Abstract
The resolution of inflammation is an active and dynamic process critical in maintaining homeostasis. Newly identified lipid mediators have been recognized as key players during the resolution phase. These specialized proresolving mediators (SPM) constitute separate families that include lipoxins, resolvins, protectins and maresins each derived from essential polyunsaturated fatty acids. New results demonstrate that SPM regulate aspects of the immune response, including reduction of neutrophil infiltration, decreased T cell cytokine production and stimulation of macrophage phagocytic activity. The actions of SPM on B lymphocytes remain unknown. Our study shows for the first time that the novel SPM 17-hydroxydosahexaenoic acid (17-HDHA), resolvin D1 (RvD1) and protectin D1 (PD1) are present in the spleen. Interestingly, 17-HDHA, RvD1 but not PD1, strongly increase activated human B cell IgM and IgG production. Furthermore, increased antibody production by 17-HDHA is due to augmented B cell differentiation towards a CD27+CD38+ antibody-secreting cell phenotype. 17-HDHA did not affect proliferation and was non-toxic to cells. Increase of plasma cell differentiation and antibody production supports the involvement of SPM during the late stages of inflammation and pathogen clearance. The present study provides new evidence for SPM activity in the humoral response. These new findings highlight the potential applications of SPM as endogenous and non-toxic adjuvants, and as anti-inflammatory therapeutic molecules.
Introduction
Omega-3 and omega-6 polyunsaturated fatty acids (PUFA) have been long praised for their beneficial roles in inflammatory disease and autoimmune disorders (1). However, little is known about the mechanisms responsible for their beneficial effects. In recent years, the identification of novel PUFA-derived specialized proresolving mediators (SPM) has generated great interest in the regulation of inflammation, particularly in the resolution phase.
The resolution of the inflammatory response is critical to maintain homeostasis and prevent disease. Once thought of as a passive process, the resolution phase of inflammation is a multifaceted and dynamic process (2). Newly-identified, endogenous lipid mediators are now recognized as important players in dampening inflammation. These SPM are synthesized through lipoxygenases (LOX) or acetylated cyclooxygenase-2 (Cox-2) mediated pathways (3). SPM constitute separate families, including lipoxins, resolvins, protectins and maresins (4, 5).
SPM play important roles during inflammation including the inhibition of neutrophil infiltration, reduction of T cell cytokine production and increased recruitment of monocytes with enhanced phagocytic activity (6–8). In addition, exogenous treatment with pro-resolving lipid mediators has been shown to alleviate symptoms in animal models of inflammatory diseases such as colitis, periodontitis, asthma as well as in autoimmune disorders like arthritis (9).
Interestingly, SPM and key intermediates have been identified in serum and in important immunological sites including tonsils and the bone marrow, where high numbers of B cells are present (10–13). Nevertheless, little is known about SPM role on lymphocyte function, particularly B cells, and their effect on the adaptive immune response. In this study we asked if SPM, particularly those found present in the spleen, influence B cell function. Our initial analysis focused on several key SPM, none of which as far as we know have been evaluated for activity on human B cells. Since B cells can respond to other lipid mediators such as prostaglandins, we asked whether certain SPM could beneficially stimulate antibody production and B cell function.
Materials and Methods
Liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based metabolo-lipidomics
FVB/NJ mouse spleens were suspended in 1.0 mL cold methanol and gently ground followed by protein precipitation for 12 hr. Samples were next extracted by SPE column and methyl formate fractions were taken for LC-MS/MS-based lipidomics. LC-MS/MS was performed with an Agilent 1100 HPLC (Agilent Technologies, Santa Clara, CA) equipped with an Agilent Eclipse Plus C-18 column (4.6 mm×50 mm×1.8 μm) paired with an ABI Sciex Instruments 5500 QRAP linear ion trap triple quadrupole mass spectrometer (Applied Biosystems, Foster City, CA). Instrument control and data acquisition were performed using AnalystTM 1.5 software (Applied Biosystems). The mobile phase consisted of methanol/water/acetic acid (55/45/0.01; v/v/v) and was ramped to 88/12/0.01 (v/v/v) after 10 min, 100/0/0.01 (v/v/v) after 18 min, and 55:45:0.01 (v/v/v) after 1 minute to wash and equilibrate the column. Mass spectrometry analyses were carried out in negative ion mode using multiple reaction monitoring (MRM) of established specific transitions for 17-HDHA (m/z 343>245) and RvD1 (m/z 375>215). Identification was matching retention time and diagnostic ions to synthetic standards (14).
B lymphocyte isolation
Human B cells were isolated from peripheral blood of healthy subjects under the ethical permission provided by the Research Subjects Review Board at the University of Rochester. B cells were isolated as described (15, 16). Briefly, the buffy coat was separated and diluted in 1x PBS. PBMCs were isolated using Ficoll-Paque (Amersham Biosciences, Piscataway, NJ) gradient centrifugation. B cells were then purified from the leukocyte layer using CD19 Dynabeads (Invitrogen, Carlsbad, CA). CD19 Dynabead-cell rosettes were disrupted using CD19 Detachabead (Invitrogen, Carlsbad, CA). Cells obtained by this method of isolation were >98% CD19+.
Reagents and culture conditions
Purified CD19+ human B cells isolated from PBMCs were cultured in RPMI 1640 (GIBCO/Invitrogen, Carlsbad, CA) supplemented with 5% fetal bovine serum, 2 mM L-glutamine, 5 × 10−5 M 2-ME, 10 mM HEPES and 50 μg/mL gentamicin. CpG ODN 2395 5′-TCGTCGTTTTCGGCGCGCGCCG-3′ was purchased from the Coley Pharmaceutical Group (Wellesley, MA) and used to stimulate B cells at a concentration of 1 μg/mL. BCR stimulation was performed using rabbit anti-human IgM antibody fragment (Jackson ImmunoResearch Laboratories, West Grove, PA) at 2 μg/mL. Both synthetic AT 15-epi lipoxin and protectin D1 were prepared as in (17). Resolvin D1 and 17 (R)-HDoHE (Cayman Chemical Company, Ann Harbor, MI) were suspended in ethanol and supplemented in culture at nanomolar concentrations. Vehicle control or SPM were added 30 min prior to B cell activation with CpG ODN 2395 plus αIgM antibody, as well as everyday for the duration of the treatment. Vehicle controls were defined as 1xPBS with 0.03 % ethanol by volume, equivalent to the highest concentration of SPM used.
Proliferation assay
Purified B cells were cultured in round-bottom 96-well plates (1×105 cells/ml, 200 μl/well). Cells were cultured in triplicate, stimulated with CpG ODN 2395 plus αIgM and treated with 17 (R)-HDoHE. 1μCi of [3H] Thymidine was added per well 12 hours prior to harvest. Samples were taken at day 1, 3, 4 and 5. [3H] Thymidine incorporation was measured by scintillation spectroscopy using a Topcount Luminometer (PerkinElmer, Boston, MA).
Enzyme-linked Immunosorbent Assays
Purified CD19+ human B cells (1 × 106 cells/ml, 200 μl/well) were cultured in triplicate in 96-well round-bottom plates for 7 days. Supernatant IgM and IgG concentrations were measured using ELISA kits as specified by the manufacturer (Bethyl Laboratories, Montgomery, TX). Cytokines were measured from purified B cells (3 × 106 cells/ml). At day 2 and 7 after activation IL-6 (BD Pharmigen, San Diego, CA), IL-10 and TNFα (BioLegend, San Diego, CA) levels were measured in the supernatants.
IgM and IgG specific ELISpot assay
Purified CD19+ human B cells (1 × 106 cells/ml, 200 μl/well) were cultured for 5 days. ELISpot plates (Millipore, Billerica, MA) were coated with goat anti-human IgM and IgG antibodies (Biosource, Carlsbad, CA) as recommended by the manufacturer. B cells were incubated in the ELISpot plates for 5 hours at 37°C. Antibody-secreting cells were detected with alkaline phosphatase-conjugated goat anti-human IgG (Jackson ImmunoResearch Laboratories) or anti-human IgM antibody (Biosource, Carlsbad, CA). Plates were developed using Vector AP substrate kit III (Vector Laboratories, Burlingame, CA) and spots were counted using a CTL plate reader and immunospot software (Cellular Technologies, Shaker Heights, OH).
Real-time PCR
Following 48 and 72 hours of culture of human B cells (3 × 106 cells/ml, 500 μl/well), total RNA was isolated using Qiagen RNAeasy mini kit (Valencia, CA). Superscript III and random primers (Invitrogen, Carlsbad, CA) were used to reverse transcribe isolated RNA to cDNA. Steady-state levels of Blimp-1, Xbp-1, Pax5, AID mRNA and 7S RNA were assessed by real-time PCR. Primers used for Blimp-1 sense 5′-GTGTCAGAACGGGATGAAC-3′ and antisense 5′-TGTTAGAACGGTAGAGGTCC-3′, Xbp-1 sense 5′-TGGCGGTATTGACTCTTCAG-3′ and antisense 5′-ACGAGGTCATCTTCTACAGG-3′, Pax5 sense 5′-TTGCTCATCAAGGTGTCAGG-3′ and antisense 5′-TAGGCACGGTGTCATTGTC-3′, AID sense 5′-CGTGACAGTGCTACATCC-3′ and antisense 5′-TGCGGTCCTCACAGAAGTAG-3′, and 7S sense 5′-ACCACCAGGTTGCCTAAGGA-3′ and antisense 5′-CACGGGAGTTTTGACCTGCT-3′. iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) was used to quantify amplified products and results were analyzed with the Bio-Rad icycler software. Blimp-1, Xbp-1 and Pax5 and AID mRNA steady-state levels were normalized to 7S expression. Changes in mRNA expression were determined by comparing mRNA steady-state levels from vehicle treated peripheral B cells to SPM treated B cells.
Western blotting
Purified human B cells were lysed in RIPA buffer (150 mM NaCl, 1% NP40, 0.5% Na Deoxycholate, 50mM Tris-base, 0.1% SDS, pH 8.0) to obtain whole cell lysate. Protein concentration was determined using Bio-Rad DC protein assay kit (BioRad, Hercules CA). Gradient SDS-PAGE gels (Pierce/Thermo Fisher Scientific, Rockford, IL) were loaded with 4 μg of protein and transferred to PVDF membranes (Millipore, Billerica, MA). Western blots were probed with mouse anti-human Blimp-1, rabbit anti-human Xbp-1 (Novus, Littleton, CO), rabbit anti-human Pax5 (Millipore, Billerica, MA), mouse anti-human AID (Cell Signaling Technology, Beverly, MA) and mouse anti-human actin control (Calbiochem/EMD Chemicals, Gibbstown, NJ). HRP conjugated goat anti-mouse or goat anti-rabbit antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) were used to detect specific probed antibodies. Western blots were visualized by autoradiography after incubation with ECL (Perkin Elmer Life Sciences Inc., Boston, MA).
Flow cytometry analysis
B cell viability was assessed by 7AAD staining using Cell Viability Solution (BD Bioscience). Cells were surface stained for CD19-V450 or FITC (BD Biosciences), CD27 PE-Cy7 (BD Biosciences), CD38-PerCP-5.5 (BD Biosciences), IgD-PE (BD Biosciences), IgG-APC (BD Biosciences). Fluorescence minus one (FMO) controls were included in each staining protocol. Cells were analyzed on a 12-color LSR II flow cytometer (BD Biosciences). Data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR).
Statistics
Each experiment was repeated using cells from five different donors unless otherwise specified. Data are expressed as mean ± SEM. Significance was determined by one-way ANOVA with a Tukey’s post-test, or a two-tailed unpaired Student t-test where applicable. A two-way ANOVA with a Bonferroni posttest was used where two or more variables were included. Probability values of p ≤ 0.05 were considered statistically significant.
Results
Specialized pro resolving mediators are present in mouse spleens
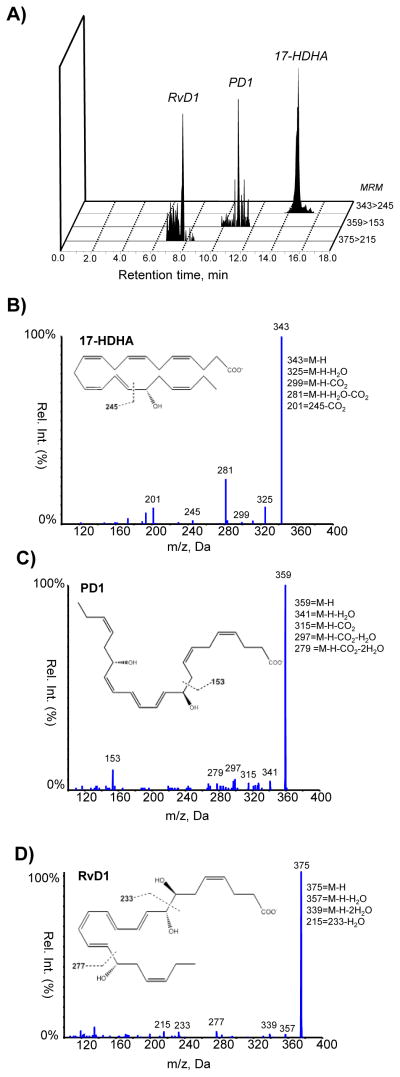
Lipid derived molecules such as PGs play important roles in B cell function (18, 19). Our laboratory has previously shown that PGE2 is not only produced by B cells, but it is also important for antibody production (15, 16, 19). In light of the critical role novel specialized proresolving mediators (SPM) play during inflammation, we first investigated the presence of SPM in the spleen, where a high number of B cells reside. Using liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based metabolo-lipidomics analysis we identified DHA-derived 17-hydroxydosahexaenoic acid (17-HDHA), resolvin D1 (RvD1) and protectin D1 (PD1) to be present in the spleen (figure 1). Interestingly, other resolvins of the D series were not detected (RvD2 and RvD5). The presence of SPM in the spleen further suggests a role for SPM and the regulation of the on the immune response.
Figure 1.
Endogenous 17-HDHA, PD1, and RvD1 are obtained in murine spleens. A) MRM chromatograms (343>245, 359>153, and 375>215) and representative tandem mass spectra of (B)17-HDHA, (C) PD1, and (D) RvD1 in the samples of mouse spleens (n=3).
Specialized proresolving mediators stimulate human B cell antibody production
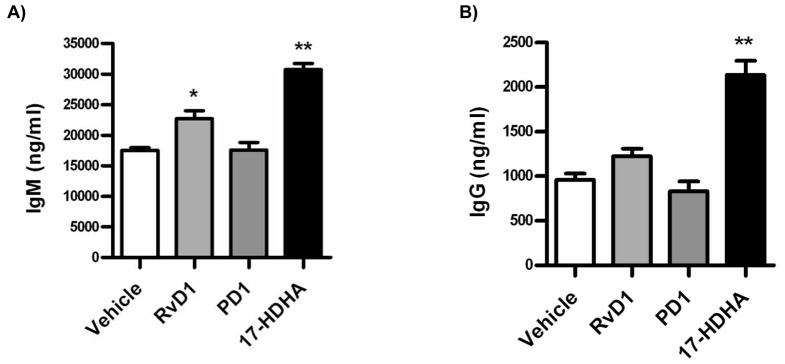
The identified 17-HDHA is a known biomarker for resolvins of the D series, as well as protectin biosynthesis (17, 20). We therefore asked if DHA-derived SPM influenced human B cell functions, particularly antibody production. Peripheral blood human B cells were purified from healthy donors by CD19 positive selection. B cells were pre-treated with either 17-HDHA, RvD1 or protectin D1. Cells were then activated with CpG ODN 2395 plus αIgM and cultured for 7 days. IgM and IgG levels were measured in the supernatants by ELISA (figure 2). Interestingly, PD1 did not affect antibody production. In contrast, the docosanoid RvD1 increased antibody production, particularly IgM. Furthermore, DHA-derived 17-HDHA, had a particularly strong effect at increasing both IgM and IgG production.
Figure 2.
Certain SPM stimulate B cell antibody production. Human CD19+ B cells were isolated by positive selection from whole units of blood from healthy subjects. Purified B cells (1 × 106 cells/ml) were treated for 30 minutes with the respective SPM (100 nM) or vehicle prior to CpG ODN 2395 (1 μg/ml) plus αIgM (2 μg/ml) activation. At day 7 of culture, supernatants were collected and antibody production measured by ELISA. Vehicle control or SPM were added to culture every day. Data were analyzed by one-way ANOVA with a Tukey’s post test, *p≤0.05, **p≤0.01 and represented as ±SEM.
Docosanoid 17-HDHA increases B cell antibody production, but does not activate naïve B cells
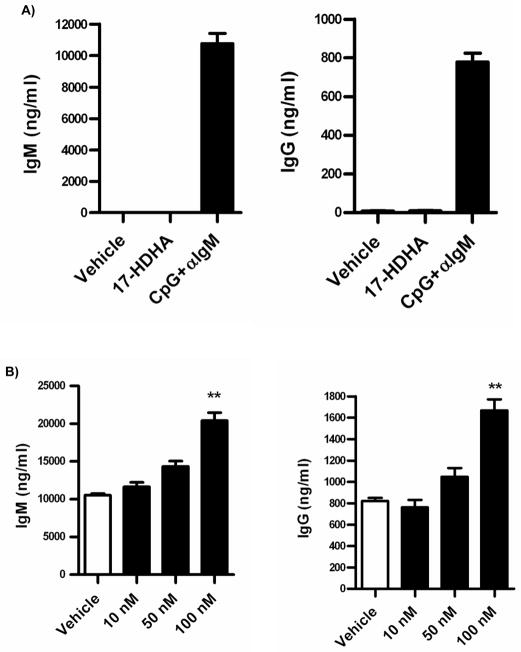
In order to determine if the effects of 17-HDHA were due to increased activation of naïve B cells, we treated freshly isolated human CD19+ B cells for 7 days with 17-HDHA alone and compared its effect to the known stimulants CpG ODN 2395 plus αIgM (15) (figure 3A). Unlike CpG plus αIgM treated cells, 17-HDHA did not induce B cell antibody production on its own.
Figure 3.
IgM and IgG production is enhanced by 17-HDHA. Purified B cells (1 × 106 cells/ml) were cultured for 7 days and antibody levels were measured by ELISA. A) Non-stimulated B cells were treated with 100 nM 17-HDHA, vehicle or with CpG ODN 2395 (1 μg/ml) plus αIgM (2 μg/ml) as a positive control. B) Purified B cells were treated for 30 minutes with 17-HDHA or vehicle prior to CpG ODN 2395 (1 μg/ml) plus αIgM (2 μg/ml) activation. Data were analyzed by one-way ANOVA with a Tukey’s post test, **p≤0.01 and represented as ±SEM.
SPM have been shown to be biologically active at nanomolar ranges on non-lymphocytic cells (21, 22). A 17-HDHA dose response curve on activated B cells was performed to determine optimal concentrations (figure 3B). B cell antibody response was enhanced in a dose dependent manner, with 100 nM concentration of 17-HDHA being the most effective at increasing IgM and IgG production. These new results demonstrate that 17-HDHA alone does not induce antibody production in peripheral human B cells, rather it significantly increases IgM and IgG production in CpG plus αIgM activated cells.
17-HDHA decreases cytokine production from activated peripheral B cells
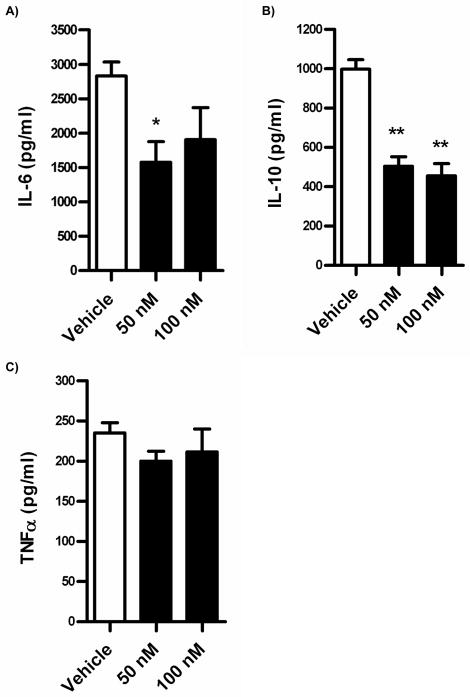
DHA-derived SPM such as protectin D1 inhibit TNFα and IFNγ production by T cells (23, 24). In addition, the eicosanoid lipoxin A4 decreases IL-12 production by dendritic cells (25, 26). Therefore, we asked if 17-HDHA influenced B cell cytokine production. Cytokine production was measured in purified human peripheral blood B cell cultures. Purified B cells were exposed to 17-HDHA followed by activation with CpG plus αIgM. IL-6, IL-10 and TNFα concentrations were measured by ELISA (figure 4). These results showed dampened production of the pro-inflammatory cytokine IL-6 by 17-HDHA. Downregulation of IL-6 was detected as early as day 2 after B cell activation (figure 4A). Surprisingly, IL-10 production was also found to be decreased by day 7 after treatment in culture (figure 4B). Earlier time points of IL-10 production showed a decreasing trend, but did not achieve statistical significance (data not shown). Lastly, there were no significant changes in TNFα production (figure 4C), a unique finding for B cells, as previous reports have shown SPM-mediated decreased TNFα production in T cells (6).
Figure 4.
17-HDHA decreases IL-6 and IL-10 production, but not TNFα. Positively selected CD19+ human B cells (3 × 106 cells/ml) were treated with 17-HDHA or vehicle 30 minutes prior to B cell stimulation with CpG ODN 2395 (1 μg/ml) plus αIgM (2 μg/ml). A) IL-10 concentrations measured by ELISA at day 7. B, C) IL-6 and TNFα concentrations measured by ELISA at day 2. Data were analyzed by one-way ANOVA with a Tukey’s post test, *p≤0.05, **p≤0.01 and represented as ±SEM.
Increased antibody production is not due to changes in B cell viability nor proliferation
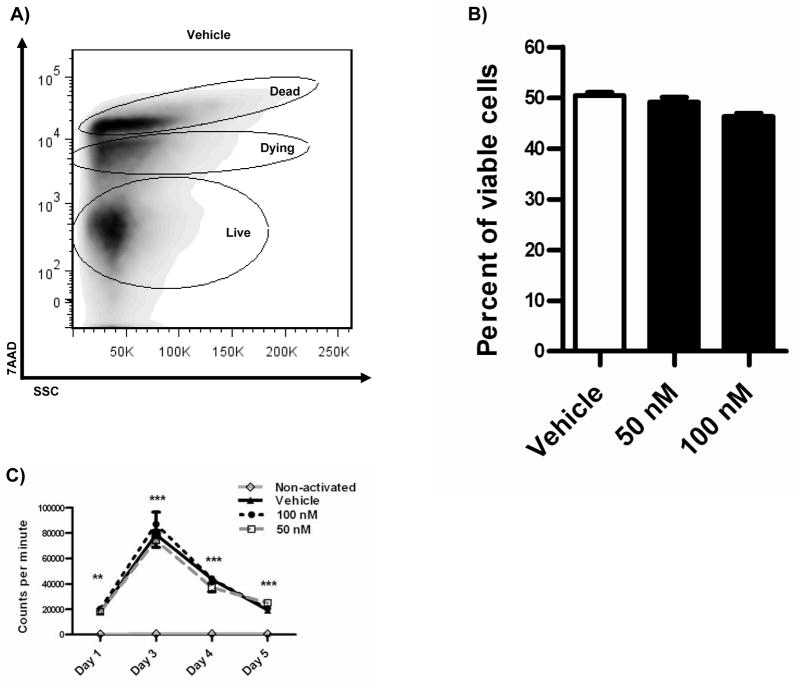
We contemplated that the changes in antibody and cytokine levels could be attributed to changes in either cell death or proliferation. Therefore, we measured B cell viability by 7AAD exclusion. CD19+ purified B cells were cultured for 7 days and viability was measured at the end of the treatment. Dying, dead and live populations were gated and compared between 17-HDHA treated and non-treated groups, with no significant changes found (figure 5A–B). Proliferation was also considered as a possible factor influencing antibody and cytokine secretion. To monitor cell proliferation, the tritiated thymidine DNA incorporation assay was performed in a time course (figure 5C). No apparent differences were found in CpG plus αIgM activated cells that had been exposed to 17-HDHA. We concluded that the endogenous 17-HDHA is not toxic to B cells nor does it have proliferative effects.
Figure 5.
17-HDHA does not significantly influence B cell viability nor proliferation. Human B cell viability was measured by 7-AAD exclusion by flow cytometry. A) Representative dot plot showing 7-AAD exclusion strategy. B) Quantification of live cells measured by 7-AAD exclusion at end of 7 day treatment. C) [3H] Thymidine incorporation assay presented as counts per minute. Data were analyzed by two-way ANOVA with a Bonferroni post test, **p≤0.01, ***p≤0.01 compared to non-activated samples, results represented as ±SEM.
17-HDHA increases the number of antibody-producing cells
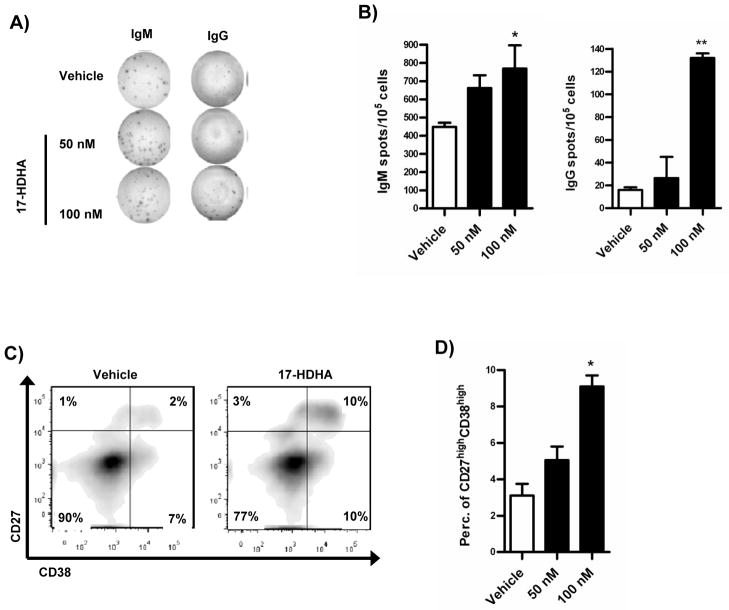
17-HDHA-mediated IgM and IgG increase production could be due to higher antibody-production levels per cell or due to a larger number of cells secreting immunoglobulin. In order to address this question, we performed ELISpot analysis. Purified B cells were treated with 17-HDHA or control vehicle and activated with CpG plus αIgM. Our results showed that 17-HDHA increased the number of cells secreting IgM and IgG in a dose dependent manner (figure 6A–B). These results suggest that the increase in antibody production is due to an increase in the number of antibody-secreting B cells.
Figure 6.
Antibody-secreting B cell differentiation is enhanced by 17-HDHA. Purified human B cells were collected on day 5 after activation. Number of antibody secreting cells was measured by ELISpot analysis. A) Representative image of IgM and IgG specific spots. B) Quantification of IgM and IgG spot forming cells. 7 days after activation, cells were harvested and stained for surface markers. A live-lymphocyte gate was used in all flow cytometry analyses as well as FMO for gating controls. C) Representative dot plot of double positive cells. D) Quantification of live IgD−CD27+CD38+ cells. Data analyzed by one-way ANOVA with Tukey’s post test, *p≤0.05, **p≤0.01 and represented as ±SEM.
Antibody-secreting B cell differentiation is enhanced by 17-HDHA
To further explore if 17-HDHA promotes cell differentiation, we analyzed characteristic B cell surface markers that change during differentiation. Purified B cells were activated and treated with 17-HDHA. Following 7 days of culture, surface marker staining and flow cytometry was performed. Cell viability was measured by 7AAD exclusion. Plasma cell precursors are defined as CD27+CD38+ cells (27, 28) therefore, B cell surface markers CD19, CD27, CD38 and IgD were used.
CD19 staining was performed at the beginning of culture in order to confirm B cell purity of >98% (data not shown). Using flow cytometry analysis, antibody-secreting cells were defined as IgD−CD27+CD38+ and pre-gated on the live-lymphocyte gate (27, 28). 17-HDHA increased the number of double positive CD27+CD38+ cells in culture (figure 6C–D). We conclude that 17-HDHA enhanced the number of antibody-secreting cells, which in turn was responsible for the increased amounts of IgM and IgG present in culture.
17-HDHA increases expression of plasma cell differentiation factors
B cell maturation and differentiation is a carefully regulated process. Blimp-1 and Xbp-1 are important transcription factors required for B cell differentiation towards plasma cells. Blimp-1 represses the transcription factor Pax-5 which is a negative regulator of Xbp-1. Expression of Xbp-1 is critical for antibody-secreting cell formation (29–31). In addition, Pax-5, which is important in early B cell lineage stages, is downregulated as B cells differentiate towards memory or plasma cells (32, 33).
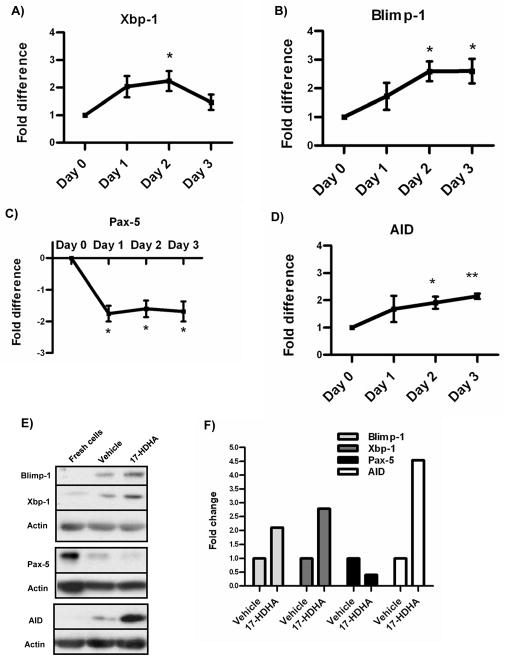
We analyzed the expression levels of Blimp-1 and Xbp-1 in 17-HDHA treated B cells. Messenger RNA steady-state levels measured by real-time PCR showed that both differentiation factors were upregulated in 17-HDHA treated B cells (figure 7A–B). In addition, Pax-5 mRNA levels were strongly downregulated as early as day 1 in culture (figure 7C). Furthermore, we measured protein expression of Blimp-1, Xbp-1 and Pax-5 by western blot (figure 7E–F). 17-HDHA increased Blimp-1 and Xbp-1 while decreasing Pax-5 protein levels. These results show that 17-HDHA promoted differentiation of activated B cells at the molecular level.
Figure 7.
17-HDHA upregulates plasma cell differentiation factors and downregulates Pax-5. RNA and protein isolated from purified B cells (3 × 106 cells/ml). A–D) Blimp-1, Xbp-1, Pax-5 and AID mRNA steady levels measured by real time PCR and quantified as the fold difference between 17-HDHA (100nM) treated cells against control vehicle samples correspondent to each day. Data were normalized to 7S RNA expression. E) Transcription factor protein levels measured by western blot. Freshly isolated B cell protein content compared to day 5 cultured cells treated with vehicle control or 100nM 17HDHA. F) Western blot densitometry analysis, samples normalized to respective vehicle control. Protein and mRNA experiments were repeated using 4 different donors. Data analyzed by Student t-test *p≤0.05, **p≤0.01 and represented as ±SEM.
Antigen experienced B cells undergo isotype switching and somatic hypermutations thus increasing specificity of the antibody-mediated immune response. The transcription factor AID is involved in both of these processes (34). Considering the observed increased antibody production by 17-HDHA-treated B cells, AID mRNA steady-state and protein levels were measured. Our results showed that AID was upregulated in 17-HDHA treated cells (figure 7D–E). Overall, these results support our hypothesis that the SPM 17-HDHA drives human B cells differentiation towards an antibody-secreting cell.
Discussion
Docosanoids have many effects on cells of the immune system including T cells, neutrophils and macrophages (6–8). In an effort to better understand SPM biological functions on the immune system, we asked if SPM are present in important lymphatic organs such as the spleen. Here we have confirmed the presence of DHA-derived SPM in mouse spleens, specifically 17-HDHA, RvD1 and PD1.
The SPM 17-HDHA is a characteristic marker of the resolvin synthesis pathway as it is a precursor to D-series resolvins and protectins (20). Therefore, we asked what their effects are, if any, on human B cells. Here, we report for the first time that SPM from the docosanoids family, RvD1 and 17-HDHA, but not PD1 increase B cell antibody production and cell differentiation. Consequently, we studied 17-HDHA’s effects on human B cells. We found that 17-HDHA alone does not activate B cells to produce antibodies. However, in CpG plus αIgM activated B cells, 17-HDHA increased antibody production without affecting cell death nor proliferation.
Based on increases in antibody production, we hypothesized that 17-HDHA influenced B cell differentiation. Here, we demonstrated that 17-HDHA increased the number of cells producing IgM and IgG, as well as the antibody-secreting phenotype characterized by CD27+CD38+ expression (27, 35). Such a phenotypic change was further confirmed at the molecular level with increased expression of Blimp-1 and Xbp-1, as well as the downregulation of Pax-5, which are key B cell differentiation factors.
An enhanced, but controlled antibody response promotes faster and more efficient antigen recognition and clearance, thus promoting the resolution of inflammation. Therefore, 17-HDHA might play an important role in B cell maturation and the adaptive immune response. Not all peripheral B cells will become antibody-secreting cells. Additional B cell subsets could be differentially responsive to certain SPM. The question of which B cell subset is differentiating towards an antibody-secreting cell will need to be addressed in the future.
Interestingly, 17-HDHA decreased B cell cytokine production, particularly IL-6 and IL-10, which have pro-inflammatory and anti-inflammatory effects, respectively. Unlike previous reports on mouse and human T cells, human B cell TNFα production was not affected by 17-HDHA (6). Decreases in both pro-inflammatory and anti-inflammatory cytokine production further suggest a preferential differentiation towards an antibody-secreting cell.
A question which has not yet been addressed is whether 17-HDHA solely promotes B cell differentiation, or if it also enhances affinity maturation or isotype switching. Our findings show 17-HDHA increases AID expression, however, we have not discerned whether this is a result of the increased number of plasma cells in culture, or if it reflects an enhanced somatic hypermutation activity within the plasma cell population. Future studies should address the effects of 17-HDHA and other SPM during an antigen-specific antibody-response and their role on the long-term immunity.
Here, we have confirmed the presence of endogenous 17-HDHA, RvD1 and PD1 in mouse spleens. The specific cells responsible for SPM biosynthesis have not yet been determined. Nevertheless, SPM such as 17-HDHA and RvD1, but not PD1, have a strong effect on B cells and the humoral response. Therefore, it is possible that SPM present in lymphoid organs, such as the spleen and bone marrow, play an important role during the humoral response. Further investigation of SPM activity in the B cell microenvironment will provide further understanding of the role of SPM during the primary and secondary immune response.
The fact that 17-HDHA and other SPM increase B cell antibody production and promote B cell differentiation to an antibody secreting cell raises interesting possibilities. For example, 17-HDHA and other SPM, might prove useful as non-toxic and non-immunogenic adjuvants. Furthermore, their ability to stimulate B cells in the nanomolar range further enhances their attractiveness as B cell activators. Overall, the current study provides the first set of evidence on SPM functions as immune regulators of B cells and their involvement in the humoral immune response. Considering the importance of polyunsaturated fatty acids in our diet and their known anti-inflammatory properties, SPM have great potential to be used as immune regulators, particularly during infection or vaccination.
Acknowledgments
We acknowledge Dr. Bruce D. Levy for providing animals and advice.
Footnotes
This work was supported by NIH ES01247 (to R.P.P.), DE011390 (to R.P.P.), 1P01GM095467 (to C.N.S.) and NIH T32 DE007202.
Disclosures
C.N.S is an inventor on patents [resolvins] assigned to BWH and licensed to Resolvyx Pharmaceuticals. CNS is a scientific founder of Resolvyx Pharmaceuticals and owns equity in the company. CNS’ interests were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
References
- 1.Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21:495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- 2.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 3.Petasis NA, Akritopoulou-Zanze I, Fokin VV, Bernasconi G, Keledjian R, Yang R, Uddin J, Nagulapalli KC, Serhan CN. Design, synthesis and bioactions of novel stable mimetics of lipoxins and aspirin-triggered lipoxins. Prostaglandins Leukot Essent Fatty Acids. 2005;73:301–321. doi: 10.1016/j.plefa.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh SF, Vickery TW, Serhan CN. Chiral lipidomics of E-series resolvins: Aspirin and the biosynthesis of novel mediators. Biochim Biophys Acta. 1811:737–747. doi: 10.1016/j.bbalip.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ariel A, Chiang N, Arita M, Petasis NA, Serhan CN. Aspirin-triggered lipoxin A4 and B4 analogs block extracellular signal-regulated kinase-dependent TNF-alpha secretion from human T cells. J Immunol. 2003;170:6266–6272. doi: 10.4049/jimmunol.170.12.6266. [DOI] [PubMed] [Google Scholar]
- 7.Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164:1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- 8.Lee TH, Horton CE, Kyan-Aung U, Haskard D, Crea AE, Spur BW. Lipoxin A4 and lipoxin B4 inhibit chemotactic responses of human neutrophils stimulated by leukotriene B4 and N-formyl-L-methionyl-L-leucyl-L-phenylalanine. Clin Sci (Lond) 1989;77:195–203. doi: 10.1042/cs0770195. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Zhang X, Wu P, Li H, Jin S, Zhou X, Li Y, Ye D, Chen B, Wan J. BML-111, a lipoxin receptor agonist, modulates the immune response and reduces the severity of collagen-induced arthritis. Inflamm Res. 2008;57:157–162. doi: 10.1007/s00011-007-7141-z. [DOI] [PubMed] [Google Scholar]
- 10.Poulsen RC, Gotlinger KH, Serhan CN, Kruger MC. Identification of inflammatory and proresolving lipid mediators in bone marrow and their lipidomic profiles with ovariectomy and omega-3 intake. Am J Hematol. 2008;83:437–445. doi: 10.1002/ajh.21170. [DOI] [PubMed] [Google Scholar]
- 11.Schneider C, Keeney DS, Boeglin WE, Brash AR. Detection and cellular localization of 12R-lipoxygenase in human tonsils. Arch Biochem Biophys. 2001;386:268–274. doi: 10.1006/abbi.2000.2217. [DOI] [PubMed] [Google Scholar]
- 12.Bafica A, Scanga CA, Serhan C, Machado F, White S, Sher A, Aliberti J. Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production. J Clin Invest. 2005;115:1601–1606. doi: 10.1172/JCI23949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong S, Porter TF, Lu Y, Oh SF, Pillai PS, Serhan CN. Resolvin E1 metabolome in local inactivation during inflammation-resolution. J Immunol. 2008;180:3512–3519. doi: 10.4049/jimmunol.180.5.3512. [DOI] [PubMed] [Google Scholar]
- 14.Yang R, Chiang N, Oh SF, Serhan CN. Metabolomics-lipidomics of eicosanoids and docosanoids generated by phagocytes. In: Coligan John E, et al., editors. Current protocols in immunology. Unit 14–26. Chapter 14. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernard MP, Phipps RP. CpG oligodeoxynucleotides induce cyclooxygenase-2 in human B lymphocytes: implications for adjuvant activity and antibody production. Clin Immunol. 2007;125:138–148. doi: 10.1016/j.clim.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan EP, Pollock SJ, Murant TI, Bernstein SH, Felgar RE, Phipps RP. Activated human B lymphocytes express cyclooxygenase-2 and cyclooxygenase inhibitors attenuate antibody production. J Immunol. 2005;174:2619–2626. doi: 10.4049/jimmunol.174.5.2619. [DOI] [PubMed] [Google Scholar]
- 17.Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot Essent Fatty Acids. 2005;73:141–162. doi: 10.1016/j.plefa.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 19.Mongini PK, Inman JK, Han H, Fattah RJ, Abramson SB, Attur M. APRIL and BAFF promote increased viability of replicating human B2 cells via mechanism involving cyclooxygenase 2. J Immunol. 2006;176:6736–6751. doi: 10.4049/jimmunol.176.11.6736. [DOI] [PubMed] [Google Scholar]
- 20.Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci U S A. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maddox JF, Serhan CN. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J Exp Med. 1996;183:137–146. doi: 10.1084/jem.183.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hachicha M, Pouliot M, Petasis NA, Serhan CN. Lipoxin (LX)A4 and aspirin-triggered 15-epi-LXA4 inhibit tumor necrosis factor 1alpha-initiated neutrophil responses and trafficking: regulators of a cytokine-chemokine axis. J Exp Med. 1999;189:1923–1930. doi: 10.1084/jem.189.12.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ariel A, Fredman G, Sun YP, Kantarci A, Van Dyke TE, Luster AD, Serhan CN. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat Immunol. 2006;7:1209–1216. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ariel A, Li PL, Wang W, Tang WX, Fredman G, Hong S, Gotlinger KH, Serhan CN. The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. J Biol Chem. 2005;280:43079–43086. doi: 10.1074/jbc.M509796200. [DOI] [PubMed] [Google Scholar]
- 25.Aliberti J, Serhan C, Sher A. Parasite-induced lipoxin A4 is an endogenous regulator of IL-12 production and immunopathology in Toxoplasma gondii infection. J Exp Med. 2002;196:1253–1262. doi: 10.1084/jem.20021183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arce S, Luger E, Muehlinghaus G, Cassese G, Hauser A, Horst A, Lehnert K, Odendahl M, Honemann D, Heller KD, Kleinschmidt H, Berek C, Dorner T, Krenn V, Hiepe F, Bargou R, Radbruch A, Manz RA. CD38 low IgG-secreting cells are precursors of various CD38 high-expressing plasma cell populations. J Leukoc Biol. 2004;75:1022–1028. doi: 10.1189/jlb.0603279. [DOI] [PubMed] [Google Scholar]
- 28.Avery DT, Ellyard JI, Mackay F, Corcoran LM, Hodgkin PD, Tangye SG. Increased expression of CD27 on activated human memory B cells correlates with their commitment to the plasma cell lineage. J Immunol. 2005;174:4034–4042. doi: 10.4049/jimmunol.174.7.4034. [DOI] [PubMed] [Google Scholar]
- 29.Bernard MP, Phipp RP. Inhibition of cyclooxygenase-2 impairs the expression of essential plasma cell transcription factors and human B-lymphocyte differentiation. Immunology. 129:87–96. doi: 10.1111/j.1365-2567.2009.03152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol. 2005;5:230–242. doi: 10.1038/nri1572. [DOI] [PubMed] [Google Scholar]
- 31.Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, Friend D, Grusby MJ, Alt F, Glimcher LH. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412:300–307. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- 32.Nutt SL, Eberhard D, Horcher M, Rolink AG, Busslinger M. Pax5 determines the identity of B cells from the beginning to the end of B-lymphopoiesis. Int Rev Immunol. 2001;20:65–82. doi: 10.3109/08830180109056723. [DOI] [PubMed] [Google Scholar]
- 33.Lin KI, Angelin-Duclos C, Kuo TC, Calame K. Blimp-1-dependent repression of Pax-5 is required for differentiation of B cells to immunoglobulin M-secreting plasma cells. Mol Cell Biol. 2002;22:4771–4780. doi: 10.1128/MCB.22.13.4771-4780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honjo T, Nagaoka H, Shinkura R, Muramatsu M. AID to overcome the limitations of genomic information. Nat Immunol. 2005;6:655–661. doi: 10.1038/ni1218. [DOI] [PubMed] [Google Scholar]
- 35.Tarte K, De Vos J, Thykjaer T, Zhan F, Fiol G, Costes V, Reme T, Legouffe E, Rossi JF, Shaughnessy J, Jr, Orntoft TF, Klein B. Generation of polyclonal plasmablasts from peripheral blood B cells: a normal counterpart of malignant plasmablasts. Blood. 2002;100:1113–1122. [PubMed] [Google Scholar]