Abstract
Background
NaV1.5 is a mechanosensitive voltage gated sodium-selective ion channel responsible for the depolarizing current and maintenance of the action potential plateau in the heart. Ranolazine is a NaV1.5 antagonist with anti-anginal and anti-arrhythmic properties.
Methods and Results
Mechanosensitivity of NaV1.5 was tested in voltage-clamped whole cells and cell-attached patches by bath flow and patch pressure, respectively. In whole cells, bath flow increased peak inward current in both murine ventricular cardiac myocytes (24±8%) and HEK cells heterologously expressing NaV1.5 (18±3%). The flow-induced increases in peak current were blocked by ranolazine. In cell-attached patches from cardiac myocytes and NaV1.5-expressing HEK cells, negative pressure increased NaV peak currents by 27±18% and 18±4% and hyperpolarized voltage dependence of activation by -11 mV and -10 mV, respectively. In HEK cells, negative pressure also increased the window current (250%) and increased late open channel events (250%). Ranolazine decreased pressure-induced shift in the voltage-dependence (IC50 54 μM) and eliminated the pressure-induced increases in window current and late current event numbers. Block of NaV1.5 mechanosensitivity by ranolazine was not due to the known binding site on DIVS6 (F1760). The effect of ranolazine on mechanosensitivity of NaV1.5 was approximated by lidocaine. However, ionized ranolazine and charged lidocaine analog (QX-314) failed to block mechanosensitivity.
Conclusions
Ranolazine effectively inhibits mechanosensitivity of NaV1.5. The block of NaV1.5 mechanosensitivity by ranolazine does not utilize the established binding site, and may require bilayer partitioning. Ranolazine block of NaV1.5 mechanosensitivity may be relevant in disorders of mechano-electric dysfunction.
Keywords: drugs, electrophysiology, ion channels, mechanics, myocytes
Introduction
Electro-mechanical coupling in the cardiac myocyte is vital for the generation of contraction. In turn, mechano-electric feedback (MEF) has an important regulatory role. Physiologic roles of MEF include stretch-related changes in sinoatrial node pacing frequency1, ventricular and atrial excitability2 and conduction velocity3. Acute mechano-electric dysfunction is a known pathophysiologic mechanism4, yet commonly, MEF pathologies are caused by chronic abnormalities of preload and afterload leading to stretch-related electrical disturbances5.
Redundancy in biological tissues exists to regulate MEF at cellular and molecular levels. Membrane tension opens stretch-activated channels (SACs), thus modulating electrical excitability6. However, molecular identities of mammalian non-selective cation stretch-activated channels remain elusive. Voltage-gated ion channels are also mechanically sensitive7. They are attractive MEF targets due to high density of expression and direct involvement in the coordination of electrical activity. NaV1.5 is the principal voltage-gated sodium channel in cardiac myocytes8. NaV1.5 is mechanosensitive and classic SACs blockers are known to inhibit this channel9, 10. Acute stretch shifts NaV1.5 voltage dependence of activation and inactivation11, resulting in accelerated kinetics11, 12, increased peak currents13 and stabilization of inactivation11. The piperazine-derivative ranolazine is a NaV1.5 antagonist and an anti-anginal with anti-arrhythmic properties14 that shows promise for management of heart failure15. The molecular mechanism of ranolazine for ischemia could be through block of persistent (i.e., late) NaV1.5 current16. Effects of ranolazine may also result from its hyperpolarizing shift in voltage-dependence of steady-state inactivation similar to the inactivated-state blocker lidocaine14. The function of lipid soluble drugs such as lidocaine17 and ranolazine may rely on partitioning into the lipid bilayer18. NaV channels are modulated by the cytoskeleton19 & amphipaths20, and the biochemical and mechanical states of the lipid bilayer21 impact mechanosensitivity of voltage-gated ion channels.
We hypothesized that ranolazine may modulate the mechanical behavior of NaV1.5. Our results show that the NaV channels native to cardiac myocytes or NaV1.5 transfected in HEK are mechanically sensitive. Ranolazine effectively blocks NaV and NaV1.5 mechanosensitivity, possibly via bilayer partitioning. Its mechanism is not due to binding to its established binding site.
Methods
Adult Murine Cardiac Myocyte Dissociation
Nine to eleven week old BALB/c mice (Harlan Sprague-Dawley, Indianapolis, IN) were used for the isolation of the ventricular cardiac myocytes as previously described22 and detailed in Supplementary Methods. The mice were maintained and the experiments were performed with approval from the Institutional Animal Care and Use Committee of the Mayo Clinic.
Cell culture
Human embryonic kidney 293 (HEK) cells were cultured and transfected as previously described11 (Supplemental Methods).
Recording solutions & Pharmacology
Recording solutions are detailed in Supplementary Methods. Ranolazine and lidocaine were diluted in bath or patch solution from 10 mM ethanol stocks. QX-314 was prepared daily from powder at 500 μM working concentration in pipette solution.
Electrophysiology
Electrodes were pulled using Sutter Instruments P-97 puller and coated with heat cured Dow Corning R6101 compound from Kimble KG-12 and Garner 8250 glass for whole-cell and patch experiments, respectively. Axon 200A amplifier, Digidata 1322A, and Clampex 9 software were used for voltage-clamp and data acquisition.
Whole cell electrophysiology
A single 18 sweep, 90 sec pulse protocol was designed to measure peak current, voltage dependence, and kinetics of activation and inactivation. Cells were held at -120 mV, stepped to test pulses from -130 mV to 30 mV at 5 or 10 mV intervals for 3 sec, then to -120 mV for 0.1 msec, then to a second test pulse at -30 mV for 100 msec. The sampling rate was 20 kHz and intersweep interval was 5 sec.
Cell-attached patches
Electrodes were fire polished specifically for patch mechano-activation as previously published11. Seals were observed for 5 minutes for stabilization. For voltage-ladder protocols, a 1 Hz stimulation frequency was used with a P/4 protocol and no interpulse delay. Cells were held at -100 mV, and at each step a 4 msec pre-pulse to -204 mV accelerated recovery from inactivation before stepping in 10 mV increments from -140 mV to 10 mV for 80 msec per step to test activation and finally a step to 0 mV for 10 msec to test availability. A 10x average was obtained for each voltage step.
For single channel experiments, currents were digitized at 10 kHz and filtered at 2 kHz using a low-pass Bessel filter. Window protocol was a sequence of 300 msec long pulses at a specific HP, determined to be at the foot of the IV curve. Pressure was applied for 100 msec in the middle of each pulse. Late current protocol was a train of depolarizations from HP -100 mV to 0 mV for 200 msec with a 1.1 sec interpulse interval.
Mechanical stimulation
Mechanical stimuli consisted of flow and patch pressure for whole cell and cell-attached patches, respectively. Flow of solution through a 0.7 mL elliptical bath chamber was calibrated at 10 mL/min. Specialized rapid pressure clamp was used to apply pressure (courtesy of Dr. Fred Sachs’ lab)23. Negative pressure produced patch stretch by displacement (Supplemental Movie 1) and was closely monitored during patch formation and pressure delivery for each of the protocols24. Length of mechanical stimuli was <100 msec to minimize structural remodeling (Supplemental Methods).
Data Analysis
Whole cell and macroscopic patch data were analyzed in Pclamp 9 while single channel data were analyzed in QUB (www.qub.buffalo.edu). Voltage dependence and dose-response curves were fit in pClamp9 and Origin 8.61 (Supplemental Methods). For whole cell experiments significance was assigned when P<0.05 by a two-way repeated measures ANOVA with Bonferroni multiple comparisons posttest in GraphPad Prism 5 (Figure 1, Figure 6). For experiments on patches significance was P<0.05 by Student's t-tests as specified in the text, and specifically two-tailed paired t-tests (Figure 4, Figure 5) and two-tailed two-sample equal variance t-tests (Figure 7, Figure 8) in Origin 8.61.
Figure 1.
Ranolazine blocks mechanosensitive response and peak currents of Na+ channels in murine cardiac myocytes and HEK cells transfected with NaV1.5. Left, A, Representative Na+ currents recorded by whole cell voltage-clamp from murine cardiac myocytes and B, HEK cells transfected with NaV1.5, elicited by stepping to -30 mV from -120 mV before (black traces, Flow OFF) or during (grey traces, Flow ON) bath flow, produced by rinsing solution through the recording chamber at 10 mL/min in the absence (Control, 0 μM) or presence (Ranolazine, 50 μM) of drug. Right, Average peak current densities in response to flow of solution without (filled symbols) or with (empty symbols) ranolazine (n=5; *P<0.05 compared to Flow OFF, †P<0.05 compared to 0 μM ranolazine, and P<0.05 interaction between flow and ranolazine blockade by two-way repeated measures ANOVA with Bonferroni multiple comparisons posttest).
Figure 6.
Ranolazine blocks a mechanosensitive response but does not reduce peak currents of NaV1.5 F1760A expressed in HEK cells. Left, Na+ currents elicited by stepping to -30 mV from -120 mV before (black, Flow OFF) or during (grey, Flow ON) bath flow, produced by rinsing solution through the recording chamber at 10 mL/min in the absence (Control, 0 μM) or presence (Ranolazine, 50 μM) of drug. Right, Average peak current densities of NaV1.5 F1760A in response to flow of solution without (filled symbols) or with (empty symbols) 50 μM ranolazine (n=6; *P<0.05 compared to Flow OFF, P>0.05 compared to 0 μM ranolazine, and P>0.05 interaction between flow and ranolazine blockade by two-way repeated measures ANOVA with Bonferroni multiple comparisons posttest).
Figure 4.
Ranolazine inhibits pressure-induced increase in window current. A are controls and B are ranolazine (50 μM). A & B, single channel Na+ window current at HP -40 mV typical (black) and overlaid first fifty 300 msec traces (grey) at 0 mmHg and -30 mmHg (bracket). Single channel activity idealized before (black bar) and during (grey bar) the pressure pulse. Bar graphs show for -30 mmHg compared to 0 mmHg percent change of open channel event number (#30/#0 × 100) for control (Ai) and 50 μM ranolazine (Bi), and open channel lifetime (τ30/τ0 × 100) for control (Aii) and 50 μM ranolazine (Bii) (n=5, *P<0.05; paired t-test for events between 0 mmHg and -30mmHg).
Figure 5.
Ranolazine inhibits pressure-induced increase in late open channel event number. A are controls and B are ranolazine (50 μM). A & B, single channel Na+ late current typical (black) and overlaid first fifty (grey) 200 msec long depolarizing pulses to 0 mV from HP -100 mV for ramp to -30 mmHg (bottom). Single channel activity in the last 100 msec of late current activity analyzed (grey bars). Bar graphs show for -30 mmHg compared to 0 mmHg percent change in open channel event number (#30/#0 × 100) for control (Ai) and 50 μM ranolazine (Bi), and for open channel lifetime (τ30/τ0 × 100) for control (Aii) and 50 μM ranolazine (Bii) (n=4, *P<0.05; paired t-test for events between 0 mmHg and -30mmHg).
Figure 7.
Ranolazine block of mechanosensitivity is independent of F1760. A, the fractional decrease in peak current [(Ictr-Irano)/Ictr] obtained from peak of IV curves in response to 50 μM ranolazine for wild-type NaV1.5 (left) and F1760A (right) (n=4; *P<0.05; two-sample t-test). B, mechanosensitivity of F1760A shown as a shift in half-point of voltage-dependence of activation (ΔV1/2a) without ranolazine and significantly reduced with 50 μM ranolazine (n=4-7, *P<0.05; two sample t-test).
Figure 8.
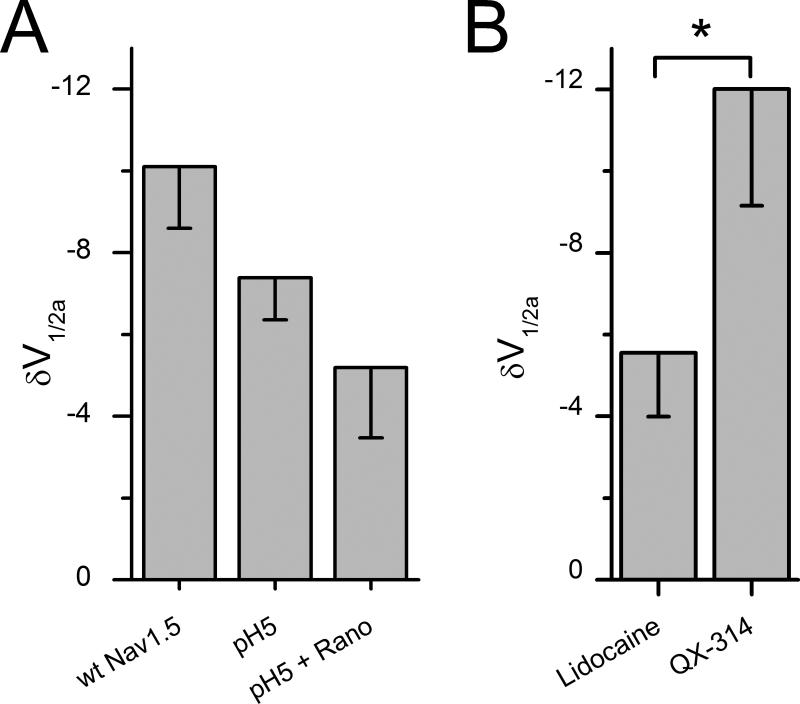
Neutral ranolazine and lidocaine block mechanosensitivity of NaV1.5. A, In cell-attached patches, pressure-induced ΔV1/2a with wt NaV1.5 in Ringer solution at pH 7.4 was not statistically different from ΔV1/2a when the pipette solution contained Ringer solution at pH 5 (n=5-7, P>0.05; two-sample t-test). ΔV1/2a was also not statistically different for pH 5 solution and ranolazine (50 μM) in pH 5 solution (n=7, P>0.05; two-sample t-test). B, Shift in V1/2a with pressure with lidocaine (50 μM) is significantly less than with QX-314 (500 μM) (n=3-5, *P<0.05; two-sample t-test).
Results
Ranolazine inhibits the flow-induced increase in peak current in murine cardiomyocytes
We used a 10 mL/min bath flow to simulate shear stress of voltage clamped murine cardiac ventricular myocytes in a control solution and then in the presence of 50 μM ranolazine. Flow of the control solution increased peak Na+ current from -28.0±4.7 pA/pF to -35.9±8.2 pA/pF, or by 24±8% (n=5, P<0.05, Figure 1A). In the same cells, 50 μM ranolazine decreased peak Na+ current from -28.0±4.7 pA/pF to -9.3±1.7 pA/pF, or by 68±3% (n=5, P<0.05). Ranolazine also blocked the flow-induced increase of peak Na+ current from -9.3±1.7 pA/pF with ranolazine to -10.2±2.4 pA with ranolazine and bath flow, (6±7%, n=5, P>0.05, Figure 1A). These data show that in cardiac myocytes ranolazine not only blocks peak NaV current but also inhibits the response of NaV channels to mechanical stimulation by bath flow.
Ranolazine decreases NaV1.5 peak current at rest and abolishes increase in current in response to bath flow in HEK cells
NaV1.5 is the predominant voltage-gated sodium channel in the adult murine25 and human8 cardiac myocytes. Next, we examined HEK cells heterologously expressing NaV1.5 α-subunit. Mechanical stimulation by flow increased NaV1.5 maximum peak Na+ current from -137±16 pA/pF for control to -161±19 pA/pF for flow, or by 18±3% (n=9, P<0.05, Figure 1B). Bath flow also accelerated the time constants of activation from 0.67±0.06 msec to 0.49±0.05 msec and inactivation from 0.87±0.08 msec to 0.77±0.07 msec (n=8, P<0.05, Supplementary Figure 1A), consistent with published data26. Subsequent 10 min exposure to 50 μM ranolazine reduced maximum peak Na+ current from -137±16 pA/pF to -90±13 pA/pF, or by 37±5% (n=9, P<0.05) and inhibited the response to flow from -90±13 pA/pF with ranolazine to -100±14 pA/pF with ranolazine and bath flow, (10±3%, n=9, P>0.05). Block by ranolazine significantly interacted with mechanical activation of NaV1.5, whereas the measurement of all other parameters such as voltage dependence and kinetics revealed no effect (P<0.05 interaction for peak current, Figure 1B); and P>0.05 for voltage dependence or kinetics, Supplemental Figure 1A). These data suggest that ranolazine can block current at rest and inhibit the mechanical activation of NaV1.5 expressed in HEK cells similar to NaV channels in cardiac myocytes.
Ranolazine reduces pressure-induced hyperpolarizing shift in the voltage sensitivity of NaV1.5
To address the possibility that the effect of ranolazine could be dependent on the stimulus, we also tested the effect of ranolazine on NaV1.5 mechanosensitivity by pressure clamp on cell patches. Since ranolazine partitions readily into cell membranes (partition coefficient 1.5318) we were able to add the drug to the bath solution with rapid effect (time constant τ=19.4±1.6 sec, Supplemental Figure 2). In NaV1.5 expressing HEK cells, addition of 50 μM ranolazine decreased peak currents by -32±8% (n=4, P<0.05, paired t-test), similar to the HEK whole-cell results above.
Before the application of ranolazine, the average peak patch current in the transfected cells was -78.6±69.2 pA (n=7). A -30 mmHg 100 msec long pressure pulse was applied at each voltage step during the subsequent ladder protocol of 17 steps from -140 to 30 mV by 10 mV increments. Patch pressure produced an increase in current amplitude of +18±4% (n=7, P<0.05, paired t-test) and accelerated kinetics at all steps as shown for -100 mV (dash dot), -50 mV (dot) and -30 mV (solid) (Figure 2A). Since pressure accelerated the kinetics of both activation and inactivation and increased peak currents, there was a significant difference current (I30-I0) (Figure 2B). For depolarization at the foot of the activation curve, pressure produced a significant increase in inward current, and a large inward difference current (dotted trace). For the large depolarization that is fully activating, the difference current was biphasic, with large early inward difference current followed by significantly decreased inward flux later in the pulse (solid trace).
Figure 2.
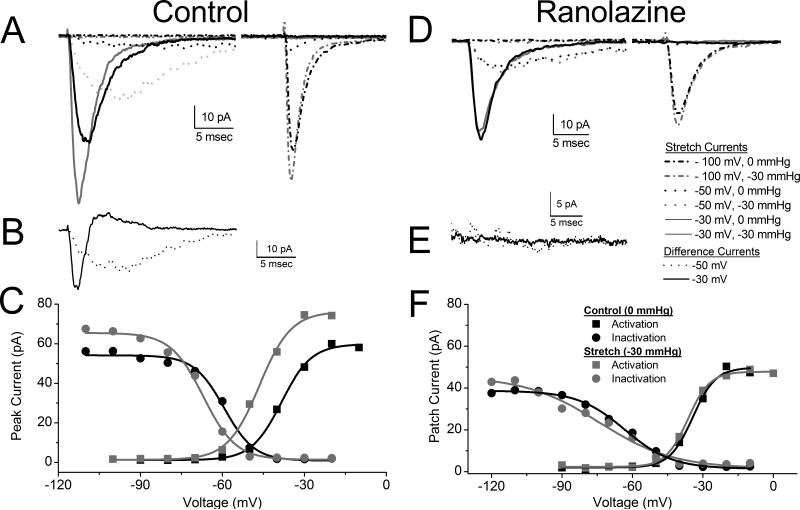
Ranolazine blocks pressure-induced increase in peak current and hyperpolarizing shift of the voltage-dependence of activation and inactivation. A, B, C are controls and D, E, F are ranolazine (50 μM). A & D, single patch average Na+ currents elicited by stepping to -100 mV (dash dot) -50 mV (dot), -30 mV (solid) from -140 mV at 0 mmHg (black) and at -30 mmHg pressure (grey). First peak is activation and second peak is inactivation (availability). B & E, same patch difference currents (I-30 – I0) shown for -50 mV (dot black) and -30 mV (solid black). C & F, peak current-voltage (IV) for this patch at 0 mmHg (black) and -30 mmHg (grey), with activation (boxes) and inactivation (circles). Solid lines are Boltzmann fits of 0 mmHg (black) and -30 mmHg (grey).
In Figure 2C peak currents from step 1 and step 2 from a typical patch are plotted against voltage, showing voltage dependence of activation (squares) and availability (circles) at rest (0 mmHg) (black) and with -30 mmHg pressure (grey). Solid lines are Boltzmann fits of the peaks at the indicated voltages (solid lines). On average, for a -30 mmHg stimulus, shift in the voltage dependence of activation (ΔV1/2a) was -10.1±1.5 mV (n=7, P<0.05, paired t-test) and shift in the voltage dependence of inactivation (ΔV1/2i) was -12.1±1.7 mV (n=6, P<0.05, paired t-test), peak Na+ current change (ΔIpeak) was 18±4% (n=7, P<0.05, paired t-test). We found no significant change in slopes (ΔdVa=0.1±0.3 mV, ΔdVi=2.9±3.2 mV, P>0.05 for both). These data demonstrate an increase in peak current, a shift in the voltage dependency of NaV1.5 current with pressure, suggesting that the channels will activate at more hyperpolarized voltages, which would make a cardiac myocyte more excitable.
After the addition of 50 μM ranolazine a -30 mmHg pressure failed to increase peak current and accelerate kinetics as for the control above, as exemplified in the raw traces (Figure 2D) and lack of difference current (Figure 2E). In the presence of 50 μM ranolazine, the half-point of the voltage dependence of activation (V1/2a) did not shift with pressure (black is 0 mmHg, grey is -30 mmHg). Pressure resulted in an average ΔV1/2a of -6.4±0.7 mV, which is smaller than with 0 μM ranolazine (n=5, P<0.05, two sample t-test) (Figure 2F). The peak current change (ΔIpeak = 14±4%) and shift in the voltage dependence of steady state inactivation (ΔV1/2i = -8.7±0.5 mV) with pressure were not statistically different from 0 μM ranolazine, (n=5, P>0.05 compared with 0 μM ranolazine, two-sample t-test). Also, no significant change was found for slope change (ΔdVa=0.11±0.29 mV, ΔdVi=2.93±3.23 mV, P>0.05, two-sample t-test).
We determined the potency of ranolazine inhibition of NaV1.5 mechanosensitivity. The voltage-dependence of activation (V1/2a) and steady-state inactivation (V1/2i) half-points from Boltzmann fits of IV curves for patches at rest (0 mmHg) and pressure (-30 mmHg) were obtained in the absence and presence of ranolazine at concentrations of 10, 50, 100, 300 μM (n≥3 per concentration). Pressure-dependence of the shift in the voltage-dependence of activation (ΔV1/2a) was fit to a dose-response curve with an IC50 of 53.6 μM and hill slope h=-0.01 (Figure 3). Ranolazine also affected the mechanosensitivity of the voltage dependence of inactivation (ΔV1/2i) and peak current (I30/I0), but these effects were more difficult to quantify since ranolazine is known to affect both of these parameters at rest14 (Supplemental Figure 4). Thus, we elected to use ΔV1/2a as an assay for pressure-effects of ranolazine on NaV1.5.
Figure 3.
Ranolazine blocks mechanosensitivity of NaV1.5 in a concentration-dependent manner. Bar graph is shift in the voltage dependence of activation (ΔV1/2a) for 0 μM ranolazine and scatter plot is ΔV1/2a versus increasing ranolazine concentrations. Solid line is a dose-response fit with IC50 of 53.6 μM.
We next examined NaV pressure sensitivity and the ranolazine effect in a native system, using cell-attached patches in murine myocytes. In cell-attached patches from adult murine ventricular cardiac myocytes voltage dependent inward Na+ current peaked at -184±223 pA and increased to -263±372 pA in response to -20 mmHg patch pressure, or by 27±18% (n=5, P<0.05, paired t-test). Patch pressure hyperpolarized the voltage-dependence of activation, shifting V1/2a by -11.4±6.7 mV (n=5, P<0.05, paired t-test). Addition of 50 μM ranolazine decreased peak currents to -65±35 pA, or by 65% percent compared to no ranolazine controls (n=6, P<0.05, two-sample t-test). In the presence of 50 μM ranolazine negative pressure (-20 mmHg) produced a 66% smaller hyperpolarizing shift in the voltage-dependence of activation V1/2a of -4.0±3.1 mV compared to no ranolazine controls (n=5, P<0.05, two-sample t-test). These data from native myocytes support pressure sensitivity of NaV1.5 and inhibition of mechanosensitivity by ranolazine.
Ranolazine reduces pressure-induced increase in the NaV1.5 window current
Steady-state window is the area underneath the overlapping feet of activation and inactivation voltage dependence curves, where an appreciable portion of NaV1.5 channels is active (Figure 2C, D). We assessed pressure-dependent changes in window current activity using single channel recording. Cell-attached patches were stepped through a voltage ladder to determine the foot of activation. For the patch in Figure 4A this was -40 mV and averaged -45±2.2 mV (n=5). For each patch, we set the holding potential (HP) to the foot of activation and recorded 300 pulses of 300 msec at the left edge of the window. We applied pressure for 100 msec (bracket) every 200 msec. Single channel openings were idealized over 50 msec intervals before (black bar) and during the -30 mmHg pressure step (grey bar). On average, the number of single channel open events increased from 85±40 at rest to 137±45 with a -30 mmHg pressure, representing a pairwise fractional increase (n=n30/n0) with pressure of 240±64% (n=5, P<0.05) (Figure 4Ai). Open channel lifetime did not change from 0.50±0.11 msec (control) to 0.54±0.13 msec (pressure), or a pairwise change (τ=τ30/τ0) by 28±29% (n=5, P>0.05) (Figure 4Aii). Since the single channel conductance does not change with pressure11, the significant increase in the number of open channel events produced a steady-state Na+ charge Q30/Q0 ([n30*τ30]/[n0*τ0]) increase of 260±31% (n=5, P<0.01, paired t-test). This significant increase in window current with pressure would depolarize the cell and likely make it more excitable.
Ranolazine (50 μM) reduced the pressure-induced increase in window current activity (Figure 4B). At HP of 40±6 mV (n=3), pressure failed to increase the number of single channel open events from 134±29 at rest to 147±44 with -30 mmHg pressure, or pairwise change of 9±14% in the window open channel numbers (n=3, P>0.05) (Figure 4Bi). Open channel lifetimes also did not change from 0.66±0.10 msec at rest to 0.68±0.057 msec, a pairwise change of 4±6% (n=3, P>0.05) (Figure 4Bii). The result was no change in steady-state Na+ charge (14±20%, n=3, P>0.05). These results suggest that pressure-induced increase in window current at resting potential is abolished by ranolazine.
Ranolazine reduces pressure-induced increase in the NaV1.5 late current open channel events
Na+ late current is the small steady-state flux during the action potential plateau. Late current abnormalities have been implicated in cardiac pathologies such as LQT327, ischemia28 and heart failure15, and are thought to be the therapeutic target for ranolazine16. In a cell-attached configuration the late current is comprised of sporadic single channel events (Figure 5A). We used a 200 msec step depolarization from -100 mV to 0 mV, and analyzed single channel events in the last 100 msec from individual patches at rest (0 mmHg, data not shown) and then using a -30 mmHg ramp pressure applied to the same patch (Figure 5A). Compared to the late single channel events at rest (19±5), -30 mmHg pressure produced an increase in event number (42±10), or a 235±44% pairwise increase (n=4, P<0.05) (Figure 5Ai). Single channel open lifetime trended to a decrease from control (0.61±0.11 msec) to pressure (0.37±0.07 msec), or a pairwise decrease by 71±18% (n=4, P>0.05) (Figure 5Aii). Assuming no change in single channel conductance11, total late steady-state Na+ charge did not change (19±70%, n=4, P>0.05). Ranolazine (50 μM) abolished the pressure-induced changes in late current (Figure 5B). The single channel event number did not change from rest (40±14) to negative pressure (28±5), or a pairwise change of 22±30% (n=5, P>0.05) (Figure 5Bi). Pressure also did not change open channel lifetime from 0.52±0.10 msec at 0 mmHg to 0.61±0.02 msec at -30 mmHg, or a pairwise change of 27±23% (n=5, P>0.05) (Figure 5Bii). Total late steady-state Na+ charge was not changed (34±79%, n=5, P>0.05). Thus, the pressure-induced increase in late single channel opening numbers is abolished with ranolazine.
F1760A mutation decreases NaV1.5 peak current block by ranolazine, but does not eliminate mechanoinhibition
Ranolazine block of NaV1.5 requires the putative local anesthetic binding site on DIVS6 F176029. Hence, we investigated whether inhibition of mechanosensitivity by ranolazine could be altered by site-directed mutagenesis of residue F1760. In whole-cell voltage clamped HEK, F1760A NaV1.5 peak currents were insensitive to 50 μM ranolazine, as current densities changed only 2±6%, from -195±29 pA/pF in control to -201±32 pA/pF with drug (n=6, P>0.05) (Figure 6). Nevertheless, F1760A NaV1.5 channels appeared to retain mechanosensitivity like wild-type NaV1.5, as F1760A currents increased 18±5% from -195±29 pA/pF to -228±32 pA/pF in response to flow of drug-free solution (n=6, P<0.05). Moreover, inhibition of flow response was also retained in F1760A channels like wild-type; F1760A peak Na+ currents were not inducible by flow of ranolazine solution, changing only 8±4% from -201±32 pA/pF with flow off to -213±30 pA/pF with flow on (n=6, P>0.05).
We confirmed these findings in cell-attached patches. At rest (0 mmHg), while the application of ranolazine decreased wild-type NaV1.5 current by 32±8% (n=4), the peak currents of F1760A NaV1.5 channels in presence of 50 μM ranolazine were decreased by only 11±9% (n=4, P<0.05 compared to wild type current) (Figure 7A). The F1760A NaV1.5 channels were mechanosensitive to a similar extent as the wild-type channels, with a -30 mmHg pulse producing ΔV1/2a of -10.8±2.5 mV (n=4), which was significantly reduced by 50 μM ranolazine to ΔV1/2a -4.7±1.8 mV (n=7) (P<0.05) (Figure 7B).
The above whole cell and cell-attached patch data confirm that F1760 is required for significant ranolazine block of NaV1.5 current at rest29. Yet, F1760A NaV1.5 retains mechanosensitivity, and ranolazine continues to inhibit F1760A NaV1.5 mechanosensitivity to a similar extent as in the wild-type NaV1.5. These results suggest that the ranolazine inhibition of NaV1.5 mechanosensitivity is not due to binding to F1760.
Ranolazine inhibition of mechanosensitivity of NaV1.5 may require drug partitioning into the lipid bilayer
Ranolazine has a pKa of 7.17, so at pH 5 the positively charged form of ranolazine is roughly 100-fold more concentrated than the neutral form. We used pH 5 and 50 μM ranolazine in the pipette only for these experiments as bath acidity is known to affect multiple cellular processes. For a -30 mmHg pressure, wild-type NaV1.5 channels shifted the half-point of voltage sensitivity of activation ΔV1/2a by -7.4±1.0 mV (n=7) at pH 5 and -10.1±1.5 mV (n=5) at pH 7 (P>0.05). With the pipette containing 50 μM ranolazine and pH 5 solution, pressure produced a shift of ΔV1/2a of -5.2±1.7 mV (n=7), compared to -7.4±1.0 mV (n=7) without ranolazine (P>0.05) (Figure 8A). Thus, it appears that ionized ranolazine does not inhibit the shift in ΔV1/2a at pH 5. Since the ionized form of ranolazine does not significantly partition into the hydrophobic core of the bilayer, these data suggest that ranolazine inhibition of NaV1.5 mechanosensitivity may require bilayer partitioning.
The similarities of ranolazine and local anesthetics with respect to the overlapping binding sites and chemical properties (structure, logP, pKa) suggested that the mechanisms of action may also overlap. It is known that local anesthetics that exist in neutral forms, such as lidocaine, partition significantly into the hydrophobic core of the bilayer. Cell-attached patches were exposed to 50 μM lidocaine by addition to the bath as before. Lidocaine (50 μM) also blocked mechanosensitivity of NaV1.5, with pressure-induced ΔV1/2a -5.6±1.6 (n=5) (Figure 8B). This ΔV1/2a is smaller than -10.1±1.5 mV shift for the controls without lidocaine (Figure 8A). Thus, it appears that NaV1.5 mechanosensitivity block is not specific for ranolazine. We next used the permanently charged lidocaine homolog QX-314 to determine the need for bilayer partitioning for mechanosensitivity block. QX-314 is membrane impermeable therefore we added 500 μM QX-314 to the pipette solution. In the presence of QX-314, NaV1.5 mechanosensitivity persisted with a ΔV1/2a -12.0±2.9 mV (n=3, P>0.05 compared to lidocaine) (Figure 8B).
Discussion
In this study we demonstrate NaV mechanosensitivity in adult murine ventricular cardiac myocytes and in HEK cells transfected with NaV1.5. We describe multiple effects of mechanical stimulation on NaV1.5 function and demonstrate effective inhibition of NaV1.5 mechanosensitivity by ranolazine and lidocaine. Furthermore, we establish that the inhibition of NaV1.5 mechanosensitivity by ranolazine does not require the established binding site, but appears to require bilayer partitioning.
Mechanical stimulation in cardiac myocytes and NaV1.5-transfected HEK cells increased peak NaV current, accelerated kinetics of activation and inactivation and in patches hyperpolarized the half-points of voltage-dependence of activation (V1/2a) and inactivation (V1/2i), thereby left-shifting the window current (Figure 1, Figure 2A-C). At the single channel level mechanical stimulation of NaV1.5 increased total window current at hyperpolarized resting potentials (Figure 4A) but not late current during depolarization to 0 mV (Figure 5A). These results suggest that NaV1.5 may contribute to MEF. In a cardiac myocyte membrane depolarization is the most consistent effect of stretch30. We showed two mechano-induced effects that would contribute to membrane depolarization: hyperpolarization of the NaV1.5 window current and a large increase in total Na+ charge density for small depolarizations (Figure 2B, C). On the other hand, for large depolarizations mechano-induced acceleration in inactivation led to a relative decrease in Na+ influx following the upstroke (Figure 2B). This may contribute to the early shortening of the action potential duration (APD) by stretch as previously shown31. However, stretch prolongs latter portions of APD30, which may predispose to development of secondary depolarization5. Stretch accelerates NaV1.5 inactivation and does not alter late current, so it is unlikely that NaV1.5 is involved in stretch-dependent ADP prolongation. Potential mechanisms include inhibition of K+ selective32 or non-selective SACs33. In contrast, mechano-induced acceleration of NaV1.5 inactivation kinetics may protect from excessive stretch-dependent action potential prolongation. A recent study of the classic LQT3 NaV1.5 mutations that have defective inactivation showed that pressure failed to accelerate inactivation in proportion to activation34, suggesting that pressure-induced acceleration of NaV1.5 inactivation may be a protective MEF response.
Ranolazine inhibited NaV1.5 mechanosensitivity in both cardiac myocytes and HEK cells. The drug diminished the mechano-induced increase in peak current, shift in voltage sensitivity (Figure 1 , Figure 2D-F), increases in the window current (Figure 4B) and late current open channel event numbers (Figure 5B). Ranolazine inhibition of NaV1.5 mechanosensitivity would indicate multiple effects on stretch-dependence in a cardiac myocyte. Most obvious is that loss of the hyperpolarizing shift in voltage-dependence of activation and window current would decrease the excitability with stretch. Block of stretch-dependence of NaV1.5 inactivation would be more complex. We confirm that ranolazine left-shifts V1/2i at rest35 (Supplemental Figure 3) but we also show that it inhibits the mechano-induced leftward V1/2i shift (Supplemental Figure 4A). Since V1/2i shift is dose-dependent with respect to stretch11, the final result of V1/2i position depends on doses of stretch and drug and would be difficult to predict.
The IC50 of ranolazine block of ΔV1/2a, the most sensitive pressure-sensitive parameter, was 54 μM (Figure 3). This value is about five-fold higher than the accepted plasma concentration36. However, there are at least two reasons not to dismiss the inhibitory effect of ranolazine on mechanosensitivity of NaV1.5. First, we do not know the true mechanical environment of the channels in situ. Previous data suggest that NaV1.5 mechanosensitivity is likely mediated by a combination of the lipid bilayer20 and the cytoskeleton26. In the native setting, NaV1.5 resides within and associates with other mechano-relevant elements. NaV1.5 channels are localized within T-tubules37 and caveolin-3 rich rafts38, which are membrane structures with high intrinsic curvature, and tend to be areas of mechanosensitivity39. The NaV1.5 channels also make extensive intracellular connections40. Some of the NaV1.5 associating proteins, such as ankyrin41, syntrophin42, 43 and telethonin44, are known to be proteins involved in cellular mechanosensitivity. Dysfunctional interaction of NaV1.5 with associating proteins is known to contribute to pathology43, 45, so these connections may serve to further focus mechanical force at the channel45. While we used a -30 mmHg stimulus, which is at the lower end of the typical pressures used to study SACs6, the degree of mechanical stimulation in situ remains to be determined. Second, the membrane concentration of the drug likely differs from the plasma concentration. The neutral form of ranolazine is the effective inhibitor of NaV1.5 mechanosensitivity and it partitions substantially into the bilayer hydrophobic core. Membrane diffusion46 and partition coefficients47 increase with the extra energy, whether in the form of stretch or temperature. As our experiments were carried out at room temperature we expect that effective membrane concentration will further increase at physiologic temperature.
Our results shed some light on the mechanism of NaV1.5 mechanosensitivity. Inhibition by ranolazine does not require the putative binding site on DIVS6 (Figure 7) and neutral lidocaine and ranolazine inhibit, but charged ranolazine and permanently charged lidocaine analog QX-314 do not impact mechanosensitivity (Figure 8). This suggests the importance of drug partitioning into the lipid bilayer, but dues not rule out other protein binding sites or cytoskeletal involvement. Previous studies support the importance of the lipid-protein interface in NaV function20 and voltage-gated channel mechanosensitivity21. Many outstanding questions remain regarding the mechanism of NaV1.5 mechanosensitivity, but it very likely requires a combination of the associating proteins, membrane domains and protein-lipid interface.
This study demonstrates the mechanosensitivity of NaV1.5 in both native cardiac myocytes and a heterologous system. We establish drugs such as ranolazine, lidocaine and their analogs as pharmacologic tools for inhibition of NaV1.5 mechanosensitivity and suggest potential mechanisms. Further exploration of these compounds in disorders associated with MEF may be warranted. Examples include novel heart failure therapy48 and atrial fibrillation related to stretch of pulmonary veins, recently shown to be suppressed by ranolazine49.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. James Rae for valuable discussions and opinions on the manuscript.
Funding Sources: This work was supported by NIDDK 52766 and P30DK84567.
Footnotes
Conflict of Interest Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cooper PJ, Lei M, Cheng LX, Kohl P. Selected contribution: Axial stretch increases spontaneous pacemaker activity in rabbit isolated sinoatrial node cells. J Appl Physiol. 2000;89:2099–2104. doi: 10.1152/jappl.2000.89.5.2099. [DOI] [PubMed] [Google Scholar]
- 2.Lerman BB, Burkhoff D, Yue DT, Franz MR, Sagawa K. Mechanoelectrical feedback: Independent role of preload and contractility in modulation of canine ventricular excitability. J Clin Invest. 1985;76:1843–1850. doi: 10.1172/JCI112177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung D, Mills RW, Schettler J, Narayan SM, Omens JH, McCulloch AD. Ventricular filling slows epicardial conduction and increases action potential duration in an optical mapping study of the isolated rabbit heart. J Cardiovasc Electrophysiol. 2003;14:739–749. doi: 10.1046/j.1540-8167.2003.03072.x. [DOI] [PubMed] [Google Scholar]
- 4.Maron BJ, Poliac LC, Kaplan JA, Mueller FO. Blunt impact to the chest leading to sudden death from cardiac arrest during sports activities. N Engl J Med. 1995;333:337–342. doi: 10.1056/NEJM199508103330602. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Taylor LK, Denney WD, Hansen DE. Initiation of ventricular extrasystoles by myocardial stretch in chronically dilated and failing canine left ventricle. Circulation. 1994;90:2022–2031. doi: 10.1161/01.cir.90.4.2022. [DOI] [PubMed] [Google Scholar]
- 6.Hu H, Sachs F. Stretch-activated ion channels in the heart. J Mol Cell Cardiol. 1997;29:1511–1523. doi: 10.1006/jmcc.1997.0392. [DOI] [PubMed] [Google Scholar]
- 7.Morris CE. Voltage-gated channel mechanosensitivity: Fact or friction? Frontiers in Physiology. 2011:2. doi: 10.3389/fphys.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gellens ME, George AL, Jr., Chen LQ, Chahine M, Horn R, Barchi RL, Kallen RG. Primary structure and functional expression of the human cardiac tetrodotoxin-insensitive voltage-dependent sodium channel. Proc Natl Acad Sci U S A. 1992;89:554–558. doi: 10.1073/pnas.89.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li GR, Baumgarten CM. Modulation of cardiac na(+) current by gadolinium, a blocker of stretch-induced arrhythmias. Am J Physiol Heart Circ Physiol. 2001;280:H272–279. doi: 10.1152/ajpheart.2001.280.1.H272. [DOI] [PubMed] [Google Scholar]
- 10.Redaelli E, Cassulini RR, Silva DF, Clement H, Schiavon E, Zamudio FZ, Odell G, Arcangeli A, Clare JJ, Alagon A, de la Vega RC, Possani LD, Wanke E. Target promiscuity and heterogeneous effects of tarantula venom peptides affecting na+ and k+ ion channels. J Biol Chem. 2010;285:4130–4142. doi: 10.1074/jbc.M109.054718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyder A, Rae JL, Bernard C, Strege PR, Sachs F, Farrugia G. Mechanosensitivity of nav1.5, a voltage-sensitive sodium channel. J Physiol. 2010;588:4969–4985. doi: 10.1113/jphysiol.2010.199034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris CE, Juranka PF. Nav channel mechanosensitivity: Activation and inactivation accelerate reversibly with stretch. Biophys J. 2007;93:822–833. doi: 10.1529/biophysj.106.101246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strege PR, Ou Y, Sha L, Rich A, Gibbons SJ, Szurszewski JH, Sarr MG, Farrugia G. Sodium current in human intestinal interstitial cells of cajal. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1111–1121. doi: 10.1152/ajpgi.00152.2003. [DOI] [PubMed] [Google Scholar]
- 14.Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: Differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116:1449–1457. doi: 10.1161/CIRCULATIONAHA.107.704890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Undrovinas AI, Belardinelli L, Undrovinas NA, Sabbah HN. Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. J Cardiovasc Electrophysiol. 2006;17:S169–S177. doi: 10.1111/j.1540-8167.2006.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belardinelli L, Shryock JC, Fraser H. Inhibition of the late sodium current as a potential cardioprotective principle: Effects of the late sodium current inhibitor ranolazine. Heart. 2006;92:iv6–iv14. doi: 10.1136/hrt.2005.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hille B. Local anesthetics: Hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977;69:497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenkey N, Karoly R, Lukacs P, Vizi ES, Sunesen M, Fodor L, Mike A. Classification of drugs based on properties of sodium channel inhibition: A comparative automated patch-clamp study. PLoS One. 2010;5:e15568. doi: 10.1371/journal.pone.0015568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Undrovinas AI, Shander GS, Makielski JC. Cytoskeleton modulates gating of voltage-dependent sodium channel in heart. Am J Physiol. 1995;269:H203–214. doi: 10.1152/ajpheart.1995.269.1.H203. [DOI] [PubMed] [Google Scholar]
- 20.Lundbaek JA, Birn P, Hansen AJ, Sogaard R, Nielsen C, Girshman J, Bruno MJ, Tape SE, Egebjerg J, Greathouse DV, Mattice GL, Koeppe RE, 2nd, Andersen OS. Regulation of sodium channel function by bilayer elasticity: The importance of hydrophobic coupling. Effects of micelle-forming amphiphiles and cholesterol. J Gen Physiol. 2004;123:599–621. doi: 10.1085/jgp.200308996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt D, Mackinnon R. Voltage-dependent k+ channel gating and voltage sensor toxin sensitivity depend on the mechanical state of the lipid membrane. Proc Natl Acad Sci U S A. 2008;105:19276–19281. doi: 10.1073/pnas.0810187105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodgson DM, Zingman LV, Kane GC, Perez-Terzic C, Bienengraeber M, Ozcan C, Gumina RJ, Pucar D, O'Coclain F, Mann DL, Alekseev AE, Terzic A. Cellular remodeling in heart failure disrupts k(atp) channel-dependent stress tolerance. Embo J. 2003;22:1732–1742. doi: 10.1093/emboj/cdg192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Besch SR, Suchyna T, Sachs F. High-speed pressure clamp. Pflugers Arch. 2002;445:161–166. doi: 10.1007/s00424-002-0903-0. [DOI] [PubMed] [Google Scholar]
- 24.Morris CE, Juranka PF, Lin W, Morris TJ, Laitko U. Studying the mechanosensitivity of voltage-gated channels using oocyte patches. Methods Mol. Biol. 2006;322:315–329. doi: 10.1007/978-1-59745-000-3_22. [DOI] [PubMed] [Google Scholar]
- 25.Haufe V, Camacho JA, Dumaine R, Gunther B, Bollensdorff C, von Banchet GS, Benndorf K, Zimmer T. Expression pattern of neuronal and skeletal muscle voltage-gated na+ channels in the developing mouse heart. J Physiol. 2005;564:683–696. doi: 10.1113/jphysiol.2004.079681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strege PR, Holm AN, Rich A, Miller SM, Ou Y, Sarr MG, Farrugia G. Cytoskeletal modulation of sodium current in human jejunal circular smooth muscle cells. Am J Physiol Cell Physiol. 2003;284:C60–66. doi: 10.1152/ajpcell.00532.2001. [DOI] [PubMed] [Google Scholar]
- 27.Bennett PB, Yazawa K, Makita N, George AL., Jr. Molecular mechanism for an inherited cardiac arrhythmia. Nature. 1995;376:683–685. doi: 10.1038/376683a0. [DOI] [PubMed] [Google Scholar]
- 28.Fraser H, Belardinelli L, Wang L, Light PE, McVeigh JJ, Clanachan AS. Ranolazine decreases diastolic calcium accumulation caused by atx-ii or ischemia in rat hearts. J Mol Cell Cardiol. 2006;41:1031–1038. doi: 10.1016/j.yjmcc.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Fredj S, Sampson KJ, Liu H, Kass RS. Molecular basis of ranolazine block of lqt-3 mutant sodium channels: Evidence for site of action. Br J Pharmacol. 2006;148:16–24. doi: 10.1038/sj.bjp.0706709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamkin A, Kiseleva I, Isenberg G. Stretch-activated currents in ventricular myocytes: Amplitude and arrhythmogenic effects increase with hypertrophy. Cardiovasc Res. 2000;48:409–420. doi: 10.1016/s0008-6363(00)00208-x. [DOI] [PubMed] [Google Scholar]
- 31.Lab MJ. Mechanically dependent changes in action potentials recorded from the intact frog ventricle. Circ Res. 1978;42:519–528. doi: 10.1161/01.res.42.4.519. [DOI] [PubMed] [Google Scholar]
- 32.Ji S, John SA, Lu Y, Weiss JN. Mechanosensitivity of the cardiac muscarinic potassium channel. A novel property conferred by kir3.4 subunit. J Biol Chem. 1998;273:1324–1328. doi: 10.1074/jbc.273.3.1324. [DOI] [PubMed] [Google Scholar]
- 33.Ravens U. Mechano-electric feedback and arrhythmias. Prog Biophys Mol Biol. 2003;82:255–266. doi: 10.1016/s0079-6107(03)00026-9. [DOI] [PubMed] [Google Scholar]
- 34.Banderali U, Juranka PF, Clark RB, Giles WR, Morris CE. Impaired stretch modulation in potentially lethal cardiac sodium channel mutants. Channels (Austin) 2010;4:12–21. doi: 10.4161/chan.4.1.10260. [DOI] [PubMed] [Google Scholar]
- 35.Rajamani S, El-Bizri N, Shryock JC, Makielski JC, Belardinelli L. Use-dependent block of cardiac late na(+) current by ranolazine. Heart Rhythm. 2009;6:1625–1631. doi: 10.1016/j.hrthm.2009.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jerling M. Clinical pharmacokinetics of ranolazine. Clin Pharmacokinet. 2006;45:469–491. doi: 10.2165/00003088-200645050-00003. [DOI] [PubMed] [Google Scholar]
- 37.Cohen SA. Immunocytochemical localization of rh1 sodium channel in adult rat heart atria and ventricle. Presence in terminal intercalated disks. Circulation. 1996;94:3083–3086. doi: 10.1161/01.cir.94.12.3083. [DOI] [PubMed] [Google Scholar]
- 38.Vatta M, Ackerman MJ, Ye B, Makielski JC, Ughanze EE, Taylor EW, Tester DJ, Balijepalli RC, Foell JD, Li Z, Kamp TJ, Towbin JA. Mutant caveolin-3 induces persistent late sodium current and is associated with long-qt syndrome. Circulation. 2006;114:2104–2112. doi: 10.1161/CIRCULATIONAHA.106.635268. [DOI] [PubMed] [Google Scholar]
- 39.Sinha B, Koster D, Ruez R, Gonnord P, Bastiani M, Abankwa D, Stan RV, Butler-Browne G, Vedie B, Johannes L, Morone N, Parton RG, Raposo G, Sens P, Lamaze C, Nassoy P. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell. 2011;144:402–413. doi: 10.1016/j.cell.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abriel H. Cardiac sodium channel na(v)1.5 and interacting proteins: Physiology and pathophysiology. J Mol Cell Cardiol. 2010;48:2–11. doi: 10.1016/j.yjmcc.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 41.Mohler PJ, Bennett V. Ankyrin-based cardiac arrhythmias: A new class of channelopathies due to loss of cellular targeting. Curr Opin Cardiol. 2005;20:189–193. doi: 10.1097/01.hco.0000160372.95116.3e. [DOI] [PubMed] [Google Scholar]
- 42.Ueda K, Valdivia C, Medeiros-Domingo A, Tester DJ, Vatta M, Farrugia G, Ackerman MJ, Makielski JC. Syntrophin mutation associated with long qt syndrome through activation of the nnos-scn5a macromolecular complex. Proc Natl Acad Sci U S A. 2008;105:9355–9360. doi: 10.1073/pnas.0801294105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ou Y, Strege P, Miller SM, Makielski J, Ackerman M, Gibbons SJ, Farrugia G. Syntrophin gamma 2 regulates scn5a gating by a pdz domain-mediated interaction. J Biol Chem. 2003;278:1915–1923. doi: 10.1074/jbc.M209938200. [DOI] [PubMed] [Google Scholar]
- 44.Mazzone A, Strege PR, Tester DJ, Bernard CE, Faulkner G, De Giorgio R, Makielski JC, Stanghellini V, Gibbons SJ, Ackerman MJ, Farrugia G. A mutation in telethonin alters nav1.5 function. J Biol Chem. 2008;283:16537–16544. doi: 10.1074/jbc.M801744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohler PJ, Rivolta I, Napolitano C, LeMaillet G, Lambert S, Priori SG, Bennett V. Nav1.5 e1053k mutation causing brugada syndrome blocks binding to ankyrin-g and expression of nav1.5 on the surface of cardiomyocytes. Proc Natl Acad Sci U S A. 2004;101:17533–17538. doi: 10.1073/pnas.0403711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oghalai JS, Zhao HB, Kutz JW, Brownell WE. Voltage- and tension-dependent lipid mobility in the outer hair cell plasma membrane. Science. 2000;287:658–661. doi: 10.1126/science.287.5453.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanchez V, Arthur GR, Strichartz GR. Fundamental properties of local anesthetics. I. The dependence of lidocaine's ionization and octanol:Buffer partitioning on solvent and temperature. Anesth Analg. 1987;66:159–165. [PubMed] [Google Scholar]
- 48.Chandler MP, Stanley WC, Morita H, Suzuki G, Roth BA, Blackburn B, Wolff A, Sabbah HN. Short-term treatment with ranolazine improves mechanical efficiency in dogs with chronic heart failure. Circ Res. 2002;91:278–280. doi: 10.1161/01.res.0000031151.21145.59. [DOI] [PubMed] [Google Scholar]
- 49.Sicouri S, Glass A, Belardinelli L, Antzelevitch C. Antiarrhythmic effects of ranolazine in canine pulmonary vein sleeve preparations. Heart Rhythm. 2008;5:1019–1026. doi: 10.1016/j.hrthm.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.