Abstract
In pre-clinical models, both dietary fat reduction and IGF-I receptor (IGF-1R) blockade individually inhibit prostate cancer xenograft growth. We hypothesized that a low-fat diet combined with IGF-1R blockade would cause additive inhibition of prostate cancer growth and offset possible untoward metabolic effects of IGF-1R blockade antibody therapy. Fifty SCID mice were injected with 22Rv1 cells subcutaneously. Ten days post-injection, the animals were randomized to four groups: 1) high fat diet + saline (HF), 2) high fat diet + IGF-1R blocking antibody, ganitumab (HF/Ab), 3) low fat diet + saline (LF), and 4) low fat diet + ganitumab (LF/Ab). After nineteen days of treatment, the animals were euthanized, serum was collected and tumors were weighed. Tumor Ki67, Akt and ERK activation, serum insulin, IGF-I and TNF-alpha were measured. In vitro, ganitumab treatment inhibited growth and induced apoptosis in several prostate cancer cell lines. In vivo, tumor weights and volumes were unaffected by the different treatments. The LF/Ab therapy significantly reduced proliferation (Ki67) and ERK activation in tumors. The HF/Ab group had significantly higher serum insulin levels than the HF group. However LF/Ab combination significantly reduced serum insulin back to normal levels as well as normalizing serum TNF-alpha level. Whereas the combination of low fat diet and IGF-1 receptor blockade did not have additive inhibitory effects on tumor weight, it led to reduced tumor cell proliferation and a reduction in serum insulin and TNF-alpha levels.
INTRODUCTION
Given the limited efficacy of current treatment regimens for advanced, castrate-resistant prostate cancer, there is strong interest in developing targeted therapies to treat men suffering from this disease (1, 2). Cancer cells are driven to proliferate by numerous pathways potentially limiting the efficacy of therapies targeting a single pathway. Additionally, targeted therapies are often associated with adverse effects on the host requiring close monitoring, possible dose reduction of the therapeutic agents, and/or additional therapies (3). Pre-clinical and clinical studies investigating the use of low-fat diets as an ”alternative therapy” for prostate cancer treatment have demonstrated a potential role for inhibiting prostate cancer progression and improving the metabolic profiles of prostate cancer patients (4–9). Dietary fat reduction inhibits cancer progression through a number of potential mechanisms including reduction in circulating IGF-I levels and inhibition of the phosphatidylinositol-3-kinase (PI3K)-Akt pathway (10–13).
IGF-I is a peptide growth factor and a potent mitogen for the growth of androgen responsive and androgen independent human prostate cancer cell lines. IGF-I plays a pivotal role in regulating cell proliferation, differentiation, and apoptosis (14, 15). IGF-I executes its biological effects by binding to the IGF-1R and activating the PI3K/Akt and RAS/RAF/mitogen-activated protein kinase (MAPK) pathways (10–12, 16). Based on the key role played by IGF-I in the progression of prostate cancer as well as other malignancies including colon and breast cancer, strong interest exists in developing targeted therapies that inhibit the IGF-I signaling pathway (17–19). An IGF-1R antibody was previously found to decrease prostate cancer xenograft growth (20). Multiple biotechnology companies have developed monoclonal antibody therapies that target the IGF-1R. These antibodies are being investigated in clinical trials for the treatment of prostate cancer, both in the neoadjuvant setting (prior to radical prostatectomy) and in patients with metastatic, castrate-resistant prostate cancer (11). Potential metabolic consequences of IGF-1R-targeted inhibition include elevation in blood glucose and insulin levels via feedback inhibition of the growth hormone IGF-I axis (21, 22). Given that dietary fat reduction appears to exert its anticancer effects, in part, through inhibition of the IGF-I axis and reduction of serum insulin levels, we hypothesized that combining dietary fat reduction with IGF-1R blocking antibody therapy would cause additive inhibition of prostate cancer progression and potentially offset the induction of insulin resistance by IGF-1R inhibition.
MATERIALS AND METHODS
Animal husbandry and feeding protocol
Fifty ;ale CB17 beige severe combined immunodeficient (SCID) mice (8 weeks old) were obtained from the UCLA Department of Laboratory Animal Medicine facility (accredited by the American Association for Accreditation of Laboratory Animal Care). The mice were housed two mice per cage. The cages were kept in a sterile and pathogen-free facility. Cages, bedding, and water were autoclaved before their use. The feeding receptacles were on top of the cages so food intake could be monitored and new feedings given without opening the cage. Sterile technique was used whenever handling the cages, mice, and food. The experiments were approved by the UCLA Chancellor’s Animal Research Committee, and animals were cared for in accordance with institutional guidelines. The diets were prepared and sterilized (by irradiation) by DYETS, Inc. (Bethlehem, PA). The high fat diet contained 43.3% calories from fat and the low fat diet contained 12.4% calories from fat (Supplementary table 1). Mice were fed ad libitum throughout the experiment. Mice were fed once a week. Feeding receptacles were filled with 50 grams of fresh feed once weekly.
Cell culture
22Rv1, DU145, and PC-3 cell lines were obtained through the ATCC (ATCC, Manassas, VA) and grown according to ATCC guidelines. Each cell line was tested and authenticated by ATCC. The ATCC Cell Biology program employs state-of-the-art technology platforms for the authentication of cells lines by applying the growth curve to determine optimal growth conditions and the seed stock scheme when expanding cell lines to minimize passaging. ATCC Routine Cell Biology Program includes: Certification that each cell line is negative for mycoplasma, bacteria, fungi contamination; Confirmation of species identity and detection of possible cellular contamination or misidentification using COI for interspecies identification and STR analysis (DNA profiling) for intraspecies identification; Conducting of additional test methods, such as cytogenetic analysis (G-banding, FISH), flow cytometry and immunocytochemistry as well as consistent refinement of cell growth conditions as well as documentation systems, ensuring traceability.
All cell lines were used within 6 months after resuscitation. 22Rv1 is a human prostate carcinoma epithelial cell line derived from a xenograft that was serially propagated in mice after castration-induced regression and relapse of the parental, androgen-dependent CWR22 xenograft. 22Rv1 cells express PSA and the androgen receptor (23). 22Rv1 were grown in RPMI 1640 supplemented with 10% FBS and 100U/ml penicillin and 100ug/ml streptomycin (Gibco/Invitrogen, Carlsbad, CA). DU145 and PC-3 cells are human prostate carcinoma epithelial cell lines derived from brain and bone metastasis respectively. These cell lines are androgen resistant and do not express prostate specific antigen. DU145 and PC-3 cells were grown in DMEM and DMEM F-12K respectively, supplemented with 10% FBS and 100U/ml penicillin and 100ug/ml streptomycin (Gibco/Invitrogen, Carlsbad, CA) respectively.
The Los Angeles Prostate Cancer 4 (LAPC-4) cell line (a generous gift from Drs. Robert Reiter and Charles Sawyers) was developed at UCLA by direct transfer of cancer cells from a patient with advanced adenocarcinoma of the prostate into the subcutaneous tissue of severe combined immunodeficiency mice. LAPC-4 produces prostate-specific antigen (PSA), has a wild-type androgen receptor, and shows features of hormone-dependent growth and metastasis (24). LAPC4 cells were authenticated in Dr Sawyers’s laboratory using cytogenetic analysis and assessing PSA expression as described by Klein et al. (24). LAPC-4 were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS, 1% penicillin/streptomycin, and 10 nmol/L R1881 (Perkin-Elmer Life Sciences, Wellesley, MA) and were used less than 4 months after resuscitation.
In vitro studies
For individual experiments, cells were seeded at a final density of 1 × 105/cm2 (24-well), or 2×105/cm2 (96-well) in plates and grown to 80% or 50% confluence, respectively. Cells were maintained in a humidified atmosphere of 5% CO2 at 37°C. 22Rv1, DU145, LAPC-4 and PC-3 were treated with 1μM ganitumab (AMG 479) (Amgen, Inc., Thousand Oaks, CA) for 24 and 72h. Apoptosis was assessed in cells growing on 24 well plates for 24h using Cell Death Detection ELISAPLUS for the determination of cytoplasmic histone-associated DNA fragments (Roche Applied Science, Indianapolis, IN) following the manufacturer’s instructions.
To assess cell growth, cells were seeded on 96-well plates, and allowed to attach overnight. Cells were incubated with 1μM IGF-1R blocking antibody (ganitumab) for 72 hours in serum free (SF) media. CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega, Madison, Wi) was performed according to manufacturer’s instructions. Mean ± SE values of the absorbance at 490 nm were plotted.
Experimental design
All mice were fed a HF diet for two weeks prior to being injected with 5×105 22Rv1 cells. On the day of subcutaneous injection, 22Rv1 were trypsinized and resuspended in serum free RPMI 1640 at a concentration of 5×105 cells/0.1ml of media. An equal volume of ice cold matrigel (BD Biosciences, Bedford, MA) was added to the cells. 0.2ml of the resulting solution containing 5×105 22Rv1cells was injected subcutaneoulsy to the mice in the lateral flank. Mice continued consuming the HF diet for ten days following injection, at which point they were randomized into four groups: 1) high fat diet with intraperitoneal saline (HF), n=12,) high fat diet with intraperitoneal IGF-1R blocking antibody, ganitumab (Amgen, Inc., Thousand Oaks, CA) (HF/Ab,), n=13,) low fat diet with intraperitoneal saline (LF), n=12, and 4) low fat diet with intraperitoneal ganitumab (LF/Ab), n=13. ganitumab antibody was administered at a dose of 20mg/kg twice weekly and intraperitoneal saline was administered twice weekly. Mice were weighed and tumor dimensions were measured twice weekly. The tumor dimensions were measured using a caliper. Tumor volumes were calculated using the formula previously described: length × width × height × 0.5236 (25). Nineteen days after initiation of treatment the mice were euthanized. Serum was collected by cardiac puncture and tumor tissue was harvested and weighed. Serum was stored at −80°C. Tumor tissue was rinsed with saline. Half of the tumor was snap frozen in liquid nitrogen while the other half was fixed for 12h in 10% neutral buffered formalin and embedded in paraffin blocks for histological sections.
Serum studies
The levels of murine IGF-I, IGFBP-1, IGFBP-3, and murine growth hormone (mGH) were measured using in-house mouse-specific ELISA as previously described (13, 26, 27). The mouse IGF-1 assay has a sensitivity of 0.1 ng/mL and has no cross-reactivity with mouse IGF-II or human IGF-I. The intraassay and interassay coefficient of variations are <10% in the range from 1 to 10 ng/mL. The mouse IGFBP-1 and IGFBP-3 assays have sensitivities of 0.2 ng/mL and no cross-reactivity with other IGFBPs or the human homologues. The intraassay and interassay coefficient of variation are <6% and <8%, respectively, in the range from 1 to 6 ng/mL. Serum concentrations of mGH were determined using an RIA kit with a sensitivity of 0.02 ng/mL (National Hormone and Pituitary Program, Harbor-UCLA Medical Center, Torrance, CA). Serum insulin and TNF-α were measured using LINCOplex technology with a multiplex assay kit (Millipore Corporation, Billerica, MA). The assay has no cross-reactivity with human insulin or human TNF-α. Interassay and intraassay coefficients of variation are <12% and <5%, respectively.
Immunohistochemistry
Four-micrometer formalin-fixed tumor sections embedded in paraffin were stained with H&E. Immunohistochemistry of representative tumor sections were performed for Ki67 (# M7240, DAKO North America Inc., Carpinteria, CA) and Pecam1/CD31 ( # sc-1506, Santa Cruz Biotechnology Inc., Santa Cruz, CA) as previously described (28). Terminal nucleotidyl transferase-mediated nick end labeling (TUNEL) assays were performed using the ApopTag® Plus Peroxidase In Situ Apoptosis Kit (#S7101, Millipore, Temecula, CA), as previously described (28). A total of 200 cells were counted for each xenograft tumor and the number of either Ki67 or TUNEL positive cells was scored by a single blinded pathologist. The number of positive nuclei was expressed as a percentage by dividing the number of positive stained nuclei by the total number of cells counted. For Pecam1/CD31, the number of vessels were counted in five 20X fields for each stained slide.
Western blot analysis
Xenograft tumor tissues were lysed using 100μl of RIPA buffer supplemented with an EDTA-free protease inhibitor cocktail and PhosSTOP tablets as recommended by the manufacturer (Roche Applied Bioscience, Indianapolis, IN) and clarified by centrifugation. Equal amounts of protein were separated on SDS gels and electrophoretically transferred to PVDF membranes for Western blotting. The IGF-1R (# 3027), Insulin Receptor (# 3025) and PTEN (# 9552) antibodies were purchased from Cell Signaling and used at a 1/1000 dilution in 5% milk blocking solution. The Phospho-Ser473-Akt antibody (# 4060) was purchased from Cell Signaling (Danvers, MA) and used at a 1/1000 dilution in 1% BSA blocking. The ERK2 (# sc-154) and phospho–ERK1/2 (#sc-7383) antibodies were purchased from Santa Cruz (Santa Cruz, CA) and used at a 1/5000 dilution in 5% milk and 1% BSA blocking solutions, respectively. All primary antibody incubations were done overnight at 4C and followed by a constant 1h incubation at room temperature with a 1:2000 dilution of the appropriate anti- mouse or anti-rabbit secondary antibody covalently coupled to horseradish peroxidase (Bio-Rad laboratories Inc. catalog # 172-1011 and # 170-6515 respectively). All immune complexes in the Western blots were visualized using the Pierce ECL Western Blotting Substrate (ThermoScientific, Rockford, IL) and exposed to film. The quantitative analysis presented was done with Image J (29).
The phospho-ERK1/2 and phospho-Ser473–Akt Western blots were stripped using a mild stripping buffer (0.2 M glycine, 0.1% SDS, and 1% Tween 20; pH 2.2) for 10 min at room temperature. After blocking, the membranes were incubated with a rabbit anti-ERK2 antibody (Santa Cruz Biotechnology, catalog # sc-154) at a 1:5000 dilution in 5% milk overnight at 4oC for the phospho-ERK1/2 blot and with a rabbit anti-Akt (Cell Signaling , catalog # 4691) at a 1:1000 dilution in 5% milk overnight at 4C for the phospho-S473-Akt blot. The immunocomplexes were detected as described above. This allowed for correction of the phospho-ERK1/2 and phospho-Akt signals for the total amount of ERK1/2 and Akt protein present.
Statistical analysis
For in vitro studies, statistical analyses were performed by using an unpaired nonparametric Mann-Whitney test. All other statistical analyses were conducted using unpaired t tests or one-way Analysis of variance (ANOVA) followed by a Tukey post-hoc test using GraphPad Prism4 (GraphPad Software Inc.). In all cases, statistical significance was considered at p< 0.05.
RESULTS
Sensitivity of prostate cancer cell lines to ganitumab
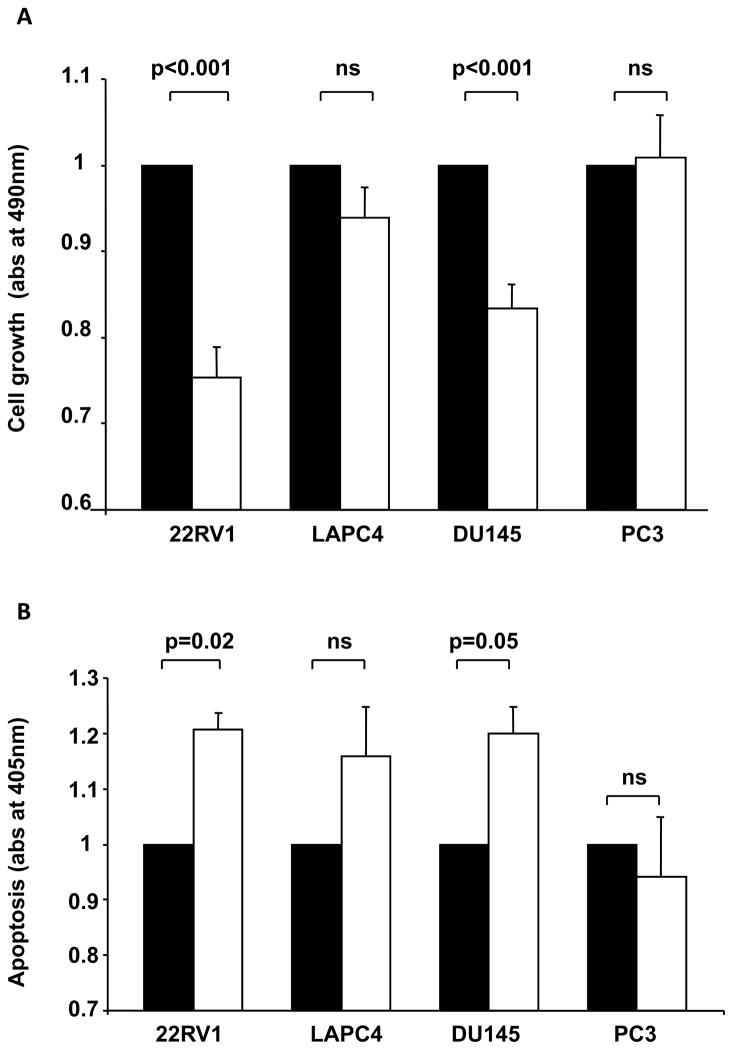
As shown in Figure 1a, the IGF-1R blocking antibody ganitumab significantly inhibited 22Rv1 and DU145 cell growth compared to serum-free media (25% and 17% reduction respectively). Ganitumab was also found to significantly induce apoptosis compared to serum free media in 22Rv1and DU145 (21% and 20%, respectively, Figure 1b). Ganitumab treatment did not result in significant change in LAPC 4 or PC-3 cells growth (Figure 1a) or apoptosis (Figure 1b). Overall the 22Rv1 cell line had the highest sensitivity to ganitumab treatment in terms of cell growth and apoptosis. Therefore 22Rv1 cell line was chosen for the xenograft study.
Figure 1. IGF-1R blocking antibody (ganitumab) effect on prostate cancer cell lines growth and apoptosis in vitro.
A/ Cell growth assay performed after 72h of incubation in serum free media (black bars) or in presence of 1uM of the IGF-1R blocking antibody ganitumab (white bars). B/ Apoptosis was measured after 24h of incubation in serum free media (black bars) or in presence of 1uM of the IGF-1R blocking antibody ganitumab (white bars). Each assay was repeated three times. Data are expressed as mean ± SEM. Statistical analysis was performed by using an unpaired nonparametric Mann-Whitney test.
Modulation of the IGF axis by IGF-1R blockade and low-fat diet
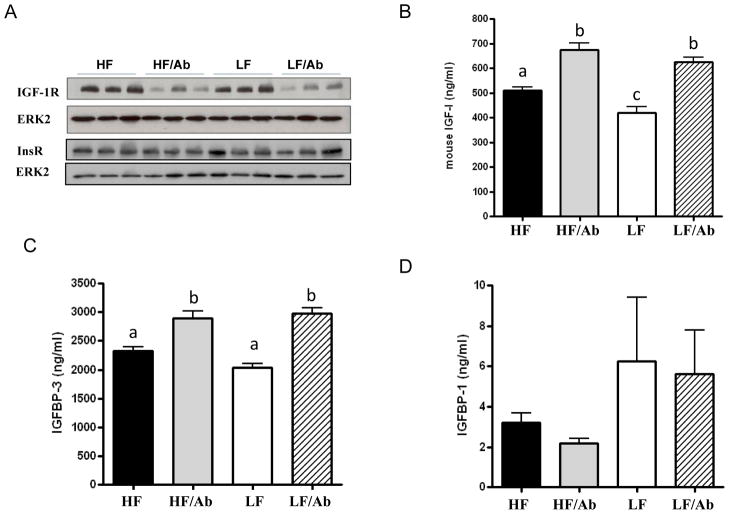
As shown in Figure 2 mice receiving ganitumab had significant reductions in IGF-1R levels in 22Rv1 xenografts as measured by Western blot analysis. No significant change in insulin receptor levels was observed (Figure 2a). Serum IGF-I and IGFBP-3 levels were significantly elevated in the HF/Ab and LF/Ab groups (Figures 2b and 2c) relative to the HF control and LF control groups, respectively. The LF group had significantly lower serum IGF-I levels relative to all other groups (Figure 2b). No changes were observed between the groups in circulating IGFBP-1 levels (Figure 2d) or murine growth hormone (mGH) levels (data not shown).
Figure 2. Modulation of the IGF axis by IGF-1R blockade and low-fat diet.
A/ IGF-IR and insulin receptor levels in 22Rv1 xenograft tissue. Total ERK2 was used as a loading control. The Western blots are representative of one experiment (n-3 animals per group). The western blots were done on a total of 5 animals per group. B, C and D/ Fasting serum IGF-I, IGFBP-3 and IGFBP-1 concentration of SCID mice on the different therapy regimen. Serum IGF-I, IGFBP-3 and IGFBP-1 levels were assessed using ELISA. Values are means ± standard errors (SE). Means with letters a,b, or c are significantly different from each other (p<0.05, One way Analysis of variance with Tukey post test).
Tumor volumes and final tumor weights
There was no significant difference in final tumor volumes or final tumor weights between the treatment groups (Table 1). At day 14 of the intervention, the mean tumor volume was significantly lower in the LF/Ab group compared to the HF group (Table 1). The animal weights were not significantly different between the groups.
Table 1.
Tumor volumes and weights
| Groups | tumor volume (mm3) at 14 days | tumor volume (mm3) at sacrifice | tumor weight at sacrifice (mg) |
|---|---|---|---|
| HF | 336.9 ± 53.7 | 450 ± 55.1 | 950.8 ± 138.4 |
| HF/Ab | 223.4 ± 40 | 333.4 ± 59.1 | 585 ± 81.1 |
| LF | 247.1± 28.6 | 366.7 ± 48.2 | 714.2 ± 4.1 |
| LF/Ab | 190.4 ± 36.6 * | 323.7 ± 39.4 | 594.2 ± 99.8 |
p<0.05
Reduced proliferation and ERK activation in the LF/Ab group
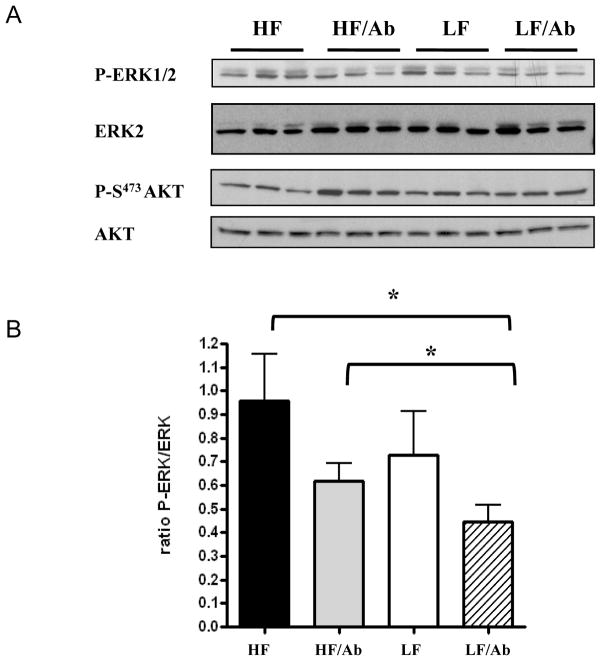
Xenografts from the LF/Ab group had a 30% decrease in proliferation as measured by Ki67 immunostaining relative to the other groups (Figure 3). Whereas there was no difference in tumor PTEN and phospho-Akt levels between the groups, phospho-ERK was significantly decreased in the LF/Ab tumors relative to the tumors in the HF and HF/Ab groups (Figures 4a and 4b). There was no difference in apoptosis or angiogenesis between the groups (data not shown).
Figure 3. Effect of the different therapies on 22Rv1 xenograft proliferation.
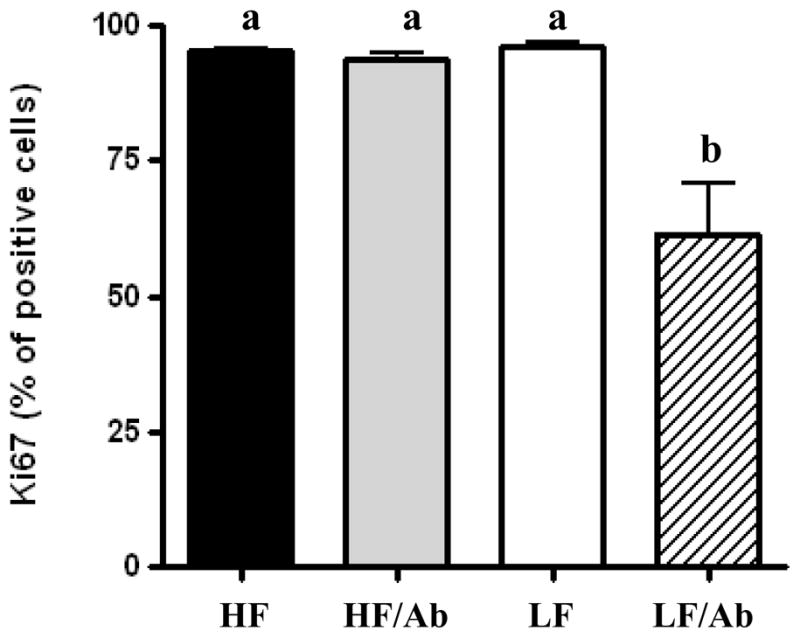
Ki67 immmunostaining was performed on xenografts from all animals in each group. 200 cells were manually counted by a blinded pathologist, the Ki67 positive cells were expressed as a percentage of total cells. Means with different letters are significantly different from each other (p<0.05, One way analysis of variance with Tukey post test).
Figure 4. Effect of the different therapies on Akt and ERK activation.
Activation of Akt and ERK was assessed by western blotting as described in material and methods. A/ Phospho-ERK and Phospho Akt and total Akt and ERK were measured on xenograft tissue lysate from 5 animals for each group. The Western blots are representative of one experiment (n-3 animals per group). B/ The LF/Ab therapy resulted in a decrease in ERK activation measured as a ratio of phosphoERK/ total ERK2. Values are means ± standard errors (SE). *: paired t-test; p<0.05.
Metabolic effects of the low fat diet and ganitumab treatment
The HF/Ab group had 57% higher serum insulin levels as compared to the HF control group (p<0.05). However, combining a low-fat diet with IGF-1R antibody therapy reduced serum insulin levels significantly by 40% relative to the HF/Ab group (Figure 5a, p<0.05). Likewise, the LF/Ab group also had significantly lower mean serum TNF-α levels relative to the HF group (p<0.05) (Figure 5b).
Figure 5. Combining a LF diet with ganitumab offset the increase in Insulin induced by the ganitumab antibody (A) and resulted in a significant reduction in TNF-α (B).
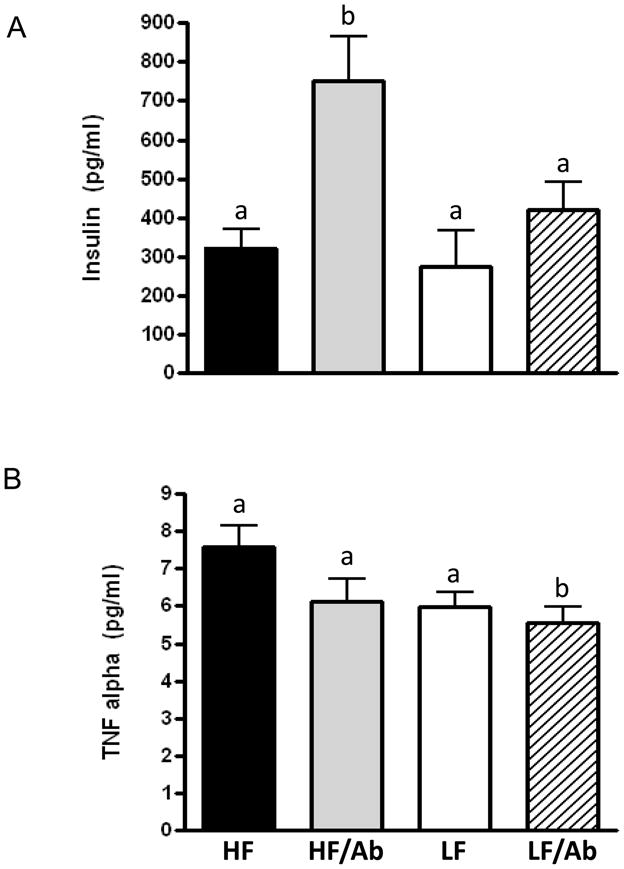
Values are means ± standard errors (SE). Means with different letters are significantly different from each other (p<0.05, One way analysis of variance with Tukey post test).
DISCUSSION
Both IGF-1R blocking therapy and dietary fat reduction were previously found to inhibit prostate cancer xenograft growth (4, 6, 20). Given that dietary fat reduction appears to exert its anticancer effects, in part, through inhibition of the IGF-I axis, we hypothesized that combining dietary fat reduction with IGF-1R blocking antibody therapy would cause additive inhibition of prostate cancer progression. Likewise, given that dietary fat reduction was previously found to decrease serum insulin levels (4), we hypothesized that combining a low fat diet with IGF-1R blocking therapy would offset the hyperinsulinemia associated with IGF-1R blocking therapy. In the present study neither dietary fat reduction, IGF-1 receptor blocking therapy or combination therapy affected tumor weight or tumor volume at the time of euthanasia. However, combining dietary fat reduction with IGF-1R blocking therapy resulted in decreased tumor proliferation and decreased ERK activation. Dietary fat reduction also offset the increased serum insulin observed with ganitumab antibody therapy.
Of the 4 cell lines tested in vitro, 22Rv1 and DU145 responded to ganitumab treatment with a significant decrease in cell growth and increase in apoptosis. The 22Rv1 cell line was chosen for the xenograft experiments because they responded similarly to the IGF-1R blocking therapy as previously described for the LNCaP cells (20) and are known to be responsive to IGF-I stimulation in vitro (30). Contrary to our in vitro experiments, and prior xenograft experiments demonstrating growth inhibition with IGF-1R blockade (20), ganitumab treatment (HF/Ab group vs. HF group) did not affect tumor weight, proliferation, or apoptosis. As expected with IGF-1R antibody therapy, ganitumab treatment resulted in significant down regulation of total IGF-1R in the xenografts, however, there was no significant effect on the activation of Akt and ERK, downstream signaling effectors of the IGF-1R. Recent studies have shown that IGF-1R inhibition can induce a resistance mechanism via the epidermal growth factor receptor (EGFR) signaling pathway or via the Insulin receptor in various cancer cell lines (31–33). Although these mechanisms were not explored in our model, the cross-talk between IGF-1R and other activators of Akt and ERK may have contributed to our findings. ganitumab therapy has been shown to inhibit cell growth and cell proliferation and induce apoptosis in vitro in other cancer cell lines such as pancreatic and endometrial cancer, and to inhibit progression of endometrial cancer xenografts in vivo (34–36). To our knowledge the present study is the first designed to assess the efficacy of ganitumab on prostate cancer progression in vivo.
Dietary fat reduction was previously found to inhibit prostate cancer xenograft progression in LNCaP and LAPC-4 xenografts in vivo, to inhibit prostate cancer development in the Hi-myc transgenic mouse model, and to inhibit proliferation in mPIN and adenocarcinoma epithelial cells in these preclinical models. (4, 6, 13). Likewise, in clinical trials, serum from subjects undergoing dietary fat reduction inhibited LNCaP and 22Rv1 cell growth in an ex-vivo bioassay (5, 37). In the current study, dietary fat reduction did not lead to a reduction in xenograft growth, proliferation or apoptosis. This difference may potentially be explained by the fact that the animals were fed ad libitum with 2 mice per cage to mirror normal human lifestyle and therefore did not receive isocaloric diets as previously described (4, 13), however, prior studies found dietary fat reduction inhibited LNCaP xenograft growth in mice fed ad-libitum (6). Since the animals were not housed one mouse per cage and we did not equalize caloric intake between the groups, we are unable to determine the total fat intake of the mice in each group – this represents a limitation of our study. In the current study dietary fat reduction induced a significant 20% decrease in serum IGF-I levels however did not result in a significant decrease in serum insulin or increase in serum IGFBP-1 as previously described (4, 13). LF group insulin and IGFBP-1 levels, while not significantly different than that of the HF group, showed a high level of variation (see error bar) between animals of the LF group. IGFBP-1 and Insulin are biomarkers of nutritional status thus are sensitive to caloric intake. This high variability was not observed in our previous study where the same diets were used in an isocaloric model (4). The lack of effect of monotherapy with dietary fat reduction or IGF-1R antibody therapy suggests that the IGF-1 pathway may not be the main pathway supporting 22Rv1 xenograft growth. The cells may have developed a compensation mechanism, possibly through activation of the insulin receptor or the epidermal growth factor receptor as shown by others in vitro (31, 33). 22RV1 are androgen dependent cells therefore the androgen pathway may be overriding the potential inhibitory effects of the IGF-1R blockade and dietary fat reduction. Given that androgens are well known to be a growth factor for prostate cancer, we have previously measured serum testosterone levels in prior high-fat low-fat mouse studies to confirm that androgen levels do not differ between the groups. In a prior manuscript from our group changing dietary fat did not alter androgen levels (13). We have also found that changing dietary fat intake does not alter serum androgen levels in human trials (5). We also have demonstrated that the low fat diet used in an isocaloric model delayed conversion from androgen-sensitive to –insensitive prostate cancer and significantly prolonged survival of severe combined immunodeficient mice bearing LAPC-4 xenografts (38). However the androgen receptor expression levels were not measured in these studies. Given the importance of androgens in prostate cancer physiology, future preclinical and clinical trials with IGF-1R blockade and dietary modification should also incorporate measurements of androgens and androgen receptors.
Despite the lack of effect on xenograft progression with dietary fat reduction or with IGF-1R blockade, combining IGF-1R blocking therapy with dietary fat reduction resulted in a significant 35% reduction of malignant epithelial cell proliferation. These data suggest a potential benefit of combining a low fat dietary intervention with IGF-1R blocking therapy to enhance efficacy in the treatment of prostate cancer. IGF-IR antibody therapy has been well tolerated in phase I and phase II clinical trials, with hyperglycemia occurring in approximately 25% of subjects (22). It is hypothesized that hyperglycemia may result from elevated human growth hormone levels (resulting from IGF-IR antibody therapy) leading to insulin resistance in insulin target tissues (39). In the present study, serum insulin levels were increased by 132% in the HF/Ab group as compared to the HF group. The serum insulin levels in the LF/Ab group were significantly lower (44% reduction) as compared to the HF/Ab group suggesting that dietary fat reduction may offset the hyperinsulinemia and associated metabolic consequences associated with IGF-IR antibody therapy. Some of the adverse effects of cancer therapy have been proposed to be related to circulating IGF-I, and reduction of IGF-I levels by fasting or genetic ablation have been shown to reduce morbidity and mortality in mouse models (40) and small human case series (41). The approach of combining pharmacological and nutritional interventions as described in our study offers a potential way of both limiting the untoward effects of drugs that raise IGF-I and insulin (such as IGF-1R antibodies) and enhancing their anti-tumor effects.
Dietary fat reduction combined with IGF-1R antibody therapy resulted in decreased serum TNF-α levels as compared to the other treatment groups. Obesity induced by a high fat diet is associated with inflammation in peripheral tissues that predisposes to insulin resistance (42). Both inflammation and an increase in serum insulin may also be involved in cancer progression (43). Thus, combining IGF-1R antibody therapy with dietary fat reduction may potentially offset the metabolic consequences of IGF-1R blockade and potentially enhance anti-tumor effects.
In summary, dietary fat reduction combined with IGF-1R antibody blockade resulted in decreased proliferation in prostate cancer xenografts and a reduction in serum insulin and TNF alpha levels. Further preclinical and clinical trials are warranted to evaluate combining dietary fat reduction with IGF-I receptor blockade to enhance the efficacy and offset the metabolic consequences of antibody therapy directed against the IGF-1 receptor.
Acknowledgments
Supported by the National Cancer Institute (NCI) Grant Number P50CA92131 (W.J. Aronson), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant number 2P30DK063491 (P. Cohen), the National Institute of Aging (NIA) grant number P01AG034906 (P. Cohen) and Wendy and Ken Ruby, President and Secretary of the Ruby Family Foundation (R. Konijeti).
We thank Colin McClean and his team for their expert assistance in the SCID mouse facility.
References
- 1.Freedland SJ. Screening, risk assessment, and the approach to therapy in patients with prostate cancer. Cancer. 2010 doi: 10.1002/cncr.25477. [DOI] [PubMed] [Google Scholar]
- 2.Bianchini D, Zivi A, Sandhu S, de Bono JS. Horizon scanning for novel therapeutics for the treatment of prostate cancer. Ann Oncol. 2010;21(Suppl 7):vii43–vii55. doi: 10.1093/annonc/mdq369. [DOI] [PubMed] [Google Scholar]
- 3.Macfarlane RJ, Chi KN. Novel targeted therapies for prostate cancer. Urol Clin North Am. 2010;37:105–19. doi: 10.1016/j.ucl.2009.11.011. Table of Contents. [DOI] [PubMed] [Google Scholar]
- 4.Ngo TH, Barnard RJ, Cohen P, Freedland S, Tran C, deGregorio F, et al. Effect of isocaloric low-fat diet on human LAPC-4 prostate cancer xenografts in severe combined immunodeficient mice and the insulin-like growth factor axis. Clin Cancer Res. 2003;9:2734–43. [PubMed] [Google Scholar]
- 5.Aronson WJ, Barnard RJ, Freedland SJ, Henning S, Elashoff D, Jardack PM, et al. Growth inhibitory effect of low fat diet on prostate cancer cells: results of a prospective, randomized dietary intervention trial in men with prostate cancer. J Urol. 2010;183:345–50. doi: 10.1016/j.juro.2009.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Corr JG, Thaler HT, Tao Y, Fair WR, Heston WD. Decreased growth of established human prostate LNCaP tumors in nude mice fed a low-fat diet. J Natl Cancer Inst. 1995;87:1456–62. doi: 10.1093/jnci/87.19.1456. [DOI] [PubMed] [Google Scholar]
- 7.Whittemore AS, Kolonel LN, Wu AH, John EM, Gallagher RP, Howe GR, et al. Prostate cancer in relation to diet, physical activity, and body size in blacks, whites, and Asians in the United States and Canada. J Natl Cancer Inst. 1995;87:652–61. doi: 10.1093/jnci/87.9.652. [DOI] [PubMed] [Google Scholar]
- 8.Gann PH, Hennekens CH, Sacks FM, Grodstein F, Giovannucci EL, Stampfer MJ. Prospective study of plasma fatty acids and risk of prostate cancer. J Natl Cancer Inst. 1994;86:281–6. doi: 10.1093/jnci/86.4.281. [DOI] [PubMed] [Google Scholar]
- 9.Giovannucci E, Rimm EB, Colditz GA, Stampfer MJ, Ascherio A, Chute CC, et al. A prospective study of dietary fat and risk of prostate cancer. J Natl Cancer Inst. 1993;85:1571–9. doi: 10.1093/jnci/85.19.1571. [DOI] [PubMed] [Google Scholar]
- 10.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–2. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 11.Chitnis MM, Yuen JS, Protheroe AS, Pollak M, Macaulay VM. The type 1 insulin-like growth factor receptor pathway. Clin Cancer Res. 2008;14:6364–70. doi: 10.1158/1078-0432.CCR-07-4879. [DOI] [PubMed] [Google Scholar]
- 12.Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. Int J Cancer. 2003;107:873–7. doi: 10.1002/ijc.11487. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi N, Barnard RJ, Said J, Hong-Gonzalez J, Corman DM, Ku M, et al. Effect of low-fat diet on development of prostate cancer and Akt phosphorylation in the Hi-Myc transgenic mouse model. Cancer Res. 2008;68:3066–73. doi: 10.1158/0008-5472.CAN-07-5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472–89. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 15.Iwamura M, Sluss PM, Casamento JB, Cockett AT. Insulin-like growth factor I: action and receptor characterization in human prostate cancer cell lines. Prostate. 1993;22:243–52. doi: 10.1002/pros.2990220307. [DOI] [PubMed] [Google Scholar]
- 16.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuen JS, Macaulay VM. Targeting the type 1 insulin-like growth factor receptor as a treatment for cancer. Expert Opin Ther Targets. 2008;12:589–603. doi: 10.1517/14728222.12.5.589. [DOI] [PubMed] [Google Scholar]
- 18.Rodon J, DeSantos V, Ferry RJ, Jr, Kurzrock R. Early drug development of inhibitors of the insulin-like growth factor-I receptor pathway: lessons from the first clinical trials. Mol Cancer Ther. 2008;7:2575–88. doi: 10.1158/1535-7163.MCT-08-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kojima S, Inahara M, Suzuki H, Ichikawa T, Furuya Y. Implications of insulin-like growth factor-I for prostate cancer therapies. Int J Urol. 2009;16:161–7. doi: 10.1111/j.1442-2042.2008.02224.x. [DOI] [PubMed] [Google Scholar]
- 20.Wu JD, Odman A, Higgins LM, Haugk K, Vessella R, Ludwig DL, et al. In vivo effects of the human type I insulin-like growth factor receptor antibody A12 on androgen-dependent and androgen-independent xenograft human prostate tumors. Clin Cancer Res. 2005;11:3065–74. doi: 10.1158/1078-0432.CCR-04-1586. [DOI] [PubMed] [Google Scholar]
- 21.Haluska P, Shaw HM, Batzel GN, Yin D, Molina JR, Molife LR, et al. Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751,871 in patients with refractory solid tumors. Clin Cancer Res. 2007;13:5834–40. doi: 10.1158/1078-0432.CCR-07-1118. [DOI] [PubMed] [Google Scholar]
- 22.Scagliotti GV, Novello S. The role of the insulin-like growth factor signaling pathway in non-small cell lung cancer and other solid tumors. Cancer Treat Rev. 2011 doi: 10.1016/j.ctrv.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Sramkoski RM, Pretlow TG, 2nd, Giaconia JM, Pretlow TP, Schwartz S, Sy MS, et al. A new human prostate carcinoma cell line, 22Rv1. In Vitro Cell Dev Biol Anim. 1999;35:403–9. doi: 10.1007/s11626-999-0115-4. [DOI] [PubMed] [Google Scholar]
- 24.Klein KA, Reiter RE, Redula J, Moradi H, Zhu XL, Brothman AR, et al. Progression of metastatic human prostate cancer to androgen independence in immunodeficient SCID mice. Nat Med. 1997;3:402–8. doi: 10.1038/nm0497-402. [DOI] [PubMed] [Google Scholar]
- 25.Gleave M, Hsieh JT, Gao CA, von Eschenbach AC, Chung LW. Acceleration of human prostate cancer growth in vivo by factors produced by prostate and bone fibroblasts. Cancer Res. 1991;51:3753–61. [PubMed] [Google Scholar]
- 26.Watson CS, Bialek P, Anzo M, Khosravi J, Yee SP, Han VK. Elevated circulating insulin-like growth factor binding protein-1 is sufficient to cause fetal growth restriction. Endocrinology. 2006;147:1175–86. doi: 10.1210/en.2005-0606. [DOI] [PubMed] [Google Scholar]
- 27.Yakar S, Bouxsein ML, Canalis E, Sun H, Glatt V, Gundberg C, et al. The ternary IGF complex influences postnatal bone acquisition and the skeletal response to intermittent parathyroid hormone. J Endocrinol. 2006;189:289–99. doi: 10.1677/joe.1.06657. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi N, Barnard RJ, Henning SM, Elashoff D, Reddy ST, Cohen P, et al. Effect of altering dietary omega-6/omega-3 fatty acid ratios on prostate cancer membrane composition, cyclooxygenase-2, and prostaglandin E2. Clin Cancer Res. 2006;12:4662–70. doi: 10.1158/1078-0432.CCR-06-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- 30.Koyama S, Cobb LJ, Mehta HH, Seeram NP, Heber D, Pantuck AJ, et al. Pomegranate extract induces apoptosis in human prostate cancer cells by modulation of the IGF-IGFBP axis. Growth Horm IGF Res. 2010;20:55–62. doi: 10.1016/j.ghir.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desbois-Mouthon C, Baron A, Blivet-Van Eggelpoel MJ, Fartoux L, Venot C, Bladt F, et al. Insulin-like growth factor-1 receptor inhibition induces a resistance mechanism via the epidermal growth factor receptor/HER3/AKT signaling pathway: rational basis for cotargeting insulin-like growth factor-1 receptor and epidermal growth factor receptor in hepatocellular carcinoma. Clin Cancer Res. 2009;15:5445–56. doi: 10.1158/1078-0432.CCR-08-2980. [DOI] [PubMed] [Google Scholar]
- 32.Buck E, Mulvihill M. Small molecule inhibitors of the IGF-1R/IR axis for the treatment of cancer. Expert Opin Investig Drugs. 2011;20:605–21. doi: 10.1517/13543784.2011.558501. [DOI] [PubMed] [Google Scholar]
- 33.Buck E, Gokhale PC, Koujak S, Brown E, Eyzaguirre A, Tao N, et al. Compensatory insulin receptor (IR) activation on inhibition of insulin-like growth factor-1 receptor (IGF-1R): rationale for cotargeting IGF-1R and IR in cancer. Mol Cancer Ther. 2010;9:2652–64. doi: 10.1158/1535-7163.MCT-10-0318. [DOI] [PubMed] [Google Scholar]
- 34.Beltran PJ, Mitchell P, Chung YA, Cajulis E, Lu J, Belmontes B, et al. AMG 479, a fully human anti-insulin-like growth factor receptor type I monoclonal antibody, inhibits the growth and survival of pancreatic carcinoma cells. Mol Cancer Ther. 2009 doi: 10.1158/1535-7163.MCT-08-1171. [DOI] [PubMed] [Google Scholar]
- 35.Mendivil A, Zhou C, Cantrell LA, Gehrig PA, Malloy KM, Blok LJ, et al. AMG 479, a Novel IGF-1-R Antibody, Inhibits Endometrial Cancer Cell Proliferation Through Disruption of the PI3K/Akt and MAPK Pathways. Reprod Sci. 2011;18:832–41. doi: 10.1177/1933719111398501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beltran PJ, Chung YA, Moody G, Mitchell P, Cajulis E, Vonderfecht S, et al. Efficacy of ganitumab (AMG 479), alone and in combination with rapamycin, in Ewing's and osteogenic sarcoma models. J Pharmacol Exp Ther. 2011;337:644–54. doi: 10.1124/jpet.110.178400. [DOI] [PubMed] [Google Scholar]
- 37.Tymchuk CN, Barnard RJ, Heber D, Aronson WJ. Evidence of an inhibitory effect of diet and exercise on prostate cancer cell growth. J Urol. 2001;166:1185–9. [PubMed] [Google Scholar]
- 38.Ngo TH, Barnard RJ, Anton T, Tran C, Elashoff D, Heber D, et al. Effect of isocaloric low-fat diet on prostate cancer xenograft progression to androgen independence. Cancer Res. 2004;64:1252–4. doi: 10.1158/0008-5472.can-03-3830. [DOI] [PubMed] [Google Scholar]
- 39.Gualberto A, Pollak M. Emerging role of insulin-like growth factor receptor inhibitors in oncology: early clinical trial results and future directions. Oncogene. 2009;28:3009–21. doi: 10.1038/onc.2009.172. [DOI] [PubMed] [Google Scholar]
- 40.Lee C, Safdie FM, Raffaghello L, Wei M, Madia F, Parrella E, et al. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 2010;70:1564–72. doi: 10.1158/0008-5472.CAN-09-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Safdie FM, Dorff T, Quinn D, Fontana L, Wei M, Lee C, et al. Fasting and cancer treatment in humans: A case series report. Aging (Albany NY) 2009;1:988–1007. doi: 10.18632/aging.100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thaler JP, Schwartz MW. Minireview: Inflammation and obesity pathogenesis: the hypothalamus heats up. Endocrinology. 2010;151:4109–15. doi: 10.1210/en.2010-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hursting SD, Berger NA. Energy balance, host-related factors, and cancer progression. J Clin Oncol. 2010;28:4058–65. doi: 10.1200/JCO.2010.27.9935. [DOI] [PMC free article] [PubMed] [Google Scholar]