Abstract
Objective
To examine default mode and salience network functional connectivity as a function of APOE ε4 status in a group of cognitively normal age, gender and education-matched older adults.
Design
Case-control study.
Setting
Community-based sample
Subjects
Fifty-six cognitively normal APOE ε4 carriers and 56 age, gender and education-matched cognitively normal APOE ε4 non-carriers.
Main Outcome Measure
Alterations in in-phase default mode and salience network connectivity in APOE ε4 carriers compared to APOE ε4 non-carriers ranging from 63 to 91 years of age.
Results
A posterior cingulate seed revealed decreased in-phase connectivity in regions of the posterior default mode network that included the left inferior parietal lobe, left middle temporal gyrus, and bilateral anterior temporal lobes in the ε4 carriers relative to APOE ε4 non-carriers. An anterior cingulate seed showed greater in-phase connectivity in the salience network, including the cingulate gyrus, medial prefrontal cortex, bilateral insular cortex, striatum, and thalamus in APOE ε4 carriers vs. non-carriers. There were no group-wise differences in brain anatomy.
Conclusions
We found reductions in posterior default mode network connectivity but increased salience network connectivity in elderly cognitively normal APOE ε4 carriers relative to APOE ε4 non-carriers at rest. The observation of functional alterations in connectivity in the absence of structural changes between APOE e4 carriers and non-carriers suggests that alterations in connectivity may have the potential to serve as an early biomarker.
INTRODUCTION
The study of intrinsic connectivity networks is rapidly emerging as a tool for understanding both normal brain function and pathologic states such as neurodegenerative disorders. The Default Mode Network (DMN) has been extensively studied and is one of the resting state networks that undergoes changes as a result of normal aging (1) and is also affected by Alzheimer's disease (2, 3). Brain regions that constitute the DMN include the posterior cingulate, lateral parietal, and medial frontal cortices and hippocampus. Many of these same regions also undergo preferential amyloid plaque formation in AD (4).
The APOE ε4 allele is a well established genetic susceptibility factor for late onset Alzheimer's Disease (5). Individuals who carry the APOE ε4 allele are at a three- to four-fold increased risk for developing late onset or sporadic Alzheimer's disease (6). There is also a strong relationship between APOE ε4 carrier status and beta amyloid deposition (7-9). Recently, studies have shown a relationship between amyloid burden, measured with Pittsburgh compound B (PiB), and disruption of functional connectivity of the DMN (10, 11).
Functional connectivity studies reveal changes in the DMN in APOE ε4 carriers decades prior to the typical age of onset of clinical symptoms of Alzheimer's disease. For example, a study of young APOE ε4 carriers (individuals in their 20s and 30s) examined the DMN at rest and found increased coactivation in retrosplenial, medial prefrontal, and medial temporal lobe (MTL) regions in the APOE ε4 carriers relative to non-carriers (12). Middle-aged and older APOE ε4 carriers also show changes in resting state DMN connectivity. Fleisher et al. found increased nodal connectivity in APOE ε4 carriers in several brain regions, including medial and dorsolateral prefrontal cortex, and temporal lobe structures while there was decreased connectivity in the precuneus and medial orbital frontal cortex.(13) A more recent study compared DMN resting state connectivity in PiB negative APOE ε4 carriers to PiB negative APOE e4 non-carriers and found decreased connectivity from the precuneus to temporal regions and the dorsal anterior cingulate as well as increased connectivity from the precuneus to the dorsal occipital cortex and anterior frontal regions (14).
Little is known about the effect of APOE ε4 status on the relationship between intrinsic connectivity networks late in life. The salience network (SN) is anti-correlated with the DMN (15). Recent evidence shows disease specificity in the interplay between the DMN and SN, and in particular, that connectivity of the SN is intensified in AD (16, 17). The objective of this study was to examine the DMN and SN during task free fMRI (TF-fMRI) in an age, gender and education-matched sample of cognitively normal elderly (CN) carriers vs. non-carriers of the APOE ε4 allele.
METHODS
Participants
Subjects were cognitively normal individuals enrolled in the Mayo Alzheimer's Disease Research Center (n = 9) which is non-population based, or the Mayo Clinic Study of Aging (n = 103) which is a prospective population based study of randomly selected residents of Olmsted County, Minnesota between the ages of 70 – 89 at the time of entry, who had undergone TF-fMRI and genotyping for the APOE ε4 allele. A group of 56 CN APOE e4 carriers were identified and matched 1:1 with a group of CN APOE e4 non-carriers by age, gender and education. This study was approved by the Mayo Clinic Institutional Review Board and followed HIPAA guidelines. Informed consent was obtained from every subject. Additional details of subject recruitment and design are described in a previous report (18).
Criteria for CN were: (1) independently functioning community dwellers, (2) did not have an active neurologic condition, (3) no cognitive complaints, (4) normal neurological and neurocognitive exam. The classification of CN was based on input from three sources: the neurologist's clinical opinion based solely on the neurologist's interview and examination of the participant, neuropsychological test results as interpreted “blindly” by the neuropsychologist, and the nurse's opinion based exclusively on information about the participant obtained from an informant and reflected in the Clinical Dementia Rating Summary Score (19). At the completion of these evaluations, the three evaluators discussed each patient and assigned a final “consensus” diagnosis. Exclusion Criteria were: (1) medical contraindications to MRI scanning, and (2) structural abnormalities (e.g., intracranial neoplasms, infarctions).
Image Acquisition Protocols
The TF-fMRI signal time series was acquired using a gradient echo-planar (EPI) sequence (TR/TE = 3000/30 ms, 90° flip angle, slice thickness 3.3, and 103 volumes). Subjects were instructed to keep their eyes open during scanning.
The structural MRI sequence was a 3D magnetization prepared rapid acquisition gradient echo (MPRAGE) imaging sequence. Parameters were: TR/TE/T1, 2300/3/900 ms; flip angle 8°, 26-cm field of view (FOV); 256 × 256 in-plane matrix with a phase FOV of 0.94 and slice thickness of 1.2 mm. All MPRAGE images underwent pre-processing correction for gradient non-linearity and intensity non-uniformity (20).
TF-fMRI Preprocessing and Analysis
Preprocessing and data analysis were performed utilizing a combination of the statistical parametric mapping (SPM5) software (Wellcome Department of Cognitive Neurology, University College London, UK), the resting-state fMRI data analysis toolkit (REST) (http://www.restfmri.net) (21) group ICA of fMRI toolbox (GIFT) software (22) and in-house developed software implemented in MATLAB (Mathworks Inc., Natick, MA, USA).
Preprocessing steps included discarding the first 3 volumes to obtain steady state magnetization, realignment, slice time correction, normalization to ICBM EPI template, smoothing with 4 mm full-width half maximum Gaussian kernel, linearly detrending to correct for signal drift, and 0.01-0.08 HZ bandpass filtering to reduce non-neuronal contributions to BOLD fluctuations. In addition, regression correction for spurious variables included rigid body transformation motion effects, global mean signal, white matter and cerebral spinal fluid (15, 23). Removal of global signal by regression improves specificity of connectivity analysis (24) and is an attractive alternative to using physiologic cardiac and respiratory inputs as regressors (25) for reducing spurious direct correlations when MR compatible physiological measuring systems are not available. This is necessary because gray matter has significantly greater capillary density than white matter (26) and this variability is not accounted for by cerebral spinal fluid and white matter regression alone (24). These preprocessed images were used for both ICA and seed-based connectivity analyses.
Independent Component Analysis
We used the TF-fMRI preprocessed data described above for ICA. The intrinsic connectivity networks were first identified using the group ICA method of GIFT (22) with a low-dimensional estimation of 20 independent components (27). The group ICA analysis on the APOE ε4 non-carrier group was run 100 times using the ICASSO function to ensure stability of the 20 estimated components. The DMN and SN were identified by visual inspection of the group independent components (IC). The individual subject ICs were derived utilizing the spatial and temporal dual regression method. The individual subjects’ components were then entered into a one-sample t-test analyzed at a threshold of a p < 0.05 corrected for multiple comparisons using the false discover rate (FDR) method. These maps were used to define seed locations described below.
Seed-based Voxel-Wise Connectivity Analysis
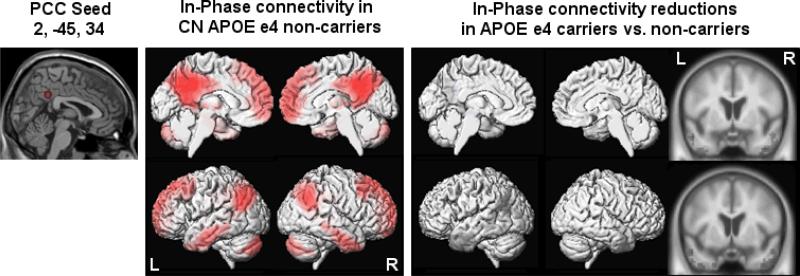
Although ICA can show group changes, we chose seed based analysis because it allows one to directly evaluate the disease effect on the connectivity of a seed to the rest of the brain. The posterior cingulate is one of the major hubs of the DMN, and the anterior cingulate is one of the major hubs of the SN (28, 29). Therefore, we selected seed locations based on the coordinates with the highest z-scores in the posterior cingulate cortex (2, -45, 34) in the DMN and in the anterior cingulate cortex (-3, 18, 42) in the SN identified with ICA in the APOE ε4 non-carrier group.
The average BOLD signal time course in each seed was correlated to every voxel in the brain for each subject using Pearson's correlation coefficient. Prior to group comparisons, the correlation coefficients were converted to z-scores using the Fischer r-to-z transformation. Regions that have positive z-scores between two fluctuating time-courses indicate “in-phase” connections and the regions with negative z-scores indicate “out-of-phase” connections. These z-score images were entered into the statistical analysis.
One sample t-tests were used to display voxel-wise connectivity maps. Two-sample two-sided t-tests were performed to compare voxel-wise connectivity between APOE ε4 non-carriers and carriers. To assess only in-phase connections, the group comparisons were masked by the out-of-phase connectivity maps identified in the one sample t-test of APOE ε4 non-carriers. The interpretation of out-of-phase connectivity is an active area of research. Conclusions based on out-of-phase results should be evaluated with the understanding that the interpretation of out-of-phase connectivity may change as the field matures.
All analyses were corrected for multiple comparisons using family wise error (FWE) at the cluster level (http://www.sph.umich.edu/nichols/JG2/CorrClusTh.m).
Structural MRI Preprocessing and Analysis
Preprocessing and data analyses were performed with a combination of voxel-based morphometry (VBM)(30), using SPM5 and in-house developed software implemented in Matlab (Mathworks Inc., Natick, MA, USA), to examine whether there were gray matter differences between APOE ε4 carriers vs. non-carriers. All MPRAGE scans were normalized to a customized template consisting of all subjects in the study, segmented using unified segmentation(31) and customized tissue probability maps into gray matter, white matter, and CSF (31), followed by the HMRF clean-up step (32). Gray matter images were modulated and smoothed at 8 mm FWHM. A two-sided t-test was used to examine whether there were any areas in which the APOE ε4 carriers showed greater gray matter loss relative to APOE ε4 non-carriers.
RESULTS
Demographics
Our sample consisted of 56 cognitively normal elderly APOE ε4 non-carriers and 56 APOE ε4 carriers matched on age, gender and education. A Wilcoxon two-sided rank sum test showed that the APOE ε4 carriers scored lower than the non-carriers on the Short Test of Mental Status though their group mean remained in the normal range (33). All APOE ε4 carriers and non-carriers had a CDR rating of 0 (see Table 1).
Table 1. Sample Demographics.
Demographics with the cognitively healthy patients matched 1:1
| Cn €4 - n = 56 | Cn €4 + n = 56 | |
|---|---|---|
| No. females (%) | 21 (38) | 21 (38) |
| No. of APOE €4 carrier (%) | ||
| No. with 2/2 (%) | 1 (2) | 0 |
| No. with 2/3 (%) | 5 (9) | 0 |
| No. with 3/3 (%) | 50 (89) | 0 |
| No. with 2/4 (%) | 0 | 1 (2) |
| No. with 3/4 (%) | 0 | 51 (91) |
| No. with 4/4 (%) | 0 | 4 (7) |
| Median age, yr. (min, max) | 79 (64, 91) | 78 (63, 90) |
| Median education, yr. (min, max) | 14 (8, 20) | 13 (8, 20) |
| Median Short Test score (min, max)* | 36 (30, 38) | 35 (29, 38) |
| Median CDR sum of boxes (min, max) | 0.0 | 0.0 |
p < .008
Posterior Cingulate Cortex Seed Connectivity
In APOE ε4 non-carriers, the posterior cingulate cortex seed (2, -45, 34) showed connectivity with retrosplenial regions including the precuneus, temporo-parietal junction, middle/inferior temporal gyri, medial temporal lobes, medial and lateral prefrontal cortex, cerebellar areas, and thalamus.
Between-group comparisons showed reduced connectivity in APOE ε4 carriers relative to APOE ε4 non-carriers in the left temporo-parietal junction, left middle temporal gyrus, and bilateral anterior temporal lobes (see Figure 1).
Figure 1.
Results from within- and between-group comparisons of the posterior cingulate cortex seed
Anterior Cingulate Cortex Seed Connectivity
In APOE ε4 non-carriers, the anterior cingulate seed (-3, 18, 42) showed connectivity within the anterior cingulate gyrus and medial prefrontal regions that were posterior to the medial prefrontal areas of connectivity with the posterior cingulate seed. There was also connectivity with the lateral prefrontal cortex, insular cortex, striatum, thalamus, and inferior parietal lobes.
Between-group comparisons showed increased connectivity in APOE ε4 carriers relative to APOE ε4 non-carriers in the cingulate gyrus, medial prefrontal cortex, bilateral insular cortex, striatum, and thalamus (see Figure 2).
Figure 2.
Results from within- and between-group comparisons of the anterior cingulate cortex seed
Voxel Based Morphometry
The VBM analysis did not detect a significant difference between the groups (FDR p < .05) suggesting that atrophy did not make a marked contribution to the present findings.
COMMENT
We investigated the effect of APOE ε4 status on intrinsic connectivity in an age, gender, and education-matched sample of elderly cognitively normal older adults. The major findings were that APOE ε4 carriers show (1) diminished connectivity of the posterior DMN, and (2) a relative increase in connectivity in the SN.
The DMN and SN are widely distributed anti-correlated neuroanatomical networks (15). The DMN is consistently shown to have relatively more activity when individuals are at rest, i.e., not performing cognitive tasks (34). The SN is involved in cognitive control functions such as attention, working memory, and response selection (35) as well as uncertainty, pain and other homeostatic challenges (29, 36, 37). When individuals engage in cognitively demanding tasks, the DMN deactivates as activity in the SN becomes more pronounced (38, 39). The right fronto-insular cortex, which is a network hub of the SN, plays a critical role in switching between task negative and task positive networks (39).
The DMN and SN are in a dynamic balance during resting states. Recent literature comparing AD with other dementias emphasizes the unique relationship between the DMN and SN and how they differ (16, 17). The DMN and SN have disease specificity, hence we interrogated both networks. Our results suggest that there is a disruption in the balance between the DMN and SN is observable even in cognitively normal asymptomatic APOE ε4 carriers. One possible interpretation, although speculative, is that a reduction in the inhibitory control of the posterior DMN may result in an aberrant relative increase in the SN, and that this may represent a loss of the ability to appropriately regulate functional networks in clinically asymptomatic individuals.
Sheline et al. recently reported on functional connectivity changes of the DMN in APOE ε4 carriers vs. non-carriers (14). All of their subjects were PiB negative which allowed for isolation of the effect of APOE status. They placed a single seed in the precuneus and similar to our results with a posterior cingulate cortex seed, they found a decrease in connectivity in regions of the posterior DMN, including the left hippocampus, left parahippocampus and middle temporal cortex in the APOE ε4 carriers relative to non-carriers. Unlike their study however, we also specifically interrogated the SN with a second seed placed in the anterior cingulate gyrus.. Another difference is that on average, our APOE ε4 carriers are 20 years older than their sample.
Several fMRI task activation studies show enhanced activation of brain regions in older APOE ε4 carriers vs. non-carriers during cognitive tasks (40-42). One of these studies used a cohort similar in age to ours and found that the APOE ε4 carriers showed greater activation during a verbal paired-associate learning task than the non-carriers in multiple regions in the right hemisphere, despite an equivalent level of memory function and comparable brain volume (43). Han and Bondi (44) revised the apolipoprotein E compensatory mechanism recruitment hypothesis and proposed that APOE ε4 carriers compensate for cognitive declines later in life by invoking additional brain regions to perform cognitive tasks with a predilection for increases in right hemisphere activity. They further suggest that frontal-executive cognitive processes might mediate these compensatory mechanisms. Our results indicate a relative increase in the SN, but suggest that rather than compensation per se, this “increase” in resting state connectivity occurs in the context of decreased posterior default mode network connectivity and thus may represent disruption of the balance between these two networks(15).
The primary biologic effect of APOE ε4 appears to be an increase in brain burden Aß. Although our subjects were clinically normal, resting state MR shows that there are subclinical consequences which are evident even in cognitively normal elderly individuals (7, 9). Our results reflect changes in resting state connectivity that may be a direct result of higher amyloid burden in APOE ε4 carriers who are more likely to harbor clinically silent amyloid plaques and who are more likely to develop AD in the future(9).
Based on the observation that regions of the DMN that are metabolically active in young adults also show a striking correlation with the pattern of amyloid deposition in older adults with AD, Buckner et al. suggested that the areas of enhanced metabolism may provide regional conditions that are conducive to amyloid deposition (4). Fillipini and colleagues (12) show that connectivity within the DMN is increased in young adult APOE ε4 carriers (i.e., mean age 28 years) relative to non-carriers, thus potentially setting the stage for earlier deposition of amyloid in regions of the DMN in carriers of the APOE ε4 allele. We propose that after an initial phase of increased resting metabolism in young adulthood, APOE ε4 carriers show a more rapid decline in DMN connectivity than APOE ε4 non-carriers as they age. Future studies of the resting state in APOE ε4 carriers and non-carriers throughout middle adulthood will ultimately provide a better understanding of the trajectory of the DMN over the life span and the age at which these declines begin to occur.
Strengths
Our study has several strengths. We have a fairly large sample size of thoroughly evaluated and well characterized individuals. Our groups are matched on age, gender, and education, effectively ruling out these variables as potential explanations for the group differences observed in resting state connectivity. Secondly, we performed both ICA and seed-based analyses, strengthening our results. Finally, results from our VBM analysis confirm that the group differences are not due to relatively greater loss of gray matter density in the APOE ε4 carriers.
Limitations
Our cohort consists of older individuals. The changes we describe may not generalize to changes in connectivity in younger cohorts. Although nearly all our patients are derived from a population-based sample, they represent a subset of individuals who are willing to undergo neuroimaging studies. This could potentially limit the generalizability of our findings. Finally, although studies clearly document an association between APOE ε4 status and amyloid, we do not have imaging evidence of amyloidosis to confirm a direct relationship.
CONCLUSION
There is a dynamic balance between the DMN and SN, and this balance is interrupted in cognitively normal APOE ε4 carriers relative to non-carriers. Specifically, there are reductions in posterior DMN connectivity but a concomitant increase in SN connectivity at rest. Our results add to our understanding of functional brain changes in individuals at risk for developing AD and suggest that prodromal alterations in connectivity may have the potential to serve as a biomarker.
Acknowledgments
Funding/Support:
Support was also provided by the Mayo Clinic Department of Psychiatry and Psychology Small Grants Program, The NIH AG11378, and the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer's Disease Research Program of the Mayo Foundation.
Footnotes
Author Contributions:
Study concept and design: Machulda, Jones, Vemuri, Jack
Acquisition of data: Jack
Analysis and interpretation of data: Machulda, Jones, Vemuri, Jack
Drafting of the manuscript: Machulda
Critical revision of the manuscript for important intellectual content: Jack, Jones, Vemuri, Boeve, Knopman
Statistical analysis: Przybelski
Obtained funding: Machulda, Jack
Administrative and technical support: Jack
Study supervision: Jack
Additional contributions: Brian Gregg, Guang Zeng, and Ankit Master also provided technical support.
Financial Disclosure:
Dr. Machulda has no disclosures.
Dr. Jones has no disclosures
Dr. Vemuri receives support from the Robert H. Smith Family Foundation Research Fellowship and NIH [R01-AG11378].
Dr. McDade has no disclosures.
Dr. Avula has no disclosures.
Mr. Przybelski has no disclosures.
Dr. Boeve has served as an investigator for clinical trials sponsored by Cephalon, Inc., and Allon Pharmaceuticals. He receives royalties from the publication of a book entitled Behavioral Neurology Of Dementia (Cambridge Medicine, 2009). He has received honoraria from the American Academy of Neurology. He receives research support from the National Institute on Aging (P50 AG16574, U01 AG06786, RO1 AG32306, and the Center for Inherited Disease Research (CIDR) (U24 AG026395).
Dr. Knopman serves as an Associate Editor of Neurology®; served on a data safety monitoring board for Sanofi Aventis; and is an investigator in a clinical trial sponsored by Elan Pharmaceutical and by Forest Laboratories.
Dr. Petersen has served as a consultant to GE Healthcare; has served on a data safety monitoring board for Elan and Wyeth Pharmaceuticals; and received research support from the NIH [P50-AG16574 and U01-AG06786].
Dr. Jack serves as a consultant for Elan, Eisai, Janssen, and GE.
Inc., the NIA [R01-AG11378 (PI), P50-AG16574 (Co-I), and U01 AG024904-01 (Co-I)], and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation.
References
- 1.Andrews-Hanna J, Snyder A, Vincent J, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Scheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer's disease: an fMRI study. Hum Brain Mapp. 2005;26:231–239. doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertram L, McQueen M, Mullin K, Blacker D, Tanzi R. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nature Genetics. 2008;29:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 6.Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 7.Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67:122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiman EM, Chen K, Liu X, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106:6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vemuri P, Wiste HJ, Weigand SD, et al. Effect of apolipoprotein E on biomarkers of amyloid load and neuronal pathology in Alzheimer disease. Ann Neurol. 2010;67:308–316. doi: 10.1002/ana.21953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedden T, Van Dijk KR, Becker JA, et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009;29:12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sperling RA, Laviolette PS, O'Keefe K, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filippini N, MacIntosh BJ, Hough MG, et al. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleisher A, Sherzai A, Taylor C, Langbaum J, Chen K, Buxton R. Resting-state BOLD networks versus task-associated functional MRI for distinguishing Alzheimer's disease risk groups. NeuroImage. 2009;47:1678–1690. doi: 10.1016/j.neuroimage.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheline Y, Morris J, Snyder A, et al. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF AB42. The Journal of Neuroscience. 2010;30:17035–17040. doi: 10.1523/JNEUROSCI.3987-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox M, Snyder A, Vincent J, Corbetta M, Van Essen D, Raichle M. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seeley W, Crawford R, Zhou J, Miller B, Greicius M. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J, Greicius M, Gennatas E, et al. Divergent network connectivity changes in behavioral variant frontotemporal dementia and Alzheimer's disease. Brain. 2010;133:1352–1367. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts R, Geda Y, Knopman D, et al. The Mayo Clinic Study of Aging: Design, sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris J. The clinical dementia rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 20.Jack CR, Jr., Bernstein MA, Fox NC, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao-Gan Y, Yu-Feng Z. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Frontiers in Systems Neuroscience. 2010;4:1–7. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C. Correlations and anti-correlations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. NeuroImage. 2009;47:1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Fox M, Zhang D, Snyder A, Raichle M. The global signal and observed anticorrelated resting state brain networks. Journal of Neurophysiology. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang C, Glover G. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. NeuroImage. 2009;47:1448–1459. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cavaglia M, Dombrowski S, Drazba J, Vasanji A, Bokesch P, Janigro D. Regional variation in brain capillary density and vascular response to ischemia. Brain Research. 2001;910:81–93. doi: 10.1016/s0006-8993(01)02637-3. [DOI] [PubMed] [Google Scholar]
- 27.Smith S, Fox P, Miller K, et al. Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences USA. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buckner RL, Sepulcre J, Talukdar T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 31.Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 33.Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC. The short test of mental status. Correlations with standardized psychometric testing. Arch Neurol. 1991;48:725–728. doi: 10.1001/archneur.1991.00530190071018. [DOI] [PubMed] [Google Scholar]
- 34.Raichle M, MacLeod A, Snyder A, Powers W, Gusnard D, Shulman G. A default mode of brain function. Proceedings of the National Academy of Sciences USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menon V, Adleman N, White C, Glover G, Reiss A. Error-related brain activation during a go/nogo response inhibition task. Human Brain Mapping. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grinblad J, Hirsch J, Ferrera V. A neural representation of categorization uncertainty in the human brain. Neuron. 2006;49:757–763. doi: 10.1016/j.neuron.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 37.Peyron R, Laurent B, CGarcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis. Clinical Neurophysiology. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 38.Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 39.Sridharan D, Levitin D, Menon V. A critical role for the right fronto-insular cortex in switching betweem the central-executive and default-mode networks. Proceedings of the National Academy of Sciences USA. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bookheimer SY, Strojwas MH, Cohen MS, et al. Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han S, Houston W, Jak A, et al. Verbal paired-associate learning by APOE genotype in non-demented older adults: fMRI evidence of a right hemispheric compensatory response. Neurobiology of Agin. 2007;28:238–247. doi: 10.1016/j.neurobiolaging.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wishart H, Saykin A, Rabin L, et al. Increased barin activation during working memory in cognitively intact adults with the APOE e4 allele. American Journal of Psychiatry. 2006;163:1603–1610. doi: 10.1176/ajp.2006.163.9.1603. [DOI] [PubMed] [Google Scholar]
- 43.Han S, Houston W, Jak A, et al. Verbal paired-associate learning by APOE genotype in non-demented older adults: fMRI evidence of a right hemispheric compensatory response. Neurobiology of Aging. 2007;28:238–247. doi: 10.1016/j.neurobiolaging.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han S, Bondi M. Revision of the apolipoprotein E compensatory mechanism recruitment hypothesis. Alzheimer's and Dementia. 2008;4:251–254. doi: 10.1016/j.jalz.2008.02.006. [DOI] [PubMed] [Google Scholar]