Abstract
Exercise training has been minimally explored as a therapy to mitigate the loss of muscle strength for individuals with Duchenne muscular dystrophy (DMD). Voluntary wheel running is known to elicit beneficial adaptations in the mdx mouse model for DMD. The aim of this study was to examine progressive resistance wheel running in mdx mice by comprehensively testing muscle function before, during, and after a 12-week training period. Male mdx mice at ~4 weeks age were randomized into three groups: Sedentary, Free Wheel, and Resist Wheel. Muscle strength was assessed via in vivo dorsiflexion torque, grip strength, and whole body tension intermittently throughout the training period. Contractility of isolated soleus muscles was analyzed at the study’s conclusion. Both Free and Resist Wheel mice had greater grip strength (~22%) and soleus muscle specific tetanic force (26%) compared with Sedentary mice. This study demonstrates that two modalities of voluntary exercise are beneficial to dystrophic muscle and may help establish parameters for an exercise prescription for DMD.
Keywords: cytoskeletal proteins, Duchenne muscular dystrophy, exercise, physical activity, skeletal muscle function
Skeletal muscles from individuals with Duchenne muscular dystrophy (DMD) are characterized by chronic weakness and susceptibility to injury. Generally defined, muscle weakness is a loss in force-producing capability and is documented in both boys with DMD and mdx mice, a mouse model for DMD.1–3 Muscle weakness is more pronounced following eccentric, lengthening contractions, indicating a susceptibility to injury that may perpetuate disease progression.4 Therapies for DMD are adopted to help individuals continue activities of daily living such as ambulation and self-care by mitigating the precipitous decline in muscle strength as the disease progresses. Because exercise training is known to improve muscle function in healthy individuals, it may be considered to be a non-invasive therapeutic modality to improve, maintain, or delay the functional decline of dystrophic muscle. However, the parameters of exercise prescription for DMD are not known, and the extent to which an appropriate exercise paradigm can avert muscle weakness and contraction-induced injury is unclear.5
The mdx mouse is commonly utilized to test exercise treatments that can be later applied in boys with DMD. In this capacity the mdx mouse responds well to voluntary wheel running. It develops increased muscle force,6–8 increased fatigue resistance,7 and desirable adaptations in muscle fiber type and size.9 At distances of ~7 km/day on free wheels, these adaptations are associated with an endurance type of exercise training that is relatively low intensity and high duration. Resistance types of exercise training (e.g., weight-lifting) that are relatively high intensity and low duration normally cause muscle hypertrophy and increased muscle strength. Indeed, resistance wheel running by non-diseased mice and rats is effective in eliciting these types of adaptations in both cardiac and skeletal muscles.10–12 Although it has been posited that resistance exercises may exploit adaptive processes in dystrophic muscle to strengthen force-bearing and -transmitting structures,13 whether or not these adaptations actually occur in dystrophic muscle has not been tested.
The common link between boys with DMD and mdx mice is the absence of dystrophin, a protein that localizes to the sarcolemma and anchors associated cytoskeletal proteins to form the dystrophin–glycoprotein complex (DGC).14,15 The DGC acts in concert with other transmembrane and subsarcolemmal proteins to form a cytoskeletal lattice termed a costamere.16 Costameres function to transmit force produced by the contractile unit laterally and longitudinally17–19 and maintain sarcolemmal integrity to facilitate unified contractions from tendon to tendon. Thus, disorganization of the costamere in dystrophin-deficient muscle is thought to contribute to muscle weakness and susceptibility to injury.20 Dystrophic muscle, albeit its predisposition, maintains some ability to adapt, as exemplified by numerous non-DGC, cytoskeletal proteins present at elevated levels (e.g., α7β1-integrin, utrophin, talin, vinculin, and γ-actin).21–23 This particular compensatory mechanism likely exists to reinforce the compromised costamere, and it has emerged as a major target of potential therapies for DMD. Furthermore, in non-diseased skeletal muscle, increases in cytoskeletal proteins are associated with resistance training.24,25 Thus, exercise-induced cytoskeletal adaptations in response to resistance wheel running in mdx mice could theoretically improve force transmission and injury resistance.
The overall aim of this study was to assess the efficacy of resistance wheel running as a non-invasive therapy to attenuate muscle weakness and susceptibility to contraction-induced injury in mdx mice. We hypothesized that 12 weeks of progressive resistance wheel running would increase muscle function as comprehensively determined in vivo through grip strength, whole body tension, and ankle dorsiflexion torque, and in vitro via soleus muscle contractility. Finally, we tested the hypothesis that resistance training would increase cytoskeletal proteins above compensatory levels in mdx skeletal muscle. This was done to determine whether the functional exercise-induced adaptations might be associated with changes in these proteins.
METHODS
Animals and Study Design
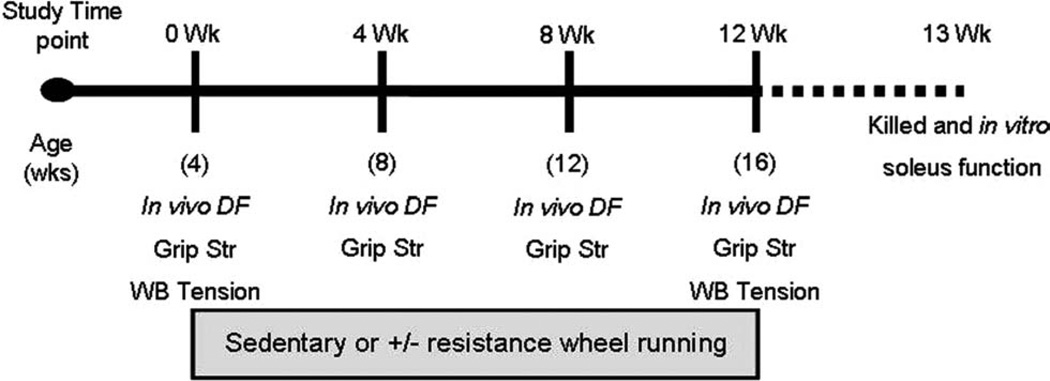
Male mdx mice (C57Bl/10ScSn-DMDmdx, n = 34), ages 4–5 weeks, were purchased from the Jackson Laboratory (Bar Harbor, Maine). Upon arrival, each mouse was tested for in vivo ankle dorsiflexion torque, forelimb grip strength, and whole body tension (Fig. 1). In vivo ankle dorsiflexion torque was always assessed on a different day than grip strength and whole body tension so that mice were not fatigued. Mice were then randomly assigned to one of three groups: (1) sedentary (Sedentary, n = 11); no resistance, voluntary wheel running (Free Wheel, n = 10); and progressively increasing resistance, voluntary wheel running (Resist Wheel, n = 13). All mice were housed individually on a 12-hour light/dark cycle, and were provided food and water ad libitum. Free Wheel and Resist Wheel mice were allowed open access to their running wheels 24 h/day. Sedentary mice remained in their individual cages without wheels throughout the 12-week study. Body masses were recorded weekly, and running distances were recorded daily. After 4 and 8 weeks of training, all mice were retested for in vivo dorsiflexion torque and forelimb grip strength. Whole body tension was only assessed at week 0 and week 12 to avoid an accommodation to the tail pinch, which is required to elicit a maximal response. After 12 weeks of training, all mice were reassessed for in vivo ankle dorsiflexion torque, forelimb grip strength, and whole body tension. Mice were returned to their individual cages and maintained their training regimen until approximately 1 week later when they were euthanized, and individual soleus muscles were excised and tested in vitro for force production and resistance to contraction-induced injury (Fig. 1). All in vivo tests were performed by the same investigator who was blinded to the exercise treatments. At this time, blood was also obtained for determining serum creatine kinase activity.26 All protocols were approved by the University of Minnesota Institutional Animal Care and Use Committee and complied with guidelines set by the American Physiological Society.
FIGURE 1.
After an initial in vivo strength assessment of dorsiflexion torque (in vivo DF), grip strength (Grip Str), and whole body tension (WB Tension), mice were randomly assigned to either the Sedentary, Free Wheel, or Resist Wheel group (0 Wk). The mice assigned to the Free and Resist Wheel groups initiated running at this time-point. At the 4- and 8-week time-points, all mice were reassessed for in vivo dorsiflexion torque and grip strength. At week 12, all mice were assessed for in vivo dorsiflexion, grip strength, and whole body tension. All mice were killed 1 week later, and skeletal muscles were harvested for in vitro contractility and injury assessment, muscle mass, and Western blot analysis.
Training Protocol
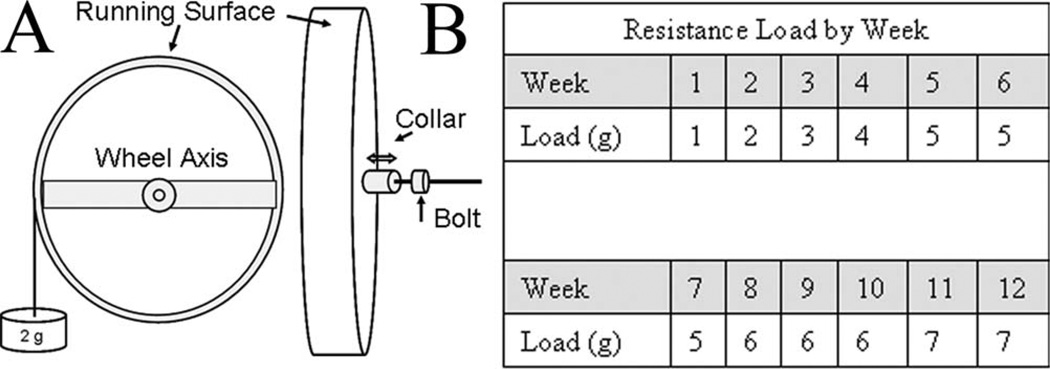
Free Wheel and Resist Wheel mice performed 12 weeks of voluntary wheel running.27 A metal bracket attached to the ceiling of the mouse cage anchored the 11-cm-diameter wheel via a horizontal rod that ran through the axis of the wheel. Resistance was added to the wheel using a partially threaded aluminum collar that screwed back and forth on the horizontal rod next to the wheel axis. A mass was loaded to the horizontal moment arm of the wheel, and the collar was adjusted until the wheel no longer turned. The collar was then locked into position with a bolt (Fig. 2A). All wheels were initially set at a resistance load of 1 g (~6% body mass). Following the first week, the resistance load was progressively increased for Resist Wheel mice (Fig. 2B), whereas the resistance load for Free Wheel mice remained at 1 g for the remainder of the study. The following criteria for increasing the resistance load were set in order to adjust wheel resistance on an individual-mouse basis. Resistance load was increased 1 g each week unless, as a result of a previous increase in resistance load, the averaged daily distance: (1) dropped 50% or (2) dropped to below 2 km/day. This final criterion was established based on results from a previous study that reported as little as 1.45 km/day free wheel running produced muscle adaptations.28 A pilot study reported that no muscle adaptations were observed in mice that ran on resistance wheels when average daily distance fell below 2 km/day.29 By the end of the study, the resistance load was either 6 g or 7 g for all Resist Wheel mice, which equated to ~20% of their body mass. External work was calculated using the following equation11: work = (torque × radians × distance) / kg body mass. Torque was calculated as: (grams of resistance loaded on the wheel) × (wheel radius) × (9.81 g/s2). Radians were calculated as: 2π radians/revolution and, therefore, 18,182 radians/km.
FIGURE 2.
Resistance wheel design and resistance load during the 12-week training period. (A) To calibrate the resistance on the wheel a mass (e.g., 2 g) was placed on the horizontal moment arm of the wheel. The metal collar was then tightened (↓) against the axis of the wheel to maintain the resistance during wheel movement. When the appropriate resistance was reached, a bolt was tightened against the collar to secure its position. The calibration mass was then removed from the horizontal moment arm. (B) Resistance load applied to the wheel of Resist Wheel mice increased progressively from week 1 to week 12.
In Vivo Functional Measurements
In Vivo Torque
In vivo maximal isometric torque of the dorsiflexors (i.e., anterior crural muscles) was assessed as previously described26 and was done so every 4 weeks. Briefly, mice were anesthetized with a cocktail of fentanyl citrate [10 mg/kg body weight (BW)], droperidol (0.2 mg/kg BW), and diazepam (5 mg/kg BW). The left hindlimb was shaved and aseptically prepared, and the foot was placed in a metal foot-plate attached to the shaft of a servomotor (Model 300B-LR; Aurora Scientific, Aurora, Ontario, Canada). Two platinum electrodes (Model E2–12; Grass Technologies, West Warwick, Rhode Island) were inserted subcutaneously on either side of the peroneal nerve. A stimulator and stimulus isolation unit (Models S48 and SIU5, respectively; Grass Technologies) stimulated the peroneal nerve via the platinum electrodes to induce a contraction of the anterior crural muscles (tibialis anterior, extensor digitorum longus, and extensor hallucis longus muscles). The parameters for stimulation were set at a 200-ms contraction duration consisting of 0.5-ms square-wave pulses at 300 Hz. The voltage was adjusted from 3.0 to 9.0 V until maximal isometric torque was achieved.
Grip Strength
Forelimb grip strength was assessed every 4 weeks. Each mouse was allowed to grab a bar attached to a force transducer as it was pulled by the tail horizontally away from the bar (Model 1027CSM; Columbus Instrument Co., Columbus, Ohio).30 Five repetitions with a 5-s pause between each were averaged and normalized to body mass (g) to determine grip strength for each mouse.
Whole Body Tension
Whole body tension was assessed at baseline and again after 12 weeks of training. Each mouse was attached to a horizontally mounted force transducer (Model SS66L; BIOPAC Systems, Inc., Goleta, California) by means of a 4-0 silk suture (3–4 inches long). The suture was securely tied to the proximal third of the tail and at the end of an aluminum S-hook attached to the force transducer. Mice were then placed in a custom-built Plexiglas apparatus that restricted movement to the forward direction. The floor of the apparatus was lined with chicken wire to prevent animals from slipping on the Plexiglas during testing. The force transducer connected to a driver (MP35; BIOPAC Systems) and laptop computer. Tension was then measured over a 300-s period, where forward movements were evoked by pinching of the tail.31 The highest forward pulling tension during the test was determined for each mouse, and results are reported normalized to body mass.
In Vitro Contractility
Approximately 1 week after completion of the in vivo testing, mice were anesthetized with sodium pentobarbital (100 mg/kg BW), and soleus muscles were excised and analyzed for force-generating capacities.26,28 The soleus muscle was selected for this study, because both proximal and distal tendons can be isolated for clamp attachment. The soleus muscle’s size facilitates oxygen diffusion to the core of the muscle, thus it is well suited for in vitro preparations. In addition, the soleus muscle responds better to exercise (i.e., increased tetanic force) than the extensor digitorum longus muscle.7,8,32 Muscles were mounted to a dual-mode muscle lever system (300B-LR; Aurora Scientific, Inc., Aurora, Ontario, Canada) with a 5-0 suture in a 0.38-ml bath assembly and incubated at 25°C in an oxygenated (95% O2) Krebs–Ringer bicarbonate buffer solution. Muscles were maintained at a resting tension (Lo) of 0.5 g, and then muscle length was measured. Following a 15-min quiescent period, muscles underwent a single passive stretch to 1.05 Lo. One minute later, two twitches separated by 30 s were elicited by stimulating the muscle with a 0.5-ms pulse at 150 V. Peak twitch force was recorded. Then a series of maximal isometric tetanic contractions (Po) were performed at 150 V and 120 Hz for 400 ms (Grass S48 stimulator delivered through a SIU5D stimulus isolation unit; Grass Telefactor, Warwick, Rhode Island) with a 1-min rest period between contractions until Po plateaued. To determine active stiffness, a sinusoidal length oscillation of 0.01% Lo at 500 Hz was applied at peak force of a single tetanic isometric contraction.33,34 One minute later, an injury protocol consisting of 15 eccentric contractions was performed. For this, muscles were passively shortened from Lo to 0.875 Lo and stimulated for 200 ms while the muscle was simultaneously lengthened to 1.125 Lo at 1.25 Lo/s before passively returning to Lo. Each eccentric contraction was separated by 3 min of rest. Three minutes after the last eccentric contraction, a final isometric tetanic contraction was performed (postPo). Eccentric contraction–induced injury was determined by eccentric force loss during the course of the injury protocol [(Contraction 15 − Contraction 1) / Contraction 1] and also by the decrement in maximal isometric tetanic force [(postPo − Po) / Po].
Muscle Mass
After in vitro preparations, the extensor digitorum longus, tibialis anterior, gastrocnemius, quadriceps, triceps, and heart, along with the soleus muscles, were snap frozen in liquid nitrogen and stored at −80°C. Masses for frozen muscles were later recorded using a microbalance (Sartorius CPA225D; Mettler Toledo, Boston, Massachusetts).
Cytoskeletal Protein Expression
Soleus, gastrocnemius, and triceps muscles from Sedentary, Free Wheel, and Resist Wheel mice were assessed for cytoskeletal protein expression. Briefly, frozen muscle was pulverized in a mortar and pestle and solubilized in 1% sodium dodecylsulfate (SDS), 5 mM ethylene-glycol tetraacetic acid (EGTA), and a cocktail of protease inhibitors.35 Solubilized muscle homogenates were assayed in triplicate to determine total protein concentration using the bicinchoninic protein assay (BCA; Pierce, Rockford, Illinois). Fifty micrograms of total protein was separated on 3–12% polyacrylamide gels at 120 V for 30 min, then 180 V for 55 min. Proteins were transferred to a nitrocellulose membrane at 106 V for 90 min. Western blotting was then performed using the following primary monoclonal antibodies (MAbs): β-dystroglycan MAb NCL-β-DG (1:50; Novocastra); α7-integrin MAb SC-81807 (1:500; Santa Cruz); vinculin MAb V4505 (1:500; Sigma); and talin MAb T-3282 (1:500; Sigma). Talin, α7-integrin, and β-dystroglycan were probed on one membrane, and vinculin was probed on a second membrane. Secondary antibodies were diluted (1:5000) and detected with the Odyssey Infrared Imaging System (Li-Cor Biosciences) using the 700- and 800-nm channels. Fluorescence intensities were determined using the Li-Cor software. Three blots were analyzed for each cytoskeletal protein (Sedentary vs. Free Wheel, Sedentary vs. Resist Wheel, Free Wheel vs. Resist Wheel). Blot intensities for Free Wheel and Resist Wheel samples were analyzed and compared with Sedentary samples, which were set to the arbitrary value of 1.0.
Statistical Analyses
Two-way repeated-measures analysis of variance (ANOVA) was used to determine whether resistance training affected wheel running distance and external work over time. Two-way repeated-measures ANOVA was also used to assess whether activity (Sedentary, Free Wheel, or Resist Wheel) affected body mass, in vivo dorsiflexion torque, grip strength, or whole body tension over time. For all two-way repeated-measures ANOVAs, the repeated factor was time and, when significant main effects were found, Tukey honestly significant difference (HSD) post hoc tests were done to determine differences among groups or times. One-way ANOVAs with Tukey HSD post hoc tests were used to detect differences among groups concerning muscle masses, in vitro soleus muscle contractility characteristics, and force loss percentage during and after eccentric injury, creatine kinase activity, and cytoskeletal protein expressions. All statistics were done using JMP (version 7) statistical software (SAS Institute, Inc., Cary, North Carolina, 1989–2007).
RESULTS
Body Mass and Wheel Running
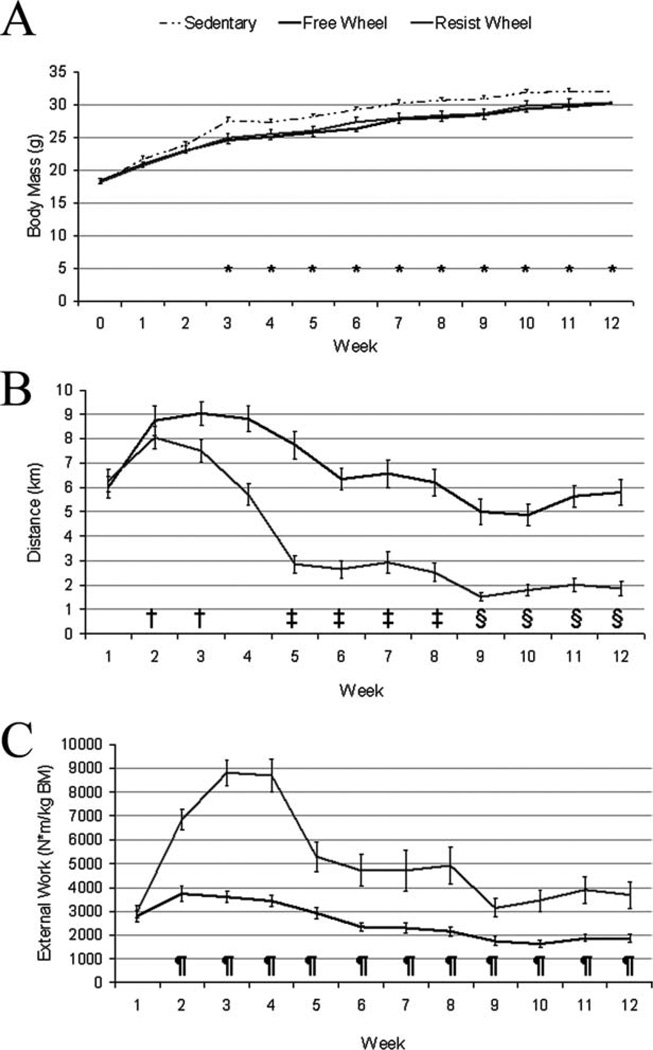
There was a significant interaction between group and time for body mass (P = 0.034; Fig. 3A). The mdx mice provided a running wheel, regardless of whether free or resisted, had lower body masses compared with mdx Sedentary mice starting at week 3 and persisting throughout the remainder of the study (P < 0.036).
FIGURE 3.
Body mass, averaged daily running distance, and averaged daily external work varied among groups and weeks during the 12-week study. (A) Starting at week 3, body mass was significantly greater in Sedentary mice compared with Free and Resist Wheel mice and stayed elevated for the remainder of the study (*Sedentary > Free and Resist Wheel). (B) Independent of group, averaged daily distance peaked at week 2 (†>week-1), then decreased at week 5 (‡<weeks 2 and 3) and week 9 (§ <weeks 1, 2, 3, and 4). (C) Averaged daily external work was greater at week 2 in Resist Wheel mice and stayed greater throughout the remainder of the study (¶>Free Wheel).
Resistance load on the wheel decreased mean daily distance run by ~40% in Resist Wheel mice compared with Free Wheel mice (P < 0.001; Fig. 3B). The mean daily distance for Resist Wheel mice over 12 weeks was 3.8 ± 0.3 km/day, whereas Free Wheel mice ran 6.7 ± 0.2 km/day. There was also a main effect of time (P < 0.001), regardless of group, as mice achieved maximum mean daily averages during week 2 (Fig. 3B). There was a significant interaction between group and time for external work (P < 0.001; Fig. 2C). Starting at week 2 and continuing through week 12, Resist Wheel mice completed 72–149% more external work per week than Free Wheel mice.
In Vivo Muscle Function Analyses
Three in vivo tests, dorsiflexion torque, grip strength, and whole body tension, were performed at intervals throughout the study. Maximal dorsiflexion torque was not different among groups, but it was greater at weeks 4, 8, and 12 compared with week 0 (Table 1). Dorsiflexion torque at week 12, independent of running, correlated with tibialis anterior muscle mass (R2 = 0.528; P < 0.001), indicating that larger tibialis anterior muscles were indeed generating more torque. However, torques normalized to tibialis anterior muscle masses were not different among groups at week 12 (P = 0.478).
Table 1.
In vivo muscle strength of Sedentary, Free Wheel, and Resist Wheel mice over time.
| Group effect | Time effect | Interaction | |||||
|---|---|---|---|---|---|---|---|
| In vivo dorsiflexion torque (N mm/kg BM) | |||||||
| Wk 0 | Wk 4* | Wk 8* | Wk 12* | ||||
| Sedentary | 44.7 ± 3.8 | 77.9 ± 2.7 | 72.2 ± 2.5 | 70.8 ± 2.4 | 0.567 | <0.001 | 0.311 |
| Free Wheel | 49.7 ± 4.0 | 79.5 ± 6.8 | 75.1 ± 5.9 | 79.8 ± 3.4 | |||
| Resist Wheel | 49.4 ± 4.3 | 69 ± 8.4 | 70.1 ± 2.8 | 67.9 ± 2.6 | |||
| Grip strength (g/g BM) | |||||||
| Wk 0 | Wk 4* | Wk 8 | Wk 12* | ||||
| Sedentary | 2.5 ± 0.2 | 2.9 ± 0.2 | 2.6 ± 0.1 | 2.8 ± 0.1 | <0.001 | <0.001 | 0.154 |
| Free Wheel† | 3.3 ± 0.2 | 3.7 ± 0.1 | 3.2 ± 0.1 | 3.7 ± 0.2 | |||
| Resist Wheel† | 2.8 ± 0.2 | 3.9 ± 0.1 | 3.1 ± 0.2 | 3.3 ± 0.1 | |||
| Whole body tension (g/g BM) | |||||||
| Wk 0 | Wk 4 | Wk 8 | Wk 12 | ||||
| Sedentary | 11.1 ± 0.5 | – | – | 11.5 ± 0.6 | 0.450 | 0.244 | 0.393 |
| Free Wheel | 11.2 ± 0.8 | – | – | 9.8 ± 0.4 | |||
| Resist Wheel | 11.3 ± 0.6 | – | – | 10.9 ± 0.5 | |||
Values expressed as mean ± SE. BM, body mass; Wk, week.
Week > week 0.
Group > Sedentary.
There was an effect of wheel running on grip strength, as both Free and Resist Wheel mice had ~22% greater forces than did Sedentary mice (Table 1). Similar to the results of the dorsiflexion torque, all mice became stronger during the study as measured by grip strength (main effect of time, Table 1). Peak force elicited during the whole body tension measurement was not different among groups or over time (Table 1).
In Vitro Soleus Muscle Contractility
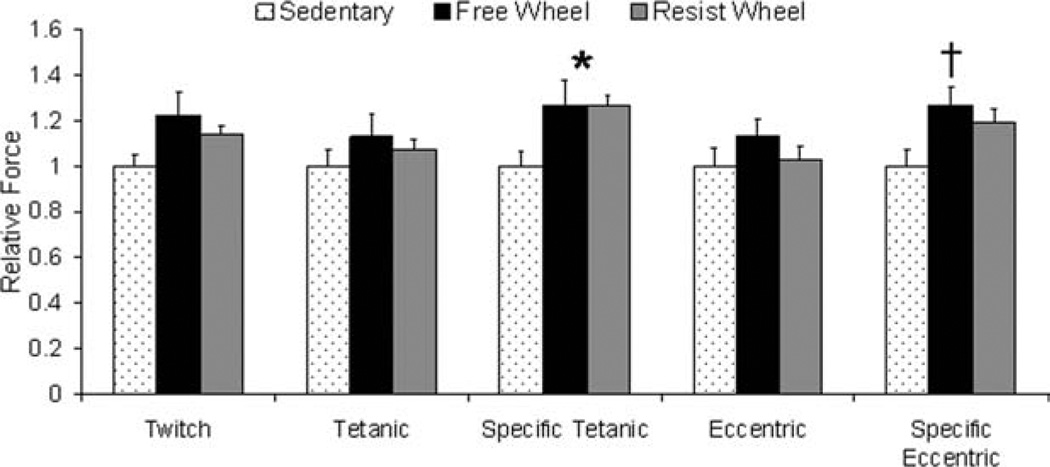
There were no differences among groups in absolute twitch, tetanic, or eccentric forces generated by isolated soleus muscles (Fig. 4). However, maximal isometric tetanic force normalized to muscle cross-sectional area (i.e., specific tetanic force) was 26% greater in Free and Resist Wheel mice compared with Sedentary mice (9.71, 9.71, and 7.68 N/cm2, respectively; P = 0.050; Fig. 4). Specific eccentric force was also 27% greater in Free Wheel compared with Sedentary mice (19.4 and 15.3 N/cm2, respectively; P = 0.031; Fig. 4).
FIGURE 4.
Free and resistance voluntary wheel running improved soleus muscle force. Data are shown relative to Sedentary values (*Free Wheel and Resist Wheel > Sedentary; †Free Wheel > Sedentary).
Twitch and tetanic force–time tracings were analyzed to determine whether wheel running affected properties indicative of how fast the soleus muscle contracted and relaxed. There were no differences in these twitch or tetanic parameters among groups (Table 2). Passive and active stiffness, which reflect the muscle’s resistance to lengthening due to non-contractile elastic elements and myosin crossbridges that are strongly bound to actin, respectively, was also not affected by wheel running (Table 2). In addition, there was no effect of exercise on force loss during and following the eccentric injury protocol (P ≥ 0.170; Table 2). Collectively, soleus muscles lost an average of 16% of their force-generating capacity from eccentric contraction numbers 1–15, and 9% of their isometric force-generating capacity (Table 2).
Table 2.
In vitro soleus muscle contractility and eccentric contraction–induced force loss from Sedentary, Free Wheel, and Resist Wheel mice at the end of the 12-week study.
| Sedentary | Free Wheel | Resist Wheel | P-value | |
|---|---|---|---|---|
| Twitch | ||||
| TPT (ms) | 36.1 ± 1.5 | 34.5 ± 2.1 | 39.1 ± 1.2 | 0.145 |
| RT1/2 (ms) | 47.8 ± 4.5 | 50.8 ± 6.1 | 51.3 ± 2.4 | 0.840 |
| Tetanic | ||||
| +dP/dt (N/s) | 1.4 ± 0.1 | 2.0 ± 0.3 | 1.7 ± 0.1 | 0.088 |
| −dP/dt (N/s) | −2.6 ± 0.1 | −3.2 ± 0.3 | −2.9 ± 0.2 | 0.352 |
| Passive stiffness (N/m) | 4.5 ± 0.6 | 13.6 ± 0.9 | 14.2 ± 0.6 | 0.734 |
| Active stiffness (N/m) | 383 ± 25 | 400 ± 28 | 405 ± 15 | 0.773 |
| Force loss | ||||
| Ecc15:Ecc1 (%) | −6.3 ± 3.9 | −20.0 ± 9.8 | −18.4 ± 4.7 | 0.170 |
| Post Po:Po (%) | −6.7 ± 3.3 | −10.83 ± 3.0 | −9.0 ± 1.8 | 0.610 |
Values expressed as mean ± SE. TPT, time to peak twitch force; RT1/2, one-half relaxation time; +dP/dt, maximal rate of tetanic force development; −dP/dt, maximal rate of relaxation; Ecc15:Ecc1, force loss during eccentric protocol, calculated as: ((Ecc15 − Ecc1)/Ecc1)·100. PostPo:Po, force loss after eccentric protocol, calculated as: ((PostPo − Po)/Po)*100.
Muscle Masses and Serum Creatine Kinase Activity
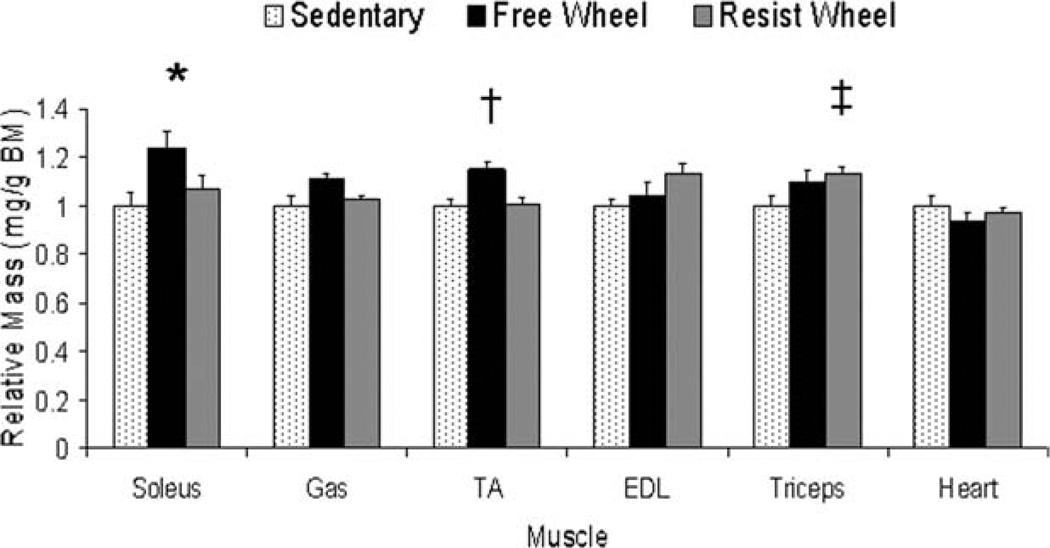
Masses of several hindlimb muscles as well as the heart were analyzed to determine whether there was a response to wheel running. Absolute masses of the muscles that were measured were not affected by wheel running (P ≥ 0.087). However, when normalized to body mass, soleus muscle masses from Free Wheel mice were 23% greater than those from Sedentary mice (P = 0.039; Fig. 5). In addition, tibialis anterior muscle masses from Free Wheel mice were 15% greater than those from both Sedentary and Resist Wheel mice (P = 0.003), and triceps muscle masses from Resist Wheel mice were 15% greater than those from Sedentary mice (P = 0.045; Fig. 5).
FIGURE 5.
Hindlimb [soleus, gastrocnemius (Gas), tibialis anterior (TA), extensor digitorum longus (EDL), and forelimb (triceps)] muscle masses were affected differently by free and resistance voluntary wheel running. Data are shown relative to Sedentary values (*Free Wheel > Sedentary; †Free Wheel > Sedentary and Resist Wheel; ‡Resist Wheel > Sedentary).
Creatine kinase activity from the serum of Sedentary, Free Wheel, and Resist Wheel mice was not different at the culmination of the study (P = 0.891). The average activity was 6906 ± 666 U/L for all mice.
Cytoskeletal Protein Expression
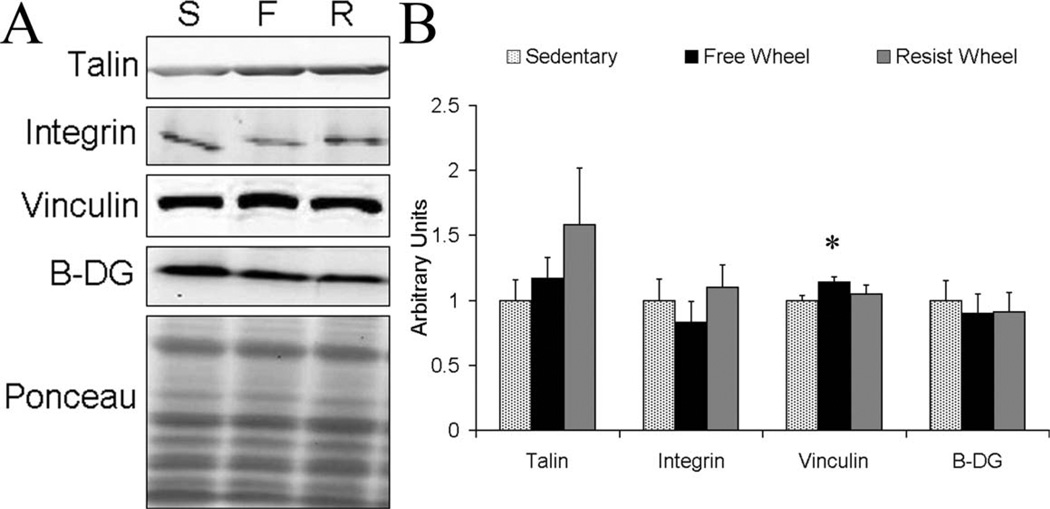
To determine whether the higher forces generated by soleus muscles from wheel runners might be attributed to increased force transmission by greater amounts of cytoskeletal protein, α7-integrin, talin, vinculin, and β-dystroglycan were analyzed by Western blot. Because the gastrocnemius muscle is a synergist to the soleus muscle and because the triceps muscle contributes to grip strength, those muscles were also analyzed. The only significant findings were that β-dystroglycan content was 35% greater in the gastrocnemius muscles of Free Wheel compared with Sedentary mice (P = 0.024), and vinculin content was 14% greater in soleus muscles of Free Wheel compared with Sedentary mice (P = 0.033; Fig. 6). α7-integrin, and talin were not different among the groups for any of the three muscles analyzed (P ≥ 0.054).
FIGURE 6.
Cytoskeletal protein expression in soleus muscle. (A) Representative blots of talin (269 kDa), α7-integrin (125 kDa), vinculin (117 kDa), and β-dystroglycan (B-DG, 43 kDa). Lane S is Sedentary, lane F is Free Wheel, and lane R is Resist Wheel. In addition, a representative Ponceau stain is shown verifying that equal amounts of total protein were loaded into each well. (B) Vinculin content was greater for Free Wheel mice compared with Sedentary mice in the soleus muscle (*). Values are represented relative to Sedentary.
DISCUSSION
The aim of this study was to determine whether a voluntary, resistance type of exercise could attenuate muscle weakness and injury susceptibility in mdx mice. Our main finding was that resistance wheel running improved muscle strength in mdx mice, although improvements were not any greater than those observed from non-resistance wheel running. This finding reinforces currently accepted principles regarding the ability of dystrophic muscle to adapt to exercise and expands our understanding of exercise parameters for improving dystrophic muscle (i.e., frequency, duration, intensity, and mode of exercise). In particular, we have shown that 12 weeks of voluntary wheel running, regardless of resistance load, improved muscle strength in terms of soleus muscle specific forces and resulted in greater grip strength forces. It is noteworthy that no functional measurements were worsened by wheel running in concurrence with previous reports that voluntary wheel running6,9,36,37 and low-intensity treadmill running38 by mdx mice do not exacerbate the disease. Also, serum creatine kinase, another marker of muscle injury, was not higher in the wheel-running mice, again indicating that additional muscle injury was not induced by exercise.
This is the first study to investigate resistance wheel running in mdx mice. The main difference between resistance and non-resistance wheel running is an increase in daily external work coupled with a decrease in daily distance, both a result of resistance load on the wheel. External work reflects the ability of a mouse to perform work on an external environment, in this case the wheel. The external work done by Resist Wheel mice in this study was roughly twice that done by Free Wheel mice and demonstrates a greater capacity and/or motivation to do work. Resistance load has been shown to affect external work similarly in non-diseased C57Bl/6 mice in a previous study that used progressive resistance wheel running.11 However, the daily distance run by the mice did not deviate in that study until the resistance load reached 7 g (~25% body mass), whereas, for the mdx mice in our study, the distance run decreased significantly with just 3 g of resistance added to the wheel (~13% body mass). This suggests that the threshold at which resistance load affects daily running distance is different between mdx and non-diseased mice. One possible explanation for this difference is that mdx mice have significantly less whole body strength compared with wild-type mice31,39 and are therefore unable to perform as much work. Another possible explanation is that there is an inability of the cardiovascular system to properly adapt to relatively high-intensity exercise in mdx mice. Exercise-induced cardiovascular adaptations have not been explored in mdx mice, but heart rate and systolic blood pressure do not increase during exercise in boys with DMD, as occurs in healthy individuals.40 As a final point, we did not attempt to quantify external work done by Sedentary mice or the wheel-running mice when they were not on the wheel. Previously we reported that cage activities, such as ambulation, rearing, and jumping, were ~40% less in mdx mice than in healthy C57BL mice9 and, more recently, we have found that cage activities of mdx mice that are and are not provided wheels are equivalent (unpublished data). These results suggest that external work done during typical cage activities by Sedentary and wheel-running mice in this study was similar.
Another notable difference between Resist Wheel mice and Free Wheel mice was an adjustment in running strategy (or form) by Resist Wheel mice. Presumably, to accommodate to increased wheel resistance, we observed that the Resist Wheel mice ran much more vertically on the wheel and utilized their forelimb muscles to initiate and maintain wheel running, as compared with Free Wheel mice, which tended to run horizontally on the wheel, that is, at the cage bottom. This running accommodation likely contributed to the greater normalized mass of the triceps in Resist Wheel mice. Resistance exercise training such as weight-lifting typically causes muscle hypertrophy and improvements in muscle strength. Although this type of exercise modality is difficult to accommodate voluntarily in rodent models, adding a resistance load to a running wheel has been used previously and somewhat successfully. For example, arm muscles (i.e., extensor carpi radialis longus and brevis) increased mass by ~20% with resistance wheel running in Sprague-Dawley rats,12 whereas similar results have been reported for the plantaris muscle in rats10 and the soleus muscle in mice.11 Our study has taken the next step in that not only were muscles masses assessed, but so too were functional outcomes. In regard to arm muscles and the altered running strategy, we have shown that increased triceps mass with resistance wheel running is associated with an improvement in grip strength. A novelty of this finding is that it was shown in mdx mice, illustrating that dystrophic muscle can positively adapt to a resistance-type exercise.
Supporting this concept further are results from the in vitro contractility analyses of soleus muscles. Previous work has shown that the soleus muscle undergoes increases in mass and improvements in force generation with non-resistance wheel running in mdx mice,6–8 and our results recapitulated these reports as well as expanded them to include resistance wheel running in mdx mice. Specifically, we have shown that maximal isometric and eccentric specific forces produced by soleus muscles were improved with wheel running by 26% relative to sedentary. The finding that specific, but not absolute, tetanic forces were greater as a result of wheel running indicates that hypertrophy was not the underlying cause of this improvement. Instead, some intrinsic capacity to generate force or the ability to transmit the force that was generated must have been bettered by the wheel running.
To begin to probe for a mechanistic explanation for the increased soleus muscle forces and grip strengths, we considered cytoskeletal protein adaptations. Structural and physiological studies have demonstrated the importance of the costamere during muscle contraction and, indeed, as much as 70% of force produced by the contractile unit is transmitted laterally through the costamere, both to adjacent myofibrils and across the sarcolemma.19,41–43 Similarly, the costamere maintains sarcolemmal integrity by ensuring that sarcolemmal adjustments that accompany forceful muscle contractions and stretches are small and periodic.16 This indicates that the inadequate costameric lattice observed in dystrophin-deficient muscle likely contributes to deficits in muscle strength and ablated sarcolemmal integrity in mdx mice and individuals with DMD.4,44–46 We hypothesized that resistance wheel running would improve muscle force and decrease muscle susceptibility to injury, in part based on the premise that resistance training increases cytoskeletal protein expression, and these increases might improve muscle strength and protect the muscle from future bouts of a similar exercise.24,47,48 This premise was also based on evidence that enhanced cytoskeletal protein expression is associated with increases in skeletal muscle force and attenuation of compromised sarcolemmal integrity.49,50 However, our hypotheses were not supported, as we observed minimal changes in cytoskeletal proteins with wheel running. Furthermore, we did not observe a benefit with regard to susceptibility to injury. These data indicate that a threshold must be surpassed regarding cytoskeletal protein expression to observe functional benefits (see Burkin et al.51 and Rebakova et al.35), and wheel running, whether or not against resistance, did not overtly elicit this adaptation in mdx mice.
In conclusion, currently, no parameters for exercise prescription exist for DMD patients. This is important to establish because young children with DMD are physically active, and activity likely plays an important role in their social and psychological well-being. At each stage of childhood development, it is imperative that appropriate activities/exercises are advocated so that no additional muscle injury occurs. As the disease progresses, being physically active/exercising becomes important in the attempt to maintain muscle function and for overall health. Thus, studies that examine the effects of exercise in the context of DMD are needed. Wheel running by mdx mice may mimic moderate physical activity in DMD inasmuch as it is a voluntary type of exercise that is not strenuous, and it does not exacerbate the dystrophic disease phenotype. Finally, we have shown that 12 weeks of resistance and non-resistance voluntary wheel running elicited beneficial adaptations, notably strength gains observed by grip strength and soleus muscle specific forces. We have established that two similar modes of voluntary exercise that differ primarily in intensity are capable of inducing comparable adaptations in skeletal muscle of mdx mice.
Acknowledgments
This study was supported by grants from the Muscular Dystrophy Association (MDA4143) and the NIH (P30-AR05722 and T32-AR07612). The authors thank Kristen Baltgalvis and Sarah Greising for technical assistance and for critically evaluating the manuscript.
Abbreviations
- ANOVA
analysis of variance
- BCA
bicinchoninic acid assay
- BM
body mass
- DMD
Duchenne muscular dystrophy
- DGC
dystrophin–glycoprotein complex
- EDL
extensor digitorum longus
- EGTA
ethylene-glycol tetraacetic acid
- MAb
monoclonal antibody
- SDS
sodium dodecylsulfate
- TA
tibialis anterior
REFERENCES
- 1.Aitkens SG, McCrory MA, Kilmer DD, Bernauer EM. Moderate resistance exercise program: its effect in slowly progressive neuromuscular disease. Arch Phys Med Rehabil. 1993;74:711–715. doi: 10.1016/0003-9993(93)90031-5. [DOI] [PubMed] [Google Scholar]
- 2.Lynch GS, Hinkle RT, Faulkner JA. Force and power output of diaphragm muscle strips from mdx and control mice after clenbuterol treatment. Neuromuscul Disord. 2001;11:192–196. doi: 10.1016/s0960-8966(00)00170-x. [DOI] [PubMed] [Google Scholar]
- 3.Sacco P, Jones DA, Dick JR, Vrbova G. Contractile properties and susceptibility to exercise-induced damage of normal and mdx mouse tibialis anterior muscle. Clin Sci (Lond) 1992;82:227–236. doi: 10.1042/cs0820227. [DOI] [PubMed] [Google Scholar]
- 4.Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grange RW, Call JA. Recommendations to define exercise prescription for Duchenne muscular dystrophy. Exerc Sport Sci Rev. 2007;35:12–17. doi: 10.1249/01.jes.0000240020.84630.9d. [DOI] [PubMed] [Google Scholar]
- 6.Carter GT, Wineinger MA, Walsh SA, Horasek SJ, Abresch RT, Fowler WM., Jr Effect of voluntary wheel-running exercise on muscles of the mdx mouse. Neuromuscul Disord. 1995;5:323–332. doi: 10.1016/0960-8966(94)00063-f. [DOI] [PubMed] [Google Scholar]
- 7.Hayes A, Williams DA. Beneficial effects of voluntary wheel running on the properties of dystrophic mouse muscle. J Appl Physiol. 1996;80:670–679. doi: 10.1152/jappl.1996.80.2.670. [DOI] [PubMed] [Google Scholar]
- 8.Wineinger MA, Abresch RT, Walsh SA, Carter GT. Effects of aging and voluntary exercise on the function of dystrophic muscle from mdx mice. Am J Phys Med Rehabil. 1998;77:20–27. doi: 10.1097/00002060-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Landisch RM, Kosir AM, Nelson SA, Baltgalvis KA, Lowe DA. Adaptive and nonadaptive responses to voluntary wheel running by mdx mice. Muscle Nerve. 2008;38:1290–1303. doi: 10.1002/mus.21141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishihara A, Roy RR, Ohira Y, Ibata Y, Edgerton VR. Hypertrophy of rat plantaris muscle fibers after voluntary running with increasing loads. J Appl Physiol. 1998;84:2183–2189. doi: 10.1152/jappl.1998.84.6.2183. [DOI] [PubMed] [Google Scholar]
- 11.Konhilas JP, Widegren U, Allen DL, Paul AC, Cleary A, Leinwand LA. Loaded wheel running and muscle adaptation in the mouse. Am J Physiol Heart Circ Physiol. 2005;289:H455–H465. doi: 10.1152/ajpheart.00085.2005. [DOI] [PubMed] [Google Scholar]
- 12.Legerlotz K, Elliott B, Guillemin B, Smith HK. Voluntary resistance running wheel activity pattern and skeletal muscle growth in rats. Exp Physiol. 2008;93:754–762. doi: 10.1113/expphysiol.2007.041244. [DOI] [PubMed] [Google Scholar]
- 13.Vrbova G. Function induced modifications of gene expression: an alternative approach to gene therapy of Duchenne muscular dystrophy. J Muscle Res Cell Motil. 2004;25:187–192. doi: 10.1023/b:jure.0000035893.59267.47. [DOI] [PubMed] [Google Scholar]
- 14.Ervasti JM, Campbell KP. Membrane organization of the dystrophin–glycoprotein complex. Cell. 1991;66:1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- 15.Ervasti JM. Dystrophin, its interactions with other proteins, and implications for muscular dystrophy. BiochimBiophys Acta. 2007;1772:108–117. doi: 10.1016/j.bbadis.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Ervasti JM. Costameres: the Achilles’ heel of Herculean muscle. J Biol Chem. 2003;278:13591–13594. doi: 10.1074/jbc.R200021200. [DOI] [PubMed] [Google Scholar]
- 17.Pardo JV, Siliciano JD, Craig SW. A vinculin-containing cortical lattice in skeletal muscle: transverse lattice elements (“costameres”) mark sites of attachment between myofibrils and sarcolemma. Proc Natl Acad Sci USA. 1983;80:1008–1012. doi: 10.1073/pnas.80.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danowski BA, Imanaka-Yoshida K, Sanger JM, Sanger JW. Costameres are sites of force transmission to the substratum in adult rat cardiomyocytes. J Cell Biol. 1992;118:1411–1420. doi: 10.1083/jcb.118.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bloch RJ, Gonzalez-Serratos H. Lateral force transmission across costameres in skeletal muscle. Exerc Sport Sci Rev. 2003;31:73–78. doi: 10.1097/00003677-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Reed P, Bloch RJ. Postnatal changes in sarcolemmal organization in the mdx mouse. Neuromuscul Disord. 2005;15:552–561. doi: 10.1016/j.nmd.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Law DJ, Allen DL, Tidball JG. Talin, vinculin and DRP (utrophin) concentrations are increased at mdx myotendinous junctions following onset of necrosis. J Cell Sci. 1994;107:1477–1483. doi: 10.1242/jcs.107.6.1477. [DOI] [PubMed] [Google Scholar]
- 22.Hodges BL, Hayashi YK, Nonaka I, Wang W, Arahata K, Kaufman SJ. Altered expression of the alpha7beta1 integrin in human and murine muscular dystrophies. J Cell Sci. 1997;110:2873–2881. doi: 10.1242/jcs.110.22.2873. [DOI] [PubMed] [Google Scholar]
- 23.Hanft LM, Rybakova IN, Patel JR, Rafael-Fortney JA, Ervasti JM. Cytoplasmic gamma-actin contributes to a compensatory remodeling response in dystrophin-deficient muscle. Proc Natl Acad Sci USA. 2006;103:5385–5390. doi: 10.1073/pnas.0600980103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woolstenhulme MT, Conlee RK, Drummond MJ, Stites AW, Parcell AC. Temporal response of desmin and dystrophin proteins to progressive resistance exercise in human skeletal muscle. J Appl Physiol. 2006;100:1876–1882. doi: 10.1152/japplphysiol.01592.2005. [DOI] [PubMed] [Google Scholar]
- 25.Stepto NK, Coffey VG, Carey AL, Ponnampalam AP, Canny BJ, Powell D, et al. Global gene expression in skeletal muscle from well-trained strength and endurance athletes. Med Sci Sports Exerc. 2009;41:546–565. doi: 10.1249/MSS.0b013e31818c6be9. [DOI] [PubMed] [Google Scholar]
- 26.Baltgalvis KA, Call JA, Nikas JB, Lowe DA. Effects of prednisolone on skeletal muscle contractility in mdx mice. Muscle Nerve. 2009;40:443–454. doi: 10.1002/mus.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorzek JF, Hendrickson KC, Forstner JP, Rixen JL, Moran AL, Lowe DA. Estradiol and tamoxifen reverse ovariectomy-induced physical inactivity in mice. Med Sci Sports Exerc. 2007;39:248–256. doi: 10.1249/01.mss.0000241649.15006.b8. [DOI] [PubMed] [Google Scholar]
- 28.Warren GL, Moran AL, Hogan HA, Lin AS, Guldberg RE, Lowe DA. Voluntary run training but not estradiol deficiency alters the tibial bone–soleus muscle functional relationship in mice. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2015–R2026. doi: 10.1152/ajpregu.00569.2007. [DOI] [PubMed] [Google Scholar]
- 29.Call JA, Baltgalvis KA, Lowe DA. Short term, progressive-resistance wheel running by C57Bl/10 mice is (not) sufficient to mimic a resistance exercise. The Physiologist. 2008;51 [Google Scholar]
- 30.Smith JP, Hicks PS, Ortiz LR, Martinez MJ, Mandler RN. Quantitative measurement of muscle strength in the mouse. J Neurosci Methods. 1995;62:15–19. doi: 10.1016/0165-0270(95)00049-6. [DOI] [PubMed] [Google Scholar]
- 31.Carlson CG, Makiejus RV. A noninvasive procedure to detect muscle weakness in the mdx mouse. Muscle Nerve. 1990;13:480–484. doi: 10.1002/mus.880130603. [DOI] [PubMed] [Google Scholar]
- 32.Hayes A, Lynch GS, Williams DA. The effects of endurance exercise on dystrophic mdx mice. I. Contractile and histochemical properties of intact muscles. Proc Biol Sci. 1993;253:19–25. doi: 10.1098/rspb.1993.0077. [DOI] [PubMed] [Google Scholar]
- 33.Gordon T, Stein RB. Comparison of force and stiffness in normal and dystrophic mouse muscles. Muscle Nerve. 1988;11:819–827. doi: 10.1002/mus.880110804. [DOI] [PubMed] [Google Scholar]
- 34.Stein RB, Gordon T. Nonlinear stiffness–force relationships in whole mammalian skeletal muscles. Can J Physiol Pharmacol. 1986;64:1236–1244. doi: 10.1139/y86-209. [DOI] [PubMed] [Google Scholar]
- 35.Rybakova IN, Patel JR, Davies KE, Yurchenco PD, Ervasti JM. Utrophin binds laterally along actin filaments and can couple costameric actin with sarcolemma when overexpressed in dystrophin-deficient muscle. Mol Biol Cell. 2002;13:1512–1521. doi: 10.1091/mbc.01-09-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dupont-Versteegden EE, McCarter RJ, Katz MS. Voluntary exercise decreases progression of muscular dystrophy in diaphragm of mdx mice. J Appl Physiol. 1994;77:1736–1741. doi: 10.1152/jappl.1994.77.4.1736. [DOI] [PubMed] [Google Scholar]
- 37.Call JA, Voelker KA, Wolff AV, McMillan RP, Evans NP, Hulver MW, et al. Endurance capacity in maturing mdx mice is markedly enhanced by combined voluntary wheel running and green tea extract. J Appl Physiol. 2008;105:923–932. doi: 10.1152/japplphysiol.00028.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaczor JJ, Hall JE, Payne E, Tarnopolsky MA. Low intensity training decreases markers of oxidative stress in skeletal muscle of mdx mice. Free Radic Biol Med. 2007;43:145–154. doi: 10.1016/j.freeradbiomed.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Deconinck N, Rafael JA, Beckers-Bleukx G, Kahn D, Deconinck AE, Davies KE, et al. Consequences of the combined deficiency in dystrophin and utrophin on the mechanical properties and myosin composition of some limb and respiratory muscles of the mouse. Neuromuscul Disord. 1998;8:362–370. doi: 10.1016/s0960-8966(98)00048-0. [DOI] [PubMed] [Google Scholar]
- 40.McDonald CM, Henricson EK, Han JJ, Abresch RT, Nicorici A, Elfring GL, et al. The 6-minute walk test as a new outcome measure in Duchenne muscular dystrophy. Muscle Nerve. 2010;41:500–510. doi: 10.1002/mus.21544. [DOI] [PubMed] [Google Scholar]
- 41.Street SF, Ramsey RW. Sarcolemma: transmitter of active tension in frog skeletal muscle. Science. 1965;149:1379–1380. doi: 10.1126/science.149.3690.1379. [DOI] [PubMed] [Google Scholar]
- 42.Street SF. Lateral transmission of tension in frog myofibers: a myofibrillar network and transverse cytoskeletal connections are possible transmitters. J Cell Physiol. 1983;114:346–364. doi: 10.1002/jcp.1041140314. [DOI] [PubMed] [Google Scholar]
- 43.Grounds MD, Sorokin L, White J. Strength at the extracellular matrix–muscle interface. Scand J Med Sci Sports. 2005;15:381–391. doi: 10.1111/j.1600-0838.2005.00467.x. [DOI] [PubMed] [Google Scholar]
- 44.Lovering RM, De Deyne PG. Contractile function, sarcolemma integrity, and the loss of dystrophin after skeletal muscle eccentric contraction-induced injury. Am J Physiol Cell Physiol. 2004;286:C230–C238. doi: 10.1152/ajpcell.00199.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lynch GS. Role of contraction-induced injury in the mechanisms of muscle damage in muscular dystrophy. Clin Exp Pharmacol Physiol. 2004;31:557–561. doi: 10.1111/j.1440-1681.2004.04026.x. [DOI] [PubMed] [Google Scholar]
- 46.Lynch GS, Rafael JA, Chamberlain JS, Faulkner JA. Contraction-induced injury to single permeabilized muscle fibers from mdx, transgenic mdx, control mice. Am J Physiol Cell Physiol. 2000;279:C1290–C1294. doi: 10.1152/ajpcell.2000.279.4.C1290. [DOI] [PubMed] [Google Scholar]
- 47.Lehti TM, Kalliokoski R, Komulainen J. Repeated bout effect on the cytoskeletal proteins titin, desmin, and dystrophin in rat skeletal muscle. J Muscle Res Cell Motil. 2007;28:39–47. doi: 10.1007/s10974-007-9102-0. [DOI] [PubMed] [Google Scholar]
- 48.Parcell AC, Woolstenhulme MT, Sawyer RD. Structural protein alterations to resistance and endurance cycling exercise training. J Strength Condit Res. 2009;23:359–365. doi: 10.1519/JSC.0b013e318198fd62. [DOI] [PubMed] [Google Scholar]
- 49.Gregorevic P, Allen JM, Minami E, Blankinship MJ, Haraguchi M, Meuse L, et al. rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat Med. 2006;12:787–789. doi: 10.1038/nm1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sonnemann KJ, Heun-Johnson H, Turner AJ, Baltgalvis KA, Lowe DA, Ervasti JM. Functional substitution by TAT-utrophin in dystrophin-deficient mice. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000083. e1000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burkin DJ, Wallce GQ, Nicol KJ, Kaufman DJ, Kaufman SJ. Enhanced expression of alpha 7 beta 1 integrin reduces muscular dystrophy and restores viability in dystrophic mice. J Cell Biol. 2001;152:1207–1218. doi: 10.1083/jcb.152.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]