Abstract
Objectives
The goal of this preliminary study was to determine if neck surface electromyography (sEMG) is sensitive to possible changes in vocal hyperfunction associated with injection laryngoplasty, particularly with respect to alterations in the degree of vocal hyperfunction.
Methods
Thirteen individuals undergoing office-based injection laryngoplasty for glottal phonatory insufficiency were prospectively studied using a battery of acoustic, aerodynamic, endoscopic, and anterior neck surface electromyographic (sEMG) assessments before the procedure and approximately one week after.
Results
Anterior neck sEMG was not significantly reduced (p < 0.05) post-procedure; however, perceptual ratings of strain and false vocal fold (FVF) compression were both significantly reduced, reflecting a decrease in vocal hyperfunction.
Conclusions
The results do not support the use of anterior neck sEMG measures to assess vocal hyperfunction, and place into question the use of some other measures (estimates of anterior-posterior (AP) supraglottal compression, quantitative measures of AP and FVF supraglottal compression, and acoustic vowel rise times) that have been considered reflective of vocal hyperfunction.
Keywords: vocal hyperfunction, injection laryngoplasty, speech acoustics, electromyography
Introduction
Vocal hyperfunction is a clinical term referring to “conditions of abuse and/or misuse of the vocal mechanism due to excessive and/or `imbalanced' muscular forces”.1 Current diagnosis of vocal hyperfunction and hyperfunctionally related voice disorders is dependent on subjective impressions of the patient's physical presentation, auditory-perceptual judgments of voice quality (in particular, vocal strain), and stroboscopy.2 Clinical care is hindered by the lack of a “gold standard” objective measure for the assessment of vocal hyperfunction.
Prior studies have suggested that neck surface electromyography (sEMG) may provide an objective metric of vocal hyperfunction.3, 4 This suggestion follows the clinical impression that individuals with vocal hyperfunction often simultaneously contract the extrinsic laryngeal muscles and other superficial neck muscles in a similar hyperfunctional manner (e.g.,5, 6–9). Although both of the previous studies comparing sEMG levels in groups with hyperfunctionally-related voice disorders and controls found higher sEMG levels in those with voice disorders, such results do not provide information about the differential sensitivity of sEMG in detecting vocal hyperfunction. A potentially more robust approach would be to track sEMG along with other measures of vocal function (including some that are believed to reflect vocal hyperfunction) over time in individuals being treated for vocal hyperfunction to determine if the measures change with successful rehabilitation. However, doing this type of longitudinal study in individuals receiving voice therapy for hyperfunctionally-related voice disorders could be somewhat challenging due to the extended time-courses of therapy and subsequent difficulties with participant retention. Patients undergoing injection laryngoplasty for glottal phonatory insufficiency seem to offer an attractive alternative because they typically display a noticeable reduction in hyperfunctional behaviors following the procedure.
Injection laryngoplasty is an established procedure for correcting glottal insufficiency (e.g.,10) through injection of material into the vocal fold. Hillman et al.1 hypothesized a relationship between glottal insufficiency and vocal hyperfunction, in which increased activity of laryngeal muscles could be used in an effort to achieve more adequate glottal closure. This agrees with the clinical observation that individuals with severe glottal insufficiency reporting for injection laryngoplasty often have concurrent vocal hyperfunction. After injection laryngoplasty, these patients can often “unload” their habituated hyperfunctional behaviors that are no longer necessary, creating a population in which vocal hyperfunction may change drastically in a short period of time.
The goal of this preliminary study is to characterize changes in anterior neck sEMG collected by multiple electrode recording sites during a variety of speech tasks before and after injection laryngoplasty. This was done by collecting neck sEMG in concert with other possible measures of vocal function in individuals before and after injection laryngoplasty, with a particular emphasis on those additional measures that are believed to reflect the presence of vocal hyperfunction. Standard measures of vocal hyperfunction included strain ratings and visual ratings (increasing compression is believed to reflect hyperfunction) of anterior-posterior (AP) and false vocal fold (FVF) supraglottal compression. New possible measures of vocal hyperfunction were also evaluated: quantitative estimates of AP and FVF supraglottal compression and measures of acoustic vowel rise times (more abrupt rise times are believed to reflect hyperfunction).
Methods
Participants and Experiment Design
Participants were 13 adults (6 males, 7 females; mean age = 56 years, SD = 15 years) undergoing office-based injection laryngoplasty using a cross-linked hyaluronic acid (Restylane) for glottal insufficiency. Table 1 lists the demographics for the injection population. Participants underwent sEMG as well as acoustic, aerodynamic, and endoscopic, assessments immediately prior to their injection laryngoplasty (PRE condition) and approximately 1 week after their procedure (POST condition). Actual times between PRE and POST measurements varied from 6 – 11 days, with most at 7 days.
Table 1.
Participant Demographics
| Participant | Age | Reason for Glottal Insufficiency |
|---|---|---|
| 1 | 75 | Unilateral left vocal fold paralysis following esophageal cancer resection |
| 2 | 77 | Presbyphonia |
| 3 | 55 | Idiopathic bilateral vocal fold paresis |
| 4 | 56 | Unilateral left vocal fold paralysis following carotidendectomy |
| 5 | 78 | Glottic insufficiency following prolonged intubation |
| 6 | 37 | Unilateral right vocal fold paralysis secondary to thyroid tumor, history of subsequent thyroidectomy |
| 7 | 40 | Unilateral left vocal fold paralysis following thyroidectomy |
| 8 | 59 | Idiopathic left unilateral vocal fold paralysis |
| 9 | 36 | Unilateral right vocal fold paralysis following thyroidectomy |
| 10 | 57 | Unilateral left vocal fold paralysis following lobectomy |
| 11 | 51 | Bilateral vocal fold sulci |
| 12 | 64 | Unilateral right vocal fold paralysis following cholecystectomy |
| 13 | 39 | Idiopathic right unilateral vocal fold paralysis |
Tasks
Participants were examined using transnasal endoscopy while they produced several iterations of the vowel /i/, speech, and a repetitive abduction-adduction maneuver (“sniff-eee”). Trans-nasal endoscopy was chosen to provide the best view of normal laryngeal function, and to avoid the biomechanical effects of tongue protrusion created by rigid endoscopy. The endoscopy procedure was digitally recorded at 30 frames per second. Average air flow rate during vowel production was measured with a Phonatory Aerodynamics System (PAS – Kay Elemetrics, Inc.) as participants produced a series of /pæ/ vocalizations at both comfortable and loud levels.
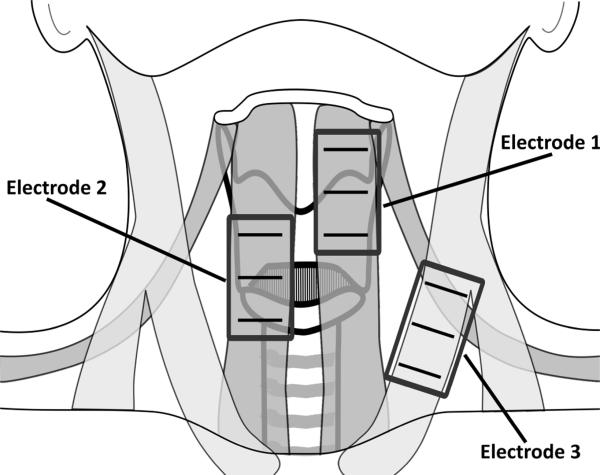
Simultaneous neck sEMG and acoustic signals from a lavalier microphone (Sennheiser MKE2-P-K, Wedemark, Germany) were filtered and recorded digitally with Delsys ™ (Boston, Massachusetts) hardware (Bagnoli Desktop System) and software (EMGworks 3.3) with a sampling frequency of 20 kHz during rest and the production of the vowels /a/ and /i/, high-pitch /a/, low-pitch /a/, six read CAPE-V sentences i.e.,11, read speech The Rainbow Passage;12, and spontaneous speech. Spontaneous speech was elicited by questions from a speech-language pathologist, asking the participant to describe their voice issues. The sEMG was recorded with three double-differential electrodes (Delsys ™ 3.1) placed parallel to the underlying muscle fibers of the 1) thyrohyoid, omohyoid, and sternohyoid muscles, 2) cricothyroid and sternohyoid muscles, 3) sternocleidomastoid muscle, and a ground electrode on the superior aspect of the participant's left shoulder (see Figure 1). Activations sampled by electrode locations 1 and 2 were likely predominated by infrahyoid musculature. sEMG signals were amplified (1000 gain) and filtered (20 – 450 Hz band-pass) using Delsys™ Bagnoli systems.. All participants performed maximal voluntary contraction (MVC) maneuvers, consisting of neck contraction against manual resistance for the purpose of normalizing sEMG data. For a subset of the participants (N = 8), the maximal force was measured with a dynamometer (Chatillon DPP-50, Ametek, Inc., Paoli, PA) during neck muscle contraction. These MVC forces ranged from 16 – 47 lbf by participant, and did not significantly differ between PRE and POST recordings.
Figure 1.
A schematic of the anterior neck with the locations of the three double differential sEMG electrodes.
Data Analysis
Using the digital endoscopy exam, short (15 – 20 seconds) video segments of speech, repeated abduction and adduction, and an /i/ vowel captured PRE and POST were created. These were rated on a 5-point scale for anterior-posterior (AP) and medial (false vocal fold; FVF) sugraglottic compression13 by a certified speech-language pathologist (SLP) and a laryngologist, both of whom re-rated approximately 25% of the samples. One image of the adducted folds during each /i/ production was extracted from the endoscopic exams and were analyzed using custom MATLAB® (Mathworks Inc., Natick, MA) software to obtain proposed quantitative parameters for AP and FVF compression.14 Specifically, images of the adducted folds were digitally labeled by the first author to obtain a numerical pixel value of the laryngeal outlet, the anterior-posterior distance, and the medial distance. The quantitative measure of AP compression was defined as the laryngeal outlet area normalized by the square of the anterior-posterior distance, whereas a measure of medial (FVF) compression as the laryngeal outlet area normalized by the square of the medial distance.14
Airflow signals were digitized and analyzed with the PAS. Glottal airflow estimates were collected from the steady-state values of the oral airflow for the /æ/ vowels produced in the /pæ/ syllable strings. Such estimates of average air flow should reflect the kinds of noticeable improvements in glottal phonatory closure that are associated with successful laryngoplasty.15
A certified speech-language pathologist (SLP) listened to each PRE and POST recording of the Rainbow Passage and /a/ vowel for each participant and perceptually rated the presence of “strain” using the CAPE-V.11 Approximately 25% of samples were re-rated by the SLP and by a second certified SLP. The rise times of the acoustic recordings of the vowels /a/ and /i/ were used as acoustic correlates of abruptness of attack, as suggested by Peters et al.16 Here, due to the disordered nature of the voice signals, the rise time of the acoustic signal was modified as the time required for an envelope of the acoustic signal to go from 20% to 80% of the maximum amplitude, which was implemented in MATLAB®, with the root-mean-squared (RMS) of the acoustic signal in 80 ms rectangular windows calculated in intervals of 2.5 ms (97% overlap).
The mean of the RMS values of anterior neck sEMG data computed in 1 s windows (no overlap) was calculated for the entire length of all completed vocal tasks using custom MATLAB® (Mathworks Inc., Natick, MA) software. Because intrinsic laryngeal musculature is most active during initiation and cessation of vowel production relative to stable vowel production (e.g.,17), for the vowels /a/ and /i/, the RMS was also calculated for the 500 ms window immediately prior to /a/ and /i/ vowel initiation. Potential variability associated with surface electrode contact and lacement between PRE and POST recordings was minimized by normalizing sEMG to the reference contraction at MVC (e.g.,18; using the maximal 1 s window), such that all sEMG data were further analyzed as RMS and reported in terms of % MVC.
Statistical Analysis
Minitab® Statistical Software (Minitab Inc., State College, PA) was used to calculate a two-factor ANOVA to assess the effects of vocal task and sEMG electrode location on POST – PRE sEMG, one-sided Student's t-tests to assess possible changes between the PRE and POST collected parameters, and Pearson's correlations between POST – PRE changes in the various measures. One-sided tests was chosen to assess possible changes between the PRE and POST collected parameters given the a priori hypotheses that post-therapy airflow measures would decrease due to increases in glottal competence, and that measures of vocal hyperfunction would change to reflect a decrease in vocal hyperfunction. Statistical testing was not adjusted for alpha inflation due to the exploratory nature and small sample size of this study. Assuming alpha = 0.05, and based on the standard deviations of the sEMG changes, Student's t-tests had ≥90% power to detect POST – PRE differences in sEMG of approximately 10% MVC, roughly half of the magnitude of the difference between individuals with vocal hyperfunction and controls seen by Redenbaugh and Reich4. Calculations of Pearson's correlations had 90% power to identify correlations with effect sizes greater than R2 ≥ 0.5. To assess the possible effects of thyroidectomy on sEMG, the mean RMS during read speech for the three participants who had previously undergone thyroidectomy were compared to the remaining 10 participants using two-sided Student's t-tests.
Results
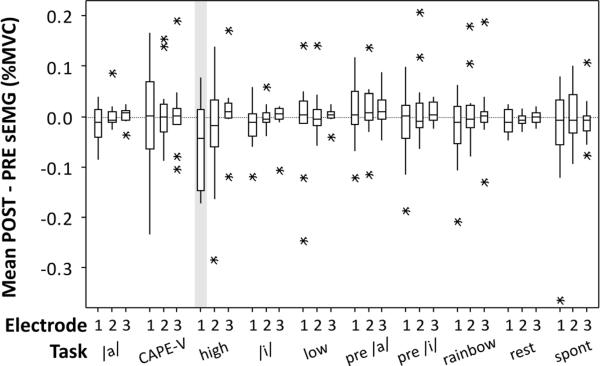
There was no statistically significant (p < 0.05) effect of vocal task (rest, /a/, /i/, preparatory /a/, preparatory /i/, high /a/, low /a/, read sentences, read paragraph, and spontaneous speech) for any anterior neck sEMG location using a two-factor ANOVA, but a statistically significant effect of electrode position (p = 0.01) was found. The mean POST – PRE change in sEMG across task was −1.7 %MVC for electrode position 1, and 0.2 and 0.4 %MVC for electrode positions 2 and 3, respectively.
Boxplots of the POST – PRE sEMG measures are shown in Figure 2. Of the sEMG measures, only one task and electrode position combination showed a statistically significant (paired, one-sided, p < 0.05) difference, which was electrode position 1 during the high-pitched /a/ task. The mean RMS during read speech for each of the three electrode positions for the three participants who had previously undergone thyroidectomy were compared to the remaining 10 participants using Student's t-tests. There were no significant differences between the two groups (p > 0.05; two-sample test, two-sided). All three electrode positions showed average differences less than or equal to 3.2 %MVC.
Figure 2.
Boxplots of the POST – PRE difference in RMS sEMG at the three electrode positions as a function of vocal task. Horizontal box lines indicate the lower and upper quartiles of the data, with the center line marking the data median. Asterisks show the location of the data means. Vertical whiskers extend from the boxes to the minimum and maximum values of each dataset. The grey shading indicates the single electrode position and vocal task combination that showed a statistically significant (p < 0.05) reduction in the POST condition relative to the PRE condition.
Table 2 shows the results of performing Student's t-tests on all other parameters between POST and PRE conditions. The following measures showed statistically significant (paired, one-sided, p < 0.05) differences: strain, FVF ratings, comfortable airflow, and loud airflow. Pearson's correlations among POST – PRE changes in the parameters thought to be associated with vocal hyperfunction are shown in Table 3. Estimates of intra- and inter-rater reliability for strain, AP compression, and FVF compression are shown in Table 4.
Table 2.
Student's t-tests for POST vs. PRE conditions of non-sEMG parameters (one-tailed, paired, N=13)
| Parameter | PRE | POST | Difference | p-value |
|---|---|---|---|---|
| Strain | 54.5 (23.0) | 26.5 (21.3) | −28.0 (24.5) | 0.001 |
| /a/ Rise Time (ms) | 112 (45) | 111 (77) | −1 (89) | 0.508 |
| /i/ Rise Time (ms) | 95 (27) | 96 (33) | 1 (41) | 0.467 |
| AP rating | 2.4 (1.1) | 2.1 (1.0) | −0.3 (0.7) | 0.060 |
| FVF rating | 3.5 (0.8) | 2.8 (0.9) | −0.7 (1.1) | 0.020 |
| AP quantitative | 0.53 (0.41) | 0.48 (0.22) | −0.05 (0.23) | 0.212 |
| FVF quantitative | 3.92 (7.01) | 3.65 (7.01) | −0.27 (0.64) | 0.076 |
| Airflow C (L/s) | 0.36 (0.19) | 0.23 (0.09) | −0.13 (0.19) | 0.014 |
| Airflow L (L/s) | 0.53 (0.23) | 0.30 (0.14) | −0.24 (0.30) | 0.008 |
PRE = mean (standard deviation) pre-injection, POST = mean (standard deviation) post-injection, Difference = mean POST − PRE, C = comfortable, L = loud, AP = anterior-posterior, FVF = false vocal fold, p-values shown in bold indicate parameters found to be statistically significantly different POST relative to PRE at a p < 0.05 level.
Table 3.
Pearson's correlations for POST - PRE changes between parameters
| Strain | /a/ Rise Time | /i/ Rise Time | AP rating | FVF rating | AP quant | |
|---|---|---|---|---|---|---|
| Strain | ||||||
| /a/ Rise Time | −0.25 | |||||
| /i/ Rise Time | − 0.56 | 0.88 | ||||
| AP rating | 0.10 | 0.49 | 0.39 | |||
| FVF rating | 0.32 | 0.42 | 0.25 | 0.60 | ||
| AP quant | −0.10 | 0.08 | 0.07 | 0.49 | 0.20 | |
| FVF quant | − 0.59 | −0.08 | 0.09 | −0.34 | −0.50 | −0.27 |
AP = anterior-posterior, FVF = false vocal fold, quant = quantitative measure. Numbers in bold indicate correlations statistically significant at a p < 0.05 level.
Table 4.
Inter- and Intra-rater reliabilities (Pearson's correlations) of perceptual measures
| Strain | AP | FVF | ||
|---|---|---|---|---|
| Intra-rater | 0.69 | Rater 1 Intra-rater | 1.00 | 0.78 |
| Inter-rater | 0.65 | Rater 2 Intra-rater | 0.94 | 0.64 |
| Inter-rater | 0.69 | 0.73 |
Discussion
Vocal Function PRE and POST Injection Laryngoplasty
Testing between PRE and POST conditions showed that, as expected, individuals displayed statistically significantly decreased airflow rates, strain ratings, and ratings of FVF supraglottic compression post-injection. These results support the a priori assumption that correction of glottal phonatory incompetence via injection laryngoplasty (indicated by reduced glottal airflow) is often associated with reduction of vocal hyperfunction, here indicated by reduced strain and FVF supraglottic compression ratings. Although the changes were not found to be significant, both AP supraglottic compression ratings and FVF quantitative measures were also reduced in the POST condition (p = 0.06, p = 0.076, respectively). The lack of a statistically significant reduction in these measures is most likely the result of relatively small differences relative to large variances (see Table 2), but could also be the result of the relatively small number of participants in this study. Overall, these findings lend more quantitative support to the clinical impressions of the relationship between glottal insufficiency and vocal hyperfunction, particularly with respect to outcomes following injection laryngoplasty.
sEMG as an Indicator of Vocal Hyperfunction
Of the 30 electrode position and vocal task combinations tested, only 1 showed statistically significant reduction in the POST condition relative to the PRE. Considering the high number of multiple comparisons, the one difference is likely spurious and due to alpha inflation. Conversely, the perceptual measures more commonly associated with vocal hyperfunction of strain and FVF ratings did show significant changes following the injection laryngoplasty.
The two past studies that have attempted to use surface electromyography (sEMG) to objectively quantify neck muscle tension were limited by task, electrode location, populations, and some methodological failings, thus the evidence of the clinical utility of sEMG as an objective indicator of vocal hyperfunction is not compelling. Redenbaugh and Reich4 measured mean neck sEMG of a single anterior neck electrode in 7 individuals with healthy normal voice and 7 ”hyperfunctional” individuals, finding that the individuals with disordered voice had significantly greater mean normalized neck sEMG during phonation than individuals with healthy normal voice. This study was limited by the single electrode position, a lack of variety in speech tasks, a disordered population that was varied in age, sex, and clinical presentation, and a rudimentary data collection in which sEMG signals were amplified, filtered, and integrated in real-time, with the integrated values displayed on-screen and recorded by hand. Hocevar-Boltezar et al.3 recorded sEMG from 18 pairs of differential electrodes on the face and anterior neck in a group of 11 women with disorders associated with vocal hyperfunction (nodules, muscle tension dysphonia) and in a group of 5 women with healthy normal voice. Although this study found significant differences between the mean sEMG of many electrode positions in the two groups, sEMG signals were not normalized. To compare sEMG signals among conditions and/or participants, normalization to a reference contraction is essential to reduce the variability due to neck surface electrode contact and participant neck mass.18 Given the findings of past studies and those of the current work, the relationship between vocal hyperfunction and extrinsic laryngeal muscle tension is still unclear. This is despite the fact that assessing and attempting to modify the tension of the extrinsic neck musculature is a primary target in voice therapy.
Vocal hyperfunction commonly associated with elevated laryngeal position (e.g.,5), which would be assumed to be a result of over-activity of the suprahyoid muscles; however activations sampled by electrode locations 1 and 2 were likely predominated by infrahyoid musculature. The electrode positions used in the current work were chosen pragmatically, to allow for comparison with past studies and to record high quality sEMG signals. Large amounts of subdermal fat act to attenuate the sEMG signal that it is possible to record. Since subdermal fat is a particular issue in the suprahyoid area, and a particular issue for individuals in the age range studied here, no submental recording locations were used. The inherent assumption was that overactivity of the suprahyoid musculature (causing laryngeal elevation and perhaps false vocal fold approximation) is accompanied by some level of compensatory activation of the infrahyoid musculature due to the biomechanical constraints of the system. However, one explanation for the lack of reduction in sEMG in the POST condition could be the absence of a recording position able to sample suprahyoid activity.
One further possibility for the discrepancy between the sEMG results of previous work and that shown here stems from the fact that three of the thirteen individuals recorded had previously undergone thyroidectomy. Even in individuals who have undergone thyroidectomy and have intact vocal fold motility, there are temporary changes in both mean fundamental frequency and in the range of fundamental frequency used during speech,19–21 most of which appear to dissipate within 3 months.19, 20 These changes could be due to swelling and/or modifications to the extrinsic laryngeal musculature. However, there is some evidence that the division of extrinsic laryngeal musculature does not have an effect on vocal function.22 The individuals who had previously undergone thyroidectomy were examined relative to the remainder of the group to ensure that they presented with similar signal integrity. This examination showed that none of the three electrode locations showed significantly different mean RMS during read speech in the three participants who had previously undergone thyroidectomy compared to the remaining 10 participants.
It is possible that relevant changes in sEMG activity associated with vocal hyperfunction were missed by this study do to the unilateral sampling paradigm. Many of the participants reported with unilateral vocal fold paralysis, and each electrode was placed on one side only (consistently) irrespective of the side of paralysis. It has been previously shown that unilateral vocal fold paresis and paralysis is associated with static contralateral false vocal fold approximation23, which could suggest asymmetrical compensatory hyperfunction. While it is possible that important changes in sEMG were missed due to asymmetrical hyperfunction associated with unilateral paralysis, this is not the view of the authors especially given that participants reporting with unilateral vocal fold paralysis were nearly equally divided by side of paralysis (5 left and 4 right). It is likely that vocal hyperfunction relates to a global extralaryngeal phenomenon, especially in light of the neural and biomechanical linkages of the systems.
Overall, the current study does not show consistent differences in RMS sEMG measures from the anterior neck PRE and POST injection laryngoplasty. Given the care taken to control for sources of measurement variability in this experimental study, the current work does not support the addition of anterior neck sEMG measures to the armamentarium of voice assessment at this time. However, given that the results of the ANOVA on the POST – PRE change in RMS sEMG indicate that there were no significant effects of vocal task and that electrode position 1 was associated with the largest POST – PRE changes, future studies are needed and should concentrate on superiorly located anterior neck sEMG electrode locations and perhaps submental locations, and need not examine a large number of vocal tasks. Additionally, given the discrepancy in findings in neck sEMG studies and the relative non-specificity of sEMG, future studies are also needed to examine the overall reliability of speech-related sEMG.
Assessment of Other Possible Indicators of Vocal Hyperfunction
Of the additional measures examined in this study that are believed to reflect hyperfunction, only strain and FVF displayed significant reductions following injection laryngoplasty. While these results serve to validate that a reduction in hyperfunction occurred post-laryngoplasty, limitations in the clinical use of these measures as indicators of vocal hyperfunction must be acknowledged. Both measures are inherently limited by a reliance on perceptual judgments. Others have shown that perceptual judgments of strain have poor reliability (e.g.,24), and the data in the current study indicate that judgments of FVF may be only slightly more reliable than strain, with both having Pearson's coefficients in the weak to moderate range. Thus there continues to be a need for more objective methods to assess vocal hyperfunction.
Ratings of AP compression are also interpreted in the clinic as indicative of vocal hyperfunction, but this work did not find a significant change between the PRE and POST conditions, and were only correlated with FVF ratings. This agrees with the previous suggestion that AP compression is more associated with typical laryngeal articulation than with vocal hyperfunction.25
Interestingly, although both strain and FVF compression ratings were significantly reduced in the POST condition relative to the PRE condition, they were not significantly correlated. The lack of a significant correlation between these two measures suggests that each is reflecting a different, and perhaps somewhat independent, manifestation of vocal hyperfunction. This view is generally in line with the existence of multiple types and/or manifestations of hyperfunction as proposed by Hillman and colleagues.1
The frequency of hard glottal attack is thought to be related to vocal hyperfunction (e.g.,26) and the rise time of the acoustic signals during vowel production is a parameter correlated with the perception of hard glottal attack.16 However, the average acoustic rise times for the vowels /a/ and /i/ did not show statistically significant increases in the POST condition. Differences in /a/ and /i/ rise times were, however, significantly (p < 0.05) correlated with one another, and the /i/ rise time was significantly correlated with perceptual ratings of strain. The lack of unequivocal evidence that this measure is sensitive to vocal hyperfunction could be a result of its inherent variability and the relatively small number of samples taken from each participant. For each condition, each participant produced each vowel a total of three times. Future work investigating this estimator should use more iterations, as well as speech materials designed to effectively capture the parameter during running speech.
The lack of significant correlations between visual perceptual estimates of compression and the quantitative measures of AP and FVF suggested by Behrman et al.14 were somewhat surprising and disappointing. Although AP ratings and quantitative measures showed a reasonably large correlation of r = 0.49, this correlation was not significant. Further, FVF ratings and quantitative measures were found to be negatively correlated, with a sizeable correlation of r = −0.50 in the opposite direction than that expected. Close examination of the equations for these measures throws doubt on their theoretical basis. The quantitative measure of AP compression used is defined as the laryngeal outlet area normalized by the square of the anterior-posterior distance, and the quantitative measure of medial (FVF) compression as the laryngeal outlet area normalized by the square of the medial distance.14 For instance, when the anterior-posterior distance is shorteneed (presumably due to AP compression), the denominator of the ratio is decreased, causing the AP measure to increase. Likewise, when the medial distance is shortened (presumably due to FVF compression), the denominator of the ratio is decreased, causing the FVF measure to increase. Unfortunately, the numerator of both ratios is not constant. The laryngeal outlet area is affected by both AP and FVF compression, meaning that the two ratios can be driven in opposite directions depending on one another. While it is true that weak interrater reliability (RANGE: r = 0.64 – 0.78) may decrease the ability to detect significant correlations, there is no reason to suppose that it would cause a negative relationship between FVF ratings and quantitative measures (r = −0.50).
Summary
This preliminary study does not show significant reductions in RMS sEMG measures from the anterior neck POST injection laryngoplasty, despite the fact that other, more established perceptual measures of strain and FVF ratings reflected significant reductions in vocal hyperfunction. The current results do not support the use of anterior neck sEMG in the assessment of vocal hyperfunction.
Acknowledgements
Thanks to Jennifer M. Bourque for technical support, Maia N. Braden for assistance with strain ratings, and Drs. Serge Roy and Robert Howe for helpful comments.
This work was supported in part by grant 5T32DC000038-17 from the National Institute on Deafness and other Communication Disorders.
References
- 1.Hillman RE, Holmberg EB, Perkell JS, Walsh M, Vaughan C. Objective assessment of vocal hyperfunction: an experimental framework and initial results. Journal of Speech and Hearing Research. 1989 Jun;32(2):373–392. doi: 10.1044/jshr.3202.373. [DOI] [PubMed] [Google Scholar]
- 2.Holmberg EB, Hillman RE, Hammarberg B, Sodersten M, Doyle P. Efficacy of a behaviorally based voice therapy protocol for vocal nodules. Journal of Voice. 2001 Sep;15(3):395–412. doi: 10.1016/S0892-1997(01)00041-8. [DOI] [PubMed] [Google Scholar]
- 3.Hocevar-Boltezar I, Janko M, Zargi M. Role of surface EMG in diagnostics and treatment of muscle tension dysphonia. Acta Otolaryngol. 1998 Sep;118(5):739–743. doi: 10.1080/00016489850183287. [DOI] [PubMed] [Google Scholar]
- 4.Redenbaugh MA, Reich AR. Surface EMG and related measures in normal and vocally hyperfunctional speakers. Journal of Speech and Hearing Disorders. 1989 Feb;54(1):68–73. doi: 10.1044/jshd.5401.68. [DOI] [PubMed] [Google Scholar]
- 5.Aronson AE. Clinical Voice Disorders: An Interdisciplinary Approach. 1 ed Thieme-Stratton, Inc.; New York: 1980. [Google Scholar]
- 6.Altman KW, Atkinson C, Lazarus C. Current and emerging concepts in muscle tension dysphonia: a 30-month review. J Voice. 2005 Jun;19(2):261–267. doi: 10.1016/j.jvoice.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Angsuwarangsee T, Morrison M. Extrinsic laryngeal muscular tension in patients with voice disorders. J Voice. 2002 Sep;16(3):333–343. doi: 10.1016/s0892-1997(02)00105-4. [DOI] [PubMed] [Google Scholar]
- 8.Morrison M. Pattern recognition in muscle misuse voice disorders: how I do it. J Voice. 1997 Mar;11(1):108–114. doi: 10.1016/s0892-1997(97)80031-8. [DOI] [PubMed] [Google Scholar]
- 9.Roy N, Ford CN, Bless DM. Muscle tension dysphonia and spasmodic dysphonia: the role of manual laryngeal tension reduction in diagnosis and management. Annals of Otology, Rhinology, & Laryngology. 1996 Nov;105(11):851–856. doi: 10.1177/000348949610501102. [DOI] [PubMed] [Google Scholar]
- 10.Zeitels SM. The evolution of the assessment and treatment of paralytic dysphonia. Otolaryngol Clin North Am. 2000 Aug;33(4):803–816. doi: 10.1016/s0030-6665(05)70245-0. [DOI] [PubMed] [Google Scholar]
- 11.Kempster GB, Gerratt BR, Verdolini Abbott K, Barkmeier-Kraemer J, Hillman RE. Consensus Auditory-Perceptual Evaluation of Voice: Development of a Standardized Clinical Protocol. Am J Speech Lang Pathol. 2009 Oct 16;18:124–132. doi: 10.1044/1058-0360(2008/08-0017). [DOI] [PubMed] [Google Scholar]
- 12.Fairbanks G. Voice and Articulation Drillbook. 2nd ed Harper and Row; New York: 1960. [Google Scholar]
- 13.Smith ME, Ramig LO, Dromey C, Perez KS, Samandari R. Intensive voice treatment in Parkinson disease: laryngostroboscopic findings. J Voice. 1995 Dec;9(4):453–459. doi: 10.1016/s0892-1997(05)80210-3. [DOI] [PubMed] [Google Scholar]
- 14.Behrman A, Dahl LD, Abramson AL, Schutte HK. Anterior-posterior and medial compression of the supraglottis: signs of nonorganic dysphonia or normal postures? J Voice. 2003 Sep;17(3):403–410. doi: 10.1067/s0892-1997(03)00018-3. [DOI] [PubMed] [Google Scholar]
- 15.McLean-Muse A, Montgomery WW, Hillman RE, et al. Montgomery Thyroplasty Implant for vocal fold immobility: phonatory outcomes. Ann Otol Rhinol Laryngol. 2000 Apr;109(4):393–400. doi: 10.1177/000348940010900410. [DOI] [PubMed] [Google Scholar]
- 16.Peters HF, Boves L, van Dielen IC. Perceptual judgment of abruptness of voice onset in vowels as a function of the amplitude envelope. J Speech Hear Disord. 1986 Nov;51(4):299–308. doi: 10.1044/jshd.5104.299. [DOI] [PubMed] [Google Scholar]
- 17.Gallena S, Smith PJ, Zeffiro T, Ludlow CL. Effects of levodopa on laryngeal muscle activity for voice onset and offset in Parkinson disease. J Speech Lang Hear Res. 2001 Dec;44(6):1284–1299. doi: 10.1044/1092-4388(2001/100). [DOI] [PubMed] [Google Scholar]
- 18.Netto KJ, Burnett AF. Reliability of normalisation methods for EMG analysis of neck muscles. Work. 2006;26(2):123–130. [PubMed] [Google Scholar]
- 19.Debruyne F, Ostyn F, Delaere P, Wellens W, Decoster W. Temporary voice changes after uncomplicated thyroidectomy. Acta Otorhinolaryngol Belg. 1997;51(3):137–140. [PubMed] [Google Scholar]
- 20.Stojadinovic A, Shaha AR, Orlikoff RF, et al. Prospective functional voice assessment in patients undergoing thyroid surgery. Ann Surg. 2002 Dec;236(6):823–832. doi: 10.1097/00000658-200212000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong KH, Kim YK. Phonatory characteristics of patients undergoing thyroidectomy without laryngeal nerve injury. Otolaryngol Head Neck Surg. 1997 Oct;117(4):399–404. doi: 10.1016/S0194-5998(97)70133-5. [DOI] [PubMed] [Google Scholar]
- 22.McIvor NP, Flint DJ, Gillibrand J, Morton RP. Thyroid surgery and voice-related outcomes. Aust N Z J Surg. 2000 Mar;70(3):179–183. doi: 10.1046/j.1440-1622.2000.01781.x. [DOI] [PubMed] [Google Scholar]
- 23.Bielamowicz S, Kapoor R, Schwartz J, Stager SV. Relationship among glottal area, static supraglottic compression, and laryngeal function studies in unilateral vocal fold paresis and paralysis. J Voice. 2004 Mar;18(1):138–145. doi: 10.1016/j.jvoice.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Kelchner LN, Brehm SB, Weinrich B, et al. Perceptual Evaluation of Severe Pediatric Voice Disorders: Rater Reliability Using the Consensus Auditory Perceptual Evaluation of Voice. J Voice. 2009 Jan 8; doi: 10.1016/j.jvoice.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Stager SV, Bielamowicz SA, Regnell JR, Gupta A, Barkmeier JM. Supraglottic activity: evidence of vocal hyperfunction or laryngeal articulation? J Speech Lang Hear Res. 2000 Feb;43(1):229–238. doi: 10.1044/jslhr.4301.229. [DOI] [PubMed] [Google Scholar]
- 26.Andrade DF, Heuer R, Hockstein NE, Castro E, Spiegel JR, Sataloff RT. The frequency of hard glottal attacks in patients with muscle tension dysphonia, unilateral benign masses and bilateral benign masses. J Voice. 2000 Jun;14(2):240–246. doi: 10.1016/s0892-1997(00)80032-6. [DOI] [PubMed] [Google Scholar]