Abstract
Hypoxia-inducible factor-1α (HIF-1α) has been reported to transactivate the expression of vascular endothelial growth factor (VEGF) and matrix metalloproteinase-2 (MMP-2), which are frequently overexpressed in numerous types of cancer and are known to be significant regulators of angiogenesis. Few studies have investigated the role of these factors in solid tumors, particularly chordomas, which are rare tumors that are thought to originate from notochordal remnants. To clarify whether HIF-1α is involved in angiogenesis in chordoma tissues, we examined the expression of HIF-1α, VEGF and MMP-2 with immunohistochemistry using a tissue microarray containing 35 chordoma samples. The results indicated that HIF-1α, VEGF and MMP-2 are expressed in the majority of chordoma samples. VEGF expression was significantly correlated with HIF-1α and MMP-2 expression, as well as with microvessel density (MVD). However, the prognosis of the chordoma patients was not significantly associated with the expression of these factors, but was associated with MVD. The results therefore showed that there is a correlation between the expression of HIF-1α, VEGF and MMP-2 in chordomas and that the angiogenic process is a potential therapeutic target for chordomas.
Keywords: chordoma, hypoxia-inducible factor-1α, vascular endothelial growth factor, matrix metalloproteinase-2
Introduction
Chordomas are rare tumors that are thought to originate from notochordal remnants. They arise from the sacrococcygeal region in approximately 50% of cases, from the sphenooccipital area in 35% and from the mobile spine in 15% (1). More males than females are affected by sacral chordoma; however, chordomas of the skull base appear to occur with equal frequency in males and females (2). Locally, these tumors are highly aggressive and frequently demonstrate local recurrence even following wide resection (3). However, there are no effective chemotherapeutic regimens available for the treatment of chordomas (4) and surgery remains the mainstay of chordoma management. Targeted molecular therapy has shown promising results in the treatment of malignancies with historically poor responses to chemotherapy. However, the expression of molecular markers in chordomas is not well understood. Thus, a better understanding of the molecular pathogenesis of this disease is required.
Hypoxia is a characteristic of all solid tumors. Under hypoxic conditions, tumor cells are deprived of oxygen due to a limited blood supply resulting from the abnormal tumor microvasculature (5). Hypoxia-inducible factor-1α (HIF-1α) is a transcription factor that regulates the pathways involved in tumor cell survival, proliferation, angiogenesis, invasion and metastasis. HIF-1α is a significant protein that directly reacts to hypoxia (6). Hypoxia leads to a rapid increase in HIF-1α protein levels, whereas HIF-1α is maintained at low levels under normoxic conditions (7). HIF-1α overexpression has been reported to correlate with tumor progression and an unfavorable prognosis in several types of cancer (8,9). In addition, HIF-1α directly activates the expression of a number of pro-angiogenic factors, including vascular endothelial growth factor (VEGF) and matrix metalloproteinase-2 (MMP-2) (10–12). Hypoxia-driven VEGF expression is considered to be a principal inducer of tumor angiogenesis. HIF-1α regulates VEGF transcription by binding to the hypoxia response element (HRE) in the VEGF promoter region (13). HIF-1α expression correlates with that of VEGF and microvessel density (MVD) in several types of tumor (14,15). Degradation and remodeling of the extracellular matrix (ECM) and basement membranes by proteolytic enzymes are essential steps in the processes of invasion and metastasis (16). MMP-2 has been shown to be one of the key enzymes regulating these processes. VEGF expression has also been found to correlate with MMP-2 expression in patients with gastric carcinoma (17).
In a previous study, we demonstrated that HIF-1α is expressed in chordomas and is correlated with multidrug resistance-associated protein 1 (MRP1) (18). There are, however, no studies available concerning the association between HIF-1α expression and clinicopathological findings in chordomas. The angiogenic process in sacral chordomas is also not fully understood. As a preliminary investigation of the molecular mechanisms of this rare disease, the aim of this study was to investigate the correlation between HIF-1α, VEGF and MMP-2 expression and to clarify their clinicopathological significance in chordomas. Paraffin-embedded tissue samples from 35 sacral chordoma patients were retrospectively obtained. We examined the expression patterns of HIF-1α, VEGF and MMP-2 by immunohistochemistry and staining was performed on a tissue microarray (TMA).
Materials and methods
Patients and tumor specimens
All cases of primary sacral chordoma diagnosed at Tangdu Hospital, The Fourth Military University (Shaanxi, China), between 1995 and 2010 were considered. The cases selected included patients with no history of chemotherapy or radiotherapy prior to surgery and a follow-up time of ≥12 months. A total of 35 patients diagnosed with sacral conventional chordoma were selected from the files. The patients ranged in age from 17 to 78 years (median, 53) and included 28 males and 7 females. The patient records were reviewed to collect the pathological data (tumor location and size and status of surgical resection margins), demographic data (patient age and gender) and follow-up information (time to local recurrence, distant metastasis and mortality) (Table I).
Table I.
Clinicopathological characteristics of study cases.
| No. | Age (years) | Gender | Sizea (cm) | Margin status | Local recurrence (m) | Metastasis (location/m) | Outcome | Follow-up (m) |
|---|---|---|---|---|---|---|---|---|
| 1 | 37 | Female | 10 | NE | 8 | Lung/49 | AWD | 49 |
| 2 | 50 | Male | 5 | − | No | No | NED | 21 |
| 3 | 60 | Male | 5 | NE | 12 | Lung/81 | DD | 86 |
| 4 | 56 | Male | 5 | + | No | No | NED | 18 |
| 5 | 53 | Male | 9 | NE | No | No | NED | 97 |
| 6 | 55 | Male | 8 | NE | 9 | L3, abdomen/45 | DD | 51 |
| 7 | 47 | Male | 11 | NE | 34 | No | NED | 89 |
| 8 | 56 | Male | 8 | + | No | No | NED | 13 |
| 9 | 44 | Female | 6 | − | 25 | No | NED | 37 |
| 10 | 54 | Male | 5 | NE | 111 | GM/111 | DD | 146 |
| 11 | 17 | Male | 9 | + | 20 | T11-12/20 | AWD | 20 |
| 12 | 58 | Male | 4 | NE | 24 | No | NED | 29 |
| 13 | 77 | Male | 5 | − | 38 | No | AWD | 66 |
| 14 | 67 | Male | 7 | NE | 17 | No | NED | 30 |
| 15 | 49 | Male | 8 | + | 75 | No | AWD | 75 |
| 16 | 55 | Male | 7 | − | 5 | No | NED | 12 |
| 17 | 51 | Female | 6 | + | 61 | No | DD | 69 |
| 18 | 60 | Female | 5 | NE | 34 | Abdomen/34 | DD | 81 |
| 19 | 51 | Female | 14 | NE | 41 | No | DD | 92 |
| 20 | 67 | Male | 8 | + | 7 | No | AWD | 38 |
| 21 | 47 | Male | 5 | − | No | No | NED | 43 |
| 22 | 52 | Female | 9 | NE | 23 | No | AWD | 23 |
| 23 | 27 | Male | 12 | NE | 25 | No | AWD | 25 |
| 24 | 74 | Male | 10 | − | 10 | No | DD | 43 |
| 25 | 78 | Male | 7 | NE | 6 | No | DD | 23 |
| 26 | 47 | Male | 4 | − | No | No | NED | 13 |
| 27 | 51 | Male | 8 | NE | 32 | No | AWD | 120 |
| 28 | 50 | Male | 9 | − | No | No | NED | 46 |
| 29 | 58 | Female | 8 | NE | 37 | No | NED | 65 |
| 30 | 59 | Male | 16 | + | 58 | No | NED | 63 |
| 31 | 62 | Male | 30 | − | 9 | No | DD | 112 |
| 32 | 56 | Male | 4 | − | 10 | No | DD | 39 |
| 33 | 50 | Male | 5 | − | No | No | NED | 73 |
| 34 | 53 | Male | 8 | NE | No | No | NED | 61 |
| 35 | 49 | Male | 6 | NE | No | No | NED | 45 |
AWD, alive with disease; DD, died of disease; NED, no evidence of disease; +, postive; −, negative; NE, not evaluable; GM, Gluteus maximus; m, months.
Largest diameter of tumor.
TMAs were prepared using a standard protocol (19). Archived chordoma tissues and representative hematoxylin and eosin (H&E) slides of each case were reviewed microscopically by a pathologist. The chordomas were identified on the corresponding H&E slides. Two tissue cores (2 mm in diameter) were removed from histologically identified representative regions of each formalin-fixed paraffin-embedded tumor. The study protocol was approved by the ethics committee of Tangdu Hospital. Informed consent was obtained from each patient in accordance with committee regulations.
Immunohistochemical staining for HIF-1α, VEGF, MMP-2 and CD34
The TMA slides were investigated by immunohistochemistry for the expression of HIF-1α (H1alpha67, dilution 1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), VEGF (VG1, dilution 1:200; Dako, Copenhagen, Denmark) and MMP-2 (polyclonal, dilution 1:150; Bioss, Beijing, China) using the ChemMate™ Envision+HRP/DAB kit (Dako). The pre-treatment of sections with heat-induced epitope retrieval was performed according to the specification for each antibody. Negative controls were prepared using phosphate-buffered solution (PBS) instead of the primary antibody. The TMA slides were independently evaluated by two pathologists who had no prior knowledge of the clinical data or histological findings.
The immunohistochemical results for the HIF-1α protein were then classified (−, no staining; +, nuclear staining in <1% of cells; ++, nuclear staining in 1–10% of cells and/or weak cytoplasmic staining; +++, nuclear staining in 11–50% of cells and/or distinct cytoplasmic staining; ++++, nuclear staining in >50% of cells and/or strong cytoplasmic staining) (20). For statistical analyses, tumors with a final staining score of − or + with a weak or moderate/strong immunoreactivity were classified as the low expression group and were compared with tumors with scores of ++, +++ or ++++ that were classified as the high expression group (20). VEGF expression was defined as positive if distinct staining of the cytoplasm was observed in ≥10% of tumor cells (14). Cytoplasmic MMP-2 staining was classified as: low expression exhibited staining in <50% of cells and high expression exhibited staining in ≥50% of cells (21).
Evaluation of MVD
Paraffin sections (4 μm) were cut from formalin-fixed paraffin-embedded tissue before tissue cores were removed for CD34 staining. CD34 (QBEnd10, Ready to use, Dako) staining was used to assess MVD by light microscopy at the site of the highest number of capillaries and small venules. Highly vascular areas were identified by scanning tumor sections at a low power (x100). MVD was determined by observing the identified six fields for each sample at a magnification of ×400, and the means were then calculated (14). A visible lumen was not necessary for a structure to be defined as a vessel (22).
Statistical analysis
Statistical analysis was performed using Microsoft Excel 2003 (Microsoft, Seattle, WA, USA) and SPSS Statistics 17.0 (SPSS, Chicago, IL, USA) for Windows. The significance of the observed associations was determined using the Mann-Whitney U test. The Spearman’s rank correlation test was used to determine the association between HIF-1α, VEGF and MMP-2. The impact of HIF-1α, VEGF and MMP-2 expression on patient survival was assessed using the Kaplan-Meier method and compared using the log-rank test. P<0.05 was considered to indicate a statistically significant result.
Results
Immunohistochemical staining of HIF-1α, VEGF and MMP-2
HIF-1α, VEGF and MMP-2 immunostaining was assessed and the samples were grouped into high-or low-grade categories. HIF-1α expression showed a nuclear and/or cytoplasmic staining pattern with diverse intensities (Fig. 1). Of the 35 chordomas, 26 (74.3%) exhibited strongly positive HIF-1α staining (score ++, +++ or ++++) and 9 (25.7%) showed weakly positive staining (score − or +). Immunohistochemical staining of VEGF and MMP-2 was observed predominantly in the cytoplasm of the tumor cells. Of the 35 chordoma cases, 25 (71.4%) exhibited high VEGF expression levels. MMP-2 expression was classified as high in 24 (68.6%) of the chordoma cases. The immunohistochemical staining of each protein and their correlation with patient age, gender, tumor size, local recurrence and distant metastases are shown in Table II. No correlation was found between any of the three factors and clinicopathological characteristics (patient age, gender, tumor size, local recurrence and distant metastases) for any patient (P>0.05).
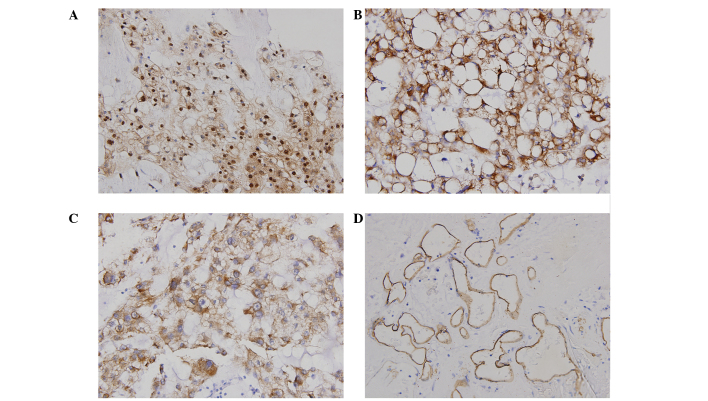
Figure 1.
Expression of HIF-1α, VEGF, MMP-2 and CD34 in primary chordoma samples is shown. (A) Strong HIF-1α immunoreactivity in the cytoplasm and nuclei of tumor cells. (B) Positive immunohistochemical staining of VEGF. VEGF immunoreactivity is present in the cytoplasm of tumor cells. (C) MMP-2 was observed in the cytoplasm of tumor cells. (D) CD34 staining of chordoma tissue. HIF-1α, hypoxia-inducible factor-1α; VEGF, vascular endothelial growth factor; MMP-2, matrix metalloproteinase-2.
Table II.
Correlation of HIF-1α, VEGF and MMP-2 expression with clinicopathological characteristics in chordoma cases.
| HIF-1α | VEGF | MMP-2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Characteristics | Case number | High | Low | P-valuea | High | Low | P-value | High | Low | P-value |
| Age (years) | ||||||||||
| >53b | 18 | 13 | 5 | 0.810 | 13 | 5 | 0.928 | 10 | 8 | 0.152 |
| ≤53 | 17 | 13 | 4 | 12 | 5 | 14 | 3 | |||
| Gender | ||||||||||
| Female | 7 | 7 | 0 | 0.239 | 6 | 1 | 0.529 | 5 | 2 | 0.903 |
| Male | 28 | 19 | 9 | 19 | 9 | 19 | 9 | |||
| Size of tumor (cm) | ||||||||||
| <8b | 16 | 11 | 5 | 0.565 | 12 | 4 | 0.733 | 10 | 6 | 0.563 |
| ≥8 | 19 | 15 | 4 | 13 | 6 | 14 | 5 | |||
| Recurrence | ||||||||||
| Yes | 25 | 18 | 7 | 0.725 | 16 | 9 | 0.240 | 16 | 9 | 0.494 |
| No | 10 | 8 | 2 | 9 | 1 | 8 | 2 | |||
| Metastasis | ||||||||||
| Yes | 6 | 4 | 2 | 0.781 | 4 | 2 | 0.872 | 4 | 2 | 0.958 |
| No | 29 | 22 | 7 | 21 | 8 | 20 | 9 | |||
Mann-Whitney U test.
Median.
HIF-1α, hypoxia-inducible factor-1α; VEGF, vascular endothelial growth factor; MMP-2, matrix metalloproteinase-2.
Correlation between HIF-1α, VEGF and MMP-2 expression
Of the 35 sacral chordoma samples, 26 (74.3%) showed intense HIF-1α immunoreactivity in the nucleus and/or cytoplasm of the tumor cells. In the 26 sacral chordomas classified as having a high level of expression of HIF-1α, the VEGF-positive and VEGF-negative expression frequency was 22/26 (84.6%) and 4/26 (15.4%), respectively. By contrast, of the 9 chordomas classified as exhibiting a low level of expression of HIF-1α, 3/9 (33.3%) were VEGF-positive and 6/9 (66.7%) were VEGF-negative. The correlation between the HIF-1α and VEGF expression levels was significant (P=0.002). In addition, of the 26 sacral chordomas classified as showing a high HIF-1α expression, the frequencies of high and low MMP-2 expression were 20/26 (76.9%) and 6/26 (23.1%), respectively. There tended to be an association between the expression levels of HIF-1α and MMP-2, but the correlation was not significant (P=0.074). The Spearman’s rank correlation analysis indicated that the expression of VEGF was positively correlated with MMP-2 expression (P=0.001).
Correlation between the protein expression and MVD
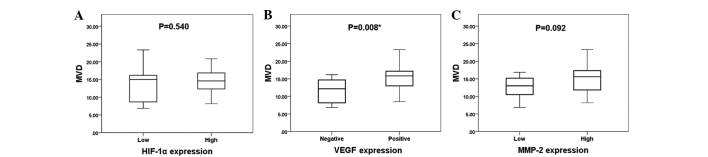
The mean MVD value was 14.2±4.0. The expression levels of HIF-1α, VEGF and MMP-2 compared with MVD are shown in Fig. 2. The mean MVD (in microvessels per field) in the high and low HIF-1α expression groups was 14.4±3.4 and 13.5±5.5, respectively. The mean MVD in the VEGF-positive and VEGF-negative groups was 15.3±3.7 and 11.5±3.6, respectively, and the mean MVD in the high and low MMP-2 expression groups was 14.9±4.0 and 12.6±3.6, respectively. VEGF expression was significantly associated with MVD (P=0.008), whereas the expression levels of HIF-1α and MMP-2 were not significantly associated with MVD, as determined by the Mann-Whitney U test (P=0.540 and P=0.092, respectively).
Figure 2.
Correlation between MVD and the expression of (A) HIF-1α, (B) VEGF and (C) MMP-2. The Mann-Whitney U test revealed that VEGF expression was significantly associated with MVD (B) (P=0.008). The expression levels of (A) HIF-1α and (C) MMP-2 were not associated with MVD (P=0.540 and P=0.092, respectively).*Statistically significant difference. HIF-1α, hypoxia-inducible factor-1α; VEGF, vascular endothelial growth factor; MMP-2, matrix metalloproteinase-2; MVD, microvessel density.
Correlation between patient survival and MVD and HIF-1α, VEGF and MMP-2 expression
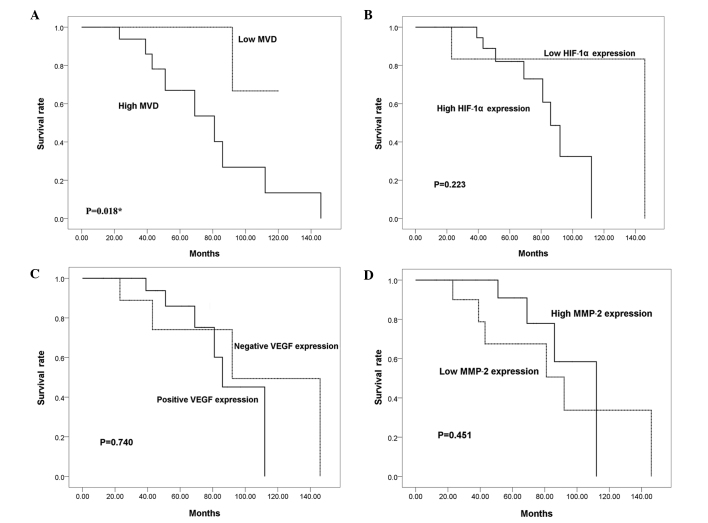
To investigate whether HIF-1α, VEGF and MMP-2 expression levels are associated with the outcome of patients with sacral chordoma, Kaplan-Meier analyses were performed. The mean duration of follow-up was 54.7 months (range, 12–146). Local recurrence occurred in 25 (71.4%) of the 35 chordoma patients during follow-up, with an average time to first local recurrence of 29.2 months (range, 5–111). Six patients presented with metastatic disease when the disease progressed to a more advanced stage (median time, 47 months; range, 20–111). The overall survival rates at 5 and 10 years were 82.3 and 29.9%, respectively. Kaplan-Meier analyses, however, indicated that there were no significant differences in the survival rates between the high and low HIF-1α expression groups, the VEGF-positive and VEGF-negative groups or the high and low MMP-2 expression groups (P=0.223, P=0.740 and P=0.451, respectively; Fig. 3). The survival of patients with a high MVD value was significantly shorter than that of patients with a low MVD value (P=0.018).
Figure 3.
Impact of MVD and HIF-1α, VEGF and MMP-2 expression on patient overall survival (log-rank test) is shown. (A) The log-rank test revealed a significantly shorter overall survival rate in patients with tumors with a high MVD (P=0.018). Kaplan-Meier analyses indicated that there were no significant differences in the survival rates between (B) the high and low HIF-1α expression groups, (C) the VEGF-positive and VEGF-negative groups or (D) the high and low MMP-2 expression groups (P=0.223, P=0.740 and P=0.451, respectively). HIF-1α, hypoxia-inducible factor-1α; VEGF, vascular endothelial growth factor; MMP-2, matrix metalloproteinase-2; MVD, microvessel density.
Discussion
Angiogenesis is an early event in carcinogenesis and one of the hallmark characteristics of tumors (23). Neovascularization is critical for tumor progression as the supply of oxygen and nutrients becomes limited in tumor cells located more than 100 μm away from a blood vessel (24). Angiogenesis is a multistep process including basement membrane degradation, endothelial cell migration, sprouting into the interstitial space, endothelial cell proliferation, lumen formation and new basement membrane and anastomosis formation (25). There are several angiogenesis-related and -promoting factors that are thought to be essential for this process during tumor development. A compelling body of evidence indicates that VEGF is one of the most significant pro-angiogenic factors involved in tumor growth and metastasis. It is widely accepted that VEGF expression is mediated by HIF-1α under hypoxic conditions (11,14,15,17). During hypoxia, HIF-1α is protected from ubiquitination and proteasomal degradation and activates the expression of a number of pro-angiogenic factors, including VEGF, by binding to the HRE in the VEGF promoter region. Furthermore, HIF-1α and VEGF expression levels have been analysed in a number of types of human cancer and their intracellular expression levels have been found to correlate with tumorigenesis and angiogenesis (14,15). Multiple proteinases, including MMPs, are also considered to be involved in the degradation of the vascular basement membrane and remodeling of the ECM during angiogenesis. Although the mechanisms underlying the HIF-1α-mediated regulation of MMP-2 protein synthesis are currently unclear, it has been demonstrated that the overexpression of HIF-1α may lead to an increase in the secretion and activation of MMP-2 (12). MMP-2 expression has also been reported to correlate with VEGF levels in patients diagnosed with gastric carcinoma (17), indicating that MMP-2 expression is closely linked with angiogenesis. However, the correlation between angiogenesis and chordomas is not well understood and has not been extensively studied. The present study was performed to retrospectively evaluate HIF-1α, VEGF and MMP-2 immunohistochemical reactivity in sacral chordoma patients and to explore the correlation of the expression of these proteins with clinicopathological characteristics and prognosis. MVD was assessed using CD34 to determine its correlation with patient outcome.
In this study, we investigated HIF-1α, VEGF and MMP-2 expression levels in human sacral chordoma using immunohistochemistry. Staining of HIF-1α was observed in the cytoplasm and/or nuclei of tumor cells. However, we found that differences in clinicopathological characteristics, including patient age, gender, tumor size, local recurrence and distant metastasis, did not correlate with the expression levels of HIF-1α, VEGF or MMP-2. Our results demonstrate that there is a significant correlation between HIF-1α and VEGF expression levels. This study is the first to demonstrate such a correlation in human chordomas. It is also the first to explore the correlation between VEGF and MMP-2 immunoreactivity in chordomas. In addition, we have determined that the expression levels of MMP-2 are correlated with the expression of HIF-1α, although this correlation was not significant. Previous studies have indicated that the three pro-angiogenic factors are associated with patient prognosis for several malignancies (17,20,21). In our study, 35 cases with follow-up data were examined to determine whether the expression levels of HIF-1α, VEGF and MMP-2 were of prognostic value. Chordoma patient outcome, however, was not significantly associated with the expression of the three proteins. We quantified tumor angiogenesis based on MVD using CD34 labeling. As a result, a significant correlation was found between the expression of VEGF and MVD. Furthermore, MVD was found to be associated with sacral chordoma patient prognosis. Chordomas are rare and the number of specimens was limited; there were only 35 samples in this study. As the group of low HIF-1α expression had only 9 patients, it is likely that the number of samples was insufficient for significance to be determined. However, the 10-year survival rate in the high HIF-1α expression group versus the low expression group was 0 versus 83.3% (Fig. 2B). As a result, a larger number of samples is required to confirm whether or not these protein expression levels correlate with prognosis.
Chen et al found that VEGF expression correlated with MMP-9 expression and was significantly higher in sacral chordoma tissue compared with adjacent normal tissue, although the difference in the disease-free survival rates between the VEGF-positive and VEGF-negative groups was not significant (26). The present study and that by Chen et al found no association between VEGF expression and disease-free survival or the survival rate; however, the number of patients in the two studies may not be sufficient to obtain prognostic information. Naka et al found that in skull base chordoma, MMP-9 was rarely expressed, but MMP-2 expression was frequently observed (27). These authors also found that MMP-2 expression was of prognostic value in non-skull base chordoma, but not in skull base chordoma (27,28). Although different results were observed in the skull base and non-skull base chordomas, together, these data indicate that the expression of VEGF and MMPs may be significant in chordoma progression.
At present, the effective treatment of chordomas is challenging. Even following wide surgical resection, the tumor frequently recurs. Chordomas have long been known to be resistant to chemotherapeutic drugs, presenting an obstacle that has to be overcome for the effective treatment of this disease. Due to its rare occurrence and a limited number of cell lines, little is known concerning the molecular basis of this disease. The results of our previous study suggest that both chordoma tissue and cell lines express HIF-1α and that the expression of HIF-1α is correlated with MRP1, which is known to be involved in hypoxia-induced chemoresistance (18). Taken together, these data indicate that HIF-1α is a significant contributor to the process of angiogenesis and hypoxia-induced chemoresistance in chordoma cells. As shown in this study, angiogenesis is a significant process in sacral chordoma. The process of angiogenesis may be a potential therapeutic target in the treatment of chordomas.
Acknowledgements
The authors wish to thank the pathologists Dr Yang Lianjia and Wen Yanhua for their assistance with the immunohistochemical analysis. We sincerely appreciate the assistance of Liu Yunyan and Ma Qiong with our experiments. We are also grateful to Wang Yuanshan for her invaluable assistance in editing the manuscript.
References
- 1.McMaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM. Chordoma: incidence and survival patterns in the United States, 1973–1995. Cancer Causes Control. 2001;12:1–11. doi: 10.1023/a:1008947301735. [DOI] [PubMed] [Google Scholar]
- 2.Anson KM, Byrne PO, Robertson ID, Gullan RW, Montgomery AC. Radical excision of sacrococcygeal tumours. Br J Surg. 1994;81:460–461. doi: 10.1002/bjs.1800810347. [DOI] [PubMed] [Google Scholar]
- 3.Tzortzidis F, Elahi F, Wright D, Natarajan SK, Sekhar LN. Patient outcome at long-term follow-up after aggressive microsurgical resection of cranial base chordomas. Neurosurgery. 2006;59:230–237. doi: 10.1227/01.NEU.0000223441.51012.9D. [DOI] [PubMed] [Google Scholar]
- 4.Chugh R, Tawbi H, Lucas DR, Biermann JS, Schuetze SM, Baker LH. Chordoma: the nonsarcoma primary bone tumor. Oncologist. 2007;12:1344–1350. doi: 10.1634/theoncologist.12-11-1344. [DOI] [PubMed] [Google Scholar]
- 5.Vaupel P, Hockel M, Mayer A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxid Redox Signal. 2007;9:1221–1235. doi: 10.1089/ars.2007.1628. [DOI] [PubMed] [Google Scholar]
- 6.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welsh SJ, Koh MY, Powis G. The hypoxic inducible stress response as a target for cancer drug discovery. Semin Oncol. 2006;33:486–497. doi: 10.1053/j.seminoncol.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Semenza GL. Evaluation of HIF-1 inhibitors as anticancer agents. Drug Discov Today. 2007;12:853–859. doi: 10.1016/j.drudis.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Dai Y, Bae K, Siemann DW. Impact of hypoxia on the metastatic potential of human prostate cancer cells. Int J Radiat Oncol Biol Phys. 2011;81:521–528. doi: 10.1016/j.ijrobp.2011.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenz GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shyu KG, Hsu FL, Wang MJ, Wang BW, Lin S. Hypoxia-inducible factor 1alpha regulates lung adenocarcinoma cell invasion. Exp Cell Res. 2007;313:1181–1191. doi: 10.1016/j.yexcr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM, Johnson RS. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res. 2000;60:4010–4015. [PubMed] [Google Scholar]
- 14.Kuwai T, Kitadai Y, Tanaka S, Elson D, Gassmann M, Arbeit JM, Johnson RS. Expression of hypoxia-inducible factor-1alpha is associated with tumor vascularization in human colorectal carcinoma. Int J Cancer. 2003;105:176–181. doi: 10.1002/ijc.11068. [DOI] [PubMed] [Google Scholar]
- 15.Kimura S, Kitadai Y, Tanaka S, Kuwai T, Hihara J, Yoshida K, Toge T, Chayama K. Expression of hypoxia-inducible factor (HIF)-1alpha is associated with vascular endothelial growth factor expression and tumour angiogenesis in human oesophageal squamous cell carcinoma. Eur J Cancer. 2004;40:1904–1912. doi: 10.1016/j.ejca.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 16.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 17.Zheng H, Takahashi H, Murai Y, Cui Z, Nomoto K, Niwa H, Tsuneyama K, Takano Y. Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res. 2006;26:3579–3583. [PubMed] [Google Scholar]
- 18.Ji Z, Long H, Hu Y, Qiu X, Chen X, Li Z, Fan D, Ma B, Fan Q. Expression of MDR1, HIF-1alpha and MRP1 in sacral chordoma and chordoma cell line CM-319. J Exp Clin Cancer Res. 2010;29:158. doi: 10.1186/1756-9966-29-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schweizer MS, Schumacher L, Rubin MA. Constructing tissue microarrays for research use. Curr Protoc Hum Genet. 2004 Feb 7;Chapter 10(Unit 10) doi: 10.1002/0471142905.hg1007s39. [DOI] [PubMed] [Google Scholar]
- 20.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- 21.Xiang ZL, Zeng ZC, Fan J, Tang ZY, Zeng HY, Gao DM. Expression profiling of fixed tissues identified hypoxia-inducible factor-1 alpha, VEGF, and matrix metalloproteinase-2 as biomarkers of lymph node metastasis in hepatocellular carcinoma. Clin Cancer Res. 2011;17:5463–5472. doi: 10.1158/1078-0432.CCR-10-3096. [DOI] [PubMed] [Google Scholar]
- 22.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis - correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 23.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Rankin EB, Giaccia AJ. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ. 2008;15:678–685. doi: 10.1038/cdd.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bussolino F, Mantovani A, Persico G. Molecular mechanisms of blood vessel formation. Trends Biochem Sci. 1997;22:251–256. doi: 10.1016/s0968-0004(97)01074-8. [DOI] [PubMed] [Google Scholar]
- 26.Chen KW, Yang HL, Lu J, Wang GL, Ji YM, Wu GZ, Zhu LF, Liu JY, Chen XQ, Gu YP. Expression of vascular endothelial growth factor and matrix metalloproteinase-9 in sacral chordoma. J Neurooncol. 2011;101:357–363. doi: 10.1007/s11060-010-0263-0. [DOI] [PubMed] [Google Scholar]
- 27.Naka T, Kuester D, Boltze C, Schulz TO, Samii A, Herold C, Ostertag H, Roessner A. Expression of matrix metallo- proteinases-1, -2, and -9; tissue inhibitors of matrix metalloproteinases-1 and -2; cathepsin B; urokinase plasminogen activator; and plasminogen activator inhibitor, type I in skull base chordoma. Hum Pathol. 2008;39:217–223. doi: 10.1016/j.humpath.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Naka T, Boltze C, Kuester D, Schulz TO, Samii A, Herold C, Ostertag H, Roessner A. Expression of matrix metalloproteinase (MMP)-1, MMP-2, MMP-9, cathepsin B, and urokinase plasminogen activator in non-skull base chordoma. Am J Clin Pathol. 2004;122:926–930. doi: 10.1309/C8T7-APJD-AUPR-8TLL. [DOI] [PubMed] [Google Scholar]