Abstract
The target of rapamycin (TOR) is a highly conserved serine/threonine kinase that controls cell growth and metabolism in response to nutrients, growth factors, cellular energy, and stress. The TOR kinase, which was originally discovered in yeast, is also expressed in human cells as mammalian TOR (mTOR). In this review, we focus on how mTOR-inducible signals function in cell protection and cell survival of effector and regulatory T cells as well as its role in endothelial cell biology. We evaluate how signaling is important for vascular endothelial cell growth, survival, and proliferation; and we consider how the function of mTOR in endothelial cells may be clinically important in the rejection process. Understanding the biology of mTOR allows clinicians to use mTOR inhibitors optimally as therapeutics following solid organ transplantation.
The Serine/Threonine kinase target of rapamycin (TOR) controls cell growth in response to nutrients and growth factors.1–6 Mammalian TOR (mTOR) exists as 2 structurally and functionally distinct multiprotein complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2).3,4 mTORC1 is rapamycin sensitive, mediating temporal control of cell growth by regulating several cellular processes, including translation, transcription, ribosome biogenesis, and nutrient transport.7–10 mTORC2 contains mTOR, rictor, SAP kinase interacting protein 1 (SIN1), proline rich repeat protein-5 (PRR)5, and mLST8 (the human homolog of yeast lethal with sec thirteen (LST)8), and is classically rapamycin insensitive. Under conditions of long-term treatment of cells in vitro, rapamycin may disrupt mTORC2 assembly, thus indirectly inhibiting mTORC2 function.9,10 mTORC2 controls phosphorylation9,10 and stability11 of the kinase Akt, and thus Akt-induced responses, including its classical role in cell survival (Fig 1). Thus, the two TOR complexes constitute an ancestral signaling network conserved throughout eukaryotic evolution to control the fundamental process of cell growth and survival.
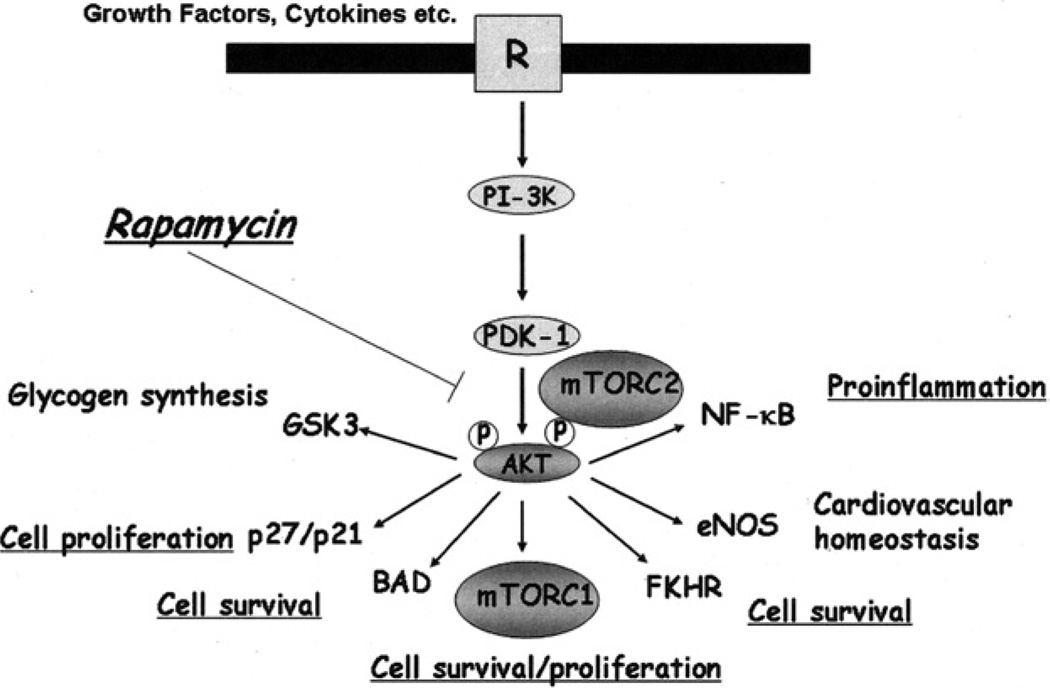
Fig 1.
The association between mTOR and the PI-3K–Akt intracellular signaling pathway in vascular endothelial cells. Assembly of the mTORC2 complex facilitates the phosphorylation and activation of Akt, and pAkt facilitates assembly of the mTORC1 complex and downstream Akt-inducible response(s). Rapamycin is known to inhibit mTORC1 function. In addition, recent studies have indicated that long-term exposure of endothelial cells to rapamycin may also inhibit the assembly of mTORC2 and thus indirectly inhibit Akt-inducible signaling.
mTOR is well established to be involved in T-cell activation responses.12–14 Inhibition of mTOR with the immunosuppressive agent rapamycin markedly inhibits effector T-cell expansion.15 This observation suggests that proliferative and survival pathways used by effector T cells require mTOR-associated signals, and perhaps Akt-inducible survival pathways. In contrast, regulatory T cells do not use mTOR signaling solely for their cell survival, but rather use additional cell growth and survival pathways, including the signal transducers and activators of transcription (STAT) signaling pathway.15 Thus, inhibition of mTOR fails to induce significant cell death in regulatory T cells after mitogen-dependent activation. This has led to the conclusion that the treatment of mitogen- or allo-activated T cells with mTOR inhibitors can lead to the selective expansion of regulatory T cells via a process of selection.15 However, it is possible that mTOR inhibitors may also promote regulatory T-cell expansion via additional mechanisms, for instance through the inhibition of Akt-dependent downregulation of FoxP3 expression.16 FoxP3 is a transcription factor that is selectively expressed in regulatory cells. These observations regarding the biology of mTOR in T cells have laid the ground work for studies in which rapamycin is used to augment immunoregulation/tolerance after clinical transplantation.
However, an underappreciated aspect of mTOR biology is that this kinase is probably expressed in all cell types within the human body.4–6 Furthermore, the relative effects and usage of mTOR signals for growth, proliferation, and protection in different cell types may be different. TOR inhibitors may alter several intracellular signals resulting in different biological responses in different cell types. This is important in terms of understanding the effects of mTOR inhibitors in clinical practice. Moreover, it is possible that mTOR expression and/or its state of activation changes according to the local microenvironment; the presence of cytokines, growth factors, and nutrients activate this pathway. Also, the levels mTOR inhibitors used clinically to inhibit T-cell activation/survival, may have different effects in non–T-cell lineages, which display high or low mTOR expression/activity or utilize alternate/compensatory pathways.
mTOR AND ENDOTHELIAL CELL BIOLOGY
For the purpose of this overview, we wish to emphasize that mTOR is expressed within and has potent functions in vascular endothelial cells.17 We and others have demonstrated that TOR and its associated signaling network is expressed and is functional in endothelial cells. TOR signaling is intricately associated with the phosphatidylinositol-3-kinase (PI3K)–Akt cell protective pathway.17,18 Although mTORC2 is classically rapamycin insensitive, in some non-endothelial cell types as well as in endothelial cells,9,10,18 rapamycin may inhibit mTORC2 assembly, thus blocking mTORC2-dependent phosphorylation of Akt and phospho Akt-induced responses. Inhibition of Akt phosphorylation/activity by rapamycin results in accelerated apoptosis of vascular endothelial cells in part via the inhibition of pAkt-induced inactivation of pro-apoptotic genes such as Foxo1 and Foxo3a.18 Although it is well established that rapamycin targets mTORC1-associated responses,7 our observations indicate that the effects of rapamycin in endothelial cells additionally involve the disruption of mTORC2-dependent responses.18 Therefore, in vascular endothelial cells, blockade of mTOR activity by rapamycin targets both upstream and downstream signals mediated by protective Akt (Fig 1).
USE OF mTOR INHIBITORS IN THE EARLY POST RENAL TRANSPLANT PERIOD
As implied by the cell biological effects of mTOR within endothelial cells, we have proposed that the use of mTOR inhibitors in the early posttransplant period is detrimental for vascular repair (Fig 2).19,20 For instance, it is possible that inhibition of mTOR within the vasculature promotes apoptosis of endothelial cells and disrupts the microcirculation. Further, we propose that in the event that microvascular loss occurs in the early posttransplant period, for instance, following kidney transplantation, the associated detrimental effects on renal tubular cells may result in nephron loss and ultimately be a factor leading to the development of chronic allograft nephropathy.
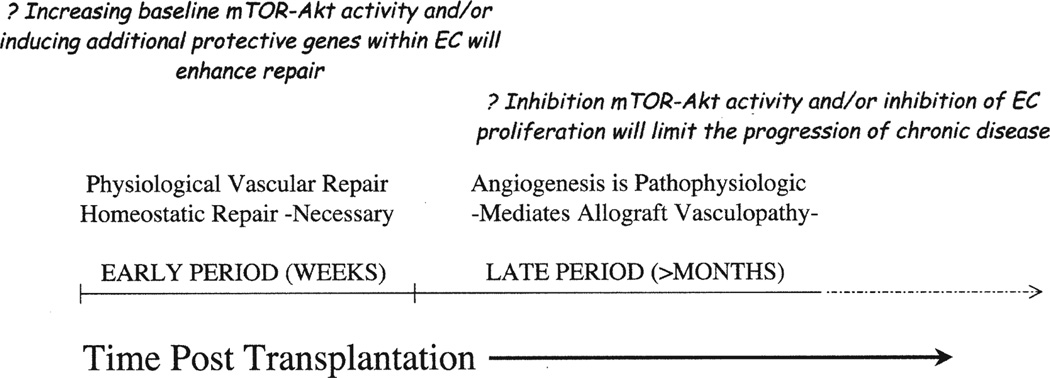
Fig 2.
A proposed model of 2 phases and time periods when the mTOR-Akt signaling in endothelial cells may be of clinical importance after transplantation. In the early posttransplant period, physiologic vascular repair is necessary to sustain long-term allograft function. In this period, increasing endothelial cell expression and activity of Akt and mTOR may facilitate vasculoprotection. In the later posttransplant period, overactivity of these signals results in pathophysiologic angiogenesis, which is associated with ischemia, sluggish blood flow, and leukocyte recruitment. At these later time points, reducing Akt activity may have therapeutic implications for the inhibition of angiogenesis and its association with the progression of chronic rejection.
In recent commentaries,19,20 we have proposed that early microvascular repair is of critical importance for long-term allograft survival. In circumstances where this repair process is dysregulated, we have suggested that chronic rejection is inevitable. In contrast, if efficient repair occurs in the absence of inflammation, then long-term graft survival is likely. In the context of kidney transplantation, Rabelink et al20,21 proposed a microvascular injury model based on the observation that tubular epithelial cells have great potential to recover function after ischemia–reperfusion injury. Indeed, nephrologists have been aware for some time that the kidney, and especially tubular epithelial cells, have the capacity to induce protective genes and recover full function after acute ischemic events. In contrast, the microvascular endothelial cell is susceptible to hypoxic injury, undergo apoptosis, and slough into the circulation. Platelets and other cell types that are recruited into the injured site bind to exposed naked basement membrane(s), Under extreme circumstances, they can mediate thrombosis. Microvascular repair may occur as a result of proliferation of local vascular cells, as well as via recruitment of endothelial progenitor cells, which migrate into the local site.20 After posttransplant ischemia–reperfusion injury, it is possible that this reparative process is compromised by the recruitment of alloreactive leukocytes into the allograft, which target the microvasculature and microvascular repair as a component of their initial interaction.20,23 Therefore, Rabelink et al21 suggest that compromised vascular repair and/or accelerated vascular injury ultimately may result in nephron loss. The use of agents that inhibit cell-protective signaling within the microvasculature during this time period, or target the repair process in the early posttransplant period, likely add to the insult and may result in detrimental effects on long-term allograft function.
Alternatively, one might propose that augmenting cell protective signals, such as Akt/mTOR-induced responses, in endothelial cells in the early posttransplant period may sustain protection and/or microvascular repair, resulting in maintenance of tubular integrity and the protection of nephron structure in the long term. This proposal requires investigation and is the subject of ongoing studies.21,22
mTOR, ANGIOGENESIS, AND THE DEVELOPMENT OF CHRONIC ALLOGRAFT REJECTION
Angiogenesis is well established to be a component of chronic inflammatory disease processes.24 It has been found to occur in association with allograft vasculopathy.20,25–28 In addition, it has been found that the inhibition of angiogenesis may attenuate the progression of chronic allograft rejection. In an established model of chronic cardiac allograft rejection, we observed that interruption therapy with an established angiogenesis inhibitor not only blocked vasculopathy, but also resulted in maintenance of allograft architechure/histology.26 Angiogenesis factors, such as vascular endothelial growth factor (VEGF), have also been well established to participate in chronic rejection, including chronic allograft nephropathy.19,20,25,29 Administration of VEGF into allografts accelerates the development of chronic rejection, and interruption of VEGF–VEGF receptor (VEGFR) signaling has been found to inhibit the degree and severity of intragraft vasculopathy within allografts.30 Together, these observations imply that angiogenesis factors, as well as the angiogenesis reaction itself, are not only associated with the development of vasculopathy, but also are of functional importance in its progression.
As discussed, mTOR-dependent signaling within endothelial cells is associated with proliferation/angiogenesis.17 Furthermore, mTOR-dependent and Akt-inducible signals are associated with VEGFR-mediated responses and VEGF-dependent angiogenesis. Pharmacologic mTOR inhibitors are potent blockers of angiogenesis responses and/or growth factor-induced angiogenesis responses. To this end, it is important to note that rapamycin targets the vasculature in vivo and inhibits Akt-inducible angiogenesis.17 One implication of these observations is that mTOR inhibitors/rapamycin may have beneficial therapeutic effects to inhibit the angiogenesis component, as well as the VEGF-dependent component of chronic allograft rejection. Another implication is that rapamycin will be efficacious in the later posttransplant period as a therapy to interrupt other angiogenesis-dependent processes such as tumor growth, which is becoming an increasing cause of morbidity and mortality.
In conclusion, mTOR is expressed in endothelial cells. It functions in cell survival and proliferation, resulting in microvascular repair and/or angiogenesis. The inhibition of mTOR in vivo after transplantation likely results in biological effects on endothelial cells. In some circumstances, this effect may be clinically detrimental, such as at times when vascular repair is necessary. In contrast, mTOR inhibition may be therapeutic in circumstances where endothelial cell proliferation and angiogenesis are components of disease etiology, such as in association with chronic allograft rejection and tumor growth. Although beyond the scope of this brief review, the development of solid tumors is an angiogenesis-dependent process. We suggest that many of the observed clinical responses following mTOR inhibitor therapy in vivo may relate to effects on endothelial cells. We propose that these effects have significant clinical implications for their introduction after early injury has resolved and/or after allograft microvascular repair. We further propose that the effects of mTOR inhibitors on endothelial cells are key to the understanding of their long term therapeutic benefits.
Acknowledgments
The studies outlined in this review were supported by the National Institutes of Health (R01 Al 46746 and R01 HL 74436), and an Investigator Originated Proposal from Wyeth Pharmaceuticals.
REFERENCES
- 1.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 2.Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- 3.Loewith R, Jacinto E, Wullschleger S, et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10:457. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- 4.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Hara K, Maruki Y, Long X, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 8.Frias MA, Thoreen CC, Jaffe JD, et al. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol. 2006;16:1865. doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 10.Sarbassov DD, Guertin DA, Ali SM, et al. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 11.Facchinetti V, Ouyang W, Wei H, et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J. 2008;27:1932. doi: 10.1038/emboj.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brazelton TR, Morris RE. Molecular mechanisms of action of new xenobiotic immunosuppressive drugs: tacrolimus (FK506), sirolimus (rapamycin), mycophenolate mofetil and leflunomide. Curr Opin Immunol. 1996;8:710. doi: 10.1016/s0952-7915(96)80090-2. [DOI] [PubMed] [Google Scholar]
- 13.Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 2003;35:7S. doi: 10.1016/s0041-1345(03)00211-2. [DOI] [PubMed] [Google Scholar]
- 14.Sho M, Samsonov DV, Briscoe DM. Immunologic targets for currently available immunosuppressive agents: what is the optimal approach for children? Semin Nephrol. 2001;21:508. doi: 10.1053/snep.2001.24946. [DOI] [PubMed] [Google Scholar]
- 15.Zeiser R, Leveson-Gower DB, Zambricki EA, et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008;111:453. doi: 10.1182/blood-2007-06-094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phung TL, Ziv K, Dabydeen D, et al. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell. 2006;10:159. doi: 10.1016/j.ccr.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dormond O, Madsen JC, Briscoe DM. The effects of mTOR-Akt interactions on anti-apoptotic signaling in vascular endothelial cells. J Biol Chem. 2007;282:23679. doi: 10.1074/jbc.M700563200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Contreras AG, Briscoe DM. Every allograft needs a silver lining. J Clin Invest. 2007;117:3645. doi: 10.1172/JCI34238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinders ME, Rabelink TJ, Briscoe DM. Angiogenesis and endothelial cell repair in renal disease and allograft rejection. J Am Soc Nephrol. 2006;17:932. doi: 10.1681/ASN.2005121250. [DOI] [PubMed] [Google Scholar]
- 21.Rabelink TJ, Wijewickrama DC, de Koning EJ. Peritubular endothelium: the Achilles heel of the kidney? Kidney Int. 2007;72:926. doi: 10.1038/sj.ki.5002414. [DOI] [PubMed] [Google Scholar]
- 22.Aydin Z, van Zonneveld AJ, de Fijter JW, et al. New horizons in prevention and treatment of ischaemic injury to kidney transplants. Nephrol Dial Transplant. 2007;22:342. doi: 10.1093/ndt/gfl690. [DOI] [PubMed] [Google Scholar]
- 23.Vos IH, Briscoe DM. Endothelial injury: cause and effect of alloimmune inflammation. Transpl Infect Dis. 2002;4:152. doi: 10.1034/j.1399-3062.2002.t01-1-02002.x. [DOI] [PubMed] [Google Scholar]
- 24.Cotran RS. Inflammation and repair. In: Cotran RS, Kumar V, Robbins SL, editors. Pathologic basis of disease. Philadelphia: W.B. Saunders; 1994. p. 51. [Google Scholar]
- 25.Reinders ME, Briscoe DM. Angiogenesis and allograft rejection. Graft. 2002;5:96. [Google Scholar]
- 26.Denton MD, Magee C, Melter M, et al. TNP-470, an angiogenesis inhibitor, attenuates the development of allograft vasculopathy. Transplantation. 2004;78:1218. doi: 10.1097/01.tp.0000137266.30134.02. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka H, Sukhova GK, Libby P. Interaction of the allogeneic state and hypercholesterolemia in arterial lesion formation in experimental cardiac allografts. Arterioscler Thromb. 1994;14:734. doi: 10.1161/01.atv.14.5.734. [DOI] [PubMed] [Google Scholar]
- 28.Libby P, Zhao DX. Allograft arteriosclerosis and immune-driven angiogenesis. Circulation. 2003;107:1237. doi: 10.1161/01.cir.0000059744.64373.08. [DOI] [PubMed] [Google Scholar]
- 29.Reinders ME, Fang JC, Wong W, et al. Expression patterns of vascular endothelial growth factor in human cardiac allografts: association with rejection. Transplantation. 2003;76:224. doi: 10.1097/01.TP.0000071363.55007.D0. [DOI] [PubMed] [Google Scholar]
- 30.Lemstrom KB, Krebs R, Nykanen Al, et al. Vascular endothelial growth factor enhances cardiac allograft arteriosclerosis. Circulation. 2002;105:2524. doi: 10.1161/01.cir.0000016821.76177.d2. [DOI] [PubMed] [Google Scholar]