Abstract
We sought to determine whether oral fluid can be used to assess serum human papillomavirus (HPV) antibody status by enrolling women who had received a prophylactic HPV-16 vaccine in a new follow-up study. After the prophylactic HPV-6/11/16/18 vaccine was licensed in the United States, we administered it to consenting participants. The sensitivity of oral fluid, treating serology as the gold standard, before and after administration of the quadrivalent vaccine was 49.6% (95% confidence interval [CI]: 42.0%–57.3%) and 100% (95% CI: 92.0%–100%), respectively. Oral fluid may have the potential to be used for monitoring of prophylactic HPV vaccines in the future.
Keywords: Human papillomavirus, Prophylactic vaccines, Antibodies, Oral fluid
Introduction
Oral fluid is viewed as an attractive alternative to serum for antibody detection in epidemiological studies. In contrast to venipuncture, oral fluid sampling is simple, noninvasive, and painless for the participants. It also does not require medically-trained personnel and participants can collect the specimen on their own.[1] The translocation of immunoglobulin G (IgG) from blood to extracellular fluid occurs most notably in the dental-capillary bed and the transudate can be obtained from fluid lying in the dental-gingival crevice.[2] This serous fluid is called oral mucosal transudate (OMT). OMT is substantially richer in IgG than saliva and constitutes a potentially valuable specimen to reflect the status of serum IgG.[2]
Previous studies have shown that OMT human papillomavirus (HPV)-specific IgG levels in natural infection are low and only modestly correlate with serum HPV-specific IgG levels.[3–7] These findings are thought to be due to the dilution of the transudated IgG from serum into the oral fluid. Serum HPV-16 IgG levels induced by prophylactic HPV vaccines are several-fold higher than those induced by natural infection with HPV-16.[8–10] Therefore, we hypothesized that HPV-16 IgG levels in OMT may strongly correlate with those in serum among vaccinated women. We conducted a study among women who had received prophylactic vaccines consisting of HPV-16 L1 virus-like particles (VLPs) to test this hypothesis.
Methods
Between October 1998 and November 1999, 2,391 women were enrolled in a multi-center double-blind phase IIb randomized controlled trial of a prophylactic HPV-16 L1 VLP vaccine in the United States (U.S.). Details of that study can be found elsewhere.[9] Of 2,391 participants in the trial, 500 women were enrolled in Seattle. Beginning in February 2006, all of these 500 women were offered participation in a new extended follow-up study with up to three visits occurring every six months to assess the long-term efficacy of the monovalent vaccine. The institutional review board of the University of Washington approved the study. One aim of this study, the focus of this report, was to assess the utilization of OMT in lieu of serum for assessment of HPV-16 IgG among women who had previously received the monovalent vaccine.
After the quadrivalent vaccine was licensed in the U.S. in 2006, we offered it to all study participants. Blood specimen collection began in March 2006. Ten milliliter (mL) of blood was drawn for assessment of HPV-16 IgG in serum. OMT collection began in June 2006. Approximately 0.5–0.8 mL of OMT was obtained for assessment of HPV-16 IgG in oral fluid. An OraSure® device (OraSure Technologies, Bethlehem, PA) was used to collect OMT specimens. The collection pad from the kit was handed to the participant. The participant was instructed to place the pad between the gum and cheek and rub the pad back and forth along the gum line until the pad was moist. The pad was left stationary against the gum for a minimum of two and maximum of five minutes. The pad was placed into the liquid in the specimen collection vial for shipment to the study-designated laboratory.
Serum and OMT specimens were defrosted and the liquid was collected by centrifugation (4000 rpm for five minutes at 4°C in an Eppendorf 5810R centrifuge, Eppendorf Inc. Westbury, NY) into two mL freezer vials for storage at −70°C until testing. HPV-16 L1 was synthesized by Blue Heron Biotechnology (Bothell, WA) to maximize expression in Escherichia coli. This sequence was subsequently cloned into a modified pGex4T vector to express L1 proteins with GST fused at the N-terminus and an 11 amino acid epitope fused to the C-terminus. Using the optimized sequence increased L1 protein expression detected by western blot; however, the level of L1 expression, measured by antibodies that recognized conformation dependent epitopes, did not increase (data not shown, sequence available upon request).
The detection of antibodies to HPV-16 L1 was performed following the methods of Waterboer et al. with modified incubation conditions [11, 12]. Compared with conventional serologic assays, this method requires less time and lower sample volume without losing sensitivity.[11] As such, this method is suited for large seroepidemiologic studies in which testing can be conducted on several samples under almost identical conditions. Briefly, HPV-16 L1 and BKV VP1 were expressed as GST fusion proteins in Rosetta cells (EMD Biosciences Inc. La Jolla, CA). An epitope tagged version of GST was also expressed. Cells were lysed by two passes through a Microfluidizer (Model M-110S, Mirofluidics Corp., Newton, MA). Polystyrene microspheres (beads) containing a unique combination of fluorescent dyes (MiraiBio, South San Francisco, CA) were covalently coupled with glutathione-(Sigma Chemical, St Louis, MO) linked casein (Sigma). Each protein preparation was bound to a different bead set, and incubated for one hour at room temperature with shaking. The beads were washed three times with PBS containing 1% casein, combined and diluted in the same buffer (final concentration = 55,000 beads/mL of each type).
Human sera were diluted 1:50 in the blocking buffer in polypropylene plates. Oral fluid specimens were diluted 1:2 in the same buffer. After blocking for one hour at room temperature with shaking, 50 microliter of diluted sera or diluted oral fluid was mixed with an equal volume of bead mixture in a 96 well filter plate (Multiscreen HTS, Millipore Corp., Bedford, MA). Plates were incubated overnight at 4°C. The following day the plates were removed from the refrigerator and incubated with shaking for one hour prior to washing. Incubations with biotin-anti-human IgG and streptavidin phycoerythrin were performed as previously described[11] and plates were read on a BioPlex 200 Instrument (BioRad Laboratories, Hercules, CA) after calibrating for the more sensitive readings. Controls included sera and oral fluid specimens[3] from women with no previous sex partners. Antibody reactivity is reported as median fluorescence intensity (MFI). Anti-HPV-16 positivity was defined as an MFI value that was at least two standard deviations above that of women with no previous sex partners; the cutoff point for serum and OMT anti-HPV-16 positivity was determined to be 2,318 and 1,262 MFI, respectively.
Sensitivity and specificity of OMT testing, treating serology as the gold standard, were calculated before and after administration of the quadrivalent vaccine. Since specimens collected before administration of the quadrivalent vaccine were not independent (i.e., one woman could have contributed more than one paired specimen), confidence intervals (CIs) were calculated using clustered sandwich estimator of the variance. We also sought to determine whether OMT and serum can be used to classify the participants in the monovalent vaccine trial according to their vaccination status. We developed receiver operating characteristic (ROC) curves to determine the sensitivity and specificity of OMT and serum for several cutoff points, treating vaccination status as the reference variable. The ROC analysis was performed using the information obtained at enrollment. All analyses were conducted using Stata 10 (Stata Corporation, College Station, TX).
Results
A total of 291 women were enrolled between March 2006 and May 2008. Fifteen women came only for one visit and in a period of time during the study when we had not started collecting OMT specimens yet; therefore, their data were excluded from the analyses. Of the remaining 276 participants, 139 and 137 women were monovalent vaccine and placebo recipients, respectively. Mean age of participants in both groups was 29 years (range: 25–33). More than 70% of participants in both groups were White.
Before administration of the quadrivalent vaccine
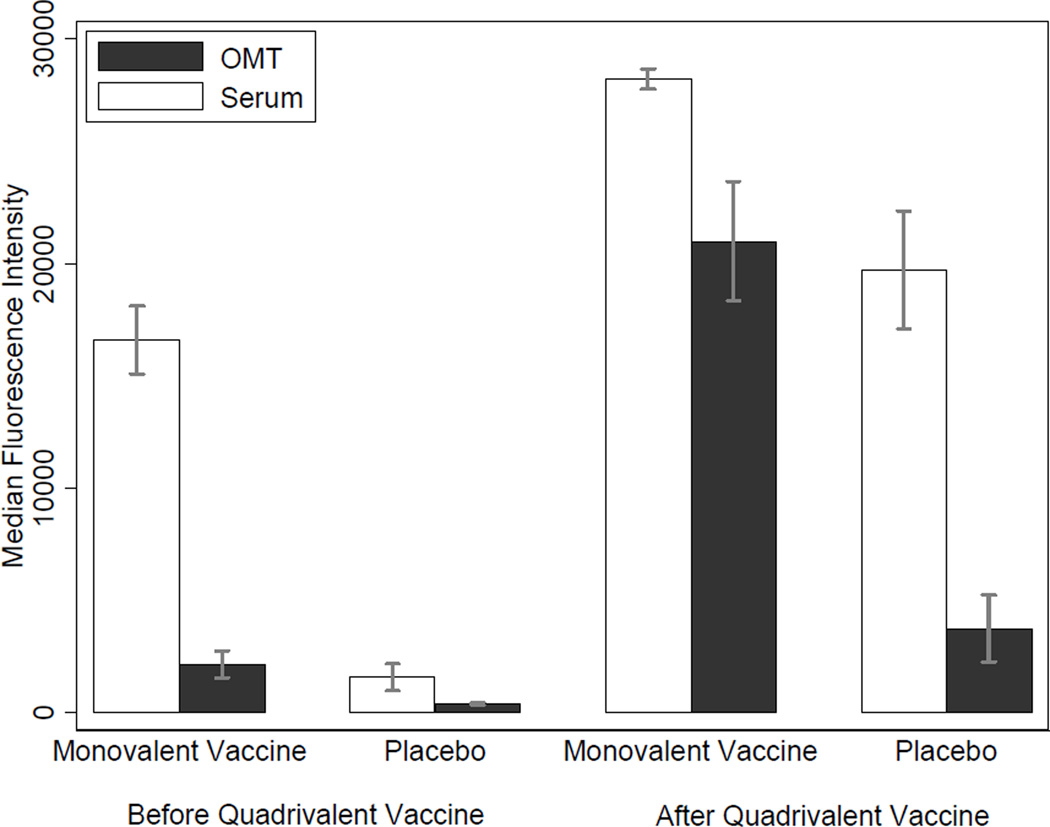
A total of 520 paired specimens were collected from 276 participants during visits before administration of the quadrivalent HPV vaccine. Serum and OMT anti-HPV-16 reactivity of monovalent vaccine recipients were 10.6 and 5.7 times higher than those of the placebo recipients, respectively. Among the monovalent vaccine recipients, anti-HPV-16 reactivity of serum was approximately 7.7 times higher than that of OMT (Figure 1). In this group, 268 (96.1%) serum specimens and 133 (47.7%) OMT specimens were anti-HPV-16 positive; corresponding serum specimens of all positive OMT specimens (n = 133) were also positive, and corresponding OMT specimens of all negative serum specimens (n = 11) were also negative. The sensitivity and specificity of OMT testing, treating serology as the gold standard, were respectively 49.6% (95% CI: 42.0%–57.3%) and 100% (95% CI: 76.1%–100%).
Figure 1.
Anti-HPV-16 reactivity in serum and oral mucosal transudate before and after administration of the quadrivalent vaccine
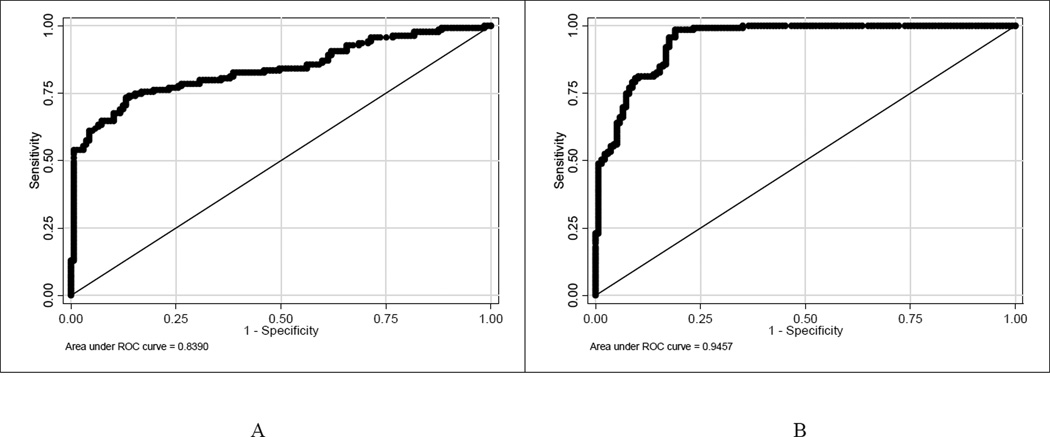
ROC analysis indicated that the OMT and serum anti-HPV-16 reactivity cutoff points of 763 and 2,045 MFI accurately classified the vaccination status of the highest proportion (79.8% and 89.9%) of participants in the monovalent vaccine trial, respectively. The sensitivity and specificity of OMT for determining the vaccination status corresponding to the cutoff point of 763 MFI were 74.1% (95% CI: 66.7%–81.5%) and 85.5% (95% CI: 80.3%–92.0%), respectively. The sensitivity and specificity of serum for determining the vaccination status corresponding to the cutoff point of 2,045 were 98.6% (95% CI: 96.6%–100%) and 81.0% (95% CI: 74.4%–87.7%), respectively. The area under the curve for OMT and serum were 0.84 (95% CI: 0.79–0.89) and 0.95 (95% CI: 0.92–0.97), respectively (Figure 2).
Figure 2.
Receiver operating characteristic curves for determining the performance of oral mucosal transudate (A) and serum (B) in classifying participants in the monovalent vaccine trial by their vaccination status
After administration of the quadrivalent vaccine
A total of 145 participants chose to receive the quadrivalent vaccine, of whom, 81 (36 monovalent vaccine recipients and 45 placebo recipients) came for one visit after vaccination with the quadrivalent vaccine. The mean follow-up time after administration of the first dose of the quadrivalent vaccine was 6.0 months (range: 2.5–7.8). After administration of this vaccine, anti-HPV-16 reactivity in serum and OMT specimens rose substantially (Figure 1). All serum and OMT specimens of monovalent vaccine recipients were anti-HPV-16 positive. In this group, the sensitivity of OMT testing for detection of HPV-16 IgG was 100% (95% CI: 92.0%–100%); specificity could not be determined due to lack of anti-HPV-16 negative serum specimens.
Discussion
To our knowledge, this is the first study to investigate the utility of oral fluid sampling in lieu of venipuncture for the determination of serum antibody status among women vaccinated with prophylactic HPV vaccines. Eight and one-half years after vaccination with the monovalent HPV-16 vaccine, OMT was 100% specific, but not highly sensitive, for the determination of serum HPV-16 IgG status. It is conceivable that the levels of transudated IgG into OMT were still not high enough to be detected by the methods used, resulting in a low sensitivity of OMT testing.
After administration of the quadrivalent vaccine, there was a several-fold increase in anti-HPV-16 reactivity in serum and OMT. The antibody reactivity was measured in a short period of time (i.e., six months) after vaccination. The utility of OMT testing for the determination of serum HPV-16 IgG status will likely depend on how long the levels of serum HPV-16 IgG will sustain after vaccination. It has been shown that a few years after administration of the quadrivalent vaccine, serum HPV-16 IgG titers remain substantially higher than those in natural infection.[10, 13] Therefore, it is conceivable that the OMT HPV-16 IgG status may accurately reflect the serum HPV-16 IgG status at least for a few years after vaccination.
This study is subject to some limitations. Antibody responses after administration of the quadrivalent vaccine in some participants may well have been beyond the maximum values that could be reported by the assay. Therefore, it was not possible to accurately calculate the difference or correlation between serum and OMT antibody levels after administration of the quadrivalent vaccine. In addition, our study was limited to a relatively homogenous population of women in Seattle; as the prophylactic HPV vaccines are introduced to different populations, it may be important to evaluate the performance of OMT testing in those groups as well.
During the past decade, oral fluid sampling has increasingly gained attention as an alternative method to venipuncture in assessing population immunity as well as in designing and evaluating vaccination programs. Widespread application of this method for epidemiologic studies of HPV in vaccinated populations requires a number of developments: the sensitivity of assays needs to be improved; validated positive and negative controls need to be developed and provided; standardized cutoff points for positivity need to be defined; and adequacy of collected specimens needs to be verified.[4, 14, 15] With such further developments, oral fluid collection may have the potential to be used in monitoring of prophylactic HPV vaccines.
Acknowledgment
Financial support: National Institute of Allergy and Infectious Diseases (Grant R01-AI38383)
Footnotes
Potential conflicts of interest
LAK has received funding from Merck through the University of Washington to conduct HPV vaccine studies. DAG has served as a consultant and member of Speakers Bureau for Merck.
This information has been presented in the Meeting of the American Association for Cancer Research, April 2009, Denver, CO (Abstract number: 2152)
References
- 1.George JR, Fitchen JH. Future applications of oral fluid specimen technology. Am J Med. 1997;102:21–25. doi: 10.1016/s0002-9343(97)00034-x. [DOI] [PubMed] [Google Scholar]
- 2.Cordeiro ML, Turpin CS, McAdams SA. A comparative study of saliva and OraSure oral fluid. Ann N Y Acad Sci. 1993;694:330–331. doi: 10.1111/j.1749-6632.1993.tb18380.x. [DOI] [PubMed] [Google Scholar]
- 3.Buchinsky FJ, Carter JJ, Wipf GC, Hughes JP, Koutsky LA, Galloway DA. Comparison of oral fluid and serum ELISAs in the determination of IgG response to natural human papillomavirus infection in university women. J Clin Virol. 2006;35:450–453. doi: 10.1016/j.jcv.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Cameron JE, Snowhite IV, Chaturvedi AK, Hagensee ME. Human papillomavirus-specific antibody status in oral fluids modestly reflects serum status in human immunodeficiency virus-positive individuals. Clin Diagn Lab Immunol. 2003;10:431–438. doi: 10.1128/CDLI.10.3.431-438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marais DJ, Best JM, Rose RC, et al. Oral antibodies to human papillomavirus type 16 in women with cervical neoplasia. J Med Virol. 2001;65:149–154. [PubMed] [Google Scholar]
- 6.Passmore JA, Marais DJ, Sampson C, et al. Cervicovaginal, oral, and serum IgG and IgA responses to human papillomavirus type 16 in women with cervical intraepithelial neoplasia. J Med Virol. 2007;79:1375–1380. doi: 10.1002/jmv.20901. [DOI] [PubMed] [Google Scholar]
- 7.Marais DJ, Sampson C, Jeftha A, et al. More men than women make mucosal IgA antibodies to Human papillomavirus type 16 (HPV-16) and HPV-18: a study of oral HPV and oral HPV antibodies in a normal healthy population. BMC Infect Dis. 2006;6:95. doi: 10.1186/1471-2334-6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367:1247–1255. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 9.Mao C, Koutsky LA, Ault KA, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006;107:18–27. doi: 10.1097/01.AOG.0000192397.41191.fb. [DOI] [PubMed] [Google Scholar]
- 10.Villa LL, Ault KA, Giuliano AR, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine. 2006;24:5571–5583. doi: 10.1016/j.vaccine.2006.04.068. [DOI] [PubMed] [Google Scholar]
- 11.Waterboer T, Sehr P, Michael KM, et al. Multiplex human papillomavirus serology based on in situ-purified glutathione s-transferase fusion proteins. Clin Chem. 2005;51:1845–1853. doi: 10.1373/clinchem.2005.052381. [DOI] [PubMed] [Google Scholar]
- 12.Waterboer T, Sehr P, Pawlita M. Suppression of non-specific binding in serological Luminex assays. J Immunol Methods. 2006;309:200–204. doi: 10.1016/j.jim.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Olsson SE, Villa LL, Costa RL, et al. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine. 2007;25:4931–4939. doi: 10.1016/j.vaccine.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 14.Cameron SO, Carman WF. The use of the OraSure collection device for hepatitis virus testing in health care settings. J Clin Virol. 2005;34(Suppl 1):S22–S28. doi: 10.1016/s1386-6532(05)80006-x. [DOI] [PubMed] [Google Scholar]
- 15.Nokes DJ, Enquselassie F, Nigatu W, et al. Has oral fluid the potential to replace serum for the evaluation of population immunity levels? A study of measles, rubella and hepatitis B in rural Ethiopia. Bull World Health Organ. 2001;79:588–595. [PMC free article] [PubMed] [Google Scholar]