Abstract
The objective of this study was to quantify the relationship of the levels of antibodies to Ostertagia ostertagi in bulk-tank milk samples from Prince Edward Island (PEI) dairy farms to milk production and to herd-management practices potentially related to gastrointestinal nematode infections. The milk samples were obtained from 289 to 322 dairy farms during 2000; production and management data were available from 197 and 200 farms, respectively. Cow exposure to pasture and whole-herd anthelmintic treatment were the only herd management variables significantly associated with antibody levels in the fall of 2000. An increase in antibody levels from the observed 25th percentile to the 75th percentile (interquartile range) was associated with a drop in milk production of 1.2 kg/cow/day. The results of this study indicate that the enzyme-linked immunosorbent assay for O. ostertagi antibody is a potentially useful technique to measure parasite exposure in adult dairy cows and that parasite burdens in lactating cattle in PEI have an important impact on milk production.
Introduction
Gastrointestinal nematode infections occur frequently in young cattle in temperate regions. Although parasite burdens tend to decrease with age, they remain present in lactating dairy cattle. Two recent slaughterhouse studies carried out in Belgium and The Netherlands in adult dairy cows have shown that more than 90% of the cows examined were infected and that some of them harbored up to 99 000 parasites (1,2). In these studies, Ostertagia ostertagi was the most prevalent parasite, and between 15% and 20% of the animals harbored more than 10 000 worms. In adult cattle, the effect of these parasites has been assessed by evaluating the milk production response after anthelmintic treatment. A review of more than 80 anthelmintic field trials using different study designs and treatment protocols suggested that after anthelmintic treatment a median increase in milk production of 0.63 kg/d could be expected (3). In addition, a recent clinical trial carried out in pastured dairy herds in 2 provinces of Canada, in which cows received at calving either placebo or eprinomectin (Ivomec Eprinex Pour-On; Merial Canada, Montreal, Quebec), showed that milk production increased by an average of 0.94 kg/cow/d during the first 6 mo of lactation (4).
In spite of the evidence that gastrointestinal nematode infection has an adverse effect on milk yield, there is considerable variability between farms in terms of milk response after anthelmintic treatment. In relation to this, Vercruysse and Claerebout (5) discussed the need for a parameter that could be used to identify animals or herds with a level of parasite infection that would justify anthelmintic treatment. A partial budgeting analysis of internal parasite control in dairy farms in Michigan recorded a benefit of US$15/head, assuming that all animals with parasite burdens were correctly diagnosed and that they responded positively to the treatment (6).
An indirect enzyme-linked immunosorbent assay (ELISA) developed in The Netherlands (7) to detect antibodies against O. ostertagi has been evaluated for the monitoring of gastrointestinal parasites in dairy cattle (8). It has a moderate correlation with fecal egg counts (FECs) when herd average optical density (OD) values are compared with herd average FECs. However, FECs in adult animals are not well correlated with parasite burdens (3). Consequently, evaluation of the ELISA requires that OD values be compared with some other, indirect estimators of parasite infection (factors that increase or reduce the risk of gastrointestinal parasitism and production measures). In 2 studies using bulk-tank milk samples, a significant positive relationship has been found between ELISA OD values and levels of exposure to pasture (housing system: confinement, yard, paddock, or pasture) (9), and a negative relationship has been found between ELISA OD values and anthelmintic treatment (10).
Finally, the relationship between ELISA OD values and production measures has been evaluated. Guitian et al (9) found that an increase in bulk tank milk OD values from 0.53 to 0.83 (the interquartile range of all observed values) was associated with a reduction in milk production of 1.25 kg/cow/d in dairy herds in Nova Scotia. In addition, Hovingh (10) found that a significant reduction in the fall milk production was associated with high levels of antibody to O. ostertagi in bulk tank milk samples from dairy herds in Prince Edward Island (PEI). The use of OD values to predict the milk-production response after anthelmintic treatment has also been investigated; Ploeger et al (11), using serum samples, and Sanchez et al (unpublished observations), using milk samples, found statistically significant associations, in which cows with high OD values had greater response to treatment. Similarly, Kloosterman et al (12) reported a trend toward a higher milk yield response in herds with high levels of antibody in bulk tank milk samples, but it was not statistically significant.
The objectives of this study were 1) to quantify the relationship between antibody levels determined by using an O. ostertagi indirect ELISA on bulk tank milk samples and herd management practices related to gastrointestinal nematode infection, and 2) to evaluate the association between antibody levels and measures of milk production.
Materials and methods
Study design and study population
A cross-sectional study, in which levels of antibody in bulk tank milk, herd management practices, and milk yield measures were determined, was conducted between January and December 2000. The study population consisted of all dairy herds in PEI.
Sample collection and laboratory methods
A complete set of bulk tank milk samples submitted to the PEI provincial milk quality laboratory in each of January, May, September, and October 2000 were used in this study. The samples were kept frozen (at −20οC) until O. ostertagi IgG levels were determined in an indirect ELISA. For the assay, crude adult antigen extracts were coated in 96-well microplates (pH 9.6) in a concentration of 1 μg/mL. Positive and negative control serum samples were diluted 1:140 in phosphate-buffered saline in quadruplicate on each plate. Anti-bovine IgG coupled to horseradish peroxidase was used as conjugate. The substrate used was in 2,2′-azino-bis-(3-ethyl-benzthiazoline-sulfonic acid (ABTS) diluted in citrate buffer (0.1 M), sodium phosphate buffer (0.2 M), and 0.09% H2O2. The OD was measured at 405/490 nm and expressed as the optical density ratio (ODR), calculated according to the following formula:
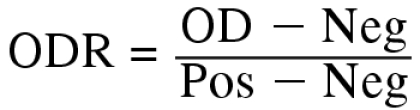 |
where OD is the sample absorbance, and Pos and Neg are the mean absorbance values of the 4 positive and 4 negative control samples, respectively, on the ELISA plate. Although this ELISA cross reacts with other helminths (mainly Cooperia spp.), this is not a serious problem when it is used for monitoring parasite burdens and an overall estimate of the effect of the gastrointestinal parasitism is desired. In addition, good reproducibility values of this ELISA have been determined by Keus et al (7) and by Sanchez et al (unpublished observations).
Farm management practices
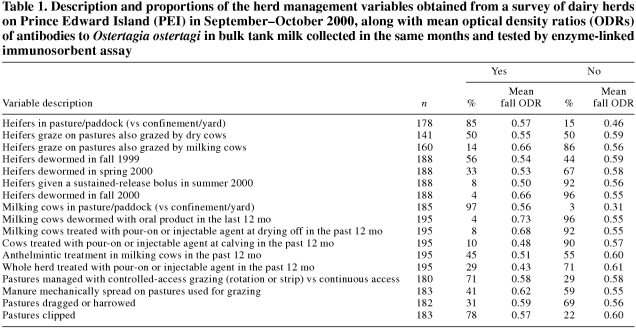
During September 2000, a 1-page closed-response questionnaire was mailed to all registered dairy producers, asking for information on factors that are hypothesized to be associated with exposure to gastrointestinal parasites. Thus, data on housing systems, pasture management, and anthelmintic treatment programs for heifers (nulliparous cows) and milking cows were obtained (descriptions of all management practice variables are presented in Table 1).
Table 1.
Milk production data
Milk yield data from January to December 2000 for individual cows were extracted from the Canadian Dairy Herd Management System (CDHMS) database for all study herds. From these data, herd average daily values per cow (kg/cow/d) were computed for annual milk production (January to December 2000), fall milk production (October to December 2000), and seasonal decline (average of October to December as a proportion of average of May to July). Herd averages for annual and fall days in milk (DIM), lactation number, and log somatic cell counts (SCCs) were also computed.
Data analyses
Mean, standard deviation (s), and ranges of bulk tank ODRs and milk yield were obtained. The variation in ODRs was evaluated by using a mixed linear regression model that was fit with the restricted iterative generalized least-square (RIGLS) algorithm in the statistical package MLwiN (13). The contribution of herd and test month to the total variance was obtained from a random intercept model containing only the intercept (null model).
The fall ODR (average of September and October ODRs) was the only ELISA measure used in the following models. The associations between the fall ODR and herd management practices, obtained from the questionnaire, and between the fall ODR and milk production were evaluated using a backwards-stepwise regression with elimination of nonsignificant effects (P > 0.05). All the main effects that were significant at P ≤ 0.05 were left in the model and 2-way interactions of these variables were evaluated. Once the final model was selected, the potential confounding effect of the eliminated variables was assessed by evaluating the change in the coefficients of the remaining variables in the model that resulted from removal of the potential confounders. Pearson correlation coefficients were used to check for collinearity among explanatory variables. Analyses of the residuals and influential observations were performed on all the models. All the analyses were carried out using Stata Statistical Software, Release 7.0 (Stata Corporation, College Station, Texas, USA).
For the model evaluating the associations between the fall ODR (dependent variable) and management practices, cow and heifer housing variables were recategorized with confinement and exercise yard combined into a single category.
Two sets of models were fit using each of the 3 milk production measures (herd average annual milk production, herd average fall milk production, and seasonal decline in milk production) as the dependent variable. One set of models included the fall ODR values, DIM, parity, and SCC as the predictors and was based on data from all herds in the province. The 2nd set also included a variable for pasture exposure, which was dichotomized as nonpastured (confinement, yard, or paddock) and pastured, and anthelmintic treatment protocols. This 2nd set was limited to herds for which a response to the questionnaire had been received.
Results
Descriptive statistics
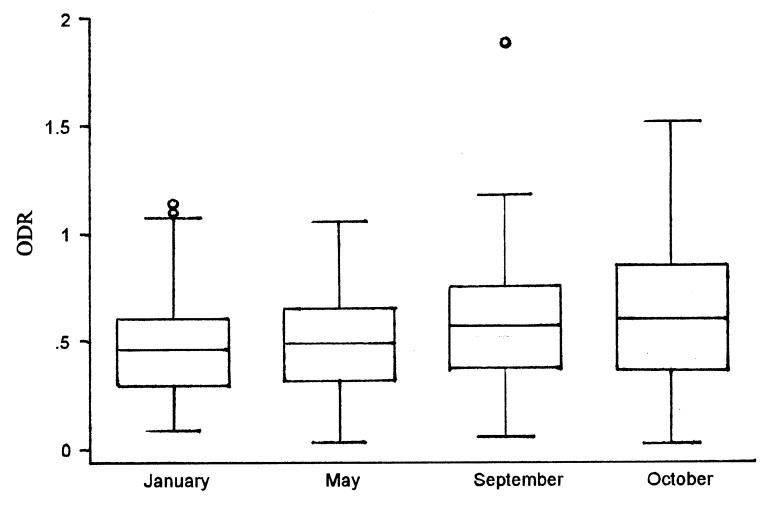
The number of farms sampled during the study period ranged from 289 to 322 per month (mean = 313), with 333 herds contributing to the fall ODR. In total, 1239 bulk-tank milk samples were tested for antibodies against O. ostertagi. The mean ODR was 0.54 (s = 0.26, range = 0.03 to 1.90). The distribution of ODRs by month is depicted in Figure 1. The proportional contributions of herd and test month to the total variance of ODRs obtained from the mixed linear model containing only the intercept were 0.64 and 0.36, respectively.
Figure 1. Box-and-whisker plot of bulk-tank milk optical density ratio (ODR) by test month from dairy farms on Prince Edward Island during January to December 2000.
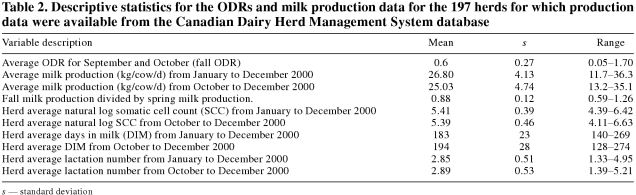
Of the 313 questionnaires mailed, 200 (64%) were returned. Milk production data were obtained for 197 of the 313 herds, but only 191 had fall ODRs. The mean fall ODRs for the responding and the nonresponding farms were 0.55 and 0.66, respectively. The fall ODR and the measures obtained from the milk production database are presented in Table 2.
Table 2.
Association between herd management factors and ELISA results
The pairwise correlation coefficients of the explanatory variables used in these models showed a moderate correlation (r = 0.69) between whole-herd treatment and lactating cow treatment, so it was decided to include the former variable. Apart from that, the highest pairwise correlation observed was 0.33, which suggested that there would be no serious multicollinearity problem.
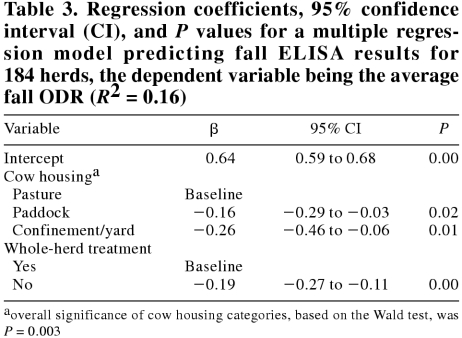
The regression coefficients, 95% confidence intervals, and P values from the final model of factors affecting the fall ODR are presented in Table 3. Cow exposure to pasture and whole-herd treatment were the only variables significantly associated with the fall ODR. The less the exposure to pasture, the lower was the fall bulk tank antibody level. Whole-herd deworming of milking cows significantly reduced the fall ODR. A model that was restricted to pastured herds showed similar results, the only variable significantly associated with the fall ODR being whole-herd treatment (β = −0.21, P = 0.00, R2 = 0.14). No heteroscedasticity was observed in the residual analysis from either model. There was only one outlying observation in each model, and it did not have a large influence on the coefficients, so was left in the model.
Table 3.
Association between milk production and ELISA results
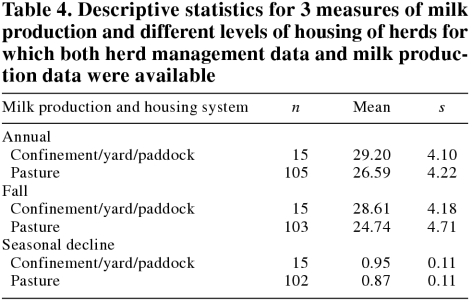
The descriptive statistics of the 3 measures of milk production by cow housing system are shown in Table 4. The Pearson correlation coefficients between the fall ODRs and annual milk production, fall milk production, and seasonal decline were −0.38, −0.44, and −0.29, respectively. Annual and fall milk production were both significantly negatively associated with the fall ODRs: a unit of increase in fall ODR was associated with a reduction of 5.82 kg/d (P = 0.00) and 6.29 kg/d (P = 0.00) in annual and fall milk production, respectively. A similar association was found when seasonal decline was the outcome variable: a unit increase in fall ODR was associated with a reduction of 9% in this parameter.
Table 4.
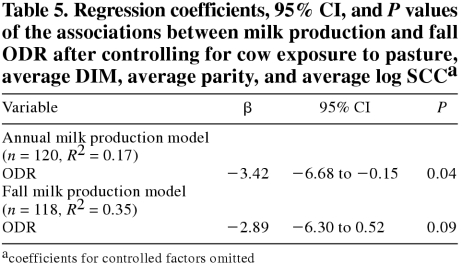
Cow exposure to pasture was the only significant management variable when herd management practices were included in the previous models. The associations between milk production measures and fall ODR are presented in Table 5. After controlling for pasture exposure, a unit of increase in fall ODR was associated with a reduction of 3.42 kg/d (P = 0.041) in annual milk production and a reduction of 2.89 kg/d in fall milk production (P = 0.096). However, these models were based on a smaller number of observations (n = 120 and n = 118) than were the models without pasture exposure (n = 189 and n = 186). For seasonal decline, no significant effect of cow exposure to pasture was observed. However, the association between fall ODR and seasonal decline was similar to the values reported here. The interaction between cow exposure to pasture and fall ODR could not be evaluated in these models owing to the small number of nonpastured herds.
Table 5.
Discussion
The overall mean ODR was higher than that found in a longitudinal study by Sanchez et al (unpublished observations) of lactating dairy cows in 4 Canadian provinces between September 1999 and October 2000. In that study, only 38 dairy herds were sampled monthly throughout the year; they had a mean bulk-tank ODR of 0.36 (range = 0.03 to 1.03).
Little variation in ODR could be observed between sampling dates (Figure 1), and it is difficult to describe a seasonal pattern in this study, because samples were not collected during the summer months, when a rise in ODR might be expected. The proportions of the variance in the fall ODR explained by herd and test day were similar to the values observed in a longitudinal study by Sanchez et al (unpublished observations) of gastrointestinal parasitism in lactating dairy cattle. This agrees with results reported by Dohoo et al (8), who suggested that ELISA OD might be a more stable indicator than FECs of gastrointestinal parasitism at the farm level. Kloosterman et al (14) have also reported that milk samples were as efficient as serum samples in discriminating between herd levels of infection. Finally, Berghen et al (15) have suggested that O. ostertagi antibody levels are the most valuable parameter for estimating the variation in levels of parasite exposure among herds.
The fall ODR of 0.60 was similar to that of 0.58 obtained by Hovingh (10) for 74 dairy herds in PEI in October 1994 and lower than that of 0.69 reported by Guitian et al (9) for 402 dairy herds in Nova Scotia in July to September 1998. However, the previous 2 studies reported “raw” ODs rather than ODRs, which makes the results less comparable. On the other hand, the higher ODs observed in the Nova Scotia study could be attributable to higher levels of parasite exposure during the summer compared with the fall.
In contrast with the study done in Nova Scotia (9), we found only cow housing system and whole-herd treatment to be significantly associated with the fall ODR. This model had an R2 of 0.16, meaning that, after controlling for cow exposure to pasture and whole herd treatment, a large amount of the variation in the fall ODR was not explained by factors in this study. In the Nova Scotia study, heifer housing system and spring anthelmintic treatment of the heifers were also significantly associated with ELISA OD. Hovingh (10) also reported a significant negative association of ELISA OD with anthelmintic treatment of mature cows.
Although the effect of the pasture grazing system (continuous vs rotational) on the fall ODR was not significant, conflicting results are found in the literature related to this factor. Stromberg and Averbeck (16) summarized the results of several parasitologic studies that evaluated the effect of the grazing system on parasite burden: although some studies found a higher parasite load with rotational systems, the others did not find such a difference for either egg or worm counts. Gasbarre et al (17), using a questionnaire on management practices in the northeastern United States, reported that a rotational program and other uses of pasture did not influence the farmer's perception of the importance of parasites in the herd. These authors concluded that “given the complexity of the parasite biology plus all the factors that regulate the egg output and larval survival on pastures, there will be no simple answer to the question of whether [a] rotational grazing system per se increases or decreases parasite transmission”.
The negative relationship between antibody levels and milk production observed in this study is in agreement with that reported in others (9,10,14). Contrary to the results reported by Guitian et al (9), the fall ODR was also significantly associated with a seasonal decline in milk production, as was reported by Hovingh (10). Sanchez et al (unpublished observations) have also found that late-lactation cows with high ODRs had a greater milk response after anthelmintic treatment at calving than cows with low ODRs.
Greater exposure to pasture has been found to be related to lower milk production (9). Similarly, Leslie et al (18), using a conjoint analysis survey of expert opinion, determined that confinement and anthelmintic treatment of replacement heifers and lactating cows was expected to increase milk production, whereas rotational grazing on pasture where manure was spread was expected to decrease milk production. Consequently, a coefficient of −3 kg/d [an intermediate value taken from models in which pasture exposure was controlled (Table 5)] would probably be a better estimate of the association between the fall ODR and milk production. With a fall ODR interquartile range of 0.38 to 0.78 and a coefficient of approximately −3 kg/d, a herd at the 75th percentile would be expected to produce 1.2 kg/cow/d [−3 × (0.78−0.38)] less than a herd at the 25th percentile. However, since exposure to pasture was relatively crudely estimated in the current study, the confounding effect on milk production may not have been totally controlled, and the effect of the ODR may still be biased upwards.
In conclusion, a high proportion of the variation in fall ODR was explained by between-herd variation (as opposed to within-herd variation between test dates). The ELISA results (ODRs) were associated with factors that biologically would increase or reduce the risk of gastrointestinal parasitism. However, it is still necessary to identify other factors that would explain the large amount of unexplained variation. Moreover, the consistently observed negative association between bulk tank milk ODRs and milk production, plus some observations that cows with high ODRs yielded more milk after anthelmintic treatment, provides evidence that cows and, or, herds with high ODRs are incurring parasite-induced losses in milk production (Sanchez et al, unpublished observations). Collectively this information supports the potential value of this ELISA for measurement of parasite burden. However, further research is needed to establish a threshold value for bulk tank milk ODR at which treatment is warranted economically.
Footnotes
Acknowledgments
The authors are grateful for all assistance provided by Judy Shepard in the technical aspects of the ELISA and Murray Myles, manager of the PEI Milk Marketing Board, and his staff for their help in handling of the questionnaires. CVJ
Address correspondence and reprint requests to Dr. Javier Sanchez.
References
- 1.Agneessens J, Claerebout E, Dorny P, et al. Nematode parasitism in adult dairy cows in Belgium. Vet Parasitol 2000;90:83–92. [DOI] [PubMed]
- 2.Borgsteede FH, Tibben J, Cornelissen JB, et al. Nematode parasites of adult dairy cattle in the Netherlands. Vet Parasitol 2000; 89:287–296. [DOI] [PubMed]
- 3.Gross SJ, Ryan WG, Ploeger HW. Anthelmintic treatment of dairy cows and its effect on milk production. Vet Rec 1999;144: 581–587. [DOI] [PubMed]
- 4.Nødtvedt A, Dohoo IR, Sanchez J, et al. The effect of eprinomectin pour-on solution in lactating dairy cows — a clinical trial in pastured dairy herds. Vet Parasitol, in press.
- 5.Vercruysse J, Claerebout E. Treatment vs non-treatment of helminth infections in cattle: defining the threshold. Vet Parasitol 2001;98:195–214. [DOI] [PubMed]
- 6.Brown MI, Lloyd JW, Kaneene JB, et al. Theoretical financial benefit of internal parasite control for Michigan dairy farms using National Animal Health Monitoring System data. Vet Prev Med 1993;17:47–56.
- 7.Keus A, Kloosterman A, Van den Brink R. Detection of antibodies to Cooperia spp. and Ostertagia spp. in calves with the enzyme-linked immunosorbent assay (ELISA). Vet Parasitol 1981;8: 229–236.
- 8.Dohoo IR, Caldwell V, Markham F, et al. Evaluation of an ELISA for monitoring parasite burdens in dairy herds. Proc 8th Int Symp Vet Epidemiol Econ 1997:31–32.
- 9.Guitian FJ, Dohoo IR, Markham RJ, et al. Relationships between bulk-tank antibodies to Ostertagia ostertagi and herd management practices and measures of milk production in Nova Scotia dairy herds. Prev Vet Med 1999;47:79–89. [DOI] [PubMed]
- 10.Hovingh E. An investigation into factors affecting summer/fall milk production and profitability in PEI dairy herds [PhD thesis]. Charlottetown, Prince Edward Island: University of Prince Edward Island, 1998.
- 11.Ploeger HW, Schoenmaker GJ, Kloosterman A, et al. Effect of anthelmintic treatment of dairy cattle on milk production related to some parameters estimating nematode infection. Vet Parasitol 1989;34:239–253. [DOI] [PubMed]
- 12.Kloosterman A, Ploeger HW, Pieke EJ, et al. The value of bulk milk ELISA Ostertagia antibody titres as indicators of milk production response to anthelmintic treatment in the dry period. Vet Parasitol 1996;64:197–205. [DOI] [PubMed]
- 13.Goldstein H, Rasbash J, Plewis I, et al. A user's guide to MLwiN. London, England: Institute of Education, University of London, 1998.
- 14.Kloosterman A, Verhoeff J, Ploeger HW, et al. Antibodies against nematodes in serum, milk and bulk milk samples as possible estimators of infection in dairy cows. Vet Parasitol 1993;47: 267–278. [DOI] [PubMed]
- 15.Berghen P, Hilderson H, Vercruysse J, et al. Evaluation of pepsinogen, gastrin and antibody response in diagnosing ostertagiasis. Vet Parasitol 1993;46:175–195. [DOI] [PubMed]
- 16.Stromberg BE, Averbeck GA. The role of parasite epidemiology in the management of grazing cattle. Int J Parasitol 1999;29:33–39. [DOI] [PubMed]
- 17.Gasbarre LC, Stout WL, Leighton EA. Gastrointestinal nematodes of cattle in the northeastern US: results of a producer survey. Vet Parasitol 2001;29–44. [DOI] [PubMed]
- 18.Leslie K, Jackson A, Duffield T, et al. Survey of selected risk factors and therapeutic strategies fro parasitism on milk production response of lactating dairy cattle. Bovine Pract 2000;34:23–31.