Summary
The maxillary sinus elevation is a standard and predictable procedure allowing the realization of dental implant rehabilitation in patients with severe bone atrophy in the lateral-posterior areas of the maxilla. Despite the presence of validated surgical methods and the broad availability of biomaterials, the procedures aimed at increasing the bone volume by lateral antrostomy still entail complications with different degrees of relevance. The prosthetic and surgical outcome is based on a successful coping with these aspects. The perforation of the Schneiderian membrane is one of the most frequent events for which a variety of protocols and approaches have been suggested by different authors. In this work is presented a case study in which a technique to repair the sinus mucosa laceration occurring during a maxillary sinus elevation procedure has been successfully adopted.
Keywords: Schneiderian membrane, maxillary sinus augmentation, sinus lift complications, underwood septa
Introduction
Nowadays, the rehabilitation by prosthetic implants, even in edentulous areas of the maxillae affected by severe bone atrophies, is an inescapable need leading to the development of standardized, predictable and safe regenerative techniques (1,2). The maxillary sinus elevation is a surgical procedure that increases vertically the available bone volume on the lateral-posterior areas of the maxilla giving the possibility to place osseointegrated implants (3,4). Thanks especially to the ample indications from the literature (1–4), this procedure represents an excellent potential for the resolution of the bone atrophies associated to edentulism. Nevertheless, such kind of intervention is still characterized by complications, often predictable and yet unavoidable, posing limitations to its successful application (5–8). The most common adverse events reported are: i) haemorrhage, mainly due to lesions of the intramural artery, an anastomoses between the infraorbital artery and the posterior superior alveolar artery, frequently localized on the site in which the surgeon makes the bone window to reach the antral cavity; ii) laceration of the Schneiderian membrane, usually occurring with a range of incidence comprised between 7% and 35% of cases (9–11). The latter may occur during different phases of the procedure: during the preparation of the antrostomy, while removing or turning over the bone window, during the membrane raising or upon grafting. Moreover, there are some anatomical risk factors, like: Underwood’s septa, which are bony walls partitioning the sinus, usually with a vertical progress; the angle between the buccal and palatal walls of the antral cavity, as analyzed on perpendicular tomographic sections, especially when below 30°; irregularities of the sinus floor due to the protrusion of the root profiles; previous sinus surgery; a decreased height of the residual alveolar ridge. Since discontinuities in the Schneiderian mucosa impair the functional homeostasis of the antral cavity and negatively affect the surgical outcome by bacterial contamination of the graft and dispersion of the particulate, several authors have studied and suggested specific repair techniques for each type of perforation (12–16).
Schneiderian membrane perforation: predisposing factors, classification and management
The maxillary sinus elevation is a standard and predictable surgical procedure to rehabilitate severe vertical bone atrophies in the lateral-posterior areas of the maxilla by placement of osseointegrated implants (6,8,17–22). However, it is known that complications like haemorrhage and perforation of the Schneiderian membrane may affect negatively the outcome of such procedure. In most reported cases, intra-operative bleeding is due to the lesion of the anastomoses between the infraorbital artery and the posterior superior alveolar arteries at an average distance of 19 mm from the apical ridge, with a tendency to superficialize in conditions of marked bone resorption. Such localization often coincides with the area where the antrostomy of access to the sinus cavity is carried out (9–11,14).
However, the most frequent unfavourable event associated to such intervention is the laceration of the sinus mucosa. Such event is often predictable, but not avoidable, since it is strictly associated to anatomo-pathological predispositions (23–26). In the literature, the following predisposing factors have been identified: previous phlogistic processes, irregularities of the sinus floor, e.g. due to root protrusions, thickness of the membrane below 1,5 mm, limited expansion of the anterior recess, angle between buccal wall and socket below 30°, former surgical treatments, reduced height of the alveolar ridge (27–34). In particular, the Underwood’s septa, present on average in 31% of the maxillary sinuses with a height of about 8 mm (range 3,5–2,2 mm), can involve all areas of the sinus. Usually they are partial, they run vertically in buccal-palatal direction and are higher at the level of the medial wall; more rarely they are multiple within the same antrum (8,24–26,29,35–37).
The laceration of the sinus mucosa affects negatively the surgical outcome by increasing the risk of iatrogenic sinusitis, impairment of functional homeostasis and dispersion of the graft material in the antral cavity as well its bacterial colonization (38–40). Among several possibilities of repair of the perforation, as reported in the international literature (8,19,20,41–45), we refer here to the study of Fugazzotto and Vlassis (2003), which classifies the lesions of the sinus mucosa in relation to size and position and associates to each class a specific therapeutic indication (46). Class 1 identifies the perforations below 5 mm in size that extend to the upper border of the antrostomy, for which it is simply required a further detachment of the membrane to allow the seal of the lesioned flaps (46,47); class 2A describes lacerations located at the borders of the osteotomy, delimited from at least 4–5 mm of intact tissue, with the suggestion to enlarge the limits of the bone window and to apply a resorbable membrane in case of failed sealing of the margins of the perforation (46–49); classes 2B and 3 correspond respectively to lacerations that develop laterally from the antrostomy, delimited by less than 4–5 mm of intact tissue, and to central lesions, often preexisting and determined by former dental avulsion or oroantral fistulae. These latter can be managed with the same treatment, known as modified Pouch Technique (46–49).
The original technique, known as “Loma Linda Pouch”, consists in covering the whole sinus with a collagen membrane simulating the natural membrane, and the graft material is completely covered in its centre by folding the membrane on the lateral wall. However, in this manner, an external barrier is created that totally isolates the biomaterial from the blood supply coming from the walls of the sinus, thus representing an obstacle to the maturation of the graft and the recovery process (46–49). In the modified method, the cover of the sinus walls is still carried out with the support of a resorbable membrane located only on the surface of the Schneiderian membrane, leaving the bone walls free so that the blood supply from the bone can favour the vascularization and thereby the integration of the graft into this virtual space. Moreover, in such technique the resorbable membrane is fastened at the superior border of the antrostomy through titanium or surgical steel pins before being reinserted in the sinus cavity; a second membrane is positioned on the antrostomy externally, to further protect the biomaterial (46). It has been demonstrated that the protection of the osteotomic window increases implant survival if some prerequisites are respected: membrane stability, sterility and optimal cohesion, compactness and handiness of the graft material (1,6,50–62).
Case report
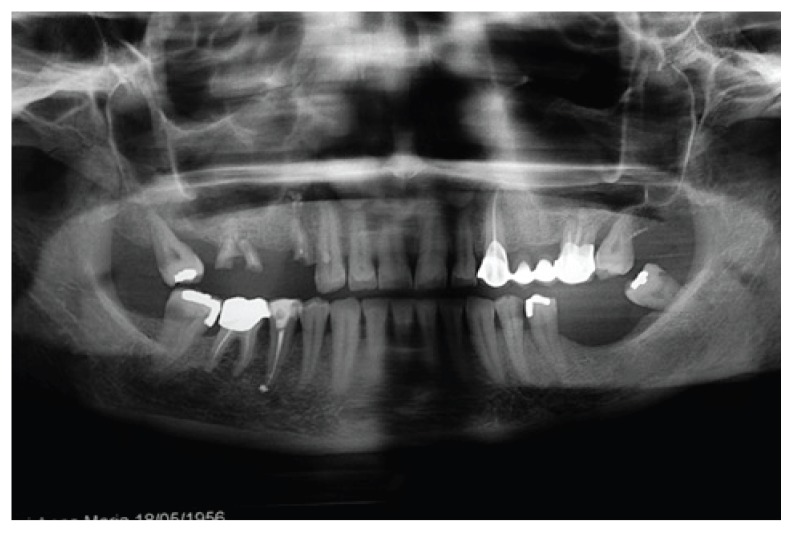
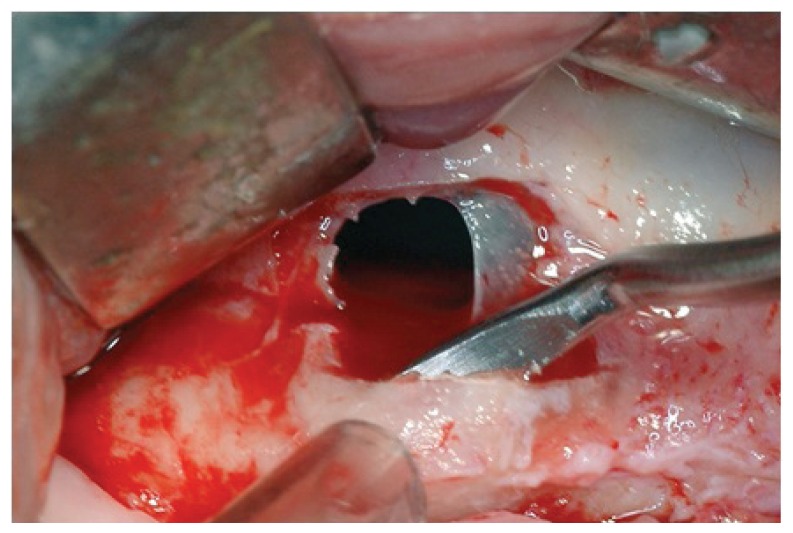
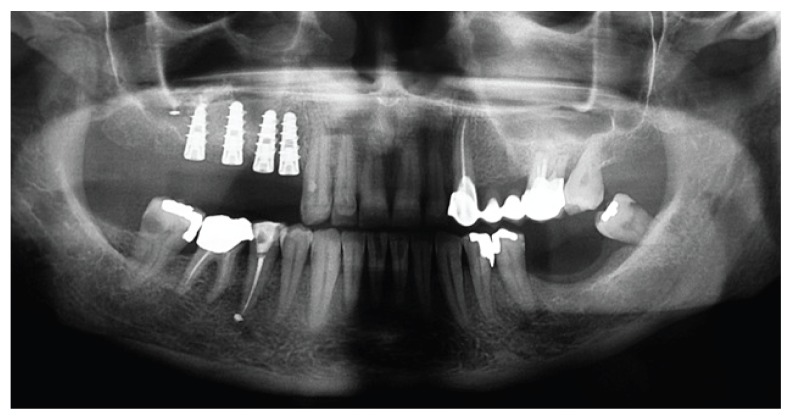
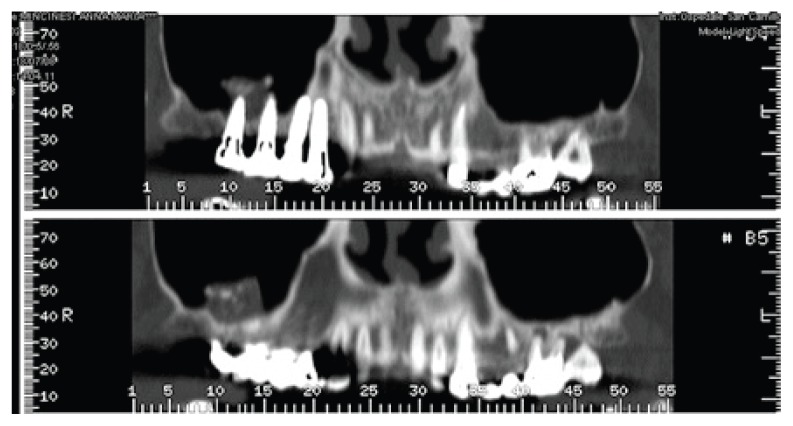
The reported clinical case has been managed in collaboration with the Departmental Unit of Odontostomatology and Maxillo-Facial Surgery of the San Camillo Forlanini Hospital (Rome, Italy). The patient (female, 45 years old) asked for a functional and aesthetical rehabilitation of the lateral posterior area of the right emi-maxilla. Personal anamnesis excluded the presence of pathologies contra-indicating the implant rehabilitation as well as attitudes such as smoking. The physical exam and the radiographic evaluation (orthopantomography) highlighted the presence of root residuals 1.4, 1.6 and of the element 1.8 compromised for periodontal evaluation (Fig. 1). In order to proceed in the best conditions such elements have been extracted. After four weeks the mucosae upon the post-extractive sockets were perfectly recovered. The anatomical state, according to Chiapasco’s classification, was attributed to class A in the area of element 1.5 and class C in the molar region (5). The individual prosthetic plan pointed towards the choice of an implant supported cemented fixed prosthesis, following optimization of the sites through surgical procedure of lateral maxillary right sinus elevation. The intervention was carried out in day hospital. Four block anaesthesias were executed at the level of the superior posterior alveolar nerve, the major palatine nerve, the infraorbital nerve and the nasopalatine nerve, and one anaesthesia by infiltration of the fornix and the palatine mucosa (articaine 4% and vasoconstrictor 1:100.000). Thereafter, a trapezoidal mucoperiosteal flap with linear main incision between distal margin 1.3 and area 1.7 has been set up, together with divergent release incisions extended 5 mm beyond the mucogingival line. The flap has been opportunistically detached and folded down to highlight the maxillary bone surface. The antrostomy, of rectangular shape with round corners and approximate size of 20 × 15 mm, has been carried out with piezoelectric devices. The detachment of the membrane was initiated with piezoelectric devices and terminated with manual devices. Despite the absence of evident anatomical abnormalities and the accuracy of the surgical procedure, a perforation of the Schneiderian membrane of about 8 × 6 mm occurred (Fig. 2).
Figure 1.
Pre-operative orthopantomogram.
Figure 2.
Maxillary sinus membrane perforation
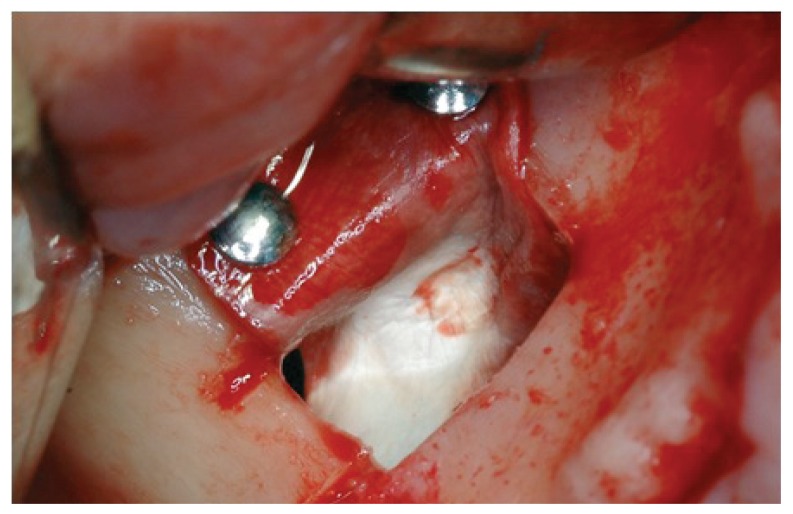
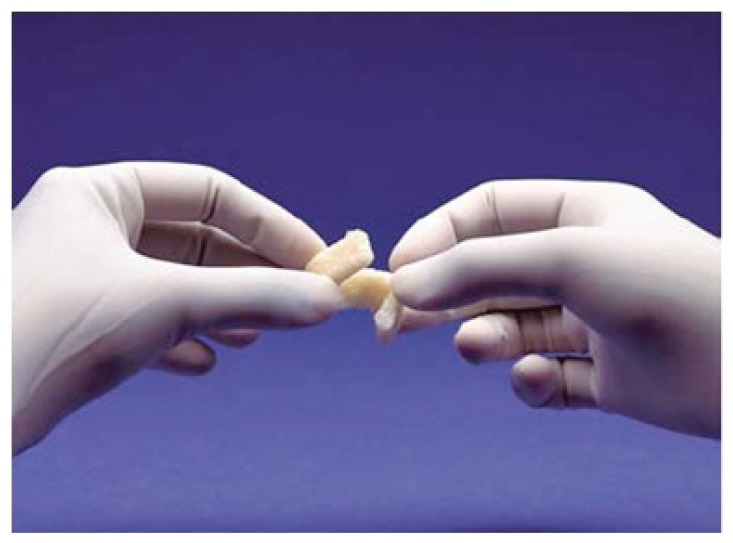
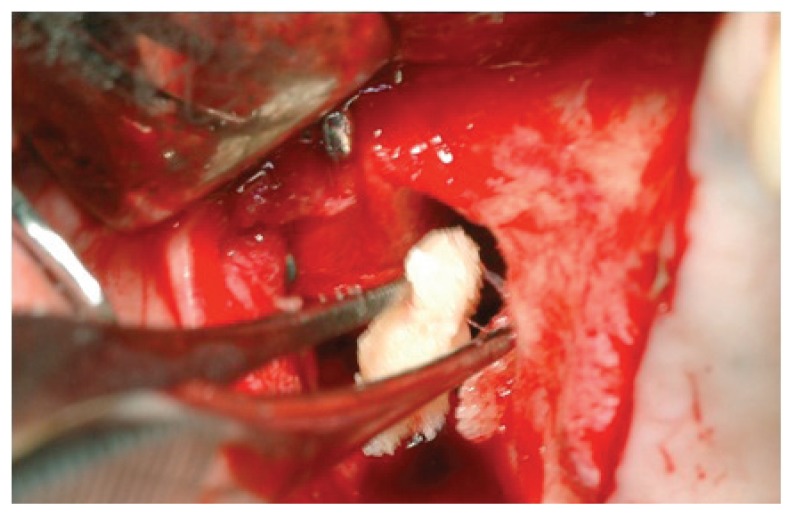
Following the suggestions of Fugazzotto and Vlassis, we chose to continue with the procedure and decided to repair the lesion through the modified Pouch Technique (46). A resorbable membrane of freeze-dried bovine pericardium (Tutopatch, Tutogen Gmbh) was modelled and blocked with titanium pins above the superior border of the antrostomy and was then folded in the inner part of the sinus to for the graft containment (Fig. 3). According to the suggestions of several authors (11,50,51,53,54,60,62,63) we used as filler a compact and consistent material in order to avoid the dispersion of particles in the sinus and thereby the possibility of phlogistic processes due to bacterial colonization of the graft. The material is a human-derived bone paste in blocks, called Bioset, available on request in Italy at Rizzoli Orthopaedic Institute (I.O.R.), that is the italian national public bank of the musculoskeletal tissue; this product contains demineralized bone matrix (DBM) and bone corticospongious particulate carried in a thermoplastic gel of suine collagen (Fig. 4).
Figure 3.
A resorbable membrane of freeze-dried bovine pericardium positioned according to modified pouch technique.
Figure 4.
A human-derived bone paste in blocks, called Bioset, containing demineralized bone matrix (DBM).
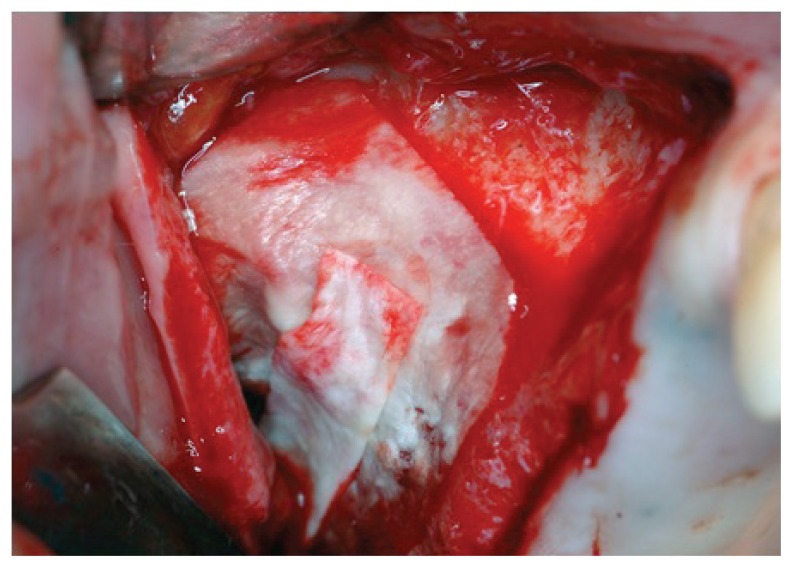
The particulate component acts as a natural osteoconductive matrix at low resorbing activity, the DBM allows the release of morphogenetic proteins, preserved by a peculiar sterilization process undergone by the material (BioCleanse®), while the carrier confers consistency and easy manipulation: it is preserved at −20°C for 6 months or at −80°C for 5 years and it becomes plastic, malleable and adhesive when warmed in hot water in its sterile package up to a temperature between 43°C and 49 °C, while becoming stable in size and consistency at body temperature (Fig. 5). We have compacted only 2 cc of bone paste internally (Fig. 6), since overfilling has been shown to be responsible of the necrosis of the sinus membrane with dispersion of material and chronic sinusitis; thereafter, a second resorbable membrane above the antrostomy has been applied (Fig. 7). Finally, mattress suture horizontal with non resorbable monophilament was carried out.
Figure 5.
Plastic consistency and easy manipulation of Bioset.
Figure 6.
Maxillary sinus filled with Bioset.
Figure 7.
A second resorbable membrane applied above the antrostomy.
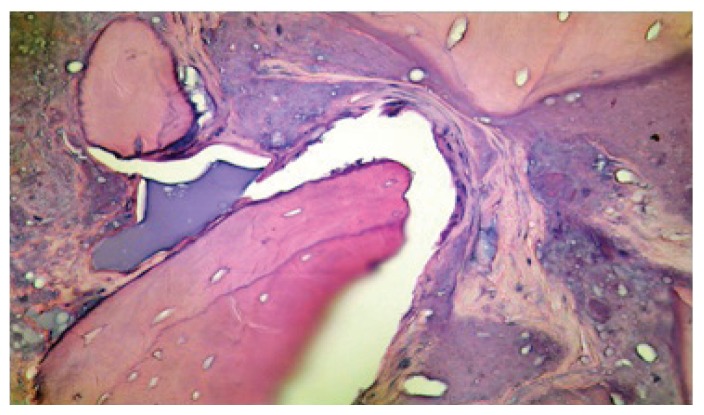
The patient underwent antibiotic, analgesic and anti-edemic therapy for seven days. After six months the case has been evaluated clinically and radiographically. We could ascertain complete recovery of the tissues, integration of the graft and absence of phlogistic complications. We therefore proceeded with the placement of three implant fixtures of conic shape, diameter 4,5 × 12 mm, and one of 4,5 × 10 mm sand blasted and acid etched (TiRADIX s.r.l.). During the preparation of the sites of implant a bone biopsy was performed with a trephine bur (internal diameter of 2 mm). The sample was fixed by buffered formalin, stained with hematoxilin-eosin and observed at the optic microscope (40x). The sections showed the presence of lamellar bone tissue with osteocyte lacunae (Fig. 8). Some lacunae appeared without cells, separated by fibro-vascular tissue containing amorphous material. We noted lamellar bone in development, deposited in proximity of young trabeculae. The active process of bone rearrangement was highlighted by the presence of osteoclasts and osteoblasts (courtesy of Prof. G. Soda, Department of Experimental Medicine, Sapienza University of Rome).
Figure 8.
Histology of bone neoformation at six months after sinus lift (40x, H&E).
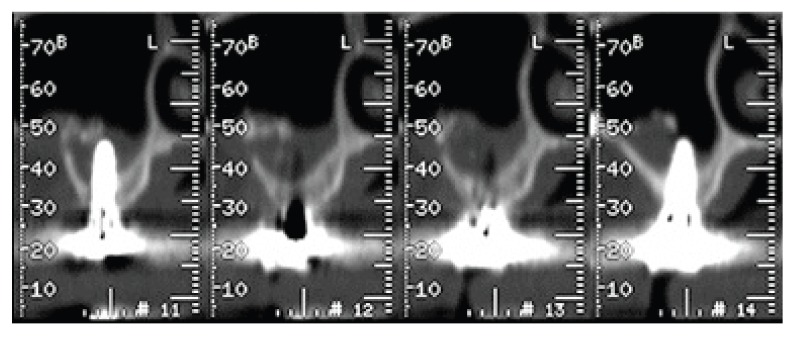
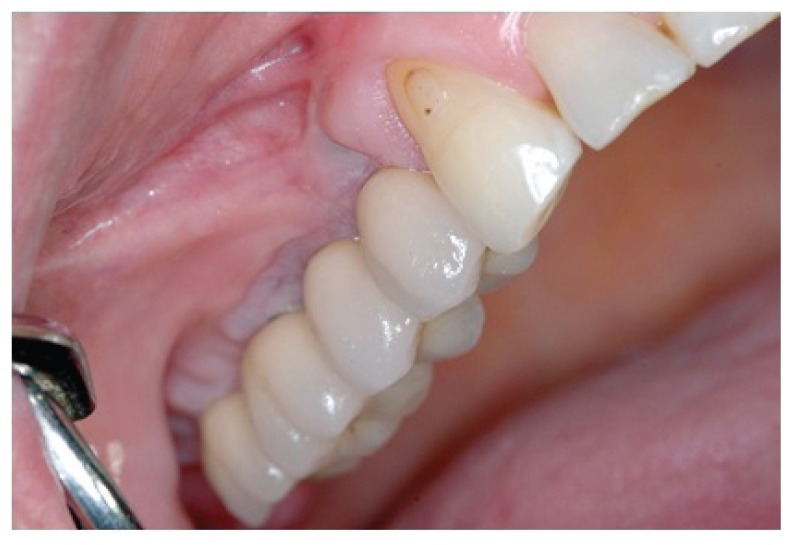
The orthopanoramic radiography and TC exam with the specific software dentascan (Figs. 9, 10, 11), performed one year after prosthetic rehabilitation, show the reorganization of the hard tissues in the antrum and around the fixtures. The case was subsequently rehabilitated with a cemented metalloceramic fixed prosthesis. The clinical result appeared aesthetically and functionally satisfactory also two years after dental implant surgery (Fig. 12).
Figure 9.
Ortopantomogram at six months after implants placement
Figure 10.
Paraxial views of TC dentascan at one year after prosthetic rehabilitation.
Figure 11.
Panoramic views of TC dentascan at one year after prosthetic rehabilitation.
Figure 12.
Clinical result at two years after implant surgery.
Conclusions
The maxillary sinus elevation is a surgical standard and highly predictable procedure allowing the positioning of osseointegrated implants also in case of serious bone atrophy of the maxilla (8,64).
However, this procedure is not devoid of complications. The most frequent is the perforation of the Schneiderian membrane, occurring in 7–35 % of cases. The factors affecting such incidence are often anatomical (8–11,24–26,28,36,37). Despite accurate pre-surgical radiographic investigations, in some cases the laceration is unavoidable even when the surgical manoeuvres are performed at best (17,21,22,27,30,32–34).
In the past some authors suggested stopping the procedure in case of perforation and postponing it after recovery (44). Despite Hernàndez-Alfaro demonstration of an inverse relationship between the size of the laceration and the implant survival, it is currently not suggested to interrupt the surgical procedure (29).
In the reported clinical case the repair of the Schneiderian membrane allowed a radiological, clinical and histological success. Therefore, the evolution of biomaterials currently available and the standardization of the techniques allow a higher predictability of success and extend the applicative possibilities of such procedure.
References
- 1.Froum SJ, Wallace SS, Elian N, Cho SC, Tarnow DP. Comparison of mineralized cancellous bone allograft (Puros) and anorganic bovine bone matrix (Bio-Oss) for sinus augmentation: histomorphometry at 26 to 32 weeks after grafting. Int J Periodontics Restorative Dent. 2006 Dec;26(6):543–51. [PubMed] [Google Scholar]
- 2.Shulman LB, Jensen OT. Sinus Graft Consensus Conference. Introduction. Int J Oral Maxillofac Implants. 1998;13(suppl):5, 6. [PubMed] [Google Scholar]
- 3.Esposito M, Grusovin MG, Rees J, Karasoulos D, Felice P, Alissa R, Worthington HV, Coulthard P. Effectiveness of sinus lift procedures for dental implant rehabilitation: a Cochrane systematic review. Eur J Oral Implantol. 2010;3(1):7–26. [PubMed] [Google Scholar]
- 4.Pignataro M, Mantovani M, Torretta S, Felisati G, Sambataro G. ENT assessment in the integrated management of candidate for (maxillary) sinus lift. Acta Otorhinolaryngol Ital. 2008;28:110–119. [PMC free article] [PubMed] [Google Scholar]
- 5.Chiapasco M, Zaniboni M. Methods to treat the edentulous posterior maxilla: implants with sinus grafting. J Oral Maxillofac Surg. 2009;67(4):867–71. doi: 10.1016/j.joms.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 6.Del Fabbro M, Bortolin M, Taschieri S, Rosano G, Testori T. Implant survival in maxillary sinus augmentation. An updated systematic review. J Osteol Biomat. 2010;1(2):69–79. [Google Scholar]
- 7.Simion M. Bone regeneration and biomaterials: state of the art and future perpectives. Quality in Implantology; SIO International Congress; Rome. 5–6 Febbraio 2010. [Google Scholar]
- 8.Testori T, Weinstein R, Wallace S. Edizioni ACME, maggio. Viterbo: 2006. La Chirurgia del Seno Mascellare e le alternative terapeutiche. [Google Scholar]
- 9.Elian N, Wallace S, Cho SC, Jalbout ZN, Froum S. Distribution of the Maxillary Artery as It Relates to Sinus Floor Augmentation. Int J Oral Maxillofac Implants. 2005;20:784–787. [PubMed] [Google Scholar]
- 10.Flanagan D. Arterial supply of the maxillary sinus and potential for bleeding complication during lateral approach sinus elevation. Implant Dent. 2005 Dec;14(4):336–8. doi: 10.1097/01.id.0000188437.66363.7c. [DOI] [PubMed] [Google Scholar]
- 11.Zijderveld SA, van den Bergh JP, Schulten EA, ten Bruggenkate CM. Anatomical and surgical findings and complications in 100 consecutive maxillary sinus floor elevation procedures. J Oral Maxillofac Surg. 2008 Jul;66(7):1426–38. doi: 10.1016/j.joms.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 12.Rosano G, Taschieri S, Gaudy JF, Lesmes D, Del Fabbro M. Maxillary sinus septa: a cadaveric study. J Oral Maxillofac Surg. 2010 Jun;68(6):1360–4. doi: 10.1016/j.joms.2009.07.069. [DOI] [PubMed] [Google Scholar]
- 13.Gosau M, Rink D, Driemel O, Draenert FG. Maxillary sinus anatomy: a cadaveric study with clinical implications. Anat Rec (Hoboken) 2009;292(3):352–4. doi: 10.1002/ar.20859. [DOI] [PubMed] [Google Scholar]
- 14.Solar P, Geyerhofer U, Traxler H, Windisch A, Ulm C, Watzek G. Blood supply to the maxillary sinus relevant to sinus floor elevation procedures. Clin Oral Implants Res. 1999;10:34–44. doi: 10.1034/j.1600-0501.1999.100105.x. [DOI] [PubMed] [Google Scholar]
- 15.Ulm CW, Solar P, Krennmair G, Matejka M, Watzek G. Incidence and suggested surgical management of septa in sinus-lift procedures. Int J Oral Maxillofac Implants. 1995;10(4):462–5. [PubMed] [Google Scholar]
- 16.Van den Bergh JP, Bruggenkate CM, Disch FJ, Tuinzing DB. Anatomical aspects of sinus floor elevations. Clin Oral Implants Res. 2000;11(3):256–65. doi: 10.1034/j.1600-0501.2000.011003256.x. [DOI] [PubMed] [Google Scholar]
- 17.Barone A, Santini S, Marconcini S, Giacomelli L, Gherlone E, Covani U. Osteotomy and membrane elevation during the maxillary sinus augmentation procedure. A comparative study: Piezoelectric device vs conventional rotative instrumentations. Clin Oral Implants Res. 2008;19:511–515. doi: 10.1111/j.1600-0501.2007.01498.x. [DOI] [PubMed] [Google Scholar]
- 18.Mandelaris GA, Rosenfeld AL. A Novel Approach to the Antral Sinus Bone Graft Technique: The Use of a Prototype Cutting Guide for Precise Outlining of the Lateral Wall. A Case Report. Int J Periodontics Restorative Dent. 2008 Dec;28(6):569–75. [PubMed] [Google Scholar]
- 19.Testori T, Del Fabbro M, Taschieri S, Francetti L, Weinstein RL. Il rialzo del seno mascellare. Italian Oral Surgery. 2005:139–48. [Google Scholar]
- 20.Tatum OH, Jr, Lebowitz MS, Tatum CA, Borgner RA. Sinus augmentation. Rationale, development, long-term results. N Y State Dent J. 1993;59(5):43–8. Review. [PubMed] [Google Scholar]
- 21.Vercellotti T, De Paoli S, Nevins M. The piezoelectric bony window osteotomy and sinus membrane elevation: Introduction of a new technique for simplification of the sinus augmentation procedure. Int J Periodontics Restorative Dent. 2001;21:561–567. [PubMed] [Google Scholar]
- 22.Wallace SS, Mazor Z, Froum SJ, Cho SC, Tarnow DP. Schneiderian membrane perforation rate during sinus elevation using piezosurgery: clinical results of 100 consecutive cases. Int J Periodontics Restorative Dent. 2007;27(5):413–9. [PubMed] [Google Scholar]
- 23.Krennmair G, Ulm CW, Lugmayr H, Solar P. The incidence, location, and height of maxillary sinus septa in the edentulous and dentate maxilla. J Oral Maxillofac Surg. 1999;57:667–71. doi: 10.1016/s0278-2391(99)90427-5. discussion 671-2. [DOI] [PubMed] [Google Scholar]
- 24.Toscano NJ, Holtzclaw D, Rosen PS. The effect of piezoelectric use on open sinus lift perforation: a retrospective evaluation of 56 consecutively treated cases from private practices. J Periodontol. 2010;81(1):167–71. doi: 10.1902/jop.2009.090190. [DOI] [PubMed] [Google Scholar]
- 25.Ulm CW, Solar P, Gsellmann B, Matejka M, Watzek G. The edentulous maxillary alveolar process in the region of the maxillary sinus: a study of physical dimension. Int J Oral Maxillofac Implants. 1995;24(4):279–82. doi: 10.1016/s0901-5027(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 26.Underwood AS. An Inquiry into the Anatomy and Pathology of the Maxillary Sinus. J Anat Physiol. 1910;44:354–69. [PMC free article] [PubMed] [Google Scholar]
- 27.Ardekian L, Oved-Peleg E, Mactei EE, Peled M. The clinical significance of sinus membrane perforation during augmentation of the maxillary sinus. J Oral Maxillofac Surg. 2006;64(2):277–82. doi: 10.1016/j.joms.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 28.Cho SC, Wallace SS, Froum SJ. Influence of anatomy on Schneiderian membrane perforations during sinus floor elevation surgery: Three dimensional analysis. Pract Periodont Aesthet Dent. 2001;13(2):160–3. [PubMed] [Google Scholar]
- 29.Hernández-Alfaro F, Torradeflot MM, Marti C. Prevalence and management of Schneiderian membrane perforations during sinus-lift procedures. Clin Oral Implants Res. 2008;19(1):91–8. doi: 10.1111/j.1600-0501.2007.01372.x. [DOI] [PubMed] [Google Scholar]
- 30.Suguimoto RM, Trindade IK, Carvalho RM. The use of negative pressure for the sinus lift procedure: a technical note. Int J Oral Maxillofac Implants. 2006;21(3):455–8. [PubMed] [Google Scholar]
- 31.Tatum OH. Maxillary and sinus implant reconstruction. Dent Clin North Am. 1986;40:207–229. [PubMed] [Google Scholar]
- 32.Ucer C. Nasal suction technique for maxillary sinus floor elevation: a report of 24 consecutive patients. Int J Oral Maxillofac Implants. 2009;24(6):1138–43. [PubMed] [Google Scholar]
- 33.Ucer TC. Use of negative air pressure by nasal suction during maxillary sinus floor lift: audit of 13 consecutive sinus grafts. Br J Oral Maxillofac Surg. 2009;47(2):151–2. doi: 10.1016/j.bjoms.2008.07.193. [DOI] [PubMed] [Google Scholar]
- 34.Vitkov L, Gellrich NC, Hannig M. Sinus floor elevation via hydraulic detachment and elevation of the Schneiderian membrane. Clin Oral Implants Res. 2005;16:615–21. doi: 10.1111/j.1600-0501.2005.01161.x. [DOI] [PubMed] [Google Scholar]
- 35.Betts NJ, Miloro M. Modification of the sinus lift procedure for septa in the maxillary antrum. J Oral Maxillofac Surg. 1994;52:332–333. doi: 10.1016/0278-2391(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 36.Kim MJ, Jung UW, Kim CS, Kim KD, Choi SH, Kim CK, Cho KS. Maxillary sinus septa: prevalence, height, location, and morphology. A reformatted computed tomography scan analysis. J Periodontol. 2006;77(5):903–8. doi: 10.1902/jop.2006.050247. [DOI] [PubMed] [Google Scholar]
- 37.Urist MR. Bone formation by autoinduction. Science. 1965;150:893–99. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 38.Chandra RK, Pearlman A, Conley DB, Kern RC, Chang D. Significance of osteomeatal complex obstruction. J Otolaryngol Head Neck Surg. 2010;39(2):171–4. [PubMed] [Google Scholar]
- 39.Halma AR, Decreton S, Bijloos JM. Density of epithelial cells in the normal human nose and the paranasal sinus mucosa: A scanning electron microscopic study. Rhinology. 1990;28:25–32. [PubMed] [Google Scholar]
- 40.Jensen OT, Shulman LB, Block MS, Iacono VJ. Report of the Sinus Consensus Conference of 1996. Int J Oral Maxillofac Implants. 1998;13(suppl):11–45. [PubMed] [Google Scholar]
- 41.Pikos MA. Maxillary sinus membrane repair: Update on technique for large and complete perforations. Implant Dent. 2008;17:24–31. doi: 10.1097/ID.0b013e318166d934. [DOI] [PubMed] [Google Scholar]
- 42.Proussaefs P, Lozada J, Kim J. Effects of sealing the perforated sinus membrane with a resorbable collagen membrane: a pilot study in humans. J Oral Implantol. 2003;29(5):235–41. doi: 10.1563/1548-1336(2003)029<0235:EOSTPS>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 43.Vina-Almunia J, Penarrocha-Diago M, Penarrocha-Diago M. Influence of perforation of the sinus membrane on the survival rate of implants placed after direct sinus lift. Literature update. Med Oral Patol Oral Cir Bucal. 2009;14(3) [PubMed] [Google Scholar]
- 44.Biglioli F, Pedrazzoli M, Colletti G. Repair of a perforated sinus membrane with a palatal fibromucosal graft: a case report. Minerva Stomatol. 2010 May;59(5):299–302. English, Italian. [PubMed] [Google Scholar]
- 45.Testori T, Stephen S, Wallace N, Del Fabbro M, Taschieri S, Trisi P, Capelli M, Weinstein RL. Riparazione di grandi perforazioni della membrana sinusale mediante membrane riassorbibili: tecniche chirurgiche con evidenza istologica e radiografica di successo. Parodontologia & Odontoiatria Ricostruttiva. 2008;28:1, 9–17. [Google Scholar]
- 46.Fugazzotto PA, Vlassis J. A simplified classification and repair system for sinus membrane perforations. J Periodontol. 2003;74(10):1534–41. doi: 10.1902/jop.2003.74.10.1534. [DOI] [PubMed] [Google Scholar]
- 47.Parenti A, Capelli M, Fumagalli L, Zuffetti F, Galli F, Taschieri S, Del Fabbro M, Castellaneta R, Testori T. Prevenzione e gestione delle complicanze. Perforazioni della membrana sinusale. Italian Oral Surgery. 2007;1:29–32. [Google Scholar]
- 48.Proussaefs P, Lozada J. The “Loma Linde Pouch”. A technique for repairing the perforated sinus membrane. Int J Periodontic Restorative Dent. 2003;23:593–597. [PubMed] [Google Scholar]
- 49.Testori T, Trisi P, Del Fabbro M, Francetti L, Taschieri S, Parenti A, Bianchi F, Zuffetti F. Gestione intraoperatoria di ampie perforazioni della membrana del seno mascellare. Italian Oral Surgery. 2007;1(6):21–28. [Google Scholar]
- 50.Arora NS, Ramanayake T, Ren YF, Romanos GE. Platelet-rich plasma in sinus augmentation procedures: a systematic literature review: Part II. Implant Dent. 2010;19(2):145–57. doi: 10.1097/ID.0b013e3181cd706d. [DOI] [PubMed] [Google Scholar]
- 51.Boyne PJ, Lilly LC, Marx RE, Moy PK, Nevins M, Spagnoli DB, Triplett RG. De novo bone induction by recombinant human bone morphogenetic protein-2 (rhBMP-2) in maxillary sinus floor augmentation. J Oral Maxillofac Surg. 2005;63(12):1693–707. doi: 10.1016/j.joms.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 52.Browaeys H, Bouvry P, De Bruyn H. A literature review on biomaterials in sinus augmentation procedures. Clin Implant Dent Relat Res. 2007;9(3):166–77. doi: 10.1111/j.1708-8208.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- 53.Tarnow DP, Wallace SS, Testori T, Froum SJ, Motroni A, Prasad HS. Maxillary sinus augmentation using recombinant bone morphogenetic protein-2/acellular collagen sponge in combination with a mineralized bone replacement graft: a report of three cases. Int J Periodontics Restorative Dent. 2010 Apr;30(2):139–49. [PubMed] [Google Scholar]
- 54.Hu Z, Peel SA, Ho SK, Sándor GK, Su Y, Clokie CM. The expression of bone matrix proteins induced by different bioimplants in a rabbit sinus lift model. J Biomed Mater Res A. 2010 Dec 15;95(4):1048–54. doi: 10.1002/jbm.a.32911. Epub 2010 Sep 28. [DOI] [PubMed] [Google Scholar]
- 55.Khoury F. Augmentation of the sinus floor with mandibular bone block and simultaneous implantation: a 6-year clinical investigation. Int J Oral Maxillofac Implants. 1999;14(4):557–64. [PubMed] [Google Scholar]
- 56.Kim SW, Lee IK, Yun KI, Kim CH, Park JU. Adult stem cells derived from human maxillary sinus membrane and their osteogenic differentiation. Int J Oral Maxillofac Implants. 2009;24(6):991–8. [PubMed] [Google Scholar]
- 57.Moore ST, Katz JM, Zhukauskas RM, Hernandez RM, Lewis CS, Supronowicz PR, Gill E, Grover SM, Long NS, Cobb RR. Osteoconductivity and osteoinductivity of Puros(R) DBM putty. J Biomater Appl. 2011 Aug;26(2):151–71. doi: 10.1177/0885328210366061. Epub 2010 Jun 21. [DOI] [PubMed] [Google Scholar]
- 58.Nkenke E, Stelzle F. Clinical outcomes of sinus floor augmentation for implant placement using autogenous bone or bone substitutes: a systematic review. Clin Oral Implants Res. 2009;20(Suppl 4):124–33. doi: 10.1111/j.1600-0501.2009.01776.x. [DOI] [PubMed] [Google Scholar]
- 59.Rickert D, Sauerbier S, Nagursky H, Menne D, Vissink A, Raghoebar GM. Maxillary sinus floor elevation with bovine bone mineral combined with either autogenous bone or autogenous stem cells: a prospective randomized clinical trial. Clin Oral Implants Res. 2011 Mar;22(3):251–8. doi: 10.1111/j.1600-0501.2010.01981.x. Epub 2010 Sep 10. Erratum in: Clin Oral Implants Res. 2011 Jul; 22 (7); 777. [DOI] [PubMed] [Google Scholar]
- 60.Sohn DS, Bae MS, Choi BJ, An KM, Shin HI. Efficacy of demineralized bone matrix paste for maxillary sinus augmentation: a histologic and clinical study in humans. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009 Nov;108(5):e30–5. doi: 10.1016/j.tripleo.2009.06.042. [DOI] [PubMed] [Google Scholar]
- 61.Urist MR. Bone formation by autoinduction. Science. 1965;150:893–99. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 62.Zhang M, Powers RM, Jr, Wolfinbarger L., Jr A quantitative assessment of osteoinductivity of human demineralized bone matrix. J Periodontol. 1997;68(11):1076–84. doi: 10.1902/jop.1997.68.11.1076. [DOI] [PubMed] [Google Scholar]
- 63.Corbi S, Meleo D, Tarquini G, Pacifici L. Impiego delle proteine ossee morfogenetiche (BMPs) nella rigenerazione ossea guidata dei mascellari. Ann Stomatol. 2009;58(4):107–116. [Google Scholar]
- 64.Misch CE. Implantologia contemporanea. 3° edizione. Elsevier Masson Editore; 2009. [Google Scholar]