Abstract
The cause of death in a postsmolt, Atlantic salmon population with elevated levels of mortalities was investigated. Diagnosis of a rickettsia-like organism was based on gross pathology, histopathology, differential staining, electron microscopy and fluorescent antibody tests. The course of the infection and response to treatment are discussed. This is the first reported occurrence of salmon rickettsias in the Atlantic coast of North or South America.
Introduction
In 1989, a large number of farmed Coho salmon (Oncorhynchus kisutch) mortalities occurred in Chile (1) and, at that time, researchers were unsure of the etiologic agent. In 1990, Fryer et al (2) isolated the organism in a chinook salmon embryo cell line and classified it as a rickettsia. The pathogenicity of this organism was confirmed in 1991 by the fulfilment of Koch's postulates (3). The Chilean isolate has since been classified as Piscirickettsia salmonis, a new rickettsial genus and species (4). In 1994, rickettsial infections were reported to have caused $50 million in losses to the Chilean salmon industry (5). Rickettsias have since been recognized as significant pathogens of farmed salmonids in many geographic areas where salmon are cultured. North America's first diagnosed case of salmonid rickettsiosis was reported in 1992 (6). In this instance, low numbers of mortalities occurred in a population of farmed Atlantic salmon (Salmo salar) in British Columbia. In addition to Chile and British Columbia, cases of infected salmonids have been reported in Scotland, Ireland, and Norway (1,2,5,6,7,8,9,10). Susceptible salmonids include Coho salmon, steelhead trout (Oncorhynchus mykiss), chinook salmon (Oncorhynchus tshawytscha), and Atlantic salmon (1,2,3,5,6,11,12). Rickettsial infections have been reported in other aquatic species, including tilapia (Oreochromis spp.) (13), dragonets (Callionymus lyra) (14), blue-eyed plecostomus (Paneque suttoni) (15), sea scallops (Pecten sp.) (16, 17), Pacific oysters (Crassostrea sp.) (18), and black abalone (Haliotis cracherodii) (19). This paper describes the first outbreak of a rickettsial disease in farmed Atlantic salmon in eastern North America.
Materials and methods
In late September 1996, an Atlantic salmon farmer complained of a slight elevation in the number of mortalities in a cohort of 40 000 animals. These fish were a November 1994 spawn and were transferred to sea pens in the spring of 1996. Fish were held in 2, 60-meter circumference plastic cages. Water temperature was 16°C and oxygen saturation between 55% and 85%. Fish were fed dry feed, ad libitum. The fish of cage A were vaccinated with an oil-based vaccine against Vibrio anguillarum, Vibrio salmonicida, and Aeromonas salmonicida. Fish in cage B were not vaccinated. The average weights of fish in the vaccinated and nonvaccinated cages were 330 and 166 g, respectively.
The vast majority of fish appeared to be behaving normally, forming a tight, cohesive school. An estimated 100 to 200 fish per cage, however, appeared lethargic, were situated away from the main school, and were oriented toward the cage netting and against the water current. The majority of fish had good appetite but the lethargic fish were nonresponsive.
Initial sample collection
Moribund fish were euthanized in tricaine methane sulfonate (TMS). Gross pathological changes were noted. Portions of brain, gill, liver, spleen, heart, pancreas, pyloric ceca, skin, muscle, and kidney were excised from affected fish and fixed in 10% neutral buffered formalin. These tissues were trimmed into cassettes, dehydrated in graded ethanol solutions, cleared in xylene, and embedded in paraffin wax. Sections were cut to 5 μm and stained with hematoxylin and eosin, Gram, and toluidine blue stains.
Kidney was cultured on 2% salt-fortified blood and tryptic soy agar and incubated for 2 wk at 22°C. Kidney was also streaked on selective kidney disease medium (20) and incubated at 15°C for 6 wk. Impressions from kidney were made on glass slides and stained for identification of Renibacterium salmoninarum by using an indirect fluorescent antibody technique (IFAT) and for general cytology by using Giemsa stain.
Follow-up sample collection
Follow-up samples were collected 1 wk after the initial sampling. Moribund fish were euthanized by an overdose of TMS. Liver and kidney with visible gross pathological changes (ecchymotic hemorrhages and liver nodules) were aseptically removed and placed in a sterile plastic bag. Impression smears of the collected organs were made on glass slides and fixed in 100% methanol. Matching pieces (1 mm3) were fixed in 2% phosphate-buffered glutaraldehyde for 1 h, then placed in phosphate-buffered saline (PBS). All pieces were placed on ice, transported to the laboratory, processed for electron microscopy, and embedded in Epo-Araldite (Canemco, Montreal, Quebec). Ultrathin sections of affected pieces were stained in uranyl acetate and lead citrate and examined by using a transmission electron microscope (Hitachi-600; Nissei Sangyo, Rexdale, Ontario) at 75 kV.
Indirect fluorescent antibody test for Piscirickettsia salmonis
A rabbit antiserum to the type strain of P. salmonis [LF89, ATCC: V(R)1361] was obtained from Dr. J.L. Fryer, Oregon State University, Corvallis, Oregon, USA. A second antiserum against LF89 was raised in rabbits by repeated immunizations with cultured LF89 cells (21). Impression smears were fixed in acetone and incubated with diluted rabbit antisera for 30 min in a moist, dark chamber at room temperature. Impression smears were rinsed with PBS and, without drying, incubated as above with a fluorescein-conjugated (FITC; Organon Teknika-Cappel, Scarborough, Ontario) goat antirabbit immunoglobulin containing Evan's blue counterstain. Imprints were rinsed and coverslipped using FA mounting medium (pH 7.2) (Difco, Detroit, Michigan, USA). The slides were viewed at 1000X using a microscope equipped for epifluorescent microscopy and filters for FITC. Non-infected Atlantic salmon from another population were used as negative controls.
Mortality rate
The rate of mortality was recorded during the period of observed clinical disease (September to March). Treatment strategies employed during the course of the investigation included 10-day in-feed administration of 100 mg/kg body weight (BW) oxytetracycline (Terramycin-Aqua; Pfizer, Pointe-Claire, Quebec) starting on September 26 for both cages and repeated in cage B starting on Novenber 4. Fish from both cages were treated with florfenicol (Aquaflor; Schering-Plough, Pointe-Claire, Quebec) at 10 mg/kg for 10 d starting on December 24.
Results
Gross pathology
At necropsy, external examination of moribund fish revealed severe dorsal fin erosions with associated transdermal ulceration of the peripheral dorsal-lateral integument, exposing, in some specimens, the superficial musculature. Low frequency occurrence of similar, but smaller, lesions (2 to 100 mm in diameter) was noted on the flanks, ventral surfaces, and around the adipose fin. The only other external finding was pale gill filaments.
Internally, affected fish showed pathological changes consistent with septicemia, characterized by copious, clear, nonviscous ascitic and pericardial fluid; an absence of feed or digesta in the alimentary tract; multifocal, small (2 to 5 mm), raised, white nodules in the liver, which, on cut section, extended into the parenchyma and were of a soft/necrotic consistency; a demarcated hepatic lobular pattern; extensive ecchymotic hemorrhages on the serosal surface of the swim bladder, peritoneal fat, pyloric ceca, and large intestine; petechiation of visceral organs; and mild splenomegaly and nephromegaly (the latter often presenting in a grey to white mottled pattern).
The gross morphologic presentation of vaccinated fish was further complicated by fibrinous peritonitis, melanization of visceral serosal surfaces, and small amounts of bacterin within the body cavity.
Culture, fluorescent antibody, and histopathologic findings
The IFAT was negative for R. salmoninarum and no growth occurred on any of the bacteriological culture media. In mild to moderately affected fish, the liver contained multifocal areas of inflammation, randomly distributed within the parenchyma and perivascular zones. The lesions were characterized by a low frequency, single-cell necrosis and a cellular infiltrate predominated by histiocytes and granulocytes. In severely affected fish, hepatic lesions had progressed to distinct areas of coagulative necrosis. Kidneys showed moderate to severe diffuse histiocytic inflammation of interstitial hematopoietic tissue, increased interstitial melanin deposition, and thickening of the glomerular basement membrane. Cardiac lesions were characterized by mild diffuse myocytic degeneration with an associated histiocytic infiltrate that was moderately intense within the epicardium surrounding the coronary vessels. A similar mild increase in numbers of splenic histiocytes was noted. Specimens from both vaccinated and unvaccinated fish showed moderately severe, diffuse granulomatous visceral steatitis and peripancreatitis.
The most significant finding was the presence of multiple, small, pleomorphic intracytoplasmic basophilic spheres in histiocytes, macrophages, and degenerating parenchymal cells in all affected tissues (Figures 1, 2). Initially, it was hypothesized that these spheres were either bacterial cocci, rickettsial agents, or coccidial oocytes. Application of selective stains to tissue sections and impression smears indicated that the cytoplasmic inclusions stained weakly gram-negative and strongly Giemsa- and toluidine blue-positive. The pleomorphic nature of the inclusions was inconsistent with bacterial cocci or coccidial oocytes, strongly suggesting a rickettsial etiology. This hypothesis was further supported by the absence of growth on bacteriological culture media.
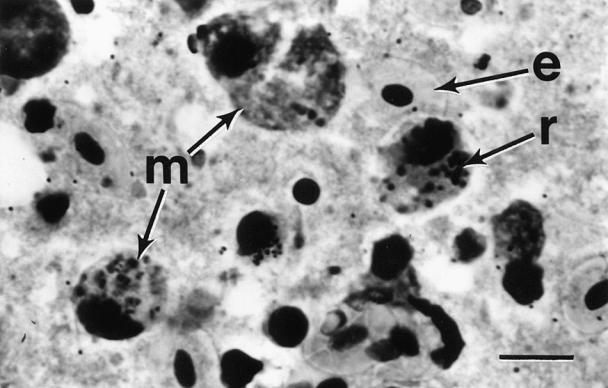
Figure 1. Photomicrograph of trunk kidney impression detailing macrophages (m) containing numerous rickettsia-like organisms (r) and erythrocytes (e). Giemsa stain; bar = 25 μm.
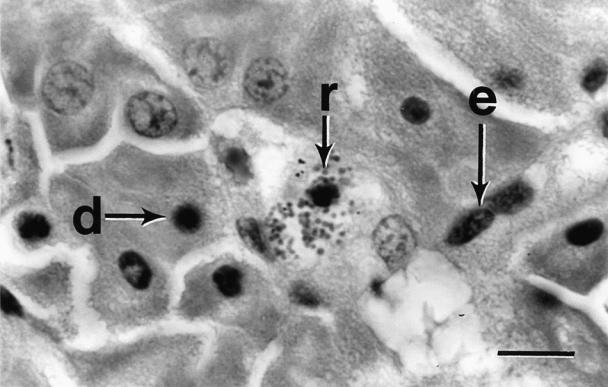
Figure 2. Photomicrograph of liver parenchyma detailing degenerating hepatocytes (d) with pyknotic nuclei and sinusoidal endothelial cells (e), some containing rickettsia-like organisms (r). Hematoxylin and eosin stain; bar = 25 μm.
Indirect fluorescent antibody test for P. salmonis
Fluorescent, small, round pleomorphic structures were observed in all sampled tissues stained with rabbit antiserum to the type strain and that made against LF89. No fluorescence was detected in tissue from uninfected salmon.
Transmission electron microscopy
Ultrastructurally, rickettsial cells were observed both intra- and extracellularly throughout kidney and liver tissue. The organisms were morphologically consistent with rickettsia-like organisms previously described by Fryer et al (2), Cvitanich et al (3), and Comps et al (22). The organisms varied in size but averaged about 1 μm in diameter, with a characteristic rippled outer membrane enclosing a smooth inner membrane, and fibrillar electron-dense material in the eccentric region (Figure 3).
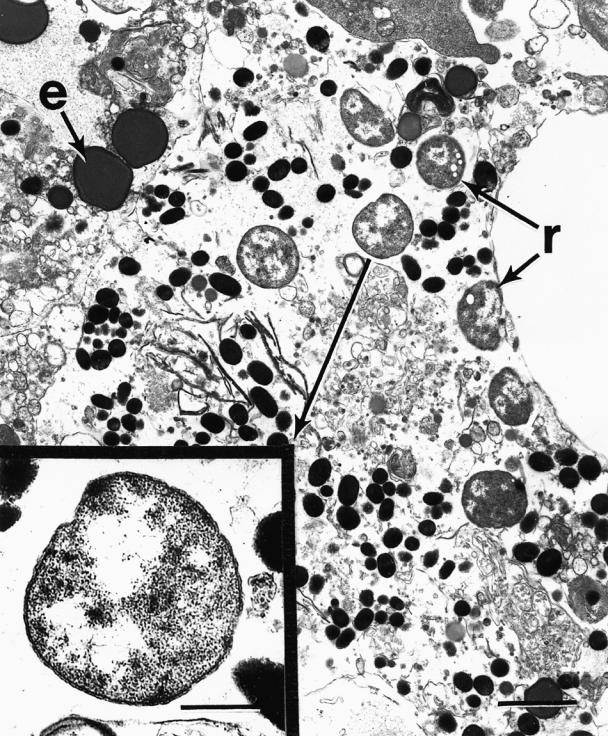
Figure 3. Electronmicrographs of a trunk kidney macrophage containing several rickettsia-like organisms (r) and erythrocytic debris (e); bar = 0.6 μm. Insert: higher magnification of rickettsia-like organism from cytoplasm of macrophage (see arrow); bar = 0.2 μm.
Mortality rate
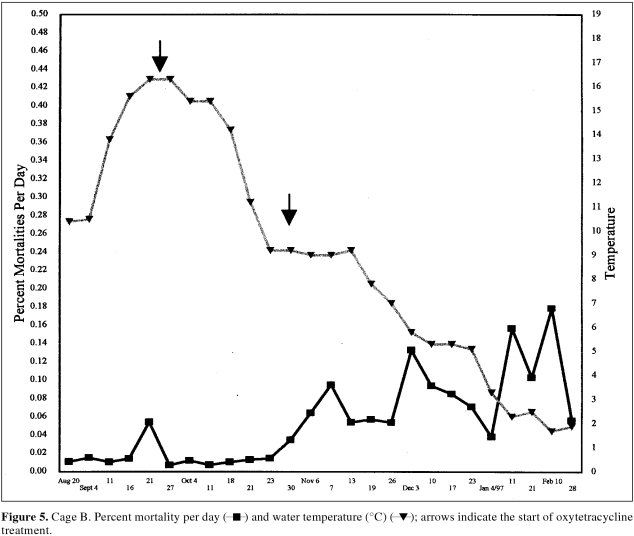
The mortality rate associated with the rickettsia-infected fish varied for the 2 affected cages (Figures 4, 5). The mortality rate began to rise much faster in cage A (vaccinated) in late September. Although fish in cage B (unvaccinated) were infected, the rate of mortality was initially much less. Treatment with oxytetracycline (100 mg/kg/d) for 10 d between September 26 and October 4 was associated with a decline in the mortality rate (Figure 4). By the end of October, however, mortality began to rise in cage B. Retreatment with oxytetracycline in this cage began in early November and was associated with a temporary reduction in the mortality rate (Figure 5). Treatment with florfenicol began on December 24. The success of this oral therapy is difficult to evaluate since the water temperature was so cold and the fish's appetite was subsequently depressed. By the spring of 1997, mortality levels returned to normal and rickettsia could no longer be detected in the suspect population.
Figure 4. Cage A. Percent mortality per day (▪) and water temperature (°C) (▴); arrow indicates the start of oxytetracycline treatment.
Figure 5. Cage B. Percent mortality per day ( ▪) and water temperature (°C) (▴); arrows indicate the start of oxytetracycline treatment.
Discussion
The combination of clinical signs and the gross and histopathologic findings has not been previously reported for salmon grown in eastern North America. Clinically, the presence of fibrinous peritonitis is a common occurrence in fish vaccinated with oil-based vaccines (23). Since nonvaccinated fish did not have this lesion, the resultant pathology could be attributed to vaccination and was not considered a problem in the construction of a rule-out list. Rule-outs for the hepatic zonal changes and nodules included exposure to toxins, severe cardiac disease, and granulomatous hepatitis caused by Renibacterium salmoninarum or fungal invasion. In eastern North America, hemorrhagic changes with resultant pericardial and peritoneal effusion usually result from bacterial septicemia due to one of many agents, including Aeromonas salmonicida, Yersinia ruckeri, Vibrio spp., and coliform and other bacteria. Viral agents causing hemorrhagic changes do occur, but in eastern North American saltwater salmonid farms these had never been reported, up to that date. Pale gills are indicative of anemia. The rule-outs for anemia include viral, bacterial, and parasitic etiologies, as well as toxic insults and neoplasms, such as lymphoblastic leukemia.
Typical bacterial agents were not isolated from suspect fish, nor was R. salmoninarum detected using the fluorescent antibody technique. Parasites or fungi were not observed in histologic sections and the gross and histopathologic findings were inconsistent with locally known viral agents (such as those causing infectious pancreatic necrosis and infectious salmon anemia). Although toxins may explain some of the lesions noted, the gross pathological, histopathologic, immunofluorescent and electron microscopic findings observed in moribund fish in this outbreak strongly support a diagnosis of rickettsiosis (1).
This is the first case of salmonid rickettsiosis along the Atlantic coast of North or South America. The observed gross and histopathologic findings described here were very similar to those described for P. salmonis in Chilean salmon by Branson and Diaz-Munoz (1) and Cvitanich et al. (3). Furthermore, the cross-reactivity of the present agent with antisera raised against P. salmonis suggested the similarity of these organisms and provided a rapid method for diagnosis. The organism described in this case was subsequently isolated in chinook embryo cells (21). Koch's postulates were fulfilled when the cultured organism was injected into Atlantic salmon, Chinook salmon (Oncorhynchus kisutch), and rainbow trout (Oncorhynchus mykiss). Mortality rates were 100%, 62%, and 22.5%, respectively (21). The organism was classified as a strain of Piscirickettsia salmonis by using the SDS-PAGE, immunoblotting, and the IFAT (21). The recent development of specific genetic primers for the identification of P. salmonis by polymerase chain reaction (PCR) (24, 25, 26) may be of further value in classification of the organism. Differentiating strains using genotyping could play a significant role in the understanding of the spread and epidemiology of rickettsial infections.
Effective oral therapy was hampered by lowering water temperatures and the presence of lethargic fish with a poor appetite. The treatments of choice for rickettsial infections in Chile are oxolinic acid and flumequine. Neither of these products are available for use in food animals in Canada. The third product of choice is florfenicol. In early December 1996, an emergency drug release for florfenicol was requested from Health Canada. On December 24, treatment began. The water temperature at the beginning of January was 3°C and the appetite of the fish was suppressed, making any further oral therapy difficult. There are no drugs approved for the treatment of rickettsial infections of fish in Canada.
The source of the infections in this outbreak remains unclear; no fish from hatcheries that supplied the site had rickettsial infections or clinical signs of this disease. Although there is no conclusive evidence regarding the source of rickettsia infecting salmonids in the Pacific Ocean, speculated sources include feral fish or shellfish species. Sources of infection in this case could also include feral salmonids, nonsalmonids, or shellfish. Processing of fresh and frozen whole fish in the same harbor as the farm may also be a source of infection. Raw effluent is passed from these processing plants directly into the Atlantic Ocean and may be a source of fish pathogens. The interspecies infectivity of rickettsia-like organisms of salmonids is not well studied.
Horizontal transmission of P. salmonis has been shown in contact and noncontact trials (27). Although experimental intraperitoneal and subcutaneous injection of P. salmonis resulted in the highest mortality rate, transmission of the pathogen across the gill or wounds in the skin are likely the most significant portals of entry (28). Temperature and stocking densities also influence mortality rates of rainbow trout infected with P. salmonis (29). Vertical transmission of P. salmonis has been shown experimentally in rainbow trout (30), but this mode of transmission seems unlikely in this case.
Predisposing stressors are often associated with rickettsial outbreaks. In Chile, storms and algal blooms have been associated with the disease (1). In the present case, strong winds associated with east coast hurricanes and record breaking rainfalls for the month of September preceded the rickettsial outbreak. In addition, the site in question is located adjacent to an industrialized area where raw sewage or industrial outfalls may occur, especially during heavy rains. These factors may play an important role in the epidemiology of the infection. Vaccines against salmonid rickettsias are not presently available in North America. Means of preventing a recurrence of this disease must include maximizing husbandry standards and the reduction of organic loading from polluting sources. Local biological factors may be important in the disease process. By the spring of 1997, mortality levels had returned to normal and rickettsias could no longer be detected in the suspect population of fish, nor has the organism been detected in Nova Scotia since the original outbreak.
In conclusion, veterinarians investigating fish mortalities at saltwater sites in northeastern North America should include rickettsias in their rule-out list. Further work to properly identify the rickettsial agent, its mode of transmission, and the source of infection is required. CVJ
Footnotes
Address correspondence and reprint requests to Dr. R.R. Cusack.
References
- 1.Branson EJ, Nieto Diaz-Munoz D. Description of a new disease condition occurring in farmed coho salmon, Oncorhynchus kisutch (Walbaum), in South America. J Fish Dis 1991;14:147–156.
- 2.Fryer JL, Lannan CN, Garcés LH, Larenas JJ, Smith PA. Isolation of a Rickettsiales-like organism from diseased coho salmon (Oncorhynchus kisutch) in Chile. Fish Pathol 1990;25:107–114.
- 3.Cvitanich JD, Garate O, Smith CE. The isolation of a rickettsia-like organism causing disease and mortality in Chilean salmonids and its confirmation by Koch's postulate. J Fish Dis 1991;14: 121–145.
- 4.Fryer JL, Lannan CN, Giovannoni SJ, Wood ND. Piscirickettsia salmonis gen. nov., sp. nov., the causative agent of an epizootic disease in salmonid fishes. Int J Sys Bacteriol 1992;42:120–126. [DOI] [PubMed]
- 5.Smith PA, Contreras JR, Garcés LH, et al. Experimental challenge of Coho salmon and rainbow trout with Piscirickettsia salmonis. J Aquat Anim Health 1996;8:130–134.
- 6.Brocklebank JR, Speare DJ, Armstrong RD, Evelyn T. Septicemia suspected to be caused by a rickettsia-like agent in farmed Atlantic salmon. Can Vet J 1992;33:407–408. [PMC free article] [PubMed]
- 7.Rodger HD, Drinan EM. Observation of a rickettsia-like organism in Atlantic salmon, Salmo salar L. in Ireland. J Fish Dis 1993;16: 361–369.
- 8.Grant AN, Brown AG, Cox DI, et al. Rickettsia-like organism in farmed salmon. Vet Rec 1996;138:423. [PubMed]
- 9.Palmer R, Ruttledge M, Callanan K, Drinan EM. A piscirickettsiosis-like disease in farmed Atlantic salmon in Ireland — isolation of the agent. Bull Eur Assoc Fish Pathol 1996;17(2): 68–72.
- 10.Olsen AB, Melby HP, Speilberg L, Evensen O, Hastein T. Piscirickettsia salmonis infection in Atlantic salmon Salmo salar in Norway — epidemiological, pathological and microbiological findings. Dis Aquat Org 1997;31:35–48.
- 11.Kent ML. Diseases of seawater netpen-reared salmonid fishes in the Pacific Northwest. Can Spec Publ Fish Aquat Sci 1992;116: 18–19.
- 12.Lannan CN, Fryer JL. Piscirickettsia salmonis a major pathogen of salmonid fish in Chile. Fish Res 1993;17:115–121.
- 13.Chern RS, Chao CB. Outbreaks of a disease caused by rickettsia-like organism in cultured tilapias in Taiwan. Fish Pathol 1994;29:61–71.
- 14.Davis AJ. A rickettsia-like organism from dragonets, Callionymus lyra L. (Teleostei: Callionymidae) in Wales. Bull Eur Assoc Fish Pathol 1986;6:103–104.
- 15.Khoo L, Dennis PM, Lewbart GA. Rickettsia-like organisms in the blue-eyed plecostomus, Paneque suttoni (Eigenmann and Eigenmann). J Fish Dis 1995;18:157–164.
- 16.LeGall G, Mialhe E, Chagot D, Grizel H. Epizootiological study of rickettsiosis of the Saint-Jacques scallop Pectin maximus. Dis Aquat Org 1991;10:139–144.
- 17.Le Gall G, Chagot D, Malhe E, Grizel H. Branchial rickettsiales-like infection associated with a mass mortality of sea scallop Pectin maximus. Dis Aquat Org 1991;10:139–145.
- 18.Meyers TR, Short S. Summer mortalities and incidental parasitisms of cultured Pacific oysters in Alaska. J Aquat Anim Health 1990;2:172–176.
- 19.Antonio DB, Andree KB, Moore JD, Friedman CS, Hedrick RP. Detection of Rickettsiales prokaryotes by in situ hybridization in black abalone, Haliotis cracherodii, with withering syndrome. J Invert Pathol 2000;75:180–182. [DOI] [PubMed]
- 20.Austin B, Embley TM, Goodfellow. Selective isolation of Renibacterium salmoninarum. FEMS Microbiol Lett 1983:111–114.
- 21.Jones SRM, Markham RJ, Groman DB, Cusack RR. Virulence and antigenic characteristics of a cultured Rickettsia-like organism isolated from Atlantic salmon Salmo salar in eastern Canada. Dis Aquat Org 1998;33(1):25–31. [DOI] [PubMed]
- 22.Comps M, Raymond JC, Plassiart GN. Rickettsia-like organism infecting juvenile sea-bass Dientrarchus labrax. Bull Eur Assoc Fish Pathol 1996;16(1):30–32.
- 23.Lillehaug A. Vaccines and vaccination procedures for Atlantic salmon. Proc Hitra Dis Workshop; St. George, New Brunswick, Canada, 1993:15–22.
- 24.Manuel MJ, Giovannoni SJ, Fryer JR. Development of polymerase chain reaction assays for detection, identification, and differentiation of Piscirickettsia salmonis. Dis Aquat Org 1996; 26:189–195.
- 25.Marshall S, Heath S, Henriquez V, Orrego C. Minimally invasive detection of Piscirickettsia salmonis in cultivated salmonids via the PCR. Appl Environ Microbiol 1998;64:3066–3069. [DOI] [PMC free article] [PubMed]
- 26.Manuel MJ, Giovannoni SJ, Fryer JR. Phylogenetic analysis of Piscirickettsia salmonis by 16S, internal transcribed spacer (ITS) and 23S ribosomal DNA sequencing. Dis Aquat Org 1999;29: 115–123. [DOI] [PubMed]
- 27.Almendras FE, Fuentealba IC, Jones SRM, Markham F, Spangler E. Experimental infection and horizontal transmission of Piscirickettsia salmonis in freshwater-raised Atlantic salmon, Salmo salar. J Fish Dis 1997;20:409–418.
- 28.Smith PA, Pizarro P, Ojeda P, Contreras J, Oyanedel S, Larenas J. Routes of entry of Piscirickettsia salmonis in rainbow trout Oncorhynchus mykiss. Dis Aquat Org 1999;29:165–172. [DOI] [PubMed]
- 29.Larenas JJ, Contreras J, Oyanedel S, Morales MA, Smith P. Efecto de la densidad poblacional y temperatura en truchas arco iris (Oncorhynchus mykiss) inoculadas con Piscirickettsia salmonis. Arch Med Vet 1997;29:113–119.
- 30.Larenas JJ, Astorga C, Contreras J, Smith P. Deteccion de Piscirickettsia salmonis en ovas fertilizadas provenientes de trucha arco iris (Oncorhynchus mykiss) experimentalmente infectadas. Arch Med Vet 1996;29:161–166.