Abstract
Low dose endotoxemia is prevalent in humans with adverse health conditions, and correlates with the pathogenesis of chronic inflammatory diseases such as atherosclerosis, diabetes, and neurological inflammation. However, the underlying molecular mechanisms are poorly understood. Here, we demonstrate that subclinical low dose lipopolysaccharide (LPS) skews macrophages into a mild pro-inflammatory state, through cell surface toll-like-receptor 4 (TLR4), interleukin-1 receptor-associated kinase-1 (IRAK-1), and the toll-interacting-protein (Tollip). Unlike high dose LPS, low dose LPS does not induce robust activation of nuclear factor kappa-B (NFκB), mitogen-activated protein kinases (MAPK), phosphoinositide 3-kinase (PI3K), or anti-inflammatory mediators. Instead, low dose LPS induces activating transcription factor 2 (ATF2) through Tollip-mediated generation of mitochondrial reactive oxygen species (ROS), allowing mild induction of pro-inflammatory mediators. Low dose LPS also suppresses PI3K and related negative regulators of inflammatory genes. Our data reveal novel mechanisms responsible for skewed and persistent low grade inflammation, a cardinal feature of chronic inflammatory diseases.
Keywords: macrophage polarization, low grade inflammation, molecular mechanisms, mitochondria
INTRODUCTION
Slightly elevated levels of circulating bacterial endotoxin (~1–100 pg/mL) persists in humans with adverse health conditions and/or life styles such as chronic infections, obesity, aging, chronic smoking and drinking (1–7). Compromised mucosal barriers, altered commensal microbiota, and vasculature leakage collectively contribute to the mild and persistent elevation of plasma endotoxin in these individuals. In contrast to high dose endotoxin that induces a robust yet transient inflammatory response, “subclinical” low dose endotoxin causes low grade yet persistent inflammatory responses from the host, as reflected in the mildly sustained levels of inflammatory mediators (1, 8–13). This may underlie the initiation and propagation of chronic pathological diseases including cardiovascular diseases, diabetes, Parkinson’s disease, and other chronic inflammatory syndromes (8, 14–16). Given the prevalence of low dose endotoxemia and low grade inflammation in humans at various stages of life, the economic and health tolls are reaching a pandemic level (17–22). As a consequence, strategies targeting at low dose endotoxemia and low grade inflammation may hold significant promise in not only the treatment, but also the prevention or reversal of these debilitating diseases. Despite its significance, the molecular mechanism underlying the effect of low grade endotoxemia is neither well studied, nor properly understood.
Host macrophages are the most potent responders to bacterial endotoxin, LPS (23). Given the fact that a high dose of circulating LPS (over 10 ng/mL) can cause acute septic shock (24–26), almost all available mechanistic studies regarding cellular responses to endotoxin utilized high dosages of LPS (>10ng/mL). High doses of LPS cause a robust induction of various pro-inflammatory mediators in macrophages through the Toll-like-receptor 4 (TLR4) pathway (27). In the meantime, high dose LPS is also capable of inducing the expression of anti-inflammatory mediators such as Interleukin-10 (IL-10). This serves as a compensatory mechanism for the host to dampen excessive inflammation(28, 29).
Molecular mechanisms responsible for the effect of LPS were largely obtained in the context of high dose LPS. Through TLR4 and other co-receptors, high dose LPS opens up a flood gate of intracellular pathways that eventually lead to the activation of diverse transcription factors such as NFκB and activated protein-1 (AP-1) (30) (31). Collectively, these transcription factors contribute to the robust induction of pro-inflammatory mediators (31). The pathway leading to the activation of NFκB is the most extensively studied and relatively well defined (32, 33). In this classical pathway, TLR4 activates the Interleukin-1 Receptor Associated Kinases (IRAK-4, 2, 1) via the MyD88 adaptor molecule (32, 33). IRAKs then recruit TRAF6/2 and contribute to the activation of MAPKKKs (e.g. TAK1, MLK3, and Tpl2), the phosphorylation of the IKKα/β complex leading to the phosphorylation and degradation of IκB, and the eventual release and nuclear translocation of p65/RelA. IRAK-1/TRAF complex is also responsible for the activation of mitogen-activated protein kinases (MAPKs) and downstream transcription factors such as AP-1. Although the potent activation of TLR4 pathway is capable of robustly inducing the expression of pro-inflammatory mediators, it also strongly induces negative regulators at multiple levels including the IκBα (the negative regulator of NFκB), PI3K, MKP-1, as well as the inactivation of IRAK-1 (33–39). Of particular note, PI3K pathway attenuates the expression of pro-inflammatory mediators, and facilitates the expression of anti-inflammatory mediators (40). CREB activation by PI3K pathway facilitates the expression of IL-10 (41), while induction of MKP-1 by PI3K contributes to the suppression of pro-inflammatory mediators (42, 43). This may serve to dampen excessive inflammation and reduce collateral damage to the host.
In contrast, low levels of circulating LPS (~1–100pg/mL) in experimental animals and humans with adverse health conditions can induce mild, yet selective expression of host pro-inflammatory mediators (1, 8–13). In well-controlled in vitro studies, low dosages of LPS (10–100pg/mL) causes a distinct effect by priming cells for more robust expression of pro-inflammatory mediators in response to a second LPS challenge (44–47). Furthermore, chronic microbial infections or injections of low level TLR agonists can exacerbate inflammatory diseases such as atherosclerosis (48, 49). Despite emerging recognition of the chronic and pathological effects of subclinical low dose endotoxin, no report is available regarding the underlying molecular mechanism. We provided the first evidence indicating that subclinical doses of LPS (<100 pg/mL) fails to activate the classical NFκB pathway and its ensuing negative feedback loops (50).
This current study aims to reveal the intracellular mechanisms responsible for the mild yet selective expression of pro-inflammatory mediators induced by low dose endotoxin. Based on previous observations that IRAK-1 and Tollip are selectively involved in mediating macrophage responses to low dose endotoxin (50, 51), we examined the molecular networks involving IRAK-1 and Tollip responsible for the selective induction of pro-inflammatory mediators by low dose endotoxin. Our study reveals that mitochondria reactive oxygen species (ROS), induced by low dose LPS through IRAK-1 and Tollip, contribute to the expression of pro-inflammatory mediators through ATF2. Furthermore, our data indicate that low dose LPS suppresses PI3K pathway involved in the expression of anti-inflammatory mediators.
METHODS
Reagents
LPS (Escherichia coli 0111:B4) was purchased from Sigma Aldrich. Anti-IκB α (#9242), pJNK (#9251), pERK (#4370), pP38 (#9211), pATF2 (#9225) antibodies were obtained from Cell Signaling Technology. Anti-Lamin B (ab-16048) was purchased from Abcam. Anti-C/EBP δ (M-17), anti-MKP-1 (M-18), anti-IRAK-1 (F-4), anti-P65 (F-6), anti-GAPDH (FL-335), anti-IRAK-M, anti-GSK3β and anti-ATF2 (C-19) antibodies were from Santa Cruz Biotechnology. Anti-mouse IgG and anti-rabbit IgG horseradish peroxidase (HRP)-linked antibodies were purchased from Cell Signaling Technology.
Mice and cell culture
Wild type (WT) C57BL/6 mice were purchased from the Charles River laboratory. IRAK-1−/− mice from C57BL/6 background were kindly provided by Dr. James Thomas from the University of Texas Southwestern Medical School. Tollip−/− mice from C57BL/6 background were provided by Dr. Jürg Tschopp from the University of Lausanne at Switzerland. All mice were housed and bred at Virginia Tech animal facility in compliance with approved Animal Care and Use Committee protocols of Virginia Tech. BMDMs were isolated from the tibias and femurs of WT, IRAK-1−/− and Tollip−/− mice by flushing the bone marrow with Dulbecco’s modified Eagle’s medium (DMEM). The cells were cultured in untreated tissue culture dishes with 50 mL DMEM containing 30% L929 cell supernatant. On the third day of culture, the cells were fed with an additional 20 mL fresh medium and cultured for another additional 3 days. Cells were harvested with phosphate-buffered saline (PBS), resuspended in DMEM supplemented with 1% fetal bovine serum, and allowed to rest overnight before further treatments. Wild type (WT) Raw264.7 and GP96 knocked-down (GP96KD) Raw264.7 cells defective in cell surface TLR4 were maintained as described previously (52).
Cell transfection
MEF cells were cultured in complete DMEM medium, as previously described. The endotoxin levels within the culture media were below detectable range. Transfections with EGFP-Tollip expression plasmid was performed using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instruction. Twenty-four hours after transfection, the stably transfected cells were selected using G418 antibiotics (400 μg/mL) for 4 weeks before use in subsequent experiments.
Confocal microscopy
The GFP-Tollip transfected MEF cells were plated in 35 mm glass bottom petri dishes (MatTek). For staining of mitochondria, cells were incubated with 75 nM Mito Tracker Red (Invitrogen) for 20 min at 37oC in darkness. After washing three times with PBS, cells were fixed with paraformaldehyde (4%) in PBS for 15 min at room temperature and then washed three times with PBS. The nuclei were stained using 4′,6-diamidino-2-phenylindole (DAPI). Fluorescence images were obtained with a laser-scanning confocal microscope Zeiss LSM510. Mito Tracker Red was excited with a 543-nm laser line and its emission was collected between 590 and 640 nm.
Analysis of protein and mRNA
Cells were harvested after specified treatments and washed with PBS. The cells were resuspended in a lysis buffer containing protease inhibitor cocktail (Sigma) and subjected to SDS-PAGE. The protein bands were transferred to an immunoblot PVDF membrane (BioRad) and subjected to immunoblot analysis with the indicated antibodies. Total RNA was extracted using an Isol-RNA lysis reagent (Invitrogen) and cDNA was generated with an High-Capacity cDNA Reverse transcription kit (Applied Biosystems) followed by analysis using SYBR Green Supermix on an IQ5 thermocycler (Bio-Rad). The relative levels of different transcripts were calculated using the ΔΔCt method and results were normalized based on the expression of Gapdh within the same experimental setting. The relative level of mRNA in untreated WT cells was adjusted to 1 and served as the basal reference value. The following primer sets were used: mouse Gapdh forward: 5′-AAC TTT GGC ATT GTG GAA GGG CTC-3′, reverse: 5′-TGG AAG AGT GGG AGT TGC TGT TGA-3′; mouse Il-6 forward: 5′-ATC CAG TTG CCT TCT TGG GAC TGA-3′, reverse: 5′-TAA GCC TCC GAC TTG TGA AGT GGT-3′; mouse Ccl22 forward: 5′-AGG CTC AGC AAG CCC TAT TCT TCT-3′, reverse: 5′-GAA TGT GTT TCC CAG CTG CCA CAT-3′; mouse Il-10 forward: 5′-GCT CTT GCA CTA CCA AAG CCA CAA-3′, reverse: 5′-AGT AAG AGC AGG CAG CAT AGC AGT-3′; mouse Tnfα forward 5′-AGC CGA TGG GTT GTA CCT TGT CTA-3′, reverse 5′-TGA GAT AGC AAA TCG GCT GAC GGT-3′.
Analysis of mitochondrial proteins and reactive oxygen species
Mitochondrial protein fractions were prepared using the Mitochondria Isolation kit from Thermo Scientific. Briefly, BMDM cells were grown on 150 mm2 tissue culture plates and treated with LPS as indicated prior to the isolation procedure as per manufacturer’s instructions. The purity of the mitochondrial fraction was determined using Western blot analysis for mitochondrial resident protein cyclooxygenase IV and cytosolic protein, GAPDH.
For the detection of mitochondrial ROS production, we utilized the mitochondrial sensitive dye MitoSOX Red (Invitrogen). BMDM and MEF cells were plated in DMEM without phenol red prior to the start of the experiment. Cells were then stained with 1 μM Hoescht 34580 (Invitrogen) for 10 min at 37°C for normalization of fluorescent data. After washing three times with 1 mL of Highly Balanced Salt Solution (HBSS, Invitrogen) cells were stained with 5 μM MitoSOX Red for 20 min at 37°C. Cells were then washed with HBSS as mentioned above prior to LPS treatment for the specified time periods. Fluorescent wavelength pairs for the individual dyes were 510/580 nm for MitoSOX Red and 392/440 nm for Hoescht 34580 on a Spectromax M2e plate reader (Molecular Devices).
Chromatin-immunoprecipitation analysis
BMDM cells were cross-linked using 1% formaldehyde solution in complete media for 15 minutes with gentle rocking every 3 minutes at room temperature. Cells were washed twice with cold PBS and treated with a glycine solution for 5 minutes to stop the cross-linking reaction. Cells were then lysed in an ice cold buffer containing SDS and protease inhibitor cocktail. The samples were then sonicated in an ice water bath to shear the chromatin for 10 minutes (30 seconds on and 30 seconds off). The sheared chromatin was processed using Chip-IT Express Kit (Active Motif™). The immuno-precipitated chromatin was analyzed by PCR using primer pairs that span the proximal promoter regions of mouse Il-6. The primer sequences used to amplify the enriched chromatin samples with the mouse Il-6 promoter are as follows: forward primer: 5′-TCC CAT CAA GAC ATG CTC AAG TGC-3′, reverse: 5′-AGC AGA ATG AGC TAC AGA CAT CCC-3′.
Statistical analysis
Results are expressed as mean ± SD. Statistical significances between groups were determined using a two-tailed Student’s t test and indicated by an asterisk in figures; p values < 0.05 were considered statistically significant.
RESULTS
Mild and selective induction of pro-inflammatory mediators by subclinical low dose LPS
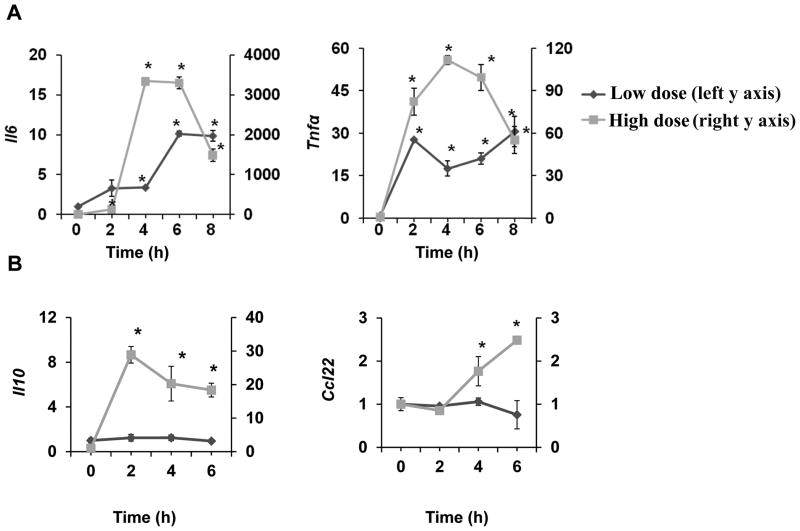
We first examined the expression profile of various pro- and anti-inflammatory genes in macrophages challenged with either a low or high dose LPS. Wild type (WT) bone marrow derived macrophages (BMDM) were treated with a low-dose (50 pg/mL) or a high-dose LPS (100 ng/mL) for the specified time periods. As expected, high dose LPS robustly induced the message expression of both pro-inflammatory (Il-6, Tnfα) and anti-inflammatory (Il-10, Ccl22) mediators (Fig. 1A,B). In contrast, low dose LPS only induced mild expression of pro-inflammatory mediators, but failed to induce any noticeable expression of anti-inflammatory mediators (Fig. 1A,B). The levels of pro-inflammatory Tnfα and Il-6 induced by high dose LPS peaked transiently at 4–6hr and dropped significantly at 8–10hr. In contrast, the expression of Tnfα and Il-6 induced by low dose LPS were prolonged and persisted throughout the time course.
Figure 1. Low dose LPS selectively induces mild and prolonged expression of pro-inflammatory mediators.
(A) WT BMDM were treated with either a low dose LPS (50 pg/mL) or a high dose LPS (100 ng/mL) for the specified time periods. Total RNA was isolated, and real-time RT-PCR assays were performed to determine the expression levels of pro-inflammatory mediators such as Il-6 and Tnfα. (B) WT BMDM cells were treated with either a low dose LPS (50 pg/mL) or a high dose LPS (200 ng/mL) for the specified time periods. The levels of anti-inflammatory Il-10 and Ccl22 were measured by real-time RT-PCR. The relative transcript levels were standardized against Gapdh levels. Data were representative of at least three independent experiments. * p < 0.05.
Subclinical dose LPS fails to induce robust activation of NFκB and MAPK as well as negative regulators
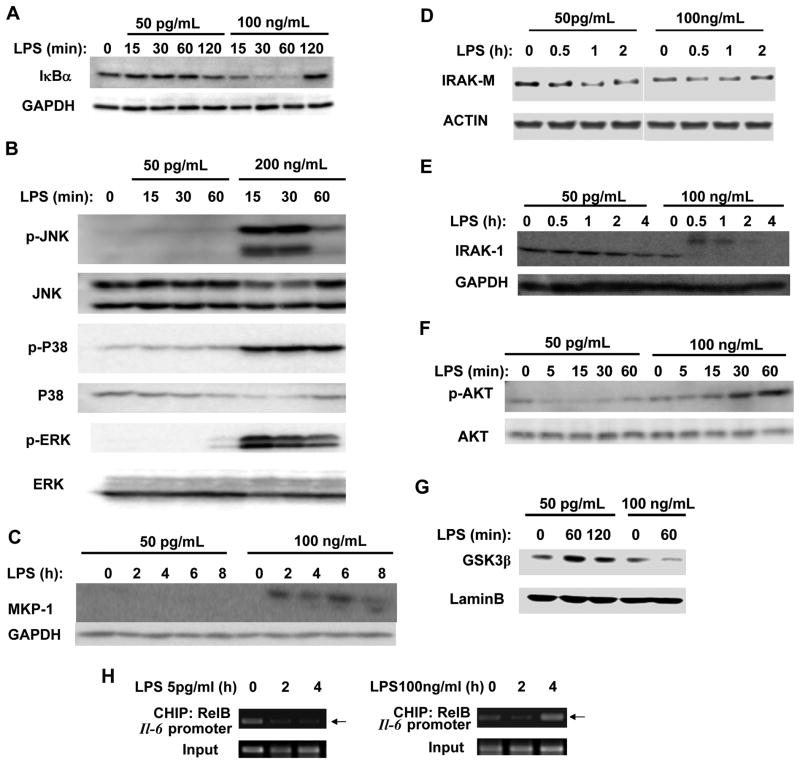
To clarify the underlying molecular mechanisms, we examined the activation status of key TLR4 downstream components including NF-κB and MAP kinases. As shown in Fig. 2A, high dose LPS induced a rapid degradation of IκB, an indication of the activation of the classical NF-κB pathway. In contrast, low dose LPS failed to induce noticeable degradation of IκB. High dose LPS also triggered robust activation of MAP kinases including p38, ERK and JNK (Fig. 2B). In contrast, low dose LPS failed to induce noticeable activation of all three MAP kinases.
Figure 2. Low dose LPS does not induce the classical NFκB and MAPK pathways, nor negative suppressors for inflammatory responses.
(A) Low dose LPS fails to induce IκBα degradation. WT BMDM were treated with either a low-dose LPS (50 pg/mL) or a high-dose LPS (100 ng/mL) for the indicated times. The levels of IkBα were determined by Western blot. The same blots were probed with Abs specific for GAPDH as loading controls. (B) Low-dose LPS failed to activate the MAP kinase pathways. Whole cell lysates from WT cells treated with either a low (50pg/mL) or high (200ng/mL) dose LPS were harvested and used to determine the levels of phosphorylated JNK, p38 and ERK. The total levels of JNK, p38 and ERK were used as loading controls. (C) Low-dose LPS fails to induce the negative regulators including MKP-1. Total levels of MKP-1 were determined by Western blot. (D) Low dose LPS reduces IRAK-M. Total levels of IRAK-M from cells treated with either a low or high dose LPS were measured by Western blot. (E) Low dose LPS fails to degrade IRAK-1. IRAK-1 levels were determined from cells treated with either low dose or high dose LPS by Western blot. Levels of GAPDH were used as loading controls in Fig C, D, and e. (F) PI3K pathway is activated by high dose LPS, and suppressed by low dose LPS. Whole cell lysates from cells treated with either low or high dose LPS were harvested and used to determine the levels of phosphorylated Akt. The total levels of Akt were used as the loading control. (G) Nuclear GSK3β is induced by low dose LPS, and reduced by high dose LPS. Nuclear lysates from cells treated with low or high dose LPS were harvested and the levels of GSK3β were determined by Western blot. The levels of Lamin B were used as a loading control. Panels were representatives of three independent experiments. (H) CHIP analysis to detect the binding of RelB to the Il-6 promoter in response to high and low dose LPS. WT BMDM cells were treated with either low or high dose LPS for the indicated time periods. The samples were immunoprecipitated using a RelB specific antibody and analyzed by PCR using primers spanning the promoter region of murine Il-6.
As well-noticed in the field, high dose LPS also induces negative regulators including MKP-1 and IRAK-M that serve to dampen the expression of pro-inflammatory mediators (43, 53). We then examined the expression of MKP-1 and IRAK-M by high versus low dose LPS. As shown in Fig. 2C, high dose LPS induced significant expression of MKP-1. In contrast, low dose LPS failed to induce MKP-1. Intriguingly, low dose LPS reduced the levels of IRAK-M (Fig. 2D). Other mechanisms of compensatory suppression/tolerance triggered by high dose LPS include degradation of IRAK-1 (33). As shown in Fig. 2E, high dose LPS led to rapid modification and degradation of IRAK-1, as reflected in the occurrence of an upper band typical of ubiquitinated IRAK-1, and later disappearance of IRAK-1. In contrast, low dose LPS failed to induce IRAK-1 ubiquitination and degradation.
The activation of PI3K pathway by high dose LPS was shown to be responsible for the induction of negative regulators including MKP-1 and degradation of IRAK-1 (42, 54). In addition, PI3K activation also leads to the expression of anti-inflammatory mediators such as IL-10 (40, 41). Thus, we evaluated the activation status of PI3K pathway in cells treated with either low or high dose LPS. As shown in Fig. 2F, high dose LPS led to robust phosphorylation of Akt, an indication of PI3K activation. Strikingly, low dose LPS not only failed to induce Akt phosphorylation, but rather reduced the residual levels of phosphorylated Akt. As a consequence, the nuclear levels of GSK3β were induced by low dose LPS, and reduced by high dose LPS (Fig. 2G).
Previous reports also indicate that high dose LPS recruits the suppressive RelB to the promoter of inflammatory genes and accounts for the endotoxin tolerance (55, 56). After an initial transient decrease in RelB, high dose LPS is well-documented to induce a late phase RelB, through NFκB as well as PI3K mediated expression (56, 57). However, no report is available regarding the status of RelB in cells challenged with low dose LPS. To further test the involvement of RelB in differential gene expression induced by low and high dose LPS, we performed chromatin immunoprecipitation analysis (ChIP) on the promoter of the Il-6 gene. As shown in Fig. 2H, high dose LPS (100 ng/mL) caused a transient decrease of suppressive RelB from the Il-6 promoter (2 hour time point), allowing for transient induction of Il-6 expression. The RelB levels at the Il-6 promoter subsequently rose significantly at the 4 hour time point, corresponding to the development of endotoxin tolerance (56). In stark contrast, low dose LPS caused prolonged removal of RelB from the Il-6 promoter at both time points (Fig. 2H).
Subclinical dose LPS preferentially activates ATF2 and C/EBPδ
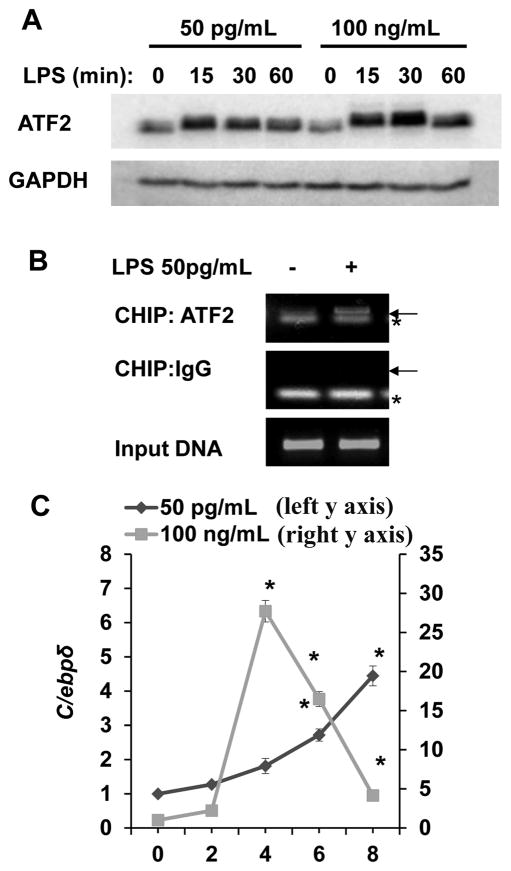
AP-1 family proteins are among potential transcription factors responsible for the mild expression of Tnfα and Il-6. We noticed a rapid and significant induction of ATF2 protein by low dose LPS. The protein levels of ATF2 were similarly and significantly induced after 30 minute of stimulation with high dose LPS (Fig. 3A). To determine whether the induction of ATF2 may be due to its gene expression, we measured mRNA levels of Atf2 and did not detect any noticeable increase in Atf2 message within 2 hr of LPS stimulation (data not shown). This suggests that LPS may selectively stabilize ATF2 protein through a novel mechanism independent of gene transcription.
Figure 3. Low dose LPS selectively induces ATF2.
(A) Low-dose LPS induces a rapid increase in ATF-2 protein levels in WT BMDM. WT cells were treated with a low (50 pg/mL) or a high dose (100 ng/mL) LPS for the indicated times. Total ATF2 protein levels were determined by Western blot and GAPDH levels were used as loading controls. (B) Low dose LPS recruits ATF2 to the proximal promoter of the pro-inflammatory gene IL-6 in WT BMDMs. BMDM were either untreated or treated with 50 pg/mL LPS for 2 h and then subjected to ChIP assay using an antibody specific to ATF-2 and primers specific to the proximal promoter of IL-6. The same samples were immunoprecipitated using IgG as a non-specific control and input DNA was analyzed as the loading control. Arrow points to the specific amplification product. * denotes a non-specific band. (C) Low-dose LPS induces a late C/ebpδ expression in WT BMDM. WT cells were treated with either a low (50 pg/mL) or a high dose (100 ng/mL) LPS for the indicated times. C/ebpδ message levels were analyzed by real-time RT-PCR. n=3; * p<0.05.
To confirm that ATF2 contributes to the expression of Il-6 induced by low dose LPS, we performed chromatin immunoprecipitation (ChIP) analyses to examine the recruitment of ATF2 to the Il-6 promoter. As shown in Fig. 3B, low dose LPS challenge led to significant recruitment of ATF2 to the proximal promoter of Il-6.
We and others also reported that a late phase C/EBPδ induction mediated by IKKε contributes to prolonged expression of pro-inflammatory mediators such as Il-6 (50, 58). To further confirm this phenomenon, we measured the levels of c/ebpδ in cells treated stimulated with low dose LPS. As shown in Fig. 3C, a late induction of c/ebpδ (4 to 8 hrs) was observed following low dose LPS stimulation. In contrast, high dose LPS led to a transient induction of c/ebpδ.
IRAK-1 and Tollip are involved in the induction of ATF-2
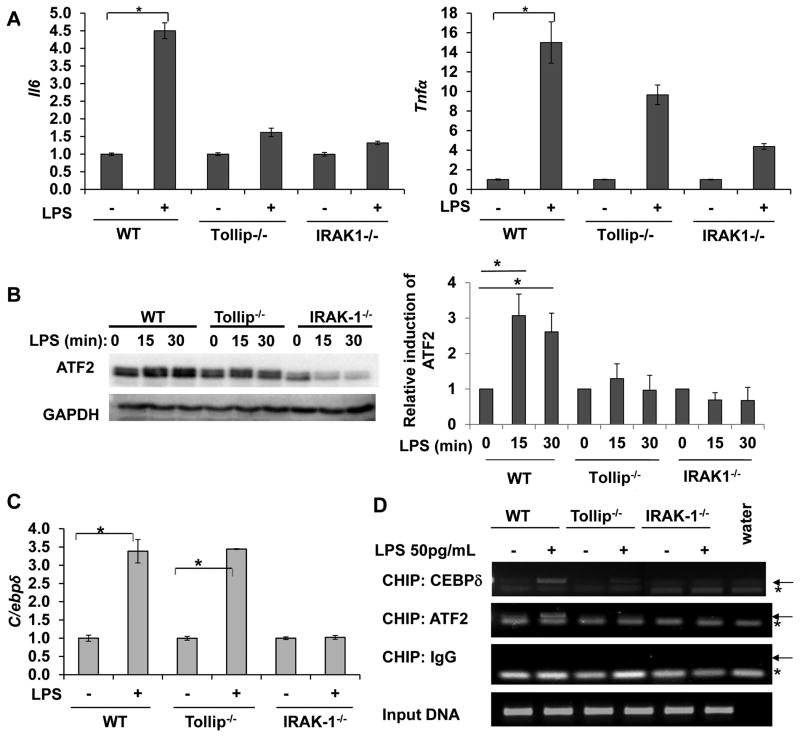
To further determine the upstream signaling pathways responsible for the mild expression of Il-6 and Tnfα, we examined the role of IRAK-1 and Tollip using macrophages harvested from IRAK-1 and Tollip deficient mice. This is based on previous reports suggesting that IRAK-1 and Tollip are involved in mediating the effect of low dose LPS (50, 51). In consistent with previous reports, the induction of Il-6 and Tnfα by low dose LPS was significantly reduced in IRAK-1 and Tollip deficient cells (Fig. 4A).
Figure 4. Low dose LPS mediated activation of ATF2 requires IRAK-1 and Tollip.
(A) Reduced induction of Il-6 and Tnfα in IRAK-1 and Tollip deficient BMDM cells. WT, IRAK-1 and Tollip deficient BMDM were treated with low dose LPS (50pg/mL) for 4 hrs and the transcript levels of Il-6 and Tnfα were analyzed by real time RT-PCR. (B) Induction of ATF2 in response to low dose LPS depends on IRAK-1 and Tollip. Whole cell lysates were prepared from WT, IRAK-1 and Tollip deficient BMDMs treated with low dose LPS for the indicated time points and analyzed by Western blot. The same blots were probed with GAPDH as loading controls. The adjusted resting levels of ATF2 in each cell type were set as 1, and the ATF2 levels in cells treated with LPS were compared and plotted. n=3; *p<0.05. (C) Low-dose LPS mediated induction of C/ebpδ message is dependent on IRAK-1. WT, IRAK-1 and Tollip deficient BMDM were treated with low dose LPS (50pg/mL) for 6 hrs and the C/ebpδ message levels were analyzed and plotted as shown. (D) Tollip and IRAK- 1 are required for low dose LPS mediated recruitment of ATF2 to the promoter of Il-6. WT, IRAK-1 and Tollip deficient BMDMs were either untreated or treated with 50 pg/mL LPS for 2 h and subjected to ChIP assay using antibodies specific to ATF-2 or C/EBPδ and primers specific to the proximal promoter of Il-6. The same samples were immunoprecipitated using IgG as specificity control and input DNA was analyzed as the loading control. Arrow points to the specific amplification product. * denotes a non-specific band.
We further examined the protein levels of ATF2 in various cells treated with low dose LPS. As shown in Fig. 4B, ATF2 protein levels were induced in WT cells treated with low dose LPS. In contrast, low dose LPS failed to increase ATF2 protein levels in either IRAK-1 or Tollip deficient cells. On the other hand, the late induction of c/ebpδ by low dose LPS is only dependent upon IRAK-1, but not Tollip (Fig. 4C).
Employing ChIP assays, we confirmed that low dose LPS failed to recruit ATF2 to the Il-6 promoter in either IRAK-1 or Tollip deficient mice (Fig. 4D). In terms of C/EBPδ, although its message induction is dependent upon IRAK-1 instead of Tollip, its active recruitment to the Il-6 promoter depend upon both IRAK-1 and Tollip. LPS induced recruitment of C/EBPδ is reduced in both IRAK-1 and Tollip deficient cells.
Tollip mediated ROS generation in mitochondria
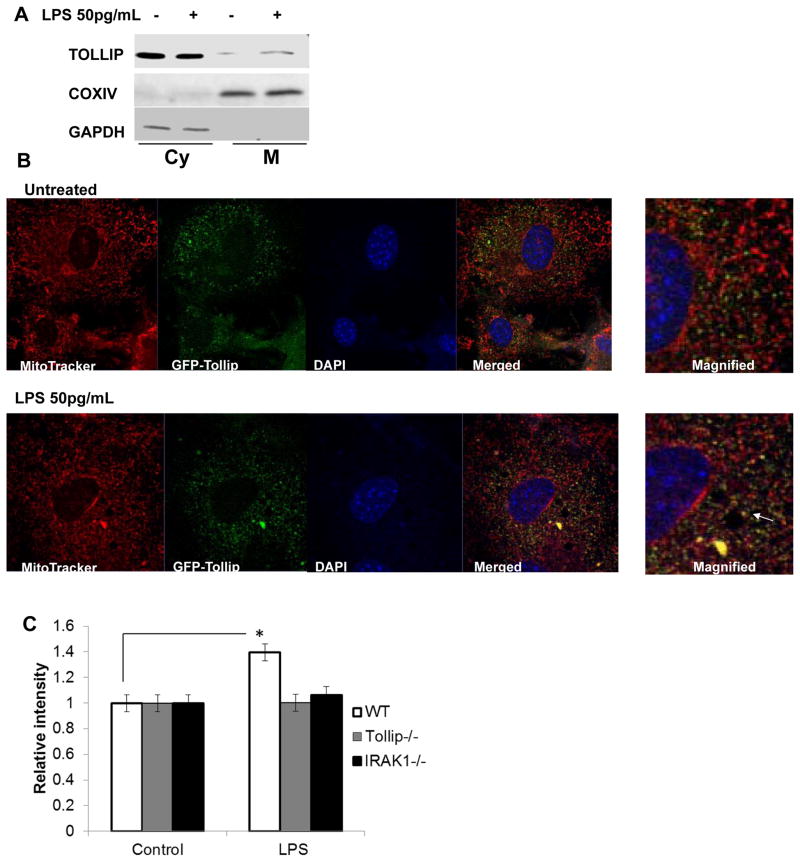
Tollip is a novel TLR intracellular adaptor molecule with poorly defined function (59). It contains a lipid-binding C2 domain and an ubiquitin-binding CUE domain (60). Limited studies suggest that Tollip might be involved in modulation of intra-cellular traffic (61, 62). To reveal the molecular mechanism responsible for Tollip-mediated activation of ATF2, we first examined cellular distribution of Tollip following LPS challenge. First, we fractionated cellular lysates from WT BMDM into soluble and mitochondrial fractions. Western blot analyses revealed that low dose LPS stimulation led to increased Tollip levels in the mitochondrial fraction (Fig. 5A). We realize that sub-cellular fractionation may contain potential contamination from other membrane sources. Thus, we also employed confocal microscopy approach to further confirm the mitochondria translocation of Tollip. To achieve this, we generated primary murine embryonic fibroblasts stably transfected with EGFP-Tollip fusion protein. Through confocal microscopy, we detected that low dose LPS induces Tollip translocation to mitochondria (Fig. 5B).
Figure 5. Low dose LPS translocates Tollip to the mitochondria and induces ROS.
(A) Mitochondrial localization of Tollip in response to low dose LPS using subcellular fractionation. WT BMDM cells were treated with low dose LPS and mitochondrial protein fractions were prepared. The purity of the mitochondrial fraction was determined using Western blot analysis for mitochondrial resident protein cyclooxygenase IV and cytosolic protein, GAPDH. (B) Mitochondrial localization of Tollip in response to low dose LPS using confocal microscopy. The GFP-Tollip transfected MEF cells were stained with Mito Tracker Red to stain the mitochondria. The nuclei were stained using DAPI (blue). The cells were visualized under laser-scanning confocal microscope. The merged images were magnified and shown on the right panel and the co-localization is indicated with an arrow. (C) Involvement of Tollip in mitochondrial ROS generation. WT, IRAK-1 and Tollip deficient BMDM were stained with mitochondrial ROS selective dye, MitoSOX Red. Cells were then washed with HBSS and treated with low dose LPS for 60 minutes. The cells were also stained with 1 μM Hoescht 34580 for normalization of fluorescent data. Fluorescent intensities were measured and quantified. n=3; * p<0.05.
Recent studies indicate that mitochondria play a key role in TLR signaling processes (63, 64). ROS generated from mitochondria induced by LPS plays a key role in the activation of TLR downstream pathway. In particular relevance, mitochondria ROS was shown to contribute to the stabilization of transcription factors including HIF1α and ATF2 (65, 66). To test whether Tollip is involved in the generation of mitochondria ROS, we measured the levels of mitochondria ROS using MitoSOX Red, a selective dye for mitochondria ROS. As shown in Fig. 5C, low dose LPS induced mitochondria ROS in WT cells, but failed to do so in either IRAK-1 or Tollip deficient cells.
Inhibition of mitochondrial ROS reduces low-grade induction of pro-inflammatory mediators
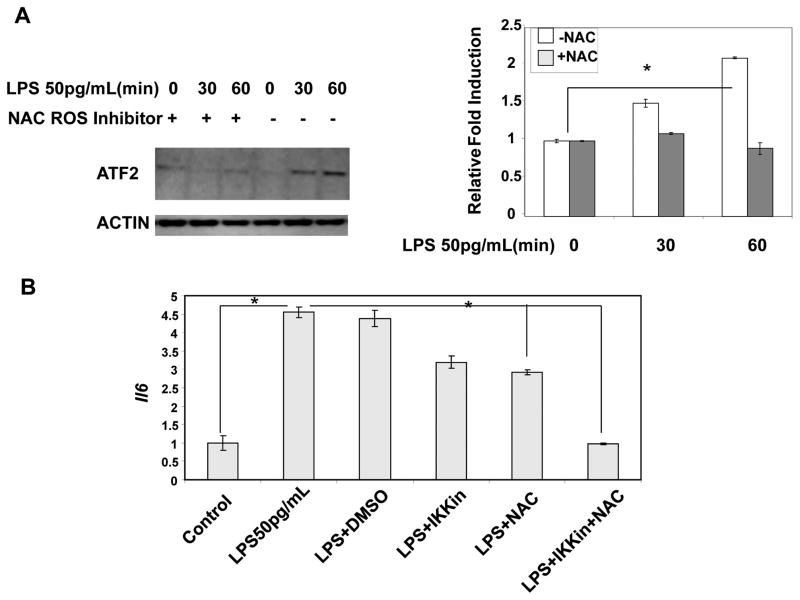
We further tested the role of ROS in the induction of IL-6 by low dose LPS by applying a selective ROS inhibitor, N-acetyl cysteine (NAC). As shown in Fig. 6a, NAC effectively blocked the induction of ATF2 by low dose LPS (Fig. 6A). Furthermore, NAC significantly reduced the expression of Il-6 induced by low dose LPS (Fig. 6B). As C/EBPδ is reported to be induced by IRAK-1 through IKKε (50, 58), we demonstrated that joint inhibition of mitochondrial ROS and IKKε completely ablated the induction of IL-6 by low dose LPS (Fig 6B).
Figure 6. Mitochondrial ROS contributes to induction of low grade inflammation.
(A) The induction of ATF2 by low dose LPS is blocked by ROS inhibitor. WT BMDM cells treated with low dose LPS (50 pg/mL) in the presence or absence of NAC for the indicated time points. Total cellular lysates were analyzed for ATF2 induction in response to low dose LPS. The band intensities were quantitated and the relative fold expressions were plotted. n=3; * p<0.05. (B) Inhibition of low dose LPS induced Il-6 by ROS inhibitor. WT BMDM cells were pretreated with DMSO, NAC or an IKKε inhibitor (IKKin) for 30 min and then treated with low dose LPS (50 pg/mL) for 4 hrs. The cells were harvested and the total RNA was used to detect the transcript levels of Il-6.
DISCUSSION
Our study reveals novel intracellular mechanisms utilized by subclinical low dose LPS in the skewed and low-grade induction of pro-inflammatory mediators. First, low dose LPS fails to activate either the classical NFκB pathway or pathways activating MAP kinases. Second, low dose LPS suppresses, instead of activating, the compensatory and anti-inflammatory PI3K pathway. Third, low dose LPS utilizes IRAK-1 and Tollip to induce mitochondrial ROS, that subsequently activates ATF2. The lack of negative feedbacks in cells challenged with a low dose LPS may enable prolonged expression of pro-inflammatory mediators, a potential culprit for chronic inflammatory diseases often associated with low grade endotoxemia.
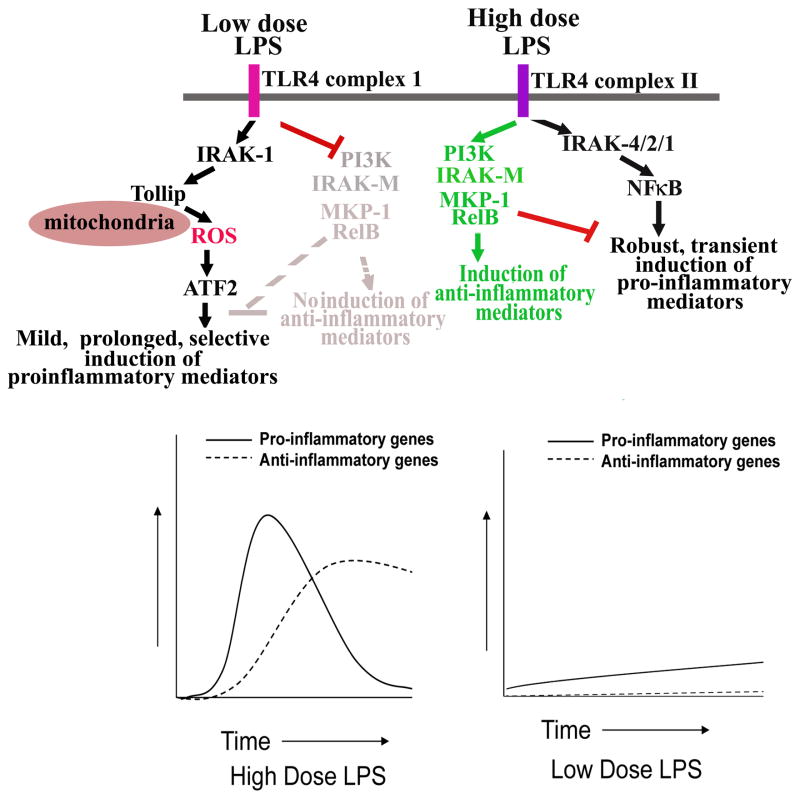
These mechanisms reconcile recent clinical findings that low dose endotoxemia closely correlates with low grade inflammation and the pathogenesis of chronic human diseases including atherosclerosis, diabetes, and neurological diseases(67) (68–70). Cardinal features of chronic low grade inflammation include mild and persistent expression of inflammatory mediators as well as skewed pro-inflammatory profiles. For example, aged individuals tend to have elevated plasma levels of pro-inflammatory mediators including CRP and IL-6 (71, 72), reduced antigen-presentation capabilities (73, 74), as well as reduced levels of anti-inflammatory TGFβ and IL-10 (75). Our finding that low dose LPS fails to induce PI3K pathway and other negative regulators of inflammatory pathways (MKP-1, SOCS1, and degradation of IRAK-1) provides a potential explanation for skewed pro-inflammatory profiles associated with low grade inflammation. When faced with a higher dose LPS, multiple pathways in macrophages are activated that lead to robust induction of both pro- and anti-inflammatory mediators. As a consequence, the coordinated and balanced portfolio of pro- and anti-inflammatory pathways ensures transient inflammatory responses and subsequent resolution. Perhaps the levels of endotoxin during low grade inflammation may fall below the “threshold of alarm” that can effectively invoke an anti-inflammatory resolution, thus undermining the long-term danger of chronic inflammatory diseases (Figure 7).
Figure 7. A schematic illustration of potential mechanisms responsible for low grade inflammation induced by low dose LPS.
Low dose LPS activates ATF2 through IRAK-1 and Tollip-mediated production of mitochondria ROS. In the meantime, low dose LPS suppresses PI3K, IRAK-M, and other related negative regulators. As a consequence, low dose LPS induces mild, prolonged, and skewed expression of pro-inflammatory mediators. In contrast, high dose LPS potently activated NFκB pathway, as well as the PI3K pathway, and causes robust yet transient expression of pro- and anti-inflammatory mediators.
The lack of robust NFκB activation and limited activation of ATF2 and C/EBPδ identified in this study may underlie the mild expression pattern of pro-inflammatory mediators. Although ATF2 and C/EBPδ may not be capable of robustly inducing the expression of pro-inflammatory mediators, they are also not capable of evoking negative feedbacks. Instead, both ATF2 and C/EBPδ have been shown to induce a positive feedback that leads to their own expression (30, 76). The selective presence of a positive feedback implies a novel “digital” or “bi-stable” toggle switch controlling the activation of macrophages by low dose endotoxin. In contrast to a linear or “analog” switch due to the activation of a linear pathway, a “digital” switch due to a positive feedback circuit can build up an “energy reservoir” and only be turned on when the concentration of stimulant reaches a critical threshold (77–79). On the other hand, once the cells are activated, the presence of the “energy reservoir” would allow the cells to stay activated with significantly less stimulant. This phenomenon, called “hysteresis”, is well-documented in relevant biological contexts, such as cell cycle and DNA annealing (77–79). This hysteresis, which has not been applied in leukocyte biology, would nicely explain several key features of leukocyte physiology and pathology. For example, our immune response is built to withstand challenges without easily getting inflammatory diseases. However, once humans are challenged with a sufficient amount of LPS, due to chronic infection, obesity or other inflammatory conditions, the inflammatory state will be easy to maintain and difficult to get rid of. Indeed, we collected data indicating the critical LPS concentration required for inducing IL-6 expression in macrophages is ~25–50pg/mL (Supplementary Figures S1, S2, S3). Once the cells are pre-treated with 50 pg/mL LPS, a much lower LPS concentration (~5pg/mL) is able to maintain the expression of IL-6. We realize that human plasma environments in vivo as well as cell culture medium in vitro include a plethora of reagents and stimulants. The exact LPS concentration that may elicit a digital activation of macrophages would most likely vary under different conditions. Nevertheless, our experiments with precisely added LPS in vitro reveal a fundamental principle for the novel nature of macrophage activation.
Our data reinforce the notion that mitochondria ROS plays a key role in innate immunity regulation by LPS. Recent studies indicate that mitochondria ROS induced by LPS participate in the expression of pro-inflammatory mediators (64, 80). Mechanistically, LPS is shown to induce the translocation of TRAF6 to mitochondria, ubiquitination of ECSIT, and disruption of electron transport system (63, 64). These events may collectively lead to the generation of mitochondria ROS. Our data reveal that IRAK-1 and Tollip are key signaling molecules responsible for the generation of mitochondria ROS. To our knowledge, Tollip is the first TLR4 intra-cellular adaptor molecule shown to translocate into mitochondria following LPS challenge. It is worth mentioning that Tollip is a novel TLR adaptor molecule capable of binding to phosphoinositides, a phenomenon first reported by our group (81). In addition, Tollip contains an ubiquitin-binding CUE domain that can potentially facilitate protein ubiquitination and degradation (82). Based on our data, future studies are clearly warranted to determine the role and mechanism of Tollip-meditated generation of mitochondria ROS. It is also important to note that the role and regulation of Tollip are highly complex. Despite its pro-inflammatory role in cells stimulated with low dose LPS, Tollip may dampen pro-inflammatory responses in cells when the NFκB pathway is activated (83). Although the mechanisms are far from clear, we hypothesize that Tollip may distribute to distinct sub-cellular compartments and form distinct protein complexes in cells treated with varying dosages of TLR agonists.
Our study reveals an intriguing paradigm of PI3K modulation by low vs. high dose LPS. Although high dose LPS is known to activate PI3K, this report first reveals that low dose LPS potently suppresses basal PI3K activation. The opposing effects of low vs high dose LPS on PI3K may be responsible for multiple un-explained paradigms. In addition to the skewed pro-inflammatory profiles in cells challenged with a low dose LPS, macrophages/monocytes can be programmed to exhibit either “LPS tolerance” or “LPS priming” dependent upon the initial dosage of LPS challenge. High dose LPS causes tolerance while low dose LPS causes priming(84). The differential modulation of PI3K by different dosages of LPS may contribute to these opposing effects. Further complementing this claim, our published data indicate that low dose LPS activates GSK3β, while high dose LPS is known to reduce GSK3β activity (41). Consistent with our finding, a recent study reported that brain tissues harvested from mice injected with a low dose LPS have increased GSK3β activity (85). This is associated with low grade inflammation and the pathogenesis of Alzheimer’s disease (85). Several other independent studies also support the conclusion that low dose endotoxemia directly contributes to chronic low grade neurological inflammation and chronic diseases (16, 67). The molecular mechanisms responsible for the opposing effect of LPS on PI3K are not clear. High dose LPS activates PI3K through activate recruitment of p85 to MyD88 (86). An intriguing recent study indicates that PKD and PKC can potently suppress basal PI3K activation by causing a distinct phosphorylation pattern of p85 (87). LPS is known to activate PKC(88). The balance and coordination between the activation of PKC and PI3K by LPS may finely modulate the pro- vs anti-inflammatory outcomes. Future studies are clearly warranted to dissect the differential regulation of PI3K by low vs high dose LPS.
We also demonstrated that low dose LPS exerts an opposing regulation of key negative regulators in TLR4 pathway as compared to high dose LPS. Specifically, we demonstrated that low dose LPS potently suppresses IRAK-M and fails to induce MKP-1. Furthermore, low dose LPS decreases the suppressive RelB on the promoter of Il-6. This is in stark contrast to increased levels of IRAK-M, MKP-1, and increased RelB recruitment to Il-6 promoter in tolerance macrophages challenged with high dose LPS. This mechanism underlies the skewed and low-grade inflammation elicited by low dose LPS.
Although it is clear that low and high dose LPS induces distinct downstream signaling networks, the responsible mechanisms at the receptor level are not well understood. TLR4 traffics between endosome and plasma membrane, and can mediate LPS signaling at both locations (89). Our data indicate that the cell surface TLR4 is essential for the effect of low dose LPS (Supplementary Figure S4). Given the fact that TLR4 may hetero-dimerize with multiple other receptors including CD36 and MAC1(90–92), we hypothesize that low dose LPS may engage a unique set of receptor configuration at the cell surface, and may prevent further receptor internalization. Future studies are needed to determine the receptor configuration and related proximal signaling network responsible for mediating the opposing effects of low vs. high doses LPS.
In summary, our study reveals novel mechanisms responsible for low grade inflammation induced by low dose LPS. Key features identified include IRAK-1 and Tollip mediated generation of mitochondria ROS as well as selective suppression of PI3K. The opposing effects of low vs. high dose LPS identified in this report explain the intriguing paradigm of endotoxin priming and tolerance, and provoke future studies paying close attention to the dosages as well as history of LPS challenges experienced by the host. Intervention of the unique cellular network activated by low dose LPS may hold significance promise in designing effective therapies for low grade and chronic inflammatory diseases.
Supplementary Material
Acknowledgments
Authorship
T.G., U.M., H.D., and B.B. conducted the experiments. L.L.,T.G., U.M., H.D. and B.B. designed the experiment, and analyzed the data. Z.L and D.C. provided critical reagent and comments. U.M, and L.L. wrote the manuscript.
We thank Matt Morris for assistance in the harvest and culture of macrophages, members of the Li laboratory for discussions.
This work was supported by grants from the American Heart Association, the National Institute of Health, and funds from Virginia Tech to L.L. Z.L. is supported by NIH grant AI070603. The online version of this article contains a data supplement.
References
- 1.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 2.Goto T, Eden S, Nordenstam G, Sundh V, Svanborg-Eden C, Mattsby-Baltzer I. Endotoxin levels in sera of elderly individuals. Clin Diagn Lab Immunol. 1994;1:684–688. doi: 10.1128/cdli.1.6.684-688.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ancuta P, Kamat A, Kunstman KJ, Kim EY, Autissier P, Wurcel A, Zaman T, Stone D, Mefford M, Morgello S, Singer EJ, Wolinsky SM, Gabuzda D. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3:e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szeto CC, Kwan BC, Chow KM, Lai KB, Chung KY, Leung CB, Li PK. Endotoxemia is related to systemic inflammation and atherosclerosis in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2008;3:431–436. doi: 10.2215/CJN.03600807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiedermann CJ, Kiechl S, Dunzendorfer S, Schratzberger P, Egger G, Oberhollenzer F, Willeit J. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease prospective results from the Bruneck Study. J Am Coll Cardiol. 1999;34:1975–1981. doi: 10.1016/s0735-1097(99)00448-9. [DOI] [PubMed] [Google Scholar]
- 6.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638–644. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lira FS, Rosa JC, Pimentel GD, Souza HA, Caperuto EC, Carnevali LC, Jr, Seelaender M, Damaso AR, Oyama LM, de Mello MT, Santos RV. Endotoxin levels correlate positively with a sedentary lifestyle and negatively with highly trained subjects. Lipids Health Dis. 2010;9:82. doi: 10.1186/1476-511X-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manco M, Putignani L, Bottazzo GF. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev. 2010;31:817–844. doi: 10.1210/er.2009-0030. [DOI] [PubMed] [Google Scholar]
- 9.Maachi M, Pieroni L, Bruckert E, Jardel C, Fellahi S, Hainque B, Capeau J, Bastard JP. Systemic low-grade inflammation is related to both circulating and adipose tissue TNFalpha, leptin and IL-6 levels in obese women. Int J Obes Relat Metab Disord. 2004;28:993–997. doi: 10.1038/sj.ijo.0802718. [DOI] [PubMed] [Google Scholar]
- 10.Sun L, Yu Z, Ye X, Zou S, Li H, Yu D, Wu H, Chen Y, Dore J, Clement K, Hu FB, Lin X. A marker of endotoxemia is associated with obesity and related metabolic disorders in apparently healthy Chinese. Diabetes Care. 2010;33:1925–1932. doi: 10.2337/dc10-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta NN, McGillicuddy FC, Anderson PD, Hinkle CC, Shah R, Pruscino L, Tabita-Martinez J, Sellers KF, Rickels MR, Reilly MP. Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes. 2010;59:172–181. doi: 10.2337/db09-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terawaki H, Yokoyama K, Yamada Y, Maruyama Y, Iida R, Hanaoka K, Yamamoto H, Obata T, Hosoya T. Low-grade endotoxemia contributes to chronic inflammation in hemodialysis patients examination with a novel lipopolysaccharide detection method. Ther Apher Dial. 2010;14:477–482. doi: 10.1111/j.1744-9987.2010.00815.x. [DOI] [PubMed] [Google Scholar]
- 13.Laugerette F, Vors C, Geloen A, Chauvin MA, Soulage C, Lambert-Porcheron S, Peretti N, Alligier M, Burcelin R, Laville M, Vidal H, Michalski MC. Emulsified lipids increase endotoxemia possible role in early postprandial low-grade inflammation. J Nutr Biochem. 2011;22:53–59. doi: 10.1016/j.jnutbio.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Moreno-Navarrete JM, Manco M, Ibanez J, Garcia-Fuentes E, Ortega F, Gorostiaga E, Vendrell J, Izquierdo M, Martinez C, Nolfe G, Ricart W, Mingrone G, Tinahones F, Fernandez-Real JM. Metabolic endotoxemia and saturated fat contribute to circulating NGAL concentrations in subjects with insulin resistance. Int J Obes (Lond) 2009 doi: 10.1038/ijo.2009.242. [DOI] [PubMed] [Google Scholar]
- 15.Wiesner P, Choi SH, Almazan F, Benner C, Huang W, Diehl CJ, Gonen A, Butler S, Witztum JL, Glass CK, Miller YI. Low doses of lipopolysaccharide and minimally oxidized low-density lipoprotein cooperatively activate macrophages via nuclear factor kappab and activator protein-1 possible mechanism for acceleration of atherosclerosis by subclinical endotoxemia. Circ Res. 2010;107:56–65. doi: 10.1161/CIRCRESAHA.110.218420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, Liu Y, Hoffmann U, Bauer DC, Newman AB, Kritchevsky SB, Harris TB, Butler J. Inflammatory markers and incident heart failure risk in older adults the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol. 2010;55:2129–2137. doi: 10.1016/j.jacc.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ridker PM. Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality implications for longevity. Nutr Rev. 2007;65:S253–259. doi: 10.1111/j.1753-4887.2007.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 19.Danesh J, Erqou S, Walker M, Thompson SG, Tipping R, Ford C, Pressel S, Walldius G, Jungner I, Folsom AR, Chambless LE, Knuiman M, Whincup PH, Wannamethee SG, Morris RW, Willeit J, Kiechl S, Santer P, Mayr A, Wald N, Ebrahim S, Lawlor DA, Yarnell JW, Gallacher J, Casiglia E, Tikhonoff V, Nietert PJ, Sutherland SE, Bachman DL, Keil JE, Cushman M, Psaty BM, Tracy RP, Tybjaerg-Hansen A, Nordestgaard BG, Frikke-Schmidt R, Giampaoli S, Palmieri L, Panico S, Vanuzzo D, Pilotto L, Simons L, McCallum J, Friedlander Y, Fowkes FG, Lee AJ, Smith FB, Taylor J, Guralnik J, Phillips C, Wallace R, Blazer D, Khaw KT, Jansson JH, Donfrancesco C, Salomaa V, Harald K, Jousilahti P, Vartiainen E, Woodward M, D’Agostino RB, Wolf PA, Vasan RS, Pencina MJ, Bladbjerg EM, Jorgensen T, Moller L, Jespersen J, Dankner R, Chetrit A, Lubin F, Rosengren A, Wilhelmsen L, Lappas G, Eriksson H, Bjorkelund C, Cremer P, Nagel D, Tilvis R, Strandberg T, Rodriguez B, Bouter LM, Heine RJ, Dekker JM, Nijpels G, Stehouwer CD, Rimm E, Pai J, Sato S, Iso H, Kitamura A, Noda H, Goldbourt U, Salonen JT, Nyyssonen K, Tuomainen TP, Deeg D, Poppelaars JL, Meade T, Cooper J, Hedblad B, Berglund G, Engstrom G, Doring A, Koenig W, Meisinger C, Mraz W, Kuller L, Selmer R, Tverdal A, Nystad W, Gillum R, Mussolino M, Hankinson S, Manson J, De Stavola B, Knottenbelt C, Cooper JA, Bauer KA, Rosenberg RD, Naito Y, Holme I, Nakagawa H, Miura H, Ducimetiere P, Jouven X, Crespo C, Garcia-Palmieri M, Amouyel P, Arveiler D, Evans A, Ferrieres J, Schulte H, Assmann G, Shepherd J, Packard C, Sattar N, Cantin B, Lamarche B, Despres JP, Dagenais GR, Barrett-Connor E, Wingard D, Bettencourt R, Gudnason V, Aspelund T, Sigurdsson G, Thorsson B, Trevisan M, Witteman J, Kardys I, Breteler M, Hofman A, Tunstall-Pedoe H, Tavendale R, Lowe GD, Ben-Shlomo Y, Howard BV, Zhang Y, Best L, Umans J, Onat A, Meade TW, Njolstad I, Mathiesen E, Lochen ML, Wilsgaard T, Gaziano JM, Stampfer M, Ridker P, Ulmer H, Diem G, Concin H, Rodeghiero F, Tosetto A, Brunner E, Shipley M, Buring J, Cobbe SM, Ford I, Robertson M, He Y, Ibanez AM, Feskens EJ, Kromhout D, Collins R, Di Angelantonio E, Kaptoge S, Lewington S, Orfei L, Pennells L, Perry P, Ray K, Sarwar N, Scherman M, Thompson A, Watson S, Wensley F, White IR, Wood AM. The Emerging Risk Factors Collaboration analysis of individual data on lipid, inflammatory and other markers in over 1.1 million participants in 104 prospective studies of cardiovascular diseases. Eur J Epidemiol. 2007;22:839–869. doi: 10.1007/s10654-007-9165-7. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol. 2007;49:2129–2138. doi: 10.1016/j.jacc.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 21.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2010 doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sturm R. The effects of obesity, smoking, and drinking on medical problems and costs. Health Aff (Millwood) 2002;21:245–253. doi: 10.1377/hlthaff.21.2.245. [DOI] [PubMed] [Google Scholar]
- 23.Gordon S. The macrophage past, present and future. Eur J Immunol. 2007;37(Suppl 1):S9–17. doi: 10.1002/eji.200737638. [DOI] [PubMed] [Google Scholar]
- 24.Schlegel N, Baumer Y, Drenckhahn D, Waschke J. Lipopolysaccharide-induced endothelial barrier breakdown is cyclic adenosine monophosphate dependent in vivo and in vitro. Crit Care Med. 2009;37:1735–1743. doi: 10.1097/CCM.0b013e31819deb6a. [DOI] [PubMed] [Google Scholar]
- 25.Scheifele DW, Olsen EM, Pendray MR. Endotoxinemia and thrombocytopenia during neonatal necrotizing enterocolitis. Am J Clin Pathol. 1985;83:227–229. doi: 10.1093/ajcp/83.2.227. [DOI] [PubMed] [Google Scholar]
- 26.Dofferhoff AS, Nijland JH, de Vries-Hospers HG, Mulder PO, Weits J, Bom VJ. Effects of different types and combinations of antimicrobial agents on endotoxin release from gram-negative bacteria an in-vitro and in-vivo study. Scand J Infect Dis. 1991;23:745–754. doi: 10.3109/00365549109024303. [DOI] [PubMed] [Google Scholar]
- 27.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Jacinto R, Hartung T, McCall C, Li L. Lipopolysaccharide- and lipoteichoic acid-induced tolerance and cross-tolerance distinct alterations in IL-1 receptor-associated kinase. J Immunol. 2002;168:6136–6141. doi: 10.4049/jimmunol.168.12.6136. [DOI] [PubMed] [Google Scholar]
- 29.Medvedev AE, Lentschat A, Wahl LM, Golenbock DT, Vogel SN. Dysregulation of LPS-induced Toll-like receptor 4-MyD88 complex formation and IL-1 receptor-associated kinase 1 activation in endotoxin-tolerant cells. J Immunol. 2002;169:5209–5216. doi: 10.4049/jimmunol.169.9.5209. [DOI] [PubMed] [Google Scholar]
- 30.Litvak V, Ramsey SA, Rust AG, Zak DE, Kennedy KA, Lampano AE, Nykter M, Shmulevich I, Aderem A. Function of C/EBPdelta in a regulatory circuit that discriminates between transient and persistent TLR4-induced signals. Nat Immunol. 2009;10:437–443. doi: 10.1038/ni.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA., Jr MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Cousart S, Hu J, McCall CE. Characterization of interleukin-1 receptor-associated kinase in normal and endotoxin-tolerant cells. J Biol Chem. 2000;275:23340–23345. doi: 10.1074/jbc.M001950200. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 35.Nahid MA, Pauley KM, Satoh M, Chan EK. miR-146a is critical for endotoxin-induced tolerance IMPLICATION IN INNATE IMMUNITY. J Biol Chem. 2009;284:34590–34599. doi: 10.1074/jbc.M109.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piao W, Song C, Chen H, Diaz MA, Wahl LM, Fitzgerald KA, Li L, Medvedev AE. Endotoxin tolerance dysregulates MyD88- and Toll/IL-1R domain-containing adapter inducing IFN-beta-dependent pathways and increases expression of negative regulators of TLR signaling. J Leukoc Biol. 2009;86:863–875. doi: 10.1189/jlb.0309189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X, Yoza BK, El Gazzar M, Hu JY, Cousart SL, McCall CE. RelB sustains IkappaBalpha expression during endotoxin tolerance. Clin Vaccine Immunol. 2009;16:104–110. doi: 10.1128/CVI.00320-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Medina EA, I, Morris R, Berton MT. Phosphatidylinositol 3-kinase activation attenuates the TLR2-mediated macrophage proinflammatory cytokine response to Francisella tularensis live vaccine strain. J Immunol. 2010;185:7562–7572. doi: 10.4049/jimmunol.0903790. [DOI] [PubMed] [Google Scholar]
- 39.Chaurasia B, Mauer J, Koch L, Goldau J, Kock AS, Bruning JC. Phosphoinositide-dependent kinase 1 provides negative feedback inhibition to Toll-like receptor-mediated NF-kappaB activation in macrophages. Mol Cell Biol. 2010;30:4354–4366. doi: 10.1128/MCB.00069-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Antoniv TT, Ivashkiv LB. Interleukin-10-induced gene expression and suppressive function are selectively modulated by the PI3K-Akt-GSK3 pathway. Immunology. 2011;132:567–577. doi: 10.1111/j.1365-2567.2010.03402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu QY, Liu Q, Chen JX, Lan K, Ge BX. MicroRNA-101 targets MAPK phosphatase-1 to regulate the activation of MAPKs in macrophages. J Immunol. 2010;185:7435–7442. doi: 10.4049/jimmunol.1000798. [DOI] [PubMed] [Google Scholar]
- 43.Androulidaki A, Iliopoulos D, Arranz A, Doxaki C, Schworer S, Zacharioudaki V, Margioris AN, Tsichlis PN, Tsatsanis C. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity. 2009;31:220–231. doi: 10.1016/j.immuni.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, Morrison DC. Lipopolysaccharide-induced selective priming effects on tumor necrosis factor alpha and nitric oxide production in mouse peritoneal macrophages. J Exp Med. 1993;177:511–516. doi: 10.1084/jem.177.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henricson BE, Manthey CL, Perera PY, Hamilton TA, Vogel SN. Dissociation of lipopolysaccharide (LPS)-inducible gene expression in murine macrophages pretreated with smooth LPS versus monophosphoryl lipid A. Infect Immun. 1993;61:2325–2333. doi: 10.1128/iai.61.6.2325-2333.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hirohashi N, Morrison DC. Low-dose lipopolysaccharide (LPS) pretreatment of mouse macrophages modulates LPS-dependent interleukin-6 production in vitro. Infect Immun. 1996;64:1011–1015. doi: 10.1128/iai.64.3.1011-1015.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shnyra A, Brewington R, Alipio A, Amura C, Morrison DC. Reprogramming of lipopolysaccharide-primed macrophages is controlled by a counterbalanced production of IL-10 and IL-12. J Immunol. 1998;160:3729–3736. [PubMed] [Google Scholar]
- 48.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naiki Y, Sorrentino R, Wong MH, Michelsen KS, Shimada K, Chen S, Yilmaz A, Slepenkin A, Schroder NW, Crother TR, Bulut Y, Doherty TM, Bradley M, Shaposhnik Z, Peterson EM, Tontonoz P, Shah PK, Arditi M. TLR/MyD88 and liver X receptor alpha signaling pathways reciprocally control Chlamydia pneumoniae-induced acceleration of atherosclerosis. J Immunol. 2008;181:7176–7185. doi: 10.4049/jimmunol.181.10.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maitra U, Gan L, Chang S, Li L. Low-Dose Endotoxin Induces Inflammation by Selectively Removing Nuclear Receptors and Activating CCAAT/Enhancer-Binding Protein {delta} J Immunol. 2011;186:4467–4473. doi: 10.4049/jimmunol.1003300. [DOI] [PubMed] [Google Scholar]
- 51.Didierlaurent A, Brissoni B, Velin D, Aebi N, Tardivel A, Kaslin E, Sirard JC, Angelov G, Tschopp J, Burns K. Tollip regulates proinflammatory responses to interleukin-1 and lipopolysaccharide. Mol Cell Biol. 2006;26:735–742. doi: 10.1128/MCB.26.3.735-742.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu B, Yang Y, Qiu Z, Staron M, Hong F, Li Y, Wu S, Hao B, Bona R, Han D, Li Z. Folding of Toll-like receptors by the HSP90 paralogue gp96 requires a substrate-specific cochaperone. Nat Commun. 2010;1:79. doi: 10.1038/ncomms1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kinjyo I, Hanada T, Inagaki-Ohara K, Mori H, Aki D, Ohishi M, Yoshida H, Kubo M, Yoshimura A. SOCS1/JAB is a negative regulator of LPS-induced macrophage activation. Immunity. 2002;17:583–591. doi: 10.1016/s1074-7613(02)00446-6. [DOI] [PubMed] [Google Scholar]
- 54.Noubir S, Hmama Z, Reiner NE. Dual receptors and distinct pathways mediate interleukin-1 receptor-associated kinase degradation in response to lipopolysaccharide. Involvement of CD14/TLR4, CR3, and phosphatidylinositol 3-kinase. J Biol Chem. 2004;279:25189–25195. doi: 10.1074/jbc.M312431200. [DOI] [PubMed] [Google Scholar]
- 55.Chen X, El Gazzar M, Yoza BK, McCall CE. The NF-kappaB factor RelB and histone H3 lysine methyltransferase G9a directly interact to generate epigenetic silencing in endotoxin tolerance. J Biol Chem. 2009;284:27857–27865. doi: 10.1074/jbc.M109.000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoza BK, Hu JY, Cousart SL, Forrest LM, McCall CE. Induction of RelB participates in endotoxin tolerance. J Immunol. 2006;177:4080–4085. doi: 10.4049/jimmunol.177.6.4080. [DOI] [PubMed] [Google Scholar]
- 57.Gustin JA, Korgaonkar CK, Pincheira R, Li Q, Donner DB. Akt regulates basal and induced processing of NF-kappaB2 (p100) to p52. J Biol Chem. 2006;281:16473–16481. doi: 10.1074/jbc.M507373200. [DOI] [PubMed] [Google Scholar]
- 58.Kravchenko VV, Mathison JC, Schwamborn K, Mercurio F, Ulevitch RJ. IKKi/IKKepsilon plays a key role in integrating signals induced by pro-inflammatory stimuli. J Biol Chem. 2003;278:26612–26619. doi: 10.1074/jbc.M303001200. [DOI] [PubMed] [Google Scholar]
- 59.Capelluto DG. Tollip: A multitasking protein in innate immunity and protein trafficking. Microbes Infect. 2011 doi: 10.1016/j.micinf.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 60.Burns K, Martinon F, Esslinger C, Pahl H, Schneider P, Bodmer JL, Di Marco F, French L, Tschopp J. MyD88, an adapter protein involved in interleukin-1 signaling. J Biol Chem. 1998;273:12203–12209. doi: 10.1074/jbc.273.20.12203. [DOI] [PubMed] [Google Scholar]
- 61.Brissoni B, Agostini L, Kropf M, Martinon F, Swoboda V, Lippens S, Everett H, Aebi N, Janssens S, Meylan E, Felberbaum-Corti M, Hirling H, Gruenberg J, Tschopp J, Burns K. Intracellular trafficking of interleukin-1 receptor I requires Tollip. Curr Biol. 2006;16:2265–2270. doi: 10.1016/j.cub.2006.09.062. [DOI] [PubMed] [Google Scholar]
- 62.Katoh Y, Imakagura H, Futatsumori M, Nakayama K. Recruitment of clathrin onto endosomes by the Tom1-Tollip complex. Biochem Biophys Res Commun. 2006;341:143–149. doi: 10.1016/j.bbrc.2005.12.156. [DOI] [PubMed] [Google Scholar]
- 63.West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol. 2011;11:389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.West AP, I, Brodsky E, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang D, Malo D, Hekimi S. Elevated mitochondrial reactive oxygen species generation affects the immune response via hypoxia-inducible factor-1alpha in long-lived Mclk1+/- mouse mutants. J Immunol. 2010;184:582–590. doi: 10.4049/jimmunol.0902352. [DOI] [PubMed] [Google Scholar]
- 66.Choi JH, Cho HK, Choi YH, Cheong J. Activating transcription factor 2 increases transactivation and protein stability of hypoxia-inducible factor 1alpha in hepatocytes. Biochem J. 2009;424:285–296. doi: 10.1042/BJ20090371. [DOI] [PubMed] [Google Scholar]
- 67.Moreno B, Jukes JP, Vergara-Irigaray N, Errea O, Villoslada P, Perry VH, Newman TA. Systemic inflammation induces axon injury during brain inflammation. Ann Neurol. 2011;70:932–942. doi: 10.1002/ana.22550. [DOI] [PubMed] [Google Scholar]
- 68.Nymark M, Pietilainen KH, Kaartinen K, Pussinen PJ, Syrjanen J, Forsblom C, Porsti I, Rissanen A, Kaprio J, Mustonen J, Groop PH, Lehto M. Bacterial Endotoxin Activity in Human Serum Is Associated With Dyslipidemia, Insulin Resistance, Obesity, and Chronic Inflammation. Diabetes Care. 2011 doi: 10.2337/dc10-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kallio KA, Buhlin K, Jauhiainen M, Keva R, Tuomainen AM, Klinge B, Gustafsson A, Pussinen PJ. Lipopolysaccharide associates with pro-atherogenic lipoproteins in periodontitis patients. Innate Immun. 2008;14:247–253. doi: 10.1177/1753425908095130. [DOI] [PubMed] [Google Scholar]
- 70.Lassenius MI, Pietilainen KH, Kaartinen K, Pussinen PJ, Syrjanen J, Forsblom C, Porsti I, Rissanen A, Kaprio J, Mustonen J, Groop PH, Lehto M. Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care. 2011;34:1809–1815. doi: 10.2337/dc10-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wikby A, Nilsson BO, Forsey R, Thompson J, Strindhall J, Lofgren S, Ernerudh J, Pawelec G, Ferguson F, Johansson B. The immune risk phenotype is associated with IL-6 in the terminal decline stage findings from the Swedish NONA immune longitudinal study of very late life functioning. Mech Ageing Dev. 2006;127:695–704. doi: 10.1016/j.mad.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 72.Tan ZS, Beiser AS, Vasan RS, Roubenoff R, Dinarello CA, Harris TB, Benjamin EJ, Au R, Kiel DP, Wolf PA, Seshadri S. Inflammatory markers and the risk of Alzheimer disease the Framingham Study. Neurology. 2007;68:1902–1908. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- 73.Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S. Innate immunity in aging impact on macrophage function. Aging Cell. 2004;3:161–167. doi: 10.1111/j.1474-9728.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- 74.Plowden J, Renshaw-Hoelscher M, Gangappa S, Engleman C, Katz JM, Sambhara S. Impaired antigen-induced CD8+ T cell clonal expansion in aging is due to defects in antigen presenting cell function. Cell Immunol. 2004;229:86–92. doi: 10.1016/j.cellimm.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 75.Santiago AF, Alves AC, Oliveira RP, Fernandes RM, Paula-Silva J, Assis FA, Carvalho CR, Weiner HL, Faria AM. Aging correlates with reduction in regulatory-type cytokines and T cells in the gut mucosa. Immunobiology. 2011;216:1085–1093. doi: 10.1016/j.imbio.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Dam H, Castellazzi M. Distinct roles of Jun Fos and Jun : ATF dimers in oncogenesis. Oncogene. 2001;20:2453–2464. doi: 10.1038/sj.onc.1204239. [DOI] [PubMed] [Google Scholar]
- 77.Tyson JJ, Novak B. Functional motifs in biochemical reaction networks. Annu Rev Phys Chem. 2010;61:219–240. doi: 10.1146/annurev.physchem.012809.103457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tyson JJ, Chen K, Novak B. Network dynamics and cell physiology. Nat Rev Mol Cell Biol. 2001;2:908–916. doi: 10.1038/35103078. [DOI] [PubMed] [Google Scholar]
- 79.Sha W, Moore J, Chen K, Lassaletta AD, Yi CS, Tyson JJ, Sible JC. Hysteresis drives cell-cycle transitions in Xenopus laevis egg extracts. Proc Natl Acad Sci U S A. 2003;100:975–980. doi: 10.1073/pnas.0235349100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, Kastner DL, Siegel RM. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) J Exp Med. 2011;208:519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li T, Hu J, Li L. Characterization of Tollip protein upon Lipopolysaccharide challenge. Mol Immunol. 2004;41:85–92. doi: 10.1016/j.molimm.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 82.Burns K, Clatworthy J, Martin L, Martinon F, Plumpton C, Maschera B, Lewis A, Ray K, Tschopp J, Volpe F. Tollip, a new component of the IL-1RI pathway, links IRAK to the IL-1 receptor. Nat Cell Biol. 2000;2:346–351. doi: 10.1038/35014038. [DOI] [PubMed] [Google Scholar]
- 83.Zhang G, Ghosh S. Negative regulation of toll-like receptor-mediated signaling by Tollip. J Biol Chem. 2002;277:7059–7065. doi: 10.1074/jbc.M109537200. [DOI] [PubMed] [Google Scholar]
- 84.Morris M, Li L. Molecular mechanisms and pathological consequences of endotoxin tolerance and priming. Arch Immunol Ther Exp (Warsz) 2012;60:13–18. doi: 10.1007/s00005-011-0155-9. [DOI] [PubMed] [Google Scholar]
- 85.Sy M, Kitazawa M, Medeiros R, Whitman L, Cheng D, Lane TE, Laferla FM. Inflammation induced by infection potentiates tau pathological features in transgenic mice. Am J Pathol. 2011;178:2811–2822. doi: 10.1016/j.ajpath.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Laird MH, Rhee SH, Perkins DJ, Medvedev AE, Piao W, Fenton MJ, Vogel SN. TLR4/MyD88/PI3K interactions regulate TLR4 signaling. J Leukoc Biol. 2009;85:966–977. doi: 10.1189/jlb.1208763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee JY, Chiu YH, Asara J, Cantley LC. Inhibition of PI3K binding to activators by serine phosphorylation of PI3K regulatory subunit p85alpha Src homology-2 domains. Proc Natl Acad Sci U S A. 2011;108:14157–14162. doi: 10.1073/pnas.1107747108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Faisal A, Saurin A, Gregory B, Foxwell B, Parker PJ. The scaffold MyD88 acts to couple protein kinase Cepsilon to Toll-like receptors. J Biol Chem. 2008;283:18591–18600. doi: 10.1074/jbc.M710330200. [DOI] [PubMed] [Google Scholar]
- 89.Barton GM, Kagan JC. A cell biological view of Toll-like receptor function regulation through compartmentalization. Nat Rev Immunol. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vogel SN, English KE, Fertsch D, Fultz MJ. Differential modulation of macrophage membrane markers by interferon analysis of Fc and C3b receptors, Mac-1 and Ia antigen expression. J Interferon Res. 1983;3:153–160. doi: 10.1089/jir.1983.3.153. [DOI] [PubMed] [Google Scholar]
- 92.Han C, Jin J, Xu S, Liu H, Li N, Cao X. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat Immunol. 2010;11:734–742. doi: 10.1038/ni.1908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.