Abstract
T cell-driven B cell hyperactivity plays an essential role in driving autoimmune disease development in systemic lupus erythematosus (SLE). IL-21 is a member of the type I cytokine family with pleiotropic activities. It regulates B cell differentiation and function, promotes T follicular helper cell (TFH) and Th17 cell differentiation and downregulates the induction of T regulatory (Treg) cells. Although IL-21 has been implicated in SLE, the relative importance of IL-21R signaling in CD4+ T cells versus B cells is not clear. To address this question we took advantage of two induced models of lupus-like chronic graft versus host disease (cGVHD) by using WT or IL-21R-/- mice as donors in the parent-into-F1 (P→F1) model and as hosts in the Bm12→B6 model. We show that IL-21R expression on donor CD4+ T cells is essential for sustaining TFH cell number and subsequent help for B cells resulting in autoantibody production and more severe lupus-like renal disease but does not alter the balance of Th17 and Treg cells. On the other hand, IL-21R signaling on B cells is critical for the induction and maintenance of germinal centers, for plasma cell differentiation and autoantibody production and the development of renal disease. These results demonstrate that IL-21 promotes autoimmunity in cGVHD through both CD4+ T cell and B cell intrinsic mechanisms and suggest that IL-21 blockade may attenuate not only the B cell hyperactivity but also the aberrant TFH cell pathway that contributes to lupus pathogenesis.
Introduction
IL-21 is a member of the type I cytokine family with pleiotropic effects on the immune system depending on the cellular context, nature of costimulation and cytokine environment (1). IL-21R is expressed on a variety of immune cells including B, T, NK and dendritic cells (DC), while IL-21 production is restricted to activated CD4+ T cells, T follicular helper cells (TFH), Th17 cells and NK T cells (1, 2). IL-21 promotes the expansion of NK cells and augments their anti-tumor activity, enhances CD8+ T cell maturation into cytotoxic T lymphocytes and promotes the differentiation and expansion of TFH cells (1, 3-5). In addition, within the T cell lineage, IL-21 regulates the reciprocal differentiation of Th17 cells and Treg cells, by promoting Th17 cells expansion and by inhibiting the generation and function of induced (i)Treg cells (6-9). Within the B cell lineage, IL-21 regulates B-cell proliferation and survival, Ig production and class switching, particularly to IgG1, germinal center (GC) formation, plasma cell (PC) differentiation and memory B cell responses (10-13). IL-21 can also induce B cell apoptosis when B cells are activated with LPS, CpG, anti-IgM and IL-4 (14).
Recent evidence suggests that IL-21 may play an important role in autoimmune diseases including SLE, rheumatoid arthritis and Sjögren syndrome (15-18). In humans, an association of IL-21 and IL-21R polymorphisms with SLE along with elevated levels of IL-21 in serum and in CD4+ T cells were reported (17-21). Studies in murine models of lupus have indicated increased production of IL-21 in MRL-Faslpr, BXSB-Yaa mice and in the knockout mouse sanroque (22-24). Furthermore, IL-21 blockade was beneficial in MRL-Faslpr mice while in BXSB-Yaa mice it had a biphasic effect, negatively influencing survival early on and positively influencing survival at later stages of disease (23, 24). In addition, IL-21R deficient BXSB-Yaa mice showed none of the autoimmune abnormalities characteristic of IL-21R-competent BXSB-Yaa mice (25).
The wide range of costimulatory and inhibitory signals delivered by IL-21 on T and B cells suggests a complex role of IL-21 in promoting autoimmunity in vivo. The relative importance of IL-21/IL-21R interaction in promoting SLE through CD4+ T cell dependent mechanisms that may affect TFH, Th17 or Treg cells or through B cell intrinsic mechanisms has not yet been determined. In the absence of conditional knockout mice, it has not been technically possible to investigate this issue in autoimmune-prone lupus models in vivo. Therefore, to address this question we took advantage of the induced lupus-like model of cGVHD that allowed us to independently manipulate T and B cell responses and dissect the requirements of IL-21/IL-21R interaction for the initiation and progression of the disease. To this end, IL-21R sufficient and deficient mice on the B6 background were used as donors in the P→F1 model or as hosts in the Bm12→B6 model of cGVHD. In addition, as the exact timing of disease onset is known, these models allowed us to perform a kinetic analysis of T and B cell activation, differentiation and effector functions (26). Our results indicate that lack of IL-21/IL-21R interaction on either B cells or Ag-specific CD4+ T cells impairs independently the development of autoimmune manifestations of cGVHD and results in an attenuated disease phenotype.
Material and methods
Mice
6-8 wk old male B6D2DF1 (BDF1), B6.C-H-2bm12Eg (Bm12) and C57BL/6J (B6) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Breeding pairs of IL-21R-/- mice on the B6 background were provided by Dr. Michael Grusby (Harvard School of Public Health, Boston, USA) (27). C57Bl/6-Tg(UBC-GFP)30Scha mice, C57Bl/6J transgenic mice that express GFP, were provided by Dr. David Trisler. IL-21R-/- mice used in this study were housed and bred at the University of Maryland Animal Care Facility. All procedures were approved by the University of Maryland School of Medicine OAWA.
Induction of GVHD
Single-cell suspensions of splenocytes were prepared in RPMI 1640, filtered through sterile nylon mesh, washed, and diluted to a concentration of 108 viable (trypan blue excluding) cells/ml. P→F1 chronic GVHD was induced with CD8+ T cell-depleted splenocytes containing 10-15×106 CD4+ donor cells from either B6 WT or B6 IL-21R-/- donors injected i.v. into BDF1 mice as described (28). Flow cytometry was used prior to injection to confirm that equal numbers of CD4+ T cells were injected into recipient F1 mice. Donor CD8+ T cells were depleted using Dynabeads Mouse CD8 (Lyt 2) (InVitrogen, San Diego, CA). Flow cytometric analysis demonstrated <1% contaminating CD8+ T cells. Controls consisted of uninjected age- and sex-matched F1 mice. Bm12→B6 chronic GVHD was induced in B6 WT or B6 IL-21R-/- recipients by i.p. injection of 1×108 Bm12 donor splenocytes. Recipient and donor mice were age- and sex-matched within each independent experiment. In all experiments we used male mice to avoid artifacts due to sex-based difference in IL-21 gene expression (29).
Cell isolation and in vitro generation and measurement of IL-17
Spleen cells from control or P→F1 cGVHD mice were pooled (three mice/pool) and CD4+ T cells were negatively selected using MACS beads (Miltenyi Biotech GmbH). Donor and host cells from cGVHD mice were further purified using biotinylated anti-H2-Kd antibodies and anti-biotin MACS beads. Purified donor CD4+ T were cultured for 5 days with plate-bound anti-CD3 (5μg/ml), anti-CD28 (1μg/ml) and IL-23 (10ng/ml) then restimulated with PMA/Ionomycin for 4h. Supernatants were tested in duplicates for IL-17A expression by ELISA. B cells were purified from control or Bm12→B6 cGVHD mice using MACS beads.
Antibodies and flow cytometry
Spleen cells were first incubated with anti-murine FcγRII/III mAb (2.4G2) for 10 min and then stained with saturating concentrations of Alexa Fluor 488-conjugated, allophycocyanin-conjugated, biotin-conjugated, PE-conjugated, FITC-conjugated, PE/Cy5-conjugated or PE/Cy7-conjugated mAb against CD4, CD8, B220, H-2Kb, H-2Kd I-Ab, I-Ad , CD80, CD86, CD69, CD44, FAS, ICOS, PD-1, GL-7, CXCR5, Annexin, FoxP3, Helios and Ki-67. Antibodies were purchased from BD Biosciences (San Jose, CA), BioLegend (San Diego, CA), eBioscience (San Diego, CA), and Sigma-Aldrich (San Louis, Mo). Biotinylated primary mAb were detected using either streptavidin-allophycocyanin (BioLegend) streptavidin-FITC, streptavidin-PE or streptavidin-PE-Cy5 (BD Biosciences). Cells were fixed in 1% paraformaldehyde before flow cytometric analysis. Intracellular staining for Foxp3, Helios and Ki-67 was performed using the FoxP3 buffer staining set from eBioscience, according to manufacturer's protocol. Annexin V staining wad performed with the FITC Annexin V Apoptosis Detection Kit I (BD Pharmingen) according to the manufacturer's protocol and analyzed by flow cytometry within 1h of staining. Multicolor flow cytometric analyses were performed using a FACScan, Accuri C6 and LSRII flow cytometer (BD Biosciences). Lymphocytes were gated by forward and side scatter, and fluorescence data were collected for a minimum of 10,000 gated cells. Studies of donor T cells in the P→F1 model were performed using a lymphocyte gate that was positive for CD4 and negative for MHC class I of the uninjected parent (H-2Kd negative).
Immunohistochemistry staining of splenic sections
Sections of formalin fixed spleen (5μm) were deparaffinized, rehydrated and then stained as previously described (30). Antibodies with their specificities and conjugations were the following: biotinylated peanut agglutinin (PNA) from Sigma-Aldrich, unlabeled rat anti-human/mouse CD45R(B220) (clone RA3-6B2) from eBioscience, unlabeled rabbit anti-GFP (Invitrogen), streptavidin horseradish peroxidase (Vector Laboratories), alkaline phosphatase-conjugated goat anti-rabbit and alkaline phosphatase-conjugated goat anti-rat Ab (Jackson ImmunoResearch Laboratories). Substrates used were AEC and Blue Alkaline Phophatase Kit (Vector Laboratories).
Preparation of CFSE -labeled donor cells
CFSE (Molecular Probes, Eugene, OR) labeling of donor splenocytes and analysis of donor cell proliferation by flow cytometry were performed as previously described (28). Cells were adjusted to 5×107/ml in PBS/0.1% BSA then incubated in the dark for 10 min at 37° C with CFSE (10mM stock solution diluted in DMSO to a final concentration of 5 μM). Staining was quenched with 5 volumes of ice-cold RPMI/10% FBS then cells were washed three times in PBS before injection into F1 mice. Proliferating CFSE+ donor CD4+ T cells were distinguished by multi-parameter flow cytometry.
ELISPOT
96-well cellulose membrane plates (MAIPS4510; Millipore) was prewet with 35% ethanol for 5 minutes then coated overnight with 100μg/ml prefiltered Herring Sperm DNA (Promega) in PBS at 4° C. Two-fold serial dilutions of spleen cells or bone marrow cells were plated in duplicate overnight on DNA-coated plates starting at 0.5 ×106 /well in 3% BSA/DMEM. After washing, the plates were incubated with anti-mouse IgG alkaline phosphatase (Sigma-Aldrich) at 1:1000 in PBS/3% BSA for 1h at 37° C. Plates were then developed with bromochloroindolyphosphate (KPL, Gaithersburg, MD). Spots were counted using an automated reader (CTL- Europe GmbH Reader System, software version 4).
ELISA for anti-ds-, ss-DNA, total IgG and IgG1 Abs
For quantitation of serum IgG and IgG1 levels of anti-ssDNA, 96 well plates were coated with heat-denatured calf thymus DNA (Sigma-Aldrich), followed by blocking with 1% BSA/PBS, and incubation with serial dilutions of experimental mouse sera beginning at a dilution of 1/40 tested in duplicate. The plates were then incubated with alkaline phosphatase-conjugated anti-mouse IgG and respectively anti-mouse IgG1 (Sigma-Aldrich) and OD was quantitated at 405 nm. For measurement of IgG and IgG1 anti-dsDNA Ab, plates were coated with 100μg/ml prefiltered Herring Sperm DNA (Promega). Following blocking with 3% BSA/PBS, sera was added to the plate at dilutions starting at 1/50. The respective secondary Abs were alkaline phosphatase-conjugated anti-mouse IgG and anti-mouse IgG1 (Sigma-Aldrich). For each experiment, murine MRL-Faslpr sera were used as a standard and results were converted to arbitrary units.
For total IgG and IgG1, goat anti-mouse IgG or IgG1 (Southern Biotech, Birmingham AL) was coated at 5μg/ml onto plates. Following blocking with 3% BSA/PBS, sera was added to the plate at dilutions starting at 1/50.000 and 1/25.000 respectively. Mouse IgG and respectively IgG1 (Rockland, Gilbertsville, PA) was used as a standard. The plates were incubated with alkaline phosphatase-conjugated anti-mouse IgG (Sigma-Aldrich) and respectively anti-mouse IgG1 (ImmunoJackson Lab).
Real time PCR
Total RNA isolation, quantitation and reverse transcription was performed as described (28). 18S rRNA was used as an internal control. All primers and probes (IL-21, IL-4, IL-10, IL-6, bcl-6, RORγt, IL-17A, Prdm1, aicda and 18S) were purchased from SABiosciences (Frederick, MD). RT-PCR was carried out on an Applied Biosystems Step1 Plus PCR machine (Applied Biosystems, Foster City, CA).
Kidney histopathology and immunofluorescence
Formalin fixed kidney sections (4 μm) were stained with hematoxylin/eosin. The sections were examined in a blinded fashion (C.C. and H.R.) for glomerular, tubular and interstitial pathology. Disease was scored on a semiquantitative scale using the published criteria with modifications (31). The severity of glomerulonephritis (GN) was graded on a 0-3 scale where 0=normal; 1= mild to moderate increase in cellularity with mesangial proliferation; 2=moderate increase in cellularity with endocapillary and mesangial proliferation, increased matrix, and/or karyorrhexis; 3=marked increase in cellularity with endocapillary proliferation, crescent formation, and/or necrosis, and/or sclerosis. Scores from 20 glomeruli were averaged to obtain a mean score for each kidney section. Deposits of IgG in the glomeruli were detected by incubating acetone fixed, 5-μm-thick cryostat sections of kidney in 20% normal goat serum for 30min, followed by a 1h incubation with FITC-conjugated goat anti-mouse IgG (1/500; Southern Biotech). Fluorescence in glomerular capillary walls and in the mesangium was subjectively scored blindly on a scale of 0–3 (0=none; 1= weak; 2= moderate; 3=strong); 10 glomeruli per section were analyzed.
Statistical analysis
Normally distributed data were analyzed by unpaired t test and nonparametric data by Mann-Whitney test using Prism 4.0 (Graphpad) software.
Results
Upregulation of IL-21 production and TFH differentiation of donor CD4+ T cells
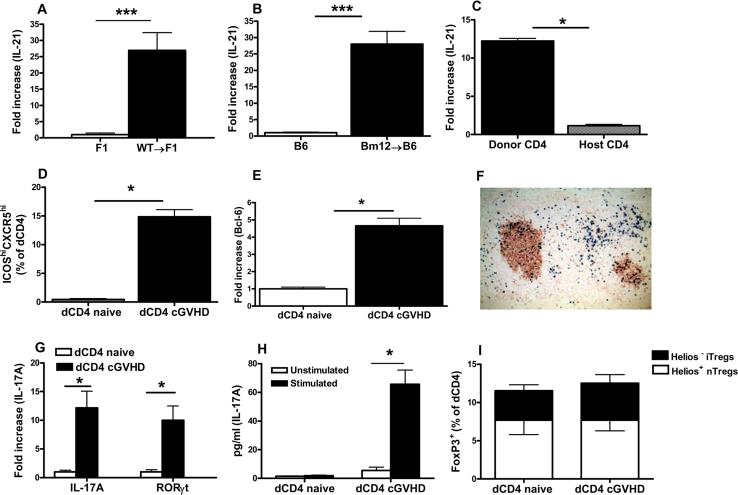
We first evaluated whether IL-21 is upregulated in the P→F1 and Bm12→B6 models of cGVHD. To this end, we measured IL-21 mRNA expression by RT-PCR in splenocytes of cGVHD mice at 2 weeks after disease induction when the autoimmune features are already present. IL-21 mRNA production increased by 30- and 28- fold compared to normal control mice in P→F1 cGVHD and Bm12→B6 cGVHD mice, respectively (Figs. 1A-B). In both models of cGVHD, donor CD4+ T cells activated by host MHC class II, expand and provide cognate help to host B cells. As activated CD4+ T cells are known to produce IL-21, we evaluated whether donor CD4+ T cells are the major source of IL-21. We addressed this question in the P→F1 model in which donor CD4+ T cells (H-2Kd-) can be distinguished by flow cytometry and separated from H-2Kd+ host cells. As seen in Figure 1C, IL-21 mRNA transcripts were significantly higher in purified donor CD4+ T cells than in host CD4+ T cells.
Figure 1. Donor CD4+ T cells produce IL-21 and display TFH, Th17 and Treg cell phenotypes.
A-B. P→F1 cGVHD (A) and Bm12→B6 cGVHD (B) were induced as described in Materials and Methods. Splenocytes were analyzed after 14 days for IL-21 mRNA expression by RT-PCR. Results were normalized to 18S RNA. C. Two weeks after P→F1 cGVHD induction, donor and host CD4+ T cells were isolated from pooled mice as described in Materials and Methods. RT-PCR for IL-21 was performed. D. Mean percentage of donor TFH cells detected by flow cytometry as ICOShiCXCR5hiCD4+H2-Kd- T cells in P→F1 cGVHD mice at 2 weeks after induction. E. Naïve donor CD4+ T cells prior to transfer and engrafted donor CD4+ T cells from cGVHD mice were isolated as described in Materials and Methods and RT-PCR for Bcl-6 was performed. F. P→F1 cGVHD was induced with GFP expressing WT donor cells. Immunohistochemistry staining of spleen section for PNA+ GCs (brown) and donor GFP+ cells (blue) was performed at 10 days after disease induction. G. mRNA expression for IL-17A and RORγt was assessed ex vivo from donor CD4+ T cells purified on day 14 after cGVHD induction. Purified, naive CD4+ T cells prior to transfer were used as controls. H. Purified donor CD4+ T cells were stimulated with anti-CD3, anti-CD28 and IL-23 for 5 days then restimulated with PMA/Ionomycin. Supernatants were tested for IL-17 expression by ELISA. I. Mean percentage of donor Treg cells identified by flow cytometry as FoxP3+ cells among uninjected, naïve donor CD4 cells and engrafted donor CD4+ cells at day 14. Helios staining was used to distinguish FoxP3+Helios+ nTregs and FoxP3+Helios– iTregs. Data are representative of two independent experiments; n=5 mice/group. *=p<0.05. ***=p<0.001
Recent studies have highlighted a role for IL-21 as a growth factor for TFH cells and as a regulator of the reciprocal differentiation of Th17 and Treg cells (6, 9, 32). To assess the effect of IL-21R signaling on these subsets, we evaluated whether TFH, Th17 and Treg cells of donor origin would be readily detectable in cGVHD mice. To this end, we first assessed whether donor CD4+ T cells differentiated into TFH cells, with upregulation of the TFH transcription factor Bcl-6 and localization in the GCs. TFH cells detected by flow cytometry as ICOShi CXCR5hi cells among CD4+H2-Kd- donor cells expand from 0.45±02% at baseline in naïve donor CD4+ T cells prior to transfer to 14±2% of donor CD4+ T cells engrafted in cGVHD mice (Fig. 1D). Furthermore, by RT-PCR we detected a significant upregulation of Bcl-6 mRNA in purified donor CD4+ T cells from cGVHD mice as compared to naïve, uninjected donor CD4+ T cell controls (Fig 1E). Consistent with their differentiation into TFH cells, in mice with cGVHD induced with GFP expressing cells, donor GFP+ cells detected by immunohistochemistry were preferentially localized in GCs as well as in the B cell area and at the T-B border (Fig. 1F). Similarly, mRNA transcripts for IL-17A and RORγt, the transcription factor required for Th17 cell differentiation, are upregulated in donor CD4+ T cells purified from cGVHD mice compared to naïve, uninjected CD4+ T cells (Fig.1G). Furthermore, purified donor CD4+ T cells from cGVHD mice, cultured in vitro with anti-CD3/ anti-CD28 Ab and IL-23, a cytokine known to expand differentiated Th17 cells, produced significant levels of IL-17 in supernatants (Fig. 1H). We could not detect IL-17 production from naïve, uninjected CD4+ T cells under the same conditions (Fig. 1H). The percentage of CD4+FoxP3+ Tregs was similar in the uninjected, naïve donor CD4+ T cells and in donor CD4+ T cells from cGVHD mice at two weeks after disease induction. Furthermore, the proportions of natural Tregs (nTregs) and iTregs determined by intracellular Helios staining was comparable in naïve, uninjected and engrafted donor CD4+ T cells. These results indicate that in cGVHD, donor CD4+ T cells upregulate IL-21 mRNA expression, differentiate into TFH and Th17 cells while maintaining the same proportion of natural and induced Treg cells.
IL-21R deficiency on donor CD4+ T cells attenuates B cell parameters and kidney disease in P→F1 cGVHD
After activation, donor CD4+ T cells provide MHC class II-restricted cognate help to host B cells resulting in chronic B cell hyperactivity and expansion. Consequently, IgG anti-ssDNA Ab levels are elevated early (day 14) after donor cell transfer and renal disease occurs after 2 month (26, 33). To assess the contribution of IL-21/IL-21R interaction on donor CD4+ T cells to the disease phenotype, we compared early and late disease parameters of P→F1 cGVHD induced with donor cells from IL-21R-/- mice, which abrogates IL-21/IL-21R interaction on donor cells but not on host B cells, versus cGVHD induced with donor cells from WT mice.
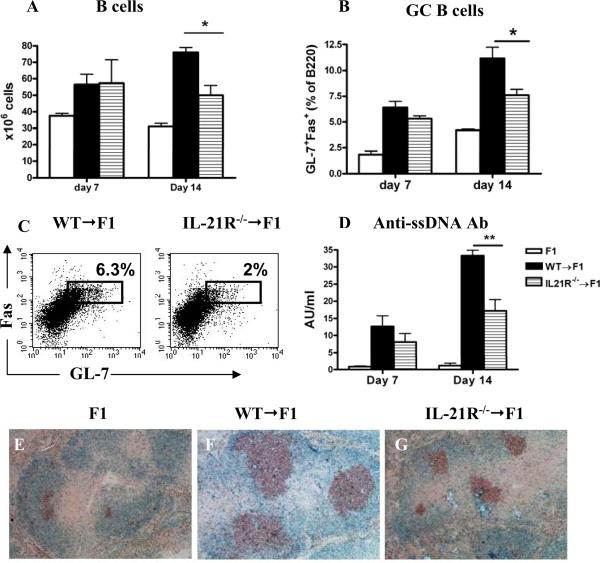
At 7 and 14 days after cGVHD induction with WT or IL-21R-/- CD8 depleted donor cells, we determined the number of B cells, B cell MHC class II expression, the proportions of GL-7+ Fas+ B220+ GC B cells and the levels of anti-ssDNA autoAb. After 7 days, no significant differences were observed in any of these parameters between the two groups. By comparison, after 14 days, mice injected with IL-21R-/- donor cells had a significantly lower number of B cells (Fig. 2A) and a trend toward decreased MHC class II expression, although this did not reach significance (data not shown). The proportion of GL-7+ Fas+ B220+ GC B cells was also significantly lower in the spleens of cGVHD mice injected with IL-21R-/- donor cells (Figs. 2B and C). Consistent with the flow cytometry data, GCs were smaller by immunohistochemistry (Figs. 2E-G). Furthermore, IgG anti-ssDNA Ab levels were significantly lower in mice injected with IL-21R-/- donor cells. These data suggest that IL-21/IL-21R interaction on donor CD4+ T cells is dispensable for the initiation of the autoimmune B cell response, but is required for the optimal expansion of host B cells and GC B cells and for maximal autoAb production.
Figure 2. IL-21R deficiency on donor CD4+ T cells attenuates B cell parameters of cGVHD.
P→F1 cGVHD was induced as described in Materials and Methods. Serum and spleens were collected at the specified time points. A. Absolute number of host B cells. B. Frequency of GL-7+Fas+GC B cells. C. Flow cytometric contour plots of GL-7+Fas+ GC B cells (B cell gate) shown. D. Anti-ssDNA Ab levels were determined by ELISA. E-G. Spleen sections obtained at two weeks were stained for PNA-R+ GC B cells (brown) and B220 (blue). Original magnification x 100. Data are representative of two independent experiments; n=5 mice/group. *=p<0.05.
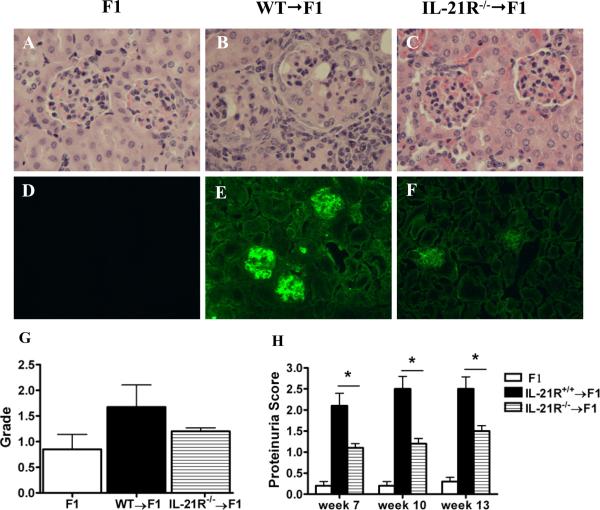
In long term studies, mice with cGVHD induced with IL-21R-/- donor cells developed an attenuated lupus-like renal disease compared to mice with cGVHD induced with WT donor cells and displayed much milder GN (Figs. 3A-C and H), significantly lower proteinuria (Fig. 3G) and decreased deposition of IgG (Figs. 3D-F).
Figure 3. IL-21R deficiency on donor CD4+ T cells ameliorates lupus-like nephritis in P→F1 cGVHD.
P→F1 cGVHD was induced as described in Materials and Methods. A-C. H&E-stained kidney sections of normal F1 control mice, cGVHD mice induced with WT or IL-21R-/- donor cells at 13 weeks after disease induction. Enlarged glomerulus with crescent formation, glomerular sclerosis and interstitial infiltrate are noted in panel B. D-F. Immunofluorescent staining of IgG deposits. Original magnification × 200. G. Proteinuria. H. Glomerulonephritis pathologic scores. Data are representative of two independent experiments; n=5 mice/group.*=p<0.05.
IL-21R deficient donor T cells exhibit diminished TFH expansion and persistence
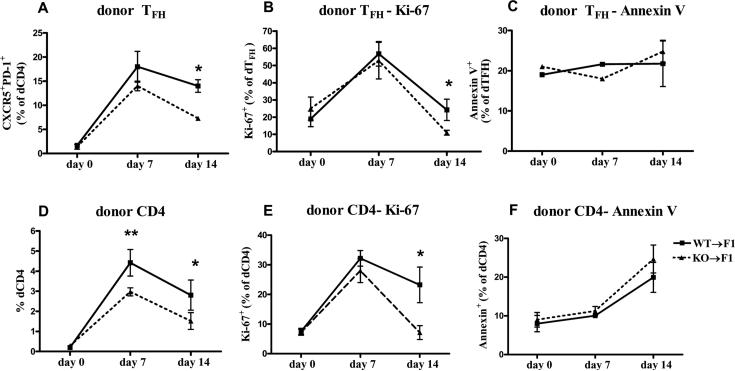
The diminished host B cell and GC expansion and decreased anti-ssDNA Ab production at two weeks after disease induction in the absence of IL-21/IL-21R interaction on donor CD4+ T cells suggests a defect in sustained CD4+ T helper function. We evaluated whether this effect is due to altered donor TFH cell differentiation and/or expansion. In mice receiving WT CD8 depleted donor cells, CXCR5+PD-1+ donor TFH cells increased from 1.4±0.5% in naïve donor CD4+ T cells at the time of transfer, to a peak of 18±3% at day 7 then decreased slightly to 14±1.3% by day 14. In mice with cGVHD induced with IL-21R-/- donor cells, the proportion of TFH cells was lower at both day 7 and 14 but reached statistical significance only on day 14 (Fig. 4A and Supplemental Fig.1B). The decrease in IL-21R-/- TFH cell frequency was mainly due to decreased proliferation as the proportion of dividing Ki-67+ donor TFH cells was significantly lower at two weeks of disease (Fig. 4B and Supplemental Fig.1D). The frequency of apoptotic dTFH cells was slightly higher in mice injected with IL-21R-/- donor cells at two weeks of disease but the difference was not statistically significant (Fig. 4C). Next we assessed whether the decreased expansion of dTFH cells is specific for this T cell subset or it affects non-TFH donor CD4+ T cells as well. As seen in Fig. 4D, the percentage of engrafted donor CD4+ T cells is significantly lower on both day 7 and 14 in the absence of IL-21R. Similar to donor TFH cells, in WT donor cells, the frequency of proliferating CD4+ T cells increased 4.2 fold from baseline at day 7 and 3 fold at day 14. By comparison, in IL-21R-/- donor cells, the percentage of proliferating donor CD4+ T cells was lower but not statistically significant on day 7 and further decreased to significantly lower levels by day 14 (Fig. 4E and Supplemental Fig. 1C). Similar to donor TFH cells, the apoptotic rate tended to be higher in IL-21R-/- injected mice, although the difference was not statistically significant (Fig. 4F). These results suggest that IL-21R is important for the sustained proliferation of TFH as well as non-TFH donor CD4+ T cells from days 7 to 14. Although we were unable to demonstrate differences in apoptotic rates, altered survival of the TFH as well as non-TTH donor CD4+ T cells cannot be completely ruled out.
Figure 4. IL-21R deficient donor CD4 and TFH cells exhibit decreased expansion and proliferation in P→F1 cGVHD.
P→F1 cGVHD was induced as described in Materials and Methods. Recipient mice were sacrificed at the times indicated. Mean percentage of donor TFH cells (A), Ki-67+ donor TFH cells (B), Annexin V+ donor TFH cells (C) , donor CD4+ T cells (D), Ki-67+ donor CD4+ T cells (E) and Annexin V+ donor CD4+ T cells (F) are shown. Data are representative of two independent experiments; n=5 mice/group. *=p<0.05.
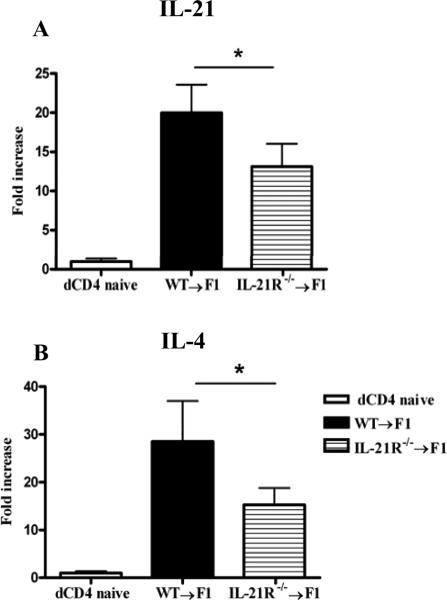
IL-21 and IL-4, known for their ability to promote GC development, are the main cytokines secreted by TFH cells (34, 35). We determined whether IL-21 and IL-4 mRNA expression are altered in donor CD4+ T cells from mice injected with IL-21R deficient donor cells. As seen in Fig. 5, both IL-21 and IL-4 mRNA transcripts are decreased in IL-21R-/- donor CD4+ T cells, suggesting that the expression of these cytokines parallels the decrease in donor CD4+ T cells and TFH cells.
Figure 5. Decreased IL-21 and IL-4 mRNA expression in IL-21R-/- donor CD4+ T cells.
Two weeks after P→F1 cGVHD induction, donor CD4+ T cells purified as described in Materials and Methods were analyzed for IL-21 (A) and IL-4 (B) mRNA expression by RT-PCR. Results were normalized to 18S RNA. Results represent mean ± SEM (n=6 mice/group). Data are representative of two independent experiments. *=p<0.05.
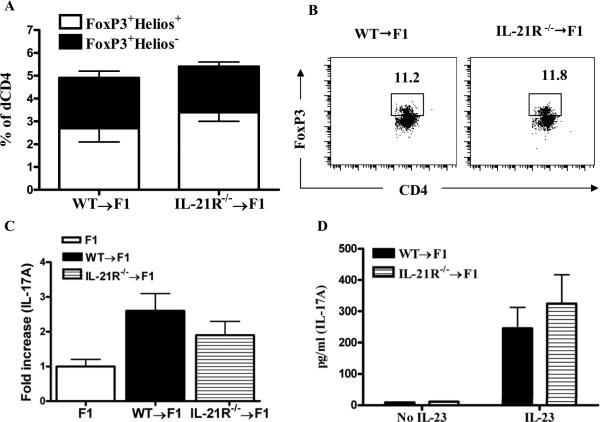
Donor Treg and Th17 cells are not altered in the absence of IL-21R signaling
To assess whether lack of IL-21R signaling on donor CD4+ T cells alters the proportion or donor Treg and donor Th17 cells, we initially compared the percentage of FoxP3+CD4+ donor cells in cGVHD mice induced with IL-21R sufficient and deficient cells. At baseline, there was no difference in the frequency of CD4+Fox P3+ Treg cells in the injected donor cells of IL-21R+/+ and IL-21R-/- origin (not shown). At two weeks after disease induction, the frequency of donor CD4+FoxP3+ Treg cells did not differ between IL-21R+/+ and IL-21R-/- donor CD4+ T cells (Figs. 6A and B). Furthermore, we did not observe a significant difference between the proportions of FoxP3+Helios+ nTregs and FoxP3+Helios– iTregs. Similarly, we observed no difference in either IL-17 mRNA expression or IL-17 secretion of in vitro stimulated donor CD4+ T cells purified from mice with cGVHD induced with IL-21R+/+ or IL-21R-/- donor cells (Figs. 6C and D).
Figure 6. Donor Treg and Th17 cells are not altered in mice with cGVHD induced with IL-21R-/- donor cells.
A. Two weeks after P→F1 cGVHD induction, spleens from recipient F1 mice were examined by flow cytometry for the frequency of FoxP3+ donor Treg cells. Helios staining was used to further identify FoxP3+Helios+ nTregs and FoxP3+Helios– iTregs. B. Flow cytometric contour plots of FoxP3+CD4+ (donor cell gate). C. mRNA expression for IL-17A was assessed ex vivo on day 14 from naïve, uninjected CD4+ T cells and donor CD4+ T cells purified from cGVHD mice. D. Purified donor CD4+ T cells from cGVHD mice at day 14 were stimulated for 5 days with plate-bound anti-CD3 (5μg/ml), anti-CD28 (1μg/ml) and IL-23 (10ng/ml) then restimulated with PMA/Ionomycin for 4 h. Supernatants were tested for IL-17 expression by ELISA. Data are representative of two independent experiments; n=5-6 mice/group. *=p<0.05.
Decreased B cell activation, (auto)Ab production and kidney disease severity in Bm12→B6 cGVHD induced in IL-21R-/- recipients
To assess the contribution of IL-21R signaling on host B cells to the disease phenotype independent of IL-21R signaling on donor CD4+ T cells, we compared early and late disease parameters in Bm12→B6 cGVHD induced in IL-21R deficient recipients, which abrogates IL-21/IL-21R interaction on host cells but not on donor cells, versus cGVHD induced in WT hosts.
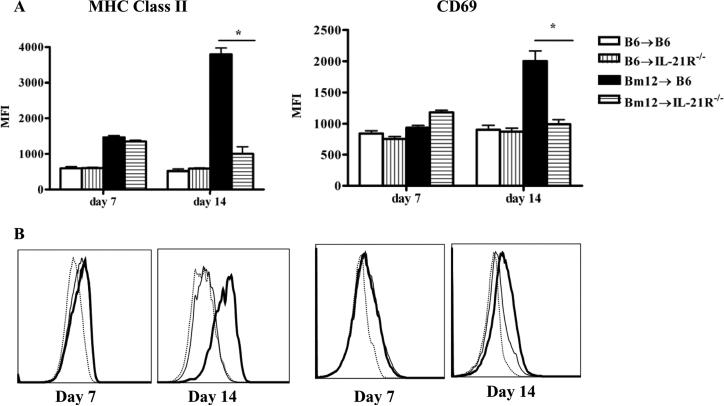
B cell activation that characterizes cGVHD is a complex multistep process involving a nonstringent step in which activation of donor T cells induces polyclonal activation and proliferation of all B cells and a stringent, cognate interaction resulting in the activation of autoreactive B cells such as anti-dsDNA B cells and autoAb production (36). We first assessed whether parameters of B cell activation were altered in the absence of IL-21R signaling on host B cells. MHC class II and CD69 were upregulated to a similar extent on B cells from IL-21R sufficient and deficient cGVHD recipients at 7 days after disease induction. However, at two weeks of disease, MHC class II expression and CD69 were further upregulated on B cells from IL-21R+/+ but not IL-21R-/- hosts (Figs. 7A and B). These data suggest that in the absence of IL-21R signaling, B cells fail to reach optimal levels of activation. However, we cannot exclude the possibility that the lack of IL-21R on activated B cells impairs their survival.
Figure 7. Activation phenotype of host B cells is attenuated in the absence of IL-21R signaling in Bm12→B6 cGVHD.
Bm12→B6 cGVHD was induced in IL-21R+/+ and IL-21R-/- recipient B6 mice as described in Materials and Methods. At 7 and 14 days after disease induction, spleens were examined by flow cytometry. A. MFI of MHC class II and CD69 on B cells. B. Flow cytometric histograms of MHC class II and CD69 on B cells from B6→ B6 mice (dotted line), Bm12→ WT B6 (thick line) and Bm12→ IL-21R-/- (thin line). Data are representative of two independent experiments for day 7 and 3 independent experiments for day 14; n=5 mice/group. *=p<0.05.
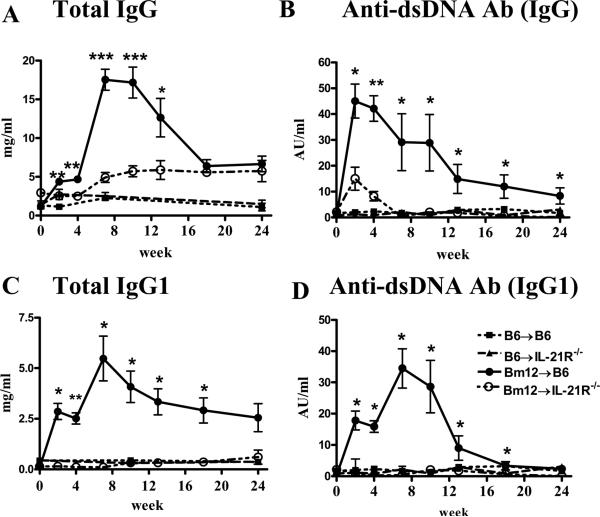
Serum levels of total IgG Abs increased significantly in IL-21 sufficient hosts as early as two weeks, peaked around week 7 then gradually decreased by week 18 to levels reached at weeks 2 and 4. IL-21R deficient hosts displayed significantly lower levels of total IgG at all time points before week 18 when they reached a plateau at levels similar to those detected in IL-21R sufficient hosts (Fig. 8A). IgG anti-dsDNA autoAb levels were only transiently detected at low titer at 2 and 4 weeks in IL-21R-/- compared to IL-21R+/+ hosts and were significantly lower at all time points (Fig. 8B). As IL-21 induces isotype switching to IgG1 we assessed the levels of total and anti-dsDNA IgG1 Abs. Both total IgG1 and anti-dsDNA IgG1 Abs were detected only in IL-21R sufficient hosts and displayed a kinetic similar to IgG Ab levels (Figs. 8C and D). IL-21R-/- hosts had total and anti-dsDNA Ab IgG1 levels similar to control groups.
Figure 8. IL-21R deficiency on host B cells impairs total and anti-dsDNA IgG and IgG1 Ab production.
Sera from Bm12→B6 cGVHD mice were assayed by ELISA for the presence of total IgG and IgG1 (A and C) and anti-dsDNA IgG and IgG1 (B and D). *=p<0.05, **=p<0.01, ***=p<0.001 comparing IL-21R sufficient and deficient cGVHD groups. Data are representative of two independent experiments; n=6-8 mice/group.
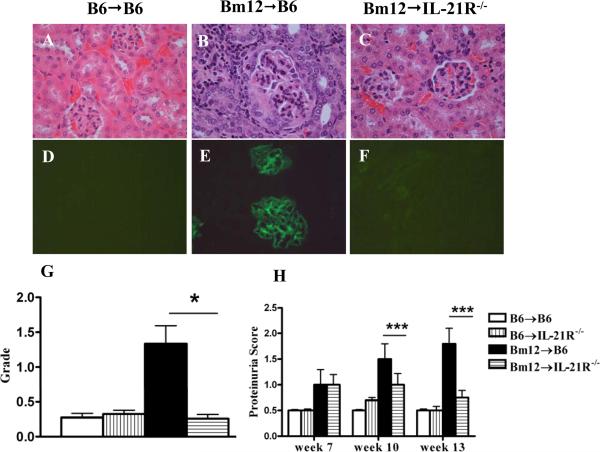
The observation that cGVHD mice have greatly reduced levels of anti-dsDNA autoAbs suggested that these mice may develop less severe renal disease. Indeed, proteinuria was significantly decreased in IL-21R-/- compared to IL-21R+/+ cGVHD mice at 10 and 13 weeks after disease induction (Fig. 9H). Furthermore, typical histological features of autoimmune GN such as mesangial and capillary cell proliferation and crescent formation were observed in the kidneys of WT but not IL-21R-/- cGVHD mice (Figs. 9A-C). The latter had glomerular histological scores similar to the control groups (Fig. 9G). In addition, WT but not IL-21R-/- recipients of Bm12 cells displayed glomerular IgG deposition at 13 wk after disease induction (Figs. 9 D-F). There was no difference between WT and KO recipient groups in the degree of perivascular lymphoid infiltration and tubulointerstitial damage (not shown).
Figure 9. IL-21R deficiency on host B cells ameliorates lupus-like nephritis in Bm12→B6 cGVHD.
Bm12→B6 cGVHD was induced as described in Materials and Methods. A-C. H&E-stained kidney sections of control mice, cGVHD mice induced in WT or IL-21R-/- hosts at 16 weeks after disease induction. Enlarged glomerulus with crescent formation, glomerular sclerosis and interstitial infiltrate are noted in panel B. D-F. Immunofluorescent staining of IgG deposits. Original magnification x 200. G. Glomerulonephritis pathologic scores. H. Proteinuria scores. *=p<0.05, **=p<0.01, ***=p<0.001. Data are representative of one experiment; n=8 mice/group.
Decreased GCs and plasma cell differentiation in IL-21R-/-cGVHD mice
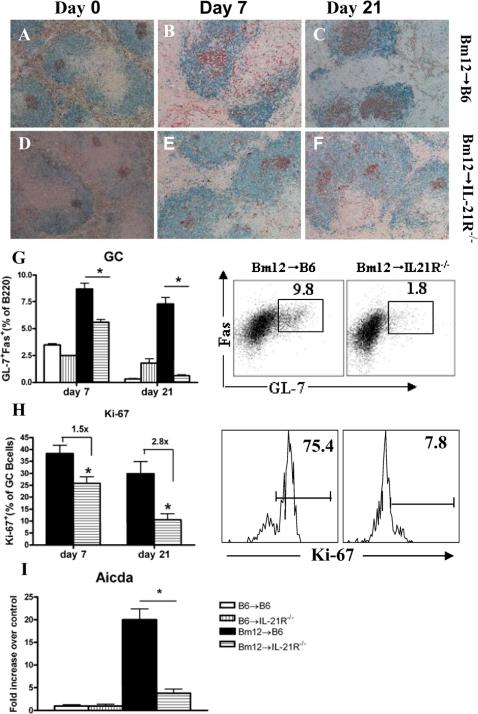
In models of protein immunization, the role of IL-21/IL-21R interaction in the initiation and/or maintenance of GCs has been controversial (5, 13, 37, 38). We assessed the importance of IL-21R signaling on B cells for GC formation and maintenance in Bm12→B6 cGVHD at one week after disease induction, when GC are initially formed and at 3 weeks when GC have already peaked. By flow cytometry, the percentages of GC B cells decreased by 40% at day 7 and by 60% at day 21 after disease induction in IL-21R deficient hosts (Fig. 10G). By immunohistochemistry, splenic GCs in IL-21R-/- hosts were smaller, ill-formed, disrupted and without apparent polarization as compared to those in IL-21R+/+ hosts, both at 7 and 21 days after disease induction (Figs. 10A-F). These differences were maintained at day 28 (not shown). These data suggest that in cGVHD the absence of IL-21R signaling on B cells reduces the magnitude of the initial germinal center response as well as its maintenance.
Figure 10. IL-21R deficiency on host B cells decreases GCs in Bm12→B6 cGVHD.
Bm12→B6 cGVHD was induced as described in Materials and Methods. Recipient mice were sacrificed at the times indicated. A-F. Spleen sections from Bm12→B6 (A-C) and Bm12→IL-21R-/- (D-F) cGVHD mice were stained for B220 (blue) and PNA (brown). Original magnification x 200. G. Flow cytometry graphic analysis and contour plots of GL-7+ Fas+ GC cells gated on B220+ lymphocytes. H. Quantification of proliferating Ki-67+ GC B cells and representative flow cytometry profiles. I. RT-PCR for Aicda in purified B cells. mRNA levels were normalized to 18S and reported as fold increase over normal control. Data are representative of two independent experiments; n=5 mice/group. *=p<0.05
Consistent with the decrease in GC B cell numbers, we observed a significant decrease in the proportion of proliferating GC B cells in the absence of IL-21R signaling. The percentage of Ki-67+ Fas+GL-7+GC B cells decreased by 1.5 fold at 7 days and by 2.8 fold at 21 days after disease induction compared to controls (Fig. 10H). Thus, although the absence of IL-21R signaling decreases GC B cell proliferation at both early and late time points, the magnitude of this reduction is greater at later time points suggesting the IL-21R signaling has a predominant role in the maintenance of GCs. Among GC B cells, centroblasts are highly proliferative cells that express AID (encoded by aicda). We detected significantly decreased aicda mRNA expression in purified B cells from IL-21R-/- hosts (Fig. 10I).
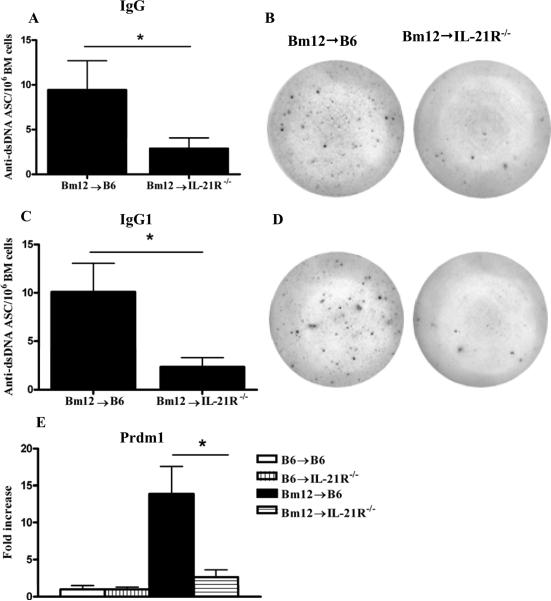
The decreased level of anti-dsDNA Abs and the attenuated GC response in IL-21R-/- hosts suggests the possibility of decreased PC differentiation in the GCs. We examined by ELISPOT the number of anti-dsDNA Ab secreting PC in the bone marrow at 28 days after disease induction when PC have already migrated out of the spleen. Anti-dsDNA secreting PC were significantly lower in the bone marrow of IL-21R-/- hosts for both IgG (Figs.11A-B) and IgG1 Abs (Figs.11C-D). Consistent with the decrease in PC formation, mRNA expression of Prdm1, the gene encoding BLIMP-1, the master regulator of PC differentiation, was significantly decreased in purified B cells from IL-21R-/- cGVHD mice (Fig.11E).
Figure 11. Lack of IL-21R on host B cells affects plasma cell formation after Bm12→B6 cGVHD induction.
Bm12→B6 cGVHD was induced as described in Materials and Methods. Bone marrow cells were collected at 28 days after immunization. The frequency of IgG (A) and IgG1(B) anti-dsDNA Ab secreting cells (ASC) was assessed by ELISPOT. The results are presented as anti-dsDNA ASC per million splenocytes. *=p<0.05. C. Prdm1 mRNA expression was determined by RT-PCR in purified B cells at 14 days after disease induction. mRNA levels were normalized to 18S and reported as fold increase over normal control. Data are representative of two independent experiments; n=5 mice/group. *=p<0.05
Normal donor CD4+ T cell priming and TFH cell formation in IL-21R-/- cGVHD mice
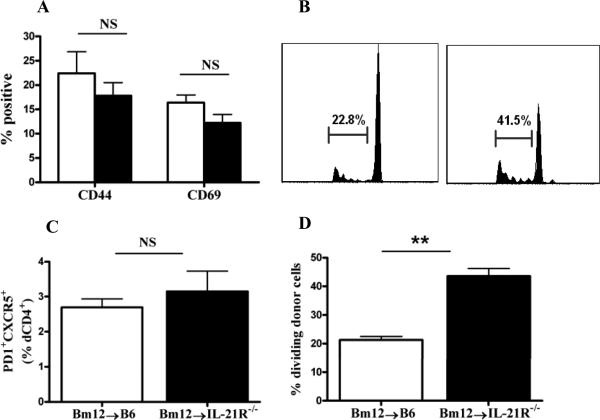
The diminished GC response and autoAb levels in the IL-21R-/- recipients raises the question of the quality of the T cell help in these mice. Therefore we assessed whether the decreased GC and auto-Ab response in IL-21R-/- cGVHD mice could be due to a failure of T cell priming or to a suboptimal donor TFH cell response. T cell priming was assessed using Bm12 donor cells labeled with CFSE prior to GVHD induction followed by detection of activation markers and proliferation rate of CFSE positive donor cells on day 3 after cGVHD induction. CD4+CFSE+ donor cells exhibited similar expression of the activation markers CD44 and CD69 whether injected into either WT or IL-21R-/- hosts (Fig. 12A). Similar results were obtained on day 5 (not shown). Interestingly, as previously reported for CD8+ T cells, the proliferative response of CFSE labeled donor CD4+ T cells was greater in IL-21R-/- than in IL-21R+/+ hosts, possibly due to increased concentration of IL-21 on donor cells in IL-21R-/- host mice (39). These data indicate that donor CD4+ T cells injected into IL-21R-/- mice were activated and expanded to the same extent, if not higher, as those transferred to the WT mice.
Figure 12. Lack of IL-21R on host B cells does not alter the priming and TFH differentiation of donor CD4+ T cells in Bm12→B6 cGVHD.
Bm12→B6 cGVHD was induced using CFSE labeled donor cells. A. Mean percentage of CD44hi and CD69hi donor cells detected as CFSE+ CD4+ T cells at 3 days after disease induction. B. Proliferation of donor CD4+ T cells detected by generational analysis of CFSE+ cells. C. Percentage of proliferating donor cells. D. Percentage of PD-1+CXCR5+ dTFH cells at 7 days after disease induction. Data are representative of two independent experiments; n=5 mice/group. **=p<0.01
Cognate interaction between primed T cells and B cells at the T-B border provide the required signals for TFH cell differentiation (5, 35, 40-42). We assessed whether lack of IL-21R signaling on B cells impairs the differentiation of TFH cells. To this end, CFSE labeled donor CD4+ T cells were assessed on day 7 after disease induction for the percentage ofPD-1+CXCR5+ TFH cells. The proportion of CFSE+ donor TFH cells was similar in both IL-21R sufficient and deficient cGVHD mice (Fig. 12D). Thus, these data demonstrate that at early time points, donor CD4+ T cells from IL-21R-/- host mice were activated, proliferated and developed into TFH cells to the same extent as the donor cells from WT mice. Therefore, the attenuated humoral response is not a consequence of a failure of donor T cell priming or TFH differentiation.
Discussion
In this study we combined the P→F1 and Bm12→B6 models of lupus-like cGVHD to investigate the importance of IL-21R signaling on CD4+ T cells independently of IL-21R signaling on B cells to the initiation and progression of the disease. Previous studies addressing this issue in bone marrow chimeras and adoptive transfer systems in recipients immunized with protein antigens have reported conflicting results. While some studies have reported that the effect of IL-21 on the immune response was exclusively CD4+ T cell intrinsic others have shown a B cell intrinsic mechanism (5, 13, 37, 38). In contrast, our data demonstrate that in cGVHD, IL-21 promotes disease parameters such as GC formation, plasma cell differentiation, autoAb production and GN through both CD4 cell dependent and B cell intrinsic mechanisms. Specifically, cGVHD induced with either IL-21R deficient donor CD4+ T cells in the P→F1 model or with IL-21R sufficient donor cells in IL-21R deficient hosts (hence IL-21R-/- B cells) in the Bm12→B6 model displayed an attenuated lupus-like phenotype with respect to GC formation, autoAb production, plasma cell differentiation and renal disease.
CD4+ T cell help provided by TFH cells is the primary limiting factor for GC formation and subsequent GC B cell responses. Conflicting data have been reported on the role of IL-21 in the differentiation or persistence of TFH cells. In the context of protein immunizations or viral infections, IL-21 promoted TFH differentiation and function in a number of studies, while in others IL-21 had a modest impact or no role (4, 5, 13, 37, 38, 43). In addition, in the autoimmune sanroque mice characterized by excessive TFH cell numbers, loss of IL-21 did not correct the increased TFH cell population (44). Our kinetic analysis of the TFH response in the P→F1 cGVHD model, demonstrates that lack of IL-21/IL-21R interaction on donor CD4+ T cells resulted in a modest decrease in the expansion of donor derived TFH cells at earlier time points and a more pronounced decline at later time points. These results indicate that in our model, IL-21 contributes primarily to the persistence of TFH cells and to a lesser extent to their differentiation and initial expansion. Furthermore, our data suggest that IL-21 promotes the persistence of TFH cells primarily by sustaining their proliferation and to a lesser extent their survival. The decrease in GCs, GC B cells and in the levels of anti-ssDNA autoAbs detected after the first week of disease in cGVHD mice that received IL-21R-/- donor cells, suggests that the decreased number of TFH cells available to provide B cell help was sufficient to attenuate the response of autoreactive IL-21R+/+ host B cells. The decreased B cell help was likely mediated by decreased production of IL-21 and IL-4 by TFH cells (34). In addition to fully differentiated TFH cells, the observed decrease in IL-21 mRNA expression in IL-21R-/- donor CD4+ T cells could have resulted from a decreased number of activated donor CD4+ T cells and Th17 cells. However, in the P→F1 model, we did not observe any changes in IL-17 mRNA expression and protein secretion in the absence of IL-21R signaling and in BXSB-Yaa mice IL-21 was not a product of Th17 cells (25). In addition, although we observed a decreased numbers of donor CD4+ T cells in the second week of disease due to a faster contraction and decrease in proliferation, it has been reported that IL-21 from non-TFH cells is less likely to contribute to GC reaction and B cell responses (45).
Although controversial, it has been suggested that IL-21 has the ability to regulate the reciprocal differentiation of Treg and Th17 cells by promoting Th17 cell differentiation and expansion and by downregulating the differentiation of iTregs and/or their suppressive capacity (6, 8, 9, 32, 46). Decreased number and/or functionally deficient Tregs as well as increased Th17 cells are well known abnormalities in murine and human lupus (47-50). An important question we sought to address is whether the attenuation of autoimmune parameters in the absence of IL-21R signaling on donor cells could be due to the correction of the balance between pathogenic Th17 and regulatory T cells. Our observation that upregulation of IL-17 mRNA expression and protein secretion are not altered in the absence of IL-21R signaling exclude the possibility that the amelioration of B cell parameters in P→F1 cGVHD is due to decreased IL-17 production. Our results parallel those obtained in BXSB-Yaa lupus-like mice, in models of bone marrow transplant, in experimental allergic encephalitis induced in IL-6 sufficient mice as well as several models of organ-related autoimmune diseases (25, 51-53). As previously reported, it is possible that the contribution of IL-21 to Th17 differentiation/expansion is less important in an autoimmune setting where IL-6 production is abundant (54). In addition, the observation that the proportion of neither natural nor induced donor Tregs was altered when IL-21R signaling was disrupted, suggests that the attenuated phenotype in this model is not due to expanded Treg cells. That does not exclude however, the possibility that IL-21 blockade may restore the function of Treg cells or reverse the resistance of responder T cells to Treg-mediated suppression (6, 9). Further studies will address this question.
In the Bm12→B6 model of cGVHD, disruption of IL-21R signaling on host B cells resulted in a significant impairment of a number of B cell parameters, such as B cell activation, GC formation, GC B cell proliferation, PC formation and autoAb production. Lupus-like GN was also attenuated. Contrasting data have been reported in different models of protein immunization with respect to the kinetics of germinal center formation and Ab response in the absence of IL-21R signaling. In the setting of SRBC immunization, IL-21R signaling was critical for both GC initiation and long term maintenance while following immunization with Keyhole Limpet Hemocyanin, GC formation was comparable to control mice at day 14 but decreased significantly at day 28 due to an accelerated resolution and increased memory B cell generation (37, 38). After immunization with NP-chicken γ globulin, early GC formation and Ab production were dependent on IL-21R but not GC maintenance or long term Ab production. Our results showing decreased GC formation and GC B cell numbers at 7 days after disease induction that is even more striking at 21 days indicate that in cGVHD, IL-21R signaling is important both for reaching the optimal initial GC response and autoAb production and for their maintenance. Consistent with the attenuated GC response we observed decreased number of anti-dsDNA Ab secreting cells in the bone marrow of IL-21R-/- hosts and decreased expression of BLIMP-1 (encoded by prdm1) in spleen B cells indicating that the differentiation of high affinity autoAb secreting PC from the follicular germinal center is impaired in the absence of IL-21R signaling. While we have not examined the effect of IL-21R signaling on the extrafollicular response in our model, the contribution of IL-21/IL-21R interaction on the extrafollicular pathway was reported in MRL-Faslpr mice, in which the extrafollicular response is the dominant pathway of autoAb production (55).
The priming of T cells by DCs in the T cell zone followed by T-B cell interaction at the T-B border resulting in TFH cell differentiation are the initial steps in the multistage, multifactorial process of GC formation (5, 35, 40-42). In view of reports showing an inhibitory effect of IL-21 on DC maturation and activation we considered the possibility that the attenuation of cGVHD parameters in IL-21R-/- hosts is not due to a direct effect on B cells but to an indirect effect on the ability of DC to prime donor T cells. Our data contradict this idea, as donor T cell priming was not impaired and was even enhanced in IL-21R-/- hosts. In addition, the expression of maturation markers MHC class II, CD80 and CD86 on CD11c+ DCs was similar in IL-21R deficient and sufficient mice (not shown). Furthermore, although a number of activation markers were decreased on host B cells, the number of TFH cells did not differ in IL-21R sufficient and deficient hosts suggesting that, at least at the early time-points examined, IL-21R deficiency on B cells did not impair TFH cell differentiation.
In conclusion, our data suggest that the genetic inactivation of IL-21R attenuates the T cell dependent B cell hyperactivity that contributes to lupus pathogenesis by impairing both the aberrant TFH cell pathway and the hyperactive B cell response. Further studies to evaluate whether in vivo blockade of the IL-21 pathway achieves similar effects are needed.
Supplementary Material
Acknowledgement
We thank Dr. Michael Grusby (Harvard School of Public Health, Boston, MA) for initially providing the IL-21R deficient mice, Dr. David Tisler (University of Maryland School of Medicine) for providing the C57Bl/6J transgenic mice that expressed GFP and Dr. Wendy Davidson (NIAID, NIH, Bethesda, MD) for helpful discussions.
This work was supported by the National Institute of Health Grant AR053704 (to V.R.), Veterans Affairs Merit Review Grant (to V.R., H.R and I.L.) and Arthritis Foundation Mid-Atlantic Chapter Grant (to V.R.).
Abbreviations used in this paper
- SLE
systemic lupus erythematosus
- B6
C57BL/6
- BDF1
B6D2F1
- Bm12
B6.C-H2bm12/KhEg
- GC
germinal center
- DC
dendritic cell
- PD-1
programmed cell death-1
- WT
wild –type
- KO
knock-out
- Treg
T regulatory cell
- nTreg
natural T reg
- iTreg
induced Treg
- TFH
T follicular helper cell
- P→F1
parent-into-F1
- PNA
peanut agglutinin
- GN
glomerulonephritis
- PC
plasma cell
References
- 1.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, Schrader S, Burkhead S, Heipel M, Brandt C, Kuijper JL, Kramer J, Conklin D, Presnell SR, Berry J, Shiota F, Bort S, Hambly K, Mudri S, Clegg C, Moore M, Grant FJ, Lofton-Day C, Gilbert T, Rayond F, Ching A, Yao L, Smith D, Webster P, Whitmore T, Maurer M, Kaushansky K, Holly RD, Foster D. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 2.Parrish-Novak J, Foster DC, Holly RD, Clegg CH. Interleukin-21 and the IL-21 receptor: novel effectors of NK and T cell responses. J Leukoc Biol. 2002;72:856–863. [PubMed] [Google Scholar]
- 3.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP, Berzofsky JA, Leonard WJ. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Peluso I, Fantini MC, Fina D, Caruso R, Boirivant M, MacDonald TT, Pallone F, Monteleone G. IL-21 counteracts the regulatory T cell-mediated suppression of human CD4+ T lymphocytes. J Immunol. 2007;178:732–739. doi: 10.4049/jimmunol.178.2.732. [DOI] [PubMed] [Google Scholar]
- 7.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 9.Fantini MC, Rizzo A, Fina D, Caruso R, Becker C, Neurath MF, Macdonald TT, Pallone F, Monteleone G. IL-21 regulates experimental colitis by modulating the balance between Treg and Th17 cells. Eur J Immunol. 2007;37:3155–3163. doi: 10.1002/eji.200737766. [DOI] [PubMed] [Google Scholar]
- 10.Good KL, Bryant VL, Tangye SG. Kinetics of human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J Immunol. 2006;177:5236–5247. doi: 10.4049/jimmunol.177.8.5236. [DOI] [PubMed] [Google Scholar]
- 11.Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, Spolski R, Leonard WJ, Lipsky PE. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175:7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 12.Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, Morse HC, 3rd, Liu C, Schwartzberg PL, Leonard WJ. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 13.Rankin AL, MacLeod H, Keegan S, Andreyeva T, Lowe L, Bloom L, Collins M, Nickerson-Nutter C, Young D, Guay H. IL-21 receptor is critical for the development of memory B cell responses. J Immunol. 186:667–674. doi: 10.4049/jimmunol.0903207. [DOI] [PubMed] [Google Scholar]
- 14.Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, Hwu P, Shaffer DJ, Akilesh S, Roopenian DC, Morse HC, 3rd, Lipsky PE, Leonard WJ. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Shen W, Kong K, Liu Z. Interleukin-21 induces T-cell activation and proinflammatory cytokine secretion in rheumatoid arthritis. Scand J Immunol. 2006;64:515–522. doi: 10.1111/j.1365-3083.2006.01795.x. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen V, Siaton B, Shorter S, Rus V. Elevated IL-21 Expression in Serum and Peripheral Blood Mononuclear Cells From Lupus Patients. Arthritis and Rheumatism. 2008;58:S816. [Google Scholar]
- 17.Wang XF, Yuan SL, Jiang L, Zhang XL, Li SF, Guo Y, Wu CL, Chen JJ. [Changes of serum BAFF and IL-21 levels in patients with systemic lupus erythematosus and their clinical significance]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2007;23:1041–1042. [PubMed] [Google Scholar]
- 18.Dolff S, Abdulahad WH, Westra J, Doornbos-van der Meer B, Limburg PC, Kallenberg CG, Bijl M. Increase in IL-21 producing T-cells in patients with systemic lupus erythematosus. Arthritis Res Ther. 13:R157. doi: 10.1186/ar3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawalha AH, Kaufman KM, Kelly JA, Adler AJ, Aberle T, Kilpatrick J, Wakeland EK, Li QZ, Wandstrat AE, Karp DS, James JA, Merrill JT, Lipsky P, Harley JB. Genetic association of IL-21 polymorphisms with systemic lupus erythematosus. Ann Rheum Dis. 2007 doi: 10.1136/ard.2007.075424. [DOI] [PubMed] [Google Scholar]
- 20.Webb R, Merrill JT, Kelly JA, Sestak A, Kaufman KM, Langefeld CD, Ziegler J, Kimberly RP, Edberg JC, Ramsey-Goldman R, Petri M, Reveille JD, Alarcon GS, Vila LM, Alarcon-Riquelme ME, James JA, Gilkeson GS, Jacob CO, Moser KL, Gaffney PM, Vyse TJ, Nath SK, Lipsky P, Harley JB, Sawalha AH. A polymorphism within IL21R confers risk for systemic lupus erythematosus. Arthritis Rheum. 2009;60:2402–2407. doi: 10.1002/art.24658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen V, Siaton B, Shorter S, Rus V. Elevated IL-21 Expression in Serum and Peripheral Blood Mononuclear Cells from Lupus Patients. Arthritis and Rheumatism. 2008;58:S816. [Google Scholar]
- 22.Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, Yu D, Domaschenz H, Whittle B, Lambe T, Roberts IS, Copley RR, Bell JI, Cornall RJ, Goodnow CC. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 23.Herber D, Brown TP, Liang S, Young DA, Collins M, Dunussi-Joannopoulos K. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J Immunol. 2007;178:3822–3830. doi: 10.4049/jimmunol.178.6.3822. [DOI] [PubMed] [Google Scholar]
- 24.Bubier JA, Bennett SM, Sproule TJ, Lyons BL, Olland S, Young DA, Roopenian DC. Treatment of BXSB-Yaa mice with IL-21R-Fc fusion protein minimally attenuates systemic lupus erythematosus. Ann N Y Acad Sci. 2007;1110:590–601. doi: 10.1196/annals.1423.063. [DOI] [PubMed] [Google Scholar]
- 25.Bubier JA, Sproule TJ, Foreman O, Spolski R, Shaffer DJ, Morse HC, 3rd, Leonard WJ, Roopenian DC. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB- Yaa mice. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0807309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Via CS. Advances in lupus stemming from the parent-into-F1 model. Trends Immunol. 31:236–245. doi: 10.1016/j.it.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasaian MT, Whitters MJ, Carter LL, Lowe LD, Jussif JM, Deng B, Johnson KA, Witek JS, Senices M, Konz RF, Wurster AL, Donaldson DD, Collins M, Young DA, Grusby MJ. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity. 2002;16:559–569. doi: 10.1016/s1074-7613(02)00295-9. [DOI] [PubMed] [Google Scholar]
- 28.Rus V, Nguyen V, Puliaev R, Puliaeva I, Zernetkina V, Luzina I, Papadimitriou JC, Via CS. T cell TRAIL promotes murine lupus by sustaining effector CD4 Th cell numbers and by inhibiting CD8 CTL activity. J Immunol. 2007;178 doi: 10.4049/jimmunol.178.6.3962. [DOI] [PubMed] [Google Scholar]
- 29.Foster AD, Haas M, Puliaeva I, Soloviova K, Puliaev R, Via CS. Donor CD8 T cell activation is critical for greater renal disease severity in female chronic graft-vs.-host mice and is associated with increased splenic ICOS(hi) host CD4 T cells and IL-21 expression. Clin Immunol. 136:61–73. doi: 10.1016/j.clim.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen V, Cudrici C, Zernetkina V, Niculescu F, Rus H, Drachenberg C, Rus V. TRAIL, DR4 and DR5 are upregulated in kidneys from patients with lupus nephritis and exert proliferative and proinflammatory effects. Clin Immunol. 2009 doi: 10.1016/j.clim.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie C, Zhou XJ, Liu X, Mohan C. Enhanced susceptibility to end-organ disease in the lupus-facilitating NZW mouse strain. Arthritis Rheum. 2003;48:1080–1092. doi: 10.1002/art.10887. [DOI] [PubMed] [Google Scholar]
- 32.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rus V, Svetic A, Nguyen P, Gause WC, Via CS. Kinetics of Th1 and Th2 cytokine production during the early course of acute and chronic murine graft-versus-host disease. Regulatory role of donor CD8+ T cells. J Immunol. 1995;155:2396–2406. [PubMed] [Google Scholar]
- 34.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol. 29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 36.Morris SC, Cheek RL, Cohen PL, Eisenberg RA. Autoantibodies in chronic graft versus host result from cognate T-B interactions. J Exp Med. 1990;171:503–517. doi: 10.1084/jem.171.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zotos D, Coquet JM, Zhang Y, Light A, D'Costa K, Kallies A, Corcoran LM, Godfrey DI, Toellner KM, Smyth MJ, Nutt SL, Tarlinton DM. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGuire HM, Vogelzang A, Ma CS, Hughes WE, Silveira PA, Tangye SG, Christ D, Fulcher D, Falcone M, King C. A subset of interleukin-21+ chemokine receptor CCR9+ T helper cells target accessory organs of the digestive system in autoimmunity. Immunity. 34:602–615. doi: 10.1016/j.immuni.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 40.Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, Fagarasan S. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 41.Ebert LM, Horn MP, Lang AB, Moser B. B cells alter the phenotype and function of follicular-homing CXCR5+ T cells. Eur J Immunol. 2004;34:3562–3571. doi: 10.1002/eji.200425478. [DOI] [PubMed] [Google Scholar]
- 42.Akiba H, Takeda K, Kojima Y, Usui Y, Harada N, Yamazaki T, Ma J, Tezuka K, Yagita H, Okumura K. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J Immunol. 2005;175:2340–2348. doi: 10.4049/jimmunol.175.4.2340. [DOI] [PubMed] [Google Scholar]
- 43.Poholek AC, Hansen K, Hernandez SG, Eto D, Chandele A, Weinstein JS, Dong X, Odegard JM, Kaech SM, Dent AL, Crotty S, Craft J. In vivo regulation of Bcl6 and T follicular helper cell development. J Immunol. 185:313–326. doi: 10.4049/jimmunol.0904023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, Schwartzberg PL, Cook MC, Walters GD, Vinuesa CG. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng SG, Wang JH, Koss MN, Quismorio F, Jr., Gray JD, Horwitz DA. CD4+ and CD8+ regulatory T cells generated ex vivo with IL-2 and TGF-beta suppress a stimulatory graft-versus-host disease with a lupus-like syndrome. J Immunol. 2004;172:1531–1539. doi: 10.4049/jimmunol.172.3.1531. [DOI] [PubMed] [Google Scholar]
- 47.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 48.Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, Yi J, Guentert T, Tousson A, Stanus AL, Le TV, Lorenz RG, Xu H, Kolls JK, Carter RH, Chaplin DD, Williams RW, Mountz JD. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 49.Garrett-Sinha LA, John S, Gaffen SL. IL-17 and the Th17 lineage in systemic lupus erythematosus. Curr Opin Rheumatol. 2008;20:519–525. doi: 10.1097/BOR.0b013e328304b6b5. [DOI] [PubMed] [Google Scholar]
- 50.Crispin JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, Kyttaris VC, Juang YT, Tsokos GC. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181:8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coquet JM, Chakravarti S, Smyth MJ, Godfrey DI. Cutting edge: IL-21 is not essential for Th17 differentiation or experimental autoimmune encephalomyelitis. J Immunol. 2008;180:7097–7101. doi: 10.4049/jimmunol.180.11.7097. [DOI] [PubMed] [Google Scholar]
- 52.Sonderegger I, Kisielow J, Meier R, King C, Kopf M. IL-21 and IL-21R are not required for development of Th17 cells and autoimmunity in vivo. Eur J Immunol. 2008;38:1833–1838. doi: 10.1002/eji.200838511. [DOI] [PubMed] [Google Scholar]
- 53.Bucher C, Koch L, Vogtenhuber C, Goren E, Munger M, Panoskaltsis-Mortari A, Sivakumar P, Blazar BR. IL-21 blockade reduces graft-versus-host disease mortality by supporting inducible T regulatory cell generation. Blood. 2009;114:5375–5384. doi: 10.1182/blood-2009-05-221135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vogelzang A, King C. The modulatory capacity of interleukin-21 in the pathogenesis of autoimmune disease. Front Biosci. 2008;13:5304–5315. doi: 10.2741/3082. [DOI] [PubMed] [Google Scholar]
- 55.Odegard JM, Marks BR, Diplacido LD, Poholek AC, Kono DH, Dong C, Flavell RA, Craft J. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008 doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.