Abstract
Determining the molecular regulators/pathways responsible for the specification of human embryonic stem cells (hESCs) into hematopoietic precursors has far-reaching implications for potential cell therapies and disease modeling. Mouse models lacking SCL/TAL1 (stem cell leukemia/T-cell acute lymphocytic leukemia 1) do not survive beyond early embryogenesis because of complete absence of hematopoiesis, indicating that SCL is a master early hematopoietic regulator. SCL is commonly found rearranged in human leukemias. However, there is barely information on the role of SCL on human embryonic hematopoietic development. Differentiation and sorting assays show that endogenous SCL expression parallels hematopoietic specification of hESCs and that SCL is specifically expressed in hematoendothelial progenitors (CD45−CD31+CD34+) and, to a lesser extent, on CD45+ hematopoietic cells. Enforced expression of SCL in hESCs accelerates the emergence of hematoendothelial progenitors and robustly promotes subsequent differentiation into primitive (CD34+CD45+) and total (CD45+) blood cells with higher clonogenic potential. Short-hairpin RNA–based silencing of endogenous SCL abrogates hematopoietic specification of hESCs, confirming the early hematopoiesis-promoting effect of SCL. Unfortunately, SCL expression on its own is not sufficient to confer in vivo engraftment to hESC-derived hematopoietic cells, suggesting that additional yet undefined master regulators are required to orchestrate the stepwise hematopoietic developmental process leading to the generation of definitive in vivo functional hematopoiesis from hESCs.
Introduction
Human embryonic stem cells (hESCs) represent a unique in vitro model for human developmental biology, drug screening, and a potential source for cell replacement strategies.1 The ability to generate cells of the hematopoietic system has immense use in several areas of clinical and experimental hematology.2,3 Unfortunately, the hematopoietic-specific differentiation potential varies among hESC lines and despite many recent efforts, the efficient generation of adequate numbers of hematopoietic cells remains poor.2,3,4,5,6,7,8,9,10,11,12,13,14,15 Hematopoietic specification of hESCs has been shown to follow a developmental progression through mesoendodermal and hemangioblastic precursors.11,12,16 However, a better understanding of the intrinsic regulators and signaling pathways driving hematopoietic specification of hESCs is highly demanded, suggesting the need to study the functional impact of early hematopoietic regulators that more closely mimic the in vivo developmental program of human hematopoietic specification.
SCL (stem cell leukemia), also known as TAL1 (T-cell acute lymphocytic leukemia 1 gene), is a transcription factor with helix–loop–helix structure that modulates the activity of other transcription factors17 and, is rearranged in several human chromosomal translocations present in both myeloid and T-cell leukemia.18,19 Most information about the developmental impact of early hematopoietic regulators comes from studies performed on invertebrate models and low vertebrates, especially the mouse. The development of genetically modified animal models for specific hematopoietic genes has immensely advanced our understanding of the cellular and molecular mechanisms underlying both normal hematopoiesis and leukemogenesis.20,21,22 Thus, Scl-deficient mice die during the embryonic development, between embryonic days 8.5 and 10.5, due to the absence of hematopoiesis.23 In addition, Porcher et al.24 demonstrated that Scl is necessary for the development of all the hematopoietic lineages in the mouse embryo. Recently, elegant studies by Lancrin et al.25 also demonstrated that Scl is indispensable for the establishment not only of the blood system but also the hemogenic endothelium. On the contrary, Scl transgenic mice26 developed T-cell acute leukemia recapitulating the effect of the human t(1;14)(p32;q11) chromosomal translocation.18 This controversial data coupled to the fact that there is a gap in our understanding between the mouse and human development should encourage studies aiming at addressing the developmental impact of SCL on human embryonic hematopoiesis.1
Despite the important advances in the field, it remains a challenge to originate functional hematopoietic stem and progenitor cells from hESCs capable of establishing human definitive hematopoiesis.7,14 Here, we have hypothesized that SCL, because of its crucial role in early hematoendothelial decisions, may regulate the early specification of hESCs into hematoendothelial precursors and subsequent blood differentiation. We demonstrate by means of gain- and loss-of-function differentiation studies that SCL expression positively modulates the specification of human hemogenic endothelium and subsequent hematopoietic differentiation.
Results
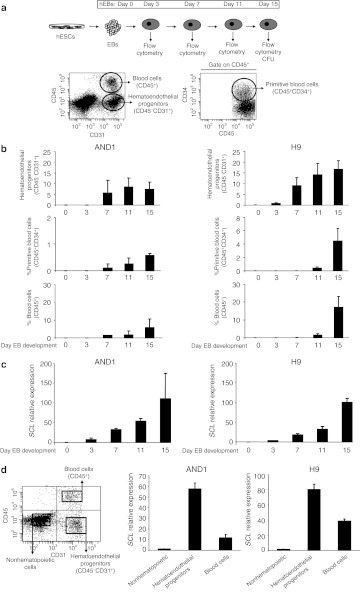
SCL expression parallels hematopoietic emergence from hESCs
To determine the developmental impact of SCL during human embryonic hematopoietic specification, hESCs were differentiated in the presence of bone morphogenetic protein-4 and hematopoietic cytokines.5,12,13,14 The kinetics of emergence of hematoendothelial progenitors (CD45−CD31+), primitive (CD45+CD34+), and total (CD45+) blood cells was analyzed throughout hEBs development for both H9 and AND1 hESC lines (Figure 1a). Hematopoietic specification from both hESC lines was very much identical with hematoendothelial progenitors appearing between days 3 and 7 of hEB development and subsequent primitive and total blood cells emerging later on around day 11 hEB (Figure 1b). Quantitative analysis of endogenous SCL throughout hEB development reveals a robust parallel trend between hematopoietic specification and SCL expression (Figure 1c), with the highest hematopoietic numbers and SCL expression levels coinciding at the end of the differentiation protocol (Figure 1c).
Figure 1.
Expression of SCL parallels hematopoietic emergence from hESCs. (a) Schematic of the hematopoietic differentiation of hESCs and representative flow cytometry dot plots showing how hematoendothelial progenitors, primitive blood cells, and total blood cells are identified and analyzed throughout EB development. (b) Kinetics of hematopoietic emergence from two different hESC lines (AND1 and H9). In the upper panels, percentage of hematoendothelial progenitors is represented. Primitive and total blood cells are shown in the middle and lower panels, respectively. (c) Quantitative real-time polymerase chain reaction (qRT-PCR) analysis of SCL demonstrating that the induction of endogenous SCL throughout EB development parallels the emergence of hematopoietic cells. Relative expression is shown normalized to undifferentiated hESCs. (d) qRT-PCR analysis of SCL in isolated cell populations reveals that SCL is specifically expressed in hematoendothelial progenitors followed by CD45+ blood cells. Relative expression is shown normalized to nonhematopoietic cells. Data represent mean ± SEM for 3–4 independent experiments from both AND1 and H9 hESC lines. CFU, colony-forming unit; hEB, human embryoid body; hESC, human embryonic stem cell; SCL, stem cell leukemia.
Next, we wanted to address which cell subset within the bulk differentiating hEB is responsible for SCL induction. Thus, the SCL expression was analyzed by qRT-PCR specifically in hematoendothelial progenitors, CD45+ blood cells and the remaining hEB cells purified by flow cytometry (purity >95%; data not shown). As shown in Figure 1d, SCL expression is restricted to hESC-derived hematopoietic cells. It is mainly expressed in hematoendothelial progenitors and at lesser extent in CD45+ blood cells (Figure 1d). Together, these results support that SCL may be a master regulator early during human embryonic hematopoiesis.
Enforced expression of SCL preserves hESC pluripotency
In order to further evaluate the developmental impact of SCL expression in the hematopoietic specification of hESCs, we generated transgenic SCL-overexpressing hESC lines as previously described.5 Three hESC lines were chosen based on their opposite intrinsic hematopoietic potential: AND1 and H9 hESC lines, which have been shown to display high predisposition to blood differentiation,5,27 and HS181, which barely gives rise to hematopoiesis.5,28 Human SCL complementary DNA was subcloned in a lentiviral vector expressing the neomycin-resistance cassette (EV; Figure 2a). Human ESCs were transduced with either the EV or the SCL-expressing vector (SCL). After 15 days of G418 selection, typical hESC colonies appeared (Supplementary Figure S1a). Stable ectopic expression of SCL/GFP was confirmed by qRT-PCR (Figure 2b and Supplementary Figure S4a), western blot (Figure 2c), and flow cytometry (Figure 2d). More than 10 weeks after G418 selection, wild-type, EV, and SCL hESC cultures were then analyzed for pluripotency markers and functional assays. SCL-expressing hESCs retained the expression of both the pluripotency markers Oct4, Nanog, Sox-2, and Rex-1 (Supplementary Figure S1a) and the hESC-associated antigens Tra-1-60, Tra-1-81, SSEA3, and SSEA4 (Supplementary Figure S1c). Functionally, SCL and EV-hESCs formed teratomas with identical efficiency (100%), latency (45–65 days), and histological composition (Supplementary Figure S2).29
Figure 2.
Enforced expression of SCL in hESCs. (a) Schematic representation of the lentiviral vectors used. (b) Quantitative real-time polymerase chain reaction analysis of SCL demonstrating specific overexpression in two independently transduced hESC lines. Relative expression is shown normalized to wt hESCs. Data represent mean ± SEM for three independent experiments. (c) Western blot detection of SCL protein in transgenic SCL hESCs. 293T cells transduced with SCL vector were used as a positive control. (d) Representative flow cytometry detection of GFP in NEO-resistant hESCs. EF1α, elongation factor 1α EGFP, enhanced green fluorescent protein; hESC, human embryonic stem cell; hSCL, human SCL; LTR, long terminal repeat; NEO, neomycin; PGK, phosphoglycerate kinase; prom, promoter; SCL, stem cell leukemia; wt, wild type.
Augmented specification of hematoendothelial progenitors from SCL hESCs
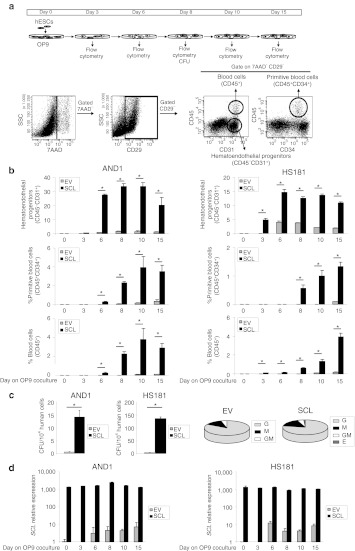
Once we confirmed that enforced SCL expression in hESCs is compatible with hESC homeostasis and pluripotency, we analyzed its developmental impact throughout embryonic hematopoietic commitment. Using the well-established hEB differentiation system5,12,13 (Figure 1a), the impact of enforced SCL was analyzed on the emergence of embryonic hematoendothelial precursors (CD45−CD31+CD34+) throughout hEB development (Figure 3a). We consistently found that SCL overexpression accelerated the kinetics of emergence of hematoendothelial precursors. During EB development, the mean frequency (for HS181 and AND1 lines) of hematoendothelial progenitors from EV-hESCs is 0% at day 3 and 1.4% at day 7, whereas the mean % of hematoendothelial progenitors from SCL hESCs is 7.3% and 6.2% at day 3 and 7, respectively (Figure 3a).
Figure 3.
Enforced expression of SCL augments hematopoietic specification from hESCs in an EB differentiation model. (a) Kinetics of hematopoietic specification from two different hESC lines (AND1 and HS181) transduced with the empty vector (EV) or SCL-expressing vector (SCL). Hematoendothelial progenitors, primitive blood cell, and total blood cells, are represented. (b) CFU read out from day 15 EBs confirming an increased hematopoietic progenitor potential in SCL cells. Scoring of CFU revealed no differences in CFU subtypes between EV- and SCL-transduced progenitors (right, pie charts). (c) Quantitative real-time polymerase chain reaction analysis of SCL demonstrates that endogenous SCL expression parallels hematopoietic specification throughout EB development in both hESC lines analyzed whereas exogenous SCL expression remains constant and high at any time during EB differentiation. Relative expression is shown normalized to undifferentiated hESCs transduced with the EV. Data represent mean ± SEM for 3–4 independent experiments. Statistical significance was assessed with Student's t-test. *P < 0.05. CFU, colony-forming unit; E, erythroid; EB embryoid body; EV, empty vector; G, granulocyte; GM, granulocyte–macrophage; hESC, human embryonic stem cell; M, macrophage; SCL, stem cell leukemia.
The increased frequency and accelerated appearance of hematoendothelial progenitors in SCL hESCs may be the consequence of either (i) SCL-mediated specification of hESCs toward hematoendothelial precursors or (ii) SCL-mediated proliferation of the emerging hemogenic progenitors. To address this, cell cycle distribution was analyzed within both the hematoendothelial precursor population and the remaining hEB cells (Supplementary Figure S3). No differences in the proportion of cycling cells or apoptotic cells (measured by 7AAD exclusion; data not shown) were observed either between SCL or EV or even wild-type hematoendothelial progenitors (cycling cells: 25% versus 27% versus 25%, respectively) or between SCL hematoendothelial precursors and the SCL remaining EB cells (25% versus 30%). These data suggest that SCL expression promotes specification, rather than selective proliferation, of hematoendothelial precursors from differentiating hEBs.
SCL enhances subsequent hematopoietic differentiation of hESC-derived hematoendothelial progenitors
We next studied whether SCL-mediated enhanced specification of hematoendothelial precursors occurs with a subsequent increase of hematopoietic commitment. The emergence of primitive (CD45+CD34+) and total hematopoietic cells (CD45+) was determined throughout hEB development (Figure 3a). The expression of SCL augmented the hematopoietic commitment in three hESC lines (Figure 3a and Supplementary Figure S4b). However, the hematopoietic differentiation was dramatically increased in HS181, an hESC line unable to give rise to blood to date.5,28 Between days 11 and 15 of hEB development, SCL HS181 gave rise to ~60–80% of CD45+ blood cells whereas EV HS181 did not give rise to blood at all (Figure 3a).
Functionally, we tested the clonogenic potential of hematopoietic progenitors derived from day 15 hEB development, measured by the ability to form CFUs in semisolid cultures (Figure 3b). Hematopoiesis generated from SCL hESCs displays a robust increased (~7- to 47-fold) clonogenic potential. Importantly, scoring of CFUs revealed no differences in the CFU types (granulocyte-, macrophage-, and granulocyte–macrophage) obtained from EV versus SCL hematopoietic progenitors (Figure 3b). However, SCL but not EV hematopoietic progenitors gave rise to ~5% erythroid colonies (burst-forming units–erythroid) in both SCL hESC lines. Supplementary Figure S5 depicts typical CFU colonies generated from both SCL and EV hematopoietic progenitors. To ensure that any developmental effect is linked to SCL expression, we confirmed by qRT-PCR stable transgene expression upon differentiation of SCL hEBs (Figure 3c). As expected, however, endogenous SCL levels in EV differentiating hEBs parallel hematopoietic emergence (Figure 3c and Supplementary Figure S4c). Together, these data confirm that ectopic SCL not only promotes the specification of hematoendothelial precursors but also the generation of blood cells and supports previous data largely linking SCL and erythropoiesis.30,31
During human embryonic specification, the population of hematoendothelial precursors is uniquely responsible for both hematopoietic and endothelial development.10,12,14 We thus wanted to address whether the SCL-mediated hematoendothelial precursors emerging earlier in development retained the ability to differentiate into mature endothelial cells as extensively reported for those hematoendothelial precursors emerging by day 10 onward.12 SCL hEBs were dissociated at day 7 and 15 of hEB development and the hematoendothelial progenitors were sorted by magnetic-activated cell sorting and cultured for 7 days in cell culture conditions promoting endothelial maturation (Supplementary Figure S6a). After this time, both day 7 and 15 SCL-overexpressing hematoendothelial precursors gave rise to endothelial-like cells, which took over the culture and expressed equal levels of the bona fide endothelial mature markers vascular endothelial-cadherin, von Willebrand factor, and endothelial nitric oxide synthase (Supplementary Figure S6b), indicating that despite the accelerated emergence of hematoendothelial precursors from SCL hESCs, they preserve the ability to generate both hematopoietic and endothelial cells.
OP9–hESC coculture differentiation system confirms a robust SCL-mediated enhanced hematopoietic specification
We next wanted to validate whether the SCL-mediated effects on hematopoietic specification observed in the hEB differentiation system is reproducible in the well-established OP9–hESC coculture system.11,32 The EV and SCL hESC lines AND1 and HS181 were allowed to differentiate on OP9 stroma and the emergence of human hematoendothelial precursors (CD45−CD31+), primitive (CD45+CD34+), and total blood cells (CD45+) was analyzed at days 3, 6, 8, 10, and 15 as depicted in Figure 4a. Regardless the hESC line, ectopic SCL accelerated the emergence of and induced a robust increase in the frequency of hematoendothelial precursors (approximately eightfold increase; Figure 4b). Consequently, further differentiation toward primitive and total blood cells was evident in both SCL-expressing hESC lines whereas EV-hESCs barely differentiate into hematopoietic cells (Figure 4b). In addition, clonogenic potential analysis of hematopoietic progenitors (CFU assays) derived from day 8 of OP9–hESC cocultures revealed a 32- to 48-fold increase in CFU potential in SCL hESCs versus EV-hESCs (Figure 4c). Despite scoring of CFUs revealed no differences in the CFU types obtained from EV versus SCL hematopoietic progenitors (Figure 4c), around 5% of erythroid colonies (burst-forming units–erythroid) were exclusively generated by SCL hematopoietic progenitors (Figure 4c and Supplementary Figure S5). Similar to the hEB differentiation system, transgene expression was stable throughout the differentiation of SCL hESCs on OP9, indicating that the observed developmental effects are linked to SCL expression (Figure 4d). Together, our data confirm that SCL positively regulates human specification into embryonic hematopoiesis regardless of the hESC line and differentiation system employed.
Figure 4.
Enforced expression of SCL augments hematopoietic specification from hESCs in an OP9 differentiation model. (a) Top panel: schematic representation of the hematopoietic differentiation of hESCs in the OP9 coculture system. Bottom panel: representative flow cytometry dot plots showing how hematoendothelial progenitors, primitive blood cells, and total blood cells were identified and analyzed. (b) Kinetics of hematopoietic specification from two different hESC lines (AND1 and HS181) transduced with the empty vector (EV) or SCL vector (SCL). The emergence of hematoendothelial progenitors, primitive blood cell, and total blood cells is shown. (c) CFU read out from differentiating day 8 hESCs confirming a robust increase in the hematopoietic progenitor potential in SCL cells. Scoring of CFU revealed no differences in CFU subtypes between EV and SCL-transduced progenitors (right pie charts). (d) Quantitative real-time polymerase chain reaction analysis of SCL demonstrates that endogenous SCL expression parallels hematopoietic specification throughout OP9 coculture in both hESC lines analyzed. In contrast, exogenous SCL expression remains constant and high at any time during differentiation. Relative expression is shown normalized to undifferentiated hESCs transduced with the EV. Data represent mean ± SEM for 3–4 independent experiments. Statistical significance was assessed with Student's t-test. *P < 0.05. CFU, colony-forming unit; E, erythroid; G, granulocyte; GM, granulocyte–macrophage; hESC, human embryonic stem cell; M, macrophage; SCL, stem cell leukemia; SSC, side scatter; 7AAD, 7-amino-actinomycin D.
SCL silencing abrogates hematopoietic specification of hESC
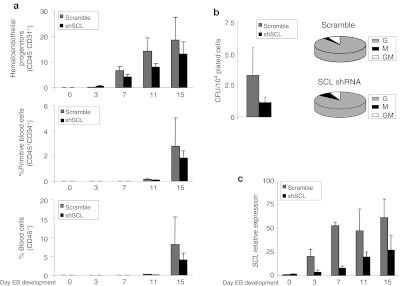
To further demonstrate that endogenous SCL modulates human embryonic hematopoietic specification, we undertook a loss-of-function approach in which endogenous SCL expression was inhibited by means of shSCL-expressing lentiviral vectors. Transgenic shSCL hESCs displayed a delayed hematopoietic specification as compared with “scramble” hESCs. As a result, a decreased of hematoendothelial precursors as well as both primitive and total blood cells was observed at any time point analyzed during hEB development (Figure 6a) or during differentiation on OP9 coculture (Supplementary Figure S7a). Similarly, hematopoietic progenitors derived from shSCL hESCs showed approximately threefold decreased hematopoietic clonogenic potential as compared with the control in both hEB (Figure 5b) and OP9 differentiation system (Supplementary Figure S7b). Interestingly, in contrast to the gain-of-function studies, no erythroid colonies were generated by shSCL hematopoietic progenitors in either differentiation system (Figure 5b and Supplementary Figure S7b). qRT-PCR revealed that endogenous SCL levels were highly reduced (~55–80%) upon shSCL expression, validating the observed SCL knock down–mediated hematopoietic developmental defects (Figure 5c).
Figure 5.
SCL silencing impairs hematopoietic specification from hESC. (a) Hematopoietic specification from hESCs transduced with an irrelevant shRNA sequence (scramble) or three different shRNA specific sequences for SCL (shSCL). Top: kinetic of emergence of hematoendothelial progenitors. Middle: emergence of primitive blood cell. Bottom: emergence of total blood cells. (b) CFU read out from day 15 EBs confirming a threefold decrease hematopoietic progenitor potential in SCL-knocked down cells. Scoring of CFUs revealed no differences in CFU subtypes between scrambled and SCL knocked-down transduced progenitors. (c) Quantitative real-time polymerase chain reaction analysis of SCL showing a highly reduced SCL induction throughout EB development in SCL-knocked down differentiating EBs. Relative expression is shown normalized to undifferentiated hESCs infected with the irrelevant shRNA sequence vector (scramble). Data represent mean ± SEM for 3–4 independent experiments. CFU, colony-forming unit; E, erythroid; EB, embryoid body; G, granulocyte; GM, granulocyte–macrophage; hESC, human embryonic stem cell; M, macrophage; SCL, stem cell leukemia; shSCL, short-hairpin SCL.
Enforced SCL expression is not sufficient to confer in vivo engraftment to hESC-derived hematopoietic cells
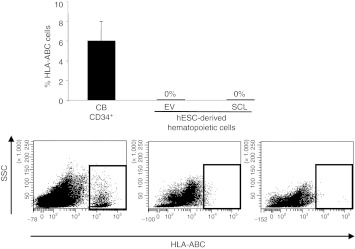
So far, our data support a role for SCL as early master human embryonic hematopoietic regulator. We thus analyzed whether enforced SCL suffices to confer in vivo engraftment to the hESC-derived SCL-expressing hematopoietic cells. Between 1 × 105 and 2.5 × 105 hESC-derived EV and SCL hematopoietic derivatives (generated by either hEB formation or OP9 coculture) were transplanted intrahepatically into newborn or intra bone marrow into adult immunodeficient mice (NSG).33 Despite that SCL clearly regulates hematopoietic specification in vitro, it did not confer on its own in vivo function/engraftment potential to hESC-derived hematopoietic cells (Figure 6), in line with previous reports suggesting that hESC hematopoietic derivatives barely repopulate immunodeficient mice. These data suggest that additional yet undefined intrinsic hematopoietic regulators or extrinsic milieu factors/signals are required for the generation of bona fide definitive and in vivo functional hematopoiesis from hESCs.
Figure 6.
Enforced expression of SCL is not sufficient to confer hESC-derived hematopoietic derivatives with in vivo engraftment potential. Anti-HLA-ABC and anti-CD45 were used to analyze the chimerism in the bone marrow, liver, spleen and peripheral blood of mice transplanted with EV or SCL-expressing hematopoietic cells. EV, empty vector; HLA, human leukocyte antigen; hESC, human embryonic stem cell; SCL, stem cell leukemia; SSC, side scatter.
Discussion
Most of the information accumulated over decades in the field of developmental hematopoiesis comes from a broad range of cell biology and genetic studies performed on invertebrate models and the mouse. The development of genetically modified animal models for specific hematopoietic genes has immensely advanced our understanding of the mechanisms underlying normal hematopoiesis and leukemogenesis.20,21,22 The derivation of mouse ESC in 1981 (ref. 34) revolutionized the field of developmental biology by providing a unique model of early mammalian development in which to address questions of lineage commitment and manipulate cell populations representing early stages of development that are inaccessible in vivo. However, despite our considerable wealth of data concerning regulation of developmental decisions in the mouse, remarkable differences between lower vertebrates and humans have left a considerable gap in our understanding. Accordingly, because of difficulties associated with the access to prenatal human tissues, the mechanisms regulating hematopoietic specification during human embryonic development remain poorly understood.35 Human ESCs are anticipated to offer an unprecedented model for deciphering the molecular and cellular mechanisms regulating early stages during human embryonic hematopoiesis.36
SCL is a transcription factor with helix–loop–helix structure with an important mesodermal patterning activity.37 The earliest marker that distinguishes mesoderm with hematopoietic potential versus nonhematopoietic potential is SCL. Originally discovered rearranged in T-cell acute lymphoblastic leukemia,38 Scl knockout studies indicate an important role for this transcription factor in invertebrate and mouse embryonic hematopoiesis.23,24 Very recently, in clustering-based transcriptional profiling studies of hESC-derived hematopoietic progenitors SCL was revealed for the first time a key master regulator of human embryonic hematopoiesis.31 Here, we have studied the developmental impact of SCL on human embryonic hematopoietic development harnessing the availability of hESCs as a working model. During hESC specification, a population of hemogenic endothelium (CD45−CD31+CD34+) is uniquely responsible for both hematopoietic and endothelial development.10,12,14 We show how endogenous SCL expression parallels hematopoietic specification of hESCs being specifically expressed in hematoendothelial progenitors and, to a lesser extent, on CD45+ hematopoietic cells.
It has been previously reported that SCL lentiviral transduction in nonhuman primate ESC lines promotes hematopoietic cell differentiation.39 Importantly, generation of SCL-overexpressing transgenic hESC lines confirmed in a human setting that SCL accelerates the emergence of hematoendothelial progenitors, which preserve both hematopoietic and endothelial differentiation potential. The increased frequency and accelerated appearance of hematoendothelial progenitors in SCL hESCs may be the consequence of either (i) SCL-mediated specification of hESCs toward hematoendothelial precursors or (ii) SCL-mediated proliferation of the emerging hematoendothelial progenitors. Cell cycle analyses indicate that SCL promotes specification, rather than selective proliferation, of hematoendothelial progenitors from differentiating hESCs. These data are in line with previous elegant studies in mouse and human also demonstrating that SCL is indispensable for the establishment of hemogenic endothelium.25,31 We next studied whether SCL-mediated enhanced specification of hematoendothelial precursors occurs with a subsequent increase of hematopoietic commitment. SCL also promotes a robust subsequent differentiation into primitive and mature blood cells with significantly higher clonogenic potential, confirming that SCL not only promotes the specification of hematoendothelial progenitors but also the generation of in vitro functional blood cells. These data are in agreement with previous data in the mouse and human suggesting that SCL is essential for hematopoietic commitment of the hemangioblast.31,40 Previous data largely linked SCL and erythropoiesis.30 Very recently, Yung et al. demonstrated that lentiviral-mediated overexpression of Scl in H1 and H9 hESC lines promoted differentiation to mesoendodermal lineages, the emergence of hematopoietic progenitors as well as enhanced erythroid differentiation.31 In accordance with these data, SCL-overexpressing HS181 and AND1 hESC lines, but not EV-hESC lines, consistently gave rise to erythroid progenitors in semisolid assays.
Worth mentioning, loss-of-function approaches for endogenous SCL in hESCs abrogate both specification of hematoendothelial precursors and subsequent blood differentiation of hESCs, confirming that during human development SCL constitutes an early hematopoietic regulator essential for both the establishment of the hemogenic endothelium and subsequent hematopoietic commitment from these hematoendothelial progenitors. Importantly, controversial information still exists in the mouse. Previous work from Keller's Lab using mouse ESCs indicates that Scl is crucial for hematopoietic commitment from the hemogenic endothelium (hemangioblast) but is not required for its development/formation.40 In contrast, a very elegant work by Lacaud's Lab recently claimed that Scl is indispensible for the establishment of such hemogenic endothelium from mouse ESCs.25 These studies reinforce the remarkable differences existing between mice and humans, further supporting the use of hESCs as a unique experimental model for studying the early stages during human embryonic hematopoiesis. Of note, a major hurdle in the hESC field is the fact that different hESC lines and differentiation protocols commonly render nonreproducible results.5 However, the developmental effects of SCL in hESCs were consistently identical regardless of the differentiation protocol (hEB system versus OP9 coculture) and the hESC lines used which, in turn, were derived under different conditions by independent laboratories, thus ruling out potential hESC-dependent results due to potential epigenetic memory.
Finally, the robust SCL-mediated hematopoietic-promoting in vitro effects encouraged us to assess whether the expression of such an early master hematopoietic regulator confers in vivo engraftment to hESC-derived SCL hematopoietic derivatives into NSG mice. Unfortunately, SCL expression on its own is not sufficient to endow hESC-derived hematopoietic cells with in vivo reconstituting function, suggesting that additional yet undefined master hematopoietic regulators or extrinsic milieu factors are required to orchestrate the stepwise hematopoietic developmental process leading to the generation of definitive in vivo functional hematopoiesis from hESCs. Lancrin et al. showed that Scl is required for the specification of hematoendothelial progenitors from mouse ESCs whereas Runx1 is critical for the generation of definitive hematopoietic cells from these hematoendothelial progenitors.25 Ongoing work in our laboratory is addressing the role of RUNX1 and extrinsic microenvironment factors, alone or in combination with SCL, in an attempt to generate bona fide in vivo functional hematopoietic cells from hESCs.
Materials and Methods
hESC culture. The hESC lines H9, AND1, and HS181 were maintained undifferentiated in a feeder-free culture following the protocol previously described.5,27,41 In brief, hESCs were cultured over Matrigel-coated (BD Bioscience, San Jose, CA) T25 flasks in human feeder-conditioned medium supplemented with 8 ng/ml basic fibroblast growth factor (Miltenyi Biotec, Bergisch Gladbach, Germany). Medium was changed daily and confluent cultures were split weekly using collagenase IV (Invitrogen, Edinburgh, Scotland). Approval from the Spanish National Embryo Ethical Committee was obtained to work with hESCs.
Lentiviral vectors and transduction. The human SCL complementary DNA (GenBank NM_003189.2) was a gift from Romeo at the Institute of Cellular and Molecular Radiobiology (CEA, France). A Flag-epitope was added at the N-terminal of the SCL by polymerase chain reaction (PCR). Then, the Flag-SCL complementary DNA was directionally subcloned into the intermediate vector KJ-EGFP-2A (kindly provided by García-Perez; GENyo, Granada, Spain), using SpeI and SacII sites. Finally, the full cassette EGFP-2A-Flag-SCL was cloned into the PmeI site in the pRRL-EF1α-PGK-NEO vector (kindly donated by Naldini, Milan, Italy). Three different RNA interference sequences for human SCL were selected from literature and commercially available (Sigma-Aldrich, St Louis, MO).42 Primers containing the hairpins and the internal loop were paired in vitro and cloned into MluI and ClaI sites in the pLVTHM vector obtained from Addgene. Primer sequences are available in Supplementary Table S1.
293T cells were transfected with either pRRL-EF1α-PGK-NEO (empty vector: EV) or pRRL-EF1α-EGFP-2A-Flag-SCL-PGK-NEO (SCL) together with the helper plasmids (psPAX and pMD2.G from Addgene) by standard calcium-phosphate transfection protocol as previously described.43 Two days after transfection, viral particles in the supernatant were collected and concentrated by ultracentrifugation. hESCs were infected overnight on the day of passage with concentrated virus in the presence of polybrene (8 µg/m; Sigma-Aldrich). The following day the viral supernatant was removed and infected hESCs were washed with fresh medium and maintained in culture. Three days later, transduced cells were selected with G418 (Invitrogen) at 100–150 µg/ml for 15 days. SCL expression was confirmed by quantitative PCR and western blot in selected cells before being used for further experiments.
Similarly, pLVTHM vectors containing the scramble or SCL-specific sequences [short-hairpin SCL (shSCL)] were used to produce viral particles on 293T cells. hESCs were infected with concentrated viral supernatant as described above. Next day, hESCs were washed and maintained in culture with no G418 selection because pLVTHM vectors contain EGFP as reporter gene. EGFP-positive colonies were plucked, expanded, and used for further experiments.
Flow cytometry characterization of hESCs. hESC colonies were disassociated with trypsin-EDTA (Invitrogen) and the cell suspension was stained with phycoerythrin-conjugated TRA-1-60, TRA-1-81, SSEA3, and SSEA4 antibodies (BD Bioscience) for 30 minutes. After washing, cells were stained with 7-amino-actinomycin D (7AAD; BD Bioscience) for 5 minutes. Live cells identified by 7AAD exclusion were analyzed using a FACS Canto II flow cytometer.5,41
RNA isolation, RT-PCR, and quantitative PCR analysis. Total RNA from undifferentiated hESCs, human embryoid bodies (hEBs), hematoendothelial precursors and CD45+ blood cells was isolated using Trizol (Invitrogen) as previously described.3,44,45 Complementary DNA was generated with the Super Script First Strand Synthesis System for real-time (RT)-PCR (Invitrogen) and analyzed by quantitative RT-PCR (qRT-PCR) using Brilliant III Ultra-Fast SYBR Green QPCR Master Mix (Agilent Technologies, La Jolla, CA) and the Mx3005P QPCR System (Applied Biosystems). The GAPDH gene was used to normalize data and relative expression was calculated using the ΔΔCT method. Primer sequences are listed in Supplementary Table S1.
Western blot analysis. hESC cultures were dissociated with trypsin-EDTA and subsequently lysed in RIPA buffer (Sigma-Aldrich) supplemented with Complete protease inhibitors cocktail (Roche Diagnostic, Basel, Switzerland) and phosphatase inhibitors (Sigma-Aldrich). Cell lysates were run on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. SCL protein was detected with the Oddyssey infrared imaging system (Li-cor, Lincoln, NE) using anti-SCL (clone C-21; Santa Cruz Biotechnologies, Santa Cruz, CA). Anti-actin antibody (clone AC-15; Sigma-Aldrich) detection was used as loading control
In vivo teratoma formation. Animal protocols were approved by the Animal Care Committee of the University of Granada. In vivo pluripotency was analyzed as previously described.29 In brief, hESCs were implanted subcutaneously in the flank of 8-week-old nonobese diabetic/severe combined immunodeficiency IL2Rγ−/− mice (NSG; Jackson Laboratories, Bar Harbor, MA). Teratoma growth was monitored weekly by palpation and mice were killed between 8 and 10 weeks after implantation. Teratomas were fixed and embedded in paraffin. Hematoxylin and eosin staining on paraffin-embedded teratoma sections was performed.29
Hematopoietic differentiation from hESCs. Undifferentiated hESCs at confluence were treated with collagenase IV and scraped off of the Matrigel attachments. To allow hEB formation, hESC clumps were transferred to low-attachment plates (Corning, Lowell, MA) and incubated overnight in differentiation medium (KO-Dulbecco's modified Eagle's medium supplemented with 20% non-heat-inactivated fetal bovine serum, 1% nonessential amino acids, 1 mmol/l L-glutamine and 0.1 mmol/l β-mercaptoethanol). Next day, medium was changed with the same differentiation medium supplemented with hematopoietic cytokines: 300 ng/ml stem cell factor, 300 ng/ml Flt3 ligand, 10 ng/ml interleukin-3, 10 ng/ml interleukin-6, and 50 ng/ml granulocyte colony–stimulating factor, and 25 ng/ml bone morphogenetic protein-4.5,8 From day 3 to 15 of EB differentiation, media change was performed twice a week. hEBs were dissociated using collagenase B (Roche Diagnostic) for 2 hours at 37 °C followed by 10 minutes incubation at 37 °C with enzyme-free Cell Dissociation Buffer (Invitrogen) at days 3, 7, 11, and 15 of development. A single-cell suspension was obtained by gentle pipetting and passage through a 70-µm cell strainer (BD Bioscience) and the dissociated cells were stained with anti-CD34-FITC or CD34-PECy7, anti-CD31-PE, and anti-CD45-APC (all from Miltenyi Biotec) antibodies and 7AAD. Live cells identified by 7AAD exclusion were analyzed using a FACSCanto II flow cytometer equipped with FACS Diva software (BD Bioscience).
OP9 hematopoietic differentiation system. hESC–OP9 cocultures were performed as previously described.11,32 Briefly, OP9 stroma cells were plated in gelatin-coated 10-cm dishes in α-minimum essential medium as basal medium supplemented with 20% non-heat-inactivated fetal bovine serum for eight days. hESCs grown in Matrigel-coated flasks were prepared as a suspension of small aggregates using Collagenase IV treatment followed by gentle scraping in differentiation medium (α-minimum essential medium as basal medium, 10% non-heat-inactivated fetal bovine serum, 100 µmol/l monothioglycerol, and 50 µg/ml ascorbic acid). One-fifth of this suspension was plated on top of the OP9 stroma in 10 ml of differentiation medium. Next day, media was replaced by 20 ml of fresh differentiation medium. From day 4 to 15 of coculture, a half-volume media change was performed every other day. Hematopoietic specification was analyzed by flow cytometry (at days 3, 6, 8, 10, and 15 of coculture) and colony-forming unit (CFU) assays (at day 8 of coculture). OP9 cells were stained with anti-mouse CD29-FITC (AbD Serotec, Düsseldorf, Germany) to exclude mouse cells from further analysis. The percentage of human hematoendothelial progenitors (CD31+CD45−), primitive blood cells (CD34+CD45+), and total blood cells (CD45+) was analyzed as previously described.5
CFU assay. Human clonogenic progenitor assays were performed by plating 5 × 104 cells from EBs and or OP9–hESC cocultures into methylcellulose H4230 (Stem Cell Technologies, Vancouver, Canada) supplemented with the recombinant human growth factors 50 ng/ml stem cell factor, 3 units/ml erythropoietin, 10 ng/ml granulocyte–macrophage colony–stimulating factor, and 10 ng/ml interleukin-3. Cells were incubated at 37 °C in a humidified atmosphere and colonies were counted between days 8 and 14 of CFU assay using standard morphological criteria.46,47
Cell cycle analysis of hematoendothelial precursors. hEBs were dissociated at day 11 of development, harvested, fixed in 70% ice-cold ethanol, and stored overnight at −20 °C. Next day, the cells were washed with PBS and incubated with anti-CD31-FITC and anti-CD34-FITC (Miltenyi Biotec) for 15 minutes. After washing, the cells were resuspended in propidium iodide buffer containing 50 µg/ml of propidium iodide and 100 µg/ml of RNAase in PBS. Cell cycle distribution was analyzed using a FACSCanto II flow cytometer equipped with Modfit software (Verity Software House, Topsham, ME).48
Endothelial differentiation from hESCs and in situ immunocytochemistry. CD34+ cells were isolated from hEBs at day 7 or 15 of development by magnetic-activated cell sorting using the hCD34 MicroBead kit and the AutoMACS Pro separator (Miltenyi Biotec) following manufacturer's instructions.47 To promote endothelial differentiation, isolated CD34+ cells were seeded into 0.1% gelatin-coated 24-well plates at 2.5 × 104 cells per well in complete endothelial growth medium-2 (Lonza, Bassel, Switzerland) for 7 days. After fixation with ethanol:methanol (1:1) for 10 minutes, cells were washed several times with PBS and stained with rabbit antihuman vascular endothelial-cadherin (Cayman Chemical, Ann Arbor, MI), mouse antihuman endothelial nitric oxide synthase (BD Bioscience), mouse antihuman von Willebrand factor (DAKO, Glostrup, Denmark) followed Cy3-conjugated anti-mouse (Jackson ImmunoResearch, Newmarket, UK) as previously described.12,49
Mice transplantation and analysis of engraftment. NSG mice were housed under sterile conditions. The Animal Care Committee of the University of Granada approved all mouse protocols. Briefly, cord blood–derived CD34+ hematopoietic stem and progenitor cells (n = 3, 3 × 104 cells in 50 µl), as well as EV (n = 5, 1 × 105 cells in 50 µ) or SCL day 15 EB hematopoietic differentiating cells or day 8 OP9-derived hematopoietic differentiating cells were transplanted intrahepatically (n = 19, 1 × 105 to 2.5 × 105 cells in 50 µl) into newborn NSG mice as previously described.50 Mice health was monitored throughout the entire experiment. Mice were killed 6–8 weeks after transplantation and bone marrow, spleen, liver, and peripheral blood were collected and analyzed for human chimerism. Cells were stained with anti-HLA-ABC-PE, anti-CD31-PE, and anti-CD45-APC (BD Bioscience) to analyzed human chimerism by flow cytometry.
Statistical analysis. All data are expressed as mean ± SEM. Statistical comparisons were performed with a paired Student's t-test. Values were considered statistically significant at P < 0.05.
SUPPLEMENTARY MATERIAL Figure S1. Enforced expression of SCL is compatible with hESC pluripotency. Figure S2. Histological analysis of teratomas formed from EV and SCL hESCs. Figure S3. Cell cycle analysis reveals that SCL expression promotes specification rather than enhanced proliferation of hESCs into hematoendothelial progenitors. Figure S4. Enforced expression of SCL augments hematopoietic specification from H9 hESCs in an EB differentiation model. Figure S5. Phase-contrast morphology of typical CFU colonies generated from both SCL and EV hematopoietic progenitors. Figure S6. SCL hematoendothelial progenitors retain the ability to differentiate towards endothelial cell fate. Figure S7. SCL silencing impairs hematopoietic specification from hESC in an OP9 differentiation model. Table Suppl 1. List of primer sequences used in the present study.
Acknowledgments
We thank J.L. García-Perez (GENyO, Granada, Spain) for the 2A-peptide vector (KJ-2A vector) and technical support for EGFP-2A-SCL design and construction; R. Rodriguez (GENyO) for help with flow cytometry cell sorting; L. Naldini (San Raffaele Telethon Institute for Gene Therapy, Milan, Italy) for pRRL-EF1α-PGK-NEO vector; P.H. Romeo (CEA, France) for human SCL complementary DNA; and Deborah Burks and Inmaculada Moreno (CIPF, Valencia, Spain) for animal assistance. This work has been funded by The Junta de Andalucía/Fondo Europeo de Desarrollo Regional (FEDER; SAS-111244 and P10-CTS-6406 to P.J.R. and P08-CTS-3678 to P.M.), The Instituto de Salud Carlos III/FEDER (PI10/00449) to P.M.; (CP09/0063) to P.J.R. and (CP07/00059 and PI11/00119) to C.B., The MICINN (PLE-2009-0111) to P.M., The Spanish Association Against Cancer/Junta Provincial de Albacete (CI110023) to P.M., and The Marie Curie IIF (PIIF-GA-2009-236430) to V.R.-M.
Supplementary Material
Enforced expression of SCL is compatible with hESC pluripotency.
Histological analysis of teratomas formed from EV and SCL hESCs.
Cell cycle analysis reveals that SCL expression promotes specification rather than enhanced proliferation of hESCs into hematoendothelial progenitors.
Enforced expression of SCL augments hematopoietic specification from H9 hESCs in an EB differentiation model.
Phase-contrast morphology of typical CFU colonies generated from both SCL and EV hematopoietic progenitors.
SCL hematoendothelial progenitors retain the ability to differentiate towards endothelial cell fate.
SCL silencing impairs hematopoietic specification from hESC in an OP9 differentiation model.
List of primer sequences used in the present study.
References
- Menendez P, Bueno C., and, Wang L. Human embryonic stem cells: a journey beyond cell replacement therapies. Cytotherapy. 2006;8:530–541. doi: 10.1080/14653240601026654. [DOI] [PubMed] [Google Scholar]
- Kaufman DS, Hanson ET, Lewis RL, Auerbach R., and, Thomson JA. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2001;98:10716–10721. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan AD, Chase JL, Lewis RL, Tian X, Kaufman DS, Thomson JA.et al. (2006Human embryonic stem cell-derived hematopoietic cells are capable of engrafting primary as well as secondary fetal sheep recipients Blood 1072180–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno C, Catalina P, Melen GJ, Montes R, Sánchez L, Ligero G.et al. (2009Etoposide induces MLL rearrangements and other chromosomal abnormalities in human embryonic stem cells Carcinogenesis 301628–1637. [DOI] [PubMed] [Google Scholar]
- Ramos-Mejia V, Melen GJ, Sanchez L, Gutierrez-Aranda I, Ligero G, Cortes JL.et al. (2010Nodal/Activin signaling predicts human pluripotent stem cell lines prone to differentiate toward the hematopoietic lineage Mol Ther 182173–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick K, Wang L, Li L, Menendez P, Murdoch B, Rouleau A.et al. (2003Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells Blood 102906–915. [DOI] [PubMed] [Google Scholar]
- Ledran MH, Krassowska A, Armstrong L, Dimmick I, Renström J, Lang R.et al. (2008Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches Cell Stem Cell 385–98. [DOI] [PubMed] [Google Scholar]
- Menendez P, Wang L, Chadwick K, Li L., and, Bhatia M. Retroviral transduction of hematopoietic cells differentiated from human embryonic stem cell-derived CD45(neg)PFV hemogenic precursors. Mol Ther. 2004;10:1109–1120. doi: 10.1016/j.ymthe.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Tian X, Woll PS, Morris JK, Linehan JL., and, Kaufman DS. Hematopoietic engraftment of human embryonic stem cell-derived cells is regulated by recipient innate immunity. Stem Cells. 2006;24:1370–1380. doi: 10.1634/stemcells.2005-0340. [DOI] [PubMed] [Google Scholar]
- Vodyanik MA, Bork JA, Thomson JA., and, Slukvin II. Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005;105:617–626. doi: 10.1182/blood-2004-04-1649. [DOI] [PubMed] [Google Scholar]
- Vodyanik MA., and, Slukvin II. Hematoendothelial differentiation of human embryonic stem cells. Curr Protoc Cell Biol. 2007;Chapter 23:Unit 23.6. doi: 10.1002/0471143030.cb2306s36. [DOI] [PubMed] [Google Scholar]
- Wang L, Li L, Shojaei F, Levac K, Cerdan C, Menendez P.et al. (2004Endothelial and hematopoietic cell fate of human embryonic stem cells originates from primitive endothelium with hemangioblastic properties Immunity 2131–41. [DOI] [PubMed] [Google Scholar]
- Wang L, Menendez P, Cerdan C., and, Bhatia M. Hematopoietic development from human embryonic stem cell lines. Exp Hematol. 2005;33:987–996. doi: 10.1016/j.exphem.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Wang L, Menendez P, Shojaei F, Li L, Mazurier F, Dick JE.et al. (2005Generation of hematopoietic repopulating cells from human embryonic stem cells independent of ectopic HOXB4 expression J Exp Med 2011603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woll PS, Grzywacz B, Tian X, Marcus RK, Knorr DA, Verneris MR.et al. (2009Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity Blood 1136094–6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodyanik MA, Yu J, Zhang X, Tian S, Stewart R, Thomson JA.et al. (2010A mesoderm-derived precursor for mesenchymal stem and endothelial cells Cell Stem Cell 7718–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lécuyer E., and, Hoang T. SCL: from the origin of hematopoiesis to stem cells and leukemia. Exp Hematol. 2004;32:11–24. doi: 10.1016/j.exphem.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Finger LR, Kagan J, Christopher G, Kurtzberg J, Hershfield MS, Nowell PC.et al. (1989Involvement of the TCL5 gene on human chromosome 1 in T-cell leukemia and melanoma Proc Natl Acad Sci USA 865039–5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley CG, Aplan PD, Davey MP, Nakahara K, Tchorz K, Kurtzberg J.et al. (1989Chromosomal translocation in a human leukemic stem-cell line disrupts the T-cell antigen receptor delta-chain diversity region and results in a previously unreported fusion transcript Proc Natl Acad Sci USA 862031–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvinsky A, Rybtsov S., and, Taoudi S. Embryonic origin of the adult hematopoietic system: advances and questions. Development. 2011;138:1017–1031. doi: 10.1242/dev.040998. [DOI] [PubMed] [Google Scholar]
- Paik EJ., and, Zon LI. Hematopoietic development in the zebrafish. Int J Dev Biol. 2010;54:1127–1137. doi: 10.1387/ijdb.093042ep. [DOI] [PubMed] [Google Scholar]
- Waltzer L, Gobert V, Osman D., and, Haenlin M. Transcription factor interplay during Drosophila haematopoiesis. Int J Dev Biol. 2010;54:1107–1115. doi: 10.1387/ijdb.093054lw. [DOI] [PubMed] [Google Scholar]
- Shivdasani RA, Mayer EL., and, Orkin SH. Absence of blood formation in mice lacking the T-cell leukaemia oncoprotein tal-1/SCL. Nature. 1995;373:432–434. doi: 10.1038/373432a0. [DOI] [PubMed] [Google Scholar]
- Porcher C, Swat W, Rockwell K, Fujiwara Y, Alt FW., and, Orkin SH. The T cell leukemia oncoprotein SCL/tal-1 is essential for development of all hematopoietic lineages. Cell. 1996;86:47–57. doi: 10.1016/s0092-8674(00)80076-8. [DOI] [PubMed] [Google Scholar]
- Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V., and, Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457:892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplan PD, Jones CA, Chervinsky DS, Zhao X, Ellsworth M, Wu C.et al. (1997An scl gene product lacking the transactivation domain induces bony abnormalities and cooperates with LMO1 to generate T-cell malignancies in transgenic mice EMBO J 162408–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes JL, Sanchez L, Ligero G, Gutierrez-Aranda I, Catalina P, Elosua C.et al. (2009Mesenchymal stem cells facilitate the derivation of human embryonic stem cells from cryopreserved poor-quality embryos Hum Reprod 241844–1851. [DOI] [PubMed] [Google Scholar]
- Catalina P, Bueno C, Montes R, Nieto A, Ligero G, Sanchez L.et al. (2009Genetic stability of human embryonic stem cells: a first-step toward the development of potential hESC-based systems for modeling childhood leukemia Leuk Res 33980–990. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Aranda I, Ramos-Mejia V, Bueno C, Munoz-Lopez M, Real PJ, Mácia A.et al. (2010Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection Stem Cells 281568–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerenyi MA., and, Orkin SH. Networking erythropoiesis. J Exp Med. 2010;207:2537–2541. doi: 10.1084/jem.20102260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung S, Ledran M, Moreno-Gimeno I, Conesa A, Montaner D, Dopazo J.et al. (2011Large-scale transcriptional profiling and functional assays reveal important roles for Rho-GTPase signalling and SCL during haematopoietic differentiation of human embryonic stem cells Hum Mol Genet 204932–4946. [DOI] [PubMed] [Google Scholar]
- Choi KD, Vodyanik M., and, Slukvin II. Hematopoietic differentiation and production of mature myeloid cells from human pluripotent stem cells. Nat Protoc. 2011;6:296–313. doi: 10.1038/nprot.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A.et al. (2004Development of a human adaptive immune system in cord blood cell-transplanted mice Science 304104–107. [DOI] [PubMed] [Google Scholar]
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavian M, Biasch K, Sinka L, Vallet J., and, Péault B. Embryonic origin of human hematopoiesis. Int J Dev Biol. 2010;54:1061–1065. doi: 10.1387/ijdb.103097mt. [DOI] [PubMed] [Google Scholar]
- Islam MS, Ni Z., and, Kaufman DS. Use of human embryonic stem cells to understand hematopoiesis and hematopoietic stem cell niche. Curr Stem Cell Res Ther. 2010;5:245–250. doi: 10.2174/157488810791824467. [DOI] [PubMed] [Google Scholar]
- Ismailoglu I, Yeamans G, Daley GQ, Perlingeiro RC., and, Kyba M. Mesodermal patterning activity of SCL. Exp Hematol. 2008;36:1593–1603. doi: 10.1016/j.exphem.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Chen Q, Yang CY, Tsan JT, Xia Y, Ragab AH, Peiper SC.et al. (1990Coding sequences of the tal-1 gene are disrupted by chromosome translocation in human T cell leukemia J Exp Med 1721403–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita R, Sasaki E, Yokoo T, Hiroyama T, Takasugi K, Imoto H.et al. (2006Tal1/Scl gene transduction using a lentiviral vector stimulates highly efficient hematopoietic cell differentiation from common marmoset (Callithrix jacchus) embryonic stem cells Stem Cells 242014–2022. [DOI] [PubMed] [Google Scholar]
- D'Souza SL, Elefanty AG., and, Keller G. SCL/Tal-1 is essential for hematopoietic commitment of the hemangioblast but not for its development. Blood. 2005;105:3862–3870. doi: 10.1182/blood-2004-09-3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes R, Ligero G, Sanchez L, Catalina P, de la Cueva T, Nieto A.et al. (2009Feeder-free maintenance of hESCs in mesenchymal stem cell-conditioned media: distinct requirements for TGF-β and IGF-II Cell Res 19698–709. [DOI] [PubMed] [Google Scholar]
- Brunet de la Grange P, Armstrong F, Duval V, Rouyez MC, Goardon N, Romeo PH.et al. (2006Low SCL/TAL1 expression reveals its major role in adult hematopoietic myeloid progenitors and stem cells Blood 1082998–3004. [DOI] [PubMed] [Google Scholar]
- Menendez P, Catalina P, Rodríguez R, Melen GJ, Bueno C, Arriero M.et al. (2009Bone marrow mesenchymal stem cells from infants with MLL-AF4+ acute leukemia harbor and express the MLL-AF4 fusion gene J Exp Med 2063131–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Real PJ, Cao Y, Wang R, Nikolovska-Coleska Z, Sanz-Ortiz J, Wang S.et al. (2004Breast cancer cells can evade apoptosis-mediated selective killing by a novel small molecule inhibitor of Bcl-2 Cancer Res 647947–7953. [DOI] [PubMed] [Google Scholar]
- Real PJ, Tosello V, Palomero T, Castillo M, Hernando E, de Stanchina E.et al. (2009Gamma-secretase inhibitors reverse glucocorticoid resistance in T cell acute lymphoblastic leukemia Nat Med 1550–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levac K, Menendez P., and, Bhatia M. Intra-bone marrow transplantation facilitates pauci-clonal human hematopoietic repopulation of NOD/SCID/β2m−/− mice. Exp Hematol. 2005;33:1417–1426. doi: 10.1016/j.exphem.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Montes R, Ayllón V, Gutierrez-Aranda I, Prat I, Hernández-Lamas MC, Ponce L.et al. (2011Enforced expression of MLL-AF4 fusion in cord blood CD34+ cells enhances the hematopoietic repopulating cell function and clonogenic potential but is not sufficient to initiate leukemia Blood 1174746–4758. [DOI] [PubMed] [Google Scholar]
- Bueno C, Montes R., and, Menendez P. The ROCK inhibitor Y-27632 negatively affects the expansion/survival of both fresh and cryopreserved cord blood-derived CD34+ hematopoietic progenitor cells: Y-27632 negatively affects the expansion/survival of CD34+HSPCs. Stem Cell Rev. 2010;6:215–223. doi: 10.1007/s12015-010-9118-5. [DOI] [PubMed] [Google Scholar]
- Bueno C, Montes R, Melen GJ, Ramos-Mejia V, Real PJ, Ayllón V.et al. (2012A human ESC model for MLL-AF4 leukemic fusion gene reveals an impaired early hematopoietic-endothelial specification Cell Resepub ahead of print). [DOI] [PMC free article] [PubMed]
- Ji J, Vijayaragavan K, Bosse M, Menendez P, Weisel K., and, Bhatia M. OP9 stroma augments survival of hematopoietic precursors and progenitors during hematopoietic differentiation from human embryonic stem cells. Stem Cells. 2008;26:2485–2495. doi: 10.1634/stemcells.2008-0642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Enforced expression of SCL is compatible with hESC pluripotency.
Histological analysis of teratomas formed from EV and SCL hESCs.
Cell cycle analysis reveals that SCL expression promotes specification rather than enhanced proliferation of hESCs into hematoendothelial progenitors.
Enforced expression of SCL augments hematopoietic specification from H9 hESCs in an EB differentiation model.
Phase-contrast morphology of typical CFU colonies generated from both SCL and EV hematopoietic progenitors.
SCL hematoendothelial progenitors retain the ability to differentiate towards endothelial cell fate.
SCL silencing impairs hematopoietic specification from hESC in an OP9 differentiation model.
List of primer sequences used in the present study.