Abstract
Prostate cancer has come to share the oncological centrestage among male cancers. The availability of Serum Prostate Specific Antigen, PSA, as a marker has encouraged it’s use to diagnose both cancer and cancer recurrence. Some clarity is required about its precise role in clinical practice. The available literature on Prostate Specific Antigen was reviewed; Articles were reviewed for content, applicability to the problem at hand, availability of data about sensitivity and specificity of values, refinements in measurements and finally for impact of screening programmes using these values on survival and quality of life. The data in the literature was critically re-evaluated and analysed to draw reasonable conclusions. Serum PSA measurements show variable reliability when it comes to diagnosis of Prostate cancer, given the dynamics of PSA physiology. Surrogate measures like PSA density, PSA velocity, free-to-complexed PSA ratio, percentage Pro-PSA, etc., have been used to improve the predictive utility of this assay for Prostate cancer. The ability of PSA to detect those cancers that will cost life, and thereby permit early curative treatment, is as yet unclear. It’s most definitive role appears to be in diagnosing recurrences after adequate surgical treatment, and in evaluating response to treatment.
Keywords: Prostate cancer, Prostate Specific Antigen (PSA), Prostate cancer diagnosis, Prostate cancer treatment
Introduction
The outstanding successes achieved in cancer treatment and patient survival in the last half of the 20th century should be credited to the “golden five” of that era: Imaging, Radiation, Chemotherapy, Radical Surgery and Screening programmes. Successes in the treatment of colon cancer, breast cancer, cervical cancer and lung cancer through a convergence of specialities and public health initiatives are well known. This success stimulated efforts to detect as many more cancers early, and save lives.
In the same period, nothing in Urologic practice captured the imagination of public and professionals alike as Prostate cancer did. The emergence of Prostate Specific Antigen (PSA) as a marker for this disease, and the increase in aging population in many countries, stimulated a campaign of early detection and treatment in the United States, followed by Europe. In this review, we take stock of the contemporary role of this glycoprotein in saving the lives of prostate cancer patients through early detection and effective treatment. We look at the physiology of PSA production, its function in the body, its significance in Prostate cancer and the various twists and turns in its journey as a premier tumour marker in Prostate cancer.
Prostate cancer is common, and a frequent cause of cancer death. In urban India, prostate cancer is the most commonly diagnosed visceral cancer [1]; in 2011, there were expected to be 1,683/1,00,000 new prostate cancer diagnoses and about 1,027/1,00,000 prostate cancer deaths [2].
Prostate specific antigen (PSA) is a glycoprotein and is expressed by both normal and neoplastic prostate tissue. The absolute value of serum PSA is useful for determining the extent of prostate cancer and assessing the response to prostate cancer treatment; its use as a screening method to detect prostate cancer is also common, although controversial.
The measurement of PSA, recommendations for clinical use and advances in PSA testing will be reviewed here. The review is divided in three sections.
Basic understanding of PSA biology
Screening of Prostate Cancer and PSA
PSA–A Marker for Recurrence
Basic Understanding of PSA Biology
PSA Expression and Processing
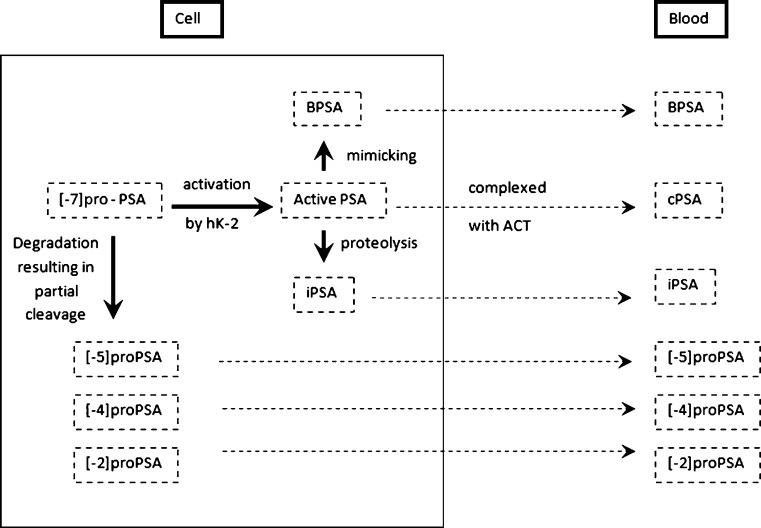
Under normal conditions, PSA is produced as a proenzyme (proPSA) by the secretory cells that line the prostate glands (acini) and secreted into the lumen, where the propeptide is removed to generate active PSA. The active PSA can then undergo proteolysis to generate inactive PSA, of which a small portion then enters the bloodstream and circulates in an unbound state (free PSA). Alternatively, active PSA can diffuse directly into the circulation where it is rapidly bound by protease inhibitors, including alpha-1-antichymotrypsin (ACT) and alpha-2-macroglobulin [3, 4]. Figure 1
Fig. 1.
Prostate-specific antigen (PSA) isoforms in cells and blood. ACT: antichymotrypsin, hK-2: human glandular kallikrein, BPSA: benign prostate-specific antigen, iPSA: initial prostate-specific antigen, cPSA: complexed prostate-specific antigen. (Adapted from Partin AW, Gretzer MB. Molecular forms of PSA: what does the future hold? Urology Times 10/2003)
In men with a normal prostate (i.e., no cancer and no major inflammation/infection), the majority of free PSA in the serum reflects the mature protein that has been inactivated by internal proteolytic cleavage. In contrast, this cleaved fraction is relatively decreased in prostate cancer. Thus, the percentage of free or unbound PSA is lower in the serum of men with prostate cancer (and conversely, the amount of complexed PSA is higher) compared with those who have a normal prostate or BPH [5–8].
Factors Affecting Normal PSA Levels
A number of factors affect the normal PSA levels and should be known before making a decision on what is elevated.
Age-Specific Reference Ranges
In men without prostate cancer, serum PSA reflects the amount of glandular epithelium, which in turn reflects prostate size. Thus as prostate size increases with increasing age, the PSA concentration also rises; it increases at a faster rate in elderly men [9, 10]. As a result, different normal reference ranges may be appropriate based upon a man’s age. The following ranges have been suggested:
| 40 to 49 years | 0 to 2.5 ng/mL |
| 50 to 59 years | 0 to 3.5 ng/mL |
| 60 to 69 years | 0 to 4.5 ng/mL |
| 70 to 79 years | 0 to 6.5 ng/mL |
Age-specific reference ranges have been proposed as a means of improving specificity and positive predictive value of the serum PSA in screening for prostate cancer. However, it should be recognized that the use of a higher upper range of normal for older men reduces the sensitivity of serum PSA testing for the detection of early prostate cancer, while increasing specificity.
Race-Specific Normal Ranges
Men without cancer from different ethnic and racial groups have different average PSA concentrations. In particular, black men without prostate cancer tend to have higher PSA values than white men without prostate cancer. It has been proposed that the definition of a “normal” PSA should vary by race using race-specific reference ranges.
Indian baseline values tend to be lower age specific PSA and higher PSAD values than those quoted in western literature. In an Indian study, a PSA level of 2.2 ng/ml and a PSAD of 0.15 ng/ml/cc in a 48 year old man has been proposed and needs further evaluation and validation [11].
Other Effects on Normal Range
Weight appears to be associated with PSA concentration. In population-based studies of men without prostate cancer, increasing body mass index (BMI) is associated with a lower mean PSA concentration [12–15].
Medications
Several classes of medication may affect serum PSA levels:
Finasteride and Dutasteride, inhibitors of 5-alpha-reductase, produce an approximately 50% or greater decrease in serum PSA during the first 3 to 6 months of therapy, which persists as long as the drug is continued. This is probably due to direct interference by these drugs with the prostatic intracellular androgen response mechanism [16], although these agents actually reduce prostate size which likely contributes to the lower PSA levels as well [17–20].
Other drugs which can lower the levels of PSA are NSAIDs [21], Statins [22, 23] and Thiazide [21] group of drugs. Whether the small changes in PSA levels seen in patients on treatment with NSAIDs, Acetaminophen, Statins, and Thiazides are clinically significant is unclear. It is also unclear whether as a corollary these drugs also reduce the risk of Prostate Cancer. This effect should, perhaps, be kept in mind when evaluating PSA changes in patients on treatment who might subsequently be prescribed one of these drugs.
Random Variation
Given the factors governing the release of PSA from the prostate, and the variability in levels that occurs with different assays, elevations seen with serial measurements may not always reflect changes prostate or disease behaviour [24].
Causes of Elevated PSA Other than Prostate
PSA has a half-life of 2.2 days [25], and levels elevated by different benign conditions will have variable recovery times [26–28]. PSA testing should be deferred accordingly:
Digital rectal examination (DRE) has minimal effect on PSA levels, leading to transient elevations of only 0.26 to 0.4 ng/mL, and PSA can be measured immediately after DRE [29, 30].
Ejaculation can increase PSA levels by up to 0.8 ng/mL, though levels return to normal within 48 h [31, 32].
Bacterial prostatitis may elevate PSA levels [33], but they generally return to baseline 6 to 8 weeks after symptoms resolve. Asymptomatic prostatic inflammation can also elevate PSA level [34], but this diagnosis is made on biopsy and so cannot generally be used to defer screening tests [33].
Prostate biopsy may elevate PSA levels by a median of 7.9 ng/mL within four to 24 h following the procedure [26]. Levels will remain elevated for 2 to 4 weeks. Similarly, a transurethral resection of the prostate (TURP) can elevate PSA levels by a median of 5.9 ng/mL [26]. Levels will remain elevated for a median time of approximately 3 weeks. A screening PSA test should not be performed for at least 6 weeks following either of these procedures.
Acute urinary retention may elevate PSA levels, but the levels can be expected to decrease by 50% within 1 or 2 days following resolution. A screening PSA test should not be performed for at least 2 weeks following an episode of acute urinary retention.
Advances in PSA Testing
Detection of PSA levels in the range of 2.5 to 10.0 ng/mL poses a diagnostic conundrum. Clinicians and patients alike would like definitive answers. Concepts related to PSA testing that have helped refine the interpretation of an elevated concentration falling in the above range include:
PSA density
PSA velocity
Free versus complexed or bound PSA
These modifications are expected to be useful in further prostate cancer screening procedures like biopsy, when the total PSA is 2.5 to 10.0 ng/mL, the range in which decisions regarding further diagnostic testing are most difficult.
PSA Density
The use of PSA density is based on the premise that a large benign adenoma may contribute to elevated PSA levels even in the absence of cancer. To compensate for BPH and prostate size, transrectal ultrasound (TRUS) has been used to measure prostate volume. Serum PSA is then divided by prostate volume to give a PSA density, with higher PSA density values (greater than 0.15 ng/mL) being more suggestive of prostate cancer while lower values are more suggestive of benign hypertrophy [35, 36].
This seemed a promising concept, subsequent work has show up limitations of this measurement, while an early study suggested that PSA density was a promising method for distinguishing patients with benign and malignant prostate disease [35, 37], subsequent reports have found considerable overlap in PSA densities between these groups [38]. One multicenter study that compared PSA density versus PSA for the early detection of prostate cancer found that almost one-half of the cancers would have been missed using 0.15 ng/mL/cc as a cut-off for biopsy [39].
PSA Velocity
Another approach has been to assess the rate of PSA change over time (the PSA velocity). An elevated serum PSA that continues to rise over time is more likely to reflect prostate cancer than one that is consistently stable [40]. In one study, a PSA velocity cut-off of 0.75 ng/mL per year distinguished patients with prostate cancer from those with either BPH or no prostate disease with a specificity of 90 and 100%, respectively [41]. A further study from the same group found that when PSA was <4 ng/mL, a PSA velocity >0.35 ng/mL per year measured over several years was associated with a high risk of death from prostate cancer 15 years later [42]. Similarly, another series of studies from a different group found that among men with prostate cancer, a PSA velocity >2 ng/mL per year in the year prior to diagnosis was associated with an increased risk of death from prostate cancer after radical prostatectomy or radiation therapy [43, 44].
In practice, the usefulness of PSA velocity is in part limited by variability in the serum PSA levels at different times in the same patient, irrespective of the presence or absence of cancer; at least three consecutive measurements should be performed [45]. A longer time over which values are measured can help reduce the general variation (i.e. “noise”) in the PSA measurements. The need for prolonging the screening process on this count can further increase anxiety about the disease.
Serum Free and Bound PSA
As noted previously, prostate cancer is associated with a lower percentage of free PSA in the serum as compared with benign conditions.
The percentage of free PSA (free/total PSA) has been used to improve the sensitivity of cancer detection when total PSA is in the normal range (<4 ng/mL) and to increase the specificity of cancer detection when total PSA in the “gray zone” (4.1 to 10 ng/mL). In this latter group, the lower the value of free to total PSA, the greater is the likelihood that an elevated PSA represents cancer and not BPH. As an example, in one study of men with PSA values in this range, the probability of cancer in men with a free-to-total PSA below 10% was 56%, compared with only 8% in men with a value >25% [46].
As with total PSA, there is no absolute cut-off ratio that reliably differentiates prostate cancer from BPH. The optimal cut-off value PSA is unclear and depends upon whether optimal sensitivity or specificity is sought [47, 48]. The higher the cut-off value, the greater the sensitivity (i.e., fewer cancers missed), but the lower the specificity (greater number of false positives).
Free PSA may be useful for risk stratification in men with prostate cancer. A lower percentage of free-to-total PSA may be associated with a more aggressive form of prostate cancer [49].
Complexed PSA
Considerable effort has been expended to develop assays for ACT-complexed PSA (cPSA); only recently has this been achieved [50–52]. Such an assay would theoretically provide a similar enhanced degree of specificity as the free-to-total PSA ratio, but require only the measurement of a single analyte. Complexed PSA has been approved for the monitoring of men with prostatic carcinoma [53].
Percent [-2] proPSA
[-2] proPSA, is unbound and potentially higher in men with prostate cancer. Based upon this observation, there has been growing interest in using the ratio of [-2] proPSA to free PSA (expressed as percent [-2] proPSA or% [-2] proPSA) for prostate cancer screening. One multicenter study of 566 men undergoing biopsy found that% [-2] proPSA significantly outperformed both total PSA and percent free PSA [54]. At 80% sensitivity,% [-2] proPSA had 52% specificity, compared to 30% for PSA and 29% for percent free PSA. Percent [-2] proPSA is currently approved by the European Union for prostate cancer detection.
Improving the Accuracy of PSA
The need for an accurate marker is driven by the fear of unnecessary biopsies on the one hand, and the more danger risk of missing a treatable cancer on the other. Given the scepticism about the utility of aggressive screening programmes, we would certainly appreciate something more accurate than what we have.
There is no consensus on using any of the PSA modifications, and none of them has been shown to reduce the number of unnecessary biopsies or improve clinical outcomes. The total PSA cut-off of 4.0 ng/mL has been the most accepted standard because it balances the trade-off between missing important cancers at a curable stage and avoiding detection of clinically insignificant disease and subjecting men to unnecessary prostate biopsies [55–57]. Ongoing efforts are targeted at identifying new serum markers that will have greater diagnostic accuracy for prostate cancer, particularly those that can predict aggressive tumours whose treatment will save lives [55, 58].
PSA & Prostate Cancer Screening: Some Issues
Prostate cancer screening has been a controversial issue because decisions were made about adopting PSA testing in the absence of efficacy data from randomized trials. Subsequently, the European Randomized Study of Screening for Prostate Cancer (ERSPC) reported a small absolute survival benefit with PSA screening after 9 years of follow-up [59]; however, 1,410 men needed to be screened and 48 additional patients would need to be diagnosed with prostate cancer to prevent one prostate cancer death. Although the report did not address quality of life outcomes, considerable data show the potential harms from aggressive treatments, including erectile dysfunction, urinary incontinence, and bowel problems [60].
Sensitivity and Specificity
The American Cancer Society systematically reviewed the literature assessing PSA performance [61]. In this pooled analysis, the estimated sensitivity of a PSA cut-off of 4.0 ng/mL was 21% for detecting any prostate cancer and 51% for detecting high-grade cancers (Gleason ≥8). Using a cut-off of 3.0 ng/mL increased these sensitivities to 32 and 68%, respectively. The estimated specificity was 91% for a PSA cut-off of 4.0 ng/mL and 85% for a 3.0 ng/mL cut-off. PSA has poorer discriminating ability in men with symptomatic benign prostatic hyperplasia [62]. Table 1
Table 1.
PSA screening test characteristics as a function of threshold for a positive test. Test positivity: (# positive/# tested) × 100; cancer detection rate: (# prostate cancer/# tested) × 100; Positive Predictive Value (PPV): (# prostate cancer/# biopsied) × 100; specificity: (true negative tests)/(true negative tests + false positive tests) × 100, estimated from: (#tested–#positive)/(#tested–#cancer) × 100, assuming that negative tests are true negatives
| Test characteristic | PSA (normal <4 NG/ML) | PSA (normal <3 NG/ML) |
|---|---|---|
| Test positivity (%) | 12 | 18 |
| Cancer detection rate (%) | 3 | 4 |
| Sensitivity (%) | 21 | 32 |
| Sensitivity (%) for high grade cancer, i.e., gleason score ≥8 | 51 | 68 |
| Specificity (%) | 91 | 85 |
| Positive predictive value (%) | 30 | 28 |
Positive Predictive Value
Overall, the positive predictive value for a PSA level >4.0 ng/mL is approximately 30%, meaning that slightly less than one in three men with an elevated PSA will have prostate cancer detected on biopsy [63–65]. For PSA levels between 4.0 and 10.0 ng/mL, the positive predictive value is about 25%. However, nearly 75% of cancers detected within the “gray zone” of PSA values between 4.0 and 10.0 ng/mL are organ confined and potentially curable [64]. The question is: how many of them would kill if not treated.
Negative Predictive Value
The Prostate Cancer Prevention Trial, which biopsied men with normal PSA levels, estimated a negative predictive value of 85% for a PSA value ≤4.0 ng/mL [66].
Effectiveness of Prostate Cancer Screening
In real terms, the efficacy of a screening programme need not necessarily mean it is effective. While efficacy will improve diagnosis, the programme can be considered “effective” only if it results in improved survival. The thrust, however, continues to be towards improving “efficacy”, in the hope perhaps that this will 1 day also be “effective”.
Effect of Lowering PSA Cut-Offs
Some investigators have suggested using a lower PSA cut-off because some men with PSA levels below 4 ng/mL and normal digital rectal examinations were found to have prostate cancer [67–70].
In a subset analysis from the placebo arm of the Prostate Cancer Prevention Trial, 449 of 2,950 men (15.2%) ages 62 to 91 years who had consistently normal PSA levels and digital rectal examinations during the 7 years of annual screening had prostate cancer on an end-of-study biopsy; 67 (2.3%) had high-grade prostate cancer with a Gleason score of 7 or higher [66]. Among men with a PSA concentration between 2.1 and 4.0 ng/mL, 24.7% had prostate cancer, and 5.2% had prostate cancer with a Gleason score of 7 or higher.
These observations indicate that there is not a clear cut-off point between “normal” and “abnormal” PSA levels. The Prostate Cancer Prevention Trial found that for biopsies performed during follow-up in the control group even a PSA cut-off of 1.1 ng/mL would miss 17% of cancers, including 5% of poorly differentiated cancers [71]. Thus, any choice of PSA cut-off involves a tradeoff between sensitivity and specificity. While lowering the PSA cut-off would improve test sensitivity, a lower PSA cut-off would also reduce specificity, leading to far more false-positive tests and unnecessary biopsies. It has been projected that if the PSA threshold were to be lowered to 2.5 ng/mL, the number of men with PSA level considered abnormal would double, to up to six million in the US [72]. Additionally, many of the cancers detected at these lower levels may never have become clinically evident, thereby leading to over diagnosis and overtreatment [57].
There is also evidence that diagnosing prostate cancer at low PSA levels does not affect outcome. A study of 875 men undergoing radical prostatectomy found only a limited association between preoperative PSA levels of 2 to 9 ng/mL and cure rates [73]. The disease-free survival curves did not significantly diverge until the preoperative PSA levels reached 7 ng/mL, suggesting that diagnosing cancers in patients with lower PSA levels may be unnecessary. Most of the PSA elevation below 7 ng/mL was attributed to benign hyperplastic tissue. The investigators emphasized the need for a better serum marker to identify early-stage aggressive cancers.
A 2010 meta-analysis summarized results from six randomized trials (including unique data from two ERSPC sites), with a total of 387,286 participants [74]. Screening with PSA with or without DRE compared to no screening did not reduce death from prostate cancer (relative risk [RR] 0.88, 95% CI 0.71-1.09). However, screening significantly increased the probability of cancer diagnosis (RR 1.46, CI 1.21-1.77). Similar results were echoed in findings of the SEER group [1].
Approach to Screening
Given the important trade-offs between potential benefits and harms involved with both screening or not screening for prostate cancer, and the lack of definitive data on screening outcomes, it is particularly important that patients make informed decisions about undergoing testing. The United States Preventive Services Task Force Guidelines [75], American College of Physicians [76], American Urology Association [77], American Cancer Society [61] all stress the importance of informed decision making. Men who are willing to accept a substantial risk of morbidity associated with treatment in return for a small reduction in mortality might reasonably choose to be screened. Men who are at increased risk of prostate cancer because of race or family history may be more likely to benefit from screening.
Efforts have focused on using decision aids to help patients understand screening issues and make informed decisions for screening [78]. The American Cancer Society provided a list of Decision Aids for Prostate Cancer Screening [61].
PSA and Prostate Cancer Recurrence
Due to the sensitivity of serum PSA as a marker for prostate cancer, serial measurements are routinely obtained to detect early disease recurrence in men who have been treated for localized disease.
Monitoring PSA after treatment of localized prostate cancer leads to the identification of men with a PSA-only (biochemical) recurrence. In this situation, increases in serum PSA over the baseline after initial treatment are not accompanied by symptoms or signs of locally recurrent or metastatic disease. Many of these men are relatively young and otherwise healthy. Thus, intense interest has been focused upon their treatment, with attention on both survival and the impact of therapy on quality of life.
Frequency of PSA Testing
The optimal frequency of PSA testing is unclear. Some authors suggest measuring PSA every 6 months for the first 2 years after treatment, and then annually [79]. Others recommend modifying the surveillance schedule according to pathologic grade and stage of disease, with decreasing frequency for low-risk disease and increasing elapsed interval following original treatment [80, 81], although the majority do not adhere to this principle [82, 83].
Definition of Biochemical Progression
The definition of PSA-only recurrence depends upon the initial treatment modality–prostatectomy or radiation therapy
Prostatectomy
All prostate tissue is removed during a successful radical prostatectomy. Postoperatively, therefore, detectable serum PSA using standard immunoassays is considered indicative of residual prostatic tissue, presumably representing locoregional or systemic cancer
Radiation Therapy
The definition of biochemical failure is more complicated after radiation therapy (RT) than following radical prostatectomy. Some normal prostatic glandular tissue remains and serum PSA levels are unlikely to fall to undetectable levels following a course of RT. The interpretation of serum PSA is also complicated by the use of androgen deprivation, in addition to radiotherapy in some patients with intermediate or high risk disease.
The decline in serum PSA following RT is gradual and the mean time for the PSA to reach its nadir is 18 months or longer [84].
To standardize serum PSA testing for outcome assessment following RT, a 1996 American Society for Radiation Oncology (ASTRO) consensus panel addressed the definition of biochemical recurrence following definitive RT. Biochemical failure was defined as occurring after three consecutive PSA rises following a nadir. The date of biochemical failure was halfway between the nadir and the date of first rise or any rise great enough to provoke the initiation of therapy [83].
A second consensus conference was held by ASTRO in 2005 to address issues that had subsequently been identified in the 1996 definition of PSA failure. Features of the revised “Phoenix” criteria [85] for PSA failure included: a) A PSA rise by 2 ng/mL or more above the nadir PSA is considered the standard definition for biochemical failure after external beam RT, regardless of whether or not a patient receives androgen deprivation therapy. b) The date of failure is defined by the time the rise in PSA is noted.
Men who normalize their serum PSA after receiving a full course of RT seem to have more durable responses than those who do not [86].
PSA Bounce
This is a phenomenon seen in prostate cancer patients treated with radiotherapy.
Serum PSA levels typically fall after RT and can then rise (“bounce”) transiently, at a median of 12 to 18 months after treatment [87]. This PSA bounce can occur in the absence of recurrent disease and is necessarily an indication for therapeutic intervention. There are no definitive methods to distinguish a PSA bounce from recurrent cancer. If an increase in the serum PSA is observed, the patient should be given reassurance, and the PSA can be repeated in 3 to 6 months. If the serum PSA continues to increase, a repeat biopsy can be considered, although the interpretation of a prostate biopsy performed following RT is difficult.
Prognosis after Biochemical Failure
The prognosis of men with prostate cancer following a PSA relapse is diverse, and biochemical failure does not necessarily predict death [88–92]. The variability in prognosis in different series ranges from estimates in the 4 to 5 year range to as long as 15 years or more.
Several parameters (e.g., PSA-doubling time) have been studied to distinguish men who are likely to develop “clinically significant” biochemical recurrence following either radical prostatectomy or RT from those who have more indolent disease [88, 93].
Conclusion
The approval of PSA testing as a screening tool by the USFDA in 1994 triggered a tremendous enthusiasm for using PSA assays to detect and treat prostate cancer, thereby reducing deaths from this disease. It became clear over the years that PSA offered diagnostic advantages when levels were above 10 ng/mL; but efforts continued to detect cancer at lower PSA levels in the belief that such detection would, logically, lead to higher survival in prostate cancer sufferers. Attempts to increase its sensitivity as a marker for detection of prostate cancer led to introduction of refinements, some of them using derived values (e.g. PSA density, PSA velocity). More cancers were certainly detected, but data on increased survival was not as forthcoming.
Over the years, several questions about its effectiveness in saving lives have been asked, as distinct from the ability of PSA to detect histological prostate cancer. Questions have also arisen above the quality of life in those that had detection through screening with consequent radical treatments. On the basis of data presently available, it would be difficult to categorically take a stand on one or the other side of this line. If and when tests that can distinguish prostate cancer patients who are likely to die of the disease from those that the disease will not kill emerge, PSA may recede into the background. Till such time, it is here to stay.
Prudence in treating PSA-detected prostate cancer patients will address many of the concerns in the interim.
References
- 1.Ries LAG, Melbert D, Krapcho M, et al. SEER cancer statistics review, 1975 2004. Bethesda: National Cancer Institute; 2007. [Google Scholar]
- 2.Cancer Incidence and Patterns in Urban Maharashtra (2001) Indian Cancer Society (http://www.indiancancersociety.org) [PubMed]
- 3.Lilja H, Christensson A, Dahlén U, et al. Prostate-specific antigen in serum occurs predominantly in complex with alpha 1-antichymotrypsin. Clin Chem. 1991;37:1618. [PubMed] [Google Scholar]
- 4.Mikolajczyk SD, Marks LS, Partin AW, Rittenhouse HG. Free prostate-specific antigen in serum is becoming more complex. Urology. 2002;59:797. doi: 10.1016/S0090-4295(01)01605-3. [DOI] [PubMed] [Google Scholar]
- 5.Björk T, Piironen T, Pettersson K, et al. Comparison of analysis of the different prostate-specific antigen forms in serum for detection of clinically localized prostate cancer. Urology. 1996;48:882. doi: 10.1016/S0090-4295(96)00486-4. [DOI] [PubMed] [Google Scholar]
- 6.Christensson A, Björk T, Nilsson O, et al. Serum prostate specific antigen complexed to alpha 1-antichymotrypsin as an indicator of prostate cancer. J Urol. 1993;150:100. doi: 10.1016/s0022-5347(17)35408-3. [DOI] [PubMed] [Google Scholar]
- 7.Partin AW, Hanks GE, Klein EA, et al. Prostate-specific antigen as a marker of disease activity in prostate cancer. Oncology (Williston Park) 2002;16:1024. [PubMed] [Google Scholar]
- 8.Balk SP, Ko YJ, Bubley GJ. Biology of prostate-specific antigen. J Clin Oncol. 2003;21:383. doi: 10.1200/JCO.2003.02.083. [DOI] [PubMed] [Google Scholar]
- 9.Crawford ED. Prostate cancer awareness week: September 22 to 28, 1997. CA Cancer J Clin. 1997;47:288. doi: 10.3322/canjclin.47.5.288. [DOI] [PubMed] [Google Scholar]
- 10.Oesterling JE, Jacobsen SJ, Chute CG, et al. Serum prostate-specific antigen in a community-based population of healthy men. Establishment of age-specific reference ranges. JAMA. 1993;270:860. doi: 10.1001/jama.1993.03510070082041. [DOI] [PubMed] [Google Scholar]
- 11.Ganpule AP, Desai MR, Manohar T, et al. Age specific prostate specific antigen and prostate specific antigen density values in a community based Indian population. Indian J Urol. 2007;23(2):122. doi: 10.4103/0970-1591.32060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baillargeon J, Pollock BH, Kristal AR, et al. The association of body mass index and prostate-specific antigen in a population-based study. Cancer. 2005;103:1092. doi: 10.1002/cncr.20856. [DOI] [PubMed] [Google Scholar]
- 13.Werny DM, Thompson T, Saraiya M, et al. Obesity is negatively associated with prostate-specific antigen in U.S. men, 2001–2004. Canc Epidemiol Biomarkers Prev. 2007;16:70. doi: 10.1158/1055-9965.EPI-06-0588. [DOI] [PubMed] [Google Scholar]
- 14.Rundle A, Neugut AI. Obesity and screening PSA levels among men undergoing an annual physical exam. Prostate. 2008;68:373. doi: 10.1002/pros.20704. [DOI] [PubMed] [Google Scholar]
- 15.Beebe-Dimmer JL, Faerber GJ, Morgenstern H, et al. Body composition and serum prostate-specific antigen: review and findings from Flint Men’s Health Study. Urology. 2008;71:554. doi: 10.1016/j.urology.2007.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang LG, Liu XM, Kreis W, Budman DR. Down-regulation of prostate-specific antigen expression by finasteride through inhibition of complex formation between androgen receptor and steroid receptor-binding consensus in the promoter of the PSA gene in LNCaP cells. Cancer Res. 1997;57:714. [PubMed] [Google Scholar]
- 17.Guess HA, Gormley GJ, Stoner E, Oesterling JE. The effect of finasteride on prostate specific antigen: review of available data. J Urol. 1996;155:3. doi: 10.1016/S0022-5347(01)66524-8. [DOI] [PubMed] [Google Scholar]
- 18.D'Amico AV, Roehrborn CG. Effect of 1 mg/day finasteride on concentrations of serum prostate-specific antigen in men with androgenic alopecia: a randomised controlled trial. Lancet Oncol. 2007;8:21. doi: 10.1016/S1470-2045(06)70981-0. [DOI] [PubMed] [Google Scholar]
- 19.Etzioni RD, Howlader N, Shaw PA, et al. Long-term effects of finasteride on prostate specific antigen levels: results from the prostate cancer prevention trial. J Urol. 2005;174:877. doi: 10.1097/01.ju.0000169255.64518.fb. [DOI] [PubMed] [Google Scholar]
- 20.Andriole GL, Bostwick D, Brawley OW, et al. The effect of dutasteride on the usefulness of prostate specific antigen for the diagnosis of high grade and clinically relevant prostate cancer in men with a previous negative biopsy: results from the REDUCE study. J Urol. 2011;185:126. doi: 10.1016/j.juro.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Chang SL, Harshman LC, Presti JC., Jr Impact of common medications on serum total prostate-specific antigen levels: analysis of the National Health and Nutrition Examination Survey. J Clin Oncol. 2010;28:3951. doi: 10.1200/JCO.2009.27.9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamilton RJ, Goldberg KC, Platz EA, Freedland SJ. The influence of statin medications on prostate-specific antigen levels. J Natl Canc Inst. 2008;100:1511. doi: 10.1093/jnci/djn362. [DOI] [PubMed] [Google Scholar]
- 23.Mondul AM, Selvin E, Marzo AM, et al. Statin drugs, serum cholesterol, and prostate-specific antigen in the National Health and Nutrition Examination Survey 2001–2004. Canc Causes Contr. 2010;21:671. doi: 10.1007/s10552-009-9494-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruun L, Becker C, Hugosson J, et al. Assessment of intra-individual variation in prostate-specific antigen levels in a biennial randomized prostate cancer screening program in Sweden. Prostate. 2005;65:216. doi: 10.1002/pros.20286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stamey TA, Yang N, Hay AR, et al. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317:909. doi: 10.1056/NEJM198710083171501. [DOI] [PubMed] [Google Scholar]
- 26.Tchetgen MB, Oesterling JE. The effect of prostatitis, urinary retention, ejaculation, and ambulation on the serum prostate-specific antigen concentration. Urol Clin North Am. 1997;24:283. doi: 10.1016/S0094-0143(05)70374-8. [DOI] [PubMed] [Google Scholar]
- 27.Yuan JJ, Coplen DE, Petros JA, et al. Effects of rectal examination, prostatic massage, ultrasonography and needle biopsy on serum prostate specific antigen levels. J Urol. 1992;147:810. doi: 10.1016/s0022-5347(17)37392-5. [DOI] [PubMed] [Google Scholar]
- 28.Nadler RB, Humphrey PA, Smith DS, et al. Effect of inflammation and benign prostatic hyperplasia on elevated serum prostate specific antigen levels. J Urol. 1995;154:407. doi: 10.1016/S0022-5347(01)67064-2. [DOI] [PubMed] [Google Scholar]
- 29.Chybowski FM, Bergstralh EJ, Oesterling JE. The effect of digital rectal examination on the serum prostate specific antigen concentration: results of a randomized study. J Urol. 1992;148:83. doi: 10.1016/s0022-5347(17)36517-5. [DOI] [PubMed] [Google Scholar]
- 30.(1995) Effect of digital rectal examination on serum prostate-specific antigen in a primary care setting. The Internal Medicine Clinic Research Consortium. Arch Intern Med 155:389 [PubMed]
- 31.Herschman JD, Smith DS, Catalona WJ. Effect of ejaculation on serum total and free prostate-specific antigen concentrations. Urology. 1997;50:239. doi: 10.1016/S0090-4295(97)00209-4. [DOI] [PubMed] [Google Scholar]
- 32.Tchetgen MB, Song JT, Strawderman M, et al. Ejaculation increases the serum prostate-specific antigen concentration. Urology. 1996;47:511. doi: 10.1016/S0090-4295(99)80486-5. [DOI] [PubMed] [Google Scholar]
- 33.Kawakami J, Siemens DR, Nickel JC. Prostatitis and prostate cancer: implications for prostate cancer screening. Urology. 2004;64:1075. doi: 10.1016/j.urology.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 34.Simardi LH, Tobias-MacHado M, Kappaz GT, et al. Influence of asymptomatic histologic prostatitis on serum prostate-specific antigen: a prospective study. Urology. 2004;64:1098. doi: 10.1016/j.urology.2004.08.060. [DOI] [PubMed] [Google Scholar]
- 35.Benson MC, Whang IS, Olsson CA, et al. The use of prostate specific antigen density to enhance the predictive value of intermediate levels of serum prostate specific antigen. J Urol. 1992;147:817. doi: 10.1016/s0022-5347(17)37394-9. [DOI] [PubMed] [Google Scholar]
- 36.Andriole GL, Telle WB, Coplen DE, Catalona WJ. PSA index (PSAI) as a predictor of prostate cancer in men with persistent serum PSA elevation (abstract) J Urol. 1992;147:387A. [Google Scholar]
- 37.Benson MC, Whang IS, Pantuck A, et al. Prostate specific antigen density: a means of distinguishing benign prostatic hypertrophy and prostate cancer. J Urol. 1992;147:815. doi: 10.1016/s0022-5347(17)37393-7. [DOI] [PubMed] [Google Scholar]
- 38.Bare R, Hart L, McCullough DL. Correlation of prostate-specific antigen and prostate-specific antigen density with outcome of prostate biopsy. Urology. 1994;43:191. doi: 10.1016/0090-4295(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 39.Catalona WJ, Richie JP, deKernion JB, et al. Comparison of prostate specific antigen concentration versus prostate specific antigen density in the early detection of prostate cancer: receiver operating characteristic curves. J Urol. 1994;152:2031. doi: 10.1016/s0022-5347(17)32299-1. [DOI] [PubMed] [Google Scholar]
- 40.Smith DS, Catalona WJ. Rate of change in serum prostate specific antigen levels as a method for prostate cancer detection. J Urol. 1994;152:1163. doi: 10.1016/s0022-5347(17)32528-4. [DOI] [PubMed] [Google Scholar]
- 41.Carter HB, Pearson JD, Metter EJ, et al. Longitudinal evaluation of prostate-specific antigen levels in men with and without prostate disease. JAMA. 1992;267:2215. doi: 10.1001/jama.1992.03480160073037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carter HB, Ferrucci L, Kettermann A, et al. Detection of life-threatening prostate cancer with prostate-specific antigen velocity during a window of curability. J Natl Canc Inst. 2006;98:1521. doi: 10.1093/jnci/djj410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D'Amico AV, Chen MH, Roehl KA, Catalona WJ. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N Engl J Med. 2004;351:125. doi: 10.1056/NEJMoa032975. [DOI] [PubMed] [Google Scholar]
- 44.D'Amico AV, Renshaw AA, Sussman B, Chen MH. Pretreatment PSA velocity and risk of death from prostate cancer following external beam radiation therapy. JAMA. 2005;294:440. doi: 10.1001/jama.294.4.440. [DOI] [PubMed] [Google Scholar]
- 45.Riehmann M, Rhodes PR, Cook TD, et al. Analysis of variation in prostate-specific antigen values. Urology. 1993;42:390. doi: 10.1016/0090-4295(93)90364-G. [DOI] [PubMed] [Google Scholar]
- 46.Catalona WJ, Partin AW, Slawin KM, et al. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. JAMA. 1998;279:1542. doi: 10.1001/jama.279.19.1542. [DOI] [PubMed] [Google Scholar]
- 47.Hoffman RM, Clanon DL, Littenberg B, et al. Using the free-to-total prostate-specific antigen ratio to detect prostate cancer in men with nonspecific elevations of prostate-specific antigen levels. J Gen Intern Med. 2000;15:739. doi: 10.1046/j.1525-1497.2000.90907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee R, Localio AR, Armstrong K, et al. A meta-analysis of the performance characteristics of the free prostate-specific antigen test. Urology. 2006;67:762. doi: 10.1016/j.urology.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 49.Carter HB, Partin AW, Luderer AA, et al. Percentage of free prostate-specific antigen in sera predicts aggressiveness of prostate cancer a decade before diagnosis. Urology. 1997;49:379. doi: 10.1016/S0090-4295(96)00629-2. [DOI] [PubMed] [Google Scholar]
- 50.Allard WJ, Zhou Z, Yeung KK. Novel immunoassay for the measurement of complexed prostate-specific antigen in serum. Clin Chem. 1998;44:1216. [PubMed] [Google Scholar]
- 51.Chichibu K, Kuroe K, Hashimoto C, Goto S. Specific quantification of gamma-seminoprotein-alpha 1 antichymotrypsin complex in serum by monoclonal antibody-based enzyme immunoassay. Rinsho Byori. 1995;43:1153. [PubMed] [Google Scholar]
- 52.Kuriyama M, Ueno K, Uno H, et al. Clinical evaluation of serum prostate-specific antigen-alpha1-antichymotrypsin complex values in diagnosis of prostate cancer: a cooperative study. Int J Urol. 1998;5:48. doi: 10.1111/j.1442-2042.1998.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 53.Allard WJ, Cheli CD, Morris DL, et al. Multicenter evaluation of the performance and clinical utility in longitudinal monitoring of the Bayer Immuno 1 complexed PSA assay. Int J Biol Markers. 1999;14:73. doi: 10.1177/172460089901400204. [DOI] [PubMed] [Google Scholar]
- 54.Sokoll LJ, Sanda MG, Feng Z, et al. A prospective, multicenter, National Cancer Institute Early Detection Research Network study of [-2]proPSA: improving prostate cancer detection and correlating with cancer aggressiveness. Canc Epidemiol Biomarkers Prev. 2010;19:1193. doi: 10.1158/1055-9965.EPI-10-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Polascik TJ, Oesterling JE, Partin AW. Prostate specific antigen: a decade of discovery–what we have learned and where we are going. J Urol. 1999;162:293. doi: 10.1016/S0022-5347(05)68543-6. [DOI] [PubMed] [Google Scholar]
- 56.Carroll P, Coley C, McLeod D, et al. Prostate-specific antigen best practice policy–part I: early detection and diagnosis of prostate cancer. Urology. 2001;57:217. doi: 10.1016/S0090-4295(00)00993-6. [DOI] [PubMed] [Google Scholar]
- 57.Carter HB. Prostate cancers in men with low PSA levels–must we find them? N Engl J Med. 2004;350:2292. doi: 10.1056/NEJMe048003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tricoli JV, Schoenfeldt M, Conley BA. Detection of prostate cancer and predicting progression: current and future diagnostic markers. Clin Cancer Res. 2004;10:3943. doi: 10.1158/1078-0432.CCR-03-0200. [DOI] [PubMed] [Google Scholar]
- 59.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 60.Wilt TJ, MacDonald R, Rutks I, et al. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148:435. doi: 10.7326/0003-4819-148-6-200803180-00209. [DOI] [PubMed] [Google Scholar]
- 61.Wolf AM, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60:70. doi: 10.3322/caac.20066. [DOI] [PubMed] [Google Scholar]
- 62.Meigs JB, Barry MJ, Oesterling JE, Jacobsen SJ. Interpreting results of prostate-specific antigen testing for early detection of prostate cancer. J Gen Intern Med. 1996;11:505. doi: 10.1007/BF02599596. [DOI] [PubMed] [Google Scholar]
- 63.Brawer MK, Chetner MP, Beatie J, et al. Screening for prostatic carcinoma with prostate specific antigen. J Urol. 1992;147:841. doi: 10.1016/s0022-5347(17)37401-3. [DOI] [PubMed] [Google Scholar]
- 64.Catalona WJ, Richie JP, Ahmann FR, et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6,630 men. J Urol. 1994;151:1283. doi: 10.1016/s0022-5347(17)35233-3. [DOI] [PubMed] [Google Scholar]
- 65.Schröder FH, Cruijsen-Koeter I, Koning HJ, et al. Prostate cancer detection at low prostate specific antigen. J Urol. 2000;163:806. doi: 10.1016/S0022-5347(05)67809-3. [DOI] [PubMed] [Google Scholar]
- 66.Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350:2239. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 67.Catalona WJ, Smith DS, Ornstein DK. Prostate cancer detection in men with serum PSA concentrations of 2.6 to 4.0 ng/mL and benign prostate examination. Enhancement of specificity with free PSA measurements. JAMA. 1997;277:1452. doi: 10.1001/jama.1997.03540420048028. [DOI] [PubMed] [Google Scholar]
- 68.Babaian RJ, Johnston DA, Naccarato W, et al. The incidence of prostate cancer in a screening population with a serum prostate specific antigen between 2.5 and 4.0 ng/ml: relation to biopsy strategy. J Urol. 2001;165:757. doi: 10.1016/S0022-5347(05)66519-6. [DOI] [PubMed] [Google Scholar]
- 69.Gilbert SM, Cavallo CB, Kahane H, Lowe FC. Evidence suggesting PSA cutpoint of 2.5 ng/mL for prompting prostate biopsy: review of 36,316 biopsies. Urology. 2005;65:549. doi: 10.1016/j.urology.2004.10.064. [DOI] [PubMed] [Google Scholar]
- 70.Porter MP, Stanford JL, Lange PH. The distribution of serum prostate-specific antigen levels among American men: implications for prostate cancer prevalence and screening. Prostate. 2006;66:1044. doi: 10.1002/pros.20417. [DOI] [PubMed] [Google Scholar]
- 71.Thompson IM, Ankerst DP, Chi C, et al. Operating characteristics of prostate-specific antigen in men with an initial PSA level of 3.0 ng/ml or lower. JAMA. 2005;294:66. doi: 10.1001/jama.294.1.66. [DOI] [PubMed] [Google Scholar]
- 72.Welch HG, Schwartz LM, Woloshin S. Prostate-specific antigen levels in the United States: implications of various definitions for abnormal. J Natl Canc Inst. 2005;97:1132. doi: 10.1093/jnci/dji205. [DOI] [PubMed] [Google Scholar]
- 73.Stamey TA, Johnstone IM, McNeal JE, et al. Preoperative serum prostate specific antigen levels between 2 and 22 ng./ml. correlate poorly with post-radical prostatectomy cancer morphology: prostate specific antigen cure rates appear constant between 2 and 9 ng./ml. J Urol. 2002;167:103. doi: 10.1016/S0022-5347(05)65392-X. [DOI] [PubMed] [Google Scholar]
- 74.Djulbegovic M, Beyth RJ, Neuberger MM, et al. Screening for prostate cancer: systematic review and meta-analysis of randomised controlled trials. BMJ. 2010;341:c4543. doi: 10.1136/bmj.c4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.U.S. Preventive Services Task Force Screening for prostate cancer: recommendation and rationale. Ann Intern Med. 2002;137:915. doi: 10.7326/0003-4819-137-11-200212030-00013. [DOI] [PubMed] [Google Scholar]
- 76.(1997) Screening for prostate cancer. American College of Physicians. Ann Intern Med 126:480 [PubMed]
- 77.Greene KL, Albertsen PC, Babaian RJ, et al. Prostate specific antigen best practice statement: 2009 update. J Urol. 2009;182:2232. doi: 10.1016/j.juro.2009.07.093. [DOI] [PubMed] [Google Scholar]
- 78.Barry MJ. Health decision aids to facilitate shared decision making in office practice. Ann Intern Med. 2002;136:127. doi: 10.7326/0003-4819-136-2-200201150-00010. [DOI] [PubMed] [Google Scholar]
- 79.Hanlon AL, Diratzouian H, Hanks GE. Posttreatment prostate-specific antigen nadir highly predictive of distant failure and death from prostate cancer. Int J Radiat Oncol Biol Phys. 2002;53:297. doi: 10.1016/S0360-3016(02)02717-7. [DOI] [PubMed] [Google Scholar]
- 80.Perez CA, Michalski JM, Lockett MA. Chemical disease-free survival in localized carcinoma of prostate treated with external beam irradiation: comparison of American Society of Therapeutic Radiology and Oncology Consensus or 1 ng/mL as endpoint. Int J Radiat Oncol Biol Phys. 2001;49:1287. doi: 10.1016/S0360-3016(00)01492-9. [DOI] [PubMed] [Google Scholar]
- 81.Yock TI, Zietman AL, Shipley WU, et al. Long-term durability of PSA failure-free survival after radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2002;54:420. doi: 10.1016/S0360-3016(02)02957-7. [DOI] [PubMed] [Google Scholar]
- 82.Ray ME, Thames HD, Levy LB, et al. PSA nadir predicts biochemical and distant failures after external beam radiotherapy for prostate cancer: a multi-institutional analysis. Int J Radiat Oncol Biol Phys. 2006;64:1140. doi: 10.1016/j.ijrobp.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 83.(1997) Consensus statement: guidelines for PSA following radiation therapy. American Society for Therapeutic Radiology and Oncology Consensus Panel. Int J Radiat Oncol Biol Phys 37:1035 [PubMed]
- 84.Crook JM, Choan E, Perry GA, et al. Serum prostate-specific antigen profile following radiotherapy for prostate cancer: implications for patterns of failure and definition of cure. Urology. 1998;51:566. doi: 10.1016/S0090-4295(97)00650-X. [DOI] [PubMed] [Google Scholar]
- 85.Roach M, Hanks G, Thames H, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 86.Shipley WU, Thames HD, Sandler HM, et al. Radiation therapy for clinically localized prostate cancer: a multi-institutional pooled analysis. JAMA. 1999;281:1598. doi: 10.1001/jama.281.17.1598. [DOI] [PubMed] [Google Scholar]
- 87.Satoh T, Ishiyama H, Matsumoto K, et al. Prostate-specific antigen ‘bounce’ after permanent 125I-implant brachytherapy in Japanese men: a multi-institutional pooled analysis. BJU Int. 2009;103:1064. doi: 10.1111/j.1464-410X.2008.08234.x. [DOI] [PubMed] [Google Scholar]
- 88.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 89.Jhaveri FM, Zippe CD, Klein EA, Kupelian PA. Biochemical failure does not predict overall survival after radical prostatectomy for localized prostate cancer: 10-year results. Urology. 1999;54:884. doi: 10.1016/S0090-4295(99)00252-6. [DOI] [PubMed] [Google Scholar]
- 90.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 91.D'Amico AV, Moul J, Carroll PR, et al. Cancer-specific mortality after surgery or radiation for patients with clinically localized prostate cancer managed during the prostate-specific antigen era. J Clin Oncol. 2003;21:2163. doi: 10.1200/JCO.2003.01.075. [DOI] [PubMed] [Google Scholar]
- 92.Kupelian PA, Buchsbaum JC, Patel C, et al. Impact of biochemical failure on overall survival after radiation therapy for localized prostate cancer in the PSA era. Int J Radiat Oncol Biol Phys. 2002;52:704. doi: 10.1016/S0360-3016(01)02778-X. [DOI] [PubMed] [Google Scholar]
- 93.Partin AW, Pearson JD, Landis PK, et al. Evaluation of serum prostate-specific antigen velocity after radical prostatectomy to distinguish local recurrence from distant metastases. Urology. 1994;43:649. doi: 10.1016/0090-4295(94)90180-5. [DOI] [PubMed] [Google Scholar]