Abstract
Some of the first human gene therapy trials targeted diseases of the lung and provided important information that will continue to help shape future trials. Here we describe both cell and gene therapies for lung diseases such as cystic fibrosis and alpha-1 antitrypsin disorder as well as fatty acid oxidation disorders that mimic sudden infant death syndrome (SIDS). Human clinical gene therapy trials for cystic fibrosis and alpha-1 antitrypsin have been performed using a variety of vectors including adenovirus, adeno-associated virus, and nonviral vectors. No human clinical gene therapy trials have been performed for disorders of fatty acid oxidation; however, important proof-of-principle studies have been completed for multiple fatty acid oxidation disorders. Important achievements have been made and have yet to come for cell and gene therapies for disorders of the lung and those mimicking SIDS.
In this review, Keeler and Flotte discuss lessons that have been learned from gene therapy clinical trials for diseases affecting the lung, such as cystic fibrosis and α1-antitrypsin deficiency, and how these lessons can be applied to new disease targets such as disorders of fatty acid oxidation.
Introduction
Genetic disorders commonly treated by pediatric and adult pulmonary physicians have been targets of great interest in the field of gene therapy, and more recently in the field of stem cell therapy as well. Most notably in the early history of human gene therapy trials, a broad initiative led by the Cystic Fibrosis (CF) Foundation drove the efforts to bring new gene therapy vectors into clinical use. Because of this, the first human use of both recombinant adenovirus (rAd) and recombinant adeno-associated virus (rAAV) vectors occurred in CF patients in 1993 and 1995, respectively (Crystal et al., 1994, 1995; Flotte et al., 1996, 2003). Many lessons were learned about vector safety and duration of effect from those early trials, even though neither has led to the development of clinically effective therapies at this point. Gene and cell therapy approaches for CF remain an active topic of research in many laboratories as more varied approaches including integrating lentivirus vectors and novel approaches to airway and lung regeneration. Most notably, artificial tracheas repopulated with human stem cells have been used clinically in Scandinavia to treat patients with surgically irreparable damage to the trachea, providing a potential proof of concept for more extensive cell-based repopulation of the respiratory tract that could eventually be applied to airway diseases like CF (Anonymous, 2011).
Meanwhile, the attention to treatment for genetic disorders within the practice of pulmonologists has broadened to include genetic emphysema due to alpha-1 antitrypsin (AAT) deficiency and genetic disorders that contributed to over 5% of cases of sudden infant death syndrome (SIDS) (Boles et al., 1998). Clinical protocols with rAAV-based vectors for AAT deficiency have progressed through phase 2 and remain currently active. This disorder mimics other deficiencies of secreted serum proteins such as hemophilias. Meanwhile, the advent of newborn screening for metabolic diseases has revealed populations of patients with disorders of fatty acid oxidation (FAO), the most frequent of which is medium chain acyl-CoA dehydrogenase (MCAD) deficiency. Without this screening, affected individuals would likely succumb to SIDS. Interventions to treat the genetic basis of FAO disorders seem more likely to affect the outcome in such infants, as compared with the previous approach to familial cases of SIDS, which was home apnea monitoring. The rAAV-based proof-of-principle studies for FAO disorders have generally been aimed at correction of cardiac and skeletal muscle (which metabolizes the largest proportion of fatty acids), the liver (which can use FAO to help generate ketones as a protection against fasting hypoglycemia), or the entire body (which could assist with both the accumulation of toxic metabolites and the lack of ATP generation caused by the disorder).
Taken together, the experiences garnered from attempts to develop gene and cell therapy for inherited diseases affecting the lung and ventilator control have both contributed to and benefited from technological advances in the field more generally. Genetic diseases in this category have been somewhat difficult as targets in the past because of the dearth of truly faithful animal models of CF and AAT deficiency and, in the case of CF, because of the truly recalcitrant nature of the target cell itself, the airway epithelial cell. Airway epithelial cells are actively replicating, making gene therapy with nonintegrating systems more challenging, and have evolved a number of barriers on the luminal surface that may limit the efficiency of entry of most vectors. The complex tissue architecture of the organ also presents challenges to cell-based therapies not encountered in the bone marrow, for instance. That said, these disorders represent several of the most common single gene defects in North Americans and Northern Europeans and so will undoubtedly continue to garner much effort and attention.
Cystic Fibrosis
Disease
CF is the most common life-threatening single gene disorder in North America, with an incidence of 1 in 3300 live births and over 30,000 CF patients known to be living on the continent (FitzSimmons 1993). It is due to defects in the CF transmembrane conductance regulator (CFTR) gene, a member of the ATP-binding cassette superfamily of transmembrane transporters. CFTR is normally present on the apical surface of airway epithelial cells and submucosal gland cells where it performs a number of vital functions, including acting as a protein kinase A–dependent chloride channel (Welsh et al., 1992; Hanrahan et al., 1998; Seibert et al., 1999; Ostedgaard et al., 2001) and a regulator of other chloride channels and the epithelial sodium channel (Briel et al., 1998; König et al., 2001; Wagner et al., 2001).
In the absence of CFTR function in the conducting airways of the respiratory tract, chloride efflux is decreased, sodium absorption is markedly increased (Olivier et al., 2002), and the net flux of water into the airway surface lining fluid is markedly diminished (Boucher, 2003). There may also be secondary effects on the biochemical composition of mucous glycoproteins and the function of antimicrobial substances at the airway surface. The overall effect is that CF patients have obstruction of small airways, inflammation, and chronic infection with specific bacteria, such as Pseudomonas aeruginosa, Staphylococcus aureus, Burkholderia cepacia, and several others. CFTR normally functions in a number of other secretory epithelia, including the pancreatic ducts, the sweat gland ducts, and the vas deferens. CFTR deficiency at these sites results in exocrine pancreatic insufficiency, elevated sweat chloride concentrations, and male infertility in the vast majority of patients. Other complications occurring more frequently in older CF patients include CF-related diabetes and osteoporosis. A subset of CF patients will develop severe intestinal obstruction prenatally or immediately after birth (known as meconium ileus), while others may develop significant cholestatic liver disease, presumably due to absence of vital CFTR functions on enterocytes and biliary duct lining cells.
Treatment for CF has continuously evolved over the past 50 years, resulting in improvements in life span from an average of less than 5 years to the current median life expectancy of over 40 years in the United States (Cystic Fibrosis Foundation, 2010). Treatment consists of nutritional support through oral administration of enteric coated pancreatic enzymes to prevent malabsorption, supplementation of fat soluble vitamins, and caloric augmentation to compensate for increase metabolic rates in these patients. Therapy for CF lung disease includes aggressive airway clearance (using a variety of physical methods and mucolytic drugs, such as rhDNase and hypertonic saline) (Lieberman, 1968; Jones and Wallis, 2010) and aggressive antibiotic therapy (including intravenous, inhaled, and oral therapies). More recently, specific small molecule therapies that correct or activate mutant CFTR chloride channels have been developed, including Kalydeco, the recently approved drug from Vertex Pharmaceuticals, which works specifically on the G551D allele to correct trafficking of mutant CFTR to the cell surface (Accurso et al., 2010; Ramsey et al., 2011). Trials are currently ongoing in which Kalydeco will be combined with a new CFTR activator (VS809) to treat patients with the most common mutation, deltaF508. The concept is that Kalydeco will correct trafficking that VX880 allows for increased activation of the mutant CFTR. While such small molecule therapies represent great progress for the field, there remains significant interest in the possibility that the primary defect may eventually be corrected definitively at the genetic level. These efforts, past and present, will be presented in following sections.
rAd-gene therapy
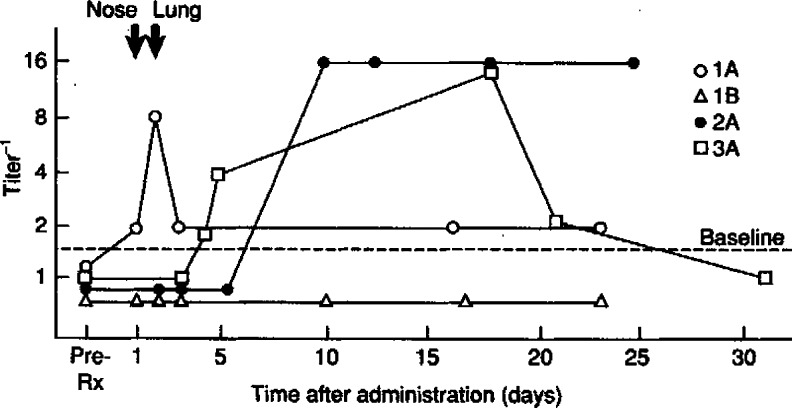
First generation rAd vectors expressing CFTR were deleted for the E1a, E1b, and E3 genes. These vectors demonstrated very robust levels of expression of either CFTR or of reporter genes in cell culture and in animal models. A number of human phase 1 clinical trials were begun in 1993, the first commencing at the National Institutes of Health (Crystal et al., 1994). Several key findings emerged from these trials. First, it became clear that there was a dose-related innate immune response to the vector (Fig. 1) as well as an adaptive immune response. These limited both the maximum tolerated dose and the duration of transgene expression. The second, somewhat surprising finding was that the efficiency of gene transfer in the airways was much lower than predicted. Ultimately, it became clear that the required receptors for rAd transduction (CAR and alphav, beta5 integrins) were more abundant on the basolateral membranes of airway epithelial cells than on the apical surface.
FIG. 1.
Serum anti-adenovirus (Ad) antibodies elicited by administration of AdCFTR to the respiratory tract. (Originally published in Crystal et al. [1994] and reprinted by permission [license number 2891420070068].) Serum was evaluated for anti-Ad antibodies before and after administration of AdCFTR. Anti-AdCFTR antibodies detected by enzyme-linked immunosorbent assay (ELISA). The ordinate is presented as the inverse of the maximum dilution of serum yielding a value higher than the highest value observed during the baseline and/or vehicle control period (pre-Rx).
These limitations further drove the development of rAd vector technology. Second-generation rAd vectors were developed with additional mutations in early genes, most notably the incorporation of a temperature-sensitive mutation in the E2a gene (Yang et al., 1994). Finally, fully deleted rAd vectors were developed. These vectors, alternatively known as gutted, gutless, high-capacity, or helper-dependent rAd (HD-rAd), were constructed such that only the noncoding sequencing of the Ad inverted terminal repeats (ITRs) were present within the vector (Palmer and Ng, 2005).
With each advancing generation of vector the duration of vector gene expression was enhanced in animal models and the adaptive immune response profiles were reduced. However, the presence of both an innate immune response to the input capsid components and the eliciting of neutralizing antibodies to the input capsid represented inherent limitations. Nonetheless, HD-rAd vectors are still in experimental use in animal models for a variety of diseases. Unfortunately, the fact that the airway epithelium is actively replicating still limits the duration of gene expression in that tissue, and the presence of neutralizing antibodies limits the ability to preserve efficiency after repeated dosing.
rAAV gene therapy
As with rAd vectors, the first in vivo animal and clinical use of rAAV gene therapy vectors were directed at CF (Flotte et al., 1993a, 1996). Unlike rAd vectors, rAAV vectors are nearly always fully deleted of vector coding sequences because of the very limited packaging capacity (<5 kb) of the virion, which necessitates the creation of space within the vector genome for the transgene, its promoter, and the polyadenylation signal. Even with deletion of both the viral genes (rep and cap), the packaging of CFTR into rAAV has represented a significant challenge, since the coding sequence of CFTR alone measures 4.4 kb in length. Therefore, the earliest efforts centered around developing vectors utilizing very small promoter sequences, such as the cryptic promoter activity of the AAV2 ITR (Flotte et al., 1993b), or on developing truncated CFTR transgenes (Carroll et al., 1995; Sirninger et al., 2004).
Based on in vivo data in rabbits and nonhuman primates, the first clinical trials of rAAV2-CFTR were launched in November 1995. The original trial consisted of combined nasal and endobronchial administration. This trial was extended several times and finally covered more than a 6-log-fold dose range, establishing safety and biological activity over that entire range. Additional studies with the original ITR promoter–driven rAAV2-CFTR vector were performed in the sinuses and by aerosol delivery to both lungs. The latter studies showed gene transfer to the lower airways that persisted for 60 days, consistent with the turnover of the airway epithelium (Moss et al., 2004). The development of neutralizing antibodies was correlated with the inability to achieve gene transfer again after subsequent doses.
It was originally in the context of lung delivery of these vectors that the episomal nature of rAAV persistence was discovered (Flotte et al., 1994; Afione et al., 1996). Ultimately, this led to the prediction that rAAV would be most effective at long-term gene expression in cells and tissues that were terminally differentiated or quiescent. This has subsequently proven to be true, as the most effective uses of rAAV vectors have been in the retina, central nervous system, and uninjured liver or muscle. In addition to the barrier presented by the turnover of airway epithelium, it was discovered that AAV2 uses receptors that are low in abundance on the apical surface of airway epithelium (Teramoto et al., 1998; Summerford et al., 1999).
Newer generations of rAAV vectors have been developed using different serotypes (rAAV5, rAAV1, rAAV-rh10) and stronger promoters, such as the cytomegalovirus (CMV) enhancer/beta actin promoter/beta globin hybrid intron (CBA or CAG) cassette. Due to the constrained packaging capacity, these vectors require either a truncated CFTR minigene (Carroll et al., 1995; Sirninger et al., 2004), or a dual vector approach that relies on dimerization between two vector constructs containing each half of the gene with a split intron (Song et al., 2009).
Lentiviral and retroviral gene therapy
Based on the potential advantage for vector integration, many studies have been performed using a variety of gamma retrovirus and lentivirus vectors to target the airway epithelium. Generally, these vectors have been pseudotyped with envelopes that are suitable for targeting receptors present on airway epithelial cells. These have included the vesicular stomatitis virus-G glycoprotein, the Gibbon ape leukemia virus envelope, and an envelope targeting the folate receptor (Bayle et al., 1993; Copreni et al., 2010). In general, the longevity and safety of such systems have been quite good. However, the gamma retrovirus vectors have generally required some form of stimulus to promote cell division within the airway epithelium in order to facilitate transduction.
Nonviral gene therapy
Much of the early work in CF gene therapy focused on the use of cationic liposomes for CFTR delivery. The most promising of these, Genzyme Lipid-67 (GL-67) was used in a trial several years ago, and the combination of lipid and DNA was found to elicit some innate immune responses that cause flu-like syndromes in some of the patients (Alton et al., 1999; Ruiz et al., 2001; Konstan et al., 2004). Further refinements of the plasmid have been carefully studied, and GL-67 is being studied again in a program undertaken by the UK CF Gene Therapy Consortium. DNA compacted with poly-l-lysine has also been brought forward into clinical trials and has shown some reproducible but transient gene expression (Ziady et al., 2003).
Cell-based therapy
Cell-based therapies for the airways have been under study for a number of years. The concept of using stem cells, derived from hematopoietic stem cells or human embryonic stem cells, has been studied by a number of groups, and the engraftment rate appears to be quite low. The use of reprogrammed cells (induced pluripotent stem cells) has also been contemplated.
More recently, tracheal grafts composed of a synthetic matrix populated with cells derived from stem cells have been used in Scandinavia for tracheal transplants following cancer or trauma (personal communication). This technology has not yet undergone clinical evaluation in the United States. It remains to be seen whether such technology could have a use in genetic disorders like CF. A similar concept has been used to produce a repopulated lung in a rodent model (Weiss et al., 2011). In this case the endogenous matrix of a lung is denuded by serial freeze–thaw cycles and then repopulated with pluripotent cells. While early results seem quite remarkable, the clinical utility of this technology remains to be seen.
AAT Deficiency
Disease
AAT is a highly abundant circulating 52 kD serum proteinase inhibitor (serpin), which is normal produced primarily in hepatocytes (to a lesser extent in macrophages and monocytes) and circulates at levels exceeding 11 μM (570 μg/ml) to protect the lung extracellular matrix from the effects of neutrophil elastase and other neutrophil products. AAT deficiency typically results in an adult onset lung disease, characterized by panacinar emphysema and airway inflammation. A small subset of patients (<10%) will develop symptomatic liver disease, which appears to be due to the effects of the common mutant protein, a Glu342Lys substitution called PiZ. PiZ-mutant AAT (Z-AAT) is hampered by the loss of a salt bridge between two beta-sheet structures, which inhibits its anti-elastase specific activity, impairs folding, and allows the reactive loop of one Z-AAT molecule to insert itself between two beta strands of an adjacent Z-AAT, enabling so-called “loop-sheet” polymerization (Elliott et al., 1998). AAT deficiency is very homogenous genetically, with over 90% of mutant alleles being PiZ. Thus, it is unclear why some PiZ homozygotes develop liver disease in response to the presence of Z-AAT, while others do not. Nonetheless, it remains reasonably clear that lung disease is treatable by replacement of wild-type AAT (M-AAT) to levels above 11 μM (570 μg/ml), while the treatment of liver disease, would likely require down-regulation of Z-AAT within hepatocytes.
Cationic lipid-based gene therapy
Cationic liposomes have been used to augment AAT levels in animal models via a number of routes. In fact, the nasal instillation of cationic lipids in a phase 1 human trial was described some years ago (Brigham et al., 2000). The lack of persistence of gene transfer with this method is a major limitation, as is the very high level of gene augmentation required to achieve therapeutic effects.
rAAV-gene therapy
Significant effort has been put toward the development of rAAV-AAT gene therapy, including both augmentation for therapy of the lung disease (which has gone through phase 2 clinical trials) and allele-specific Z-AAT liver-directed knockdown (which has only been studied in animal models at this point).
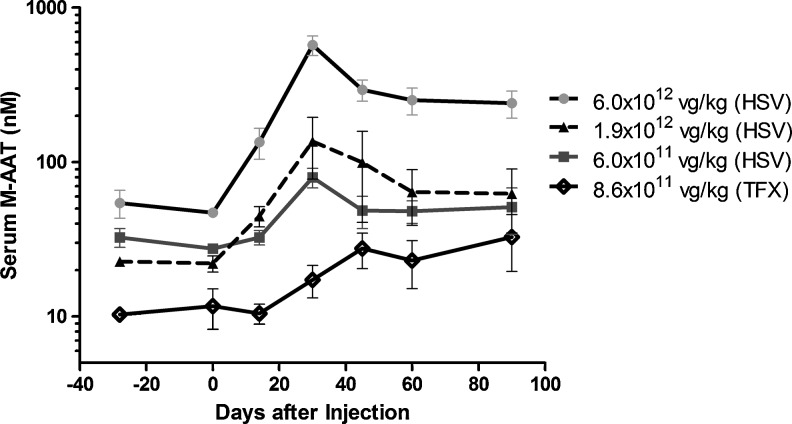
The study of gene augmentation for M-AAT is greatly aided by the fact that C57Bl6 (B6) mice naturally have tolerance for hAAT (Song et al., 1998, 2001). Thus rAAV-hAAT vectors were studied directly in B6 mice. Studies of intramuscular (IM), portal vein, airway delivery, and intrapleural delivery have all led to the production of stable, high-level hAAT expression (Conlon et al., 2005; Virella-Lowell et al., 2005). Additional preclinical studies in nonhuman primates have assisted in the clinical translation of IM AAT delivery. An initial phase 1 trial was performed using a serotype 2 rAAV-AAT vector, but the levels were too low to justify further pursuit of this avenue (Brantly et al., 2006). Subsequently, both phase 1 and phase 2 trials of rAAV1-AAT IM delivery have been performed (Brantly et al., 2009; Flotte et al., 2011). Dose-related expression, persisting for up to 1 year has been seen, even in the face of T-cell responses to the AAV capsid and in the absence of immune suppression. The levels in the phase 2 trial (at doses of up to 6×1012 viral genome [vg]/kg or 5×1014 vg per patient) were approximately 3% of the therapeutic target (Fig. 2). Future trials will seek to further increase the dose by moving to limb perfusion methods, which will allow for a greater volume of vector to be delivered over a greater mass of muscle. This is particularly necessary since the top dose in the most recent trial entailed 100 injections during a single procedure.
FIG. 2.
Serum M-specific alpha1-antitrypsin (AAT) concentration after injection of rAAV1-CB-hAAT produced by plasmid transfection (TFX) or the herpes simplex virus (HSV) method. (Originally published in Flotte et al. [2011] and reprinted with permission by Flotte et al.) Values shown represent means±SD. The dose of vector administered to subjects is indicated in the figure legend. Values for the TFX group are from a previous study (Brantly et al., 2009). Values for the 6×1011 vg/kg HSV group do not include results for subject 303 who had an AAT phenotype of SZ; the monoclonal antibody used to determine serum M-specific AAT concentrations had little cross-reactivity with Z-type AAT but cross-reacted strongly with S-type AAT, causing results for this assay in this subject to be spuriously high. vg, viral genome.
Other gene therapy
AAT augmentation has also been accomplished in animal models using retrovirus vectors, SV40 vectors, and nonviral vectors (Zern et al., 1999). First and second generation rAd vectors and HD-Ad vectors have also been used to achieve high levels of AAT augmentation in animal models but have faced the same limitations described above. The clinical utility of such approaches has yet to be tested.
Cell-based therapy
Cell-based therapy of AAT lung and liver disease might be accomplished if one could identify a population of cells, which could be genetically corrected and then used to repopulate the liver. This has been modeled in mice using oval cells (an endogenous liver cell progenitor found in mice). Using oval cells for ex vivo rAAV-AAT gene therapy, repopulation was demonstrated by Song et al. (2004), resulting in stable AAT expression over several months. The clinical practicality of such approaches remains in question, particularly since the human equivalent of oval cells has yet to be identified. However, the availability of reprogramming technology has restored some interest in this approach.
Disorders of FAO
Background of FAO
FAO is a cyclic process in which fatty acids are broken down to produce energy. FAO is also important in removing toxic accumulations of certain fatty acids, such as short branched-chain fatty acid created by degradation of branched-chain amino acids. Under normal fed conditions, many organs prefer to get most of their energy from glycolysis, but under certain conditions, such as fasting, exercise, or metabolic stress, FAO is an important source of energy. However, the heart's preferred energy source, from which it derives 70%–95% of its energy, is FAO (Bing et al., 1954; Neely and Morgan, 1974). In utero a fetus receives a constant supply of glucose from the placenta, but after birth an infant receives 60% of it calories from fat in breast milk, so highly oxidative tissues must rely on FAO for energy (Goetzman 2011). During periods of fasting the liver uses FAO to produce acetyl-CoA to produce ketones. The ketones can then be used for energy in the brain, where glucose is the preferred energy source. Skeletal muscle also uses FAO as an energy source when energy is in high demand during exercise.
FAO can take place either in the mitochondria or in the peroxisomes, but generally peroxisomes only handle unusual fatty acid species and those with chain lengths greater than 20 carbons, which are shortened, and then oxidation is finished in the mitochondria. Both α- and β-oxidation can occur in the peroxisomes, but mitochondria can only perform β-oxidation. ω-Oxidation occurs in the endoplasmic reticulum. The focus of this article will be on mitochondrial β-oxidation and disorders of β-oxidation, primarily acyl-CoA dehydrogenase deficiencies.
In order for β-oxidation of fatty acids to occur, fatty acids must first be transported into the mitochondria. Free fatty acids are esterified with coenzyme-A to produce acyl-CoA molecules inside the cell, which are the precursors for β-oxidation. Long-chain fatty acids, which contain 12 or more carbons, are believed to be actively transported into the mitochondrial membrane. On the outer mitochondria membrane, carnitine palmitoyltransferase-1 converts long-chain acyl-CoA to acyl-carnitine, translocating it to in the inner mitochondria membrane. To cross the inner mitochondria membrane, carnitine acyl-carnitine translocase carries the acyl-carnitine and exchanges it for free carnitine. Finally carnitine palmitoyltransferase-2 converts the acyl-carnitines back to acyl-CoA. In order for FAO to occur, transportation into the mitochondria is essential. In the case of defects of β-oxidation, the transport system also works in reverse, except the fatty acids remain as acyl-carnitine in the cytosol from which they transfuse out of the cell and into the blood (Goetzman, 2011).
Next β-oxidation occurs in the following steps: dehydrogenation in which acyl-CoA–dehydrogenases (ACADs) catalyze a double bond between C-2 and C-3 and are noncovalently bound to flavin adenine dinucleotide (FAD) to accept electrons; hydration of the bond between C-2 and C-3 by enoyl CoA hydratase, which is sterospecific and forms only the l-isomer; oxidation by L-β-hydroxyacyl-CoA-dehydrogenase, converting the hydro group to a keto group and using NAD+ as an electron acceptor; and thiolysis by β-ketothiolase cleaving the thiol group of CoA using free CoA. The results are an acetyl-CoA molecule and an acyl-CoA molecule that is two carbons shorter. This process continues shortening the acyl-CoA molecule two carbons at a time until two molecules of acetyl-CoA are produced. Acetyl-CoA can then go to the TCA cycle and electron transport chain and produce 10 ATP. With the additional electrons captured during steps 1 and 3 of β-oxidation, more ATP is made from FAO than would be for the same carbon length glucose chains. The electrons from the reduced FAD are removed from the ACADs by a mechanism involving electron transferring flavoprotein, which only recently has become better understood (Chohan et al., 2001; Leys et al., 2003; Toogood et al., 2004).
ACADs are a family of flavoenzymes that are responsible for catalyzing α,β-dehydrogenation of acyl-CoA and using electron transferring flavoprotein to transfer elections. There are at least 11 known family members that function in either straight chain β-oxidation of fatty acids or metabolism of branched-chain amino acids. All ACADs are processed into a mature form in the mitochondria but are encoded in the nuclear genome and translated in the cytoplasm. The enzymes involved in straight chain β-oxidation include very long chain acyl-CoA dehydrogenase (VLCAD), acyl-CoA dehydrogenase-9 (ACAD9), long-chain acyl-coA dehydrogenase (LCAD), medium-chain acyl-coA dehydrogenase (MCAD), and short-chain acyl-coA dehydrogenase (SCAD). They are responsible for catalyzing the first steps of mitochondrial FAO. As their names suggest, each enzyme has specificity for a particular chain length; for example, VLCAD is specific for palmitoyl-CoA (C16), making it the rate-limiting enzyme of long-chain fatty acid oxidation.
VLCAD gene therapy
VLCAD differs from the rest of the acyl-CoAs not only because it is structurally active in the homodimer form, but also because it is associated with the inner mitochondrial membrane. VLCAD can catabolize long-chain fatty acids from 14–24 carbons in size, and it has optimal specificity for chain lengths 14–16 (Izai et al., 1992). The gene is approximately 5.4 kb with 20 introns located on chromosome 17 between bands p11.2 and p11.13105. The resulting protein encoded is 67 kD in size. Prevalence of VLCAD disorder has been reported to be from 1:30,000 to 1:85,000 (Lindner et al., 2010).
ACADs are responsible for catalyzing the first step in FAO, and VLCAD is the rate-limiting ACAD. In the absence of functional VLCAD enzyme, long-chain fatty acids are accumulated and an energy deficiency is created. Three phenotypes have been associated with VLCAD disorder, representing energy deficiencies in different highly metabolic organs. Of the three phenotypes, the earliest onset is also the most severe. It often presents as a cardiac phenotype, with cardiomyopathy as well as heptamegaly, hypotonia, and hypoglycemia within the first few months of life. Another phenotype presents during childhood, is associated with the liver, and is characterized by reoccurring hypoketotic hypoglycemia. The final phenotype is the most mild, occurring in late childhood/early adulthood with a mostly muscle phenotype such as rhabdomyolysis and myopathy. Since the establishment of newborn screening, patients with the two more severe phenotypes are surviving to adulthood and are now described as having exercise-induced rhabdomyolysis. There has been a genotype–phenotype correlation that has been recorded with VLCAD deficiency that is not seen with MCAD deficiency (Andresen et al., 1999). Null mutations are more likely to present with severe symptoms in early childhood, and patients with missense mutations present with milder phenotypes. However, since VLCAD does not have a predominating mutation, and many different mutations have been identified some missense mutations have been associated with severe disease. Residual enzyme activities of specific mutations have also been useful in predicting clinical outcomes. Patients with less than 10% activity will develop clinical features in absence of treatment, and patients with less than 20% may also be at risk for serious clinical disease (Hoffmann et al., 2012). For example, one fetal case occurred in a patient with a genotype encoding a protein that retains residual activity. This mutation is often found in many asymptomatic patients (Coughlin and Ficicioglu, 2010).
VLCAD deficiency results in fatty acids being transported out of the mitochondria and accumulating in the blood in the form of acyl-carnitines at a high frequency, creating a metabolic block. These acyl-carnitine species are readily detectable by tandem mass spectrometry (MS/MS). Screening by MS/MS for specific chain length accumulations provides clear evidence of a metabolic disorder; in the case of VLCAD, C14:1, C14:2, C14, and C12:1. VLCAD screening has been added to the standard panel as part of the newborn screening program across all 50 states. Accumulations of acyl-carnitine are measured from a dried blood spot taken shortly after birth. Confirmation can by made by analysis of VLCAD enzyme activity from various tissues as well as analysis of β-oxidation of fibroblast cells. Since the advent of newborn screening, the prevalence of VLCAD deficiency has been revealed to be higher than originally expected and many patients remain asymptomatic, suggesting they would have gone undiagnosed prior to newborn screening.
During periods of metabolic derangement patients are given intravenous glucose, often with insulin, and to prevent this metabolic status, patients are to avoid fasting, myocardial irritation, dehydration, high fat diets, and anesthetics containing high doses of long-chain fatty acids. Patients with the severe forms of VLCAD deficiency are placed on medium-chain triglyceride (MCT) supplementation and often have nocturnal gastric drip feedings. However, although MCT supplementation is considered safe, many question its use and long-term effects (Tucci et al. 2010, 2011). Uncooked cornstarch is often given as a source of sustained release glucose. Alternative experimental therapies have included supplementation with carnitine, branched chain fatty acids, and benzafibrate.
Early efforts at designing a gene therapy for VLCAD deficiency began with modeling deficiency of LCAD, which does not actually exist in humans, but which encompasses an overlapping range of substrates in other mammals. Unlike humans, mice express LCAD at high levels in metabolically active tissues such as liver and skeletal and cardiac muscle (Chegary et al., 2009; Maher et al., 2010). The LCAD mouse model has a more severe phenotype then VLCAD mice, which mimics the severe clinical phenotype of VLCAD-deficient patients (Cox et al., 2001). Correction of LCAD+/− mice, which display an intermediate phenotype, was shown by both a muscle-targeted approach (rAAV1 injected intramuscularly) and a liver-targeted approach (rAAV8 through the portal vein). Reduction of the lipid peak by magnetic resonance spectrometry (MRS) was observed in the muscle 10 weeks after IM injection or rAAV1 as well as systemic effect on decreased macrosteatosis in the liver by Oil Red O staining in female mice. Muscle-targeted transduction, however, was not able to reduce acyl-carnitine accumulation in the serum. Reduction of both macrosteatosis and microsteatosis was observed by Oil Red O staining after portal vein injection of rAAV8.
Hydrodynamic injection of VLCAD into VLCAD-deficient mice was associated with expression of functional VLCAD protein, and in vitro correction was observed in human VLCAD-deficient fibroblast cells by reduction of acyl-carnitine accumulation (Merritt et al., 2006). rAAV8 gene therapy, using the CMV promoter, by systemic tail vein injection was published by the same group (Merritt et al., 2009). Expression was shown in the liver of these mice at an early time point (11 days post-injection) but was not seen at a later 102 day post-injection time point although vector genomes were still present. Expression of VLCAD in the heart increased between 11 and 102 days, and muscle transduction was generally poor. Correction of acyl-carnitine accumulation in the blood as well as fasting-induced hypoglycemia were seen throughout the study.
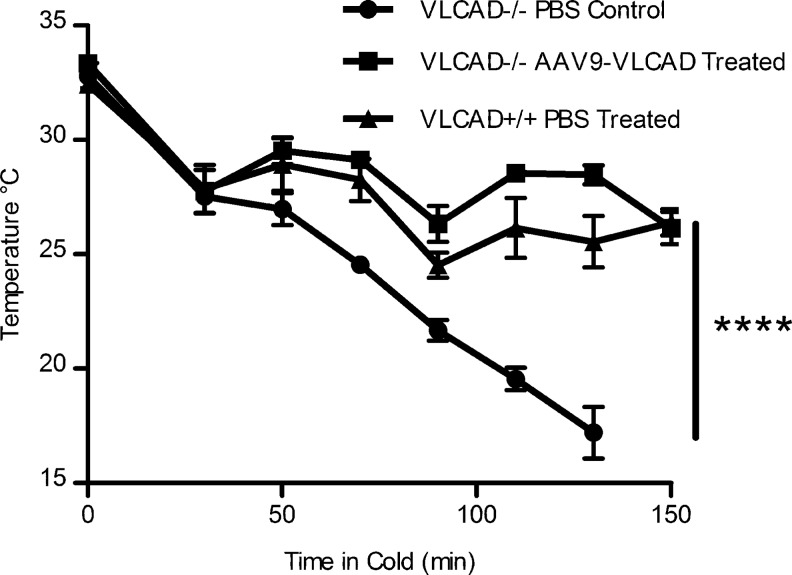
Most recently, a study performed using a systemic correction of VLCAD-deficient mice using rAAV9 vectors expressing VLCAD under a CMV enhancer/CB promoter. In this study long-term expression of VLCAD protein, 147–182 days post injection, was observed in the liver and skeletal and cardiac muscle as well as brown fat (Keeler et al., 2012). Systemic correction was seen in reduction of blood acyl-carnitine accumulation, but tissue-specific accumulation was measured in the liver and heart and muscle ex vivo and in the liver and muscle in vivo by MRS. Also for the first time disease-specific phenotypes of cold intolerance and cold-induced hypoglycemia and hypotonia were observed after correction, and animals receiving AAV9-expressing VLCAD behaved like wild-type animals (Keeler et al., 2012) (Fig. 3). This study shows significant proof of principle for gene therapy for VLCAD deficiency, and taken together, these studies have important clinical applications for diseases of mitochondrial fatty acid oxidation.
FIG. 3.
Phenotypic correction with rAAV9-very long-chain acyl-coA dehydrogenase (VLCAD)- treated mice after cold fast challenge. (Originally published in Keeler et al. [2012] and reprinted with permission by Keeler et al.) Core body temperature of mice undergoing cold fast challenge, temperatures were recorded every 20 min by rectal thermometer. Mice were humanely sacrificed if body temperatures dropped below 20°C, n=4. Error bars are SEM. p=value by two-way analysis of variance (ANOVA) ****p<0.0001.
Gene therapy for MCAD deficiency and other FAO disorders
In 1997, Kelly et al. (1997) created a liver-specific SCAD knock-in mouse in the SCAD-deficient mouse model. They were able to show decreased lipid accumulation in the liver by Oil Red O staining and systemic correction of decreased accumulation of butyrylcarnitine in the urine. These studies provided a foundation for gene therapy through specific tissue targeting being possible for disorders of fatty oxidation, and also showed that overexpression of therapeutic protein was not detrimental. Holm et al. (2003) next showed gene transfer to livers of SCAD-deficient mice by hydrodynamic transfer. Although gene transfer occurred and 5% of liver cells expressed functional SCAD proteins 31 days post injection, systemic correction by reduced blood butyrylcarnitine after fast was not seen. Conlon et al. (2006) published a gene therapy for SCAD disorder using IM injection of rAAV1 and rAAV2 encoding SCAD showing both correction of SCAD-deficient patient cells in vitro and in vivo in SCAD-deficient mice. This targeted muscle gene therapy using rAAV1 showed systemic correction of serum butyrylcarnitine levels after fasting 10 weeks post injection. A method of detecting lipid accumulation within specific tissues noninvasively in vivo was also validated using MRS. Next Beattie et al. (2008) used a liver-directed approach, like Kelly et al. (1997), but with rAAV5 and rAAV8 for gene therapy in the SCAD-deficient mouse. Using portal vein injections or rAAV8, protein expression was targeted to the liver where decreases in lipid accumulation were observed by both Oil Red O staining and MRS 10 weeks post injection. Systemic correction was also seen by reduction of serum butyrylcarnitines after fasting.
In vitro correction of MCAD deficiency using a rAd vector has been performed, but so far no gene therapy in vivo has been published for MCAD deficiency. MCAD-deficient human fibroblasts were infected with rAd vectors encoding MCAD, and whole cell medium showed reductions of C6, C8, and C10 fatty acids (Schowalter et al., 2005). These results are promising for future studies of an in vivo MCAD gene therapy.
Summary
In summary, lessons learned from gene therapy clinical trials for diseases affecting the lung such as CF and AAT deficiency can be applied to new disease targets like disorders of FAO, which were associated with SIDS prior to newborn screening. Clinical trials have been investigated for these disorders using a variety of vectors such as adenovirus, AAV, and nonviral vectors. Phase 2 clinical trials for AAT have had promising results, as well as proof of concept for gene therapies for acyl-CoA dehydrogenase deficiencies.
Author Disclosure Statement
No competing financial interests exist.
References
- Accurso F.J. Rowe S.M. Clancy J.P., et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N. Engl. J. Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afione S.A. Conrad C.K. Kearns W.G., et al. In vivo model of adeno-associated virus vector persistence and rescue. J. Virol. 1996;70:3235–3241. doi: 10.1128/jvi.70.5.3235-3241.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alton E.W. Stern M. Farley R., et al. Cationic lipid-mediated CFTR gene transfer to the lungs and nose of patients with cystic fibrosis: a double-blind placebo-controlled trial. Lancet. 1999;353:947–954. doi: 10.1016/s0140-6736(98)06532-5. [DOI] [PubMed] [Google Scholar]
- Andresen B.S. Olpin S. Poorthuis B.J., et al. Clear correlation of genotype with disease phenotype in very-long-chain acyl-CoA dehydrogenase deficiency. Am. J. Hum. Genet. 1999;64:479–494. doi: 10.1086/302261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous. The implantation of a stem cell-seeded artificial trachea in a human patient. Regen. Med. 2011;6:542. [PubMed] [Google Scholar]
- Bayle J.Y. Johnson L.G. St George J.A., et al. High-efficiency gene transfer to primary monkey airway epithelial cells with retrovirus vectors using the gibbon ape leukemia virus receptor. Hum. Gene Ther. 1993;4:161–170. doi: 10.1089/hum.1993.4.2-161. [DOI] [PubMed] [Google Scholar]
- Beattie S.G. Goetzman E. Conlon T., et al. Biochemical correction of short-chain acyl-coenzyme A dehydrogenase deficiency after portal vein injection of rAAV8-SCAD. Hum. Gene Ther. 2008;19:579–588. doi: 10.1089/hum.2007.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing R.J. Siegel A. Ungar I. Gilbert M. Metabolism of the human heart II. Studies on fat, ketone and amino acid metabolism. Am. J. Med. 1954;16:504–515. doi: 10.1016/0002-9343(54)90365-4. [DOI] [PubMed] [Google Scholar]
- Boles R.G. Buck E.A. Blitzer M.G., et al. Retrospective biochemical screening of fatty acid oxidation disorders in postmortem livers of 418 cases of sudden death in the first year of life. J. Pediatr. 1998;132:924–933. doi: 10.1016/s0022-3476(98)70385-3. [DOI] [PubMed] [Google Scholar]
- Boucher R. Regulation of airway surface liquid volume by human airway epithelia. Plugers Arch. 2003;445:495–498. doi: 10.1007/s00424-002-0955-1. [DOI] [PubMed] [Google Scholar]
- Brantly M.L. Spencer L.T. Humphries M., et al. Phase I trial of intramuscular injection of a recombinant adeno-associated virus serotype 2 alphal-antitrypsin (AAT) vector in AAT-deficient adults. Hum. Gene Ther. 2006;17:1177–1186. doi: 10.1089/hum.2006.17.1177. [DOI] [PubMed] [Google Scholar]
- Brantly M.L. Chulay J.D. Wang L., et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc. Natl. Acad. Sci. U. S. A. 2009;106:16363–16368. doi: 10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briel M. Greger R. Kunzelmann K. Cl- transport by cystic fibrosis transmembrane conductance regulator (CFTR) contributes to the inhibition of epithelial Na+ channels (ENaCs) in Xenopus oocytes co-expressing CFTR and ENaC. J. Physiol. 1998;508:825–836. doi: 10.1111/j.1469-7793.1998.825bp.x. (Pt 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigham K.L. Lane K.B. Meyrick B., et al. Transfection of nasal mucosa with a normal alpha1-antitrypsin gene in alpha1-antitrypsin-deficient subjects: comparison with protein therapy. Hum. Gene Ther. 2000;11:1023–1032. doi: 10.1089/10430340050015338. [DOI] [PubMed] [Google Scholar]
- Carroll T.P. Morales M.M. Fulmer S.B., et al. Alternate translation initiation codons can create functional forms of cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 1995;270:11941–11946. doi: 10.1074/jbc.270.20.11941. [DOI] [PubMed] [Google Scholar]
- Chegary M. Brinke H. Ruiter J.P., et al. Mitochondrial long chain fatty acid beta-oxidation in man and mouse. Biochim. Biophys. Acta. 2009;1791:806–815. doi: 10.1016/j.bbalip.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chohan K.K. Jones M. Grossmann J.G., et al. Protein dynamics enhance electronic coupling in electron transfer complexes. J. Biol. Chem. 2001;276:34142–34147. doi: 10.1074/jbc.M101341200. [DOI] [PubMed] [Google Scholar]
- Conlon T.J. Cossette T. Erger K., et al. Efficient hepatic delivery and expression from a recombinant adeno-associated virus 8 pseudotyped alpha1-antitrypsin vector. Mol. Ther. 2005;12:867–875. doi: 10.1016/j.ymthe.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Conlon T.J. Walter G. Owen R., et al. Systemic correction of a fatty acid oxidation defect by intramuscular injection of a recombinant adeno-associated virus vector. Hum. Gene Ther. 2006;17:71–80. doi: 10.1089/hum.2006.17.71. [DOI] [PubMed] [Google Scholar]
- Copreni E. Palmieri L. Castellani S. Conese M. A VSV-G pseudotyped last generation lentiviral vector mediates high level and persistent gene transfer in models of airway epithelium in vitro and in vivo. Viruses. 2010;2:1577–1588. doi: 10.3390/v2081577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin C.R., 2nd Ficicioglu C. Genotype-phenotype correlations: sudden death in an infant with very-long-chain acyl-CoA dehydrogenase deficiency. J Inher. Metab. Dis. 2010 doi: 10.1007/s10545-009-9041-6. [DOI] [PubMed] [Google Scholar]
- Cox K.B. Hamm D.A. Millington D.S., et al. Gestational, pathologic and biochemical differences between very long-chain acyl-CoA dehydrogenase deficiency and long-chain acyl-CoA dehydrogenase deficiency in the mouse. Hum. Mol. Genet. 2001;10:2069–2077. doi: 10.1093/hmg/10.19.2069. [DOI] [PubMed] [Google Scholar]
- Crystal R.G. McElvaney N.G. Rosenfeld M.A., et al. Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Nat. Genet. 1994;8:42–51. doi: 10.1038/ng0994-42. [DOI] [PubMed] [Google Scholar]
- Crystal R.G. Jaffe A. Brody S., et al. A phase 1 study, in cystic fibrosis patients, of the safety, toxicity, and biological efficacy of a single administration of a replication deficient, recombinant adenovirus carrying the cDNA of the normal cystic fibrosis transmembrane conductance regulator gene in the lung. Hum. Gene Ther. 1995;6:643–666. doi: 10.1089/hum.1995.6.5-643. [DOI] [PubMed] [Google Scholar]
- Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry 2010. 2010. www.cff.org/UploadedFiles/LivingWithCF/CareCenterNetwork/PatientRegistry/2010-Patient-Registry-Report.pdf. [Apr 19;2012 ]. www.cff.org/UploadedFiles/LivingWithCF/CareCenterNetwork/PatientRegistry/2010-Patient-Registry-Report.pdf
- Elliott P.R. Bilton D. Lomas D.A. Lung polymers in Z alpha1-antitrypsin deficiency-related emphysema. Am. J. Respir. Cell Mol. Biol. 1998;18:670–674. doi: 10.1165/ajrcmb.18.5.3065. [DOI] [PubMed] [Google Scholar]
- FitzSimmons S.C. The changing epidemiology of cystic fibrosis. J. Pediatr. 1993;122:1–9. doi: 10.1016/s0022-3476(05)83478-x. [DOI] [PubMed] [Google Scholar]
- Flotte T.R. Afione S.A. Conrad C., et al. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc. Natl. Acad. Sci. U. S. A. 1993a;90:10613–10617. doi: 10.1073/pnas.90.22.10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotte T.R. Afione S.A. Solow R., et al. Expression of the cystic fibrosis transmembrane conductance regulator from a novel adeno-associated virus promoter. J. Biol. Chem. 1993b;268:3781–3790. [PubMed] [Google Scholar]
- Flotte T.R. Afione S.A. Zeitlin P.L. Adeno-associated virus vector gene expression occurs in nondividing cells in the absence of vector DNA integration. Am. J. Respir. Cell Mol. Biol. 1994;11:517–521. doi: 10.1165/ajrcmb.11.5.7946381. [DOI] [PubMed] [Google Scholar]
- Flotte T. Carter B. Conrad C., et al. A phase I study of an adeno-associated virus-CFTR gene vector in adult CF patients with mild lung disease. Hum. Gene Ther. 1996;7:1145–1159. doi: 10.1089/hum.1996.7.9-1145. [DOI] [PubMed] [Google Scholar]
- Flotte T.R. Zeitlin P.L. Reynolds T.C., et al. Phase I trial of intranasal and endobronchial administration of a recombinant adeno-associated virus serotype 2 (rAAV2)-CFTR vector in adult cystic fibrosis patients: a two-part clinical study. Hum. Gene Ther. 2003;14:1079–1088. doi: 10.1089/104303403322124792. [DOI] [PubMed] [Google Scholar]
- Flotte T.R. Trapnell B.C. Humphries M., et al. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing alpha1-antitrypsin: interim results. Hum. Gene Ther. 2011;22:1239–1247. doi: 10.1089/hum.2011.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzman E.S. Modeling disorders of fatty acid metabolism in the mouse. Prog. Mol. Biol. Transl. Sci. 2011;100:389–417. doi: 10.1016/B978-0-12-384878-9.00010-8. [DOI] [PubMed] [Google Scholar]
- Hanrahan J.W. Kone Z. Mathews C.J., et al. Patch-clamp studies of cystic fibrosis transmembrane conductance regulator chloride channel. Methods Enzymol. 1998;293:169–194. doi: 10.1016/s0076-6879(98)93014-2. [DOI] [PubMed] [Google Scholar]
- Hoffmann L. Haussmann U. Mueller M. Spiekerkoetter U. VLCAD enzyme activity determinations in newborns identified by screening: a valuable tool for risk assessment. J. Inherit. Metab. Dis. 2012;35:269–277. doi: 10.1007/s10545-011-9391-8. [DOI] [PubMed] [Google Scholar]
- Holm D.A. Dagnaes-Hansen F. Simonsen H., et al. Expression of short-chain acyl-CoA dehydrogenase (SCAD) proteins in the liver of SCAD deficient mice after hydrodynamic gene transfer. Mol. Genet. Metab. 2003;78:250–258. doi: 10.1016/s1096-7192(03)00038-6. [DOI] [PubMed] [Google Scholar]
- Izai K. Uchida Y. Orii T., et al. Novel fatty acid beta-oxidation enzymes in rat liver mitochondria I. Purification and properties of very-long-chain acyl-coenzyme A dehydrogenase. J. Biol. Chem. 1992;267:1027–1033. [PubMed] [Google Scholar]
- Jones A.P. Wallis C. Dornase alfa for cystic fibrosis. Cochrane Database of Systematic Reviews. 2010;3:CD001127. doi: 10.1002/14651858.CD001127.pub2. [DOI] [PubMed] [Google Scholar]
- Keeler A.M. Conlon T. Walter G., et al. Long-term correction of very long-chain acyl-CoA dehydrogenase deficiency in mice using AAV9 gene therapy. Mol. Ther. 2012;20:1131–1138. doi: 10.1038/mt.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C.L. Rhead W.J. Kutschke W.K., et al. Functional correction of short-chain acyl-CoA dehydrogenase deficiency in transgenic mice: implications for gene therapy of human mitochondrial enzyme deficiencies. Hum. Mol. Genet. 1997;6:1451–1455. doi: 10.1093/hmg/6.9.1451. [DOI] [PubMed] [Google Scholar]
- König J. Schreiber R. Voelcker T., et al. The cystic fibrosis transmemebrane conductance regulator (CFTR) inhibits ENaC through an increase in the intracellular Cl- concentration. EMBO Rep. 2001;2:1047–1051. doi: 10.1093/embo-reports/kve232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstan M.W. Davis P.B. Wagener J.S., et al. Compacted DNA nanoparticles administered to the nasal mucosa of cystic fibrosis subjects are safe and demonstrate partial to complete cystic fibrosis transmembrane regulator reconstitution. Hum. Gene Ther. 2004;15:1255–1269. doi: 10.1089/hum.2004.15.1255. [DOI] [PubMed] [Google Scholar]
- Leys D. Basran J. Talfournier F., et al. Extensive conformational sampling in a ternary electron transfer complex. Nat. Struct. Biol. 2003;10:219–225. doi: 10.1038/nsb894. [DOI] [PubMed] [Google Scholar]
- Lieberman J. Dornase aerosol effect on sputum viscosity in cases of cystic fibrosis. JAMA. 1968;205:312–313. [PubMed] [Google Scholar]
- Lindner M. Hoffmann G.F. Matern D. Newborn screening for disorders of fatty-acid oxidation: experience and recommendations from an expert meeting. J. Inher. Metab. Dis. 2010;33:521–526. doi: 10.1007/s10545-010-9076-8. [DOI] [PubMed] [Google Scholar]
- Maher A.C. Mohsen A.W. Vockley J. Tarnopolsky M.A. Low expression of long-chain acyl-CoA dehydrogenase in human skeletal muscle. Mol. Genet. Metab. 2010;100:163–167. doi: 10.1016/j.ymgme.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt J.L., 2nd Matern D. Vockley J., et al. In vitro characterization and in vivo expression of human very-long chain acyl-CoA dehydrogenase. Mol. Genet. Metab. 2006;88:351–358. doi: 10.1016/j.ymgme.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Merritt J.L., 2nd Nguyen T. Daniels J., et al. Biochemical correction of very long-chain acyl-CoA dehydrogenase deficiency following adeno-associated virus gene therapy. Mol. Ther. 2009;17:425–429. doi: 10.1038/mt.2008.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R.B. Rodman D. Spencer L.T., et al. Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: a multicenter, double-blind, placebo-controlled trial. Chest. 2004;125:509–521. doi: 10.1378/chest.125.2.509. [DOI] [PubMed] [Google Scholar]
- Neely J.R. Morgan H.E. Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu. Rev. Physiol. 1974;36:413–459. doi: 10.1146/annurev.ph.36.030174.002213. [DOI] [PubMed] [Google Scholar]
- Olivier R. Scherrer U. Horisberger J.D., et al. Selected contribution: limiting Na(+) transport rate in airway epithelia from alpha-ENaC transgenic mice: a model for pulmonary edema. J. Appl. Physiol. 2002;93:1881–1887. doi: 10.1152/japplphysiol.00413.2002. [DOI] [PubMed] [Google Scholar]
- Ostedgaard L.S. Baldursson O. Welsh M.J. Regulation of the cystic fibrosis transmembrane conductance regulator Cl- channel by its R domain. J. Biol. Chem. 2001;276:7689–7692. doi: 10.1074/jbc.R100001200. [DOI] [PubMed] [Google Scholar]
- Palmer D.J. Ng P. Helper-dependent adenoviral vectors for gene therapy. Hum. Gene Ther. 2005;16:1–16. doi: 10.1089/hum.2005.16.1. [DOI] [PubMed] [Google Scholar]
- Ramsey B.W. Davies J. McElvaney N.G., et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Engl. J. Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz F.E. Clancy J.P. Perricone M.A., et al. A clinical inflammatory syndrome attributable to aerosolized lipid-DNA administration in cystic fibrosis. Hum. Gene Ther. 2001;12:751–761. doi: 10.1089/104303401750148667. [DOI] [PubMed] [Google Scholar]
- Schowalter D.B. Matern D. Vockley J. In vitro correction of medium chain acyl CoA dehydrogenase deficiency with a recombinant adenoviral vector. Mol. Genet. Metab. 2005;85:88–95. doi: 10.1016/j.ymgme.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Seibert F.S. Chang X.B. Aleksandrov A.A., et al. Influence of phosphorylation by protein kinase A on CFTR at the cell surface and endoplasmic reticulum. Biochim. Biophys. Acta. 1999;1461:275–283. doi: 10.1016/s0005-2736(99)00163-7. [DOI] [PubMed] [Google Scholar]
- Sirninger J. Muller C. Braag S., et al. Functional characterization of a recombinant adeno-associated virus 5-pseudotyped cystic fibrosis transmembrane conductance regulator vector. Hum. Gene Ther. 2004;15:832–841. doi: 10.1089/hum.2004.15.832. [DOI] [PubMed] [Google Scholar]
- Song S. Morgan M. Ellis T., et al. Sustained secretion of human alpha-1-antitrypsin from murine muscle transduced with adeno-associated virus vectors. Proc. Natl. Acad. Sci. U. S. A. 1998;95:14384–14388. doi: 10.1073/pnas.95.24.14384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S. Embury J. Laipis P.J., et al. Stable therapeutic serum levels of human alpha-1 antitrypsin (AAT) after portal vein injection of recombinant adeno-associated virus (rAAV) vectors. Gene Ther. 2001;8:1299–1306. doi: 10.1038/sj.gt.3301422. [DOI] [PubMed] [Google Scholar]
- Song S. Witek R.P. Lu Y., et al. Ex vivo transduced liver progenitor cells as a platform for gene therapy in mice. Hepatology. 2004;40:918–924. doi: 10.1002/hep.20404. [DOI] [PubMed] [Google Scholar]
- Song Y. Lou H.H. Boyer J.L., et al. Functional cystic fibrosis transmembrane conductance regulator expression in cystic fibrosis airway epithelial cells by AAV6.2-mediated segmental trans-splicing. Hum. Gene Ther. 2009;20:267–281. doi: 10.1089/hum.2008.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerford C. Bartlett J.S. Samulski R.J. AlphaVbeta5 intergrin: a co-receptor for adeno-associated virus type 2 infection. Nat. Med. 1999;5:78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- Teramoto S. Bartlett J.S. McCarty D., et al. Factors influencing adeno-associated virus-mediated gene transfer to human cystic fibrosis airway epithelial cells: comparison with adenovirus vectors. J. Virol. 1998;72:8904–8912. doi: 10.1128/jvi.72.11.8904-8912.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toogood H.S. van Thiel A. Basran J., et al. Extensive domain motion and electron transfer in the human electron transferring flavoprotein.medium chain acyl-CoA dehydrogenase complex. J. Biol. Chem. 2004;279:32904–32912. doi: 10.1074/jbc.M404884200. [DOI] [PubMed] [Google Scholar]
- Tucci S. Primassin S. Ter Veld F. Spiekerkoetter U. Medium-chain triglycerides impair lipid metabolism and induce hepatic steatosis in very long-chain acyl-CoA dehydrogenase (VLCAD)-deficient mice. Mol. Genet. Metab. 2010;101:40–47. doi: 10.1016/j.ymgme.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Tucci S. Flogel U. Sturm M., et al. Disrupted fat distribution and composition due to medium-chain triglycerides in mice with a beta-oxidation defect. Am. J. Clin. Nutr. 2011;94:439–449. doi: 10.3945/ajcn.111.012948. [DOI] [PubMed] [Google Scholar]
- Virella-Lowell I. Zusman B. Foust K., et al. Enhancing rAAV vector expression in the lung. J. Gene Med. 2005;7:842–850. doi: 10.1002/jgm.759. [DOI] [PubMed] [Google Scholar]
- Wagner C.A. Ott M. Klingel K., et al. Effects of the serine/threonine kinase SGK1 on the epithelial Na(+) channel (ENaC) and CFTR: implications for cystic fibrosis. Cell Physiol. Biochem. 2001;11:209–218. doi: 10.1159/000051935. [DOI] [PubMed] [Google Scholar]
- Weiss D.J. Bertoncello I. Borok Z., et al. Stem cells and cell therapies in lung biology and lung diseases. Proc. Am. Thoracic Soc. 2011;8:223–272. doi: 10.1513/pats.201012-071DW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M.J. Anderson M.P. Rich D.P., et al. Cystic fibrosis transmembrane conductance regulator: a chloride channel with novel regulation. Neuron. 1992;8:821–829. doi: 10.1016/0896-6273(92)90196-k. [DOI] [PubMed] [Google Scholar]
- Yang Y. Nunes F.A. Berencsi K., et al. Inactivation of E2a in recombinant adenoviruses improves the prospect for gene therapy in cystic fibrosis. Nat. Genet. 1994;7:362–369. doi: 10.1038/ng0794-362. [DOI] [PubMed] [Google Scholar]
- Zern M.A. Ozaki I. Duan L., et al. A novel SV40-based vector successfully transduces and expresses an alpha 1-antitrypsin ribozyme in a human hepatoma-derived cell line. Gene Ther. 1999;6:114–120. doi: 10.1038/sj.gt.3300793. [DOI] [PubMed] [Google Scholar]
- Ziady A.G. Gedeon C.R. Miller T., et al. Transfection of airway epithelium by stable PEGylated poly-L-lysine DNA nanoparticles in vivo. Mol. Ther. 2003;8:936–947. doi: 10.1016/j.ymthe.2003.07.007. [DOI] [PubMed] [Google Scholar]