Abstract
Primary liver cancer (PLC) represents approximately 4% of all new cancer cases diagnosed worldwide. The purpose of this review is to describe some of the latest international patterns in PLC incidence and mortality, as well as to give an overview of the main etiological factors. We used two databases, GLOBOCAN 2002 and the World Health Organization (WHO) mortality database to analyze the incidence and mortality rates for PLC in several regions around the world. The highest age adjusted incidence rates (>20 per 100,000) were reported from countries in Southeast Asia and sub-Saharan Africa that are endemic for HBV infection. Countries in Southern Europe have medium-high incidence rates, while low-incidence areas (<5 per 100,000) include South and Central America, and the rest of Europe.
Cirrhosis is present in about 80–90% of HCC patients and is thereby the largest single risk factor. Main risk factors include HBV, HCV, aflatoxin and possibly obesity and diabetes. Together HBV and HCV account for 80–90% of all HCC worldwide. HBV continues to be the major HCC risk factor worldwide, although its importance will most likely decrease during the coming decades due to the widespread use of the HBV vaccine in the newborns. HCV has been the dominant viral cause in HCC in North America, some Western countries and Japan. Obesity and diabetes are increasing at a fast pace throughout the world, and if they are proven to be HCC risk factors, they would account for more HCC cases in the future.
Keywords: Cirrhosis, Epidemiology, GLOBOCAN, Hepatitis, Liver cancer
1. Overview
Primary liver cancer (PLC) represents approximately 4% of all new cancer cases diagnosed worldwide. PLC is the third most common cause of cancer-related death among men and the sixth among women, respectively. Annually, more than 560,000 people are diagnosed with PLC and approximately the same number die with it, demonstrating the dismal prognosis of this cancer [1]. Hepatocellular carcinoma (HCC) accounts for 85–90% of all PLC and these two entities are often used interchangeably [2]. The etiology of HCC, with >80% arising in patients infected by hepatitis B virus (HBV) or hepatitis C virus (HCV) [3], explains the distinct geographic distribution of liver cancer, with the majority of cases seen in the developing world where these infections are or have been endemic. During the past few decades much attention has been drawn to the rising HCC incidence rates seen in several regions throughout the world, but data are also starting to congregate from some countries that the trend might be changing. In Italy and Denmark, for instance, the HCC incidence is already decreasing, or projected to start decreasing within the next few years [4,5]. These trends are likely related to the prevalence and patterns of HBV and HCV in the underlying population.
The purpose of this review is to describe some of the latest international patterns in PLC incidence and mortality, as well as to give an overview of the main etiological factors.
2. Global trends in primary liver cancer
We used two databases, GLOBOCAN 2002 and the World Health Organization (WHO) mortality database [6,7] to extract, analyze and report the incidence and mortality rates for PLC in several regions around the world. The incidence and mortality data in GLOBOCAN 2002 was compiled by the International Agency for Research on Cancer (IARC). GLOBOCAN 2002 uses data from national cancer registries when available and, if such data is not available, rates for incidence and mortality have been estimated using earlier data or data from neighbouring regions. Rates have been computed for the year 2002, but data are generally from 2–5 years earlier. The WHO mortality database provides annual mortality statistics that were obtained from civil registration systems in countries with a high level of certification of cause-of-death. Both databases identify PLC cases using the International Classification of Diseases 10th revision (ICD-10) code C22, and both report age-standardized rates calculated using the 1960 world standard population.
3. Incidence of PLC
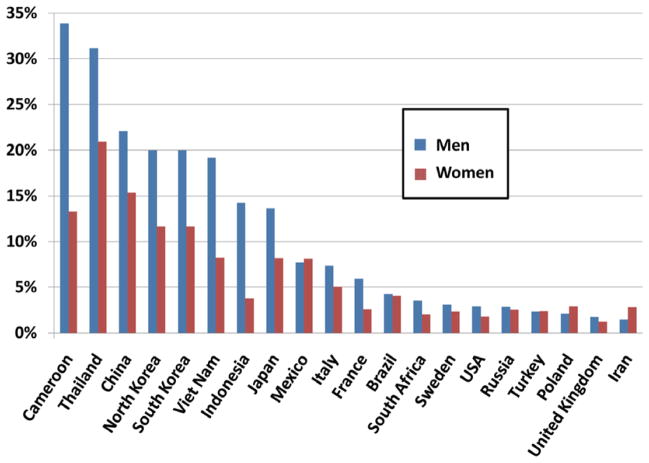
Figure 1 shows the considerable regional heterogeneity in the incidence of PLC worldwide. This geographical variation is illustrated more in detail in Fig. 2, where select examples of age-standardized incidence rates (ASR, cases per 100,000) of PLC in several regions, calculated separately for men and women are shown. The highest ASRs (>20) were reported from countries in Southeast Asia (North and South Korea, China, Vietnam). These regions are endemic for HBV infection with most PLC in these regions constituted by HCC. An exception to this is Thailand where cholangiocarcinoma due to high exposure to liver flukes is the predominant form of PLC. Another exception is Japan where HCV is the predominant risk factor for HCC.
Fig. 1.
Age-standardized incidence rates of primary liver cancer worldwide, estimated for 2002. Source: GLOBOCAN 2002, with estimates for 2002. The Age-Standardized Rate is calculated using the 1960 world standard population in 5 age-groups 0–14, 15–44, 45–54, 55–64, 65+.
Fig. 2.
Age-standardized incidence rates of primary liver cancer, per 100,000 population at risk. Source: GLOBOCAN 2002, with estimates for 2002. The Age-Standardized Rate is calculated using the 1960 world standard population in 5 age-groups 0–14, 15–44, 45–54, 55–64, 65+.
Due to its very large population and high ASRs, China alone sees more than 55% of all PLCs worldwide, with rates of 37.9 in males and 14.2 in females (Fig. 2). Other high-incidence areas outside Southeast Asia include sub-Saharan African countries such as Cameroon and Mozambique. In general, countries in Southern Europe have medium-high incidence rates (ASR in males: 10–15), with Italy on the high end with an ASR of 15.9 in men and 5.1 in women. Low-incidence areas include South and Central America, Europe excluding the Mediterranean countries and North America (ASRs in males: <6). In the United States, the ASR more than doubled during the past two decades, an increase most likely due to HCV-related cirrhosis [2].
4. Mortality
PLC accounted for around 1% of all deaths worldwide in 2004 [8]. There is considerable regional variation in PLC mortality that tend to mirror those reported for incidence rates. In addition, there are also significant differences in the proportion of total cancer mortality attributable to PLC in different regions (Fig. 3). For example, in Cameroon and Thailand, about one third of all cancer deaths are due to PLC, compared to less than 5% in most Western countries in Europe and North America.
Fig. 3.
Percentage of age-standardized cancer mortality due to primary liver cancer in selected countries, by sex. Source: GLOBOCAN 2002, with estimates for 2002. The Age-Standardized Rate is calculated using the 1960 world standard population in 5 age-groups 0–14, 15–44, 45–54, 55–64, 65+.
Due to the poor survival for PLC patients, the main burden of PLC is due to premature mortality rather than long-term illness. Estimates from the Global Burden of Disease Study1 cancer-related YLDs among males and 0.7% among females [8].
5. Survival
The similarity between mortality rates and incidence rates for PLC within a given population is indicative of the poor survival prospects for most PLC patients. The median survival of PLC patients is estimated at less than 1 year [9]. Without effective treatment the reported median survival is dismal, at less than 5 months [10,11]. In a study using the SEER cancer registry data from the US, in 1992–1999, median survival was 3.5 months and survival rates after 1 and 3 years of diagnosis 20.9% and 5.7%, respectively [12]. However, clinic and hospital based studies often report much higher survival rates. For example, a study from Italy from the 1990s, reported an 18 month median survival [13]. This reflects the notion that survival in population-based registries is often worse than that observed in the clinical setting [9].
The low PLC survival rates are related to the lack of observable symptoms for early stage PLC, aggressive disease nature, concomitant hepatic decompensation, and limited availability of potential therapeutic options. Patients presenting with symptomatic PLC usually have well advanced disease and treatment options are limited. Transplantation and surgical resection remain the most effective treatment for early localized tumours, however, only a minority is diagnosed at that stage and of these, only a minority receives potentially curative therapy [12].
5.1. Age
The global age distribution of HCC varies by region, incidence rate, gender and, possibly, by etiology [14]. In most Western low-risk populations, the highest age-specific rates occur among persons aged 75 and older (Fig. 4). A similar pattern is seen among most high-risk Asian populations (e.g., Hong Kong). In contrast, male rates in high-risk African populations (e.g., Gambia, Mali) tend to peak between ages 60 and 65 before declining; while female rates peak between 65 and 70 before declining. Countries such as South Africa and Egypt show more similarity to low-risk population areas than high-risk African populations with rates that peak over age 85 [6]. These variable age-specific patterns are likely related to differences in the dominant hepatitis virus in the population, the age at viral infection and the existence of other risk factors. Notably, while most HCV carriers become infected as adults, most HBV carriers become infected at very young ages.
Fig. 4.
Age distribution of primary liver cancer mortality (per 100,000) in select examples of high-, middle- and low-incidence countries. Source: WHO mortality database. M: Male, F: female.
5.2. Sex
In almost all populations, males have higher liver cancer rates than females, with male:female ratios usually averaging between 2:1 and 4:1. The male predominance in PLC is depicted in Fig. 2, where the highest ratios are seen especially in high- and medium-incidence areas. For example, the sex ratio is 5:1 (males:females) in France. However, PLC is more equally distributed among men and women in low-incidence countries in South and Central America. Similar to the sex differences seen in incidence rates, men are predominantly affected with PLC-related deaths in high- and medium-incidence areas. When the PLC mortality is expressed as proportion of total cancer mortality for a given region, the sex ratio tends to be less pronounced, especially in medium- and low-incidence areas, with the sex ratio in Italy, for example, decreasing from 3.1 to 1.4. (Fig. 3) This indicates that some of the male predominance seen in HCC can be explained by higher cancer rates in males in general.
However, the remaining sex differences still need to be explained. The reasons for higher rates of liver cancer in males may relate to gender-specific differences in exposure to risk factors. Men are more likely to be infected with HBV and HCV, consume alcohol, smoke cigarettes, have higher body mass index (BMI) and also have increased iron stores. A possible role for sex hormones in the development of HCC has also been suggested [15]. However, available data do not fully explain these observed sex differences in HCC incidence rates.
5.3. Ethnicity
HCC incidence rates vary not only geographically, but also vary substantially among different populations living in the same region. In a study from the United States, 6% of Whites diagnosed with HCC were HBsAg-positive, compared to 16% of Blacks and 50% of Asians. HCV seropositivity showed an opposite pattern with 30% of Asians being infected compared to around 50% of Whites and Blacks [16]. In another study from the United States, the age-adjusted HCC incidence rates in individuals of Asian/Pacific Islander ethnicity was almost three times higher than in Whites (ASR 11.7 and 3.9, respectively), with Hispanics and Blacks in between these two extremes (ASRs 8.0 and 7.0, respectively) [17]. In the United States a large proportion of the individuals that have been studied may be first generation immigrants, and so the differences noticed could in part be due to early environmental factors rather than ethnicity alone. However, in Malaysia where the proportion of recent immigrants is much lower, there is still an unexplained difference in HCC rates among the ethnic groups, with Chinese males (ASR 8.0/100,000 years) having almost twice as high a risk as Indians and Malays (ASRs 4.2 and 4.8/100,000 years, respectively) [18].
5.4. Cirrhosis
Cirrhosis is present in about 80–90% of HCC patients and is thereby the largest single risk factor [19,20]. The risk of developing HCC in cirrhosis patients varies considerably with the underlying condition [21]. The highest 5-year cumulative risks are seen in HCV cirrhosis (30% in Japan and 17% in the West), hemochromatosis (21%), HBV cirrhosis (15% in Asia and 10% in the West), alcoholic cirrhosis (8%) and biliary cirrhosis (4%) [22]. Furthermore, the stage of cirrhosis also seems to be an independent risk factor in itself. In an Italian cohort study, those who at study entry had Child-Pugh stage B or C had a threefold increased risk of HCC compared to those with stage A [23].
6. Risk factors for HCC
Main risk factors include HBV, HCV, aflatoxin and possibly obesity and diabetes. Together HBV and HCV account for 80–90% of all HCC worldwide [3]. The attributable fractions for HBV and HCV are shown in Table 1.
Table 1.
Estimated cases of primary liver cancer in 2002, attributable to HBV and HCV
| Primary liver cancer cases | HBV
|
HCV
|
Cases attributable to HBV or HCV | |||
|---|---|---|---|---|---|---|
| Attributable fraction (%) | Attributable cases | Attributable fraction (%) | Attributable cases | |||
| Developed countries | 110,800 | 23.3 | 25,800 | 19.9 | 22,000 | 48,000 |
| Developing countries | 515,300 | 58.8 | 303,000 | 33.4 | 172,000 | 475,000 |
| Total | 626,100 | 54.4 | 340,600 | 31.1 | 195,000 | 535,000 |
Source: Adapted from Parkin, 2006 [25].
6.1. HBV
According to the WHO, around 350 million people around the world are estimated to be chronically infected by HBV [24]. Worldwide, chronic HBV infection accounts for approximately 50% of the cases [25]. However, the regional variation is enormous. In South Korea about 70% of the HCC cases are attributed to HBV compared to only 15% in Japan [26]. Rates in Europe and the United States vary as well, with estimates varying from a 3% HBsAg seroprevalence in HCC cases in Sweden, 9% in the United States, 19% in Italy, to 55% in Greece [27]. HCC associated with HBV results from chronic inflammation and repeated cellular regeneration with the HCC typically being diagnosed after 25–30 years of infection [28]. It has been estimated that the lifetime risk of HCC for a person with a chronic HBV infection is between 10% and 25% [29]. In two meta-analyses of case-control and cross-sectional studies the relative risk for lifetime HCC among HBV infected individuals was estimated at 15.6 and 20.4, respectively [30,31].
The great majority, between 70% and 90%, of HBV-related HCC develops in livers already affected by cirrhosis [2]. HBV DNA is found in the host genome of both infected and malignant hepatic cells. HBV might thus exert its carcinogenic potential by increasing the likelihood of viral DNA insertion in or near proto-oncogenes or tumour-suppressor genes. However, despite initial attention drawn by this discovery, subsequent research has failed to explain the mechanism by which integration of HBV DNA leads to HCC.
HBV is a highly contagious virus, that is transmitted by percutaneous and permucosal exposure to infected blood or other body fluids. The increased HCC risk associated with HBV infection particularly applies to areas where HBV is endemic. In these areas, it is usually transmitted from mother to newborn (vertical transmission) and up to 90% of infected persons follow a chronic course. This pattern is different in areas with low HCC incidence rates where HBV is acquired in adulthood through sexual and parenteral routes (horizontal transmission) with >90% of acute infections resolving spontaneously.
Several other factors have been reported to increase HCC risk among HBV carriers including: male gender, older age (or longer duration of infection), Asian or African race, cirrhosis, family history of HCC, exposure to aflatoxin, alcohol or tobacco or co-infection with HCV or HDV. HCC risk is also increased in patients with higher levels of HBV replication, as indicated by high HBV DNA levels and presence of HBeAg. Using sensitive amplification assays, many studies have demonstrated that HBV DNA persists as “occult HBV infection” for decades among persons with serological recovery (HBsAg negative) from acute infection. Occult HBV is associated with anti-HBc and/or anti-HBs [32]. However, in a significant proportion of individuals, neither anti-HBc nor anti-HBs can be detected. A single multinational investigation found prevalence of occult HBV in liver tissue to be 11% in Italy, 5–9% in Hong Kong, and 0% in the UK. Supporting an association with occult HBV, a high proportion of individuals with HCV infection who develop HCC have demonstrable HBV DNA and proteins in their neoplastic and adjacent non-neoplastic liver tissue. However, although some studies have linked development of HCC in individuals with chronic HCV infection to occult HBV, others have not found an association.
Since 1982 there is a safe and effective HBV vaccine, but due to the vaccine being relatively expensive (2USD/dose) it took more than 20 years for the vaccine to be used worldwide [33]. As of 2006, 164 countries vaccinate infants against HBV in national immunization programs, compared to 31 countries in 1992 [24]. Frequently cited reports from Taiwan have described how the introduction of a universal vaccination program against HBV started in the 1980s resulted in reduction of HCC incidence rates in children a decade later [34].
6.2. Dietary aflatoxin
Aflatoxins are naturally occurring potent hepatocarcinogenic mycotoxins produced by some Aspergillus species. They are weedy molds that grow on a large number of substrates, including grains, corn, cassava, peanuts, and fermented soy beans, particularly under high moisture conditions in parts of sub-Saharan Africa and eastern Asia. In areas where ingestion of aflatoxin-contaminated food is common, the incidence rates of HCC tend to be high, most likely due to a characteristic mutation in the p53 tumour suppressor gene that has been found in 30–60% of all HCCs in these areas [35]. Furthermore, it has been found that individuals infected by HBV and who are exposed to aflatoxin have an even higher risk of liver cancer, suggesting a synergistic effect between HBV and aflatoxin. A prospective cohort study from China found that among individuals with chronic HBV infection the adjusted relative risk (RR) for HCC increased from 7.3 in HBV-infected with no aflatoxin exposure to 60 in those who were also exposed to aflatoxin [36].
6.3. HCV
The association between HCV infection and increased HCC risk is well established and has been shown in case-control as well as cohort studies. Markers of HCV infection are found in a variable proportion of HCC cases; for example, 44–66% in Italy [37,38], and in 80–90% of HCC cases in Japan [39], and constitutes the major HCC risk factor in Western countries and Japan. The global HCV prevalence, according to WHO, is estimated to be 2%, or one third of the prevalence of HBV [40]. If PCR testing of liver tissue and/or serum had been used, a higher proportion of HCC patients might have had HCV, even if the antibody to HCV (anti-HCV) was non-detectable. In a meta-analysis of 21 case-control studies in which second generation enzyme immunoassay tests for anti-HCV were used, HCC risk was increased 17-fold in HCV-infected patients compared with HCV-negative controls [41].
In low-rate HCC areas, there are increasing numbers of persons living with cirrhosis related to HCV as well as a general improvement in survival among all cirrhosis patients. It has been estimated that HCV began to infect large numbers of young adults in Japan in 1920s, in southern Europe in 1940s and in North America in the 1960s and 1970s as a result of contaminated needles and/or injection drug use [42]. The virus then migrated into national blood supplies and circulated until a screening test was developed in 1990, after which time the rates of new infection dropped dramatically.
The rate of HCC development in HCV-infected persons ranges from 1 to 3% after 30 years [43]. HCV increases HCC risk by promoting fibrosis and eventually cirrhosis. Once cirrhosis is established, the annual incidence of HCC is 1 to 4% [21]. Rates of cirrhosis 25–30 years post-infection range between 15 and 35% [44], and after initial HCV exposure it takes an average of about 30 years to develop HCC [45]. In HCV-infected patients, factors related to host and environment/lifestyle appear to be more important than viral factors in determining progression to cirrhosis. These factors include: older age overall as well as older age at the time of acquisition of infection, male gender, heavy alcohol intake (>50 g/day), diabetes, obesity, and co-infection with HIV or HBV [46].
6.4. Alcohol
Heavy alcohol intake, defined as ingestion of >50–70 g/day for prolonged periods, is a well-established HCC risk factor. It is unclear whether risk of HCC is significantly altered in those with low or moderate alcohol intake. Most data point to the conclusion that alcohol does not have a carcinogenic effect in itself; the increased risk is rather through the cirrhosis that prolonged alcohol intake can cause. However, there is evidence for a synergistic effect of heavy alcohol ingestion with HCV or HBV, with these factors presumably operating together to increase HCC risk by more actively promoting cirrhosis. In one study it was reported that among alcohol drinkers, HCC risk increased in a linear fashion with daily intake >60 g, a risk that doubled with concomitant presence of HCV infection [41].
6.5. Cryptogenic
Many studies conducted in Western countries fail to identify a major risk factor (HBV, HCV, alcohol) for chronic liver disease or HCC in a large proportion of patients (30–40%). It has been suggested that many cryptogenic cirrhosis and HCC cases in fact represent more severe forms of non-alcoholic fatty liver disease (NAFLD), namely non-alcoholic steatohepatitis (NASH). Potential risk factors such as diabetes and obesity increase HCC risk at least partly by promoting NAFLD and NASH which then increases cirrhosis risk.
6.6. Obesity
The increasing incidence in HCC has paralleled that of obesity and type 2 diabetes in many countries. It has further been estimated that up to 90% of all obese individuals (BMI >30 kg/m2) and up to 70% of all people with type II diabetes have some type of fatty liver disease [47]. The effect of obesity on HCC risk has been examined in several cohort studies. In a large prospective cohort study of more than 900,000 individuals from around the USA followed for a 16-year period, liver cancer mortality rates were 4.5 higher in men with a BMI >35 and 1.7 higher in women with a BMI >35 compared to normal-weight individuals [48]. Two other population-based cohort studies from Sweden and Denmark found a 2–3-fold increased HCC risk in obese men and women compared to those with normal BMI [49,50]. The greatest increase in risk with obesity was observed with concomitant HCV infection (Hazard Ratio = 4.1, 95% CI 1.4–12.4). While a more than twofold excess risk that approached significance was also observed among persons who were negative for both HBV and HCV infection, obesity conveyed only a very modest and non-significant 1.4-fold excess risk among persons with HBV infection. There was, however, evidence of a very strong synergism between obesity and diabetes which, when both conditions occurred together, conveyed a 100-fold excess HCC risk with obesity in the context of either HBV or HCV infection.
6.7. Diabetes
Diabetes, particularly type II, has been proposed to be a risk factor for both chronic liver disease and HCC possibly through development of NAFLD and NASH. Several case-control studies have examined the association between diabetes, mostly type II, and HCC. The majority found a statistically significant association between HCC and 50% to 100% increased diabetes. However, reverse causality is a concern in all these studies because in some cases diabetes might itself be a result of cirrhosis. A few cohort studies, better suited to evaluate temporality, have been conducted, showing that individuals with type II diabetes had on average a doubled risk of developing HCC, with one showing an association between longer duration of diabetes and increased HCC risk [38,51]. Additional research is needed to examine how any excess risk conveyed by diabetes is mediated by duration and treatment of diabetes, family history of diabetes, obesity and physical activity.
6.8. Tobacco
The relationship between cigarette smoking and HCC has been examined in more than 50 studies in both low- and high-rate areas. In almost all countries, both positive association and lack of association findings have been reported. Taken together, available evidence suggests that any effect of smoking on HCC is likely to be weak and limited to a subset of the general population.
6.9. Diet
The role of diet, except for alcohol drinking and aflatoxin contamination, in the etiology of HCC in human populations is largely unknown. Dietary anti-oxidants including selenium as well as retinoic acid and beta-carotene have been shown to inhibit hepatocarcinogenesis in animals. However, epidemiologic data are fairly limited and in some places conflicting. Several studies performed in Southern Europe, predominantly in Italy, have evaluated various dietary factors as potential risk or protective factors for HCC. A positive, but weak, effect of high intake of specific foods including milk and yogurt, white meats, eggs and fruits and of selected macronutrients including beta-carotene was reported by a multi-center hospital based case-control study in Italy [52].
6.10. Coffee drinking
Coffee drinking has been studied extensively in relation to HCC. Several epidemiological studies have previously reported that coffee drinking reduces risk of elevated liver enzymes and of cirrhosis, while animal studies suggest that coffee reduces liver carcinogenesis. Further, coffee drinking has also been associated with reduced insulin levels as well as reduced risk of type II diabetes, in itself considered to be a risk factor for HCC [53]. Both case-control and cohort studies conducted in Japan and Southern Europe specifically evaluated the relationship between coffee consumption and HCC risk, most reporting a significantly reduced risk of HCC with increased consumption [54–56].
7. Future trends
HBV continues to be the major HCC risk factor worldwide, although its importance will most likely decrease during the coming decades due to the widespread use of the HBV vaccine in newborns in HBV endemic and HCC high-incidence areas. This effect may become more tangible as the first individuals to get immunized grow older. HCV has been the dominant viral cause in HCC in North America, some Western countries and Japan. Obesity and diabetes are increasing at a fast pace throughout the world, and if they are established as HCC risk factors, whether independently or in the presence of viral hepatitis or alcohol abuse, these conditions would plausibly account for more HCC cases in the future.
Footnotes
The Global Burden of Disease Study is a project originally commissioned by the World Bank in 1991 to provide a comprehensive assessment of the burden of the most common diseases and injuries introducing the disability-adjusted life years measure, a time-based measure that combines years of life lost due to premature mortality and years of life lost due to time lived in reveal that PLC caused nearly 7 million years of life lost (YLL) accounting for 11.6% and 5.9% of cancer-related YLLs in men and women, respectively. In contrast, PLC was estimated to be responsible for approximately 60,000 years lost due to disability (YLD) during 2004, comprising 2.7% of health states less than ideal health. The Global Burden of Disease report was last updated by WHO in 2004.
Conflict of interest
The corresponding author has received a fee from Bayer HealthCare for his contribution to this supplement. Bayer HealthCare played no role in the preparation, review, or approval of the manuscript. The co-authors have no conflict of interest to report.
References
- 1.Stewart BW, Kleihues P, editors. World Cancer Report. Lyon: IARC Press; 2003. [Google Scholar]
- 2.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 3.Bosch FX, Ribes J, Cleries R, et al. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9(2):191–211. doi: 10.1016/j.cld.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Bosetti C, Bianchi C, Negri E, et al. Estimates of the incidence and prevalence of hepatocellular carcinoma in Italy in 2002 and projections for the years 2007 and 2012. Tumori. 2009;95(1):23–7. doi: 10.1177/030089160909500104. [DOI] [PubMed] [Google Scholar]
- 5.Jepsen P, Vilstrup H, Tarone RE, et al. Incidence rates of hepatocellular carcinoma in the U.S. and Denmark: recent trends. Int J Cancer. 2007;121(7):1624–6. doi: 10.1002/ijc.22860. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Mortality database. WHO Statistical Information System; 2008. http://www.who.int/whosis. [Google Scholar]
- 7.GLOBOCAN. International Agency for Research on Cancer (IARC); 2002. http://www-dep.iarc.fr. [Google Scholar]
- 8.Global burden of disease (The): 2004 update. World Health Organization; 2008. [Google Scholar]
- 9.Nguyen VT, Law MG, Dore GJ. Hepatitis B-related hepatocellular carcinoma: epidemiological characteristics and disease burden. J Viral Hepat. 2009;16(7):453–63. doi: 10.1111/j.1365-2893.2009.01117.x. [DOI] [PubMed] [Google Scholar]
- 10.Yeung YP, Lo CM, Liu CL, et al. Natural history of untreated nonsurgical hepatocellular carcinoma. Am J Gastroenterol. 2005;100(9):1995–2004. doi: 10.1111/j.1572-0241.2005.00229.x. [DOI] [PubMed] [Google Scholar]
- 11.Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56(4):918–28. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 12.El-Serag HB, Siegel AB, Davila JA, et al. Treatment and outcomes of treating of hepatocellular carcinoma among Medicare recipients in the United States: a population-based study. J Hepatol. 2006;44(1):158–66. doi: 10.1016/j.jhep.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Lerose R, Molinari R, Rocchi E, et al. Prognostic features and survival of hepatocellular carcinoma in Italy: impact of stage of disease. Eur J Cancer. 2001;37(2):239–45. doi: 10.1016/s0959-8049(00)00354-3. [DOI] [PubMed] [Google Scholar]
- 14.Parkin DM, Whelan SL, Ferlay J, et al. Cancer Incidence in Five Continents. VIII. IARC Scientific Publication; 2002. p. 155. [Google Scholar]
- 15.Yu MW, Chang HC, Chang SC, et al. Role of reproductive factors in hepatocellular carcinoma: Impact on hepatitis B- and C-related risk. Hepatology. 2003;38(6):1393–400. doi: 10.1016/j.hep.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 16.Di Bisceglie AM, Lyra AC, Schwartz M, et al. Hepatitis C-related hepatocellular carcinoma in the United States: influence of ethnic status. Am J Gastroenterol. 2003;98(9):2060–3. doi: 10.1111/j.1572-0241.2003.t01-1-07641.x. [DOI] [PubMed] [Google Scholar]
- 17.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27(9):1485–91. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim GCC, Yahaya H, Lim TO. The first report of the national cancer registry. [Accessed Nov 10th 2009];Cancer incidence in Malaysia. 2002–2003 http://www.crc.gov.my.
- 19.Colombo M, de Franchis R, Del Ninno E, et al. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med. 1991;325(10):675–80. doi: 10.1056/NEJM199109053251002. [DOI] [PubMed] [Google Scholar]
- 20.Tiribelli C, Melato M, Croce LS, et al. Prevalence of hepatocellular carcinoma and relation to cirrhosis: comparison of two different cities of the world – Trieste, Italy, and Chiba, Japan. Hepatology. 1989;10(6):998–1002. doi: 10.1002/hep.1840100618. [DOI] [PubMed] [Google Scholar]
- 21.Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112(2):463–72. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 22.Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5 Suppl 1):S35–50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Bolondi L, Sofia S, Siringo S, et al. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut. 2001;48(2):251–9. doi: 10.1136/gut.48.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO. [Accessed Nov 10th 2009];Hepatitis B. 2008 (Fact sheet No. 204 ). http://www.who.int/mediacentre/factsheets/fs204/en/
- 25.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118(12):3030–44. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 26.Kim SR, Kudo M, Hino O, et al. Epidemiology of hepatocellular carcinoma in Japan and Korea. A review Oncology. 2008;75(Suppl 1):13–6. doi: 10.1159/000173419. [DOI] [PubMed] [Google Scholar]
- 27.Raza SA, Clifford GM, Franceschi S. Worldwide variation in the relative importance of hepatitis B and hepatitis C viruses in hepatocellular carcinoma: a systematic review. Br J Cancer. 2007;96(7):1127–34. doi: 10.1038/sj.bjc.6603649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337(24):1733–45. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 29.Seeger C, Mason WS. Hepatitis B virus biology. Microbiol Mol Biol Rev. 2000;64(1):51–68. doi: 10.1128/mmbr.64.1.51-68.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi J, Zhu L, Liu S, Xie WF. A meta-analysis of case-control studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma in China. Br J Cancer. 2005;92(3):607–12. doi: 10.1038/sj.bjc.6602333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donato F, Boffetta P, Puoti M. A meta-analysis of epidemiological studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma. Int J Cancer. 1998;75(3):347–54. doi: 10.1002/(sici)1097-0215(19980130)75:3<347::aid-ijc4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 32.Torbenson M, Thomas DL. Occult hepatitis B. Lancet Infect Dis. 2002;2(8):479–86. doi: 10.1016/s1473-3099(02)00345-6. [DOI] [PubMed] [Google Scholar]
- 33.Hepatitis B vaccines. Wkly Epidemiol Rec. 2004;79(28):255–63. [PubMed] [Google Scholar]
- 34.Chang MH, Chen CJ, Lai MS, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336(26):1855–9. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 35.Zhang YJ, Chen Y, Ahsan H, et al. Silencing of glutathione S-transferase P1 by promoter hypermethylation and its relationship to environmental chemical carcinogens in hepatocellular carcinoma. Cancer Lett. 2005;221(2):135–43. doi: 10.1016/j.canlet.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 36.Qian GS, Ross RK, Yu MC, et al. A follow-up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, People’s Republic of China. Cancer Epidemiol Biomarkers Prev. 1994;3(1):3–10. [PubMed] [Google Scholar]
- 37.Fasani P, Sangiovanni A, De Fazio C, et al. High prevalence of multinodular hepatocellular carcinoma in patients with cirrhosis attributable to multiple risk factors. Hepatology. 1999;29(6):1704–7. doi: 10.1002/hep.510290604. [DOI] [PubMed] [Google Scholar]
- 38.Stroffolini T, Andreone P, Andriulli A, et al. Gross pathologic types of hepatocellular carcinoma in Italy. Oncology. 1999;56(3):189–92. doi: 10.1159/000011963. [DOI] [PubMed] [Google Scholar]
- 39.Yoshizawa H. Hepatocellular carcinoma associated with hepatitis C virus infection in Japan: projection to other countries in the foreseeable future. Oncology. 2002;62(Suppl 1):8–17. doi: 10.1159/000048270. [DOI] [PubMed] [Google Scholar]
- 40.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5(9):558–67. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 41.Donato F, Tagger A, Gelatti U, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002;155(4):323–31. doi: 10.1093/aje/155.4.323. [DOI] [PubMed] [Google Scholar]
- 42.Armstrong GL, Alter MJ, McQuillan GM, et al. The past incidence of hepatitis C virus infection: implications for the future burden of chronic liver disease in the United States. Hepatology. 2000;31(3):777–782. doi: 10.1002/hep.510310332. [DOI] [PubMed] [Google Scholar]
- 43.Hassan MM, Frome A, Patt YZ, et al. Rising prevalence of hepatitis C virus infection among patients recently diagnosed with hepatocellular carcinoma in the United States. J Clin Gastroenterol. 2002;35(3):266–9. doi: 10.1097/00004836-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 44.Freeman AJ, Dore GJ, Law MG, et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology. 2001;34(4 Pt 1):809–16. doi: 10.1053/jhep.2001.27831. [DOI] [PubMed] [Google Scholar]
- 45.Tong MJ, el-Farra NS, Reikes AR, et al. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332(22):1463–66. doi: 10.1056/NEJM199506013322202. [DOI] [PubMed] [Google Scholar]
- 46.Cramp ME. Hbv + Hcv = Hcc? Gut. 1999;45(2):168–9. doi: 10.1136/gut.45.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37(5):1202–19. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 48.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 49.Moller H, Mellemgaard A, Lindvig K, et al. Obesity and cancer risk: a Danish record-linkage study. Eur J Cancer. 1994;30A(3):344–50. doi: 10.1016/0959-8049(94)90254-2. [DOI] [PubMed] [Google Scholar]
- 50.Wolk A, Gridley G, Svensson M, et al. A prospective study of obesity and cancer risk (Sweden) Cancer Causes Control. 2001;12(1):13–21. doi: 10.1023/a:1008995217664. [DOI] [PubMed] [Google Scholar]
- 51.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126(2):460–8. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 52.Talamini R, Polesel J, Montella M, et al. Food groups and risk of hepatocellular carcinoma: A multicenter case-control study in Italy. Int J Cancer. 2006;119(12):2916–21. doi: 10.1002/ijc.22267. [DOI] [PubMed] [Google Scholar]
- 53.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4(3):369–80. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 54.Bravi F, Bosetti C, Tavani A, et al. Coffee drinking and hepatocellular carcinoma risk: a meta-analysis. Hepatology. 2007;46(2):430–5. doi: 10.1002/hep.21708. [DOI] [PubMed] [Google Scholar]
- 55.Montella M, Polesel J, La Vecchia C, et al. Coffee and tea consumption and risk of hepatocellular carcinoma in Italy. Int J Cancer. 2007;120(7):1555–9. doi: 10.1002/ijc.22509. [DOI] [PubMed] [Google Scholar]
- 56.Shimazu T, Tsubono Y, Kuriyama S, et al. Coffee consumption and the risk of primary liver cancer: pooled analysis of two prospective studies in Japan. Int J Cancer. 2005;116(1):150–4. doi: 10.1002/ijc.20989. [DOI] [PubMed] [Google Scholar]