Abstract
Inguinal lymph node involvement is an important prognostic and predictive factor in various neoplasms of the genitalia and lower limb. As part of the multimodality approach, these patients undergo surgery and adjuvant radiotherapy. Morbidity of inguinal lymphadenectomy includes lymphedema, lymphorrhea and infection; however the most common distressing complication is skin necrosis. Myocutaneous flaps have been the most popular form of primary or delayed groin reconstruction. This paper aims to critically review the different myocutaneous flaps used in groin reconstruction, discuss evidence based data on the versatility and utility of these flaps and discuss ways in which modifications maybe incorporated in treatment and radiation planning following groin reconstruction. A comprehensive search of the scientific literature was carried out using PubMed to access all publications related to groin reconstruction. The search focused specifically on current management, technique, safety and complications of these procedures. Keywords searched included “inguinal lymphadenectomy”, “primary reconstruction”, “musculocutaneus flap”, “myocutaneous flap”, “tensor fascia lata flap”, “anterolateral thigh flap”, “rectus abdominis flap”. Low to middle income countries witness a huge burden of locally advanced genital malignancies and melanoma of the lower extremity. Higher tumor burden both at the primary site as well as the inguinal basin requires surgery as the primary modality of treatment. Groin reconstruction is required not only to prevent femoral blowouts but also for early administration of adjuvant radiation. The versatility of tensor fascia lata, anterolateral thigh, and rectus abdominis flaps is useful to cover the defect, provide radiation, eradicate pain and achieve good palliation. Assessment of aesthetic and functional outcomes of one flap over the other and the “ideal” form of reconstruction for groin defects needs additional investigation.
Keywords: Inguinal lymphadenectomy, Primary reconstruction, Musculocutaneus flap
Introduction
Metastases to the inguinal lymph node arise from primary epithelial cancers and/or melanomas of the penis, vulva, vagina, urethra, scrotum, anorectum as well as lower extremities. Lymph node involvement is an important prognostic and predictive marker in these tumors. The clinical presentation of locally advanced primary and nodal disease is not uncommon in low to middle income countries (LMC). Surgery may be curative or palliative (in case of ulcerative/fungating or bleeding tumors) and consists of radical surgery for the primary and en bloc inguinal or ilioinguinal lymphadenectomy. Various modifications of groin dissection have been described by Whitmore et al. in early 1980’s [1]. Prophylactic inguinal node dissection has been adopted though not frequently in few instances of epithelial cancers. Similarly with a better understanding of the natural history of melanomas and epithelial cancers as well as growing surgeon interest in minimally invasive procedures, sentinel lymph node biopsy (SLNB) has been increasingly adopted for these tumors. While the management of inguinal nodes has moved from radical dissection to SLNB to reduce morbidity, the role of radical inguinal lymphadenectomy is not completely extinct. In the absence of screening programs in developing countries for several genital cancers, advanced stage at presentation and the lack of effective systemic modalities for cutaneous melanomas, “therapeutic inguinal or ilioinguinal dissection” is still an accepted practice. Radical groin dissection is also indicated in positive SLNB.
Thus surgery remains the most effective modality of treatment for these patients in terms of both cure and local disease control. That said, this procedure is associated with high morbidity. In an attempt to reduce this morbidity, various reconstructive procedures such as muscle transposition, skin grafts and myocutaneous flaps are used for groin reconstruction. This paper aims to review the commonly used pedicled flaps in groin reconstruction, vascular anatomy of each alongwith their advantages and disadvantages.
Utility of Flaps: Is There a Need for Primary Reconstruction?
Complications of inguinal block dissection include infection (6–20 %), lymphorrhea (6–40 %), lymphedema (8–69 %) and more importantly skin flap necrosis (27–85 %) [2]. The blood supply of skin flaps in the inguinal region arises from the superficial branches of the inferior epigastric, external pudendal and circumflex iliac arteries, which run parallel to the inguinal ligament. After removal of the adipofascial layer in a groin dissection, the fascial plexus and the oblique vessels supplying the subdermal plexus are damaged, leading to skin flap necrosis. This is particularly important as it delays institution of adjuvant radiation. The presence of regional nodal disease that has spread outside the lymph node capsule is associated with regional recurrence rates of 24–63 % in cutaneous melanomas [3, 4]. Addition of post operative radiation decreases the local recurrence to 5–19 % and helps in long term survival with improved quality of life [3, 5]. However with addition of adjuvant radiation, edema, fibrosis, wound healing complications or skin necrosis are common. In a skin flap that already has compromised blood supply, treatment related morbidity can be an issue. Hence primary reconstruction of the groin is a reasonable approach for such patients. Flap closure of the groin has the following advantages [6]: (1) The flap provides protection to the femoral vessels. (2) The flap brings well-vascularized tissue from a distant area to the groin. (3) It covers the dead space in the femoral triangle and decreases seroma formation. (4) It helps in wound closure without tension. (5) Early radiotherapy can be safely given. (6) It shortens the hospital stay.
Conventionally a variety of reconstructive options (skin grafts, gracilis or sartorius flap and omental flap, tensor fascia lata flap, anterolateral thigh flap, rectus abdominis flap and rectus femoris flap) have been suggested for covering groin defects. Historically a simple sartorius transposition was used to cover the femoral vessels followed by split thickness graft when feasible. Simple skin grafting is not sufficient to cover exposed bones, nerves and vessels to prevent septic rupture or recurrent ulceration. Free flaps require enhanced microsurgical expertise and techniques as also may require venous interposition grafts and overburden patients in critical condition with progressive malignant disease. In these situations, sufficient soft tissue coverage needs to be achieved by simple and reliable techniques with moderate donor site morbidity. Hence pedicled flap reconstruction is preferred as it not only facilitates postoperative hip function and cosmesis but also tolerates postoperative radiotherapy. Myocutaneous flaps have the following advantages [6]:
A blood supply that is based out of the field of resection or radiation.
A blood supply precisely known, as is the exact location of the vascular pedicle.
A single-stage procedure.
The most commonly used flaps include the tensor fascia lata, anterolateral thigh and vertical rectus abdominis flap. All these flaps are reliable and provide good soft tissue cover but at the expense of sacrifice of a functioning muscle.
Tensor Fascia Lata Pedicled (TFL) Flap
First described in 1934 by Wangensteen et al., TFL flap is a fasciomyocutaneous flap initially used for abdominal wall reconstruction, which was later popularized by Nahai et al. for many more applications (primarily to reconstruct pressure sores and for complications following block dissections) [7, 8]. Typically the work horse for reconstruction of groin defects over the years, this flap has been used as a pedicled flap (with its various modifications) rather than a free tissue transfer.
Vascular Anatomy of TFL Flap
The TFL flap is based on the transverse branch of the lateral circumflex femoral artery, anterior branches through profunda femoris and forms a longitudinal anastomosis along the iliotibial tract and skin covering that area and is marked about 8–10 cm below the anterior superior iliac spine (ASIS) (Fig. 1). The lateral circumflex femoris artery is identified by its course running between the rectus femoris and the vastus lateralis, where it gives the transverse branch that pierces the TFL muscle and is accompanied by venae commitantes. The perforators of the TFL are classified as septocutaneous or musculocutaneous. The septocutaneous perforators are observed between 8 cm and 12 cm from the ASIS. Thus the possible pedicle length of a flap based on these vessels is 8.1 cm. The motor supply of the muscle is via the superior gluteal nerve which enters the posterior surface of the muscle and the sensory supply is by the lateral cutaneous branch of T12 and the lateral cutaneous nerve of the thigh. A skin island up to 15 cm in width from the pedicle site to around 8 cm above the knee can be reliably elevated. Further mobilization of the flap or lengthening of the pedicle if needed, requires division of the muscle branches of the lateral circumflex femoral artery and the flap can be completely islanded which allows rotation upto 180°. Donor site can usually be closed primarily if the size of the defect is less than 9 cm or a split thickness skin graft may be applied. To prevent the limitation of the arc of rotation, free TFL flap has been used by some surgeons. The maximum pedicle length obtained by this technique is 10 cm. The advantages of a free TFL flap are preservation of the motor function of the TFL muscle, a relatively less bulky flap and a donor site that can be concealed. However, the poor vascular recipient area, difficult dissection of the muscle perforator and a short vascular pedicle (in case of a perforator based free flap) alongwith the need of adjuvant radiation or sometimes the need to cover the inguinal defect following radiation induced necrosis, precludes widespread use of a microvascular tissue transfer.
Fig. 1.
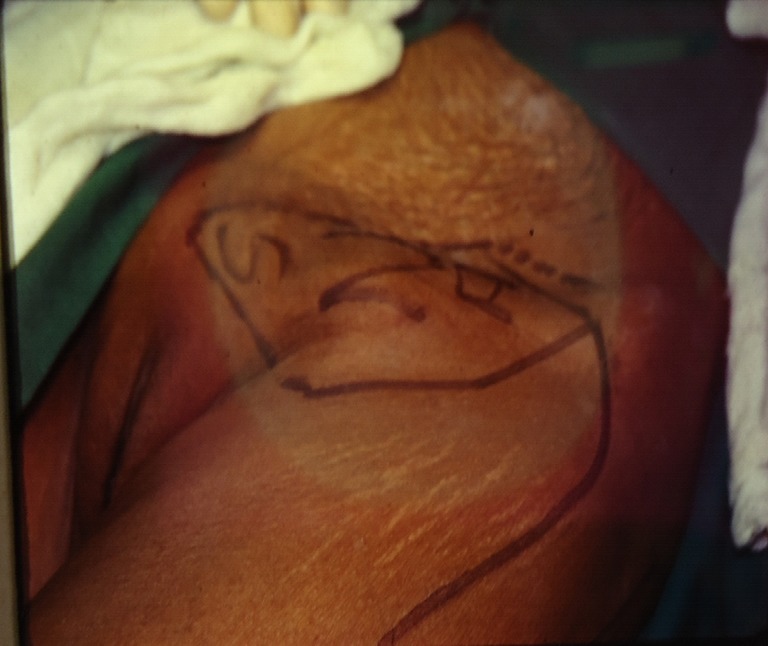
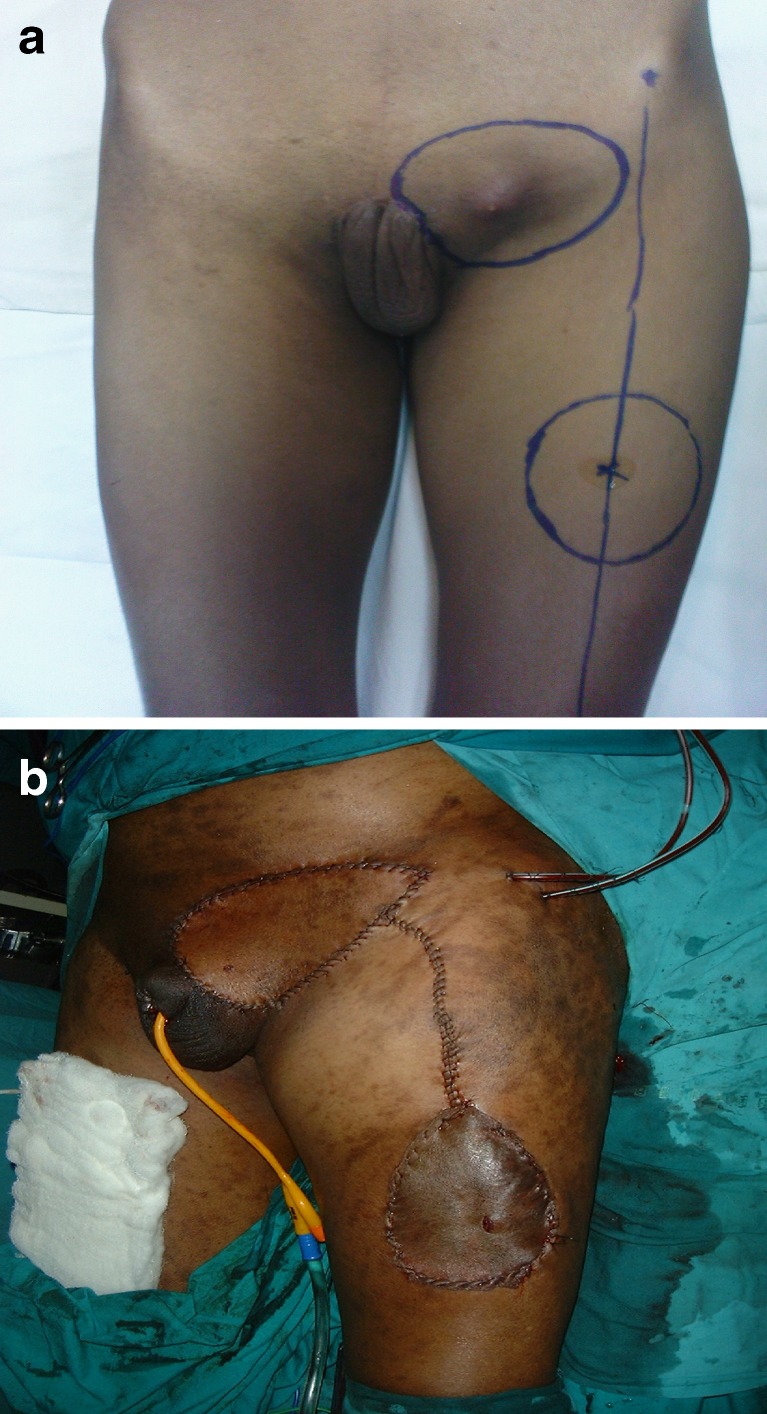
Preoperative view of left sided inguinal region with enlarged lymph nodes. Note the marking of the site of inguinal lymphadenectomy along with the overlying skin and the marking of the TFL flap on the thigh
Table 1 lists the studies of genital malignancies and melanomas using pedicled TFL flap for groin reconstruction reported in literature.
Table 1.
Published reports of Tensor fascia lata flap in groin reconstruction following inguinal node dissection
| Study, Year | Number of patients | Site of primary malignancy | Partial flap necrosis % (n=) | Flap infection % (n=) | Lymphedema % (n=) | Adjuvant radiation % (n=) |
|---|---|---|---|---|---|---|
| Gopinath et al. [29], 1988 | 20 | SCC Penis, melanoma of foot | 5 % (1) | 15 % (3) | 5 % (1) | 30 % (6) |
| Schoeman et al. [30], 1995 | 11 | SCC Penis, SCC of leg, melanoma of foot | 27.2 % (3) | 27.2 % (3) | 18.2 % (2) | 100 % (11) |
| Agarwal et al. [31], 2009 | 15 | SCC Penis, melanoma of foot | 13.3 % (2) | 13.3 % (2) | 20 % (3) | 100 % (15) |
| Hubmer et al. [32], 2011 | 17 | SCC of leg, melanoma of foot | 5.8 % (1) | 11.76 % (2) | 0 % (0) | NR |
SCC, squamous cell carcinoma; NR not reported
Advantages of the TFL flap
TFL flap can be used in many locations based on its blood supply, arc of rotation, transposition and potential as a free flap. It provides reliable cutaneous coverage, durability, consistent vascular pedicle, neurosensation when needed, good mobility and ease of positioning. TFL flap has been adopted both as a pedicled myocutaneous flap as well as a free flap. Pedicled TFL flap has been used for primary or delayed reconstruction of abdominal wall defect, inguinal/groin defects, trochanteric or ischial pressure sores, radical vulvectomy defect, pelvic floor defect, while free TFL flaps have been used in head and neck reconstructions (including maxillectomy and orbital floor defects, skull base reconstruction, cheek and palate reconstruction).
Disadvantages of the TFL Flap
Potential drawbacks of this flap include a thin distal flap which covers the defect and bulkiness proximally (the flap rotation creates an unsightly dog ear which may interfere with sitting), a depressed scar (especially when the donor area is grafted), and potential loss of knee stability. Excessive tension and sometimes even suture separation at the confluence of the donor site and the TFL flap; alongwith marginal skin necrosis is another reported complication.
Modifications of the TFL Flap
The main aim of modifications of the original TFL flap has been to prevent or reduce the complication rate. The region of most potential complication is in the donor area when rotating the TFL flap which is susceptible to necrosis due to the undermining necessary for advancement because of its distance from the blood supply and the inherent degree of tension necessary to close this portion of the defect primarily. Hence Lynch first described the “bilobed TFL flap”, a modification that additionally utilizes a triangular lobe medially partly with the fascia and partly random [9]. For anterior rotation of the TFL flap, a posterior triangular segment could be similarly designed. Lewis Jr et al. have described the “TFL V-Y retroposition” modification where the flap is elevated to the standard length; however the tip is tapered so that the donor site can be closed in V-Y fashion [10]. This retroposition was first evaluated in 10 patients over 2 years with no necrosis of flap or bulky rotation point. Siddiqui et al. performed V-Y retroposition flaps on 70 patients with minimal wound complications and concluded that it is a reliable, durable and technically straightforward treatment option for reconstruction of groin defects [11]. The “duck” modification of the TFL flap was introduced by Aslan et al. in which on the anterior aspect of the flap, a V-shaped triangular flap is prepared and included in the classic flap [12]. The triangular flap is sutured to the confluence of the flaps covering the donor site, where possible excessive tension is expected. They used the duck modification in 27 patients with no flap loss and only one patient developed a hematoma which was successfully evacuated without flap loss. The authors concluded that the duck modification provided a tension free closure, dead space and dog ear formation at the flap base was prevented which resulted in a more acceptable esthetic result.
Anterolateral Thigh (ALT) flap
Based on his septocutaneous theory, this flap was first described by Song [13] in 1984 (alongwith the anteromedial and posterior thigh flap) and later popularized by Koshima et al. [14]. It was originally used for reconstruction of complex head and neck defects but later extended to include lower extremity and trunk. A versatile flap used in a variety of indications from oncologic defects to burns and trauma. The skin territory of this flap is very wide and can be raised as a very thin flap, but is technically more demanding.
Vascular Anatomy of ALT Flap
Anterolateral thigh flap is a perforator flap based on the branches of the descending branch of the lateral circumflex femoral artery, a branch of the profunda femoris. At the proximal level of the descending branch, the arterial diameter is approximately 2 mm. Typically 2 venae comitantes, accompany the artery. The veins are typically larger than the artery, with one slightly larger vein demonstrating its dominant flow. Proximally, in type 1 (as described by Song), the septum between the vastus lateralis and rectus femoris muscles retains the branching point of the descending branch off of the lateral circumflex femoral artery. The perforating vessels then course through the vastus lateralis, but will occasionally be maintained within the septum. Pedicle length is a strong-suite of this flap and is upto 12 cm. In type 2 (as described by Koshima et al.), septocutaneous perforator derives independently from the profunda femoris artery and not from the lateral circumflex femoral artery, a pattern found in about 38 % population. This suggests that the vascular pedicle of the ALT flap is not as constant as Song et al. suggested. The flap may be sensate by including branch (es) of the lateral femoral cutaneous nerve. This can be particularly advantageous in reconstructing areas of the body where protective sensation is imperative. A line drawn from the anterior superior iliac spine to the upper outer edge of the patella represents the surface marking of the intermuscular septum between rectus femoris medially and vastus lateralis laterally (Fig. 2a). Vastus lateralis perforators are usually within 3 cm inferolateral to the midpoint of this line. An elliptical flap of appropriate size and orientation is designed around the provisionally selected perforator(s) such that its predicted arc of rotation will reach the defect to be closed. The flap is then raised in a suprafascial or subfascial plane. Perforators are identified as dissection proceeds, and the most appropriate perforator(s) is selected and dissected. Dissection proceeds along the descending branch of the lateral circumflex femoral vessels to the lateral circumflex femoral vessels themselves. The nerve to vastus lateralis can be intimately related to the vascular pedicle during this part of the dissection and should be protected. Pedicle length and some arcs of rotation in this flap can be increased by ligation of the main rectus femoris pedicle and by tunneling under the rectus femoris muscle. The donor site is closed primarily in layers or by using a split-thickness skin graft, depending on the width of the flap harvested and patient characteristics (Fig. 2b). Primary closure of the fascia is required if suprafascial flap harvesting has been performed.
Fig. 2.
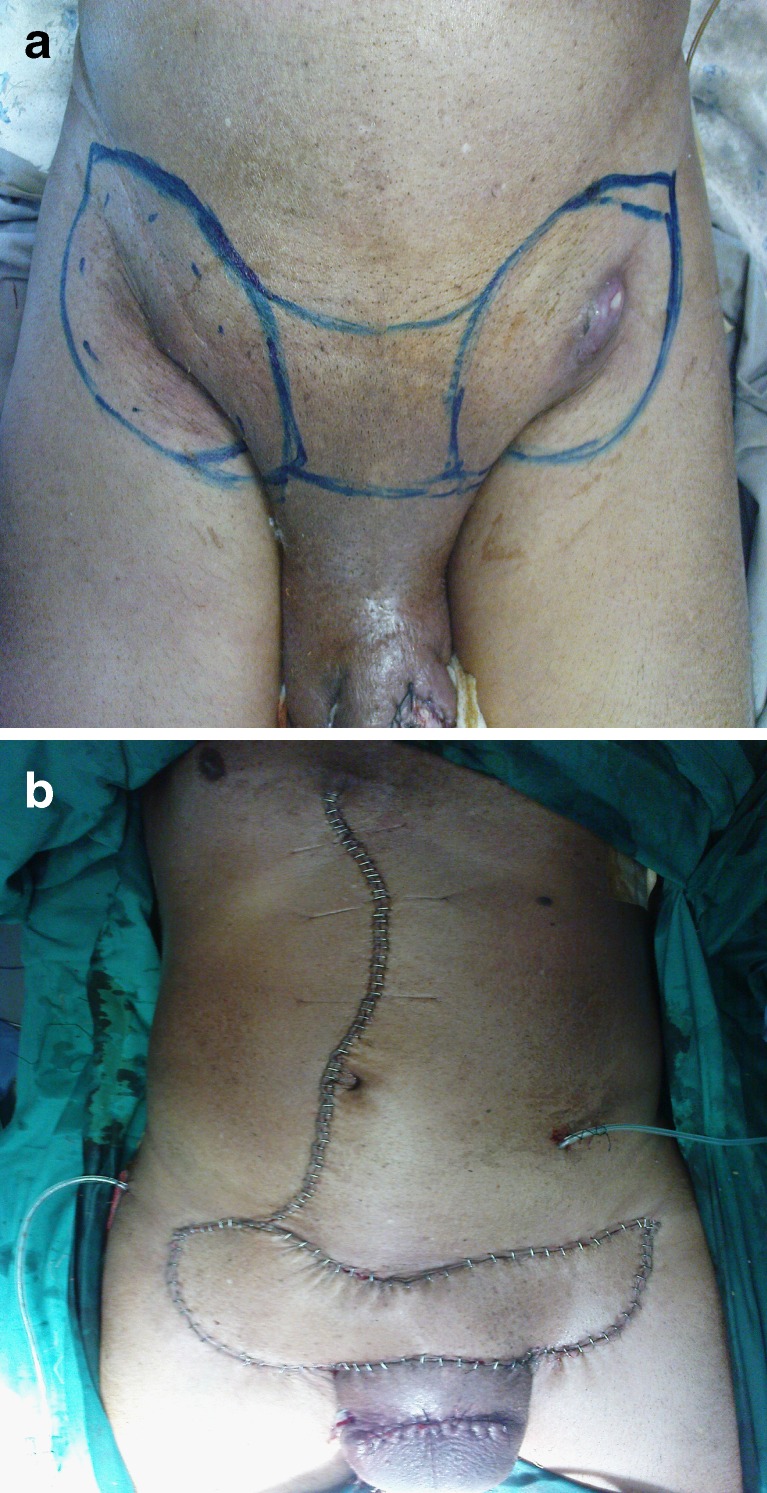
a Preoperative view of left sided inguinal region with enlarged lymph nodes in a male patient following partial amputation in a case of carcinoma penis. Note the marking of the site of inguinal lymphadenectomy along with the involved overlying skin and the marking of the anterolateral thigh flap on the thigh. b Showing an immediate postoperative view of the anterolateral flap covering the inguinal defect in Fig. 2a and the skin graft over the donor site
Table 2 lists the studies of genital malignancies and melanomas using pedicled ALT flap for groin reconstruction reported in literature.
Table 2.
Published reports of Anterolateral thigh flap in groin reconstruction following inguinal node dissection
| Study, Year | Number of patients | Site of primary malignancy | Partial flap necrosis % (n=) | Flap infection % (n=) | Lymphedema % (n=) | Adjuvant radiation % (n=) |
|---|---|---|---|---|---|---|
| Luo et al. [33], 1999 | 16 | SCC penis, SCC vagina, SCC lower extremity | 0 % (0) | 0 % (0) | NR | NR |
| Ahmad et al. [34], 2004 | 16 | SCC penis | 0 % (0) | 0 % (0) | 37.5 % (6) | 100 % (16) |
| Evriviades et al. [35], 2007 | 6 | SCC penis, melanoma lower extremity | 0 % (0) | 33.3 % (2) | NR | 100 % (6) |
| Lannon et al. [17], 2011 | 28 | SCC penis, SCC lower extremity | 7.1 % (2) | 14.2 % (4) | NR | 100 % (28) |
SCC squamous cell carcinoma; NR, not reported
Advantages of ALT flap
It is a safe flap because there are usually accessory branches deriving from the lateral circumflex femoral vessels which can be included in the pedicle. A long vascular pedicle can be obtained by dissection up to the deep femoral vessels and the diameter of the proximal end of the vascular pedicle is as large as that of the latissimus dorsi muscle. The flap skin is thin and pliable enough to be folded, tubed, or packed into cavities, and the ease with which it is harvested makes it an excellent first-line emergency flap. Furthermore, its skin territory is very wide and the donor scar, even with split skin graft, is less noticeable than those of some other flaps. If the pedicle is found to be absent, the flap can safely be converted to a TFL or anteromedial thigh flap. The flap will reach to 8 cm above the umbilicus, the contralateral lower abdomen and inguinal area, the entire groin, and perineum to anus. The ALT flap is useful to cover thin defects in mobile areas such as the face, neck, extremities or intraoral region. In addition, the island flap can be used to repair defects in the abdominal or genital region. Its harvest can be significantly simplified using a basic step-by-step approach. Total harvest time averages between 30 min and 45 min, without the need for preoperative computed tomographic angiography or Doppler imaging to identify the perforator location. It is also suitable for providing a free vascularised fascial graft for reconstruction of dural or abdominal muscle defects. It has the potential of being innervated by linking up the lateral or anterior cutaneous nerve of the thigh with a nerve at the recipient site. In combination with vascularised iliac bone, ALT can be used as an osteocutaneous flap with double vascular pedicle, for reconstruction of bone with wide skin defect.
Primary closure of the donor site results in minimal pain or paresthesia, acceptable scar cosmesis, good thigh contour, and rapid mobilization. Pedicled ALT flap has been used for primary or delayed reconstruction of abdominal wall defect, inguinal/groin defects, coverage of groin vessels, coverage of exposed bone or orthopedic hardware around the hip and pelvis, knee defects, extremity reconstruction, partial penile reconstruction, vulvar defect and trochanteric sores, while free ALT flaps have been used in oromandibular reconstructions (including upper aerodigestive tract defects and scalp defect).
Disadvantages of ALT Flap
Flaps with perforators that arise directly from the profunda femoris artery are difficult to combine with other free flaps. Because the perforators are extremely small and tend to thrombose soon after congestion develops, these flaps are difficult to salvage with recirculation surgery [15]. Therefore, several perforators should be included with the flap, if possible. Pain and weakness of the thigh are significantly related to sacrifice of the deep fascia through its elevation with the flap. Cautious tunnel creation and tunneling of the flap is required in morbid obesity.
Modifications of the ALT Flap
The ALT flap may be defined by its vascular supply as either musculocutaneous or septocutaneous. Alternatively, this flap may be defined by its tissue components: skin, fat, fascia, tendon, muscle, or nerve (suprafascial, fasciocutaneous, adipofascial, and musculocutaneous anterolateral thigh flaps) [16]. Each variant is equipped with well defined indications according to the recipient site [17]:
Suprafascial dissection of the flap is performed to minimize donor-site morbidity when fascia is not required for reconstruction. Pain and weakness of the thigh are significantly related to sacrifice of the deep fascia through its elevation with the flap.
Extended fascial harvest is performed in patients in whom larger areas of fascia are required for reconstruction of abdominal wall integrity. It is performed by undermining beyond the cutaneous perimeter of the flap for several centimeters, and then incising around the larger area of fascia.
Buffering of the pedicle with an interposition of fascia is performed in patients when the flap is placed on top of prosthetic mesh. Without this fascial interposition there is a high risk of damage to the pedicle by abrasion against the mesh during abdominal movement with respiration.
Combined use of a pedicled ALT flap and a sartorius “switch”: It is a useful flap to use in combination with a pedicled ALT flap, especially in patients who are prone to wound dehiscence, such as those with radiated groins.
Vertical Rectus Abdominis Myocutaneous (VRAM) Flap
The pedicled rectus abdominis myocutaneous flap represents a valuable option to treat defects located in its arc of rotation, which reaches from the jugulum to the axilla, the lower dorsum, the pelvis, the perineum and the groin to the proximal thigh. After the first report of the successful transposition of a VRAM flap for abdominal wall reconstruction, by Mathes et al., it has become accepted as workhorse in defect coverage of the trunk and the proximal thigh [18]. Due to its axial vascular supply based on the superior and inferior epigastric vessels with large calibre, the flap can be rotated superiorly or inferiorly based, whereas the inferior pedicle seems to be more reliable.
Vascular Anatomy of Rectus Abdominis Flap
It is a vertically oriented muscle which extends from the costal margins to the pubic ramus. It has a Type III (two dominant pedicles) pattern of vasculature. Its main vascular supply is from:
Deep Superior epigastric artery (DSEA) and vein.
Deep Inferior epigastric artery (DIEA) and vein.
Usually, one of the vascular pedicles is sufficient for perfusion of the entire musculocutaneous flap. The DIEA is the dominant artery of the abdominal wall (average size, 2.5–3.8 mm), which is double the size of the DSEA. Thus, rectus abdominis musculocutaneous flaps can be transferred superiorly based on the DSEA, as well as inferiorly pedicled or free based on the DIEA. The DIEA arises from the external iliac artery immediately above the inguinal ligament, and follows a fairly constant anatomical course ascending obliquely to reach the posterior surface of the rectus abdominis muscle roughly 7 cm superior to its insertion. It is more reliable as the inferiorly based VRAM flap due to the thinner diameter of the DSEA, particularly in pre radiated patients (Fig. 3a). The flap is raised as an inferiorly based flap with vertical or oblique skin paddle. The skin paddle is incised above the external oblique fascia laterally until the anterior rectus sheath is identified. A careful dissection above the fascia is performed to preserve the lateral and medial perforator rows and anterior rectus sheath opened similar to the fascia-sparing transverse rectus abdominis musculocutaneous flap dissection technique. The inferior epigastric vessels are exposed and identified and the main trunk of the vessels skeletonized. Depending on the location of the perforators and the appearance of the inferior epigastric artery, the full width of the rectus is dissected or a muscle-sparing dissection performed. Then, the full width of the rectus muscle is incised superiorly and raised off the posterior sheath to the inferior epigastric vessels. The rectus muscle is cut off inferiorly below the insertion of inferior epigastric vessels to the muscle. The flap is then tunneled subcutaneously and inset into the defect. Double layer closure of the anterior rectus fascia, and in case of too much tension, the use of resorbable or non-resorbable meshes must be performed (Fig. 3b).
Fig. 3.
a Preoperative view of bilateral enlarged inguinal lymph nodes in a male patient following partial amputation in a case of carcinoma penis. Note the marking of the site of inguinal lymphadenectomy alongwith the involved overlying skin. b. Postoperative view of vertical rectus abdominis flap covering the bilateral inguinal defect after the flap has been rotated. Note prolene mesh has been used to cover the defect and the donor site has been closed primarily
Table 3 lists the studies of genital malignancies and melanomas using pedicled VRAM flap for groin reconstruction reported in literature.
Table 3.
Published reports of Rectus abdominis flap in groin reconstruction following inguinal node dissection
| Study, Year | Number of patients | Site of primary malignancy | Partial flap necrosis % (n=) | Flap infection % (n=) | Lymphedema % (n=) | Adjuvant radiation % (n=) |
|---|---|---|---|---|---|---|
| Deo et al. [36], 2001 | 8 | SCC penis, melanoma lower extremity | 0 % (0) | 0 % (0) | 0 % (0) | 25 % (2) |
| Küntscher et al. [37], 2006 | 15 | SCC penis, SCC vulva, melanoma lower extremity | 6.6 % (1) | 1.3 % (2) | NR | NR |
| Parrett et al. [38], 2007 | 50 | SCC penis, SCC lower extremity | 4 % (2) | 16 % (8) | 4 % (2) | NR |
| Daigeler et al. [23], 2011 | 78 | SCC penis, melanoma lower extremity | 12.8 % (10) | 35. % (28) | 32.6 % (17) | 66.6 % (52) |
SCC squamous cell carcinoma; NR not reported
Advantages of Rectus Abdominis Flap
The straightforward harvesting and a safe vascular supply result in low rates of flap losses. VRAM flap is versatile and sturdy flap with a wide arc of rotation and it can reach diverse anatomical sites like torso, chest wall, thigh and perineum to cover large defects following radical resection for tumors. The VRAM flap has the skin paddle centered over the rectus abdominis muscle. Studies do not demonstrate differences in complication rates between free and pedicle flaps for sarcoma defects in the lower extremity [19, 20]. However, the pedicled RAM flap can be elevated and inset rapidly, is voluminous, has a wide arc of rotation, does not require a change in the patient’s position, does not require dissection of irradiated vessels, acceptable donor site morbidity and allows transfer of muscle with a large skin island. Because the donor site is not in the lower extremity, extremity function, leg vasculature, and lymphatic drainage is not affected. In addition, the VRAM flap can be used in a contralateral fashion if the ipsilateral epigastric vessels have been ligated. Another advantage is the versatility of flap design, with extended, oblique, and vertically oriented skin paddles available for defects of varying dimensions. Pedicled VRAM flap has been used for primary or delayed reconstruction of abdominal wall defect, inguinal/groin defects, sacral defects, neophallus reconstruction, vulvar, vaginal, sternal and breast reconstruction, while free VRAM flaps have been used in head and neck reconstructions (including hypopharyngeal and scalp defect).
Disadvantages of Rectus Abdominis Flap
Only the contralateral muscle can be used, because the inferior epigastric vessels on the ipsilateral side are divided during inguinal block dissection. Previous exploratory laparotomy or caesarean sections are a contraindication for a VRAM flap. In VRAM flap patients, herniation or abdominal wall fascia dehiscence has been reported in 9 % to 17 % [21, 22]. Stabilization of the donor site using artificial mesh is highly recommended after any VRAM flap procedure. Hence double layer closure of the anterior rectus fascia, and in case of too much tension, the use of resorbable or non-resorbable meshes must be performed. Improper healing of the donor site can cause abdominal wall incompetency and herniation. Although a manageable complication, this may represent a debilitating sequela without a second operative endeavor for correction. However, in case of abdominal hernia or bulging, a revision is not always necessary. When no incarceration is pending, an abdominal brace is often sufficient to support abdominal stability. A decrease in maximum isometric flexion torque after pedicled RAM flaps is reported to be approximately 20 % 1 year after the operation [23]. Lymphedema is also known to be reported in upto 32 % patients with increase in those patients with poor wound healing or recurrent disease. Hence a cutaneous pedicle to improve lymphatic drainage in case of covering defects in the groin has been recommended by Parkash et al. [24], with the idea that the skin and subcutaneous tissue including lymphatic collectors can drain to the contralateral side by connection to dissected collectors of the operated groin.
Modifications of the VRAM Flap
In an effort to alter the skin paddle design to increase the amount of skin and subcutaneous fat included with the flap or to extend the reach of the flap, various modifications of the VRAM flap have been used.
The extended deep inferior epigastric flap, first described by Taylor et al. can include a skin paddle extending to the midaxillary line. [25]The extended RAM flap has an oblique skin paddle supplied by paraumbilical perforators. The extended flap design can be adjusted to include vertical and oblique components for larger more complex defects. The cranial aspect of the extended VRAM flap’s skin paddle extends superolaterally beyond the costal margin toward the axilla, providing considerably longer flap reach and greater distal flap volume [26].
The oblique rectus abdominal myocutaneous flap is a flap design based on perforating vessels exiting the rectus near the umbilicus [27]. The skin flap is angled obliquely, utilizing preferential blood flow through periumbilical perforating arteries in the direction of the tip of the scapula. This flap has considerable technical advantages over other flaps used to fill dead space in the pelvis, including easier flap elevation, easier closure of the donor site, and less muscle taken from the abdominal wall to perform the flap [28].
Conclusion
Low to middle income countries witness a huge burden of locally advanced genital malignancies as well as advanced stage cutaneous melanomas of the lower extremity. Higher tumor burden both at the primary site as well as the inguinal basin requires surgery as the primary modality of treatment. Groin reconstruction is required not only to prevent femoral blowouts but also for early administration of adjuvant radiation. The versatility of tensor fascia lata, anterolateral thigh, and rectal abdominis flaps make them useful to cover the defect, provide radiation, eradication of pain and achieve good palliation. Several factors have been associated with an increased rate of postoperative complications: smoking, age, overweight, multi-morbidity, radiation at the donor site, and previous operations at the donor and the recipient site. Multiple modifications of the conventional myocutaneous flaps can be used to tailor the procedure to each individual patient and can be successfully used to reconstruct groin and abdominal wall integrity in a high proportion of patients and can be used in combination with the other muscle flaps for reliable coverage of groin vessels in radiated patients. However assessment of aesthetic and functional outcomes (such as decreasing lymphedema) of one flap over the other and the “ideal” form of reconstruction for groin defects needs additional investigation.
Acknowledgments
Special acknowledgement to Dr.Shivanada Swamy, Dr.Amarendra, Dr.Anand who are involved in management of the cases and Suresh J. for preparing the manuscript.
References
- 1.Whitemore WF, Jr, Vagavala MR. A technique of ilionguinal dissection for carcinoma of penis. Surg Gynecol Obstet. 1984;159(6):573–578. [PubMed] [Google Scholar]
- 2.Swan MC, Furniss D, Cassell OCS. Surgical management of metastatic inguinal lymphadenectomy. BMJ. 2004;329:1272–1276. doi: 10.1136/bmj.329.7477.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee RJ, Gibbs JF, Proulx GM, Kollmorgen DR, Jia C, Kraybill WG. Nodal basin recurrence following lymph node dissection for melanoma: implications for adjuvant radiotherapy. Int J Radiat Oncol Biol Phys. 2000;46:467–474. doi: 10.1016/S0360-3016(99)00431-9. [DOI] [PubMed] [Google Scholar]
- 4.Calabro A, Singletary SE, Balch CM. Patterns of relapse in 1001 consecutive patients with melanoma nodal metastases. Arch Surg. 1989;124:1051–1055. doi: 10.1001/archsurg.1989.01410090061014. [DOI] [PubMed] [Google Scholar]
- 5.Gopinath KS, Chandrashekhar M, Vijaya Kumar M, Murthy SK, Prabhakaran PS, Srikanta KC. Primary reconstruction of groin defects by tensor fascia latae flap after surgery. Indian J Surg. 1991;53(6):260–264. [Google Scholar]
- 6.Gupta AK, Kingsly PM, Jeeth Ij. Groin reconstruction after inguinal block dissection. Indian J Urol. 2006;22(4):355–359. doi: 10.4103/0970-1591.29125. [DOI] [Google Scholar]
- 7.Wangensteen OH. Repair of recurrent and difficult hernias and other large defects of the abdominal wall employing the iliotibial tract of fascia lata as a pedicled flap. Surg Gynecol Obstet. 1934;59:766–780. [Google Scholar]
- 8.Nahai F, Hill HL, Hester TR. Experiences with the tensor fascia lata flap. Plast reconstr Surg. 1979;63:788–799. doi: 10.1097/00006534-197963060-00004. [DOI] [PubMed] [Google Scholar]
- 9.Lynch SM. The bilobed tensor fascia lata myocutaneous flap. Plast Reconstr Surg. 1981;67(6):796–798. doi: 10.1097/00006534-198106000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Lewis VL, Jr, Cunningham BL, Hugo NE. The tensor fascia lata V-Y retroposition flap. Ann Plast Surg. 1981;6(1):34–37. doi: 10.1097/00000637-198101000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Siddiqui A, Wiedrich T, Lewis VL., Jr Tensor fascia lata V-Y retroposition myocutaneous flap: clinical experience. Ann Plast Surg. 1993;31(4):313–317. doi: 10.1097/00000637-199310000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Aslan G, Tuncali D, Bingul F, et al. The“duck” modification of the tensor fascia lata flap. Ann Plast Surg. 2005;54(6):637–639. doi: 10.1097/01.sap.0000162519.25430.1a. [DOI] [PubMed] [Google Scholar]
- 13.Song YG, Chen GZ, Song YL. The free thigh flap: a new free flap concept based on the septocutaneous artery. Br J Plast Surg. 1984;37:149. doi: 10.1016/0007-1226(84)90002-X. [DOI] [PubMed] [Google Scholar]
- 14.Koshima I, Fukuda H, Utonomiya R, et al. The anterolateral thigh flap: variations in its vascular pedicle. Br J Plast Surg. 1989;42:260. doi: 10.1016/0007-1226(89)90142-2. [DOI] [PubMed] [Google Scholar]
- 15.Kimata Y, Uchiyama K, Ebihara S, et al. Anatomic variations and technical problems of the anterolateral thigh flap: a report of 74 cases. Plast Reconst Surg. 1998;102(5):1517–1523. doi: 10.1097/00006534-199810000-00026. [DOI] [PubMed] [Google Scholar]
- 16.Ali RS, Bluebond-Langner R, Rodriguez ED, et al. The versatility of the anterolateral thigh flap. Plast Reconstr Surg. 2009;124(6 Suppl):e395–e407. doi: 10.1097/PRS.0b013e3181bcf05c. [DOI] [PubMed] [Google Scholar]
- 17.Lannon DA, Ross GL, Addison PD, et al. Versatility of the proximally pedicled anterolateral thigh flap and its use in complex abdominal and pelvic reconstruction. Plast Reconstr Surg. 2011;127(2):677–688. doi: 10.1097/PRS.0b013e3181fed714. [DOI] [PubMed] [Google Scholar]
- 18.Mathes SJ, Bostwick J. A rectus abdominis myocutaneous flap to reconstruct abdominal wall defects. Br J Plast Surg. 1977;30(4):282–283. doi: 10.1016/0007-1226(77)90118-7. [DOI] [PubMed] [Google Scholar]
- 19.Serletti J, Carras AJ, O’Keefe RJ, et al. Functional outcome after soft-tissue reconstruction for limb salvage after sarcoma surgery. Plast Reconstr Surg. 1998;102:1576. doi: 10.1097/00006534-199810000-00036. [DOI] [PubMed] [Google Scholar]
- 20.Hoy E, Granick M, Benevenia J, et al. Reconstruction of musculoskeletal defects following oncologic resection in 76 patients. Ann Plast Surg. 2006;57:190. doi: 10.1097/01.sap.0000216255.18106.e1. [DOI] [PubMed] [Google Scholar]
- 21.Lefevre JH, Parc Y, Kerneis S, et al. Abdomino-perineal resection for anal cancer: impact of a vertical rectus abdominis myocutaneus flap on survival, recurrence, morbidity, and wound healing. Ann Surg. 2009;250(5):707–711. doi: 10.1097/SLA.0b013e3181bce334. [DOI] [PubMed] [Google Scholar]
- 22.Butler CE, Gundeslioglu AO, Rodriguez-Bigas MA. Outcomes of immediate vertical rectus abdominis myocutaneous flap reconstruction for irradiated abdominoperineal resection defects. J Am Coll Surg. 2008;206(4):694–703. doi: 10.1016/j.jamcollsurg.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Daigeler A, Simidjiiska-Belyaeva M, Drücke D, Goertz O, et al. The versatility of the pedicled vertical rectus abdominis myocutaneous flap in oncologic patients. Langenbecks Arch Surg. 2011;396(8):1271–1279. doi: 10.1007/s00423-011-0823-6. [DOI] [PubMed] [Google Scholar]
- 24.Parkash S. The use of myocutaneous flaps in block dissections of the groin in cases with gross skin involvement. Br J Plast Surg. 1982;35(4):413–419. doi: 10.1016/0007-1226(82)90038-8. [DOI] [PubMed] [Google Scholar]
- 25.Taylor GI, Corlett R, Boyd JB. The extended deep inferior epigastric flap: a clinical technique. Plast Reconstr Surg. 1983;72:751. doi: 10.1097/00006534-198312000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Villa M, Saint-Cyr M, Wong C, et al. Extended vertical rectus abdominis myocutaneous flap for pelvic reconstruction: three-dimensional and four-dimensional computed tomography angiographic perfusion study and clinical outcome analysis. Plast Reconstr Surg. 2011;127(1):200–209. doi: 10.1097/PRS.0b013e3181f95a54. [DOI] [PubMed] [Google Scholar]
- 27.Abbott DE, Halverson AL, Wayne JD, et al. The oblique rectus abdominal myocutaneous flap for complex pelvic wound reconstruction. Dis Colon Rectum. 2008;51(8):1237–1241. doi: 10.1007/s10350-008-9359-4. [DOI] [PubMed] [Google Scholar]
- 28.Lee MJ, Dumanian GA. The oblique rectus abdominis musculocutaneous flap: revisited clinical applications. Plast Reconstr Surg. 2004;114:367–373. doi: 10.1097/01.PRS.0000131878.52740.EA. [DOI] [PubMed] [Google Scholar]
- 29.Gopinath KS, Chandrashekar M, Kumar MV, et al. Tensor fascia latae musculocutaneous flaps to reconstruct skin defects after radical inguinal lymphadenectomy. Br J Plast Surg. 1988;41(4):366–368. doi: 10.1016/0007-1226(88)90075-6. [DOI] [PubMed] [Google Scholar]
- 30.Schoeman BJ. The tensor fascia lata myocutaneous flap in reconstruction of inguinal skin defects after radical lymphadenectomy. S Afr J Surg. 1995;33(4):175–178. [PubMed] [Google Scholar]
- 31.Agarwal AK, Gupta S, Bhattacharya N, et al. Tensor fascia lata flap reconstruction in groin malignancy. Singapore Med J. 2009;50(8):781–784. [PubMed] [Google Scholar]
- 32.Hubmer MG, Justich I, Haas FM, et al. Clinical experience with a tensor fasciae latae perforator flap based on septocutaneous perforators. Plast Reconstr Aesthet Surg. 2011;64(6):782–789. doi: 10.1016/j.bjps.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Luo S, Raffoul W, Luo J, et al. Anterolateral thigh flap: a review of 168 cases. Microsurgery. 1999;19:232–238. doi: 10.1002/(SICI)1098-2752(1999)19:5<232::AID-MICR5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 34.Ahmad QG, Reddy M, Shetty KP, Prasad R, Hosi JS, Bhathena M. Groin reconstruction by anterlateral thigh flap: a review of 16 cases. Indian J Plast Surg. 2004;37:34–39. [Google Scholar]
- 35.Evriviades D, Raurell A, Perks AG. Pedicled anterolateral thigh flap for reconstruction after radical groin dissection. Urology. 2007;70(5):996–999. doi: 10.1016/j.urology.2007.07.062. [DOI] [PubMed] [Google Scholar]
- 36.Deo SV, Nootan KS, Niranjan B, et al. Vertical rectus abdominis myocutaneous flap cover for lower abdomen, chest wall, groin and thigh defects following resection of malignant tumours. Indian J Cancer. 2001;38(1):33–37. [PubMed] [Google Scholar]
- 37.Küntscher MV, Mansouri S, Noack N, et al. Versatility of vertical rectus abdominis musculocutaneous flaps. Microsurgery. 2006;26(5):363–369. doi: 10.1002/micr.20253. [DOI] [PubMed] [Google Scholar]
- 38.Parrett BM, Winograd JM, Garfein ES, et al. The vertical and extended rectus abdominis myocutaneous flap for irradiated thigh and groin defects. Plast Reconstr Surg. 2008;122(1):171–177. doi: 10.1097/PRS.0b013e3181774330. [DOI] [PubMed] [Google Scholar]