SUMMARY
A 3′ overhang is critical for the protection and maintenance of mammalian telomeres. How these overhangs are generated and whether different processing steps modify telomeres synthesized by leading- and lagging-strand DNA replication was not known. Here we evaluate changes in the telomeric overhangs through the cell cycle and at leading- and lagging-end telomeres in mouse cells lacking relevant genes. Apollo, a nuclease bound to the shelterin subunit TRF2, initiated formation of the 3′ overhang at leading-, but not lagging-end telomeres. Hyper-resection by Apollo was blocked at both ends by the shelterin protein POT1b. Exo1 extensively resected both telomere ends, generating long 3′ overhangs that transiently occurred in S/G2. CST/AAF, a DNA polymeraseα. primase accessory factor related to yeast CST, bound POT1b and shortened the extended overhangs produced by Exo1, most likely through fill-in synthesis. The results establish 3′ overhang formation as a multi-step, shelterin-controlled process that ensures functional telomeric overhangs at all chromosome ends.
Keywords: Apollo, CST, Exo1, overhang, POT1, shelterin, telomere, telomerase, TRF2
INTRODUCTION
A conserved feature of telomeres is a 3′ overhang composed of G-rich repeats that protrude beyond the complementary C-rich telomeric repeat strand. TTAGGG repeat overhangs of 30–400 nt are present at both ends of each mammalian chromosome (Makarov et al., 1997; McElligott and Wellinger, 1997) and contribute to telomere function by binding the POT1 components of the telomeric shelterin complex; serving as primers for telomerase; and forming the t-loop structure (Palm and de Lange, 2008). At the telomeres generated by lagging-strand DNA synthesis (lagging-end telomeres), overhangs of up to 200 nt could potentially originate from an inability of the DNA polymeraseα•primase complex to initiate Okazaki fragment synthesis efficiently at the end of a linear DNA template, a deficiency that has been observed in vitro (Ohki and Ishikawa, 2004). However, leading-strand DNA synthesis is expected to generate a blunt end that requires additional processing to mature into a functional telomere terminus.
The significance of overhang generation is not only in the maintenance of a protected telomere state but also relates to cellular aging. The 5′ end resection needed to generate a 3′ overhang at leading-end telomeres could contribute significantly to telomere shortening, compounding the “end-replication problem”, which refers to incomplete replication by lagging-strand synthesis. In agreement, the rate of telomere shortening in telomerase-deficient human cells correlates with the average length of the telomeric overhang (Huffman et al., 2000). Since telomere attrition rates determine the replicative lifespan of telomerase-deficient human cells, the post-replicative processing of telomere ends could affect cellular aging and thus govern the telomere tumor suppressor pathway (Artandi and DePinho, 2010).
Despite a wealth of information on telomere end-processing in yeast (Longhese et al., 2010), relatively little is known about how the mammalian telomeric overhangs are generated. In telomerase-negative human cells, overhangs at leading-end telomeres appear to be shorter than those at lagging-end telomeres, while the presence of telomerase equalizes this size distribution (Chai et al., 2006). The terminal nucleotides on the C-rich strand are remarkably precise, ending in 3′-CCAATC-5′ at >80% of leading- and lagging-end telomeres (Sfeir et al., 2005). This suggests that 5′ end-processing of leading- and lagging-end telomeres is highly regulated and includes a common final step (Sfeir et al., 2005). In contrast, the last nucleotides of the G-rich strand are variable, although TAG-3′ ends predominate when telomerase is active (Sfeir et al., 2005). While telomeric overhangs can be detected in all stages of the cell cycle, the telomeric overhang signal increases in late S/G2 phase, presumably due to resection of the C-rich strand (Dai et al., 2010).
The Apollo/SNM1B nuclease has been proposed to affect telomere end-processing, specifically at leading-end telomeres (Wu et al., 2010; Lam et al., 2010). Apollo is recruited to telomeres by the shelterin subunit TRF2 (Chen et al., 2008; van Overbeek and de Lange, 2006; Lenain et al., 2006; Bae et al., 2008). Absence of Apollo results in a 25–35% reduction in the overhang signal, which was proposed to be due to diminished processing of the leading-end telomeres because of the propensity of Apollo-depleted leading-end telomeres to fuse (Wu et al., 2010; Lam et al., 2010).
A second factor regulating the overhang is POT1, the single-stranded (ss) DNA binding factor in shelterin. Knockdown of human POT1 abolishes the specification of the telomeric 5′ end and reduces the telomeric overhang signal by 20–30% (Hockemeyer et al., 2005). However, the interpretation of this data is confounded by the concomitant activation of the DNA damage response, which could induce aberrant processing at the POT1-depleted telomeres. Less ambiguous information emerged from the analysis of mouse shelterin, which unlike human telomeres has two POT1 proteins (POT1a and POT1b) that evolved distinct functions, such that POT1b regulates the telomeric overhang while POT1a represses ATR signaling (Hockemeyer et al., 2006; Denchi and de Lange, 2007; Guo et al., 2007; Wu et al., 2006). POT1b deletion results in long ss telomeric overhangs and accelerated telomere shortening but no DNA damage signal (Hockemeyer et al., 2008; He et al., 2009; Hockemeyer et al., 2006). POT1b was proposed to limit degradation of the telomeric C-rich strand but the nuclease(s) responsible for aberrant processing have not been identified.
A third factor involved in modulating the telomeric overhang is the CST/AAF complex, composed of the OB-fold containing proteins Ctc1, Stn1 (also referred to as OBFC1), and Ten1 (Surovtseva et al., 2009; Miyake et al., 2009; Goulian et al., 1990). Based on structural similarities, CST/AAF is proposed to be the ortholog of the budding yeast telomeric CST complex (also known as t-RPA), an RPA-like complex composed of Cdc13, Stn1, and Ten1 (Gao et al., 2007). Human Ctc1 and Stn1 were originally identified as the AAF132 and AAF44 accessory factors of DNA polα.primase, which stimulate de novo RNA primer synthesis as well as primer-dependent elongation in reconstituted DNA replication systems (Goulian et al., 1990; Casteel et al., 2009). Human CST/AAF can localize to telomeres, potentially through an interaction with the shelterin protein TPP1 (Wan et al., 2009) and its depletion increases the ss telomeric DNA (Dai et al., 2010; Miyake et al., 2009; Surovtseva et al., 2009).
Here we document the combinatorial action of Apollo, POT1b, CST, and the 5′ exonuclease Exo1 in post-replicative telomere end-processing in mouse cells, clarifying the mechanism by which the mammalian telomeric 3′ overhang is generated and modulated.
RESULTS
Apollo specifically affects the overhangs of leading-end telomeres
To further define the role of Apollo in telomere end-processing, we adapted the use of CsCl density gradient equilibrium centrifugation to separate the telomeres synthesized by leading- and lagging-strand DNA synthesis based on their different incorporation of BrdU (Fig. 1A,B) (Chai et al., 2006). Fractionated DNA from BrdU-labeled MEFs revealed distinct peaks of telomeric signal intensity corresponding to the unreplicated, lagging-end, leading-end, and (rarely) doubly-replicated telomeres (Fig. 1B). The densities of unreplicated and doubly-substituted telomeric DNA were confirmed with DNA isolated from untreated cells or cells incubated with BrdU for 48 h (Fig. S1A,B).
Figure 1. Apollo contributes to overhang generation at leading-end telomeres.
(A and B) Separation of leading- and lagging-end telomeres. DNA from BrdU labeled MEFs (e.g. ApolloF/F at 120 h after Cre or without Cre) was digested and fractionated by CsCl density gradient equilibrium centrifugation. (B) Telomeric signals in slot-blotted gradient fractions (plotted in arbitrary units) and CsCl densities calculated from the refractive index. Fractions pooled for overhang analyses are indicated. (C) Overhang analysis of separated leading- and lagging-end telomeres fractionated by pulse-field gel electrophoresis. ss telomeric signal was detected by annealing a 32P-[AACCCT]4 probe to native DNA. After capture of the signal, the DNA was denatured in situ and rehybridized with the same probe to capture the total telomeric DNA signal for normalization of each lane. The bracketed region was used for quantification of all the gels; the trends were the same when the entire lanes were used for quantification. Asterisks: presumed interstitial telomeric fragments that differentially fractionate with the leading- or lagging-end telomeres providing an internal control for the gradients. Numbers under the lanes indicate the relative normalized overhang signals with the underlined lane set to 1. (D) Quantification of the telomeric overhang signal as detected in (C). Mean and SDs of three or more independent experiments. ** indicates p<0.05 (paired student’s t-test). Bar with ref is the reference value (set to 1). See also Figure S1.
Pooled fractions representing leading- and lagging-end telomeres were analyzed by in-gel native DNA hybridization with an oligonucleotide complementary to TTAGGG repeats to detect the 3′ overhangs (Fig. 1C). After capture of the signal, the DNA was denatured in situ and re-hybridized with the same probe in order to determine the total telomeric signal for normalization of the ss TTAGGG signal in each lane. Although this method does not directly evaluate the length of the telomeric overhangs, it is generally assumed that changes in the normalized overhang signals reflect changes in overhang lengths.
As the separated leading- and lagging-end telomere fractions represent fully replicated TTAGGG repeat arrays, they are not expected to contain replication intermediates. Therefore, the ss TTAGGG signal should be primarily derived from the 3′ overhang. Indeed, in the fractions containing leading- and lagging-end telomeres, there is very little ss telomeric signal outside of the bracketed (quantified) regions, whereas the bulk telomeres show additional signal smearing upward that may represent replication intermediates (Fig. 1C).
Detection of the telomeric overhang signal revealed that in Apollo-deficient cells, the overhangs at leading-end telomeres were reduced by ~50% while lagging-end overhangs were unaffected (Fig. 1C,D). The severity of the overhang defect at leading-end telomeres in the absence of any defect in lagging-end overhangs is consistent with the 20–35% reduction in overhang signal detected in bulk DNA isolated from Apollo-deficient cells (Wu et al., 2010; Lam et al., 2010). Thus, Apollo contributes to overhang generation specifically at leading-end telomeres.
In cells with normal Apollo levels, the ratio of the overhangs at leading- and lagging-end telomeres was affected by the telomerase status. When telomerase was absent (mTR−/− cells), leading- and lagging-end telomeres show equal overhangs, whereas in cells with telomerase activity (mTR+/+ cells), the overhangs at leading-end telomeres were 30–50% longer than the overhangs at the lagging-end telomeres (Fig. 1D, Fig. S1C). The studies below on the impact of Exo1, CST, and POT1b on the bulk overhang signals were performed in both telomerase-proficient and -deficient cells with essentially the same results. The effect of Apollo on the telomeric overhang was previously shown to be independent of telomerase (Wu et al., 2010).
Exo1 mediates formation of transiently extended overhangs after DNA replication
It was previously shown that the overhang signal in mouse and human cells transiently increases ~2-fold in S/G2 (Dai et al., 2010; Wu et al., 2010) (Fig. 2C below). Using a previously developed FUCCI-based system to isolate cells in G1 and S/G2 (Wu et al., 2010), we verified that the 3′ overhang signal was 1.5–2 fold greater in S/G2 than in G1, and found this to be the case regardless of telomerase status (Fig. 2A–C). The signals were sensitive to digestion with E. coli 3′ exonuclease, confirming that they represented the 3′ overhang rather than internal ss DNA formed during replication (Fig. 2A).
Figure 2. Exo1 contributes to transient overhang elongation in late S phase.
(A, B) Overhang assay on Exo1+/+mTR−/− and Exo1−/−mTR−/− MEFs in G1 and S/G2 isolated by FUCCI-FACS. See legend of Fig. 1 for details. (C) Quantification of relative overhang signals in Exo1+/+mTR+/+ and Exo1−/−mTR+/+ MEFs in G1 and S/G2 (as in (A, B)). (D, E) Overhang analysis of leading-, lagging-end and unreplicated telomeres from Exo1+/+mTR−/− and Exo1−/−mTR−/− MEFs. (F,G) Overhang analysis of ApolloF/FExo1+/+ and ApolloF/FExo1−/− MEFs at 120 h after Hit&Run Cre. (H, I) Overhang analysis of leading- and lagging-end telomeres from ApolloF/F and ApolloF/FExo1−/−MEFs without Cre or at 120 h after Hit&Run Cre. Slot blots of the gradients are shown in Fig. S2. (J) Relative overhang size in WT, Apollo KO, Exo1 KO, and Apollo/Exo1 DKO MEFs. See also Figure S2 and S3.
Exonuclease 1 has been implicated as one of the nucleases mediating 5′ end resection at DNA double strand breaks (DSBs) (Mimitou and Symington, 2008; Zhu et al., 2008; Gravel et al., 2008) and in the generation of ss DNA at chromosome ends in late-generation telomerase knockout mice (Schaetzlein et al., 2007). Although we and others previously reported that Exo1 has no effect on the telomeric overhang signal in mouse fibroblasts (Hockemeyer et al., 2008; Schaetzlein et al., 2007), we found that absence of Exo1 from asynchronous mTR−/− and mTR+/+ MEFs resulted in a 30–40% reduction in the overhang signal (Fig. 2A–C). Furthermore, Exo1-deficiency significantly altered the cell cycle dependent changes in overhang signal, resulting in a minimal increase in the overhang signal in S/G2 (Fig. 2A–C). The transient elongation of the overhangs in S/G2 depends on Exo1 in both telomerase-proficient and -deficient cells (Fig. 2B,C). Thus, Exo1 is largely responsible for the telomerase-independent increase in ss telomeric DNA that occurs after DNA replication. The residual transient increase in the overhang signal that occurs in S/G2 in Exo1−/− mTR−/− cells may reflect the processing of leading-end telomeres by Apollo and/or the formation of an extended overhang at lagging-end telomeres as a consequence of incomplete replication. Prior studies suggesting that Exo1 status does not affect the overhang signal may have used cell populations with a low S phase index.
Unlike Apollo, Exo1 appeared to exert its effect on both leading- and lagging-end telomeres. Exo1 deficiency resulted in a 40% reduction in the telomeric overhang signal at both newly synthesized telomeres (Fig. 2D,E). In contrast, the overhang signal at the unreplicated telomeres, representing the overhang status in G1, showed no decrease in Exo1-deficient cells.
We derived ApolloF/FExo1−/− conditional double knockout (DKO) cells to determine the effect of the combined absence of Apollo and Exo1. Whereas absence of either Apollo or Exo1 alone resulted in a 30–50% reduction in the overhang signal, cells lacking both nucleases had an overhang signal that was reduced by 70% compared to wild type cells (Fig. 2F,G). Co-deletion of Apollo and Exo1 had an additive effect on the overhang signal at leading- but not lagging-end telomeres, as expected from the leading-end specificity of Apollo (Fig. 2H–J; Fig. S2A–D).
We also explored the role in telomere end-processing of NBS1 and BLM, whose orthologs contribute to DSB processing in budding yeast (Gravel et al., 2008; Mimitou and Symington, 2008; Zhu et al., 2008). However, deletion of either NBS1 or BLM from MEFs did not reduce the overhang signal significantly (Fig. S2E,F) even when Exo1 was absent (Fig. S2G,H).
Interestingly, Exo1-deficient cells showed no signs of telomere dysfunction as reflected by the phosphorylation of Chk2, formation of telomere dysfunction induced foci (TIFs), or telomere fusions (Fig. S3A–D). This is in contrast to the phenotype associated with loss of Apollo, which results in the appearance of ATM-dependent TIFs at a subset of telomeres in S phase and gives rise to fusions between leading-end telomeres (Wu et al., 2010; Lam et al., 2010) (Fig. S3C–E). These results would be consistent with Exo1 acting at a step subsequent to the initial processing steps required to maintain end protection or could be explained by Exo1 being redundant with other processing factors.
In addition, Exo1 deficiency did not exacerbate the telomere dysfunction phenotypes associated with Apollo deletion (Fig. S3C–E). Taken together, these results suggest that Exo1 does not require Apollo to act at telomeres. However, the apparent Apollo-independent action of Exo1 may in part be due to the DNA damage response (DDR) at leading-end telomeres in cells lacking Apollo. In the absence of Apollo, the DDR at leading-end telomeres could mediate the initial resection needed for Exo1 to act (Mimitou and Symington, 2008; Zhu et al., 2008) and/or facilitate Exo1 recruitment.
The data above suggested that after Exo1 generates extended overhangs in S/G2, additional events decrease the overhang length resulting in the lowered overhang signal in G1. Consistent with this interpretation of the data, there was no attenuation of the telomere shortening rate in cells deficient in both Exo1 and mTR compared to cells lacking telomerase only (Fig. S3F,G). Thus, the amount of sequence lost with every cell division does not primarily depend on Exo1-mediated processing, implying subsequent step(s) in telomere processing that removes the long overhangs generated in late S/G2 and shortens them to their G1 size.
CST contributes to the post-replicative correction of the overhang
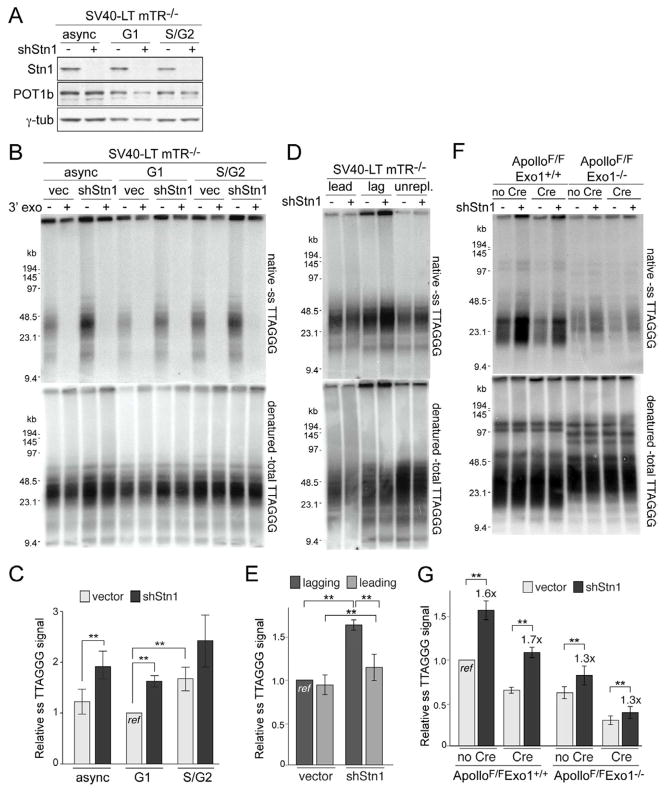
Because the shortening of the overhangs could be due to fill-in synthesis, we examined the role of CST/AAF, which has been implicated in both overhang regulation and DNA polα.primase function (Dai et al., 2010; Miyake et al., 2009; Surovtseva et al., 2009; Goulian et al., 1990; Casteel et al., 2009). To inhibit Stn1, we used an shRNA that resulted in an obvious reduction in Stn1 levels based on immunoblotting with a mouse polyclonal antibody raised against full-length recombinant mouse Stn1 (Fig. 3A) but did not affect the cell cycle profile or proliferation at the early time points used for this analysis. Importantly, the shRNA treated cells did not display TIFs (<3% cells with >5 TIFs; n>50) or chromosome end fusions (<1% of chromosomes; n>1000), indicating that their telomere function was not overtly compromised. However, Stn1-depletion in both telomerase-deficient and –proficient cells showed a nearly 2-fold increase in the relative ss telomeric DNA detected by in-gel hybridization (Fig. 3B,C; Fig. S4A; see Fig. 3F,G below). The signal could be attributed to the 3′ overhang, since it was removed by E. coli 3′ exonuclease. Furthermore, repression of Ctc1 with an shRNA increased the overhang signal (Fig. S4A).
Figure 3. Stn1 restores overhangs to their G1 size.
(A) Immunoblot for Stn1 and POT1b in asynchronous mTR−/− MEFs or in cells in G1 and S/G2 isolated by FUCCI-FACS at 96 h after Stn1 shRNA. (B,C) Overhang analysis of the cells described in (A). See legend of Fig. 1 for details. (D, E) Overhang analysis of leading- and lagging-end telomeres from mTR−/− MEFs at 96 h after Stn1 shRNA. See Fig. S4B CsCl gradient slot blots. (F, G) Overhang assay of ApolloF/F and ApolloF/FExo1−/− MEFs at 96 h after Stn1 shRNA to Stn1 and at 120 h after Hit&Run Cre. See also Figure S4.
To address whether Stn1 was involved in the correction of the overhangs created in late S/G2, telomerase-deficient cells depleted of Stn1 were sorted using the FUCCI system (Fig. 3B,C). The results indicated that Stn1-depleted cells had aberrantly high overhang signals in G1 (Fig. 3C). Meanwhile, the increase in overhang signal in S/G2 was modest and not significantly different from the control (p>0.05) and Stn1-depleted cells did not show a significant difference in overhang signals in G1 and S/G2 (p>0.05) (Fig. 3C). Thus, the depletion of Stn1 leads to aberrant overhangs primarily in G1, which is consistent with Stn1 contributing to the restoration of the transiently elongated overhangs formed in S/G2.
Stn1 depletion did not overtly compromise the semi-conservative replication of bulk telomeres, as there were no changes in the CsCl profiles (Fig. S4B). However, depleting Stn1 resulted in a 1.6-fold increase in the lagging-end overhangs and a 1.2-fold increase in the leading-end overhangs (Fig. 3D,E). Thus, the data suggest that Stn1 acts at both newly synthesized telomeres to correct the excessive overhangs generated in late S phase. Although the shRNA experiments are unlikely to reveal the full extent of the null phenotype of Stn1, the same conclusion was reached based on experiments in which Stn1 was blocked from associating with telomeres (see below).
Since these results suggested that Apollo and Exo1 contribute to the generation and lengthening of overhangs in S phase, while CST restores the long overhangs to their G1 size, we investigated the effect of depleting Stn1 in cells lacking both Apollo and Exo1. When Stn1 was depleted from cells lacking Apollo, a 1.7-fold increase in the overhang signal was observed, similar to what occurs in wild type cells depleted of Stn1 (Fig. 3F,G). However, in cells lacking Exo1, the increase in overhang signal upon Stn1 depletion was only 1.3-fold, a result that was independent of the status of Apollo (Fig. 3F,G). These data suggest that a major role for CST is to correct the excessive overhangs generated by Exo1. However, even in the absence of Exo1 and Apollo, CST contributes to limiting overhang length, possibly by fill-in synthesis that restores excessive overhangs generated by other, still unidentified nucleases and/or incomplete lagging strand DNA synthesis.
POT1b controls the overhang at both newly synthesized telomeres
Prior work has shown that POT1b deletion increases the overhang signal in a manner that is independent of telomerase (Hockemeyer et al., 2008). We determined whether POT1b functions, like Apollo, Exo1, and CST in the regulation of post-replicative telomere end processing. Consistent with such a role, removal of POT1b resulted in an increase in the telomeric overhang signal in cycling cells but not in contact-inhibited, serum-starved primary MEFs kept in G0 (Fig. 4A,B). Importantly, no difference was observed in the overhang signal in cells in POT1b-deficient cells in G1 or S/G2 (Fig. 4C,D). Separation of leading- and lagging-end telomeres showed that POT1b deletion induced a ~2-fold increase in the overhang signal at leading-end telomeres and a ~3-fold increase in the overhang at lagging-end telomeres (Fig. 4E,F; Fig. S4C), indicating that POT1b plays a role at both newly synthesized telomeres.
Figure 4. POT1b regulates overhangs at both sister telomeres.
(A, B) Overhang assay of asynchronous, G0 arrested, and released 1° POT1bF/F ROSA26-Cre-ERT2 MEFs with and without Cre induction (4-OHT). Values represent the mean of two experiments using independent POT1bF/F ROSA-Cre-ERT2 MEF lines and SEM. See legend of Fig. 1 for details. (C, D) Overhang analysis of POT1bF/− cells at 120 h post Cre in G1 and S/G2 isolated by FUCCI-FACS. (E, F) Overhang analysis of leading- and lagging-end telomeres from POT1bF/− MEFs at 120 h post-Cre (or withouit Cre). For slot blots of the CsCl gradient, see Fig. S5C. (G) Quantification of overhang analyses of POT1bF/− MEFs at 96 h following lentiviral shRNA to Stn1 and 120 h after Hit&Run Cre treatment. The signal in POT1bF/− MEFs without Cre is set to 1. Mean of 3 independent experiments and SDs. See also Figure S5.
The defect in the post-replicative restoration of overhangs in POT1b deficient cells is reminiscent of the effect of Stn1 depletion but more pronounced. We therefore asked whether Stn1 exerts its effect independent of POT1b. Whereas Stn1 depletion with shRNA in POT1b proficient cells showed the expected ~ 2-fold increase in the overhang signal, no increase was observed in POT1b deficient cells (Fig. 4G; Fig. S4D,E), suggesting that Stn1 depends on POT1b to restore the overhang size.
CST interacts with POT1b
Consistent with the finding that the effect of Stn1 depends on POT1b (Fig. 4G; Fig. S4E), a robust interaction between CST and POT1b was observed in co-IP experiments (Fig. 5A, Fig. S5A–C). In contrast, co-IP experiments revealed no interaction of CST with TRF1, TRF2, Rap1, TIN2, and POT1a (Fig. S5A–C). CST showed a weak interaction with mouse TPP1 in some experiments (Fig. S5A), consistent with a report on the binding of human TPP1 to CST (Wan et al., 2009), but this was not observed in other experiments (Fig. S5C), and co-transfection of TPP1 was not required for the interaction of POT1b with CST (Fig. S5B). All three members of the CST complex could be detected in co-IPs with POT1b (Fig. S5A,B) and conversely, IP of CST brought down POT1b (Fig. 5A). Both Ctc1 and Stn1 appeared to be required for the interaction of CST with POT1b, whereas Ten1 was not required for the interaction of POT1b with Ctc1 and Stn1 (Fig. S5D).
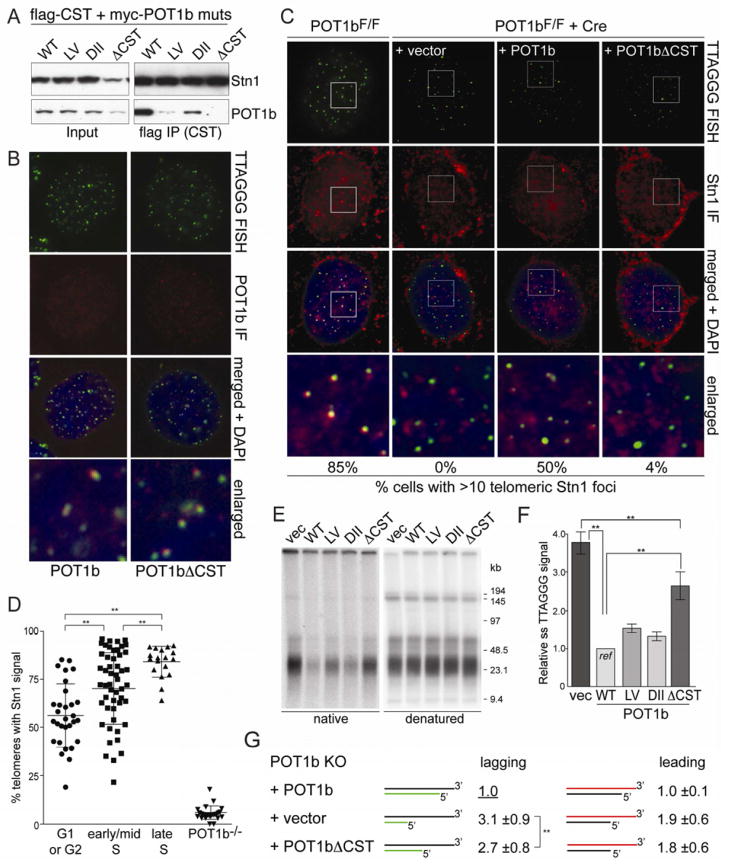
Figure 5. The telomeric function of CST requires its interaction with POT1b.
(A) Co-immunoprecipitation of POT1b mutants with CST from 293T cells transfected with myc-POT1b alleles and flag-tagged mouse Ctc1, Stn1, and Ten1. FLAG IPs were immunoblotted with FLAG (top) and myc (bottom) Abs. (B) Telomeric localization of POT1b alleles detected by myc IF (red) in POT1bF/− MEFs after deletion of endogenous POT1b with Cre. Telomeres detected by FISH (C-rich probe, green). (C) IF-FISH for co-localization of Stn1 with telomeres in POT1bF/F MEFs (left) and in the same cells expressing the indicated POT1b alleles or vector control, after deletion of endogenous POT1b with Cre. Stn1 (red) was detected with an anti-mStn1 antibody. Telomeres (green) are detected by FISH. Cells with 10 or more mStn1 signals co-localizing with telomeres were scored (bottom) (n>100 nuclei). (D) Quantification of the telomeric localization of Stn1 at different cell cycle phases. Each plotted value represents the percentage of telomeres in an individual cell that contain Stn1 signal detected by IF (n>100 cells). Mean and SDs are shown; ** indicates p<0.05. Cell cycle phases were determined based on BrdU IF pattern. (E, F) Overhang analysis of POT1bF/− MEFs expressing the indicated POT1b alleles at 120 h after Cre. See Fig. 1 for details. (G) Relative overhang size of separated leading- and lagging-end telomeres from POT1bF/− MEFs expressing vector control, wild type POT1b, or POT1bΔCST, after deletion of endogenous POT1b with Cre. Mean values and SDs of 4 independent experiments. See also Fig S6.
We next analyzed the POT1b-CST interaction with the objective of creating a mutant of POT1b defective in CST binding. The region(s) in POT1b required for binding to CST were mapped using previously characterized chimeras between human POT1 and mouse POT1a and -b (Palm et al., 2009). Co-IPs of the chimeric POT1 proteins and CST indicated that the interaction involved aa 300–350 of POT1b, which is one of two regions in POT1b required for 3′ overhang control (Fig. S5E,F). However, aa 300–535 of POT1b were not sufficient to confer the CST interaction to POT1a, indicating an additional interaction site near the C-terminus of POT1b. Therefore, residues within aa 300–350 and 535–640 in POT1b were mutated to the equivalent POT1a residues (Fig. 5A, Fig. S5G–I). Two mutations in POT1b, L329P/V332E and D638N/I639V/I640V, weakened the interaction with CST (Fig. S5G–I) while mutating all five residues in POT1b, resulting in a mutant we refer to as POT1bΔCST, completely abolished CST binding (Fig. 5A; Fig. S6A–E). POT1bΔCST retained its ability to bind TPP1, was expressed at the same level as wild type POT1b, and showed the same subcellular fractionation (Fig. S6B–D). Importantly, POT1bΔCST was detected at telomeres similar to wild type POT1b, although the staining for both proteins was weak and only detectable in ~40% of the cells (Fig. 5B).
POT1b-mediated recruitment is required for overhang regulation by CST
In the presence of wild type POT1b, ~85% of cells contained >10 Stn1 foci that co-localized with telomeres (Fig. 5C). The percentage of telomeres containing Stn1 foci was significantly increased in late S phase (Fig. 5D). However, in cells lacking POT1b or expressing POT1bΔCST, the telomeric localization of Stn1 was strongly reduced (Fig. 5C,D), even though the POT1bΔCST protein was expressed at the same level as wild type POT1b (Fig. S6B) and localized to telomeres (Fig. 5B). These results argue that the interaction with POT1b is the primary mechanism by which CST localizes to telomeres.
Importantly, while wild type POT1b completely abolishes the 3–4 fold excess overhang signal associated with POT1b deletion, in the presence of POT1bΔCST, the telomeric overhang signal remained elevated by ~2 fold (Fig. 5E,F; Fig. S6F). POT1bΔCST affected the overhangs at both newly synthesized telomeres (Fig. 5G; Fig. S6G), consistent with the results obtained with Stn1 shRNA. In the presence of POT1bΔCST, the overhangs at both leading- and lagging-end telomeres were increased by ~2-fold compared to those in the presence of wild type POT1b (Fig. 5G; Fig. S6H). Similar results were obtained when POT1bΔCST was expressed in POT1b−/−mTR−/− cells (Fig. S6I). These data argue that the role of CST in limiting overhang size depends on its recruitment by POT1b and is independent of telomerase.
Although the POT1bΔCST mutant mimicked the depletion of Stn1, the elevated overhang signal observed in the absence of POT1b could not be attributed entirely to compromised CST function as POT1b KO cells had overhangs signals that were 1.5 fold greater than for cells expressing POT1bΔCST (Fig. 5E,F). Furthermore, the overhang signals at lagging-end telomeres in cells lacking POT1b were significantly greater than those in cells expressing POT1bΔCST (Fig. 5G), indicating that POT1b was fulfilling a CST-independent function at the lagging-end telomeres.
POT1b blocks Apollo from hyper-resecting lagging- and leading-end telomeres
Since the data pointed to a CST-independent role of POT1b, we asked whether POT1b protects the C-rich strand from excessive degradation by Apollo using ApolloF/FPOT1bF/F MEFs from which Apollo and POT1b could be co-deleted with Cre (Fig. 6, Fig. S7A,B). The increase in overhang signal observed following Cre treatment was substantially attenuated in ApolloF/FPOT1bF/F compared to POT1bF/F MEFs (Fig. 6A,B). Exogenous Apollo reversed the overhang phenotype in Cre-treated ApolloF/FPOT1bF/F cells to that associated with deletion of POT1b alone whereas expression of an Apollo mutant (ApolloΔTRF2 (Wu et al., 2010)) that is unable to localize to telomeres had no effect (Fig. S7C,D). Importantly, the overhang signal in Apollo/POT1b DKO cells expressing the POT1bΔCST mutant was not significantly different from that in cells with the vector control (Fig. 6C,D). This result indicated that, in the absence of Apollo, POT1b regulates overhang length primarily through CST.
Figure 6. POT1b inhibits hyper-resection by Apollo.
(A, B) Overhang analysis of ApolloF/F, POT1bF/F, and ApolloF/FPOT1bF/F MEFs without and at 120 h after Hit&Run Cre. See Fig. 1 for details. (C, D) Overhang analysis of ApolloF/FPOT1bF/F MEFs expressing the indicated POT1b alleles at 120 h after Cre. (E) Overhang analysis of the leading- and lagging-end telomeres from ApolloF/FPOT1bF/F MEFs in the absence of Cre and at 120 h post-Cre. (F) Relative overhang size in different genetic backgrounds. Values for Apollo/POT1b DKO cells are the mean and SDs of 3 independent experiments (see Figs. 2G, 3F and 6E for data on Apollo KO and POT1b KO). See also Figure S7.
To determine whether POT1b inhibits Apollo at both newly synthesized telomeres, the leading- and lagging-end telomeres were separated following deletion of Apollo and POT1b (Fig. S7E,F). At lagging-end telomeres the overhang signal was increased by approximately 2 fold in the Apollo/POT1b DKO setting compared to wild type (Fig. 6E,F), which is less of an increase than observed after deletion of POT1b alone (Fig. 6F and 4F). Thus, POT1b limits overhang size at lagging-end telomeres in part through the inhibition of Apollo. Meanwhile, the absence of Apollo completely abolished the effect of POT1b deletion on the overhangs of leading-end telomeres. Instead of a ~2 fold increase as in POT1b KO cells, the leading-end telomeres in the Apollo/POT1b DKO cells showed a ~30–40% reduction in overhang signal compared to wild type (Fig. 6E,F), indicating that at leading-end telomeres, the increase in overhang signal induced by POT1b deletion is mediated by Apollo. Consistent with the reduced overhang signal at leading-end telomeres, approximately 10% of chromosomes in Apollo/POT1b DKO cells were engaged in leading-end fusions (Fig. S6G). Thus, POT1b inhibits excessive resection by Apollo at both leading- and lagging-end telomeres.
Combinatorial action of POT1b, Apollo, and Exo1
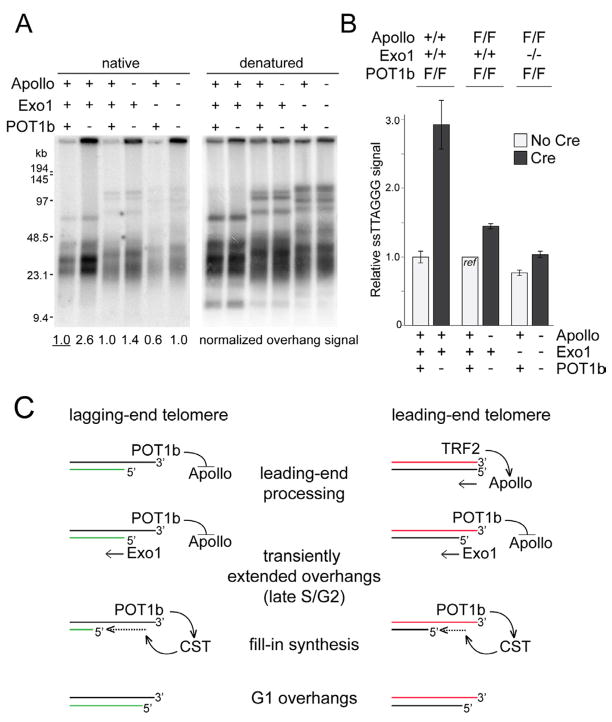
The data above indicate that POT1b has two functions at telomeres: it inhibits excessive resection by Apollo and recruits CST. As the CST complex primarily functions to correct to extended overhangs generated by Exo1, it is expected that the deletion of POT1b from Exo1 deficient cells will have no effect on this aspect of overhang processing. However, since POT1b also functions to block excessive resection by Apollo, the test of this prediction must be performed in an Apollo deficient setting. We therefore generated ApolloF/FPOT1bF/F MEFs with and without Exo1 and assayed the telomeric overhang following co-deletion of Apollo and POT1b with Cre (Fig. 7A,B). As predicted, the deletion of POT1b from Exo1-deficient cells that also lack Apollo resulted in an overhang signal that was not significantly different from that of wild type cells containing all three factors (Fig. 7A,B). These data define the role of POT1b-recruited CST as correcting the extended overhang generated in S phase and Exo1 as the primary source of these excessive overhangs. Furthermore, the data describe the dual role of POT1b in overhang regulation. POT1b recruits CST and thereby ensures the correction of the overhangs generated by Exo1. In addition, POT1b inhibits inappropriate processing of the leading- and lagging-end telomeres by Apollo (Fig. 7C).
Figure 7. Combinatorial action of Apollo, Exo1, CST, and POT1b.
(A) Overhang assay on ApolloF/FPOT1bF/F and ApolloF/FExo1−/−POT1bF/F MEFs at 96 h after Hit&Run Cre. (B) Quantification of overhang analysis in (A). Mean and SDs of 3 independent experiments. Genotypes of the MEFs (top) and status of each gene (bottom) are indicated. (C) Model for the generation of the telomeric overhang. Apollo, recruited by TRF2, initiates overhang generation at leading-end telomeres while at lagging-end telomeres an overhang originates from incomplete lagging-strand DNA synthesis. POT1b loaded on the terminal overhang inhibits resection by Apollo at the lagging-end telomere and limits resection by Apollo at leading-end telomeres once Apollo has generated a POT1b binding site. Exo1 acts on both ends to generate transiently elongated overhangs. POT1b then recruits CST which facilitates fill-in synthesis of the C-rich strand at both ends.
DISCUSSION
How shelterin orchestrates telomere end-processing
These data establish the mechanism by which the 3′ telomeric overhang is generated and illuminate how shelterin controls this process (Fig. 7C). The generation of the overhang critically depends on factors involved in DNA replication and repair, including the Apollo/SNM1B nuclease, which initiates overhang formation at leading-end telomeres; Exo1, which transiently elongates overhangs at all telomeres through 5′ end resection in S/G2; and CST/AAF, a DNA polα•primase accessory factor needed to restore the elongated overhangs to their G1 size.
Within shelterin, TRF2 and POT1b are the main players in orchestrating post-replicative telomere end-processing. The primary role of TRF2 is to recruit Apollo and thus ensure correct formation of the overhang at all leading-end telomeres. When Apollo is absent, the overhang signal at leading-end telomeres is severely reduced and at least some of the leading-end telomeres fail to protect the chromosome ends. Since TRF2 binds to double-stranded telomeric DNA and has a preference for DNA ends in vitro, it seems well suited to position Apollo at or near the leading-end telomere terminus. Whereas TRF2 mediates the telomeric localization of Apollo, POT1b acts as its negative regulator, ensuring that Apollo’s action is limited. POT1b may not be active as a regulator of end-processing when telomere ends are blunt. Although POT1b can bind to the TRF1/TIN2/TRF2 complex on the duplex telomeric DNA, we imagine that its binding to ss TTAGGG repeats is required for POT1b to inhibit Apollo. Therefore, at the leading-end telomeres, resection by Apollo may first have to generate a POT1b binding site before POT1b can block further resection by Apollo. At the other sister telomere, however, POT1b should be able to bind immediately to the overhang resulting from lagging-strand DNA synthesis and inhibit Apollo without requirement for initial resection.
In addition to regulating Apollo at both telomere ends, POT1b contributes to telomere end-processing through CST. The interaction with CST is specific to POT1b, explaining why POT1a lacks the ability to regulate the telomeric overhang. CST is crucial for the correction of the extended overhangs generated by Exo1 and its recruitment by POT1b ensures the efficiency of this process. As CST interacts with the DNA polα•primase complex, it is also expected to arrive at the telomere with the replication fork. However, recruitment by POT1b may be necessary as CST presumably acts after DNA replication has been completed.
Telomere end processing by the Apollo and Exo1: comparison to DSB processing
According to this data, Apollo and Exo1 are the main nucleases acting on telomeres after DNA replication. Although Apollo/Exo1 DKO cells do show a residual overhang signal, some ss telomeric DNA is expected to arise from lagging-strand synthesis. In addition, nucleolytic activities that are activated by the DNA damage response may be operational at telomeres lacking Apollo since a subset of telomeres in Apollo deficient cells activate the ATM kinase signaling pathway.
The combined action of Apollo and Exo1 at leading-end telomeres is remarkable because one might have anticipated that leading-end telomeres (and perhaps also lagging-end telomeres) are processed in the same way as DSBs. However, the DNA damage response, including the ATM kinase and the MRN complex, which facilitates CtIP-mediated 5′ end resection at DSBs, does not appear to have a prominent role at wild type mouse telomeres. Indeed, deficiency in either ATM or Nbs1 is not accompanied by an overhang defect or other telomere dysfunction phenotypes (Dimitrova and de Lange, 2009; Celli and de Lange, 2005; Deng et al., 2009; Attwooll et al., 2009) and our data did not reveal a role in telomere-end processing for the BLM helicase, which has been implicated in DSB resection in yeast and mammalian cells.
Transient elongation of the telomeric overhangs
Exo1 appears to be involved in a futile step that creates transiently elongated overhangs, which are later reset by CST dependent fill-in synthesis. The lack of an obvious defect in telomere protection in the absence of Exo1 indicates that a protective telomere structure can be formed without it. Furthermore, Exo1 has no net effect on the loss of terminal sequences due to telomere end processing since Exo1 deficiency does not alter the rate of telomere shortening in telomerase-deficient cells. What then might be the purpose of the transient elongation of the overhangs at leading- and lagging-end telomeres? On the one hand, Exo1 action at telomere ends may be a fail-safe mechanism. By allowing Exo1 to resect the 5′ ends without interference by shelterin, overhangs could be generated at every daughter telomere, even if other systems fail. An interesting additional possibility is that the transient long overhangs generated by Exo1 ensure that all telomeres can be elongated by telomerase.
The role of CST at mammalian telomeres
The Cdc13 component of budding yeast CST was first identified based on the cell cycle arrest induced by deprotected telomeres (Garvik et al., 1995). Budding yeast CST was subsequently shown to protect telomeres from excessive 5′ resection, promote the recruitment of telomerase, and act as a negative regulator of telomere length (reviewed in (Bertuch and Lundblad, 2006; Price et al., 2010)). Similarly, in fission yeast, CST is crucial for the maintenance of the telomeric DNA, perhaps facilitating its semi-conservative replication. By comparison, the protective role described here for mammalian CST is much less pronounced and some of the functions of CST in yeast are relegated to shelterin. However, both yeast and mammalian CST interacts with DNA polymerase α (Goulian et al., 1990; Casteel et al., 2009; Grossi et al., 2004; Qi and Zakian, 2000) and our data suggest that this is the key feature relevant to overhang regulation at mammalian telomeres.
How CST is regulated remains to be determined. Apart from the recruitment of CST by POT1b, it is anticipated that the fill-in synthesis mediated by CST is subject to control because while CST corrects the overhangs, it does not remove them altogether. It is possible that the remaining overhang is due to an intrinsic aspect of this type of fill-in synthesis. On the other hand, a regulatory step could explain the precise ATC-5′ ends of the C-rich telomeric strand (Sfeir et al., 2005).
Implications for the mechanism of telomere attrition
The elucidation of multiple regulatory mechanisms that govern the proper terminal structure of mammalian telomeres is hoped to provide information that may someday guide the treatment of human diseases in which the status of telomere function modifies pathogenesis or prognosis. Because telomere attrition is largely due to telomere end processing, it has been of interest to understand the details of this process and perhaps to identify means of altering the rate of telomere shortening. A slower rate of telomere shortening is predicted to extend the life-span of primary human cells with potential clinical implications. On the other hand, more rapid telomere attrition could synergize with the targeting of telomerase in cancer treatment. Our data reveal that the generation of the telomeric overhang in mammalian cells is a complex process involving at least two shelterin proteins and three DNA processing factors, resulting in highly regulated steps as well as redundancies. Although the results do not nominate a single nuclease whose inhibition or activation is predicted to alter telomere attrition rates, POT1b has emerged as a discrete regulatory node with strong effects on telomere dynamics. The translation of this insight into human telomere biology will be a challenge because the single POT1 protein in human cells incorporates functions of both POT1a and POT1b that will need to be deconvolved. Indeed, the great advantage of mouse telomeres has been that the regulation of the telomeric overhang by POT1b can be dissected separately from the repression of ATR kinase signaling by POT1a. This evolutionary oddity has revealed aspects of telomere biology that remain opaque in human cells.
EXPERIMENTAL PROCEDURES
Genetically altered MEFs
Genetically altered mice harboring Apollo, mTR, Exo1, POT1b, NBS1, BLM, and Rosa26-Cre-ERT2 targeted alleles were described previously. See Supplemental Materials for details. MEFs were derived and immortalized with SV40-LT using standard procedures. For synchronization of primary MEFs in G0, primary MEFs were grown to confluency on 10 cm dishes in DMEM/10% FBS. Medium was changed daily according to the following serum withdrawal protocol: 10% FBS (day 1), 5% FBS (day 2–3), 1% FBS (day 4–5), 0.5% FBS (from day 6 on). FACS analysis showed that the cells were in G0 on day 8.
Separation of leading- and lagging-end telomeres
The procedure is based on the method of Chai et al. (Chai et al., 2006). Details are given in the supplemental materials. Briefly, MEFs were cultured with 100 μM of BrdU for 16 h and processed with minimal exposure to light. Genomic DNA was isolated, digested with MboI and AluI, loaded onto 5 ml CsCl (final density of 1,800 mg/ml) in 0.3 ml, and ultracentrifuged at 55,000 rpm in a NVT90 rotor for 20 h at 25°C. 100 μl fractions were collected, denatured in 0.1 M NaOH for 20–30 min at 37°C, neutralized with one volume of 12×SSC and slot blotted onto Hybond-N+. The membrane was washed in 20×SSC, dried, baked for 2 h at 80°C, pre-hybridized at 55°C in Church mix, hybridized at 55° C with a 32P-[TTAGGG]4 probe in Church mix o/n, and washed 3x with 4×SSC for 30 min, once with 4×SSC/0.1% SDS, and exposed to a PhosphorImager screen. Pooled DNAs from each peak were surface-dialyzed by rocking on 2% agarose in a 50 ml tube for 30 min at rt, precipitated with EtOH, and resuspended in TE (10 mM Tris-HCl, pH 8.0, 1 mM EDTA) for overhang analysis.
Overhang analysis and FUCCI-FACS sorting
FUCCI-FACS sorting and determination of telomeric overhang signals were performed using previously described methods (Wu et al., 2010). Overhang signals were normalized to the total telomeric DNA signal in the same lane. Quantification of the normalized overhang signals was done using data from three or more independent experiments.
Supplementary Material
Highlights.
Telomeric 3′ overhangs are formed by a shelterin-controlled multistep process
Leading- and lagging-end telomeres are initially processed differently
5′ end resection is primarily executed by the Apollo and Exo1 nucleases
After S phase, over-resected telomere ends are filled in by shelterin-bound CST
Acknowledgments
We are thankful to W. Wright, J. Shay, Y. Zhao, and T. Chow at UTSW in Dallas for teaching one of us (PW) how to separate leading- and lagging-end telomeres. We thank D. White for expert mouse husbandry and the RU Flow Cytometry Resource Center for help with FACS sorting. De Lange lab members are thanked for advice. PW was supported by the NIA/NIH Ruth L. Kirschstein NRSA Individual Fellowship F30AG034744 and NIH MSTP grant GM07739 to the Weill Cornell/RU/MSKCC Tri-Institutional MD-PhD Program. HT was supported by a grant from the Breast Cancer Research Foundation. TdL is an American Cancer Society Research Professor. This work was supported by a grant from the NIH (CA076027).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Artandi SE, DePinho RA. Telomeres and telomerase in cancer. Carcinogenesis. 2010;31:9–18. doi: 10.1093/carcin/bgp268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwooll CL, Akpinar M, Petrini JH. The mre11 complex and the response to dysfunctional telomeres. Mol Cell Biol. 2009;29:5540–5551. doi: 10.1128/MCB.00479-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae JB, Mukhopadhyay SS, Liu L, Zhang N, Tan J, Akhter S, Liu X, Shen X, Li L, Legerski RJ. Snm1B/Apollo mediates replication fork collapse and S Phase checkpoint activation in response to DNA interstrand cross-links. Oncogene. 2008;27:5045–5056. doi: 10.1038/onc.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertuch AA, Lundblad V. The maintenance and masking of chromosome termini. Curr Opin Cell Biol. 2006;18:247–253. doi: 10.1016/j.ceb.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Casteel DE, Zhuang S, Zeng Y, Perrino FW, Boss GR, Goulian M, Pilz RB. A DNA polymerase-{alpha}{middle dot}primase cofactor with homology to replication protein A-32 regulates DNA replication in mammalian cells. J Biol Chem. 2009;284:5807–5818. doi: 10.1074/jbc.M807593200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celli GB, de Lange T. DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nat Cell Biol. 2005;7:712–718. doi: 10.1038/ncb1275. [DOI] [PubMed] [Google Scholar]
- Chai W, Du Q, Shay JW, Wright WE. Human telomeres have different overhang sizes at leading versus lagging strands. Mol Cell. 2006;21:427–435. doi: 10.1016/j.molcel.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yang Y, van Overbeek M, Donigian JR, Baciu P, de Lange T, Lei M. A shared docking motif in TRF1 and TRF2 used for differential recruitment of telomeric proteins. Science. 2008;319:1092–1096. doi: 10.1126/science.1151804. [DOI] [PubMed] [Google Scholar]
- Dai X, Huang C, Bhusari A, Sampathi S, Schubert K, Chai W. Molecular steps of G-overhang generation at human telomeres and its function in chromosome end protection. EMBO J. 2010;29:2788–2801. doi: 10.1038/emboj.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denchi EL, de Lange T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature. 2007;448:1068–1071. doi: 10.1038/nature06065. [DOI] [PubMed] [Google Scholar]
- Deng Y, Guo X, Ferguson DO, Chang S. Multiple roles for MRE11 at uncapped telomeres. Nature. 2009;460:914–918. doi: 10.1038/nature08196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova N, de Lange T. Cell cycle dependent role of MRN at dysfunctional telomeres: ATM signaling-dependent induction of NHEJ in G1 and resection-mediated inhibition of NHEJ in G2. Mol Cell Biol. 2009;29:5552–5563. doi: 10.1128/MCB.00476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Cervantes RB, Mandell EK, Otero JH, Lundblad V. RPA-like proteins mediate yeast telomere function. Nat Struct Mol Biol. 2007;14:208–214. doi: 10.1038/nsmb1205. [DOI] [PubMed] [Google Scholar]
- Garvik B, Carson M, Hartwell L. Single-stranded DNA arising at telomeres in cdc13 mutants may constitute a specific signal for the RAD9 checkpoint. Mol Cell Biol. 1995;15:6128–6138. doi: 10.1128/mcb.15.11.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulian M, Heard CJ, Grimm SL. Purification and properties of an accessory protein for DNA polymerase alpha/primase. J Biol Chem. 1990;265:13221–13230. [PubMed] [Google Scholar]
- Gravel S, Chapman JR, Magill C, Jackson SP. DNA helicases Sgs1 and BLM promote DNA double-strand break resection. Genes Dev. 2008;22:2767–2772. doi: 10.1101/gad.503108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossi S, Puglisi A, Dmitriev PV, Lopes M, Shore D. Pol12, the B subunit of DNA polymerase alpha, functions in both telomere capping and length regulation. Genes Dev. 2004;18:992–1006. doi: 10.1101/gad.300004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Deng Y, Lin Y, Cosme-Blanco W, Chan S, He H, Yuan G, Brown EJ, Chang S. Dysfunctional telomeres activate an ATM-ATR-dependent DNA damage response to suppress tumorigenesis. EMBO J. 2007;26:4709–4719. doi: 10.1038/sj.emboj.7601893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Wang Y, Guo X, Ramchandani S, Ma J, Shen MF, Garcia DA, Deng Y, Multani AS, You MJ, Chang S. Pot1b deletion and telomerase haploinsufficiency in mice initiate an ATR-dependent DNA damage response and elicit phenotypes resembling dyskeratosis congenita. Mol Cell Biol. 2009;29:229–240. doi: 10.1128/MCB.01400-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockemeyer D, Daniels JP, Takai H, de Lange T. Recent expansion of the telomeric complex in rodents: Two distinct POT1 proteins protect mouse telomeres. Cell. 2006;126:63–77. doi: 10.1016/j.cell.2006.04.044. [DOI] [PubMed] [Google Scholar]
- Hockemeyer D, Palm W, Wang RC, Couto SS, de Lange T. Engineered telomere degradation models dyskeratosis congenita. Genes Dev. 2008;22:1773–1785. doi: 10.1101/gad.1679208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockemeyer D, Sfeir AJ, Shay JW, Wright WE, de Lange T. POT1 protects telomeres from a transient DNA damage response and determines how human chromosomes end. EMBO J. 2005;24:2667–2678. doi: 10.1038/sj.emboj.7600733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman KE, Levene SD, Tesmer VM, Shay JW, Wright WE. Telomere shortening is proportional to the size of the G-rich telomeric 3′-overhang. J Biol Chem. 2000;275:19719–19722. doi: 10.1074/jbc.M002843200. [DOI] [PubMed] [Google Scholar]
- Lam YC, Akhter S, Gu P, Ye J, Poulet A, Giraud-Panis MJ, Bailey SM, Gilson E, Legerski RJ, Chang S. SNMIB/Apollo protects leading-strand telomeres against NHEJ-mediated repair. EMBO J. 2010;29:2230–2241. doi: 10.1038/emboj.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenain C, Bauwens S, Amiard S, Brunori M, Giraud-Panis MJ, Gilson E. The Apollo 5′ exonuclease functions together with TRF2 to protect telomeres from DNA repair. Curr Biol. 2006;16:1303–1310. doi: 10.1016/j.cub.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Longhese MP, Bonetti D, Manfrini N, Clerici M. Mechanisms and regulation of DNA end resection. EMBO J. 2010;29:2864–2874. doi: 10.1038/emboj.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarov VL, Hirose Y, Langmore JP. Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening. Cell. 1997;88:657–666. doi: 10.1016/s0092-8674(00)81908-x. [DOI] [PubMed] [Google Scholar]
- McElligott R, Wellinger RJ. The terminal DNA structure of mammalian chromosomes. EMBO J. 1997;16:3705–3714. doi: 10.1093/emboj/16.12.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimitou EP, Symington LS. Sae2, Exo1 and Sgs1 collaborate in DNA double- strand break processing. Nature. 2008;455:770–774. doi: 10.1038/nature07312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake Y, Nakamura M, Nabetani A, Shimamura S, Tamura M, Yonehara S, Saito M, Ishikawa F. RPA-like mammalian Ctc1-Stn1-Ten1 complex binds to single-stranded DNA and protects telomeres independently of the Pot1 pathway. Mol Cell. 2009;36:193–206. doi: 10.1016/j.molcel.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Ohki R, Ishikawa F. Telomere-bound TRF1 and TRF2 stall the replication fork at telomeric repeats. Nucleic Acids Res. 2004;32:1627–1637. doi: 10.1093/nar/gkh309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- Palm W, Hockemeyer D, Kibe T, de Lange T. Functional dissection of human and mouse POT1 proteins. Mol Cell Biol. 2009;29:471–482. doi: 10.1128/MCB.01352-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CM, Boltz KA, Chaiken MF, Stewart JA, Beilstein MA, Shippen DE. Evolution of CST function in telomere maintenance. Cell Cycle. 2010;9:3157–3165. doi: 10.4161/cc.9.16.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Zakian VA. The Saccharomyces telomere-binding protein Cdc13p interacts with both the catalytic subunit of DNA polymerase alpha and the telomerase-associated est1 protein. Genes Dev. 2000;14:1777–1788. [PMC free article] [PubMed] [Google Scholar]
- Schaetzlein S, Kodandaramireddy NR, Ju Z, Lechel A, Stepczynska A, Lilli DR, Clark AB, Rudolph C, Kuhnel F, Wei K, Schlegelberger B, Schirmacher P, Kunkel TA, Greenberg RA, Edelmann W, Rudolph KL. Exonuclease-1 deletion impairs DNA damage signaling and prolongs lifespan of telomere-dysfunctional mice. Cell. 2007;130:863–877. doi: 10.1016/j.cell.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfeir AJ, Chai W, Shay JW, Wright WE. Telomere-end processing the terminal nucleotides of human chromosomes. Mol Cell. 2005;18:131–138. doi: 10.1016/j.molcel.2005.02.035. [DOI] [PubMed] [Google Scholar]
- Surovtseva YV, Churikov D, Boltz KA, Song X, Lamb JC, Warrington R, Leehy K, Heacock M, Price CM, Shippen DE. Conserved telomere maintenance component 1 interacts with STN1 and maintains chromosome ends in higher eukaryotes. Mol Cell. 2009;36:207–218. doi: 10.1016/j.molcel.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Overbeek M, de Lange T. Apollo, an Artemis-related nuclease, interacts with TRF2 and protects human telomeres in S phase. Curr Biol. 2006;16:1295–1302. doi: 10.1016/j.cub.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Wan M, Qin J, Songyang Z, Liu D. OB fold-containing protein 1 (OBFC1), a human homolog of yeast Stn1, associates with TPP1 and is implicated in telomere length regulation. J Biol Chem. 2009;284:26725–26731. doi: 10.1074/jbc.M109.021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Multani AS, He H, Cosme-Blanco W, Deng Y, Deng JM, Bachilo O, Pathak S, Tahara H, Bailey SM, Deng Y, Behringer RR, Chang S. Pot1 deficiency initiates DNA damage checkpoint activation and aberrant homologous recombination at telomeres. Cell. 2006;126:49–62. doi: 10.1016/j.cell.2006.05.037. [DOI] [PubMed] [Google Scholar]
- Wu P, van Overbeek M, Rooney S, de Lange T. Apollo Contributes to G Overhang Maintenance and Protects Leading-End Telomeres. Mol Cell. 2010;39:1–12. doi: 10.1016/j.molcel.2010.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Chung WH, Shim EY, Lee SE, Ira G. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell. 2008;134:981–994. doi: 10.1016/j.cell.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.