Abstract
Current evidence suggests that multiple neural mechanisms contribute to the fatal lethal event in SIDS. The processes may develop from a range of otherwise seemingly-innocuous circumstances, such as unintended external airway obstruction or accidental extreme flexion of the head of an already-compromised structure of the infant upper airway. The fatal event may occur in a sleep state which can suppress muscle tone essential to restore airway patency or exert muscle action to overcome a profound loss of blood pressure. Neural processes that could overcome those transient events with reflexive compensation appear to be impaired in SIDS infants. The evidence ranges from subtle physiological signs that appear very early in life, to autopsy findings of altered neurotransmitter, including serotonergic, systems that have extensive roles in breathing, cardiovascular regulation, and thermal control. Determination of the fundamental basis of SIDS is critical to provide biologic plausibility to SIDS risk reduction messages and to develop specific prevention strategies.
Keywords: Apnea, brainstem, cerebellum, chemoreception, hypotension, serotonin
INTRODUCTION
Any discussion of mechanisms underlying the Sudden Infant Death Syndrome (SIDS) needs to relate those processes to the developmental period of most risk (2–4 months), state of the infant during the fatal event (during sleep, or in close proximity to a sleeping period), and ancillary circumstances (enhanced risk with prone sleeping position, diminished risk with use of a pacifier, and increased risk with prenatal exposure to tobacco, alcohol, and other drugs of abuse). All of these factors contribute to risk of an event that occurs suddenly in an otherwise “healthy” infant, i.e., without obvious cause after autopsy and examination of the death scene [1]. Subtle indicators of risk appear as early as within a few days after birth in infants at risk for SIDS or who later succumb to SIDS, manifested, among other characteristics, as distortions in sleep state organization, periods of tachycardia, diminished influence of respiratory modulation of heart rate, a loss of momentary respiratory pauses, an increased incidence of obstructive apnea, and decreased overall motility [2–8]. Although those indicators provide insights into underlying pathology, the characteristics are rather inconspicuous, with none being so extreme as to seemingly precipitate a fatal event, particularly as isolated events.
The suddenness of the final event suggests a process of catastrophic failure of ventilation or cardiovascular collapse. Although numerous theories about the potential mechanisms resulting in SIDS have been put forward since the original NIH definition in 1969, the most enduring and widely accepted is the cardiorespiratory hypothesis involving central mechanisms [9–14] (Table 1). The hypothesis concerning central (brain) mechanisms of cardiorespiratory failure, the focus of this review, has been considerably strengthened over the years by: 1) normative physiological data indicating the first year of human postnatal life, particularly the first six months, is a vulnerable/critical period in the development and integration of central cardiorespiratory control; 2) abnormal physiological data in infants at risk for SIDS or who subsequently die of SIDS (in prospective studies) indicating subclinical deficits in autonomic function, respiration, and/or arousal; 3) neuropathologic studies of infants dying of SIDS indicating abnormalities in brain regions involved in cardiorespiratory control; and 4) an explosion in our understanding of central cardiorespiratory and arousal mechanisms at the molecular, cellular, neurochemical, and systems level through the neuroscientific analysis of human neuroimaging, whole animal models, reduced (brainstem) preparations, and cell culture [15–20]. Cardiovascular failure results from arrhythmia or other centrally-mediated autonomic processes, especially shock, culminating in hypotension with failure to perfuse vital organs. Failure of ventilation results from external airflow blockage or upper airway obstruction, loss of the drive to breathe, or failure of gasping to recover from hypoxic or hypoxemic events. An important issue for understanding SIDS mechanisms is that “cardiovascular” or “respiratory” failures are not mutually exclusive: rather, breathing mechanisms interact with the cardiovascular system. Consequently, a loss of blood pressure immediately triggers enhanced breathing efforts to restore vascular integrity (in addition to tachycardia and enhanced muscle tone). A transient increase in blood pressure, on the other hand, suppresses respiratory muscle tone [21], and does so preferentially to the upper airway musculature [22] possibly precipitating central apnea in the case of both diaphragmatic and upper airway muscle atonia, or an obstructive event if the suppression is principally to the upper airway. SIDS appears to result from a combination of circumstances of an exceptional cardiovascular or respiratory challenge, occurring in a compromised infant at a particular period of development [12]. The triad of conditions suggests that evaluation of neurotransmitter abnormalities that could interfere with multiple physiological aspects in SIDS infants would be valuable. The subsequent discussion considers cardiorespiratory processes in detail, with an emphasis on brain mechanisms that lead to cardiorespiratory failure, or alternatively, fail to give rise to compensatory mechanisms that overcome cardiovascular or respiratory failure. Evidence is drawn from physiological of infants at risk of dying of SIDS and neuropathological studies of SIDS infants, as well as developmental conditions such as congenital central hypoventilation syndrome (CCHS) which illustrate physiological characteristics relevant to the investigation of defective central cardiorespiratory mechanisms in SIDS [23].
Table 1.
Potential Mechanisms of State-Dependent Failure in Cardiovascular and Respiratory Control, Alone or in Combination, in SIDS
| Cardiovascular Mechanisms | |
| Bradycardia | |
| Hypotension (shock-like episode) | |
| Centrally-induced or -modulated arrythmia | |
| Adverse postural influences upon blood pressure control | |
| Respiratory Mechanisms | |
| External upper airway obstruction Impaired motor control of the head in the prone sleep position |
|
| Obstructive apnea | |
| Central apnea | |
| Impaired gasping | |
| Arousal Mechanisms | |
| Impaired state-related modulation of cardiorespiratory reflexes | |
| Failure to arouse in response to life-threatening challenge | |
CARDIOVASCULAR MECHANISMS
Cardiovascular collapse may be a scenario for SIDS based on evidence from physiologic characteristics detected by monitoring in infants who subsequently die of SIDS in days prior to and the moments immediately preceding the fatal event [9, 10, 24, 25]. Findings in such infants include a high incidence of tachycardia-bradycardia sequences before central respiratory efforts cease, a sequence that parallels the two-stage initial sympathetic followed by parasympathetic activity pattern in shock [26]. Cardiovascular collapse has been suggested as a failure mechanism in rare cases of SIDS where blood pressure was shown to be impaired prior to the fatal event [27]. Signs of autonomic dysregulation appear in SIDS infants in the days and weeks prior to the SIDS event, including trains of tachycardia [28], increased numbers of autonomic, but decreased full (i.e., with EEG activation) arousals [29], profuse sweating (i.e., excessive sympathetic activation), and reduced respiratory-related heart rate variation [30]. An absence of short respiratory pauses, which are most likely a consequence of momentary blood pressure effects on breathing, has also been noted [6, 21, 31, 32]. Overheating has often accompanied the fatal event [33]; vasodilation associated with overheating makes compensation for low blood pressure more difficult. A primary risk factor for SIDS, the prone sleeping position, diminishes vestibular contributions to blood pressure recovery from hypotension [34, 35], and hampers heart rate and breathing compensation to such blood pressure manipulations as head-up tilt [36–38]. Vestibular influences on responses to pressor, hypercapnic, and hypoxic challenges are largely mediated through the cerebellar cortex and deep nuclei [39, 40]. Several processes can induce a shock or shock-like sequence; the most common causative processes being blood loss, infection or deep visceral pain or irritation. Blood loss can be ruled out in SIDS, but visceral irritation [10] or shock following infection remain possibilities; the relationship of infection to SIDS is being actively pursued [41].
Arrhythmia
A more-commonly postulated process for cardiovascular collapse is cardiac arrhythmia, with congenital prolonged QT syndrome a principally-proposed mechanism [42, 43]. Prolongation and variability in QT interval develops from mutations in any of several genes, each of which encodes cardiac ion channels [11]. The potential for induction of excessively-prolonged QT intervals is enhanced with excessive sympathetic outflow, with such expression resulting in a potentially fatal arrhythmia of torsades de pointes which can degenerate into ventricular fibrillation [44]. Nearly 10% of a sample of Norwegian SIDS infants showed genetic predispositions for prolonged QT intervals [45]. Genetic cardiac channelopathies are now thought to account for 5–10% of infants who die suddenly, i.e., fall under the rubric of sudden and unexpected infant death (SUID). Given that a specific cause of death has now been determined in these infants, they are no longer classified as SIDS, but rather as explained deaths [11] Nevertheless, it is possible that a larger proportion of SIDS deaths will ultimately be related to a cardiac arrhythmia with continued molecular research in SIDS. Even if SIDS infants have not inherited the genetic processes which lead to prolonged QT intervals, it is important to emphasize that generation of the sympathetic processes that contribute to cardiac arrhythmia can depend on excessively-activated central autonomic processes derived from seizure discharge [46], or from damaged brain structures which normally limit sympathetic and parasympathetic outflow or regulate extent of output in each system [47]. Several central structures limit sympathetic outflow and recovery from hypotension, among which are brainstem and cerebellar areas. Damage to the fastigial nucleus, the major autonomic roof nucleus of the cerebellum, can lead to death from hypotension in animal models [47]; Other models of exaggerated sympathetic tone [23] show significant cerebellar injury upon neuroimaging studies [48] and long QT intervals [49].
Although a set of findings suggests that SIDS results from a “cardiovascular failure”, spontaneous restorative mechanisms from cardiovascular collapse often depend on respiratory efforts, frequently exaggerated, such as gasping. Indeed, the capacity of the autonomic system to interact with breathing processes is critical to recovery. Thus, deficiencies in breathing mechanisms, or interactions between breathing and cardiovascular processes, must be considered in any fatal failure mechanism. Moreover, the integrated nature of the vital functions suggests the usefulness of considering overall regulatory systems affecting both vital processes.
RESPIRATORY FAILURE
External Airway Obstruction
A potential threat to infant survival develops with failure to recover from external airway obstruction, such as facedown positioning in a pillow or other soft bedding, resulting in excessive carbon dioxide (CO2) exposure and hypoxia [11, 50]. Active promulgation of the “Back-to-Sleep” message, i.e., recommendation to place infants supine for sleep, has contributed substantially to the decline in the SIDS rates in recent years. The supine sleep position reduces the propensity for external airway obstruction. The mechanism of failure from such obstruction is thought, at least in part, to result from a developmental or potentially acquired inability to appropriately self-position the head and airway for free gas exchange. The loss of head movement can stem from several processes, including impaired carbon dioxide (CO2) or oxygen (O2) sensing, i.e., inadequate detection of extreme hypercarbia or hypoxia, due to deficits in central processing systems, deficient integration of sensory processes with appropriate motor reflexes, and/or failure of arousal mechanisms to restore motor tone or activate appropriate motor responses. Inadequate CO2 or O2 sensing or integration is an intense focus of investigation, with aberrations in development of neurotransmitter systems involved in that signal transduction, including prenatal nicotine exposure that can modify neurotransmitter development, or early hypoxic exposure which can “condition” or otherwise adapt afferent systems (see below). Multiple motor integrative systems participate in recovery from external airway obstruction, including structures in the brainstem. Another possibility involves cerebellar structures, since a principal function of the cerebellum is coordination of motor activity, including certain reflex actions.
Upper Airway Obstruction
Upper airway obstruction results from loss of tone to the upper airway musculature in association with continued diaphragmatic movements. These movements, in turn, generate repetitive negative thoracic pressures, enhancing airway collapse through the Venturi principle of accelerated airflow through a reduced diameter passage [51, 52]. Atonia of respiratory muscles can be induced by rapid transient elevation of blood pressure [21]; such atonia is preferentially exerted on the upper airway relative to the diaphragm [22]. The consequence is that impaired blood pressure responses to challenges can exert unexpected effects on breathing. Repeated obstructive events pose a significant risk for infants, first, from multiple exposures to intermittent hypoxia with successive obstructions, and secondly from repeated extreme changes in arterial pressure. The potential for obstruction is enhanced by atonia of the upper airway musculature during rapid eye movement (REM) sleep, a condition in which most of the body musculature, with the exception of the eye musculature and the diaphragm, lose tone. Rapid eye movement sleep also imposes an additional risk for breathing in infants, since intercostal muscles lose tone during that state. Since the ribs require a period of time to calcify, the intercostal muscles provide much of the stiffness of the infant thoracic wall cage. However, the atonia of intercostal muscles during REM sleep increases compliance, resulting in a “floppy” thoracic wall that collapses with each inspiratory effort [53]. The thoracic wall collapse leads to a substantial loss of intrathoracic volume with inspiration, leaving very little room for inspired air. The result is a potential for rapid desaturation with any process that might interfere with airflow, such as airway obstruction. Thus, the natural atonia of intercostal muscles during REM sleep introduces circumstances which can enhance SIDS risk. The potential for upper airway obstruction is also enhanced by the unique structure of the upper airway in the infant, with a relatively large tongue and airway dimensions which predispose to obstruction, particularly if the head is flexed, as shown by Tonkin [54] That head position can be particularly a risk condition from certain body positions for sleeping in automobile seats that allow extreme forward head flexion [55]. The circumstances under which head flexion in a developmentally “normal” but morphologically-compromised airway, combined with the atonia of REM sleep state, leads to a fatal event could be considered accidental, but may be further compromised by deficient hypoxia-sensing or motor reflex pathways, possibly involving brainstem and/or cerebellar processes.
Central Apnea
Failure of respiratory drive to both upper airway and diaphragmatic musculature, or central apnea, has occupied a central focus for attention in proposed mechanisms underlying the fatal event in SIDS. That failure can result from several components of the breathing process, including impaired sensory transduction or integration of either CO2 or O2, or non-recruitment of gasping mechanisms, the final restorative mechanism to low oxygen. Since breathing failure is presumed to occur during sleep, a principal concern is loss of the “wakefulness drive to breathe,” i.e., the waking state activates processes which maintain breathing, while during sleep; those influences are suppressed, or not recruited. A consistent loss of drive to breathe during sleep, especially during quiet sleep, occurs in congenital central hypoventilation syndrome (CCHS) [23], a rare disorder resulting from mutation of PHOX2B, a gene responsible for cell differentiation, with autonomic ganglia and neurons near the parafacial nuclei especially affected, as well as maldevelopment of the locus coeruleus, nucleus of the solitary tract, and retrotrapezoid nucleus at the ventral medullary surface [15, 56–60]. In addition to hypoventilation during sleep, breathing in CCHS infants is unresponsive to higher levels of CO2 or low O2. CCHS is not a model for SIDS, since, although a PHOX2B polymorphism appears in SIDS infants, that polymorphism is unrelated to that found in CCHS [61, 62], and affected CCHS infants show a wide range of profound autonomic deficiencies much more extreme than apparent in SIDS infants prior to death. However, impaired central chemosensitivity and breathing drive during sleep are major concerns in SIDS, and the loss of central chemosensitivity provides a useful model to illustrate processes other than chemical drive which contribute to maintaining breathing. Moreover, by comparing brain responses to high CO2 in CCHS and control children, brain structures involved in mediating neural responses to chemoreception can be determined.
The implications from CCHS studies for understanding SIDS mechanisms are that processes used to sustain breathing depend on multiple inputs, including thermal, affect, and kinesthetic cues, in addition to chemosensitive input and intrinsic oscillatory activity of medullary structures. Moreover, contributions from different influences vary by sleep or waking state; temperature drive to breathe, for example, is lost during REM sleep [63], and control of ventral medullary surface neural structures on blood pressure are altered during that state [64]. The atonia of REM sleep modifies upper airway and other muscle function, and hence, kinesthetic feedback. CCHS breathing deficiencies appear preferentially during quiet sleep; REM sleep is more protected, again indicating that determination of mechanisms underlying breathing requires consideration of influences from forebrain as well as medullary sites. The implication for SIDS from CCHS studies is that both rostral brain and brainstem mechanisms are involved in breathing control, and different mechanisms may contribute to state-related drive to breathe. Of note, a recent in-depth neuropathologic case study of Haddad syndrome (CCHS combined with Hirschsprung’s disease) revealed hypoplasia of the locus coeruleus which mimics that seen in Phox2b knockout mice, in addition to other brainstem and forebrain developmental anomalies [65].
Gasping
The final defense to hypoxic exposure is gasping, a sequence of respiratory efforts triggered by activation of structures in the brainstem. Gasping is frequently found in monitored respiratory signals in infants who succumb during home monitoring [25]. Because a successful outcome to gasping is obviously vital, determining the underlying triggering and neuromodulatory processes for this respiratory pro-cess are objects of considerable interest. Blockade of 5-HT and noradrenergic receptors suppresses gasping; 5-HT alone appears to be less effective, suggesting that an integrated participation of multiple systems triggers gasping efforts [66, 67].
AROUSAL MECHANISMS AND CARDIORESPIRATORY CONTROL
A pervasive aspect through all attempts to understand mechanisms underlying SIDS is that the fatal event apparently occurs during sleep, with the possibility that restoration of the “wakefulness” stimulus has the potential to restore vital function. The processes underlying arousal are complex, since “arousal” exists at several neuroanatomic levels, from activation of muscle tone, autonomic regulation, electroencephalographic activity, and cognition. Each of these processes differs in underlying neural pathways and neurotransmitter action, many of which interact to produce an integrated response. Normally, arousal processes are integrated in time, with near-simultaneous recruitment of muscle activity, autonomic enhancement, such as heart rate and blood pressure, and electroencephalogram activation [68]. However, individual components of the arousal process can be separated, showing that arousal is not a unitary phenomenon. Electroencephalographic (EEG) synchronized slow wave activity can appear in cortical structures in an alert animal with atropine-induced cholinergic blockade [69], desynchronized EEG activity appears in REM sleep, and cognitive processes can be blocked during waking by serotonergic blockade [70]. Different components of the arousal response emerge in infant sleep, with SIDS infants showing more “autonomic” arousals and fewer “full” arousals, i.e., with cortical desynchronization [29]. The implication for SIDS is that an “arousal” failure has the potential to result from impaired action in any of a number of separate systems.
BRAIN STUDIES IN SIDS INFANTS RELEVANT TO THE CENTRAL CARDIORESPIRATORY HYPOTHESIS
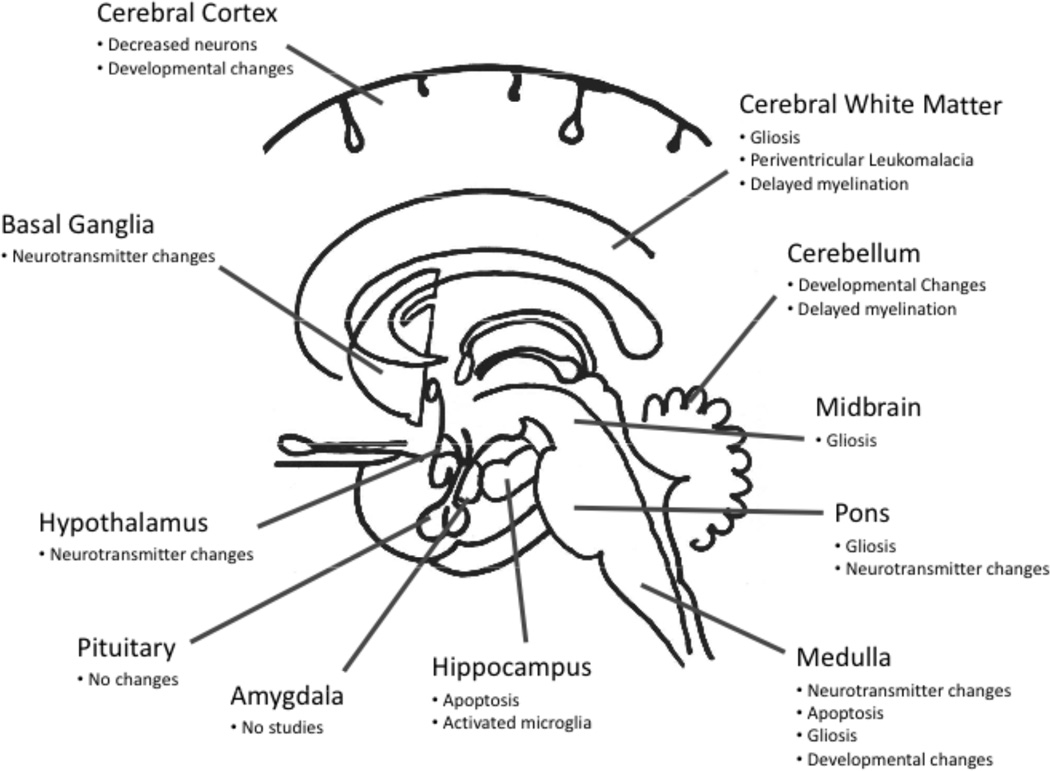
The central cardiorespiratory hypothesis in SIDS has led to multiple neuroanatomic studies of relevant brain regions in SIDS infants at autopsy [11, 12, 71, 72] (Fig. 1). The dilemma of brain research in SIDS, however, is that the brains in general “look normal” under the light microscope, the tool of standard histopathology. At the very most, there are nonspecific and subtle indications of cell injury that are not limited to cardiorespiratory related regions. Moreover, certain abnormalities may reflect secondary consequences of chronic, prior, or repetitive hypoxia-ischemia, e.g., apoptosis and microglial activation in the hippocampus, brainstem gliosis and apoptosis, periventricular leukomalacia, cerebral white matter gliosis, and cerebral cortical injury, recently reviewed in depth [11, 72]. In addition, certain brain abnormalities suggest subtle developmental anomalies originating in utero that may point to abnormal maturational factors in the overall neuropathology of SIDS [72], e.g., increased number and density of leptomeningeal neurons [73]. The overall cardiorespiratory hypothesis of brain studies in SIDS is that there are lethal abnormalities in one or more brain structures critical for state-dependent autonomic and respiratory control in SIDS infants at autopsy which are detectable only by quantitative and/or special molecular, cellular, and/or neurochemical research tools at autopsy. To date, virtually all cardiorespiratory-related brain regions have been scrutinized in SIDS infants, including the brainstem, cerebellum, hypothalamus, and hippocampus, as recently reviewed by us [11, 12, 71, 72] (Fig. 1). Here we highlight neuropathologic findings in two brain regions that have received perhaps the greatest attention, i.e., the brainstem and cerebellum. Of note, the potential definition of SIDS-specific neuropathology in arousal-related pathways is a special challenge, because virtually all of the principal identified neurotransmitter systems in the brain are involved in arousal responses, with the major participation of cholinergic, adrenergic, serotonergic (5-HT), and dopaminergic neurotransmitter systems, and a range of neuropeptides, including orexin (hypocretin) [17]. Neural structures responsible for arousal characteristics also lie in multiple brain areas, especially the basal forebrain, hypothalamus, and brainstem (ventral tegmental area of Tsai, lateral tegmental pons, locus coeruleus, and midline raphé). Future research is needed in SIDS brains that attempts to integrate potential pathologic findings across these widespread and diverse neurochemical and neuroanatomic systems.
Fig. (1).
Brain regions and abnormalities in SIDS in one or more published reports. See text for references.
Brainstem Findings in SIDS Infants and the Central Cardiorespiratory Hypothesis
To date, the most robust, reproducible, and in-depth findings related to the central cardiorespiratory hypothesis in SIDS have been reported in SIDS brainstems, as recently reviewed by us [12, 72] (Fig. 1). These abnormalities involve (although are not necessarily specific to) regions critical to central cardiorespiratory control, modulation, and/or integration. These regions include the hypoglossal nucleus (airway patency, particularly during sleep), nucleus of the solitary tract (visceral sensory input), dorsal motor nucleus of the vagus (preganglionic parasympathetic outflow), rostral ventrolateral medulla, including the putative homologous site of the preBötzinger complex involved in respiratory rhythm generation, vestibular nuclei (head control and hypotensive reflexes), and caudal raphé complex (cardiorespiratory integration). The types of abnormalities included gliosis enhanced by the immunomarker glial fibrillary acidic protein for reactive astrocytes, neurotransmitter deficits detected by immunocytochemistry or tissue receptor autoradiography, and apoptosis detected by relevant immunomarkers, e.g., caspase 3 [12, 72].
Reported neurotransmitter/neuromodulator defects in different brainstem sites in SIDS infants include catecholaminergic, nicotinic and muscarinic cholinergic, glutamatergic, serotonergic (5-HT), and neuropeptide systems, suggesting that no single neurotransmitter system is at fault, but that a combination of systems are most likely involved [12, 72]. Nevertheless, we found that the majority of SIDS infants show abnormalities in several markers of 5-HT function in the medulla oblongata (caudal brainstem) in regions that are critically related to state-dependent modulation of cardiorespiratory control and that are mediated by medullary 5-HT neurons, the so-called medullary 5-HT system [74–77]. These abnormalities, now detected in four independent (nonoverlapping) datasets by us, included alterations in 5-HT receptor binding, including for the 5-HT1A receptor [74–77], in nuclei that contain 5-HT neurons as well as receive 5-HT projections, decreased binding to the 5-HT transporter relative to 5-HT cell density [76], increased density of 5-HT neurons [76], and 5-HT neuronal immaturity [76]. The finding of decreased 5-HT1A receptors has also been reported by independent investigators in different laboratories [78, 79]. Recently, a deficit in 5-HT levels detectable by high performance liquid chromatography, and in levels of tryptophan hydroxylase (TPH2), the key biosynthetic enzyme for 5-HT, have been reported in the same SIDS medullae and in the same regions of the medullary 5-HT system that demonstrate 5-HT1A receptor binding abnormalities [77]. Of note, the medullary 5-HT profile differed between infants dying of SIDS and those dying with known chronic oxygenation disorders, suggesting that chronic hypoxia does not necessarily play a major role in the pathogenesis of the impairments in the 5-HT tissue markers [74, 77]. The data now suggest that SIDS is associated with a brainstem (medullary) disorder of 5-HT deficiency rather than 5-HT over-production [77]. Thus, experimental paradigms that attempt to mimic SIDS should consider modeling a medullary 5-HT deficiency, as found in various 5-HT related knockout mice, e.g., PET1 and Lmx1b knockouts [77]. The medullary 5-HT system is involved in the modulation and integration of diverse homeostatic functions according to the level of arousal, including upper airway control, ventilation and gasping, autonomic control, thermoregulation, responses to CO2 and O2, arousal from sleep, and hypoxia-induced plasticity [11, 12]. Given the wide array of these homeostatic functions, sudden death in infants with 5-HT defects with all or parts of the 5-HT system may result from a convergence of defects in protective responses to homeostatic stressors during sleep. These responses are modulated by 5-HT, probably in conjunction with other neurotransmitters and interacting (rostral) systems [11]. In SIDS cases, we propose that insufficient 5-HT levels are produced early in development, potentially as early as the first or second trimester, resulting in a compensatory increase in immature 5-HT neurons with immature (decreased) 5-HT1A binding and 5-HT transporter levels. The key factor in the sequence of neurochemical events in SIDS may be impaired regulation of TPH2, with subsequent reduced 5-HT levels and increased 5-HT cell density due to impaired feedback inhibition of 5-HT levels upon 5-HT cell number [80]. The partial, rather than total defect in 5-HT markers could help explain why medullary 5-HT-mediated pathways function reasonably well at baseline or during waking, but are unable to respond to homeostatic stressors during the sleep period when the partial deficit is unmasked in some unknown but important way by sleep itself, thereby resulting in sudden death.
Cerebellar Findings in SIDS Infants and the Central Cardiorespiratory Hypothesis
The cerebellum is a focus of active neuropathologic research in SIDS due to its recognized role in central cardiorespiratory control, particularly as it relates to vestibular reflexes and head position in the prone versus supine sleep position and positional influences upon blood pressure regulation. Maldevelopment or acquired lesions of the cerebellum, for example, could lead to an uncompensated action to recover blood pressure loss during hypotensive challenges, and would similarly be unable to restrain excessive sympathetic outflow, thereby enhancing the potential for arrhythmia, as well as to lead to inadequate head positioning during sleep (see above). The neuropathologic evidence for cerebellar involvement in SIDS include reports of: 1) increase in apoptosis with (albeit not specific to) vestibular nuclei [81] that project via vestibulo-cerebellar pathways to mediate the influences of the vestibular system upon respiration, blood pressure regulation, and head position during sleep; 2) delayed maturation of the external granular layer which contains precursor cells of the internal granular layer that migrate inward up to the end of the first postnatal year, i.e., the time frame of SIDS, and receive mossy fibers from many incoming brainstem and spinal cord systems [10, 82] an underpopulation of neurons, reflected in decreased density, in neurons within the inferior olive which provide the sole source of climbing fibers to the cerebellum [83]; and delayed myelination in cerebellar-related pathways in the context of generalized hypomyelination in several brainstem and forebrain sites [84]. How these different acquired and developmental processes inter-relate to produce potential cerebellar dysfunction in SIDS is uncertain. Also uncertain are the mechanisms leading to dysfunctional processes. The well-recognized susceptibility of the human fetal and infant cerebellum to hypoxia-ischemia suggests this insult plays a role [83]. Impaired action of 5-HT projections to the cerebellum and/or inferior olive (cerebellar-relay) from abnormalities in the medullary 5-HT system, particularly the caudal raphé complex, is likely to contribute to altered motor responsiveness to compromised airways. Further neuropathologic research into cerebellar-brainstem interconnecting pathways in SIDS infants is needed.
Developmental-Dependent Neural Organization in Central Cardiorespiratory Control
A defining characteristic of SIDS is a developmental period of high risk, with relative protection in the first postnatal month and in the second six months. It is thus useful to examine central time sequences of responses to chemoreceptor and blood pressure stimuli, and thus determine what structures may place an infant at risk. As one example, the deep cerebellar nuclei play a role in CO2 regulation [40], and help mediate compensation for extremes in blood pressure loss or excessive sympathetic outflow. The latter role is age-dependent in animals, and may be similarly subject to developmental processes in infants. Animal functional magnetic resonance imaging studies suggest that cerebellar structures serve essential roles for regulating blood pressure very early in life, but that a transition occurs, with more-rostral brain structures assuming a greater role with development [85]. Similarly, ventral medullary surface activity, measured with optical procedures, increases to pressor challenges in young felines, but that activity reverses after day 24 [86]. Substantial reorganization of brainstem neurotransmitter systems takes place shortly after the 12th day of life in the rat, with several of those systems playing significant roles in metabolism, breathing, baroreceptor gain, and blood pressure [87–90]. Since infants are relatively protected from SIDS in early life [1], some neural developmental process likely underlies the failure process. An analogous pattern of neural regulatory mechanisms in autonomic and respiratory control may be operating in humans as shown in rodent models. Substantial evidence exists that prenatal exposure to nicotine, alcohol, cocaine or heroin alters developmental processes which significantly increase the risk for SIDS [11, 12]; the best documented is nicotine exposure with risk factors of 1.9 [91]. Such a remarkable increase in risk could only result from significant interference with vital cardiac or breathing systems. The systemic interference likely results from an interaction of nicotine with 5-HT neurotransmitter components, such as the demonstration of reduced 5-HT receptor binding following prenatal nicotine exposure [80, 92]. In addition to effects of nicotine exposure, evidence exists that low maternal hematocrit values are linked to enhanced SIDS risk [93].
CONCLUSIONS
The available evidence suggests that multiple neural mechanisms contribute to the fatal lethal event in SIDS. The processes may develop from a range of otherwise seemingly-innocuous circumstances, such as external airway obstruction or accidental extreme flexion of the head of an already-compromised structure of the infant upper airway. The fatal event may occur during rapid eye movement sleep, which imposes a paralysis of muscles necessary to restore airway patency or activate reflexes or motor activity to overcome a profound loss of blood pressure. Neural processes that could overcome those transient events with reflexive compensation appear to be impaired in SIDS. The evidence ranges from subtle physiological signs that appear very early in life, to autopsy findings of altered neurotransmitter systems that have extensive roles in breathing, cardiovascular regulation, and thermal control. Cardiovascular and respiratory systems are closely integrated to support vital functions, and it is useful to consider interdependencies between these functions rather than exclusive roles for either system. The vast extent of medullary 5-HT influences on vital physiologic functions in particular suggests a significant potential for that system to contribute to the failing mechanism, likely in conjunction with other neurotransmitter systems and neuroanatomic sites, e.g., cerebellum. The determination of the fundamental basis of SIDS is critical to provide biologic plausibility to SIDS risk reduction messages so that they are closely followed. More importantly, the determination of the biologic basis of the mechanisms of failure in SIDS is essential if we are to develop specific diagnostic and therapeutic strategies to eradicate all SIDS deaths-the goal of all SIDS research.
ACKNOWLEDGEMENTS
Dr. Harper was supported by NIH HD-22695. Dr. Kinney was supported by NIH HD-20991.
REFERENCES
- 1.Willinger M, James LS, Catz C. Defining the sudden infant death syndrome (SIDS): Deliberations of an expert panel convened by the National Institute of Child Health and Human Development. Pediatr Pathol. 1991;11:677–684. doi: 10.3109/15513819109065465. [DOI] [PubMed] [Google Scholar]
- 2.Harper RM, Leake B, Hoffman H, et al. Periodicity of sleep states is altered in infants at risk for the sudden infant death syndrome. Science. 1981;213:1030–1032. doi: 10.1126/science.7268406. [DOI] [PubMed] [Google Scholar]
- 3.Schechtman VL, Harper RM, Wilson AJ, Southall DP. Sleep state organization in normal infants and victims of the sudden infant death syndrome. Pediatrics. 1992;89:865–870. [PubMed] [Google Scholar]
- 4.Kluge KA, Harper RM, Schechtman VL, Wilson AJ, Hoffman HJ, Southall DP. Spectral analysis assessment of respiratory sinus arrhythmia in normal infants and infants who subsequently died of sudden infant death syndrome. Pediatr Res. 1988;24:677–682. doi: 10.1203/00006450-198812000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Schechtman VL, Raetz SL, Harper RK, et al. Dynamic analysis of cardiac R-R intervals in normal infants and in infants who subsequently succumbed to the sudden infant death syndrome. Pediatr Res. 1992;31:606–612. doi: 10.1203/00006450-199206000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Schechtman VL, Lee MY, Wilson AJ, Harper RM. Dynamics of respiratory patterning in normal infants and infants who subsequently died of the sudden infant death syndrome. Pediatr Res. 1996;40:571–577. doi: 10.1203/00006450-199610000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Hoppenbrouwers T, Jensen D, Hodgman J, Harper R, Sterman M. Body movements during Quiet Sleep (QS) in subsequent siblings of sids. Clin Resear. 1982;30:A136. [Google Scholar]
- 8.Kato I, Groswasser J, Franco P, et al. Developmental characteristics of apnea in infants who succumb to sudden infant death syndrome. Am J Respir Crit Care Med. 2001;164:1464–1469. doi: 10.1164/ajrccm.164.8.2009001. [DOI] [PubMed] [Google Scholar]
- 9.Harper RM. Sudden infant death syndrome: A failure of compensatory cerebellar mechanisms? Pediatr Res. 2000;48:140–142. doi: 10.1203/00006450-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Harper RM, Bandler R. Finding the failure mechanism in Sudden Infant Death Syndrome. Nat Med. 1998;4:157–158. doi: 10.1038/nm0298-157. [DOI] [PubMed] [Google Scholar]
- 11.Kinney HC, Thach BT. The sudden infant death syndrome. N Engl J Med. 2009;361:795–805. doi: 10.1056/NEJMra0803836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinney HC, Richerson GB, Dymecki SM, Darnall RA, Nattie EE. The brainstem and serotonin in the sudden infant death syndrome. Annu Rev Pathol. 2009;4:517–550. doi: 10.1146/annurev.pathol.4.110807.092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahni R, Fifer WP, Myers MM. Identifying infants at risk for sudden infant death syndrome. Curr Opin Pediatr. 2007;19:145–149. doi: 10.1097/MOP.0b013e32808373b6. [DOI] [PubMed] [Google Scholar]
- 14.Hunt CE, Brouillette RT. Sudden infant death syndrome: 1987 perspective. J Pediatr. 1987;110:669–678. doi: 10.1016/s0022-3476(87)80001-x. [DOI] [PubMed] [Google Scholar]
- 15.Guyenet PG, Bayliss DA, Stornetta RL, Fortuna MG, Abbott SBG, DePuy SD. Retrotrapezoid nucleus, respiratory chemosensitivity and breathing automaticity. Respir Physiol Neurobiol. 2009;168:59–68. doi: 10.1016/j.resp.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cechetto DF, Shoemaker JK. Functional neuroanatomy of autonomic regulation. Neuroimage. 2009;47:795–803. doi: 10.1016/j.neuroimage.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 17.Siegel JM. The neurobiology of sleep. Semin Neurol. 2009;29:277–296. doi: 10.1055/s-0029-1237118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuller PM, Gooley JJ, Saper CB. Neurobiology of the sleep-wake cycle: Sleep architecture, circadian regulation, and regulatory feedback. J Biol Rhythms. 2006;21:482–493. doi: 10.1177/0748730406294627. [DOI] [PubMed] [Google Scholar]
- 20.Doi A, Ramirez J-M. Neuromodulation and the orchestration of the respiratory rhythm. Respir Physiol Neurobiol. 2008;164:96–104. doi: 10.1016/j.resp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trelease RB, Sieck GC, Marks JD, Harper RM. Respiratory inhibition induced by transient hypertension during sleep in unrestrained cats. Exp Neurol. 1985;90:173–186. doi: 10.1016/0014-4886(85)90050-0. [DOI] [PubMed] [Google Scholar]
- 22.Marks JD, Harper RM. Differential inhibition of the diaphragm and posterior cricoarytenoid muscles induced by transient hypertension across sleep states in intact cats. Exp Neurol. 1987;95:730–742. doi: 10.1016/0014-4886(87)90312-8. [DOI] [PubMed] [Google Scholar]
- 23.American Thoracic Society. Idiopathic congenital central hypoventilation syndrome: Diagnosis and management. Am J Respir Crit Care Med. 1999;160:368–373. doi: 10.1164/ajrccm.160.1.16010. [DOI] [PubMed] [Google Scholar]
- 24.Meny RG, Carroll JL, Carbone MT, Kelly DH. Cardiorespiratory recordings from infants dying suddenly and unexpectedly at home. Pediatrics. 1994;93:44–49. [PubMed] [Google Scholar]
- 25.Poets CF, Meny RG, Chobanian MR, Bonofiglo RE. Gasping and other cardiorespiratory patterns during sudden infant deaths. Pediatr Res. 1999;45:350–354. doi: 10.1203/00006450-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Ludbrook J. Haemorrhage and shock. In: Hainsworth R, Mark A, editors. Cardiovascular reflex control in health and disease. London: Saunders; 1993. pp. 463–490. [Google Scholar]
- 27.Ledwidge M, Fox G, Matthews T. Neurocardiogenic syncope: A model for SIDS. Arch Dis Child. 1998;78:481–483. doi: 10.1136/adc.78.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Southall DP, Stevens V, Franks CI, Newcombe RG, Shinebourne EA, Wilson AJ. Sinus tachycardia in term infants preceding sudden infant death. Eur J Pediatr. 1988;147:74–78. doi: 10.1007/BF00442617. [DOI] [PubMed] [Google Scholar]
- 29.Kato I, Franco P, Groswasser J, et al. Incomplete arousal processes in infants who were victims of sudden death. Am J Respir Crit Care Med. 2003;168:1298–1303. doi: 10.1164/rccm.200301-134OC. [DOI] [PubMed] [Google Scholar]
- 30.Schechtman VL, Harper RM, Kluge KA, Wilson AJ, Hoffman HJ, Southall DP. Cardiac and respiratory patterns in normal infants and victims of the sudden infant death syndrome. Sleep. 1988;11:413–424. doi: 10.1093/sleep/11.5.413. [DOI] [PubMed] [Google Scholar]
- 31.Fukumizu M, Kohyama J. Central respiratory pauses, sighs, and grosses body movements during sleep in children. Physiol Behav. 2004;82:721–726. doi: 10.1016/j.physbeh.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Kohyama J, Shimohira M, Itoh M, Fukumizu M, Iwakawa Y. Phasic muscle activity during REM sleep in infancy-normal maturation and contrastive abnormality in SIDS/ALTE and West syndrome. J Sleep Res. 1993;2:241–249. doi: 10.1111/j.1365-2869.1993.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 33.Fleming PJ, Levine MR, Azaz Y, Wigfield R, Stewart AJ. Interactions between thermoregulation and the control of respiration in infants: possible relationship to sudden infant death. Acta Paediatr Suppl. 1993;82(Suppl 389):57–59. doi: 10.1111/j.1651-2227.1993.tb12878.x. [DOI] [PubMed] [Google Scholar]
- 34.Chong A, Murphy N, Matthews T. Effect of prone sleeping on circulatory control in infants. Arch Dis Child. 2000;82:253–256. doi: 10.1136/adc.82.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yiallourou SR, Walker AM, Horne RS. Prone sleeping impairs circulatory control during sleep in healthy term infants: Implications for SIDS. Sleep. 2008;31:1139–1146. [PMC free article] [PubMed] [Google Scholar]
- 36.Fifer WP, Greene M, Hurtado A, Myers MM. Cardiorespiratory responses to bidirectional tilts in infants. Early Hum Dev. 1999;55:265–279. doi: 10.1016/s0378-3782(99)00026-2. [DOI] [PubMed] [Google Scholar]
- 37.Fifer WP, Myers MM. Sudden fetal and infant deaths: Shared characteristics and distinctive features. Semin Perinatol. 2002;26:89–96. doi: 10.1053/sper.2002.29854. [DOI] [PubMed] [Google Scholar]
- 38.Kinney HC, Myers MM, Belliveau RA, et al. Subtle autonomic and respiratory dysfunction in sudden infant death syndrome associated with serotonergic brainstem abnormalities: A case report. J Neuropathol Exp Neurol. 2005;64:689–694. doi: 10.1097/01.jnen.0000174334.27708.43. [DOI] [PubMed] [Google Scholar]
- 39.Giuditta M, Ruggiero DA, Del Bo A. Anatomical basis for the fastigial pressor response. Blood Press. 2003;12:175–180. doi: 10.1080/08037050310010912. [DOI] [PubMed] [Google Scholar]
- 40.Hernandez JP, Xu F, Frazier DT. Medial vestibular nucleus mediates the cardiorespiratory responses to fastigial nuclear activation and hypercapnia. J Appl Physiol. 2004;97:835–842. doi: 10.1152/japplphysiol.00134.2004. [DOI] [PubMed] [Google Scholar]
- 41.Blood-Siegfried J, Rambaud C, Nyska A, Germolec DR. Evidence for infection, inflammation and shock in sudden infant death: Parallels between a neonatal rat model of sudden death and infants who died of sudden infant death syndrome. Innate Immun. 2008;14:145–152. doi: 10.1177/1753425908090730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwartz PJ. The congenital long QT syndromes from genotype to phenotype: Clinical implications. J Intern Med. 2006;259:39–47. doi: 10.1111/j.1365-2796.2005.01583.x. [DOI] [PubMed] [Google Scholar]
- 43.Franco P, Groswasser J, Scaillet S, et al. QT interval prolongation in future SIDS victims: A polysomnographic study. Sleep. 2008;31:1691–1699. doi: 10.1093/sleep/31.12.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz PJ, Stramba-Badiale M, Segantini A, et al. Prolongation of the QT interval and the sudden infant death syndrome. N Engl J Med. 1998;338:1709–1714. doi: 10.1056/NEJM199806113382401. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz PJ. Cardiac sympathetic innervation and the sudden infant death syndrome. A possible pathogenetic link. Am J Med. 1976;60:167–172. doi: 10.1016/0002-9343(76)90425-3. [DOI] [PubMed] [Google Scholar]
- 46.Surges R, Thijs RD, Tan HL, Sander JW Medscape. Sudden unexpected death in epilepsy: Risk factors and potential pathomechanisms. Nat Rev Neurol. 2009;5:492–504. doi: 10.1038/nrneurol.2009.118. [DOI] [PubMed] [Google Scholar]
- 47.Lutherer LO, Lutherer BC, Dormer KJ, Janssen HF, Barnes CD. Bilateral lesions of the fastigial nucleus prevent the recovery of blood pressure following hypotension induced by hemorrhage or administration of endotoxin. Brain Res. 1983;269:251–257. doi: 10.1016/0006-8993(83)90134-8. [DOI] [PubMed] [Google Scholar]
- 48.Kumar R, Macey PM, Woo MA, Alger JR, Harper RM. Diffusion tensor imaging demonstrates brainstem and cerebellar abnormalities in congenital central hypoventilation syndrome. Pediatr Res. 2008;64:275–280. doi: 10.1203/PDR.0b013e31817da10a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gronli JO, Santucci BA, Leurgans SE, Berry-Kravis EM, Weese-Mayer DE. Congenital central hypoventilation syndrome: PHOX2B genotype determines risk for sudden death. Pediatr Pulmonol. 2008;43:77–86. doi: 10.1002/ppul.20744. [DOI] [PubMed] [Google Scholar]
- 50.Lijowska AS, Reed NW, Chiodini BA, Thach BT. Sequential arousal and airway-defensive behavior of infants in asphyxial sleep environments. J Appl Physiol. 1997;83:219–228. doi: 10.1152/jappl.1997.83.1.219. [DOI] [PubMed] [Google Scholar]
- 51.Harper RM, Sauerland EK. The role of the tongue in sleep apnea. In: Guilleminault C, Dement WC, editors. Sleep Apnea Syndromes. New York: Alan R. Liss; 1978. pp. 219–234. [Google Scholar]
- 52.Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- 53.Henderson-Smart DJ, Read DJ. Reduced lung volume during behavioral active sleep in the newborn. J Appl Physiol. 1979;46:1081–1085. doi: 10.1152/jappl.1979.46.6.1081. [DOI] [PubMed] [Google Scholar]
- 54.Tonkin SL, Gunn TR, Bennet L, Vogel SA, Gunn AJ. A review of the anatomy of the upper airway in early infancy and its possible relevance to SIDS. Early Hum Dev. 2002;66:107–121. doi: 10.1016/s0378-3782(01)00242-0. [DOI] [PubMed] [Google Scholar]
- 55.Tonkin SL, Vogel SA, Bennet L, Gunn AJ. Apparently life threatening events in infant car safety seats. BMJ. 2006;333:1205–1206. doi: 10.1136/bmj.39021.657083.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature. 1999;399:366–370. doi: 10.1038/20700. [DOI] [PubMed] [Google Scholar]
- 57.Pattyn A, Goridis C, Brunet JF. Specification of the central noradrenergic phenotype by the homeobox gene Phox2b. Mol Cell Neurosci. 2000;15:235–243. doi: 10.1006/mcne.1999.0826. [DOI] [PubMed] [Google Scholar]
- 58.Dauger S, Pattyn A, Lofaso F, et al. Phox2b controls the development of peripheral chemoreceptors and afferent visceral pathways. Development (Cambridge, England) 2003;130:6635–6642. doi: 10.1242/dev.00866. [DOI] [PubMed] [Google Scholar]
- 59.Dubreuil V, Ramanantsoa N, Trochet D, et al. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnea, and specific loss of parafacial neurons. Proc Natl Acad Sci USA. 2008;105:1067–1072. doi: 10.1073/pnas.0709115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spengler CM, Gozal D, Shea SA. Chemoreceptive mechanisms elucidated by studies of congenital central hypoventilation syndrome. Respir Physiol. 2001;129:247–255. doi: 10.1016/s0034-5687(01)00294-8. [DOI] [PubMed] [Google Scholar]
- 61.Weese-Mayer DE, Berry-Kravis EM, Ceccherini I, Rand CM. Congenital central hypoventilation syndrome (CCHS) and sudden infant death syndrome (SIDS): Kindred disorders of autonomic regulation. Respir Physiol Neurobiol. 2008;164:38–48. doi: 10.1016/j.resp.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 62.Weese-Mayer DE, Ackerman MJ, Marazita ML, Berry-Kravis EM. Sudden Infant Death Syndrome: Review of implicated genetic factors. Am J Med Genet A. 2007;143A:771–788. doi: 10.1002/ajmg.a.31722. [DOI] [PubMed] [Google Scholar]
- 63.Ni H, Schechtman VL, Zhang J, Glotzbach SF, Harper RM. Respiratory responses to preoptic/anterior hypothalamic warming during sleep in kittens. Reprod Fertil Dev. 1996;8:79–86. doi: 10.1071/rd9960079. [DOI] [PubMed] [Google Scholar]
- 64.Richard CA, Rector DM, Harper RK, Harper RM. Optical imaging of the ventral medullary surface across sleep-wake states. Am J Physiol. 1999;277:R1239–R1245. doi: 10.1152/ajpregu.1999.277.4.R1239. [DOI] [PubMed] [Google Scholar]
- 65.Tomycz ND, Haynes RL, Schmidt EF, Ackerson K, Kinney HC. Novel neuropathologic findings in the Haddad syndrome. Acta Neuropathol. 119:261–269. doi: 10.1007/s00401-009-0599-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tryba AK, Pena F, Ramirez JM. Gasping activity in vitro: A rhythm dependent on 5-HT2A receptors. J Neurosci. 2006;26:2623–2634. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Toppin VA, Harris MB, Kober AM, Leiter JC, St-John WM. Persistence of eupnea and gasping following blockade of both serotonin type 1 and 2 receptors in the in situ juvenile rat preparation. J Appl Physiol. 2007;103:220–227. doi: 10.1152/japplphysiol.00071.2007. [DOI] [PubMed] [Google Scholar]
- 68.Jouvet M. Neurophysiology of the states of sleep. Physiol Rev. 1967;47:117–177. doi: 10.1152/physrev.1967.47.2.117. [DOI] [PubMed] [Google Scholar]
- 69.Wikler A. Pharmacologic dissociation of behavior and EEG "sleep patterns" in dogs; morphine, n-allylnormorphine, and atropine. Proc Soc Exp Biol Med. 1952;79:261–265. doi: 10.3181/00379727-79-19345. [DOI] [PubMed] [Google Scholar]
- 70.Vanderwolf CH, Baker GB. Evidence that serotonin mediates non-cholinergic neocortical low voltage fast activity, non-cholinergic hippocampal rhythmical slow activity and contributes to intelligent behavior. Brain Res. 1986;374:342–356. doi: 10.1016/0006-8993(86)90428-2. [DOI] [PubMed] [Google Scholar]
- 71.Kinney HC, Filiano JJ. Brain research in the Sudden Infant Death Syndrome. In: Byard RW, Krous HF, editors. Sudden Infant Death Syndrome; A Diagnostic Approach. London: Arnold; 2001. [Google Scholar]
- 72.Kinney HC. Neuropathology provides new insight in the pathogenesis of the sudden infant death syndrome. Acta Neuropathol. 2009;117:247–255. doi: 10.1007/s00401-009-0490-7. [DOI] [PubMed] [Google Scholar]
- 73.Rickert CH, Gros O, Nolte KW, Vennemann M, Bajanowski T, Brinkmann B. Leptomeningeal neurons are a common finding in infants and are increased in sudden infant death syndrome. Acta Neuropathol. 2009;117:275–282. doi: 10.1007/s00401-009-0489-0. [DOI] [PubMed] [Google Scholar]
- 74.Panigrahy AM, Filiano JM, Sleeper LAS, et al. Decreased serotonergic receptor binding in rhombic lip-derived regions of the medulla oblongata in the sudden infant death syndrome. J Neuropath Exp Neurol. 2000;59:377–384. doi: 10.1093/jnen/59.5.377. [DOI] [PubMed] [Google Scholar]
- 75.Kinney HCM, Randall LLRM, Sleeper LAES, et al. Serotonergic brainstem abnormalities in northern plains indians with the sudden infant death syndrome. J Neuropath Exp Neurol. 2003;62:1178–1191. doi: 10.1093/jnen/62.11.1178. [DOI] [PubMed] [Google Scholar]
- 76.Paterson DS, Trachtenberg FL, Thompson EG, et al. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA. 2006;296:2124–2132. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- 77.Duncan JR, Paterson DS, Hoffman JM, et al. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA. 2010;303:430–437. doi: 10.1001/jama.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ozawa Y, Okado N. Alteration of serotonergic receptors in the brain stems of human patients with respiratory disorders. Neuropediatrics. 2002;33:142–149. doi: 10.1055/s-2002-33678. [DOI] [PubMed] [Google Scholar]
- 79.Machaalani R, Say M, Waters K. Serotoninergic receptor 1A in the sudden infant death syndrome brainstem medulla and associations with clinical risk factors. Acta Neuropathol. 2009;117:257–265. doi: 10.1007/s00401-008-0468-x. [DOI] [PubMed] [Google Scholar]
- 80.Duncan JR, Garland M, Myers MM, et al. Prenatal nicotine-exposure alters fetal autonomic activity and medullary neurotransmitter receptors: implications for sudden infant death syndrome. J Appl Physiol. 2009;107:1579–1590. doi: 10.1152/japplphysiol.91629.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Waters KA, Meehan B, Huang JQ, Gravel RA, Michaud J, Cote A. Neuronal apoptosis in sudden infant death syndrome. Pediatr Res. 1999;45:166–172. doi: 10.1203/00006450-199902000-00002. [DOI] [PubMed] [Google Scholar]
- 82.Cruz-Sanchez FF, Lucena J, Ascaso C, Tolosa E, Quinto L, Rossi ML. Cerebellar cortex delayed maturation in sudden infant death syndrome. J Neuropathol Exp Neurol. 1997;56:340–346. doi: 10.1097/00005072-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 83.Kinney HC, McHugh T, Miller K, Belliveau RA, Assmann SF. Subtle developmental abnormalities in the inferior olive: An indicator of prenatal brainstem injury in the sudden infant death syndrome. J Neuropathol Exp Neurol. 2002;61:427–441. doi: 10.1093/jnen/61.5.427. [DOI] [PubMed] [Google Scholar]
- 84.Kinney HC, Brody BA, Finkelstein DM, Vawter GF, Mandell F, Gilles FH. Delayed central nervous system myelination in the sudden infant death syndrome. J Neuropathol Exp Neurol. 1991;50:29–48. doi: 10.1097/00005072-199101000-00003. [DOI] [PubMed] [Google Scholar]
- 85.Henderson LA, Macey PM, Richard CA, Runquist ML, Harper RM. Functional magnetic resonance imaging during hypotension in the developing animal. J Appl Physiol. 2004;97:2248–2257. doi: 10.1152/japplphysiol.00297.2004. [DOI] [PubMed] [Google Scholar]
- 86.Gozal D, Dong XW, Rector DM, Harper RM. Maturation of kitten ventral medullary surface activity during pressor challenges. Dev Neurosci. 1995;17:236–245. doi: 10.1159/000111292. [DOI] [PubMed] [Google Scholar]
- 87.Liu Q, Fehring C, Lowry TF, Wong-Riley MT. Postnatal development of metabolic rate during normoxia and acute hypoxia in rats: Implication for a sensitive period. J Appl Physiol. 2009;106:1212–1222. doi: 10.1152/japplphysiol.90949.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Q, Wong-Riley MT. Postnatal changes in the expressions of serotonin 1A, 1B, and 2A receptors in ten brain stem nuclei of the rat: implication for a sensitive period. Neuroscience. 165:61–78. doi: 10.1016/j.neuroscience.2009.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Q, Lowry TF, Wong-Riley MT. Postnatal changes in ventilation during normoxia and acute hypoxia in the rat: Implication for a sensitive period. J Physiol. 2006;577:957–970. doi: 10.1113/jphysiol.2006.121970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu Q, Wong-Riley MT. Developmental changes in the expression of GABAA receptor subunits alpha1, alpha2, and alpha3 in the rat pre-Botzinger complex. J Appl Physiol. 2004;96:1825–1831. doi: 10.1152/japplphysiol.01264.2003. [DOI] [PubMed] [Google Scholar]
- 91.Anderson ME, Johnson DC, Batal HA. Sudden Infant Death Syndrome and prenatal maternal smoking: rising attributed risk in the Back to Sleep era. BMC Med. 2005;3:4. doi: 10.1186/1741-7015-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Duncan JR, Paterson DS, Kinney HC. The development of nicotinic receptors in the human medulla oblongata: inter-relationship with the serotonergic system. Auton Neurosci. 2008;144:61–75. doi: 10.1016/j.autneu.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bulterys MG, Greenland S, Kraus JF. Chronic fetal hypoxia and sudden infant death syndrome: interaction between maternal smoking and low hematocrit during pregnancy. Pediatrics. 1990;86:535–540. [PubMed] [Google Scholar]