Abstract
BACKGROUND
Trauma and transfusion can both alter immunity, and while transfusions are common among traumatically injured patients, few studies have examined their combined effects on immunity.
STUDY DESIGN AND METHODS
We tracked the plasma levels of 41 immunomodulatory proteins in 56 trauma patients from time of injury up to 1 year later. In addition, a murine model was developed to distinguish between the effects of transfusion and underlying injury and blood loss.
RESULTS
Thirty-one of the proteins had a statistically significant change over time after traumatic injury, with a mixed early response that was predominantly anti-inflammatory followed by a later increase in proteins involved in wound healing and homeostasis. Results from the murine model revealed similar cytokine responses to humans. In mice, trauma/hemorrhage caused early perturbations in a number of the pro- and anti-inflammatory mediators measured, and transfusion blunted early elevations in IL-6, IL-10, MMP-9, and IFN-γ. Transfusion caused or exacerbated changes in MCP-1, IL-1α, IL-5, IL-15, and soluble E-selectin. Finally, trauma/hemorrhage alone increased KC and IL-13.
CONCLUSIONS
This work provides a detailed characterization of the major shift in the immunological environment in response to trauma and transfusion and clarifies which immune mediators are affected by trauma/hemorrhage and which by transfusion.
Keywords: Allogeneic Transfusion, Trauma/Hemorrhage, Mouse model, Cytokines, Immune Dysregulation
INTRODUCTION
Traumatic injury represents a major health concern, resulting in approximately 2.7 million hospital admissions each year in the United States.1,2 Transfusion of allogeneic blood products is a common intervention following traumatic injury, with about 9% of trauma patients transfused, and approximately one-third of these given massive transfusions.1,3 As a result, 9% of RBC units in the US are used in the acute support of traumatically injured patients.4
Traumatic injury has been shown to be a major immunological event, leading to immune dysregulation that can contribute to further tissue damage as well as compromise the host’s ability to fight infection. Severe injury is thought to lead initially to a proinflammatory response or systemic inflammatory response syndrome (SIRS) which may be important as part of the normal healing process, but can cause excessive tissue damage and in some cases contribute to multiple organ failure.5–7 This initial proinflammatory state is thought to be followed by a compensatory anti-inflammatory response syndrome, or CARS, which while important for controlling inflammation, may also lead to an increased susceptibility to infection.5–7 This model has recently been questioned as gene expression profiles of trauma patients suggest that the pro- and anti-inflammatory responses occur simultaneously.8 Human studies of peripheral cytokines after trauma have observed increases in IL-6, IL-10, IL-1Ra, and IL-8 at various time-points after injury, while reports of increases in TNF-α and IL-4 have been inconsistent.9–22 Changes in levels of different antibody isotype levels have also been reported, with increased IgE and decreased IgM.22–24 Human ex vivo cellular assays with peripheral blood from trauma patients have found decreased HLA-DR expression and increased IL-6 and IL-10 production by monocytes, increased regulatory T cell activity, and altered T cell effector functions.25–32 Mouse models of traumatic injury using various combinations of femur fractures, hemorrhagic shock, laparotomy, or burn (all under anesthesia) have found increases in serum IL-6, IL-10, and other cytokines, as well as increased regulatory T cell activity, reduced ex vivo dendritic cell activation, and altered ex vivo T cell cytokine profiles.33–41 In mouse and rat models of hemorrhage where animals are anesthetized and bled either a fixed volume or to a fixed reduced blood pressure, short-term defects in IL-2 production and T cell proliferative capacity have been observed.42–44
Gender and age are also contributing factors to the immune response to trauma. In humans several studies have found that men have an increased risk of death, sepsis, and multiple organ dysfunction syndrome compared to women and that this gender difference is age-dependent.45–49 Mouse models of trauma utilizing ovariectomised females, castrated males, and administration of sex hormones show that the gender differences can be overcome by changing the balance of sex hormones, with androgens suppressing responses to septic challenges and estrogens enhancing these responses.50,51
Transfusion of whole blood or blood components is perhaps the most commonly performed type of allogeneic transplantation and in itself represents a major immunological intervention. In some contexts, transfusion has been shown to have an immunosuppressive effect. This immunosuppression can contribute to positive clinical outcomes such as reduced transplant graft failure, but has also been suggested to increase cancer growth and susceptibility to infection in some patient populations and animal models.52–57 In spite of the immunological consequences of both trauma and transfusion, and the prevalence of transfusion among injured patients, very few studies have directly examined the combined effect of traumatic injury and allogeneic transfusion on immunity. Transfusion has been shown to lead long-term survival of allogeneic donor cells in 10–15% of trauma patients, but this is not observed in surgical patients receiving transfusions, suggesting that there is some form of unique immunosuppression occurring with traumatic injury58. Mouse burn models have observed reduced resistance to infection and altered NK cell activity following burn and transfusion as compared with burn alone,59,60 and a murine model of hemorrhage found that transfusion modulated the ex vivo cytokine production of T cells from hemorrhaged mice,61 but all of these studies used anesthetized animals, potentially missing some of the effects of the stress response to traumatic injury.
In the current study a wide range of immunomodulatory plasma proteins were evaluated in serial samples collected from transfused and non-transfused trauma patients, enrolled upon arrival in the emergency room and followed for up to 1 year after injury. A mouse model was developed to investigate the distinct contributions of traumatic blood loss (without anesthesia) and transfusion on immunity.
MATERIALS AND METHODS
Human Subjects
Trauma patients were recruited from the University of California Davis Medical Center (UCDMC, Sacramento, CA) as part of a larger study of microchimerism, the persistence of donor blood cells in transfusion recipients. From this larger cohort, 56 subjects (39 transfused and 17 non-transfused) were selected for cytokine analysis based on serial sample availability. The University of California Davis Institutional Review Board approved the human subjects portion of our study. Upon arrival to the UCDMC Emergency Department, all injured patients meeting specific institutional triage criteria (Figure S1) associated with a relatively high likelihood of severe injury between November 2006 and August 2010 were evaluated for enrollment. We excluded subjects less than 12 years of age, prisoners, patients from whom we were unable to obtain a blood sample prior to the first transfusion of blood products, and patients who had undergone previous transplantation (solid organ or hematopoietic transplant). Because the focus of the microchimerism study was on subjects likely to survive long-term following traumatic injury, we also excluded patients who died within seven days after injury. We collected an initial blood sample on all eligible subjects, and then retained in the study those subjects who subsequently provided informed consent. We approached for consent all transfused subjects and a subset of non-transfused subjects with comparable significant injuries, enrolled at an approximate ratio of one non-transfused subject per four transfused subjects over the life of the study. Transfused blood products were all leukoreduced.
Modeling traumatic blood loss and transfusion in mice
Female BALB/cJ and C57Bl/6J mice were purchased from The Jackson Laboratory (Bar Harbor, Maine), and allowed to acclimate for a minimum of 2 weeks before use at age 9 weeks and 2–3 months, respectively. Mice were maintained in a specific-pathogen-free vivarium under barrier conditions at Blood Systems Research Institute (San Francisco, CA). All research was performed with approval and oversight of the Institutional Animal Care and Use Committee at ISIS Services LLC (San Carlos, CA).
To model traumatic blood loss, BALB/cJ mice were bled 25–30% of their total estimated blood volume based on weight. The volume of blood to be removed was calculated as 25% (in μL) = (weight in g) × (20); 30% (in μL) = (weight in g) × (24). Mice were bled at the submandibular vein cluster using a sterile lancet without anesthesia. This method allows for collection of high blood volumes as well as rapid control of bleeding once the desired volume is collected.62 After bleeding, mice were placed in a recovery area with gentle heat and close monitoring until able to resume grooming and other light activity. C57Bl/6J blood-donor mice were exsanguinated via orbital enucleation under deep anesthesia. Blood was collected from multiple mice into a single tube containing CPDA-1 anticoagulant (taken from a 500 mL WBF Double CPDA-1 Blood Bag Unit, PALL Medical, East Hills, NY) at 14% final volume and gently mixed between collections. Donor blood was centrifuged, then a portion of the plasma fraction was removed to bring to a hematocrit of ~75% and gently mixed. Blood transfusions consisting of 100 μL fresh packed red cells (<6hrs old) and 400 μL sterile 0.9% sodium cloride (Baxter Healthcare Corporation, Deerfield, IL) were administered IV by tail vein, as were injections of normal saline alone (500μL). Blood was administered on the same day as collection to simplify the model and avoid introducing further variation that might be associated with age of blood products.
Sample collection and processing
Human blood samples were collected into 10 mL Plastic Vacutainer® spray-coated K2EDTA tubes (BD) at UCDMC and shipped via overnight courier service (FedEx) to Blood Systems Research Institute. Upon arrival, the plasma fraction was isolated, aliquoted, and stored at −80°C until use. Murine blood samples were collected by exsanguination via orbital enucleation under anesthesia (Isofluorane) into tubes without anticoagulant additives. Blood samples were allowed to clot for 20–30 minutes after collection, and then centrifuged at high speed to isolate serum. Serum was aliquoted and stored at −80°C until use.
Cytokine detection
Cytokines were measured using a Luminex 100 platform (Luminex, Austin, TX) and the BioManager Software (BioRad, Hercules, CA) for analysis. The following multiplexing kits were purchased from Millipore (Billerica, MA): the Milliplex Map Human Cytokine/Chemokine Kit containing IL-1α, IL-1Ra, IL-9, IL-12p40, IL-15, IL-17, EGF, eotaxin, FGF-2, fractalkine, IP-10 (CXCL10), MCP-1, monocyte chemotactic protein-3, MDC, MIP-1α, macrophage inflammatory protein 1 beta (MIP-1β), sIL-2Rα, TNF-β, VEGF; the Milliplex Map High Sensitivity Human Cytokine Kit containing IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p70, IL-13, IFN-γ, granulocyte-macrophage colony stimulating factor (GM-CSF), and TNF-α; the Milliplex Map Human Sepsis/Apoptosis Kit containing sVCAM-1, sICAM-1, sFas, sFasL, MIF, and tPAI-1; the Milliplex Map Human Cardiovascular Disease (CVD) Panel 1 Kit containing sE-Selectin, MMP-9, and MPO; the Milliplex Map Mouse Cytokine/Chemokine Panel 1 Kit containing eotaxin, GM-CSF, IFN-γ, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IP-10, KC (CXCL1), M-CSF, MCP-1, MIP-1α, MIP-1β, MIP-2, TNF-α; and the Milliplex MAP Mouse Cardiovascular Disease (CVD) Panel 1 containing MMP-9, tPAI-1, sE-Selectin, sICAM-1, sVCAM-1. Samples were run in duplicate according to manufacturer instructions. For the human studies, 4 plates were required for each kit so the same lots were used for all plates from the same kit, and additional internal controls were added to all plates to confirm minimal plate-to-plate variation. For the murine studies, each experiment was fit on a single plate for each kit to avoid plate-to-plate variation.
Statistical Analysis
Human data
Cytokine and clinical data were loaded into a SQL database (MySQL version 5.1.7) using SQuirreLSQL (client version 2.6.9). Undetectable values were assigned a value of zero for the purposes of analysis. Data were then imported into Stata Special Edition version 10.1 (College Station, Texas) for analysis. While all follow-up samples were processed within 24 hours from collection, a subset of index samples were (out of necessity) collected over weekends and holidays and were therefore delayed in processing for an extra 24–48 hours. To address this, 10 additional trauma index samples received within 24 hours after collection were subaliquoted with portions processed immediately, 24, 48, and 72 hours after arrival, and analyzed for cytokine levels.63 For all proteins with a significant change over time, older index samples were dropped from the analysis. Concentration of all proteins in pg/mL were log transformed using ln(concentration+1). For reference, untransformed median values with interquartile ranges are reported for all cytokines at day 0 in Table S1. Age, ISS, number of units transfused in the first 48 hours, and time were converted into categorical variables. GEE was used to model the changes in each cytokine over time. This method allows for repeated measures, accounting both for variation between individuals and differences between individuals over time. Exchangeable correlation was used to allow for gaps in sample collection or loss to follow up over time. For modeling time since trauma, linear, quadratic, and cubic functions were evaluated for each protein, and the function with the best overall fit was chosen. In addition to time since trauma, the following independent variables were included in the model: age (<25, 25–34, 35–44, or >44 years), gender (male or female), ISS (1–9, 10–14, 16–24, 25–43, 45–75), number of units transfused in the first 48 hours [expressed as the indicator variables “no transfusion” and “large transfusion” (>4 units) each compared against “modest transfusion” (≤4units)], injury type (blunt or penetrating), and microchimeric (yes or no). The Wald χ2 statistic was used to assess overall significance with a cutoff of p < 0.05. This tests against the hypothesis that none of the variables have an effect on the protein concentrations. A coefficient for an individual variable was considered statistically significant if p < 0.05. Graphs were generated in Stata Special Edition version 10.1. Maximum and minimum values over a set range for each time function generated by the model were calculated and a percent increase was calculated as 100 × (eMaximum − eMinimum)/[(eMinimum)−1].
Animal data
Concentrations of each protein analyzed were compared between treatment groups using one-way ANOVA; each treatment group was then compared to the untreated control group using Dunnett’s multiple comparison post-test. Prism Version 5.0a (GraphPad Software, Inc., La Jolla, CA) was used for analysis and graphing.
RESULTS
Human response to trauma and transfusion
To assess the human immune response to trauma and transfusion, serial blood samples were collected from trauma patients at UCDMC. The first blood sample was collected upon arrival in the emergency room, with follow-up samples collected at regular intervals up to one year after injuries. Of the 56 subjects included in the analysis, 75% were male, 73% suffered blunt trauma, and 70% were transfused. The median age was 30.5 years, the median injury severity score (ISS) was 17, and a median of 4 RBC units was given in the first 48 hours after injury to those who received a transfusion (Table I).
Table 1.
Characteristics of injured patients
| Characteristic | Transfused (n=39) | Not Transfused (n=17) |
|---|---|---|
| Age, years (mean ± SD) | 34 ± 14 | 38 ± 16 |
| Male gender, n (%) | 33 (85) | 9 (53) |
| Blunt mechanism of injury, n (%) | 26 (67) | 14 (82) |
| Mechanism of injury, n (%) | ||
| Motor vehicle collision | 12 (31) | 7 (41) |
| Motorcycle collision | 7 (18) | 1 (6) |
| Automobile versus pedestrian/bicycle | 2 (5) | 2 (12) |
| Fall | 1 (2) | 2 (12) |
| Firearm wound | 8 (20) | 2 (12) |
| Stab wound | 4 (10) | 1 (6) |
| Other | 5 (13) | 2 (12) |
| Revised Trauma Score† | 7.84 (7.11, 7.84) | 7.84 (6.38, 7.84) |
| Injury Severity Score (mean ± SD) | 20 ± 12 | 13 ± 6 |
| Injury Severity Score components† | ||
| Abbreviated Injury Scale—Head/neck | 2 (0, 2) | 2 (0, 3) |
| Abbreviated Injury Scale—Face | 0 (0, 0) | 0 (0, 0) |
| Abbreviated Injury Scale—Chest | 2 (0, 3) | 0 (0, 3) |
| Abbreviated Injury Scale—Abdomen | 2 (0, 2) | 0 (0, 2) |
| Abbreviated Injury Scale—Extremities | 3 (0, 3) | 1 (0, 2) |
| Abbreviated Injury Scale—External | 0 (0, 1) | 0 (0, 0) |
| Predicted probability of survival†* | 0.98 (0.85, 0.99) | 0.98 (0.96, 0.99) |
| Initial systolic blood pressure† | 103 (93, 118) | 130 (108, 140) |
| Initial international normalized ratio (INR)† | 1.06 (1.00, 1.20) | 0.99 (0.97, 1.03) |
| Initial partial thromboplastin time (PTT)† | 24.0 (22.6, 26.1) | 23.7 (22.8, 24.8) |
| PRBC transfused (units)† ‡ | 4 (2, 8) | — |
| Platelets transfused (units)† ‡ | 0 (0, 0) | — |
| Cryoprecipitate transfused (units)† ‡ | 0 (0, 0) | — |
| Plasma transfused (units)† ‡ | 1 (0, 2) | — |
| Crystalloid infused (liters)† ‡ | 10.4 (7.6, 16.5) | 5.6 (3.9, 7.8) |
| Colloid infused (liters)† ‡ | 0 (0, 0.8) | 0 (0, 0) |
| Length of stay (days)† | 17 (10, 34) | 8 (5, 14) |
Determined from the Trauma and Injury Severity Score (TRISS)
Median (IQR)
Within the first 48 hours after injury
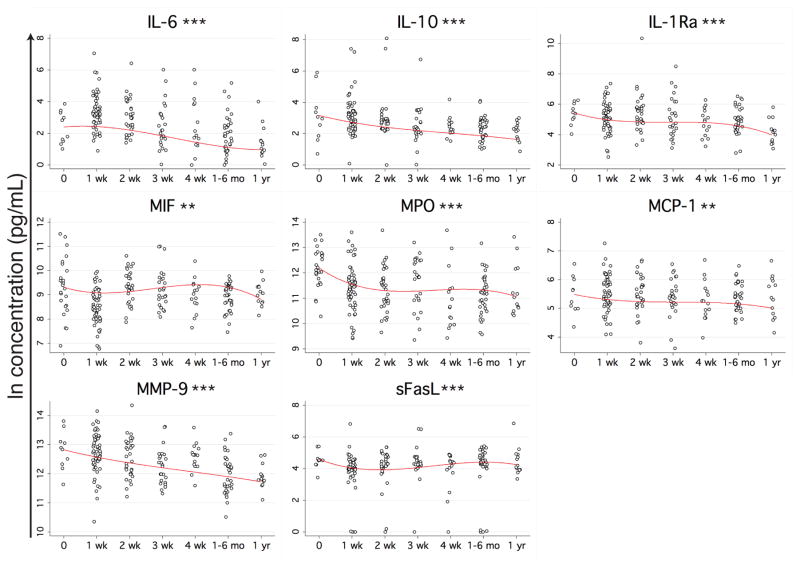
Plasma was isolated from the blood and assayed for 41 cytokines, chemokines, and other immunomodulating proteins using multiplexing techniques. Generalized estimating equations (GEE) models were used to examine the change in protein concentrations over time for each protein, controlling for the included clinical data. Thirty-one of the proteins had a statistically significant change over time after trauma, controlling for the other variables in the model. Of these, 8 proteins were elevated at the time of arrival in the emergency room: IL-6, IL-10, IL-1Ra, macrophage migration inhibitory factor (MIF), myeloperoxidase (MPO), monocyte chemotactic protein-1 (MCP-1), MMP-9, and sFasL (Figure 1). The elevations observed for IL-6 and IL-10 were the highest, with the models predicting a percent increase at maximum (over minimum) of 550% and 450%, respectively. IL-1Ra showed the next largest increase at 330%, followed by MPO (210%), MMP-9 (200%), sFasL (98%), MIF (83%), and MCP-1 (61%).
Figure 1. Proteins with early elevation following trauma.
Blood samples were collected from trauma patients beginning with arrival to the ER and up to 1 year after injury. Multiplexing techniques were used to measure the levels of 41 immunomodulatory proteins in the plasma. Multivariable GEE models were generated using the natural log of the concentration of each protein as the dependent variable and time since trauma, ISS, injury type, size of transfusion, age, sex, and microchimerism as the independent variables. Concentration of proteins with a statistically significant change in concentration over time since trauma (p<0.05) are plotted in black (raw data) with the model’s prediction of the influence of time since trauma on concentration (controlling for all other independent variables) overlaid in red. **p<0.01, ***p<0.001.
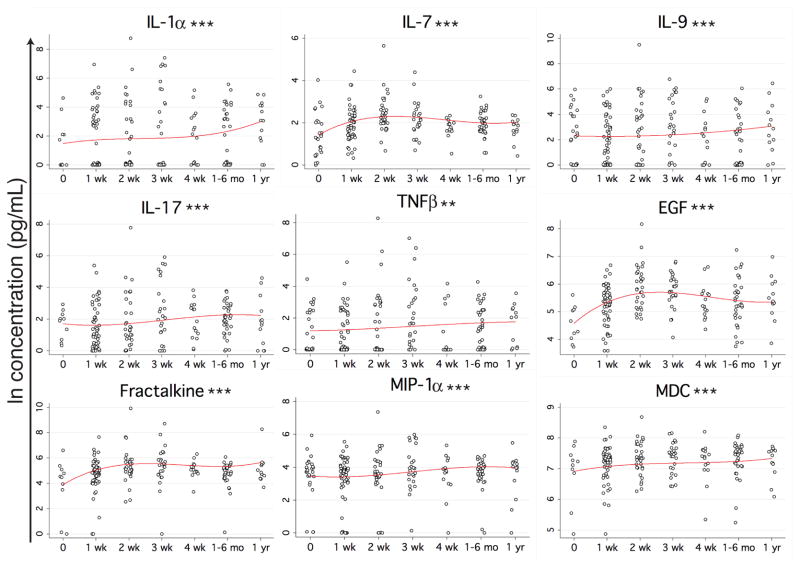
Nine of the proteins with a significant change over time since trauma showed a depression at the time of arrival in the emergency room (Figure 2). The largest decrease based on the percent change of the predicted maximum value over minimum was fractalkine (490%), followed by IL-1α (460%), epidermal growth factor (EGF) (210%), IL-7 (190%), IL-9 (130%), IL-17 (120%), tumor necrosis factor-beta (TNFβ) (110%), macrophage inflammatory protein 1 alpha (MIP-1α) (90%), and macrophage derived chemokine (MDC) (52%).
Figure 2. Proteins with early depression following trauma.
See Figure 1 for experimental details. **p<0.01, ***p<0.001.
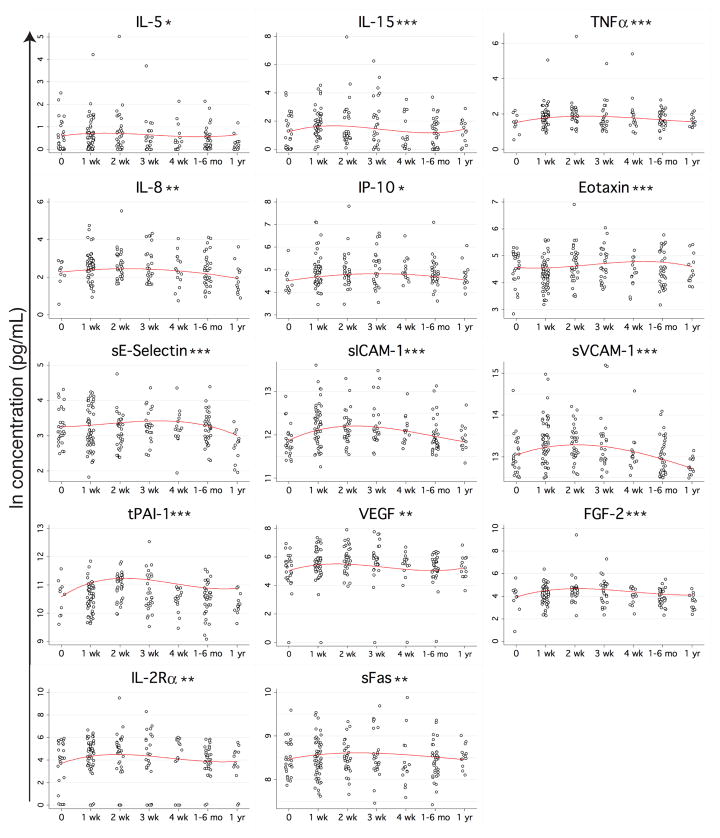
The remaining 14 proteins with a statistically significant change over time since trauma had a delayed elevation pattern, peaking between 1 and 4 weeks post trauma (Figure 3). The range of these elevations was not as wide as what was observed for the early responses; most of the proteins with this late elevation pattern had an increase less than 2-fold. The largest elevation was observed with IL-2Rα (120%), followed by fibroblast growth factor-2 (FGF-2) (110%), total plasminogen activator inhibitor-1 (tPAI-1) (93%), IL-15 (92%), soluble vascular cell adhesion molecule-1 (sVCAM-1) (76%), vascular endothelial growth factor (VEGF) (60%), TNFα (56%), soluble E-Selectin (sE-Selectin) (54%), IL-8 (47%), soluble inter-cellular adhesion molecule 1 (sICAM-1) (44%), IL-5 (42%), IP-10 (36%), eotaxin (28%), and sFas (17%). Examples of trends for five different cytokines within three representative patients are shown in Figure S2.
Figure 3. Proteins with late elevation following trauma.
See Figure 1 for experimental details. *p<0.05, **p<0.01, ***p<0.001.
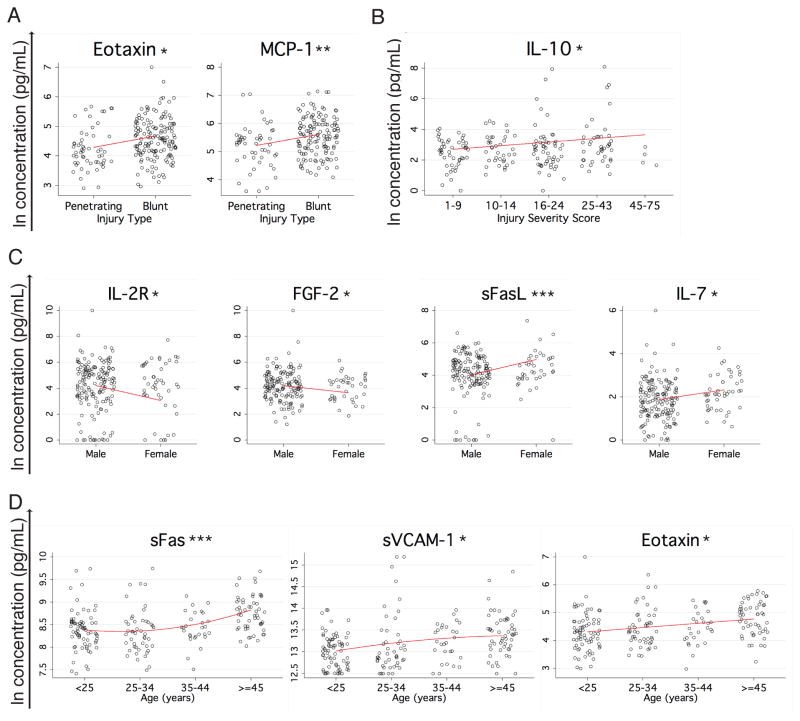
Only three of the proteins studied showed a statistically significant association between concentration and injury type or severity. Higher levels of eotaxin and MCP-1 were associated with blunt as opposed to penetrating injury when controlling for the other variables included in the model (Figure 4A). Somewhat surprisingly, only IL-10 had a significant association with injury severity in our multivariate model, with higher concentrations associated with increasing ISS (Figure 4B).
Figure 4. Proteins associated with injury type or severity, gender, and age.
(A) Proteins with a statistically significant difference (p<0.05) between blunt and penetrating injury are plotted in black (raw data) with the model’s prediction of the influence of injury type on concentration (controlling for all other independent variables) overlaid in red. (B) IL-10, the only protein measured with a statistically significant association (p<0.05) with injury severity score is plotted in black (raw data) with the model’s prediction of the influence of injury severity on concentration (controlling for all other independent variables) overlaid in red. (C) Proteins with a statistically significant difference (p<0.05) between male and female patients are plotted in black (raw data) with the model’s prediction of the influence of gender on concentration (controlling for all other independent variables) overlaid in red. (D) Proteins with a statistically significant association (p<0.05) with age are plotted in black (raw data) with the model’s prediction of the influence of age on concentration (controlling for all other independent variables) overlaid in red. *p<0.05, **p<0.01. ***p<0.001.
Significant associations were also observed between cytokines and age and gender, controlling for the other covariates. FGF-2 and IL-2Rα responses were higher in males, while IL-7 and sFasL responses were higher in females (Figure 4C). Higher concentrations of eotaxin, sFas, and sVCAM-1 were associated with increasing age (Figure 4D).
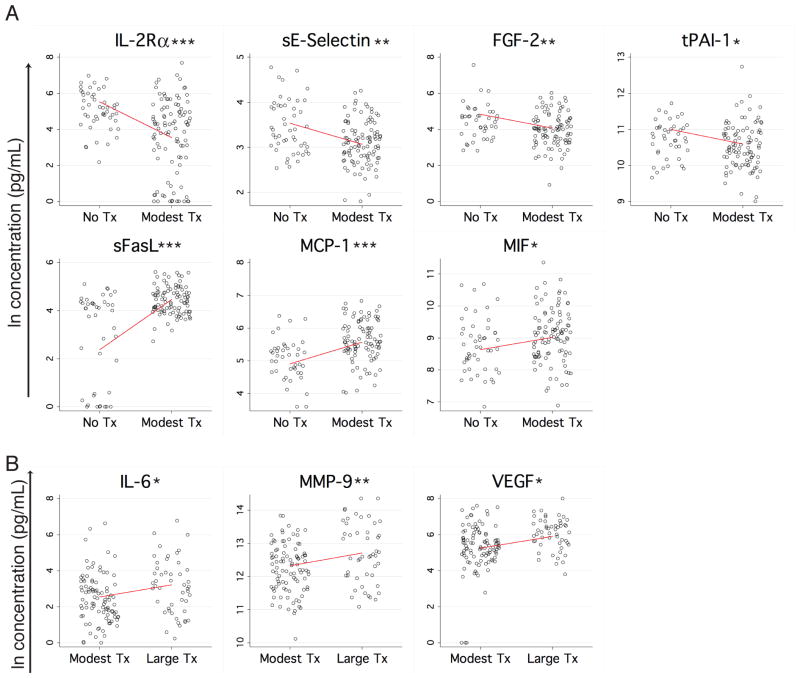
Ten of the proteins measured had a statistically significant relationship with transfusion size when controlling for the other covariates. IL-2Rα, sE-Selectin, FGF-2, and tPAI-1 were lower in patients receiving a modest transfusion (1–4 units in the first 48 hours) compared with no transfusion (Figure 5A, upper panels). sFasL, MCP-1, and MIF had the opposite relationship, with higher levels in the group receiving a modest transfusion as opposed to no transfusion (Figure 5A, lower panels). Increased concentrations of IL-6, MMP-9, and VEGF were associated with large transfusion (>4 units in the first 48 hours) as compared to modest transfusion (Figure 5B). These effects could be driven by the transfusion itself, or by the corresponding blood loss or injury type that generates a need for transfusion.
Figure 5. Proteins associated with transfusion.
(A) Proteins with a statistically significant difference (p<0.05) between no transfusion and a modest transfusion (≤4 units in the first 48 hours after trauma) are plotted in black (raw data) with the model’s prediction of the influence of no versus modest transfusion on concentration (controlling for all other independent variables) overlaid in red. (B) Proteins with a statistically significant difference (p<0.05) between modest transfusion and a large transfusion (≥5 units in the first 48 hours after trauma) are plotted in black (raw data) with the model’s prediction of the influence of modest versus large transfusion on concentration (controlling for all other independent variables) overlaid in red. *p<0.05, **p<0.01, ***p<0.001.
Murine model of trauma and transfusion
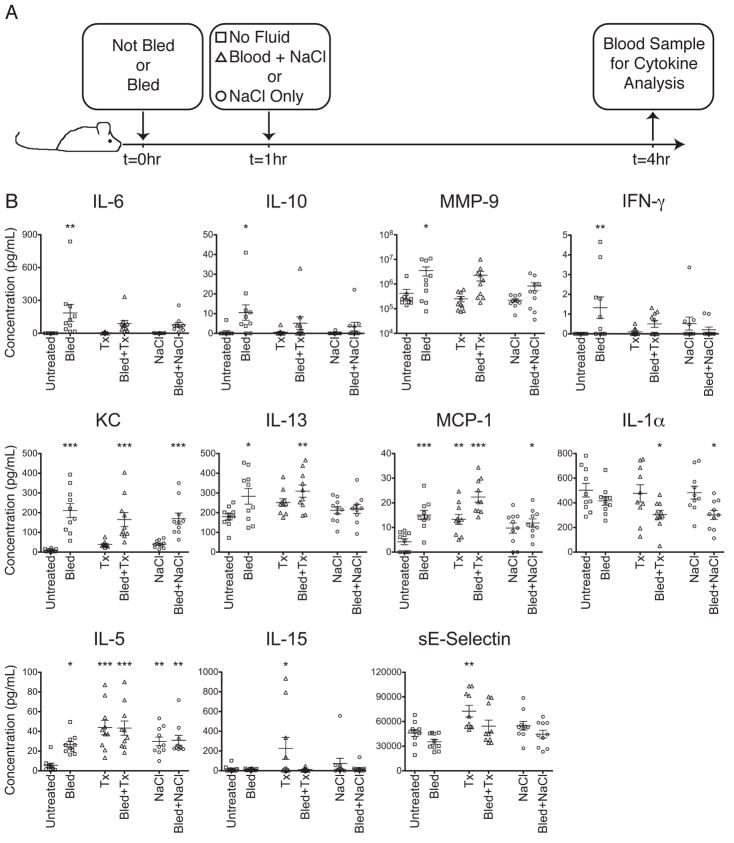
Although transfusion size was associated with different plasma concentrations for a number of the proteins examined in humans, it was not possible to separate the effects of the injuries from the effects of transfusion itself using the human data. To determine the mechanism behind the changes observed in protein concentration in transfused trauma patients, a mouse model was established to compare the individual and combined roles of traumatic blood loss and allogeneic blood transfusion on circulating cytokine levels. To model traumatic blood loss, BALB/cJ mice were bled 25–30% of their total estimated blood volume by submandibular bleeding. Bleeds were conducted without anesthesia to avoid anesthesia effects and to mimic more closely the stress of an unplanned traumatic injury in a human. Transfusions consisted of tail vein injection of fresh allogeneic C57Bl/6J red cells diluted in saline. As an additional control of fluid resuscitation alone, infusions of saline without blood were given. Mice were first bled or not at t=0, then at t=1 hour were given no treatment, allogeneic blood transfusion, or fluids alone. Blood samples were collected at t=4 hours and the concentrations of serum proteins were measured using multiplexing techniques (Figure 6A). Groups were compared using one-way ANOVA, and each treatment group was compared to the untreated controls with Dunnett’s multiple comparison post-test. A number of different patterns of cytokine response to trauma and transfusion emerged.
Figure 6. Traumatic blood loss and transfusion in mice.
(A) Time-line of traumatic blood loss, transfusion, and blood sample collection. BALB/cJ mice were bled 25–30% of their total blood volume or not at t=0, then given no transfusion (squares), 100 μL allogeneic C57Bl/6J packed red cells + 400 μL 0.9% NaCl (triangles), or 500 μL 0.9% NaCl (circles) at t=1hr. At t=4hr, peripheral blood was harvested. (B) Serum was screened for cytokines using multiplexing techniques. Pooled data from 2 representative experiments with 5 mice per group each are shown. Experiments were repeated 6 times. Concentrations were compared between treatment groups with one-way ANOVA, and Dunnett’s multiple comparisons post-test was used to compare each treatment group to the untreated controls. *p<0.05, **p<0.01, ***p<0.001.
Four proteins, IL-6, IL-10, MMP-9 and interferon-gamma (IFN-γ), were significantly elevated in the bled mice compared with untreated controls. Transfusion or fluids alone had no effect on these analytes, and transfusion or fluids given 1 hour after traumatic blood loss prevented the elevation in levels caused by trauma (Figure 6B, upper panels). KC was significantly elevated in all bled mice, regardless of transfusion or fluid resuscitation, and IL-13 was elevated in both bled only mice and bled and transfused mice (Figure 6B, middle panels), implying that transfusion could blunt some (IL-6, IL-10, MMP-9, IFN-γ) but not all (KC, IL-13) cytokine perturbation induced by trauma.
For some analytes trauma and transfusion induced additive immune modulating effects. MCP-1 was elevated in all bled or transfused mice, with the highest levels seen in mice that were both bled and transfused, while IL-1α was significantly depressed in mice that were bled and transfused or bled and given fluids only (Figure 6B, middle panels). Finally, a few analytes showed the strongest dependence on transfusion status, with trauma playing a less significant or no role. IL-5 was significantly elevated in all treatment groups compared with untreated controls, but the highest levels were seen in mice given blood transfusions (Figure 6B, lower panels). Significant elevations in IL-15 and sE-Selectin were only observed in unbled mice given blood transfusions (Figure 6B, lower panels).
DISCUSSION
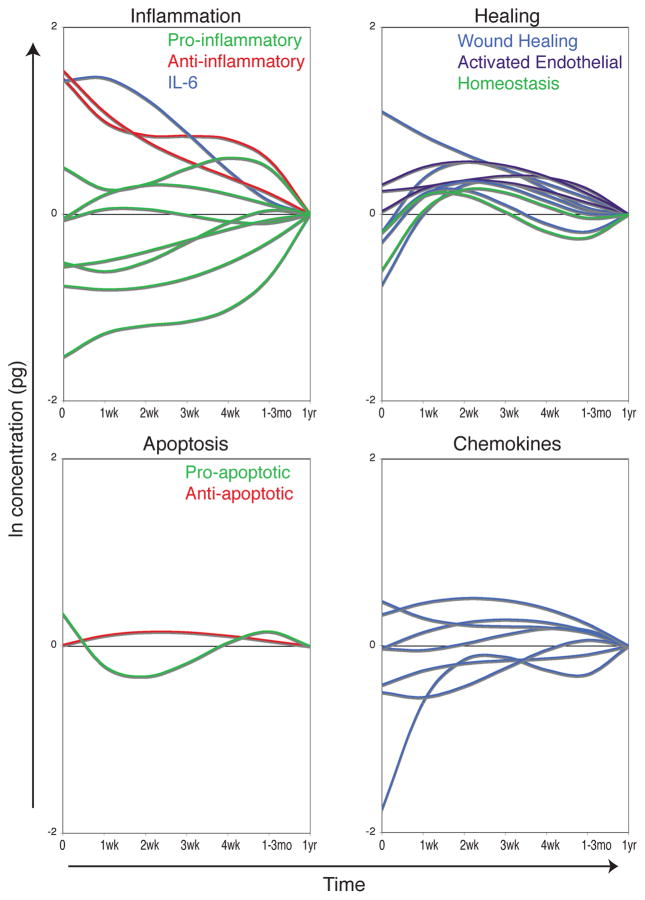
The heterogeneity of a human population, including differences in baseline health, genetics, nature of the injury, and treatments administered, makes it very difficult to determine the specific driving forces behind the many components of the immune response to trauma and transfusion. In spite of this tremendous patient variability, we have seen a clear response to traumatic injury in this cohort. Of the 41 proteins measured, 31 had a statistically significant change over time after trauma. Early responses were dominated by an anti-inflammatory profile, though not exclusively, as some pro-inflammatory cytokines were also elevated (Figure 7, upper left). Proteins involved in tissue remodeling, activated endothelial tissues, and lymphocyte homeostasis were elevated 1–4 weeks after injury (Figure 7, upper right). An initial pro-apoptotic balance of sFas/sFasL was followed by a shift towards an anti-apoptotic state 1–4 weeks after injury (Figure 7, lower left). Changes in blood concentrations of different chemokines were more mixed with some elevations and depressions at different times after trauma (Figure 7, lower right). Using a novel mouse trauma/transfusion model, we found that both traumatic blood loss and transfusion contribute to the early immune response to trauma. Trauma caused early perturbations in a number of the pro- and anti-inflammatory mediators measured, and transfusion blunted early elevations in IL-6, IL-10, MMP-9, and IFN-γ. Transfusion caused or exacerbated changes in MCP-1, IL-1α, IL-5, IL-15, and soluble E-selectin. Finally, trauma alone increased murine KC (a human IL-8 analog) and IL-13 (Table II).
Figure 7. Kinetics of immune response to trauma.
Overlays of the models’ prediction of the influence of time since trauma controlling for the other covariates are plotted by protein type. Predicted values at 1 year after trauma are set as the baseline (0) for each cytokine to show elevation or depression relative to this value. The inflammation plot includes the pro-inflammatory cytokines IL-1α, IL-5, IL-9, IL-17, TNFα, TNFβ, and MIF, the anti-inflammatory cytokines IL-1Ra and IL-10, and IL-6, which has both pro- and anti-inflammatory properties. The healing plot includes the wound healing proteins EGF, FGF-2, VEGF, MMP-9, and tPAI-1, the activated endothelial markers sE-Selectin, sICAM-1, and sVCAM-1, and the homeostasis cytokines IL-7 and IL-15. The apoptosis plot includes the pro-apoptotic sFasL and the anti-apoptotic sFas. The chemokine plot includes IP-10, IL-8, MIP-1α, MCP-1, eotaxin, fractalkine, and MDC.
Table 2.
Summary of the effects of trauma and transfusion on protein levels*
| Protein | Human Trauma | Tx | Mouse Mechanism: Trauma or Tx |
|---|---|---|---|
| IL-6 | ↑ | ↑‡ | ↑ trauma drives, fluids rescue |
| IL-10 | ↑ | ↑ trauma drives, fluids rescue | |
| MMP-9 | ↑ | ↑‡ | ↑ trauma drives, fluids rescue |
| INF-γ | ↑ trauma drives, fluids rescue | ||
| IL-8/KC | ↑† | ↑ trauma drives | |
| IL-13 | ↑ trauma drives | ||
| MCP-1 | ↑ | ↑ | ↑ both trauma and tx but not NaCl drive, highest with both |
| IL-1α | ↓ | ↓ requires both trauma and fluids | |
| IL-5 | ↑† | ↑ both trauma and fluids drive, highest levels with tx | |
| IL-15 | ↑† | ↑ tx drives | |
| sE-Selectin | ↑† | ↓ | ↑ tx drives |
| TNFα | ↑† | ||
| Eotaxin | ↑† | ||
| IP-10 | ↑† | ||
| sICAM-1 | ↑† | ||
| sVCAM-1 | ↑† | ||
| tPAI-1 | ↑† | ↓ | |
| IL-7 | ↓ | ||
| IL-17 | ↓ | ||
| MIP-1α | ↓ |
Includes all proteins measured in both human and mouse where there was a significant association with trauma or transfusion (tx)
Delayed change
> 4 units compared with ≤ 4 units in first 48 hours
Earlier studies have not looked at such a large array of cytokines over a comparably long study period after traumatic injury. Previously published reports of the cytokine response to trauma that overlap with this study are, for the most part, consistent with our findings. Early systemic elevation of IL-6, IL-10, and IL-8 has been seen fairly consistently in a number of studies of trauma.9–13,16,17,19–21,64,65 Elevations in IL-1Ra, sICAM-1, MMP-9, sFas, and have also been reported by multiple groups.11,20,64,66–71 Individual reports of elevations in other proteins we found associated with time since trauma such as PAI-1, sE-Selectin, sFasL, MCP-1, and MPO can also be found.20,64,71–73 These results are all consistent with our findings. Finally, we did not see the early increases in TNFα or IL-4 reported by some other groups. Reports of the role of these two cytokines in the immune response to trauma have been conflicting and often differ between different subsets of trauma patients and different animal models.10,12–15,18,19,22,38,39,74–78 The current study significantly broadens our understanding of the breadth of immune modulation after trauma and transfusion, as to our knowledge there are no previous reports in human trauma patients of our observed early increases in MIF, later increases in IL-5, IL-15, IP-10, eotaxin, sVCAM-1, VEGF, FGF-2, IL-2Rα and early decreases in IL-1α, IL-7, IL-9, IL-17, TNFβ, EFG, Fractalkine, MIP-1α, and MDC.
Our findings show that a vigorous anti-inflammatory response occurs within hours following trauma, in contrast with the SIRS/CARS model popularized by Moore et al., which describes a strong initial pro-inflammatory state followed by a later anti-inflammatory state.7 While our paper was under review, a study examining gene expression profiles in trauma patients in the first month post-injury was published that also fits with this revised model of the immune response to trauma. Xiao et al. demonstrated that early anti-inflammatory responses do not follow the pro-inflammatory response, but instead these responses overlap in a state of immune dysregulation.8
The levels of anti-inflammatory cytokines observed in our study appear to be high enough to have biological consequence. The median concentration at time zero for IL-10, for example, was 18.6 μg/L (Table S1), or approximately 1.5–13.0 μg/kg. A clinical trial evaluating the use of recombinant human IL-10 in the treatment of psoriasis found immunosuppressive effects with subcutaneous injection of 8 μg/kg per day.79 Recombinant human IL-1Ra has also been evaluated in for the treatment of rheumatoid arthritis. One study focused on rheumatoid arthritis compared the doses of 30, 75, or 150 mg per day (approximately 429–2140 μg/kg) injected subcutaneously and found improvement over placebo with treatment, regardless of dose.80 Our levels were lower with a median concentration of 242 μg/L, or approximately 19.4–169 μg/kg. While subcutaneous injection of a recombinant is not equivalent to endogenous circulating cytokines, it seems reasonable to expect that the combination of these two cytokines alone at the doses we observed should be sufficient to have an immunosuppressive effect.
Separating the roles of trauma and transfusion in modulating the immune response was not possible in our human subjects, as transfused patients likely have different types and severity of injuries than non-transfused patients. Using a murine model, we found that the overall response to traumatic injury with blood loss was very similar to what we observed in the human trauma subjects (Table II). IL-6, IL-10, MMP-9, and MCP-1 were all elevated, and IL-1α was lowered, after trauma in both humans and mice. IL-6, IL-10, IFN-γ, and MMP-9 levels were lower in mice that received blood transfusions or saline after blood loss, and the absence of any elevation in response to transfusion or saline suggest that their elevation was driven solely by trauma and hemorrhage. We did not see any evidence for an IFN-γ response to trauma in our human subjects; over half of the samples had undetectable levels of IFN-γ. Based on the mouse data we would predict that transfusion blunted the IFN-γ response induced by trauma in humans. MCP-1 was elevated in response to trauma and was associated with both blunt injury and transfusion in our human subjects, suggesting that a number of factors influence MCP-1 production. This is consistent with our mouse model, which demonstrated significant upregulation of MCP-1 with either traumatic blood loss or transfusion (but not saline), and maximum upregulation with both. In contrast, IL-1α, which was depressed early after trauma in our human subjects, did not show any significant change in mice after blood loss, transfusion, or saline alone, but did show a significant depression after blood loss combined with either transfusion or saline, suggesting a two-hit mechanism of induction. KC, which has a similar role to IL-8 in humans, was elevated following traumatic blood loss, but unaffected by transfusion or saline. The IL-8 response to trauma in humans did not peak until 1–2 weeks after injury, but was still higher at the time of injury than at 1 year later. In mice IL-5 levels were driven higher by all interventions, but the highest levels were seen after transfusion. This is consistent with our observations in humans in that IL-5 was elevated in response to trauma, but this difference was subtle in humans, where it peaked later (1–2 weeks after injury), and showed no association with transfusion. Similarly, IL-13 was elevated in bled or bled and transfused mice, but not in our human cohort. This may be the result of the more distinct Th2-type responses to trauma in mice compared with humans that has been previously reported.39,74–76,78 Finally, IL-15 and sE-Selectin were both elevated in the mice given transfusion alone, but not the other groups. There was no transfusion only group for the human subjects, and the elevations in the proteins seen in humans were at later time points than we examined in the mice. Furthermore, while our human subjects were given leukoreduced blood, leukoreduction was not used in the mouse model, which may have contributed to this observed response to transfusion alone in the mice.
Generally, saline infusion had a similar “rescue” effect as transfusion (Table II). This was seen with IL-6, IL-10, MMP-9 and IFN-γ, where administration of either blood or saline alone after trauma brought concentrations down to a level where they were no longer significantly different from untreated controls. Similarly, the reduction in IL-1α seen after trauma and transfusion was also seen with trauma and saline. For other cytokines, the effects driven by transfusion could not be replicated by saline, suggesting that transfusion of fresh allogeneic blood can modulate even early immune responses (Table II). This pattern was seen with MCP-1 and IL-5, where saline effects were weaker than what was observed with blood, and with IL-15 and sE-Selectin, where elevation was only seen after blood transfusion alone.
Our murine model differs from existing animal models of traumatic injury in that it involves less tissue damage than those involving femur fracture or large lacerations, enabling us to ethically bleed the mice without anesthesia. The disadvantage of our system is that the tissue damage is probably less than that of our clinical patients, though the bleeding process does involve a 5mm deep stab requiring moderate force for delivery. The advantage of this model is that it more closely mimics the stress of accidental injury than those requiring anesthesia, which arguably mimic surgical injury. Our study examined a larger array cytokines and involved a different type of injury than has been previously assessed in other murine models of trauma, making it difficult to fully compare our results with models using anesthetized animals. While trends of elevated IL-6, IL-10, MCP-1, and KC were similar to what has been observed in models utilizing anesthesia,36,38 further work is required to determine if anesthesia modulates the immune response to trauma.
One of the unanticipated findings of the current study was that IL-1α, IL-7, IL-9, IL-17, TNFβ, EGF, fractalkine, MIP-1α, and MDC were depressed early after trauma in humans (the early levels were lower than late “baseline” time-points) (Figure 2). We believe this is the first report of reduced concentrations of circulating cytokines after trauma, though there are reports of reduced capacity of cells from trauma patients or injured mice to produce cytokines ex vivo.32,37,75 Since it is not possible to collect samples prior to injury in humans, and because variation is high between individuals, we determined that samples collected months after injury were likely to be the closest to baseline for these patients. An alternative, less likely explanation to the observed trends is that they are actually delayed responses to trauma that persists for up to one year. It is worth noting, however, that of the cytokines measured both in mice and humans, the cytokine with the largest percent decrease in our trauma patients, IL-1α, was also significantly reduced in our mouse model after trauma and transfusion.
In our cohort the severity (measured by ISS) and type of injury (blunt versus penetrating) did not have a significant effect on the concentrations of most of the cytokines we evaluated. A number of studies have linked concentrations of various cytokines with injury severity including IL-10 as we observed here, but also IL-6, IL-8, and IL-4.10,15,17,21,22 One explanation for the lack of significant statistical association between severity of injury and most of the cytokines we evaluated is that our multivariable model controlled for potential confounders. Alternatively, the difference might be explained by the overall severity of injury in our cohort compared with others. For example, the median ISS in our study was 17, and Hoch et al. saw significant increases in IL-6 and IL-8 with ISS ≥ 25.15 The lower ISS in our cohort may be partially explained by the selection criteria used for our study. Our cohort was selected for long-term assessment of microchimerism, so patients who died in the first 7 days were excluded. Three proteins, IL-6, MMP-9, and VEGF were seen at higher levels in patients receiving large transfusions of 5 or more units in the first 48 hours after injury. While our model controlled for the severity and type of injury to some extent by including ISS and blunt versus penetrating injury, the differences seen in these patients may still be due more to the type of injury that results in a massive transfusion than from the transfusion itself. All three of these proteins have been associated with ischemia/reperfusion injuries and/or hypoxia in different clinical and experimental settings.20,81–86
We also observed a few differences associated with age and gender in our trauma patient cohort. We saw increased sFasL in women compared with men, and increasing sFas with age, which is consistent with a study from Kavathia et al.87 Eotaxin and sVCAM-1 also increased with age in our cohort, as found in studies in humans and rats.88–92 Similarly, the higher IL-7 levels we observed in women are consistent with a study that found 40% higher levels of IL-7 in HIV+ women compared with HIV+ men.93 Differences in the cytokine response to trauma between men and women may help to explain the reduced rates of death, sepsis, and organ failure observed among women compared with men following trauma.45–49 These findings support the observation in mouse models that sex hormones alter the immune response to trauma.50,51
Overall, we have demonstrated that there is a massive change in the cytokine environment following traumatic injury and transfusion in humans. Furthermore, we have been able to effectively model this change in mice, allowing us to distinguish between those factors regulated by traumatic blood loss, transfusion, or both. This work suggests that the immune response to trauma is significant, and that transfusion and fluid administration have the potential to alter the immunological consequences of trauma. Given the rates of transfusion among trauma patients, the interplay of the responses to trauma and transfusion has important implications for immunologically driven clinical complications such as multiple organ failure, sepsis, transfusion-related acute lung injury, and alloimmunization.
Supplementary Material
Patients in the 911 and 922 triage groups were included in the study.
Levels of five cytokines for three representative patients (0013, 0114, and 0153) are plotted as examples of the observed trends within individuals. All three patients are transfused males with blunt wounds. 0013 is age 34 with an injury severity score of 32, 0114 is age 43 with an injury severity score of 14, and 0153 is age 69 with an injury severity score of 14. (A) IL-6 and IL-10 are shown as examples of cytokines elevated early after trauma. (B) IL-17 is shown as an example of a cytokine that is depressed after trauma. (C) tPAI-1 is shown as an example of a cytokine that has a later elevation after trauma.
Acknowledgments
Support: this work was supported by NIH RO1 HL-083388-01A1.
We would like to thank Dr. Marina E. Fomin for her assistance with animal care, and Dr. Brian S. Custer for consultation on statistical methods.
Footnotes
Competing interests: the authors have no competing interests.
References
- 1.Hess JR, Hiippala S. Optimizing the use of blood products in trauma care. Crit Care. 2005;9 (Suppl 5):S10–4. doi: 10.1186/cc3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trunkey DD. History and development of trauma care in the United States. Clin Orthop Relat Res. 2000:36–46. doi: 10.1097/00003086-200005000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Como JJ, Dutton RP, Scalea TM, Edelman BB, Hess JR. Blood transfusion rates in the care of acute trauma. Transfusion. 2004;44:809–13. doi: 10.1111/j.1537-2995.2004.03409.x. [DOI] [PubMed] [Google Scholar]
- 4.Department of Health and Human Services U. The 2009 National Blood Collection and Utilization Survey Report. 2009. [Google Scholar]
- 5.Giannoudis PV. Current concepts of the inflammatory response after major trauma: an update. Injury. 2003;34:397–404. doi: 10.1016/s0020-1383(02)00416-3. [DOI] [PubMed] [Google Scholar]
- 6.Mannick JA, Rodrick ML, Lederer JA. The immunologic response to injury. J Am Coll Surg. 2001;193:237–44. doi: 10.1016/s1072-7515(01)01011-0. [DOI] [PubMed] [Google Scholar]
- 7.Moore FA, Sauaia A, Moore EE, Haenel JB, Burch JM, Lezotte DC. Postinjury multiple organ failure: a bimodal phenomenon. J Trauma. 1996;40:501–10. doi: 10.1097/00005373-199604000-00001. discussion 10–2. [DOI] [PubMed] [Google Scholar]
- 8.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Brownstein BH, Mason PH, Baker HV, Finnerty CC, Jeschke MG, Lopez MC, Klein MB, Gamelli RL, Gibran NS, Arnoldo B, Xu W, Zhang Y, Calvano SE, McDonald-Smith GP, Schoenfeld DA, Storey JD, Cobb JP, Warren HS, Moldawer LL, Herndon DN, Lowry SF, Maier RV, Davis RW, Tompkins RG. A genomic storm in critically injured humans. J Exp Med. 2011;208:2581–90. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foex BA, Lamb WR, Roberts TE, Brear SG, Macartney I, Hammer M, Brenchley PE. Early cytokine response to multiple injury. Injury. 1993;24:373–6. doi: 10.1016/0020-1383(93)90098-q. [DOI] [PubMed] [Google Scholar]
- 10.Mimasaka S, Funayama M, Hashiyada M, Nata M, Tsunenari S. Significance of levels of IL-6 and IL-8 after trauma: a study of 11 cytokines post-mortem using multiplex immunoassay. Injury. 2007;38:1047–51. doi: 10.1016/j.injury.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 11.Partrick DA, Moore FA, Moore EE, Biffl WL, Sauaia A, Barnett CC, Jr, Jack A. Barney Resident Research Award winner. The inflammatory profile of interleukin-6, interleukin-8, and soluble intercellular adhesion molecule-1 in postinjury multiple organ failure. Am J Surg. 1996;172:425–9. doi: 10.1016/s0002-9610(96)00252-8. discussed 9–31. [DOI] [PubMed] [Google Scholar]
- 12.Martin C, Boisson C, Haccoun M, Thomachot L, Mege JL. Patterns of cytokine evolution (tumor necrosis factor-alpha and interleukin-6) after septic shock, hemorrhagic shock, and severe trauma. Crit Care Med. 1997;25:1813–9. doi: 10.1097/00003246-199711000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Endo S, Inada K, Yamada Y, Takakuwa T, Kasai T, Nakae H, Yoshida M, Ceska M. Plasma endotoxin and cytokine concentrations in patients with hemorrhagic shock. Crit Care Med. 1994;22:949–55. doi: 10.1097/00003246-199406000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson KL, Taheri P, Rodriguez J, Tonapi V, Cardellio A, Dechert R. Tumor necrosis factor activity increases in the early response to trauma. Acad Emerg Med. 1997;4:1035–40. doi: 10.1111/j.1553-2712.1997.tb03676.x. [DOI] [PubMed] [Google Scholar]
- 15.Hoch RC, Rodriguez R, Manning T, Bishop M, Mead P, Shoemaker WC, Abraham E. Effects of accidental trauma on cytokine and endotoxin production. Crit Care Med. 1993;21:839–45. doi: 10.1097/00003246-199306000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Biffl WL, Moore EE, Moore FA, Peterson VM. Interleukin-6 in the injured patient. Marker of injury or mediator of inflammation? Ann Surg. 1996;224:647–64. doi: 10.1097/00000658-199611000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giannoudis PV, Smith RM, Perry SL, Windsor AJ, Dickson RA, Bellamy MC. Immediate IL-10 expression following major orthopaedic trauma: relationship to anti-inflammatory response and subsequent development of sepsis. Intensive Care Med. 2000;26:1076–81. doi: 10.1007/s001340051320. [DOI] [PubMed] [Google Scholar]
- 18.Rabinovici R, John R, Esser KM, Vernick J, Feuerstein G. Serum tumor necrosis factor- alpha profile in trauma patients. J Trauma. 1993;35:698–702. doi: 10.1097/00005373-199311000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Roumen RM, Hendriks T, van der Ven-Jongekrijg J, Nieuwenhuijzen GA, Sauerwein RW, van der Meer JW, Goris RJ. Cytokine patterns in patients after major vascular surgery, hemorrhagic shock, and severe blunt trauma. Relation with subsequent adult respiratory distress syndrome and multiple organ failure. Ann Surg. 1993;218:769–76. doi: 10.1097/00000658-199312000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seekamp A, Jochum M, Ziegler M, van Griensven M, Martin M, Regel G. Cytokines and adhesion molecules in elective and accidental trauma-related ischemia/reperfusion. J Trauma. 1998;44:874–82. doi: 10.1097/00005373-199805000-00022. [DOI] [PubMed] [Google Scholar]
- 21.Sherry RM, Cue JI, Goddard JK, Parramore JB, DiPiro JT. Interleukin-10 is associated with the development of sepsis in trauma patients. J Trauma. 1996;40:613–6. doi: 10.1097/00005373-199604000-00016. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 22.DiPiro JT, Howdieshell TR, Goddard JK, Callaway DB, Hamilton RG, Mansberger AR., Jr Association of interleukin-4 plasma levels with traumatic injury and clinical course. Arch Surg. 1995;130:1159–62. doi: 10.1001/archsurg.1995.01430110017004. discussion 62–3. [DOI] [PubMed] [Google Scholar]
- 23.DiPiro JT, Hamilton RG, Howdieshell TR, Adkinson NF, Jr, Mansberger AR., Jr Total IgE in plasma is elevated after traumatic injury and is associated with sepsis syndrome. Ann Surg. 1992;215:460–5. doi: 10.1097/00000658-199205000-00008. discussion 5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjornson AB, Altemeier WA, Bjornson HS. Host defense against opportunist microorganisms following trauma. II. Changes in complement and immunoglobulins in patients with abdominal trauma and in septic patients without trauma. Ann Surg. 1978;188:102–8. doi: 10.1097/00000658-197807000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faist E, Kupper TS, Baker CC, Chaudry IH, Dwyer J, Baue AE. Depression of cellular immunity after major injury. Its association with posttraumatic complications and its reversal with immunomodulation. Arch Surg. 1986;121:1000–5. doi: 10.1001/archsurg.1986.01400090026004. [DOI] [PubMed] [Google Scholar]
- 26.O’Mahony JB, Palder SB, Wood JJ, McIrvine A, Rodrick ML, Demling RH, Mannick JA. Depression of cellular immunity after multiple trauma in the absence of sepsis. J Trauma. 1984;24:869–75. doi: 10.1097/00005373-198410000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Keane RM, Birmingham W, Shatney CM, Winchurch RA, Munster AM. Prediction of sepsis in the multitraumatic patient by assays of lymphocyte responsiveness. Surg Gynecol Obstet. 1983;156:163–7. [PubMed] [Google Scholar]
- 28.Livingston DH, Appel SH, Wellhausen SR, Sonnenfeld G, Polk HC., Jr Depressed interferon gamma production and monocyte HLA-DR expression after severe injury. Arch Surg. 1988;123:1309–12. doi: 10.1001/archsurg.1988.01400350023002. [DOI] [PubMed] [Google Scholar]
- 29.Szabo G, Kodys K, Miller-Graziano CL. Elevated monocyte interleukin-6 (IL-6) production in immunosuppressed trauma patients. I. Role of Fc gamma RI cross-linking stimulation. J Clin Immunol. 1991;11:326–35. doi: 10.1007/BF00918798. [DOI] [PubMed] [Google Scholar]
- 30.Faist E, Schinkel C, Zimmer S, Kremer JP, Von Donnersmarck GH, Schildberg FW. Inadequate interleukin-2 synthesis and interleukin-2 messenger expression following thermal and mechanical trauma in humans is caused by defective transmembrane signalling. J Trauma. 1993;34:846–53. doi: 10.1097/00005373-199306000-00016. discussion 53–4. [DOI] [PubMed] [Google Scholar]
- 31.Lyons A, Kelly JL, Rodrick ML, Mannick JA, Lederer JA. Major injury induces increased production of interleukin-10 by cells of the immune system with a negative impact on resistance to infection. Ann Surg. 1997;226:450–8. doi: 10.1097/00000658-199710000-00006. discussion 8–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacConmara MP, Maung AA, Fujimi S, McKenna AM, Delisle A, Lapchak PH, Rogers S, Lederer JA, Mannick JA. Increased CD4+ CD25+ T regulatory cell activity in trauma patients depresses protective Th1 immunity. Ann Surg. 2006;244:514–23. doi: 10.1097/01.sla.0000239031.06906.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy TJ, Ni Choileain N, Zang Y, Mannick JA, Lederer JA. CD4+CD25+ regulatory T cells control innate immune reactivity after injury. J Immunol. 2005;174:2957–63. doi: 10.4049/jimmunol.174.5.2957. [DOI] [PubMed] [Google Scholar]
- 34.Kelly JL, Lyons A, Soberg CC, Mannick JA, Lederer JA. Anti-interleukin-10 antibody restores burn-induced defects in T-cell function. Surgery. 1997;122:146–52. doi: 10.1016/s0039-6060(97)90003-9. [DOI] [PubMed] [Google Scholar]
- 35.Ni Choileain N, MacConmara M, Zang Y, Murphy TJ, Mannick JA, Lederer JA. Enhanced regulatory T cell activity is an element of the host response to injury. J Immunol. 2006;176:225–36. doi: 10.4049/jimmunol.176.1.225. [DOI] [PubMed] [Google Scholar]
- 36.Hsieh CH, Frink M, Hsieh YC, Kan WH, Hsu JT, Schwacha MG, Choudhry MA, Chaudry IH. The role of MIP-1 alpha in the development of systemic inflammatory response and organ injury following trauma hemorrhage. J Immunol. 2008;181:2806–12. doi: 10.4049/jimmunol.181.4.2806. [DOI] [PubMed] [Google Scholar]
- 37.Kawasaki T, Fujimi S, Lederer JA, Hubbard WJ, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Trauma-hemorrhage induces depressed splenic dendritic cell functions in mice. J Immunol. 2006;177:4514–20. doi: 10.4049/jimmunol.177.7.4514. [DOI] [PubMed] [Google Scholar]
- 38.Kobbe P, Vodovotz Y, Kaczorowski DJ, Mollen KP, Billiar TR, Pape HC. Patterns of cytokine release and evolution of remote organ dysfunction after bilateral femur fracture. Shock. 2008;30:43–7. doi: 10.1097/SHK.0b013e31815d190b. [DOI] [PubMed] [Google Scholar]
- 39.Mack VE, McCarter MD, Naama HA, Calvano SE, Daly JM. Dominance of T-helper 2-type cytokines after severe injury. Arch Surg. 1996;131:1303–8. doi: 10.1001/archsurg.1996.01430240057007. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 40.Kang SC, Matsutani T, Choudhry MA, Schwacha MG, Rue LW, Bland KI, Chaudry IH. Are the immune responses different in middle-aged and young mice following bone fracture, tissue trauma and hemorrhage? Cytokine. 2004;26:223–30. doi: 10.1016/j.cyto.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Wichmann MW, Ayala A, Chaudry IH. Severe depression of host immune functions following closed-bone fracture, soft-tissue trauma, and hemorrhagic shock. Crit Care Med. 1998;26:1372–8. doi: 10.1097/00003246-199808000-00024. [DOI] [PubMed] [Google Scholar]
- 42.Abraham E, Chang YH. Cellular and humoral bases of hemorrhage-induced depression of lymphocyte function. Crit Care Med. 1986;14:81–6. doi: 10.1097/00003246-198602000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Abraham E, Freitas AA. Hemorrhage produces abnormalities in lymphocyte function and lymphokine generation. J Immunol. 1989;142:899–906. [PubMed] [Google Scholar]
- 44.Stephan RN, Kupper TS, Geha AS, Baue AE, Chaudry IH. Hemorrhage without tissue trauma produces immunosuppression and enhances susceptibility to sepsis. Arch Surg. 1987;122:62–8. doi: 10.1001/archsurg.1987.01400130068010. [DOI] [PubMed] [Google Scholar]
- 45.Morris JA, Jr, MacKenzie EJ, Damiano AM, Bass SM. Mortality in trauma patients: the interaction between host factors and severity. J Trauma. 1990;30:1476–82. [PubMed] [Google Scholar]
- 46.Oberholzer A, Keel M, Zellweger R, Steckholzer U, Trentz O, Ertel W. Incidence of septic complications and multiple organ failure in severely injured patients is sex specific. J Trauma. 2000;48:932–7. doi: 10.1097/00005373-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 47.Schroder J, Kahlke V, Staubach KH, Zabel P, Stuber F. Gender differences in human sepsis. Arch Surg. 1998;133:1200–5. doi: 10.1001/archsurg.133.11.1200. [DOI] [PubMed] [Google Scholar]
- 48.Wichmann MW, Inthorn D, Andress HJ, Schildberg FW. Incidence and mortality of severe sepsis in surgical intensive care patients: the influence of patient gender on disease process and outcome. Intensive Care Med. 2000;26:167–72. doi: 10.1007/s001340050041. [DOI] [PubMed] [Google Scholar]
- 49.Wohltmann CD, Franklin GA, Boaz PW, Luchette FA, Kearney PA, Richardson JD, Spain DA. A multicenter evaluation of whether gender dimorphism affects survival after trauma. Am J Surg. 2001;181:297–300. doi: 10.1016/s0002-9610(01)00582-7. [DOI] [PubMed] [Google Scholar]
- 50.Choudhry MA, Bland KI, Chaudry IH. Trauma and immune response--effect of gender differences. Injury. 2007;38:1382–91. doi: 10.1016/j.injury.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yokoyama Y, Schwacha MG, Samy TS, Bland KI, Chaudry IH. Gender dimorphism in immune responses following trauma and hemorrhage. Immunol Res. 2002;26:63–76. doi: 10.1385/ir:26:1-3:063. [DOI] [PubMed] [Google Scholar]
- 52.Burrows L, Tartter P. Effect of blood transfusions on colonic malignancy recurrent rate. Lancet. 1982;2:662. doi: 10.1016/s0140-6736(82)92764-7. [DOI] [PubMed] [Google Scholar]
- 53.Okuno K, Ozaki M, Shigeoka H, Nakajima I, Nakamura K, Hirohata T, Jinnai H, Yasutomi M. Effect of packed red cell and whole blood transfusion on liver-associated immune function. Am J Surg. 1994;168:340–4. doi: 10.1016/s0002-9610(05)80161-8. [DOI] [PubMed] [Google Scholar]
- 54.Opelz G, Sengar DP, Mickey MR, Terasaki PI. Effect of blood transfusions on subsequent kidney transplants. Transplant Proc. 1973;5:253–9. [PubMed] [Google Scholar]
- 55.Pirenne J, Kitade H, Kawai M, Koshiba T, Van Damme B, Mathieu C, Waer M. Regulatory cells, TH1/TH2 unbalance, and antibody-induced chronic rejection in operational tolerance induced by donor-specific blood transfusion. Transplantation. 2005;79:S25–7. doi: 10.1097/01.tp.0000153295.51565.f1. [DOI] [PubMed] [Google Scholar]
- 56.Salvatierra O, Jr, Vincenti F, Amend W, Potter D, Iwaki Y, Opelz G, Terasaki P, Duca R, Cochrum K, Hanes D, Stoney RJ, Feduska NJ. Deliberate donor-specific blood transfusions prior to living related renal transplantation. A new approach. Ann Surg. 1980;192:543–52. doi: 10.1097/00000658-198010000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singal DP, Joseph S. Role of blood transfusions on the induction of antibodies against recognition sites on T lymphocytes in renal transplant patients. Hum Immunol. 1982;4:93–108. doi: 10.1016/0198-8859(82)90010-6. [DOI] [PubMed] [Google Scholar]
- 58.Utter GH, Reed WF, Lee TH, Busch MP. Transfusion-associated microchimerism. Vox Sang. 2007;93:188–95. doi: 10.1111/j.1423-0410.2007.00954.x. [DOI] [PubMed] [Google Scholar]
- 59.Gianotti L, Pyles T, Alexander JW, Babcock GF, Carey MA. Impact of blood transfusion and burn injury on microbial translocation and bacterial survival. Transfusion. 1992;32:312–7. doi: 10.1046/j.1537-2995.1992.32492263443.x. [DOI] [PubMed] [Google Scholar]
- 60.Winslow GA, Shelby J, Nelson EW, Saffle JR. Influence of allogeneic blood transfusion on natural killer cell activity in burn-injured mice. J Burn Care Rehabil. 1996;17:117–23. doi: 10.1097/00004630-199603000-00005. [DOI] [PubMed] [Google Scholar]
- 61.Sullivan DJ, Barton RG, Edelman LS, Shao Y, Nelson EW, Shelby J. Distinct effects of allogeneic blood transfusion on splenocyte cytokine production after hemorrhagic shock. J Surg Res. 1998;75:54–60. doi: 10.1006/jsre.1997.5254. [DOI] [PubMed] [Google Scholar]
- 62.Golde WT, Gollobin P, Rodriguez LL. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Anim (NY) 2005;34:39–43. doi: 10.1038/laban1005-39. [DOI] [PubMed] [Google Scholar]
- 63.Jackman RP, Utter GH, Heitman JW, Hirschkorn DF, Law JP, Gefter N, Busch MP, Norris PJ. Effects of blood sample age at time of separation on measured cytokine concentrations in human plasma. Clin Vaccine Immunol. 2011;18:318–26. doi: 10.1128/CVI.00465-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gardlund B, Sjolin J, Nilsson A, Roll M, Wickerts CJ, Wretlind B. Plasma levels of cytokines in primary septic shock in humans: correlation with disease severity. J Infect Dis. 1995;172:296–301. doi: 10.1093/infdis/172.1.296. [DOI] [PubMed] [Google Scholar]
- 65.Perl M, Gebhard F, Knoferl MW, Bachem M, Gross HJ, Kinzl L, Strecker W. The pattern of preformed cytokines in tissues frequently affected by blunt trauma. Shock. 2003;19:299–304. doi: 10.1097/00024382-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 66.Law MM, Cryer HG, Abraham E. Elevated levels of soluble ICAM-1 correlate with the development of multiple organ failure in severely injured trauma patients. J Trauma. 1994;37:100–9. doi: 10.1097/00005373-199407000-00017. discussion 9–10. [DOI] [PubMed] [Google Scholar]
- 67.Grossetete M, Phelps J, Arko L, Yonas H, Rosenberg GA. Elevation of matrix metalloproteinases 3 and 9 in cerebrospinal fluid and blood in patients with severe traumatic brain injury. Neurosurgery. 2009;65:702–8. doi: 10.1227/01.NEU.0000351768.11363.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ulrich D, Noah EM, von Heimburg D, Pallua N. TIMP-1, MMP-2, MMP-9, and PIIINP as serum markers for skin fibrosis in patients following severe burn trauma. Plast Reconstr Surg. 2003;111:1423–31. doi: 10.1097/01.PRS.0000049450.95669.07. [DOI] [PubMed] [Google Scholar]
- 69.Crespo AR, Da Rocha AB, Jotz GP, Schneider RF, Grivicich I, Pinheiro K, Zanoni C, Regner A. Increased serum sFas and TNFalpha following isolated severe head injury in males. Brain Inj. 2007;21:441–7. doi: 10.1080/02699050701311125. [DOI] [PubMed] [Google Scholar]
- 70.Lenzlinger PM, Marx A, Trentz O, Kossmann T, Morganti-Kossmann MC. Prolonged intrathecal release of soluble Fas following severe traumatic brain injury in humans. J Neuroimmunol. 2002;122:167–74. doi: 10.1016/s0165-5728(01)00466-0. [DOI] [PubMed] [Google Scholar]
- 71.Paunel-Gorgulu A, Flohe S, Scholz M, Windolf J, Logters T. Increased serum soluble Fas after major trauma is associated with delayed neutrophil apoptosis and development of sepsis. Crit Care. 2011;15:R20. doi: 10.1186/cc9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Semple BD, Bye N, Rancan M, Ziebell JM, Morganti-Kossmann MC. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2−/− mice. J Cereb Blood Flow Metab. 2010;30:769–82. doi: 10.1038/jcbfm.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pincemail J, Deby-Dupont G, Deby C, Thirion A, Torpier G, Faymonville ME, Damas P, Tomassini M, Lamy M, Franchimont P. Fast double antibody radioimmunoassay of human granulocyte myeloperoxidase and its application to plasma. J Immunol Methods. 1991;137:181–91. doi: 10.1016/0022-1759(91)90023-9. [DOI] [PubMed] [Google Scholar]
- 74.De AK, Kodys KM, Pellegrini J, Yeh B, Furse RK, Bankey P, Miller-Graziano CL. Induction of global anergy rather than inhibitory Th2 lymphokines mediates posttrauma T cell immunodepression. Clin Immunol. 2000;96:52–66. doi: 10.1006/clim.2000.4879. [DOI] [PubMed] [Google Scholar]
- 75.O’Sullivan ST, Lederer JA, Horgan AF, Chin DH, Mannick JA, Rodrick ML. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg. 1995;222:482–90. doi: 10.1097/00000658-199522240-00006. discussion 90–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Puyana JC, Pellegrini JD, De AK, Kodys K, Silva WE, Miller CL. Both T-helper-1- and T-helper-2-type lymphokines are depressed in posttrauma anergy. J Trauma. 1998;44:1037–45. doi: 10.1097/00005373-199806000-00017. discussion 45–6. [DOI] [PubMed] [Google Scholar]
- 77.Stylianos S, Wakabayashi G, Gelfand JA, Harris BH. Experimental hemorrhage and blunt trauma do not increase circulating tumor necrosis factor. J Trauma. 1991;31:1063–7. [PubMed] [Google Scholar]
- 78.Wick M, Kollig E, Muhr G, Koller M. The potential pattern of circulating lymphocytes TH1/TH2 is not altered after multiple injuries. Arch Surg. 2000;135:1309–14. doi: 10.1001/archsurg.135.11.1309. [DOI] [PubMed] [Google Scholar]
- 79.Asadullah K, Docke WD, Ebeling M, Friedrich M, Belbe G, Audring H, Volk HD, Sterry W. Interleukin 10 treatment of psoriasis: clinical results of a phase 2 trial. Arch Dermatol. 1999;135:187–92. doi: 10.1001/archderm.135.2.187. [DOI] [PubMed] [Google Scholar]
- 80.Nuki G, Bresnihan B, Bear MB, McCabe D. Long-term safety and maintenance of clinical improvement following treatment with anakinra (recombinant human interleukin-1 receptor antagonist) in patients with rheumatoid arthritis: extension phase of a randomized, double-blind, placebo-controlled trial. Arthritis and rheumatism. 2002;46:2838–46. doi: 10.1002/art.10578. [DOI] [PubMed] [Google Scholar]
- 81.Caron A, Desrosiers RR, Beliveau R. Ischemia injury alters endothelial cell properties of kidney cortex: stimulation of MMP-9. Exp Cell Res. 2005;310:105–16. doi: 10.1016/j.yexcr.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 82.de Vries DK, Lindeman JH, Tsikas D, de Heer E, Roos A, de Fijter JW, Baranski AG, van Pelt J, Schaapherder AF. Early renal ischemia-reperfusion injury in humans is dominated by IL-6 release from the allograft. Am J Transplant. 2009;9:1574–84. doi: 10.1111/j.1600-6143.2009.02675.x. [DOI] [PubMed] [Google Scholar]
- 83.Detmar M, Brown LF, Berse B, Jackman RW, Elicker BM, Dvorak HF, Claffey KP. Hypoxia regulates the expression of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) and its receptors in human skin. J Invest Dermatol. 1997;108:263–8. doi: 10.1111/1523-1747.ep12286453. [DOI] [PubMed] [Google Scholar]
- 84.Kukielka GL, Youker KA, Hawkins HK, Perrard JL, Michael LH, Ballantyne CM, Smith CW, Entman ML. Regulation of ICAM-1 and IL-6 in myocardial ischemia: effect of reperfusion. Ann N Y Acad Sci. 1994;723:258–70. [PubMed] [Google Scholar]
- 85.Kuyvenhoven JP, Molenaar IQ, Verspaget HW, Veldman MG, Palareti G, Legnani C, Moolenburgh SE, Terpstra OT, Lamers CB, van Hoek B, Porte RJ. Plasma MMP-2 and MMP-9 and their inhibitors TIMP-1 and TIMP-2 during human orthotopic liver transplantation. The effect of aprotinin and the relation to ischemia/reperfusion injury. Thromb Haemost. 2004;91:506–13. doi: 10.1160/TH03-05-0272. [DOI] [PubMed] [Google Scholar]
- 86.Proczka RM, Malecki M, Chorostowska-Wynimko J, Polanski JA. Vascular-endothelial growth factor (VEGF) in patients with peripheral ischemia. J Physiol Pharmacol. 2006;57 (Suppl 4):305–11. [PubMed] [Google Scholar]
- 87.Kavathia N, Jain A, Walston J, Beamer BA, Fedarko NS. Serum markers of apoptosis decrease with age and cancer stage. Aging (Albany NY) 2009;1:652–63. doi: 10.18632/aging.100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shurin GV, Yurkovetsky ZR, Chatta GS, Tourkova IL, Shurin MR, Lokshin AE. Dynamic alteration of soluble serum biomarkers in healthy aging. Cytokine. 2007;39:123–9. doi: 10.1016/j.cyto.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 89.Miller SJ, Watson WC, Kerr KA, Labarrere CA, Chen NX, Deeg MA, Unthank JL. Development of progressive aortic vasculopathy in a rat model of aging. Am J Physiol Heart Circ Physiol. 2007;293:H2634–43. doi: 10.1152/ajpheart.00397.2007. [DOI] [PubMed] [Google Scholar]
- 90.Richter V, Rassoul F, Purschwitz K, Hentschel B, Reuter W, Kuntze T. Circulating vascular cell adhesion molecules VCAM-1, ICAM-1, and E-selectin in dependence on aging. Gerontology. 2003;49:293–300. doi: 10.1159/000071710. [DOI] [PubMed] [Google Scholar]
- 91.Zou Y, Jung KJ, Kim JW, Yu BP, Chung HY. Alteration of soluble adhesion molecules during aging and their modulation by calorie restriction. Faseb J. 2004;18:320–2. doi: 10.1096/fj.03-0849fje. [DOI] [PubMed] [Google Scholar]
- 92.Li L, Smith A, Hagen TM, Frei B. Vascular oxidative stress and inflammation increase with age: ameliorating effects of alpha-lipoic acid supplementation. Ann N Y Acad Sci. 2010;1203:151–9. doi: 10.1111/j.1749-6632.2010.05555.x. [DOI] [PubMed] [Google Scholar]
- 93.Napolitano LA, Burt TD, Bacchetti P, Barron Y, French AL, Kovacs A, Anastos K, Young M, McCune JM, Greenblatt RM. Increased circulating interleukin-7 levels in HIV-1-infected women. J Acquir Immune Defic Syndr. 2005;40:581–4. doi: 10.1097/01.qai.0000187442.53708.b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patients in the 911 and 922 triage groups were included in the study.
Levels of five cytokines for three representative patients (0013, 0114, and 0153) are plotted as examples of the observed trends within individuals. All three patients are transfused males with blunt wounds. 0013 is age 34 with an injury severity score of 32, 0114 is age 43 with an injury severity score of 14, and 0153 is age 69 with an injury severity score of 14. (A) IL-6 and IL-10 are shown as examples of cytokines elevated early after trauma. (B) IL-17 is shown as an example of a cytokine that is depressed after trauma. (C) tPAI-1 is shown as an example of a cytokine that has a later elevation after trauma.