Abstract
Drug development for nicotinic acetylcholine receptors (nAChR) is challenged by subtype diversity arising from variations in subunit composition. On-target activity for neuronal heteromeric receptors is typically associated with CNS receptors that contain α4 and other subunits, while off-target activity could be associated with ganglionic-type receptors containing α3β4 binding sites and other subunits, including β4, β2, α5, or α3 as a structural subunit in the pentamer. Additional interest in α3 β4 α5-containing receptors arises from genome-wide association studies linking these genes, and a single nucleotide polymorphism (SNP) in α5 in particular, to lung cancer and heavy smoking. While α3 and β4 readily form receptors in expression system such as the Xenopus oocyte, since α5 is not required for function, simple co-expression approaches may under-represent α5-containing receptors. We used a concatamer of human α3 and β4 subunits to form ligand-binding domains, and show that we can force the insertions of alternative structural subunits into the functional pentamers. These α3β4 variants differ in sensitivity to ACh, nicotine, varenicline, and cytisine. Our data indicated lower efficacy for varenicline and cytisine than expected for β4-containing receptors, based on previous studies of rodent receptors. We confirm that these therapeutically important α4 receptor partial agonists may present different autonomic-based side-effect profiles in humans than will be seen in rodent models, with varenicline being more potent for human than rat receptors and cytisine less potent. Our initial characterizations failed to find functional effects of the α5 SNP. However, our data validate this approach for further investigations.
Keywords: Smoking cessation, drug screening, ganglionic receptors, partial agonists
1.0 Introduction
Early single channel studies of the nicotinic acetylcholine receptors of autonomic neurons revealed a rich diversity of channel subtypes (Papke, 1993). Although it is now appreciated that, at least in embryonic ganglia, rapidly desensitizing α7-containing nAChRs are of functional importance, blocking α7 receptors in adult animals generally does not impair ganglionic function, and it is likely that the early single-channel studies only detected an array of more slowly desensitizing heteromeric receptor subtypes. We now know, based on recent studies using knockout animals, that ganglionic receptors are primarily assembled as pentameric complexes containing varying arrangements of α3, β2, β4, and α5 subunits (David et al., 2010).
Although ganglionic blockers were the first drugs used clinically to target neuronal nAChR, most current drug development programs intended to target nAChR in the CNS, either for therapeutics or nicotine dependence, view ganglionic receptors containing α3 in various combinations with β2, β4, and α5 as potential sites for off-target side effects. The human α3-β4-α5 genes are in a cluster at chromosomal location 15q24, and recent genome-wide association studies indicated strong correlations between single nucleotide polymorphisms in the α3-β4-α5 gene cluster and risk for both cancer and nicotine dependence (Chen et al., 2009; Stevens et al., 2008). Nicotine addiction and dependence has been clearly linked to α4* and α6* receptors (Wu and Lukas, 2011), and α5 co-assembly into α4* receptors also promotes high sensitivity to nicotine, suggesting a link between nicotine use and α4β2α5 receptors (Kuryatov et al., 2011). However, recent studies have also demonstrated a link between α3β4α5-containing receptors in the medial habenula and nicotine-related behavior, promoting receptors with combinations of these subunits as an alternative target for the development of smoking cessation drugs (Fowler et al., 2011; Frahm et al., 2011; Gallego et al., 2011; Salas et al., 2009).
It has been shown both in vivo (Grady et al., 2009) and in heterologous expression systems (Boulter et al., 1990; Gerzanich et al., 1998) that α3 will form receptors in various combinations with β2, β4 and α5 subunits. However, α3 and β4 subunits readily form functional receptors without additional subunits, and functional effects of α5 co-expression are much more easily detectable in β2- containing than in β4- containing receptors (Gerzanich et al., 1998). Therefore, since most effectively targeted drug development relies on the use of receptors with known subunit composition, we adopted a strategy previously shown to be useful for controlling the subunit composition of α4* receptors (Zhou et al., 2003), by constructing a concatamer of β4 and α3 (β4–6–α3), suitable for co-expression with monomeric α3, β2, β4, or α5 subunits. The β4–6–α3 construct will provide ligand-binding domains with α3–β4 interfaces, so that co-expressed subunit monomers will, with high likelihood, take the fifth position as a structural subunit in the assembled pentamer.
We provide pharmacological validation of hypothesized subunit compositions and characterize the agonist and partial-agonist profiles of the α3β4 receptor subtypes for ACh, nicotine, and the smoking cessation agents, cytisine and varenicline. Cytisine and varenicline have been proposed to have therapeutic utility through potent partial agonist effects on CNS α4-containing receptors. However, it has been a concern that the reportedly high efficacy of these agents on ganglionic α3-containing receptors might be a source of autonomic side effects. We reevaluate those data and show significant differences from the previously reported data based on the use of rodent receptor subtypes and our current studies based on the use of human receptor clones. Additionally, we used the β4–6–α3 construct to study the D376N variant of α5, specifically associated with smoking and cancer risks.
2.0 Methods and materials
2.1 ACh receptor clones
Human nAChR clones were obtained from Dr. Jon Lindstrom (University of Pennsylvania, Philadelphia PA). Alpha3 and β4 were subcloned into the pSGEM vector, obtained from Dr. Michael Hollmann (Ruhr University, Bochum, Germany), which contains Xenopus β-globin untranslated regions to aid Xenopus oocyte expression. Rat nAChR clones were obtained from Dr. Jim Boulter (University of California, Los Angeles).
2.2 Concatamer construction
As the C terminus of β4 is of similar length as that of β2, we followed the scheme of Zhou et al, 2003 (Zhou et al., 2003), and prepared the concatamer with, in sequence: β4 signal, mature β4, 6(AGS) linker, then α3 mature (without signal sequence), all in frame, which should assemble with the α3-β4 binding pocket intact (Zhou et al., 2003). With this approach, co-injected subunits should co-assemble into the structural, non-ligand-binding-domain position.
Specifically, β4 was mutated silently to introduce a DraIII restriction recognition site just before the stop codon. The site-directed mutagenesis was performed using the QuikChange kit (Agilent Technologies, Santa Clara CA). Long (100 bp) complementary oligos (sense strand: GCTGGAAGGCACAACGTGACGCTGGAAGTGCTGGAAGTGCTGGAAGTGCTGGAAGTGCTGGAAGTGCTGGAAGTGCAGAGGCTGAGCTCGAGACTGAAGC) incorporating the DraIII recognition sequence at the end of β4 before the stop codon, 6(AGS), the first 13 bases of mature α3 coding region including the unique BlpI site, and an XhoI recognition site were annealed following the protocol of Integrated DNA Technologies: Each 4nmole oligo was dissolved in 40 µl of 100mM potassium acetate, 30mM HEPES, pH 7.5. 10µl of each was combined in a 1.5 ml microcentrifuge tube and placed in a heat block set at 94°; after 5 min, the heat block was turned off and allowed to slowly return to room temperature, about 2 hours. Beta4 and the annealed oligo were digested with DraIII and XhoI. Beta4 was gel-purified, and the oligo was purified with QIAQuick PCR purification kit (Qiagen, Valencia CA) to remove the 12 and 15 bp unwanted pieces while retaining the 73 bp insert. After ligation and plasmid miniprep the construct was confirmed with restriction diagnostics (BlpI). The β4 construct was then subcloned into α3 at the SacII and BlpI sites, thereby creating the β4–6–α3 concatamer. The final construct was confirmed with automated fluorescent sequencing (University of Florida Biotechnology core facility) as well as by restriction diagnostics.
The α5 single nucleotide polymorphism affecting amino acid translation, α5D376N, was constructed using the QuikChange kit.
2.3 Expression in Xenopus laevis oocytes
Xenopus laevis oocytes were surgically removed from frogs (Nasco, Ft. Atkinson WI) and treated with Type I collagenase (Worthington Biochemical Corporation, Freehold NJ) in calcium-free Barth’s solution (88 mM NaCl, 1 mM KCl, 2.38 mM NaHCO3, 0.82 mM MgS04, 15 mM HEPES (pH 7.6), 12 mg/l tetracycline) in order to remove the follicular layer. Stage-5 oocytes were isolated and injected with 50 nl (3–20 ng) of each cRNA. After linearization and purification of cloned cDNAs, RNA transcripts were prepared in vitro using the appropriate mMessage mMachine kit (Ambion, Austin TX). Recordings were conducted 2–10 days post-injection.
2.4 Electrophysiology
Experiments were conducted using OpusXpress6000A (Molecular Devices, Union City, CA). OpusXpress is an integrated system that provides automated impalement and voltage clamp of up to eight oocytes in parallel. Both the voltage and current electrodes were filled with 3M KCl. The oocytes were clamped at a holding potential of −60 mV. Data were collected at 50 Hz and filtered at 5 Hz. The oocytes were bath-perfused with Ringer’s solution (115 mM NaCl, 10 mM HEPES, 2.5 mM KCl, 1.8 mM CaCl2) containing 1µM atropine to block muscarinic acetylcholine receptors which may be native in the oocytes. Agonist solutions were delivered from 96-deepwell plates using disposable tips. Flow rates were set at 4 ml/min.
2.5 Experimental protocols and data analysis
Responses were calculated as both net charge and peak currents. Each oocyte received initial control applications of 100µM acetylcholine (ACh), then experimental drug applications, and follow-up control applications of ACh. Responses to experimental drug applications were calculated relative to the preceding ACh control responses in order to normalize the data, compensating for the varying levels of channel expression among the oocytes. A second normalization step was applied to adjust for the empirically determined difference between the 100 µM ACh control responses and the observed ACh maximum for each receptor subtype. Average values and standard errors (SEM) were calculated from the normalized responses of at least four oocytes for each experimental condition. For concentration-response relations, data were plotted using Kaleidagraph 3.0.2 (Abelbeck Software; Reading, PA), and curves were generated from the Hill equation:
Imax denotes the maximal response for a particular agonist/subunit combination, and n represents the Hill coefficient. Imax, n, and the EC50 were all unconstrained for the fitting procedures, except in the case of the ACh response curves. Since ACh is our reference full agonist, for the ACh concentration-response curves the data were normalized to the observed ACh maximum, and the Imax of the ACh curve fits were constrained to equal one.
3.0 Results
3.1 Experiments confirming the incorporation of specific structural subunits
When β4–6–α3 was expressed alone it was capable of forming functional receptors with properties similar to those formed when was β4–6–α3 co-expressed with β4, suggesting the assembly of α3(2)β4(3) receptors with tethered supernumerary α3 subunits. That is, the ACh concentration-response curves and the recoveries from TMPH and BTMPS responses were similar (data not shown). In order to obtain better control of structural subunit identity, the RNAs for the monomeric constructs were co-expressed at a five-fold excess to the concatamer. We confirmed pharmacologically that this approach was successful, as shown in Figure 1.
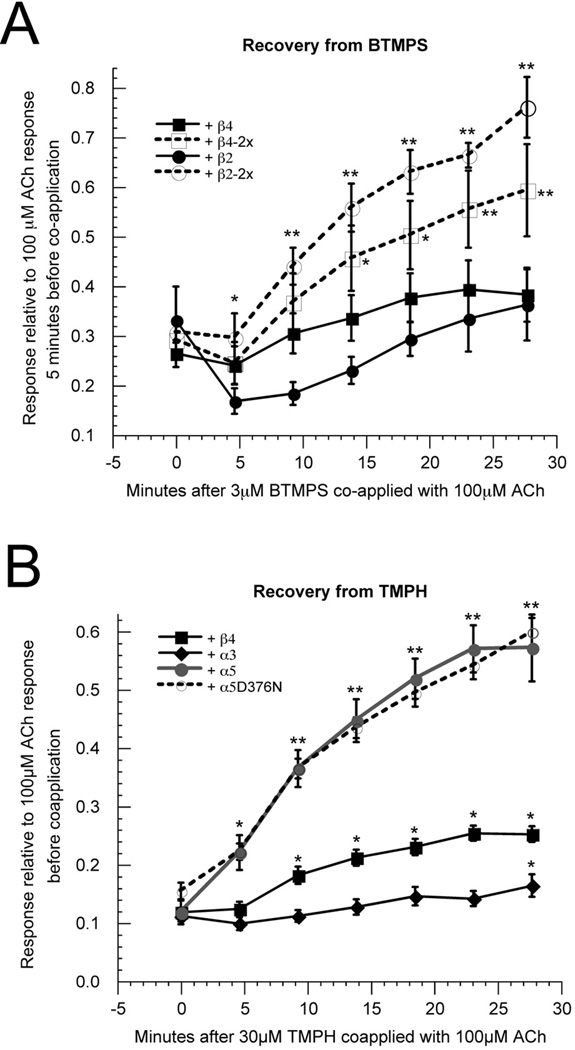
Figure 1.
BTMPS and TMPH confirm the functional incorporation of structural subunits. A) The β4–6–α3 concatamer was co-expressed at 1:5 RNA ratio with either wild type β4 or β2 or with beta subunits containing mutations at the 6' and 10' sites (Miller, 1989) in the second transmembrane domain (TM2) (β2-2x and β4-2x). These sites have previously been shown to regulate the reversibility of the use-dependent antagonist BTMPS (Francis et al., 1998). After obtaining two control responses to the application of 100 µM ACh, 3 µM BTMPS was co-applied with 100 µM ACh. While cells expressing wild-type beta subunits showed no significant increase in response between the original co-application response and responses recorded 14–28 minutes later, cells expressing the beta subunit mutants showed progressive recovery, and responses after 9.2 minutes (β2-2x) and 18.5 minutes (β4-2x) were significantly larger than those recorded during the co-application, * indicates p < 0.05, ** indicates p < 0.001. All points represent the average data (± SEM) for at least four cells. B) TMPH is an amphipathic non-competitive antagonist of heteromeric neuronal nAChR, which produces long-lived inhibition of receptors containing only α3 and β4 or α4 and β2 subunits and more readily-reversible inhibition of receptors containing α5 as an accessory subunit. The β4–6–α3 concatamer was co-expressed at 1:5 RNA ratio with either wild-type β4, β2, or α5 subunits or with the α5D376N variant. After obtaining two control responses to the application of 100 µM ACh, 30 µM TMPH was co-applied with 100 µM ACh. Cells expressing α3 or β4 subunits showed much less recovery after the co-application response than the cells expressing the α5 subunits, where responses after 4.6 minutes were significantly larger than those recorded during the co-application. Cells expressing α3 or β4 subunits required 28 minutes and 9.2 minutes, respectively, for responses to be significantly larger than those recorded during the co-application, * indicates p < 0.05, ** indicates p < 0.001. At all points after the co-applications the responses of the α5-containing receptors were significantly larger than those of the α3 or β4-containing receptors (p < 0.01). All points represent the average data (± SEM) for at least four cells.
When mutations known to increase the reversibility of inhibition by the non-competitive antagonist BTMPS (Francis et al., 1998) were present in the β2 or β4 subunits expressed as monomers, the receptors assembled with the concatamers showed more rapid recovery from inhibition by BTMPS (Figure 1A), confirming the functional incorporation of the mutant subunits in the accessory subunit position with the concatamer providing the ligand binding domains.
We have previously shown that receptors containing α5 subunits have reduced sensitivity to prolonged inhibition by the antagonist TMPH (Papke et al., 2005), and the co-expression of α5 with the β4–6–α3 concatamer yielded reduced sensitivity compared to receptors formed with other subunit compositions (Figure 1B). We also confirmed that the β4–6–α3 concatamer could be used to generate functional receptors containing the potentially important α5 single nucleotide polymorphism (SNP) which generates a D376N mutation.
3.2 Pharmacological characterization of α3β4 receptor subtypes
3.2.1 ACh responses
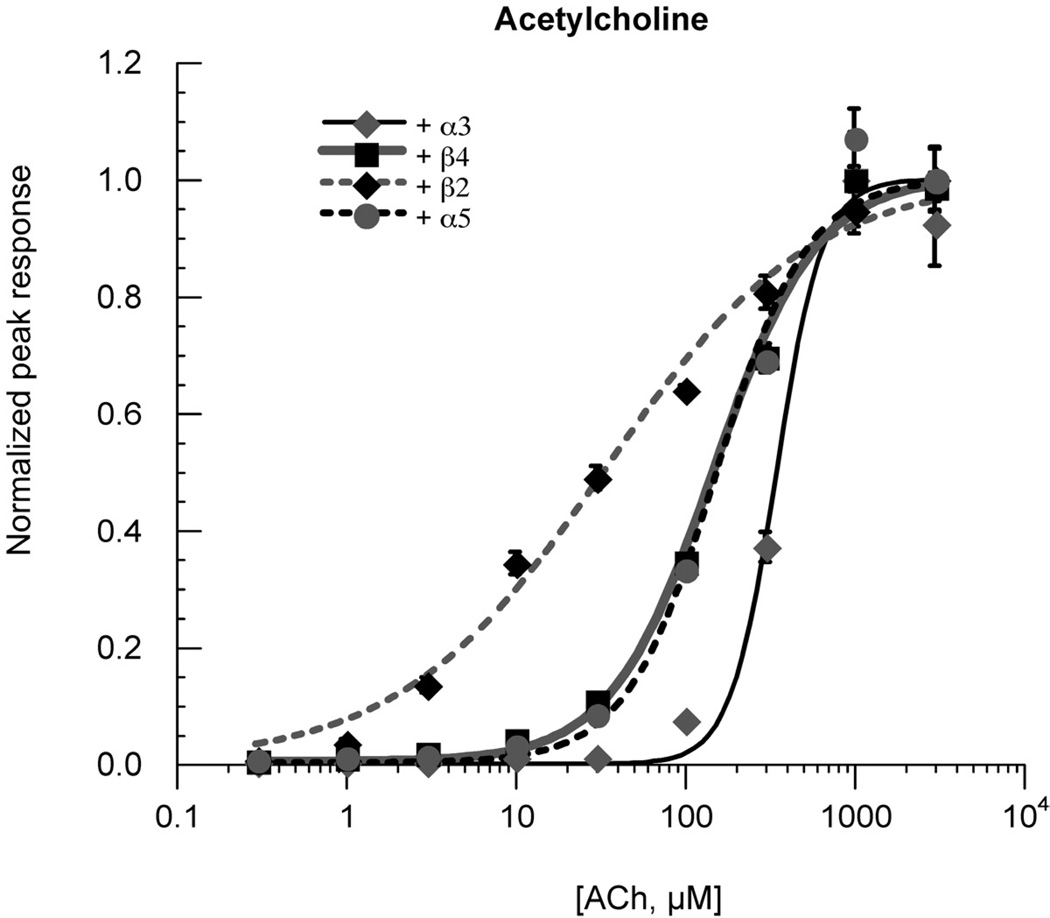
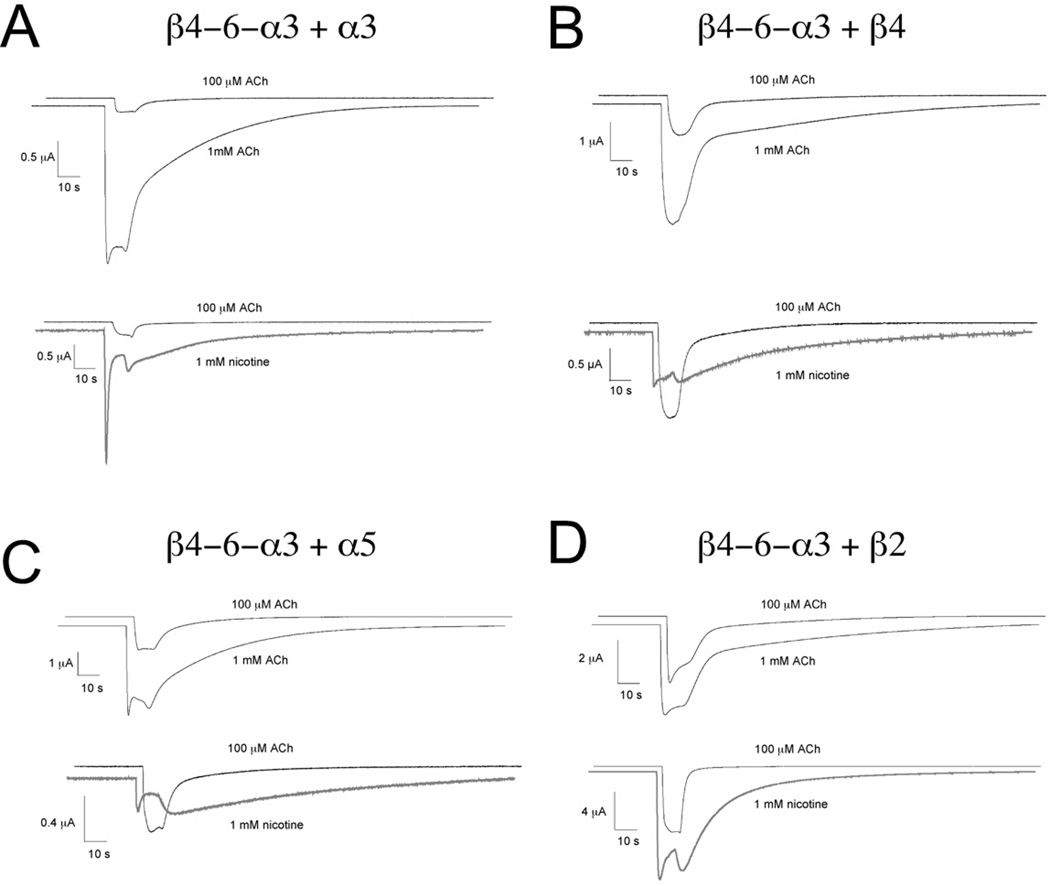
We conducted studies of the ACh concentration-response relationships of the various α3β4 subtypes. The data indicated that substitution of α3, but not α5, for the β4 subunit in the accessory position produced a decrease in ACh potency, while the substitution of a β2 subunit produced receptors with increased ACh sensitivity (Figure 2 and Table 1). For all of the combinations studied, the kinetics of the ACh-evoked responses were similar with both high and low concentrations of ACh (Figure 3), so the results based on either peak currents or net charge were equivalent (not shown). However, as shown in Figure 3, the currents of α3 and α5 containing receptors evoked by 1 mM ACh showed decay during the agonist application pulse. This was most likely due to channel block by ACh, consistent with the appearance of rebound current when the ACh began to be washed out of the chamber.
Figure 2.
ACh concentration-response curves. The β4–6–α3 concatamer was co-expressed at 1:5 RNA ratio with either α3, β4, β2, or α5. Data were initially normalized to 100 µM ACh control responses obtained immediately prior to the test responses and subsequently adjusted to the empirically determined ACh maximum responses. ACh controls remained of stable amplitude throughout each experiment. All points represent the average data (± SEM) for at least four cells. EC50 values (Table 1) were determined by fits of the data to the Hill equation (Methods).
Table 1.
Acetylcholine potency, peak current data
| Accessory subunit | EC50, µM |
|---|---|
| α3 | 349.4 ± 22.0 |
| β4 | 153.9 ± 9.9 |
| β2 | 34.1 ± 3.9 |
| α5 | 157.7 ± 16.4 |
| α5D376N | 126.8 ± 8.0 |
Figure 3.
Sample traces of high ACh and nicotine responses of the receptors formed by concatamer and monomer co-expression, compared to 100 µM ACh controls obtained from the same cells. Note that the waveforms of the 1 mM ACh-evoked responses are relatively similar to the 100 µM ACh control responses, with only small indications of channel block for the α3(3)β4(2) (A) and α3(2)β4(2)α5 (C) receptors. In contrast, there is strong indication of channel block and rebound for the nicotine-evoked responses of all the subtypes.
The curves for α3-containing and β2-containing receptors were fit with Hill slopes that were distinctly different than the curves for the β4 and α5 containing receptors. It is difficult to interpret the Hill slopes for macroscopic current responses since multiple factors can affect the amplitude and kinetics of the responses (Papke, 2009). For example, a steep Hill slope, as seen for the α3(3)β4(2) receptors, could be produced if channel block by agonist became a limiting factor to the ACh-evoked responses, thereby creating a narrow range for effectively increasing ACh-evoked current. In this case of the α3(3)β4(2) receptors the potency for activation is low and does approach the expected potency of ACh for channel block (Lape et al., 2008). The shallow Hill slope of β2-containing receptors could be due to a mixed population of receptors or possibly alternative subunit interfaces functioning as low potency agonist binding sites.
3.2.2 Nicotine responses
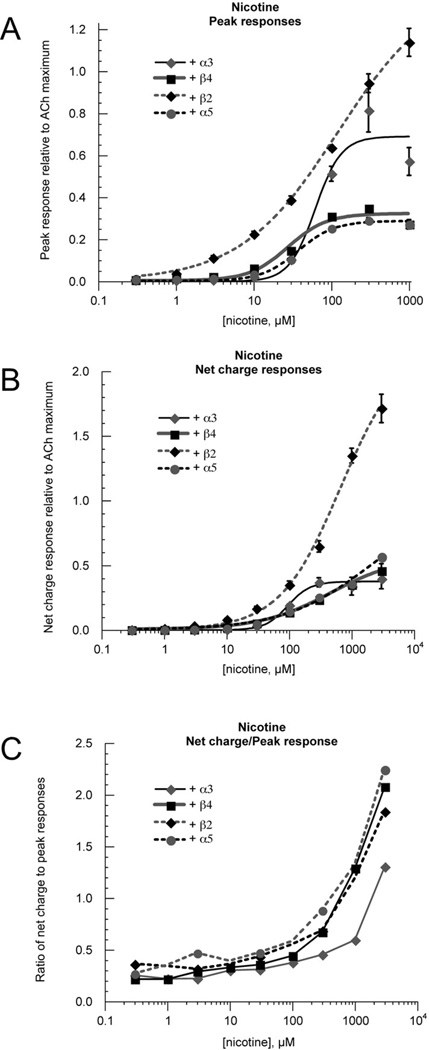
For each of the receptors studied, the waveforms of the nicotine-evoked responses were different from the ACh evoked responses (Figure 3), so that concentration-response data based on peak currents and net charge were significantly different (Figure 4). Analysis of peak currents suggested that nicotine was a full agonist for α3(2)β4(2)β2 receptors, a 30% partial agonist for α3(2)β4(2)α5 and α3(2)β4(3) receptors, and with intermediate efficacy for α3(3)β4(2)receptors (Table 2). At high concentrations of nicotine, evoked responses were protracted for all subunit combinations. For the α3(2)β4(2)β2 receptor net charge data, this effect had the appearance of making nicotine appear more efficacious than ACh, possibly because nicotine was retained at the binding site of these receptors and remained continuously active when the free drug concentrations were being reduced.
Figure 4.
Nicotine peak current and net charge responses. A) Nicotine peak current concentration-response curves. The β4–6–α3 concatamer was co-expressed at a 1:5 RNA ratio with either α3, β4, β2, or α5. Data were initially normalized to 100 µM ACh control responses obtained immediately prior to the test responses and subsequently adjusted to the empirically determined ACh maximum responses. B) Nicotine net-charge concentration-response curves. The β4–6–α3 concatamer was co-expressed at a 1:5 RNA ratio with either α3, β4, β2, or α5. Data were initially normalized to 100 µM ACh control responses obtained immediately prior to the test responses and subsequently adjusted to the empirically determined ACh maximum responses. C) The ratio of net charge to peak current (relative to 100 µM ACh controls) for nicotine-evoked responses. A–C Data for progressive increases in nicotine concentration were used from a single experiment only under conditions when ACh controls remained of stable amplitude throughout each experiment. In some cases, nicotine applications prevented the full recovery of ACh controls; in those cases, responses evoked by nicotine relative to ACh were determined in a series of single concentration experiments using new sets of cells for each nicotine concentration. All points represent the average data (± SEM) for at least four cells.
Table 2.
Nicotine potency and efficacy
| Peak | Net Charge | |||
|---|---|---|---|---|
| Accessory subunit |
EC50 µM |
Imax relative to AChmax |
EC50 µM |
Imax relative to AChmax |
| α3 | 62.0 ± 15.6 | 0.69 ± 0.06 | 95.3 ± 8.36 | 0.38 ± 0.01 |
| β4 | 28.3 ± 6.7 | 0.32 ± 0.02 | 488.8 ± 77.2 | 0.57 ± 0.03 |
| β2 | 121.8 ± 21.6 | 1.4 ± 0.07 | 685.4 ± 184.9 | 2.21 ± 0.21 |
| α5 | 35.6 ± 4.5 | 0.27 ± 0.01 | 1996 ± 2019 | 0.99 ± 0.31 |
| α5D376N | 24.9 ± 1.9 | 0.28 ± 0.01 | 366.41 ± 96.379 | 0.55 ± 0.04 |
It should also be noted that from the appearance of rapid peak currents and subsequent rebounds for several receptors, channel block by nicotine may have also played a role in shaping the unique waveforms of the responses and limiting the apparent efficacy for all subtypes other than the α3(2)β4(2)β2 receptors.
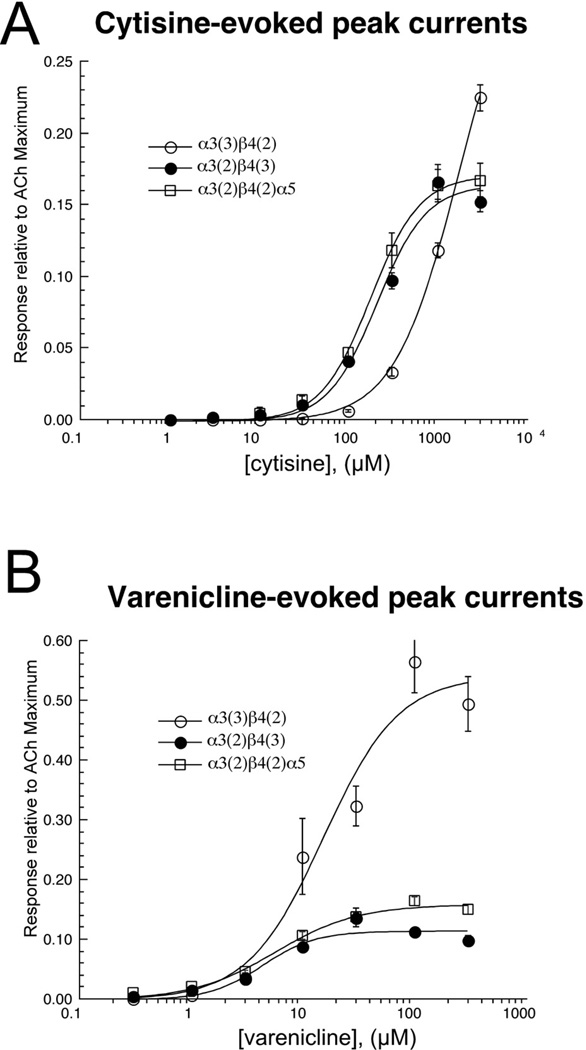
3.2.3 Responses to cytisine and varenicline
Cytisine and varenicline are agents presently in use as smoking cessation aids (Rollema et al., 2010). Their efficacy for this indication is believe to be related to a potent but weak partial agonism of the α4β2 nAChR subtype(s) (Mihalak et al., 2006; Papke and Heinemann, 1994; Papke et al., 2011; Papke et al., 2010) which are highly expressed in the CNS. However, these agents have also been reported to be strong activators of ganglionic α3β4* nAChR (Mihalak et al., 2006; Papke and Heinemann, 1994), a hypothetically off-target effect assumed to be a potential source of autonomic side effects. We evaluated the activity of these agents on the α3β4 receptors formed with different accessory subunits (Figure 5). Contrary to expectations based on prior literature, we found these agents to be only partial agonists of the α3β4 receptor subtypes. Cytisine was most efficacious but least potent for α3(3)β4(2) receptors and had an efficacy of no more than 20% that of ACh for the other subtypes tested. The pattern was similar for varenicline except that in all cases it was at least 30-fold more potent than cytisine (Table 3). Additionally we noted that that at concentrations higher than 100 µM varenicline, but not cytisine had effects on the response waveforms (not shown) similar to those of nicotine (Figure 3),(Shytle et al., 2011) suggesting limiting effects of channel block.
Figure 5.
Concentration-response curves for (A) Cytisine and (B) Varenicline. The β4–6–α3 concatamer was co-expressed at a 1:5 RNA ratio with either α3, β4, or α5. Data were initially normalized to 100 µM ACh control responses obtained immediately prior to the test responses and subsequently adjusted to the empirically determined ACh maximum responses. ACh controls remained of stable amplitude throughout each experiment. All points represent the average data (± SEM) for at least four cells. EC50 values (Table 3) were determined by fits of the data to the Hill equation (Methods).
Table 3.
Cytisine and Varenicline potency and efficacy, peak current data
| Cytisine | Varenicline | |||
|---|---|---|---|---|
| Accessory subunit |
EC50 µM |
Imax relative to AChmax |
EC50 µM |
Imax relative to AChmax |
| α3 | 1750 ± 300 | 0.34 ± 0.03 | 16 ± 5 | 0.54 ± 0.06 |
| β4 | 214 ± 32 | 0.16 ± 0.01 | 4.5 ± 1.4 | 0.11 ± 0.01 |
| α5 | 180 ± 8 | 0.17 ± 0.01 | 5.8 ± 0.9 | 0.16 ± 0.01 |
| α5D376N | 309 ± 43 | 0.21 ± 0.01 | 6.5 ± 1.5 | 0.15 ± 0.01 |
3.3 Species-specific features of cytisine and varenicline activation of α3β4 receptors
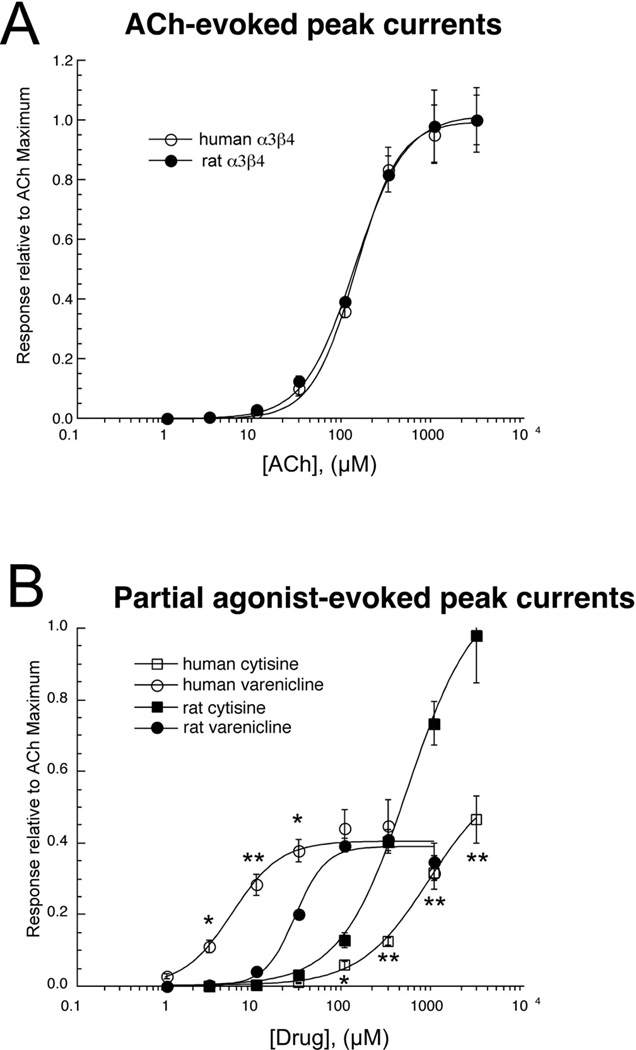
The initial studies (Mihalak et al., 2006; Papke and Heinemann, 1994) which reported high efficacy of cytisine and varenicline for α3β4 receptors utilized the standard procedure of injecting equal ratios of α3 and β4 RNAs and were conducted using rat cDNA clones. More recently, we reported that for mouse α3β4 receptors, cytisine was a full agonist, while varenicline had an efficacy approximately 50% that of ACh (Papke et al., 2010). In order to confirm that the low efficacy we observed for cytisine and varenicline was a characteristic of human α3β4, we conducted direct comparisons between human and rat α3β4 receptors with the standard procedure of monomer co-expression.
3.3.1 ACh responses
As shown in Figure 6A, the ACh concentration response curves were nearly identical for rat and human α3β4 receptors, with an ACh potency like that of the α3(2)β4(3) receptors generated with controlled accessory subunit (Table 4).
Figure 6.
A) ACh concentration-response curves for rat and human α3β4 nAChR formed with the conventional method of co-expressing RNA for the subunit monomers at a 1:1 ratio. B) Cytisine and varenicline concentration-response curves for rat and human α3β4 nAChR formed with the conventional method of co-expressing RNA for the subunit monomers at a 1:1 ratio. All points represent the average data (± SEM) for at least four cells. Statistical analysis based on t-tests between the normalized responses of rat and human receptors indicated significance values of p <0.01 (*), or p <0.001 (**).
Table 4.
Species dependent effects on α3β4 responses using monomeric constructs
| Acetylcholine potency, peak current data | |
|---|---|
| Species | EC50, µM |
| human α3β4 | 131 ± 7 |
| rat α3β4 | 127 ± 6 |
| Cytisine | Varenicline | |||
|---|---|---|---|---|
|
Species |
EC50 µM |
Imax relative to AChmax |
EC50 µM |
Imax relative to AChmax |
| human α3β4 | 890 ± 120 | 0.59 ± 0.03 | 5.6 ± 1.8 | 0.40 ± 0.03 |
| rat α3β4 | 520 ± 50 | 1.10 ± 0.04 | 28 ± 3.2 | 0.39 ± 0.02 |
3.3.2 Responses to cytisine and varenicline
Consistent with previous data on rat α3β4 (Papke and Heinemann, 1994) we found (Figure 6B) cytisine to be a full agonist for rat α3β4 receptors, although the potency was rather low (520 ± 50 µM). At all concentrations of cytisine > 30 µM, the responses of human α3β4 receptors formed by the expression of monomers were significantly (p < .001) lower than the responses of rat receptors, with curve fits for the concentration-response curves indicating both lower Imax and higher EC50 values for human α3β4 receptors compared to rat. It should be noted, however that the expression of monomers did suggest a higher cytisine Imax relative to ACh than was seen in the concatamer experiments.
The potency and efficacy of varenicline for human α3β4 in the monomer co-expression experiments (Figure 6B) were inbetween the values for putative α3(3)β4(2) and α3(2)β4(3) receptors obtained with the concatamer (Table 3). Although the efficacy of varenicline was similar for rat and human α3β4, the potency of varenicline was approximately 5-fold greater for human than for rat, so that the evoked responses at concentrations from 3 µM to 30 µM varenicline were significantly larger for human than for rat receptors, in striking contrast to the results obtained with cytisine.
3.4 Evaluations of the functional effects of the α5 SNP
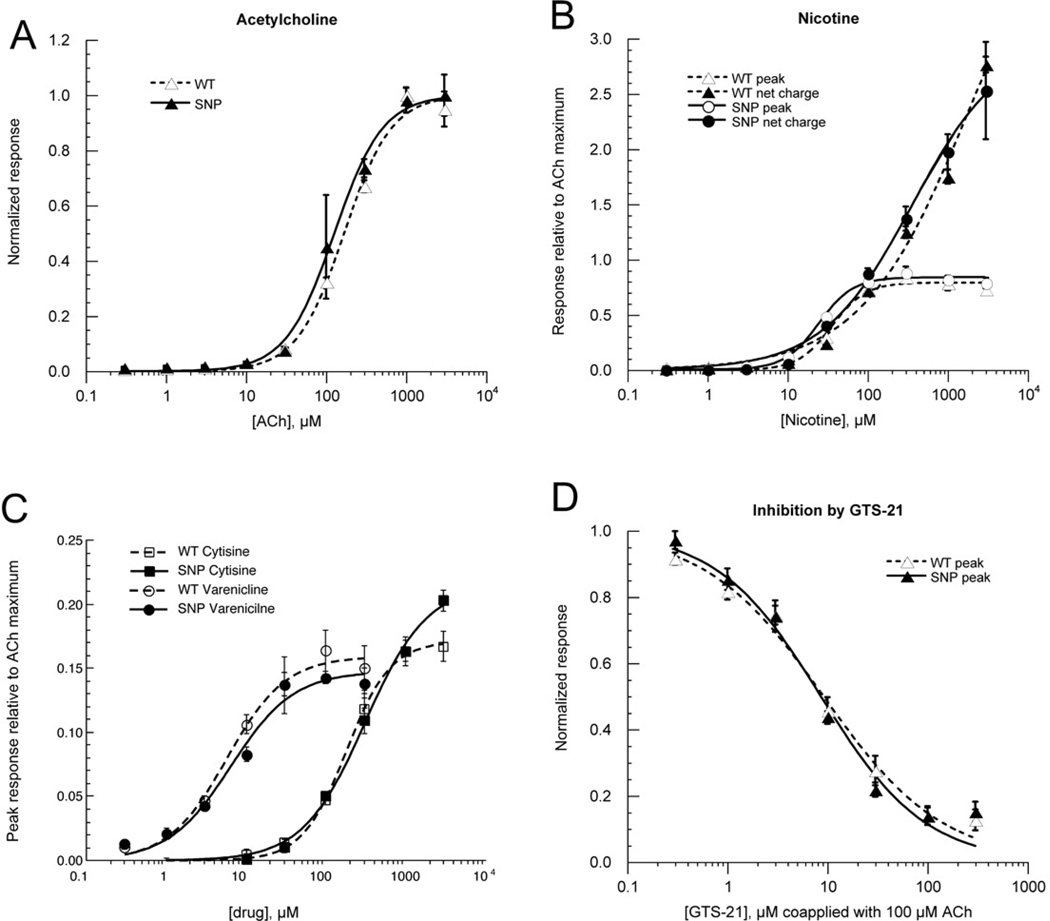
3.4.1 Agonist-evoked responses
The α5 SNP D376N was studied along with the wild-type α5 in all of the agonist concentration-response studies (Figure 7 A–C). No functional effects of the α5 SNP were detected in those experiments (Tables 1–3). Although the curve fit values for nicotine net charge data suggest that there might be an effect, it should be noted that the curve for the wild-type α5 data is not well fit by the Hill equation since no clear plateau response was achieved at concentrations ≤ 3 mM nicotine.
Figure 7.
Data for α3β4α5 receptors containing either wild-type α5 or the α5D376N SNP. A) ACh peak current responses. B) Nicotine peak current and net charge responses. C) Cytisine and varenicline peak current responses. D) Inhibition of ACh-evoked peak current responses by co-applications of GTS-21. (A–C) Data were initially normalized to 100 µM ACh control responses obtained immediately prior to the test responses and subsequently adjusted to the empirically determined ACh maximum responses. ACh controls remained of stable amplitude throughout each experiment. (A–D) All points represent the average data (± SEM) for at least four cells.
3..4.2 Inhibition by an α7-selective agonist
Orthosteric ligands that are selective for the α7 nAChR have been proposed for a variety of indications from Alzheimer's disease to asthma. In addition to producing selective activation of α7 receptors, most of these drugs also inhibit other nAChR subtypes such as α3β4* receptors (Horenstein et al., 2008). We evaluated the inhibitory activity of a prototypical agent in this class, GTS-21 (3-(2,4dimethoxybenzylidene)anabaseine) on α3β4 receptors incorporating either wild-type α5 or the α5 SNP as the accessory subunit (Figure 7D). The IC50 for the GTS-21 inhibition of the concatamer co-expressed with wild-type α5 was 9.17 ± 1.08 µM, not significantly different than when co-expressed with α5D376N (8.63 ± 1.41 µM).
4.0 Discussion
It has been shown both in vivo and in heterologous expression systems that α3 will form receptors in various combinations with β2, β4, and α5 subunits. Unconstrained expression, when all of these subunits are present, results in a heterogeneous population of receptor subtypes both in neurons and in oocytes. We adopted the strategy of co-expressing a concatamer of β4 and α3 (β4–6–α3), with monomeric β2, β4, or α5 subunits and were able to confirm that we obtained pharmacologically distinct populations of receptors useful for drug characterization.
For the subtypes specifically associated with the α3–β4–α5 gene cluster, our approach to co-expression appeared to be largely successful at generating distinct, potentially homogeneous, populations of receptors. However, it may be noted that the acetylcholine response curve of the putative α3(2)β4(2)β2 receptors (Figure 2) appears as though it may contain two populations of receptors. There appears to be a high sensitivity component, likely to contain β2 subunits, and an incomplete suppression of the α3β4 type receptors that occur when the concatamers were expressed alone.
We found that nicotine responses were affected by the identity of the α3β4* structural subunits, as previously reported for α4β2* receptors (Kuryatov et al., 2008). Receptors containing β2 structural subunits were most sensitive to low concentrations of nicotine. Our data also indicate that structural subunits will affect the channel-blocking activity of nicotine, as well as other noncompetitive antagonists.
Our data indicate that the specific α3β4 receptor subtypes will have unique profiles of response to cytisine and related agents that are in development for smoking cessation. Importantly, our data highlighted a disparity between the activity of the agents on human α3β4 receptor subtypes and previous findings based on rodent receptors. It is particularly interesting that the species-specific differences for the two agents tested were in opposite directions and therefore likely to rely on different elements in the receptors. We typically use rodent models for preclinical testing of whole animal drug effects. In the context of our previous studies of mouse nAChR (Papke et al., 2010), we noted that cytisine and varenicline have similar activity profiles for rodent and human α4* and α7* type receptors. However, data for human α3β4* receptors were not available at that time. Our current results therefore highlight the potential importance of comprehensive cross-species validation of pharmacology before the translation of preclinical results to human therapeutics.
Constipation is a commonly reported side effect of varenicline-based smoking cessation programs. This side effect might easily be associated with depolarizing block of autonomic ganglia, consistent with the high sensitivity to varenicline we see for human α3β4 receptors. Cytisine (Tabex®) is commonly used as a smoking cessation agent in Europe, and reportedly has only mild side-effects, which may in part be due to the low sensitivity of human α3β4 receptors to cytisine. Interestingly, α3β4 receptors in the central nervous system (CNS) have also been linked to nicotine's effects on appetite and weight loss (Mineur et al., 2011), and so differences in the α3β4 activity of specific smoking cessation agents may also affect the process of weight gain that often occurs following successful smoking cessation. Additionally, there have been numerous reports of adverse neuropsychiatric side effects for varenicline, while there have been fewer such reports for cytisine (Moore et al., 2011; Shytle et al., 2011). The potential importance of α3β4 receptors in the medial habenula and other parts of the brain associated with mood and behavior might suggest that the differences in the neuropsychiatric side effect profiles of varenicline and cytisine could also be related to their differing activity profiles for human α3β4* receptors.
The gene for the nAChR α5 subunit was first identified as part of the gene cluster with α3 and β4 in 1990 (Boulter et al., 1990). The predicted gene product was classified as an alpha subunit based on sequence similarity to other nAChR alpha subunits, most notably the presence of the two vicinal cystines in the structural subdomain of the ligand binding site currently identified as the C-loop. The α5 gene was subsequently confirmed to be expressed in autonomic ganglia (Wang et al., 2002), hippocampus (Sudweeks and Yakel, 2000) and the cortex (Han et al., 2000), as well as the particularly nAChR-rich brain structures of the medial habenula and interpeduncular nucleus (Fowler et al., 2011; Grady et al., 2009). However, for many years the identification of the protein as a putative alpha subunit was something of a puzzle since it was not functional in heterologous expression systems either alone or in combinations with β2 or β4, subunits known to form functional receptors with neuronal alpha subunits. The α5 subunit was subsequently confirmed to be an obligatory structural subunit that could form functional receptors in combination with α4 and β2, or α3 and β4, and possibly other combinations of subunits which would not require α5 for function (Ramirez-Latorre et al., 1996) (Grinevich et al., 2005). Although α5 subunits were not required for function, the presence of α5 subunits has significant effects on receptor pharmacology, in most cases increasing the agonist sensitivity of the receptors formed.
Based on immunoprecipitation and Western blot studies of wild-type and knockout mutant mice, it has been estimated that the nAChR of mouse autonomic ganglia contain approximately 55% α3β4, 21% α3β4β2, and 24% α3β4α5 nAChRs (David et al., 2010). Therefore, although α5-containing receptors play a role in autonomic transmission, the knockout of α5 does not produce a serious deficit in autonomic function, as seen with α3 or β4 knockouts (Wang et al., 2002). Knockout of α5 however, does appear to impact nicotine-mediated behaviors relevant to development of nicotine dependence (Jackson et al., 2011) and adult brain circuitry required for attentional performance (Bailey et al., 2011). It may be the case that the role of α5, in combination with α3 and β4 in the habenular/interpeduncular circuit, accounts for the knockout phenotype related to nicotine associated behavior, while the phenotype associated with attentional performance may relate to receptors containing α4 and β2 in combination with α5 in the cortex, hippocampus, and other parts of the brain associated with cognitive function (Bailey et al., 2011).
The polymorphisms in the α3–β4–α5 gene cluster associated with cancer risk and smoking behavior include several variations in untranslated sequence and a single salient mutation in the sequence coding for the α5 subunit protein. This SNP changes an aspartic acid to an asparagine at amino acid 376 (mature protein numbering, or 398 from start codon), a site within the putative amphipathic helix of the intracellular domain. In co-expression studies with α4–β2 concatamers, Lindstrom and co-workers (Kuryatov et al., 2011) found this mutant in α5 lowered the calcium permeability of the receptors compared to wild-type and may have also affected the receptor’s kinetic properties. They studied the effects of the α5 SNP in co-expression with α3 and β4 using the more conventional approach of expressing monomeric subunits at ratios hypothesized to give enriched populations of α5-containing receptors. Using that approach they found no clear effect of the α5 SNP on the calcium permeability of the putative α3β4α5 receptors. Functional effects were also undetected when these subunits were co-expressed in HEK calls (Li et al., 2011). Consistent with these previous studies, no functional effects of the α5 SNP were detected in the current study on the agonist response profiles of the α3β4α5 receptors. However, as with the introduction of any new experimental tool, our experiments touch upon only a small fraction of the questions that might be addressed.
5.0 Conclusion
Receptors containing α3β4 nAChR subunits have long been considered strictly off target for nAChR targeting CNS therapeutics. However, as new roles are being discovered for these receptors in the CNS, in regard to nicotine use and appetite control (Mineur et al., 2011), it becomes important to characterize the α3β4 receptor subtypes and understand how to target them selectively. Therefore programs are being developed for high throughput screening of α3β4-containing receptors, and the use of the β4–6–α3 concatamer will permit detailed follow up characterizations of potential new drug candidates that may be applied to smoking and other novel CNS indications. Our data additionally highlight the importance of cross characterization of potentially useful therapeutic agents with in vitro tests of both the receptors relevant to animals models and the human receptor subtypes that will ultimately be the molecular targets of therapeutics.
Highlights.
A concatamer of human α3 and β4 nAChR subunits was constructed for expression in Xenopus oocytes.
Insertion of specific subunits was confirmed with selective antagonists and resistant mutants.
No effects of the α5 D376N SNP were observed on agonist-evoked responses of α3β4α5 receptors.
Compared to rat, human α3β4 receptors respond more to varenicline and less to cytisine.
Our data will potentially impact the side-effect liability of agents used for smoking cessation.
Acknowledgements
We thank Shehd Abdullah Abbas Al Rubaiy, Sara Copeland, Matthew Kimbrell, and Matthew Isaacson for conducting OpusXpress experiments and Dr. Lynn Wecker (University of South Florida) for the use of an additional OpusXpress 6000.
Supported by a supplement to GM57481 and James and Esther King Biomedical Research award 1KG12.
Abbreviations
- BTMPS
bis-(2,2,6,6-tetramethyl-4-piperidinyl)-sebacate
- TMPH
2,2,6,6-tetramethylpiperidin-4-yl heptanoate
- GTS-21
3-(2,4dimethoxybenzylidene)anabaseine
- SNP
single nucleotide polymorphism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bailey CD, De Biasi M, Fletcher PJ, Lambe EK. The nicotinic acetylcholine receptor alpha5 subunit plays a key role in attention circuitry and accuracy. J Neurosci. 2011;30:9241–9252. doi: 10.1523/JNEUROSCI.2258-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter J, O'Shea-Greenfield A, Duvoisin RM, Connolly J, Wada E, Jensen A, Gardner PD, Ballivet M, Deneris ES, McKinnon D, Heinemann S, Patrick J. a3, a5, and b4: Three members of the rat neuronal nicotinic acetylcholine receptor-related gene family form of gene cluster. J. Biol. Chem. 1990;265:4472–4482. [PubMed] [Google Scholar]
- Chen X, Chen J, Williamson VS, An SS, Hettema JM, Aggen SH, Neale MC, Kendler KS. Variants in nicotinic acetylcholine receptors alpha5 and alpha3 increase risks to nicotine dependence. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:926–933. doi: 10.1002/ajmg.b.30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David R, Ciuraszkiewicz A, Simeone X, Orr-Urtreger A, Papke RL, McIntosh JM, Huck S, Scholze P. Biochemical and functional properties of distinct nicotinic acetylcholine receptors in the superior cervical ganglion of mice with targeted deletions of nAChR subunit genes. Eur J Neurosci. 2010;31:978–993. doi: 10.1111/j.1460-9568.2010.07133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm S, Slimak MA, Ferrarese L, Santos-Torres J, Antolin-Fontes B, Auer S, Filkin S, Pons S, Fontaine JF, Tsetlin V, Maskos U, Ibanez-Tallon I. Aversion to nicotine is regulated by the balanced activity of beta4 and alpha5 nicotinic receptor subunits in the medial habenula. Neuron. 2011;70:522–535. doi: 10.1016/j.neuron.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Francis MM, Choi KI, Horenstein BA, Papke RL. Sensitivity to voltage-independent inhibition determined by pore-lining region of ACh receptor. Biophys. J. 1998;74:2306–2317. doi: 10.1016/S0006-3495(98)77940-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego X, Molas S, Amador-Arjona A, Marks MJ, Robles N, Murtra P, Armengol L, Fernandez-Montes RD, Gratacos M, Pumarola M, Cabrera R, Maldonado R, Sabria J, Estivill X, Dierssen M. Overexpression of the CHRNA5/A3/B4 genomic cluster in mice increases the sensitivity to nicotine and modifies its reinforcing effects. Amino Acids. 2011 doi: 10.1007/s00726-011-1149-y. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Gerzanich V, Wang F, Kuryatov A, Lindstrom J. alpha 5 Subunit alters desensitization, pharmacology, Ca++ permeability and Ca++ modulation of human neuronal alpha 3 nicotinic receptors. J. Pharmacol. Exp. Ther. 1998;286:311–320. [PubMed] [Google Scholar]
- Grady SR, Moretti M, Zoli M, Marks MJ, Zanardi A, Pucci L, Clementi F, Gotti C. Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. J Neurosci. 2009;29:2272–2282. doi: 10.1523/JNEUROSCI.5121-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinevich VP, Letchworth SR, Lindenberger KA, Menager J, Mary V, Sadieva KA, Buhlman LM, Bohme GA, Pradier L, Benavides J, Lukas RJ, Bencherif M. Heterologous expression of human {alpha}6{beta}4{beta}3{alpha}5 nicotinic acetylcholine receptors: binding properties consistent with their natural expression require quaternary subunit assembly including the {alpha}5 subunit. J Pharmacol Exp Ther. 2005;312:619–626. doi: 10.1124/jpet.104.075069. [DOI] [PubMed] [Google Scholar]
- Han ZY, Le Novere N, Zoli M, Hill JA, Jr, Champtiaux N, Changeux JP. Localization of nAChR subunit mRNAs in the brain of Macaca mulatta. Eur J Neurosci. 2000;12:3664–3674. doi: 10.1046/j.1460-9568.2000.00262.x. [DOI] [PubMed] [Google Scholar]
- Horenstein NA, Leonik FM, Papke RL. Multiple pharmacophores for the selective activation of nicotinic alpha7-type acetylcholine receptors. Mol Pharmacol. 2008;74:1496–1511. doi: 10.1124/mol.108.048892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Marks MJ, Vann RE, Chen X, Gamage TF, Warner JA, Damaj MI. Role of alpha5 nicotinic acetylcholine receptors in pharmacological and behavioral effects of nicotine in mice. J Pharmacol Exp Ther. 2011;334:137–146. doi: 10.1124/jpet.110.165738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Berrettini W, Lindstrom J. Acetylcholine receptor (AChR) alpha5 subunit variant associated with risk for nicotine dependence and lung cancer reduces (alpha4beta2)alpha5 AChR function. Mol Pharmacol. 2011;79:119–125. doi: 10.1124/mol.110.066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Onksen J, Lindstrom J. Roles of accessory subunits in alpha4beta2(*) nicotinic receptors. Mol Pharmacol. 2008;74:132–143. doi: 10.1124/mol.108.046789. [DOI] [PubMed] [Google Scholar]
- Lape R, Colquhoun D, Sivilotti LG. On the nature of partial agonism in the nicotinic receptor superfamily. Nature. 2008;454:722–727. doi: 10.1038/nature07139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, McCollum M, Bracamontes J, Steinbach JH, Akk G. Functional characterization of the alpha5(Asn398) variant associated with risk for nicotine dependence in the alpha3beta4alpha5 nicotinic receptor. Mol Pharmacol. 2011;80:818–827. doi: 10.1124/mol.111.073841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Miller C. Genetic manipulation of ion channels: a new approach to structure and mechanism. Neuron. 1989;2:1195–1205. doi: 10.1016/0896-6273(89)90304-8. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Abizaid A, Rao Y, Salas R, DiLeone RJ, Gundisch D, Diano S, De Biasi M, Horvath TL, Gao XB, Picciotto MR. Nicotine decreases food intake through activation of POMC neurons. Science. 2011;332:1330–1332. doi: 10.1126/science.1201889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TJ, Furberg CD, Glenmullen J, Maltsberger JT, Singh S. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6:e27016. doi: 10.1371/journal.pone.0027016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL. The kinetic properties of neuronal nicotinic receptors: genetic basis of functional diversity. Prog. in Neurobio. 1993;41:509–531. doi: 10.1016/0301-0082(93)90028-q. [DOI] [PubMed] [Google Scholar]
- Papke RL. Tricks of Perspective: Insights and limitations to the study of macroscopic currents for the analysis of nAChR activation and desensitization. Journal of Molecular Neuroscience. 2009;40:77–86. doi: 10.1007/s12031-009-9261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Buhr JD, Francis MM, Choi KI, Thinschmidt JS, Horenstein NA. The effects of subunit composition on the inhibition of nicotinic receptors by the amphipathic blocker 2,2,6,6-tetramethylpiperidin-4-yl heptanoate. Mol Pharmacol. 2005;67:1977–1990. doi: 10.1124/mol.105.011676. [DOI] [PubMed] [Google Scholar]
- Papke RL, Heinemann SF. The partial agonist properties of cytisine on neuronal nicotinic receptors containing the beta2 subunit. Mol. Pharm. 1994;45:142–149. [PubMed] [Google Scholar]
- Papke RL, Trocme-Thibierge C, Guendisch D, Abbas Al Rubaiy SA, Bloom SA. Electrophysiological perspectives on the therapeutic use of nicotinic acetylcholine receptor partial agonists. J Pharmacol Exp Ther. 2011;337:367–379. doi: 10.1124/jpet.110.177485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Wecker L, Stitzel JA. Activation and inhibition of mouse muscle and neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 2010;333:501–518. doi: 10.1124/jpet.109.164566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Latorre J, Yu C, Qu X, Perin F, Karlin A, Role L. Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature. 1996;380:347–351. doi: 10.1038/380347a0. [DOI] [PubMed] [Google Scholar]
- Rollema H, Shrikhande A, Ward KM, Tingley FD, 3rd, Coe JW, O'Neill BT, Tseng E, Wang EQ, Mather RJ, Hurst RS, Williams KE, de Vries M, Cremers T, Bertrand S, Bertrand D. Pre-clinical properties of the alpha4beta2 nicotinic acetylcholine receptor partial agonists varenicline, cytisine and dianicline translate to clinical efficacy for nicotine dependence. Br J Pharmacol. 2010;160:334–345. doi: 10.1111/j.1476-5381.2010.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Sturm R, Boulter J, De Biasi M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J Neurosci. 2009;29:3014–3018. doi: 10.1523/JNEUROSCI.4934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shytle R, Sheehan D, Sanberg PR, Arias HR. Neuronal nicotinic receptors as therapeutic targets for mood disorders. In: Arias HR, editor. Pharmacology of Nicotinic Acetylcholine Receptors from the Basic and Therapeutic Perspectives. India: Research Signpost; 2011. pp. 187–198. [Google Scholar]
- Stevens VL, Bierut LJ, Talbot JT, Wang JC, Sun J, Hinrichs AL, Thun MJ, Goate A, Calle EE. Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer Epidemiol Biomarkers Prev. 2008;17:3517–3525. doi: 10.1158/1055-9965.EPI-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudweeks SN, Yakel JL. Functional and molecular characterization of neuronal nicotinic ACh receptors in rat CA1 hippocampal neurons. J Physiol. 2000;527(Pt 3):515–528. doi: 10.1111/j.1469-7793.2000.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Orr-Urtreger A, Korczyn AD. The role of neuronal nicotinic acetylcholine receptor subunits in autonomic ganglia: lessons from knockout mice. Prog Neurobiol. 2002;68:341–360. doi: 10.1016/s0301-0082(02)00106-5. [DOI] [PubMed] [Google Scholar]
- Wu J, Lukas RJ. Naturally-expressed nicotinic acetylcholine receptor subtypes. Biochem Pharmacol. 2011;82:800–807. doi: 10.1016/j.bcp.2011.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Nelson ME, Kuryatov A, Choi C, Cooper J, Lindstrom J. Human alpha4beta2 acetylcholine receptors formed from linked subunits. J Neurosci. 2003;23:9004–9015. doi: 10.1523/JNEUROSCI.23-27-09004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]