Abstract
Southwest Mexico City (SWMC) air pollution is characterized by high concentrations of ozone and particulate matter < 10 μm (PM10) containing lipopolysaccharides while in the North PM2.5 is high. These intra-city differences are likely accounting for higher CD14 and IL-1β in SWMC v NMC mice myocardial expression. This pilot study was designed to investigate whether similar intra-city differences exist in the levels of myocardial inflammatory genes in young people. Inflammatory mediator genes and inflammasome arrays were measured in right and left autopsy ventricles of 6 southwest/15 north (18.5 ± 2.6 years) MC residents after fatal sudden accidental deaths. There was a significant S v N right ventricle up-regulation of IL-1β (p=0.008), TNF-α (p=0.001), IL-10 (p=0.001), and CD14 (p=0.002), and a left ventricle difference in TNF-α (p=0.007), and IL-10 (p=0.02). SW right ventricles had significant up-regulation of NLRC1, NLRP3 and of 29/84 inflammasome genes, including NOD factors and caspases. There was significant degranulation of mast cells both in myocardium and epicardial nerve fibers. Differential expression of key inflammatory myocardial genes and inflammasomes are influenced by the location of residence. Myocardial inflammation and inflammasome activation in young hearts is a plausible pathway of heart injury in urbanites and adverse effects on the cardiovascular system are expected.
Keywords: cardiovascular risk factor, children, particulate matter, inflammasomes, mast cells, myocardial inflammation, urban pollution
Introduction
Inhalation of particulate matter (PM) air pollution increases the risk for adverse clinical cardiovascular (CV) events as well as both short- and long-term cardiovascular mortality1, 2. Three generalized intermediary pathways have been hypothesized to explain the detrimental effects on the CV system in response to PM inhalation: pulmonary and systemic oxidative stress and inflammation, pulmonary receptor mediated alterations in autonomic balance, and direct effects of PM or its constituents on the vasculature and/or blood elements after translocation from the lung1. Yet, uncertainty remains whether exposure to different air pollution profiles causes distinct myocardial inflammatory responses in humans.
Residents of Mexico City are exposed year-round to air pollutant concentrations frequently above the National Air Ambient Quality Standards (NAAQS) for the United States3,4. High concentrations of fine particulate matter (PM2.5) as well as significant levels of PM associated with lipopolysaccharides (PM-LPS) are present in Mexico City’s air, and marked regional differences in the air pollutants concentrations and composition have been reported within metropolitan Mexico City3,4,5,6,7.
Previously, these well-established regional differences in air pollution between southwest Mexico City (SWMC) and north Mexico City (NMC) were exploited to assess differential health effects in mice exposed for 16 months to ambient air8. Tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and cluster designation antigen 14 (CD14) gene mRNA myocardial expression increased in mice exposed in SWMC when compared to mice from NMC8. The observed differences were attributed to the regional differences in ambient air PM-LPS concentration3,4,5,6,7.
The goal for this work was to test the hypothesis that lifetime exposures linked to one of the two distinctly polluted Mexico City target atmospheres modifies the myocardial inflammatory gene profile and inflammasome activation in specimens obtained from autopsied children and young adults with long life residency in the target areas. Given that the pattern recognition Toll-like receptor-4 (TLR-4) is a signaling receptor for lipopolysaccharides (LPS) and that the Asp299Gly TLR-4 polymorphism is associated with a blunted response to lipopolysacharides, we selected individuals with a negative polymorphism9,10. Specifically, based on previous in vivo mice studies8, myocardial inflammation in SWMC residents exposed to an atmosphere with high concentrations of LPS is predicted to be significantly higher than inflammation in NMC residents. Myocardial mRNA was measured for two key inflammatory genes: IL-1β and TNF-α. Measurements were also made of the LPS receptor CD14 and IL-10, an anti-inflammatory cytokine; and two inflammasomes: nucleotide-binding oligomerization domain NOD containing 1 (NLRC1) and pyrin domain containing 3 (NLRP3). A cDNA array targeted for the expression of 84 genes involved in the function of inflammasomes, protein complexes involved in innate immunity and nucleotide oligomerization binding domain (NOD)-like receptors (NLR) signaling was run. Light and electron microscopy of the hearts were also done.
Methods and Procedures
Study city and air quality
Mexico City is an example of extreme urban growth and accompanying environmental pollution3,4. The metropolitan area of over 2,000 km2 lies in an elevated basin 2,240 meters above sea level surrounded on three sides by mountain ridges. Mexico City’s 20 million inhabitants, over 40,000 industries, and 4 million vehicles consume more than 40 million liters of petroleum fuels per day, producing an estimated annual emission of 2.6 tons of particulate and gaseous air pollutants11. Mexico City’s metropolitan area motor vehicles produce abundant amounts of primary fine PM, elemental carbon, particle-bound polycyclic aromatic hydrocarbons, carbon monoxide, and a wide range of air toxics, including formaldehyde, acetaldehyde, benzene, toluene, and xylenes11,12,13,14,15. The high altitude and tropical climate facilitate ozone production all year and contribute to the formation of fine secondary particulate matter. Air quality is generally worse in the winter, when rain is less common and thermal inversions are more frequent. LPS detected in the coarse fraction of PM (PM10) from SWMC show the highest LPS concentrations at 59 EU/mg PM105, 6. SWMC historically exhibits the highest endotoxin concentrations, with PM10-LPS ranging from 15.3 to 20.6 ng/mg, while NMC contains less than 70% of the SW Mexico City values5. On the other hand, ozone concentrations spatial distribution peak towards the downwind SW area in the afternoon as a result of the typical diurnal wind transport of polluted air masses from the urban area. Hourly levels higher than 0.12 ppm as well as 8-hour ozone average values above 0.075 ppm, the respective US EPA air quality standards, are typically registered in SWMC.
Selection of subjects from SWMC versus NMC was made based on the significant differences between outdoor environments in “northern-industrialized” zones in comparison with “southern-residential” zones, which illustrate the contribution from the industry in the north4,7,13. SWMC residents have been exposed to significant concentrations of ozone, secondary tracers (NO3ˉ) and PM-LPS, while NMC residents have been exposed to higher concentrations of volatile organic compounds (VOCs), PM2.5, and its constituents: organic and elemental carbon including VOCs, secondary inorganic aerosols (SO42ˉ, NO3ˉ, NH4+), and metals (Zn, Cu, Pb, Ti, Mn, Sn, V, Ba) 3,4,7,15. Recent studies on the composition of PM2.5 with regards to sites and samples collected in 1997 show that composition has not changed during the last decade4.
Heart samples
The Human Studies Committees of the involved institutions in Mexico City approved the study and the research protocol. Twenty-one clinically healthy, non-smoking, non-obese children and young adults who died suddenly, accidentally, and without chest or head trauma were included. Six subjects were residents in SWMC and 15 in NMC. Their major everyday activities, including work and school took place within 10 miles of their residency. All subjects had documented instant deaths related to their accidents and were pronounced death at the scene immediately after the accident by the pertinent authorities. Autopsies were performed 3.7 ± 1.7 hours after death. Subjects had no pathological evidence of recent or long-term inflammatory processes or pathological findings such as myocardial infarction, valve pathology, coronary artery disease, ventricle or atrial dilatation or hypertrophy, large vessel gross abnormalities, chest trauma, cerebral ischemia, head injury, or stroke. Toxicological studies were negative and included drug alkaline and acid/neutral screen, amphetamines, benzodiazepines, cocaine/opiates, alcohol, volatiles and cannabinoids. All subjects were negative for the Asp299Gly TLR4 polymorphism. The mean age of the SWMC subjects was 19.1 ± 2.9 years (mean ± standard deviation [SD]) and 17.8 ± 2.3 years for the NMC subjects (p=0.93). Representative sections of the heart muscle including the left and right ventricles and the inter-ventricular septum were fixed in 10% neutral formaldehyde for 48 hours and transferred to 70% alcohol for histopathology. Heart tissues were fixed in 2% paraformaldehyde and 2% glutaraldehyde in sodium phosphate buffer (0.1 M, pH 7.4) for electron microscopy. The remaining heart tissues were quickly frozen and stored at –80°C and transmural sections of the left and right ventricular wall were selected for the RT-PCR and array studies.
Light and electron microscopy
Paraffin sections 7μm thick were cut and stained with hematoxylin eosin (H&E) and toluidine blue, and underwent immunohistochemistry for tryptase (Novocastra laboratories Ltd., NCL-MCTRYP 1:50, Newcastle upon Tyne NE12 8EW, UK). Three board-certified pathologists (GD, RDC, LCG), including a Forensic pathologist (GD) without access to the identification codes reviewed the sections. Electron microscopy was performed in 8 age-matched cases: 4 NMC and 4 SWMC. Tissues were post-fixed in 1% osmium tetraoxide and embedded in Epon. Semi-thin sections (0.5 to 1 μm) were cut and stained with toluidine blue for light microscopic examination. Ultra-thin sections (60–90 nm) were cut and collected on slot grids previously covered with formvar membrane. Sections were stained with uranyl acetate and lead citrate, and examined with a JEM-1011 (Japan) microscope.
Estimation of mRNA Abundance by RT-PCR
To determine the expression of mRNA from IL-1β, TNF-α, IL-10, CD14, NLRC1 and NLRP3, total RNA was extracted from the heart samples using Trizol Reagent (InVitrogen Corp). RNA integrity, concentration, and purity were determined by spectrophotometry using the NanoDrop ND-1000, keeping only samples with the OD A260/A280 and the OD A260/A230 ratios close to 2.0. Small fragments of myocardium, while in ice, were homogenized in 1 mL of Trizol, and the tissue homogenate was centrifuged at 12,000 × g for 15 min at a temperature of 4°C. Relative abundances of mRNAs encoding the genes of interest were estimated by quantitative fluorogenic 5’ nuclease (TaqMan) assay of the first strand cDNAs using Platinum-qPCR Supermix-UDG reagent (Invitrogen) and the proper oligonucleotide primers and probes.
PCR arrays
Microarray analysis was conducted with SABiosciences (Frederick, MD, USA) Human Inflammasome Array RT2 ProfilerTM. RNA was pooled from each sample inside each group and cDNA was synthesized using the C-03 first strand kit (SABiosciences). Relative gene expression was normalized to five housekeeping genes in each PCR array plate. The fold change for each gene was calculated as 2(ΔΔCt) and shown as up-regulated if expression was >2 or down-regulated if expression was <–2. Included in the array were 84 genes distributed in three groups: 1) Inflammasomes: i. negative regulation, ii. down-stream signaling, 2) NOD-Like Receptors: i. down-stream signaling, and 3) Pro-inflammatory caspases: CASP1, CASP4, and CASP5.
Statistics
Statistical analyses were performed using the SAS statistical software 9.0 version. The student’s test, the sign test and/or the Wilcoxon signed rank test were used to test whether there were significant differences in the expression of the selected genes and in the difference of the annual PM2.5 between the 2 geographical regions of interest, NMC and SWMC. Significance was assumed at p<0.05. Data are expressed as mean values ± SD.
Results
Air quality data
Mexico City residents are exposed year-round to air pollutant concentrations above PM2.5 and ozone United States’ National Air Ambient Quality Standards (NAAQS). Panels 1A and 1B in Fig. 1 show the trend of PM2.5 concentrations from 1997 to 2010 in two representative Metropolitan Mexico City areas located at NMC (Tlalnepantla) and SWMC (Pedregal). The PM2.5 annual air quality standard of 15 µg/m3 has been historically exceeded across the metropolitan area, including the selected target areas (Table 1). Statistically higher levels of fine PM have been observed in NMC v SWMC due mostly to industry and heavy truck traffic (p=0.0001). During the dry season extending from November to May, PM2.5 levels as high as ~ 90 µg/m3 are common in NMC.
Fig. 1.
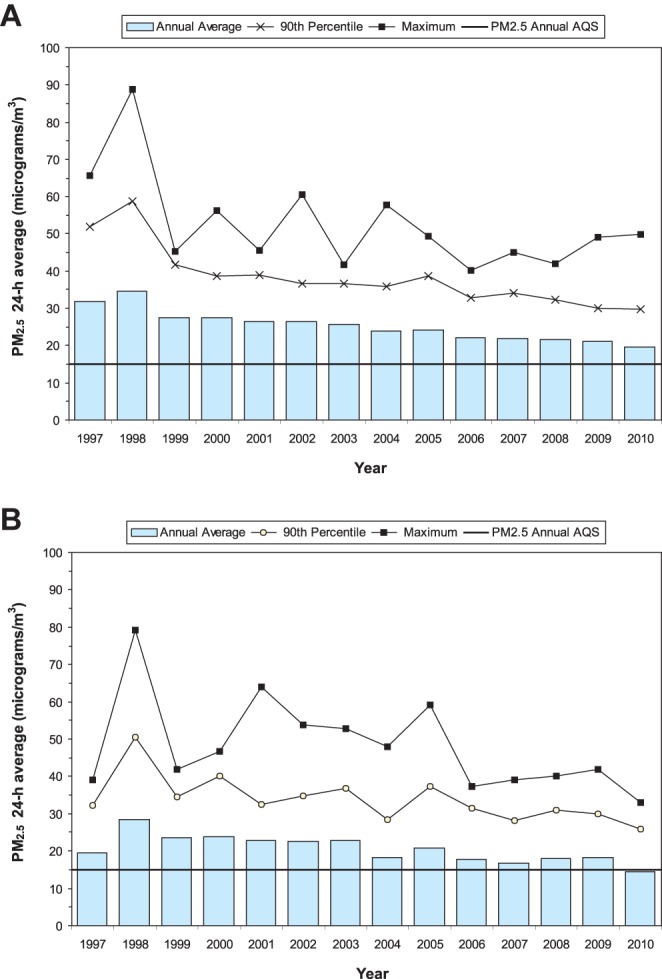
A: Trends of annual averages, maxima and 90th percentiles of 24-hr PM2.5 levels in the North Mexico City Tlalnepantla (NMC) monitoring station for the period 1997–2010. The levels for the period 1997–2003 were estimated from PM10 data using a regression equation (R2 = 0.71) for simultaneous PM10 and PM2.5 data registered between 2003 and 2007 at the same site. PM data were obtained from the Secretaría del Medio Ambiente, Gobierno del Distrito Federal (SMA-GDF, 2011). B: Trends of annual averages, maxima and 90th percentiles of 24-hr PM2.5 levels in the Mexico City Southwest Pedregal monitoring station for the period 1997–2010. The levels for the period 1997–2003 were estimated from PM10 data using a regression equation (R2 = 0.65) for simultaneous PM10 and PM2.5 data registered between 2003 and 2007 in the same site. PM data were obtained from the Secretaría del Medio Ambiente, Gobierno del Distrito Federal (SMA-GDF, 2011).
Table 1. PM2.5 Annual Concentrations in μg/m3 for NMC versus SWMC Stations.

Heart histopathology and electron microscopy
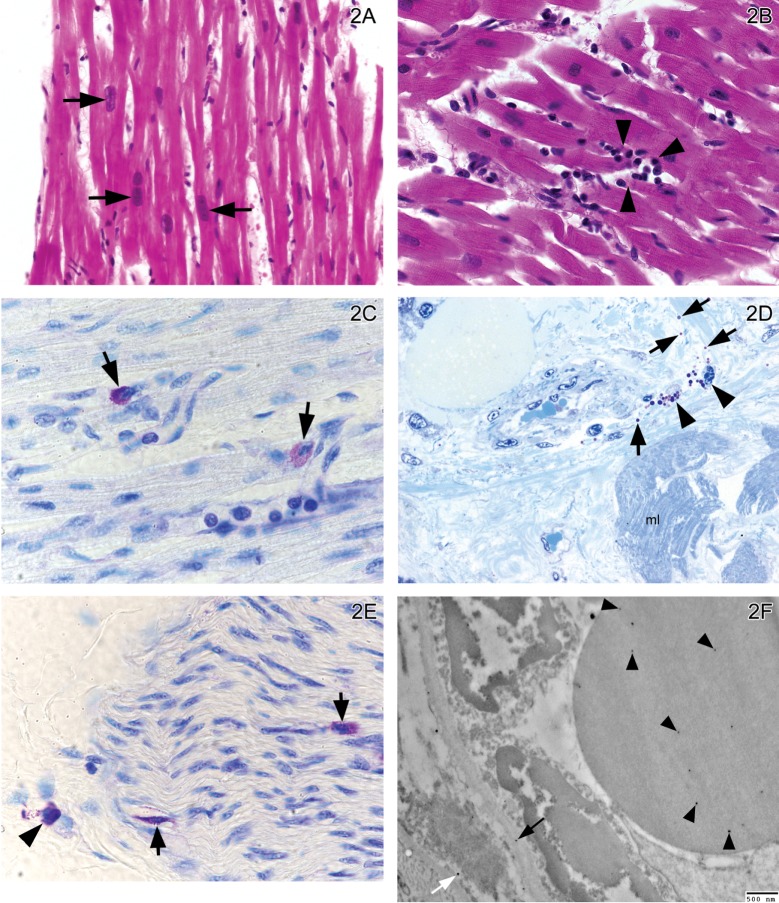
An average of 12 sections were stained and examined for each block, including hematoxilyn-eosin, toluidine blue, Gomori’s Trichrome stain, Verhoeff’s Elastic Stain, and tryptase. Mild variation in nuclear size in myocardial fibers (Fig. 2A), isolated mononuclear cell infiltrates (Fig. 2B), mast cell degranulation both in myocardium (Fig. 2C), perivascular (Fig. 2D), and in epicardial nerves (Fig. 2E) were observed in all subjects regardless of residency area. Gomori’s Trichrome and Verhoeff’s elastic stains showed no pathological findings. Particle-like material average size 28 nm was seen in erythrocytes, endothelial and smooth muscle cells and basement membranes of myocardial arterioles of hearts from NMC and SWMC subjects (2/4 in each group)(Fig. 2F). Tryptase and toluidine blue stained sections provided an assessment of mast cell number. Tryptase positive cells counted per 10x field yielded 10.8 ± 2.2 and 10.03 ± 3.7 for SWMC and NMC respectively (p=0.69). For toluidine blue, positive cells counted per 40x were 1.34 ± 0.45 and 0.94 ± 0.54 for SWMC and NMC respectively (p=0.13), and for the percent of degranulated cells using tryptase, we recorded 45 ± 26 and 42 ± 27 for SWMC and NMC respectively (p=0.92). Thus, no differences were seen between south versus north residents in any of the mast cell endpoints. All participating pathologists concluded that H&E, Gomori’s Trichrome and Verhoeff’s elastic stains showed no significant myocardial pathology.
Fig. 2.
A: Left ventricle in a 14 year old NMC boy. There is mild variation in the nuclei size of the myocardial fibers (short arrows). H&E × 40. B: Left ventricle in a 14 year old SWMC girl. Isolated small foci of mononuclear cells are observed (head arrows). H&E × 60. C: Right ventricle in a NMC 11 month old boy. Two mast cells are observed with different degrees of degranulation. The mast cell on the right (short arrow) exhibits significant decrease in the number of cytoplasmic granules. The mast cell on the left is well granulated. Toluidine blue × 100. D: Right ventricle in a 19 year old SWMC male. There are perivascular degranulated mast cells (head arrows). The granules can be observed in the interstitial space (short arrows). Myocardial fibers are labeled mI. Toluidine blue 1 μm section × 40. E: Right ventricle epicardial nerve in a 14 year old SWMC girl. There are mast cells located in the epineurial (arrowhead) and endoneurial (short arrows) spaces. Endoneurial mast cells (short arrows) are partially degranulated. Toluidine blue 7 μm section × 100. F: Electron micrograph of right ventricle arteriole in a SWMC 19 years old male. The luminal red blood cell (RBC) shows several nanosize particle-like material (average size 28 nm) (head arrows) and similar size particulate-like material is seen in the endothelial basement membrane (long arrow), and the smooth muscle cell nucleus and cytoplasm (white arrow). EM × 20,000.
Real-time PCR mRNA analysis of target genes
Real-time PCR analysis of IL-1β, TNF-α, IL-10, CD14, NLRC1, and NLRP3 in the heart samples indicated that the corresponding mRNA was present in each of the samples analyzed. Table 2 and Fig. 3 illustrate the RT-PCR results expressed as an index where the values of the target genes were normalized to the amount of the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase, (GAPDH) cDNA (expressed in molecules per attomol/GAPDH) for residents of SWMC v NMC. Up-regulation of IL-1β (p=0.008), TNF-α (p=0.001), IL-10 (p=0.001), and CD14 (p=0.002) was significantly greater in the right ventricle of SWMC v NMC residents. In contrast to the right ventricle, only TNF-α (p=0.007) and IL-10 (p=0.02) where up-regulated in the left ventricle in SWMC v NMC residents.
Table 2. RT-PCR Results of Target Genes Normalized to the Amount of GADPH cDNA for SWMC and NMC Subjects.

Fig. 3.
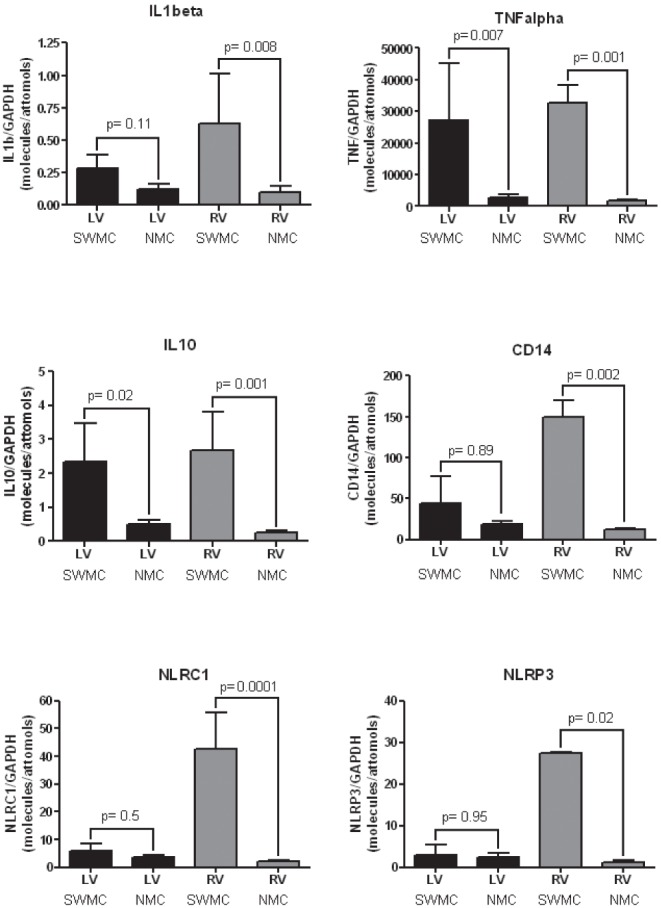
RT-PCR left and right ventricular mRNA statistical results for IL1β, TNF α, IL10, CD14, NLCR1 and NLRP3 in SWMC and NMC subjects.
For the inflammasomes, NLRC1 (42.6 ± 18.4 versus 2.2 ± 1.7, p=0.0001) and NLRP3 (27.4 ± 0.41 versus 1.1 ± 1.6, p=0.02) were up-regulated significantly in the right ventricle from SWMC v NMC residents. No differences were measured between SWMC v NMC residents for the left ventricle for either NLRC1 or NLRP3 (p=0.5 and 0.95, respectively).
Signaling pathways related to inflammasomes
Myocardial samples from SWMC residents exhibited a significant up-regulation of 29 of 84 genes in the array. The up-regulated genes fell into the following categories: 16 downstream from the NOD-like receptors, 5 inflammasome negative regulation, 5 downstream from inflammasome pathways, two inflammatory caspases, and one NOD-like receptor (Table 3). The four genes with the higher expression were: IRF-1 binding to the upstream regulatory region of type I IFN and IFN-inducible MHC class I genes, CCL-2 (a monocytic and basophil chemotactic factor), CXCL1 (a chemotactic neutrophil factor), and TNF.
Table 3. Inflammasomes Signaling Pathway Genes Up-regulated in Myocardial SWMC versus NMC Samples.

Discussion
Exposures to distinct air pollution environments within Mexico City are associated with significant differences in the expression of key inflammatory and anti-inflammatory cytokines including IL-1β, TNF-α, IL-10, the LPS receptor CD14 and two key inflammasomes NLRC1 and NLRP3 within the hearts of a unique young autopsy cohort. Specifically, significant differences were observed between SWMC v NMC residents, two areas characterized by sustained differences in the outdoor concentration of PM associated with LPS and in PM 2.5 concentrations. The results suggest a residency effect similar to the one observed in our SWMC v NMC mice studies8.
Inflammation involves a coordinated immune response to stimuli related to infections, oxidative stress, or tissue damage16,17. The innate immune system rapidly detects invading pathogenic microbes and eliminates them. In the case of SWMC residents PM with LPS likely initiates an inflammatory response having detrimental effects8. Toll-like receptors sense “extracellular microbes” (i.e., PM-LPS) and trigger anti-pathogen signaling cascades17. Heart tissues require up-regulation of several inflammasome components in order to assemble functional inflammasomes. Although the issue of myocardial inflammasomes has not yet been discussed in air pollution-related human cardiotoxicity, the finding of increased IL-1β, CD14, NLRC1 and NLRP3 strongly suggests that inflammasome activation plays a role in exposed subjects.
Up-regulation of two key NOD-like receptor (NLR) family members in the right ventricles from clinically healthy children and young adults is critical in the context of air pollution exposures. NLRC1 enhances caspase-9-mediated apoptosis, induces NF-kappa-B activity via RIPK2 and IKK-gamma and confers responsiveness to intracellular bacterial LPS, while NLRP3 functions as an upstream activator of NF-kappa-B signaling and activates caspase-1 in response to a number of triggers including LPS which leads to processing and release of IL1β16, 17. The central role of the NLR family as pathogen sensors and activators of inflammatory caspases and transcriptional regulation of immune response genes17,18,19,20, including pro-inflammatory cytokines with detrimental cardiac effects, raises the issue of the long-term impact of the innate immune altered responses and inflammation upon the cardiovascular system of highly exposed young individuals.
Exposures to micro-particles, including asbestos and silica, activate the NALP3 inflammasome, and NALP3 deficient mice have decreased lung inflammatory responses21,22,23. Cholesterol crystals acting as an endogenous danger signal activate NLRP3 inflammasomes in a mice atherogenesis model24. NLRP3/NALP3 activators produce reactive oxygen species (ROS) and are essential secondary messengers signaling inflammasome activation18. Thus, inflammasomes are rapidly emerging in air pollution related mechanisms as novel and important cellular complexes involved in inflammatory responses.
As expected, based on the higher historical south Mexico City exposures to endotoxins, the mRNA expression of CD14 was significantly higher in SWMC residents and the right ventricle was the target of the up-regulation. Indeed, the right ventricle expression of the target genes was significantly different for all selected genes, while the left ventricle differences between cohorts were only significant for TNF-α and IL-10. Cardiac chambers differ in their morphologic and contractile properties25 and in myocardial circulation26. In a heart with increased afterload there is a sharp arterial plethora of the left ventricle and sharp blood stasis in the microcirculatory bed in case of increased right ventricle afterload26. These contractile and microcirculatory changes might contribute to the sharp differences between ventricular gene expression in subjects exposed to high levels of pollution. Moreover, PM exposure increases pulmonary arterial pressure related to the production of endothelin-1 (ET-1) 27,28,29,30,31,32,33, thus right ventricular pressure-overload potentially could modify the targeted genes responses. The issue is key for young urban residents, as we have seen sustained elevations of ET-1 and pulmonary arterial pressures in clinically healthy children in Mexico City32. To complicate matters further, the vasoconstrictor responses upon LPS administration are associated to ET-1 production34.
Degranulation of mast cells was observed in the epicardial left and right ventricle nerve bundles. Because portions of autonomic nerves and receptors are located on the epicardial surface of the heart35,36,37,38, it is possible that the mast cell degranulation may alter autonomic neurotransmission39 providing a potential mechanism by which the nervous system impacts on inflammatory ventricular responses. Mast cells had been largely neglected and remain as low key players in the air pollution literature, however there are significant associations between ET-1 and cardiac mast cell degranulation30, their role limiting ET-1 toxicity 40,41 and modulation of cardiac contractility through both ET-1 and myocardial mast cell degranulation42. Our canine studies in Mexico City versus controls from low polluted areas showed apoptotic myocytes and degranulated mast cells associated with scattered foci of mononuclear cells in both ventricles and the interventricular septum 43. Thus, the potential inflammatory and physiopathological impact of the mast cell degranulation both in the myocardium and epicardial nerves are critical.
We have shown in young Mexico City residents, a distinct right versus left inflammatory response of the carotid portion of the vagus nerve, specifically the right vagus has a marked up-regulation of COX-244. The role of the right vagus nerve in the innervation of key structures (i.e., liver and bowel) involved in the detoxification and clearance of foreign and altered-self substances including PM and PM-LPS was discussed. Thus, the cardiac vagal innervation could also play a role in the differential ventricular responses44.
Pro-inflammatory mediators, such as TNF-α and IL-1β have been implicated in the pathogenesis of myocardial dysfunction and cardiomyocyte death in ischemia-reperfusion injury, sepsis, chronic heart failure, viral myocarditis, and cardiac allograft rejection45,46,47. TNF-α was strongly up-regulated in SWMC exposed residents (p=0.001). The importance of TNF in cardiovascular morbidity and mortality is well known46,47. In a healthy heart TNF-α is mainly located in the endothelium and in resident mast cells47. Apoptosis, inflammation and oxidative stress are pivotal TNF-mediated responses that are independently linked to pathological remodeling46. During myocardial ischemia and after myocardial infarction (MI), preformed TNF-α is released within minutes and contributes both to contractile dysfunction and irreversible myocardial injury47. Interestingly, preconditioning with TNF-α decreases infarct size48. The issue of TNF-α cardioprotective properties depends on a number of factors, including its concentration, the localization of the increased TNF-α levels, the concentrations of the TNF receptors, particularly TNFR1, and the myocardial duration of exposures to detrimental factors47. In an infarcted myocardium, TNF-α contributes to cardiomyocyte apoptosis, whereas in the peri-infarct area could stimulate fibroblasts, stabilize the infarcted area and attract stem cells for cardiac repair and decrease inflammation49,50. Given that TNF-α has an ambivalent role in case of myocardial infarction and that sustained post-infarction TNF-α contributes to chronic left ventricular dysfunction, it was concluded that myocardial TNF-α biventricular up-regulation in the context of severe exposures to air pollution is not beneficial to the exposed subjects.
The higher level of CD14 mRNA in SWMC residents who are exposed to a higher dose of LPS-PM is a novel and potentially important observation. CD14 is a surface differentiation antigen capable of binding LPS51 and the high expression seen in SWMC subjects is likely related to the high concentrations of LPS detected in south Mexico City PM10 samples5,6,14. The toxic effects of LPS include the release of cytokines, nitric oxide, and reactive oxygen species (ROS) by vascular endothelial cells52,53. LPS-induced cardiac dysfunction may be in part due to reactive oxygen species mediated by inflammatory mediators like TNF-α53,54. LPS acts through the CD14 receptor to release TNF-α, deregulates the intracellular calcium, and gives rise to the apoptotic death program55. LPS internalization depends on CD14, as shown in Panaro et al. in an in vitro model of myocardial cells exposed to endotoxin51..
The production of pro-inflammatory mediators occurs in the myocardium exposed to endotoxin, a situation that is critical in septic patients. In a model of low-grade chronic inflammation with the administration of low doses of LPS, there was a significant increase in myocardial fibrosis, infiltration of mononuclear cells, and changes in arteries and arterioles, a finding consistent with vascular disease56. Given that SWMC residents are exposed to high concentrations of PM-LPS, the observation of their significant up-regulation of CD14, TNF-α and IL-1β suggests a key role of environmental endotoxin on the health of the southern Mexico City population. The issue is key for the understanding of how the sensing of “microbial invaders” (i.e., PM-LPS) could translate into signaling pathways that culminate in the transcriptional regulation of immune responsive genes and how the activation of inflammasomes17,19,57 could be a contributing factor for myocardial cardiotoxicity58.
Thus, this study’s observations in SWMC young adults are potentially important for elderly SWMC residents due to the fact that IL-1β reduces cardiac muscle function and inhibits angiogenesis in cardiac endothelial cells59. An increase in myocardial IL-1β could translate in a reduced capacity for proliferation of microvascular endothelial cells and a consequent fault in the myocardial repair after a myocardial infarction. IL-1β is also important to myocardial remodeling60, and its increased levels are associated with worsening of interstitial fibrosis61. The roles of IL-1β in atherothrombotic disease and after myocardial infarction when it critically regulates the inflammatory response62 are also potentially clinically important for SWMC residents.
Chronic inflammation leads to an increase in cardiovascular disease risk1. The findings of significant differences in up-regulation of key inflammatory myocardial genes and inflammasomes in healthy young adults provide potential important mechanistic pathways to explain the higher risk of cardiovascular disease in susceptible urban populations. These findings are relevant to susceptible residents in the study areas, since the outcome of cardiac ischemic events depends not only on the intensity and duration of the ischemic stimulus but also on the myocardial intrinsic tolerance to ischemic injury58, even in the absence of manifest cardiovascular disease. Thus, the concept of occult cardiotoxicity as described by Golomb et al. should be taken into account in subjects exposed to significant concentrations of air pollutants.
We recognized that given our strict inclusion criteria including the negative Asp299Gly TLR4 polymorphism, the autopsy time restricted to 3.7 ± 1.7 hours after death, and the age range of the subjects, the group is small, however the endpoint results are significantly different to warrant that residency likely plays a key factor in the myocardial responses. Additional characterization of the particle-like material observed in blood vessels by energy filtered TEM63 would have benefited these studies.
In summary, exposure to air pollution produces differential up-regulation of key inflammatory genes in young urban residents. Extensive degranulation of mast cells complete the picture in seemingly healthy hearts from children and young adults. Exposure to particulate matter can trigger cardiovascular disease64and long exposures increase the risk for cardiovascular mortality and reduces life expectancy1, thus our findings of myocardial inflammation in young urbanites may have deleterious CV effects, especially in view of our reports that sustained endothelin-1 increases are associated with elevated mean pulmonary artery pressure in clinically Mexico City healthy children32.
Hopefully this research will contribute: i) to awareness that within-city sustained exposures to a distinct profile of air pollutants produce differential responses in myocardial gene expression, thus the clinical outcomes of cardiac events could be different ii) sustained myocardial up-regulation of IL-1β and TNF-α, fundamental in the pathogenesis of myocardial dysfunction, ventricular remodeling, and angiogenesis in cardiac endothelial cells—among their other roles—likely will have long term cardiovascular effects on the exposed populations, iii) occult cardiotoxicity is an important concept in the context of air pollution exposure, and iv) inflammasome myocardial activation is a novel pathway of heart injury in exposed individuals.
Acknowledgments
This work was supported in part by the NCRR Grant # P20RRO15583. This work was presented in part at the 2010 FASEB meeting at Anaheim, CA by Rodolfo Villarreal-Calderon.
References
- 1.Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Jr, Whitsel L, Kaufman JD. Particulate matter air pollution and cardiovascular disease. An update to the scientific statement from the American Heart Association. Circulation. 121: 2331–2378 2010. [DOI] [PubMed] [Google Scholar]
- 2.Franchini M, Mannucci PM. Thrombogenicity and cardiovascular effects of ambient air pollution. Blood. 118: 2405–2412 2011. [DOI] [PubMed] [Google Scholar]
- 3.Bravo-Alvarez HR, Torres-Jardón RJ. Air pollution levels and trends in the Mexico city metropolitan area. In: Urban Air Pollution and Forests: Resources at Risk in the Mexico City Air Basin Ecological Studies. M Fenn, L Bauer, T Hernández (eds). Vol 156. Springer-Verlag; New York: 121–159. 2002 [Google Scholar]
- 4.Molina LT, Madronich S, Gaffney JS, Apel E, de Foy B, Fast J, Ferrare R, Herndon S, Jimenez JL, Lamb B, Osorio-Vargas AR, Russell P, Schauer JJ, Stevens PS, Volkamer R, Zavala M. An overview of the MILAGRO 2006 Campaign: Mexico City emissions and their transport and transformation. Atmos Chem Phys. 10:8697–8760 2010. [Google Scholar]
- 5.Osornio-Vargas AR, Bonner JC, Alfaro-Moreno E, Martinez L, Garcia-Cuellar C, Ponce-de-Leon-Rosales S, Miranda J, Rosas I. Proinflammatory and cytotoxic effects of Mexico City air pollution particulate matter in vitro are dependent on particle size and composition. Environ Health Perspect. 111:1289–1293 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosas-Pérez I, Serrano J, Alfaro-Moreno E, Baumgardner D, Garcia-Cuellar C, Martin del campo JM, Raga GB, Castillejos M, Colin RD, and Osornio-Vargas AR. Relations between PM10 composition and cell toxicity: A multivariate and graphical Approach. Chemosphere. 67: 1218–1228 2007. [DOI] [PubMed] [Google Scholar]
- 7.Querol X, Pey J, Minguillón MC, Pérez N, Alastuey A, Viana M, Moreno T, Bernabe RM, Blanco S, Cardenas B, Vega E, Sosa G, Escalona S, Ruiz H, Artiñano B. PM speciation and sources in Mexico during the MILAGRO-2006 Campaign. Atmos Chem Phys. 8: 111–128 2008. [Google Scholar]
- 8.Villarreal-Calderon R, Reed W, Keefe S, Herritt L, Brooks D, Torres-Jardón R, Calderón-Garcidueñas L. Urban air pollution produces up-regulation of myocardial inflammatory genes and dark chocolate provides cardioprotection. Exp Tox Path. 2010. October 5 E Pub. [DOI] [PMC free article] [PubMed]
- 9.Garantziotis S, Hollingsworth JW, Zaas AK, Schwartz DA. The effect of toll-like receptors and toll-like receptors genetics in human disease. Ann Rev Med. 59: 343–359 2008. [DOI] [PubMed] [Google Scholar]
- 10.Panaro MA, Gagliardi N, Saponaro C, Calvello R, Mitolo I, Cianciulli A. Toll-like receptor 4 mediates LPS-induced release of nitric oxide and tumor necrosis factor-alpha by embryonal cardiomyocytes:biological significance and clinical implications in human pathology. Curr Pharm Des. 16: 766–774 2010. [DOI] [PubMed] [Google Scholar]
- 11.SMA Secretaría del Medio Ambiente del Gobierno del Distrito Federal Dirección General de Gestión de la Calidad del Aire. Sistema de monitoreo Atmosférico de la ciudad de México. Dirección de Monitoreo Atmosférico. 2008. Indicadores 2010. http://www.sma.df.gob.mx/simat2/
- 12.Calderón-Segura ME, Gómez-Arroyo S, Villalobos-Pietrini R, Butterworth FM, Amador-Munoz O. The effects of seasonal weather on the genotoxicity, cytokinetic properties, cytotoxicity and organochemical content of extracts of airborne particulates in Mexico City. Mutat Res. 558: 7–17 2004. [DOI] [PubMed] [Google Scholar]
- 13.Dzepina K, Arey J, Marr L, Worsnop DR, Salcedo D, Zhang Q, Onasch TB, Molina LT, Molina MJ, Jimenez JL. Detection of particle-phase polycyclic aromatic hydrocarbons in Mexico City using an aerosol mass spectrometer. International Journal of Mass Spectrometry. 263: 152–170 2007. [Google Scholar]
- 14.Estrada-Garcia T, Cerna JF, Thompson MR, López-Saucedo C. Fecal contamination and enterotoxigenic Escherichia coli in street-vended chili sauces in Mexico and its public relevance. Epidemiology and Infection. 129: 223–226 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riveros-Rosas H, Pfeifer GD, Lynam DR, Pedroza JL, Julian-Sanchez A, Canales O, Garfias J.Personal exposure to elements in Mexico city air. Sci Total Environ. 198: 79–96 1997. [DOI] [PubMed] [Google Scholar]
- 16.Stutz A, Golenbock DT, Latz E. Inflammasomes: too big to miss. J Clin Invest. 119:3502–3511 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinon F, Mayor A, Tschopp J.The inflammasomes: guardians of the body. Annual Review of Immunology. 27: 229–265 2009. [DOI] [PubMed] [Google Scholar]
- 18.Martinon F.Signaling by ROS drives inflammasome activation. Eur J Immunol. 40: 616-619 2010. [DOI] [PubMed] [Google Scholar]
- 19.Yin Y, Yan Y, Jiang X, Mai J, Chen NC, Wang H, Yang XF. Inflammasomes are differentially expressed in cardiovascular and other tissues. International Journal of Immunopathology and Pharmacology. 22:311–322 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bryant C, Fitzgerald KA. Molecular mechanisms involved in inflammasome activation. Trends in Cell Biology. 19: 455–464 2009. [DOI] [PubMed] [Google Scholar]
- 21.Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci USA. 105: 9035–9040 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dostert C, Petrilli V, van Bruggen R, Steel C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 320: 674–677 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H. Silica crystals and aluminum salts activate the NAP3 inflammasome through phagosomal destabilization. Nat Immunol. 9: 847–856 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 464: 1357–1361 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holland MR, Gibson AA, Kirshner CA, Hicks D, Ludomirsky A, Singh GK. Intrinsic myoarchitectural differences between the left and right ventricles of fetal human hearts: an ultrasonic backscatter feasibility study. Journal of the American Society of Echocardiography. 22: 170–176 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tverskaya MS, Sukhoparova VV, Karpova VV, Raksha AP, Kadyrova MK, Abdulkerimova NZ, Bobrova NA. Pathomorphology of myocardial circulation: comparative study in increased left or right ventricle overload. Bulletin of Experimental Biology and Medicine. 145: 377–381 2008. [DOI] [PubMed] [Google Scholar]
- 27.Bouthillier L, Vincent R, Goegan P, Adamson IY, Bjarnason S, Stewart M, Guénette J, Potvin M, Kumarathasan P. Acute effects of inhaled urban particles and ozone: lung morphology, macrophage activity, and plasma endothelin-1. Am J Pathol. 153:1873–1884 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang YJ, Li Y, Zhou Z, Roberts AM, Cai L, Myers SR, Wang L, Schuchke DA. Elevation of serum endothelins and cardiotoxicity induced by particulate matter (PM2.5) in rats with acute myocardial infarction. Cardiovasc Toxicol. 2: 253–261 2002. [DOI] [PubMed] [Google Scholar]
- 29.Douthwaite JA, Lees DM, Corder R. A role for increased mRNA stability in the induction of endothelin-1 synthesis by lipopolysaccharide. Biochem Pharmacol. 66: 589–594 2003. [DOI] [PubMed] [Google Scholar]
- 30.Murray DB, Gardner JD, Brower GL, Janicki JS. Endothelin-1 mediates cardiac mast cell degranulation, matrix metalloproteinase activation, and myocardial remodeling in rats. Am J Physiol Heart Circ Physiol. 287: H2295–2299 2004. [DOI] [PubMed] [Google Scholar]
- 31.Thomson E, Kumarathasan P, Goegan P, Aubin RA, Vincent R. Differential regulation of the lung endothelin system by urban particulate matter and ozone. Toxicol Sci. 88: 103–113 2005. [DOI] [PubMed] [Google Scholar]
- 32.Calderón-Garcidueñas L, Vincent R, Mora-Tiscareño A, Franco-Lira M, Henríquez-Roldán C, Garrido-García L, Camacho-Reyes L, Valencia-Salazar G, Paredes R, Romero L, Osnaya N, Villarreal-Calderon R, Torres-Jardón R, Hazucha MJ, Reed W. Elevated plasma endothelin-1 and pulmonary arterial pressure in children exposed to air pollution. Environ Health Perspect. 115: 1248–1253 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deacon K, Knox AJ. Endothelin-1 (ET-1) increases the expression of remodeling genes in vascular smooth muscle through linked calcium and cyclic-adenosine mono-phosphate (CAMP) pathways: role of a phospholipase A2(cPLA2)/cyclo-oxygenase-2 (COX-2)/prostacyclin receptor dependent autocrine loop. J Biol Chem. 285: 25913–25927 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mansart A, Ruff LJ, Arians MP, Ross JJ, Reilly CS, Brown NJ, Kaufman S, Brookes ZL. Constriction of rat extra-splenic veins to lipopolysaccharide involves endothelin-1. Naunyn Schmiedebergs Arch Pharmacol. 381: 555–562 2010. [DOI] [PubMed] [Google Scholar]
- 35.Marron K, Wharton J, Sheppard MN, Fagan D, Royston D, Kuhn DM, de Leval MR, Whitehead BF, Anderson RH, Polak JM. Distribution, morphology, and neurochemistry of endocardial and epicardial nerve terminal arborizations in the human heart. Circulation. 92: 2343–2351 1995. [DOI] [PubMed] [Google Scholar]
- 36.Sola OM, Shi Q, Vernon RB, Lazzara RR. Cardiac denervation after transmyocardial laser. Ann Thorac Surg. 71: 732 2001. [PubMed] [Google Scholar]
- 37.Accord RE, van Suylen RJ, van Brakel TJ, Maessen JG. Post-mortem histological evaluation of microwave lesions alter epicardial pulmonary vein isolation for atrial fibrillation. Ann Thoracic Surg. 80: 881–887 2005. [DOI] [PubMed] [Google Scholar]
- 38.Kulboka A, Lekas R, Veikutis V, Civinskiene G, Pavilonis A.Changes of cardiac electrophysiological parameters after destruction of pericardial nervous plexi innervating sinoatrial model. Kardiologiia. 45: 11–14 2005. [PubMed] [Google Scholar]
- 39.Levick SP, Murray DB, Janicki JS, Brower GL. Sympathetic nervous system modulation of inflammation and remodeling in the hypertensive heart. Hypertension. 55: 270–276 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maurer M, Wedemeyer J, Metz M, Piliponsky AM, Weller K, Chatterjea D, Cloutheir DE, Yanagisawa MM, Tsai M, Galli SJ. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature. 432: 512–516 2004. [DOI] [PubMed] [Google Scholar]
- 41.Walsh SK, Kane KA, Wainwright CL. Mast cell degranulation-a mechanism for the anti-arrhythmic effect of endothelin-1? Br J Pharmacol. 157: 716–723 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eszlári E, Czóbel M, Molnar G, Kaszaki J, Nagy S, Boros M.Modulation of cardiac contractility through endothelin-1 release and myocardial mast cell degranulation. Acta Physiol Hung. 95: 267–285 2008. [DOI] [PubMed] [Google Scholar]
- 43.Calderón-Garcidueñas L, Gambling TM, Acuna H, Garcia R, Osnaya N, Monroy S, Villarreal-Calderon A, Carson J, Koren HS, Devlin RB. Canines as sentinel species for assessing chronic exposures to air pollutants: part 2. Cardiac pathology. Toxicol Sci. 61: 356–367 2001. [DOI] [PubMed] [Google Scholar]
- 44.Calderón-Garcidueñas L, Solt AC, Henríquez-Roldán C, Torres-Jardón R, Nuse B, Herritt L, Villarreal-Calderon R, Osnaya N, Garcia R, Brooks DM, Gonzalez-Maciel A, Reynoso-Robles R, Delgado-Chavez R, Reed W. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol. 36: 289–310 2008. [DOI] [PubMed] [Google Scholar]
- 45.Cain BS, Meldrum DR, Dinarello CA, Meng X, Joo KS, Banerjee A, Harken AH. Tumor necrosis factor-alpha and interleukin-1-beta synergistically depress human myocardial function. Critical Care Medicine. 27: 1309–1318 1999. [DOI] [PubMed] [Google Scholar]
- 46.Hamid T, Gu Y, Ortines RV, Bhattacharya C, Wang G, Xuan YT, Prabhu SD. Divergent tumor necrosis factor receptor-related remodeling responses in heart failure: role of nuclear factor kappa B and inflammatory activation. Circulation. 119:1386–1397 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schulz R, Heusch G.Tumor necrosis factor-alpha and its receptors 1 and 2. Circulation. 119: 1355–1357 2009. [DOI] [PubMed] [Google Scholar]
- 48.Schulz R.TNF α in myocardial ischemia/reperfusion: Damage vs. protection. Journal of Molecular and Cell Cardiology. 45: 712–714 2008. [DOI] [PubMed] [Google Scholar]
- 49.Chen Y, Ke Q, Yang Y, Rana JS, Tang J, Morgan JP, Xiao YF. Cardiomyocytes overexpressing TNF-alpha attract migration of embryonic stem cells via activation of p38 and c-Jun amino-terminal kinase. FASEB J. 17: 2231–2239 2003. [DOI] [PubMed] [Google Scholar]
- 50.Bao C, Guo J, Lin G, Hu Z.TNFR gene-modified mesenchymal stem cells attenuate inflammation and cardiac dysfunction following MI. Scandinavian Cardiovascular Journal. 42: 56–62 2008. [DOI] [PubMed] [Google Scholar]
- 51.Panaro MA, Cianciulli A, Gagliardi N, Mitolo I, Acquafredda A, Cavallo P, Mitolo V. CD14 major role during lipopolysacchride-induced inflammation in chick embryo cardiomyocytes. Federation European Microbiological Societies Immunology and Medical Microbiology. 53: 35–45 2008 [DOI] [PubMed] [Google Scholar]
- 52.Fitzgerald KA, Rowe DC, Golenbock DT. Endotoxin recognition and signal transduction by the TLR4/MD2-complex. Microbes and Infection. 6: 1361–1367 2004. [DOI] [PubMed] [Google Scholar]
- 53.Rudiger A, Singer M.Mechanisms of sepsis-induced cardiac dysfunction. Critical Care Medicine. 35:1599–1608 2007. [DOI] [PubMed] [Google Scholar]
- 54.Meldrum DR. Tumor necrosis factor in the heart. American Journal of Physiology. 274: R577–R595 1998/ [DOI] [PubMed] [Google Scholar]
- 55.Comstock KL, Krown KA, Page MT. LPS-induced TNF-alpha release from and apoptosis in rat cardiomyocytes: obligatory role for CD14 in mediating the LPS response. J Mol Cell Cardiol. 30: 2761–2775 1998. [DOI] [PubMed] [Google Scholar]
- 56.Smith BJ, Lightfoot SA, Lerner MR, Denson KD, Morgan DL, Hanas JS, Bronze MS, Postier RG, Brackett DJ. Induction of cardiovascular pathology in a novel model of low-grade chronic inflammation. Cardiovascular Pathology. 18: 1–10 2009. [DOI] [PubMed] [Google Scholar]
- 57.Lamkanfi M, Dixit VM. Inflammasomes: guardians of cytosolic sanctity. Immunological Reviews. 227: 95–105 2009. [DOI] [PubMed] [Google Scholar]
- 58.Golomb E, Nyska A, Schwalb H.Occult cardiotoxicity-toxic effects on cardiac ischemic tolerance. Toxicologic Pathology. 37:572–593 2009. [DOI] [PubMed] [Google Scholar]
- 59.Mountain DJH, Singh M, Singh K.Interleukin-1β-mediated inhibition of the processes of angiogenesis in cardiac microvascular endothelial cells. Life Sciences. 82:1224–1230 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hwang MW, Matsumori A, Furukawa Y, Ono Y, Okada M, Iwasaki A, Hara M, Miyamoto T, Touma M, Sasayama S. Neutralization of IL1β in the acute phase of myocardial infarction promotes the promotion of progression of left ventricular remodeling. Journal of American College of Cardiology. 38: 1546–1553 2001. [DOI] [PubMed] [Google Scholar]
- 61.Ono K, Matsumori A, Shioi T, Furukawa Y, Sasayuma S.Cytokine gene expression after myocardial infarction in rat hearts: possible implication in left ventricular remodeling. Circulation. 98: 149–156 1998. [DOI] [PubMed] [Google Scholar]
- 62.Bujak M, Frangogiannis NG. The role of IL-1 in the pathogenesis of heart disease. Archivum Immunologiae et Therapiae Experimentalis. 57: 165–176 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brandenberger C, Clift MJD, Vanhecke D, Mühlfeld C, Stone V, Gehr P, Rothen-Rutishauser B. Intracellular imaging of nanoparticles: Is it an elemental mistake to believe what you see? Part Fibre Toxicol. 7: 15–20 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nawrot TS, Perez L, Künzli N, Munters E, Nemery B. Public Health importance of triggers of myocardial infarction: a comparative risk assessment. Lancet. 377: 732–740 2011. [DOI] [PubMed] [Google Scholar]