SUMMARY
Objectives
To evaluate whether augmenting neuronal protective mechanisms might slow or arrest experimental diabetic peripheral neuropathy (DPN). DPN is one of the most common neurodegenerative disorders and is rising in prevalence. How it targets sensory neurons is uncertain; the disorder is irreversible and untreatable. We explored the intrinsic protective properties of overexpressed human HSP27 on experimental DPN. HSP27 is a small pro-survival heat shock protein that also increases axonal regeneration.
Methods
Experimental diabetes was superimposed on mice overexpressing a human HSP27 transgene and its impact was evaluated on epidermal innervation, behavioral tests of sensation and electrophysiological indices of DPN.
Results
Mice that overexpress human HSP27 in their sensory and motor neurons and that were made diabetic for 6 months by streptozotocin treatment were protected from a range of neuropathic abnormalities, including loss of footpad thermal sensation, mechanical allodynia, loss of epidermal innervation, and slowing of sensory conduction velocity. The protection was selective for sensory neurons in comparison to motor neurons and at 6 months provided better protection in female than male mice. Markers of RAGE-NFκB activation were attenuated by the transgene.
Conclusions
The findings support the idea that diabetic polyneuropathy involves a unique, sensory-centric neurodegenerative process which can be reduced by overexpressing a single gene, an important starting point for new disease-modifying therapeutic approaches.
Keywords: Diabetic polyneuropathy, Heat shock protein 27, Peripheral nerve, Diabetes mellitus
Diabetic polyneuropathy (DPN) may be among the most common neurodegenerative disorders, involving approximately 50% of all persons with diabetes mellitus, now ranked at 6–10% of the global population and rising in prevalence (Wild et al., 2004; Yang et al., 2010). The mechanisms of DPN remain uncertain and the disorder is currently irreversible. Roles for excessive polyol flux, nerve microangiopathy, impaired growth factor availability and free radical stress, among others, have all been considered (Obrosova, 2008; Tomlinson, 2008; Zochodne, 2007). While controversial, a common thread among these ideas is that there is neuronal targeting by a unique and gradual neurodegenerative cascade that involves sensory neurons early. If correct, an approach that augments intrinsic survival pathways would prevent the range of abnormalities that develop during neuropathy in the peripheral nervous system.
Heat shock proteins (HSPs) are candidates for protecting sensory neurons from neurotoxic insults. As inducible molecular chaperones, they assist in the proper folding and assembly of nascent polypeptides and help to target abnormal proteins for lysosomal degradation (Ohtsuka and Suzuki, 2000). HSPs provide constitutive protection to peripheral neurons, since HSP27 gene mutations, for example, are associated with inherited forms of polyneuropathy (Houlden et al., 2008). An association between the presence of polyneuropathy and circulating HSP27 has also been identified in a large cohort of human subjects with type 1 diabetes mellitus (Gruden et al., 2008). HSP27 expression promotes survival of adult sensory and motor neurons after axonal injury, and its expression is elevated in sensory neurons of diabetic rodent models (Benn et al., 2002; Zochodne et al., 2001). HSP27 (or its mouse analog, HSP25) knockdown or overexpression are respectively associated with attenuated or improved axon regeneration in adult sensory neurons (Lewis et al., 1999; Ma et al., in press).
We hypothesized that overexpression of a human transgene of HSP27 in mice rendered Type 1 diabetic with streptozotocin (STZ) might protect peripheral neurons from progressive degeneration. The findings identified an interesting form of apparent selective sensory neuron protection over a range of behavioral, electrophysiological, structural and molecular neuropathic abnormalities.
Methods
Human HSP27 transgenic mice and breeding
Mice overexpressing hHSP27 were generated in the Woolf lab by microinjecting the prepared transgenic construct into fertilized B6C3F1 mouse oocytes which were subsequently bred with C57BL/6 mice to establish the transgenic line as recently described with overexpression in both motor and sensory neurons (Ma et al., in press). In this transgenic line hHSP27 is overexpressed under a neuronal specific promoter (Thy1.2). hHSP27 overexpressing mice were transferred to the University of Calgary for breeding and diabetes induction. Heterozygous hHSP27 male mice were bred with C57B6J/L female mice.
The protocol was reviewed and approved by the Animal Care Committee, University of Calgary. All groups studied included male and female mice to render gender balance among the study groups.
Prior to assignment of mice to experimental groups at the University of Calgary, mice underwent genotyping to confirm expression of the transgene. For genotyping DNA extraction was performed on ear notch samples using quick gDNA extraction buffer (Sakurai et al., 2008) adding 300 μl of buffer mix to each sample and incubating at 56 °C for 2 hrs. After tissues were dissolved they were incubated at 95–98 °C for 15 min to inactivate the proteinase K enzyme. The samples were spun at 12000 rpm and 200 μl of supernatant was collected. 3 μl of this was used as template for the PCR using standard protocol for ABI7000 sequence amplifier and SybrGreen as ampliication marker. The standard dissociation protocol shows clear single peak of product amplification for the each duplicate sample containing the transgene. An internal control gene was used to test gDNA quality. The sequence of primers was follows:
hHsp27 F5 5′-GTCCCTGGATGTCAACCACT-3′
Thy1.2 R4 5′-GGGTTCCTTAGGAGGGACAA-3′
TRCD F 5′-CAAATGTTGCTTGTCTGGTG-3′
TRCD R 5′-GTCAGTCGAGTGCACAGTTT-3′
While not quantitated, we confirmed endpoint hHSP27 overexpression at the 6 month endpoint (data not shown) by immunohistochemistry. While protein–protein interactions of the overexpressed transgene may have changed over the 6 month life span of these mice, this work and previous studies have not noted loss of overexpression (Ma et al., in press). hHSP27 overexpressing transgenic mice and littermate mice received intraperitoneal injections of STZ with citrate buffer vehicle or citrate buffer alone at 2 months of age as previously described. Three consecutive daily doses of 55, 70 and 85 mg/kg STZ in citrate buffer vehicle or citrate buffer vehicle alone (pH=4.8) were administered. Subsequent fasting tail blood samples were obtained to determine diabetic status. Diabetic status was defined as blood glucose greater than 16.0 mmol/L following an 8-hr fast. STZ injected mice not demonstrating diabetic range fasting glucose measurements were excluded from the study. Fasting blood glucose was re-assessed at 3 and 6 months following induction of diabetes to confirm persistence of diabetes. Glycated hemoglobin was measured at time of harvesting. Glycated hemoglobin testing was performed at Calgary Laboratory Services (Calgary, AB).
Four groups of mice were established through genotyping for presence of the hHSP27 transgene and presence of diabetes: 1. Littermate control group (no transgene, no diabetes); 2. Littermate diabetic group (no transgene, diabetes present); 3. Transgenic control group (transgene present, no diabetes); 4. Transgenic diabetic group (transgene present, diabetes present). Results were given in gender balanced (similar numbers of males and females) in each experimental group. Later subgroup analysis was conducted to compare the impact of gender on some of the endpoints (Supplemental data).
Electrophysiology, behavioral testing
Electrophysiology was performed in mice anesthetized with isofluorane (99.9%; Halocarbon Products Corporation, River Edge, New Jersey) as previously described (Toth et al., 2008) with near nerve temperature at 37.0 °C. Thermal algesia was tested by hindpaw withdrawal latencies to a light heat source (Hargreaves apparatus) and mechanical allodynia by esthesiometer testing (force threshold to induce hindpaw withdrawal) (Calcutt, 2004; Toth et al., 2010; Truong et al., 2003). For mechanical sensitivity, 12 stimulations were averaged for each of the 3 and 6 month timepoints consisting of 2 tests daily per hindpaw for three days. For thermal sensitivity, 6 stimulations were averaged at each of the 3 and 6 month timepoints that consisted of one test per hindpaw daily for three days. Single averaged values for both mechanical and thermal sensitivity at each time point were used for each mouse in subsequent statistical analysis.
Footpad immunohistochemistry
Bilateral hind footpad samples were excised at the time of harvesting. Samples were placed in 2% PLP (paraformaldehyde, lysine and periodate) fixative as previously described. Samples were cryoprotected overnight in 20% glycerol/20% 0.4 M Sorrenson buffer at 4 °C. Samples were then cut in series at 25 μM intervals with a freezing microtome (Polydefkis et al., 2004). Samples were then washed with PBS (1×), 1% Triton × 100/1× PBS then 10% Goat Serum. Samples were stained with PGP9.5, 1:1000 (Rabbit polyclonal); (Encor Biotechnology Inc., Gainesville, FL), washed with PBS 1× then stained with CY3:1:100 (Goat anti-rabbit); (Thermo Fisher Scientific Inc., Rockford, IL). Images were captured using an Olympus laser scanning confocal microscope equipped with Epifluorescence at 100× magnification 512×512 resolution with a scanning step size of 1 μm. For each mouse, six footpad sections were made and 5 adjacent fields examined for each section. Mean counts were arrived at from 30 individual images/mouse. Areas of the epidermis analyzed were measured (mm2) for each field using Image J software and axon densities calculated.
Dorsal root ganglion immunohistochemistry
Slides were rinsed 3× with PBS for 5 min. Blocking was performed with 1% Normal goat serum, 0.3% Triton-X in PBS for 30 min at room temperature. Antibodies applied were: NFκb p-50 (H-119), rabbit polyclonal Ig/G from Santa Cruz Biotechnology at the dilution of 1:200, RAGE (ab3611) rabbit polyclonal to RAGE from Abcam (Dilution 1:800), activated Caspase-3 (1:100; Anti-cleaved Caspase-3 Rabbit Polyclonal IgG; Trevigen Inc., Gaithersburg, MD) with Alexa Flour 488 Goat anti-rabbit IgG as secondary antibody from Invitrogen (secondary antibody 1:200). Antibodies were applied and left overnight. In the morning, slides were rinsed 3× with PBS for 5 min. Secondary antibody was applied at 1:200 (Alexa Flour 488 Goat anti-rabbit IgG (Invitrogen, Carlsbad, CA)) and then rinsed with 3× PBS for 5 min.
Image analysis was performed using Adobe Photoshop CS4 (Adobe Systems Incorporated, San Jose, CA). To better characterize direct neuronal expression we divided neurons into categories of arbitrary intensity levels: >70 (arbitrary units) were considered positive, medium (70–100), strong (100–150) and very strong (>150). We counted the total number of neurons, number of positive or negative neurons, number of neurons with cytoplasmic staining, number of neurons with nuclear staining. For counting, each mouse had L4 or L5 lumbar ganglia sectioned and counting included 8–12 individual sections (analyzing approximate 250–450 neurons/mouse). This analysis was limited to control nontransgenic, diabetic nontransgenic and diabetic transgenic groups only.
Analysis
Values were calculated as mean ± sem (standard error of mean). One-way analysis of variance (ANOVA) was used to compare mean values between groups with a priori pairwise comparisons between all groups using Tukey’s HSD method.
Results
Diabetic mice overexpressing hHSP27
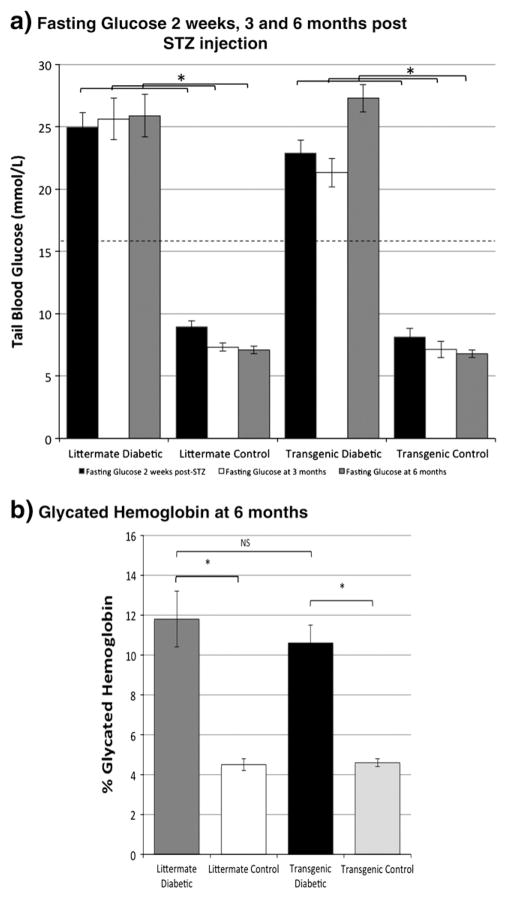
To render a group of mice with sufficient exposure to diabetes to produce peripheral diabetic neuropathy, we chose the STZ type 1 model over a prolonged 6 month period, an exposure designed to mimic long term human diabetes. We compared hHSP27 transgenic non-diabetic and diabetic mice with littermate non-diabetic and diabetic mice (four groups) and all had a similar balance of male and females in all of the measurements reported below. Diabetic mice maintained a fasting glucose level of >16.0 mmol/L and had elevated glycated haemoglobin (Fig. 1). Transgenic and littermate diabetic models had hyperglycaemic exposure of comparable severity but transgenics had a lower mortality rate (Supplemental Fig. 1).
Fig. 1.
a. Fasting glucose was elevated in diabetics at 2 weeks, 3 months and 6 months after injection of STZ in both littermate and transgenics and normal in control group mice. The difference in group mean fasting glucose between diabetic and control groups was significant at all timepoints (*p<.01; 1-way ANOVA with post-hoc Tukey HSD test; littermate diabetic mean 6 month glucose 25.9±1.7 mmol/L (2 week, 3 and 6 month n =22,22,14 respectively); littermate control 7.1± 0.3 mmol/L (n =22,22,21); transgenic diabetic 27.3±1.1 mmol/L (n =26,26,20); transgenic control 6.8±0.3 mmol/L (n =16,16,14)) whereas no significant difference between transgenic and littermate diabetic groups was observed at any timepoint (dotted line-cutoff for definition of diabetes; ± sem). b. Mean percent glycated hemoglobin was significantly higher in diabetic groups than respective control groups (*p<.01; 1-way ANOVA with post-hoc Tukey HSD test; n =6 littermate diabetic, n =4 littermate control; n =6 transgenic diabetic; n =5 transgenic control). No significant difference in mean percent glycated hemoglobin was observed between lit-termate and transgenic diabetic groups (p =NS; 1-way ANOVA).
Preservation of epidermal innervation in diabetic mice overexpressing hHSP27
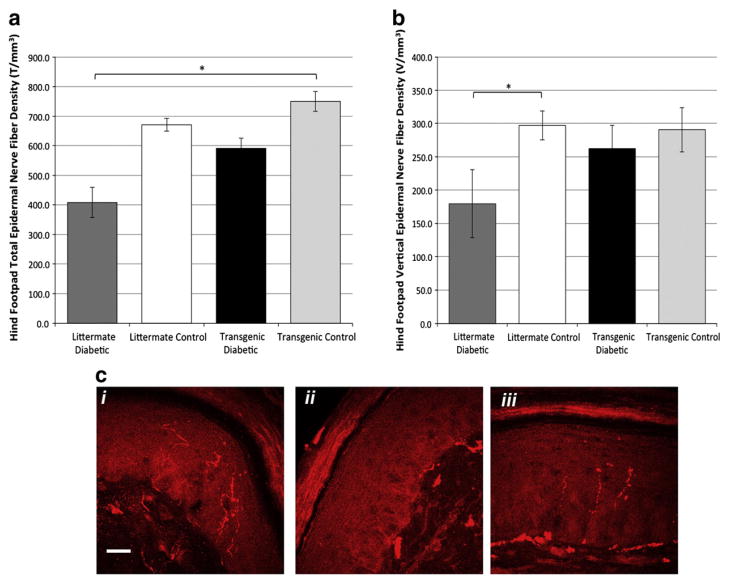
Diabetes in humans and mice produces sensory loss that is associated with retraction of distal axon terminals and loss of C-fiber epidermal axon innervation (Kennedy and Zochodne, 2005; Kennedy et al., 1996). To test the influence of the hHSP27 transgene on this feature of diabetic sensory damage, we analyzed hindpaw footpad intraepidermal axon density at 6 months. Littermate diabetic mice had intraepidermal axonal terminal densities that were significantly reduced in comparison to nondiabetic mice, as expected. However, in transgenic diabetic mice, densities at 6 months were preserved and were not significantly different from those of the control groups (Fig. 2). In nondiabetic mice, the presence of the hHSP27 transgene did not alter epidermal axon density relative to littermate controls. A separate analysis considering vertically directed axons identified similar trends, but differences were only borderline significant.
Fig. 2.
Hind foodpad epidermal nerve fiber density (NFD) was examined at 6 months of diabetes. The littermate diabetic group had significantly reduced NFD compared to the lit-termate and transgenic control groups (a; *p=.016; 1-way ANOVA and post-hoc Tukey HSD test; n=4 mice/group). The transgenic diabetic group was not significantly different from the other groups. A separate analysis of vertical axon only epidermal density is given that show similar trends, only borderline significant (b; 1-way ANOVA among the groups NS; *p=0.04; one tailed student’s t-test littermate control vs. diabetic). Examples of footpad sections of mice (c) stained with PGP 9.5 (red) to label axons. i—nondiabetic littermate; ii—diabetic littermate; iii—transgenic diabetic mouse (nondiabetic transgenic not shown) [Bar=20 microns].
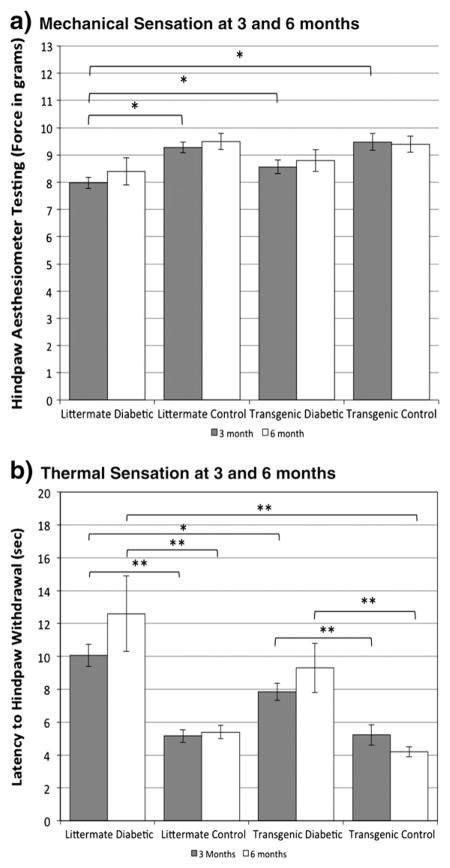
hHSP27 overexpression attenuates loss of thermal sensation and mechanical allodynia
To evaluate whether hHSP27 overexpression impacted behavioral measures of pain sensation in diabetic mice, we evaluated thermal and mechanical sensitivity in our gender-equalized long term diabetic cohorts (Calcutt, 2004; Francis et al., 2009). Littermate diabetic mice at 3 months had negative symptoms reflecting impaired sensation, with significantly reduced (2-fold) thermal sensitivity, which deteriorated further at 6 months (2.6 fold) (Fig. 3a). In the hHSP27 transgenic mice the relative impairment was less at 3 months (1.6 fold). Littermate diabetic mice at 3 months also had positive symptoms reflecting pain hypersensitivity, showing mechanical allodynia, withdrawal elicited by stimulation with a lower mechanical force (Fig. 3b). In contrast, the transgenic hHSP27 diabetic mice at this time had a mechanical threshold that was not statistically different from non-diabetic controls. Following 6 months of diabetes, there was ongoing mechanical allodynia in littermate diabetic mice, with a trend toward partial protection in transgenic hHSP27 mice. The difference between littermate diabetic and transgenic diabetic was significant when female mice were considered separately (Supplemental Fig. 2). Similarly, reduced thermal sensation was still present in littermate diabetic mice at 6 months, and its preservation in transgenic hHSP27 mice at this time was also accounted for by greater protection in female mice (Supplemental Fig. 3).
Fig. 3.
a. Hindpaw mechanical sensation was measured at 3 and 6 months using an esthesiometer. Significantly increased mechanical sensation (lower latency to withdrawal; mechanical allodynia) was observed in the littermate diabetic group in comparison to the other three groups (*p<.01; 1-way ANOVA with post-hoc Tukey test; n=26, 12 littermate diabetic at 3 and 6 months respectively; n=24,18 littermate control; n=16,12 transgenic diabetic; n=10,10 transgenic control; gender equal groups). The transgenic diabetic group was not significantly different from transgenic and litter-mate control groups. b. Thermal sensation was measured at 3 and 6 months using a light heat source. Thermal sensation was significantly reduced (thermal hypoalgesia) in littermate and transgenic diabetic groups compared to littermate and transgenic control groups respectively (p<.01 (**); 1-way ANOVA with post-hoc Tukey test; n=20, 12 littermate diabetic at 3 and 6 months; n=24,18 littermate control; n=16, 14, transgenic diabetic; n=12, 12 transgenic control). The littermate diabetic group demonstrated significantly more loss of thermal sensation compared to the transgenic diabetic group (p<.05 (*); 1-way ANOVA with post-hoc Tukey HSD test).
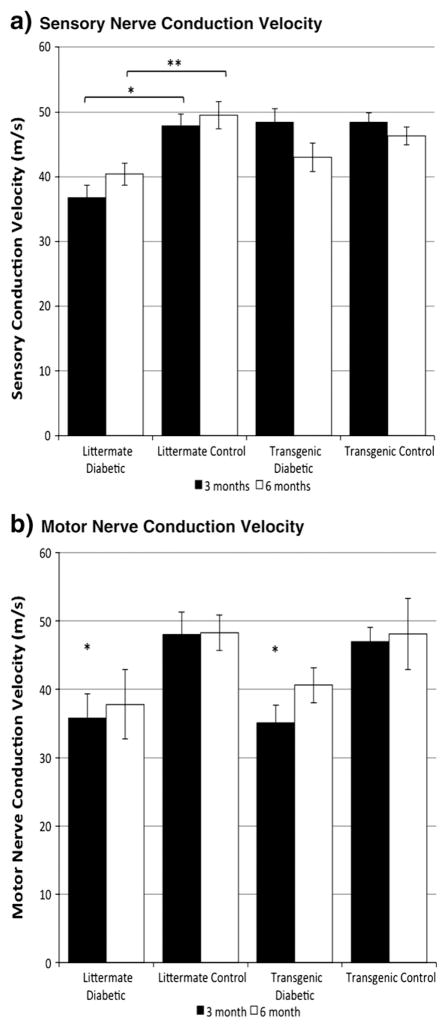
hHSP27 selectively protects sensory conduction
To evaluate whether hHSP27 alters the cardinal electrophysiological abnormalities of myelinated axons in diabetes, we carried out serial multifiber sensory and motor recordings. At 3 months of diabetes duration there were expected changes indicative of neuropathy as evidenced by slowing of sensory and motor myelinated fiber conduction velocities in nontransgenic diabetic littermate controls (Figs. 4a, b). However diabetic mice with the hHSP27 transgene did not develop sensory conduction slowing at 3 months and results were comparable to nondiabetic control values. In contrast, motor conduction velocities were slowed in these diabetic transgenic mice, comparable to diabetic littermate values. After 6 months of diabetes transgenic mice continued to exhibit relative protection from sensory electrophysiological abnormalities comparable to nondiabetic controls but it was less robust.
Fig. 4.
a. Mean sciatic-tibial sensory nerve conduction velocities were significantly slower in the littermate diabetic group than in the littermate control group at 3 and 6 months (p<.01 (*) and pb.05 (**) respectively; 1-way ANOVA with post-hoc Tukey HSD test; n=10,12 littermate diabetic at 3 and 6 months respectively; n=14,10 litter-mate control; n=15,10 transgenic diabetic; n=10,10 transgenic control) while no significant difference was observed between transgenic diabetic and nondiabetic groups. b. Mean sciatic motor nerve conduction velocity was significantly slowed in the litter-mate diabetic group compared to the littermate control group, and in the transgenic diabetic group compared to the transgenic control group at 3 months (*p<.05; 1-way ANOVA with post-hoc Tukey HSD test; n=12 littermate diabetic; n=14 littermate control; n=16 transgenic diabetic; n=10 transgenic control). At 6 months there was no difference between the four groups (p=NS; 1-way ANOVA; n=12 littermate diabetic; n=12 littermate control; n=10 transgenic diabetic; n=10 transgenic control).
hHSP27 partly protects sensory neurons from RAGE and NF-κB neuron upregulation during diabetes
Activation of AGE-RAGE signalling in neurons is thought to contribute toward neurodegeneration in diabetes. RAGE was expressed both within the cytoplasm and nucleus of DRG sensory neurons. Diabetes, as expected (Toth et al., 2008), was associated with expression in approximately 90% of neurons from nontransgenic mice, with a corresponding 10% proportion of neurons that failed to express it (RAGE negative). In contrast, in nondiabetic mice, a larger proportion of neurons did not express RAGE. In the transgenic diabetic mice, a larger proportion of sensory neurons were RAGE negative than in wildtype diabetic littermates (Figs. 5a, 6). Activation of NF-κB, a pro-inflammatory transcription factor, is triggered by expression of RAGE in the setting of diabetes (Bierhaus et al., 2001). We confirmed upregulation of sensory neuron specific protein expression of NF-κB in several ways: a much larger percentage of neurons with expression in the highest intensity luminosity category, a small percentage of negative neurons and a much larger proportion of neuronal nuclei that expressed NF-κB (Figs. 5b–d, 6). In transgenic mice, all of these indicators were attenuated, with values very similar to control nondiabetic levels. Activated caspase-3 was expressed in the cytoplasm and nucleus of sensory neurons. Cytoplasmic expression did not differ among the groups (Figs. 5e, 6). In contrast, nuclear expression, traditionally linked to apoptosis of neurons, but instead a marker of cytoxic stress in chronic diabetes, was upregulated in diabetic mice with partial attenuation in transgenic mice overexpressing hHSP27 (Figs. 5f, 6). Taken together, the markers studied in this analysis did support partial protection by the transgene. However the changes were relatively modest and likely indicate that additional mechanisms, not analyzed, were impacted by hHSP27.
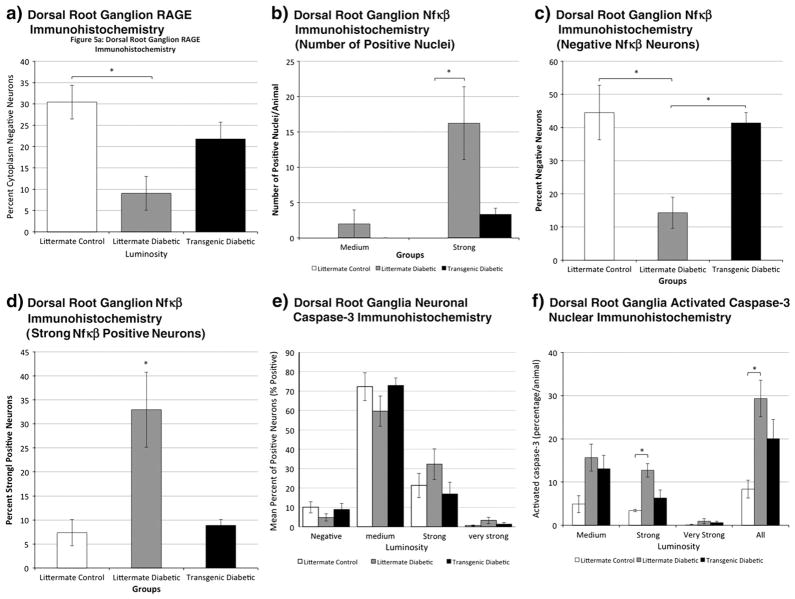
Fig. 5.
Analysis of dorsal root ganglion neuronal immunohistochemistry at 6 months of diabetes for control nontransgenic (littermate), diabetic nontransgenic (littermate) and diabetic transgenic mice. Luminosity intensity in neurons considered to have positive expression was further into medium, strong and very strong categories using arbitrary but standardized criteria (see methods). a. RAGE immunohistochemistry at 6 months of diabetes. Significant relative protection in the transgenic diabetic group against cytoplasmic expression within DRG neurons in comparison to littermate diabetics was observed (p<.05 (*); 1-way ANOVA with post-hoc Tukey HSD test; n=5 littermate control, n=5 littermate diabetic, n=4 transgenic diabetic). b. Nuclear NF-κB immunohistochemistry indicated relative protection in the transgenic diabetic group against strong nuclear expression in comparison to littermate diabetics (p<.05 (*); 1-way ANOVA with post-hoc Tukey HSD test; n=3 littermate control; n=4 littermate diabetic; n=3 transgenic diabetic). c. NF-κB immunohistochemistry also indicated relative protection in the transgenic diabetic group against neuronal NF-κB expression in comparison to littermate diabetics observed by analyzing the percentage of negative neurons (p<.05 (*); 1-way ANOVA with post-hoc Tukey HSD test; n=3 littermate control; n=4 littermate diabetic; n=3 transgenic diabetic). d. Overall high intensity (strong positive) NF-κB immunohistochemistry indicated that littermate diabetic mice had a substantial proportion of neurons with measurements of fluorescence intensity in the ‘strong’ range whereas transgenic littermates had results comparable to nondiabetic controls (p<.05 (*); 1-way ANOVA with post-hoc Tukey HSD test; n=3 littermate control; n=4 littermate diabetic; n=3 transgenic diabetic). e. Neuronal activated caspase-3 cytoplasmic expression by percentage of positive neurons was not significantly different among the groups (n=3 littermate control; n=5 littermate diabetic; n=3 transgenic diabetic). f. Nuclear activated caspase-3 immunohistochemistry at 6 months of diabetes indicated that diabetic mice had a higher percentage of labelled nuclei than nondiabetics (p=0.018 (*); ANOVA with post-hoc Tukey; n=3 littermate control; n=5 littermate diabetic; n=3 transgenic diabetic). There was blunting of its expression in transgenic diabetic mice, with expression values not significantly different than controls.
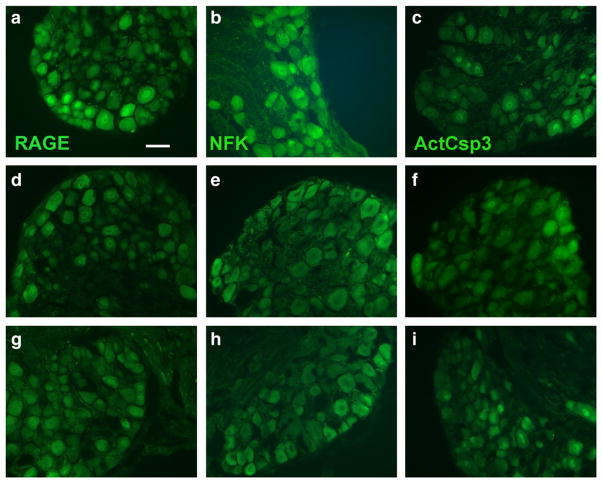
Fig. 6.
Dorsal root ganglion neuronal caspase-3 immunohistochemistry at 6 months of diabetes. Examples of dorsal root ganglion sections immunostained for RAGE (a, d, g), NFκ B (b, e, h) and activated caspase-3 (c, f, i). The top panels (a–c) are from diabetic littermates, the middle panels (d-f) from control nondiabetic littermates and the lower panels (g–i) from transgenic diabetic mice. Note that each of the markers has more prominent staining in diabetic littermates [Bar=50 μM].
Discussion
We believe that several novel insights into the pathogenesis of diabetic polyneuropathy emerged from this work. The major findings were (i) a human HSP27 transgene prevented retraction of epidermal sensory axons in the skin, a key mechanism of sensory loss in diabetes; (ii) the transgene similarly blunted early mechanical allodynia and loss of thermal sensitivity; protection was significant but not complete; (iii) early electrophysiological abnormalities expected of diabetes were prevented in sensory, but not motor axons; (iv) the protection accompanied blunting of recently identified molecular mechanisms of neuronal dysfunction in diabetes. Overall these findings indicate a selective slowing of neurodegeneration rather than complete protection. The analysis did not include autonomic neurons, also well recognized targets of diabetes.
Despite these caveats, the most impressive result is the finding that overexpression of a single gene, hHSP27, has a widespread impact on many of the key pathological abnormalities that define diabetic polyneuropathy. While the full impact of overexpression of hHSP27 on a wide range of protein expression and activity is not known, in nondiabetic mice the transgene did not alter essential machinery responsible for peripheral nerve physiology or behavior. Nondiabetic wild type mice and transgenic mice did not have differing phenotypes. However, it is likely that there was an important and differing interaction between hHSP27 and altered neuronal properties in diabetes, accounting for its protection. Altered sensation with retraction and damage involving C-fiber epidermal sensory axons in the skin is a critical feature of DPN in both models and humans (Kennedy and Zochodne, 2005; Kennedy et al., 1996; Toth et al., 2008). We suspect that the reduction in thermal sensitivity in diabetic littermates is a functional correlate of the reduced epidermal innervation, and that the protection of the innervation of the skin by hHSP27 may be responsible for retention of greater thermal sensitivity in diabetic transgenic mice.
Hypotheses that a “dying-back” of nerve terminals develops from an isolated targeting only of distal sensory and motor axons with ischemia or radical stress have not proven to be sufficient to explain this disorder. Evidence has instead been mounted to implicate concurrent derangement of perikaryal function and expression of genes that support distal axonal physiologic integrity (Zochodne, 2007; Zochodne et al., 2008). It is remarkable that the hHSP27 transgene reduced the vulnerability of sensory axons remote from their perikarya. Surprisingly however, slowing of motor conduction velocity was not prevented in diabetic mice expressing the hHSP27 transgene. While experimental and human diabetes does spare motor neurons from axon or neuron loss, conduction slowing has been a constant feature in all experimental diabetes models, and was seen in the present model (Obrosova, 2008; Ramji et al., 2007; Sima et al., 2000; Tomlinson, 2008). The findings are not explained by a difference in transgene expression of hHSP27 between motor and sensory neurons (Ma et al., in press). Similarly it is less likely that the impact on conduction velocity altered myelination, since myelin changes have been small to absent in many diabetic models including that studied here. It is more likely that selective improvement in sensory conduction velocity was related to a change in sensory axon structure or excitability. The data suggest that the mechanisms for motor axon deficits in the disease or of the actions of hHSP27 differ from those of sensory neurons, in keeping with prior work indicating differential susceptibility of these peripheral neurons to diabetes (Ramji et al., 2007).
In patients, loss of sensitivity to thermal stimuli is accompanied by appearance of spontaneous burning pain, presumably reflecting development of ectopic firing in terminally atrophic C-fiber afferents. The mechanism responsible for the mechanical allodynia in diabetic mice is uncertain but may reflect a secondary consequence to loss of C-fiber terminals, such as induction of central sensitization by spontaneously firing C-fibers and perhaps an indirect measure therefore of spontaneous activity in C-fibers and the pain it causes. In any case we find that protection of C-fiber terminals in the skin by hHSP27 ameliorated both the negative symptoms (thermal hyposensitivity) and positive symptoms (mechanical allodynia) that are features of the STZ diabetic model in mice.
Preliminary evidence suggests hHSP27 overexpression may support diabetic neurons by attenuating caspase-3, RAGE and NFκB signaling (Toth et al., 2008). HSP27 has been also demonstrated through protein–protein interaction to activate Akt which is deficient in sensory dorsal root ganglia in diabetics with diabetic polyneuropathy (Rane et al., 2003) (Havasi et al., 2008). HSP27 also promotes actin polymerization in a phosphorylation-dependent fashion and is distributed to the tips of filopodia in growth cones, where it accelerates axon regeneration. An additional, an unexpected finding was that after 6 months of diabetes, apparent protection by the transgene was less robust in male than female mice. This finding was observed in the setting of overall protection that was significant but was incomplete. While not explored in human and experimental diabetes the specific role of gender is a key topic for future work and some findings suggest an impact of estrogens on HSP27 action (Lu et al., 2002; Rayner et al., 2009).
Prior work has demonstrated that direct effects of HSP27 on components of cellular apoptosis may result in inhibition of cell death signalling through interaction with cytochrome C and pro-caspase 3 (Bruey et al., 2000). While early and extensive apoptosis is probably not a feature of diabetic neuropathy, markers of apoptosis are indeed expressed in models of DPN and indicate an ongoing level of ‘neurotoxic stress’ (Cheng and Zochodne, 2003; Kamiya et al., 2005; Kennedy and Zochodne, 2005; Russell et al., 1999; Zochodne et al., 2001). This is compatible with the upregulation of nuclear activated caspase-3 we identified. Increased expression of RAGE in sensory diabetic neurons is linked to the development of neuropathic abnormalities in diabetes. For example, RAGE null mice have attenuated features of DPN (Toth et al., 2008). Furthermore, NF-κB is activated in diabetes through increased RAGE expression and signaling resulting in translocation of NF-κB into the nucleus followed by DNA binding and transcriptional activation (Barnes and Karin, 1997; Bierhaus et al., 2001, 2005; Cameron and Cotter, 2008). We noted downregulated expression of both RAGE and especially NFκB expression in diabetic mice with the hHSP27 transgene. These results suggest that transgene protection may be upstream of their activation and that their response to diabetes is secondary to a primary HSP27 sensitive diabetic alteration in cellular function.
Conclusions
We show that in a mouse model of diabetes mellitus, overexpression of a single protein, human HSP27, important for the integrity of several neuronal intracellular pathways, delays and prevents development of key manifestations of sensory polyneuropathy. In addition to providing insight into the development of diabetic neuropathy, specific pharmacologic induction of HSP27 for treatment and prevention may warrant investigation, particularly since there are no other disease-modifying interventions for this common neurodegenerative disorder.
Supplementary Material
Acknowledgments
Work in the laboratory of CJW was supported by NIH (National Institutes of Health) NS038253. Work in the laboratory of DWZ was supported by CIHR (Canadian Institutes of Health Research) 184584 and CDA (Canadian Diabetes Association). LK was supported as a clinical fellow by AHFMR (Alberta Heritage Foundation for Medical Research) and DWZ has been supported by AHFMR as a scientist.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nbd.2012.04.017.
References
- Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- Benn SC, Perrelet D, Kato AC, et al. Hsp27 upregulation and phosphorylation is required for injured sensory and motor neuron survival. Neuron. 2002;36:45–56. doi: 10.1016/s0896-6273(02)00941-8. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Schiekofer S, Schwaninger M, et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes. 2001;50:2792–2808. doi: 10.2337/diabetes.50.12.2792. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Humpert PM, Morcos M, et al. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med. 2005;83:876–886. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- Bruey JM, Ducasse C, Bonniaud P, et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000;2:645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- Calcutt NA. Modeling diabetic sensory neuropathy in rats. Methods Mol Med. 2004;99:55–65. doi: 10.1385/1-59259-770-x:225. [DOI] [PubMed] [Google Scholar]
- Cameron NE, Cotter MA. Pro-inflammatory mechanisms in diabetic neuropathy: focus on the nuclear factor kappa B pathway. Curr Drug Targets. 2008;9:60–67. doi: 10.2174/138945008783431718. [DOI] [PubMed] [Google Scholar]
- Cheng C, Zochodne DW. Sensory neurons with activated caspase-3 survive long-term experimental diabetes. Diabetes. 2003;52:2363–2371. doi: 10.2337/diabetes.52.9.2363. [DOI] [PubMed] [Google Scholar]
- Francis G, Martinez J, Liu W, et al. Intranasal insulin ameliorates experimental diabetic neuropathy. Diabetes. 2009;58:934–945. doi: 10.2337/db08-1287. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gruden G, Bruno G, Chaturvedi N, et al. Serum heat shock protein 27 and diabetes complications in the EURODIAB prospective complications study: a novel circulating marker for diabetic neuropathy. Diabetes. 2008;57:1966–1970. doi: 10.2337/db08-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havasi A, Li Z, Wang Z, et al. Hsp27 inhibits Bax activation and apoptosis via a phosphatidylinositol 3-kinase-dependent mechanism. J Biol Chem. 2008;283:12305–12313. doi: 10.1074/jbc.M801291200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlden H, Laura M, Wavrant-De VF, et al. Mutations in the HSP27 (HSPB1) gene cause dominant, recessive, and sporadic distal HMN/CMT type 2. Neurology. 2008;71:1660–1668. doi: 10.1212/01.wnl.0000319696.14225.67. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Zhangm W, Sima AA. Apoptotic stress is counterbalanced by survival elements preventing programmed cell death of dorsal root ganglions in subacute type 1 diabetic BB/Wor rats. Diabetes. 2005;54:3288–3295. doi: 10.2337/diabetes.54.11.3288. [DOI] [PubMed] [Google Scholar]
- Kennedy JM, Zochodne DW. Experimental diabetic neuropathy and spontaneous recovery: Is there irreparable damage? Diabetes. 2005;54:830–837. doi: 10.2337/diabetes.54.3.830. [DOI] [PubMed] [Google Scholar]
- Kennedy WR, Wendelschafer-Crabb G, Johnson T. Quantitation of epidermal nerves in diabetic neuropathy. Neurology. 1996;47:1042–1048. doi: 10.1212/wnl.47.4.1042. [DOI] [PubMed] [Google Scholar]
- Lewis SE, Mannion RJ, White FA, et al. A role for HSP27 in sensory neuron survival. J Neurosci. 1999;19:8945–8953. doi: 10.1523/JNEUROSCI.19-20-08945.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A, Ran RQ, Clark J, et al. 17-beta-estradiol induces heat shock proteins in brain arteries and potentiates ischemic heat shock protein induction in glia and neurons. J Cereb Blood Flow Metab. 2002;22:183–195. doi: 10.1097/00004647-200202000-00006. [DOI] [PubMed] [Google Scholar]
- Ma CHE, Omura T, Cobos EJ, et al. Accelerating axonal growth promotes motor recovery after peripheral nerve injury in mice. J Clin Invest. doi: 10.1172/JCI58675. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrosova IG. Diabetes and the peripheral nerve. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbadis.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Ohtsuka K, Suzuki T. Roles of molecular chaperones in the nervous system. Brain Res Bull. 2000;53:141–146. doi: 10.1016/s0361-9230(00)00325-7. [DOI] [PubMed] [Google Scholar]
- Polydefkis M, Hauer P, Sheth S, et al. The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain. 2004;127:1606–1615. doi: 10.1093/brain/awh175. [DOI] [PubMed] [Google Scholar]
- Ramji N, Toth C, Kennedy J, et al. Does diabetes mellitus target motor neurons? Neurobiol Dis. 2007;26:301–311. doi: 10.1016/j.nbd.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Rane MJ, Pan Y, Singh S, et al. Heat shock protein 27 controls apoptosis by regulating Akt activation. J Biol Chem. 2003;278:27828–27835. doi: 10.1074/jbc.M303417200. [DOI] [PubMed] [Google Scholar]
- Rayner K, Sun J, Chen YX, et al. Heat shock protein 27 protects against athero-genesis via an estrogen-dependent mechanism: role of selective estrogen receptor beta modulation. Arterioscler Thromb Vasc Biol. 2009;29:1751–1756. doi: 10.1161/ATVBAHA.109.193656. [DOI] [PubMed] [Google Scholar]
- Russell JW, Sullivan KA, Windebank AJ, et al. Neurons undergo apoptosis in animal and cell culture models of diabetes. Neurobiol Dis. 1999;6:347–363. doi: 10.1006/nbdi.1999.0254. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Kamiyoshi A, Watanabe S, et al. Rapid zygosity determination in mice by SYBR Green real-time genomic PCR of a crude DNA solution. Transgenic Res. 2008;17:149–155. doi: 10.1007/s11248-007-9134-7. [DOI] [PubMed] [Google Scholar]
- Sima AA, Zhang W, Xu G, et al. A comparison of diabetic polyneuropathy in type II diabetic BBZDR/Wor rats and in type I diabetic BB/Wor rats. Diabetologia. 2000;43:786–793. doi: 10.1007/s001250051376. [DOI] [PubMed] [Google Scholar]
- Tomlinson DRGNJ. Diabetic neuropathies: components of etiology. J Peripher Nerv Syst. 2008;13:112–121. doi: 10.1111/j.1529-8027.2008.00167.x. [DOI] [PubMed] [Google Scholar]
- Toth C, Rong LL, Yang C, et al. RAGE and experimental diabetic neuropathy. Diabetes. 2008;57:1002–1017. doi: 10.2337/db07-0339. [DOI] [PubMed] [Google Scholar]
- Toth CC, Jedrzejewski NM, Ellis CL, et al. Cannabinoid-mediated modulation of neuropathic pain and microglial accumulation in a model of murine type I diabetic peripheral neuropathic pain. Mol Pain. 2010;6:16. doi: 10.1186/1744-8069-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong W, Cheng C, Xu QG, et al. Mu opioid receptors and analgesia at the site of a peripheral nerve injury. Ann Neurol. 2003;53:366–375. doi: 10.1002/ana.10465. [DOI] [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- Zochodne DW. Diabetes mellitus and the peripheral nervous system: manifestations and mechanisms. Muscle Nerve. 2007;36:144–166. doi: 10.1002/mus.20785. [DOI] [PubMed] [Google Scholar]
- Zochodne DW, Verge VMK, Cheng C, et al. Does diabetes target ganglion neurons? Progressive sensory neuron involvement in long term experimental diabetes. Brain. 2001;124:2319–2334. doi: 10.1093/brain/124.11.2319. [DOI] [PubMed] [Google Scholar]
- Zochodne DW, Ramji N, Toth C. Neuronal targeting in diabetes mellitus: a story of sensory neurons and motor neurons. Neuroscientist. 2008;14:311–318. doi: 10.1177/1073858408316175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.