Abstract
The effectiveness of methadone as a treatment for opioid abuse and nicotine preparations as treatments for tobacco smoking has led to an interest in developing a similar strategy for treating psychostimulant abuse. The current study investigated the effects of three such potential therapies on i.v. methamphetamine self-administration (1 - 30 µg/kg/injection) in rhesus monkeys. When given as a pre-session i.m. injection, a high dose of methamphetamine (1.0 mg/kg) decreased i.v. methamphetamine self-administration but did not affect responding for a food reinforcer during the same sessions. However, the dose of i.m. methamphetamine required to reduce i.v. methamphetamine self-administration exceeded the cumulative amount taken during a typical self-administration session, and pretreatment with a low dose of methamphetamine (0.3 mg/kg) actually increased self-administration in some monkeys at the lower self-administration dose. Like pretreatment with methamphetamine, pretreatment with bupropion (3.2 mg/kg) decreased methamphetamine self-administration but did not affect responding for food. Pretreatment with methylphenidate (0.56 mg/kg) did not significantly alter methamphetamine self-administration. These results suggest that some agonist-like agents can decrease methamphetamine self-administration. Although the most robust effects occurred with a high dose of methamphetamine, safety and abuse liability considerations suggest that bupropion should also be considered for further evaluation as a methamphetamine addiction treatment.
Keywords: Methamphetamine, self-administration, bupropion, methylphenidate, rhesus monkeys
Despite the fact that substantial research effort has been focused on developing a pharmaceutical for the treatment of psychomotor stimulant abuse, no clearly effective medications have been developed for the treatment of cocaine or methamphetamine abuse (Vocci and Elkashef, 2005). Noting the effectiveness of methadone treatment for opioid abuse and the use of nicotine replacement therapies in smoking-cessation treatments, a number of investigators have suggested that replacement therapy might also be useful for treating stimulant abuse (Grabowski et al., 2004; Herin et al., 2010; Rothman et al., 2008). Since certain actions within the CNS dopamine system are thought to mediate the abuse of these drugs (Wise, 2008), most research in this area has focused on the use of direct and indirect dopamine agonists in the treatment of cocaine and methamphetamine abuse (Karila et al., 2010).
When investigating indirect agonists, the non-specific monoamine releaser (Heal et al., 2009) amphetamine has been a popular choice for demonstrating the potential usefulness of this treatment approach. For example, Negus and Mello (2003a, 2003b) showed that chronic treatment with amphetamine can decrease cocaine self-administration in rhesus monkeys under a variety of schedule conditions. The effects of amphetamine appeared to be specific for drug self-administration, as the effects of chronic amphetamine treatment were not as evident when food was used as the reinforcer. Grabowski et al. (2001) conducted a double-blind clinical trial in cocaine abusers using amphetamine as a treatment compound. Using a sustained release formulation of oral d-amphetamine they found both increases in treatment retention and decreases in urines positive for cocaine in the amphetamine treatment groups.
Because agents such as amphetamine carry with them clear abuse potential, a number of investigators have focused on treatments that might have less abuse potential. Two of these drugs are bupropion and methylphenidate. Bupropion is a dopamine and norepinephrine uptake inhibitor (Dwoskin et al., 2006) that is used clinically as an antidepressant and in smoking cessation therapy. Reichel et al. (2009) used bupropion as a pretreatment in rats self-administering methamphetamine or in separate groups of rats responding for sucrose. A 30 mg/kg dose of bupropion selectively reduced methamphetamine self-administration. At a higher bupropion dose (60 mg/kg) both methamphetamine- and sucrose-reinforced responding were reduced. In clinical studies, Newton et al. (2006) showed that bupropion treatment can reduce some subjective effects of methamphetamine, and Elkashef et al. (2008) and Shoptow et al. (2008) showed an increase in abstinence in methamphetamine users classified as having lower methamphetamine use at the beginning of the treatment.
Like bupropion, methylphenidate is an inhibitor of the reuptake of dopamine and norepinephrine (Heal et al., 2009). It is used clinically in the treatment of ADHD. In rats treated for 2 months with 2 mg/kg methylphenidate, Thanos et al. (2007) showed reduced rates of cocaine self-administration. However, Thanos et al. did not investigate the specificity of this effect by testing the effects of methylphenidate on responding maintained by another reinforcer. Tiihonen et al. (2007) reported that in amphetamine abusers, treatment with methylphenidate led to a reduction in amphetamine positive urines.
The results of these studies support the continued investigation of both bupropion and methylphenidate for the treatment of stimulant abuse. For comparison, we also tested methamphetamine (which releases and blocks the reuptake of both dopamine and norepinephrine and, to a lesser extent, serotonin) as a pretreatment. To determine the specificity of any observed effects of these pretreatment compounds, the monkeys were also trained to respond to receive food pellets. Ideally, a treatment medication should be able to decrease the self-administration of methamphetamine while not affecting responding for other reinforcers (Mello and Negus, 1996). That is, the treatment drug should show some specificity for methamphetamine self-administration. The rhesus monkeys used in these studies had been self-administering methamphetamine over a number of years. Since changes in sensitivity to the behavioral effects of methamphetamine, both sensitization and tolerance, have been demonstrated with repeated administration, we also tracked the stability of methamphetamine dose-effect functions over a number of months. If these functions were to change over time, it would complicate the use of the within-subject design in testing treatment drugs for methamphetamine abuse.
Methods
Subjects
The subjects were 8 adult male rhesus monkeys (Macaca mulatta). Each monkey was fed twice daily and maintained on a diet of monkey biscuits (Monkey Diet 5038, LabDiet, Brentwood, MO) and a variety of fresh fruit/vegetables in addition to banana-flavored pellets delivered during operant sessions (see below). The amount of food provided each day (typically 12-20 biscuits, with 8 banana-flavored pellets substituting for one biscuit) was adjusted according to the animal's size to maintain a normal body weight. Water was freely available at all times. The monkeys were housed in a humidity and temperature controlled room with a 12 hr light-dark cycle (lights on from 6 a.m. to 6 p.m., E.S.T.).
Monkeys were surgically implanted (under aseptic conditions) with Silicone rubber catheters (inside diameter 0.8 mm; outside diameter 2.4 mm). Catheters were implanted into a jugular or femoral vein and exited in the midscapular region. The intravenous (i.v.) catheter was protected by a tether system consisting of a custom-fitted nylon vest and stainless steel harness, connected to a flexible stainless steel cable and fluid swivel (Lomir Biomedical, Montreal, Canada). This flexible tether system permitted the monkeys to move freely. Catheter patency was occasionally evaluated by i.v. administration of ketamine (5 mg/kg). The catheter was considered patent if ketamine produced a loss of muscle tone within 10 seconds after its administration. Monkeys were also anesthetized with ketamine (10 mg/kg, i.m.) every other week to allow for cage changes and physical examination by a veterinarian. On these days, experimental sessions were suspended. When a catheter failed during the experiment, the catheter was surgically removed, the monkey was allowed to recover for at least 2 weeks, and then surgery was performed to implant a catheter in another available vein.
Animal maintenance and research were conducted in accordance with the NIH Guidelines for Using Animals in Intramural Research. The facility was accredited by the AAALAC, and protocols were approved by the NIDA-IRP Institutional Animal Care and Use Committee. Monkeys received environmental enrichment and had visual, auditory and olfactory contact with other monkeys throughout the study.
Apparatus
Each monkey was housed individually in a well-ventilated stainless steel cage (81 X 75 X 86 cm, Britz-Heidbrink, Weatland, WY). The cages were arranged in a one-over-one configuration with all the monkeys housed in a single room. The tether was mounted to the fluid swivel in the center of the back panel 62 cm above the cage floor. An 18 cm shelf ran along the back wall, 25 cm off the floor. This shelf allowed the monkeys to sit off the floor of the cage. The home cages of all monkeys were modified to include an operant-training panel (38 X 37 cm) mounted in the upper left corner of the front wall. Two circular translucent response keys (4.5 cm, PPC-003, BRS/LVE Laurel, MD) were arranged 9.0 cm apart (center to center) in a horizontal row 55 cm from the cage floor. The left key was 15 cm from the left wall. Each key could be transilluminated by a red or green stimulus light. The operant panel also supported an externally-mounted pellet dispenser (Gerbrands, Model G5310 or BRS/LVE series PDC/PPD) that delivered 1 gm banana-flavored food pellets (F0022, Bioserv, Frenchtown, NJ) to a food receptacle mounted on the cage beneath the operant response panel. Two infusion pumps (Model 55-7766, Harvard Apparatus, South Natick, MA) were situated behind each cage for delivery of saline or drug solutions through the intravenous catheter. The tubing from the pumps were connected to a single line through a series of stopcocks that then connected to the fluid swivel on the back of the cage. The capacity of this single line, including the catheter implanted in the monkey was approximately 1 ml. One pump operated continuously (except during drug self-administration components) to deliver saline at a rate of 0.03 ml/min to maintain catheter patency. A second pump delivered the self-administered drug. Operation of the operant panels, pumps and data collection were accomplished with a PC-compatible computer. The experimental sessions were controlled by customized software written in MedState Notation (Med Associates, St. Albans, VT).
Procedure
Food and i.v. injections of methamphetamine were available during three alternating schedule components: an initial 5-min food component, a drug component (90- or 180-min), and a second 5-min food component. Most monkeys were initially trained with the 180-min drug component. During this time, saline was frequently substituted for methamphetamine to facilitate extinction training. The drug component time was then reduced to 90-min. Some monkey's extinction behavior was disrupted by this change, and their drug component length was again increased to 180 min. Frequent saline substitutions continued throughout training and testing (at least once every 2–3 weeks). Both food and i.v. injections were available under a fixed-ratio (FR 10) schedule of reinforcement. During the two food components, the response key was transilluminated red. During the drug component, the response key was transilluminated green. Which key (left or right) was illuminated and available for responding was counterbalanced across monkeys. Following the delivery of each food pellet or drug injection, there was a 10-sec timeout period, during which the stimulus light illuminating the response key was turned off and responding has no scheduled consequence. The food and drug components were separated by 5-min timeout periods, during which the response key was dark and responding had no scheduled consequences. One min prior to the start of the drug component, the saline pump was turned off and 2 drug injections were given separated by 30 sec to fill the dead space in the catheter. Sessions began at noon, 7 days per week.
During training, the methamphetamine solution available for self-administration during the drug component was either 5 μg/kg/injection (monkeys C219, C253) or 10 μg/kg/injection (monkeys C254, C265, C166, RQ4988, RQ5017, RQ4339). The training dose was chosen based on the stability of responding maintained at that dose. All self-administration injections were given in a volume of 0.5 ml and at a rate of 3.0 ml/min. Dose was adjusted by changing the concentration of the solution.
Responding by monkeys was maintained on methamphetamine injections throughout training. Throughout initial training and during maintenance, saline was routinely substituted for methamphetamine to insure the rapid extinction of methamphetamine self-administration. They were tested with various pretreatment doses of methamphetamine (i.m., 15 min prior to the session), bupropion (i.m., 20 min prior to the session), methylphenidate (i.m., 20 min prior to the session) or saline. The doses and pretreatment times for methamphetamine where chosen based on preliminary data from our laboratory. Starting doses for bupropion (0.32 mg/kg) and methylphenidate (0.1 mg/kg) were selected based on results from prior drug discrimination testing. Both compounds had been evaluated, using a 20 minute pretreatment time, in rhesus monkeys trained to discriminate 0.4 mg/kg cocaine from saline (unpublished NIDA contract data). The selected starting doses were slightly below the ED50 values observed for substitution in the cocaine drug discrimination monkeys. During dose-effect testing of the treatment compounds, the dose of methamphetamine available for self-administration was fixed at either 3 or 5 μg/kg/injection. Subsequently, saline or a fixed pretreatment dose of methamphetamine (0.3 or 1.0 mg/kg), bupropion (3.2 mg/kg), or methylphenidate (0.56 mg/kg) was tested during individual sessions in which varying doses of self-administered methamphetamine were substituted for the training dose. All dose testing was done in a random order within pretreatment drugs. At least 2 days of stable (± 20%) baseline training separated each test session. Not all animals were tested with every pretreatment drug. The order of pretreatment testing was methylphenidate, bupropion and methamphetamine. Methamphetamine was tested after studies investigating cannabinoid antagonists (Schindler et al., 2010), and a slightly different set of self-administered methamphetamine doses (1, 3 10 and 30 μg/kg/injection) was used for determining the methamphetamine pretreatment dose-effect function than was used when studying bupropion (1, 2.5, 5, 10 and 25 μg/kg/injection) or methylphenidate (1, 2.5, 5, 10, 25 and 50 μg/kg/injection)
Data Analysis
The principal dependent variables were the response rates during each of the food and drug components. The response rate during each component was calculated as the total responses during the component divided by the component time exclusive of timeouts. As responding during the second food component was affected by the amount of methamphetamine taken during the drug component, response rates in the second food component were not included in the analysis. The procedure of Tabatchnick and Fidell (2007) was used to screen for outliers in the data. One outlier was removed from the methamphetamine pretreatment data at the lowest methamphetamine self-administration dose. One outlier was removed from the bupropion pretreatment data at the highest methamphetamine self-administration dose, and one outlier was removed from the methylphenidate pretreatment data at the lowest methamphetamine self-administration dose. Data were then analyzed using the Mixed procedure (SAS Institute, Cary, NC) ANOVA, which is capable of analyzing data sets for which some subjects were not tested under all conditions. Appropriate follow-up tests for individual effects were performed using the Tukey-Kramer or Dunnett (for comparisons only to a control) tests, maintaining a 0.05 significance level within each set of comparisons. Separate analyses were performed for the results for food and methamphetamine. Only the first 90 min of the drug component were used in the calculation of results. This was done to maintain consistency in analysis between animals with different drug component durations.
Drugs
Methamphetamine hydrochloride (Sigma Chemical, St. Louis, MO) for self-administration was dissolved in sterile saline in a stock concentration of 50 mg/ml. Solutions (in 500 or 1000 ml sterile saline bags) were prepared from this stock solution for each monkey based on the animal's weight. Care was taken to maintain the sterility of the solution. Depending on overall intake, a solution bag could last 1 to 2 weeks. Doses are for the salt. For pretreatments, methamphetamine was given in a volume of 0.02 ml/kg. Bupropion hydrochloride (NIDA, Bethesda) and methylphenidate hydrochloride (NIDA, Bethesda) were both dissolved in sterile saline and given in a volume not exceeding 0.05 ml/kg. All pretreatment injections were given i.m. in the leg.
Results
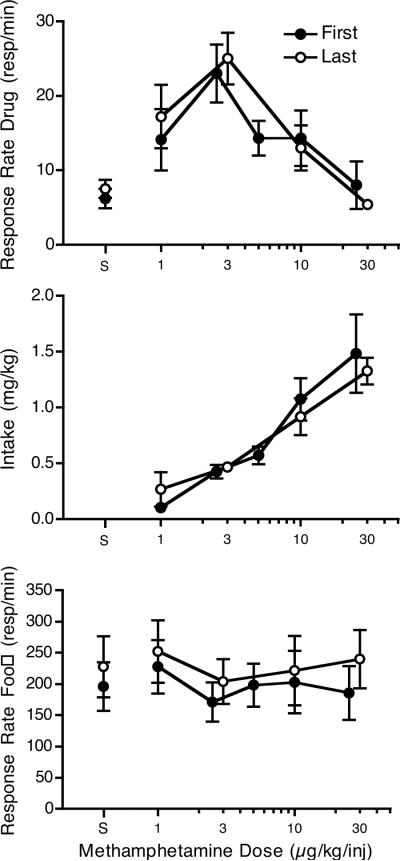
Six monkeys completed the methamphetamine pretreatment and at least one of the other 2 pretreatments. Data from these monkeys were analyzed to assess potential changes in the methamphetamine dose-effect function due to long-term exposure to methamphetamine and the self-administration procedure. These monkeys had an average of 40.7 ± 9.9 months (range 20 to 72) between their first and last methamphetamine dose-effect determination. During that time the monkeys self-administered an average of 0.63 ± 0.12 mg/kg methamphetamine per session (days of saline substitution or time between surgeries were not included). Figure 1 shows average dose-effect functions for the monkey's first and last determinations. The dose-effect functions were remarkably similar. For statistical comparisons, the 2.5 and 3 μg/kg/injection doses and the 25 and 30 μg/kg/injection doses were analyzed as a single dose and the 5 μg/kg/injection dose was dropped from the analysis. This analysis showed that for response rate, neither the time (first or last determination) nor time x dose factors were significant. A significant dose effect was observed (F4,20 = 10.83, p < 0.001), with the 3/2.5 μg/kg/injection dose being significantly different in follow-up tests from saline and the 10 and 25/30 μg/kg/injection doses. As with response rate, only the dose factor was significant for intake (F3,15 = 19.61, p < 0.001). Follow-up tests revealed that both the 10 and 25/30 μg/kg/injection doses were significantly different from the 1 and 2.5/3 μg/kg/injection doses. For responding during the first food component, there were no significant effects of time or dose.
Figure 1.
Comparison of the first and last methamphetamine dose-effect determination in six monkeys that were treated with i.m. methamphetamine as a pretreatment prior to the last determination on drug response rate (top panel), drug intake (middle panel) and response rate during the first food component (bottom panel). Saline was given as a pretreatment for the two dose-effect functions presented. There was an average of 40.7 ± 9.9 months (range 20 to 72) between their first and last methamphetamine dose-effect determination.
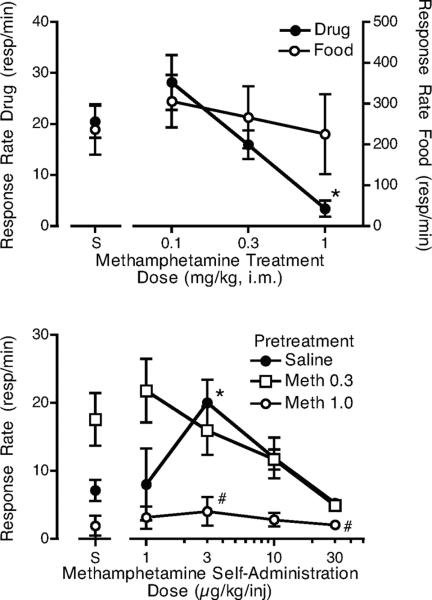
When various doses of methamphetamine were given prior to sessions in which the monkeys self-administered 3 μg/kg/injection methamphetamine (Figure 2, top panel), a significant effect of the pretreatment was observed (F3,12 = 15.6, p <0.01). The highest dose of methamphetamine significantly reduced methamphetamine self-administration. Responding during the food component, which preceded the self-administration component, was not significantly affected by methamphetamine pretreatment. When 0.3 and 1.0 mg/kg methamphetamine were given as pretreatments prior to the determination of the methamphetamine dose-effect function, responding for food was not affected (average rates for pretreatment with saline: 236.7 ± 48.7; 0.3 mg/kg: 279.7 ± 76.6; 1.0 mg/kg: 217.2 ± 71.8). However, the highest methamphetamine pretreatment dose significantly reduced methamphetamine self-administration at two self-administration doses (Figure 2, bottom panel, F2,24 = 4.5, p < 0.01). The lower methamphetamine dose appeared to increase self-administration at the lowest methamphetamine self-administration doses and for saline, but these effects were not significant. Of the 4 monkeys tested at a pretreatment dose of 0.3 mg/kg, 3 showed increases in responding for saline and 2 showed increases in responding for 1 μg/kg/injection methamphetamine. For the other monkeys, responding was similar to saline pretreatment (i.e., not reduced). As would be expected with the relatively large changes in response rate, when intake was calculated (Figure 3, top panel) the 1.0 mg/kg methamphetamine pretreatment dose significantly reduced methamphetamine intake during the self-administration component (F6,17 = 10.5, p < 0.01). The lower pretreatment dose of methamphetamine did not affect intake at any self-administration dose.
Figure 2.
The top panel shows the effects of pretreatment with three doses of i.m. methamphetamine or saline on responding for 3 μg/kg/injection i.v. methamphetamine or food. Each curve is the mean of 4–5 monkeys (*p < 0.05 from saline pretreatment). The bottom panel shows the effects of pretreatment with saline or with either 0.3 or 1.0 mg/kg i.m. methamphetamine on responding for various self-administration doses of i.v. methamphetamine or saline (S). Each curve is the mean of five monkeys. *p < 0.05 from saline self-administration following saline pretreatment, #p < 0.05 from saline pretreatment at the comparable i.v. methamphetamine dose.
Figure 3.
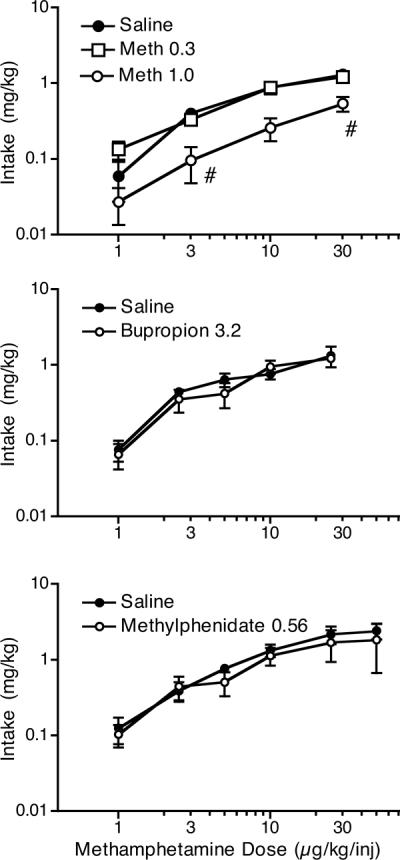
Methamphetamine intake at various i.v. methamphetamine self-administration doses following pretreatment with saline, or 0.3 or 1.0 mg/kg i.m. methamphetamine (top panel); following pretreatment with saline or 3.2 mg/kg i.m. bupropion (middle panel); or following pretreatment with saline or 0.56 mg/kg i.m. methylphenidate. #p < 0.05 from saline pretreatment at the comparable i.v. methamphetamine dose.
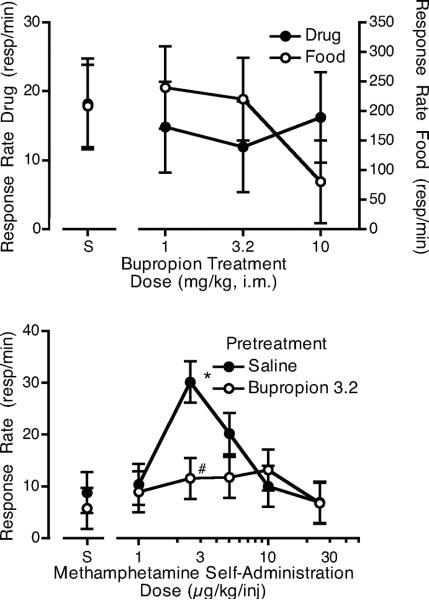
The 10 mg/kg dose of bupropion appeared to reduce responding for food in at least some animals (Figure 4). Therefore, the 3.2 mg/kg dose of bupropion was chosen as a pretreatment for the methamphetamine dose-effect function. The 3.2 mg/kg dose of bupropion did not decrease responding for food (average rates for pretreatment with saline: 204.3 ± 50.8; bupropion: 267.3 ± 78.8). When 3.2 mg/kg bupropion was given as a pretreatment during the determination of the methamphetamine dose-effect function, the methamphetamine dose-effect function appeared to be flattened in comparison to the function following saline pretreatment. While the bupropion x methamphetamine dose interaction was not significant (F5,14 = 2.75, p = 0.06), planned comparisons confirmed that bupropion reduced responding for methamphetamine compared to saline pretreatment at the 2.5 μg/kg/injection dose. Of the 4 monkeys tested, 3 showed evidence of a reduction in responding following bupropion for at least 1 dose of methamphetamine. Intake of methamphetamine was not significantly different from the saline pretreatment condition (Figure 3, middle panel).
Figure 4.
The top panel shows the effects of pretreatment with 3 doses of i.m. bupropion or saline on responding for 5 μg/kg/injection i.v. methamphetamine or food. Each curve is the mean of four monkeys. The bottom panel shows the effects of pretreatment with saline or 3.2 mg/kg i.m. bupropion on responding for various self-administration doses of i.v. methamphetamine or saline (S). Each curve is the mean of four monkeys. *p < 0.05 from saline self-administration following saline pretreatment, #p < 0.05 from saline pretreatment at the comparable i.v. methamphetamine dose.
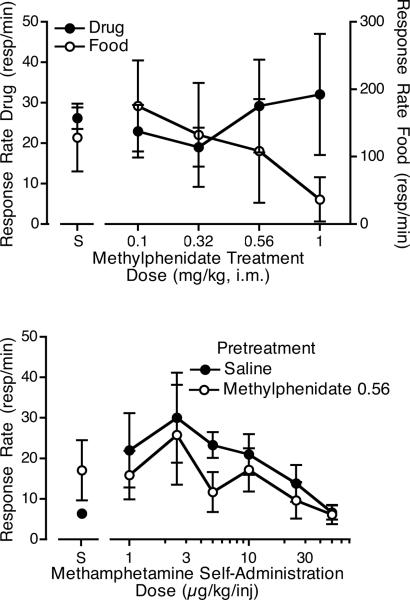
When various doses of methylphenidate were evaluated as a pretreatment (Figure 5, top panel), the 1 mg/kg dose of methylphenidate substantially reduced rates of responding for food. For this reason, the 0.56 mg/kg dose of methylphenidate was chosen for evaluation as a pretreatment to the methamphetamine dose-effect determination. When 0.56 mg/kg methylphenidate was given as a pretreatment during the determination of the methamphetamine dose-effect function, responding for food was not affected (average rates for pretreatment with saline: 129.6 ± 33.4; methylphenidate: 119.5 ± 31.0). Overall responding for methamphetamine was slightly reduced; however, the methylphenidate x methamphetamine dose interaction was not significant (F6,18 = 2.25, p = 0.068), and planned comparisons did not show any significant changes in responding for methamphetamine compared to saline pretreatment at any dose. Intake of methamphetamine was not significantly different from the saline pretreatment condition following methylphenidate pretreatment (Figure 3, bottom panel).
Figure 5.
The top panel shows the effects of pretreatment with four doses of i.m. methylphenidate or saline on responding for 5 μg/kg/injection i.v. methamphetamine or food. Each curve is the mean of three monkeys. The bottom panel shows the effects of pretreatment with saline or 0.56 mg/kg i.m. methylphenidate on responding for various self-administration doses of i.v. methamphetamine or saline (S). Each curve is the mean of results with four monkeys.
Discussion
The most robust effect of the pretreatments was the reduction in i.v. methamphetamine self-administration by pretreatment with high-dose i.m. methamphetamine. The dose of 1.0 mg/kg methamphetamine reduced self-administration at every methamphetamine dose, but did not significantly decrease responding for food. The lower dose of methamphetamine (0.3 mg/kg) did not reduce self-administration, and in the majority of subjects it actually increased self-administration of saline or of the lowest dose of methamphetamine (a dose that was not reinforcing under normal conditions). Although these increases in responding did not reach statistical significance in this small-N study, the possibility of such reinstatement-like effects is an important consideration in evaluating agonist type treatments. These results suggest that pretreatment with a drug that directly mimics the effects of self-administered methamphetamine can reduce self-administration if the dose is sufficient. If too low a dose is used, then there may be a risk of increasing self-administration, possibly due to priming effects like those that can occur following extinction of self-administration and subsequent administration of a stimulant.
While it appears that the effects of methamphetamine pretreatment on methamphetamine self-administration have not been previously studied, the present results do agree with other studies using amphetamines as pretreatments. For example, d-amphetamine has been shown to decrease self-administration of i.v. cocaine in rats (Chiodo et al., 2008; Chiodo and Roberts, 2009; Lynch et al. 1998; Peltier et al., 1996) and monkeys (Foltin and Evans, 1999; Glowa et al., 1995; Herling et al., 1979; Mansbach and Balster 1993; Negus, 2003; Negus and Mello, 2003a, 2003b). Methamphetamine has also been shown to decrease cocaine self-administration in both rats (Peltier et al., 1996) and monkeys (Negus et al., 2007). Another psychostimulant, cocaine, has been shown to decrease cocaine self-administration in monkeys (Herling et al., 1979; Mansbach and Balster, 1993; Panlilio et al. 1998). In humans, amphetamine has been shown to reduce use of both amphetamine (Charnaud and Griffiths, 1998; Fleming and Roberts, 1994; White, 2000) and cocaine (Grabowski et al., 2001; Rush et al., 2009; Shearer et al., 2003). In addition, methamphetamine has been show to reduce cocaine use in humans (Mooney et al., 2009).
While a number of studies have shown the potential utility of amphetamines in reducing the self-administration of cocaine and amphetamine, the specificity of that effect to drug self-administration has not always been clear. A number of studies that showed decreases in self-administration also showed similar decreases in responding for alternative reinforcers such as food (Foltin and Evans, 1999; Herling et al., 1979; Mansbach and Balster, 1993). One factor that might account for this difference is the development of tolerance to the rate-reducing effect of methamphetamine on food responding that could have occurred in the present study. Monkeys responded for food during the second food component after they had completed their methamphetamine self-administration component, potentially allowing tolerance to develop. Some support for this interpretation can be found by comparing rates in the second food component following responding for either 25 or 30 μg/kg/injection in the sessions shown in Figure 1. During the first determination of the dose effect function rates in the second food component were 85.5 ± 25.1 responses/min, while for the second determination they were 152.0 ± 74.7 responses/min. These results may indicate a development of tolerance although, as the large variability in the second determination indicates, some monkeys still had very low levels of responding in the second food component following extended training. The changes would not reflect any change due to experience with the FR schedule or age as responding in the second food component, like that in the first food component, following saline self-administration was not changed (first determination: 193.9 ± 11.4, second determination: 230.6 ± 29.7).
In other studies that have shown specificity for the effects of amphetamine on drug self-administration, similar multiple schedules or choice schedules have been used (Glowa et al., 1995; Negus, 2003) or amphetamine treatment has been continued during food sessions (Negus and Mello, 2003a, 2003b). In contrast, Herling et al. (1979) trained different groups of monkeys on cocaine self-administration and food. In the Foltin and Evans (1999) study, monkeys responded for oral cocaine or food in separate chambers, possibly negating the development of tolerance due to the different contexts. However, Mansbach and Balster (1993) used a procedure very similar to that used here and found that amphetamine treatment produced similar effects on both cocaine self-administration and on responding for food. This result suggests that factors other than tolerance may contribute to whether amphetamine pretreatment decreases food reinforced responding. An additional factor that might determine whether specificity is observed is the rate of responding maintained by the two reinforcers. In the current study, food maintained much higher rates of responding than did methamphetamine (see Figure 2). Typically, a drug pretreatment would be expected to reduce a high rate behavior more than a low rate behavior, an effect known as rate dependency (Dews, 1977). However, the higher rate of food responding may also indicate that the behavior is under stronger stimulus control, which may negate the effect of the pretreatment since behavior under stronger stimulus control is typically less sensitive to the effects of psychostimulants (Thompson and Moerschbaecher, 1979).
Since the effects of methamphetamine pretreatment were so striking, it might be expected that other treatments that lead to the activation of the dopamine system would also decrease methamphetamine self-administration. It should be noted that both methamphetamine and the other drugs tested also affect norepinephrine, so a contributing, or even primary, role for norepinephrine in these effects cannot be ruled out. In fact, bupropion, like methamphetamine, also decreased methamphetamine self-administration. Bupropion has not been tested as extensively as the amphetamines as a treatment for psychomotor stimulant self-administration, but there is some evidence for its usefulness. Reichel et al. (2009) showed that bupropion could decrease methamphetamine self-administration in rats with less of an effect on responding for sucrose. However, in an earlier study, Reichel et al. (2008) failed to observe specificity when testing bupropion on the acquisition of methamphetamine self-administration. In an early study of the effects of bupropion on human methamphetamine users, Newton et al. (2005) showed that bupropion could decrease the subjective effects of methamphetamine. Two recent double-blind clinical trials (Elkashef et al., 2008; Shoptow et al., 2008) failed to show a significant effect of bupropion on methamphetamine use in all subjects; however, in both trials bupropion significantly reduced methamphetamine use in a subgroup of subjects with a lower baseline level of use. In clinical trials looking at the effectiveness of bupropion in cocaine dependence, Margolin et al. (1995) failed to observe a positive effect, while Poling et al. (2006) reported that bupropion improved treatment outcomes. Therefore, while the effectiveness of bupropion may not match that of the amphetamines, the data presented here and from previous research do support the potential use of this drug in treatment settings.
In contrast to bupropion, there was no evidence that methylphenidate decreased methamphetamine self-administration. If anything, methylphenidate appeared to have a larger effect on food responding than it did on methamphetamine self-administration. In a study in rats, Thanos et al. (2007) reported that methylphenidate decreased the acquisition of cocaine self-administration and Levin et al. (1998) reported positive effects of methylphenidate in a non-controlled human trial. In a controlled clinical trial, Tiihonen et al. (2007) also reported that methylphenidate decreased amphetamine positive urines. However, no effect of methylphenidate was observed in two other controlled clinical trials (Grabowski et al., 1997; Schubiner et al., 2002). Therefore, the use of methylphenidate in the treatment of psychomotor stimulant abuse is not supported by the current results.
It is not clear why methamphetamine treatment was so effective in reducing methamphetamine self-administration while other agonists that share some of the same effects (e.g., bupropion and methylphenidate) were arguably less effective. The simplest explanation is that the closer a drug's overall effects are to the abused drug, the more likely it is to reduce the self-administration of the abused drug when given as a pretreatment. Obviously, i.m. methamphetamine would share many subjective and physiological effects with i.v. methamphetamine. Even with methamphetamine, however, a low dose was not effective at decreasing self-administration. At the peak of the dose-effect function (3 μg/kg/injection), intake of methamphetamine was less than 0.5 mg/kg over the course of the session. To block that intake required a 1 mg/kg dose of methamphetamine that clearly exceeded intake. This result agrees with a previous study looking at the effect of cocaine as a pretreatment to cocaine self-administration in rhesus monkeys (Panlilio et al., 1998). When cocaine was delivered non-contingently as a slow infusion, Panlilio et al. found that self-administration of cocaine “… was decreased [only] when the total non-contingent dose was 2–3 times the amount usually self-administered during a session.” These results are consistent with the hypothesis that drug intake in highly-experienced drug users is regulated largely by the drug's subjective effects (see Panlilio et al., 2008), such that an agonist-like treatment must produce a level of subjective effect comparable to, or exceeding that of the abused drug to substantially reduce drug seeking. Thus, when testing potential replacement-type therapies, it may not be surprising that drugs that only partially mimic the spectrum of effects produced by methamphetamine are only partially effective at decreasing methamphetamine self-administration. Nevertheless, in treatment settings, factors such as safety and abuse liability must be taken into consideration and these drugs may be preferred over methamphetamine itself.
A number of the monkeys in the current study had self-administered methamphetamine over a long period of time. Despite this long-term use, there was no evidence that the behavioral effects of methamphetamine as measured by self-administration were altered. It is possible that any chronic effects of methamphetamine that would alter this behavior occurred prior to the determination of the first methamphetamine dose-effect function. However, these results do support the continued use of within-subjects designs in monkeys in testing potential treatments for methamphetamine abuse. The long-term use of methamphetamine did not alter the dose-effect function for methamphetamine after the first dose-effect determination, negating the need to account for any baseline change over the course of training.
In conclusion, pretreatment with i.m. methamphetamine reduced i.v. methamphetamine self-administration without affecting responding for food reinforcement. This effect of i.m. methamphetamine was evident only at a relatively high dose. At a lower pretreatment dose, i.m. methamphetamine actually increased self-administration of i.v. saline and a sub-reinforcing dose of i.v. methamphetamine in some monkeys, suggesting that, like cocaine (Panlilio et al., 1998), methamphetamine pretreatment may prime methamphetamine self-administration under some conditions. Bupropion significantly reduced methamphetamine self-administration at one dose but showed no evidence of stimulating or priming self-administration. Given its lower abuse potential, these results indicate that an agonist-like treatment like bupropion can be effective in specifically decreasing methamphetamine self-administration and, therefore, some version of this treatment strategy may prove effective in treating methamphetamine abuse.
Acknowledgement
This research was supported by the Intramural Research Program of the NIH, NIDA and by the NIDA Division of Pharmacotherapies and Medical Consequences of Drug Abuse.
References
- Charnaud B, Griffiths V. Levels of intravenous drug misuse among clients prescribed oral dexamphetamine or oral methadone: a comparison. Drug Alcohol Depend. 1998;52:79–84. doi: 10.1016/s0376-8716(98)00052-0. [DOI] [PubMed] [Google Scholar]
- Chiodo KA, Lack CM, Roberts DC. Cocaine self-administration reinforced on a progressive ratio schedule decreases with continuous D-amphetamine treatment in rats. Psychopharmacology (Berl) 2008;200:465–473. doi: 10.1007/s00213-008-1222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodo KA, Roberts DC. Decreased reinforcing effects of cocaine following 2 weeks of continuous D-amphetamine treatment in rats. Psychopharmacology (Berl) 2009;206:447–456. doi: 10.1007/s00213-009-1622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dews PB. Rate-dependency hypothesis. Science. 1977;198:1182–1183. doi: 10.1126/science.563103. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Rauhut AS, King-Pospisil KA, Bardo MT. Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent. CNS Drug Rev. 2006;12:178–207. doi: 10.1111/j.1527-3458.2006.00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33:1162–1170. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- Fleming PM, Roberts D. Is the prescription of amphetamine justified as a harm reduction measure? J R Soc Health. 1994;114:127–131. doi: 10.1177/146642409411400303. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Evans SM. The effects of d-amphetamine on intake of food and a sweet fluid containing cocaine. Pharmacol Biochem Behav. 1999;62:457–464. doi: 10.1016/s0091-3057(98)00217-2. [DOI] [PubMed] [Google Scholar]
- Glowa JR, Wojnicki FHE, Matecka D, Rice KC, Rothman RB. Effects of dopamine reuptake inhibitors on food- and cocaine-maintained responding: II. Comparisons with other drugs and repeated administration. Exp Clin Psychopharmacology. 1995;3:232–239. [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, et al. Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J Clin Psychopharmacol. 2001;21:522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Roache JD, Schmitz JM, Rhoades H, Creson D, Korszun A. Replacement medication for cocaine dependence: methylphenidate. J Clin Psychopharmacol. 1997;17:485–488. doi: 10.1097/00004714-199712000-00008. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav. 2004;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Cheetham SC, Smith SL. The neuropharmacology of ADHD drugs in vivo: insights on efficacy and safety. Neuropharmacology. 2009;57:608–618. doi: 10.1016/j.neuropharm.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Herin DV, Rush CR, Grabowski J. Agonist-like pharmacotherapy for stimulant dependence: preclinical, human laboratory, and clinical studies. Ann N Y Acad Sci. 2010;1187:76–100. doi: 10.1111/j.1749-6632.2009.05145.x. [DOI] [PubMed] [Google Scholar]
- Herling S, Downs DA, Woods JH. Cocaine, d-amphetamine, and pentobarbital effects on responding maintained by food or cocaine in rhesus monkeys. Psychopharmacology (Berl) 1979;64:261–269. doi: 10.1007/BF00427508. [DOI] [PubMed] [Google Scholar]
- Karila L, Weinstein A, Aubin H-J, Benyamina A, Reynaud M, Batki SL. Pharmacological approaches to methamphetamine dependence: a focused review. Brit. J. Clin. Pharmacol. 2010;69:578–592. doi: 10.1111/j.1365-2125.2010.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin FR, Evans SM, McDowell DM, Kleber HD. Methylphenidate treatment for cocaine abusers with adult attention-deficit/hyperactivity disorder: a pilot study. J Clin Psychiatry. 1998;59:300–305. doi: 10.4088/jcp.v59n0605. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Heaser WA, Carroll ME. Effects of amphetamine, butorphanol, and morphine pretreatment on the maintenance and reinstatement of cocaine-reinforced responding. Exp Clin Psychopharmacol. 1998;6:255–263. doi: 10.1037//1064-1297.6.3.255. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Balster RL. Effects of mazindol on behavior maintained or occasioned by cocaine. Drug Alcohol Depend. 1993;31:183–191. doi: 10.1016/0376-8716(93)90071-w. [DOI] [PubMed] [Google Scholar]
- Margolin A, Kosten TR, Avants SK, Wilkins J, Ling W, Beckson M, et al. A multicenter trial of bupropion for cocaine dependence in methadone-maintained patients. Drug Alcohol Depend. 1995;40:125–131. doi: 10.1016/0376-8716(95)01198-6. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Herin DV, Schmitz JM, Moukaddam N, Green CE, Grabowski J. Effects of oral methamphetamine on cocaine use: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2009;101:34–41. doi: 10.1016/j.drugalcdep.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:919–931. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a second-order schedule in rhesus monkeys. Drug Alcohol Depend. 2003a;70:39–52. doi: 10.1016/s0376-8716(02)00339-3. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a progressive-ratio schedule in rhesus monkeys. Psychopharmacology (Berl) 2003b;167:324–332. doi: 10.1007/s00213-003-1409-y. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB. Monoamine releasers with varying selectivity for dopamine/norepinephrine versus serotonin release as candidate “agonist” medications for cocaine dependence: studies in assays of cocaine discrimination and cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320:627–636. doi: 10.1124/jpet.106.107383. [DOI] [PubMed] [Google Scholar]
- Newton TF, Roache JD, De La Garza R, 2nd, Fong T, Wallace CL, Li SH, et al. Safety of intravenous methamphetamine administration during treatment with bupropion. Psychopharmacology (Berl) 2005;182:426–435. doi: 10.1007/s00213-005-0102-8. [DOI] [PubMed] [Google Scholar]
- Newton TF, Roache JD, De La Garza R, 2nd, Fong T, Wallace CL, Li SH, et al. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacology. 2006;31:1537–1544. doi: 10.1038/sj.npp.1300979. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Goldberg SR, Gilman JP, Jufer R, Cone EJ, Schindler CW. Effects of delivery rate and non-contingent infusion of cocaine on cocaine self-administration in rhesus monkeys. Psychopharmacology (Berl) 1998;137:253–258. doi: 10.1007/s002130050618. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Thorndike EB, Schindler CW. A stimulus-control account of regulated drug intake in rats. Psychopharmacology (Berl) 2008;196:441–450. doi: 10.1007/s00213-007-0978-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier RL, Li DH, Lytle D, Taylor CM, Emmett-Oglesby MW. Chronic d-amphetamine or methamphetamine produces cross-tolerance to the discriminative and reinforcing stimulus effects of cocaine. J Pharmacol Exp Ther. 1996;277:212–218. [PubMed] [Google Scholar]
- Poling J, Oliveto A, Petry N, Sofuoglu M, Gonsai K, Gonzalez G, et al. Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Arch Gen Psychiatry. 2006;63:219–228. doi: 10.1001/archpsyc.63.2.219. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Linkugel JD, Bevins RA. Bupropion differentially impacts acquisition of methamphetamine self-administration and sucrose-maintained behavior. Pharmacol Biochem Behav. 2008;89:463–472. doi: 10.1016/j.pbb.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Murray JE, Grant KM, Bevins RA. Bupropion attenuates methamphetamine self-administration in adult male rats. Drug Alcohol Depend. 2009;100:54–62. doi: 10.1016/j.drugalcdep.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Dual dopamine/serotonin releasers: potential treatment agents for stimulant addiction. Exp Clin Psychopharmacol. 2008;16:458–474. doi: 10.1037/a0014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Hays LR. Cocaine effects during D-amphetamine maintenance: a human laboratory analysis of safety, tolerability and efficacy. Drug Alcohol Depend. 2009;99:261–271. doi: 10.1016/j.drugalcdep.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Panlilio LV, Gilman JP, Justinova Z, Vemuri VK, Makriyannis A, et al. Effects of cannabinoid receptor antagonists on maintenance and reinstatement of methamphetamine self-administration in rhesus monkeys. Eur J Pharmacol. 2010;633:44–49. doi: 10.1016/j.ejphar.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubiner H, Saules KK, Arfken CL, Johanson CE, Schuster CR, Lockhart N, et al. Double-blind placebo-controlled trial of methylphenidate in the treatment of adult ADHD patients with comorbid cocaine dependence. Exp Clin Psychopharmacol. 2002;10:286–294. doi: 10.1037//1064-1297.10.3.286. [DOI] [PubMed] [Google Scholar]
- Shearer J, Wodak A, van Beek I, Mattick RP, Lewis J. Pilot randomized double blind placebo-controlled study of dexamphetamine for cocaine dependence. Addiction. 2003;98:1137–1141. doi: 10.1046/j.1360-0443.2003.00447.x. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Heinzerling KG, Rotheram-Fuller E, Steward T, Wang J, Swanson AN, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96:222–232. doi: 10.1016/j.drugalcdep.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Experimental design using ANOVA. Duxbury; Belmont, CA: 2007. [Google Scholar]
- Thanos PK, Michaelides M, Benveniste H, Wang GJ, Volkow ND. Effects of chronic oral methylphenidate on cocaine self-administration and striatal dopamine D2 receptors in rodents. Pharmacol Biochem Behav. 2007;87:426–433. doi: 10.1016/j.pbb.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Thompson DM, Moerschbaecher JM. An experimental analysis of the effects of d-amphetamine and cocaine on the acquisition and performance of response chains in monkeys. J Exp Anal Behav. 1979;32:433–444. doi: 10.1901/jeab.1979.32-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J, Kuoppasalmi K, Föhr J, Tuomola P, Kuikanmäki O, Vorma H, et al. A comparison of aripiprozole, methylphenidate and placebo for amphetamine dependence. Am. J. Psychiatry. 2007;164:160–162. doi: 10.1176/ajp.2007.164.1.160. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Elkashef A. Pharmacotherapy and other treatments for cocaine abuse and dependence. Curr Opin Psychiatry. 2005;18:265–270. doi: 10.1097/01.yco.0000165596.98552.02. [DOI] [PubMed] [Google Scholar]
- White R. Dexamphetamine substitution in the treatment of amphetamine abuse: an initial investigation. Addiction. 2000;95:229–238. doi: 10.1046/j.1360-0443.2000.9522299.x. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res. 2008;14:169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]