Abstract
A transcription factor network that includes STAT4, T-bet and Runx3 promotes the differentiation of T helper type 1 (Th1) cells and inflammatory immune responses. How additional transcription factors regulate the function of Th1 cells has not been defined. In this report we show that the negative regulatory factor Twist1 decreases expression of T-bet, Runx3 and IL-12Rβ2 as it inhibits IFN-γ production. Ectopic expression of Runx3, but not T-bet or IL-12Rβ2 compensates for the effects of Twist1 on IFN-γ production, and Twist1 regulation of Ifng depends upon complex formation with Runx3. Twist1 decreases Runx3 and T-bet binding at the Ifng locus, and decreases chromatin looping within the Ifng locus. These data define an IL-12/STAT4-induced negative regulatory loop that impacts multiple components of the Th1 transcriptional network and provides further insight into regulation of Th1 differentiation.
Keywords: T helper 1 cells, transcription factors, differentiation, interferon-gamma, Twist1, Runx3, STAT4
Introduction
A network of transcription factors regulates the development of Type 1 helper T cells (Th1), cells that play a crucial role in anti-bacterial and inflammatory immunity. IL-12 and IFN-γ promote Th1 differentiation by the respective activation of STAT4 and STAT1 leading to the induction of additional transcription factors including T-bet and Runx3. Th1 differentiation requires both T-bet and STAT4 for complete development of the Th1 genetic program (1) and is mediated by the interlinked and sequential effects of TCR-IFN-γ-STAT1-T-bet and IL-12-STAT4-T-bet signaling pathways (2). Runx3 cooperates with T-bet to activate Ifng and silence Il4 in Th1 cells, but can regulate Ifng expression independently of T-bet and STAT4 (3, 4). Although some factors that impact this network, including GATA3 (5–7), have been described, regulation of these pathways is still not clearly defined.
Twist1 is a transcriptional repressor and a member of the basic helix-loop-helix (bHLH) family of proteins that plays a positive role in dorso-ventral patterning in Drosophila embryos (8). Although the role of Twist1 in developmental and cancer biology has been explored (9), the function of Twist1 in the immune response is only beginning to be understood. Twist1 regulates cytokine production in macrophages through a negative feedback loop by targeting NF-κB activation resulting in decreased TNF-α and IL-1β (10, 11). The repressive mechanism may involve binding of Twist1 to E-boxes in cytokine promoters to inhibit the transcriptional activity of NF-κB (10, 11). In Th1 cells, NF-κB, NFAT and IL-12-STAT4 signaling can induce Twist1 expression, and Twist1 limits inflammation by suppressing IFN-γ and TNF-α production (12). However, a detailed mechanism of how Twist1 regulates cytokine production in Th1 cells has not been described.
In this report, we define the role of Twist1 in the Th1 cell transcriptional network. Using retroviral transduction studies, we demonstrate that Twist1 negatively regulates Th1 gene expression by decreasing expression and function of transcription factors including T-bet, STAT4, and Runx3. Twist1 functions through several mechanisms including physical interaction with Runx3 and interfering with the association of T-bet and Runx3 at the Ifng locus. The results suggest that Twist1 acts as a modulator that regulates multiple transcription factors in Th1 cells to limit potentially harmful inflammatory responses.
Materials and Methods
Mice
C57BL/6 mice were purchased from Harlan Sprague Dawley (Indianapolis, IN, USA). Stat4−/− mice were previously described (13). Twist1fl/fl mice (14) were crossed with CD4-Cre transgene mice to generate Twist1fl/fl CD4-Cre+ mice with Cre-negative littermates as WT mice. Twist1cc/wt mice (15) were crossed with Twist1fl/fl CD4-Cre+ mice to generate Twist1fl/cc CD4-Cre+ and Twist1fl/wt CD4-Cre+ as WT control. Mice were maintained under specific pathogen-free conditions. All experiments were performed with the approval of the Indiana University Institutional Animal Care and use Committee.
In vitro T cell differentiation
Naïve CD4+CD62L+ T cells were isolated from spleen and lymph nodes using a MACS isolation system (Miltenyi Biotec). CD4+ T cells were activated with plate-bound anti-CD3 (2 ug/ml 145-2C11) and soluble anti-CD28 (0.5 ug/ml BD Pharmingen for Th0, Th1, Th2, and Th17 or 1 ug/ml for Th9 and Treg) with additional cytokines (all from PeproTech) and antibodies to generate Th1 (5 ng/ml IL-12; and 10 ug/ml anti-IL-4 11B11), Th2 (10ng/ml IL-4; and 10ug/ml anti-IFN-γ XMG), Th9 (20 ng/ml IL-4; 2 ng/ml TGF-β; and 10 ug/ml anti-IFN-γ XMG) or Th17 (100 ng/ml IL-6; 10 ng/ml IL-23,; 10 ng/ml IL-1β; 2 ng/ml TGF-β;10 ug/ml anti-IL-4, 11B11; and 10 ug/ml anti-IFN-γ, XMG) or Treg (2ng/ml TGF-β, and 10ug/ml anti-IL-4, 11B11) culture conditions. Cells were expanded after 3 days without additional cytokines (Th0, Th1 and Th2), with 50 U/ml human-IL-2 (Treg), full concentration (Th9) or half concentration of IL-6 (Th17) of the original cytokines in fresh medium. Cells were harvested on day 5 for analysis.
Retroviral expression vectors and retroviral transduction
Bicistronic retrovirus expressing EGFP only (MIEG) and T-bet and EGFP (T-bet) were previously described (16, 17). PBMN-IRES-GFP-IL-12Rb2c was a kind gift from Dr. Takashi Usui (Kyoto University). Il12rb2c and Twist1 (Open Biosystems) cDNAs were digested and sub-cloned into MIEG3-EGFP or MIEG3-hCD4. Flag-tagged Twist1 (18) and Flag-tagged Twist1cc pCDNA3.1 that was made using QuikChange Site-Directed Mutagenesis Kit (Stratagene) with primer pair Forward 5′-TCAGCTACGCCTTCCCCGTCTGGAGGATG-3′ and Reverse 5′-CATCCTCCAGACGGGGAAGGCGTAGCTGA-3′, were digested, and subcloned into MIEG3-EGFP. Runx3 cDNA (Open Biosystems) was amplified, digested and sub-cloned into MSCV-Thy1.1. Twist1-targeting shRNA oligo was designed as described (12) and introduced into RNAi-Ready pSIREN-RetroQ-ZsGreen according to the manufacture manual (Clonetech). Retroviral stocks were prepared by calcium phosphate transfection of Phoenix GP cells. The medium was replaced after 12 h, and viral supernatants were collected after 24 h and 48 h later. Purified CD4+ T cells were cultured under Th1 cell differentiation condition. On day 2, cells were transduced with retrovirus.. expressing vector control or gene of interest by centrifugation at 2000 rpm at 25°C for 1 h in the presence of 8 μg/ml polybrene. Viral supernatant was replaced with the former culture supernatant supplemented with 50 U/ml human IL-2. After spin infection, cells were expanded on day 3 and analyzed on day 5.
Cell sorting, analysis of gene expression, and flow cytometry
Transduced cells were collected on day 5, stained with anti-human CD4 Alexa Fluor 647 (BD) and anti-rat CD90/mouse CD90.1 FITC Abs (Biolegend), and sorted using a FACSVantageSE cell sorter (BD Biosciences). Sorted cells were rested or re-stimulated with 2ug/ml anti-CD3 for RT-qPCR (6 h) and ELISA (24 h). Quantitative RT-PCR and ELISA were performed as previously described (17). For surface staining, resting T cells were stained with anti-IL12Rβ2-PE (R&D) or anti-IL-18Rα-Alexa Fluor 647 (Biolegend) and fixed with 2% paraformaldehyde for 10min before analysis. For phospho-STAT4 and T-bet analyses, cells were fixed, permeabilized using 100% ice cold methanol, and stained for anti-phospho-Stat4-PE (BD Pharmingen) and anti-T-bet Alexa Fluor 647 (Biolegend) before analysis.
Immunoblot, Immunoprecipitation and DNA affinity precipitation assay
Whole-cell protein lysates were extracted from sorted T cells and immunoblotted with Runx3 (6821C3a), Stat4 (C-40), Twist1 (Twist2C1a), T-bet (4B10) or β-Actin (C4) (Santa Cruz) as a control. For immunoprecipitation, whole-cell protein lysates were generated from Th1 cells, or 293T cells transfected with constructs expressing Twist1, Runx3, T-bet, Flag-tagged Twist1, or Flag-tagged- Twist1cc using calcium phosphate. Whole-cell protein lysates were pre-cleared with Protein A agarose (Thermo Scientific), before immunoprecipitation of equal amounts of protein with Runx3, T-bet (Santa Cruz) or Flag (M2, Sigma Aldrich) Abs overnight at 4°C. Immunocomplexes were captured by Protein A agarose and eluted at 100°C for 5 min in Laemmli’s sample buffer. Immunoprecipitates were separated by 10% SDS-PAGE and immunoblotted with Twist1, Runx3 and T-bet Abs. DAPA assay was performed as described (19). Oligonucleotides containing a Twist1 binding site were described previously (20). Additional oligonucleotides (biotinylated at 5′) containing wild type or mutated (bold) Runx3 and T-bet binding sites (underline) are: wild type Runx3 and T-bet 5′-ACCTATGTGGTCTGCCTTTTCTTCTTTCTGGGCACGTTGA-3′, mutant Runx3 5′-ACCTAAGAGGACTGCCTTTTCTTCTTTCTGGGCACGTTGA-3′, mutant T-bet 5′-ACCTATGTGGTCTGCCTTTTCTTCTTTCTGGGCTCGTTGA-3′, and mutant Runx3-T-bet 5′-ACCTAAGAGGACTGCCTTTTCTTCTTTCTGGGCTCGTTGA-3′. Biotinylated oligonucleotides were incubated with streptavidin-agarose beads for 30min at 4°C. The complex was washed with pull-down buffer (25mM HEPES, 15mM NaCl, 0.5mM DTT0.5% NP-40, 0.1mM EDTA pH 7.5, 10% glycerol). Nuclear extracts were added and incubated at 4°C for 2 h. The complex was washed with pull-down buffer, eluted, and separated with 10% SDS-PAGE.
Chromatin Immunoprecipitation (ChIP)
ChIP assay was performed as described (1). In brief, resting Th1 cells were cross-linked for 10 min with 1% formaldehyde and lysed by sonication. After pre-clearing with salmon sperm DNA, bovine serum albumin, and Protein A agarose bead slurry (50%), cell extracts were incubated with either rabbit polyclonal T-bet (4B10), PEBP2β (FL-182) (Santa Cruz) or normal rabbit IgG (12–370, Milipore) overnight at 4°C. The immunocomplexes were precipitated with protein A agarose beads at 4°C for 2 h, washed, eluted, and reversed cross-links at 65°C overnight. DNA was purified, resuspended in H20 and analyzed by quantitative PCR with Taqman or SYBR primers as previously described (19, 21, 22)
Chromosome conformation capture assay (3C)
3C assay was performed as described (23–25) with some modifications. 107 cells were cross-linked with 2% formaldehyde for 10 min at room temperature and quenched with 0.125M Glycine for 5 min. Cells were lysed with ice-cold lysis buffer (10mM Tris-HCl pH 8, 10mM NaCl, 0.2% NP-40, Protease inhibitor cocktail) for 30 min. Nuclei were resuspended in 0.5 ml of restriction enzyme buffer (NEB3) containing 0.3% SDS and shaken for 1h at 37°C. Triton X-100 (final concentration 1.8%) was added and shaken for 1h at 37°C to sequester the SDS. Crosslinked DNA was digested overnight with 400–800U BglII containing 1mM ATP that has been shown to enhance digestion efficiency (26). Enzyme was inactivated by addition of SDS (final concentration 1.6%) and samples were shaken for 20min at 65°C. The reaction was diluted with 7ml of ligation buffer (50mM Tris-HCl pH 8, 10mM MgCl2, 10mM DTT, 1mM ATP, 1mg/ml BSA), and Triton X-100 (final concentration 1%) was added and shaken for 1h at 37°C. DNA fragments were ligated with 4000U T4 ligase (NEB) for 4 h at 16°C followed by 40 min at room temperature. Crosslinks were reversed by incubation with 300ug proteinase K overnight at 65°C. The samples were further incubated with 300ug RNase for 30–45min at 37°C, and the DNA were purified by phenol-chloroform extraction and ethanol precipitation. Ligation products were quantified by quantitative TaqMan real-time PCR using primers as described (23, 25). Additional primers and Taqman probes with the location in kb from Ifng transcription start site in the parenthesis are as follows: (+35) 5′-GGAGACACAGAAGTTCGAAGTTAGAA-3′, (+46) 5′-AAAAACCAACCTGTGTTATTTTG-3′, (−35) 6FAM-CCTCTGCAAGCCTCACAGAGCA-TAMRA, and (+51) 6FAM-CCATCTACTGCAAAAAGAAGCT-TAMRA. To generate control templates for the positive controls and to correct for differences in ligation and PCR efficiency between different templates, BAC clones were used to generate control template containing all possible ligation products. Equimolar amounts of three BAC clones spanning the mouse Ifng locus (RP23-353P23, RP23-138P22, and RP23-55O21) and BAC spanning the mouse Gapdh locus (RP23-410F11) from CHORI were mixed, digested, phenol chloroform extracted and ethanol precipitated, and ligated at a DNA concentration of 300ng/ul. This sample was used as the DNA reference standard. Relative crosslinking frequencies between the analyzed pairs were calculated as described (23) and were normalized to control interaction frequencies using primer pairs within the Gapdh locus.
Results
Twist1 is induced by Th1 cell activation
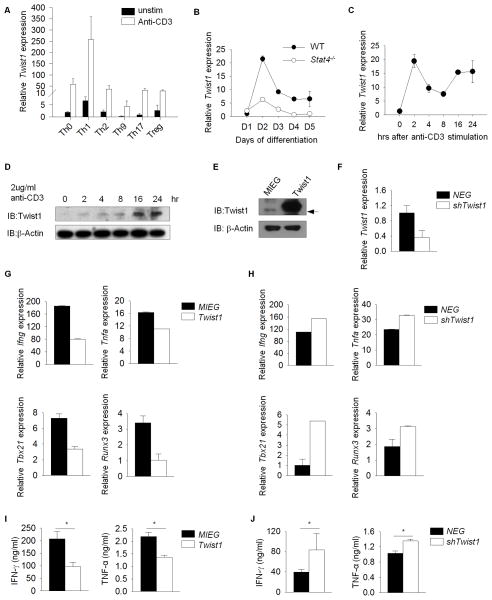
Twist1 regulates Th1 cytokine production through an unidentified mechanism (12). To begin to define that mechanism, we determined the expression of Twist1 in T cell subsets. Twist1 mRNA expression was highest in resting and activated Th1 cells, compared to other T cell subsets (Figure 1A). Previous reports identified Twist1 as a STAT4 target gene (1, 12). Although Twist1 mRNA expression was detected during differentiation, expression was lower in Stat4−/− than wild type Th1 cells (Figure 1B). Stimulation of Th1 cells with anti-CD3 resulted in the induction of Twist1 mRNA and protein (Figure 1C, D). Thus, Twist1 is expressed in the greatest amounts in Th1 cells compared to other T cell subsets and is induced by TCR stimulation.
Figure 1. Twist1 negatively regulates Th1 gene expression and cytokine production.
A, Naive wild-type (WT) lymphocytes were differentiated under neutral conditions (Th0) or Th1 (IL-12 + anti-IL-4), Th2 (IL-4 + anti-IFN-γ), Th9 (IL-4 + TGF-β), Th17 (IL-6 + TGF-β) or Treg (TGF-β)-polarizing conditions in vitro. Twist1 mRNA expression in resting or anti-CD3 activated Th1 cells was determined by RT-qPCR. Data represent mean ± S.D. of 3 mice; and representative of two independent experiments. B, Kinetics of Twist1 mRNA expression during Th1 differentiation in WT and Stat4−/− cells. Average of replicate samples ± S.D. (C–D) Kinetics of Twist1 mRNA expression (C) and Twist1 protein (D) in Th1 cells after stimulation with 2ug/ml anti-CD3 for 2, 4, 8, 16 and 24 h. Results are representative of two to three independent experiments with similar results. (E–J) Naïve WT T cells were stimulated under Th1-polarizing condition. On day 2, cells were transduced with control EGFP (MIEG) or retroviral vector expressing Twist1 and EGFP (Twist1)(E, G, I), or control or short hairpin Twist1 (shTwist1)(F, H, J). On day 5, GFP+ cells were sorted for analysis. (E–F) Altered Twist1 protein expression in cells transduced with Twist1 (E) and altered Twist1 expression in shRNA transduced Th1 cells (F). (G–H) Th1 gene expression in ectopic Twist1- (G) or short hairpin- (H) transduced cells was assessed by RT-qPCR before (Tbx21 and Runx3) or after (Ifng and Tnfa) 6 h re-stimulation with 2ug/ml anti-CD3. (I, J) Sorted GFP+ cells were stimulated with 2ug/ml anti-CD3 for 24 h, supernatants were collected and analyzed for IFN-γ and TNF-α by ELISA. Data are (I) mean ± S.D. of three independent experiments or (J) mean of replicate samples ± S.D. and representative of three independent experiments with similar results. *p<0.05
Twist1 negatively regulates the Th1 transcription factor network
To define how Twist1 regulates Th1 cell function, Twist1 was ectopically expressed or targeted by shRNA in Th1 cells using retroviral transduction (Figure 1E–F). In agreement with a previous report (12), ectopic Twist1 expression in Th1 cells reduced IFN-γ and TNF-α mRNA and protein levels, and decreasing Twist1 expression resulted in increased IFN-γ and TNF-α production (Figure 1G–J). The difference in IFN-γ and TNF-α production in Th1 cells was not due to altered expression of other cytokines, including Il4 and Il17a (data not shown). Coincident with decreased cytokine production, the expression of Th1-related transcription factors such as T-bet, Hlx1 and Runx3 were decreased upon ectopic Twist1 expression and increased upon Twist1 shRNA transduction (Figure 1G–H and data not shown).
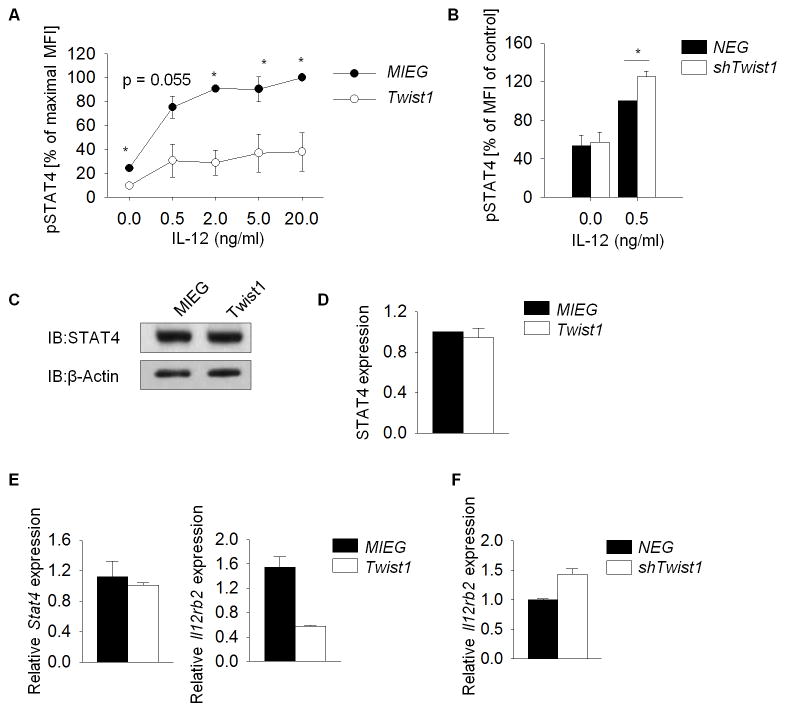
Since STAT4 is required for the expression of many Th1-specific genes (1), we examined whether Twist1 was a component in a feedback mechanism to control STAT4 activation. Phospho-STAT4, assessed by flow cytometry, was lower in Th1 cells transduced with Twist1-expressing retrovirus compared to vector control, following re-stimulation with increasing doses of IL-12 (Figure 2A). Decreased induction of pSTAT4 correlated with reduction in IL-18rα expression, a STAT4-target gene (data not shown) (19, 27). Consistent with this result, reduced Twist1 expression results in increased IL-12-induced STAT4 phosphorylation (Figure 2B). We next examined the expression of genes that contribute to STAT4 activation. STAT4 mRNA and protein expression, and mRNA of suppressors of cytokine signaling (SOCS) and protein tyrosine phosphatase-Basophil like (PTP-BL) that negatively regulate STAT4 activation (28, 29) were not altered. However, Il12rb2 mRNA expression was decreased approximately 50% by ectopic Twist1 expression compared to vector control (Figure 2C–E and data not shown). In parallel, transduction of Th1 cells with retroviral Twist1 shRNA resulted in increased Il12rb2 expression (Figure 2F).
Figure 2. Twist1 regulates Il12rb2 expression and STAT4 activation.
A, Naïve WT T cells were stimulated and transduced as described in Figure 1. Transduced Th1 cells were stimulated with IL-12 for 4 h and GFP+ cells were analyzed for phosphorylated STAT4 (pSTAT4). B, Phospho-STAT4 were determined in control or shRNA transduced Th1 cells following sorting for GFP expression. (C–D) Total cell extracts were immunoblotted for STAT4 and β-Actin as a control (C), and densitometry analysis of STAT4 protein is indicated (D). (E–F) Stat4 and Il12rb2 gene expression in Twist1 (E) or shTwist1 (F) transduced Th1 cells sorted for GFP expression. Data are mean of three to four independent experiments ± S.D. (A, B) or the average of replicate samples ± S.D. and representative of two to three independent experiments with similar results (C–F). *p<0.05
Runx3 rescues the inhibitory effect of Twist1 independent of T-bet and STAT4
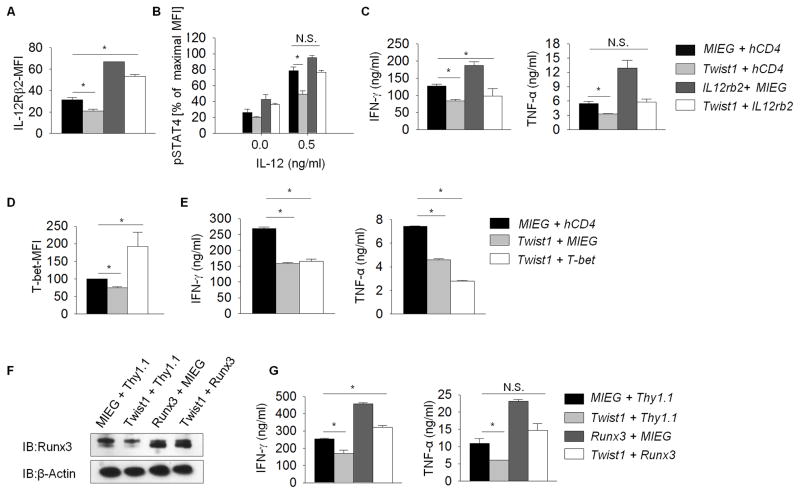
Since Twist1 regulates multiple genes in Th1 cells, we wanted to determine if a single factor could rescue the inhibitory effect of Twist1. Initially, we asked whether ectopic expression of IL-12Rβ2 could compensate for the repressive effect of Twist1 by co-transducing Th1 cells using transduction of both IL-12Rβ2 and Twist1. IL-12Rβ2 surface expression was decreased in cells with ectopic Twist1 expression but was higher in double transduced cells, compared to vector control (Figure 3A). Recovery of IL-12Rβ2 expression resulted in increased IL-12-induced phospho-STAT4 (Figure 3B). Despite recovery of T-bet expression and phospho-STAT4 (Figure 3B and data not shown), ectopic IL-12Rβ2 expression was unable to rescue IFN-γ production, although there was recovery of TNF-α production (Figure 3C). Co-expression of T-bet and Twist1 resulted in higher T-bet expression compared to vector control but failed to recover IFN-γ and TNF-α production (Figure 3D, E). Thus, although recovery of IL-12 signaling was able to induce TNF-α in the presence of Twist1, neither recovery of IL-12 signaling nor T-bet expression was able to compensate for the effects of Twist1 on IFN-γ production.
Figure 3. Ectopic Runx3 expression compensates for the repressive activity of Twist1 in Th1 cells.
Naïve WT T cells were stimulated under Th1-polarizing conditions. On day 2, cells were transduced with control retrovirus vectors or (A–C) retroviral vector expressing Twist1-GFP and IL-12Rβ2-hCD4 (D, E) Twist1-hCD4 and T-bet-GFP (F, G) Twist1-GFP and Runx3-Thy1.1. Th1 cells were stained for IL-12Rβ2 by surface staining (A) or phospho-STAT4 with or without IL-12 stimulation by ICS (B), or T-bet by ICS (D). Analysis was performed by gating on doubly transduced cells. (E, G) Double positive cells were sorted and re-stimulated with 2ug/ml anti-CD3 for 24 h. Supernatants were collected before IFN-γ and TNF-α production was measured by ELISA. F, Whole cell lysates were extracted from sorted double positive Th1 cells and were immunoblotted for Runx3 and β-Actin as a control. Data are mean of three to four independent experiments ± S.D (A, B) or averages of replicate samples ± S.D. and representative of two to three independent experiments with similar results (C–G). *p<0.05
Since Runx3 regulates IFN-γ independently of T-bet and STAT4 (4), we hypothesized that regulation of Runx3 by Twist1 might be a critical target. Ectopic Twist1 expression resulted in decreased Runx3, and retroviral expression of Runx3 resulted in higher Runx3 level in double transduced cells compared to control cells (Figure 3F). IFN-γ and TNF-α production in Runx3/Twist1-transduced cells was comparable to that of control transduced cells (Figure 3G). The recovery of IFN-γ and TNF-α production by Runx3 in Th1 cells was independent of T-bet and STAT4 since there were no recovery of T-bet expression or phospho-STAT4-positive cells (data not shown). Thus, although Twist1 can negatively regulate many Th1 genes, the recovery of T-bet and IL-12Rβ2 expression (and as a consequence, STAT4 phosphorylation) did not compensate for the effects of Twist1. These results suggested that Runx3, or a Runx3 induced-gene, is at least one of the important Twist1 targets in the regulation of IFN-γ.
Twist1 exists in a complex with Runx3 or T-bet
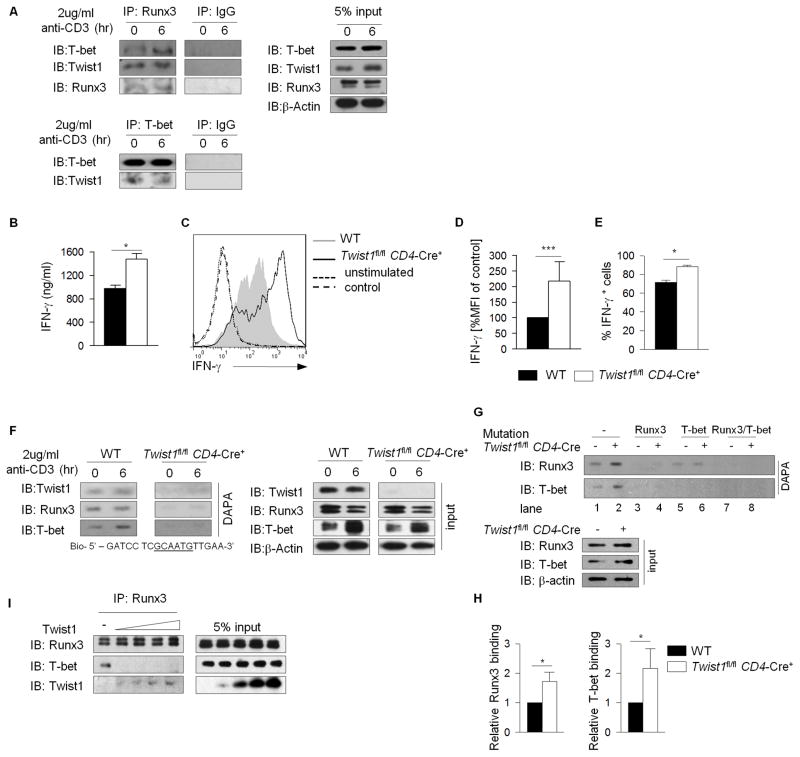
Twist1 inhibits osteoblast differentiation by interacting with the Runt domain of Runx2, a highly conserved domain shared with related proteins including Runx3 (15). We hypothesized that Twist1 physically interacts with Runx3 and inhibits its regulatory function. Using immunoprecipitation of extracts from resting and early activated Th1 cells we observed that Twist1 co-purified with Runx3, and confirmed the association between Runx3 and T-bet in Th1 cells (3). We also found an association between T-bet and Twist1 following precipitation with T-bet antibody (Figure 4A).
Figure 4. Twist1 physically interacts with Runx3 and T-bet.
A, Whole cell lysates were extracted from activated WT Th1 cells. Immunoblots were performed for the indicated proteins following immunoprecipitation with Runx3- or T-bet-or control antibodies as indicated. Input for immunoprecipitation is indicated on the right. (B–E) Naïve CD4+CD62Lhi WT or Twist1fl/fl CD4-Cre+ T cells were cultured under Th1-polarizing conditions. IFN-γ production was measured by ELISA (B) after reactivating with 2ug/ml anti-CD3 for 24h or ICS (C–E) after reactivating with PMA/IONO for 6 h. (DE) Graphs indicate the mean fluorescence intensity (D) or average percent IFNγ-positive cells (E) of Th1 cells from mice of the indicated genotype. (F–H) Nuclear extracts from activated (F) or resting (G) WT and Twist1fl/fl CD4-Cre+ Th1 cells were incubated with biotinylated oligonucleotides containing Twist1-specific binding site (F) or Runx3-T-bet specific binding sites (wild type or mutant as indicated) (G). H, Immunoblots of precipitated proteins, with densitometry measurements for (G). I, Mixture of whole cell lysates collected from 293T cells transfected with constructs expressing Runx3, T-bet or Twist1 were incubated at 4°C overnight. Immunoblots indicate proteins immunoprecipitated with Runx3. Results are average ± S.E.M (B) or ± S.D. (D, E, H) of three independent experiments, *p<0.05, ***p<0.001, or representative of three or more independent experiments with similar results (A, C, F, I).
To examine the function of Runx3 and T-bet in the absence of Twist1, Twist1fl/fl mice (14) were mated with mice carrying a CD4-Cre transgene to generate mice with Twist1-deficient T cells (Twist1fl/fl CD4-Cre+). We confirmed the absence of Twist1 expression in Twist1-deficient Th1 cells (Figure S1). Twist1-deficiency did not alter normal lymphocyte development in thymus, spleen and lymph nodes (Figure S1). Twist1-deficient Th1 cells produced significantly greater amounts of IFN-γ on a per cell basis and in frequency of IFN-γ-positive cells compared to wild type cells (Figure 4B–E). We also confirmed the increase in gene expression of Tbx21, Runx3, and Il12rb2 in Twist1-deficient Th1 cells, compared to WT Th1 cells. There was no significant difference in Eomes expression between WT and Twist1-deficient Th1 cells (data not shown).
We next tested the effect of Twist1 on Runx3 and T-bet DNA binding by DNA affinity precipitation assay using extracts from Twist1-deficient mice and littermate control (WT) activated Th1 cells. Extracts from WT and Twist1-deficient Th1 cells were incubated with biotinylated oligonucleotides containing a Twist1-binding sequence. Although T-bet and Runx3 were precipitated with DNA-bound Twist1 in WT cell extracts, T-bet and Runx3 were absent from precipitates of Twist1-deficient extracts, further supporting the interaction of these transcription factors and suggesting that interactions can occur when Twist1 is bound to DNA (Figure 4F). We observed that Runx3 and Tbx21 mRNA expression increased in Twist1-deficient Th1 cells compared to WT cells, although there was a modest increase at protein level, suggesting the importance of protein complex formation in the function of Twist1. Since there is an association between Twist1, Runx3, and T-bet, we tested if, in the absence of Twist1, there would be greater binding of Runx3 and T-bet to the oligonucleotides containing Runx3 and T-bet specific binding sites. In the absence of Twist1, we detected greater binding of Runx3 and T-bet to the respective oligonucleotides compared to WT samples (Figure 4G, H, lane 2 vs. 1). When the binding sites of Runx3, T-bet or both were mutated, the binding of Runx3 and T-bet was diminished, regardless of Twist1 expression (Figure 4G). We then tested if Twist1 mediates its inhibitory effect in Th1 cells by interfering with the T-bet-Runx3 interaction. Using Runx3 immunoprecipitation, we detected a Runx3 and T-bet interaction in the absence of Twist1 (first lane), although this interaction was diminished in the presence of Twist1 (Figure 4I). These results suggested that the decrease in IFN-γ production in Th1 cells might not only be due to the reduction in Runx3 gene expression but also due to diminished association and DNA binding activity of Runx3 and T-bet.
Twist1 interferes with the binding of Runx3 and T-bet to the Ifng locus
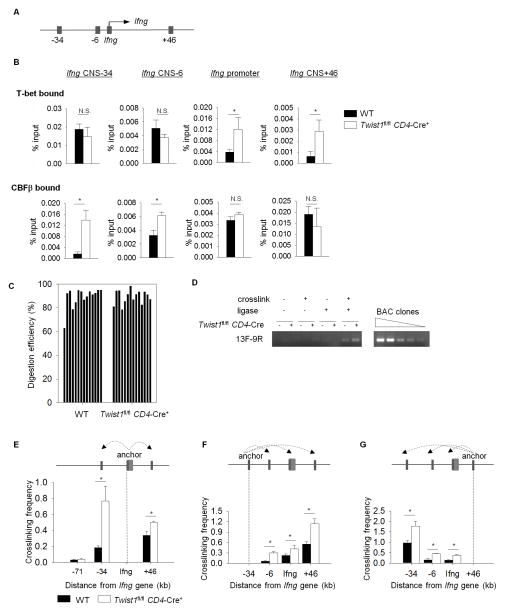
Since Twist1 interferes with T-bet and Runx3 DNA binding, and we observed only modest binding of Twist1 to the Tbx21, Ifng or other Th1 gene promoters (data not shown), we hypothesized that the interaction of Twist1 with Th1 transcription factors decreased their binding to target genes including Ifng. T-bet and Runx3 regulate Ifng gene expression by binding to several conserved non-coding sequences (CNS) (4). To examine T-bet and Runx3 binding in the absence of Twist1, we performed chromatin Immunoprecipitation (ChIP) assay using wild type and Twist1-deficient Th1 cells and examined the binding at previously documented regions including CNS-34, CNS-6, Ifng promoter, and CNS+46 of the Ifng gene (Figure 5A)(4, 22), using ChIP for T-bet or CBF-β, the binding partner of Runx3 (4). Our data showed that more T-bet was bound in Twist1-deficient Th1 cells than in wild type cells at the Ifng promoter and CNS+46 while there were no difference at CNS-34 and CNS-6 (Figure 5B). In contrast, more CBF-β was bound at CNS-34 and CNS-6 in Twist1-deficient Th1 cells compared to littermate controls (Figure 5B). This result suggested that Twist1 interferes with T-bet and CBF-β-Runx3 complex binding at specific regulatory regions of Ifng gene. Since T-bet and Runx3 bind to many CNS regions of the Ifng locus, and the association between T-bet and Runx3 is required for optimal Ifng expression (3, 4), it is likely that Runx3 binding to the Ifng locus enhances T-bet binding to regulatory elements. This is consistent with the recovery of IFN-γ production in the Runx3 transduction experiment (Figure 3G).
Figure 5. Twist1 interferes with transcription factor binding and chromatin conformation at the Ifng locus in Th1 cells.
A, Representation of Ifng locus indicating conserved non-coding sequences (CNS) used for analysis. (B–G) Naïve CD4+CD62hi T cells from WT and Twist1fl/fl CD4-Cre+ were stimulated under Th1-polarizing conditions. B, ChIP assays were performed on Th1 cells using T-bet and CBFβ antibodies. C, Th1 cells were fixed and digested with BglII enzyme. Undigested and digested samples were subjected for qPCR using primer pairs spanning the restriction sites. %Digestion was calculated using the formula: 100 – 100/2((CtR – CtC)D – (CtR – CtC) UND); D: digested, UND: undigested, R: restriction site, C: internal control. D, Th1 cells as described above were fixed (crosslinked) and/or ligased as indicated. qPCR was performed using primer pair 13F-9R. PCR products were run on 2% agarose gel. BAC clones were titrated for qPCR and used as control. (E–G), 3C assay showing the relative cross-linking frequencies between the Ifng promoter (E), CNS-34 (F) or CNS+46 (G) as the fixed anchor fragments and other BglII fragments containing the indicated CNS regions. Results are the average ± S.D. of replicated samples and are representative of four independent experiments with similar results. *p<0.05
The effect of Twist1 on T-bet and CBF-β-Runx3 binding at distinct elements suggested that interfering with T-bet-Runx3 interactions might also alter chromatin looping at the Ifng locus (24, 25). To determine whether Twist1 interferes with chromatin looping at the Ifng locus, a chromosome conformation capture (3C) assay was performed with wild type and Twist1-deficient Th1 cells that examined the interactions among Ifng CNS regions using an established assay (23, 25)(Figure 5C, D). Using three different anchor points, our results showed increased crosslinking frequency between CNS-34, CNS+46, and the Ifng promoter, and increased crosslinking of CNS-6 with CNS-34 and CNS+46 (Figure 5E–G). The distance between the Ifng promoter and CNS-6 is too short to provide consistent results in this assay. Crosslinking of the CNS region at −71 was not altered. Together, these results suggest that Twist1 regulates Ifng expression by altering the binding of the Th1 transcription factors T-bet and Runx3, and altering the conformation of the Ifng locus.
Twist1-Runx3 interactions are required to regulate Ifng
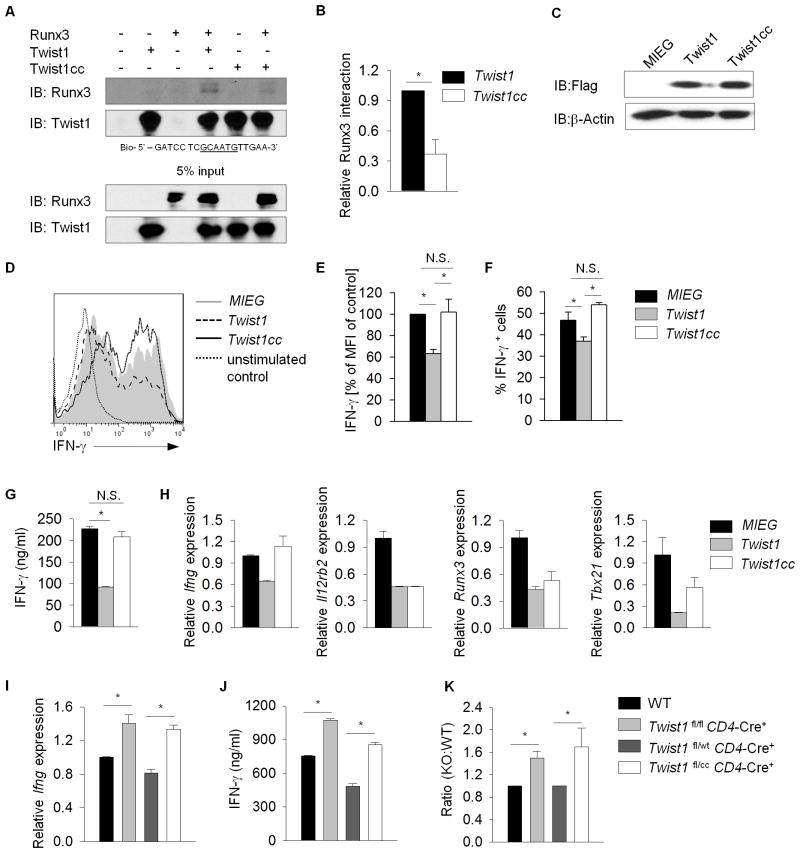
A mutation in Twist1 at amino acid 192 from Serine to Proline (Twist1cc) results in diminished interaction between Twist1 and Runx2 (15). Thus we wanted to examine whether Twist1 S192P (Twist1cc) could interact with Runx3. DAPA using extracts from cells transfected with Runx3, WT Twist1 (Twist1), and Twist1cc expressing vectors that were incubated with biotinylated oligonucleotides containing a Twist1-binding sequence demonstrated decreased interaction between Runx3 and Twist1cc (Figure 6A, B). The mutation in Twist1cc did not affect the association of Twist1 with T-bet (data not shown).
Figure 6. The Twist1-Runx3 interaction is required for regulation of Ifng.
(A, B) Nuclear extracts from 293T cells co-transfected with either Flag-tagged Twist1 or Flag-tagged Twist1cc and with or without Runx3 were incubated with biotinylated oligonucleotides containing Twist1-specifc sequence. Immunoblot demonstrates protein expression of Twist1 and Runx3 (A) with densitometry measurements (B). (C–H) Naïve CD4+CD62hi T cells from WT were cultured under Th1-polarizing conditions and were infected with retrovirus expressing Twist1 or Twist1cc. C, Twist1 protein was assessed in transduced Th1 cells using immunoblot, with β-actin as a control. (D–G) IFN-γ production was assessed by intracellular staining and gating on GFP+ populations following 6h of activation with anti-CD3 (D–F) or by ELISA following 24 h stimulation with anti-CD3 of sorted GFP+ populations (G). (E–F) Graphs indicate the mean fluorescence intensity (E) or average percent IFNγ-positive cells (F) of each transduced population. H, Th1 gene expression in ectopic Twist1 or Twist1cc expression was assessed by RT-qPCR before (Il12rb2, Runx3 and Tbx21) or after (Ifng) 6 h re-stimulation with anti-CD3. (I–K), Naïve CD4+CD62hi T cells from mice of the indicated genotypes were cultured under Th1-polarizing conditions. Th1 cells were stimulated with anti-CD3 for 6 h or 24 h respectively for testing Ifng expression by RT-qPCR (I) or IFN-γ production by ELISA with the ratio of production from Twist1-deficient or Twist1 mutant Th1 cells to the respective controls (J, K). Data are mean of three to four independent experiments ± S.D, *p<0.05 (A–F), or are mean of replicate samples ± S.D. and representative of two independent experiments with similar results (G–K). *p<0.05
The mutant Twist1 provided a tool to mechanistically distinguish the effects of Twist1 that rely on interactions with Runx3 at the protein level, versus effects independent of Runx3 binding. To test the effects of mutant Twist1, a retrovirus expressing Twist1cc was introduced into Th1 cells, and cytokine production and gene expression were analyzed. Introduction of Twist1cc did not repress IFN-γ production in Th1 cells compared to vector control and wild type Twist1-transduced cells (Figure 6D–H). Importantly, Twist1cc was able to repress expression of several Th1 genes including Il12rb2, Runx3 and Tbx21 as effectively as wild type Twist1 (Figure 6H). To further define the function of Twist1cc in Th1 cells, we utilized a mouse mutant strain termed Charlie Chaplin (Twist1cc/wt) that encodes Twist1 S192P and results in hindlimb polydactyly (15, 30). We mated Twist1cc/wt with Twist1fl/fl CD4-Cre+ mice generating Twist1fl/wt CD4-Cre+ and Twist1fl/cc CD4-Cre+ (Twist1fl/cc) mice that have T cells expressing one wild type or one mutated allele of Twist1, respectively. IFN-γ production in Twist1fl/cc Th1 cells was increased compared to control cells (Figure 6I–K). These results demonstrate although Twist1cc retains some repressive function in Th1 cells independent of Runx3, Twist1 control of IFN-γ production is primarily through association with Runx3 (Figure 7).
Figure 7. Transcriptional regulatory network in Th1 cells.
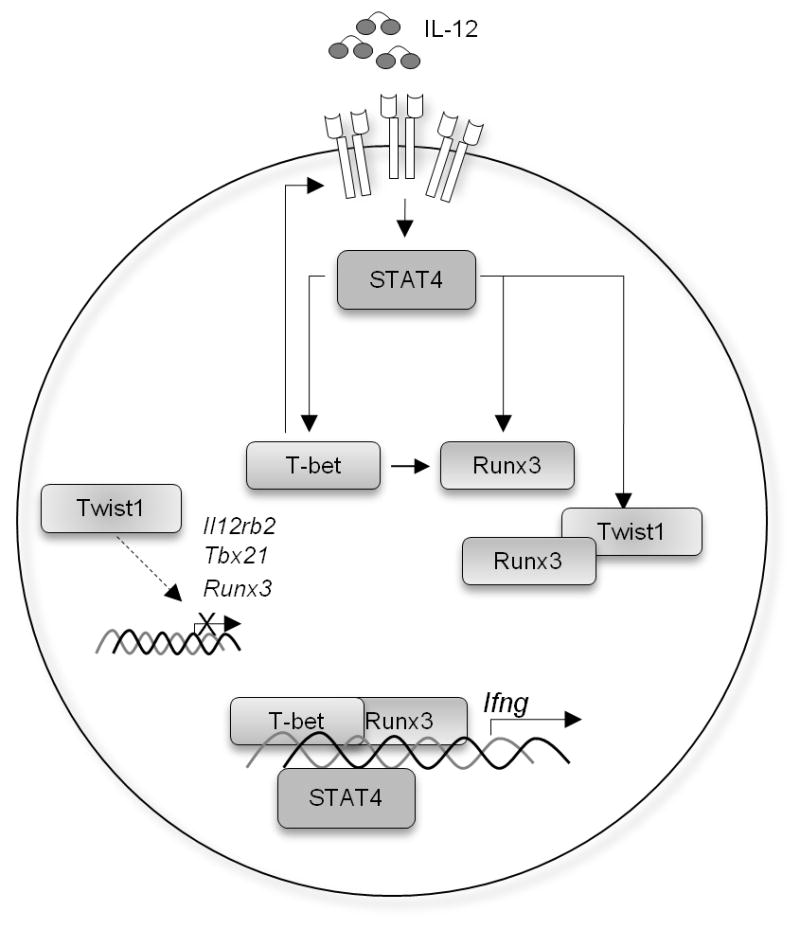
IL-12-IL-12Rβ2-STAT4 induces T-bet expression that positively regulates IL-12Rβ2 expression. Both STAT4 and T-bet are required for the induction of Runx3 that contribute to the optimal IFN-γ production. Twist1, a transcription repressor that is induced by IL-12-STAT4, suppresses IFN-γ production by two mechanisms: the repression of Il12rb2, Tbx21, and Runx3 expression potentially by directly binding to the conserved motif E-box (CANNTG) in gene regulatory regions, and the interference with the function of Runx3 through physical interaction. The latter mechanism is critical for regulation of Ifng since complementation of Il12rb2 nor Tbx21 expression did not rescue IFN-γ production.
Discussion
Twist1 is expressed in Th1 effector memory cells and limits Th1-mediated inflammation in mouse models of delayed-type hypersensitivity and antigen-induced arthritis (12). In this report, we have identified a mechanism by which Twist1 modulates inflammatory cytokine production in Th1 cells. We showed that Twist1 negatively regulates transcription factors including T-bet, STAT4 and Runx3 leading to decreased IFN-γ production in Th1 cells. The formation of the Twist1-Runx3 complex is required for Twist1 to decrease IFN-γ production. In the absence of Twist1, there is increased transcription factor binding and increased looping at the Ifng locus resulting in increased IFN-γ production. Thus, Twist1 impairs the activity of the Th1 transcription factor network.
Our data suggest that Twist1 regulates Th1 gene expression through several mechanisms. First, it negatively regulates the expression of Th1 genes. In macrophages, Twist1 has been shown to regulate TNF-α and IL-1β by binding to E-boxes in gene promoters (15). However, we did not observe Twist1 binding to the promoters of Ifng, Tnfa, or Il12rb2, and only minimal binding to the promoter of Tbx21. It is still possible that Twist1 binds directly to Th1 genes in regions other than the promoter that we tested. The second mechanism of Twist1-dependent gene regulation is through complex formation with Runx3. This mechanism appears to be the primary mechanism for regulating Ifng. Yet, the Twist1 S192P mutant that reduced interactions with Runx3 was still able to transcriptionally regulate other Th1 genes, suggesting that Twist1 has gene regulatory activity independent of Runx3. The direct regulation of genes other than Ifng might further impact the Th1 phenotype and further analysis will be required to further refine this regulatory network.
In this report, we showed that Twist1 is expressed highest in resting Th1 cells compared to other T cell subsets, although its expression increased in all T cell subsets upon TCR stimulation. During differentiation, Twist1 expression peaked at day 2 in both Th1 and Th2 and gradually decreased, though Twist1 expression was lower in Th2 cells than Th1 cells after day 3. Thus Twist1 might be a negative regulator of Th1 cytokine production in other T cell subsets.
Although Twist1 is a STAT4 target in Th1 cells, it might be targeted by other STATs in other T helper lineages. In breast cancer cell lines, STAT3 binds directly to the Twist1 promoter, and amounts of phospho-STAT3 correlate with expression of Twist1 (31). Since STAT3 has a critical role in Th2 and Th17 differentiation, and STAT3-deficient Th2 cultures show increased IFN-γ production compared to wild-type cultures, Twist1 might play a role in regulating cytokine production in these lineages as well (32–35). Twist2 promotes IL-10 production by activating the transcription factor c-Maf in myeloid cells, and since c-maf is an important transcription factor for Th2 and Th17 cells Twist1 might play an analogous role in these cells (36, 37). The function of Twist1 in other Th subsets will be the focus of further study.
Twist1 requires dimerization to bind DNA (38). In Drosophila, dimerization partners determine the activity of Twist1 (39). Twist1 homodimers have opposing effects on mesoderm gene expression as heterodimers of Twist1 and the Drosophila E protein homologue, Daughterless (39). The Daughterless gene has 79% identity to E12 and E47, two alternative splice products of E2a gene (40, 41). Th1 cells differentiated from E2a-deficient mice showed a decrease in IFN-γ production compared to WT cells (42). It is possible that E12/E47 sequesters Twist1 in heterodimer complexes to inhibit its repressive function in Th1 cells. In the absence of E2A gene products, there is more “free” Twist1 to form homodimers and thus inhibit cytokine production. E47 and the HLH inhibitor Id3 regulate the balance between Treg and Th17 cell differentiation (43). The balance among Twist1, Id, and E proteins is likely important in defining Twist1 activity as Id proteins interacting and titrating E proteins would favor the formation of Twist1 homodimers (44, 45). Understanding how this balance is regulated in T helper cells will lead to greater insight into the networks of transcription factors that regulate differentiation.
Supplementary Material
Acknowledgments
This work was supported by Public Health Service grants AI045515 to MHK and AR061392 and HL061677 to ABF. DP was supported by T32 HL007910.
The authors thank A.L. Dent and L.D. Mayo for critical reading of this manuscript. We thank R. Berringer for providing Twist1fl/fl mice, and T. Usui for plasmid.
References
- 1.Thieu VT, Yu Q, Chang HC, Yeh N, Nguyen ET, Sehra S, Kaplan MH. Signal transducer and activator of transcription 4 is required for the transcription factor T-bet to promote T helper 1 cell-fate determination. Immunity. 2008;29:679–690. doi: 10.1016/j.immuni.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schulz EG, Mariani L, Radbruch A, Hofer T. Sequential polarization and imprinting of type 1 T helper lymphocytes by interferon-gamma and interleukin-12. Immunity. 2009;30:673–683. doi: 10.1016/j.immuni.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Djuretic IM, Levanon D, Negreanu V, Groner Y, Rao A, Ansel KM. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nat Immunol. 2007;8:145–153. doi: 10.1038/ni1424. [DOI] [PubMed] [Google Scholar]
- 4.Yagi R, I, Junttila S, Wei G, Urban JF, Jr, Zhao K, Paul WE, Zhu J. The transcription factor GATA3 actively represses RUNX3 protein-regulated production of interferon-gamma. Immunity. 2010;32:507–517. doi: 10.1016/j.immuni.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005;307:430–433. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]
- 6.Ouyang W, Ranganath SH, Weindel K, Bhattacharya D, Murphy TL, Sha WC, Murphy KM. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9:745–755. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- 7.Yagi R, Zhu J, Paul WE. An updated view on transcription factor GATA3-mediated regulation of Th1 and Th2 cell differentiation. Int Immunol. 2011;23:415–420. doi: 10.1093/intimm/dxr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnes RM, Firulli AB. A twist of insight - the role of Twist-family bHLH factors in development. Int J Dev Biol. 2009;53:909–924. doi: 10.1387/ijdb.082747rb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Sosic D, Richardson JA, Yu K, Ornitz DM, Olson EN. Twist regulates cytokine gene expression through a negative feedback loop that represses NF-kappaB activity. Cell. 2003;112:169–180. doi: 10.1016/s0092-8674(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 11.Sharif MN, Sosic D, Rothlin CV, Kelly E, Lemke G, Olson EN, Ivashkiv LB. Twist mediates suppression of inflammation by type I IFNs and Axl. J Exp Med. 2006;203:1891–1901. doi: 10.1084/jem.20051725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niesner U, Albrecht I, Janke M, Doebis C, Loddenkemper C, Lexberg MH, Eulenburg K, Kreher S, Koeck J, Baumgrass R, Bonhagen K, Kamradt T, Enghard P, Humrich JY, Rutz S, Schulze-Topphoff U, Aktas O, Bartfeld S, Radbruch H, Hegazy AN, Lohning M, Baumgart DC, Duchmann R, Rudwaleit M, Haupl T, Gitelman I, Krenn V, Gruen J, Sieper J, Zeitz M, Wiedenmann B, Zipp F, Hamann A, Janitz M, Scheffold A, Burmester GR, Chang HD, Radbruch A. Autoregulation of Th1-mediated inflammation by twist1. J Exp Med. 2008;205:1889–1901. doi: 10.1084/jem.20072468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 14.Chen YT, Akinwunmi PO, Deng JM, Tam OH, Behringer RR. Generation of a Twist1 conditional null allele in the mouse. Genesis. 2007;45:588–592. doi: 10.1002/dvg.20332. [DOI] [PubMed] [Google Scholar]
- 15.Bialek P, Kern B, Yang X, Schrock M, Sosic D, Hong N, Wu H, Yu K, Ornitz DM, Olson EN, Justice MJ, Karsenty G. A twist code determines the onset of osteoblast differentiation. Dev Cell. 2004;6:423–435. doi: 10.1016/s1534-5807(04)00058-9. [DOI] [PubMed] [Google Scholar]
- 16.Chang HC, Zhang S, Thieu VT, Slee RB, Bruns HA, Laribee RN, Klemsz MJ, Kaplan MH. PU.1 expression delineates heterogeneity in primary Th2 cells. Immunity. 2005;22:693–703. doi: 10.1016/j.immuni.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Mathur AN, Chang HC, Zisoulis DG, Kapur R, Belladonna ML, Kansas GS, Kaplan MH. T-bet is a critical determinant in the instability of the IL-17-secreting T-helper phenotype. Blood. 2006;108:1595–1601. doi: 10.1182/blood-2006-04-015016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Firulli BA, Krawchuk D, Centonze VE, Vargesson N, Virshup DM, Conway SJ, Cserjesi P, Laufer E, Firulli AB. Altered Twist1 and Hand2 dimerization is associated with Saethre-Chotzen syndrome and limb abnormalities. Nat Genet. 2005;37:373–381. doi: 10.1038/ng1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu Q, V, Thieu T, Kaplan MH. Stat4 limits DNA methyltransferase recruitment and DNA methylation of the IL-18Ralpha gene during Th1 differentiation. EMBO J. 2007;26:2052–2060. doi: 10.1038/sj.emboj.7601653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Firulli BA, Redick BA, Conway SJ, Firulli AB. Mutations within helix I of Twist1 result in distinct limb defects and variation of DNA binding affinities. J Biol Chem. 2007;282:27536–27546. doi: 10.1074/jbc.M702613200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang S, Aune TM. Dynamic changes in histone-methylation ‘marks’ across the locus encoding interferon-gamma during the differentiation of T helper type 2 cells. Nat Immunol. 2007;8:723–731. doi: 10.1038/ni1473. [DOI] [PubMed] [Google Scholar]
- 22.Schoenborn JR, Dorschner MO, Sekimata M, Santer DM, Shnyreva M, Fitzpatrick DR, Stamatoyannopoulos JA, Wilson CB. Comprehensive epigenetic profiling identifies multiple distal regulatory elements directing transcription of the gene encoding interferon-gamma. Nat Immunol. 2007;8:732–742. doi: 10.1038/ni1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagege H, Klous P, Braem C, Splinter E, Dekker J, Cathala G, de Laat W, Forne T. Quantitative analysis of chromosome conformation capture assays (3C-qPCR) Nat Protoc. 2007;2:1722–1733. doi: 10.1038/nprot.2007.243. [DOI] [PubMed] [Google Scholar]
- 24.Hadjur S, Williams LM, Ryan NK, Cobb BS, Sexton T, Fraser P, Fisher AG, Merkenschlager M. Cohesins form chromosomal cis-interactions at the developmentally regulated IFNG locus. Nature. 2009;460:410–413. doi: 10.1038/nature08079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sekimata M, Perez-Melgosa M, Miller SA, Weinmann AS, Sabo PJ, Sandstrom R, Dorschner MO, Stamatoyannopoulos JA, Wilson CB. CCCTC-binding factor and the transcription factor T-bet orchestrate T helper 1 cell-specific structure and function at the interferon-gamma locus. Immunity. 2009;31:551–564. doi: 10.1016/j.immuni.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Court F, Baniol M, Hagege H, Petit JS, Lelay-Taha MN, Carbonell F, Weber M, Cathala G, Forne T. Long-range chromatin interactions at the mouse Igf2/H19 locus reveal a novel paternally expressed long non-coding RNA. Nucleic Acids Res. 2011;39:5893–5906. doi: 10.1093/nar/gkr209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu Q, Chang HC, Ahyi AN, Kaplan MH. Transcription factor-dependent chromatin remodeling of Il18r1 during Th1 and Th2 differentiation. J Immunol. 2008;181:3346–3352. doi: 10.4049/jimmunol.181.5.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto K, Yamaguchi M, Miyasaka N, Miura O. SOCS-3 inhibits IL-12-induced STAT4 activation by binding through its SH2 domain to the STAT4 docking site in the IL-12 receptor beta2 subunit. Biochem Biophys Res Commun. 2003;310:1188–1193. doi: 10.1016/j.bbrc.2003.09.140. [DOI] [PubMed] [Google Scholar]
- 29.Nakahira M, Tanaka T, Robson BE, Mizgerd JP, Grusby MJ. Regulation of signal transducer and activator of transcription signaling by the tyrosine phosphatase PTP-BL. Immunity. 2007;26:163–176. doi: 10.1016/j.immuni.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Krawchuk D, Weiner SJ, Chen YT, Lu BC, Costantini F, Behringer RR, Laufer E. Twist1 activity thresholds define multiple functions in limb development. Dev Biol. 2010;347:133–146. doi: 10.1016/j.ydbio.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng GZ, Zhang WZ, Sun M, Wang Q, Coppola D, Mansour M, Xu LM, Costanzo C, Cheng JQ, Wang LH. Twist is transcriptionally induced by activation of STAT3 and mediates STAT3 oncogenic function. J Biol Chem. 2008;283:14665–14673. doi: 10.1074/jbc.M707429200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O’Malley JT, Kapur R, Levy DE, Kansas GS, Kaplan MH. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178:4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 33.Stritesky GL, Muthukrishnan R, Sehra S, Goswami R, Pham D, Travers J, Nguyen ET, Levy DE, Kaplan MH. The transcription factor STAT3 is required for T helper 2 cell development. Immunity. 34:39–49. doi: 10.1016/j.immuni.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 35.Stritesky GL, Muthukrishnan R, Sehra S, Goswami R, Pham D, Travers J, Nguyen ET, Levy DE, Kaplan MH. The transcription factor STAT3 is required for T helper 2 cell development. Immunity. 2011;34:39–49. doi: 10.1016/j.immuni.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, Kuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ho IC, Lo D, Glimcher LH. c-maf promotes T helper cell type 2 (Th2) and attenuates Th1 differentiation by both interleukin 4-dependent and -independent mechanisms. J Exp Med. 1998;188:1859–1866. doi: 10.1084/jem.188.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Massari ME, Murre C. Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol. 2000;20:429–440. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castanon I, Von Stetina S, Kass J, Baylies MK. Dimerization partners determine the activity of the Twist bHLH protein during Drosophila mesoderm development. Development. 2001;128:3145–3159. doi: 10.1242/dev.128.16.3145. [DOI] [PubMed] [Google Scholar]
- 40.Caudy M, Vassin H, Brand M, Tuma R, Jan LY, Jan YN. daughterless, a Drosophila gene essential for both neurogenesis and sex determination, has sequence similarities to myc and the achaete-scute complex. Cell. 1988;55:1061–1067. doi: 10.1016/0092-8674(88)90250-4. [DOI] [PubMed] [Google Scholar]
- 41.Cronmiller C, Schedl P, Cline TW. Molecular characterization of daughterless, a Drosophila sex determination gene with multiple roles in development. Genes Dev. 1988;2:1666–1676. doi: 10.1101/gad.2.12a.1666. [DOI] [PubMed] [Google Scholar]
- 42.Pan L, Bradney C, Zheng B, Zhuang Y. Altered T-dependent antigen responses and development of autoimmune symptoms in mice lacking E2A in T lymphocytes. Immunology. 2004;111:147–154. doi: 10.1111/j.0019-2805.2003.01802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maruyama T, Li J, Vaque JP, Konkel JE, Wang W, Zhang B, Zhang P, Zamarron BF, Yu D, Wu Y, Zhuang Y, Gutkind JS, Chen W. Control of the differentiation of regulatory T cells and T(H)17 cells by the DNA-binding inhibitor Id3. Nat Immunol. 2011;12:86–95. doi: 10.1038/ni.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Firulli AB, Conway SJ. Phosphoregulation of Twist1 provides a mechanism of cell fate control. Curr Med Chem. 2008;15:2641–2647. doi: 10.2174/092986708785908987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayashi M, Nimura K, Kashiwagi K, Harada T, Takaoka K, Kato H, Tamai K, Kaneda Y. Comparative roles of Twist-1 and Id1 in transcriptional regulation by BMP signaling. J Cell Sci. 2007;120:1350–1357. doi: 10.1242/jcs.000067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.