Abstract
Aims: We investigated the molecular mechanism by which ethyl pyruvate (EP) induces heme oxygenase-1 (HO-1) in RAW 264.7 cells and its effect on survival rate in cecal ligation and puncture (CLP)-induced wild-type (WT) and HO-1 knockout (HO-1−/−) septic mice. Results: EP induced HO-1 in a dose- and time-dependent manner, which was mediated through p38 mitogen-activated protein kinase (MAPK) and NF-E2-related factor 2 (Nrf2) signaling cascade in RAW 264.7 cells. EP significantly inhibited the lipopolysaccharide (LPS)-stimulated inducible nitric oxide synthase (iNOS) expression and high-mobility group box 1 (HMGB1) release in RAW 264.7 cells. The inhibitory effect of EP on LPS-stimulated iNOS expression and HMGB1 release was reversed by transfection with siHO-1RNA in RAW 264.7 cells, but EP failed to reduce them in HO-1−/− peritoneal macrophages treated with LPS. Moreover, treatment of cells with glutathione ethyl ester (GSH-Et), SB203580 (p38 MAPK inhibitor), siHO-1, or p38-siRNA transfection inhibited anti-inflammatory effect of EP. Interestingly, both HO-1 induction and phosphorylation of p38 by EP were reversed by GSH-Et, and antioxidant redox element-luciferase activity by EP was reversed by SB203580 in LPS-activated cells. EP increased survival and decreased serum HMGB1 in CLP-WT mice, whereas it did not increase survival or decrease circulating HMGB1 in HO-1−/− CLP-mice. Innovation and Conclusion: Our work provides new insights into the understanding the molecular mechanism by showing that EP induces HO-1 through a p38 MAPK- and NRF2-dependent pathway by decreasing GSH cellular levels. We conclude that EP inhibits proinflammatory response to LPS in macrophages and increases survival in CLP-induced septic mice by upregulation of HO-1 level, in which p38 MAPK and Nrf2 play an important role. Antioxid. Redox Signal. 17, 878–889.
Introduction
Sepsis is a serious medical condition, which is an extreme immune system response to an infection that has spread throughout the blood and tissues and is characterized by a generalized inflammatory response and activation of the coagulation and fibrinolytic cascades, resulting in endothelial injury (7). It occurs in 750,000 patients per year in the United States and is fatal in 20%–40% of cases (43). The pathological sequence of sepsis is mediated by proinflammatory cytokines, such as tumor necrosis factor (TNF), interleukin-1 (IL-1), and high-mobility group box 1 (HMGB1), that are released from macrophages, neutrophils, and other cells of innate immune system (26). HMGB1 was first identified as a nuclear protein that participates in maintenance of nucleosome structure and regulation of gene transcription (25). Recently, it has been discovered that HMGB1 can be released from immune cells under proinflammatory stimulus and acts as a late mediator in sepsis (45). Administration of neutralizing antibodies to HMGB1 significantly improved survival in septic animals, suggesting a potential window for therapeutic intervention (43).
Innovation.
Improvement of sepsis by EP has been well known in the mouse model, but the underlying mechanisms of its down-regulation of sepsis are poorly understood. In this study, the ameliorating mechanisms of EP in the sepsis are clearly demonstrated. EP causes the depletion of glutathione, which activates p38 MAPK leading to HO-1 induction. EP-induced HO-1 plays an important role in improvement of the survival rate for septic mice.
Heme oxygenase-1 (HO-1) is a stress-responsive protein that can be induced by stimulants such as cytokines, heat shock, heavy metals, and oxidants, and it degrades heme into three products: Fe++, biliverdin, and carbon monoxide (CO) (27). It has been already documented that the HO-1/CO system provides a therapeutic effect in many experimental pathological conditions (2, 31). Recently, we and others demonstrated that HO-1/CO can improve survival and decrease the circulating HMGB1 level in septic animals, which further supports beneficial effects of HO-1 in inflammatory disorders (37, 38, 40).
Ethyl pyruvate (EP) is a simple aliphatic ester of the metabolic intermediate pyruvate, and it has been demonstrated as a potent anti-inflammatory agent in a variety of in vivo and in vitro model systems, including sepsis (8, 10, 34, 41). Importantly, anti-inflammatory effects of EP may be related to inhibition of HMGB1 secretion (41). Although HO-1 induction by EP was recently reported in macrophages and ameliorated murine colitis (8) and endotoxic rats (20), no report is available for the signal mechanisms by which EP induces HO-1, and this induction of HO-1 brings about the improvement of survival in septic animals by reduction of HMGB1 release. In this report, we claim that EP is able to induce HO-1 by depletion of glutathione (GSH), which activates p38 mitogen-activated protein kinase (MAPK) and NF-E2-related factor 2 (Nrf2) expression in macrophages, and it improves survival rates in septic mice via reduction of HMGB1.
Results
EP significantly attenuates inflammatory response to endotoxin in macrophages
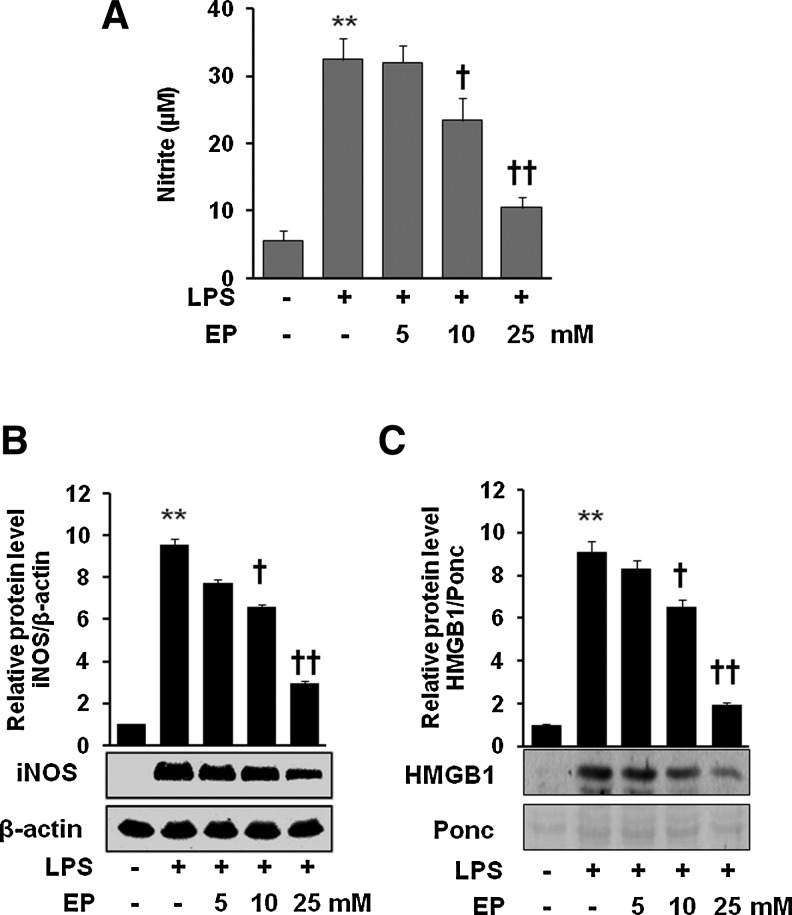
To identify the potential anti-inflammatory effect of EP, cells were incubated with EP in the presence of lipopolysaccharide (LPS). As shown in Figure 1, EP significantly inhibited production of nitric oxide (NO) (Fig. 1A), inducible nitric oxide synthase (iNOS) protein expression (Fig. 1B), and HMGB1 release (Fig. 1C) in LPS-activated macrophages. Thus, we confirmed the previous report that EP can inhibit the expression of pro-inflammatory proteins such as iNOS and HMGB1 (8, 20).
FIG. 1.
Effect of EP on the expression of iNOS/NO and HMGB1 release in LPS-stimulated macrophages (A–C). Cells were pretreated with EP for 1 h at doses 5, 10, and 25 mM. Then, the cells were stimulated with LPS (1 μg/ml) for 16 h. The culture medium was collected and subjected to NO assay (A) and HMGB1 analysis (C). Cells were lysed and harvested and subjected to western blot for iNOS detection (B) as described in the Materials and Methods section. Data are presented as±SD of three independent experiments. Significance compared with control, **p<0.01; significance compared with LPS, †p<0.05 and ††p<0.01. EP, ethyl pyruvate; HMGB1, high-mobility group box 1; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; NO, nitric oxide.
EP induces HO-1 through p38 MAPK but not ERK or JNK
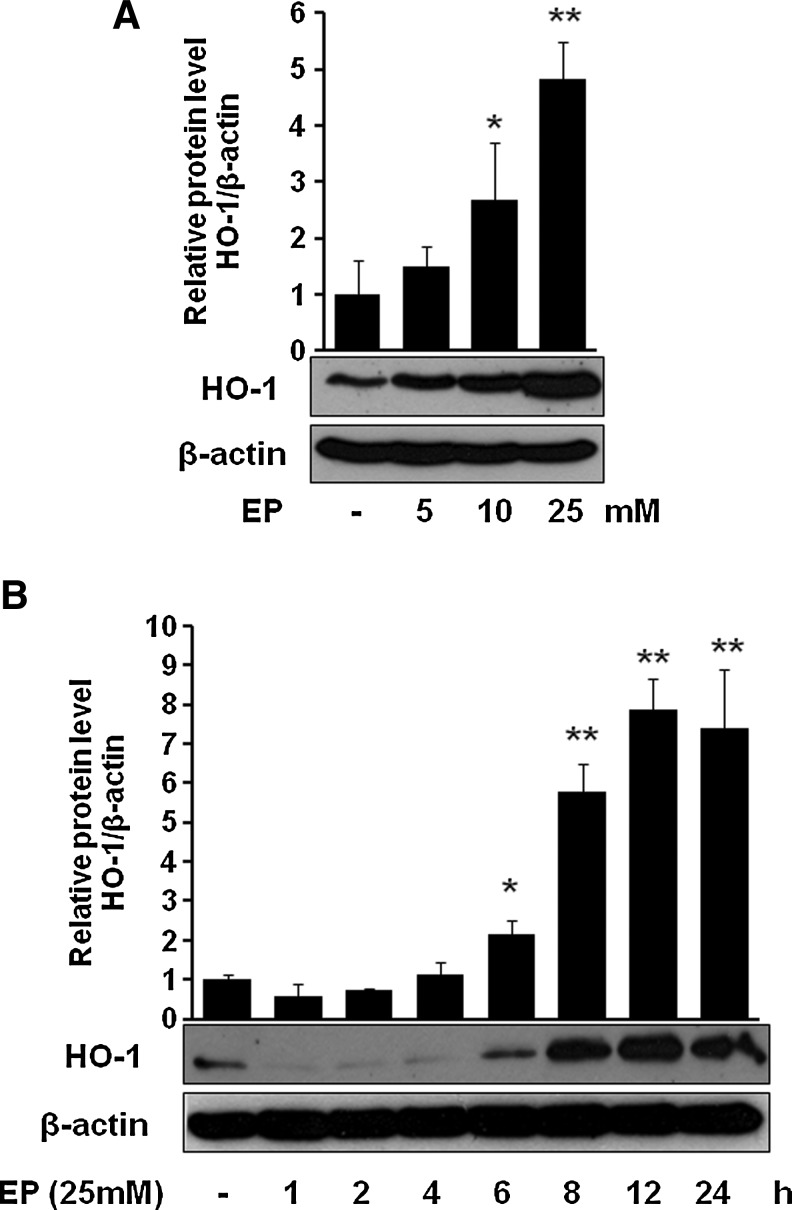
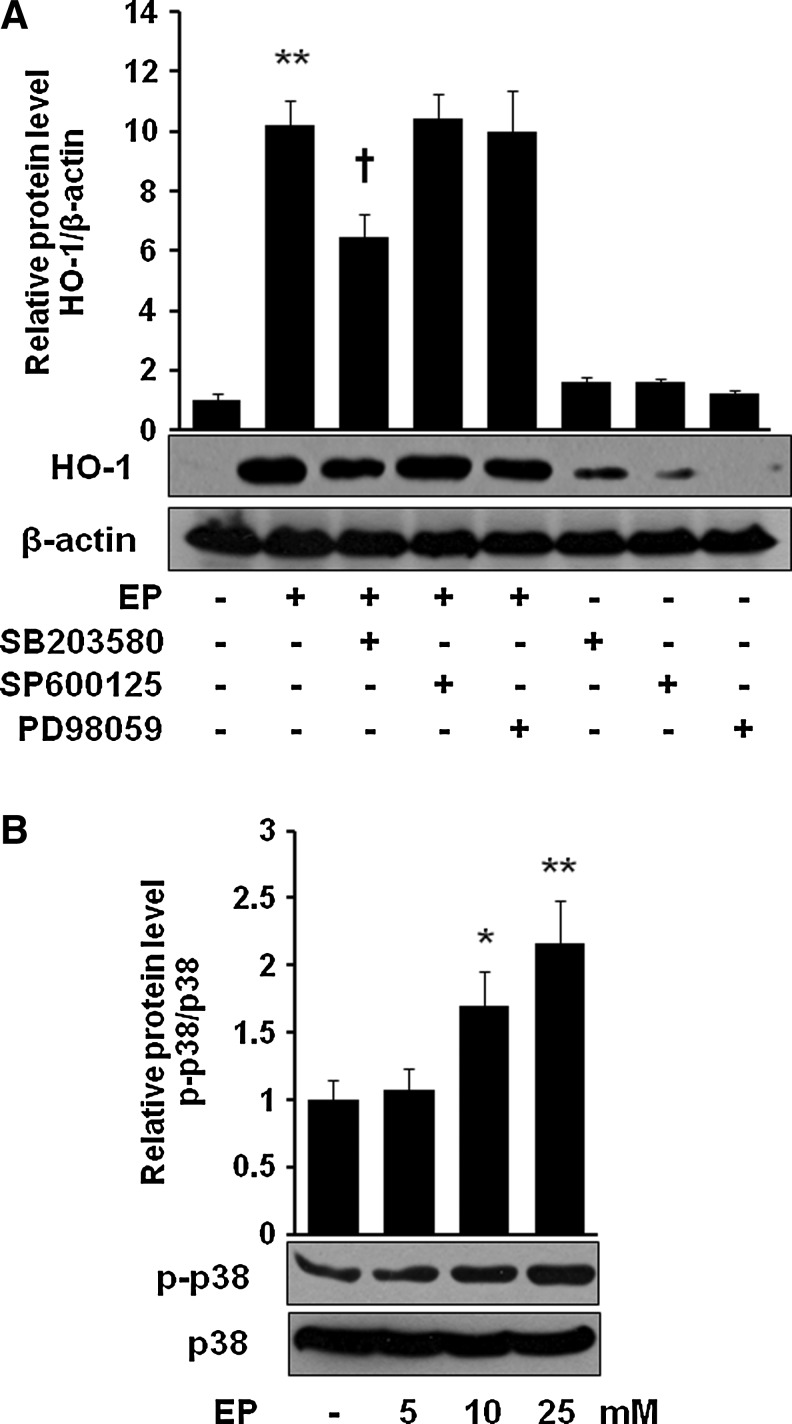
Next, we asked whether EP can induce HO-1 in macrophages. Figure 2A and B clearly show that EP significantly induced HO-1 level in a dose-dependent (Fig. 2A) and time-dependent (Fig. 2B) fashion. EP potentiated the LPS-induced HO-1 expression (Supplementary Fig. S1; Supplementary Methods and Data are available online at www.liebertonline.com/ars). To identify through which MAPK signaling cascade EP upregulates HO-1, we used different pharmacological signal inhibitors. As shown in Figure 3A, EP potently induced HO-1 in macrophages, which was inhibited only by SB203580 (p38 inhibitor) but not by either SP600125 (JNK inhibitor) or PD98059 (ERK inhibitor), suggesting the involvement of p38, but not ERK or JNK, in HO-1 regulation by EP. However, signal inhibitors by themselves (SB203580, SP600125, and PD98059) did not affect HO-1 expression (Fig. 3A). Furthermore, we observed that EP increased phosphorylation of p38 MAPK (Fig. 3B).
FIG. 2.
Effect of EP on the expression of HO-1 in macrophages. Cells were treated with EP at doses 5, 10, and 25 mM for 8 h (A) or at dose 25 mM for 1, 2, 4, 6, 8, 12, and 24 h (B). After incubation, cells were harvested and subjected to western blot as described in the Materials and Methods section. Data are presented as±SD of three independent experiments. Significance compared with control, *p<0.05 and **p<0.01. HO-1, heme oxygenase-1.
FIG. 3.
EP induces HO-1 through p38 MAPK. Cells were pretreated with SB203580 (10 μM), SP600125 (10 μM), and PD98059 (10 μM) for 1 h, and then cells were treated with EP (25 mM) for 8 h (A, for HO-1) and for 6 h (B, for p-p38). After incubation, cells were harvested and subjected to western blot as described in the Materials and Methods section. Data are presented as±SD of three independent experiments. Significance compared with control, *p<0.05 and **p<0.01; significance compared with EP alone, †p<0.05. MAPK, mitogen-activated protein kinase.
EP depletes GSH and activates P38 MAPK and Nrf2 in LPS-activated RAW 264.7 cells
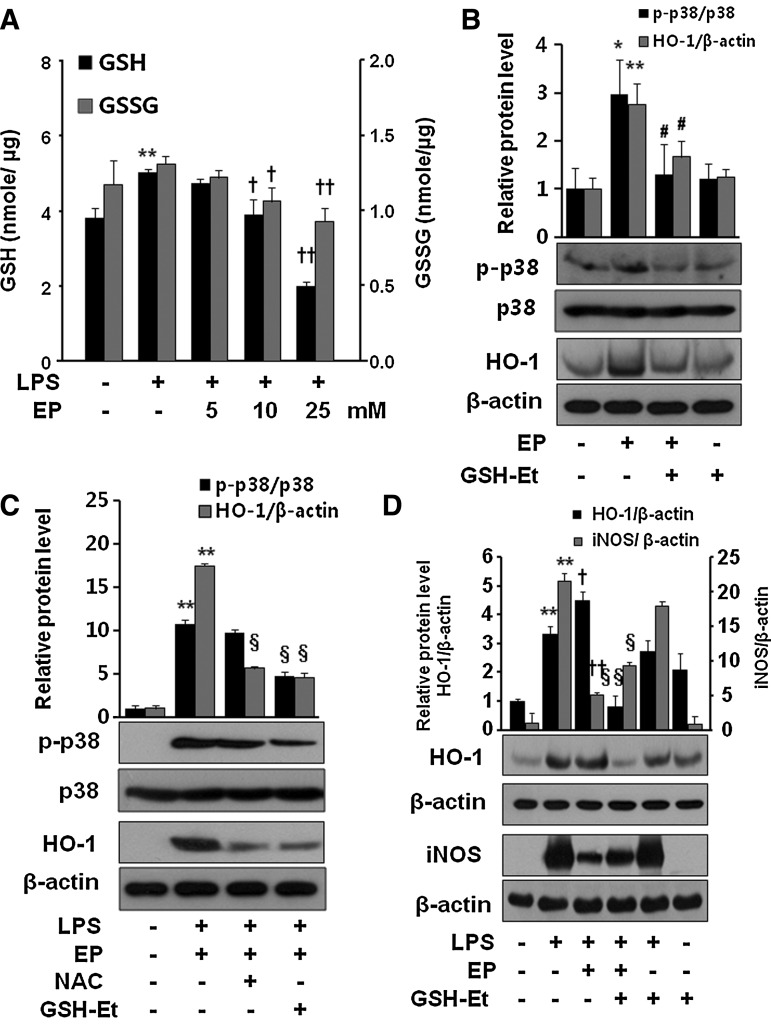
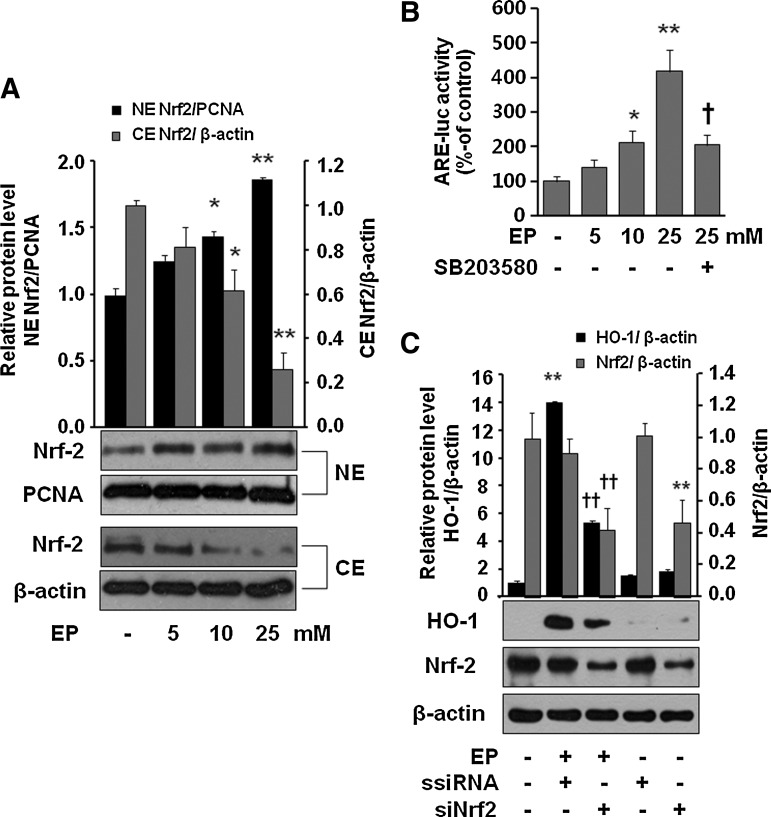
Then what is the molecular mechanism by which EP induces HO-1? Recently, it has been demonstrated that a chemical that depletes GSH induced HO-1 by activation of p38 MAPK in airway smooth muscle cells (33). In addition, EP has also been shown to deplete GSH in LPS-stimulated RAW 264.7 cells (36). Thus, we were interested whether EP is able to induce HO-1 by activation of P38 MAPK in macrophages due to depletion of GSH. As shown in Figure 4A, EP concentration dependently reduced the intracellular level of both reduced (GSH) and oxidized forms of glutathione (GSSG) in LPS-activated RAW 264.7 cells. Next, we asked whether GSH depletion increases phosphorylation of p38 MAPK and HO-1 expression, and reduces iNOS expression in response to LPS. We found that EP increased p-p38 and HO-1 expression, which was reduced by GSH-ethyl ester (GSH-Et) treatment (Fig. 4B). The expression of p-p38 was further intensified by the presence of LPS, which was again reduced by N-acetyl cysteine (NAC) or GSH-Et (Fig. 4C). In addition, treatment with GSH-Et reversed the effect of EP on the expression of iNOS and HO-1 in LPS-activated RAW 264.7 cells (Fig. 4D). To understand whether p38 MAPK plays a key role for induction of HO-1 via Nrf2 activation, we checked Nrf2 translocation and antioxidant redox element (ARE)-luciferase activity by EP. Figure 5A clearly showed that EP stimulated nuclear accumulation of Nrf2 in macrophages. Moreover, EP increased ARE-luciferase activity, which was significantly reduced by SB203580, indicating that Nrf2 to DNA-binding site by EP is dependent on p38 activity (Fig. 5B). P38 MAPK inhibitor, SB203580, itself did not affect the ARE-luciferase activity and EP also increased ARE-luciferase activity in the presence of LPS (Supplementary Fig. S2). To further identify whether EP-mediated HO-1 expression underlies Nrf2 activation, we used siNrf2. As shown in Figure 5C, EP significantly reduced HO-1 expression in siNrf2-transfected cells suggesting the critical role of p38/Nrf2 signaling in HO-1 expression. To verify that Nrf2 is a target gene for EP, we investigated the expression of another Nrf2-dependent gene, NQO1. Indeed, EP induced HO-1 protein and NQO1 mRNA expression, which was significantly reduced by siNrf2 transfection (Supplementary Fig. S3).
FIG. 4.
Effect of EP on intracellular GSH levels and p38 phosphorylation in RAW 264.7 cells. The cells were incubated for 24 h without or with LPS, or LPS with different concentrations of EP (5, 10, and 25 mM). Intracellular levels of reduced (GSH) and oxidized (GSSG) glutathione were assayed (A) as described in the Materials and Methods section. To see the effect of EP on p38 phosphorylation and HO-1 expression, cells were incubated with EP (25 mM) in the presence or absence of GSH-Et (10 mM) for 8 h (B). To check the expression of HO-1 and p38 phosphorylation by NAC and GSH-Et, cells were incubated for 8 h in the presence of LPS+EP (C). To see whether GSH-Et reverses the EP effect on LPS-activated iNOS and HO-1 expression, cells were treated with EP (25 mM) or EP with GSH-Et (10 mM) for 8 h (HO-1) or for 16 h (HMGB1) in the presence of LPS (D). After incubation, cells were harvested and subjected to western blot as described in the Materials and Methods section. Data are presented as means±SD of three independent experiments. Significance compared with control, *p<0.05 and **p<0.01; significance compared with LPS, †p<0.05 and ††p<0.01; significance compared with EP, #p<0.05; significance compared with LPS+EP, §p<0.05 and §§p<0.01. GSH-Et, glutathione ethyl ester; NAC, N-acetyl cysteine.
FIG. 5.
Involvement of Nrf2 in EP-mediated HO-1 expression. Cells were treated for 1 h with EP at 5, 10, and 25 mM, and then harvested and subjected to cytosol/nuclear fractionation (A). Cells were transiently transfected with the ARE-luc vector (B). After incubation, cells were treated with EP (5, 10, and 25 mM) or EP (25 mM)+SB203580 (10 μM, which was pretreated 1 h before addition of EP) for 1 h. After incubation, cells were subjected to luciferase assay as described in the Materials and Methods section. Cells were transfected with scramble (siRNA) or siNrf2 (C) as described in the Materials and Methods section. After 8-h incubation with EP (25 mM), cells were harvested and subjected to western blot for HO-1 expression. Transfection efficiency was confirmed by checking Nrf2 expression. Data are presented as±SD of three independent experiments. Significance compared with control, *p<0.05 and **p<0.01; significance compared with EP alone or EP+ssiRNA, †p<0.05 and ††p<0.01. ARE, antioxidant redox element; Nrf2, NF-E2-related factor 2.
Anti-inflammatory effect of EP is mediated through HO-1
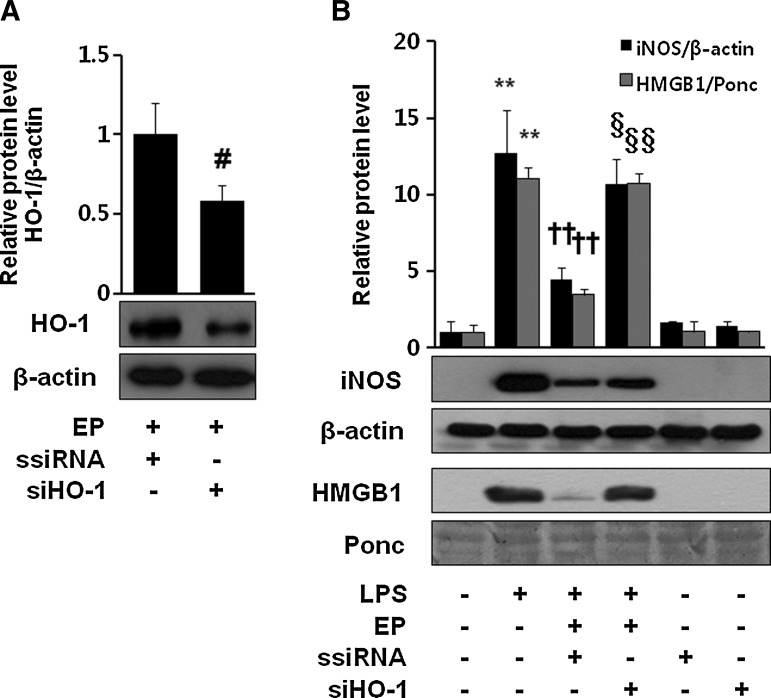
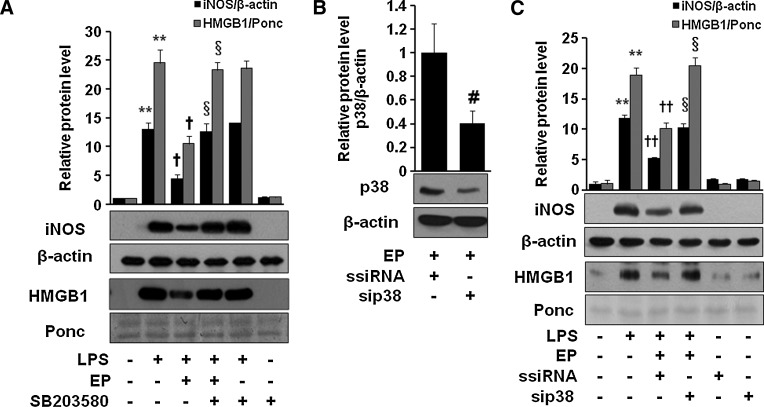
EP possesses potent anti-inflammatory activities (8, 10, 15, 20, 34, 36, 41). We investigated how HO-1 induction is important for EP-mediated anti-inflammation. As shown in Figure 6A, first, we checked siHO-1 RNA transfection efficiency by western blot of EP-induced HO-1 expression. Next we investigated whether EP is able to inhibit LPS-induced iNOS expression or HMGB1 release in macrophages transfected with HO-1 siRNA. We identified that siHO-1 RNA transfection significantly reversed the anti-inflammatory effect of EP (Fig. 6B). Interestingly, EP-mediated inhibition of iNOS and HMGB1 was also reversed in the presence SB203580 (Fig. 7A) or p38-siRNA (Fig. 7C), suggesting the importance of p38/HO-1 signaling in anti-inflammatory properties of EP. Figure 7B shows the p38-siRNA transfection efficiency.
FIG. 6.
Importance of HO-1 in the anti-inflammatory action of EP. Cells were transfected with ssiRNA or siHO-1 RNA, which were subjected to western blot to confirm siHO-1 efficiency by EP (25 mM) for 8 h (A). Cells were stimulated with LPS (1 μg/ml) in the presence or absence of EP (25 mM) for 16 h. Then, cell extract and culture medium samples were subjected to western blot for iNOS and HMGB1, respectively (B). Significance compared with control, **p<0.01; significance compared with LPS, ††p<0.01; significance compared with LPS+EP+ssiRNA, §p<0.05 and §§p<0.01; significance compared with EP+ssiRNA, #p<0.05.
FIG. 7.
SB203580 and p38-siRNA reverse anti-inflammatory effect of EP in macrophages. Cells were incubated with EP (25 mM) with or without SB203580 (10 μM) for 1 h and stimulated with LPS (1 μg/ml) for 16 h (A), or cells were transfected with p38-siRNA or ssiRNA (B, C), which were incubated with EP (25 mM) for 1 h and stimulated with LPS (1 μg/ml) for 16 h. Cell extract and culture medium samples were subjected to western blot for iNOS and HMGB1 analysis, respectively. Data are presented as±SD of three independent experiments. Significance compared with control, **p<0.01; significance compared with LPS, †p<0.05 and ††p<0.01; significance compared with LPS+EP or LPS+EP+ssiRNA, §p<0.05; significance compared with EP+ssiRNA, #p<0.05.
Therapeutic effect of EP in cecal ligation and puncture-induced sepsis model is mediated through HO-1
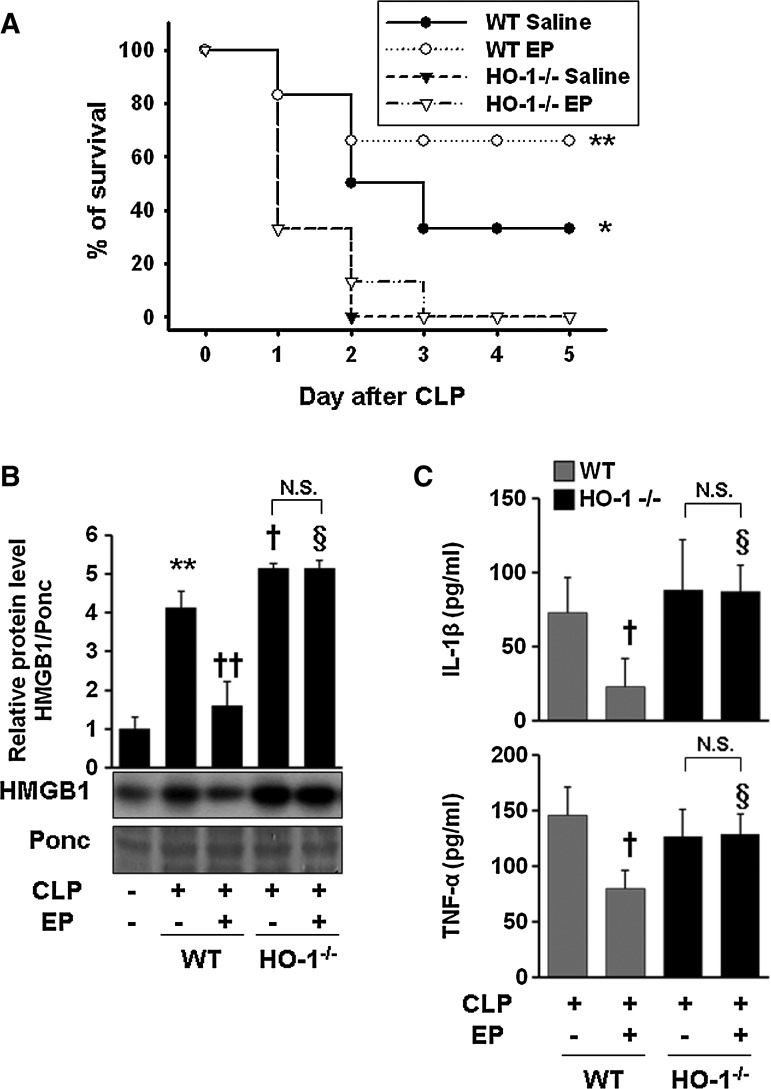
Although HO-1 induction by EP is responsible for amelioration of acute lung injury in the sepsis model (20), it has not yet been reported whether HO-1 induction by EP is accounted for protection from lethal death in septic animals. We observed that EP significantly improved the survival rate and decreased serum HMGB1 levels in cecal ligation and puncture (CLP)-induced wild-type (WT) septic mice. In WT animals, survival in vehicle-treated controls for 5 days after CLP was 30%, whereas treatment with EP significantly increased survival to 70% (Fig. 8A). The survival rate was drastically reduced to 20% in WT animals that cotreated EP with ZnPPIX (data not shown). However, EP failed to increase the survival rate in HO-1−/− mice (Fig. 8A). In peritoneal macrophages isolated from HO-1−/− mice, EP treatment did not reduce the iNOS, HMGB1, and NO production in response to LPS (Supplementary Fig. S4). As expected, circulating HMGB1 in blood by EP significantly reduced in CLP-treated WT but not in HO-1−/− animals (Fig. 8B). Likewise, EP significantly reduced pro-inflammatory cytokines (TNF-α and IL-1β) in the serum of CLP-induced WT mice but not in HO-1−/− mice (Fig. 8C). Finally, CLP operation by itself increased the level of p-p38 in lung tissues in WT animals, but EP treatment further increased the phosphorylation of p38 (Supplementary Fig. S5). Thus, we showed direct evidence that the therapeutic effect of EP is mediated via HO-1.
FIG. 8.
EP fails to increase the survival rate in the CLP-induced sepsis model of HO-1−/− mice. BALB/c WT (n=12) and HO-1−/− mice (n=12) were subjected to CLP with EP (n=6, 40 mg/kg i.p.) or an equal volume of vehicle (n=6, saline) treatment at 0 and 24 h, after onset of sepsis. Survival was monitored daily for up to 5 days (A). To determine serum HMGB1 levels, WT and HO-1−/− mice were treated with EP (40 mg/kg i.p., n=5) or an equal volume of vehicle (n=4, saline) at 0 and 12 h after onset of sepsis (CLP). Twenty-four hours later, blood was collected by cardiac puncture and subjected to HMGB1 (B) and ELISA (C) analysis. The Kaplan–Meier program was utilized to compare the differences in mortality rates between groups. **p=0.009, WT+EP versus WT+saline or HO-1−/−+EP or HO-1−/−+saline. *p=0.047, WT+saline versus HO-1−/−+saline or HO-1−/−+EP (A). Data are presented as mean±SD of three independent experiments. In (B) and (C), significance compared to sham, **p<0.01; significance compared to CLP, †p<0.05 and ††p<0.01; significance to compared to WT+EP, §p<0.05. N.S., not significant; CLP, cecal ligation and puncture; WT, wild-type.
Discussion
In the present study, we provided direct evidence that HO-1 induction in macrophages by EP contributed to anti-inflammatory action. EP induces HO-1 by depletion of intracellular GSH, which, in turn, activates p38 MAPK and Nrf2. Moreover, EP treatment improved survival in CLP-induced septic animals, whereas this effect was abrogated either in HO-1−/− animals or in WT animals that were co-treated with ZnPPIX (data not shown). Accordingly, EP could neither inhibit inflammatory response (inflammatory cytokines and HMGB1) nor improve survival in HO-1-deficient animals, demonstrating concrete evidence that anti-inflammatory action of EP is dependent on HO-1. That depletion of intracellular GSH induces Nrf2 expression as a cellular defense mechanism has been proposed (35). In fact, previous studies have been shown that depletion of GSH induced HO-1 expression in different cells (9), including RAW 264.7 cells (12, 24), and inhibited LPS-induced NO production (12, 24). Moreover, EP has been reported to deplete GSH levels in LPS-activated RAW 264.7 cells, which is responsible for the anti-inflammatory effect (36). Thus, it is quite plausible to think that cellular depletion of GSH by EP could trigger HO-1 induction. Indeed, we found that supplemetation of GSH (either GSH-Et or NAC) diminished expression of HO-1 by EP. Then what is the signal mechanism by which EP induces HO-1? Because activation of MAPKs plays a central role for the induction of HO-1 gene expression (1), and JNK and p38 are primarily induced by stress-related stimuli (21, 42), it is of great interest to investigate which MAPKs are responsible for EP-mediated HO-1 induction. We found that SB203580 (p38 MAPK inhibitor), but neither SP600125 (JNK inhibitor) nor PD98059 (ERK inhibitor), significantly inhibited EP-induced HO-1 induction, indicating that p38 MAPK plays a crucial role for HO-1 induction. Numerous reports suggested that activation of p38 MAPK mediates an anti-inflammatory action. Indeed, involvement of p38 MAPK is well documented in many diseases, including inflammatory disorders. Especially, it has been shown that CO, one of the byproducts of HO-1, provides an anti-inflammatory effect through activation of p38 MAPK (3, 19, 32). Because both induction of HO-1 and anti-inflammatory effect of EP were reversed by the presence of SB203580, we believe that p38 plays a key role for EP-mediated HO-1 induction. In contrast to the present results, equally compelling evidence has been shown previously that EP specifically inhibited the activation of p38 MAPK in LPS-activated RAW 264.7 cells (41) and in a rat model of an LPS-induced acute lung injury (20). Again, it has been demonstrated that p38 MAPK activation found to be critically involved in LPS-induced iNOS expression and NO release in RAW 264.7 macrophages and in other cells (5). Although we cannot properly explain this discrepancy at the present time, the concentration of the p38 MAPK inhibitor (SB203580) or different methods or animal models may have caused this difference. It has been demonstrated that SB203580 (1 μM) augmented nitrite accumulation in RAW 264.7 cells in response to LPS (29). Likewise, Lahti et al. (22) reported that the p38 MAPK inhibitor SB203580 has a bidirectional effect on iNOS expression and NO production in J774 mouse macrophages and T-84 human colon epithelial cells when activated with LPS. For example, a high concentration of SB203580 (30 μM) showed >90% NO inhibition without affecting LPS-activated nuclear factor kappa B (NF-κB) activation. Actually, the concentration of SB203580 by Chen et al.'s (5) studies was 30 μM, which may have resulted in iNOS inhibition, but we used 10 μM SB203580 in the present study. Consistent with the present result, our previous study also showed that 10 μM SB203580 did not inhibit iNOS expression and NO production in LPS-activated RAW 264.7 cells (39). An interesting finding is that inhibition of p38 MAPK enhances JNK activity, which led to stabilization of iNOS mRNA, so it enhanced iNOS expression and increased NO production in murine macrophages (23). On the other hand, there are reports that p38 MAPK is not involved in iNOS induction or NO production in RAW 264.7 cells under inflammatory stimulation (30). For example, both p42/44 MAP kinases and p38 MAP kinase involve in the regulation of COX-2 but not iNOS induction following exposure to LPS in RAW 264.7 cells (30), or ERK and p38 MAPK cascades are both rate-limiting for LPS- and IFN-γ-stimulated arginine uptake, but not for iNOS synthesis in RAW 264.7 cells (4). However, different subunits of p38 can impact differently and even sometimes contrary. For example, the α and β isoforms of p38 play counter-regulatory roles to the p38γ and p38δ isoforms in the induction of HO-1 by sodium arsenite (14) and LPS (44). These findings indicate the complexity of the role of p38 MAPK in the regulation of iNOS (NO) in different cells, including RAW 264.7 cells, under inflammatory stimuli.
Previously, the potential therapeutic effect of EP in the experimental model of sepsis was clearly demonstrated (41). Interestingly, the HMGB1-inhibitory effect of EP was due to the blockade of signaling proteins such as NF-κB and p38 MAPK (41). On the other hand, others suggested that neither NF-κB nor p38 MAPK is involved in HMGB1 release in LPS-treated macrophages, making a strong emphasis on the critical role of HO-1, Janus kinase 2/signal transducer and activator of transcription-1 (STAT-1), and iNOS/NO signaling in HMGB1 release (11, 16, 40). We found that EP strongly inhibited iNOS (NO) and HMGB1 in LPS-activated RAW 264.7 cells and CLP-induced septic mice that were depended on HO-1 induction, confirming earlier reports that HO-1 inducers inhibit HMGB1 release in LPS- or CLP-induced septic animals (37, 40). In addition, EP has been reported to show an anti-inflammatory effect by inhibition of JAK2/STAT1 and STAT3 through Rac1 inactivation and SOCS1 induction (15). In this sense, the JAK/STAT pathway is essential for LPS or Escherichia coli–induced HMGB1 release (16). Thus, it needs further study whether the JAK/STAT pathway attributes to inhibit HMGB1 through induction of HO-1 by EP.
Nrf2 is the transcription factor that can activate the transcription of antioxidant proteins, including HO-1. Under the Nrf2 activation, it dissociates from Kelch-like ECH-associated protein 1 (Keap1) and translocates into the nucleus, where it binds ARE (18). Different pathways may activate Nrf2, including PI3K/Akt and MAPKs (6, 13, 38). We found that EP can activate Nrf2 by enhancing Nrf2 nuclear accumulation and the ARE-luciferase activity. This effect was dependent on p38 MAPK (Fig. 5), further emphasizing the importance of p38 MAPK in EP-mediated HO-1 induction. Moreover, silencing of Nrf2 protein expression led to the attenuation of HO-1 expression by EP, signifying the critical role of Nrf2 in induction of HO-1 by EP (Fig. 5C). Thus, the p38 MAPK/Nrf2 signal plays an important role for induction of HO-1 by EP in RAW 264.7 cells. These findings are further supported by previous reports that GSH depletion increased the phoshorylation of p38 MAPK in C6 glioma cells (17), and increased the expression of the Nrf2 protein in RAW 264.7 cells (12). We provided firm evidence that Nrf2 is a target gene for EP by showing that it also increased expression of NQO1, another Nrf2 target gene. Indeed, increased expression of both HO-1 and phosphorylation of p38 MAPK and decreased expression of iNOS by EP in the presence of LPS were significantly diminished by GSH-Et. In addition, treatment with SB203580 and transfection with p38-siRNA also reversed the effect of EP on iNOS and HMGB1 expression in LPS-activated RAW 264.7 cells. These results indicate that the p38/Nrf2/HO-1 signal is critical for the anti-inflammatory action of EP (Fig. 9). The increased phosphorylation of p38 MAPK by EP was not limited to RAW 264.7 cells in vitro. We found that EP augmented p-p38 MAPK in CLP-induced septic mouse lung tissues (Supplementary Fig. S5), indicating that tight regulation of the p38 and HO-1 activity in the anti-inflammatory action of EP in vivo.
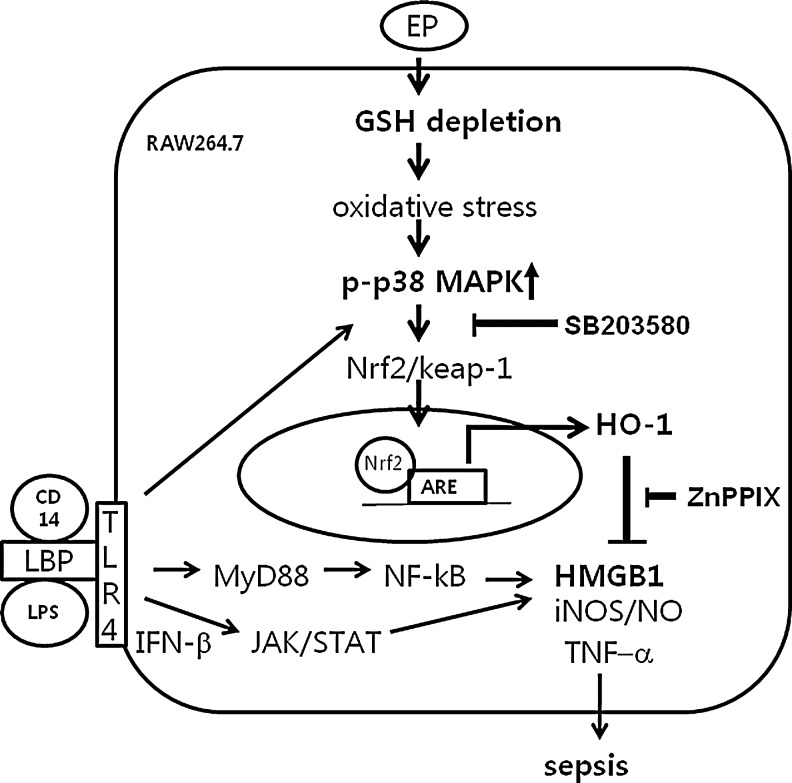
FIG. 9.
Possible mechanism by which EP reduces HMGB1 in sepsis. EP depletes intracellular GSH levels that change redox states of the cell, which, in turn, stimulates p38 MAPK. The activation of p38 MAPK by phosphorylation triggers to activate Nrf2, which dissociates from keap1 and then moves into the nucleus, where it binds to ARE binding sites to induce the HO-1 gene. Thus, SB203580, p38 inhibitor, inhibits EP-mediated HO-1 induction. On the other hand, LPS binds to LPS binding protein (LBP) along with CD14, a recognition molecule for LPS, which activates toll-like receptor 4 (TLR4). The activated TLR4 stimulates p38 MAPK, which then induces HO-1. However, LPS activates NF-κB through MyD88-dependent signal pathways, and IFN-β, generated by LPS through TRIF-dependent signal pathways, activates the JAK/STAT signal pathway to induce inflammatory gene expression, such as iNOS, TNF-α, IL-1β, and release of HMGB1. The induction of HO-1 by EP inhibits these inflammatory cytokines and release of HMGB1 and NO production in LPS-activated RAW 264.7 cells. ZnPPIX, a HO-1 inhibitor, reverses the anti-inflammatory effect of EP (data not shown). Administration of EP also inhibits iNOS expression and circulating TNF-α, IL-1β, and HMGB1 in CLP-induced septic mice, which are dependent on HO-1 induction via activation of p38 MAPK. The schema describes possible signal pathways by which EP activates HO-1 induction in RAW 264.7 cells. IL-1, interleukin-1; NF-κB, nuclear factor kappa B; STAT1, signal transducer and activator of transcription 1; TNF, tumor necrosis factor.
Furthermore, we clearly showed that treatment with EP of peritoneal macrophages from HO-1−/− mice did not reduce LPS-activated expression of iNOS (NO) and HMGB1 in vitro. Likewise, administration of EP failed to increase survival rate and reduced circulating pro-inflammatory cytokines, including HMGB1 in HO-1−/− mice in vivo. In contrast, EP significantly reduced the survival rate along with circulating pro-inflammatory cytokines (TNF-α and IL-1β) and HMGB1 in HO-1+/+ mice. We confirmed previous report (37) that circulating HMGB1 levels are higher in HO-1−/− mice than in HO-1+/+ mice by CLP-induced sepsis. Although CLP is a commonly used experimental animal model of sepsis, investigators must rigorously control the operational conditions, such as distance of the CLP size. Also, strains influence the mortality rate: ICR mice are more sepsis tolerant than Balb c strain (unpublished data) in CLP-induced mortality.
In summary, we found that EP is able to induce HO-1 in macrophages and CLP-induced mice tissues, and this effect is mediated through p38 MAPK/Nrf2/HO-1 signaling. Thus, we suggest that the HO-1-inducible effect of EP is necessary to manifest its anti-inflammatory properties in vitro and in vivo.
Materials and Methods
Materials
Anti-HMGB1 was purchased from Abcam, anti-iNOS from Transduction Laboratories, and anti-β-actin, anti-HO-1, and anti-Nrf2 from Santa Cruz Biotechnology. Enhanced chemiluminescence (ECL) western blotting detection reagent was from Amersham. SB203580, SP600125, and PD98059 were obtained from Calbiochem. All other chemicals, including LPS (E. coli 0111:B4), were purchased from Sigma-Aldrich.
Cell culture
RAW 264.7 cells were obtained from the American Type Culture Collection. The cells were grown in the RPMI-1640 medium supplemented with 25 mM N-(2-hydroxyethyl)piperazine-N-2-ethanesulfonic acid (HEPES), 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% heat-inactivated fetal calf serum.
NO assay
NO was measured as its stable oxidative metabolite, nitrite (NOx) as described previously (38). At the end of incubation, 100 μl of the culture medium was mixed with the same volume of Griess solution (0.1% naphthylethylenediamine and 1% sulfanilamide in 5% phosphoric acid). Light absorbance was measured at 550 nm, and the nitrite concentration was determined using a curve calibrated on sodium nitrite standards.
Cytokine assays
The concentration of TNF-α or IL-1β in culture supernatants was determined using enzyme-linked immunosorbent assay kits (R &D Systems) according to the instructions provided by the manufacturer. Concentrations of TNF-α or IL-1β were calculated with standard curves.
HMGB1 analysis
HMGB1 was measured as described previously (38). In brief, the culture medium or blood samples were briefly centrifuged. Same volumes of samples were then concentrated 40-fold with Amicon Ultra-4-10000 NMWL (Millipore). Centrifugation conditions were fixed angle (35°) and 7500 g for 20 min at 4°C. The concentrated samples were subjected to sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis.
Western blot
Western blot was performed as previously described (40). In brief, whole-cell lysates were performed using buffer containing 0.5% SDS, 1% Nonidet P-40, 1% sodium deoxycholate, 150 mM NaCl, 50 mM Tris-Cl (pH 7.5), and protease inhibitors. Concentrated supernatants, to detect HMGB1, and whole-cell lysates, to detect iNOS, β-actin, and HO-1 were subjected to electrophoresis in different percentage polyacrylamide gels depending on the size of the interested protein. The gels were transferred to polyvinylidene difluoride (PVDF) membranes by semi-dry electrophoretic transfer at 15 V for 60–75 min. The membranes were stained with the Ponceau S solution (2 μg/ml) for 5 min to determine efficiency of transfer and/or protein loading levels per track. Then, the PVDF membranes were blocked overnight at 4°C in 5% bovine serum albumin (BSA). The cells were incubated with primary antibodies diluted 1:500 in Tris–buffered saline–Tween 20 (TBS-T) containing 5% BSA for overnight in 4°C and then incubated with secondary antibody at room temperature for 1 h. The signals were detected by ECL.
siRNA technique
siHO-1 and scramble siRNA were purchased from Invitrogen. siNrf2 and p38-siRNA were acquired from Santa Cruz Biotechnology. siRNA was transfected into RAW 264.7 cells according to the manufacturer's protocol using the transfection reagent Superfect© from Qiagen. The cells were incubated with 100 nM of target siRNA or scramble siRNA for 4 h in serum- and antibiotic-free media. Then, the cells were incubated for 16 h in media containing antibiotics and FBS, and cells were washed and pretreated with or without nicotine, following LPS stimulation.
ARE-luciferase assay
RAW 264.7 cells were plated at a confluence of 50% density in a 6-well plate and grown in DMEM supplemented with 10% heat-inactivated horse serum at 37°C in a humidified atmosphere of 5% CO2/95% air. The cells were cotransfected with 2 μg of the luciferase reporter gene fusion construct (pTi-luciferase), WT ARE, and 0.5 μg of the pCMV-β-galactosidase control vector with Superfect© from Qiagen according to the manufacturer's instructions. After 24-h transfection, the cells were treated with EP for additional 6 h, and the cells were lysed with the 1× reporter lysis buffer (Promega). After mixing the cell extract with a luciferase substrate (Promega), the luciferase activity was measured by a TD-20/20 luminometer (Tuner Designs) according to the manufacturer's protocol. The β-galactosidase assay was done according to the supplier's instructions (Promega β-galactosidase Enzyme Assay System) for normalizing the luciferase activity.
Determination of cellular levels of reduced and oxidized glutathione
The GSH recycling methods were performed as described previously (28) with a slight modification. In brief, cells were washed twice with cold phosphate-buffered saline and scraped in 100 μl of 4.3% sulfosalicylic acid and 392.5 μl of 0.1 M phosphate buffer. The mixture was centrifuged, and the supernatants were neutralized with 7.5 μl 5 M KOH per 500 μl total volume. Fifty-microliter samples of cell lysates or standards were transferred into the wells of microtiter plates. After addition of 100 μl of reaction mixture [0.1 M sodium phosphate buffer, pH 7.5, containing 2.5 mM EDTA, 0.15 mM 5,5′-dithio-bis(2-nitrobenzoic acid), 0.2 mM NADPH, and 1.0 U/ml GSH reductase], absorbance at 405 nm was read at 30-s intervals over 5 min using the MRX Revelation microplate reader. The GSH standards were prepared in the background buffer and treated in the same way as the samples. To measure GSSG concentration, 100 μl of the above cell lysate was incubated with 100 μl of 35 mM N-ethylmaleimide (NEM) for 0.5 h at room temperature to sequester all free (reduced) GSH. Remaining excess NEM was sequestered by adding 100 μl of 35 mM L-cysteine. Fifty microliters of the cell lysates or standards was assayed as described above. The concentrations of total GSH and GSSG were calculated on the basis of a calibration curve obtained with authentic GSH as standards. Protein concentration was determined using the Bio-Rad protein assay kit with BSA as a standard. Results are expressed as nmol/μg protein.
Animal model of sepsis
BALB/c HO-1 knockout mice (HO-1−/−) were generously provided by Dr. Perrella MA (Harvard Medical Center). To induce sepsis, mice were anesthetized with ketamine (30 mg/kg) and xylazine (6 mg/kg). Next, a 2-cm midline incision was performed to allow exposure of the cecum with adjoining intestine. The cecum was tightly ligated with a 3.0-silk suture at 5.0 mm from the cecal tip and punctured once with a 22-gauge needle (top and bottom) and then gently squeezed to extrude a small amount of feces from the perforation sites and returned to the peritoneal cavity. The laparotomy site was then stitched with 4.0-silk sutures. In sham control animals, the cecum was exposed but not ligated or punctured and then returned to the abdominal cavity. Mice were maintained in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication 85-23, revised 1996) and were treated ethically. The protocol was approved in advance by the Animal Research Committee of the Gyeongsang National University.
Statistical evaluation
Scanning densitometry was performed using an Image Master® VDS (Pharmacia Biotech, Inc.). Data are expressed as mean±SD of results obtained from the number of replicate treatments. Differences between data sets were assessed by one-way analysis of variance followed by Newman–Keuls tests. The Kaplan–Meier method was used to compare the differences in mortality rates between groups. p<0.05 was accepted as statistically significant.
Supplementary Material
Abbreviations Used
- ARE
antioxidant redox element
- BSA
bovine serum albumin
- CLP
cecal ligation and puncture
- CO
carbon monoxide
- ECL
enhanced chemiluminescence
- EP
ethyl pyruvate
- GSH-Et
glutathione ethyl ester
- HEPES
N-(2-hydroxyethyl)piperazine-N-2-ethanesulfonic acid
- HMGB1
high-mobility group box 1
- HO-1
heme oxygenase-1
- IL-1
interleukin-1
- iNOS
inducible nitric oxide synthase
- Keap1
Kelch-like ECH-associated protein
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- NAC
N-acetyl cystein
- NEM
N-ethylmaleimide
- NF-κB
nuclear factor kappa B
- Nrf2
NF-E2-related factor 2
- NO
nitric oxide
- PVDF
polyvinylidene difluoride
- SDS
sodium dodecyl sulfate
- STAT1
signal transducer and activator of transcription 1
- TNF
tumor necrosis factor
- WT
wild-type
Acknowledgments
This work was supported by grant from NRF (03-2010-0298), MRC program from MOST/KOSEF (R13-2005-012-01003-0), and KRF grant funded by the Korean Government (MOEHRD) (BRL-2009-0087350).
Author Disclosure Statement
There is no conflict of interest to declare.
References
- 1.Alam J. Cook JL. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am J Respir Cell Mol Biol. 2007;36:166–174. doi: 10.1165/rcmb.2006-0340TR. [DOI] [PubMed] [Google Scholar]
- 2.Bains SK. Foresti R. Howard J. Atwal S. Green CJ. Motterlini R. Human sickle cell blood modulates endothelial heme oxygenase activity: effects on vascular adhesion and reactivity. Arterioscler Thromb Vasc Biol. 2010;30:305–312. doi: 10.1161/ATVBAHA.109.196360. [DOI] [PubMed] [Google Scholar]
- 3.Brugger J. Schick MA. Brock RW. Baumann A. Muellenbach RM. Roewer N. Wunder C. Carbon monoxide has antioxidative properties in the liver involving p38 MAP kinase pathway in a murine model of systemic inflammation. Microcirculation. 2010;17:504–513. doi: 10.1111/j.1549-8719.2010.00044.x. [DOI] [PubMed] [Google Scholar]
- 4.Caivano M. Role of MAP kinase cascades in inducing arginine transporters and nitric oxide synthetase in RAW264 macrophages. FEBS Lett. 1998;429:249–253. doi: 10.1016/s0014-5793(98)00578-x. [DOI] [PubMed] [Google Scholar]
- 5.Chen CC. Chiu KT. Sun YT. Chen WC. Role of the cyclic AMP-protein kinase A pathway in lipopolysaccharide-induced nitric oxide synthase expression in RAW 264.7 macrophages. Involvement of cyclooxygenase-2. J Biol Chem. 1999;274:31559–31564. doi: 10.1074/jbc.274.44.31559. [DOI] [PubMed] [Google Scholar]
- 6.Cheng SE. Lee IT. Lin CC. Kou YR. Yang CM. Cigarette smoke particle-phase extract induces HO-1 expression in human tracheal smooth muscle cells: role of the c-Src/NADPH oxidase/MAPK/Nrf2 signaling pathway. Free Radic Biol Med. 2010;48:1410–1422. doi: 10.1016/j.freeradbiomed.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 8.Dave SH. Tilstra JS. Matsuoka K. Li F. DeMarco RA. Beer-Stolz D. Sepulveda AR. Fink MP. Lotze MT. Plevy SE. Ethyl pyruvate decreases HMGB1 release and ameliorates murine colitis. J Leukoc Biol. 2009;86:633–643. doi: 10.1189/jlb.1008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson HE. Kulkarni A. Lehmann GM. Garcia-Bates TM. Thatcher TH. Huxlin KR. Phipps RP. Sime PJ. Electrophilic peroxisome proliferator-activated receptor-gamma ligands have potent antifibrotic effects in human lung fibroblasts. Am J Respir Cell Mol Biol. 2009;41:722–730. doi: 10.1165/rcmb.2009-0006OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fink MP. Ethyl pyruvate: a novel treatment for sepsis. Curr Drug Targets. 2007;8:515–518. [PubMed] [Google Scholar]
- 11.Jiang W. Pisetsky DS. The role of IFN-alpha and nitric oxide in the release of HMGB1 by RAW 264.7 cells stimulated with polyinosinic-polycytidylic acid or lipopolysaccharide. J Immunol. 2006;177:3337–3343. doi: 10.4049/jimmunol.177.5.3337. [DOI] [PubMed] [Google Scholar]
- 12.Jin XY. Lee SH. Park PH. Hur J. Kim SA. Kim HS. Sohn DH. 2′-Methoxy-4′6′-bis(methoxymethoxy)chalcone inhibits nitric oxide production in lipopolysaccharide-stimulated RAW 264.7 macrophages. Basic Clin Pharmacol Toxicol. 2010;106:454–460. doi: 10.1111/j.1742-7843.2009.00524.x. [DOI] [PubMed] [Google Scholar]
- 13.Joung EJ. Li MH. Lee HG. Somparn N. Jung YS. Na HK. Kim SH. Cha YN. Surh YJ. Capsaicin induces heme oxygenase-1 expression in HepG2 cells via activation of PI3K-Nrf2 signaling: NAD(P)H:quinone oxidoreductase as a potential target. Antioxid Redox Signal. 2007;9:2087–2098. doi: 10.1089/ars.2007.1827. [DOI] [PubMed] [Google Scholar]
- 14.Kietzmann T. Samoylenko A. Immenschuh S. Transcriptional regulation of heme oxygenase-1 gene expression by MAP kinases of the JNK and p38 pathways in primary cultures of rat hepatocytes. J Biol Chem. 2003;278:17927–17936. doi: 10.1074/jbc.M203929200. [DOI] [PubMed] [Google Scholar]
- 15.Kim HS. Cho IH. Kim JE. Shin YJ. Jeon JH. Kim Y. Yang YM. Lee KH. Lee JW. Lee WJ. Ye SK. Chung MH. Ethyl pyruvate has an anti-inflammatory effect by inhibiting ROS-dependent STAT signaling in activated microglia. Free Radic Biol Med. 2008;45:950–963. doi: 10.1016/j.freeradbiomed.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Kim JH. Kim SJ. Lee IS. Lee MS. Uematsu S. Akira S. Oh KI. Bacterial endotoxin induces the release of high mobility group box 1 via the IFN-beta signaling pathway. J Immunol. 2009;182:2458–2466. doi: 10.4049/jimmunol.0801364. [DOI] [PubMed] [Google Scholar]
- 17.Kim SM. Park JG. Baek WK. Suh MH. Lee H. Yoo SK. Jung KH. Suh SI. Jang BC. Cadmium specifically induces MKP-1 expression via the glutathione depletion-mediated p38 MAPK activation in C6 glioma cells. Neurosci Lett. 2008;440:289–293. doi: 10.1016/j.neulet.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 18.Kim YM. Pae HO. Park JE. Lee YC. Woo JM. Kim NH. Choi YK. Lee BS. Kim SR. Chung HT. Heme oxygenase in the regulation of vascular biology: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2011;14:137–167. doi: 10.1089/ars.2010.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohmoto J. Nakao A. Stolz DB. Kaizu T. Tsung A. Ikeda A. Shimizu H. Takahashi T. Tomiyama K. Sugimoto R. Choi AM. Billiar TR. Murase N. McCurry KR. Carbon monoxide protects rat lung transplants from ischemia-reperfusion injury via a mechanism involving p38 MAPK pathway. Am J Transplant. 2007;7:2279–2290. doi: 10.1111/j.1600-6143.2007.01940.x. [DOI] [PubMed] [Google Scholar]
- 20.Kung CW. Lee YM. Cheng PY. Peng YJ. Yen MH. Ethyl pyruvate reduces acute lung injury via regulation of iNOS and HO-1 expression in endotoxemic rats. J Surg Res. 2011;167:e323–e331. doi: 10.1016/j.jss.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Kyriakis JM. Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 22.Lahti A. Kankaanranta H. Moilanen E. P38 mitogen-activated protein kinase inhibitor SB203580 has a bi-directional effect on iNOS expression and NO production. Eur J Pharmacol. 2002;454:115–123. doi: 10.1016/s0014-2999(02)02490-1. [DOI] [PubMed] [Google Scholar]
- 23.Lahti A. Sareila O. Kankaanranta H. Moilanen E. Inhibition of p38 mitogen-activated protein kinase enhances c-Jun N-terminal kinase activity: implication in inducible nitric oxide synthase expression. BMC Pharmacol. 2006;6:5. doi: 10.1186/1471-2210-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SH. Seo GS. Kim JY. Jin XY. Kim HD. Sohn DH. Heme oxygenase 1 mediates anti-inflammatory effects of 2′,4′,6′-tris(methoxymethoxy) chalcone. Eur J Pharmacol. 2006;532:178–186. doi: 10.1016/j.ejphar.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Lotze MT. Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 26.Marshall JC. Inflammation, coagulopathy, and the pathogenesis of multiple organ dysfunction syndrome. Crit Care Med. 2001;29:S99–S106. doi: 10.1097/00003246-200107001-00032. [DOI] [PubMed] [Google Scholar]
- 27.Morse D. Choi AM. Heme oxygenase-1: the “emerging molecule” has arrived. Am J Respir Cell Mol Biol. 2002;27:8–16. doi: 10.1165/ajrcmb.27.1.4862. [DOI] [PubMed] [Google Scholar]
- 28.Novelli GP. Role of free radicals in septic shock. J Physiol Pharmacol. 1997;48:517–527. [PubMed] [Google Scholar]
- 29.Patel R. Attur MG. Dave MN. Kumar S. Lee JC. Abramson SB. Amin AR. Regulation of nitric oxide and prostaglandin E2 production by CSAIDS (SB203580) in murine macrophages and bovine chondrocytes stimulated with LPS. Inflamm Res. 1999;48:337–343. doi: 10.1007/s000110050469. [DOI] [PubMed] [Google Scholar]
- 30.Paul A. Cuenda A. Bryant CE. Murray J. Chilvers ER. Cohen P. Gould GW. Plevin R. Involvement of mitogen-activated protein kinase homologues in the regulation of lipopolysaccharide-mediated induction of cyclo-oxygenase-2 but not nitric oxide synthase in RAW 264.7 macrophages. Cell Signal. 1999;11:491–497. doi: 10.1016/s0898-6568(99)00018-2. [DOI] [PubMed] [Google Scholar]
- 31.Ryter SW. Alam J. Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 32.Schwer CI. Mutschler M. Stoll P. Goebel U. Humar M. Hoetzel A. Schmidt R. Carbon monoxide releasing molecule-2 inhibits pancreatic stellate cell proliferation by activating p38 mitogen-activated protein kinase/heme oxygenase-1 signaling. Mol Pharmacol. 2010;77:660–669. doi: 10.1124/mol.109.059519. [DOI] [PubMed] [Google Scholar]
- 33.Seidel P. Goulet S. Hostettler K. Tamm M. Roth M. DMF inhibits PDGF-BB induced airway smooth muscle cell proliferation through induction of heme-oxygenase-1. Respir Res. 2010;11:145. doi: 10.1186/1465-9921-11-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shang GH. Lin DJ. Xiao W. Jia CQ. Li Y. Wang AH. Dong L. Ethyl pyruvate reduces mortality in an endotoxin-induced severe acute lung injury mouse model. Respir Res. 2009;10:91. doi: 10.1186/1465-9921-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shih AY. Johnson DA. Wong G. Kraft AD. Jiang L. Erb H. Johnson JA. Murphy TH. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci. 2003;23:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song M. Kellum JA. Kaldas H. Fink MP. Evidence that glutathione depletion is a mechanism responsible for the anti-inflammatory effects of ethyl pyruvate in cultured lipopolysaccharide-stimulated RAW 264.7 cells. J Pharmacol Exp Ther. 2004;308:307–316. doi: 10.1124/jpet.103.056622. [DOI] [PubMed] [Google Scholar]
- 37.Takamiya R. Hung CC. Hall SR. Fukunaga K. Nagaishi T. Maeno T. Owen C. Macias AA. Fredenburgh LE. Ishizaka A. Blumberg RS. Baron RM. Perrella MA. High-mobility group box 1 contributes to lethality of endotoxemia in heme oxygenase-1-deficient mice. Am J Respir Cell Mol Biol. 2009;41:129–135. doi: 10.1165/rcmb.2008-0331OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsoyi K. Jang HJ. Kim JW. Chang HK. Lee YS. Pae HO. Kim HJ. Seo HG. Lee JH. Chung HT. Chang KC. Stimulation of alpha7 nicotinic acetylcholine receptor by nicotine attenuates inflammatory response in macrophages and improves survival in experimental model of sepsis through heme oxygenase-1 induction. Antioxid Redox Signal. 2011;14:2057–2070. doi: 10.1089/ars.2010.3555. [DOI] [PubMed] [Google Scholar]
- 39.Tsoyi K. Kim HJ. Shin JS. Kim DH. Cho HJ. Lee SS. Ahn SK. Yun-Choi HS. Lee JH. Seo HG. Chang KC. HO-1 and JAK-2/STAT-1 signals are involved in preferential inhibition of iNOS over COX-2 gene expression by newly synthesized tetrahydroisoquinoline alkaloid, CKD712, in cells activated with lipopolysacchride. Cell Signal. 2008;20:1839–1847. doi: 10.1016/j.cellsig.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 40.Tsoyi K. Lee TY. Lee YS. Kim HJ. Seo HG. Lee JH. Chang KC. Heme-oxygenase-1 induction and carbon monoxide-releasing molecule inhibit lipopolysaccharide (LPS)-induced high-mobility group box 1 release in vitro and improve survival of mice in LPS- and cecal ligation and puncture-induced sepsis model in vivo. Mol Pharmacol. 2009;76:173–182. doi: 10.1124/mol.109.055137. [DOI] [PubMed] [Google Scholar]
- 41.Ulloa L. Ochani M. Yang H. Tanovic M. Halperin D. Yang R. Czura CJ. Fink MP. Tracey KJ. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci U S A. 2002;99:12351–12356. doi: 10.1073/pnas.192222999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner EF. Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 43.Wang H. Ward MF. Sama AE. Novel HMGB1-inhibiting therapeutic agents for experimental sepsis. Shock. 2009;32:348–357. doi: 10.1097/SHK.0b013e3181a551bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wijayanti N. Huber S. Samoylenko A. Kietzmann T. Immenschuh S. Role of NF-kappaB and p38 MAP kinase signaling pathways in the lipopolysaccharide-dependent activation of heme oxygenase-1 gene expression. Antioxid Redox Signal. 2004;6:802–810. doi: 10.1089/ars.2004.6.802. [DOI] [PubMed] [Google Scholar]
- 45.Yang H. Ochani M. Li J. Qiang X. Tanovic M. Harris HE. Susarla SM. Ulloa L. Wang H. DiRaimo R. Czura CJ. Roth J. Warren HS. Fink MP. Fenton MJ. Andersson U. Tracey KJ. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.