049/jimmunol.1000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quezada SA, Fuller B, Jarvinen LZ, Gonzalez M, Blazar BR, Rudensky AY, Strom TB, Noelle RJ. Mechanisms of donor-specific transfusion tolerance: preemptive induction of clonal T-cell exhaustion via indirect presentation. Blood. 2003;102(5):1920–6. doi: 10.1182/blood-2003-02-0586. [DOI] [PubMed] [Google Scholar]
- 49.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–28. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.