Abstract
Objectives
To determine the impact of evaluative care guideline compliance on surgical intervention for BPH.
Methods
From Medicare claims data, we developed a cohort of men new to a urologist with a diagnosis of BPH. We determined urologists’ compliance with guideline recommended care (3 months) and their time- and geography-standardized average monthly Medicare expenditures (1 year). At the level of the urologist, we assessed the impact of these measures on the use of surgical therapy within 1 year of the new patient visit.
Results
Of 10,248 patients in the cohort, 675 received surgical intervention (6.7%). Guideline compliance (2% received surgery in highest quintile; 11% lowest quintile) was associated with surgical intervention. The results were robust to adjustment for patient and surgeon factors (Guideline Compliance - OR 0.09; 95% CI 0.06 to 0.15 highest to lowest adherence).
Conclusions
Urologists who tend to follow the AUA best practice guidelines for BPH evaluation perform surgical interventions on their BPH patients less frequently than urologists who do not follow these guidelines.
Introduction
Urologists serve as the medical and surgical specialists for men with lower urinary tract symptoms (LUTS). These symptoms, including urgency, frequency, intermittency, and nocturia, are most often attributed to benign prostatic hyperplasia (BPH),1 a symptom complex which affects up to 48% of men in the United States by their eighth decade of life.2 Depending on the degree of symptoms, and prior BPH therapies, patients new to a urologist may receive a variety of interventions, ranging from simple testing and medication adjustment to invasive testing and surgical therapy. These diagnostic and screening procedures are used to stratify patients by the severity of their LUTS,1,3 and have been advocated to guide the use of therapy.4,5 While absolute indications for surgical management exist (e.g. urinary retention, intractable hematuria),6 for the vast majority of patients surgical interventions are elective.7,8
Elective, or discretionary, surgical therapies are subject to variable use depending on local practice patterns. Indeed, local variation in use of transurethral resection of the prostate (TURP) for BPH was an initial impetus for the study of small area variation,9 and for development of patient centered decision making.7 Given these developments, variability in practice patterns could be expected to decrease over time.10 However, as seen in other discretionary surgical conditions11, extensive variations continue in the use of therapies for BPH among urologists in the United States.12
Guidelines developed for the management of BPH might help attenuate some the practice variation among urologists, and help improve care. Indeed evidence from other specialties supports this premise. Increased use of guidelines has been associated with lower costs, and improved care, in acute stroke victims,13,14 schizophrenia patients,15 and in cardiovascular disease.16 However, evidence that use of guidelines for diagnostic procedures is associated with subsequent use of discretionary surgical therapy is lacking.
As guidelines proliferate in medical practice, objective evidence of their impact on clinical care is needed. In this paper, we use the care of men new to urologists as an example of how guidelines can influence care. We explore whether a urologist’s compliance with BPH guidelines is associated with the delivery of surgical care for men new to their practice with BPH. We hypothesize that high guideline compliance is associated with decreased surgical intervention.
Materials and Methods
Data Source
We created our cohort using a 5% random sample of Medicare beneficiaries from 1999 to 2007. The Medicare program provides health care for Americans over age 65, patients with end stage renal disease, and patients with long term disability. Over 95% of Americans over age 65 use Medicare as their primary insurance.17 We limited our study to male Medicare beneficiaries over 65 years of age.
Study population
We selected patients with International Classification of Disease Ninth Edition (ICD-9) diagnosis codes (Appendix 1) consistent with a BPH diagnosis based on physician billing records. We determined the specialty of the physician billing for the service from the Medicare records, and confirmed with data from the AMA Masterfile. All patients with an initial visit to a urologist for a BPH diagnosis were included in the initial cohort. We excluded patients from the cohort if they lacked continuous enrollment in Medicare parts A and B or if they were enrolled in a Medicare HMO for any time in the two years prior through one year after the initial visit with the urologist (the three-year period of study for each man). The two year period prior to diagnosis allowed exclusion of patients who had already established urologic care for non-BPH conditions. We also excluded patients with diagnoses suggesting prior surgical BPH therapy, prostate cancer, or neurologic disease that could contribute to LUTS (Appendix 2). These restrictions resulted in a study population of 40,483 patients. We established the primary urologist responsible for the patient’s care by assessing the Unique Provider Identification Number (UPIN) for the patient visits and laboratory testing. For most patients, a single urologist provided the BPH-related care. In cases with multiple urologists, the primary urologist was classified as the urologist who provided the plurality of patient care services. Next, to examine differences in care at the level of the urologist, we restricted the cohort to patients seen by urologists who cared for a panel of at least 10 BPH/LUTS patients between 1999 and 2007. Because we were using a 5% sample of patients, the restriction to at least 10 patients per urologist equated to at least 200 new BPH patients seen by each urologist in our cohort over the 9 years of study. This yielded a final study population of 10,248 patients treated by 748 urologists.
Characterization of Practice Style Intensity
We have previously defined a measure of practice style intensity.18 In brief, we used appropriate Healthcare Common Procedure Coding System (HCPCS) and ICD-9 codes to determine the BPH related tests for patients. Testing was included for up to one year after the initial visit with a urologist or until the patient received surgical therapy for BPH. We then calculated the Medicare expenditures on testing for each patient, and totaled this expenditure at the level of the urologist (adjusted to 2007 dollars). Finally, the average Medicare expenditure per month for each urologist was calculated ($35 to $527 per month). Urologists were then divided into quintiles based on this practice style expenditure calculation (Median $92, 20th percentile $72, 40th percentile $85, 60th percentile $100, 80th percentile $123).
Characterization of Compliance with Guidelines
We have previously outlined an index of compliance with the 2003 American Urological Association Best Practice Guidelines for BPH care.19 In brief, this index was based on a urologist’s average use of testing considered recommended, optional, and not-recommended by the guidelines using appropriate ICD-9 and HCPCS codes. Use of recommended care was valued with 1 point, not-recommended care testing received minus 1 point, and optional care received no points. For this index, BPH related testing was assessed within 3 months of the initial visit with the urologist, and each type of test was allowed to count more than once in the 3 month period. We then summed the points for the patient and divided by the total amount of testing provided in the three month period. Each individual patient score was then aggregated at the level of the treating urologist, and the average index of compliance for the urologist was calculated. If a urologist performed only not-recommended care for a patient, the index value would be −1. Conversely if a urologist performed only recommended care, the index would be 1. Provision of only optional care would result in an index score of 0. We then categorized providers into quintile groups based on their average compliance score across all patients in their panel.
Comparison of Practice Style Intensity and Compliance with Guidelines
We assessed the independence of the two measures of urologist practice with a Pearson correlation coefficient. The correlation between the two measures was weak, −0.31.
Outcome
The primary outcome of interest was receipt of BPH surgery. The 12 months after the initial urology visit were assessed for surgical procedures as defined by appropriate CPT codes (Appendix 2). We then explored the impact of urologist guideline compliance on patient receipt of surgical care.
Statistical Analysis
Differences in patient populations by guideline compliance categories were assessed with Chi-squared tests.
We assessed the impact of guideline compliance on receipt of surgery with unadjusted logistic regression analysis. We then adjusted for urologist and patient characteristics in a multivariate logistic regression model. Patient factors included age (67–70, 71–74, 75–78, and 79+), socioeconomic status (zip code level using the methodology of Diez-Roux),20 race (white, black, and other), and comorbidity (Klabunde modification of the Charlson comorbidity index).21 Physician factors, derived from the AMA Masterfile and Medicare records, included region of practice (Northeast, Midwest, South, West), practice structure (solo, group, hospital based), and years in practice (<15, 15–30, >30). When analyzing the impact of guideline compliance on patient’s receipt of surgery, we adjusted for the urologist’s practice style expenditures. We also tested the interactions between practice style intensity and guideline compliance. Because these interactions were not significant, they were not included in the final models.
Results
Receipt of surgical care for BPH was related to the urologist’s compliance with guidelines (Figure 1). Overall, 6.7% of men newly seen by urologists for BPH received surgical intervention within one year of their initial visit to the urologist. A linear trend of decreasing use of surgery with increasing compliance with evaluative care guidelines was seen. Surgery was performed in 10.9% of men seen by urologists in the lowest quintile of guideline compliance. This proportion decreased to 9.1%, 6.2%, 4.6% and 2.4% in the low to highest guideline compliance quintiles (p < 0.01).
Figure 1. Percentage of Patients Receiving Surgery across the Quintiles of Guideline Compliance.
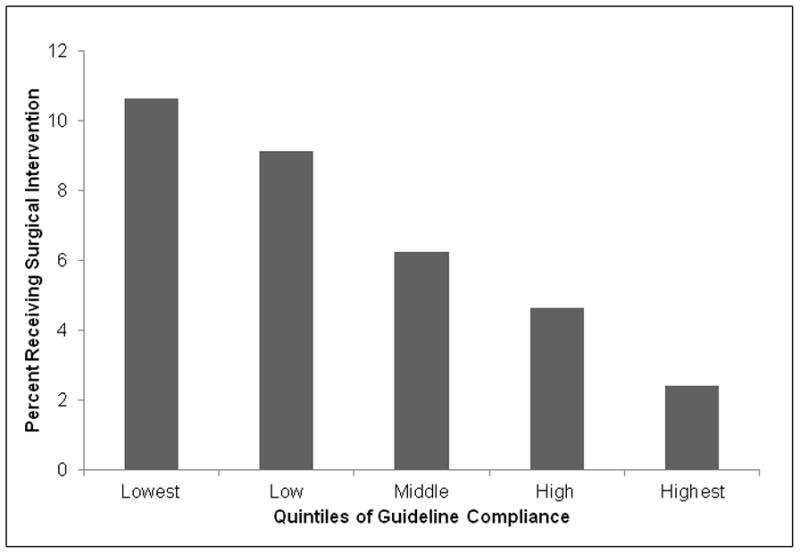
Overall, 6.7% of patients in the study received a surgical intervention. 2% of patients treated by urologists in the highest quintile of guideline compliance received surgery compared to 11% treated by urologists in the lowest quintile.
Patient populations differed by physician practice style intensity and with the urologist’s level of guideline compliance (Table 1). As previously reported,18,19 patient race, comorbidity, socioeconomic status, and age were associated with urologist’s practice style and compliance with guidelines. In addition, urologist factors of region of practice, employment status, and years in practice were also associated with practice style intensity and compliance with guidelines.18,19
Table 1.
Patient Populations by Level of Evaluative Care Intensity and Level of Compliance with Best Practice Guidelines
| Quintile of Evaluative Care Intensity | Quintile of Compliance with Best Practice Guidelines | |||||
|---|---|---|---|---|---|---|
| Lowest | Highest | p-value | Lowest | Highest | p-value | |
| Patient | ||||||
| Age | P = 0.02 | P = 0.02 | ||||
| 67–70 | 21.3 | 19.7 | 18.2 | 21.9 | ||
| 71–74 | 18.5 | 20.6 | 19.4 | 20.7 | ||
| 75–78 | 21.0 | 20.6 | 18.6 | 21.6 | ||
| 79+ | 19.5 | 19.4 | 21.6 | 19.2 | ||
| Race | P < 0.01 | P = 0.70 | ||||
| White | 20.5 | 19.5 | 19.7 | 20.7 | ||
| Black | 21.0 | 17.8 | 16.9 | 19.4 | ||
| Other | 11.2 | 30.4 | 18.4 | 22.7 | ||
| Comorbidity | P< 0.01 | P<0.01 | ||||
| 0 | 21.3 | 19.0 | 19.3 | 21.6 | ||
| 1 | 17.7 | 23.2 | 18.6 | 20.1 | ||
| 2+ | 15.3 | 21.5 | 23.7 | 16.1 | ||
| Socioeconomic Status | P <0.01 | P < 0.01 | ||||
| Lowest | 20.3 | 21.2 | 16.6 | 23.9 | ||
| Middle | 25.2 | 18.8 | 22.1 | 18.5 | ||
| Upper Middle | 17.0 | 20.0 | 18.9 | 21.0 | ||
| Highest | 5.2 | 22.0 | 22.5 | 18.3 | ||
| Surgeon | ||||||
| Region | P < 0.01 | p < 0.01 | ||||
| Northeast | 10.5 | 19.2 | 27.4 | 10.9 | ||
| Midwest | 29.0 | 12.5 | 27.1 | 17.5 | ||
| South | 19.5 | 21.8 | 12.7 | 26.8 | ||
| West | 17.2 | 25.5 | 19.2 | 17.2 | ||
| Employment | P <0.01 | P < 0.01 | ||||
| Solo | 18.2 | 23.6 | 17.3 | 19.4 | ||
| Group | 21.6 | 16.2 | 19.6 | 21.3 | ||
| Hospital | 13.5 | 23.1 | 20.5 | 9.8 | ||
| Years in Practice | P < 0.01 | p < 0.01 | ||||
| 0–15 | 13.3 | 28.3 | 19.8 | 18.8 | ||
| 15–30 | 20.7 | 18.6 | 20.6 | 20.7 | ||
| >30 | 22.9 | 17.1 | 17.2 | 21.3 | ||
In adjusted analysis, the urologist’s level of guideline compliance was associated with receipt of surgical care. We found a linear trend in decreasing use of surgical therapy with increasing guideline compliance quintile that was robust to adjustment for patient and physician factors, and for the urologist’s practice style intensity (Figure 2). Comparing the lowest guideline compliance urologists to the highest, there was a 91% decrease in the adjusted odds of receiving surgery with high guideline compliance (OR 0.09; 95% CI 0.05, 0.15). A linear decline in the use of surgical therapy is seen from the lowest to highest quintile of guideline compliance, with significant overlap in the confidence intervals around the odds ratios exists for each step in the analysis.
Figure 2. Adjusted Odds Ratios of Receiving Surgical Therapy by Compliance with Guidelines.
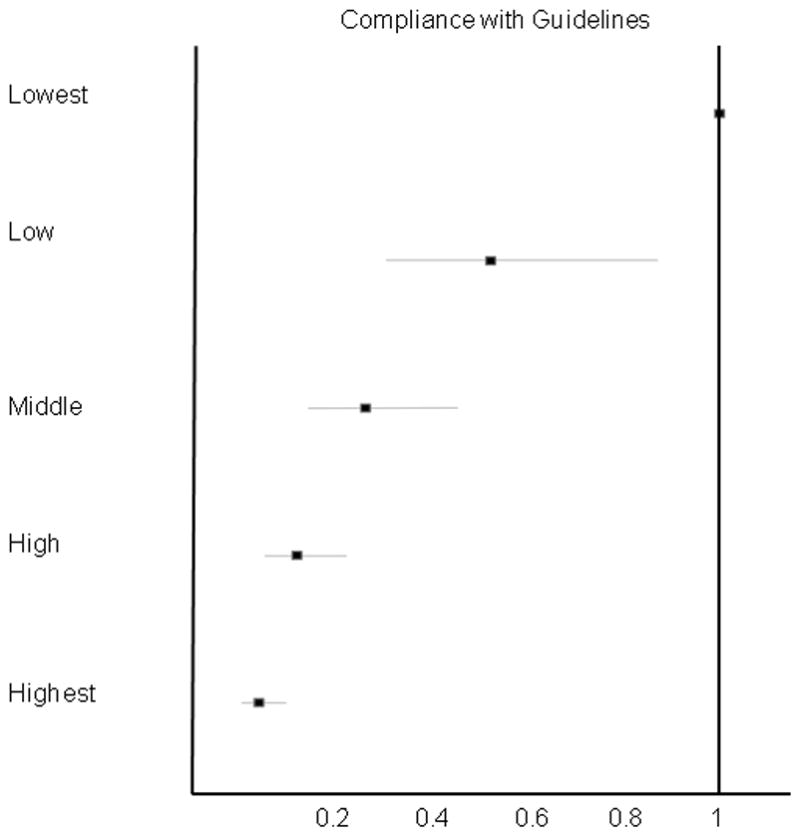
The adjusted model incorporates expenditures on evaluative care and guideline compliance and adjusts for patient (age, race, comorbidity, and socioeconomic status) and urologist (type of practice, practice location, and years in practice) factors. Adjustment was made for temporal trends by adding a term for year to the model. Interactions between intensity and guideline adherence were assessed, but were not significant, and were excluded from the final model.
Comment
Overall, 6.7% of BPH patients newly seen by a urologist for BPH receive surgical intervention within one year of their initial visit to a urologist. Urologists who followed guideline recommendations performed fewer surgical procedures for BPH than urologists with lower guideline compliance, varying from 2.4% in the highest guideline compliance quintile to 10.9% in the lowest. After adjusting for patient and physician characteristics we still found that urologists with the highest guideline compliance were almost ten times less likely to perform surgical interventions than were urologists with the lowest guideline compliance.
Our results suggest there are significant variations among urologists in their approach to men with BPH. Such variation has been seen across surgical and medical procedures,22 especially in situations with discretionary indications for intervention,23 and are not related to patient preferences.24 In the management of BPH, variation in use of surgical therapy has been well documented.25 Despite consensus panels,25 outcome assessments,1 and development of practice guidelines,26,27 variations in the use of surgical therapy among men with BPH continue to be found,28 and new variations are developing.12
Despite the success of surgical interventions in relieving symptoms, well-informed patients rarely choose a surgical intervention for their BPH.29 Such data suggest men are reluctant to pursue intervention, and that surgery for BPH is often discretionary. Indeed, consistent with this supposition, only 6.7% of men in our study received a surgical intervention for BPH. Despite this small overall percentage of patients receiving surgical intervention, we found large variations in use of surgery across urologists.
Clinical guidelines may provide a mechanism to help standardize care and eliminate variations amongst practitioners. Thus, many studies have examined physician compliance with guidelines, and the economic impact of guideline compliance.13,14 Such studies help to show the large gaps between optimal care, and care that is actually provided in practice. However, the impact of compliance with clinical guidelines for diagnostic testing is less well defined.
The impact of clinical guidelines for diagnostic testing varies based on the intensity of the practice pattern called for in the guideline compared to standard practice in the community. In the case of BPH, guidelines call for a less intensive work up than performed by many urologists. In our study, we found that lower compliance with guidelines was associated with greater use of surgical therapy. Our study provides the first evidence, to our knowledge, suggesting modifiable features of clinical practice are associated with variations in delivery of a discretionary surgical procedure. We have previously established that physician factors including geographic location, experience, and type of practice setting are related to how a physician uses the testing available to evaluate men with BPH.18 Yet, after adjusting for these factors, guideline compliance remains an independent factor associated with delivery of surgery.
Physician compliance with guidelines is a potentially modifiable feature of health care delivery for men with BPH. For some physicians, a practice pattern with higher levels of testing is reflective of an intensive practice pattern where surgery would be performed regardless of testing performed. For many other physicians however, test results influence their decision about when to perform surgical intervention. Among these physicians, each additional test obtained increases the chance of finding an abnormality that could prompt surgical intervention. In BPH care for instance, post void residual urine measurements are highly variable from test to test, and have not been shown to relate to failure of medical management of BPH.6 Yet, post-void residual urine measurements are advocated by some authorities in the evaluation of men with LUTS,30 and may be used to prompt surgical intervention. By identifying significant variations in the use of guideline recommended care, efforts to redirect practice style can be made through residency training, continuing medical education, regulatory changes, or reimbursement changes.
Our study needs to be considered in light of certain limitations. By using Medicare claims data, we do not have access to the patients’ symptoms or prior treatment histories. We attempted to attenuate this issue by excluding patients with diseases other than BPH that may contribute to LUTS. A common argument to explain our findings would be that more extensive and expensive tests are necessary in the patient with severe BPH. Such patients may be best managed with surgery due to their advanced disease. The evaluation of patients being considered for BPH surgery may justly include certain tests normally considered not indicated (e.g. serum creatinine) or optional and thus incorrectly indicate poor quality care. However, the design of our study controls for this by determining the quality and intensity of care as well as the receipt of surgery variables at the level of the urologist. In other words, the intensity of the evaluation would not correlate with the receipt of surgery for any one patient because the unit of analysis in this study is the urologist, not the patient. In order for guideline compliance to be associated with surgery, the entire panel of patients seen by that urologist would have to present with more severe BPH. Furthermore, by excluding patients with a urologist visit for any condition for 2 years prior to the index BPH visit we limited the possibility of referral from one urologist to another for specialized care. Despite these techniques, some providers may see a different panel of patients, and the study design cannot fully control for these occurrences. Thus, we are limited in making causal statements that better guideline compliance would result in fewer surgeries for men with BPH.
Other limitations result from changes in guidelines and limitations to the data. During the study period, guidelines on BPH management were updated in 2003. Only the recommendation status of serum creatinine measurements changed, making the use of 2003 guidelines applicable across the entire study period. Our study lacks pharmacy data. We do not know if the use of medical therapy shows similar or different variations based on levels of guideline compliance. Finally, our results best apply to patients seen in the Medicare fee-for-service population. We cannot determine if the practice patterns and surgery utilization among urologists would be the same in younger men, or if urologists practicing in HMO environments would have different utilization of BPH testing and surgery.
Conclusion
Urologists vary extensively in their use of surgical treatments for men with BPH. These variations appear to be associated with urologist’s compliance with guidelines for testing. These data provide evidence that compliance with clinical guidelines is associated with discretionary surgical therapy.
Supplementary Material
Acknowledgments
Funding Source: National Institutes of Health (NIH)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barry MJ, Fowler FJ, Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–57. doi: 10.1016/s0022-5347(17)36966-5. discussion 1564. [DOI] [PubMed] [Google Scholar]
- 2.Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol. 2005;173:1256–61. doi: 10.1097/01.ju.0000155709.37840.fe. [DOI] [PubMed] [Google Scholar]
- 3.Jepsen JV, Bruskewitz RC. Comprehensive patient evaluation for benign prostatic hyperplasia. Urology. 1998;51:13–8. doi: 10.1016/s0090-4295(98)00050-8. [DOI] [PubMed] [Google Scholar]
- 4.d’Ancona FC, Francisca EA, Hendriks JC, et al. The predictive value of baseline variables in the treatment of benign prostatic hyperplasia using high-energy transurethral microwave thermotherapy. Br J Urol. 1998;82:808–13. doi: 10.1046/j.1464-410x.1998.00880.x. [DOI] [PubMed] [Google Scholar]
- 5.Walden M, Dahlstrand C, Schafer W, et al. How to select patients suitable for transurethral microwave thermotherapy: a systematic evaluation of potentially predictive variables. Br J Urol. 1998;81:817–22. doi: 10.1046/j.1464-410x.1998.00656.x. [DOI] [PubMed] [Google Scholar]
- 6.Roehrborn C, McConnell J, Barry M. American Urological Association Guideline on the Management of Benign Prostatic Hyperplasia (BPH) American Urological Association; 2003. [Google Scholar]
- 7.Barry MJ, Mulley AG, Jr, Fowler FJ, Wennberg JW. Watchful waiting vs immediate transurethral resection for symptomatic prostatism. The importance of patients’ preferences. JAMA. 1988;259:3010–7. [PubMed] [Google Scholar]
- 8.Fowler FJ, Jr, Wennberg JE, Timothy RP, et al. Symptom status and quality of life following prostatectomy. JAMA. 1998;259:3018–22. [PubMed] [Google Scholar]
- 9.Wennberg J. Wrestling with variation: an interview with Jack Wennberg [interviewed by Fitzhugh Mullan] Health Aff (Millwood) Suppl Web Exclusives. 2004:VAR73–80. doi: 10.1377/hlthaff.var.73. [DOI] [PubMed] [Google Scholar]
- 10.Griggs JJ, Sorbero ME, Ahrendt GM, et al. The pen and the scalpel: effect of diffusion of information on nonclinical variations in surgical treatment. Med Care. 2009;47:749–57. doi: 10.1097/MLR.0b013e31819748b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birkmeyer JD, Sharp SM, Finlayson SR, et al. Variation profiles of common surgical procedures. Surgery. 1998;124:917–23. [PubMed] [Google Scholar]
- 12.Yu X, McBean AM, Caldwell DS. Unequal use of new technologies by race: the use of new prostate surgeries (transurethral needle ablation, transurethral microwave therapy and laser) among elderly Medicare beneficiaries. J Urol. 2006;175:1830–5. doi: 10.1016/S0022-5347(05)00997-3. discussion 1835. [DOI] [PubMed] [Google Scholar]
- 13.Quaglini S, Cavallini A, Gerzeli S, et al. Economic benefit from clinical practice guideline compliance in stroke patient management. Health Policy. 2004;69:305–15. doi: 10.1016/j.healthpol.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Micieli G, Cavallini A, Quaglini S. Guideline compliance improves stroke outcome: a preliminary study in 4 districts in the Italian region of Lombardia. Stroke. 2002;33:1341–7. doi: 10.1161/01.str.0000013663.27776.db. [DOI] [PubMed] [Google Scholar]
- 15.Dickey B, Normand SL, Eisen S, et al. Associations between adherence to guidelines for antipsychotic dose and health status, side effects, and patient care experiences. Med Care. 2006;44:827–34. doi: 10.1097/01.mlr.0000215806.11805.6c. [DOI] [PubMed] [Google Scholar]
- 16.Guadagnoli E, Landrum MB, Normand SL, et al. Impact of underuse, overuse, and discretionary use on geographic variation in the use of coronary angiography after acute myocardial infarction. Med Care. 2001;39:446–58. doi: 10.1097/00005650-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER—Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 18.Strope SA, Elliott SP, Smith A, et al. Urologist practice styles in the initial evaluation of elderly men with benign prostatic hyperplasia. Urology. 2011;77:535–40. doi: 10.1016/j.urology.2010.07.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strope SA, Elliott SP, Saigal CS, et al. Urologist compliance with AUA best practice guidelines for benign prostatic hyperplasia in Medicare population. Urology. 2011;78:3–9. doi: 10.1016/j.urology.2010.12.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345:99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 21.Klabunde CN, Legler JM, Warren JL, et al. A refined comorbidity measurement algorithm for claims—based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17:584–90. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Wennberg JE, O’Connor AM, Collins ED, et al. Extending the P4P agenda, part 1: how Medicare can improve patient decision making and reduce unnecessary care. Health Aff (Millwood) 2007;26:1564–74. doi: 10.1377/hlthaff.26.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sirovich B, Gallagher PM, Wennberg DE, et al. Discretionary decision making by primary care physicians and the cost of U.S. Health care. Health Aff (Millwood) 2008;27:813–23. doi: 10.1377/hlthaff.27.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anthony DL, Herndon MB, Gallagher PM, et al. How much do patients’ preferences contribute to resource use? Health Aff (Millwood) 2009;28:864–73. doi: 10.1377/hlthaff.28.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wennberg JE. On the status of the Prostate Disease Assessment Team. Health Serv Res. 1990;25:709–16. [PMC free article] [PubMed] [Google Scholar]
- 26.McConnell JD, Barry MJ, Bruskewitz RC. Benign prostatic hyperplasia: diagnosis and treatment. Agency for Health Care Policy and Research. Clin Pract Guidel Quick Ref Guide Clin. 1994:1–17. [PubMed] [Google Scholar]
- 27.Roehrborn CG, McConnell JD, Barry MJ, et al. American Urological Association. Guideline on the Management of Benign Prostatic Hyperplasia (BPH) American Urological Association; 2003. [Google Scholar]
- 28.Sung JC, Curtis LH, Schulman KA, et al. Geographic variations in the use of medical and surgical therapies for benign prostatic hyperplasia. J Urol. 2006;175:1023–7. doi: 10.1016/S0022-5347(05)00409-X. [DOI] [PubMed] [Google Scholar]
- 29.Wagner EH, Barrett P, Barry MJ, et al. The effect of a shared decisionmaking program on rates of surgery for benign prostatic hyperplasia. Pilot results. Med Care. 1995;33:765–70. doi: 10.1097/00005650-199508000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Madersbacher S, Alivizatos G, Nordling J, et al. EAU 2004 guidelines on assessment, therapy and follow—up of men with lower urinary tract symptoms suggestive of benign prostatic obstruction (BPH guidelines) Eur Urol. 2004;46:547–54. doi: 10.1016/j.eururo.2004.07.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


