Abstract
This report describes a spontaneous hybrid cyst in a Sprague-Dawley (SD) rat. A 52-week-old, male SD rat had a cutaneous cyst on the left mystacial pad. Histologically, the cyst wall showed infundibular differentiation with keratohyalin granules in the granular layer and matrical differentiation comprising basaloid epithelial cells with trichohyalin granules. The cyst cavity was filled with lamellar, flaky keratin and aggregates of shadow cells. Immunohistochemically, the infundibular-type epithelium was positive for cytokeratin (CK) AE1/AE3, CK KL1 and CK14 but negative for CK15, whereas the matrical-type epithelium was negative for all four CK isoforms examined. These immunohistochemical properties of the infundibular- and matrical-type epithelia were similar to those of the infundibulum and inferior segment of normal hair follicles, respectively. Based on these findings, the cyst was diagnosed as a hybrid cyst, comprising more than one type of cyst arising from various parts of the pilosebaceous unit.
Keywords: kin, hybrid cyst, epidermal inclusion cyst, cytokeratin, Sprague-Dawley rat
Epidermal inclusion cysts are common lesions that have been reported in many animals, including rodents1. Histopathologically, an epidermal inclusion cyst is considered to be derived from the hair follicle and is also referred to as an infundibular, epidermal or epidermoid cyst2. However, it is rarely complicated by pilomatrical changes with the appearance of basophilic and ghost cells within its luminal epithelium. In domestic animals, epidermal inclusion cysts are generally classified as follicular cysts3, which are further categorized on the basis of the epithelial lining or the preexisting structure from which the cyst arose. The categories include infundibular, isthmus, matrical and hybrid cysts. The concept of a follicular hybrid cyst was first proposed in humans and includes any type of cyst arising from various parts of the pilosebaceous unit4,5,6. Similarly, in domestic animals, a hybrid cyst is composed of two or three types of epithelial lining3 and is also referred to as a panfollicular (trichoepitheliomatous) cyst2. Hybrid cysts are not particularly common in humans, are common in dogs and are rare in cats3, 7. However, to our knowledge, hybrid cysts have not been reported in rodents, although they frequently develop epidermal inclusion cysts.
Cytokeratins (CKs) are classified according to their molecular weight and isoelectric point8, and the isoforms are used as differentiation markers for luminal epithelial cells of follicular cysts9. In this report, we describe the pathological features of a hybrid cyst in a male Sprague-Dawley (SD) rat.
A 52-week-old, clinically normal, male SD rat was euthanized in accordance with the guidelines approved by the Animal Research Committee of Azabu University. At necropsy, a dome-shaped, hard cutaneous nodule (10 mm × 10 mm in diameter) was observed on the left mystacial pad and was attached to the skin. In a sliced section, the nodule showed a large cystic structure containing white-to-gray desiccated material, and a pore connecting the cyst to the overlying epidermis was observed. The other organs showed no gross abnormalities.
The cystic lesion was fixed in 10% neutral-buffered formalin, embedded in paraffin and sectioned and stained with hematoxylin and eosin (HE) and periodic acid-Schiff (PAS). Immunohistochemical staining was performed using the immunoenzyme polymer method with the primary antibodies shown in Table 1. Peroxidase-conjugated anti-mouse immunoglobulin G (Histofine Simple Stain MAX-PO(M); Nichirei, Tokyo, Japan) was used as a secondary antibody. After immunoreaction, the sections were colorized with diaminobenzidine and counterstained with Mayer’s hematoxylin. Intact skin samples from five rats (n = 5) were used as controls to compare the immunohistochemical properties of CK expression with hair follicles in normal rats.
Table 1. Immunohistochemistry.

Histologically, a single dermal-based cyst had developed and extended into the underlying fat (Fig. 1). The upper portion of the cyst was open, forming a C-shaped structure that connected with the overlying epidermis. The epithelial lining and keratinization pattern of the cyst wall was divided into two types: infundibular and matrical (Fig. 2). The former comprised an infundibular-type epithelium with keratohyalin granules in the granular layer resulting in keratinization with laminated corneocytes. The latter, which was adjacent to the infundibular-type, showed a matrical-type epithelium, which was lined by basophilic basaloid epithelial cells with scant cytoplasm and hyperchromatic nuclei, and was abruptly keratinized, forming aggregates of shadow cells. Trichohyalin granules and PAS-positive cells containing glycogen were observed within the matrical-type, indicating differentiation into the inner root sheath. The two types of epithelium in turn formed a line, and the transition was irregularly developed. The cyst cavity was filled with lamellar or flaky keratin in addition to the aggregates of shadow cells, but no hair fragments were observed. Several lymphocytes and neutrophils were observed inside the cyst and the outer layer of the cyst wall.
Fig. 1.
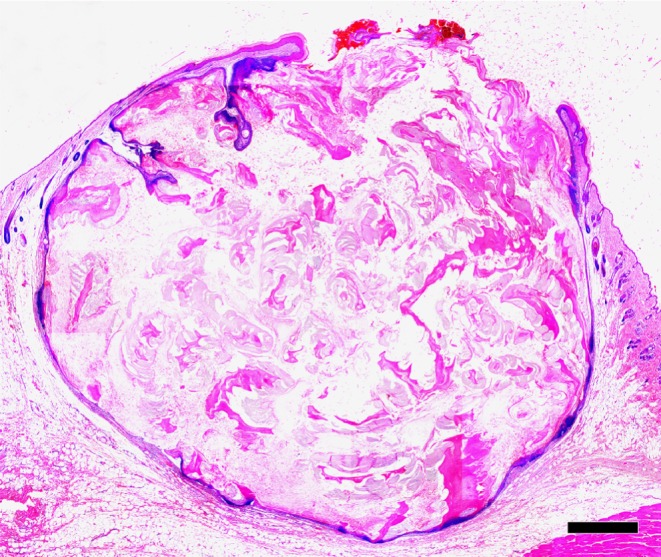
Low-power view of the cutaneous cyst. The cyst develops and extends into the underlying fat. The upper portion opens into the overlying epidermis. HE. Bar = 1 mm.
Fig. 2.

The cyst wall is lined with a mixture of the infundibular-type epithelium (right) and matrical-type epithelium (left). The former shows infundibular keratinization with lamellar keratin and keratohyalin granules (arrows), and the latter shows abrupt keratinization forming aggregates of shadow cells (*) and trichohyalin granules (arrow heads). HE. Bar = 200 μm.
The results of immunohistochemical examinations of the cyst in this case and normal tissues are summarized in Table 2. The expression of CK isoforms in the cyst wall differed between the types of epithelium lining. The infundibular-type epithelium was positive for CK AE1/AE3, CK KL1, and CK14, but was negative for CK15 (Fig. 3). In contrast, the matrical-type epithelium was negative for all four CK isoforms examined (Fig. 3). The cystic lesion was finally diagnosed as a hybrid cyst on the basis of morphological and immunohistological characterization.
Table 2. Results of Immunohistochemical Examination on the Present Case and Normal Tissues.

Fig. 3.

Immunostaining of cytokeratin (CK) in the cyst wall. The lining consists of the infundibular-type epithelium (right) and matrical type epithelium (left). The former is positive for CK AE1/AE3 (A), CK KL1 (B) and CK14 (C) but negative for CK15 (D). The latter is negative for all four CK isoforms examined.
Hybrid cysts comprise more than two structures of the pilosebaceous unit, and the combination of these structures can vary. The following combinations have been described in humans: infundibular cyst-trichilemmal (isthmic) cyst, infundibular cyst-matrical cyst, trichilemmal cyst-matrical cyst, eruptive vellus hair cyst-steatocystoma, trichilemmal cyst-eruptive vellus hair cyst, and infundibular cyst-apocrine hidrocystoma7. The most common combination in humans, as well as dogs, is the infundibular cyst-trichilemmal cyst3, 7. Histologically, the combination type of the present case was infundibular cyst-matrical cyst, which was also confirmed by immunohistochemical examinations. The immunohistochemical properties of the infundibular- and matrical-type epithelia within the cyst in this case were similar to those of the infundibulum and inferior segment in normal rat hair follicles, respectively (Table 2). This strongly suggests that the epithelial lining of this cyst showed divergent differentiation to an infundibulum and inferior segment of the hair follicle.
The etiology of hybrid cysts is unclear, but they probably originate from follicular stem cells. Rodins et al.10 suggested that β-catenin, which is an important requirement for follicular stem cells that are involved in hair follicle development11, plays a role in the pathogenesis of hybrid cysts. Papilloma virus has also been demonstrated in epidermal inclusion cysts of European harvest mice, which indicates a papillomaviral etiology for this cyst12.
McMartin et al. reported that epidermal inclusion cyst can occur spontaneously in 1.7% of male and 0.3% of female SD rats13. The frequency of hybrid cysts in rats is unclear, but a certain proportion of hybrid cysts may be diagnosed as epidermal inclusion cysts during routine examinations. Investigation of the combinations of different types of epithelium in this disease is useful to understand the pathogenesis of pilosebaceous-derived tumors in rats, and immunohistochemical examination using CK isoforms could help to clarify the differentiation of the epithelium lining of hybrid cysts.
Acknowledgments
This research was partially supported by a research project grant awarded by the Azabu University.
References
- 1.Stephen G, Lake LH-E, Robert EM, Barry PS. Nonneoplaspic lesions. In: Integument and Mammary Glands (Monographs on Pathology of Laboratory Animals), Springer-Verlag, Heidelberg, Germany. 130–157. 1989 [Google Scholar]
- 2.Goldschmidt MH, Dunstan RW, Stannard AA. Cyst. In: Histological Classifcation of Epithelial and Melanocytic Tumors of the Skin of Domestic Animals, Armed Forces Institute of Pathology, Washington DC, USA. 33–35. 1998 [Google Scholar]
- 3.Gross TL, Ihrke PJ, Walder EJ, Affolter VK. Follicular Tumors. In: Skin Diseases of the Dog and Cat, 2nd ed. TL Gorss, PJ Ihrke, EJ Walder, and VK Affolter (eds). Blackwell Publishing, Oxford, UK. 604–640. 2005 [Google Scholar]
- 4.Brownstein MH. Hybrid cyst: a combined epidermoid and trichilemmal cyst. J Am Acad Dermatol. 9: 872–875 1983. [DOI] [PubMed] [Google Scholar]
- 5.McGavran MH, Binnington B. Keratinous cysts of the skin. Identification and differentiation of pilar cysts from epidermal cysts. Arch Dermatol. 94: 499–508 1966. [PubMed] [Google Scholar]
- 6.Requena L, Sanchez Yus E.Follicular hybrid cysts. An expanded spectrum. Am J Dermatopathol. 13: 228–233 1991. [DOI] [PubMed] [Google Scholar]
- 7.Takeda H, Miura A, Katagata Y, Mitsuhashi Y, Kondo S.Hybrid cyst: case reports and review of 15 cases in Japan. J Eur Acad Dermatol Venereol. 17: 83–86 2003. [DOI] [PubMed] [Google Scholar]
- 8.Cooper D, Schermer A, Sun TT. Classification of human epithelia and their neoplasms using monoclonal antibodies to keratins: strategies, applications, and limitations. Lab Invest. 52: 243–256 1985. [PubMed] [Google Scholar]
- 9.Tsuji N, Kurokawa I, Tokime K, Omoto Y, Senba Y, Habe K, Yamanaka K, Isoda K, Tsubura A, Mizutani H.Epidermal cyst with pilomatricoma (follicular hybrid cyst): immunohistochemical study with epithelial keratins and filaggrin. J Dermatol. 37: 922–925 2010. [DOI] [PubMed] [Google Scholar]
- 10.Rodins K, Baillie L.Hybrid follicular cyst (pilomatrical and infundibular) arising within a sebaceous nevus. Pediatr Dermatol. 29: 213–216 2012. [DOI] [PubMed] [Google Scholar]
- 11.Gat U, DasGupta R, Degenstein L, Fuchs E.De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 95: 605–614 1998. [DOI] [PubMed] [Google Scholar]
- 12.Sundberg JP, O’Banion MK, Shima A, Knupp C, Reichmann ME. Papillomas and carcinomas associated with a papillomavirus in European harvest mice (Micromys minutus). Vet Pathol. 25: 356–361 1988. [DOI] [PubMed] [Google Scholar]
- 13.McMartin DN, Sahota PS, Gunson DE, Hsu HH, Spaet RH. Neoplasms and related proliferative lesions in control Sprague-Dawley rats from carcinogenicity studies. Historical data and diagnostic considerations. Toxicol Pathol. 20: 212–225 1992. [DOI] [PubMed] [Google Scholar]


