Summary
How CD4-CD8 expression is maintained in mature T cells is largely unknown. The present study has examined the role in this process of the zinc finger protein Zbtb7b, a critical factor for the commitment of MHC II-restricted thymocytes to the CD4 lineage, that remains expressed in mature CD4 cells. We show that Zbtb7b acts in peripheral CD4 cells to suppress CD8-lineage gene expression, including that of CD8 and cytotoxic effector genes perforin and Granzyme B, and is important for the proper repression of IFNγ during effector differentiation. The inappropriate expression of IFNγ by Zbtb7b-deficient CD4 cells required the activities of Eomesodermin and Runx3 transcription factors. Runx activity was needed for Granzyme B expression, indicating that Runx proteins control expression of the cytotoxic program. We conclude that a key function of Zbtb7b in the mature CD4 T cell compartment is to repress CD8-lineage gene expression.
Introduction
T lymphocytes (T cells) are critical for adaptive immune responses and are distributed into multiple lineages, the two predominant ones being defined by their reactivity against peptide antigens bound to classical MHC molecules and by their mutually exclusive expression of CD4 or CD8 surface glycoproteins (Janeway and Bottomly, 1994). CD4 T cells typically recognize MHC-II peptide complexes and upon effector differentiation control the function of other immunocompetent cells, either positively or negatively; in contrast, CD8 T cells generally recognize MHC-I peptide complexes and differentiate into cytotoxic effectors able to lyze virtually any nucleated cell given the broad pattern of MHC-I expression. Once established in the thymus (Singer and Bosselut, 2004), CD4-CD8 lineage differentiation is stably maintained in peripheral T cells and is inherited through cell division after activation and during effector differentiation.
Several transcription factors orchestrate the differentiation of CD4 and CD8 cells from bipotent precursors that express both CD4 and CD8 (double positive [DP] thymocytes). Two members of the Runx family, Runx1 and Runx3, contribute to CD8 cell differentiation and notably to the termination of CD4 expression (Taniuchi et al., 2002a; Woolf et al., 2003), whereas the HMG protein Tox and the zinc finger transcription factors Gata3 and Zbtb7b (Thpok, cKrox) promote the generation of CD4 T cells (Aliahmad and Kaye, 2008; Hernandez-Hoyos et al., 2003; Pai et al., 2003; Zhu et al., 2004; He et al., 2005; Sun et al., 2005, and Wang et al., submitted).
Little is known on how CD4-CD8 differentiation is maintained in peripheral T cells. Some of the factors that promote lineage-differentiation in the thymus carry distinct functions in post-thymic T cells. In the mature CD4 compartment, Runx3 contributes to IFNγ production and IL-4 repression during Type 1 effector differentiation (Djuretic et al., 2007; Naoe et al., 2007), whereas Runx1 promotes cell survival (Egawa et al., 2007). Gata3 promotes Type 2 effector differentiation and does not appear required to maintain the CD4 lineage (Zheng and Flavell, 1997; Pai et al., 2004; Zhu et al., 2004).
In differentiating thymocytes, Zbtb7b both promotes CD4-helper and represses CD8-cytotoxic differentiation (He et al., 2005; Sun et al., 2005). Thus, because Zbtb7b expression is CD4-lineage specific in peripheral T cells, it was possible that it promoted the maintenance of CD4-helper gene expression or the repression of CD8-cytotoxic gene expression, or both, in CD4 cells. In the present study, we tested these hypotheses using hypomorphic and conditional Zbtb7b alleles. We demonstrate that the key function of Zbtb7b in CD4 T cells is to prevent expression of CD8 and cytotoxic genes.
Results
Expression of Zbtb7b in mature CD4 T cells
We and others previously reported that Zbtb7b was expressed in CD4 but not CD8 peripheral T cells (He et al., 2005; Sun et al., 2005; Setoguchi et al., 2008). We more extensively examined the pattern of Zbtb7b expression in peripheral T cells using a BAC reporter transgene in which Zbtb7b cis-regulatory elements control the expression of a GFP cDNA inserted in the first Zbtb7b coding exon (Fig. 1A; Wang et al., submitted). This BAC drives unimodal GFP expression in spleen and lymph node (LN) CD4 but not CD8 cells, at levels similar to those observed in mature CD4 SP thymocytes (Fig. 1B). GFP levels were similar in naïve (CD44lo) and effector or memory-type cells (CD44hi), and in CD4+CD25+ Treg cells (Fig. 1C). GFP expression in CD4 T cells persisted after T cell activation and during in vitro effector differentiation under Type 1 (Th1) or Type 2 (Th2) culture conditions (Fig. 1D). These experiments indicate that Zbtb7b expression is maintained throughout the life of peripheral CD4 T cells, and prompted us to evaluate the role of Zbtb7b in CD4 T cell function.
Figure 1. Expression of Zbtb7b is CD4-specific.
(A). The transcriptional activity of Zbtb7b cis-regulatory elements was assessed in mice carrying a BAC reporter transgene in which the first coding exon of Zbtb7b was replaced by an eGFP cDNA (white box) (Wang et al., submitted), so that GFP fluorescence is a readout of Zbtb7b expression. Thick boxes depict coding sequences within the second and third Zbtb7b exons.
(B). Overlaid histograms show GFP fluorescence (plain lines) in CD4 and CD8 LN T cells and in TCRβhi CD4 and CD8 SP thymocytes that had down-regulated CD24 (Heat Stable Antigen), an event characteristic of the most mature SP thymocyte subset.
(C) Two parameter contour plots show CD44 or CD25 vs. GFP expression on gated CD4+CD8−peripheral T cells from Zbtb7b+/+ mice carrying the GFP BAC reporter. Numbers indicate the percentage of cells in each quadrant.
(D) Overlaid histograms show GFP fluorescence (plain lines) in CD4 T cells differentiating in vitro under Th1, Th2 or ThN conditions (see Methods), 1 to 4 days after activation (set as day 0). Dotted histograms show GFP fluorescence of fresh CD4 T cells analyzed in parallel. Gray shaded histograms in B, D show background fluorescence on non-transgenic cells of the same subset subject to the same treatment.
CD4 T cells with reduced Zbtb7b protein expression
Germline Zbtb7b inactivation prevents CD4 T cell development (He et al., 2005, and Wang et al., submitted) and therefore cannot be used to study the role of this factor in post-thymic CD4 cells. As an alternative strategy, we took advantage of the targeted allele (Zbtb7bt) we generated to disrupt Zbtb7b (Wang et al., submitted). This allele is engineered to reduce Zbtb7b protein expression through the insertion, immediately upstream of the first Zbtb7b coding exon, of a Neo cassette flanked by splice acceptor and transcription termination signals (Fig. SF1).
Mice carrying one or two copies of this allele (Zbtb7bt/− or Zbtb7bt/t) were fertile and did not display any gross developmental defect. The number of peripheral CD4 T cells was modestly reduced in Zbtb7bt/t mice, and most of them were naïve CD44lo cells (Fig. 2AB and Fig. SF2). The number of CD8 T cells was slightly increased, and analyses with TCR transgenic Zbtb7bt/t mice indicated that this resulted from the partial CD8-redirection of MHC II-restricted thymocytes (Fig. 2DE). Expression of Zbtb7b protein in peripheral CD4 cells was lower in Zbtb7bt/t than in wild-type mice (Fig. 2C); thus, the inserted Neo cassette impairs rather than prevents Zbtb7b protein expression, and the Zbtb7bt allele is hypomorphic. Changes in CD4 and CD8 single positive (SP) thymocyte subsets paralleled those seen in the periphery (Fig. 2AB and DE). Zbtb7bt/t thymocytes had normal expression of CD5 and CD69 (data not shown), suggesting that TCR signal transduction operated normally in these cells, as in thymocytes with germline Zbtb7b inactivation (Azzam et al., 1998; Swat et al., 1993; He et al., 2005).
Figure 2. CD4 cell development in mice hypomorphic for Zbtb7b.
(A, B). Reduced CD4 T cell generation in mice hypomorphic for Zbtb7b. (A) Thymocytes and splenocytes from wild-type, Zbtb7bt/t and Zbtb7bt/− mice were analyzed by 4-color flow cytometry; Zbtb7b−/− mice (Wang et al., submitted) are shown as a control. CD4 SP thymocytes (left) are gated on CD4 vs. CD8 contour plots and analyzed for expression of TCRβ and CD24; the box indicates the mature TCRhi CD24lo subset. CD4 and CD8 subsets are gated on a two color contour plot of CD4 and CD8 expression on all live or TCR+ LN cells (right). Numbers indicate the percentage of cells within boxes. (B) Bar graphs display the absolute numbers of mature CD4 (left, filled bars) and CD8 SP (right, open bars) thymocytes (TCRhiCD24lo, top) and of CD4 and CD8 spleen T cells (bottom). Note the x-axis scales. Error bars indicate SEM. Data is from 4–6 mice of each genotype analyzed in distinct experiments.
(C). Expression of Zbtb7b protein was analyzed by immunoprecipitation and immunoblotting on bead-purified CD4 T cells from either Zbtb7bt/t or Zbtb7bt/− mice (1 × 107/lane), or from wild-type controls (0.1–1 × 107/lane, as indicated). The leftmost lane is an antibody-only control.
(D, E). CD8-lineage redirection in mice hypomorphic for Zbtb7b. Four-parameter flow cytometry was performed on thymocytes from Zbtb7bt/t and Zbtb7bt/− mice, and from wild-type and Zbtb7b−/− mice (Wang et al., submitted) as controls, all carrying the MHC II-restricted AND TCR transgene that, in I-Ab-expressing mice, promotes the generation of CD4 SP cells expressing the transgenic Vα11 TCRα chain (Kaye et al., 1989). (D) Contour plots of CD4 and CD8 expression gated on all (left) or mature thymocytes (Vα11hi CD24lo, right) show the presence of both CD4 and CD8 SP subsets in Zbtb7b hypomorphic mice, unlike in wild-type mice that only have mature CD4-lineage AND cells. (E) Absolute numbers of mature thymocytes and splenocytes are displayed as in (B); data from Zbtb7b+/t mice was obtained similarly and show that the Zbtb7bt allele does not exert any dominant negative effect on AND TCR cell development. Data is from 3 or 4 mice of each genotype analyzed in distinct experiments.
Zbtb7b protein expression was further reduced in Zbtb7bt/− mice (Fig. 2C), in which T cell development and homeostasis were more severely affected. There were few mature CD4 cells (Fig. 2AB), most of which CD44hi (Fig. SF2), and a near complete redirection of MHC II-restricted thymocytes to the CD8 lineage (Fig. 2DE). Thus, even though Zbtb7bt/− peripheral CD4 cells were mostly MHC II-restricted (data not shown), they were not representative of their wild-type counterparts. Consequently, we used Zbtb7bt/t mice to explore the function of Zbtb7b in mature CD4 cells.
CD8-lineage gene expression in Zbtb7bt/t CD4 T cells
Although most peripheral CD4 cells in Zbtb7bt/t mice did not express CD8, these animals had TCRhi CD4+CD8int cells that were not found in wild-type mice (Fig. 2A, second row, right column). To evaluate if such CD4+CD8int cells were post-thymically derived from CD4+CD8− cells, we adoptively transferred wild-type and Zbtb7bt/t CD4+CD8− cells (containing less than 0.1% contaminating CD8-expressing cells, Fig. SF3A) into Rag2-deficient recipients (Shinkai et al., 1992). While wild-type donors remained CD4+CD8− after transfer, a subset of Zbtb7bt/t cells re-expressed CD8, indicating impaired CD8 gene repression (Fig. 3A). CD8 expression on transferred cells was bi-modal, suggesting loss of epigenetic CD8 repression in a subset of cells. Of note, these CD8-expressing cells did not terminate CD4 expression. These results prompted us to evaluate how reduced Zbtb7b levels affected CD8-lineage gene expression.
Figure 3. inappropriate CD8-lineage gene expression in Zbtb7b hypomorphic CD4 cells.
(A). Bead-purified CD4+CD8− cells (2 × 106) from wild-type or Zbtb7bt/t mice were adoptively transferred into Rag2−/− recipients. Left: starting populations. Right: splenocytes from recipient mice were analyzed two weeks later by 4 color flow cytometry for expression of CD4, CD8α, TCRβ and CD44. Single parameter histograms identify TCR+ splenocytes, on which contour plots show expression of CD4 vs. CD8α and of CD4 vs. CD44. Data is representative of two separate experiments (the second one with β2m-deficient recipients), each including 5 recipients for each donor genotype.
(B). Expression of T-bet, Eomes, Perforin and GzmB mRNAs was assessed by qRT-PCR in sorted naïve (CD44lo) CD4+CD8− LN T cells from wild-type and Zbtb7bt/t mice and in CD4−CD8+ LN cells from wild-type mice. Messenger RNA levels are normalized to β-actin mRNA and expressed as a ratio over those in wild-type CD8 cells (set to 1, not depicted). Error bars indicate experimental variation among triplicates within the experiment shown; data is representative of three separate such experiments.
(C). Expression of Runx3 mRNAs initiated at the distal (dis-Runx3) and proximal (prox-Runx3) promoters was analyzed by conventional RT-PCR in sorted naïve (CD44lo) CD4+CD8− LN T cells from wild-type and Zbtb7bt/t mice and in CD4−CD8+ LN cells from wild-type mice. Wedges represent 3 fold dilutions (1.2; 0.4 and 0.12 × 104 cells, respectively). β-actin mRNA expression was assessed in parallel as a control for mRNA preparation (bottom row). Data is representative of three separate experiments.
(D-F). Sorted CD44lo CD4+CD8− LN T cells from wild-type or Zbtb7bt/t mice were activated by anti-CD3 and anti-CD28 on irradiated APCs in the presence of IL-2, IL-4 and antibodies against IL-12 and IFNγ (Th2 conditions) or IL-2 only (ThN conditions), and analyzed five days after stimulation. (D) Cells were stained for CD4, CD8 and intra-cellular GzmB; GzmB expression is shown on gated CD4+CD8− Zbtb7bt/t (plain line) or wild-type (gray filled histograms); the dashed lines show GzmB expression on wild-type CD8 cells stimulated in parallel in the same conditions. (E) IL-4 and IFNγ production was assessed by flow cytometry on cells restimulated with PMA and Ionomycin. Cells were stained for CD4, CD8 and intra-cellular IL-4 and IFNγ; contour plots are gated on CD4+CD8− cells. Numbers indicate the percent of cells within quadrants. (F) Analyses of gene expression were conducted on Th2-differentiated effectors as in panel (B). Data is representative of three or more separate experiments for each panel.
We analyzed gene expression by quantitative RT-PCR (qRT-PCR) in purified CD4+CD8− CD44lo T cells from wild-type and Zbtb7bt/t mice. Zbtb7bt/t CD4 cells had modestly elevated (twofold) expression of the cytotoxic marker perforin and of Eomes, a Tbox transcriptional activator of the cytotoxic effector program in the CD8 lineage (Fig. 3B) (Pearce et al., 2003). A larger increase (3–10 fold) was seen for the cytotoxic gene Granzyme B (GzmB), but flow cytometry failed to detect GzmB protein expression in both Zbtb7bt/t CD4 and wild-type CD8 naïve cells (data not shown). We assessed expression of Runx3, which has been reported to promote CD8 expression (Sato et al., 2005), by conventional RT-PCR to distinguish mRNAs initiated at the distal (dis-Runx3) and proximal promoters (Bangsow et al., 2001). In resting T cells, the former transcripts are both characteristic of the CD8 lineage and considered to be the main source of Runx3 protein (Egawa et al., 2007). While in wild-type mice dis-Runx3 transcripts were found in CD8 but not CD4 cells, they were detected in CD4 cells from Zbtb7bt/t mice (Fig. 3C). In contrast, transcripts initiated at the proximal promoter, that do not appear to be efficiently translated in resting T cells (Egawa et al., 2007), were present in all three cell subsets (Fig. 3C). Thus, Zbtb7b hypomorphic CD4 T cells fail to normally repress CD8-lineage genes.
To determine if reduced Zbtb7b levels would result in cytotoxic gene expression during effector differentiation, we activated wild-type and Zbtb7bt/t CD44lo CD4 T cells with anti-TCR and anti-CD28 antibodies, and cultured them for five days in the presence of irradiated APCs and exogenous IL-2 (non polarizing, ThN conditions). In such conditions, GzmB protein, normally expressed in CD8 but not CD4 cells, was detected in Zbtb7bt/t CD4 T cells (Fig. 3D, right). Analyses of cytokine production showed abnormal effector differentiation in Zbtb7bt/t cells. Whereas wild-type CD4 cells were skewed towards IL-4 in contrast to IFNγ in CD8 cells (Fig. SF4), most Zbtb7bt/t CD4 cells produced IFNγ, and a notable fraction of those also made IL-4 (Fig. 3E). Of note, both wild-type and Zbtb7bt/t effector cells maintained a strict CD4+CD8− pattern of coreceptor expression after in vitro activation (Fig. SF5 and data not shown). CD4 cells from Zbtb7b+/t littermates behaved like wild-type controls in these and subsequent analyses, indicating that the skewing towards cytotoxic differentiation did not result from a dominant effect of the Zbtb7bt allele.
Both Zbtb7bt/t and wild-type CD4 cells produced of IL-4 and no IFNγ when activated in Th2 conditions (IL-4 and antibodies against IL-12 and IFNγ) (Fig. 3E). Nonetheless, Zbtb7bt/t Th2 effectors had higher GzmB protein and mRNA expression than their wild-type counterparts cells (Fig. 3D, left and 3F); GzmB mRNA levels were more than 1000 times greater in effector than in resting cells (Fig. SF6, presumably accounting for the lack of detectable GzmB protein expression in naïve Zbtb7bt/t cells). Activation in Th1 conditions (IL-12 and anti IL-4) similarly found higher expression of GzmB protein in Zbtb7bt/t than in wild-type CD4 effectors (Fig. SF7). The increased GzmB expression of GzmB by Zbtb7b effectors, irrespective of their activation conditions, supports the conclusion that the expression of ‘cytotoxic’ genes by Zbtb7bt/t is not simply the reflect of a Th2 to Th1 population shift. Of note, perforin mRNA levels remained well below those in CD8 effectors, suggesting that they would be insufficient to allow cytolytic killing. We conclude from these experiments that Zbtb7b molecules act to prevent the aberrant expression of cytotoxic genes both in resting and effector CD4 cells.
The Zbtb7b hypomorphic allele has minimal effects on CD4-lineage gene expression
We next examined CD4-lineage gene expression in Zbtb7bt/t CD4 cells. While expression of CD4 in resting CD4+CD8− splenocytes or LN cells was minimally (86 ± 4 %) but reproducibly lower in Zbtb7bt/t cells than on their wild-type counterparts, expression on activated cells was back to wild-type levels (Fig. SF5 and data not shown). Gata3 gene expression, analyzed by qRT-PCR on naïve CD44lo CD4+CD8− cells was similar in Zbtb7bt/t and wild-type mice (Fig. SF8A), and so was the activity of the cis-regulatory elements that normally control Zbtb7b gene expression (Fig. 4A).
Figure 4. Expression of CD4-lineage genes in Zbtb7b hypomorphic CD4 T cells.
(A). Transcriptional activity of Zbtb7b cis-regulatory elements in Zbtb7b hypomorphic CD4 cells. Expression of GFP was assessed by flow cytometry in gated CD4+CD8− LN T cells from wild-type, Zbtb7bt/t or Zbtb7bt/− mice carrying a BAC GFP reporter for Zbtb7b expression, in which GFP expression is driven by endogenous Zbtb7b cis-regulatory elements in an unmodified context. Contour plots show expression of GFP against CD44. Numbers indicate the frequency of cells within quadrants. Data is from a least three mice of each genotype analyzed in more than two experiments.
(B). Surface expression of CD40L (CD154) was assessed by flow cytometry on B-cell depleted LN cells stimulated four hours with PMA and Ionomycin. Overlaid histograms are gated on CD4+CD8− cells and show CD40L expression on treated (plain lines) or untreated (gray-shaded histograms) cells. Dashed lines indicate CD40L expression on gated wild-type CD4−CD8+ cells in the same culture. Numbers indicate the mean fluorescence intensity of CD40L staining on stimulated CD4+CD8− cells. Data is from three determinations; the mean intensity of CD40L staining in Zbtb7bt/t CD4 cells was 47% of that in their wild-type counterparts.
(C). LN cells from wild-type, Zbtb7bt/t or Zbtb7b−/− mice were stained for CD4, CD8, CD25 and intra-cellular Foxp3. Contour plots of CD4 and CD8 expression are shown for all cells (left) or CD25+ Foxp3+ cells (right) as gated on middle-column plots. Data is from three experiments.
To analyze how Zbtb7b affected CD4 cell effector functions, we assessed expression of CD40 ligand (CD154), a helper molecule upregulated in CD4 cells upon TCR engagement or after treatment by the surrogate drug combination of PMA and Ionomycin (Foy et al., 1996; Roy et al., 1993; Lesley et al., 2006). In wild-type mice, PMA and Ionomycin-stimulated up-regulation of CD40L was considerably higher in CD4 than in CD8 cells, and this response was reduced by half in Zbtb7bt/t CD4 cells (Fig. 4B). When activated in the proper stimulation conditions, Zbtb7bt/t CD4 T cells differentiated not only into IFNγ- or IL-4-producers (Figs. 3E and SF7B), but also could express IL-17 or the transcription factor Foxp3, that in wild-type mice promotes T-regulatory differentiation (Fig. SF8B)(Bettelli et al., 2006; Veldhoen et al., 2006; Reiner, 2007; Zheng and Rudensky, 2007; Sakaguchi et al., 2008). Accordingly, Zbtb7bt/t mice had subnormal numbers of CD4+CD25+ T cells expressing Foxp3 (Fig. 4C). Although in reduced numbers, CD25+ Foxp3+ cells were present in Zbtb7b−/− mice, demonstrating that Zbtb7b is not required for Foxp3 expression. Remarkably, even though most Zbtb7b-deficient MHC II-restricted T cells are CD4−CD8+ T cells (He et al., 2005, and L.W. and R.B., unpublished data), Foxp3 expression was largely restricted to the residual CD4+ population (Fig. 4C). These findings suggest that Zbtb7b deficiency had a lesser impact on CD4 than on CD8 markers, although their amplitude in Zbtb7bt/t cells was not negligible given that it may be limited by the residual expression of Zbtb7b.
Zbtb7b is required in peripheral CD4 T cells to repress CD8-lineage genes
The present findings suggested that Zbtb7b acted in peripheral CD4 T cells to repress the cytotoxic program; however, they could not exclude that CD8-lineage gene expression by Zbtb7bt/t CD4 cells resulted from impaired intrathymic development. To distinguish between these possibilities, it was necessary to examine mature Zbtb7b-deficient CD4 T cells that had developed as Zbtb7b-sufficient thymocytes. To this end, we generated a conditional (‘floxed’) Zbtb7b allele (Zbtb7bfl, Fig. SF1) in which the Zbtb7b coding sequence is flanked by two loxP sites and can be excised by the Cre recombinase. Zbtb7bfl/fl mice had normal numbers of CD4 T cells (Fig. SF9), indicating that the floxed allele was functional.
Deletion of the floxed allele in vitro by a Cre-expressing retrovirus did not affect CD4 or CD8 surface expression, but de-repressed GzmB (data not shown). We excised the floxed sequences in vivo by using the IFNα-responsive Mx1 promoter to express the Cre recombinase (Kuhn et al., 1995). Treatment of Mx-Cre transgenic Zbtb7bfl/fl mice with poly-IC (pIC), an RNA mimic that promotes IFNα production, resulted in a substantial, although incomplete, deletion of the floxed allele as assessed by PCR on genomic DNA (Fig. SF10AB). Despite the IFNα secretion, pIC-treated Zbtb7bfl/fl and Mx-Cre Zbtb7bfl/fl mice retained substantial numbers of CD44lo CD4 T cells (Fig. SF10C).
CD4 cells from pIC treated Mx-Cre Zbtb7bfl/fl mice re-expressed CD8 after adoptive transfer into Rag2-deficient hosts, similar to Zbtb7bt/t cells (Fig. 5A and Fig. SF3BC). Thus, post-thymic Zbtb7b expression is required in CD4 T cells for the sustained repression of CD8 genes. However, these CD8 re-expressing cells remained CD4+, suggesting that Zbtb7b is not required to maintain CD4 expression. To assess the role of Zbtb7b in effector differentiation, we stimulated CD44lo CD4+CD8− cells from pIC-treated Zbtb7bfl/fl and Mx-Cre transgenic Zbtb7bfl/fl mice. Under both ThN and Th2 differentiation conditions, peripheral Zbtb7b excision resulted in GzmB production, even though it did not affect CD4 or CD8 surface expression (Fig. 5B and SF10D). Post-thymic Zbtb7b deletion resulted in the same skewing towards IFNγ production in ThN conditions as that observed in Zbtb7bt/t hypomorphic cells (Fig. 5C). These observations demonstrate that Zbtb7b post-thymically represses CD8-lineage genes in CD4 T cells.
Fig. 5. peripheral deletion of Zbtb7b re-activates CD8 lineage gene expression.
(A). CD4+CD8− LN cells were bead-purified from pIC-treated Zbtb7bfl/fl and Mx-Cre transgenic Zbtb7bfl/fl mice, seven days after the first pIC injection, and adoptively transferred into Rag2−/− mice. Left: contour plots show expression of CD4 and CD8α on ex vivo LN cells and on cells purified for injection. Right: Recipient spleens were harvested two weeks after transfer and analyzed by 3 color flow cytometry for expression of CD4, CD8α and TCRβ. Contour plots (right column) show expression of CD4 and CD8α on gated TCR+ cells (gating on left column). Note that because of the incomplete MxCre-induced deletion (Fig. SF8A), the bimodal expression of CD8 on transferred cells cannot be interpreted conclusively; attempts to identify cells that had undergone efficient deletion using Rosa26-flox-GFP reporter mice (Mao et al., 2001) showed no strict correlation between Rosa26 activation and Zbtb7b deletion (data not shown) and were not pursued further.
(B, C). Sorted CD44lo CD4+CD8− LN T cells from pIC-treated Zbtb7bfl/fl and Mx-Cre transgenic Zbtb7bfl/fl mice were activated in Th2 or ThN conditions as in Fig. 3 and analyzed for expression of CD4, CD8 and intra-cellular GzmB (B) or of CD4, CD8 and intra-cellular IL-4 and IFNγ after PMA-ionomycin restimulation (C). Data is representative of three such experiments.
Runx3 mediates cytotoxic gene expression in Zbtb7b-deficient CD4 cells
Because Eomes but not Runx3 activates the cytotoxic program in mature CD8 cells (Pearce et al., 2003; Intlekofer et al., 2005; Taniuchi et al., 2002a), we predicted that aberrant cytotoxic gene expression by Zbtb7b-insufficient cells was caused by Eomes, and would be prevented by inhibiting Eomes activity. To evaluate this prediction, we retrovirally transduced Zbtb7bt/t cells with a dominant negative version of T-bet (T-bet DN) that represses Eomes and T-bet target genes (Pearce et al., 2003). As expected, expression of this virus in ThN-activated Zbtb7bt/t CD4 cells impaired production of IFNγ, a prototypical target of Tbox proteins (Szabo et al., 2002; Pearce et al., 2003) (Fig. 6A). However, the T-bet DN virus had little or no effect on GzmB expression, both in ThN and Th2 conditions (Fig. 6B and data not shown). Thus, the de-repression of GzmB in Zbtb7b-deficient cells was at least in part independent from T-box protein activity.
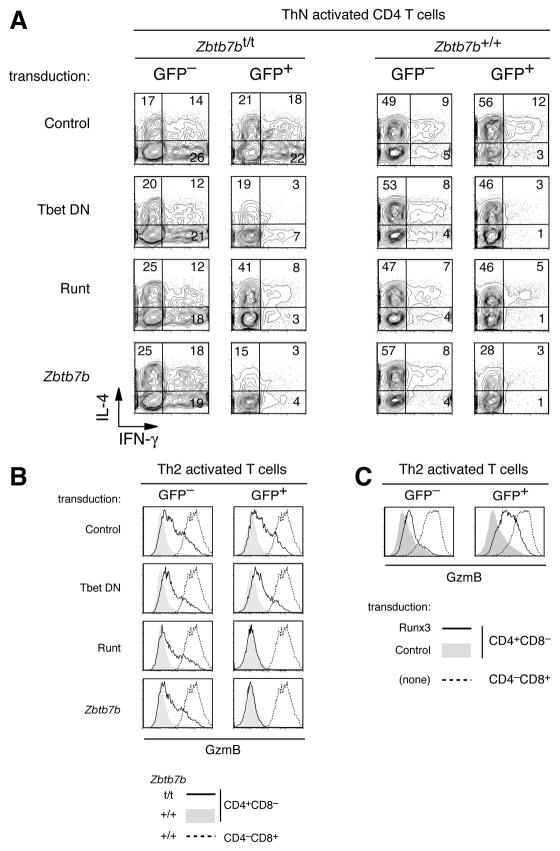
Figure 6. Expression of cytotoxic genes by Zbtb7b hypomorphic CD4 cells depends on Runx and Tbox protein activities.
(A, B). Bead-purified CD4+CD8− cells from Zbtb7bt/t (left panels) or wild-type (right panels) mice were activated and transduced with retroviral vectors encoding a T-bet-engrailed chimeric protein that inhibits both T-bet and Eomes activities (T-bet DN), the Runt domain of Runx3 (Runt) or wild-type Zbtb7b (Zbtb7b), or with a control vector encoding GFP only, and analyzed after a five-day culture in the indicated conditions for expression of CD4, CD8 and intra-cellular IL-4 and IFNγ (A), or of CD4, CD8 and intra-cellular GzmB (B). (A) Contour plots of IFNγ vs. IL-4 expression are gated on CD4+CD8− cells and are shown for transduced (GFP+) and untransduced (GFP−) cells. Numbers indicate the percentage of cells within each quadrant. (B) Overlaid histograms show expression of GzmB on transduced (GFP+) and untransduced (GFP−) Zbtb7bt/t CD4+CD8− cells (plain lines). Dashed lines show GzmB expression on untransduced CD8 cells stimulated in parallel in the same conditions. None of these retroviral vectors affected GzmB expression in wild-type cells (gray-shaded histograms). Data is representative of at least two separate experiments for each panel.
(C). Wild-type CD4 or CD8 T cells were activated under Th2 conditions and transduced with either a Runx3-encoding retroviral vector (plain lines) or the GFP-only control (grey filled). Overlaid histograms show GzmB expression on GFP+ and GFP− cells (dashed lines: CD8 cells activated in the same conditions).
Consequently, we examined if Runx3 was involved in cytotoxic gene expression by Zbtb7b-deficient CD4 cells. We expressed in Zbtb7bt/t CD4 cells a truncated version of Runx3, that includes its Runt domain and inhibits endogenous Runx activity by occupying Runx target sites in association with Cbfβ molecules (Hayashi et al., 2000). Transduction of the Runt virus into Zbtb7bt/t CD4 cells inhibited their production of IFNγ (Fig. 6A) and reversed their aberrant GzmB expression as efficiently as Zbtb7b transduction (Fig. 6B). Conversely, retroviral transduction of Runx3 in wild-type CD4 cells promoted GzmB protein expression (Fig. 6C). Together, these observations support the conclusion that the expression of cytotoxic genes in Zbtb7bt/t CD4 cells is caused in part by Runx3 de-repression.
In summary, the present study demonstrates that the transcription factor Zbtb7b is required in post-thymic CD4 T cells to repress CD8-lineage gene expression.
Discussion
Most conventional TCRαβ T cells (i.e. those restricted by classical MHC molecules) are distributed into CD4 and CD8 lineages that diverge in the thymus from DP precursors, are characterized by stable expression of CD4 or CD8 molecules and harbor distinctive functional properties. Maintaining CD4-CD8 lineage differentiation in the peripheral repertoire, and specifically the correspondence between MHC specificity, coreceptor expression and effector differentiation, is essential to the proper function of the immune system. How this is achieved has been largely unknown.
The present study demonstrates that Zbtb7b, a key CD4-committing factor in the thymus, is required is peripheral CD4 T cells to prevent the inappropriate expression of CD8 lineage genes, including CD8 and the cytotoxic effectors perforin and GzmB, and of the cytokine IFNγ. Zbtb7b also regulates the expression of the transcription factors Runx3 and Eomes that promote cytotoxic gene expression. These findings demonstrate the importance of direct transcription factor control in the maintenance of lineage differentiation in the mature T cell compartment. They agree with our previous observation that Zbtb7b represses cytotoxic differentiation, including perforin and GzmB expression, when introduced into mature CD8 T cells (Jenkinson et al., 2007). Such a function of Zbtb7b in peripheral CD4 T cells is conceptually similar to the repression of aberrant gene expression by Pax5, a transcription factor critical to establish and maintain B cell commitment (Cobaleda et al., 2007).
Two arguments suggest that Zbtb7b acts as a repressor of CD8 genes. First, Zbtb7b belongs to a family of proteins that generally functions as transcriptional repressors, notably through the recruitment of multiprotein complexes that covalently modify histones or contribute to chromatin remodeling (Dhordain et al., 1997; Fujita et al., 2004; Bilic and Ellmeier, 2007). Second, Zbtb7b functionally acts as CD8 repressor, as its expression in CD8 T cells represses CD8 transcription whereas its disruption in peripheral CD4 cells results in CD8 re-expression (Jenkinson et al., 2007, and this study). It is possible that Zbtb7b binds the CD8 locus and directly represses transcription, that it acts indirectly by repressing activators of CD8 expression, possibly including Runx3 (Sato et al., 2005), or that is promotes epigenetic CD8 silencing. It is difficult at present to distinguish between these possibilities, and the unavailability of ChIP-grade antibodies against Zbtb7b has so far precluded us from investigating the first hypothesis. However, the bi-modal CD8 re-expression by Zbtb7b-insufficient CD4 cells, a pattern typical of epigenetic control (Kioussis and Ellmeier, 2002), suggests that Zbtb7b contributes to maintain epigenetic CD8 repression. Further supporting this idea, CD8 de-repression after Zbtb7b deletion was observed only after cell expansion in adoptively transferred recipients, suggesting that it required extensive proliferation to dilute repressing epigenetic marks.
That CD8 re-expression by Zbtb7b-deficient cells was only observed in vivo also raises the possibility that it requires T cell ligands not provided in vitro. IL-7 regulates CD8 expression and is an obvious candidate for such a function (Park et al., 2007). However, we do not think that the absence of IL-7 accounts for the lack of CD8 re-expression by Zbtb7b-deficient CD4 T cells in vitro; indeed, no CD8 expression was observed on these cells when cultured in the presence of IL-2 or IL-4, which both promote CD8 expression similarly to IL-7 (Park et al., 2007).
That CD8 silencing in mature CD4 cells depends on the continuous activity of Zbtb7b raises the possibility that coreceptor silencing in peripheral T cells is maintained along distinct mechanisms in each lineage. CD4 expression is controled by an enhancer active in both CD4 and CD8 lineages and a silencer that recruits Runx proteins and so far unknown additional CD4 repressors to terminate CD4 expression in CD8-differentiating thymocytes (Sawada et al., 1994; Siu et al., 1994; Taniuchi et al., 2002a; Taniuchi et al., 2002b). However, while the silencer is required to establish silencing, it is not needed to maintain it in mature CD8 cells (Zou et al., 2001). This strongly suggests that the repressors that bind the silencer in thymocytes no longer contribute to silencing in post-thymic CD8 cells. In contrast, there is no known silencer element in the CD8 locus and Zbtb7b is required both to establish (in the thymus) and maintain (in post-thymic cells) CD8 silencing in the CD4 lineage.
Unlike this effect on CD8 expression, peripheral Zbtb7b inactivation did not reveal any Zbtb7b requirement for sustained CD4 expression. It is possible that this is due to technical limitations of our assays (for instance because cells that terminate CD4 expression in vivo after Zbtb7b inactivation would do so early, before the time they were harvested). Alternatively, this may reflect the primary role of Zbtb7b as a transcriptional repressor. We previously proposed that Zbtb7b indirectly promotes CD4 expression in CD4-differentiating thymocytes by preventing the expression of CD4 repressors (Wildt et al., 2007). It is possible that such repressors are not expressed in post-thymic T cells, therefore explaining the persistence of CD4 expression despite Zbtb7b disruption and Runx3 expression. In agreement with this possibility, physiological or ectopic expression of Runx3 fails to repress CD4 in mature CD4 cells (Telfer et al., 2004; Grueter et al., 2005; Kohu et al., 2005; Djuretic et al., 2007; Naoe et al., 2007). Alternatively, epigenetic mechanisms may ‘lock’ the CD4 locus in an open, active configuration in peripheral CD4 cells, in a mirror image of its inactive configuration in CD8 cells (Delaire et al., 2004; Merkenschlager et al., 2004).
As for CD8 genes, it is possible that Zbtb7b directly represses cytotoxic genes by binding to their cis-regulatory elements; the multiplicity of conserved non coding sequences with potential or demonstrated regulatory functions within such genes (Glimcher et al., 2004; Pipkin et al., 2007) and the absence of ChIP-grade antibodies against Zbtb7b currently preclude a detailed investigation of this hypothesis. However, we document that Zbtb7b acts at least in part by preventing or limiting the expression of trans-activators of cytotoxic gene expression. In CD4 T cells, IFNγ, GzmB and perforin are responsive to Eomes, a trans-activator normally expressed during cytotoxic differentiation (Pearce et al., 2003). Thus, limiting or delaying Eomes expression during the effector differentiation of CD4 cells appears as a key function of Zbtb7b in restraining the production of components of the cytotoxic program that remain ‘accessible’ in CD4 cells, including IFNγ and GzmB.
Given the overlap between Th1 and cytotoxic gene expression programs, the effect of Zbtb7b on cytotoxic gene expression has the consequence of constraining the expression of Th1 effector genes. We note however that Zbtb7b deficiency resulted in GzmB expression under both Th1 and Th2 differentiation conditions, even though it did not affect the IL-4 vs. IFNγ polarization. Together with the re-expression of CD8 by Zbtb7b-deficient cells, this supports the interpretation that repressing cytotoxic gene expression is a key target of Zbtb7b.
Previous studies (Wargnier et al., 1995; Babichuk et al., 1996), pointed to a potential role of Runx proteins in GzmB expression. Our findings, using both loss and gain-of-function approaches, document that this is the case. While Runx3, normally the predominant Runx factor in CD8 cells, is not required for perforin expression or cytotoxic function (Taniuchi et al., 2002a), there is substantial redundancy between Runx1 and Runx3 to promote CD8 cell generation (Woolf et al., 2003; Egawa et al., 2007; Setoguchi et al., 2008), and it is possible that such redundancy has so far masked the role of Runx activity in cytotoxic effector differentiation.
Runx molecules, presumably Runx3, repress Zbtb7b expression during CD8-lineage differentiation in the thymus, by binding to a cis-regulatory element (‘silencer’) upstream of the Zbtb7b promoter (Setoguchi et al., 2008). Given that Zbtb7b-hypomorphic cells inappropriately express Runx3, it is tempting to speculate that, conversely, Zbtb7b directly represses Runx3. Future studies will determine whether that is the case, in developing thymocytes and mature T cells. It is possible that a two-way cross-repression mechanisms operates in thymocytes and contributes to decide CD4-CD8 commitment by terminating expression of one of these factors. However, it is unlikely that similar rules apply in mature T cells, as Runx3 is up-regulated in Th1 effectors but does not force the termination of Zbtb7b expression. In fact, Runx-repression of Zbtb7b expression is context-dependent, as Runx complexes (presumably involving Runx1) are bound to the Zbtb7b ‘silencer’ in CD4 cells (Setoguchi et al., 2008).
If Zbtb7b prevents cytotoxic differentiation in part through repressing cytotoxic effector regulators such as Runx3 or Eomes, why is it that wild-type Th1-differentiating cells, that express both Runx3 and Eomes do not enter cytotoxic differentiation (Djuretic et al., 2007; Egawa et al., 2007; Suto et al., 2006) ? We think of two non mutually excusive possibilities to explain this apparent paradox. First, Zbtb7b may control the kinetics of expression of such factors, and specifically prevent their expression in resting cells and during initial activation. Effector T cell differentiation is typically determined by the cytokine and cell-cell interaction milieu that affect the balance of transcription factor activities present during early activation (Jenkins et al., 2001; Reiner, 2007). Thus, it is conceivable that expression of Runx3 or Eomes in naïve Zbtb7b-insufficient cells promotes cytotoxic gene expression, whereas the transcriptional circuitry is no longer permissive to this effect when they are expressed at a later time point in wild-type cells. The second possibility is that Zbtb7b acts by impairing Runx3 or Tbox protein function, possibly by directly binding to and repressing cytotoxic genes.
It is notable that Zbtb7b levels that allow CD4 cell development are potentially insufficient for the proper effector differentiation of peripheral T cells. Future studies will determine whether this has functional consequences in vivo during immune responses to infectious agents. However, excessive IFNγ production by CD4 T cells has been implicated in tissue damage in several infectious models and in autoimmune diseases, including type 1 diabetes (Christen and von Herrath, 2004) and future studies will examine if insufficient Zbtb7b expression contributes to inappropriate IFNγ production in such circumstances.
Finally, the present study reveals a need for persistent repression of the cytotoxic CD8 program in mature CD4 T cells. One interpretation of this observation is that CD8-lineage differentiation depends on factors that are available in peripheral lymphoid organs [such as IL-7 (Singer, 2002)] and must therefore be actively prevented in peripheral CD4 cells. We propose that Zbtb7b is expressed in all CD4-lineage cells to counteract such factors and prevent the resurgence of the CD8-cytotoxic program.
Experimental procedures
Mice
The Zbtb7b gene targeting strategy and the generation of the Zbtb7bt allele were described (Wang et al., submitted) and are schematically summarized in Fig. SF1 Deletion of the Frt flanked segments was obtained by crossing mice heterozygous for the targeted allele with mice expressing Flpe under the control of a β-actin promoter. The genotype of recombinant animals was verified by Southern blotting for the first two generations, and subsequently by PCR on tail DNA. Mice carrying a GFP BAC reporter for Zbtb7b expression were previously reported (Wang et al., submitted). Rag2−/− (Shinkai et al., 1992) and AND TCR transgenic (Kaye et al., 1989) mice were obtained from Taconic, and MxCre transgenic (Kuhn et al., 1995) mice from Jax. Mice carrying the indicated genotypes were generated by appropriate intercrosses or backcrosses. Animals were genotyped by PCR on tail DNA or phenotyped by staining of peripheral blood cells, and were analyzed between 4 and 12 weeks of age except when indicated otherwise. All transgenic animals were heterozygous for the transgenic allele. All mice were housed in Specific Pathogen Free facilities. Animal procedures used in the present study were approved by NCI Animal Care and Use committees, as appropriate.
Animal procedures
For deletion of the Zbtb7bfl allele in peripheral cells, young adult mice were injected intraperitoneally with pIC (0.25 mg in 0.2 ml sterile water) three times at two days intervals. LN T cells were harvested for analysis 7 days after the first injection. For adoptive transfer experiments, bead purified CD4+CD8− thymocytes (2 × 106) were resuspended in 0.25 ml PBS and tail vein-injected into unmanipulated Rag2−/− recipients. Recipient splenocytes were harvested and analyzed 2 weeks after transfer.
T cell activation and effector differentiation cultures
Antigen-presenting cells (APCs) were obtained from C57BL/6 splenocytes by Thy1.2 magnetic bead depletion (Dynal) and irradiated at 2500 rad. Sorted CD44lo CD4+CD8− LN or spleen T cells (0.5 × 106) were mixed with 2 × 106 irradiated APCs in complete culture medium (RPMI 1640 supplemented with 10% FCS) and activated with anti-CD3 (2C11, 1 μg/ml) and anti-CD28 (3 μg/ml) in the presence of either (i) 50 U/ml IL-2 (“ThN conditions”); (ii) 50 U/ml IL-2, 10 ng/ml IL-12, 10 μg/ml anti-IL-4 (“Th1 conditions” (iii); 50 U/ml IL-2, 100 U/ml IL-4, 10 μg/ml anti IL-12 and 10 μg/ml anti IFNγ (“Th2 conditions”), (iv) 10ng/ml IL-6, 5ng/ml TGF-β1, 10 μg/ml anti IL-4, 10 μg/ml anti IFNγ (“Th17 conditions”) or (v) 50 U/ml IL-2, 5ng/ml TGF-β1, 10 μg/ml anti IL-4, 10 μg/ml anti IFNγ (“Treg conditions”). All cytokines were from Preprotech. Except where indicated otherwise, cells were analyzed five days after activation. For retroviral transductions, T cells activated as above were infected with supernatants from Plat-E packaging cells (Morita et al., 2000) transfected with retroviral constructs as described (Jenkinson et al., 2007). Cells were replated in their original medium after infection.
Antibodies and retroviral constructs
Antibodies used for staining were from eBiosciences (anti-Foxp3) or BD-Pharmingen (all others) and were used according to the manufacturer’s instructions. The anti-Zbtb7b antiserum has been described (Wang et al., submitted). Anti-cytokine antibodies used in T cell effector differentiation cultures were from BD-Pharmingen.
pMRX (Saitoh et al., 2002) derivatives encoding mouse Runx3 (exon 1 containing isoform) or its 204 amino-terminal residues (Runt construct) were constructed using conventional cloning procedures (details available on requests). MSCV-derived retroviral vectors for Cre, the T-bet-Engrailed fusion protein (T-betDN) and Gata3 were previously described (Pearce et al., 2003; Zhu et al., 2004).
Cell preparation, purification and flow cytometry
Cells were prepared from thymus, spleen or lymph nodes (LN), counted and assessed for surface or intra-cellular protein expression by immunofluorescence and flow cytometry as described (Liu et al., 2003), using either an LSR II flow cytometer (BD Biosciences) or a modified (Cytek) FacsCalibur cytometer (BD Biosciences). CD4+CD8− cells were purified from LN cells with an antibody mix including anti-CD8α and magnetic beads (CD4 Negative Isolation kit, Dynal-Invitrogen 114.15D). CD8 bead cell purification was performed similarly using the CD8 separation kit from Dynal. Where indicated, CD44lo cells were sorted from bead-purified CD4+CD8− cells using Facs Vantage or Facs Aria instruments (BD Biosciences) as described (Liu and Bosselut, 2004).
Gene and protein expression analyses
Analyses of gene expression by RT-PCR were performed as described (Liu and Bosselut, 2004) from RNA extracted with RNAqueous (Ambion) and reverse-transcribed with Oligo dT priming (superscript III, Invitrogen). Analyses by qRT-PCR were performed using Taqman reagents, probes (Gata3: Mm00484683_m1; GzmB: Mm00442834_m1; Perforin: Mm00812512_m1; T-bet: Mm00450960_m1, Eomes: Mm01351985_m1) and an ABI PRISM 7500HT sequence detection system, all from Applied Biosystems (Jenkinson et al., 2007). Gene expression values are normalized to β-actin in the same sample. Analyses of Zbtb7b protein expression were performed on cells lyzed in 1% Triton containing buffer as previously described (Sun et al., 2005).
Supplementary Material
Figure SF1: Generation and genotyping of Zbtb7b hypomorphic mice
(A) The Zbtb7b locus is schematically depicted. The targeting strategy inserted a LoxP site (filled triangle) 3′ of the Zbtb7b open reading frame, and a LoxP and Frt (open triangle) flanked cassette 5′ of the first Zbtb7b coding exon (exon 2), that includes: (i) a neomycin resistance gene (black box) driven by the pGK promoter (yellow), (ii) a splice acceptor (SA, green box)-intron (white box) sequence derived from pLMT330 (Wang et al., submitted) and (iii) a poly-adenylation site (pA, red box). The targeted allele (Zbtb7bt) was obtained by homologous recombination in ES cells and is engineered to promote premature transcription termination at the inserted pA and aberrant mRNA splicing towards the inserted splice acceptor. The floxed allele (Zbtb7bfl) was generated by Flpe deletion of the Frt-flanked Neo cassette using Flpe-expressing mice, and the deleted allele (Zbtb7b−) by Cre-mediated deletion of the floxed sequence. EcoRV restriction sites and the position of probes C and D used for genotyping are indicated. Two-arrowhead lines depict the restriction fragments shown in (B). The bold-faced line indicates the extent of the recombination arms in the targeted and null alleles.. Homologous recombination on the 5′ side was checked using HincII digestion (Wang et al., submitted).
(B, C). Southern blot analyses of tail DNA from Zbtb7b+/fl and Zbtb7b+/t mice. EcoRV-digested genomic DNA was resolved on agarose gels, transferred to Hybond-XL membranes (Amersham) and hybridized with probes C or D. Arrowheads point to restriction specific fragments generated from wild-type (filled) or modified (dashed) alleles. Lane 1 in each blot shows the digestion pattern from the targeted ES cell cells.
Figure SF2: Peripheral CD4 T cell populations in Zbtb7b hypomorphic mice.
Contour plots of CD4 vs. CD44 expression are shown on gated CD4+CD8− splenocytes from wild-type, Zbtb7bt/t and Zbtb7bt/− mice stained with antibodies against CD4, CD8 and CD44 and analyzed by 3-color flow cytometry. Numbers indicate the percentage of cells within boxes.
Figure SF3: Purity of CD4+CD8− populations used in adoptive transfer experiments.
LN T cells were obtained from wild-type and Zbtb7bt/t mice (A) or from pIC-treated MxCre transgenic or non-transgenic Zbtb7bfl/fl mice (B, C) and bead-purified into CD4+CD8− T cells as described in Experimental Procedures. (A, B) Cell purity was assessed by staining for surface expression of TCRβ, CD4 and CD8β (none of which was targeted by antibodies used for purification). Contour plots show CD4 and CD8 expression on all and gated TCRβ+ cells, before (left) or after (right) purification. (C) Dot plots of CD8α vs. CD8β expression verify that pIC treatment did not result in the presence of CD8α+CD8β− cells. Numbers indicate the percentage of cells within quadrants.
Figure SF4: Preferential expression of IFNγ in ‘ThN’-stimulated CD8 cells.
Wild-type sorted CD44lo CD4+CD8− and bead-purified CD4−CD8+ LN T cells were activated by anti-CD3 and anti-CD28 on irradiated APCs in the presence of IL-2 (‘ThN’ conditions) and assessed for IFNγ and IL-4 production as in Fig. 3D.
Figure SF5: in vitro differentiating Zbtb7bt/t effectors maintain a CD4+CD8− coreceptor expression pattern.
Wild-type and Zbtb7bt/t sorted CD44lo CD4+CD8− LN T cells were cultured in vitro under Th2 conditions and assessed for CD4 and CD8 expression five days after activation.
Figure SF6: Relative expression of GzmB in naïve and differentiated effector cells.
GzmB mRNA expression was analyzed by qRT-PCR in naïve (CD44lo, left) and Th2-activated (right) CD4 cells from wild-type or Zbtb7bt/t mice, and in resting or Th2-activated wild-type CD8 cells, and is expressed in arbitrary units relative to β-actin. Data is the average of at least four determinations; error bars indicate SEM.
Figure SF7: Increased expression of GzmB in Zbtb7bt/t Th1 effectors.
Wild-type and Zbtb7bt/t sorted CD44lo CD4+CD8− and wild-type CD8 LN T cells were cultured in vitro under Th1 conditions and assessed for GzmB expression by intra-cellular staining and flow cytometry. (A) Overlaid histograms show GzmB fluorescence gated on CD4+CD8− cells from wild-type (gray-shaded) and Zbtb7bt/t (plain line) cells. The dashed trace shows GzmB expression in wild-type CD8 T cells activated in the same conditions. (B) Contour plots show IFNγ vs. IL-4 expression on the same cultures.
Fig. SF8: Effector differentiation of Zbtb7bt/t CD4 T cells.
(A): Expression of Gata3 was assessed by qRT-PCR in naïve (CD44lo, left) and Th2-activated (right) CD4 cells from wild-type or Zbtb7bt/t mice, and is expressed relative to that of wild-type cells in each condition, after normalization to β-actin mRNA. Data is representative of three independent experiments.
(B): Bead purified CD4+CD8− LN T cells from wild-type (top) or Zbtb7bt/t (bottom) mice were activated in Th17 or Treg conditions. Cytokine production was assessed by intra-cellular staining and flow cytometry on PMA and Ionomycin re-stimulated cells (left), and expression of Foxp3 molecules on cells not subject to re-stimulation (right). Cells were stained for CD4, CD8 and the indicated cytokines or Foxp3. Contour plots (left) and histograms of Foxp3 expression (right) are gated on CD4+CD8− cells.
Figure SF9: T cell development in Zbtb7bfl/fl mice.
Thymocytes and splenocytes from wild-type and Zbtb7bfl/fl mice were analyzed by 4-color flow cytometry as described in Fig. 2A. Numbers indicate the percentage of cells within boxes.
Figure SF10: Analyses of CD4 T cells after in vivo deletion of the floxed Zbtb7b allele.
(A,B). Genomic DNA was prepared from CD44lo CD4+CD8− LN cells from pIC-treated Zbtb7bfl/fl and Mx-Cre transgenic Zbtb7bfl/fl mice, seven days after the first pIC injection (lanes 3, 4 and 7, 8), and analyzed by PCR for the presence of the wild-type (Zbtb7b+), floxed (Zbtb7bfl) (bottom panel, lanes 5–8) or deleted (Zbtb7b−) alleles (top panel, lanes 1–4). (A) The position of primers used for these analyses is schematically depicted. (B) Amplified DNA fragments were separated by agarose gel electrophoresis and revealed by Ethydium Bromide staining of the gel. Tail DNAs from wild-type mice (lanes 2 and 6), Zbtb7b−/− mice (lane 1) and unmanipulated Zbtb7bfl/fl mice (lane 5) were used as controls for the position of amplified bands.
(C). CD4+CD8− LN T cells were analyzed by 3-color flow cytometry for CD4, CD8 and CD44 expression. Two parameter contour plots of CD4 vs. CD8 and CD4 vs. CD44 are shown. Numbers indicate the percentage of cells within boxes.
(D). Sorted CD44lo CD4+CD8− LN T cells from pIC-treated Zbtb7bfl/fl mice, carrying or not the Mx-Cre transgene were activated under Th2 conditions and assessed by flow cytometry for CD4 and CD8 expression five days later.
Acknowledgments
We thank Eilen Southon for ES cell recombination, Genevieve Sanchez for mouse technical assistance, Barbara Taylor for expert cell sorting; Steve Reiner, Bill Paul and Jinfang Zhu for reagents; Jinfang Zhu for helpful discussions and T cell activation protocols; and Jon Ashwell, Yasmine Belkaid and Steve Reiner for reading the manuscript. This work was supported by the Intramural Research Program of the National Cancer Institute, Center for Cancer Research, NIH.
References
- Aliahmad P, Kaye J. Development of all CD4 T lineages requires nuclear factor TOX. J Exp Med. 2008;205:245–256. doi: 10.1084/jem.20071944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med. 1998;188:2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babichuk CK, Duggan BL, Bleackley RC. In vivo regulation of murine granzyme B gene transcription in activated primary T cells. J Biol Chem. 1996;271:16485–16493. doi: 10.1074/jbc.271.28.16485. [DOI] [PubMed] [Google Scholar]
- Bangsow C, Rubins N, Glusman G, Bernstein Y, Negreanu V, Goldenberg D, Lotem J, Ben-Asher E, Lancet D, Levanon D, Groner Y. The RUNX3 gene--sequence, structure and regulated expression. Gene. 2001;279:221–232. doi: 10.1016/s0378-1119(01)00760-0. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Bilic I, Ellmeier W. The role of BTB domain-containing zinc finger proteins in T cell development and function. Immunol Lett. 2007;108:1–9. doi: 10.1016/j.imlet.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Christen U, von Herrath MG. Manipulating the type 1 vs type 2 balance in type 1 diabetes. Immunol Res. 2004;30:309–325. doi: 10.1385/IR:30:3:309. [DOI] [PubMed] [Google Scholar]
- Cobaleda C, Schebesta A, Delogu A, Busslinger M. Pax5: the guardian of B cell identity and function. Nat Immunol. 2007;8:463–470. doi: 10.1038/ni1454. [DOI] [PubMed] [Google Scholar]
- Delaire S, Huang YH, Chan SW, Robey EA. Dynamic repositioning of CD4 and CD8 genes during T cell development. J Exp Med. 2004;200:1427–1435. doi: 10.1084/jem.20041041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhordain P, Albagli O, Lin RJ, Ansieau S, Quief S, Leutz A, Kerckaert JP, Evans RM, Leprince D. Corepressor SMRT binds the BTB/POZ repressing domain of the LAZ3/BCL6 oncoprotein. Proc Natl Acad Sci U S A. 1997;94:10762–10767. doi: 10.1073/pnas.94.20.10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djuretic IM, Levanon D, Negreanu V, Groner Y, Rao A, Ansel KM. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nat Immunol. 2007;8:145–153. doi: 10.1038/ni1424. [DOI] [PubMed] [Google Scholar]
- Egawa T, Tillman RE, Naoe Y, Taniuchi I, Littman DR. The role of the Runx transcription factors in thymocyte differentiation and in homeostasis of naive T cells. J Exp Med. 2007;204:1945–1957. doi: 10.1084/jem.20070133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy TM, Aruffo A, Bajorath J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand GP39. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- Fujita N, Jaye DL, Geigerman C, Akyildiz A, Mooney MR, Boss JM, Wade PA. MTA3 and the Mi-2/NuRD complex regulate cell fate during B lymphocyte differentiation. Cell. 2004;119:75–86. doi: 10.1016/j.cell.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Glimcher LH, Townsend MJ, Sullivan BM, Lord GM. Recent developments in the transcriptional regulation of cytolytic effector cells. Nat Rev Immunol. 2004;4:900–911. doi: 10.1038/nri1490. [DOI] [PubMed] [Google Scholar]
- Grueter B, Petter M, Egawa T, Laule-Kilian K, Aldrian CJ, Wuerch A, Ludwig Y, Fukuyama H, Wardemann H, Waldschuetz R, Moroy T, Taniuchi I, Steimle V, Littman DR, Ehlers M. Runx3 Regulates Integrin {alpha}E/CD103 and CD4 Expression during Development of CD4−/CD8+ T Cells. J Immunol. 2005;175:1694–1705. doi: 10.4049/jimmunol.175.3.1694. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Natsume W, Watanabe T, Abe N, Iwai N, Okada H, Ito Y, Asano M, Iwakura Y, Habu S, Takahama Y, Satake M. Diminution of the AML1 transcription factor function causes differential effects on the fates of CD4 and CD8 single-positive T cells. J Immunol. 2000;165:6816–6824. doi: 10.4049/jimmunol.165.12.6816. [DOI] [PubMed] [Google Scholar]
- He X, He X, Dave VP, Zhang Y, Hua X, Nicolas E, Xu W, Roe BA, Kappes DJ. The zinc finger transcription factor Th-POK regulates CD4 versus CD8 T-cell lineage commitment. Nature. 2005;433:826–833. doi: 10.1038/nature03338. [DOI] [PubMed] [Google Scholar]
- Hernandez-Hoyos G, Anderson MK, Wang C, Rothenberg EV, Alberola-Ila J. GATA-3 expression is controlled by TCR signals and regulates CD4/CD8 differentiation. Immunity. 2003;19:83–94. doi: 10.1016/s1074-7613(03)00176-6. [DOI] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- Janeway CAJ, Bottomly K. Signals and signs for lymphocyte responses. Cell. 1994;76:275–285. doi: 10.1016/0092-8674(94)90335-2. [DOI] [PubMed] [Google Scholar]
- Jenkins MK, Khoruts A, Ingulli E, Mueller DL, McSorley SJ, Reinhardt RL, Itano A, Pape KA. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol. 2001;19:23–45. doi: 10.1146/annurev.immunol.19.1.23. [DOI] [PubMed] [Google Scholar]
- Jenkinson SR, Intlekofer AM, Sun G, Feigenbaum L, Reiner SL, Bosselut R. Expression of the transcription factor cKrox in peripheral CD8 T cells reveals substantial postthymic plasticity in CD4-CD8 lineage differentiation. J Exp Med. 2007;204:267–272. doi: 10.1084/jem.20061982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye J, Hsu ML, Sauron ME, Jameson SC, Gascoigne NR, Hedrick SM. Selective development of CD4+ T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature. 1989;341:746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- Kioussis D, Ellmeier W. Chromatin and CD4, CD8A and CD8B gene expression during thymic differentiation. Nat Rev Immunol. 2002;2:909–919. doi: 10.1038/nri952. [DOI] [PubMed] [Google Scholar]
- Kohu K, Sato T, Ohno S, Hayashi K, Uchino R, Abe N, Nakazato M, Yoshida N, Kikuchi T, Iwakura Y, Inoue Y, Watanabe T, Habu S, Satake M. Overexpression of the Runx3 transcription factor increases the proportion of mature thymocytes of the CD8 single-positive lineage. J Immunol. 2005;174:2627–2636. doi: 10.4049/jimmunol.174.5.2627. [DOI] [PubMed] [Google Scholar]
- Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- Lesley R, Kelly LM, Xu Y, Cyster JG. Naive CD4 T cells constitutively express CD40L and augment autoreactive B cell survival. Proc Natl Acad Sci U S A. 2006;103:10717–10722. doi: 10.1073/pnas.0601539103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Adams A, Wildt KF, Aronow B, Feigenbaum L, Bosselut R. Restricting Zap70 expression to CD4+CD8+ thymocytes reveals a T cell receptor-dependent proofreading mechanism controlling the completion of positive selection. J Exp Med. 2003;197:363–373. doi: 10.1084/jem.20021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Bosselut R. Duration of TCR signaling controls CD4-CD8 lineage differentiation in vivo. Nat Immunol. 2004;5:280–288. doi: 10.1038/ni1040. [DOI] [PubMed] [Google Scholar]
- Mao X, Fujiwara Y, Chapdelaine A, Yang H, Orkin SH. Activation of EGFP expression by Cre-mediated excision in a new ROSA26 reporter mouse strain. Blood. 2001;97:324–326. doi: 10.1182/blood.v97.1.324. [DOI] [PubMed] [Google Scholar]
- Merkenschlager M, Amoils S, Roldan E, Rahemtulla A, O’connor E, Fisher AG, Brown KE. Centromeric repositioning of coreceptor loci predicts their stable silencing and the CD4/CD8 lineage choice. J Exp Med. 2004;200:1437–1444. doi: 10.1084/jem.20041127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
- Naoe Y, Setoguchi R, Akiyama K, Muroi S, Kuroda M, Hatam F, Littman DR, Taniuchi I. Repression of interleukin-4 in T helper type 1 cells by Runx/Cbf beta binding to the Il4 silencer. J Exp Med. 2007;204:1749–1755. doi: 10.1084/jem.20062456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai SY, Truitt ML, Ho IC. GATA-3 deficiency abrogates the development and maintenance of T helper type 2 cells. Proc Natl Acad Sci U S A. 2004;101:1993–1998. doi: 10.1073/pnas.0308697100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai SY, Truitt ML, Ting C, Leiden JM, Glimcher LH, Ho IC. Critical Roles for Transcription Factor GATA-3 in Thymocyte Development. Immunity. 2003;19:863–875. doi: 10.1016/s1074-7613(03)00328-5. [DOI] [PubMed] [Google Scholar]
- Park JH, Adoro S, Lucas PJ, Sarafova SD, Alag AS, Doan LL, Erman B, Liu X, Ellmeier W, Bosselut R, Feigenbaum L, Singer A. ‘Coreceptor tuning’: cytokine signals transcriptionally tailor CD8 coreceptor expression to the self-specificity of the TCR. Nat Immunol. 2007;8:1049–1059. doi: 10.1038/ni1512. [DOI] [PubMed] [Google Scholar]
- Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, Reiner SL. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- Pipkin ME, Ljutic B, Cruz-Guilloty F, Nouzova M, Rao A, Zuniga-Pflucker JC, Lichtenheld MG. Chromosome transfer activates and delineates a locus control region for perforin. Immunity. 2007;26:29–41. doi: 10.1016/j.immuni.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Reiner SL. Development in motion: helper T cells at work. Cell. 2007;129:33–36. doi: 10.1016/j.cell.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Roy M, Waldschmidt T, Aruffo A, Ledbetter JA, Noelle RJ. The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells. J Immunol. 1993;151:2497–2510. [PubMed] [Google Scholar]
- Saitoh T, Nakano H, Yamamoto N, Yamaoka S. Lymphotoxin-beta receptor mediates NEMO-independent NF-kappaB activation. FEBS Lett. 2002;532:45–51. doi: 10.1016/s0014-5793(02)03622-0. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Sato T, Ohno S, Hayashi T, Sato C, Kohu K, Satake M, Habu S. Dual functions of Runx proteins for reactivating CD8 and silencing CD4 at the commitment process into CD8 thymocytes. Immunity. 2005;22:317–328. doi: 10.1016/j.immuni.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Sawada S, Scarborough JD, Killeen N, Littman DR. A lineage-specific transcriptional silencer regulates CD4 gene expression during T lymphocyte development. Cell. 1994;77:917–929. doi: 10.1016/0092-8674(94)90140-6. [DOI] [PubMed] [Google Scholar]
- Setoguchi R, Tachibana M, Naoe Y, Muroi S, Akiyama K, Tezuka C, Okuda T, Taniuchi I. Repression of the transcription factor Th-POK by Runx complexes in cytotoxic T cell development. Science. 2008;319:822–825. doi: 10.1126/science.1151844. [DOI] [PubMed] [Google Scholar]
- Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Singer A. New perspectives on a developmental dilemma: the kinetic signaling model and the importance of signal duration for the CD4/CD8 lineage decision. Curr Opin Immunol. 2002;14:207–215. doi: 10.1016/s0952-7915(02)00323-0. [DOI] [PubMed] [Google Scholar]
- Singer A, Bosselut R. CD4/CD8 coreceptors in thymocyte development, selection, and lineage commitment: analysis of the CD4/CD8 lineage decision. Adv Immunol. 2004;83:91–131. doi: 10.1016/S0065-2776(04)83003-7. [DOI] [PubMed] [Google Scholar]
- Siu G, Wurster AL, Duncan DD, Soliman TM, Hedrick SM. A transcriptional silencer controls the developmental expression of the CD4 gene. EMBO J. 1994;13:3570–3579. doi: 10.1002/j.1460-2075.1994.tb06664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Liu X, Mercado P, Jenkinson SR, Kypriotou M, Feigenbaum L, Galera P, Bosselut R. The zinc finger protein cKrox directs CD4 lineage differentiation during intrathymic T cell positive selection. Nat Immunol. 2005;6:373–381. doi: 10.1038/ni1183. [DOI] [PubMed] [Google Scholar]
- Suto A, Wurster AL, Reiner SL, Grusby MJ. IL-21 inhibits IFN-gamma production in developing Th1 cells through the repression of Eomesodermin expression. J Immunol. 2006;177:3721–3727. doi: 10.4049/jimmunol.177.6.3721. [DOI] [PubMed] [Google Scholar]
- Swat W, Dessing M, von BH, Kisielow P. CD69 expression during selection and maturation of CD4+8+ thymocytes. Eur J Immunol. 1993;23:739–746. doi: 10.1002/eji.1830230326. [DOI] [PubMed] [Google Scholar]
- Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- Taniuchi I, Osato M, Egawa T, Sunshine MJ, Bae SC, Komori T, Ito Y, Littman DR. Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development. Cell. 2002a;111:621–633. doi: 10.1016/s0092-8674(02)01111-x. [DOI] [PubMed] [Google Scholar]
- Taniuchi I, Sunshine MJ, Festenstein R, Littman DR. Evidence for distinct CD4 silencer functions at different stages of thymocyte differentiation. Mol Cell. 2002b;10:1083–1096. doi: 10.1016/s1097-2765(02)00735-9. [DOI] [PubMed] [Google Scholar]
- Telfer JC, Hedblom EE, Anderson MK, Laurent MN, Rothenberg EV. Localization of the domains in Runx transcription factors required for the repression of CD4 in thymocytes. J Immunol. 2004;172:4359–4370. doi: 10.4049/jimmunol.172.7.4359. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Wargnier A, Legros-Maida S, Bosselut R, Bourge JF, Lafaurie C, Ghysdael CJ, Sasportes M, Paul P. Identification of human granzyme B promoter regulatory elements interacting with activated T-cell-specific proteins: implication of Ikaros and CBF binding sites in promoter activation. Proc Natl Acad Sci U S A. 1995;92:6930–6934. doi: 10.1073/pnas.92.15.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildt KF, Sun G, Grueter B, Fischer M, Zamisch M, Ehlers M, Bosselut R. The transcription factor zbtb7b promotes CD4 expression by antagonizing runx-mediated activation of the CD4 silencer. J Immunol. 2007;179:4405–4414. doi: 10.4049/jimmunol.179.7.4405. [DOI] [PubMed] [Google Scholar]
- Woolf E, Xiao C, Fainaru O, Lotem J, Rosen D, Negreanu V, Bernstein Y, Goldenberg D, Brenner O, Berke G, Levanon D, Groner Y. Runx3 and Runx1 are required for CD8 T cell development during thymopoiesis. Proc Natl Acad Sci U S A. 2003;100:7731–7736. doi: 10.1073/pnas.1232420100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- Zhu J, Min B, Hu-Li J, Watson CJ, Grinberg A, Wang Q, Killeen N, Urban JFJ, Guo L, Paul WE. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat Immunol. 2004;5:1157–1165. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- Zou YR, Sunshine MJ, Taniuchi I, Hatam F, Killeen N, Littman DR. Epigenetic silencing of CD4 in T cells committed to the cytotoxic lineage. Nat Genet. 2001;29:332–336. doi: 10.1038/ng750. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure SF1: Generation and genotyping of Zbtb7b hypomorphic mice
(A) The Zbtb7b locus is schematically depicted. The targeting strategy inserted a LoxP site (filled triangle) 3′ of the Zbtb7b open reading frame, and a LoxP and Frt (open triangle) flanked cassette 5′ of the first Zbtb7b coding exon (exon 2), that includes: (i) a neomycin resistance gene (black box) driven by the pGK promoter (yellow), (ii) a splice acceptor (SA, green box)-intron (white box) sequence derived from pLMT330 (Wang et al., submitted) and (iii) a poly-adenylation site (pA, red box). The targeted allele (Zbtb7bt) was obtained by homologous recombination in ES cells and is engineered to promote premature transcription termination at the inserted pA and aberrant mRNA splicing towards the inserted splice acceptor. The floxed allele (Zbtb7bfl) was generated by Flpe deletion of the Frt-flanked Neo cassette using Flpe-expressing mice, and the deleted allele (Zbtb7b−) by Cre-mediated deletion of the floxed sequence. EcoRV restriction sites and the position of probes C and D used for genotyping are indicated. Two-arrowhead lines depict the restriction fragments shown in (B). The bold-faced line indicates the extent of the recombination arms in the targeted and null alleles.. Homologous recombination on the 5′ side was checked using HincII digestion (Wang et al., submitted).
(B, C). Southern blot analyses of tail DNA from Zbtb7b+/fl and Zbtb7b+/t mice. EcoRV-digested genomic DNA was resolved on agarose gels, transferred to Hybond-XL membranes (Amersham) and hybridized with probes C or D. Arrowheads point to restriction specific fragments generated from wild-type (filled) or modified (dashed) alleles. Lane 1 in each blot shows the digestion pattern from the targeted ES cell cells.
Figure SF2: Peripheral CD4 T cell populations in Zbtb7b hypomorphic mice.
Contour plots of CD4 vs. CD44 expression are shown on gated CD4+CD8− splenocytes from wild-type, Zbtb7bt/t and Zbtb7bt/− mice stained with antibodies against CD4, CD8 and CD44 and analyzed by 3-color flow cytometry. Numbers indicate the percentage of cells within boxes.
Figure SF3: Purity of CD4+CD8− populations used in adoptive transfer experiments.
LN T cells were obtained from wild-type and Zbtb7bt/t mice (A) or from pIC-treated MxCre transgenic or non-transgenic Zbtb7bfl/fl mice (B, C) and bead-purified into CD4+CD8− T cells as described in Experimental Procedures. (A, B) Cell purity was assessed by staining for surface expression of TCRβ, CD4 and CD8β (none of which was targeted by antibodies used for purification). Contour plots show CD4 and CD8 expression on all and gated TCRβ+ cells, before (left) or after (right) purification. (C) Dot plots of CD8α vs. CD8β expression verify that pIC treatment did not result in the presence of CD8α+CD8β− cells. Numbers indicate the percentage of cells within quadrants.
Figure SF4: Preferential expression of IFNγ in ‘ThN’-stimulated CD8 cells.
Wild-type sorted CD44lo CD4+CD8− and bead-purified CD4−CD8+ LN T cells were activated by anti-CD3 and anti-CD28 on irradiated APCs in the presence of IL-2 (‘ThN’ conditions) and assessed for IFNγ and IL-4 production as in Fig. 3D.
Figure SF5: in vitro differentiating Zbtb7bt/t effectors maintain a CD4+CD8− coreceptor expression pattern.
Wild-type and Zbtb7bt/t sorted CD44lo CD4+CD8− LN T cells were cultured in vitro under Th2 conditions and assessed for CD4 and CD8 expression five days after activation.
Figure SF6: Relative expression of GzmB in naïve and differentiated effector cells.
GzmB mRNA expression was analyzed by qRT-PCR in naïve (CD44lo, left) and Th2-activated (right) CD4 cells from wild-type or Zbtb7bt/t mice, and in resting or Th2-activated wild-type CD8 cells, and is expressed in arbitrary units relative to β-actin. Data is the average of at least four determinations; error bars indicate SEM.
Figure SF7: Increased expression of GzmB in Zbtb7bt/t Th1 effectors.
Wild-type and Zbtb7bt/t sorted CD44lo CD4+CD8− and wild-type CD8 LN T cells were cultured in vitro under Th1 conditions and assessed for GzmB expression by intra-cellular staining and flow cytometry. (A) Overlaid histograms show GzmB fluorescence gated on CD4+CD8− cells from wild-type (gray-shaded) and Zbtb7bt/t (plain line) cells. The dashed trace shows GzmB expression in wild-type CD8 T cells activated in the same conditions. (B) Contour plots show IFNγ vs. IL-4 expression on the same cultures.
Fig. SF8: Effector differentiation of Zbtb7bt/t CD4 T cells.
(A): Expression of Gata3 was assessed by qRT-PCR in naïve (CD44lo, left) and Th2-activated (right) CD4 cells from wild-type or Zbtb7bt/t mice, and is expressed relative to that of wild-type cells in each condition, after normalization to β-actin mRNA. Data is representative of three independent experiments.
(B): Bead purified CD4+CD8− LN T cells from wild-type (top) or Zbtb7bt/t (bottom) mice were activated in Th17 or Treg conditions. Cytokine production was assessed by intra-cellular staining and flow cytometry on PMA and Ionomycin re-stimulated cells (left), and expression of Foxp3 molecules on cells not subject to re-stimulation (right). Cells were stained for CD4, CD8 and the indicated cytokines or Foxp3. Contour plots (left) and histograms of Foxp3 expression (right) are gated on CD4+CD8− cells.
Figure SF9: T cell development in Zbtb7bfl/fl mice.
Thymocytes and splenocytes from wild-type and Zbtb7bfl/fl mice were analyzed by 4-color flow cytometry as described in Fig. 2A. Numbers indicate the percentage of cells within boxes.
Figure SF10: Analyses of CD4 T cells after in vivo deletion of the floxed Zbtb7b allele.
(A,B). Genomic DNA was prepared from CD44lo CD4+CD8− LN cells from pIC-treated Zbtb7bfl/fl and Mx-Cre transgenic Zbtb7bfl/fl mice, seven days after the first pIC injection (lanes 3, 4 and 7, 8), and analyzed by PCR for the presence of the wild-type (Zbtb7b+), floxed (Zbtb7bfl) (bottom panel, lanes 5–8) or deleted (Zbtb7b−) alleles (top panel, lanes 1–4). (A) The position of primers used for these analyses is schematically depicted. (B) Amplified DNA fragments were separated by agarose gel electrophoresis and revealed by Ethydium Bromide staining of the gel. Tail DNAs from wild-type mice (lanes 2 and 6), Zbtb7b−/− mice (lane 1) and unmanipulated Zbtb7bfl/fl mice (lane 5) were used as controls for the position of amplified bands.
(C). CD4+CD8− LN T cells were analyzed by 3-color flow cytometry for CD4, CD8 and CD44 expression. Two parameter contour plots of CD4 vs. CD8 and CD4 vs. CD44 are shown. Numbers indicate the percentage of cells within boxes.
(D). Sorted CD44lo CD4+CD8− LN T cells from pIC-treated Zbtb7bfl/fl mice, carrying or not the Mx-Cre transgene were activated under Th2 conditions and assessed by flow cytometry for CD4 and CD8 expression five days later.