Abstract
Dysferlin (DYSF) is involved in the membrane-repair process, in the intracellular vesicle system and in T-tubule development in skeletal muscle. It interacts with mitsugumin 53, annexins, caveolin-3, AHNAK, affixin, S100A10, calpain-3, tubulin and dihydropyridine receptor. Limb-girdle muscular dystrophy 2B (LGMD2B) and Miyoshi myopathy (MM) are muscular dystrophies associated with recessively inherited mutations in the DYSF gene. The diseases are characterized by weakness and muscle atrophy that progress slowly and symmetrically in the proximal muscles of the limb girdles. LGMD2B and MM, which are collectively termed “dysferlinopathy”, both lead to abnormalities in vesicle traffic and membrane repair at the plasma membrane in muscle fibers. SJL/J (SJL) and A/J mice are naturally occurring animal models for dysferlinopathy. Since there has been no an approach to therapy for dysferlinopathy, the immediate development of a therapeutic method for this genetic disorder is desirable. The murine models are useful in verification experiments for new therapies and they are valuable tools for identifying factors that accelerate dystrophic changes in skeletal muscle. It could be possible that the genetic or immunological background in SJL or A/J mice could modify muscle damage in experiments involving these models, because SJL and A/J mice show differences in the progress and prevalent sites of skeletal muscle lesions as well as in the gene-expression profiles of their skeletal muscle. In this review, we provide up-to-date information on the function of dysferlin, the development of possible therapies for muscle dystrophies (including dysferlinopathy) and the detection of new therapeutic targets for dysferlinopathy by means of experiments using animal models for dysferlinopathy.
Keywords: SJL/J mouse, A/J mouse, dysferlin, complement, dysferlinopathy
Introduction
Muscular dystrophy is a generic term that is used to refer to a group of hereditary muscular disorders characterized clinically by progressive muscular weakness and muscle atrophy and histopathologically by degeneration/necrosis and regeneration of skeletal muscle fibers. This group of inherited muscular disorders includes Duchenne muscular dystrophy (DMD), Becker muscular dystrophy (BMD), Emery–Dreifuss muscular dystrophy, limb-girdle muscular dystrophy (LGMD), distal muscular dystrophy including Miyoshi myopathy (MM) and Fukuyama/non-Fukuyama congenital muscular dystrophy among others1. Of these disorders, LGMD is a muscular disorder in which weakness and atrophy of muscles progress slowly and symmetrically in the proximal muscles of the limb girdle. Because LGMD includes many types of unclassified muscular dystrophy and is etiologically heterogeneous, a locus-based classification has been proposed by a consortium that met under the auspices of the European Neuromuscular Centre. In this classification, the dominant LGMD loci are designated LGMD1A, LGMD1B, LGMD1C, etc., and the recessive loci are designated as LGMD2A, LGMD2B, LGMD2C, etc., in the order that they were identified2.
LGMD2B and MM are both caused by recessively inherited mutations in the dysferlin (DYSF) gene1. LGMD2B is characterized by progressive wasting and weakness of the muscles of the proximal lower limb girdle, whereas MM mostly affects the distal muscle groups of the limb girdle. Both disorders are considered to result from a loss of dysferlin (DYSF) protein from the plasma membrane of muscle fibers, leading to abnormalities in vesicle traffic and membrane repair3,4; this process is collectively referred to as “dysferlinopathy”.
The prevalence of progressive muscular dystrophy among the general population is about four cases in 100,000 people5. The relative frequency by the type of disease is 60% DMD, 30% LGMD and 10% facioscapulohumeral dystrophy. Among the 1420 patients diagnosed with muscular dystrophies at the National Center of Neurology and Psychiatry (Japan) in 2004, dystrophinopathy (DMD/BMD) (56%) was the most common condition followed by LGMD (19%). Of these LGMD patients, 18% were diagnosed with LGMD2B, which occurs at a relatively higher frequency in Japan than elsewhere6. At the same facility in 2010, dysferlinopathy was identified as the most frequent type of LGMD present among the Japanese population7.
The progression of muscular dystrophy confines patients to a wheelchair or a ventilator, and it detracts from their quality of life. Various approaches to definitive therapy of muscular dystrophy have been attempted. These include gene therapies, such as gene transfer using plasmids or adeno-associated virus (AAV) vectors, or exon skipping using antisense oligonucleotides (AVI-4658 or GSK-2402968 [PRO-051])8,9,10; cell-based therapies11; therapies using small-molecule drugs that act through a read-through mechanism, such as aminoglycosides12,13,14, ataluren (PTC124)15 or TG00316; and an antibody therapy using anti-myostatin antibody17. Clinical studies for some of these therapies are ongoing in DMD/BMD patients or others. Since there has been no an approach to therapy for dysferlinopathy, the immediate development of a therapeutic method for this genetic disorder is desirable.
As described above, although dysferlinopathy is a type of muscular dystrophy that has a relatively high prevalence in Japan, the development of a standard therapy has been slow, and therefore, animal models of dysferlinopathy remain useful for verification experiments on novel therapies. Additionally, because the histopathological lesions are localized in the limb-girdle muscles, despite a systemic deficiency in dysferlin protein, accelerating factors that produce the muscular damage in only the limb-girdle muscles must be present in patients with dysferlinopathy, and animal models of dysferlinopathy are useful as tools for exploring these accelerating factors.
This review provides up-to-date information on the function of dysferlin, the development of possible therapies for muscle dystrophies (including dysferlinopathy) and the detection of new therapeutic targets for dysferlinopathy by means of experiments using animal models for dysferlinopathy. It also discusses the effectiveness and the problems of using animal models for dysferlinopathy.
The Function of Dysferlin
The role of dysferlin in membrane-repair processes and in the intracellular vesicular system
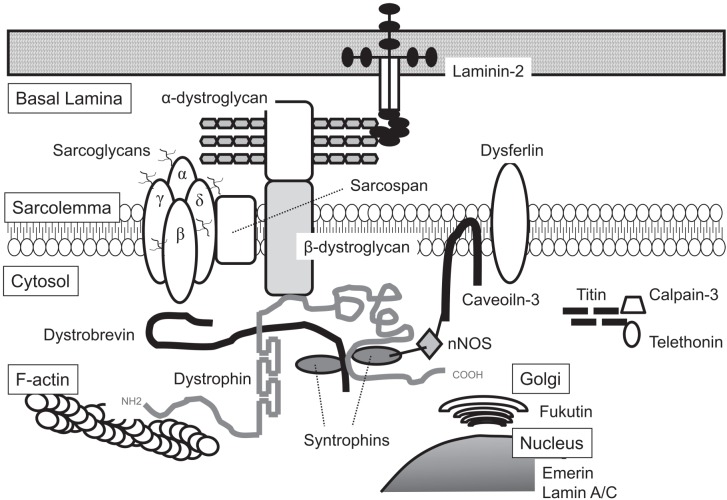
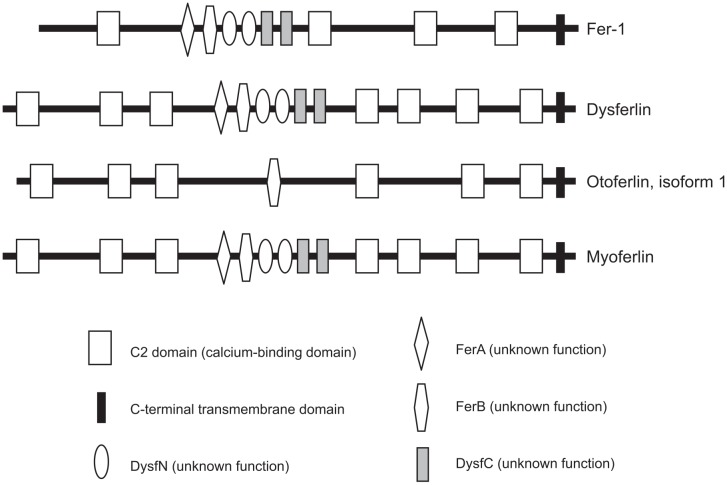
DYSF is a 230-kDa transmembrane protein that has a C-terminal transmembrane domain. The DYSF gene is abundantly expressed in skeletal and cardiac muscle18, and its gene product is distinct from the dystrophin–glycoprotein complex (Fig. 1)19. DYSF belongs to the ferlin family, which includes otoferlin, myoferlin and Fer-1 (identified in Caenorhabditis elegans4). The proteins of the ferlin family have several calcium-binding C2 domains associated with calcium-dependent membrane fusion and repair4 (Fig. 2). Fer-1 mediates calcium-dependent membrane fusion of multiple intracellular vesicles (called “membranous organellas”) to the spermatid plasma membrane during spermatogenesis in C. elegans4. DYSF has seven C2 domains and is implicated in Ca-dependent resealing after disruption of the sarcolemma. It is known that the membrane-repair process requires intracellular vesicles20 that deliver excess membrane to form a “membrane patch” through calcium-triggered vesicular exocytosis21,22. It has been suggested that these intracellular vesicles are initially transported to the site of damage by sequential actions of motor proteins, including kinesin and nonmuscle myosin IIA and IIB23,24. It has also been suggested that DYSF is distributed to these intracellular vesicles together with caveolin-325, and it has been reported that DYSF is also associated with annexins A1 and A2 in a Ca2+- and membrane injury-dependent manner26.
Fig. 1.
Schematic showing the organization of some of the integral and peripheral components involved in muscular dystrophies in skeletal muscles.
Fig. 2.
Conserved structure of the ferlin family. Proteins of the ferlin family have highly homologous structures. The proteins have a variable number of tandem C2 domains and a C-terminal transmembrane domain.
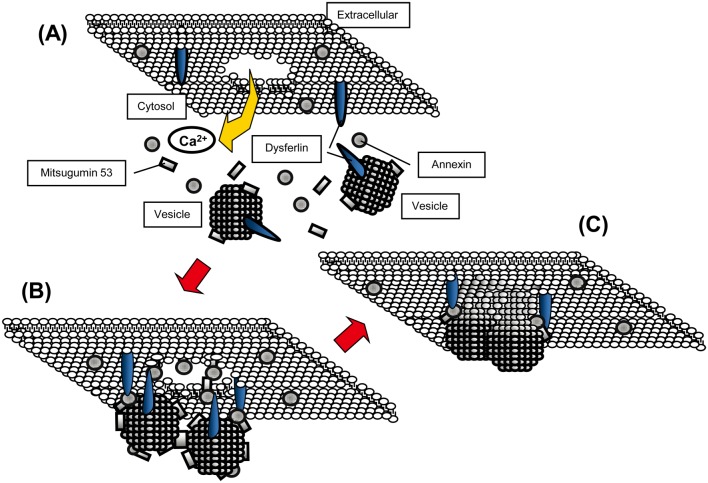
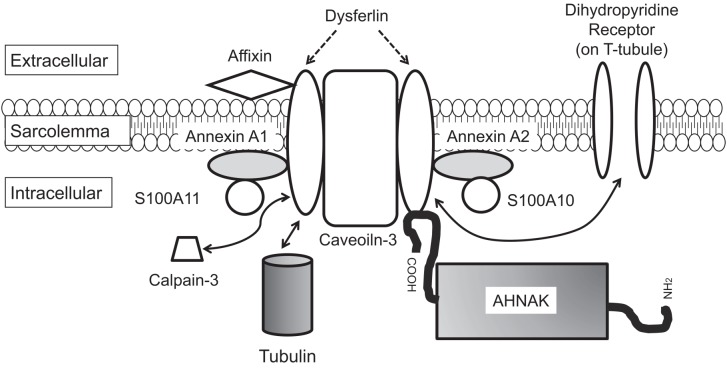
Some studies have suggested that mitsugumin 53 (MG53) plays a role in facilitated vesicle translocation for muscle membrane repair27,28,29,30,31,32. The interactions between DYSF, MG53 and caveolin-3 have been examined in vitro by means of immunoprecipitation experiments27. Accordingly, DYSF is considered to act with MG53, annexins and other proteins in the accumulation of vesicles at the site of damage following membrane disruption (Fig. 3). In addition to MG53, annexins and caveolin-3, human neuroblast differentiation-associated protein (AHNAK)33, affixin34, S100A1035, calpain-336, tubulin37, and dihydropyridine receptor (DHPR)38 have been reported to interact with DYSF. Figure 4 shows a schema of the proteins that interact with DYSF at the sarcolemma in skeletal muscle.
Fig. 3.
Schema for a model of the membrane-resealing processes associated with dysferlin in vitro. (A) shows the intact condition. Membrane disruption leads to an influx of calcium ions (B). Transport of intracellular vesicles toward the damaged site by the motor proteins kinesin and myosin may be facilitated by mitsugumin 53 (MG53) in an oxidation/cholesterol-dependent manner. The vesicles dock through oxidized MG53 and fuse with each other and the plasma membrane, possibly with mediation by annexin, soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) and dysferlin in the presence of the calcium ion. Dysferlin interacts with annexins A1 and A2 and mediates wound healing of the sarcolemma. A membrane patch is consequently formed and reseals the membrane lesion (C). Although the proteins dysferlin and MG53 are known to be involved in muscle repair, there is still no direct evidence of an in vivo interaction between them.
Fig. 4.
Schema showing the interactions of proteins with dysferlin at the sarcolemma in skeletal muscle.
The role of dysferlin in T-tubule development
Some recent studies have shown that DYSF is active in with the development of T-tubules39,40. DYSF has been observed to be associated with developing T-tubules and also to interact with DHPR at the sarcolemma of T-tubules40. Because a deficiency in DYSF induces ultrastructural abnormalities in primary T-tubules in skeletal muscle, it has been suggested that DYSF is required for the maintenance or development of T-tubules.
The role of dysferlin in ATP release and intracellular Ca2+ signaling
Another study has indicated that DYSF might be involved in ATP release and intercellular Ca2+ signaling41. It has been suggested that DYSF mediates Ca2+-triggered intercellular signaling in response to membrane wounding in sea urchin embryos, as it has been shown that morpholino knockdown of DYSF mRNA expression in sea urchin embryos effectively blocks the release of ATP after membrane damage and thereby suppresses the consequent intercellular Ca2+ signaling41. In contrast, it has been hypothesized that a deficiency of DYSF in mammalian skeletal muscles results in the release of ATP or other endogenous danger/alarm signals such as high-mobility group box-1 (HMGB1), S100 proteins or heat-shock proteins (HSPs), possibly through a compensatory vesicle-trafficking pathway mediated by the synaptotagmin-like protein Slp2a and the small GTPase Rab27A. It has also been suggested that the released factors activate an inflammatory pathway by means of toll-like receptors or a P2X7 receptor (a mammalian ATP-gated nonselective cation channel)42. In fact, P2 receptor antagonist lowered serum levels of CK and reduced muscle damage in dystrophin-deficient mdx mice and in sarcoglycan-deficient BIO 14.6 hamsters43. Further studies are necessary to elucidate the relationship between releases of danger/alarm molecules and DYSF deficiency.
The role of dysferlin in phagocytosis
Monocytes derived from DYSF-deficient mice and from human patients with dysferlinopathy have been shown to promote phagocytic activity44. Knockdown of DYSF mRNA expression by RNA interference in the J774 macrophage cell line significantly enhanced this phagocytosis. Therefore, the accumulation of macrophages in muscles showing dystrophic changes may be a primary lesion caused by DYSF deficiency rather than a secondary lesion that occurs after muscle degeneration/necrosis. However, because muscle-specific transgenic expression of DYSF at appropriate levels suppresses the progression of dystrophic changes in dysferlin-deficient mice45,46, enhanced phagocytic activity alone in DYSF-deficient monocytes is considered to be insufficient to cause muscle damage.
Animal Models for Dysferlinopathy
Two naturally occurring animal models for LGMD2B have been identified: SJL/J (SJL) mice and A/J mice. These have been shown to have mutations in the DYSF gene associated with phenotypical features of progressive muscular dystrophy18,47. SJL mice have a splice site mutation in which part of the highly conserved C2E domain in DYSF is removed47. A/J mice bear a unique ETn retrotransposon insertion near the 5’ end (intron 4) of the DYSF gene47.
Characteristics of animal models for dysferlinopathy
(1) Histopathological findings: Distribution of histopathological lesions:
Histopathological characteristics of animal models of dysferlinopathy include degeneration/necrosis of muscle fibers, variations in the size of muscle fibers, atrophy of muscle fibers, inflammatory cell infiltration, centronuclear fibers, fatty infiltration and fibrosis in the limb girdle (mainly the rectus femoris and lateral longissimus muscles)47,48,49.
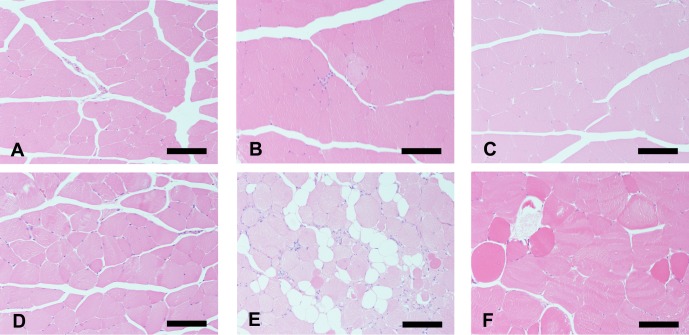
Studies have also shown that there are differences between SJL and A/J mice in terms of the progress and prevalent sites of skeletal muscular lesions47,48. In particular, the difference between SJL and A/J mice in their sensitivity to muscular dystrophic lesions was most apparent in their lumbar (longissimus and sublumbar) muscles. These findings support the hypothesis that, as well as a deficiency in DYSF, additional enhancers or modifiers might be involved in the progression of skeletal muscle lesions in dysferlinopathy. Figure 5 shows typical histopathological findings for the femoral muscles of SJL and A/J mice aged 10 and 30 weeks in comparison with normal BALB/c mice of the same age.
Fig. 5.
Histopathology of the rectus femoris in BALB/c, SJL/J and A/J mice. At 10 weeks of age (upper figures), no significant changes were observed in the skeletal muscle fibers of BALB/c (A) and A/J mice (C), and a few muscle fibers showed minimal degeneration with mononuclear cell infiltration in SJL/J mice (B). At 30 weeks of age (lower figures), BALB/c mice did not exhibit histopathological changes in any skeletal muscles (D). The histopathological lesions of skeletal muscles in SJL/J mice progressed in severity with age and were characterized by the following findings: degenerative/necrotic muscle fibers, centronuclear muscle fibers, fatty infiltration and variations in the size of the muscle fibers (E). The muscle fibers in A/J mice showed only degenerative/necrotic features and variations in size (F). Hematoxylin and eosin (HE) staining. Bar: 100 μm.
(2) Histochemical findings: Typing of damaged muscle fibers:
In dysferlin-deficient patients with an advanced-stage dystrophic pattern, a predominance (in excess of 80%) of Type 1 (slow-twitch) fibers has been observed, suggesting a selective loss of Type 2 (fast-twitch) fibers or a process of conversion of Type 1 fibers into Type 2 fibers50. Similarly, histochemical staining for NADH-TR and SDH enzymes revealed that degeneration of fast-twitch muscle fibers was a predominant characteristic of SJL mice48. However, the medial vastus and iliocostalis muscles, which originally consisted mainly of fast-twitch muscle fibers, showed little degeneration/necrosis of muscle fibers. These findings indicate that the sensitivity of Type 2 fibers to injury might be site-specific, as seen in the rectus femoris and lateral longissimus muscles of SJL mice.
(3) Immunohistochemical findings: Identification of infiltrating cells:
Mononuclear cells found in or around degenerative and/or necrotic muscle fibers show a positive reaction to mouse F4/80 antigen (widely used to identify mouse macrophages in lesions51,52) and must therefore be macrophages. A previous immunohistochemical analysis using an antibody to the Mac-1 α-chain (also known as CD11b or integrin αM chain) showed that macrophages were the predominant type of infiltrating cell in the muscles of SJL mice49. It is not known whether this macrophage infiltration at the muscle lesions occurs solely as a result of the uptake of cellular debris (such as necrotic muscle fibers) or is a consequence of enhanced phagocytosis of the target cells as a result of opsonization of the sarcolemma by C3b.
Gene expression profiling of animal models for dysferlinopathy
(1) Endoplasmic reticulum stress-associated gene:
It has been reported that a novel mutant (L1341P) DYSF spontaneously aggregates in the endoplasmic reticulum (ER), resulting in phosphorylation of the eukaryotic translation-initiation factor subunit eIF2α and conversion of microtubule-associated protein light chain 3 (LC3) until ER-stress-related cell death eventually occurs53. Moreover, it has been reported that ER dysfunction plays a significant role in the pathophysiology of several myopathies54,55,56. Therefore, to examine the relationship between ER stress and skeletal muscle lesions in SJL or A/J mice, we have conducted a semiquantitative analysis of expression levels of spliced XBP1 mRNA as an ER stress marker in SJL mice48; we have also conducted an analysis of ER stress-associated genes (HSP5, Grp78, Atf6, and Chop) in SJL and A/J mice by means of a quantitative real-time polymerase chain reaction (qRT-PCR) using TaqMan® Gene Expression Assays57. Because these ER stress-associated gene expression analyses did not show any increases in gene expression, we consider that ER stress does not affect the progression of skeletal muscle lesions in SJL or A/J mice in advanced stages of dysferlinopathy.
(2) Lipid metabolism associated gene:
A qRT-PCR study demonstrated that SJL mice tend to show increased expression of uncoupling protein 2 (Ucp2) in the rectus femoris and longissimus lumborum at 30 weeks of age, which is when dystrophic lesions become histopathologically pronounced 57.
Forced expression of Ucp2 in pancreatic islets resulted in a decreased content of ATP, and the islet cells of Ucp2 knockout mice showed increased levels of ATP58. Overexpression of UCP2 in primary cardiomyocytes led to a significant decline in the ATP level and an enhanced sensitivity to hypoxia–reoxygenation59. Ucp2-mediated energy loss may be related to muscle degeneration/necrosis in SJL mice. On the other hand, Tbc1d1 gene-deficient cells exhibited inhibited trafficking of the glucose transporter GLUT4 from intracellular vesicles to the plasma membrane60, suggesting a decrease in intracellular glucose levels and a subsequent enhancement of fatty acid oxidation. These results suggest that the Tbc1d1 gene-deficient skeletal muscles in SJL mice61 are likely to show uncoupling.
(3) Anti-oxidative stress-associated genes and heat-shock protein genes:
Heme oxygenase 1 (Hmox1) was upregulated in the rectus femoris, longissimus lumborum and abdominal muscles at 30 weeks of age; dystrophic lesions occur more commonly in these muscles in SJL mice57. The gene expression levels of HSP70 in most muscles of A/J mice were lower than those in BALB/c mice used as controls.
Hmox1 provides the first line of defense against oxidative stress, because it responds rapidly to oxidants62. However, Txnrd1, which (together with Hmox1) is a part of the anti-oxidation system, was not upregulated in any muscles of SJL mice. Recently, Ca-dependent upregulation of Hsp70 and Hmox-1 in skeletal muscle cells and in hepatocytes has been reported63,64. Because DYSF-null muscle fibers are defective in Ca2+-dependent resealing of disruptions of the sarcolemma19, these muscle fibers may cause persistent influx of Ca into the cytoplasm after membrane injury. The gene expression levels of Hmox1 were correlated with the severity of histopathological lesions in femoral (rectus femoris), lumbar (longissimus lumborum) and abdominal muscles; therefore, Ca influx into the cytoplasm following muscle injury may induce Hmox1 gene expression. However, Hsp70 was also upregulated in the diaphragms of SJL mice, where few histopathological changes were observed at any stage when this was examined. In addition, no change in the level of expression of the Hsp70 gene was observed in abdominal muscles of SJL mice in where histopathological changes were observed at 30 weeks of age. The muscles and hearts in histopathologically normal BALB/c mice exhibited upregulation of the expression of this gene from 15 weeks of age57. It has been reported that significant increases in Hsp70 are observed at 12 weeks postpartum in normal rats65. The physiological mechanism of the expression of the Hsp70 gene may develop somewhat later in noncardiac muscles of SJL mice; therefore, an unknown factor in addition to persistent Ca influx may cause Hmox1 induction in these muscles of SJL mice.
In contrast, the levels of expression of the Hsp70 gene in most muscles of A/J mice were lower than those in the control. Loss of fer-1, a DYSF homolog, in C. elegans causes downregulation of hsp-7066. It is possible that the downregulation of Hsp70 gene expression in the skeletal muscles of A/J mice is caused by a functional loss of DYSF.
(4) Ca-binding protein gene:
S100 calcium-binding protein A4 (S100A4) was upregulated in the rectus femoris, longissimus lumborum and abdominal muscles in SJL mice at 30 weeks of age57; these are the muscles in which dystrophic lesions occur most commonly in these mice48. These upregulations of S100A4 coincide approximately with the occurrence of dystrophic changes in the associated lesions. Most of the infiltrating cells in muscle lesions in SJL mice are F4/80 antigen-positive macrophages48. Recently, it has been demonstrated that S100A4 mediates macrophage recruitment and chemotaxis in vivo67. Upregulation of S100A4 in the rectus femoris and longissimus lumborum of SJL mice may be linked to the pathological characteristics of muscle in SJL mice.
(5) Complement control factor gene:
In comparison with BALB/c mice, SJL mice of all ages showed a marked lowering of the expression of Daf1/CD55 gene in all studied muscles, except for the heart57. In contrast, there was no predominant difference in the levels of Daf1/CD55 gene expression in A/J mice compared with those in BALB/c mice57. As previously indicated, it has been reported that gene expression of Daf1/CD55 as a complement inhibitor is downregulated in the skeletal muscles of LGMD2B patients and in those of SJL mice68. Moreover, the serum concentration of C5 in SJL mice is known to be significantly greater than that in other strains69. On the other hand, A/J mice are genetically deficient in C570. At the time of the study, we understood that the difference in phenotype between the two DYSF-deficient mice was related to the presence or absence of C5; however, it was later revealed that genetic ablation of C5 had a minimal effect on muscle lesions in DYSF-deficient mice45.
Table 1 lists gene expression and prospective events in SJL and A/J mice. It has also been reported that differential gene expression profiles of proximal and distal muscle groups are altered in prepathological C57BL/10.SJL-Dysf mice71. Furthermore, the expression profiles of 10,012 genes in the quadriceps femoris muscles of control and SJL mice have been established by means of a cDNA microarray analysis, with the aim of identifying genes that are involved in the degeneration and regeneration process and in the functional network of dysferlin72. However, it cannot be stated with confidence that common factors associated with muscular changes in dysferlinopathy were discovered in these studies (including our own), and further investigations are required to determine whether alterations in gene expression levels are the cause of dystrophic changes or they occur as a result of damage to the muscle.
Table 1. Changes of Gene Expression and Prospective Events in SJL and A/J Mice.

Problems associated with animal models for dysferlinopathy
In general, murine models for muscular dystrophies, including SJL and A/J mice, do not show any significant muscle weakness. This makes it difficult to evaluate improved muscle strength, and therefore, the screening of therapies by using murine models depends exclusively on histopathological examination.
Because it is simple to induce autoimmune diseases in SJL mice, they have been used as animal models for autoimmunological diseases such as experimental autoimmune encephalomyelitis73, experimental autoimmune myositis74, and experimental autoimmune hypophysitis75. In addition, it has been reported that SJL mouse-derived monocytes increase phagocytic activity76 and that dysferlin deficiency induces an upregulation of inflammasome42. However, a more recent study using C57BL/10-SJL.Dysf mice, which have a more controlled genetic background, did not find any change in the phagocytic activity of dysferlin-deficient monocytes77. A/J mice, on the other hand, show later onset of muscle lesions than do SJL mice or DYSF-deficient mice with the genetic background 129SvJ, and the lumbar muscles of SJL and A/J mice showed a difference in their sensitivity to muscular dystrophic lesions. There is a concern therefore that differences in the genetic or immunological background of SJL and A/J mice could cause modifications in the nature of any muscle damage.
As pointed out above, the two strains show phenotypic divergences. A/J mice display a later onset and a slower progression of muscular disease compared with SJL mice47,48, and the two strains show differences in their gene expression profiles. Similarly, dysferlinopathies in humans are a clinically heterogeneous group of disorders78. It has been considered that through probing of the causes of interstrain differences, A/J and SJL strains of mice might prove useful in providing clues regarding the causes of clinical heterogeneity in humans and in identifying targets for stopping or slowing the progression of the disease. At present, however, we have little knowledge of the causes of interstrain differences.
Development of Therapies for Muscular Dystrophies, Including Dysferlinopathy
Transfection of cDNA by plasmid vectors
Plasmid vectors can transfer large cDNA fragments (for example, full-length dystrophin cDNA) and they are noninfectious and nonimmunogenic8. Although these characteristics are major advantages compared with viral vectors, plasmid vectors provide less-efficient transfection than do viral vectors.
Transfection of cDNA by viral vectors
The AAV vector is currently one of the most promising viral vectors for transfection of cDNA. AAV can package and protect recombinant DNA strands as large as 6.0 kb, but virions carrying larger DNA strands are preferentially degraded by the proteasome79. Furthermore, AAV vectors can induce immune reactions80,81.
In gene therapy for LGMD2B, the size of the dysferlin cDNA prevents its direct incorporation into an AAV vector for therapeutic gene transfer into muscle. To bypass this limitation, Lostal et al. have split dysferlin cDNA at the exon 28/29 junction and have cloned it into two independent AAV vectors, each carrying the appropriate splicing sequences82. Intramuscular injection of the corresponding vectors into a dysferlin-deficient mouse led to the expression of full-length dysferlin for at least 1 year.
Exon skipping by antisense oligonucleotides
Exon skipping by antisense oligonucleotides is among the most advanced therapies for muscular dystrophy (especially DMD). GSK-2402968 is currently advancing to Phase III clinical trials (http://clinicaltrials.gov/ct2/results?term=GSK-2402968), and a Phase II study of AVI-4658 is ongoing (http://clinicaltrials.gov/ct2/results?term=AVI-4658). However, exon skipping by antisense oligonucleotides has some disadvantages8 in that the therapy needs regular repeated administration because the method modifies only the process of mRNA splicing, and different antisense oligonucleotides are needed for different types of dystrophin gene deletion.
Stem or progenitor cell transplantation
Myoblasts/satellite cells, hematopoietic stem cells, mesenchymal stem cells, bone-marrow side population, muscle side population, muscle-derived stem cells, adipose-derived stem cells, endothelial progenitor cells and vessel-associated stem cells have all been studied for their possible use in cell-based therapies11,83. Several clinical trials involving injection of myoblasts or pericytes from HLA-matched donors have begun or are planned, and the number of such trials will increase in the near future11; however, little information is currently available regarding the safety and efficacy of these techniques in clinical use.
In a study of a cell-based therapy for LGMD2B, human and mouse dysferlin proteins were detected one month after transplantation in all SCID mice transplanted with normal human myoblasts and in SJL mice transplanted with allogeneic primary mouse muscle cell cultures84. The number of dysferlin-positive fibers was 40–50% and 20–30% in SCID and SJL muscle sections, respectively. In another study, it was shown immunohistochemically that a small number of human cells from human umbilical cord blood became grafted into recipient SJL mice muscle and expressed both dysferlin and human-specific dystrophin 12 weeks after transplantation85.
Read-through by small-molecule drugs
Phase IIb trials of ataluren (PTC124), which causes read-through for the nonsense mutation DMD/BMD, has been suspended or terminated because the primary endpoint did not reach statistical significance within the 48-week duration of the study (http://clinicaltrials.gov/ct2/results?term=PTC124).
Antibody treatment
A safety trial of a neutralizing antibody to myostatin, MYO-029, has been conducted in adult muscular dystrophies (Becker muscular dystrophy, facioscapulohumeral dystrophy and LGMD)86. Although MYO-029 has good safety and high tolerability, no improvements were noted in the exploratory end points of muscle strength and function. This probably occurred because the study was not designed to seek efficacy.
In a study of an antibody therapy for LGMD2B, administration of an antibody for tumor necrosis factor (TNF) resulted in dose-dependent reductions in inflammatory change, necrosis and fatty/fibrous change. These findings suggest that TNF does indeed play a role in damage to muscle in SJL mice87.
Because these therapeutic methods for dysferlinopathy are still not under evaluation in clinical studies, immediate development of a therapy for dysferlinopathy is a desirable goal.
Identification of New Therapeutic Targets for Dysferlinopathy by Using Animal Models
Complement-associated factors
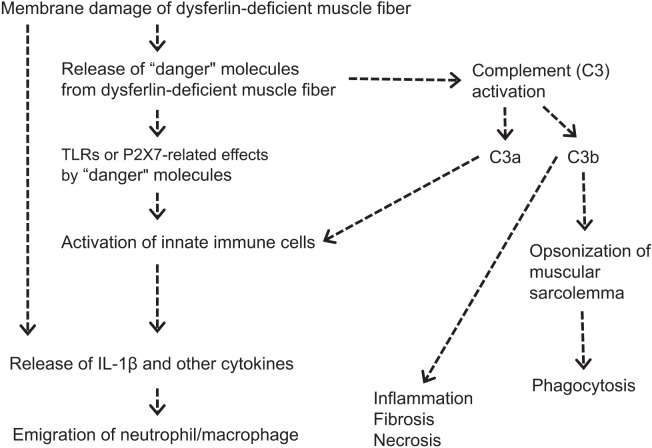
Complement factors are upregulated or activated in DYSF-deficient muscles from mice and humans45,68. This upregulation of complement factors was observed in DYSF-deficient mice before the onset of the obvious pathological markers and was normalized by muscle-specific expression of the DYSF transgene45. To confirm whether or not an activated complement system causes skeletal muscle damage, a gene for complement factor C3 or C5 was disrupted in DYSF-deficient mice45. Whereas genetic ablation of C5 had a minimal effect on muscle lesions in DYSF-deficient mice, a deficiency in C3 ameliorated histopathological changes in the skeletal muscles. In addition, deposition of C3 on the sarcolemma of quadriceps muscles in DYSF-deficient mice was confirmed by means of immunofluorescence analysis, whereas this staining pattern was not observed in DYSF/C3 double-deficient or wild-type mice. These results suggest that activated C3 is responsible for muscle damage in dysferlinopathy. Han88 has proposed a mechanism for muscle damage caused by active C3, in which C3 is cleaved into C3a and C3b on activation of the complement system. C3a is an anaphylotoxin that produces a local inflammatory response, whereas C3b serves as an opsonizing agent by coating the sarcolemma of dysferlin-deficient muscle. Opsonization of the sarcolemma, either with or without C5, enhances the phagocytosis of the target cell by macrophages, which are the predominant infiltrating cells in dysferlin-deficient muscles48,89. Figure 6 shows an estimated inflammatory process in dysferlin-deficient skeletal muscle.
Fig. 6.
Estimated inflammatory process in dysferlin-deficient skeletal muscle. Plasma membrane damage to dysferlin-deficient muscle fibers causes a release of “danger” molecules (heat-shock proteins, high mobility group box-1, ATP, etc.). These “danger” molecules are recognized by receptors on innate immune cells and muscle cells and they stimulate the production of proinflammatory cytokines. Moreover, the released “danger” molecules activate the complement system and stimulate C3a or opsonizing C3b. The proinflammatory mediator C3a can trigger the production of proinflammatory cytokines from cells and render the local vascular endothelium “leaky”; this is followed by attraction of the migration of neutrophils and macrophages. C3b binds to the negatively charged sarcolemma, stimulating phagocytosis. These processes cause more-severe muscular degeneration/necrosis.
Downregulation of decay-accelerating factor 1 (Daf1; also known as CD55), which acts as the complement inhibitor, induces increased susceptibility to complement attack in DYSF-deficient muscle cells68. However, in our study, SJL mice of all ages showed a marked downregulation of Daf1/CD55 gene expression in skeletal muscles lacking obvious histopathological lesions57. Additionally, A/J mice showed no abnormality in Daf1/CD55 gene expression levels in their skeletal muscles with histopathological lesions57. For these reasons, downregulation of Daf1/CD55 alone cannot explain muscle damage in DYSF-deficient mice.
Endogenous danger/alarm factors
As previously mentioned, it has been proposed that DYSF deficiency in skeletal muscles results in release of endogenous danger/alarm signals, including HSPs, HMGB1 and ATP. These factors bind to the toll-like receptor P2X7, activating inflammasome41, nuclear factor kappa-B and the complement pathways45. It is known that HSPs are antibody-dependently and antibody-independently activated in the complement system90. It has been reported that HMGB1 causes an irreversible decrease in the release of Ca2+ from the sarcoplasmic reticulum in vitro91. If the role of these factors in dysferlinopathy can be elucidated, the resulting knowledge may be helpful in preventing the progression of muscular lesions.
Conclusion
DYSF is involved in the membrane-repair process, intracellular vesicle system and development of T-tubules in skeletal muscles, and it interacts with MG53, annexins, caveolin-3, AHNAK, affixin, S100A10, calpain-3, tubulin and DHPR. In humans, a deficiency in DYSF induces dystrophic changes in the skeletal muscles of the limb girdle, the so-called LGMD2B and MM (which are collectively known as dysferlinopathy). LGMD2B occurs at a relatively high frequency in Japan, to the extent that the National Center of Neurology and Psychiatry stated in 2010 that dysferlinopathy has become the most common type of LGMD among the Japanese population. Because no therapeutic methods for dysferlinopathy are currently under evaluation in clinical studies, the immediate development of a therapy for dysferlinopathy is desirable. SJL and A/J mice are known to be naturally occurring animal models for dysferlinopathy. These models are therefore useful in verification experiments for new therapies and are valuable tools for identifying factors that accelerate dystrophic changes in skeletal muscle. However, it should be borne in mind that the genetic or immunological background of SJL or A/J mice could result in modifications of muscle damage in experiments involving these animal models.
Acknowledgments
The authors thank Dr Kimiaki Hirakawa (Shin Nippon Biomedical Laboratories, Ltd.) for his valuable comments.
References
- 1.Cohn RD, Campbell KP. Molecular basis of muscular dystrophies. Muscle Nerve. 23: 1456–1471 2000. [DOI] [PubMed] [Google Scholar]
- 2.Bushby KM. Making sense of the limb-girdle muscular dystrophies. Brain. 122: 1403–1420 1999. [DOI] [PubMed] [Google Scholar]
- 3.Han R, Bansal D, Miyake K, Muniz VP, Weiss RM, McNeil PL, Campbell KP. Dysferlin-mediated membrane repair protects the heart from stress-induced left ventricular injury. J Clin Invest. 117: 1805–1813 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glover L, Brown RH., JrDysferlin in membrane trafficking and patch repair. Traffic. 8: 785–794 2007. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Prevalence of Duchenne/Becker muscular dystrophy among males aged 5–24 years — four states, 2007. Morb Mortal Wkly Rep. 58: 1119–1122 2009 [PubMed] [Google Scholar]
- 6.Sunada Y.Limb-girdle muscular dystrophy: Update. Rinsho Shinkeigaku. 44: 995–997 2004. [PubMed] [Google Scholar]
- 7.Hayashi S, Ohsawa Y, Takahashi T, Suzuki N, Okada T, Rikimaru M, Murakami T, Aoki M, Sunada Y.Rapid screening for Japanese dysferlinopathy by fluorescent primer extension. Intern Med. 49: 2693–2696 2010. [DOI] [PubMed] [Google Scholar]
- 8.Park KS, Oh D. Gene therapy for muscular dystrophy: Progress and challenges. J Clin Neurol. 6: 111–116 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cirak S, Arechavala-Gomeza V, Guglieri M, Feng L, Torelli S, Anthony K, Abbs S, Garralda ME, Bourke J, Wells DJ, Dickson G, Wood MJ, Wilton SD, Straub V, Kole R, Shrewsbury SB, Sewry C, Morgan JE, Bushby K, Muntoni F. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: An open-label, Phase 2, dose-escalation study. Lancet. 378: 595–605 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammond SM, Wood MJ. PRO-051, an antisense oligonucleotide for the potential treatment of Duchenne muscular dystrophy. Curr Opin Mol Ther. 12: 478–486 2010. [PubMed] [Google Scholar]
- 11.Quattrocelli M, Cassano M, Crippa S, Perini I, Sampaolesi M.Cell therapy strategies and improvements for muscular dystrophy. Cell Death Differ. 17: 1222–1229 2010. [DOI] [PubMed] [Google Scholar]
- 12.Arakawa M, Shiozuka M, Nakayama Y, Hara T, Hamada M, Kondo S, Ikeda D, Takahashi Y, Sawa R, Nonomura Y, Sheykholeslami K, Kondo K, Kaga K, Kitamura T, Suzuki-Miyagoe Y, Takeda S, Matsuda R.Negamycin restores dystrophin expression in skeletal and cardiac muscles of mdx mice. J Biochem. 134: 751–758 2003. [DOI] [PubMed] [Google Scholar]
- 13.De Luca A, Nico B, Rolland JF, Cozzoli A, Burdi R, Mangieri D, Giannuzzi V, Liantonio A, Cippone V, De Bellis M, Nicchia GP, Camerino GM, Frigeri A, Svelto M, Camerino DC. Gentamicin treatment in exercised mdx mice: Identification of dystrophin-sensitive pathways and evaluation of efficacy in work-loaded dystrophic muscle. Neurobiol Dis. 32: 243–253 2008. [DOI] [PubMed] [Google Scholar]
- 14.Nudelman I, Rebibo-Sabbah A, Cherniavsky M, Belakhov V, Hainrichson M, Chen F, Schacht J, Pilch DS, Ben-Yosef T, Baasov T. Development of novel aminoglycoside (NB54) with reduced toxicity and enhanced suppression of disease-causing premature stop mutations. J Med Chem. 52: 2836–2845 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finkel RS. Read-through strategies for suppression of nonsense mutations in Duchenne/Becker muscular dystrophy: Aminoglycosides and ataluren (PTC124). J Child Neurol. 25: 1158–1164 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishida A, Kataoka N, Takeshima Y, Yagi M, Awano H, Ota M, Itoh K, Hagiwara M, Matsuo M.Chemical treatment enhances skipping of a mutated exon in the dystrophin gene. Nat Commun. 2: 308 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS, Khurana TS. Functional improvement of dystrophic muscle by myostatin blockade. Nature. 420: 418–421 2002. [DOI] [PubMed] [Google Scholar]
- 18.Bittner RE, Anderson LV, Burkhardt E, Bashir R, Vafiadaki E, Ivanova S, Raffelsberger T, Maerk I, Hoger H, Jung M, Karbasiyan M, Storch M, Lassmann H, Moss JA, Davison K, Harrison R, Bushby KM, Reis A. Dysferlin deletion in SJL mice (SJL-Dysf) defines a natural model for limb girdle muscular dystrophy 2B. Nat Genet. 23: 141–142 1999. [DOI] [PubMed] [Google Scholar]
- 19.Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 423: 168–172 2003. [DOI] [PubMed] [Google Scholar]
- 20.McNeil PL, Miyake K, Vogel SS. The endomembrane requirement for cell surface repair. Proc Natl Acad Sci USA. 100: 4592–4597 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bi GQ, Alderton JM, Steinhardt RA. Calcium-regulated exocytosis is required for cell membrane resealing. J Cell Biol. 131: 1747–1758 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyake K, McNeil PL. Vesicle accumulation and exocytosis at sites of plasma membrane disruption. J Cell Biol. 131: 1737–1745 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bi GQ, Morris RL, Liao G, Alderton JM, Scholey JM, Steinhardt RA. Kinesin- and myosin-driven steps of vesicle recruitment for Ca2+ regulated exocytosis. J Cell Biol. 138: 999–1008 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Togo T, Steinhardt RA. Nonmuscle myosin IIA and IIB have distinct functions in the exocytosis-dependent process of cell membrane repair. Mol Biol Cell. 15: 688–695 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cacciottolo M, Belcastro V, Laval S, Bushby K, di Bernardo D, Nigro V.Reverse engineering gene network identifies new dysferlin-interacting proteins. J Biol Chem. 286: 5404–5413 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lennon NJ, Kho A, Bacskai BJ, Perlmutter SL, Hyman BT, Brown RH., JrDysferlin interacts with annexins A1 and A2 and mediates sarcolemmal wound-healing. J Biol Chem. 278: 50466–50473 2003. [DOI] [PubMed] [Google Scholar]
- 27.Weisleder N, Takeshima H, Ma J.Mitsugumin 53 (MG53) facilitates vesicle trafficking in striated muscle to contribute to cell membrane repair. Commun Integr Biol. 2: 225–226 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai C, Weisleder N, Ko JK, Komazaki S, Sunada Y, Nishi M, Takeshima H, Ma J. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3 and dysferlin. J Biol Chem. 284: 15894–15902 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai C, Masumiya H, Weisleder N, Pan Z, Nishi M, Komazaki S, Takeshima H, Ma J.MG53 regulates membrane budding and exocytosis in muscle cells. J Biol Chem. 284: 3314–3322 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai C, Masumiya H, Weisleder N, Matsuda N, Nishi M, Hwang M, Ko JK, Lin P, Thornton A, Zhao X, Pan Z, Komazaki S, Brotto M, Takeshima H, Ma J. MG53 nucleates assembly of cell membrane repair machinery. Nat Cell Biol. 11: 56–64 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Xie W, Zhang Y, Lin P, Han L, Han P, Wang Y, Chen Z, Ji G, Zheng M, Weisleder N, Ziao RP, Takeshima H, Ma J, Cheng H. Cardioprotection of ischemia/reperfusion injury by cholesterol-dependent MG53-mediated membrane repair. Circ Res. 107: 76–83 2010. [DOI] [PubMed] [Google Scholar]
- 32.Cao CM, Zhang Y, Weisleder N, Ferrante C, Wang X, Lv F, Song R, Hwang M, Jin L, Guo J, Peng W, Li G, Nishi M, Takeshima H, Ma J, Xiao RP. MG53 constitutes a primary determinant of cardiac ischemic preconditioning. Circulation. 121: 2565–2574, 2010. [DOI] [PubMed] [Google Scholar]
- 33.Huang Y, Laval SH, van Remoortere A, Baudier J, Benaud C, Anderson LV, Straub V, Deelder A, Frants RR, den Dunnen JT, Bushby K, van der Maarel SM. AHNAK, a novel component of the dysferlin protein complex, redistributes to the cytoplasm with dysferlin during skeletal muscle regeneration. FASEB J. 21: 732–742 2007. [DOI] [PubMed] [Google Scholar]
- 34.Matsuda C, Kameyama K, Tagawa K, Ogawa M, Suzuki A, Yamaji S, Okamoto H, Nishino I, Hayashi YK. Dysferlin interacts with affixin (beta-parvin) at the sarcolemma. J Neuropathol Exp Neurol. 64: 334–340 2005. [DOI] [PubMed] [Google Scholar]
- 35.Rezvanpour A, Shaw GS. Unique S100 target protein interactions. Gen Physiol Biophys. 28 Spec No Focus: F39–F46 2009. [PubMed] [Google Scholar]
- 36.Huang Y, de Morrée A, van Remoortere A, Bushby K, Frants RR, Dunnen JT, van der Maarel SM. Calpain 3 is a modulator of the dysferlin protein complex in skeletal muscle. Hum Mol Genet. 17: 1855–1866 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azakir BA, Di Fulvio S, Therrien C, Sinnreich M. Dysferlin interacts with tubulin and microtubules in mouse skeletal muscle. PLoS One. 5: e10122 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ampong BN, Imamura M, Matsumiya T, Yoshida M, Takeda S. Intracellular localization of dysferlin and its association with the dihydropyridine receptor. Acta Myol. 24: 134–144 2005. [PubMed] [Google Scholar]
- 39.Klinge L, Laval S, Keers S, Haldane F, Straub V, Barresi R, Bushby K.From T-tubule to sarcolemma: Damage-induced dysferlin translocation in early myogenesis. FASEB J. 21: 1768–1776 2007. [DOI] [PubMed] [Google Scholar]
- 40.Klinge L, Hams J, Sewry C, Charlton R, Anderson L, Laval S, Chiu YH, Homsey M, Straub V, Barresi R, Lochmüller H, Bushby K. Dysferlin associates with the developing T-tubule system in rodent and human skeletal muscle. Muscle Nerve. 41: 166–173 2010. [DOI] [PubMed] [Google Scholar]
- 41.Covian-Nares JF, Koushik SV, Puhl HL, 3rd, Vogel SS. Membrane wounding triggers ATP release and dysferlin-mediated intercellular calcium signaling. J Cell Sci. 123: 1884–1893 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rawat R, Cohen TV, Ampong B, Francia D, Henriques-Pons A, Hoffman EP, Nagaraju K. Inflammasome up-regulation and activation in dysferlin-deficient skeletal muscle. Am J Pathol. 176: 2891–2900 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iwata Y, Katanosaka Y, Hisamitsu T, Wakabayashi S.Enhanced Na+/H+ exchange activity contributes to the pathogenesis of muscular dystrophy via involvement of P2 receptors. Am J Pathol. 171: 1576–1587 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagaraju K, Rawat R, Veszalovszky E, Thapliyal R, Kesari A, Sparks S, Raben N, Plotz P, Hoffman EP. Dysferlin deficiency enhances monocyte phagocytosis: A model for the inflammatory onset of limb-girdle muscular dystrophy 2B. Am J Pathol. 172: 774–785 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han R, Frett EM, Levy JR, Rader EP, Lueck JD, Bansal D, Moore SA, Ng R, Beltrán-Valero de Bernabe D, Faulkner JA, Campbell KP. Genetic ablation of complement C3 attenuates muscle pathology in dysferlin-deficient mice. J Clin Invest. 120: 4366–4374 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Millay DP, Maillet M, Roche JA, Sargent MA, McNally EM, Bloch RJ, Molkentin JD. Genetic manipulation of dysferlin expression in skeletal muscle: Novel insights into muscular dystrophy. Am J Pathol. 175: 1817–1823 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ho M, Post CM, Donahue LR, Lidov HG, Bronson RT, Goolsby H, Watkins SC, Cox GA, Brown RH., JrDisruption of muscle membrane and phenotype divergence in two novel mouse models of dysferlin deficiency. Hum Mol Genet. 13: 1999–2010 2004. [DOI] [PubMed] [Google Scholar]
- 48.Kobayashi K, Izawa T, Kuwamura M, Yamate J.The distribution and characterization of skeletal muscle lesions in dysferlin-deficient SJL and A/J mice. Exp Toxicol Pathol. 62: 509–517 2010. [DOI] [PubMed] [Google Scholar]
- 49.Kostek CA, Dominov JA, Miller JB. Up-regulation of MHC class I expression accompanies but is not required for spontaneous myopathy in dysferlin-deficient SJL/J mice. Am J Pathol. 160: 833–839 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fanin M, Angelini C.Muscle pathology in dysferlin deficiency. Neuropathol Appl Neurobiol. 28: 461–470 2002. [DOI] [PubMed] [Google Scholar]
- 51.Dixon JE, Allan JE, Doherty PC, Hume DA. Immunohistochemical analysis of the involvement of F4/80 and Ia-positive macrophages in mouse liver infected with lymphocytic choriomeningitis virus. J Leukoc Biol. 40: 617–628 1986. [DOI] [PubMed] [Google Scholar]
- 52.Sunderkötter C, Kunz M, Steinbrink K, Meinardus-Hager G, Goebeler M, Bildau H, Sorg C.Resistance of mice to experimental leishmaniasis is associated with more rapid appearance of mature macrophages in vitro and in vivo. J Immunol. 151: 4891–4901 1993. [PubMed] [Google Scholar]
- 53.Fujita E, Kouroku Y, Isoai A, Kumagai H, Misutani A, Matsuda C, Hayashi YK, Momoi T. Two endoplasmic reticulum-associated degradation (ERAD) systems for the novel variant of the mutant dysferlin: Ubiquitin/proteasome ERAD(I) and autophagy/lysosome ERAD(II). Hum Mol Genet. 16: 618–629 2007. [DOI] [PubMed] [Google Scholar]
- 54.Ikezoe K, Furuya H, Ohyagi Y, Osoegawa M, Nishino I, Nonaka I, Kira J.Dysferlin expression in tubular aggregates: Their possible relationship to endoplasmic reticulum stress. Acta Neuropathol. 105: 603–609 2003. [DOI] [PubMed] [Google Scholar]
- 55.Ikezoe K, Nakamori M, Furuya H, Arahata H, Kanemoto S, Kimura T, Imaizumi K, Takahashi MP, Sakoda S, Fujii N, Kira J. Endoplasmic reticulum stress in myotonic dystrophy type 1 muscle. Acta Neuropathol. 114: 527–535 2007. [DOI] [PubMed] [Google Scholar]
- 56.Vattemi G, Engel WK, McFerrin J, Askanas V. Endoplasmic reticulum stress and unfolded protein response in inclusion body myositis muscle. Am J Pathol. 164: 1–7 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kobayashi K, Izawa T, Kuwamura M, Yamate J.Comparative gene expression analysis in the skeletal muscles of dysferlin-deficient SJL/J and A/J mice. J Toxicol Pathol. 24: 49–62 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Langin D.The role of uncoupling protein 2 in the development of type 2 diabetes. Drugs Today (Barc). 39: 287–295 2003. [DOI] [PubMed] [Google Scholar]
- 59.Bodyak N, Rigor DL, Chen YS, Han Y, Bisping E, Pu WT, Kang PM. Uncoupling protein 2 modulates cell viability in adult rat cardiomyocytes. Am J Physiol Heart Circ Physiol. 293: H829–H835 2007. [DOI] [PubMed] [Google Scholar]
- 60.Roach WG, Chavez JA, Mîinea CP, Lienhard GE. Substrate specificity and effect on GLUT4 translocation of the Rab GTPase-activating protein Tbc1d1. Biochem J. 403: 353–358 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chadt A, Leicht K, Deshmukh A, Jiang LQ, Scherneck S, Bernhardt U, Dreja T, Vogel H, Schmolz K, Kluge R, Zierath JR, Hultschig C, Hoeben RC, Schürmann A, Joost HG, Al-Hasani H. Tbc1d1 mutation in lean mouse strain confers leanness and protects from diet-induced obesity. Nat Genet. 40: 1354–1359 2008. [DOI] [PubMed] [Google Scholar]
- 62.Islam T, McConnell R, Gauderman WJ, Avol E, Peters JM, Gilliland FD. Ozone, oxidant defense genes, and risk of asthma during adolescence. Am J Respir Crit Care Med. 177: 388–395 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jorquera G, Juretić N, Jaimovich E, Riveros N.Membrane depolarization induces calcium-dependent upregulation of Hsp70 and Hmox-1 in skeletal muscle cells. Am J Physiol Cell Physiol. 297: C581–C590 2009. [DOI] [PubMed] [Google Scholar]
- 64.Silomon M, Bauer I, Bauer M, Nolting J, Paxian M, Rensing H.Induction of heme oxygenase-1 and heat shock protein 70 in rat hepatocytes: The role of calcium signaling. Cell Mol Biol Lett. 12: 25–38 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Neill DE, Noble EG. Constitutive expression of inducible Hsp70 is linked to natural shifts in skeletal muscle phenotype. Acta Physiol Scand. 181: 35–41 2004. [DOI] [PubMed] [Google Scholar]
- 66.Krajacic P, Hermanowski J, Lozynska O, Khurana TS, Lamitina T. The C. elegans dysferlin homolog fer-1 is expressed in muscle and fer-1 mutations initiate altered gene expression of muscle enriched genes. Physiol Genomics. 40: 8–14 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li ZH, Dulyaninova NG, House RP, Almo SC, Bresnick AR. S100A4 regulates macrophage chemotaxis. Mol Biol Cell. 21: 2598–2610 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wenzel K, Zabojszcza J, Carl M, Taubert S, Lass A, Harris CL, Ho M, Schulz H, Hummel O, Hubner N, Osterziel KJ, Spuler S. Increased susceptibility to complement attack due to down-regulation of decay-accelerating factor/CD55 in dysferlin-deficient muscular dystrophy. J Immunol. 175: 6219–6225 2005. [DOI] [PubMed] [Google Scholar]
- 69.Lynch DM, Kay PH. Studies on the polymorphism of the fifth component of complement in laboratory mice. Exp Clin Immunogenet. 12: 253–260 1995. [PubMed] [Google Scholar]
- 70.Rhodes JC, Wicker LS, Urba WJ. Genetic control of susceptibility to Cryptococcus neoformans in mice. Infect Immun. 29: 494–499 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.von der Hagen M, Laval SH, Cree LM, Haldane F, Pocock M, Wappler I, Peters H, Reitsamer HA, Hoger H, Wiedner M, Oberndorfer F, Anderson LV, Straub V, Bittner RE, Bushby KM. The differential gene expression profiles of proximal and distal muscle groups are altered in pre-pathological dysferlin-deficient mice. Neuromuscul Disord. 15: 863–877 2005. [DOI] [PubMed] [Google Scholar]
- 72.Suzuki N, Aoki M, Hinuma Y, Takahashi T, Onodera Y, Ishigaki A, Kato M, Warita H, Tateyama M, Itoyama Y.Expression profiling with progression of dystrophic change in dysferlin-deficient mice (SJL). Neurosci Res. 52: 47–60 2005. [DOI] [PubMed] [Google Scholar]
- 73.Bernard CC, Carnegie PR. Experimental autoimmune encephalomyelitis in mice: Immunologic response to mouse spinal cord and myelin basic proteins. J Immunol. 114: 1537–1540 1975. [PubMed] [Google Scholar]
- 74.Rosenberg NL, Ringel SP, Kotzin BL. Experimental autoimmune myositis in SJL/J mice. Clin Exp Immunol. 68: 117–129 1987. [PMC free article] [PubMed] [Google Scholar]
- 75.Tzou SC, Lupi I, Landek M, Gutenberg A, Tzou YM, Kimura H, Pinna G, Rose NR, Caturegli P. Autoimmune hypophysitis of SJL mice: Clinical insights from a new animal model. Endocrinology. 149: 3461–3469 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nagaraju K, Rawat R, Veszelovszky E, Thapliyal R, Kesari A, Sparks S, Raben N, Plotz P, Hoffman EP. Dysferlin deficiency enhances monocyte phagocytosis: A model for the inflammatory onset of limb-girdle muscular dystrophy 2B. Am J Pathol. 172: 774–785 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chiu YH, Hornsey MA, Klinge L, Jørgensen LH, Laval SH, Charlton R, Barresi R, Straub V, Lochmüller H, Bushby K. Attenuated muscle regeneration is a key factor in dysferlin-deficient muscular dystrophy. Hum Mol Genet. 18: 1976–1989 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nguyen K, Bassez G, Krahn M, Bernard R, Laforêt P, Labelle V, Urtizberea JA, Figarella-Branger D, Romero N, Attarian S, Leturcq F, Pouget J, Lévy N, Eymard B. Phenotypic study in 40 patients with dysferlin gene mutations: High frequency of atypical phenotypes. Arch Neurol. 64: 1176–1182 2007. [DOI] [PubMed] [Google Scholar]
- 79.Grieger JC, Samulski RJ. Packaging capacity of adeno-associated virus serotypes: Impact of larger genomes on infectivity and postentry steps. J Virol. 79: 9933–9944 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yuasa K, Sakamoto M, Miyagoe-Suzuki Y, Tanouchi A, Yamamoto H, Li J, Chamberlain JS, Xiao X, Takeda S. Adeno-associated virus vector-mediated gene transfer into dystrophin-deficient skeletal muscles evokes enhanced immune response against the transgene product. Gene Ther. 9: 1576–1588 2002. [DOI] [PubMed] [Google Scholar]
- 81.Mingozzi F, High KA. Immune responses to AAV clinical trials. Curr Gene Ther. 7: 316–324 2007. [DOI] [PubMed] [Google Scholar]
- 82.Lostal W, Bartoli M, Bourg N, Roudaut C, Bentaïb A, Miyake K, Guerchet N, Fougerousse F, McNeil P, Richard I.Efficient recovery of dysferlin deficiency by dual adeno-associated vector-mediated gene transfer. Hum Mol Genet. 19: 1897–1907 2010. [DOI] [PubMed] [Google Scholar]
- 83.Sancricca C, Mirabella M, Gliubizzi C, Broccolini A, Gidaro T, Morosetti R.Vessel-associated stem cells from skeletal muscle: From biology to future uses in cell therapy. World J Stem Cells. 2: 39–49 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leriche-Guérin K, Anderson LV, Wrogemann K, Roy B, Goulet M, Tremblay JP. Dysferlin expression after normal myoblast transplantation in SCID and in SJL mice. Neuromuscul Disord. 12: 167–173 2002. [DOI] [PubMed] [Google Scholar]
- 85.Kong KY, Ren J, Kraus M, Finklestein SP, Brown RH., JrHuman umbilical cord blood cells differentiate into muscle in SJL muscular dystrophy mice. Stem Cells. 22: 981–993 2004. [DOI] [PubMed] [Google Scholar]
- 86.Wagner KR, Fleckenstein JL, Amato AA, Barohn RJ, Bushby K, Escolar DM, Flanigan KM, Pestronk A, Tawil R, Wolfe GI, Eagle M, Florence JM, King WM, Pandya S, Straub V, Juneau P, Meyers K, Csimma C, Araujo T, Allen R, Parsons SA, Wozney JM, Lavallie ER, Mendell JR. A phase I/II trial of MYO-029 in adult subjects with muscular dystrophy. Ann Neurol. 63: 561–571 2008. [DOI] [PubMed] [Google Scholar]
- 87.Nemoto H, Konno S, Sugimoto H, Nakazora H, Nomoto N, Murata M, Kitazono H, Fujioka T.Anti-TNF therapy using etanercept suppresses degenerative and inflammatory changes in skeletal muscle of older SJL/J mice. Exp Mol Pathol. 90: 264–270 2011. [DOI] [PubMed] [Google Scholar]
- 88.Han R.Muscle membrane repair and inflammatory attack in dysferlinopathy. Skelet Muscle. 1: 10 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Confalonieri P, Oliva L, Andreetta F, Lorenzoni R, Dassi P, Mariani E, Morandi L, Mora M, Comelio F, Mantegazza R.Muscle inflammation and MHC class I up-regulation in muscular dystrophy with lack of dysferlin: An immunopathological study. J Neuroimmunol. 142: 130–136 2003. [DOI] [PubMed] [Google Scholar]
- 90.Prohászka Z, Singh M, Nagy K, Kiss E, Lakos G, Duba J, Füst G.Heat shock protein 70 is a potent activator of the human complement system. Cell Stress Chaperones. 7: 17–22 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grundtman C, Bruton J, Yamada T, Östberg T, Pisetsky DS, Harris HE, Andersson U, Lundberg IE, Weserblad H. Effects of HMGB1 on in vitro responses of isolated muscle fibers and functional aspects in skeletal muscles of idiopathic inflammatory myopathies. FASB J. 24: 743–757 2010 [DOI] [PubMed] [Google Scholar]