Abstract
To review our experience of RCC with IVC thrombus in terms of clinical presentation, principles of surgical management in contemporary era, also an impact of clinico-pathological factors on prognosis. Total 100 patients who underwent radical nephrectomy and IVC thrombectomy between 1991–2008 were included in this retrospective analysis. Data was analysed in terms of clinical pathological factors, survivals and compared with contemporary literature. The extent tumour thrombus was infrahepatic in 58 retro hepatic in 28 and suprahepatic in 14 patients including 6 with right atrial thrombus. The immediate postoperative mortality was 2% and incidence of major postoperative non fatal complications was 38%, which were managed conservatively. The overall and disease free 5 year survival was 63% and 55%. Further amongst the histological types, patients with clear cell tumours had the best (DFS- 71.42%), and those with papillary had the poor (DFS- 30.76%) outcome. Grade II tumors had better survivals as compared to grade IV (DFS 75.39% vs 23.52%, p < 0.05). Loco- regional extent wise 74% patients without perinephric fat invasion were free from disease at 5 years as compared to 30% of those who had perinephric fat invasion (p < 0.01). Similarly 5 year DFS was 76.11% in patients with negative nodes as compared to 12% in positive nodes (p < 0.01). In conclusion radical nephrectomy with IVC thrombectomy still remains the most effective therapeutic option in management in this clinical setting. Although this is complicated surgery success with multi disciplinary approach excellent survival outcome can be obtained. Further pathological factors, such as loco-regional spread and grade of tumor, rather than clinical factors influence long term survival.
Keywords: RCC, IVC thrombus, Clinical presentation, Surgical management, Complications & prognosis, Imunotherapy
Introduction
The tumour thrombus formation and it’s migration in to venous system like renal vein or Inferior vena cava are unique features of Renal Cell Carcinoma (RCC) [1, 2]. Further venous system invasion with RCC is reported to occur in 4–10% of patients [3] .Some of them (2–10%) may present with tumour thrombus extending up to right atrium [4]. There has been increase in incidence of RCC following wide spread use of non invasive imaging techniques like sonography for non urological symptoms. The incidence of asymptomatic patients of RCC with venous involvement is rising steadily as well. Further in the past patients with IVC invasion with metastasis were not operated because of poor survival [5], However some series have reported that patients with solitary metastasis can be operated with good results [6, 7],especially if one is planning for immunotherapy.
Management of such a clinical situation is not only diagnostic (accurate level of thrombus) and surgical challenge but has prognostic implications [2].The current review will focus on literature of clinical presentation, principles of surgical management in the contemporary era & report our experience and outcome data of IVC thrombectomy in 100 patients of RCC.
Materials & Methods
Hospital records of 100 patients of radical nephrectomy with IVC thrombectomy done for RCC from 1991–2008 were queried & analysed. Of them-a cohort of 63 patients has been reported earlier [8]. Further a systematic review of literature was performed using keywords like RCC, IVC thrombus, clinical presentation, surgical management, complications & prognosis. American Joint Committee on Cancer Staging system (TNM) [9] was used for staging & Fuhrman criteria [10] were used for histopathological grading. The operative procedure was a joint venture combining urologist & cardio- vascular surgeon & performed in cardiac theatre with continuous arterial & central venous pressure & end tidal carbon dioxide monitoring throughout the series.. All patients underwent surgery by midline abdominal incision from xiphisternum to symphysis pubis. Those with difficult retro hepatic extension (such as gross hepatomegaly or very obese patients) & those with supra hepatic extension had additional midline sternotomy for control &/or temporary occlusion of intrapericardial IVC. For patients with supra diaphragmatic or right atrial thrombus extensions, cardiopulmonary bypass (CPB) with deep hypothermic circulatory arrest were used. The standard techniques were followed for mobilisation & exposure, thrombectomy & repair [8]. The patients were followed up every 3 monthly for first 2 years and semi annually till 5 years and on annual basis thereafter. Follow up studies included complete clinical examination, biochemical studies and imaging studies like IVU and X ray chest in earlier years and later IVU was replaced by sonography. CAT Scans were used selectively. Data was analysed using SPSS (Statistical Package for Social Sciences) 12 soft ware. Meticulous recording of the clinical findings, operative details and complications was done and analysed. Patients with only renal vein thrombus were excluded from the analysis and data included all patients with IVC thrombus. Survivals were censored at 5 years and both overall survival (OAS) and disease free survival (DFS) were calculated using Kaplan Meyers and students test
Results
Table 1 gives demographic and clinical data of 100 IVC thrombectomies performed over a period of 19 years (1991–2008). During this period inferior vena caval extension of tumor was seen in 11% of all patients of RCC. Mean age of patient was 71 years-(range 23–90 year) with male outnumbering females-ratio 2:1.There was almost 2:1 ratio of right: left side tumors. Majority of patients presented with one or more features of the symptom triad- hematuria, mass or pain, however 36 patients had symptoms and signs of para neoplastic syndrome and were diagnosed on imaging. The average duration of symptoms was 3–4 months. On physical examination, majority (85.7%) had palpable mass, about a third had varicocoele &/or oedema. Since our institution is a tertiary referral centre and majority had investigations done elsewhere also the long study period (19 years) hence the imaging studies were not uniform. However level of thrombus on imaging was consistent with operative findings in last decade.
Table 1.
RCC with IVC thrombectomy—our data with literature review
| Our series | Literature | |
|---|---|---|
| IVC extension | 11% | 4–10% |
| Total no. | 100 | 10–100 |
| Male:female ratio | 2:1 | 1:5.1–4:1 |
| Age-23–94 year | Mean 71 year | 55–70 year |
| R:L | 63:37 | 1:1–4:1 |
| Paraneoplastic syndrome | ||
| Increased ESR | 66.6 | 55.6 |
| Hypertension | 27.7 | 37.5 |
| Anemia | 38.8 | 36.3 |
| Cachexia | 22.2 | 34.5 |
| PUO | 13.8 | 17.2 |
| Raised LFT | 30.5 | 14.4 |
| Hypercalcemia | 5.5 | 4.9 |
| IVC Thrombus-Mayo’s staging | ||
| A | – | – |
| B | 58% | 50% |
| C | 28% | 30% |
| D | 14% | 20% |
| Histopathology type | ||
| Clear cell | 44% | 25–30% |
| Clear + Granular | 26% | 30–40% |
| Granular | 17% | 20–25% |
| Papillary | 13% | 10–15% |
| Tumor grade | ||
| I | – | – |
| II | 40 | |
| III | 35 | |
| IV | 25 | |
| Local stage | ||
| Perinephric positive | 40 | |
| Lymph node positive | 31 | |
| Adrenal positive | 08 | |
| IVC wall positive | 10 | |
Table 2 gives the operative details. Midline abdominal incision was used only in 72 patients & additional sternotomy in 28 patients, of which 8 patient with deep hypothermic circulatory arrest needed cardiopulmonary bypass. The mean operative time for midline abdominal approach was 4.1 h, 5.3 h for additional sternotomy cases & 6.8 h when cardiopulmonary bypass (CPB) used for atrial thrombus. The mean blood transfusion replacement was 3.1 units, 3.8 units & 5.6 units respectively for these 3 levels of approaches & average hospital stay was 8.2,10.6 & 12.2 days for 3 levels of approaches. Of the total 38 (38%) patients with post operative complications 2 died (2% mortality)—1 due to fulminant hepatitis due to sepsis leading to multi organ failure (was suffering from stable chronic hepatitis pre operatively). The other died of CVA. Of the 34 patients with early postoperative morbidity 17 had transient renal dysfunction, 13 had sepsis, another 3 had CVA & 1 had prolonged fever which turned out to be malaria. Late morbidity occurred in 2 patients had intestinal obstruction at 6 month & 2 year requiring reoperation in one (Table 3).
Table 2.
Level of thrombus vs surgical approach (n = 100)
| Approach | Level of thrombus | Number | % of total |
|---|---|---|---|
| Midline abdomen | Intrahepatic | 58 | 72% |
| Retrohepatic | 14 | ||
| Additional sternotomy only | Retrohepatic | 14 | 20% |
| Suprahepatic | 6 | ||
| Additional CPB & deep hypothermia | Suprahepatic | 2 | 8% |
| Right atrial | 6 |
Table 3.
Perioperative complications (n = 100)
| Septicemia/Mods | 13(1 death) | 13% |
|---|---|---|
| Transient renal dysfunction | 17 | 17% |
| Malaria | 3 | 3% |
| Intestinal obstruction | 2 | 2% |
| CVA | 3(1 death) | 3% |
| Total morbidity | 38 | 38% |
| Total mortality | 2 | 2% |
The histopathological report showed majority had Clear cell histology −70% with or without granular differentiation, where as 17 patients had pure granular type & 13 had papillary type. Further grade II tumours were seen in 40%, grade III in 35% and grade IV in 25% patients. The average size of tumour was 11.1 cm (ranging from 5 to 18 cm) with 40 patients had tumour reaching up to perinephric fat and adrenal gland was involved in 8% while wall of the IVC in 10% patients of which 3 patients with gross involvement of IVC wall underwent partial resection of the involved segment with primary repair. Lastly 31% had positive lymph nodes (Table 1).
Five Year Survival
Over all (OAS) and Disease free survival (DFS) was calculated by censoring the data at 5 years. Five patients were lost to follow up. The data showed at 5 year (censored) follow up, overall survival was 63%, of those 55% patients were alive without disease, and 08% were alive with metastasis. Further 2 patients died in immediate postoperative period while 30 had died in follow up due to metastasis. Survival rates in our series were comparable to those mentioned in literature (Table 4).
Table 4.
Literature review
| Author/reference | Year | No. | Survival |
|---|---|---|---|
| Skinner [5] | 1989 | 43 | 5-year OS 57% |
| Glazer [11] | 1996 | 18 | 5-year OS 57% |
| 5-year CSS 60% | |||
| Moinzadeh [12] | 2004 | 140 | 10-year OS |
| Renal vein 66% | |||
| IVC 29% | |||
| Kim [13] | 2004 | 81 | 3-year CSS |
| Renal vein 36% | |||
| IVC 35% | |||
| T3c 12% | |||
| Lubahn [14] | 2006 | 44 | 5-year OS 56% |
| Ciancio [15] | 2007 | 56 | 2-year PFS 53% |
| Wagner [16] | 2007 | 1192 | Median survival, mo |
| Renal vein: 52 | |||
| IVC below diaphragm: 25.8 | |||
| IVC above diaphragm: 18 | |||
| Kulkarni et al. (Our series) | 2011 | 100 | 5 year survival |
| OS- 63% | |||
| DFS- 55% |
*CSS Cancer Specific Survival, **PFS Progression Free Survival, ***OS Overall Survival, ****DFS Disease Free Survival
When survival was correlated with the pathological variables, 71.42% patients with clear cell carcinoma were alive without metastasis, whereas only 30% of papillary cell type had DFS. Further grade wise 75.39% of patients with grade II tumours were alive without metastasis which dropped down to 23.52% in grade IV. However this difference was not statistically significant. Pathologically when the local extent was correlated patients with or without perinephric fat invasion, 5 year DFS was 31% and 76% respectively while only 8 patients out of 18 with either Adrenal or IVC wall involvement were alive at 5 years (44.4%). Similarly in patients with positive and negative lymph node involvement had 12% and 76% had 5 year DFS. These all pathological factors had statistical significance (Table 5).
Table 5.
Pathologic variable v/s disease free survival (excluding 2 postoperative deaths)
| Pathologic factor | No. of patients | No. of patients with DFS | % DFS | P value |
|---|---|---|---|---|
| Overall | 98 | 55 | 56.12% | |
| Tumor type | ||||
| Clear cell | 42 | 30 | 71.42% | Not significant |
| Clear + Granular | 26 | 15 | 57.69% | |
| Granular | 17 | 06 | 35.29% | |
| Papillary | 13 | 04 | 30.76% | |
| Grade | ||||
| II | 39 | 30 | 75.39% | <0.05 |
| III | 34 | 19 | 55.88% | |
| IV | 25 | 06 | 23.52% | |
| Local stage | ||||
| Perinephric negative | 58 | 43 | 74.13% | <0.01 |
| Perinephric positive | 40 | 12 | 30% | |
| Node negative | 67 | 51 | 76.11% | <0.01 |
| Node positive | 31 | 04 | 12.8% | |
| Adrenal positive/IVC wall invasion positive | 18 | 08 | 44.4% | N. A. |
| IVC thrombus level | ||||
| A | – | – | – | – |
| B | 58 | 34 | 57.75 | Not significant |
| C | 28 | 16 | 57.14 | |
| D | 12 | 05 | 40.08 | |
Discussion
Ever since the first report of radical surgery for RCC with IVC thrombus in 1970 [17], several authors have reported their success and long term survivals. Thus today excision of venacaval thrombus excision has become a standard of care. Although surgical mortality of these operations has reduced significantly morbidity is still a challenge in spite of technical advances. Current review focuses on some of the contemporary issues like patient selection criteria, accuracy of imaging techniques, surgical volume and role of IVC thrombectomy in metastatic setting and adjuvant therapy. Additionally we try to compare our results (100 cases of IVC thrombectomy) with literature.
Patient Selection Criteria
Age above 70 year and associated comorbidites like diabetes, cardiac, renal and liver disorders is known to be high risk for this surgery [18]. However with proper selection & risk balancing there is beneficial impact on reduction on morbidity. In our series we had about 10% patients above 70 years and oldest was 94 years. Secondly patients with metastasis were generally not operated because of poor survival rates [5] but now with advent of immunotherapy those who have resectable solitary metastasis[6, 7] or those who are symptomatic for caval thrombi can be operated with good results. We have operated only non metastatic patients but we believe patients with solitary metastasis could be benefited by surgery but cost of adjuvant therapy is an issue.
Surgical Issues
Surgeon & Hospital Volume
Over last 5 year, there has been some debate on the issue of surgical learning curve both at personal & institutional level vis-a-vis surgical volume [18, 19]. Although it’s a complex subject, it is accepted where expertise & proper facilities for major surgeries are available including Cardiopulmonary bypass & deep hypothermic circulatory arrest can undertake these surgeries.
Level of Thrombus
An aggressive surgical resection is proposed when there is IVC involvement because those patients who undergo only nephrectomy, all die within 1 year [5, 20]. IVC involvement is more common on right than on left because of shorter renal vein. Identifying the extent of IVC involvement is crucial for staging & planning. Although the extent of IVC thrombus can be identified by abdominal sonography & computed tomography, the sensitivity of MRI is superior to these techniques (96–100%) & is now the gold standard for assessing the level of thrombus [21, 22]. Currently MRIscan is the Gold Standard for delineating the level & extent of tumor thrombi in the IVC & staging of RCC with a Sensitivity of 96–100%. It may even rule out caval wall invasion so that exact surgical procedure can be planned [21, 22]. Infrahepatic thrombi were present in about 58% of patients & retro hepatic & suprahepatic in about 28% & 14% respectively. With the recent advance in endovascular tools, angioscopically guided luminal intervention has become an increasingly useful approach to many vascular problems [23]. Although this appears an attractive concept to date, no studies reported the possibility of a therapeutic role for vascular endoscopy in IVC tumor thrombectomy.
Staging
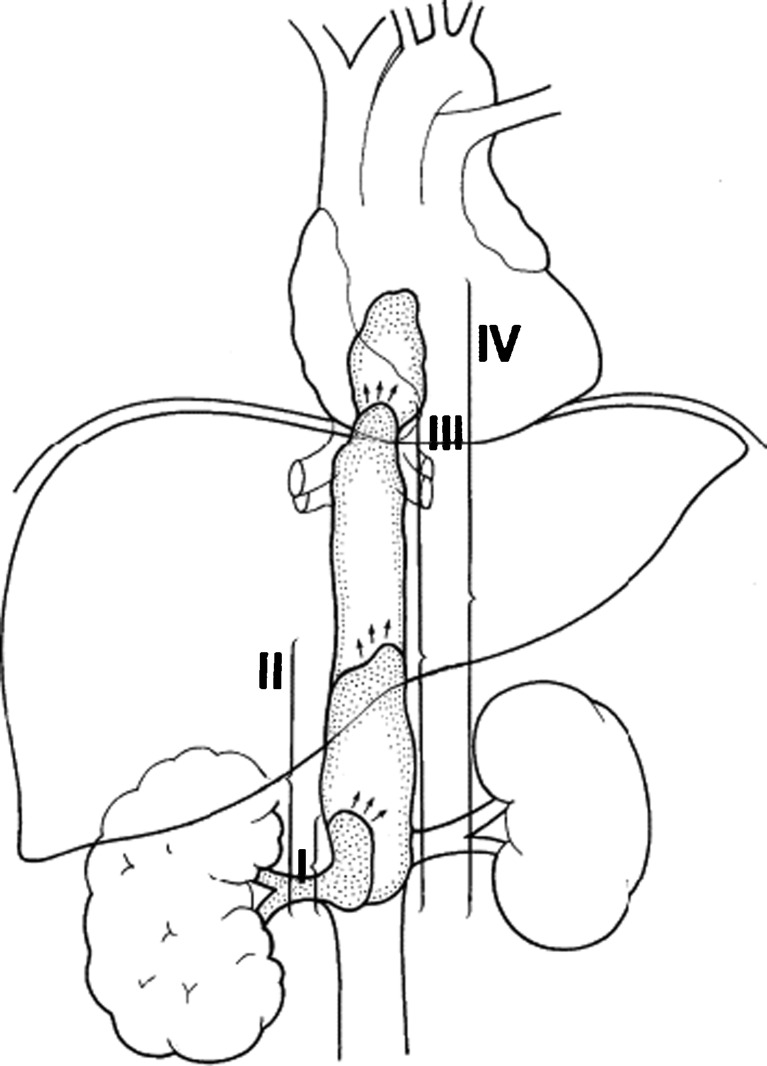
A number of classification systems are used for the macroscopic vascular involvement in RCC. The Mayo classification is commonly used. We have used Mayo classification in our series (Fig. 1).
Fig. 1.
The Mayo classification of venous invasion in renal cell carcinoma [24]. Level I: Tumor thrombus is either at the entry of the renal vein or within the inferior vena cava (IVC) < 2 cm from the confluence of the renal vein and the IVC. Level II: Thrombus extends within the IVC > 2 cm above the confluence of the renal vein and IVC but still remains below the hepatic veins. Level III: Thrombus involves the intrahepatic IVC. The size of the thrombus ranges from a narrow tail that extends into the IVC to one that fills the lumen and enlarges the IVC. Level IV: Thrombus extends above the diaphragm or into the right atrium
Primary TNM tumor staging for renal cell carcinoma has also been under constant evolution. Current staging, revised in 2010, differentiates IVC involvement below diaphragm (T3b) and above diaphragm (T3c), or invasion into wall of vena cava at any level as T3c, to be considered as an important adverse factor.
Surgical Technique
Tumors with Infra hepatic involvement are approached by midline abdominal incision. After mobilisation of bowel & caudate lobe of Liver, gentle complete dissection of Kidney is done. Proximal & distal control of IVC & opposite renal vein is obtained. Tourniquets snugged & IVC is opened & thrombus is milked out. IVC repair done after flushing out air followed by radical nephrectomy. RCC with tumor thrombi extension into Hepatic or suprahepatic IVC requires median sternotomy for control of suprahepatic vena cava & Pringle’s manoeuvre. Few patients with thrombi extending into right atrium require Cardiopulmonary bypass with hypothermic circulatory arrest [11, 25, 26]. Some authors advocate alternative technique for suprahepatic IVC involvement like Temporary occlusion of Intrapericardial IVC or veno venous bypass or application of supradiaphragmatic aortic cross clamp. But they have serious side effects as hypotension, increased bleeding from hepatic veins or paraplegia & intestinal gangrene respectively [15, 18, 27]. We concur with the benefits of team approach of urologist, cardiac surgeon, cardiac anaesthetist and cardiac theatre facilities. In our series we successfully performed IVC thombectomy as a second surgery after primary nephrectomy done elsewhere [8].
Minimal Invasive Techniques
There has been considerable interest generated in developing surgical approach using minimal invasive either pure laparoscopic or robotic assisted surgery for nephrectomy with vena caval thrombectomy. Some have questioned its benefits [28–31] while others have used laparoscopy for IVC tumor thrombi using open incisions for tumor thrombectomy after laparoscopic mobilization and dissection [32–34] or pure laparoscopy with hand assistance for the thrombectomy only [35]. Single case reports have also been described using hand-assisted or pure laparoscopy for short thrombi [36, 37], recently few case reports and one case series of robotic IVC thrombectomy has been reported in the literature [38].We await results of larger series since robotic and laparoscopic IVC tumor thrombectomy remain a challenging procedure and should not be embarked on lightly and hence open surgery remains the predominant method for addressing RCC involving the IVC.
Mortality and Morbidity
Various centres have reported mortality rates of 2.7%–13% for IVC extension of RCC [39]. The major causes of deaths reported to be are pulmonary embolism and myocardial infarction or due to complications related to the bypass procedures [3, 5, 6, 9, 20, 22, 25]. The complication rates increase with the higher extension of the caval thrombus. The most common complication is significant blood loss. Reported transfusion amounts vary between 3and 70 units [5, 22]. The average blood loss is higher in patients with left-sided tumors and with higher however, with better perioperative management and standardization of the surgical techniques, the mortality rates can be decreased considerably [4]. In our series in spite of the best efforts 30% non fatal complications were seen and required longer hospitalization. Since our series expands nearly two decades the complication were higher in earlier period. Minimal invasive techniques are being propagated as an effective alternative in reducing morbidity [32–38].
Five Year Survival and Pathological Variables
Several pathologic prognostic factors are mentioned in the literature [6, 11, 40–43], including mainly the local stage of the tumor, histological type and grade of tumor and presence of vascular invasion, vessel wall invasion, adrenal and pelvicalyceal involvement.
Comparing the pathologic prognostic factors with survival we noted that Clear cell tumors had the best prognosis, those with papillary had the worst prognosis (DFS of 71.4% vs 30.76%) and those with granular and mixed had intermediate prognosis. Moreover as in literature [10, 41] grade II tumors had better prognosis as compared to grade IV (75.39% vs 23.52%, P < 0.05, statistically significant). Local stage is probably most important prognostic factor; (40, 41, 42) in our series, 74.13% patient without perinephric fat involvement were free from disease as compared to 30% with perinephric fat involvement.(P < 0.01, statistically significant). In addition, 76.11% without LN involvement were free from disease as against 12.8% those with LN invasion. (P < 0.01, statistically significant). Another important prognostic factor is involvement of IVC wall or adrenal. Hatcher et al. noted that vessel wall is more important than the level of thrombus, and resection of IVC wall improved survival [40, 44]. In our series ten patients had invasion of either renal vein or IVC wall, which was resected, of which six were alive at follow up. And out of 8 patients with adrenal involvement only 2 were alive. It may be difficult to draw the conclusion from the study as the number of patients with vessel wall or adrenal involvement was small.
Adjuvant Therapy
Adjuvant therapy for RCC with IVC thrombus is still in evolving phase, but cost remains an important issue. Randomized trials of adjuvant radiation, hormonal therapy and, more recently, immunotherapy has shown no clinical benefit. Autologous tumor vaccines may decrease recurrence rates for patients with Locally Advanced RCC, but additional prospective, randomized studies need to be undertaken [45].
Prognosis
The prognostic significance of IVC thrombi in RCC patients is still evolving because factors predicting the prognosis have not been well defined and unpredictable clinical behaviour [46, 47]. Some have reported increased risk of metastasis with more cranial extent of the thrombus [5] while others doubt extent of thrombus as prognostic factor.[10, 38].Absence of local spread to perinephric tissue and negative nodes are favourable factors with cures of 45–70% with surgery alone [48]. Patients with incomplete resections (excluding patients with metastatic disease) have a significantly worse prognosis, a finding that reflects the importance of surgical eradication of disease [4]. Hatcher noted that the prognosis was determined by the ability to perform a complete resection of the tumor, not by the level of tumor thrombus [40, 49].
Conclusion
In conclusion, all patients with RCC and IVC tumor thrombi (even those with distant metastasis) should be considered for operation (as there is no other therapeutic modality). Further these tumors can be totally resected by an aggressive approach with an acceptable morbidity and mortality, and satisfactory long-term survival rates [46]. Our series spanning over nearly 2 decades endorses the view of aggressive approach of radical surgery with acceptable morbidity in this clinical setting.
References
- 1.Ciancio G, Soloway M. Resection of the abdominal IVC for complicated renal cell carcinoma with tumor thrombus. BJU Int. 2005;96:815–18. doi: 10.1111/j.1464-410X.2005.05719.x. [DOI] [PubMed] [Google Scholar]
- 2.Zisman A, Wieder J, Pantuck A. Renal cell carcinoma with tumor thrombus extension: biology, role of nephrectomy and response to immunotherapy. J Urol. 2003;169:909–16. doi: 10.1097/01.ju.0000045706.35470.1e. [DOI] [PubMed] [Google Scholar]
- 3.Mootha RK, Butler R, Laucirica R. Renal cell carcinoma with infra-renal vena caval tumor thrombus. J Urol. 1999;54:561–5. doi: 10.1016/s0090-4295(99)00136-3. [DOI] [PubMed] [Google Scholar]
- 4.Nesbitt JC, Soltero ER, Dinney CP. Surgical management of renal cell carcinoma with IVC tumor thrombus. Ann Thorac Surg. 1997;63:1592–600. doi: 10.1016/S0003-4975(97)00329-9. [DOI] [PubMed] [Google Scholar]
- 5.Skinner DG, Pritchett TR, Lieskovsky G, Boyd SD, Stiles QR. Vena caval involvement by renal cell carcinoma. Surgical resection provides meaningful long-term survival. Ann Surg. 1989;210:387–94. doi: 10.1097/00000658-198909000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golimbu M, Joshi P, Sperber A, Tessier A, Al-Askari S, Morales P, et al. Renal cell carcinoma -Survival and prognostic factors. J Urol. 1986;27:291–301. doi: 10.1016/0090-4295(86)90300-6. [DOI] [PubMed] [Google Scholar]
- 7.Zisman A, Pantuck AJ, Chao DH, Wieder JA, Dorey F, Said JW, et al. Renal cell carcinoma with tumor thrombus: is cytoreductive nephrectomy for advanced disease associated with an increased complicztion rate? J Urol. 2002;168:962–67. doi: 10.1016/S0022-5347(05)64552-1. [DOI] [PubMed] [Google Scholar]
- 8.Kulkarni JN, Acharya PU, Rizvi SJ, Somaya AC. Surgical management of RCC with IVC thrombus : a teaching hospital experience. Indian J Canc. 2007;44:45–50. doi: 10.4103/0019-509X.35810. [DOI] [PubMed] [Google Scholar]
- 9.Guinan P, Sobin LH, Algaba F, Badellino F, Kameyama S, Mac Lennan G, et al. TNM staging of renal cell carcinoma. Workgroup No 3Union International Contre le Cancer(UICC) and the American Joint Committee on Cancer(AJCC) Cancer. 1997;80:992–3. doi: 10.1002/(SICI)1097-0142(19970901)80:5<992::AID-CNCR26>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 10.Fuuhrman SA, Lasky LC, Limas C. Prognostic significance of morphological parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–63. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Glazer AA, Novick AC. Long-term follow-up after surgical treatment for renal cell carcinoma extending into the right atrium. J Urol. 1996;155:448–50. doi: 10.1016/S0022-5347(01)66415-2. [DOI] [PubMed] [Google Scholar]
- 12.Moinzadeh A, Libertino JA. Prognostic significance of tumor thrombus level in patients with renal cell carcinoma and venous tumor thrombus extension. Is all T3b the same? J Urol. 2004;171:598–601. doi: 10.1097/01.ju.0000108842.27907.47. [DOI] [PubMed] [Google Scholar]
- 13.Kim HL, Zisman A, Han K. Prognostic significance of venous thrombus in renal cell carcinoma. Are renal vein and inferior vena cava involvement different? J Urol. 2004;171:588–91. doi: 10.1097/01.ju.0000104672.37029.4b. [DOI] [PubMed] [Google Scholar]
- 14.Lubahn JG, Sagalowsky AI, Rosenbaum DH. Contemporary techniques and safety of cardiovascular procedures in the surgical management of renal cell carcinoma with tumor thrombus. J Thorac Cardiovasc Surg. 2006;131:1289–95. doi: 10.1016/j.jtcvs.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 15.Ciancio G, Livingstone AS, Soloway M. Surgical management of renal cell carcinoma with tumor thrombus in the renal and inferior vena cava: the University of Miami experience in using liver transplantation techniques. Eur Urol. 2007;51:988–95. doi: 10.1016/j.eururo.2006.11.055. [DOI] [PubMed] [Google Scholar]
- 16.Wagner B, Patard JJ, Méjean A. Prognostic value of renal vein (RV) and inferior vena cava (IVC) involvement in renal cell carcinoma (RCC) Eur Urol Suppl. 2007;6:159. doi: 10.1016/S1569-9056(07)60544-2. [DOI] [PubMed] [Google Scholar]
- 17.Marshall VF, Middleton RG, Holswade GR, Goldsmith EI. Surgery for renal cell carcinoma in the vena cava. J Urol. 1970;103:414–20. doi: 10.1016/s0022-5347(17)61970-0. [DOI] [PubMed] [Google Scholar]
- 18.Konety BR, Allareddy V, Modak S, Smith B (2006) Mortality after major surgery for urology cancer in specialised urology hospitals;are they any better? J Clin Oncol 24-2006-12 [DOI] [PubMed]
- 19.Hollenbeek BK, Dunn RL, Miller DC, Diagnault S, Taub DA, Wei JT. Volume based referral for cancer surgery:informing debate. J Clin Oncol. 2007;25:91–6. doi: 10.1200/JCO.2006.07.2454. [DOI] [PubMed] [Google Scholar]
- 20.Sosa RE, Muecke EC, Vaughan ED., Jr Renal carcinoma extending into the inferior vena cava: the prognostic significance of the level of vena caval involvement. J Urol. 1984;132:1097–100. doi: 10.1016/s0022-5347(17)50050-6. [DOI] [PubMed] [Google Scholar]
- 21.Lawrentschuk N, Gani J, Riordan R, Esler S, Bolton DM. Multidetector computed tomography vs magnetic resonance imaging for defi ning the upper limit of tumour thrombus in renal cellcarcinoma: a study and review. BJU Int. 2005;96:291–5. doi: 10.1111/j.1464-410X.2005.05617.x. [DOI] [PubMed] [Google Scholar]
- 22.Oto A, Herts BR, Remer EM. Inferior vena cava tumor thrombus in renal cell carcinoma: staging by MR imaging and impact on surgical treatment. Am J Roentgenol. 1998;171:1619–24. doi: 10.2214/ajr.171.6.9843299. [DOI] [PubMed] [Google Scholar]
- 23.White JV, Eid I. Diagnostic and interventional angioscopy. J Surg Clin North Am. 1998;78(4):539–59. doi: 10.1016/S0039-6109(05)70333-9. [DOI] [PubMed] [Google Scholar]
- 24.Neves RJ, Zincke H. Surgical treatment of renal cancerwith vena cava extension. Br J Urol. 1987;59:390–95. doi: 10.1111/j.1464-410X.1987.tb04832.x. [DOI] [PubMed] [Google Scholar]
- 25.Montie JE, Jackson CL, Cosgrove DM. Resection of large inferior vena caval thrombi from renal cell carcinoma with the use of circulatory arrest. J Urol. 1988;139:25–8. doi: 10.1016/s0022-5347(17)42279-8. [DOI] [PubMed] [Google Scholar]
- 26.Novick AC, Kaye MC, Cosgrove DM. Experience with cardiopulmonary bypass and hypothermic arrest in the management of retroperitoneal tumors with large vena cava thrombi. Ann Surg. 1990;212:472–7. doi: 10.1097/00000658-199010000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciancio G, Hawke C, Soloway M. The use of liver transplant techniques to aid in the surgical management of urological tumors. J Urol. 2000;164:665–72. doi: 10.1016/S0022-5347(05)67277-1. [DOI] [PubMed] [Google Scholar]
- 28.Novick AC. Laparoscopic and partial nephrectomy. Clin Cancer Res. 2004;10:6322S–7S. doi: 10.1158/1078-0432.CCR-050003. [DOI] [PubMed] [Google Scholar]
- 29.Eisenberg MS, Meng MV, Master VA. Laparoscopic versus open cytoreductive nephrectomy in advanced renal-cell carcinoma. J Endourol. 2006;20:504–8. doi: 10.1089/end.2006.20.504. [DOI] [PubMed] [Google Scholar]
- 30.Rabets J, Kaouk J, Fergany A. Laparoscopic versus open cytoreductive nephrectomy for metastatic renal cell carcinoma. J Urol. 2004;64:930–4. doi: 10.1016/j.urology.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 31.Matin S, Madsen L, Wood C. Laparoscopic cytoreductive nephrectomy: the M. D. Anderson Cancer Center experience. J Urology. 2006;68:528–32. doi: 10.1016/j.urology.2006.03.076. [DOI] [PubMed] [Google Scholar]
- 32.Disanto V, Pansadoro V, Portoghese F. Retroperitoneal laparoscopic radical nephrectomy for renal cell carcinoma with infrahepatic vena caval thrombus. Eur Urol. 2005;47:352–6. doi: 10.1016/j.eururo.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Varkarakis IM, Bhayani SB, Allaf ME. Laparoscopic-assisted nephrectomy with inferior vena cava tumor thrombectomy: preliminary results. J Urol. 2004;64:925–9. doi: 10.1016/j.urology.2004.05.044. [DOI] [PubMed] [Google Scholar]
- 34.Hoang AN, Vaporcyian AA, Matin SF. Laparoscopy-assisted radical nephrectomy with inferior vena caval thrombectomy for level II to III tumor thrombus: a single institution experience and review of the literature. J Endourol. 2010;24:1005–12. doi: 10.1089/end.2009.0532. [DOI] [PubMed] [Google Scholar]
- 35.Martin GL, Castle EP, Martin AD. Outcomes of laparoscopic radical nephrectomy in the setting of vena caval and renal vein thrombus: seven-year experience. J Endourol. 2008;22:1681–6. doi: 10.1089/end.2008.0035. [DOI] [PubMed] [Google Scholar]
- 36.Sundaram CP, Rehman J, Landman J. Hand assisted laparoscopic radical nephrectomy for renal cell carcinoma with inferior vena caval thrombus. J Urol. 2002;168:176–9. doi: 10.1016/S0022-5347(05)64855-0. [DOI] [PubMed] [Google Scholar]
- 37.Romero FR, Muntener M, Bagga HS. Pure laparoscopic radical nephrectomy with level II vena caval thrombectomy. Urology. 2006;68:1112–4. doi: 10.1016/j.urology.2006.08.1084. [DOI] [PubMed] [Google Scholar]
- 38.Abaza R. Robotic IVC thrombectomy. Eur Urol. 2011;59:652–659. doi: 10.1016/j.eururo.2010.08.038. [DOI] [PubMed] [Google Scholar]
- 39.Terakawa T, Miyake H, Takenaka A, Hara I, Fujisawa M. Clinical outcome of surgical management for patients with renal cell carcinoma involving the inferior vena cava. Int J Urol. 2007;14:781–4. doi: 10.1111/j.1442-2042.2007.01749.x. [DOI] [PubMed] [Google Scholar]
- 40.Hatcher PA, Anderson EE, Paulson DF, Carson CC, Robertson JE. Surgical management and prognosis of renal cell carcinoma invading the vena cava. J Urol. 1991;145:20–4. doi: 10.1016/s0022-5347(17)38235-6. [DOI] [PubMed] [Google Scholar]
- 41.Thrasher JB, Paulson DF. Prognostic factors in renal cancer. Urol Clin North Am. 1993;20:247–62. [PubMed] [Google Scholar]
- 42.Tongaonkar HB, Dandekar NP, Datak AV, Kulkarni JN, Kamat MR. Renal cell carcinoma extending to renal vein and inferior vena cava: results of surgical treatment and prognostic factors. J Surg Oncol. 1995;59:94–100. doi: 10.1002/jso.2930590205. [DOI] [PubMed] [Google Scholar]
- 43.Uzzo RG, Cherullo EE, Myles J, Novick AC. Renal cell carcinoma invading the urinary collecting system: implications for staging. J Urol. 2002;167:2392–6. doi: 10.1016/S0022-5347(05)64991-9. [DOI] [PubMed] [Google Scholar]
- 44.Montie JE, Ammar R, Pontes JE, Mendendorp SV, Novick AC, Streem SB, et al. Renal cell carcinoma with inferior vena cava thrombi. Surg Gynecol Obstet. 1991;173:107–15. [PubMed] [Google Scholar]
- 45.Belgrano E, Trombetta C. Surgical Management of Renal Cell Carcinoma (RCC) with vena cava tumour thrombus. Eur Urol Suppl. 2006;5:610–618. doi: 10.1016/j.eursup.2006.04.001. [DOI] [Google Scholar]
- 46.Kaplan S, Ekici S, Dogan R, Demircin M, Ozen H, Pasaoglu I. Surgical management of renal cell carcinoma with IVC tumor thrombus. Am J Surg. 2002;183:292–9. doi: 10.1016/S0002-9610(02)00782-1. [DOI] [PubMed] [Google Scholar]
- 47.Leibovich BC, Cheville JC, Lohse CM. Cancer specific survival for patients with pT3 renal cell carcinoma-can the 2002 primary tumor classification be improved. J Urol. 2005;173:716–19. doi: 10.1097/01.ju.0000151830.27750.d2. [DOI] [PubMed] [Google Scholar]
- 48.Wszolek MF, Wotkowicz C, Libertino JA. Surgical management of large renal tumors. Nat Clin Pract Urol. 2008;5:35–46. doi: 10.1038/ncpuro0963. [DOI] [PubMed] [Google Scholar]
- 49.Boorjian SA, Sengupta S, Blute ML. Renal cell carcinoma: vena caval involvement. BJU Int. 2007;99:1239–44. doi: 10.1111/j.1464-410X.2007.06826.x. [DOI] [PubMed] [Google Scholar]