Abstract
Many different masses can involve the kidney other than the commonly encountered renal cell carcinoma (RCC). The purpose of this article is to review the characteristic clinical and imaging findings of common and uncommon masses that predominantly present unilaterally in the adult patient, other than RCC. Awareness of such lesions and knowing the clinical scenario is important for appropriate diagnosis and management, especially in a multidisciplinary care setting.
Keywords: Unilateral adult renal masses, transitional cell carcinoma, oncocytoma, angiomyolipoma, renal hematoma, renal infection, renal infarction, multilocular cystic renal tumor, renal medullary neoplasms, metanephric adenoma, renal leiomyoma, renal hemangioma, renal lymphangioma, renal sarcoma, renal medullary interstitial tumor, renal neuroendocrine tumor, juxtaglomerular apparatus tumor
Introduction
Renal cell carcinoma (RCC) is the most common adult renal epithelial cancer, accounting for more than 90% of all renal malignancies[1]. It is the most lethal of all urologic cancers and most commonly presents unilaterally. However, there are differential diagnostic considerations. For example, transitional cell carcinoma, oncocytoma, angiomyolipoma, hematoma, infection, and infarction/ischemia also can present as unilateral masses. In addition, a number of less common renal lesions can present unilaterally including multilocular cystic renal tumor, renal medullary neoplasms, metanephric adenoma, leiomyoma, hemangioma, lymphangioma, sarcoma, renal medullary interstitial tumor, renal neuroendocrine tumor, and juxtaglomerular apparatus tumor (Tables 1 and 2).
Table 1.
Summary of more common adult unilateral renal masses other than RCC
| Mass | Distinguishing features |
|---|---|
| Transitional cell carcinoma | Sessile filling defect, pelvicaliceal irregularity, mural thickening, or obstructed calices on CT urography |
| Infiltrative pattern when invasive, without deforming the renal contour | |
| Oncocytoma | Low attenuation mass that demonstrates spoke-wheel enhancement and central scar |
| Benign slow growing tumor | |
| Angiomyolipoma | Composed of blood vessels, smooth muscle and adipose tissue in varying proportions |
| Adipose tissue allows imaging diagnosis | |
| Involves the renal parenchyma | |
| Hematoma | Acute hematomas are hyperattenuating in appearance on noncontrast-enhanced CT, become iso- to hypoattenuating and smaller over time |
| Large subcapsular hematoma may cause renovascular hypertension, page kidney | |
| Renal abscess | Result of liquefactive necrosis in the setting of acute pyelonephritis |
| Appears as a wall-enhancing focal fluid collection, may contain bubbles of gas | |
| Xanthogranulomatous pyelonephritis | Chronic kidney infection secondary to renal obstruction; usually associated with staghorn calculus |
| Presents clinically with: malaise, low-grade fever, and recurrent urinary tract infection | |
| Kidneys diffusely or focally enlarged, containing multiple low-attenuation masses with rim enhancement representing dilated calyces or foci of parenchymal destruction |
Table 2.
Summary of less common adult unilateral renal masses
| Mass | Distinguishing features |
|---|---|
| Multilocular cystic renal tumor | Bimodal age distribution, young boys and middle-aged women |
| Large, multiseptated, cystic mass separated by thick, enhancing septations | |
| Renal medullary carcinoma | Predominance in black patients with sickle cell trait |
| Aggressive, infiltrating neoplasm arising from the medulla | |
| Collecting duct carcinoma | Aggressive neoplasm arising from the medulla |
| Metanephric adenoma | Produces erythropoietin and is associated with polycythemia in 12% of cases |
| Benign neoplasm related histologically to Wilms tumor | |
| Leiomyoma | Commonly found in women in the 2nd to 5th decade of life |
| Hypervascular | |
| Benign | |
| Hemangioma | Demonstrates early intense enhancement on the arterial phase that persists on the delayed phase |
| Hyperintense on T2-weighted MR images | |
| Lymphangioma | Well-defined uni-or multiloculated mass arising from the renal sinus or perinephric space; locules do not enhance |
| Communicating cysts with lymphoid cells in their septa | |
| Sarcoma | Different histologic subtypes: leiomyosarcoma, angiosarcoma, rhabdomyosarcoma, etc. |
| Nonspecific imaging characteristics | |
| Renomedullary interstitial tumor | Benign tumors arising from renomedullary interstitial cells |
| Commonly found on autopsies as small lesions, but clinically are rare and not seen | |
| Carcinoid | Neuroendocrine tumor that can manifest with carcinoid syndrome on liver metastasis |
| Somatostatin receptor scintigraphy can aid in staging, but not often in locating the renal tumor | |
| Small cell carcinoma | Resembles its counterparts arising from the trachea-bronchial tree |
| Aggressive behavior | |
| Juxtaglomerular apparatus tumor | May produce elevated renin, associated with hypertension |
The purpose of this article is to review the clinical and imaging characteristics of these common and uncommon adult masses that predominantly present unilaterally, other than RCC. It is known that between 10% and 30% of renal masses are benign at surgery. Thus, knowledge of their imaging characteristics can aide in accurate diagnosis and treatment and potentially avoid unnecessary invasive procedures.
More common unilateral renal masses
Transitional cell carcinoma
Transitional cell carcinoma (TCC) is most commonly encountered in the urinary bladder, but up to 10% of transitional cell carcinomas occur in the upper urinary tract[2]. Upper tract TCC typically occurs in the sixth or seventh decades of life, and has a male preponderance of 3:1. Risk factors include smoking, chemical carcinogens such as aniline, benzidine and azo dyes, cyclophosphamide, heavy caffeine consumption, and analgesic abuse, especially phenacetin[2]. These substances are considered carcinogenic to the urothelium when excreted in urine. Families affected with Balkan endemic nephropathy are also at an increased risk.
Patients with TCC typically present with microscopic or gross hematuria. Eighty-five percent of upper tract TCCs are low-grade, broad-based, superficial, papillary neoplasms that are small at the time of detection, demonstrate slow growth, and have a relatively benign course[3]. Fifteen percent of upper tract TCCs are pedunculated or diffusely infiltrative, commonly advanced at detection, and have a more aggressive course. Both synchronous and metachronous TCCs may occur.
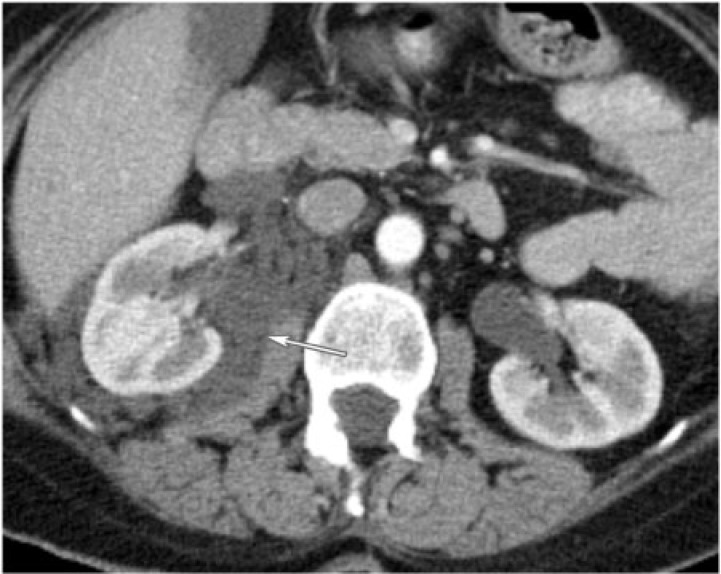
Renal pelvis TCC most frequently arises in the extrarenal part of the pelvis followed by the infundibulocaliceal region. Filling defects within dilated calices may result secondary to tumor obstruction of the infundibulum, which may lead to what has been called caliceal amputation. When invading the renal parenchyma, often they do so in an infiltrative pattern without deforming the renal contour. Other findings include pelvicaliceal irregularity, focal or diffuse mural thickening, focally obstructed calices, and calices dilated with tumor, also called oncocalices. Computed tomography (CT), especially with the advent of CT urography, is well established in preoperative staging and assessment of upper tract TCC (Fig. 1). Smaller lesions less than 1 cm or carcinoma in situ may be difficult to detect with CT imaging. Treatment depends on the stage of the disease, but if surgical, ipsilateral nephrectomy and ureterectomy are performed.
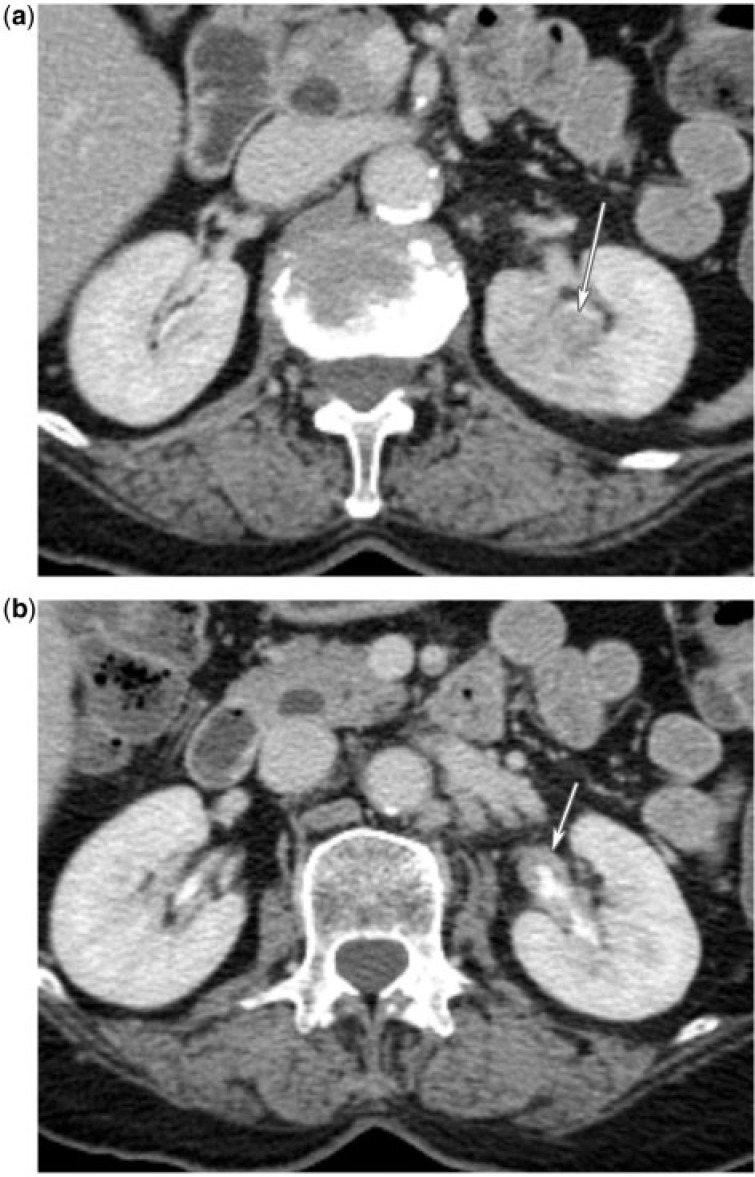
Figure 1.
(a) A 75-year-old woman with upper tract urothelial carcinoma. CT urogram reveals a poorly enhancing filling defect involving and infiltrating the upper pole left renal calices (arrow). (b) A 75-year-old woman with upper tract urothelial carcinoma. CT urogram reveals additional urothelial thickening in the left renal pelvis (arrow).
Oncocytoma
Oncocytoma is the most common benign renal tumor, accounting for 3–10% of all renal tumors. It has a 2:1 male preponderance. It is usually asymptomatic and rarely associated with hypertension, flank pain and hematuria. Oncocytoma is usually unilateral and solitary in occurrence.
Classically, oncocytomas are low attenuation masses that demonstrate spoke-wheel like enhancement and a central scar (Fig. 2). However, these imaging features are found in just a small percentage of the tumors (Fig. 3). Oncocytomas are often resected because they can be indistinguishable from renal cell carcinoma on imaging and even biopsy. Conservative management includes close observation to assess for increase in size[4].
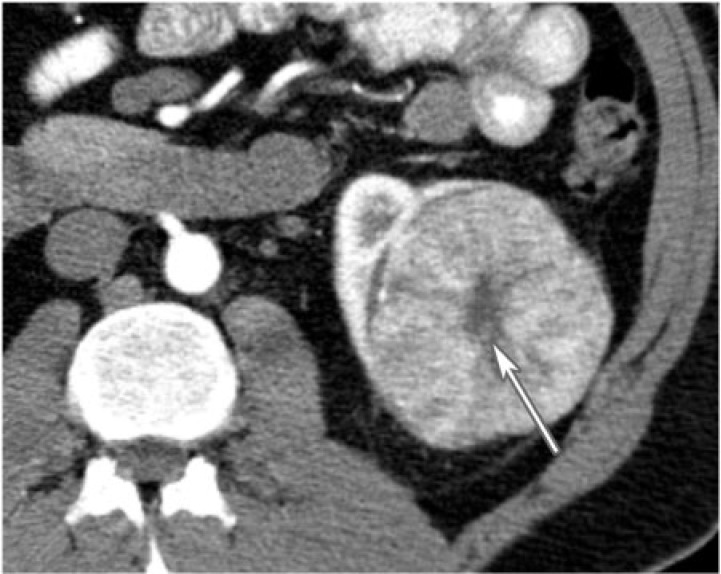
Figure 2.
A 42-year-old man with left renal oncocytoma. CT with contrast displays a large left enhancing renal mass with central low density scar (arrow).
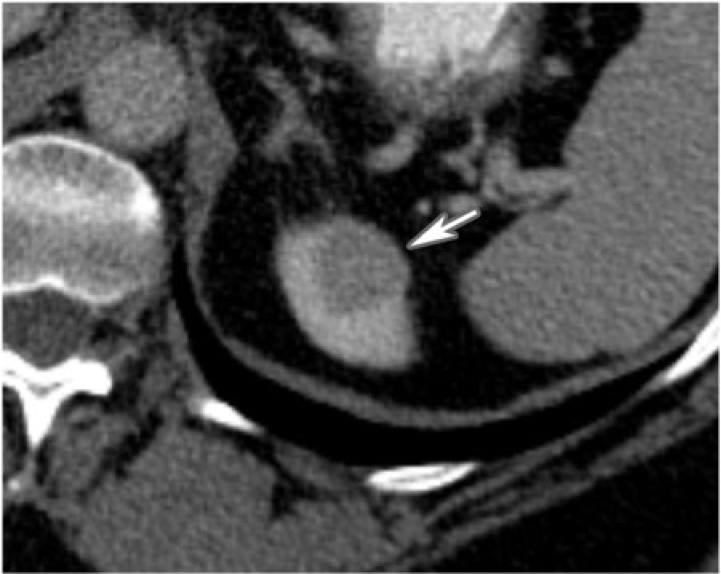
Figure 3.
A 59-year-old woman with oncocytoma. CT with contrast shows a poorly enhancing homogenous mass involving the upper pole of the left kidney (arrow). It lacks the classic spokewheel appearance.
Mesenchymal fat-containing neoplasms
Fat-containing renal tumors include the fairly common angiomyolipomas (AML) and less common lipomas and liposarcomas. Angiomyolipomas are the most common benign renal mesenchymal neoplasms. They are composed of blood vessels, smooth muscle and adipose tissue in varying proportions[5]. Angiomyolipomas are classified into two subtypes: the common classic triphasic subtype and the rare monotypic epithelioid subtype. The latter is infrequent, more aggressive and potentially malignant; it can be associated with recurrence, metastasis and death[6]. The classic triphasic subtype is most often sporadic, solitary (80%) and symptomatic with a 4:1 female preponderance[5]. Triphasic refers to the pathologic appearance containing thick-walled blood vessels, adipose tissue, and myxoid elements. Tuberous sclerosis is associated with small, multicentric, asymptomatic angiomyolipomas in 80% of patients.
Angiomyolipomas can often be distinguished from other fat-containing renal and peri-renal neoplasms such as renal lipomas, and retroperitoneal lipomas and liposarcomas based on imaging findings. Angiomyolipomas manifest as fat-containing renal masses with common soft tissue components as well as enhancing blood vessels within them; they almost always alter the renal contour (Fig. 4). Fatty components of lipid-containing tumors show fat attenuation on CT and on magnetic resonance imaging (MRI), are usually hyperintense on T1-weighted images, hypointense on T2-weighted images, and lose signal on fat-suppressed images. Frequency selective fat suppression should be used for fat suppression instead of in- and out-of-phase imaging due to the presence of macroscopic fat.
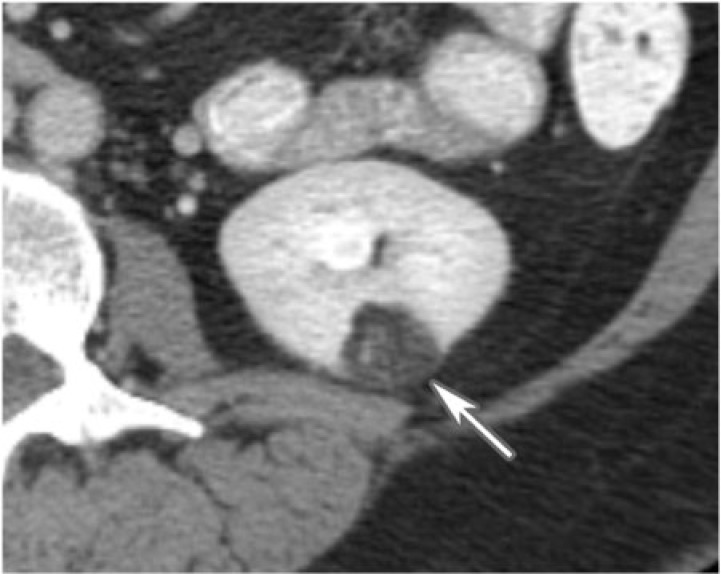
Figure 4.
A 50-year-old woman with left renal angiomyolipoma. A small fat-containing mass lies in the lower pole of the left kidney, consistent with angiomyolipoma as seen on CT imaging (arrow).
Primary renal lipoma is a very rare renal neoplasm with only 20 reported cases in the published literature[7]. Liposarcomas are also rare. Retroperitoneal lipomas and liposarcomas occur outside the kidneys and may demonstrate perinephric space involvement, but tend to preserve the renal contour and do not invade the renal parenchyma (Fig. 5).
Figure 5.
A 44-year-old woman with retroperitoneal liposarcoma. The retroperitoneal liposarcoma manifests as a poorly enhancing mass involving the left perinephric space as demonstrated by CT with intravenous contrast (arrows). It does not invade the renal parenchyma.
Management of asymptomatic angiomyolipomas less than 4 cm entails annual or semi-annual follow-up. AML greater than 4 cm as well as symptomatic angiomyolipomas manifesting as acute abdominal or flank pain and bleeding are treated with surgical resection or selective embolization. Simple lipomas require no treatment while complex masses suspicious for liposarcomas are routinely resected.
Hematoma
Renal hematomas can be spontaneous or post-traumatic. They are more commonly unilateral rather than bilateral. Most renal hematomas are subcapsular and appear crescentic when small and biconvex if large. The hematomas may extend into the perinephric space if the renal capsule is interrupted or lacerated. Rarely, hematomas have a mass-like appearance. Acute hematomas are hyperattenuating in appearance on noncontrast CT, become iso- to hypoattenuating and commonly smaller over time.
Spontaneous renal hematomas can be secondary to an underlying neoplasm, vasculitides, infection, or bleeding dyscrasias (Fig. 6). Hence, a careful search for underlying pathology should be made if a spontaneous hematoma is detected without a history of trauma. A large subcapsular hematoma may cause renovascular hypertension by compression and ischemia of the underlying renal parenchyma, known as a page kidney, often necessitating emergent evacuation. Treatment of hematomas depends on the underlying cause and symptoms.
Figure 6.
A 74-year-old woman with left perinephric hematoma from a bleeding angiomyolipoma. CT without intravenous contrast shows a high attenuation fluid collection surrounding the left kidney. The high attenuation fluid collection is an acute hematoma (arrow). The fat attenuation mass is the bleeding angiomyolipoma (arrowhead).
Infection
Renal abscess
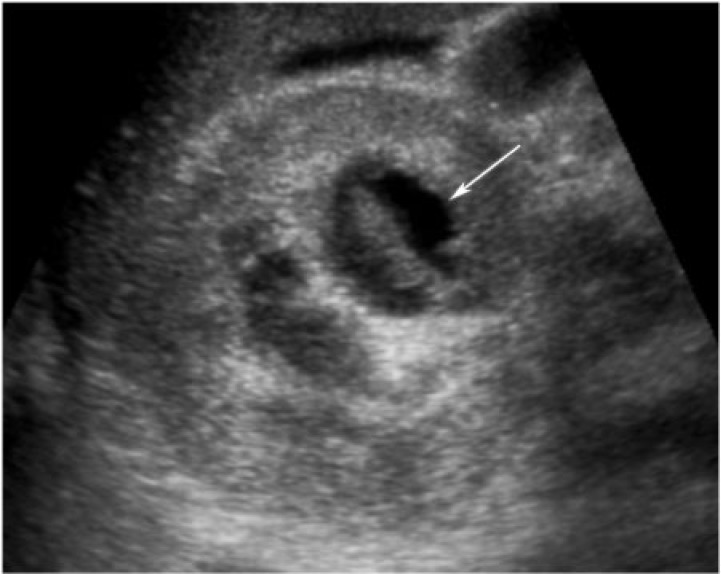
Renal abscess is a result of liquefactive necrosis in the setting of acute pyelonephritis[8]. Abscesses may be associated with ascending infection with organisms such as Escherichia coli or Proteus, commonly with obstruction; by hematogenous spread or by direct extension from an extrarenal inflammatory process[8]. Fever, flank pain, hematuria and leukocytosis are some of the presenting symptoms. Significantly, urine culture is negative in 67% of patients and blood cultures are negative in 50% of patients.
On cross-sectional imaging, an abscess usually appears as a focal fluid collection with enhancing walls that may contain bubbles of gas (Fig. 7). Abscesses are hyperechoic on ultrasonography in the acute stage with a subsequent isoechoic or hypoechoic appearance with or without clear through transmission, septations or internal echoes reflecting bubbles of gas (Fig. 8). Abscesses can extend into the perirenal space causing thickening of the Gerota fascia, and can extend into adjacent structures, including the psoas muscle[8]. Treatment involves antibiotics and commonly drainage.
Figure 7.
A 33-year-old man with gastric cancer and renal abscess. CT with intravenous contrast reveals a central hypodense mass with peripheral enhancement and perinephric stranding in the left kidney (arrow).
Figure 8.
A 23-year-old woman with metastatic clear cell carcinoma of the cervix and renal abscess. Renal ultrasonography reveals a complex heterogeneous cystic mass in the right kidney (arrow). Aspiration demonstrated purulent material.
Xanthogranulomatous pyelonephritis
Xanthogranulomatous pyelonephritis (XGP) is an uncommon reaction of the kidney to chronic renal infection secondary to renal obstruction; it is characterized by destruction and replacement of renal parenchyma by lipid-laden macrophages[9]. A staghorn calculus is the most common cause of the long-standing obstruction. XGP is more common in middle-aged women. E. coli and Proteus mirabilis are the most common causative organisms.
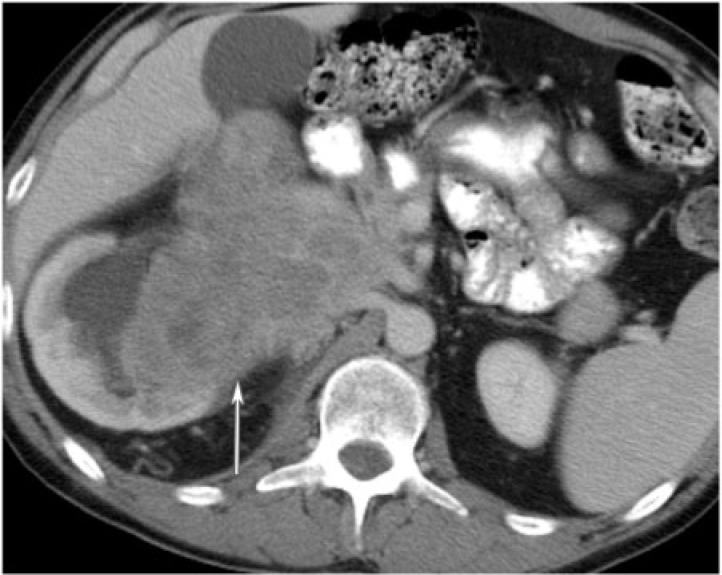
XGP more commonly presents as diffuse renal involvement or less often as a localized, tumefactive form that can sometimes be confused with a renal neoplasm on imaging. Intravenous pyelogram can demonstrate complete or focally absent nephrogram, commonly with the presence of a staghorn calculus on the scout view. Ureteropelvic or infundibulocalyceal obstruction may be seen on retrograde pyelogram. The calyces are dilated on ultrasonographs, often with fluid levels and echogenic rim, with or without the loss of corticomedullary differentiation. On CT, the kidney is diffusely enlarged by multiple, low attenuation masses with rim enhancement representing dilated calyces or foci of parenchymal destruction compressing the surrounding renal parenchyma (Fig. 9). Other findings may include the presence of a staghorn calculus, contracted renal pelvis and extrarenal extension into the perinephric space or adjacent organs. Cross-sectional imaging can also show extension of the inflammatory process beyond the kidney.
Figure 9.
A 29-year-old woman with xanthogranulomatous pyelonephritis. The left renal parenchyma is replaced with a heterogeneous low density and infiltrating mass (arrow). There are associated perinephric stranding and coarse calcifications (arrowhead) in the upper pole as seen on CT with intravenous contrast.
Significantly, urine cultures may be negative in 26–39% of cases and renal parenchymal cultures may be misleading. Treatment includes antibiotic therapy for controlling infection, lithotripsy for the staghorn calculus and partial or total nephrectomy.
Less common unilateral renal masses
Multilocular cystic renal tumor
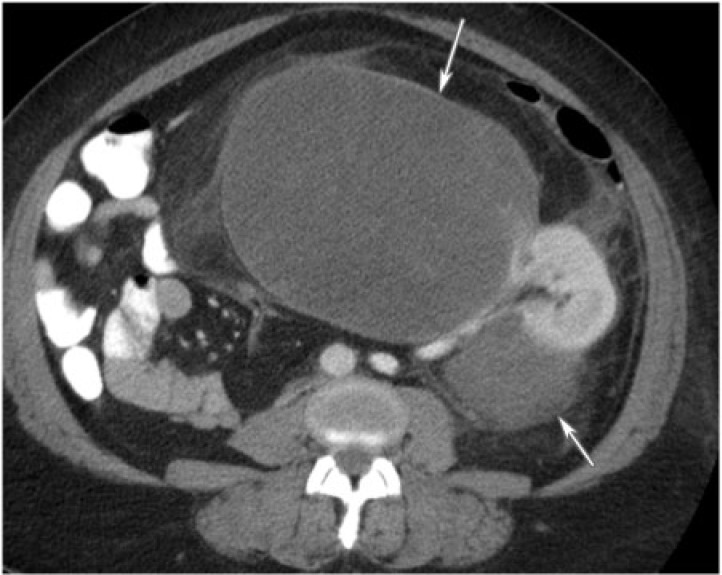
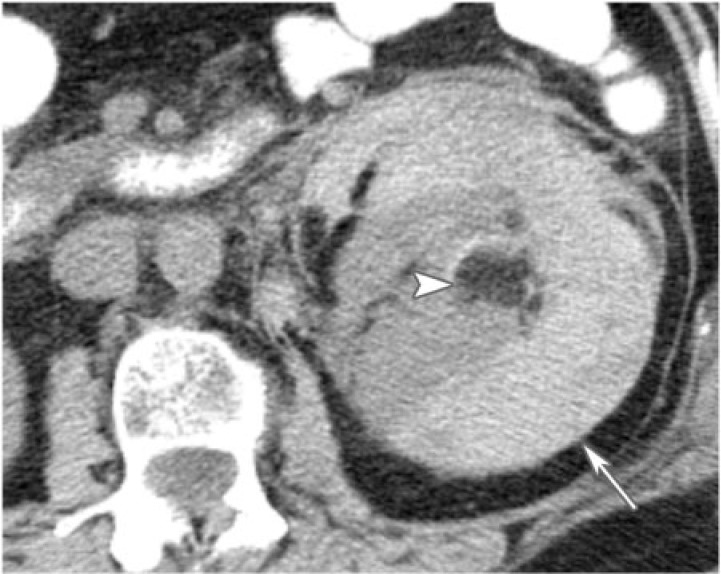
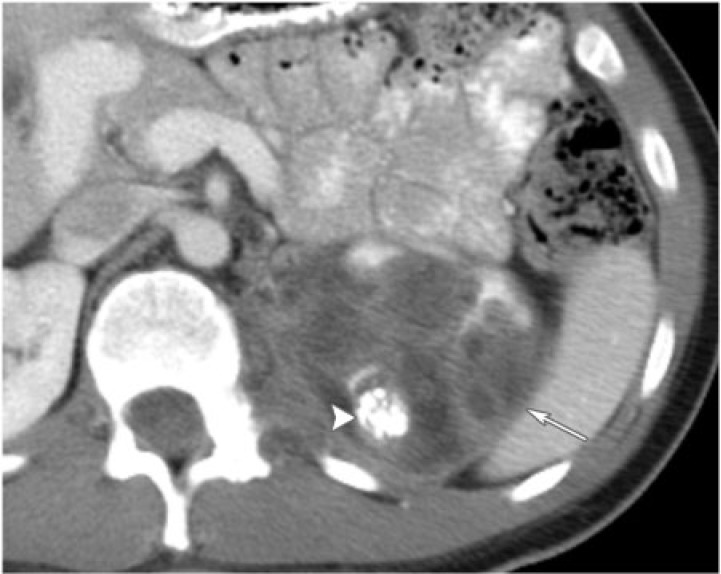
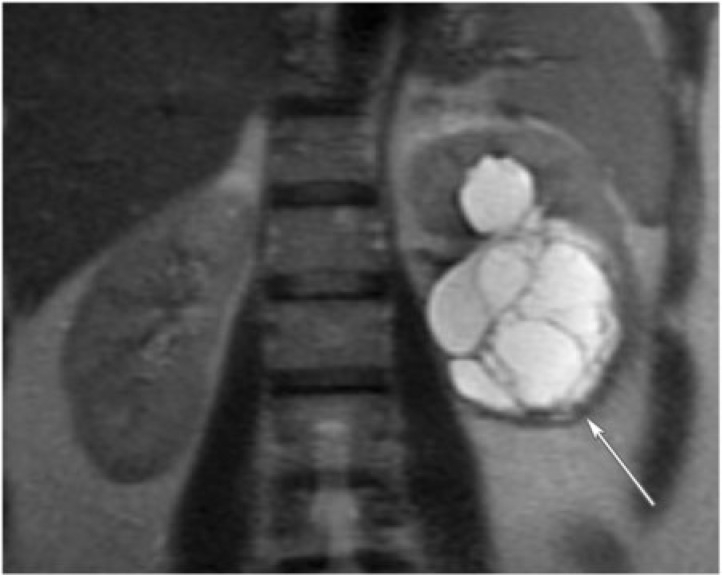
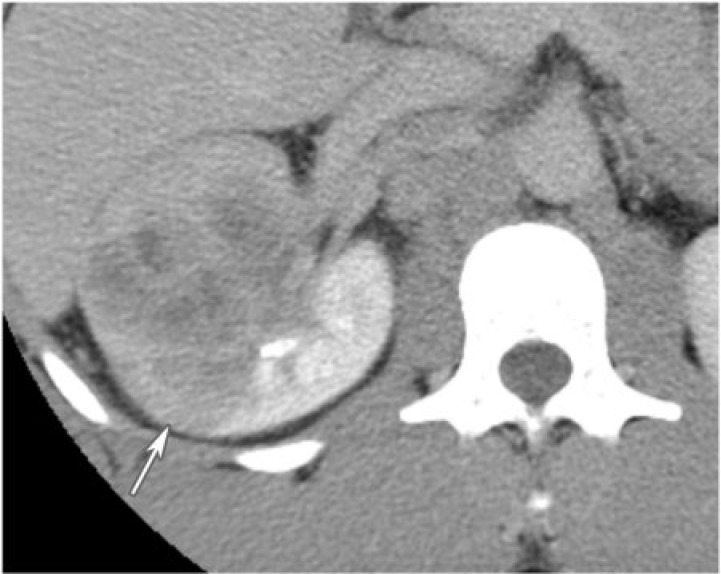
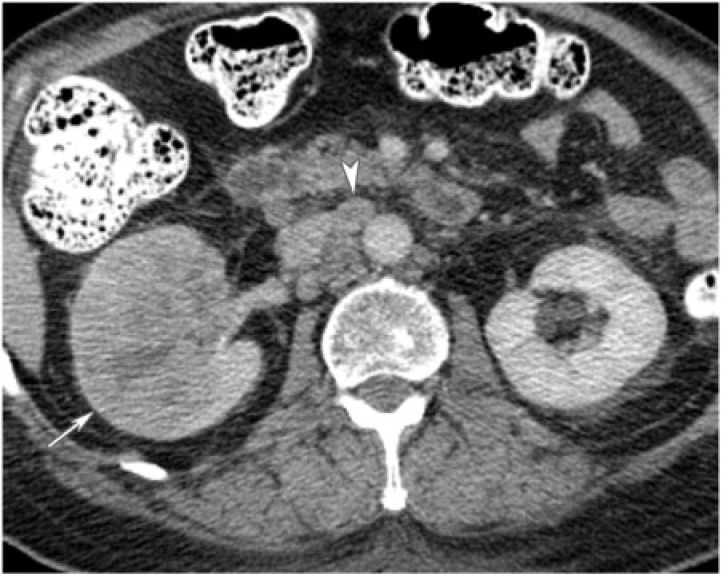
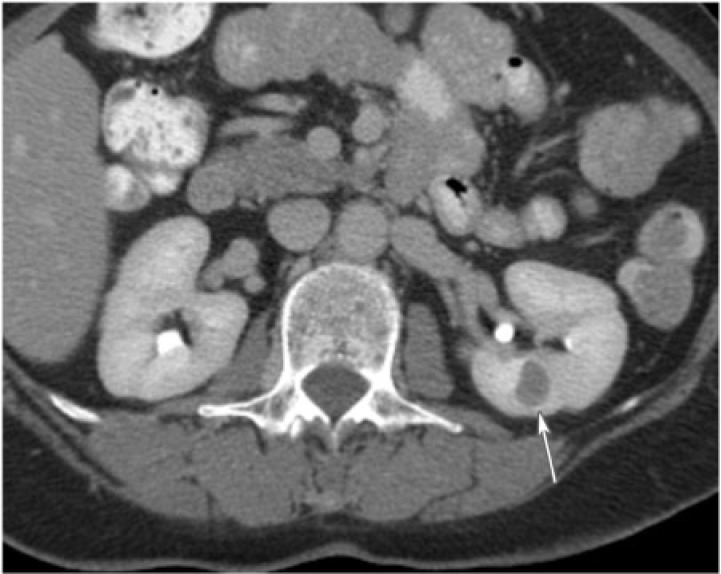
Cystic nephroma is a rare benign tumor composed of cysts lined by epithelium and fibrous septa containing mature tubules . It belongs to the same spectrum and can be anatomically and radiologically indistinguishable from cystic partially differentiated nephroblastoma (CPDN), which contains blastemal cells in the epithelial lining. Cystic nephroma and CPDN represent two extremes of the same spectrum of disease and can be lumped under the term multilocular cystic renal tumor[10]. It has a bimodal age and sex distribution occurring in young boys aged 3 months to 4 years (usually CPDN) and older women aged 40 to 60 years (usually cystic nephroma). It typically presents as a painless abdominal mass in children and as abdominal pain and hematuria in adults.
Multilocular cystic renal tumor manifests as a large (average size 10 cm), well-circumscribed, multiseptated cystic mass with various sized cysts separated by thick enhancing septations. Calcifications of septae or capsule are uncommon. The mass may entirely replace one pole of the kidney. On CT, the cysts have slightly higher attenuation than water. When the cysts are small, the tumor may appear as a complex echogenic mass. On MRI, the cysts are hyperintense on T2-weighted images and can have variable signal on T1-weighted images, depending on the presence of hemorrhagic and proteinaceous material within them (Fig. 10). The septations are typically hypointense on T1-weighted and T2-weighted images.
Figure 10.
A 60-year-old woman with multilocular cystic nephroma. Coronal T2-weighted single shot fast spin echo of the abdomen reveals a multilocular and multisepated, cystic left renal mass (arrow).
No treatment is needed, but this lesion may be excised if it cannot be distinguished from a cystic renal cell carcinoma. Close post-surgical surveillance is required if the pathology reveals CPDN because potentially it can behave aggressively due to the presence of blastemal cells.
Renal medullary neoplasms
Renal medullary carcinoma
Renal medullary carcinoma is an uncommon, aggressive, infiltrative neoplasm arising from the renal medulla. It occurs primarily in patients younger than 40 years of age and predominantly in black patients with sickle cell[11]. Histologically, the tumors arise from the renal medulla, either from the distal collecting ducts or from the epithelium of the renal papillae. On cross-sectional imaging, these tumors appear as large, often infiltrative, central masses with varying amounts of hemorrhage and necrosis (Fig. 11). The prognosis is dismal, with the majority of patients presenting with metastasis to regional lymph nodes, lung or liver at the time of diagnosis. Renal medullary carcinoma may represent a particularly aggressive form of collecting duct carcinoma[12].
Figure 11.
A 23-year-old African American male with sickle cell trait. CT with contrast shows an infiltrative mass invading the right kidney with associated asymmetric renal enlargement (arrow). Renal medullary carcinoma was diagnosed after right nephrectomy.
Collecting duct carcinoma
Collecting duct carcinoma, also called duct of Bellini carcinoma, is an aggressive, rare neoplasm arising from the renal medulla. Over 100 cases have been reported in the literature. The cell origin of the tumor is uncertain, although the tumor is composed of collecting ducts on histology. The mean age at diagnosis is 55 years and there is a male preponderance (2:1). Forty percent of patients present with metastatic disease at diagnosis and only one-third of patients survive beyond 2 years from diagnosis[12].
On CT, collecting duct carcinomas manifest as large infiltrative masses centered in the renal medulla. They encroach the renal sinus fat and are often associated with an expansile component representing ductal dilatation. Collecting duct carcinomas are usually hyperechoic relative to renal parenchyma on ultrasonography, hypovascular on angiogram and hypointense on T2-weighted MR imaging (Fig. 12). They may be indistinguishable from transitional cell carcinoma. However, it is important to make the distinction based on tissue diagnosis, if possible, because TCC is treated with nephroureterectomy rather than nephrectomy.
Figure 12.
A 64-year-old man with collecting duct carcinoma. An ill-defined, infiltrative and poorly enhancing mass invades the right renal parenchyma as seen on CT with contrast (arrow). Associated retroperitoneal lymphadenopathy is present (arrowhead).
Metanephric adenoma
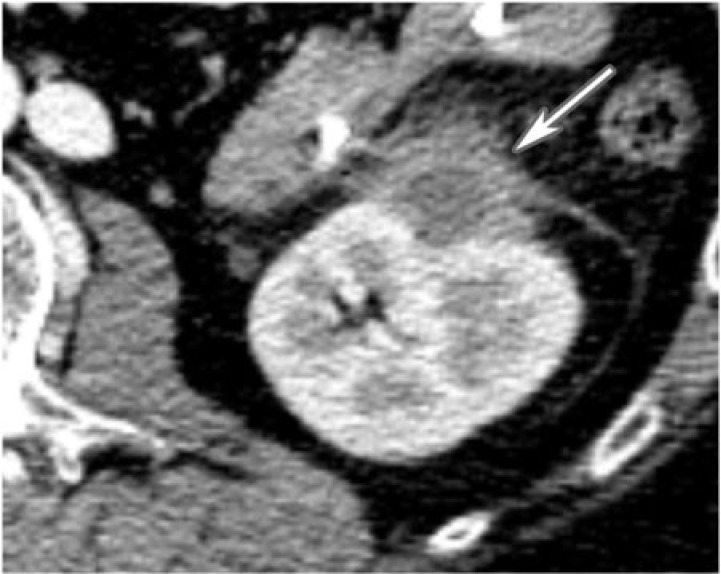
Metanephric adenoma is a rare, benign renal neoplasm. There are over 100 reported cases. It has a reported 2:1 female preponderance, with a peak occurrence in the fifth and sixth decades of life. It is histologically related to Wilms tumor and is thought to represent the hyperdifferentiated benign end of the nephroblastoma spectrum[13]. Histologically, the tumor is composed of acinar sheet-like or tubular arrangements of monotonous small blue embryonal cells. A distinguishing feature is that these tumors produce erythropoietin and are associated with polycythemia in 12% of cases.
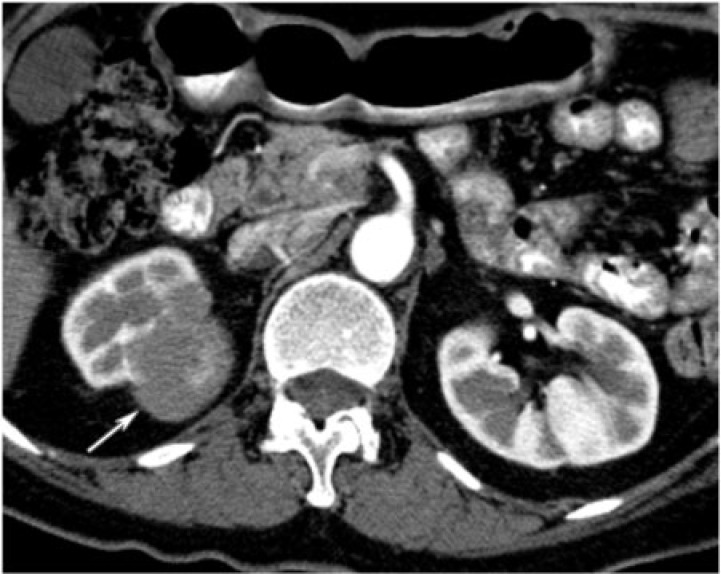
The tumor typically appears as a well-defined unencapsulated mass on unenhanced CT that is often exophytic, solid-cystic, and hyperattenuating. Calcifications are seen in 20% of cases. Larger masses may be heterogeneous and hypovascular, with areas of hemorrhage and necrosis. On ultrasonography, they may appear as hyperechoic masses with relatively good through transmission. Since they are generally indistinguishable from renal cell carcinoma on imaging, they are commonly treated surgically (Fig. 13). Polycythemia or percutaneous biopsy may be helpful in making the diagnosis. No treatment is needed if asymptomatic.
Figure 13.
A 36-year-old woman with metanephric adenoma. CT with contrast exposes a low density, well-circumscribed, poorly enhancing mass in the upper pole of the right kidney (arrow).
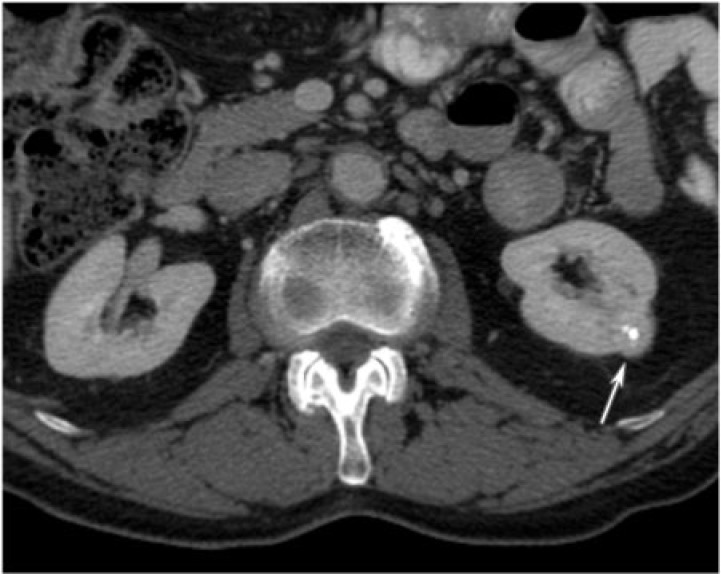
Leiomyoma
Renal leiomyoma is a rare benign mesenchymal tumor with an incidence of 5% on autopsy. It has been categorized into three types: subcortical lesions, lesions originating from the renal vessels or capsule, and those originating in the renal pelvis. Small subcortical lesions are the most common. The lesions originating from the capsule and renal vessels may be large in size and cause obstructive or compressive symptoms. The type originating from the renal pelvis is rare[14]. Histologically, the tumors are composed of intersecting fascicles of spindle cells that are positive for immunoreactivity to actin or desmin. They more commonly occur in women in the second to fifth decade of life.
On imaging, leiomyomas are usually indistinguishable from the renal parenchyma and if seen, are commonly indistinguishable from RCCs or TCCs. Small lesions may appear as homogenously enhancing, well-circumscribed, partially exophytic, solid masses. Larger lesions have a variable appearance and may be heterogeneous, solid-cystic or completely solid tumors and can have areas of hemorrhage or necrosis (Figs. 14 and 15). Leiomyomas are typically hypervascular and they often derive their blood supply from the capsular vessels, which may be demonstrated angiographically. These tumors have an excellent prognosis.
Figure 14.
A 62-year-old female with renal leiomyoma. CT with contrast delineates a homogenously enhancing, exophytic, cortical mass in the upper pole of the right kidney (arrow).
Figure 15.
A 77-year-old man with left renal leiomyoma. CT with contrast reveals a homogenously enhancing, cortical mass near the mid-pole of the left kidney with central calcifications (arrow). Its enhancement is isodense to the surrounding normal renal parenchyma.
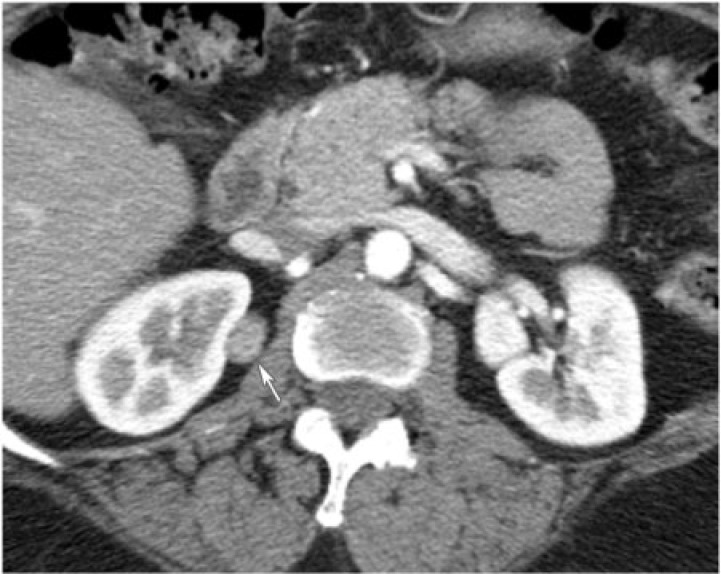
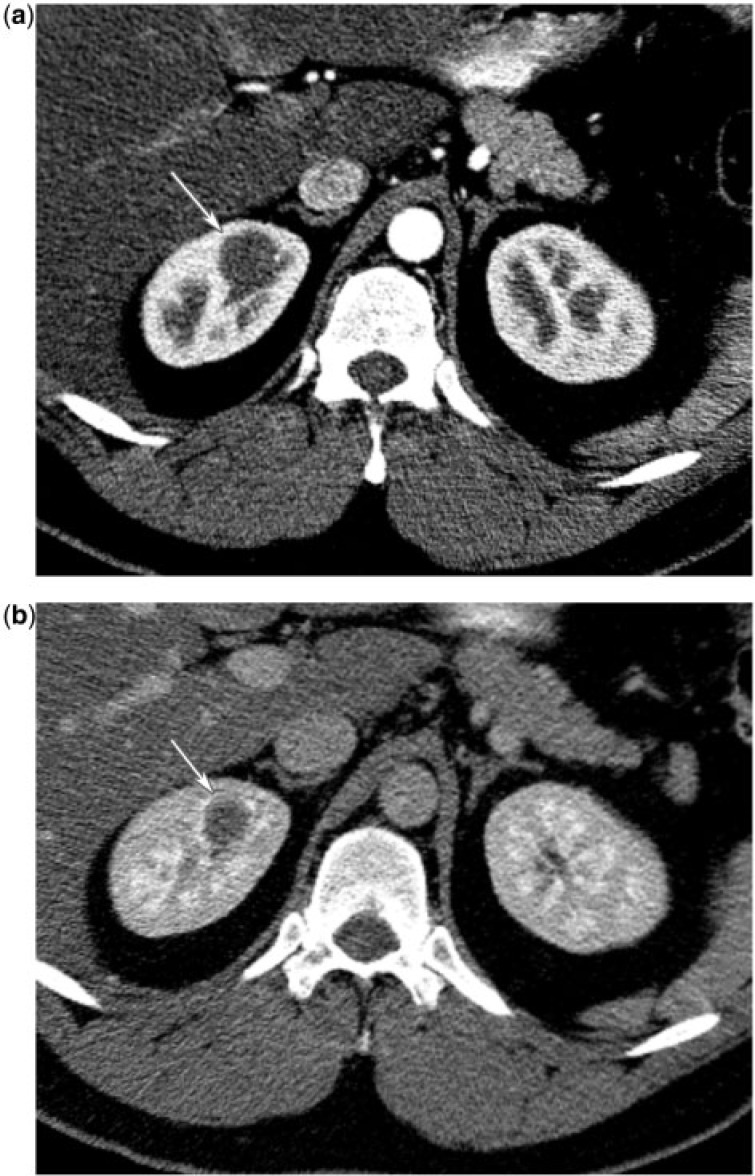
Hemangioma
Renal hemangioma is a rare, benign mesenchymal neoplasm composed of blood-filled vascular spaces lined by endothelium. It commonly affects young adults[6]. It typically presents as recurrent hematuria, renal colic or can be asymptomatic and incidentally found. Renal hemangiomas are often associated with syndromes such as Sturge–Weber, Klippel–Trenaunay, and systemic angiomatosis. Cavernous hemangiomas are more common than capillary variants[6].
Renal hemangioma is typically a solitary, unilateral, unencapsulated mass arising from the renal pyramids or the pelvis. On ultrasonography, they have variable echogenicity. As in other organs, hemangiomas can demonstrate early intense enhancement on contrast-enhanced CT that persists on the delayed phase, and are hyperintense on T2-weighted MR images[6]. They can also present atypically without the characteristic enhancement pattern (Fig. 16).
Figure 16.
(a) A 35-year-old man with right renal hemangioma. CT renal protocol in the corticomedullary phase reveals a poorly enhancing mass in the upper pole medulla of the right kidney (arrow). (b) A 35-year-old man with a right renal hemangioma. CT renal protocol in the nephrographic phase reveals a poorly enhancing mass in the upper pole medulla of the right kidney (arrow).
Lymphangioma
Renal lymphangioma is a rare, benign, cystic neoplasm arising from the peripelvic region or the renal sinus, and occasionally from the lymphatics of the capsule or the cortex. Histologically, lymphangiomas are composed of communicating, endothelial spaces with clear fluid that may have lymphoid cells in their septae[6]. Renal lymphangioma may occur as an isolated lesion or in association with perinephric or systemic lymphangiomatosis and may be localized or diffuse. A lymphangioma typically appears as a well-defined uni- or multiloculated cystic mass most commonly arising from the renal sinus or perinephric space (Fig. 17).
Figure 17.
A 49-year-old woman with lymphangioma. Cystic masses involve the right perinephric space and sinus as seen with CT with intravenous contrast enhancement (arrow).
Sarcoma
Primary renal sarcomas are rare malignant mesenchymal neoplasms that constitute about 1% of malignant renal tumors[15]. Renal sarcomas carry a poor prognosis, depending on the biological behavior of different histologic subtypes. The subtypes of sarcoma include; leiomyosarcoma, angiosarcoma, hemangiopericytoma, rhabdomyosarcoma, fibrosarcoma and osteosarcoma. Leiomyosarcoma constitutes over 50% of primary renal sarcomas. Diagnosis requires exclusion of sarcomatoid renal carcinoma and direct extension from a primary retroperitoneal sarcoma, both of which are more common than primary renal sarcoma (Fig. 18).
Figure 18.
A 53-year-old man with retroperitoneal sarcoma. CT of the abdomen with contrast displays a heterogeneously enhancing, irregular, large retroperitoneal mass engulfing the right renal sinus and extending into the right kidney causing obstructive hydronephrosis (arrow).
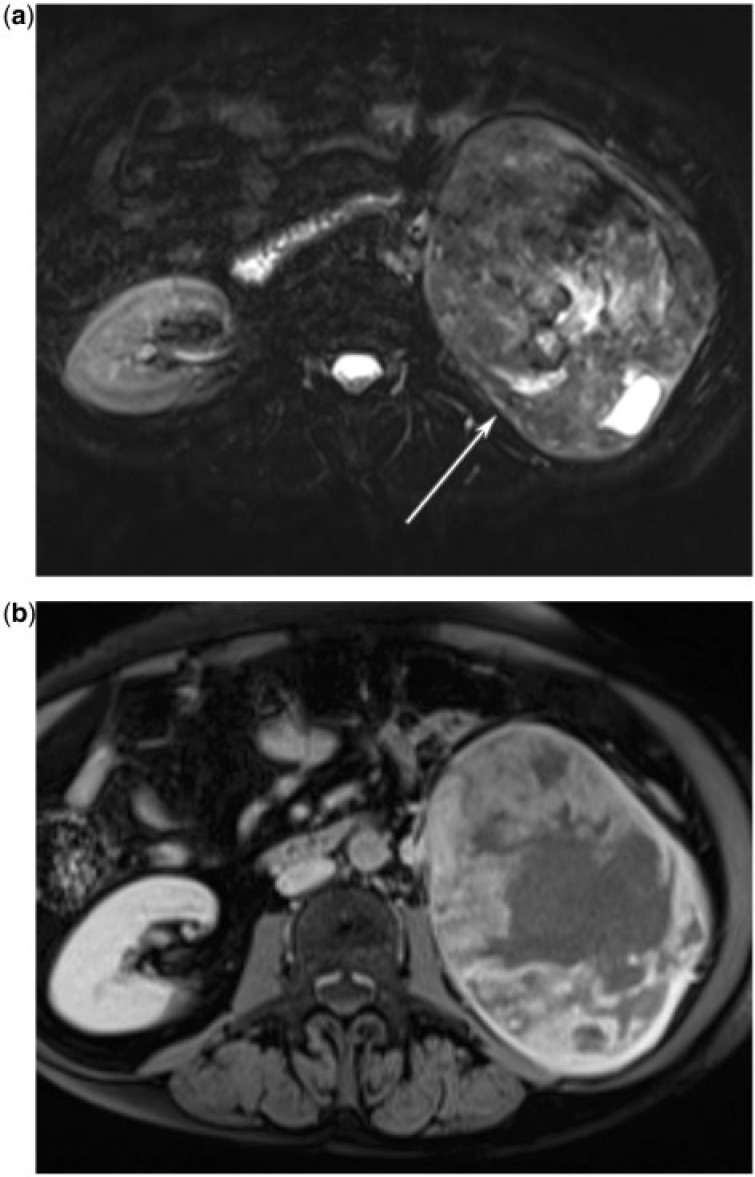
Leiomyosarcoma (Fig. 19) and most other renal sarcomas typically present as a well-defined, expansile mass; whereas rhabdomyosarcomas and angiosarcomas tend to have an infiltrative pattern[16]. The imaging appearance of renal sarcomas is variable and depends on their cellular constituents.
Figure 19.
(a) A 71-year-old woman with renal leiomyosarcoma. T-2 weighted fat-saturates axial MR image reveals a mixed signal, well-circumscribed mass replacing the left kidney (arrow). (b) A 71-year-old woman with renal leiomyosarcoma. T1-weighted fat-saturated post intravenous contrast axial MR image shows that the mass has heterogeneous enhancement.
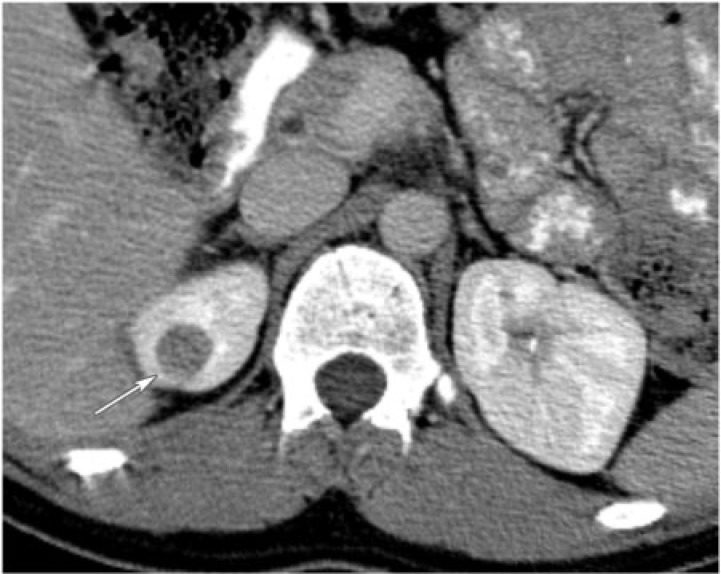
Renomedullary interstitial tumor
Also known as medullary fibromas, these are benign tumors arising from renomedullary interstitial cells. Normally, these cells play a role in blood pressure homeostasis . If symptoms are present, they are usually due to torsion of the tumor about its pedicle, resulting in infarct and hematuria[17]. Renomedullary interstitial tumors are common incidental findings noted in approximately 50% of adults at autopsy[6]. However, clinically they are rare because most are small. They can reach up to 5 cm, and are characteristically located in the renal pyramids. Imaging findings are nonspecific. These tumors appear as nonenhancing, hypoattenuating, solid lesions without calcifications (Fig. 20)[6].
Figure 20.
A 68-year-old woman with left renal medullary fibroma. CT with intravenous contrast reveals a well-defined, homogeneous, poorly enhancing mass in the left kidney (arrow).
Renal neuroendocrine tumors
Carcinoid
Carcinoid tumors are low-grade malignant tumors that arise from neuroendocrine enterochromaffin or amine precursor uptake and decarboxylation cells. Primary renal carcinoid is a rare tumor of the kidney, with approximately 60 cases reported in the English literature[18]. If renal carcinoid is found, it is more commonly a metastasis from a primary carcinoid arising elsewhere and often as part of late-stage disease. Their pathogenesis is uncertain since neuroendocrine cells are not normally found in the renal parenchyma or the collecting systems. Hypotheses explaining the origin of primary renal carcinoids include misplaced neural crest tissue in the hilar aspect of the kidney during embryogenesis, and neuroendocrine cells arising as part of intestinal metaplasia of the renal pelvicalyceal system as a sequela of chronic inflammation. The mean age of the patients at the time of diagnosis is 50 years with a range of 23–79 years. Approximately 20% of the reported patients are asymptomatic at the time of diagnosis. Others have symptoms such as abdominal pain, flank pain, hematuria and rarely carcinoid syndrome.
Renal carcinoids can manifest as solid masses, contain cystic components, necrosis, and dystrophic calcifications. Findings on cross-sectional imaging are nonspecific and often indistinguishable from other solid tumors, which commonly results in surgical excision (Fig. 21). Somatostatin receptor scintigraphy is an integral diagnostic and staging modality due to the relative lack of specificity and sensitivity of findings on CT or MR, but is more useful for detecting metastases rather than renal carcinoid since physiologic excretion of the radiopharmaceutical often obscures the renal lesion.
Figure 21.
A 53-year-old woman with primary renal carcinoid tumor. CT with contrast demonstrates a solid enhancing mass growing exophytically from the upper pole of the right kidney (arrow). Case courtesy of Dr Aparna Balachandran.
Complete surgical resection is the treatment of choice for localized tumors. Primary renal carcinoids have a more indolent course compared with renal cell carcinomas, even in the presence of metastases.
Small cell carcinoma
Small cell carcinoma of the kidney is an extremely rare neoplasm with a female preponderance of 3.4:1. The median age at diagnosis is 62 years[19]. Renal small cell carcinoma resembles its counterparts arising from the tracheobronchial tree or other extrapulmonary sites in its aggressive behavior and high propensity of locoregional spread and distant metastasis. Clinical presentation is usually late in the course of the disease. Presenting symptoms include flank pain, abdominal pain and hematuria.
Small cell carcinoma has imaging characteristics indistinguishable from renal cell carcinoma. It carries a poor prognosis with a median survival of 8 months[19]. Treatment includes surgical resection and chemotherapy.
Juxtaglomerular apparatus tumor
Juxtaglomerular apparatus (JGA) tumor is a rare benign tumor. There are less than 50 such cases reported in the literature. They are distinguishable when they produce renin. Although uncommon, JGA tumor presents a distinct clinical presentation that should allow correct preoperative diagnosis. Typically this includes headaches, polyuria, and/or severe hypertension. The average age at initial presentation is 22 years. The plasma rennin levels are commonly increased two- to sevenfold greater than the normal value. In the presence of hyperreninism, it is important to differentiate a primary source such as secretion by a renal tumor from a secondary cause such as renal arterial stenosis or renal parenchymal disease. Lung carcinoma, pancreatic adenocarcinoma and fallopian tube adenocarcinoma can also be causes of increased renin secretion.
Nearly all JGA tumors are visible on CT scan as weakly enhancing, isodense or hypodense lesions compared with the renal medulla. Although JGA tumors are benign, with no reports of recurrence or metastases, they can be potentially lethal if untreated due to hypertension-related complications[20]. Treatment may include pharmacologic management of hypertension and/or curative resection.
Conclusion
A wide spectrum of unilateral neoplasms occurs in the kidney, besides renal cell carcinoma. Although many of these neoplasms have overlapping features and may be indistinguishable on imaging, others have characteristic history, imaging findings and distribution. Knowledge of histology, pathogenesis, clinical and imaging features of both common and uncommon adult unilateral renal masses helps in more confidently suggesting differential diagnostic possibilities and in some cases can obviate the need for unnecessary nephrectomies.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Prasad SR, Humphrey PA, Catena JR, et al. Common and uncommon histologic subtypes of renal cell carcinoma: imaging spectrum with pathologic correlation. Radiographics. 2006;26:1795–1806. doi: 10.1148/rg.266065010. [DOI] [PubMed] [Google Scholar]
- 2.Hall MC, Womack S, Sagalowsky AI, Carmody T, Erickstad MD, Roehrborn CG. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology. 1998;52:594–601. doi: 10.1016/S0090-4295(98)00295-7. [DOI] [PubMed] [Google Scholar]
- 3.Urban BA, Buckley J, Soyer P, Scherrer A, Fishman EK. CT appearance of transitional cell carcinoma of the renal pelvis: Part 1. Early-stage disease. AJR Am J Roentgenol. 1997;169:157–161. doi: 10.2214/ajr.169.1.9207517. [DOI] [PubMed] [Google Scholar]
- 4.Zhang G, Monda L, Wasserman NF, Fraley EE. Bilateral renal oncocytoma: report of 2 cases and literature review. J Urol. 1985;133:84–86. doi: 10.1016/s0022-5347(17)48798-2. [DOI] [PubMed] [Google Scholar]
- 5.Eble JN. Angiomyolipoma of kidney. Semin Diagn Pathol. 1998;15:21–40. [PubMed] [Google Scholar]
- 6.Prasad SR, Surabhi VR, Menias CO, Raut AA, Chintapalli KN. Benign renal neoplasms in adults: cross-sectional imaging findings. AJR Am J Roentgenol. 2008;190:158–164. doi: 10.2214/AJR.07.2724. [DOI] [PubMed] [Google Scholar]
- 7.Chiang IC, Jang MY, Tsai KB, Hsieh TJ. Huge renal lipoma with prominent hypervascular non-adipose elements. Br J Radiol. 2006;79:e148–e151. doi: 10.1259/bjr/28725217. [DOI] [PubMed] [Google Scholar]
- 8.Demertzis J, Menias CO. State of the art: imaging of renal infections. Emerg Radiol. 2007;14:13–22. doi: 10.1007/s10140-007-0591-3. [DOI] [PubMed] [Google Scholar]
- 9.Hayes WS, Hartman DS, Sesterbenn IA. From the archives of the AFIP. Xanthogranulomatous pyelonephritis. Radiographics. 1991;11:485–498. doi: 10.1148/radiographics.11.3.1852939. [DOI] [PubMed] [Google Scholar]
- 10.Lowe LH, Isuani BH, Heller RM, et al. Pediatric renal masses: Wilms tumor and beyond. Radiographics. 2000;20:1585–1603. doi: 10.1148/radiographics.20.6.g00nv051585. [DOI] [PubMed] [Google Scholar]
- 11.Davis CJ, Jr, Mostofi FK, Sesterhenn IA. Renal medullary carcinoma. The seventh sickle cell nephropathy. Am J Surg Pathol. 1995;19:1–11. doi: 10.1097/00000478-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Srigley JR, Eble JN. Collecting duct carcinoma of kidney. Semin Diagn Pathol. 1998;15:54–67. [PubMed] [Google Scholar]
- 13.Argani P. Metanephric neoplasms: the hyperdifferentiated, benign end of the Wilms tumor spectrum? Clin Lab Med. 2005;25:379–392. doi: 10.1016/j.cll.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Yusim IE, Neulander EZ, Eidelberg I, Lismer LJ, Kaneti J. Leiomyoma of the genitourinary tract. Scand J Urol Nephrol. 2001;35:295–299. doi: 10.1080/003655901750425873. [DOI] [PubMed] [Google Scholar]
- 15.Pickhardt PJ, Siegel CL, McLarney JK. Collecting duct carcinoma of the kidney: are imaging findings suggestive of the diagnosis? Am J Roentgenol. 2001;176:627–633. doi: 10.2214/ajr.176.3.1760627. [DOI] [PubMed] [Google Scholar]
- 16.Srinivas V, Sogani PC, Hajdu SI, Whitmore WF., Jr Sarcomas of the kidney. J Urol. 1984;132:13–16. doi: 10.1016/s0022-5347(17)49441-9. [DOI] [PubMed] [Google Scholar]
- 17.Cormier P, Patel SK, Turner DA, Hoeksema J. MR imaging findings in renal medullary fibroma. AJR Am J Roentgenol. 1989;153:83–84. doi: 10.2214/ajr.153.1.83. [DOI] [PubMed] [Google Scholar]
- 18.Lane BR, Jour G, Zhou M. Renal neuroendocrine tumors. Indian J Urol. 2009;25:155–160. doi: 10.4103/0970-1591.52905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Majhail NS, Elson P, Bukowski RM. Therapy and outcome of small cell carcinoma of the kidney: report of two cases and a systematic review of the literature. Cancer. 2003;97:1436–1441. doi: 10.1002/cncr.11199. [DOI] [PubMed] [Google Scholar]
- 20.Gherardi GJ, Arya S, Hickler RB. Juxtaglomerular body tumor: a rare occult but curable cause of lethal hypertension. Human Pathol. 1974;5:236–240. doi: 10.1016/S0046-8177(74)80070-5. [DOI] [PubMed] [Google Scholar]