Abstract
Purpose
To report the esophageal toxicity from single-fraction paraspinal stereotactic radiosurgery (SRS) and identify dosimetric and clinical risk factors for toxicity.
Methods and Materials
204 spinal metastases abutting the esophagus (182 patients) were treated with high-dose single-fraction SRS during 2003-2010. Toxicity was scored using NCI CTCAE 4.0. Dose-volume histograms (DVHs) were combined to generate a comprehensive atlas of complication incidence that identifies risk factors for toxicity. Correlation of dose-volume factors with esophageal toxicity were assessed using Fishers exact test and logistic regression. Clinical factors were correlated with toxicity.
Results
The median dose to the planning treatment volume was 24 Gy. Median follow-up was 12 months (range 3-81). There were 31 (15%) acute and 24 (12%) late esophageal toxicities. The rate of grade ≥3 acute or late toxicity was 6.8% (14 patients). Fisher’s exact test resulted in significant median splits for grade ≥3 toxicity at V12 = 3.78 cm3 (relative risk [RR] 3.7, p = 0.05), V15 = 1.87 cm3 (RR 13, p = 0.0013), V20 = 0.11 cm3 (RR = 6, p = 0.01), and V22 = 0.0 cm3 (RR 13, p = 0.0013). The median split for D2.5 cm3 (14.02 Gy) was also a significant predictor of toxicity (RR6; p=0.01). A highly significant logistic regression model was generated based on D2.5 cm3. 100% (n = 7) of grade ≥4 toxicities were associated with radiation recall reactions after adriamycin or gemcitabine chemotherapy or iatrogenic manipulation of the irradiated esophagus.
Conclusions
High dose, single fraction paraspinal SRS has a low rate of grade ≥ 3 esophageal toxicity. Severe esophageal toxicity is minimized with careful attention to esophageal doses during treatment planning. Iatrogenic manipulation of the irradiated esophagus and systemic agents classically associated with radiation recall reactions are associated with development of grade ≥4 toxicity.
Keywords: Spine radiosurgery, esophageal toxicity, paraspinal SBRT, IGRT, spine tumors
INTRODUCTION
High-dose, single-fraction paraspinal SRS is transforming the management of metastatic spine tumors. SRS delivers highly conformal treatment plans with steep dose gradients between target volumes and adjacent normal tissues. This is particularly desirable for spine tumors, where target volumes commonly abut critical normal structures such as the spinal cord, esophagus, and brachial plexus. After initial reports demonstrating the feasibility of paraspinal SRS (1), numerous authors reported local control and progression-free survival rates for spinal metastases of ≈90% with single-fraction treatment (2, 3). SRS also yields excellent palliative results, with symptomatic improvement in 80%-90% of patients (3, 4). These rates of local control and palliation for SRS alone compare favorably to historical controls using conventionally fractionated radiotherapy (5). These results support the use of radiosurgery in appropriately selected patients with metastatic spinal tumors.
The toxicity profile of paraspinal SRS remains incompletely defined. Although several publications report a <1% risk of radiation myelopathy (6, 7), few studies address esophageal toxicity associated with SRS. Esophageal toxicity is a concern due to its anatomic proximity to radiosurgery target volumes and because the esophagus is a serial organ where focal dysfunction can cause global organ failure. The esophagus courses from the cricoid cartilage to the gastroesophageal junction, is approximately 25 cm long, and abuts the anterior portion of the vertebral bodies from approximately C7 to T10. Esophageal toxicities can cause morbidity and mortality, and negatively affect quality of life, all of which are highly undesirable in the palliative setting.
This represents the largest known series reporting rates of esophageal toxicity with single-fraction SRS for spinal metastases. A comprehensive atlas of complication incidence and a dose-response model for predicting esophageal toxicity are presented. The atlas is provided in the electronic supplement for the purposes of meta-analysis. Dosimetric, volumetric, and clinical risk factors are identified.
MATERIALS AND METHODS
During 2003-2010, 249 patients were treated with single-fraction SRS to the cervical or thoracic spine at XXXXXXX. Institutional Review Board approval was obtained to retrospectively study this population. All patients had histological confirmation of a solid tumor and radiographic evidence of spinal metastases. Treatments were excluded from analysis if the target volume was not encompassing any portion of the C5-T10 vertebrae, the portion of the planning target volume (PTV) nearest the esophagus was >2 cm away, or there was prior radiotherapy to the region. After excluding 29 patients, 182 patients with 204 treatment sites were analyzed.
Paraspinal SRS simulation, planning, and delivery was performed as previously described 8). Patients underwent supine computed tomography simulation preceded by a myelogram. When myelography was not possible, pretreatment MRI images were fused to the treatment-planning scan. Immobilization was with a thermoplastic mold and a custom cradle as previously described for patients with lesions at or below T4 and with a thermoplastic mold with five-point mask for patients with lesions above T4. Axial CT images were obtained at 2 mm intervals. The gross tumor volume was defined as any gross tumor visible on available imaging studies, including epidural and paraspinal components of disease. The clinical tumor volume (CTV) included abnormal marrow signal suspicious for microscopic involvement and a margin of normal bone to account for subclinical spread. There were no epidural or paraspinal CTV expansions. The PTV was generated by expanding the CTV ≥2 mm. The PTV never violated the spinal cord but could violate the esophageal contours. The esophagus was defined as a solid structure including all layers of the esophageal wall and luminal contents and extended at least 2 cm cephalad and caudad to the PTV. Identification of the esophagus was based on CT simulation imaging alone without MRI fusion. In the rare instances where the esophagus was not visible on each axial image, interpolation was used to bridge between clearly visible slices of esophagus.
Inverse treatment planning with intensity modulation was performed using in-house software as previously described (9, 10). Treatment plans were generated using a fluence-based, gradient search optimization algorithm and typically used 7-9 coplanar beams. Delivery used dynamic multileaf collimation. Plans were normalized to the 100% isodose line to maximize percentage of PTV receiving prescription dose without exceeding normal tissue dose limits. Treatment was prescribed to the 100% isodose line, delivered with 6 and/or 15 MV photons. The dose to the spinal cord was limited to a maximum point dose of 12-14 Gy. Early in the cohort, dose-volume constraints for esophagus were at the discretion of attending physicians. After analysis of initial toxicity (11), formal dose-volume limits were instituted, with ≤15 Gy permissible to 2 cm3 of esophagus. If not achievable, the limit could be increased to no more than 20 Gy to 2 cm3 at the discretion of the attending physician. This second requirement was modified in April 2010 to ≤20 Gy to 2 cm3 and ≤14 Gy to 4 cm3.
At treatment, patients were immobilized and aligned to in-room lasers before performing pretreatment three-dimensional (3D) kV cone beam CT (CBCT) imaging to match regional internal target bony anatomy to the simulation scan. Rotational and translational errors were corrected with further CBCT imaging. 2D kV orthogonal verification scans were obtained immediately prior to treatment to confirm patient alignment. Infrared imaging was used to monitor patient movement. For motion >2 mm, treatment was stopped and the positioning process repeated. Patients were generally premedicated with dexamethasone to prevent acute treatment-related edema and pain. Patients were restricted from receiving cytotoxic systemic therapy for 7-10 days before and after radiosurgery.
Patients were evaluated 8 weeks after treatment and then at 3-4 month intervals thereafter. An MRI of the total spine was generally obtained at each post-treatment visit. For the analysis, all patients were re-graded using CTCAE 4.0 toxicity criteria. The entire medical record was used to assess esophageal toxicity, including but not limited to clinical encounters, radiographic imaging, operative reports, and endoscopy reports.
All treatment plans were restored and the accuracy of the esophageal contours confirmed. An atlas of the incidence of esophageal complications, based on absolute volume dose-volume histograms (DVHs), was generated as previously published (12). Fisher’s exact tests (split at median values) were performed for absolute esophageal volumes exposed to at least 10, 12, 15, 20, and 22 Gy and for minimum doses to the 2.5 cm3 of esophagus receiving the greatest dose to identify dosimetric and volumetric predictors of grade ≥3 toxicity. Logistic regression models were created based on Vx (the volume in cm3 receiving at least x Gy) and Dx (the minimum dose to the x cm3 receiving the highest dose).
RESULTS
The study cohort characteristics are summarized in Table 1. 182 patients were included in the study, 22 for more than one spinal site; 60% were men. Metastatic lesions from all primary sites were included in the analysis, with renal cell carcinomas (18%) and sarcomas (14%) the most common. 37% of cases were carcinomas and 33% were adenocarcinomas with the remainder sarcomas, melanomas, or other tumors. The median pretreatment Karnofsky Performance Status of the cohort was 90% (range 50%-90%). 26 treated lesions (13%) were in the cervical spine and 178 (87%) in the thoracic spine. The median prescribed dose for single fraction was 24 Gy. The institutional dose prescription for single-fraction spine radiosurgery increased over the treatment period for this cohort, from 1600 cGy (<1%) to 1800 cGy (12%) to 2100 cGy (5%) to 2200 cGy (3%) to 2300 cGy (< 1%) to our current practice of 2400 cGy (81%). The mean dose for single-fraction radiosurgery was 2310 cGy. The median follow-up was 12 months (range, 3-81 months). At last follow-up, 56% of the cohort was alive.
Table 1.
Patient Characteristics
| n | Percent | |
|---|---|---|
| Patients | 182 | 100% |
| Total number of lesions | 204 | 100% |
| Patients with ≥2 treatment sites | 22 | 12% |
| Gender | ||
| Female | 73 | 40% |
| Male | 109 | 60% |
| Age (years) | ||
| Median | 61 | |
| Age | 21-88 | |
| Baseline KPS | ||
| Median | 90% | |
| Range | 50%-90% | |
| Spinal Region | ||
| Cervical | 26 | 13% |
| Thoracic | 178 | 87% |
| Follow-Up (months) | ||
| Median | 12 | |
| Range | 3-81 | |
| Status at Last Follow-up | ||
| Alive | 102 | 56% |
| Deceased | 80 | 44% |
| Primary Tumor Site | ||
| Breast | 11 | 6% |
| Upper GI (esophagus, pancreas, gallbladder) | 4 | 2% |
| Lower GI (anal, rectal, colon) | 13 | 7% |
| Hepatocellular | 8 | 4% |
| Sarcoma | 25 | 14% |
| Melanoma | 12 | 6% |
| Lung | 18 | 10% |
| Prostate | 20 | 11% |
| Renal Cell | 33 | 18% |
| Other GU (penile, testicular, bladder) | 3 | 2% |
| Thyroid | 13 | 7% |
| H&N SCC | 3 | 2% |
| CNS | 7 | 4% |
| Other | 12 | 7% |
| Histological Category | ||
| Adenocarcinoma | 59 | 33% |
| Carcinoma | 68 | 37% |
| Melanoma | 14 | 7% |
| Sarcoma | 25 | 14% |
| Other | 16 | 9% |
| Prescribed Dose | ||
| 1600 cGy | 1 | < 1% |
| 1800 cGy | 24 | 12% |
| 2100 cGy | 10 | 5% |
| 2200 cGy | 3 | 1% |
| 2300 cGy | 1 | < 1% |
| 2400 cGy | 165 | 81% |
| Median (cGy) | 2400 | |
| Mean (cGy) | 2310 |
CNS, central nervous system; GI, gastrointestinal; GU, genitourinary; H&N, head and neck; KPS, Karnofsky performance status; SCC, squamous cell carcinoma.
Using CTCAE 4.0, the crude overall rate of any acute or late esophageal toxicity of any grade was 27% (n = 55/204). 75% (n = 41/55) were grade 1 or 2. Thirty-one (15%) were acute (within 90 days of treatment): 28 (14%) grade 1 or 2, one (< 1%) grade 3, and two (1%) grade 4 toxicities. No grade 5 acute toxicity was noted. The most common acute toxicity was transient esophagitis (n = 28, 90%), followed by esophageal ulcer (n = 2, 7%) and esophageal edema (n = 1, 3%). There were 24 (12%) late toxicities (≥90 days after treatment): 13 (6%) grade 1 or 2, 6 (3%) grade 3, 4 (2%) grade 4, and one (< 1%) grade 5. The late toxicities were categorized as esophagitis (n = 12, 50%), esophageal stenosis (n = 4, 17%), esophageal fistula formation (n = 4, 17%), and esophageal ulcer (n = 4, 17%). Overall crude esophageal toxicity rates are summarized in Table 2.
Table 2.
Esophageal Toxicity with Single-Fraction Radiosurgery
| n | Percent | |
|---|---|---|
| Acute Toxicities (≤3 months) | ||
| Overall | 31 | 15 % |
| Grade 1-2 | 28 | 14% |
| Grade 3 | 1 | < 1% |
| Grade 4 | 2 | 1% |
| Grade 5 | 0 | 0% |
| Esophagitis | 28 | 90% |
| Esophageal ulcer | 2 | 7% |
| Esophageal edema | 1 | 3% |
| Late (≥ 3 months) | ||
| Overall | 24 | 12% |
| Grade 1-2 | 13 | 6% |
| Grade 3 | 6 | 3% |
| Grade 4 | 4 | 2% |
| Grade 5 | 1 | < 1% |
| Esophagitis | 12 | 50% |
| Esophageal stenosis | 4 | 17% |
| Esophageal fistula | 4 | 17% |
| Esophageal ulcer | 4 | 17% |
The crude rate for late ≥3 esophageal toxicity was 5% (n=11). The crude rate of grade ≥3 acute or late toxicity combined was 6.8% (n = 14). Grade ≥3 toxicities were esophagitis (n = 2) and esophageal ulcer (n = 1). The 11 late ≥3 toxicities were esophageal stenosis (n = 5), esophageal fistula (n = 4, all tracheoesophageal fistulas), and esophageal ulcer (n = 2). Actuarial analysis for any grade ≥3 acute or late esophageal toxicity is shown in Figure 1. Median time to development of acute and late grade ≥3 toxicity was 2.5 months and 12.7 months, respectively. 100% (n = 7/7) of grade ≥4 complication was proceeded by either adriamycin or gemcitabine chemotherapy (n = 4/7, 57%), iatrogenic manipulation of the esophagus (n = 6/7), or both (3/7). Table 3 summarizes the relevant clinical characteristics of patients with grade ≥4 toxicity.
Fig. 1.
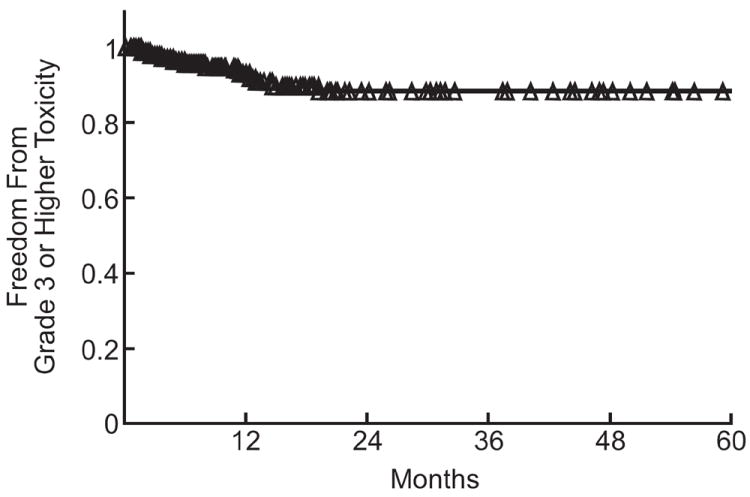
Actuarial analysis of grade ≥3 esophageal toxicity. The number of treated sites at risk for toxicity at 3 month intervals during the first 24 months was: 0 months, 204; 3 months, 165; 6 months, 142; 9 months, 122; 12 months, 102; 15 months, 85; 18 months, 64; 21 months, 50; 24months, 40.
Table 3.
Characteristics of Patients with Grade ≥4 Toxicity
| Patient | Site | Dose (cGy) | Acute vs. Late | Grade | Toxicity Class | Time to Maximum Toxicity (months) | Iatrogenic Manipulation Before Maximum Toxicity | Radiation Recall Reaction (Agent) |
|---|---|---|---|---|---|---|---|---|
| 1 | T4 | 2400 | Acute | 4 | Esophagitis | 2.1 | No | Adriamycin |
| 2 | C7 | 2400 | Acute | 4 | Esophageal Ulcer | 2.8 | Biopsy | Liposomal Adriamycin |
| 3 | T3 | 2400 | Late | 4 | Esophageal Fistula | 14.8 | Dilation | No |
| 4 | T3 | 2400 | Late | 4 | Esophageal Fistula | 5.0 | Biopsy | No |
| 5 | T7 | 2400 | Late | 4 | Esophageal Fistula | 12.7 | Stent | Gemcitabine |
| 6 | C4-T2 | 2400 | Late | 4 | Esophageal Stenosis | 4.3 | Dilation | Adriamycin |
| 7 | T2 | 2100 | Late | 5 | Esophageal Fistula | 19.5 | Stent | No |
An atlas of complication incidence was created using absolute volume DVHs from all treatment plans in this cohort. For the purposes of meta-analysis, this is provided in the electronic supplement (appendices e1 and e2) as recommended by QUANTEC (13, 14). The atlas identifies several dose-volume factors predictive of significant toxicity. Figure 2 shows maps of the complication rate for patients whose DVHs pass above a given point, for each point in the dose-volume plane (a) and the probability that the true grade ≥3 complication rate is >10%, given the observed complications (b). These demonstrate that probability of grade ≥3 toxicity is a function of dose and volume of irradiated esophagus. For any fixed volume of esophagus, particularly for ≤8 cm3, probability of toxicity clearly increases with dose. There is also a high probability of complication for large volumes of esophagus exposed to low doses of radiation (eg, a 70% probability that the true complication rate is >10% when 13.5 cm3 esophagus receives ≥6 Gy). Areas of low toxicity probability are identified in the lower left atlas. Figures 2c and d are maps of the lower and upper 68% confidence limit on the grade ≥3 complication rate, respectively. These maps show the uncertainty on the observed complication rate in Figure 2a. The large value of the lower 68% confidence limit at high doses (on the order of 25 Gy) and very small volumes of esophagus (~1 cm3) indicate that exposure to such doses increases risk for grade ≥3 toxicity. It should be understood, however, that there are very few DVHs in this region. The apparent decrease in complication rate seen at volumes > 10 cm3 for doses > 10 Gy is also likely due to the low statistics in that region. The total number of treatments with DVHs passing over the point (15 Gy, 10 cm3) is only 6. We refer the reader to the atlas in the electronic supplement e1 for complete details of the number of DVHs and associated complications passing over any position in the DVH plane.
Fig. 2.
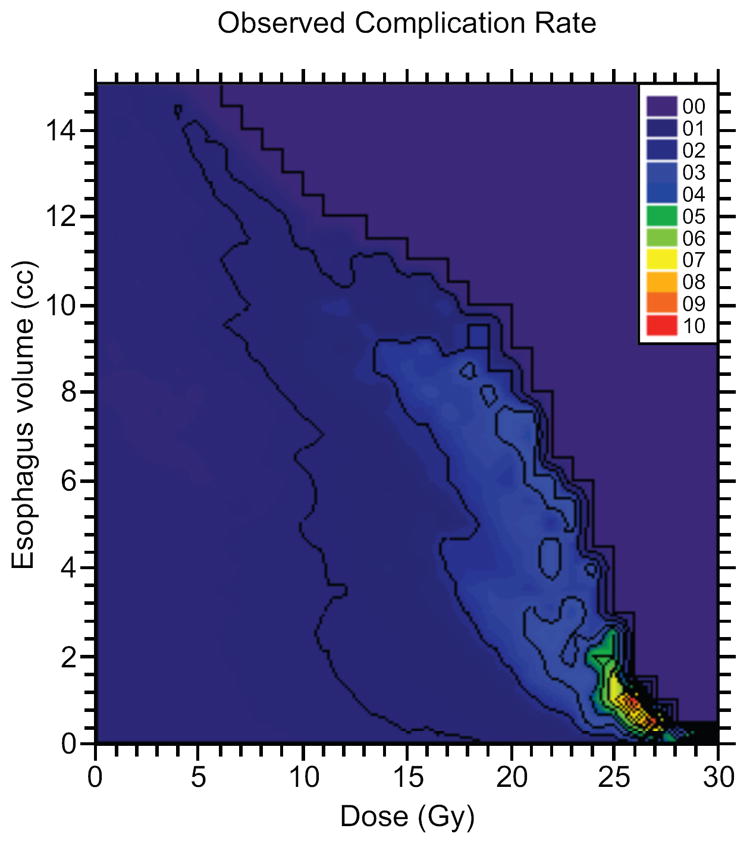
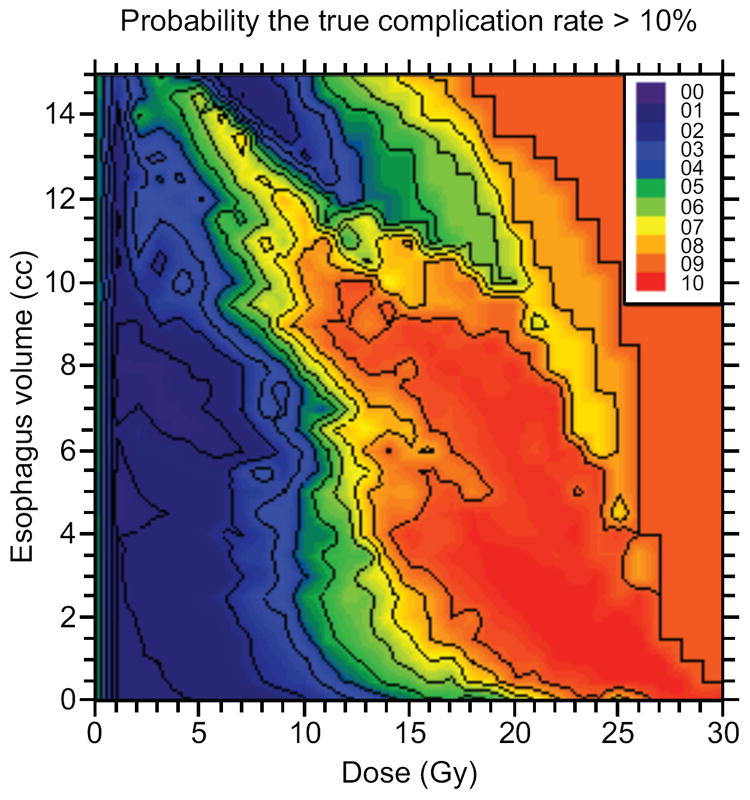
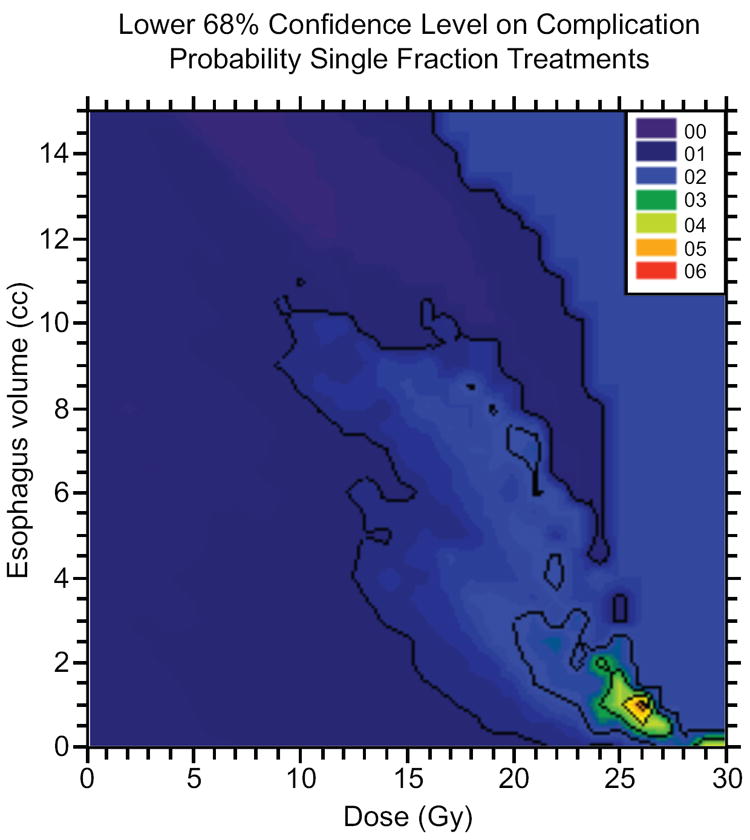

a) Observed severe esophageal complication rate for DVHs passing over a given position in the dose-volume plane, plotted as a function of that position. b) Probability of a true esophageal complication rate >10% for DVHs passing over a point in the dose-volume plane for the esophagus, plotted at that point. C) Represents the lower 68% confidence level of complication probability for single-fraction treatments for DVHs passing over a point in the dose-volume plane for the esophagus. Esophageal doses of greater than 25 Gy to even small volumes of esophagus yields a high probability of esophageal complication. D) demonstrates the upper 68% confidence level for complication rate for DVHs passing over a given position in the dose/volume plane, plotted as a function of that position.
In dose-volume analysis, Fisher’s exact test resulted in significant median splits for grade ≥3 toxicity at V12 = 3.78 cm3(relative risk [RR] 3.7, p = 0.05), V15 = 1.87 cm3 (RR 13, p = 0.0013), V20 = 0.11 cm3 (RR = 6, p = 0.01), and V22 = 0.0 cm3 (RR 13, p = 0.0013). The median split for D2.5 cm3 (14.02 Gy) was also a highly significant predictor of toxicity (RR, 6; p=0.01.) Table 4 summarizes the results using median dosimetric and volumetric splits to predict risk of grade ≥3 toxicity.
Table 4.
Dosimetric and Volumetric Predictors of Grade ≥3 Esophageal Toxicity
| Dosimetric Variable | Median Split | Toxicity Incidence Below Median Split | Toxicity Incidence Above Median Split | RR Grade ≥3 Toxicity | p Value | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| n | % | n | % | ||||
| D2.5 cm3 | 14.02 Gy | 2/102 | 2% | 12/102 | 12% | 12/2 = 6 | 0.01 |
| V10 Gy | 4.77 cc | 4/102 | 4% | 10/102 | 10% | 10/4 = 2.5 | 0.16 |
| V12 Gy | 3.78 cc | 3/102 | 3% | 11/102 | 11% | 11/3 = 3.7 | 0.05 |
| V15 Gy | 1.87 cc | 1/102 | 1% | 13/102 | 13% | 13/1 = 13 | 0.0013 |
| V20 Gy | 0.11 cc | 2/102 | 2% | 12/102 | 12% | 12/2 = 6 | 0.01 |
| V22 Gy | 0.0 cc | 1/102 | 1% | 13/102 | 13% | 13/1 = 13 | 0.0013 |
RR, relative risk.
Figure 3 demonstrates the dose response as modeled and as observed in quartiles in terms of D2.5 cm3 (minimum dose to the hottest 2.5 cm3 of the esophagus), the most significant logistic regression model (p<0.0006). This model suggests that keeping the dose to the hottest 2.5 cm3 of esophagus <14.5 Gy yields a grade ≥3 toxicity rate of <5% with a steep increase in toxicity after further increases in dose. There is a 10% risk of grade ≥3 toxicity if the dose to this volume is increased to 18 Gy and a 15% risk of ≥3 toxicity if 20 Gy is delivered.
Fig. 3.

Dose-response model for grade ≥3 esophagitis with single-fraction spine radiosurgery, from logistic regression using D 2.5 cm3. When the D 2.5 cm3 <14 Gy, there is a <5% probability of grade ≥3 esophageal toxicity, with a steep increase in toxicity at higher doses (p<0.01). For comparison, observed complication rates in quartiles in D 2.5 cm3 are plotted at the median value of the quartile. Black vertical lines, 68% confidence limits on the observed complication rates; horizontal lines, central 68% of the dose values in the quartile.
DISCUSSION
All patients evaluated in this study were at an elevated risk of toxicity due to target volumes directly abutting the esophagus. This study reports a crude late grade 3 esophageal toxicity rate of 5% for high-dose, single-spine radiosurgery. Radiation-induced esophageal complications from spine radiosurgery include pain, ulceration, stenosis, and tracheoesophageal fistula formation, which may lead to dehydration, malnutrition, and subsequent complications requiring intervention, including death. Dosimetric and volumetric parameters clearly predict for development of esophageal toxicity. Importantly, no grade ≥4 esophageal toxicity occurred in the absence of iatrogenic manipulation of the esophagus or systemic agents classically associated with radiation recall reactions such as adriamycin and gemcitabine.
Based on this analysis, we have modified our radiosurgery practice. We limit no more than 14 Gy to 2.5 cm3 of esophagus. We further attempt to limit V12Gy to <3.78 cm3, V15Gy to <1.87 cm3, and V20Gy to <0.11 cm3, and the maximum point dose to the esophagus to <22 Gy. We carefully evaluate each patient’s pretreatment swallowing function, rigorously delineate the esophagus on the treatment simulation scan, and actively educate the patient’s multidisciplinary care team regarding post-radiosurgery risk factors.
Limited publications are available regarding esophageal toxicity of single-fraction radiosurgery. A QUANTEC review identified a variety of parameters that predict for radiation esophageal injury (15). The findings have limited applicability to radiosurgery because of a high proportion of conventionally fractionated regimens and concurrent chemotherapy. Other series have reported similar parameters predictive for injury (16, 17). Gomez et al. reported an early low rate of esophageal toxicity with single-fraction intrathoracic SRS (11). This analysis was limited by a small cohort and limited dosimetric analysis. There are no other publications reporting esophageal toxicity from spinal SRS with the exception of limited dosimetry-based studies without outcomes data (18). Several publications report esophageal toxicity for lung-cancer patients treated with stereotactic body radiotherapy (19, 20). However, these studies have limited applicability to single-fraction spine radiosurgery due to anatomic differences and lung stereotactic body radiotherapy being given in fractionated regimens.
It is essential to delineate the toxicities of modern spine radiosurgery. SRS is an attractive alternative to aggressive surgical en bloc resection or conventionally fractionated radiotherapy and offers high rates of durable local control (2, 3). There is superior local progression–free survival with high-dose single-fraction radiation to a dose of 24 Gy compared with doses of 18-23 Gy (2). Even for radioresistant histologies, aggressive en bloc resection strategies do not offer superior local control rates and carry medical and functional risks that spine radiosurgery does not (21). Conventionally fractionated radiation provides substantially worse rates of local control (5). Therefore, as spine radiosurgery is more widely applied, the careful balance between high-dose target coverage and safe normal tissue dose-volume limits must be optimized. These findings clearly demonstrate that esophageal toxicity must be considered during spine radiosurgery treatment planning, in addition to potential toxicities of radiation myelopathy (6, 7) and vertebral body fracture (22).
Strengths of this study include a large, consecutive, homogeneously treated cohort identified from a prospectively maintained database. Comprehensive follow-up was available, with regular clinical evaluation and serial imaging after treatment. This is the first study in the literature utilizing atlases of esophageal complication incidences, allowing a complete and unbiased summary of dose-volume distributions and toxicities in the cohort. This robust method of dosimetric analysis yields systematic information about the safety of regions of dose-volume exposure and allows logistic regression.
Limitations of this study include the use of retrospective analysis and grading of toxicities, and the limited follow-up of this patient cohort. Median follow-up for treatments in the study was 12 months; however, the median time to the onset of late grade ≥3 complications was 11.3 months. With further follow-up, the crude rate of severe complications may increase.
In summary, there is a low overall rate of grade ≥3 toxicity with high-dose, single-fraction spine radiosurgery that can be minimized through careful evaluation of the esophageal DVH during treatment planning. Toxicity can further be minimized by avoiding unnecessary iatrogenic manipulation of the esophagus, such as dilatation, biopsy, and stent placement, and systemic agents classically associated with radiation recall reactions.
Supplementary Material
Figure 4.
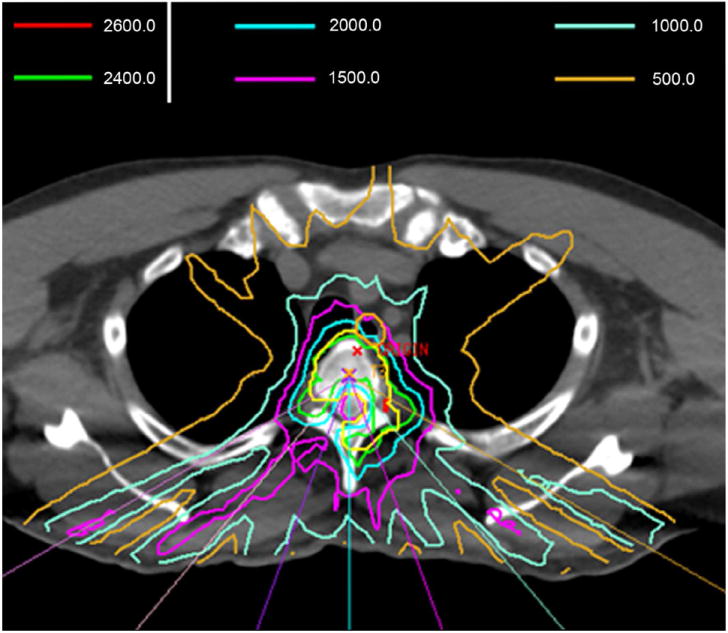
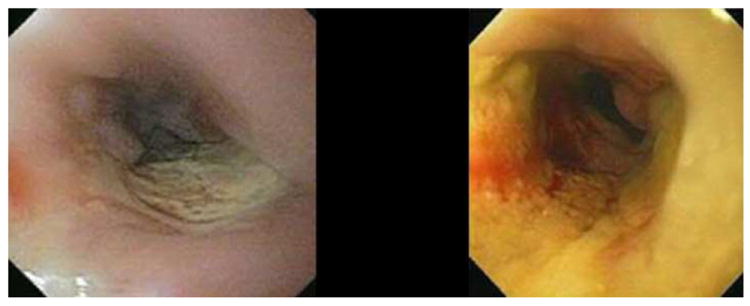
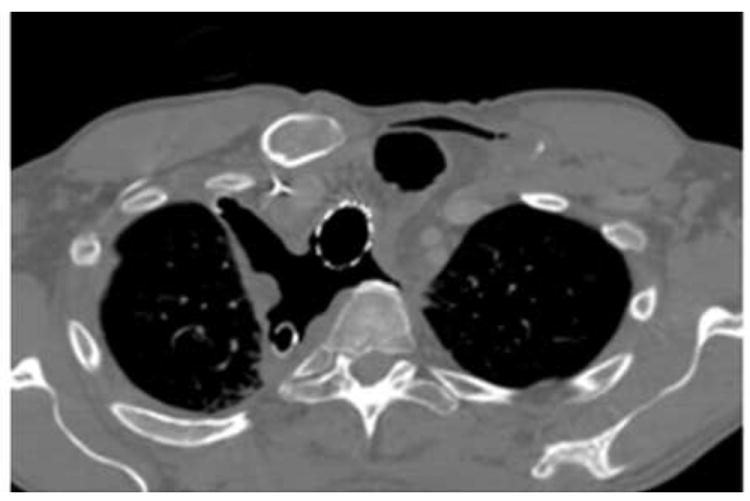
Representative grade 4 esophageal complication after spine radiosurgery showing the role of iatrogenic manipulation in development of high-grade toxicity. Patient was a 45-year-old man with oligometastatic renal cell carcinoma treated with 24 Gy to a symptomatic T3 lesion. a) shows representative isodose distributions. Esophageal planning constraints kept the D2.0 cm3 esophagus <15 Gy. The patient experienced grade 2 esophagitis at 4 months, and an esophagogastroduodenoscopy (EGD) showed a 3 cm nonbleeding ulcer (b) that was biopsied. Pain immediately worsened, and repeat EGD at 6 months showed increase in size, extent, and severity with superinfection (c). Biopsy and dilation was performed in the absence of stricture. Two weeks later the patient acutely developed a tracheoesophageal fistula (TEF) requiring multiple stent and repair procedures. d) A thoracic CT image demonstrating multiple stigmata of TEF formation, including tracheal and esophageal stents in place, pneumomediastinum, and soft-tissue defects. The patient died from distant progression of disease at 11 months.
Acknowledgments
NIH/NCI grant 1RO1CA129182 provides direct funding to Dr, Jackson and indirect funding to Memorial Sloan-Kettering Cancer Center.
Dr. Hunt’s institution has received research grants from Varian Medical Systems, and has received lecturing fees from Varian Medical Systems.
Dr. Yamada is a consultant to Varian Medical Systems and Funcacion Hospital Provincial Castellon, and has received lecturing fees from the Institute for Medical Education.
Footnotes
Based on work accepted for oral presentation at the 2011 ASTRO annual meeting.
Conflict of Interest Notification
Drs. Cox and Bilsky have no actual or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ryu SI, Chang SD, Kim DH, et al. Image-guided hypo-fractionated stereotactic radiosurgery to spinal lesions. Neurosurgery. 2001;49:838–846. doi: 10.1097/00006123-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Yamada Y, Bilsky MH, Lovelock DM, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71:484–490. doi: 10.1016/j.ijrobp.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 3.Gerszten PC, Burton SA, Ozhasoglu C, et al. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine (Phila Pa 1976) 2007;32:193–199. doi: 10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]
- 4.Gibbs IC, Kamnerdsupaphon P, Ryu MR, et al. Image-guided robotic radiosurgery for spinal metastases. Radiother Oncol. 2007;82:185–190. doi: 10.1016/j.radonc.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 5.Katagiri H, Takahashi M, Inagaki J, et al. Clinical results of nonsurgical treatment for spinal metastases. Int J Radiat Oncol Biol Phys. 1998;42:1127–1132. doi: 10.1016/s0360-3016(98)00288-0. [DOI] [PubMed] [Google Scholar]
- 6.Gibbs IC, Patil C, Gerszten PC, et al. Delayed Radiation-Induced Myelopathy after Spinal Radiosurgery. Neurosurgery. 2009;64:A67–A72. doi: 10.1227/01.NEU.0000341628.98141.B6. [DOI] [PubMed] [Google Scholar]
- 7.Sahgal A, Ma L, Gibbs I, et al. Spinal cord tolerance for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;77:548–553. doi: 10.1016/j.ijrobp.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 8.Lovelock DM, Hua C, Wang P, et al. Accurate setup of paraspinal patients using a noninvasive patient immobilization cradle and portal imaging. Med Phys. 2005;32:2606–2614. doi: 10.1118/1.1951042. [DOI] [PubMed] [Google Scholar]
- 9.Ling CC, Burman C, Chui CS, et al. Conformal radiation treatment of prostate cancer using inversely-planned intensity-modulated photon beams produced with dynamic multileaf collimation. Int J Radiat Oncol Biol Phys. 1996;35:721–730. doi: 10.1016/0360-3016(96)00174-5. [DOI] [PubMed] [Google Scholar]
- 10.Mohan R, Barest G, Brewster LJ, et al. A comprehensive three-dimensional radiation treatment planning system. Int J Radiat Oncol Biol Phys. 1988;15:481–495. doi: 10.1016/s0360-3016(98)90033-5. [DOI] [PubMed] [Google Scholar]
- 11.Gomez DR, Hunt MA, Jackson A, et al. Low rate of thoracic toxicity in palliative paraspinal single-fraction stereotactic body radiation therapy. Radiother Oncol. 2009;93:414–418. doi: 10.1016/j.radonc.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson A, Yorke ED, Rosenzweig KE. The atlas of complication incidence: a proposal for a new standard for reporting the results of radiotherapy protocols. Semin Radiat Oncol. 2006;16:260–268. doi: 10.1016/j.semradonc.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Jackson A, Marks LB, Bentzen SM, et al. The lessons of QUANTEC: recommendations for reporting and gathering data on dose-volume dependencies of treatment outcome. Int J Radiat Oncol Biol Phys. 2010;76:S155–160. doi: 10.1016/j.ijrobp.2009.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deasy JO, Bentzen SM, Jackson A, et al. Improving normal tissue complication probability models: the need to adopt a “data-pooling” culture. Int J Radiat Oncol Biol Phys. 2010;76:S151–154. doi: 10.1016/j.ijrobp.2009.06.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Werner-Wasik M, Yorke E, Deasy J, et al. Radiation dose-volume effects in the esophagus. Int J Radiat Oncol Biol Phys. 2010;76:S86–93. doi: 10.1016/j.ijrobp.2009.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahn SJ, Kahn D, Zhou S, et al. Dosimetric and clinical predictors for radiation-induced esophageal injury. Int J Radiat Oncol Biol Phys. 2005;61:335–347. doi: 10.1016/j.ijrobp.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Maguire PD, Sibley GS, Zhou SM, et al. Clinical and dosimetric predictors of radiation-induced esophageal toxicity. Int J Radiat Oncol Biol Phys. 1999;45:97–103. doi: 10.1016/s0360-3016(99)00163-7. [DOI] [PubMed] [Google Scholar]
- 18.Ma L, Sahgal A, Cozzi L, et al. Apparatus-dependent dosimetric differences in spine stereotactic body radiotherapy. Technol Cancer Res Treat. 2010;9:563–574. doi: 10.1177/153303461000900604. [DOI] [PubMed] [Google Scholar]
- 19.Chang JY, Balter PA, Dong L, et al. Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;72:967–971. doi: 10.1016/j.ijrobp.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taremi M, Hope A, Dahele M, et al. Stereotactic Body Radiotherapy for Medically Inoperable Lung Cancer: Prospective, Single-Center Study of 108 Consecutive Patients. Int J Radiat Oncol Biol Phys. 2011 doi: 10.1016/j.ijrobp.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 21.Bilsky MH, Laufer I, Burch S. Shifting paradigms in the treatment of metastatic spine disease. Spine (Phila Pa 1976) 2009;34:S101–107. doi: 10.1097/BRS.0b013e3181bac4b2. [DOI] [PubMed] [Google Scholar]
- 22.Rose PS, Laufer I, Boland PJ, et al. Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases. J Clin Oncol. 2009;27:5075–5079. doi: 10.1200/JCO.2008.19.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


